Abstract
Background and Purpose
The severity of aphasic impairment in chronic stroke survivors is typically thought to be stable by 6 months postonset. However, a recent study showed that stroke survivors with aphasia experience language improvement or decline in the chronic phase, years beyond onset. Little is known about why some individuals improve whereas others remain stable or decline. Additionally, no study has tracked changes in aphasia from assessments completed at multiple time points across many years. The current study offers a comprehensive analysis of potential predictive demographic and health information to determine which factors predict dynamic changes in aphasia severity in chronic stroke.
Methods
Individuals in the chronic stage of a single-event, left-hemisphere ischemic stroke were identified from an archival database and included for study (N = 39). Participants were included if they had undergone 2 or more standardized language assessments acquired at time points at least 6 months apart, with the 1st assessment at least 6 months postinjury. A linear mixed-effects model was used to determine the impact of treatment and a variety of demographic and health factors on language change.
Results
Over time, half of the participants improved (51%), whereas approximately a quarter (26%) decreased, and a quarter (23%) remained stable. A greater number of aphasia treatment hours significantly predicted language improvement (p = .03), whereas older stroke age was associated with long-term decline (p = .04). Two interactions were found to be significant in predicting improvement in individuals with diabetes: Increased exercise and younger age at stroke were significant in predicting outcomes (p < .05).
Conclusions
Factors that significantly influence language recovery in chronic aphasia include stroke age and receiving aphasia treatment. For those with diabetes, increased exercise was shown to improve outcomes. Results from this study offer clinicians greater insight into the influence of patient factors on long-term recovery from stroke aphasia while suggesting a potential adjunct to language therapy: exercise.
Supplemental Material
Convention assumes that language deficits in stroke aphasia remain relatively stable after the initial phase of spontaneous recovery, particularly about 6 months post–stroke onset, thus entering the chronic stage of aphasia (Allen, Mehta, Mcclure, & Teasell, 2012; Breitenstein et al., 2017; Robey, 1998). This notion has clear clinical implications, with some advocating that treatment should focus on the early stages where plasticity appears most active (Krakauer & Marshall, 2015). Growing evidence, however, suggests that persons in the chronic stage of aphasia may be more fluid in recovery than previously thought (Holland, Fromm, Forbes, & MacWhinney, 2017; Hope et al., 2017). Treatment studies involving intensive speech-language therapy (e.g., Basso & Macis, 2011; Breitenstein et al., 2017; Fridriksson, Richardson, Fillmore, & Cai, 2012; Moss & Nicholas, 2006; Pulvermüller et al., 2001; Smania et al., 2010), involvement with interactive technology (Aftonomos, Steele, & Wertz, 1997), neural stimulation (Fridriksson, Richardson, Baker, & Rorden, 2011; Turkeltaub, 2015), and changes in neural organization (Elkana, Frost, Kramer, Ben-Bashat, & Schweiger, 2013; Hope et al., 2017; Naeser et al., 1998) are among the most persuasive evidence for recovery in the chronic stage of aphasia. Although promising evidence has shown that language abilities in individuals with chronic aphasia can improve with language treatment, transcranial cortical stimulation, and participation in intervention-based research studies, it is still relatively uninvestigated whether there are certain predispositions to language recovery, decline, or stability.
Previous small group and case studies investigating language improvement in chronic aphasia have found that speech-language treatment results in language improvement for some individuals (Aftonomos, Appelbaum, & Steele, 1999; Robey, 1998). Few studies exist that investigate long-term aphasia change with larger samples. Basso, Capitani, and Vignolo (1979) reported that rehabilitation and time between aphasia onset and first examination were significantly related to long-term aphasia recovery in a sample of 281 persons with aphasia (PWAs). Similarly, in a Phase III trial that included a large sample of individuals with chronic aphasia (N = 156), Breitenstein et al. (2017) found that participants benefited significantly from 3 weeks of intensive language therapy. Those with chronic aphasia enhanced verbal communication in everyday life scenarios (using the Amsterdam–Nijmegen Everyday Language Test) after participating in intensive speech and language therapy (Breitenstein et al., 2017). Although aphasia treatment studies suggest chronic aphasia recovery is more dynamic in nature than previously thought, research employing relatively large sample sizes and detailed health and demographic data have been few and far between. One exception was an epidemiological study published by Plowman, Hentz, and Ellis (2012), who related potential prognostic factors (sex, handedness, education, socioeconomic status [SES], and age) to language recovery among patients who had a stroke in both the chronic and acute stages. Although the results showed no significant effect regarding the relationship between demographic factors and recovery, stroke-related factors such as lesion size, lesion location, and initial aphasia severity appeared to significantly influence long-term outcomes (Plowman et al., 2012). However, this study did not include health-related factors that could shed light on possible predispositions to successful language recovery in the chronic stage. Price, Seghier, and Leff (2010) have relied on a comprehensive data set including brain imaging, aphasia testing, basic demographic information, stroke status, and other comorbidities to predict long-term aphasia recovery (Predicting Language Outcome and Recovery After Stroke). Using the Predicting Language Outcome and Recovery After Stroke data set, Hope, Seghier, Leff, and Price (2013) predicted speech production skills in 270 patients who had a stroke. Hope and colleagues report that demographic information alone (handedness, gender, age at stroke, and time since stroke occurred) did not produce a statistically significant model of predicting chronic aphasia recovery. More recently, in a study that aimed to evaluate long-term changes in chronic (at least 6 months poststroke) aphasia, Holland et al. (2017) evaluated a group of participants who had completed two assessments of aphasia at least 1 year apart. Using the Western Aphasia Battery–Revised (WAB-R) created by Kertesz (2007), participants were divided into three groups based on whether their aphasia severity improved, declined, or remained stable between the initial and final assessment sessions. Aphasia severity was defined by the WAB–Aphasia Quotient (WAB-AQ), a 100-point scale on the WAB-R, which encompasses several submeasures of language ability, including spontaneous speech, speech repetition, naming, and verbal comprehension. Overall, most participants showed improvement on WAB-AQ, and it was reported that there were no statistically significant correlations found between age and WAB-AQ change score (r = .2; Holland et al., 2017). Holland and colleagues did not address additional demographic and health-related data, so it is unclear if such factors lead to language improvement.
As far as we can tell, no study has evaluated changes in chronic aphasia severity using language testing at more than two time points. In addition to evaluating aphasia testing at multiple time points, this study also evaluated potential prognostic factors to determine long-term recovery from aphasia.
Method
Participants
Participant data were extracted from an archival database collected at the Center for the Study of Aphasia Recovery at the University of South Carolina and the Medical University of South Carolina. Written informed consent was obtained from all participants when admitted into initial and subsequent studies. These data represent a convenience sample and are based on recruitment through advertising, referrals from neurologists and speech pathologists, and active recruitment within support/aphasia groups. Available data included demographic information and a variety of behavioral test scores collected at varying poststroke intervals. WAB administrations typically coincided with baseline testing for studies in which the PWA participated; therefore, some of the participants who participated in more than one study were tested at multiple time points (M = 2.5 times). For this reason, and because the WAB is a common clinical measure, the WAB was used here as the behavioral measure of interest. See Supplemental Material S1 for individual participant data.
This study included individuals in the chronic stage of stroke at the time of both the initial assessment and subsequent assessments who (a) had two or more WAB assessments acquired at least 6 months apart; (b) had no accompanying neuropsychological disorders (self-report); (c) had a single-event, left-hemisphere ischemic stroke; and (d) were less than 85 years of age. Thirty-nine individuals (13 women, 26 men) with a mean age of 54.7 years (SD = 9.8) at the time of stroke met the inclusion criteria. At initial assessment, the group had a mean WAB-AQ of 53.5 (range = 5.6–88.8, SD = 21.5) and a mean time (in months) poststroke of 38.0 (range = 6.0–224.0, SD = 47.2). The average number of months between assessments was 27.2 (range = 6–86, SD = 20.3). A summary of participant data is presented in Table 1. Twenty-six participants in this sample completed two WABs, six participants completed three WABs, six participants completed four WABs, and one participant completed five WABs. In total, 99 WAB assessments met the inclusion criteria and were used in analysis.
Table 1.
Demographic information of participants.
| Demographic variables | Overall (N = 39) | Improving (n = 20) | Stable (n = 9) | Declining (n = 10) |
|---|---|---|---|---|
| Age at stroke (years) | ||||
| M | 54.7 | 53.3 | 52.6 | 59.5 |
| SD | 9.8 | 8.9 | 12.0 | 8.8 |
| Range | 39.0–71.0 | 39.0–69.0 | 39.0–68.0 | 46.0–71.0 |
| Sex, n (%) | ||||
| Female | 13 (33.3) | 6 (30) | 6 (66.7) | 1 (10) |
| Male | 26 (66.7) | 14 (70) | 3 (33.3) | 9 (90) |
| Diabetes, n (%) | ||||
| Presence | 10 (25.6) | 5 (25) | 2 (22.2) | 3 (30) |
| Absence | 29 (74.4) | 15 (75) | 7 (77.8) | 7 (70) |
| Education (years) | ||||
| M | 15.0 | 15.2 | 14.4 | 15.1 |
| SD | 2.8 | 2.6 | 2.5 | 3.6 |
| Range | 10.0–22.0 | 10.0–20.0 | 12.0–18.0 | 12.0–22.0 |
| Time poststroke a (months) | ||||
| M | 38.0 | 21.3 | 56.4 | 54.6 |
| SD | 47.2 | 16.5 | 67.5 | 59.5 |
| Range | 6.0–224.0 | 6.0–59.0 | 7.0–224.0 | 7.0–201.0 |
| No. of WABs | ||||
| M | 2.5 | 2.9 | 2.2 | 2.4 |
| SD | 0.9 | 1.0 | 0.4 | 0.8 |
| Range | 2.0–5.0 | 2.0–5.0 | 2.0–3.0 | 2.0–4.0 |
| WAB-AQ a | ||||
| M | 53.5 | 46.4 | 63.2 | 58.9 |
| SD | 21.5 | 21.8 | 23.1 | 15.3 |
| Range | 5.6–88.8 | 5.6–88.8 | 20.9–87.6 | 25.2–73.5 |
Note. WAB = Western Aphasia Battery; WAB-AQ = Western Aphasia Battery–Aphasia Quotient.
At initial assessment.
Behavioral Assessment
Because participant recruitment to populate our database began in 2006, 16 of the 99 assessments were administered using the original WAB (Kertesz, 1982), whereas the remaining 83 assessments relied on the WAB-R (revised in 2007; Kertesz, 2007). Both versions of the WAB include the same behavioral measures, including spontaneous speech, auditory comprehension, speech repetition, and naming. Additionally, both versions of the WAB estimate overall aphasia severity (AQ) by totaling the four aforementioned subscores (spontaneous speech, auditory verbal comprehension, speech repetition, and naming and word finding). Participant AQ scores below 93.8 are classified as having aphasia, and aphasia subtypes are determined based on the patterns of subscores. Table 2 provides details of WAB assessment for all participants. All language assessments were administered by or under the supervision of an American Speech-Language-Hearing Association certified speech-language pathologist with extensive experience working with individuals with aphasia. Video recordings of assessment administration were collected for offline scoring in order for the certified speech-language pathologist to verify WAB scoring. None of the WAB assessments was used as an outcome measure in treatment studies but was merely used to classify aphasia type and measure severity at the beginning of individual studies. Therefore, changes in severity measured on the WAB were not reflective of immediate posttreatment effects as the mean time between repeated WAB assessment after the enrollment in any treatment study was 33 months (SD = 20.18, range = 6–18 months). The focus of this study was on changes in overall aphasia severity over time; therefore, WAB-AQ scores were used in the outcome analyses.
Table 2.
Western Aphasia Battery–Aphasia Quotient (AQ) data.
| WAB data | Overall (N = 39) | Improving (n = 20) | Stable (n = 9) | Declining (n = 10) |
|---|---|---|---|---|
| AQ: initial | ||||
| M | 53.5 | 46.4 | 63.2 | 58.9 |
| SD | 21.5 | 21.8 | 23.1 | 15.3 |
| Range | 5.6–88.8 | 5.6–88.8 | 20.9–87.6 | 25.2–73.5 |
| AQ: final | ||||
| M | 56.6 | 56.6 | 63.4 | 50.3 |
| SD | 20.7 | 21.6 | 23.3 | 15.8 |
| Range | 20.0–97.6 | 20.1–97.6 | 20.7–88.6 | 20.5–68.9 |
| AQ: change | ||||
| M | 3.1 | 10.2 | 0.2 | −8.6 |
| SD | 9.3 | 5.7 | 0.9 | 5.0 |
| Range | −19.8 to 23.0 | 3.2 to 23.0 | −1.6 to 1.3 | −19.8 to −3.8 |
| Fluency a | ||||
| Fluent | 17 | 8 | 4 | 5 |
| Nonfluent | 22 | 12 | 5 | 5 |
| Aphasia types a | ||||
| Anomic | 5 | 3 | 2 | NA |
| Broca's | 20 | 9 | 7 | 4 |
| Conduction | 6 | 3 | NA | 3 |
| Global | 3 | 2 | NA | 1 |
| Transcortical sensory | 2 | NA | NA | 2 |
| Wernicke's | 3 | 3 | NA | NA |
At initial assessment.
Demographic Information
To accompany the aphasia severity assessments, our database included comprehensive demographic information on each participant. In the current analysis, the variables of interest were chosen based on previous literature investigating demographic and health variables as pertaining to aphasia severity and outcomes: age at stroke (Breitenstein et al., 2017; Laska, Hellblom, Murray, Kahan, & Von Arbin, 2001), education level (Hillis & Tippett, 2014), aphasia treatment hours (Fridriksson, Richardson, et al., 2012; Moss & Nicholas, 2006; Pulvermüller et al., 2001; Smania et al., 2010), number of days exercised per week poststroke (Harnish et al., 2018), presence of diabetes (Megherbi et al., 2003), use of antidepressants (Hillis & Tippett, 2014), and SES (Connor, Obler, Tocco, Fitzpatrick, & Albert, 2001). The number of treatment hours was quantified by totaling the number of hours the PWA was involved in treatment research studies at either the Aphasia Lab at the University of South Carolina or the Medical University of South Carolina. Treatment hours that a participant may have received include group-based intensive language action therapy (Pulvermüller et al., 2001), speech entrainment treatment (Fridriksson, Basilakos, Hickok, Bonilha, & Rorden, 2015; Fridriksson, Hubbard, et al., 2012), cueing hierarchy (Fridriksson, Richardson, et al., 2012), computerized naming treatment (Fridriksson et al., 2009, 2018), phonological-based treatment, or semantic-based treatment. The total treatment hours do not include aphasia treatment hours a person may have completed in other settings (i.e., inpatient or outpatient rehabilitation programs). Although chronic patients typically do not receive aphasia treatment in traditional rehabilitation settings, the number of aphasia treatment hours included here is likely an underestimate of the overall number of treatment hours completed by participants.
Participants were only included in this analysis if the previously listed variables of interest had been provided (i.e., there were no missing data points).
Statistical Analyses
To accommodate the variation between and within subjects, linear mixed-effects models (LMEs) were used to predict changes in WAB-AQ based on demographic information: age at stroke onset, age at WAB testing, sex, education, time poststroke of WAB testing (in months), fluent versus nonfluent aphasia type, days of exercise per week poststroke, use of antidepressants, presence of diabetes, and number of hours of participation in aphasia treatment studies. Information on presence of diabetes, years of education, and days exercised poststroke were derived from a demographic questionnaire completed by participants at the time of initial assessment. Speech fluency (i.e., fluent, nonfluent) was determined by the type of aphasia an individual was classified as having at the initial assessment. For statistical analyses of WAB-AQ scores over time, p values < .05 were considered significant. All statistical analyses were done in statistical software R (R Core Team, 2017). Linear mixed models were applied using R package NLME (Pinheiro, Bates, DebRoy, Sarkar, & R Core Team, 2017), and all figures were made using package GGPLOT2 (Wickham, 2009).
Results
Data detailing WAB assessments are included in Table 2. The mean time postonset at initial WAB administration was 3.2 years, which is well within what is typically considered the chronic stage of aphasia. To better estimate language change, each participant was assigned a group based on the WAB-SEM (2.5 points). 1 Similar to Holland et al. (2017), we rounded up and used ±3 points to determine improvement (+3 points), stability (−3 < x < 3), and decline (−3 points). The estimated test–retest reliability of the WAB-R, which was reported in the WAB-R manual (Kertesz, 2007) for 35 individuals with chronic aphasia (initial assessment mean of 2.05 years poststroke and final assessment at 3.91 years poststroke on average), indicates high test–retest reliability (r = .99), with a mean AQ change between tests of 0.12 AQ points. This mean change is vastly less than the WAB-SEM that we adopted as a metric of change.
Tables 1 and 2 include participant demographic information for the three subgroups (improvers, decliners, and those who remained stable) as well as the group as a whole. Summary statistics for WAB-AQ at baseline were calculated between the whole group and subgroups using two-tailed paired t tests to determine if there were any statistically significant differences between the groups. Results showed that there were no statistically significant differences for AQ at baseline between improvers and decliners (p = .08), between stable and decliners (p = .65), and between stable and improvers (p = .09). In addition, there were no statistical differences for age of stroke between improvers and decliners (p = .08), stable and decliners (p = .88), and stable and improvers (p = .18). Although these group differences are not statistically significant, results should be interpreted with caution until replication with a larger sample of participants.
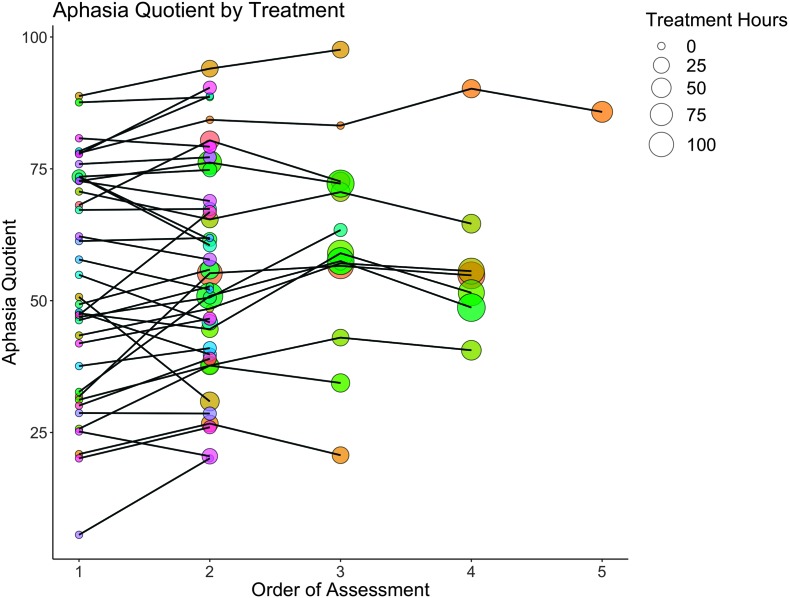
Figure 1 provides a graphical display showing the WAB-AQ by the number of aphasia treatment hours (indicated by the size of the circle) that each participant (indicated by different colors) completed at the time of their WAB assessment (x-axis). All but one participant completed their first WAB before the initiation of aphasia treatment administered in the chronic phase.
Figure 1.
Spaghetti plot of all included participants' aphasia quotient (AQ; y-axis) as a function of treatment. The x-axis depicts the order of Western Aphasia Battery assessment. Point size is indicative of treatment hours, where larger points indicate more treatment hours (see inset legend). Colors are used to distinguish between different participants.
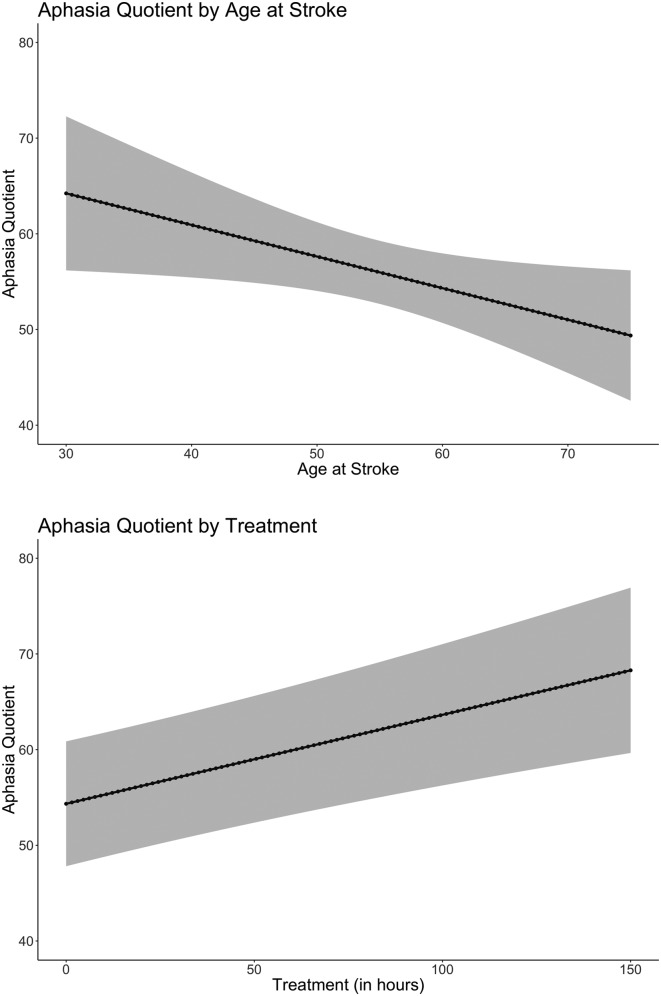
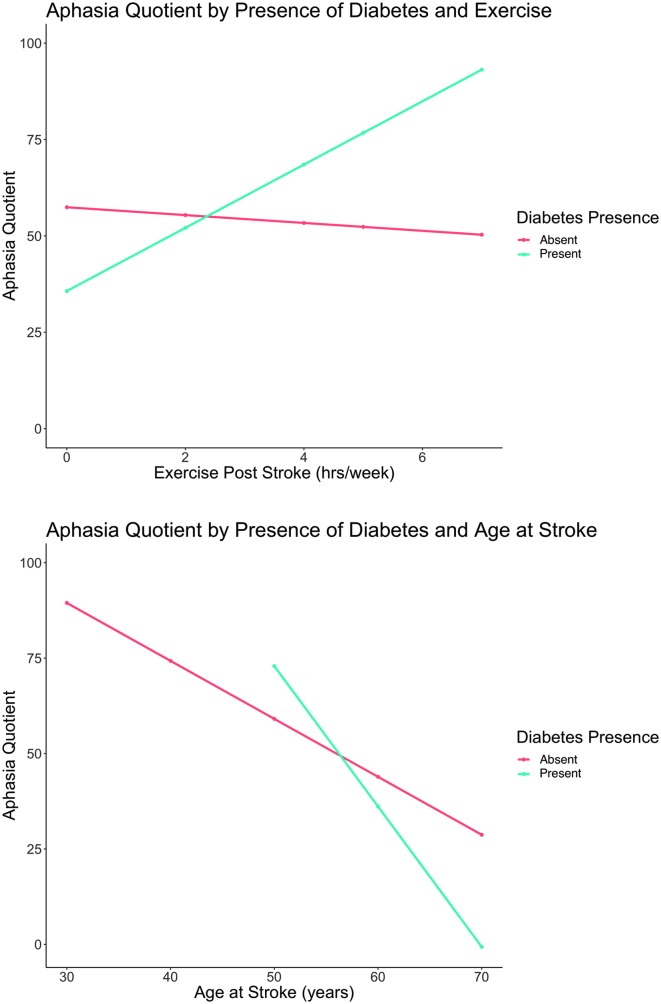
Table 3 provides statistics for significant effects for the LME results. Two main effects, illustrated in Figure 2, were statistically significant: age at stroke (p = .04) and cumulative number of aphasia treatment hours (p = .03). The results reveal that, for every year increase in age at stroke, WAB-AQ decreased by 1.51 points, and for every additional hour of aphasia treatment, WAB-AQ increased 0.07 points on average. Two factor interactions (illustrated in Figure 3) reached statistical significance: the interaction between exercise poststroke and presence of diabetes and the interaction between age at stroke and presence of diabetes. For individuals with diabetes, results suggest that, for every extra day one exercises per week, WAB-AQ is expected to go up, on average, by approximately 8.16 points. The second significant interaction suggests that, for every year a person with diabetes gets older, WAB-AQ is expected to decrease by approximately 3.60 points.
Table 3.
Mixed linear effects model including all demographic factors.
| Demographic factors | Value | SE | df | t | p |
|---|---|---|---|---|---|
| (Intercept) | 50.21 | 30.77 | 53 | 1.63 | .12 |
| Age at stroke | −1.51 | 0.72 | 53 | −2.10 | .04* |
| Exercise poststroke | −1.34 | 1.73 | 32 | −0.78 | .44 |
| Sex (female) | 0.83 | 30.39 | 32 | 0.03 | .98 |
| Age at WAB | 1.72 | 0.89 | 53 | 1.93 | .06 |
| Diabetes (present) | 89.09 | 48.26 | 32 | 1.85 | .07 |
| Time poststroke of WAB | −0.19 | 0.10 | 53 | −1.91 | .06 |
| Treatment cumulative | 0.07 | 0.03 | 53 | 2.22 | .03* |
| Fluency (fluent) | 3.44 | 3.61 | 53 | 0.95 | .35 |
| Months between test | 0.05 | 0.08 | 53 | 0.67 | .51 |
| Education | −0.28 | 1.17 | 53 | −0.24 | .81 |
| Sex (female):age at WAB | 0.00 | 0.50 | 53 | 0.00 | 1.00 |
| Exercise poststroke:diabetes (present) | 9.51 | 3.76 | 32 | 2.53 | .02* |
| Age at stroke:diabetes (present) | −2.09 | 1.01 | 32 | −2.08 | .05* |
Note. WAB = Western Aphasia Battery.
p < .05.
Figure 2.
(Top) Main effect of age at stroke by aphasia quotient with 95% confidence intervals (p = .04). (Bottom) Main effect of estimated treatment by aphasia quotient with 95% confidence intervals (p = .03).
Figure 3.
(Top) Interaction between diabetes presence and days exercised per week (p = .02). (Bottom) Interaction between diabetes presence and age at stroke (p = .05). yrs = years.
Although direct information on SES was not available in our database, an estimate of SES was calculated based on a U.S. Census Bureau method from González-Fernández, Christian, Davis, and Hillis (2013). In this post hoc analysis, mean household income was not correlated with change between the first and last WAB assessments (r = .20, p = .22), nor was it a significant predictor when entered into an LME model (F = −0.00003, p = .83); therefore, this factor was not included in our final model.
Discussion
In this study, 20 of the 39 participants showed improvements on WAB-AQ by more than 3 points, providing further evidence that many individuals experience aphasia recovery in the chronic phase of stroke. By using multiple WABs collected in the chronic stage, we were able to investigate the effects of demographic factors on language recovery after the effects of early spontaneous recovery have run their course.
Although there are no studies that have investigated test–retest reliability of the WAB across greater than two time points, test–retest reliability of the WAB has been shown to be excellent across a variety of intervals between two administrations assessments (Kertesz & McCabe, 1977; Shewan & Kertesz, 1980). In a study investigating test–retest reliability in 22 PWAs in the chronic stage of recovery (Pedersen, Vinter, & Olsen, 2001), Kertesz and McCabe (1977) reported excellent within-subject variability using Pearson r correlation (r = .99). Similarly, Shewan and Kertesz (1980) reported WAB test–retest reliability across all subtests and AQ as having a Pearson r correlation of .88 or higher (WAB-AQ Pearson r = .968) in a sample of 38 persons with chronic aphasia with time interval between assessment anywhere from 6 months to 6.5 years apart (M = 12–23 months). Finally, in a study by Pederson et al. (2001), test–retest reliability of the WAB in Pedersen with chronic aphasia was reported to have a Pearson r correlation of .96 with assessment interval at 3.5 months, with no significant change in WAB scores between the two time points. Nevertheless, it is possible that practice effects contributed slightly to changes in AQ for those who had greater than two evaluations.
Age-Related Changes
Based on previous literature, it is unclear which demographic factors are reliable predictors of aphasia recovery (Breitenstein et al., 2017; Harnish et al., 2018; Hillis & Tippett, 2014; Laska et al., 2001; Megherbi et al., 2003). Language changes in healthy aging are heavily debated, and despite rapid, multiple computations required, core aspects of speech comprehension are left relatively preserved as individuals age (Shafto & Tyler, 2014). However, older adults have been reported to have worse accuracy on a comprehension task compared to younger adults when speech is rapid or presented in noise (Tun, 1998). Age differences in healthy older and younger adults are smaller when words appear in context (Thornton & Light, 2006; Tun, 1998). Healthy older adults have also been reported to perform similarly to their younger counterparts on speech comprehension tasks, suggesting that task difficulty and the cognitive processes the task might recruit are reasons for conflicting results in the literature (DeDe, Caplan, Kemtes, & Waters, 2004; Tyler, Cobb, & Graham, 1992; Tyler, Wright, Randall, Marslen-Wilson, & Stamatakis, 2010; Waters & Caplan, 2001). Contrary to speech comprehension, literature on speech production has produced reliable age-related declines (Shafto & Tyler, 2014). In healthy older adults, speech is simplified, more vague, and slower as word-finding difficulties become apparent (Bortfeld, Leon, Bloom, Schober, & Brennan, 2001; Kemper & Sumner, 2001; Neumann-Werth, Obler, Gomes, & Shafer, 2009). Age has also been reported as a factor of aphasia recovery across a range of symptoms (Ellis & Urban, 2016; Ferro & Crespo, 1988; Hillis et al., 2018; Hillis & Tippett, 2014; Holland, Greenhouse, Fromm, & Swindell, 1989; Ogrezeanu, Voinescu, Mihăilescu, & Jipescu, 1994). Our results provide further evidence of this, suggesting that older age at stroke onset has negative effects on language recovery (p = .04).
Effects of Exercise
Previous studies have shown that aerobic exercise can positively affect recovery poststroke in both motor and cognitive abilities (Cumming, Tyedin, Churilov, Morris, & Bernhardt, 2012; Ploughman, McCarthy, Bossé, Sullivan, & Corbett, 2008). More specifically, exercise has been shown to benefit executive functioning (Kluding, Tseng, & Billinger, 2011; Rand, Eng, Liu-Ambrose, & Tawashy, 2010), speed of processing (Quaney et al., 2009), memory (Pyöriä et al., 2007; Rand et al., 2010), and visuospatial processing (Pyöriä et al., 2007). In a study investigating cognitive and functional capacity in individuals who had suffered a stroke, Pyöriä et al. (2007) reported that language was shown to improve significantly for those who received physiotherapy. The influence of exercise on language recovery in aphasia due to stroke has been relatively unexplored. One study suggested that exercise may positively influence language recovery when used as an adjunct to aphasia therapy (Harnish et al., 2018). Although no main effect of exercise was found in our current analysis, there was an interaction between exercise and presence of diabetes, showing that the more days exercised per week in individuals who have diabetes, the more their WAB-AQ improved over time (p = .02). It is important to consider that approximately a quarter of our participants (n = 10) had diabetes. Accordingly, the results related to diabetes should be interpreted in the context of this clear limitation. Nevertheless, further research on the effects of exercise is needed to determine if exercise could be beneficial for improving cognitive function in acute or chronic aphasia, perhaps eventually used as an adjuvant to aphasia therapy.
Effects of Diabetes/Health
Evidence from neurocognitive examinations suggests that cognitive declines should be listed along with neuropathy, nephropathy, and cardiovascular disease as one of the many complications of diabetes (Kodl & Seaquist, 2008). Cognitive declines that accompany diabetes include slower processing speed (Brands et al., 2006; Ryan, Geckle, & Orchard, 2003; Ryan, Williams, Finegold, & Orchard, 1993; Wessels et al., 2007) and more deficits in vocabulary (Hershey, Craft, Bhargava, & White, 1997; Northam, Anderson, Werther, Warne, & Adler, 1998; Schoenle, Schoenle, Molinari, & Largo, 2002; Weinger et al., 2008; Wessels et al., 2007), attention (Wessels et al., 2007), memory (Weinger et al., 2008), and executive function compared to controls (Northam et al., 1998; Weinger et al., 2008). A meta-analysis by Brands, Biessels, de Haan, Kappelle, and Kessels (2005) revealed that overall cognition, fluid intelligence, and speed of processing were reduced in patients with diabetes compared to controls. Few studies have investigated the influence of diabetes on poststroke recovery. One study evaluating language poststroke reported that presence of diabetes predicts presence of dysarthria and reduced cognitive outcomes (Megherbi et al., 2003). However, others have failed to find a significant influence for diabetes in predicting poststroke motor recovery (Nannetti, Paci, Baccini, Rinaldi, & Taiti, 2009). In this study, there was a numerical trend associated with diabetes (p = .07), and an interaction between age at stroke and presence of diabetes was statistically significant (p = .05). Diabetes often presents with other chronic conditions (i.e., obesity, hypertension, depression, chronic kidney disease, cardiovascular disease, sleep disorders, and cancer) that may affect performance on cognitively demanding tasks. It has been reported that most individuals with diabetes have at least one additional comorbid disease (Druss et al., 2001) and 40% have at least three (Maddigan, Feeny, & Johnson, 2005; Wolff, Starfield, & Anderson, 2002). One of the most prevalent comorbidities seen in adults with diabetes is hypertension. It has been reported the 75% of adults with diabetes present with hypertension, thus further provoking the microvascular and macrovascular complications that are initially present in diabetes alone and increasing the risk of neurological damage (Long & Dagogo-Jack, 2011). In language production and comprehension, high-level cognitive processing abilities are critical for successful communication. One explanation for why those with diabetes are predicted not to recover as well as those without is due to the general cognitive declines experienced by those with diabetes (leading to reduced cognitive reserve). Results from the current study provide preliminary evidence that age-related cognitive decline and diabetes have negative effects on long-term aphasia recovery. Again, our sample of individuals with aphasia and diabetes was relatively small (N = 10), which limits the potential impact of these preliminary findings. Clearly, further research including a larger sample size is needed to verify the potential effects of diabetes on aphasia recovery.
Role of Speech-Language Therapy
Speech-language therapy is an important factor in aphasia recovery (Aftonomos et al., 1999; Breitenstein et al., 2017; Fridriksson, Richardson, et al., 2012; Moss & Nicholas, 2006; Pulvermüller et al., 2001; Smania et al., 2010). In a comprehensive meta-analysis of aphasia recovery, Robey (1998) highlighted aphasia treatment effects in both the acute and chronic stages, albeit to a varying degree. Pulvermüller, Hauk, Zohsel, Neininger, and Mohr (2005) reported improved language performance as measured by clinical assessments after only 2 weeks of therapy in individuals with chronic aphasia. Several other studies have reported similar findings (Breitenstein et al., 2017; Fridriksson, Richardson, et al., 2012; Moss & Nicholas, 2006; Pulvermüller et al., 2001; Smania et al., 2010), and the current research further supports the claim that aphasia treatment supports long-term recovery in chronic patients. These results provide further evidence that policies that deny reimbursement on treatment during the chronic stage of aphasia should be reevaluated.
Limitations
This study aimed to determine what health-related or demographic factors are associated with changes in aphasia severity in chronic stroke. Although this study involved health factors that have often not been considered when investigating aphasia recovery, it is important to note that this study would benefit from a denser sample with a variety of health conditions, also complete with a measure of initial severity in the acute stage of stroke. For example, 10 participants in our study had diabetes, which constitutes only a quarter of the sample. Additionally, because this study was retrospective in nature, changes to participant case history may have occurred and have been underreported. Factors of interest were based exclusively on information collected at admission into our database; therefore, it is unclear if and how much exercise habits or other pertinent health information changed between WAB assessments. As mentioned previously, it is important to note that the factor “therapy hours” only reflected the number of treatment hours a participant received while participating in a research study in our laboratory. The potential number a participant used aphasia therapy apps or websites, or attended in-person speech therapy, is not included in our database, making it possible that we underestimated the amount of treatment participants received between assessments. It is unclear whether participant-identifying factors that influence aphasia recovery is a complex question that involves a large, demographically sparse data set. Investigating these factors in a larger sample is certainly warranted to verify the current findings.
Conclusions
The main strengths of our study lie in a relatively large sample size as well as inclusion of demographic factors not examined in previous studies. By analyzing results of multiple language assessments in relation to demographic factors, we were able to explore what are important predictors of aphasia severity changes in chronic stroke. Clinical tradition typically assumes that the severity of behavioral impairment is stable among chronic patients; however, results from our study suggest that continuing treatment is a reliable predictor of aphasia recovery, even in the chronic stage. Maintaining good, overall health by exercising—and thus managing diabetes if present—may also lead to greater recovery. Additionally, further studies investigating how initial aphasia severity in the acute stage interacts with these health factors are also necessary. Further investigation is needed to determine how other health demographics not reflected in our database influence long-term aphasia recovery in stroke. In summary, patients continuing to seek out and receive aphasia treatment have a greater likelihood of recovery, suggesting that language impairment and its severity in the chronic phase of stroke are much more dynamic than previously reported.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute on Deafness and Other Communication Disorders to Julius Fridriksson (R03 DC005915, R01 DC008355, R01 DC009571, R03 DC010262, R01 DC011739, R21 DC014170, and P50 DC014664) and to Alexandra Basilakos (T32 DC014435).
Funding Statement
This work was supported by grants from the National Institute on Deafness and Other Communication Disorders to Julius Fridriksson (R03 DC005915, R01 DC008355, R01 DC009571, R03 DC010262, R01 DC011739, R21 DC014170, and P50 DC014664) and to Alexandra Basilakos (T32 DC014435).
Footnote
The WAB-SEM was calculated in the same fashion as described by Holland et al.'s (2017) study: data provided in the WAB-R manual for the second standardization group of 141 “aphasics with infarcts” (Kertesz, 2007). The standard deviation (29.9) was divided by the square root of the sample size (141), resulting in an SEM of 2.52.
References
- Aftonomos L. B., Appelbaum J. S., & Steele R. D. (1999). Improving outcomes for persons with aphasia in advanced community-based treatment programs. Stroke, 30(7), 1370–1379. https://doi.org/10.1161/01.STR.30.7.1370 [DOI] [PubMed] [Google Scholar]
- Aftonomos L. B., Steele R. D., & Wertz R. T. (1997). Promoting recovery in chronic aphasia with an interactive technology. Archives of Physical Medicine and Rehabilitation, 78(8), 841–846. https://doi.org/10.1016/S0003-9993(97)90197-0 [DOI] [PubMed] [Google Scholar]
- Allen L., Mehta S., Mcclure J. A., & Teasell R. (2012). Therapeutic interventions for aphasia initiated more than six months post stroke: A review of the evidence. Topics in Stroke Rehabilitation, 19, 523–535. https://doi.org/10.1310/tsr1906-523 [DOI] [PubMed] [Google Scholar]
- Basso A., Capitani E., & Vignolo L. A. (1979). Influence of rehabilitation on language skills in aphasic patients: A controlled study. Archives of Neurology, 36(4), 190–196. https://doi.org/10.1001/archneur.1979.00500400044005 [DOI] [PubMed] [Google Scholar]
- Basso A., & Macis M. (2011). Therapy efficacy in chronic aphasia. Behavioural Neurology, 24(4), 317–325. https://doi.org/10.3233/BEN-2011-0342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortfeld H., Leon S. D., Bloom J. E., Schober M. F., & Brennan S. E. (2001). Disfluency rates in conversation: Effects of age, relationship, topic, role, and gender. Language and Speech, 44(2), 123–147. https://doi.org/10.1177/00238309010440020101 [DOI] [PubMed] [Google Scholar]
- Brands A. M. A., Biessels G. J., de Haan E. H. F., Kappelle L. J., & Kessels R. P. C. (2005). The effects of type 1 diabetes on cognitive performance. Diabetes Care, 28(3), 726–735. Retrieved from http://care.diabetesjournals.org/content/28/3/726.abstract [DOI] [PubMed] [Google Scholar]
- Brands A. M. A., Kessels R. P. C., Hoogma R. P. L. M., Henselmans J. M. L., van der Beek Boter J. W., Kappelle L. J., … Biessels G. J. (2006). Cognitive performance, psychological well-being, and brain magnetic resonance imaging in older patients with type 1 diabetes. Diabetes, 55(6), 1800–1806. Retrieved from http://diabetes.diabetesjournals.org/content/55/6/1800.abstract [DOI] [PubMed] [Google Scholar]
- Breitenstein C., Grewe T., Flöel A., Ziegler W., Springer L., Martus P., … Baumgaertner A. (2017). Intensive speech and language therapy in patients with chronic aphasia after stroke: A randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. The Lancet, 389(10078), 1528–1538. https://doi.org/10.1016/S0140-6736(17)30067-3 [DOI] [PubMed] [Google Scholar]
- Connor L. T., Obler L. K., Tocco M., Fitzpatrick P. M., & Albert M. L. (2001). Effect of socioeconomic status on aphasia severity and recovery. Brain and Language, 78(2), 254–257. https://doi.org/10.1006/brln.2001.2459 [DOI] [PubMed] [Google Scholar]
- Cumming T. B., Tyedin K., Churilov L., Morris M. E., & Bernhardt J. (2012). The effect of physical activity on cognitive function after stroke: A systematic review. International Psychogeriatrics, 24(4), 557–567. https://doi.org/10.1017/S1041610211001980 [DOI] [PubMed] [Google Scholar]
- DeDe G., Caplan D., Kemtes K., & Waters G. (2004). The relationship between age, verbal working memory, and language comprehension. Psychology and Aging, 19, 601–616. https://doi.org/10.1037/0882-7974.19.4.601 [DOI] [PubMed] [Google Scholar]
- Druss B. G., Marcus S. C., Olfson M., Tanielian T., Elinson L., & Pincus H. A. (2001). Comparing the national economic burden of five chronic conditions. Health Affairs, 20(6), 233–241. https://doi.org/10.1377/hlthaff.20.6.233 [DOI] [PubMed] [Google Scholar]
- Elkana O., Frost R., Kramer U., Ben-Bashat D., & Schweiger A. (2013). Cerebral language reorganization in the chronic stage of recovery: A longitudinal fMRI study. Cortex, 49(1), 71–81. https://doi.org/10.1016/j.cortex.2011.09.001 [DOI] [PubMed] [Google Scholar]
- Ellis C., & Urban S. (2016). Age and aphasia: A review of presence, type, recovery and clinical outcomes. Topics in Stroke Rehabilitation, 23(6), 430–439. https://doi.org/10.1080/10749357.2016.1150412 [DOI] [PubMed] [Google Scholar]
- Ferro J. M., & Crespo M. (1988). Young adult stroke: Neuropsychological dysfunction and recovery. Stroke, 19(8), 982–986. https://doi.org/10.1161/01.STR.19.8.982 [DOI] [PubMed] [Google Scholar]
- Fridriksson J., Baker J. M., Whiteside J., Eoute D., Moser D., Vesselinov R., & Rorden C. (2009). Treating visual speech perception to improve speech production in non-fluent aphasia. Stroke, 40(3), 853–858. https://doi.org/10.1161/STROKEAHA.108.532499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J., Basilakos A., Hickok G., Bonilha L., & Rorden C. (2015). Speech entrainment compensates for Broca's area damage. Cortex, 69, 68–75. https://doi.org/10.1016/j.cortex.2015.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J., Hubbard H. I., Hudspeth S. G., Holland A. L., Bonilha L., Fromm D., & Rorden C. (2012). Speech entrainment enables patients with Broca's aphasia to produce fluent speech. Brain, 135(12), 3815–3829. https://doi.org/10.1093/brain/aws301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J., Richardson J. D., Baker J. M., & Rorden C. (2011). Transcranial direct current stimulation improves naming reaction time in fluent aphasia. Stroke, 42(3), 819–821. Retrieved from http://stroke.ahajournals.org/content/42/3/819.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J., Richardson J. D., Fillmore P., & Cai B. (2012). Left hemisphere plasticity and aphasia recovery. NeuroImage, 60(2), 854–863. https://doi.org/10.1016/j.neuroimage.2011.12.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J., Rorden C., Elm J., Sen S., MS G., & Bonilha L. (2018). Transcranial direct current stimulation vs sham stimulation to treat aphasia after stroke: A randomized clinical trial. JAMA Neurology, 75(12), 1470–1476. https://doi.org/10.1001/jamaneurol.2018.2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Fernández M., Christian A. B., Davis C., & Hillis A. E. (2013). Role of aphasia in discharge location after stroke. Archives of Physical Medicine and Rehabilitation, 94(5), 851–855. https://doi.org/10.1016/j.apmr.2012.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnish S. M., Rodriguez A. D., Blackett D. S., Gregory C., Seeds L., Boatright J. H., & Crosson B. (2018). Aerobic exercise as an adjuvant to aphasia therapy: Theory, preliminary findings, and future directions. Clinical Therapeutics, 40(1), 35.e6–48.e6. https://doi.org/10.1016/j.clinthera.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey T., Craft S., Bhargava N., & White N. H. (1997). Memory and insulin dependent diabetes mellitus (IDDM): Effects of childhood onset and severe hypoglycemia. Journal of the International Neuropsychological Society, 3(6), 509–520. [PubMed] [Google Scholar]
- Hillis A. E., Beh Y. Y., Sebastian R., Breining B., Tippett D. C., Wright A., … Fridriksson J. (2018). Predicting recovery in acute poststroke aphasia. Annals of Neurology, 83(3), 612–622. https://doi.org/10.1002/ana.25184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis A. E., & Tippett D. C. (2014). Stroke recovery: Surprising influences and residual consequences. Advances in Medicine, 2014, 1–10. https://doi.org/10.1155/2014/378263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland A. L., Fromm D., Forbes M., & MacWhinney B. (2017). Long-term recovery in stroke accompanied by aphasia: A reconsideration. Aphasiology, 31(2), 152–165. https://doi.org/10.1080/02687038.2016.1184221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland A. L., Greenhouse J. B., Fromm D., & Swindell C. S. (1989). Predictors of language restitution following stroke: A multivariate analysis. Journal of Speech and Hearing Research, 32(2), 232–238. https://doi.org/10.1044/jshr.3202.232 [DOI] [PubMed] [Google Scholar]
- Hope T. M. H., Leff A. P., Prejawa S., Bruce R., Haigh Z., Lim L., … Price C. (2017). Right hemisphere structural adaptation and changing language skills years after left hemisphere stroke. Brain, 140, 1718–1728. https://doi.org/10.1093/brain/awx086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope T. M. H., Seghier L., Leff A. P., & Price C. J. (2013). Predicting outcome and recovery after stroke with lesions extracted from MRI images. Clinical, 2, 424–433. https://doi.org/10.1016/j.nicl.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper S., & Sumner A. (2001). The structure of verbal abilities in young and older adults. Psychology and Aging, 16(2), 312–322. https://doi.org/10.1037/0882-7974.16.2.312 [PubMed] [Google Scholar]
- Kertesz A. (1982). Western Aphasia Battery test manual. New York, NY: Grune & Stratton. [Google Scholar]
- Kertesz A. (2007). Western Aphasia Battery–Revised. New York, NY: Grune & Stratton. [Google Scholar]
- Kertesz A., & Mccabe P. (1977). Recovery patterns and prognosis in aphasia. Brain, 100(1), 1–18. https://doi.org/10.1093/brain/100.1.1 [DOI] [PubMed] [Google Scholar]
- Kluding P. M., Tseng B. Y., & Billinger S. A. (2011). Exercise and executive function in individuals with chronic stroke: A pilot study. Journal of Neurologic Physical Therapy, 35(1), 11–17. https://doi.org/10.1097/NPT.0b013e318208ee6c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodl C. T., & Seaquist E. R. (2008). Cognitive dysfunction and diabetes mellitus. Endocrine Reviews, 29(4), 494–511. https://doi.org/10.1210/er.2007-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer J. W., & Marshall R. S. (2015). The proportional recovery rule for stroke revisited. Annals of Neurology, 78(6), 845–847. https://doi.org/10.1002/ana.24537 [DOI] [PubMed] [Google Scholar]
- Laska A. C., Hellblom A., Murray V., Kahan T., & Von Arbin M. (2001). Aphasia in acute stroke and relation to outcome. Journal of Internal Medicine, 249(5), 413–422. https://doi.org/10.1046/j.1365-2796.2001.00812.x [DOI] [PubMed] [Google Scholar]
- Long A. N., & Dagogo-Jack S. (2011). Comorbidities of diabetes and hypertension: Mechanisms and approach to target organ protection. The Journal of Clinical Hypertension, 13(4), 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddigan S. L., Feeny D. H., & Johnson J. A. (2005). Health-related quality of life deficits associated with diabetes and comorbidities in a Canadian national population health survey. Quality of Life Research, 14, 1311–1320. [DOI] [PubMed] [Google Scholar]
- Megherbi S. E., Milan C., Minier D., Couvreur G., Osseby G. V., Tilling K., … European BIOMED Study of Stroke Care Group. (2003). Association between diabetes and stroke subtype on survival and functional outcome 3 months after stroke: Data from the European BIOMED Stroke Project. Stroke, 34(3), 688–694. https://doi.org/10.1161/01.STR.0000057975.15221.40 [DOI] [PubMed] [Google Scholar]
- Moss A., & Nicholas M. (2006). Language rehabilitation in chronic aphasia and time postonset: A review of single-subject data. Stroke, 37(12), 3043–3051. https://doi.org/10.1161/01.STR.0000249427.74970.15 [DOI] [PubMed] [Google Scholar]
- Naeser M. A., Palumbo C. L., Prete M. N., Fitzpatrick P. M., Mimura M., Samaraweera R., & Albert M. L. (1998). Visible changes in lesion borders on CT scan after five years poststroke, and long-term recovery in aphasia. Brain and Language, 62(1), 1–28. https://doi.org/10.1006/brln.1997.1866 [DOI] [PubMed] [Google Scholar]
- Nannetti L., Paci M., Baccini M., Rinaldi L. A., & Taiti P. G. (2009). Recovery from stroke in patients with diabetes mellitus. Journal of Diabetes and Its Complications, 23(4), 249–254. https://doi.org/10.1016/j.jdiacomp.2008.02.008 [DOI] [PubMed] [Google Scholar]
- Neumann-Werth Y., Obler L., Gomes H., & Shafer V. (2009). Phonological vs sensory contributions to age effects in naming: An electrophysiological study. Aphasiology, 23, 1028–1039. https://doi.org/10.1080/02687030802661630 [Google Scholar]
- Northam E. A., Anderson P. J., Werther G. A., Warne G. L., Adler R. G., & Andrewes D. (1998). Neuropsychological complications of IDDM in children 2 years after disease onset. Diabetes Care, 21(3), 379–384. Retrieved from http://care.diabetesjournals.org/content/21/3/379.abstract [DOI] [PubMed] [Google Scholar]
- Ogrezeanu V., Voinescu I., Mihăilescu L., & Jipescu I. (1994). “Spontaneous” recovery in aphasics after single ischaemic stroke. Romanian Journal of Neurology and Psychiatry, 32(2), 77–90. [PubMed] [Google Scholar]
- Pedersen P. M., Vinter K., & Olsen T. S. (2001). The Communicative Effectiveness Index: Psychometric properties of a Danish adaptation. Aphasiology, 15(8), 787–802. https://doi.org/10.1080/02687040143000195 [Google Scholar]
- Pinheiro J., Bates D., DebRoy S., Sarkar D., & Core Team R. (2017). nlme: Linear and nonlinear mixed effects models. (R package version 3.1–131) [Electronic resource]. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Ploughman M., McCarthy J., Bossé M., Sullivan H. J., & Corbett D. (2008). Does treadmill exercise improve performance of cognitive or upper-extremity tasks in people with chronic stroke? A randomized cross-over trial. Archives of Physical Medicine and Rehabilitation, 89(11), 2041–2047. https://doi.org/10.1016/j.apmr.2008.05.017 [DOI] [PubMed] [Google Scholar]
- Plowman E., Hentz B., & Ellis C. (2012). Post-stroke aphasia prognosis: A review of patient-related and stroke-related factors. Journal of Evaluation in Clinical Practice, 18(3), 689–694. https://doi.org/10.1111/j.1365-2753.2011.01650.x [DOI] [PubMed] [Google Scholar]
- Price C. J., Seghier M. L., & Leff A. P. (2010). Predicting language outcome and recovery after stroke: The PLORAS system. Nature Reviews: Neurology, 6(4), 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F., Hauk O., Zohsel K., Neininger B., & Mohr B. (2005). Therapy-related reorganization of language in both hemispheres of patients with chronic aphasia. NeuroImage, 28(2), 481–489. https://doi.org/10.1016/j.neuroimage.2005.06.038 [DOI] [PubMed] [Google Scholar]
- Pulvermüller F., Neininger B., Elbert T., Mohr B., Rockstroh B., Koebbel P., & Taub E. (2001). Aphasia after stroke. Stroke, 32, 2–7. https://doi.org/10.1161/01.STR.32.7.1621 [DOI] [PubMed] [Google Scholar]
- Pyöriä O., Talvitie U., Nyrkkö H., Kautiainen H., Pohjolainen T., & Kasper V. (2007). The effect of two physiotherapy approaches on physical and cognitive functions and independent coping at home in stroke rehabilitation. A preliminary follow-up study. Disability and Rehabilitation, 29(6), 503–511. https://doi.org/10.1080/09638280600902497 [DOI] [PubMed] [Google Scholar]
- Quaney B. M., Boyd L. A., McDowd J. M., Zahner L. H., He J., Mayo M. S., & Macko R. F. (2009). Aerobic exercise improves cognition and motor function poststroke. Neurorehabilitation and Neural Repair, 23(9), 879–885. https://doi.org/10.1177/1545968309338193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand D., Eng J. J., Liu-Ambrose T., & Tawashy A. E. (2010). Feasibility of a 6-month exercise and recreation program to improve executive functioning and memory in individuals with chronic stroke. Neurorehabilitation and Neural Repair, 24(8), 722–729. https://doi.org/10.1177/1545968310368684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2017). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Robey R. R. (1998). A meta-analysis of clinical outcomes in the treatment of aphasia. Journal of Speech, Language, and Hearing Research, 41(1), 172–187. https://doi.org/10.1044/jslhr.4101.172 [DOI] [PubMed] [Google Scholar]
- Ryan C. M., Geckle M. O., & Orchard T. J. (2003). Cognitive efficiency declines over time in adults with Type 1 diabetes: Effects of micro- and macrovascular complications. Diabetologia, 46(7), 940–948. https://doi.org/10.1007/s00125-003-1128-2 [DOI] [PubMed] [Google Scholar]
- Ryan C. M., Williams T. M., Finegold D. N., & Orchard T. J. (1993). Cognitive dysfunction in adults with type 1 (insulin-dependent) diabetes mellitus of long duration: Effects of recurrent hypoglycaemia and other chronic complications. Diabetologia, 36(4), 329–334. https://doi.org/10.1007/BF00400236 [DOI] [PubMed] [Google Scholar]
- Schoenle E. J., Schoenle D., Molinari L., & Largo R. H. (2002). Impaired intellectual development in children with Type I diabetes: Association with HbA1c , age at diagnosis and sex. Diabetologia, 45(1), 108–114. https://doi.org/10.1007/s125-002-8250-6 [DOI] [PubMed] [Google Scholar]
- Shafto M. A., & Tyler L. K. (2014). Language in the aging brain: The network dynamics of cognitive decline and preservation. Science, 346(6209), 583–587. Retrieved from http://science.sciencemag.org/content/346/6209/583.abstract [DOI] [PubMed] [Google Scholar]
- Shewan C. M., & Kertesz A. (1980). Reliability and validity characteristics of the Western Aphasia Battery (WAB). Journal of Speech & Hearing Disorders, 45(3), 308–324. https://doi.org/10.1044/jshd.4503.308 [DOI] [PubMed] [Google Scholar]
- Smania N., Gandolfi M., Aglioti S. M., Girardi P., Fiaschi A., & Girardi F. (2010). How long is the recovery of global aphasia? Twenty-five years of follow-up in a patient with left hemisphere stroke. Neurorehabilitation and Neural Repair, 24(9), 871–875. https://doi.org/10.1177/1545968310368962 [DOI] [PubMed] [Google Scholar]
- Thornton R., & Light L. L. (2006). Language comprehension and production in normal aging. In Birren J. E. & Warner Schaie K. (Eds.), Handbook of the psychology of aging (pp. 262–287). Burlington, MA: Elsevier. [Google Scholar]
- Tun P. A. (1998). Fast noisy speech: Age differences in processing rapid speech with background noise. Psychology and aging, 13(3), 424–434. https://doi.org/10.1037/0882-7974.13.3.424 [DOI] [PubMed] [Google Scholar]
- Turkeltaub P. E. (2015). Brain stimulation and the role of the right hemisphere in aphasia recovery. Current Neurology and Neuroscience Reports, 15(11), 72 https://doi.org/10.1007/s11910-015-0593-6 [DOI] [PubMed] [Google Scholar]
- Tyler L. K., Cobb H., & Graham N. (1992). Spoken language comprehension: An experimental approach to disordered and normal processing. Cambridge, MA: MIT Press. [Google Scholar]
- Tyler L. K., Wright P., Randall B., Marslen-Wilson W. D., & Stamatakis E. A. (2010). Reorganization of syntactic processing following left-hemisphere brain damage: Does right-hemisphere activity preserve function? Brain, 133(11), 3396–3408. https://doi.org/10.1093/brain/awq262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters G. S., & Caplan D. (2001). Age, working memory, and on-line syntactic processing in sentence comprehension. Psychology and Aging, 16(1), 128–144. https://doi.org/10.1037/0882-7974.16.1.128 [DOI] [PubMed] [Google Scholar]
- Weinger K., Jacobson A. M., Musen G., Lyoo I. K., Ryan C. M., Jimerson D. C., & Renshaw P. F. (2008). The effects of type 1 diabetes on cerebral white matter. Diabetologia, 51(3), 417–425. https://doi.org/10.1007/s00125-007-0904-9 [DOI] [PubMed] [Google Scholar]
- Wessels A. M., Rombouts S. A. R. B., Remijnse P. L., Boom Y., Scheltens P., Barkhof F., … Snoek F. J. (2007). Cognitive performance in type 1 diabetes patients is associated with cerebral white matter volume. Diabetologia, 50(8), 1763–1769. https://doi.org/10.1007/s00125-007-0714-0 [DOI] [PubMed] [Google Scholar]
- Wickham H. (2009). ggplot2: Elegant graphics for data analysis. New York, NY: Springer-Verlag. [Google Scholar]
- Wolff J. L., Starfield B., & Anderson G. (2005). Prevalence, Expenditures, and Complications of Multiple Chronic Conditions in the Elderly. Archives of Internal Medicine, 162(20), 2269–2276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.