Alcoholic hepatitis (AH) and Nonalcoholic steatohepatitis (NASH) are two major causes of liver related morbidity and mortality. While AH is a major cause of liver-related hospitalizations, NASH is projected to surpass hepatitis C virus infection as the leading etiology of end stage liver disease requiring liver transplantation 1. NASH is also the leading etiology driving the burden of hepatocellular cancer 2. Despite their obvious public health relevance, there are currently no drugs approved for either condition. There is thus a need to consider the barriers that have contributed to this situation and the potential pathways to overcome such barriers to bring effective therapies for these conditions to afflicted individuals.
A. General principles of drug approval
The core principle that drives drug approval is the demonstration of safety and clinically meaningful benefit for patients when a drug is given for a specific indication 3. Clinically meaningful benefit is broadly defined as an improvement in terms of how patients feel, function or survive following an intervention 3. While theoretically, drugs may be approved on the basis of how they affect functionality or how patients feel, the relative lack of specificity of these findings make it difficult to attribute changes clearly to administration of a drug especially in complex chronic diseases with multiple comorbidities. Therefore, most development efforts have focused on establishing the efficacy of drugs by demonstration of clinical benefit either by hard outcomes or the use of surrogate endpoints.
Mortality is a well-recognized measure of clinical benefit or “hard endpoint” which can be quantified and reflects how a patient survives. However, for many chronic conditions such as NASH, the disease leads to mortality over a course of years or even decades. When considered over such a prolonged period of time, other causes also contribute to mortality further confounding assessment of drug effects on mortality. The logistic and fiscal challenges of performing a large enough study to document an improvement in survival for such chronic conditions represent a barrier for sponsors from engaging in such trials.
In order to deal with the difficulties of long trials designed to demonstrate clinically meaningful benefit, specific mechanisms have been established by the Food and Drug Administration to expedite drug approval while still meeting the regulatory burden of evidence to support efficacy and safety of the drug as required by law as well as ethics. One such pathway involves the use of generally accepted surrogate endpoints. These are endpoints that have been extensively validated to reflect ultimate clinically meaningful benefit. Generally accepted surrogate endpoints can form the basis for full approval of a therapeutic agent. There are currently no such endpoints that meet the evidentiary burden to qualify as a generally accepted endpoint for either AH or NASH.
A second pathway is also known as the Accelerated Approval pathway (subpart H for drugs and subpart E for biologics) and allows a sponsor to apply for approval with trials of relatively shorter duration using a surrogate endpoint(s) that are considered reasonably likely to predict clinical benefit such as a clinical endpoint(s). Reasonably accepted surrogate endpoints differ from generally accepted surrogate endpoints in the amount of supporting literature validating them as reflective of changes in clinically meaningful benefit. They are often measured earlier than irreversible morbidity and mortality (IMM) and reflect an effect on IMM or other clinical benefit. They may be clinical endpoints or biomarkers. The need for biomarker development to identify populations of interest and validate endpoints have been recently reviewed 3. Interested readers are also referred to FDA publications on such endpoints:
See the Guidance for Industry - Expedited Programs for Serious Conditions - Drugs and Biologics, at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm358301.pdf
There is however a caveat when using the accelerated approval pathway and reasonably accepted surrogate endpoints that sponsors must complete a post-marketing trial to demonstrate that the improvement in the surrogate endpoints actually translates in to clinically meaningful benefit to patients.
The issue of surrogate endpoints is of considerable relevance for both AH and NASH because it drives the populations to be studied, the study design itself, endpoint construction and analysis. The acceptance of an endpoint as a generally accepted versus reasonably likely to predict surrogate is largely in the domain of regulatory agencies. Current interactions between academia and regulatory authorities particularly in the area of drug development for liver diseases have been productive and the addition of the Liver Forum (http://www.hivforum.org/projects/drug-development/liver-forum) to promote communication and collaboration between multiple stakeholders especially academia and regulatory agencies has become a powerful catalyst to promote drug development for these unmet medical needs.
B. Integrating regulatory perspectives to drug development for AH
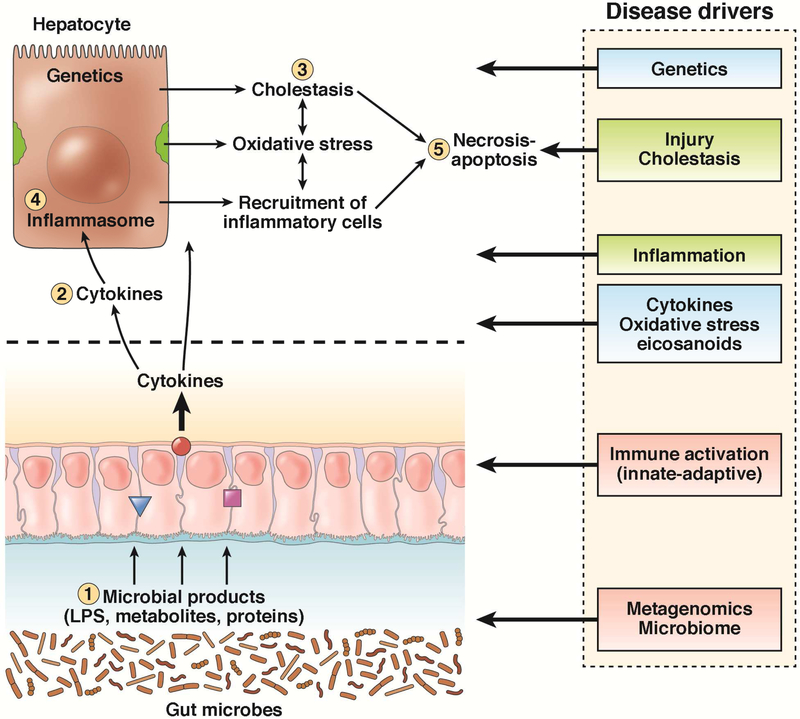
AH covers a clinical spectrum including relatively asymptomatic individuals who engage in risky drinking behavior and have a liver biopsy demonstrating steatohepatitis to severely ill individuals with jaundice, hepatomegaly, neutrophilia and a biopsy showing florid steatohepatitis and cholestasis and profound inflammatory and cellular injury 4-6. A large body of basic scientific literature on AH that have provided a potential plethora of therapeutic targets some of which are currently under evaluation (Figure 1). However, there remain gaps in knowledge some of which are particularly germane to the development of therapeutics for AH 7.
FIGURE 1:
Pathophysiological drivers of alcoholic hepatitis are shown. Several of these are potentially targetable for therapeutics. Alcohol impairs the integrity of the intestinal barrier leading to enhanced endotoxemia and systemic exposure to bacterial products which activate the innate immune system and sensitize hepatic macrophages. Frequently following a bout of binge drinking there is increased endotoxemia and activation of severe hepatic inflammation and cholestasis along with liver injury and cell death. The intestinal barrier function (1), cytokines (2), bile acid-related cholestatic and immune-inflammatory pathways (3), genetics and inflammasome (4), and cell death modifying pathways (5) have been or are under active clinical investigation.
A key first step in formal drug development for AH is to define the condition clearly and determine the specific target population to be studied to gain clarity on what can be reasonably expected from a therapeutic intervention 7. While many patients already have AH superimposed in alcohol-related cirrhosis, others may develop it in pre-cirrhotic stages of alcohol-related liver disease 5. The latter can be associated with variable clinical and laboratory features 8. Indeed typical steatohepatitis due to alcohol can be seen is relatively asymptomatic individuals in an ambulatory care setting. On the other hand, the typical subject who is hospitalized with AH is often jaundiced and has hepatomegaly, neutrophilia and right upper quadrant tenderness. The liver histology in such cases not only has features of steatohepatitis but also has additional findings of cholestasis and more florid inflammation 9. A major barrier for regulatory development is a common nomenclature system that does not clearly separate these rather different phenotypes let alone identify intermediate phenotypes. Furthermore, a lack of high quality longitudinal data linking clinically meaningful patient-centered outcomes to the initial clinical-histological-molecular-genetic subpopulation adds to potential heterogeneity in terms of disease severity, disease mechanisms and response to therapy making it more difficult to cleanly demonstrate clinically meaningful benefit in this population.
Current therapeutic approaches, largely in academic centers, have focused on the sickest patients with AH using the Maddrey index and MELD score 10. While these scores identify the sickest individuals, it is not always feasible to exclude decompensated cirrhosis with sepsis without a liver biopsy. There are several challenges with performing these in this sick population which makes it more difficult to engage in large-scale trials. Furthermore, the emphasis on these scores have been a disservice to those with lesser severity of disease who are often allowed to progress to more florid disease. Better delineation of the varying clinical-histological phenotypes and the underlying genetic and physiological mechanisms driving disease severity and progression is now required to develop focused trials of specific interventions in specific populations with focused specific objectives that are linked to disease biology and the target of intervention. These will also open new therapeutic opportunities for those with AH and a Maddrey index of 20-31, development of primary and secondary prophylaxis of AH and the use of anti-fibrotic agents to reduce severe fibrosis in those who have recovered from acute AH.
Both behavior and physiology determine the development of AH and its ultimate outcome. While sepsis and inflammation are critical determinants of short-term outcomes, the long-term outcomes are linked to continued alcohol consumption 11, 12. Thus, drugs targeting inflammation alone without additional interventions to reduce long-term recidivism are unlikely to have longterm benefit. The regulatory implications of this is that the study duration, choice of endpoint and when it is measured must be related to the mechanism of action of the intervention. The lack of a regulatory pathway to evaluate integrated therapeutic approaches remains a major barrier to drug development for AH. It is hoped that greater collaboration between regulatory agencies, academia and other stakeholders in the future will allow these issues to be resolved. Clinical trials for AH must also include assessment of drug safety. While general guidance for assessment of drug safety is important, a critical unmet need at this time is the development of minimal evidentiary thresholds for stopping further therapeutic development based on safety concerns in early phase trials for AH. Also, there is no formal guidance of the types of safety data and the time course over which it has to be studied when the intestinal microbiota are targeted for AH.
C. Integrating regulatory science in to drug development for NASH
In contrast to AH, there has been considerable recent activity in developing pathways for drug approval for NASH. This was catalyzed by a joint workshop between the American Association for Study of Liver Diseases and the FDA the outcomes of which have been published 3. Briefly, two broad categories of patients with NASH with varying treatment objectives are being targeted for therapeutic development. These include those with established cirrhosis and those with precirrhotic stages of NASH.
In those with cirrhosis, there is an approximately 4% annual risk of decompensation 13. Individuals who are otherwise clinically compensated but have a MELD score > 10 or have a hepatic venous pressure gradient > 10 mm Hg have a higher risk of decompensation up to 20% within 2 years and can be targeted for therapeutics in this context 14, 15. Clinically meaningful benefit can be evaluated in such patients from mortality or by rates of hepatic decompensation which is characterized by onset of ascites or variceal hemorrhage or encephalopathy. Drugs targeting oncogenesis will need to demonstrate a decrease in hepatocellular cancers in this high risk population. Current literature suggests that while the total number of subjects at risk due to NASH are higher than those with hepatitis C, the rates of cancer development in NASH are somewhat lower than in hepatitis C 2, 16. There is also a possibility of a subpart H accelerated approval for individuals with compensated cirrhosis based on the use of reasonably accepted surrogate endpoints such as hepatic venous pressure gradient rise from below 10 mm Hg to above 10 mm Hg but would necessitate a post-marketing trial to demonstrate clinically meaningful benefit.
In those with pre-cirrhotic stages of NASH, there is controversy about the best populations to be targeted and how to demonstrate clinically meaningful benefit given that the natural course of the disease progresses over decades and is marked by both progression and spontaneous regression. Given these difficulties, progression to cirrhosis is being considered as a generally accepted endpoint for full approval. However, even the progression to cirrhosis takes many years and many trials are using the subpart H pathway with a histology based endpoint as a reasonably accepted surrogate endpoint for approval. Composite endpoints of resolution of steatohepatitis without any worsening of fibrosis or one point or more improvement in fibrosis score (NASH/CRN fibrosis scale) with no worsening of steatohepatitis, have been suggested as minimal requirements for surrogate endpoints in NASH trials in precirrhostic populations3. Recently, the FDA has agreed to the use of a co-primary endpoint consisting of the two composite endpoints listed above for a pivotal trial for obeticholic acid for NASH (from NCT # 02548351, clinical trials.gov).
Current literature indicates that fibrosis stage is a robust biomarker of the risk of disease progression to cirrhosis and mortality 17. Therefore, subjects with NASH and some degree of fibrosis with enrichment of the population with those with bridging fibrosis is a strategy being employed in some advanced level clinical trials. This will permit both the shorter term assessment of liver histology for subpart H approval but also set the stage for long-term extension trials to demonstrate decreased risk of progression to cirrhosis as required for approval via the subpart H pathway.
D. Future trends in regulatory science development for AH and NASH
There is considerable momentum driving drug development for NASH. It is related to the success of recent clinical trials, interest by the pharmaceutical sector and a serious engagement between the FDA, European Medical Agency, academic and industry partners to identify barriers and to develop solutions to overcome such barriers via The Liver Forum. It is anticipated that current efforts to harmonize methods and case definitions across trials will allow pooling of placebo data to develop a robust model of disease progression across multiple stages of disease and in the presence of varying combinations of comorbidities that may impact progression. When this is accomplished, the thresholds for improvement that would obviate the need for long placebo controlled trials could be defined. Also, growth in biomarker science is also anticipated to further speed up development by allowing assessment of both efficacy and safety in shorter periods of time. Much of the advances in regulatory science can also be applied to AH although the challenges there are different than those in NASH. The recent efforts by the National Institutes of Addiction and Alcohol Abuse to address these issues in partnership with the FDA is very encouraging and will hopefully allow effective therapies for AH to also be brought to patients within the next decade.
TABLE 1:
SOME KEY BARRIERS TO DRUG DEVELOPMENT FOR AH AND NASH AND POTENTIAL SOLUTIONS
| Barrier | Alcoholic Hepatitis | NASH | Solution |
|---|---|---|---|
| General Barriers | • Social stigma • Lack of awareness • Lack of clear regulatory pathway |
• Lack of awareness | Education |
| Populations | • Lack of consensus Related to definitions and nomenclature • Heterogeneity in terms of clinical-histological-molecular phenotypes • Lack of data linking well definited phenotypes to specific outcomes |
• Heterogeneity of populations with respect to risk of progression and natural history of precirrhotic versus cirrhotic stages of the disease | • Alcoholic Hepatitis: collaborative efforts between NIAAA, academia and regulatory agencies. • NASH: distinct trial design and endpoints for those with precirrhotic vs cirrhotic NASH |
| Endpoints | • Endpoints not cleanly linked to drivers of outcomes • Timing of outcome measurement affects assessment of drug interventions • Lack of validated endpoints other than mortality |
• Need for a liver biopsy to assess short-term outcomes • Lack of validated surrogate endpoints |
• Multi-stakeholder collaboration to validate clinical endpoints and natural course of disease for specific populations of subjects with alcoholic liver disease or NASH |
| Safety | • DILI assessment is unclear • Lack of clarity on safety assessment related to intestinal microbiome |
• DILI assessment is unclear • Lack of clarity on safety assessment related to intestinal microbiome |
• Collaborative multi-stakeholder effort to generate the evidence base to support guidance on safety assessment • Ongoing FDA efforts to generate guidance |
| Biomarkers | • Gaps in knowledge related to distinct clinical-histological-molecular subpopulations | • Lack of point of care assessment tool • Lack of validated PRO |
• Large cohort and case control studies accompanied by biomarker assessment • Assessment of novel biomarkers in circulation, urine, breath or stool |
Acknowledgement:
The authors would like to express their gratitude to Dr. Lara Dimick from the Food and Drug Administration of the US Government for providing critical review of this document.
Funding: This manuscript was supported in part by a grant RO1 DK 10596 and T32 07150.
Biographies

Dr. Sanyal has stock options in Genfit and is the President of Sanyal Biotechnologies. He has served as a consultant to Merck, Lilly, Bristol Myers, Novartis, Abbvie, Astra Zeneca, Gilead, Intercept, Genfit, Zafgen, Enanta, Immuron, Galmed, Nitto Denko, Durect, Ikaria and Salix. His institution receives grant support from Intercept, Merck, Astra Zeneca, Bristol Myers and Gilead.

Veronica Miller is an employee of the Forum for Collaborative HIV Research, which receives unrestricted educational or research grants from : Abbot; Abbvie; Achilion; Alere; Astellas; Astra-Zeneca; Biogen; BioRAD, Boeheringer Ingelheim, Bristol-Myers Squib; Cell Medica; Chimerix; Cocrystal Pharma, Inc.; Covance; DDL Diagnostics; DeuteRx; DiaPharma; DS Biopharma; Echosens; Exalenz; Fibrogen; Fractyl; Galectin Therapeutics; Genentech; Genfit; Gilead Sciences; GlaxoSmithKline; Icon; Illumina; Immuron; Intercept; Janssen; Kaiser Permanente; LabCorp; Lilly; Madrigal Pharmaceuticals; Mallinckrodt Pharmaceuticals; MediciNova; Medivir; Merck Laboratories; Microbiotix; Monogram; Mylan; NGM Biopharmaceuticals; Nimbus Therapeutics; Nitto Denko Technical Corp; Novartis; Novo Nordisk Inc.; Nusirt; Orasure; OWL Metabolomics; Pacific Biosciences; Pfizer; PPD Inc.; Presidio; Qiagen; Quest; Quintiles; Raptor Pharmaceuticals; Resonance Health; Roche Molecular Systems; Rooivant Sciences; RuiYi; SeraCare; Schinazi Family Foundation; Shire; Takeda; Tobira; ViiV Healthcare; VLVBio; and Zafgen
Veronica Miller has served as an advisory board member to ViiV Healthcare
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Arun J. Sanyal, MCV Box 980341, Richmond, VA 23298-0341, Phone: (804) 828 6314, Fax: (804) 929 2992, arun.sanyal@vcuhealth.org, Dept. of Medicine, VCU School of Medicine, Richmond, VA 23298.
Veronica Miller, 1608 Rhode Island Ave, Suite 21, Washington DC 20036, Phone: 202-974-6290, veronicam@berkeley.edu, Univ. California School of Public Health, Berkeley, CA..
REFERENCES
- 1.Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011;141:1249–53. [DOI] [PubMed] [Google Scholar]
- 2.Sanyal A, Poklepovic A, Moyneur E, et al. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin 2010;26:2183–91. [DOI] [PubMed] [Google Scholar]
- 3.Sanyal AJ, Friedman SL, McCullough AJ, et al. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology 2015;61:1392–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galambos JT. Natural history of alcoholic hepatitis. 3. Histological changes. Gastroenterology 1972;63:1026–35. [PubMed] [Google Scholar]
- 5.Lischner MW, Alexander JF, Galambos JT. Natural history of alcoholic hepatitis. I. The acute disease. Am J Dig Dis 1971;16:481–94. [DOI] [PubMed] [Google Scholar]
- 6.Casanova J, Bataller R. Alcoholic hepatitis: Prognosis and treatment. Gastroenterol Hepatol 2014;37:262–8. [DOI] [PubMed] [Google Scholar]
- 7.Sanyal AJ, Gao B, Szabo G. Gaps in Knowledge and Research Priorities for Alcoholic Hepatitis. Gastroenterology 2015;149:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singal AK, Kamath PS, Gores GJ, et al. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol 2014;12:555–64; quiz e31-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altamirano J, Miquel R, Katoonizadeh A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology 2014;146:1231–9 e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005;41:353–8. [DOI] [PubMed] [Google Scholar]
- 11.Louvet A, Wartel F, Castel H, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology 2009;137:541–8. [DOI] [PubMed] [Google Scholar]
- 12.Damgaard Sandahl T Alcoholic hepatitis. Dan Med J 2014;61:B4755. [PubMed] [Google Scholar]
- 13.Sanyal AJ, Banas C, Sargeant C, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology 2006;43:682–9. [DOI] [PubMed] [Google Scholar]
- 14.Ripoll C, Groszmann R, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 2007;133:481–8. [DOI] [PubMed] [Google Scholar]
- 15.Ripoll C, Lastra P, Rincon D, et al. Comparison of MELD, HVPG, and their changes to predict clinically relevant endpoints in cirrhosis. Scand J Gastroenterol 2012;47:204–11. [DOI] [PubMed] [Google Scholar]
- 16.Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972–8. [DOI] [PubMed] [Google Scholar]
- 17.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015;149:389–97 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]