Abstract
Purpose
As pulse rate increases beyond a few hundred Hertz, younger normal-hearing (NH) participants' ability to encode temporal information in band-limited acoustic pulse trains decreases, demonstrating a rate limitation in processing rapid temporal information. Rate discrimination abilities, however, have yet to be investigated in older NH participants—a population that experiences age-related temporal processing deficits. It was hypothesized that age-related temporal processing deficits lead to decreased temporal rate discrimination abilities in older compared with younger NH participants, which could be observed in both perceptual and electrophysiological measurements.
Method
Fifteen younger and 15 older NH participants were presented acoustic pulse trains with a 4-kHz center frequency and 1-kHz bandwidth at 75 dB SPL monaurally. The pulse rate was 80, 200, or 400 Hz. Just noticeable differences were obtained using an adaptive procedure that instructed the participants to identify the pulse train with the highest pitch. Auditory steady-state responses (ASSRs) were recorded to the same pulse trains with 2 additional rates—20 and 40 Hz. The Digit Symbol Coding and Digit Symbol Search subtests of the Wechsler Adult Intelligence Scale (Wechsler, 1997) were measured as correlates to domain-general cognitive processing speed.
Results
As rate increased from 80 to 400 Hz, performance on the perceptual rate discrimination task worsened in both groups. ASSR spectral energy also decreased, but only in the older group. Perceptual performance was equivalent between groups across rates. The older group had lower ASSR spectral energy (lower signal-to-noise ratios) at the 400-Hz rate than the younger group, but there were no group differences for the other rates. The overall strength of neural rate representation, along with speed of processing performance, predicted perceptual performance for the 400-Hz rate.
Conclusion
These results suggest that neural representation at early levels of the auditory system and processing speed are factors in perceptual auditory temporal processing performance, especially in older adults.
As people age, their ability to encode rapid acoustic information degrades, which has consequences for understanding similarly rapid temporal changes that are characteristic of running speech (Gordon-Salant & Fitzgibbons, 1993; Gordon-Salant, Yeni-Komshian, & Fitzgibbons, 2008; Gordon-Salant, Yeni-Komshian, Fitzgibbons, & Barrett, 2006). It is thought that these age-related temporal auditory processing deficits manifest from peripheral, central, and cognitive origins (Committee on Hearing, Bioacoustics, and Biomechanics [CHABA], 1988). The purpose of this study was to examine age-related changes in temporal encoding, both perceptually and electrophysiologically.
Although not assessed previously, decline in temporal rate discrimination may be one dimension of temporal processing deficits that can be observed in older adults. The ability to detect changes in the rate of a signal relates to the ability to distinguish the fundamental frequency of voices (i.e., speaker and gender identification; Grimault, Micheyl, Carlyon, Bacon, & Collet, 2003), to the use of prosodic cues in speech communication (Mitchell & Kingston, 2014), and to the ability to make judgments about musical pitch (i.e., melody recognition; Kong, Cruz, Jones, & Zeng, 2004). In perceptual studies, rate limitations have been demonstrated for both monaural and binaural processing of timing information. For example, younger normal-hearing (YNH) participants can monaurally discriminate pulse trains presented at different rates around 100 Hz with a 3%–7% difference (Carlyon & Deeks, 2002; Carlyon, Long, & Deeks, 2008). However, as rate increases beyond 300 Hz, performance decreases rapidly. Similar 300-Hz rate limitations have been observed in binaural processing of interaural time differences using acoustic pulse trains and other amplitude-modulated stimuli (e.g., Bernstein & Trahiotis, 2002; Brown & Stecker, 2010; Goupell, Laback, & Majdak, 2009; Hafter & Buell, 1990; Hafter, Dye, & Wenzel, 1983). An effect of rate has also been observed in interaural level difference processing using acoustic pulse trains (Hafter et al., 1983), and the rate effect was recently explained mostly with peripheral mechanisms, namely, auditory nerve adaptation (Laback, Dietz, & Joris, 2017). Rate limitations for monaural or binaural processing using band-limited acoustic pulse trains, however, have not yet been evaluated in older normal-hearing (ONH) adults or older adults with hearing impairment.
Although the effects of aging on rate limitations have been studied in normal-hearing (NH) participants with more complex stimuli such as speech, the use of highly controlled and basic stimuli such as acoustic pulse trains is limited. Temporal processing abilities as measured with acoustic pulse trains have been studied in middle-aged and older individuals to a great degree in a clinical population—cochlear implant (CI) users. Comparisons between NH and CI participants have been limited (e.g., Carlyon & Deeks, 2002; Macherey & Carlyon, 2014), but previous studies suggest that NH participants may have a higher rate limitation around 700–800 Hz compared with CI participants around 300 Hz (with an exception of the CI users with very high rate limitations). Any comparison within or across studies, however, has a few clear confounds. First, differences in the processing of acoustic and electric stimulation, where for example, ringing of the cochlear filters is bypassed for CI participants, could contribute to differences in performance between NH and CI listeners. Second, the NH participants were mostly younger (< 45 years old); the CI participants were mostly middle-aged and older participants (> 45 years old). Because there is evidence of age-related temporal processing deficits across many types of paradigms, it could be that some of the difference in NH versus CI rate limitation may be a result of the aging process. Therefore, we sought to determine if an age-related difference in temporal rate discrimination could be found in YNH versus ONH participants.
The previous studies have focused on perceptual data; however, electrophysiological measurements may help quantify and better understand rate limitations and age-related temporal processing deficits. The level of the auditory system at which these rate limitations manifest remains unclear. One way to determine potential sources of age-related declines would be to record the auditory steady-state response (ASSR) at different rates. The ASSR is a scalp-recorded evoked potential to amplitude-modulated (AM) or frequency-modulated auditory stimuli (Picton, John, Dimitrijevic, & Purcell, 2003). The energy of the periodic waveforms elicited by these stimuli occurs predominantly at the modulation rate of the stimuli. The ASSR has been recorded to varying rates from 1 to 400 Hz, with the largest response occurring to the 40-Hz AM rate (Picton et al., 2003; Rees, Green, & Kay, 1986). Herdman and colleagues (2002) used brain electrical source analysis to determine neural origins of ASSR recorded to different AM rates and found that brainstem activity was larger than cortical activity for the higher AM rate (88 Hz), whereas lower rates represented combined activation from both brainstem and cortical sources. A few previous studies have compared aging effects on ASSR amplitudes at different AM rates and have found lower amplitudes in older adults compared with younger adults, but only at relatively higher rates (80–128 Hz) and not at lower rates (≤ 40 Hz; Goossens, Vercammen, Wouters, & van Wieringen, 2016; Grose, Mamo, & Hall, 2009). Further understanding of changes in spectral energy at different rates may help to inform whether age-related deficits in rate discrimination abilities arise from central or peripheral origins.
In this study, we sought to reveal age-related temporal processing deficits using perceptual and electrophysiological approaches. The underlying hypotheses for this experiment were as follows: (a) Faster pulse rates result in poorer performance for all participants, (b) increasing age results in poorer temporal processing abilities that limit the ability to perceive rate changes or to encode fast pulse trains, (c) faster pulse rates significantly tax the auditory system of ONH participants to a greater degree than YNH participants (i.e., we expect an age by rate interaction), (d) neural representation of rate can predict rate discrimination performance, and (e) speed of information processing can partially predict rate discrimination performance.
Materials and Method
Participants
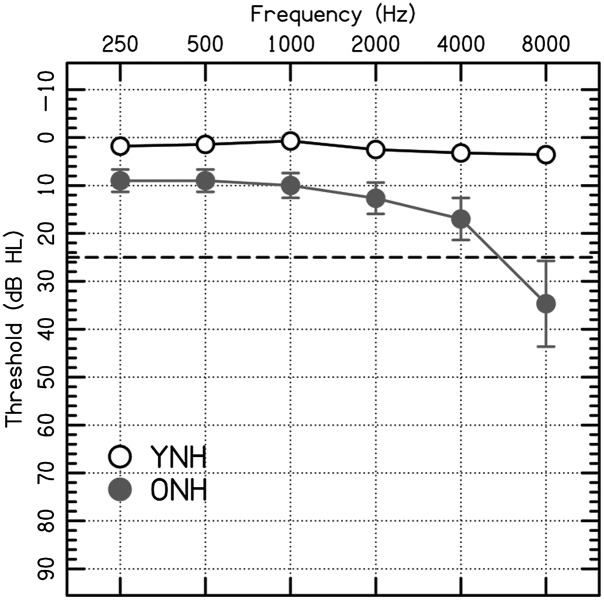
The study included two participant groups: 15 YNH (age: 18–29 years, M = 22.3 years, SD = 2.7 years) and 15 ONH (age: 63–78 years, M = 70.3 years, SD = 3.8 years) participants. Normal hearing was defined as thresholds ≤ 25 dB HL at octave frequencies from 250 to 4000 Hz. Average thresholds for both participant groups are shown in Figure 1. The thresholds were lower for YNH than ONH participants at all frequencies (two-tailed t tests; all ps < .003). The Montreal Cognitive Assessment Version 7.1 (original version) was administered to ensure that all participants had normal to near-normal cognitive function (score ≥ 22; YNH: M ± SD = 29.2 ± 1.0; ONH: M ± SD = 27.2 ± 2.6; Dupuis et al., 2015; Nasreddine et al., 2005).
Figure 1.
Average pure-tone thresholds in dB HL (American National Standards Institute, 2010). Normal hearing thresholds were defined at ≤ 25 dB HL (dashed horizontal line). Mean thresholds are plotted as open circles for younger normal-hearing (YNH) participants and closed circles for older normal-hearing (ONH) participants. Error bars show ±1 SE.
General Stimulus Information
The stimuli were band-limited pulse trains with a stimulus duration of 300 ms. The pulse rates were 20, 40, 80, 200, or 400 Hz. Broadband pulses were forward-backward bandpass filtered between 3.5 and 4.5 kHz using fifth-order Butterworth filters, resulting in a 4-kHz center frequency and 1-kHz bandwidth. A 5-ms Tukey window was applied to the pulse train to avoid onset and offset transients generated by the filtering. The pulse trains were presented monaurally at 75 dB SPL.
Electrophysiological Measurement
Equipment
Pulse trains were presented via the Intelligent Hearing Systems Smart EP continuous acquisition module (IHS SEPCAM) with a standard montage of four electrodes (Cz active, one forehead ground, and two earlobe references). Stimuli were presented monaurally to the right ear via electromagnetically shielded insert earphones in a double-walled electrically shielded sound-attenuating chamber. Data recorded with IHS SEPCAM were analyzed in MATLAB (Version 2011b; MathWorks).
Stimuli
Participants were tested with five AM rates to obtain responses from relatively higher (20 and 40 Hz) and lower (80–400 Hz) levels of the auditory system. The presentation rate for the full pulse train occurred at 1.66 Hz or a period of 600 ms, twice the duration of the pulse train. Responses were recorded at a sampling frequency of 10 kHz with an online bandpass filter setting from 1 to 5 kHz. Level roving and low-frequency masking noise were excluded from all electrophysiological pulse-train stimuli.
Procedure
During the recording period, participants were seated in a reclining chair and were given a silent captioned movie to watch to facilitate a relaxed but awake state. The ASSR was used to measure the neural activity generated from the fast-rate repetitive pulse-train stimuli (Presacco, Bohórquez, Yavuz, & Özdamar, 2010). A minimum of 1,024 artifact-free sweeps were collected for each rate (1,024 sweeps × 5 rates = 5,120 total sweeps). Any response exceeding 30 μV was considered artifact and excluded from the recording average. ASSR responses for each rate were averaged offline using MATLAB.
Perceptual Task
Equipment
Pulse trains were presented via a personal computer running MATLAB (Version 2011b; MathWorks) connected to a real-time processor (RP2.1; Tucker-Davis Technologies), headphone buffer (HB7; Tucker-Davis Technologies), and programmable attenuator (PA4; Tucker-Davis Technologies). Stimuli were presented monaurally to the right ear using insert earphones (ER2; Etymotic) in a double-walled sound-attenuating chamber.
Stimuli
Participants were tested on a subset of the rates: 80, 200, and 400 Hz. The sampling rate of the stimuli was 100 kHz. To prevent the use of loudness instead of pitch cues, the overall level was roved over ±10 dB (i.e., 20-dB range; random uniform distribution). To eliminate the use of low-frequency distortion products to perform the task, low-frequency masking noise was mixed with the pulse-train stimuli. The masking noise began 300 ms before the first interval and ended 300 ms after the third interval. A 10-ms Tukey window was applied to the noise to avoid onset and offset transients generated by the filtering. The masking noise started as wideband white noise, was then low-pass filtered using a −3-dB/octave filter with a 200-Hz cutoff, and then was low-pass filtered using a −5-dB/octave filter with a 1000-Hz cutoff. The masking noise was presented at a 30-dB spectrum level or 61.1 dB SPL.
Procedure
A three-interval, two-alternative forced choice task was used with a three-down, one-up adaptive rule to target the 79.4% correct threshold. Three staircases, one for each of the three rates (80, 200, and 400 Hz), were simultaneously tested. On each trial, the staircase was randomly chosen. A trial consisted of two reference intervals and one target interval. The target interval was never the first interval. The target pulse train had a higher pulse rate than the reference intervals. The initial rate difference between the reference and target rates was 40%. Step size decreased by a factor of 2 until the participant reached four reversals for each staircase. After the initial four reversals, step size decreased by a factor of the √2.
A fixed number of 60 trials was presented for each simultaneous staircase (3 × 60 = 180 trials). Three testing blocks were obtained, with each block lasting 15 min in duration (180 × 3 = 540 trials in total).
During testing, the monitor displayed four boxes. One box read “Begin Trial,” followed by the presentation of three boxes, side-by-side, below. The three smaller boxes were labeled with numbers in order from 1 to 3. The participant was instructed to click on the “Begin Trial” box to start each presentation. Each trial was initiated and self-paced by the participant. Upon beginning each presentation, the participant heard three pulse trains. Participants were instructed to choose the sound with the highest pitch and to ignore any changes in loudness. They were also told that the first sound would never be the one with the highest pitch. Participants were provided with correct answer feedback after each response. Perceptual responses to stimuli were recorded using MATLAB software. Just noticeable differences (JNDs) were averaged over all the reversals in the adaptive staircase procedure; the average JND over three staircases was calculated for each condition.
Cognitive Testing
In addition to the Montreal Cognitive Assessment, the Digit Symbol Coding and Digit Symbol Search (Wechsler Adult Intelligence Scale–Third Edition; Wechsler, 1997) were administered, which are nonauditory measurements of processing speed (Deary, Johnson, & Starr, 2010). These measures were used to assess additional cognitive components that may affect the participants' performance on the perceptual task. These subtests are commonly used to test speed of processing in aging and other studies (e.g., Deary et al., 2010). They both assess aspects of processing speed, but the Coding test draws on short-term memory, whereas the Search test draws on perceptual organization. Note that administration of the Wechsler Adult Intelligence Scale measures was initiated after the study began, so they were obtained in a subset of 11 YNH and 14 ONH participants.
Data Analysis
Electrophysiological
The averaged response was filtered from 10 to 500 Hz using a zero-phase fourth-order Butterworth filter. A time-frequency analysis utilizing continuous complex Morlet wavelets was completed (Tallon-Baudry, Bertrand, Delpuech, & Pernier, 1996). The spectral energy was calculated by taking the square of the absolute value of the convolution of the signal complex using 10-Hz bins around the 20-, 40-, and 80-Hz rates from 50 to 250 ms and using 80-Hz bins from 12 to 250 ms and from 5 to 250 ms for the 200- and 400-Hz rates, respectively. The 80-Hz bins and earlier time regions for the 200- and 400-Hz rates reflected wider spread of spectral energy and earlier onset latencies for these rates. Because of previous evidence of higher neural noise levels in older adults (Anderson, Parbery-Clark, White-Schwoch, & Kraus, 2012), we calculated baseline neural noise levels by quantifying spectral energy for the same frequency ranges for each rate during the period of silence corresponding to the 350- to 550-ms time region. A repeated-measures analysis was conducted to compare noise levels between the two groups at different rates. The signal-to-noise ratios (SNRs) were calculated by dividing the energy in the response regions by the energy in the silent regions.
The SNR values were modeled with a generalized linear model containing the effects of rate (four levels: 40, 80, 200, and 400 Hz; within subjects) and age group (two levels: YNH and ONH; between subjects). The generalized linear model, which used a gamma regression with a logit link, was appropriate due to the high positive skew of the SNR data. The 20-Hz rate was excluded from the model due to missing electrophysiological data in that condition.
Perceptual
Rate discrimination JNDs were modeled with a generalized linear mixed-effects model containing the fixed effects of rate (three levels: 80, 200, and 400 Hz; within subjects) and age group (two levels: YNH and ONH; between subjects) as well as their interaction. The model also contained the random intercept of subject and the random slope of rate. A second generalized linear model containing the additional fixed effects of ASSR SNR values at 80, 200, and 400 Hz (all between subjects) as well as their interactions with rate and age group was conducted to determine if data from the electrophysiological experiment helped explain the perceptual results. Both models used a gamma regression with a logit link, which was appropriate due to the high positive skew of the data obtained for the perceptual task.
Correlations Among Perceptual, Cognitive, and Electrophysiological Measures
Pearson correlations were calculated to compare the relationships among the perceptual (400-Hz relative JND), cognitive (Digit Symbol Coding and Search raw scores), 4-kHz threshold (corresponding to the stimulus frequency), and ASSR SNR values. There was insufficient power in the subset of 25 participants who had cognitive testing to obtain results with a mixed-effects linear regression model that included perceptual, cognitive, and ASSR variables. Therefore, we used a multiple linear regression (enter method) with a limited number of cognitive and neural variables to determine if they contributed to the variance of the relative JNDs for the 400-Hz rate. The two processing speed scores were highly correlated (r = .92), and we could not include both of the measures in the model due to problems with collinearity. Therefore, we chose only the Digit Symbol Coding raw score because it contains a component of short-term memory. Digit Symbol Coding was entered on the first step of the regression, and the 80- and 400-Hz SNRs (representing high and low brainstem, respectively) were entered on the second step. In all models, we checked residuals for normality to ensure that the linear regression analysis was appropriate for the data set. We ran collinearity diagnostics with satisfactory variance inflation factor (highest = 1.7) and tolerance (lowest = 0.61) scores, indicating the absence of strong correlations between two or more predictors.
Results
Electrophysiological Model
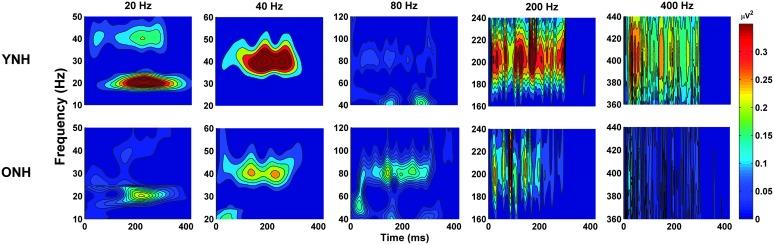
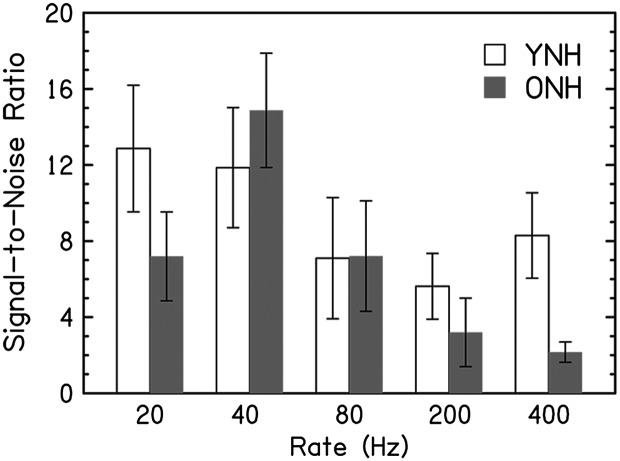
ASSR spectral energy for YNH and ONH participants is displayed in the time-frequency domain in Figure 2, and the data are summarized in Figure 3. A log transform was used to normalize the neural noise data for each rate. The repeated-measures analysis of variance on these log-transformed data indicated that neural noise levels were significantly lower in YNH than in ONH participants—main effect of group: F(1, 29) = 4.9, p = .035, ηp 2 = .15. Subsequent analyses were conducted using the SNRs for each rate to avoid inflating spectral values with higher baseline noise levels in the older adults. The figures show equivalent SNRs for YNH and ONH participants except at 400 Hz, where the SNRs were higher in YNH than in ONH participants. Figure 3 shows that SNRs decreased as rate increased in the ONH participants but not in the YNH participants.
Figure 2.
Electroencephalography spectral energy for all five pulse rates, represented in the time and frequency domain. Mean responses to pulse trains as a function of rate per each panel are displayed for younger normal-hearing (YNH; Row A) and older normal-hearing (ONH; Row B) participants.
Figure 3.
Average signal-to-noise ratios (SNRs) as a function of pulse rate. Bar graphs showing the SNR values for younger normal-hearing (YNH; white) and older normal-hearing (ONH; gray) participants in response to pulse trains at five rates (20, 40, 80, 200, and 400 Hz). Error bars show ±1 SE.
These observations were confirmed with statistical analysis. SNRs in the electrophysiological data were analyzed with a generalized linear model using SPSS 25 (IBM Corp.). The model utilized a gamma distribution with a log link due to the high positive skew in the distribution of the outcome data. The model contained the factors of rate (four levels: 400, 200, 80, and 40 Hz, with 400 Hz as the referent category; within subjects), age group (two levels: YNH and ONH, with YNH as the referent category; between subjects), and their interaction.
The overall model was significant, χ2(7) = 38.6, p < .001 (likelihood ratio). Results from the model are presented in Table 1. The effect of age group was significant (B = −1.34, Wald χ2 = 14.37, p < .001), as were the interactions of Age Group × 80 Hz (B = 1.36, Wald χ2 = 3.98, p = .046) and Age Group × 40 Hz (B = 1.57, Wald χ2 = 10.73, p = .001). The referent categories in this model were YNH (for age group) and 400 Hz (for rate). The intercept therefore represented the expected SNR (in logit form) for a YNH participant presented at a 400-Hz rate. The coefficients for 200-, 80-, and 40-Hz rates represented expected differences in YNH participant SNRs compared with the 400-Hz rate. Thus, the nonsignificant effects of 200-Hz (p = .326), 80-Hz (p = .758), and 40-Hz (p = .329) rates indicated that, for YNH participants, SNRs in the different rate conditions were not significantly different from the SNRs at the 400-Hz rate. In other words, YNH participants experienced no effects of rate on SNRs.
Table 1.
Final generalized linear model for signal-to-noise ratios (SNRs) of auditory steady-state response.
| Factors | Coefficient | SE | Wald χ2 | p |
|---|---|---|---|---|
| Intercept | 2.12 | 0.26 | 65.61 | < .001 |
| 200 Hz (compared with 400 Hz) | −0.39 | 0.40 | 0.97 | .326 |
| 80 Hz (compared with 400 Hz) | −0.16 | 0.51 | 0.10 | .758 |
| 40 Hz (compared with 400 Hz) | 0.36 | 0.37 | 0.95 | .329 |
| ONH (compared with YNH) | −1.34 | 0.35 | 14.37 | < .001 |
| Interactions | ||||
| ONH × 200 Hz | 0.78 | 0.71 | 1.20 | .274 |
| ONH × 80 Hz | 1.36 | 0.68 | 3.98 | .046 |
| ONH × 40 Hz | 1.57 | 0.48 | 10.73 | .001 |
Note. ONH = older normal-hearing participants; YNH = younger normal-hearing participants.
The significant age group effect, reported above, indicated that ONH participants had significantly lower SNRs than YNH participants at the 400-Hz rate (p < .001), with the latter value being represented by the intercept of the model. The significant interactions of age group with 80- and 40-Hz rates, also reported above, indicated that ONH participants did experience an effect of rate on SNRs. Compared with SNRs at the 400-Hz rate, ONH participants' SNRs at the 80- and 40-Hz rates were significantly higher (p = .046 and p = .001, respectively). SNRs at the 200-Hz rate were not significantly different from SNRs at the 400-Hz rate for ONH participants (p = .274).
Perceptual Model
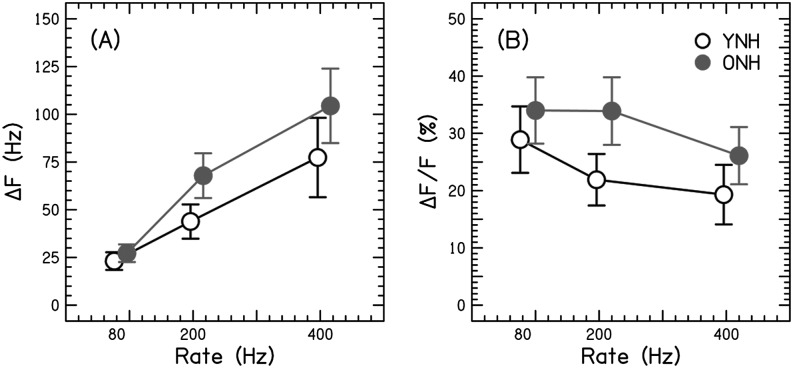
The perceptual data are displayed in Figure 4; absolute JNDs in Hertz are shown in Panel A, and relative JNDs in percentage are shown in Panel B. Both panels show equivalent performance in the YNH and ONH participants. Figure 4a shows that thresholds in Hertz increased as rate increased, and the change was equivalent between groups.
Figure 4.
Perceptual just noticeable differences (JNDs) as a function of rate, in Hertz (A) or percentage change (B). Mean JNDs are plotted as open circles for younger normal-hearing (YNH) participants and closed circles for older normal-hearing (ONH) participants. Error bars show ±1 SE.
These observations from Figure 4 were confirmed with statistical analysis. Relative JND values for each participant from the perceptual experiment were modeled with a generalized linear mixed-effects model. The distribution of participants' relative JND values was not normal and best modeled using a gamma regression with a logit link. Model building followed recommendations from Hox, Moerbeek, and Van de Schoot (2017). An intercept-only model was first fitted to the relative JND values, containing a random subject intercept. The intraclass correlation coefficient from this model equaled .66, meaning 66% of the variance in relative JND values was explained by the clustering of outcomes among participants. Next, the fixed effect of rate (three levels: 400, 200, and 80 Hz, with 400 Hz as the referent; within subjects), the fixed effect of age group (two levels: YNH and ONH, with YNH as the referent; between subjects), the cross-level interaction of rate and age group, and the random slope of rate (which allowed the effect of rate to vary among participants) were added to the model. This final model is presented in Table 2. Compared with the 400-Hz rate, slower rates elicited significantly larger relative JNDs (400 vs. 80 Hz: B = 0.80, t = 3.23, p = .001; 400 vs. 200 Hz: B = 0.50, t = 2.41, p = .017). Neither the main effect of age group nor the Age Group × Rate interaction was significant, possibly due to insufficient power. It was of interest whether electrophysiological results from the present experiment may help explain additional variance in the relative JND outcomes; thus, electrophysiological predictors were added to the model in the next analysis.
Table 2.
Final generalized linear mixed-effects model for relative just noticeable differences.
| Fixed effects | Coefficient | SE | t | p | Odds ratio |
|---|---|---|---|---|---|
| Intercept | −2.32 | 0.35 | −6.55 | < .001 | 0.10 |
| 80 Hz (compared with 400 Hz) | 0.80 | 0.25 | 3.23 | .001 | 2.23 |
| 200 Hz (compared with 400 Hz) | 0.50 | 0.21 | 2.41 | .017 | 1.65 |
| ONH (compared with YNH) | 0.73 | 0.40 | 1.84 | .067 | 2.08 |
| ONH × 80 Hz | −0.50 | 0.26 | −1.89 | .06 | 0.61 |
| ONH × 200 Hz |
−0.25 |
0.23 |
−1.08 |
.283 |
0.78 |
|
Random effects
|
Variance
|
SE
|
p
|
||
| Subject (intercept) | 0.63 | 0.19 | .001 | ||
| Subject (Hz) | 0.11 | 0.04 | .003 | ||
| Residual | 0.24 | 0.03 | < .001 |
Note. ONH = older normal-hearing participants; YNH = younger normal-hearing participants.
Combined Electrophysiological/Perceptual Model
A generalized linear mixed-effects model with a gamma distribution and a logit link was used to model relative JNDs with both electrophysiological and perceptual predictors. The model contained the fixed predictors of rate (three levels: 400, 200, and 80 Hz, with 400 Hz as the referent; within subjects), age group (two levels: ONH and YNH, with YNH as the referent category; between subjects), SNRs (three levels: measured at 400-, 200-, and 80-Hz rates, which were derived from the electroencephalography experiment and specific to each participant), and the interactions of age group with the three SNRs. The SNR variables were each centered at their median values, so that the coefficients for these variables would be predictive of outcomes for a participant with median SNRs. Median values were more representative of actual participant performance than were mean values, due to the positive skew in the data. The SNRs at 400-, 200-, and 80-Hz rates were chosen to be included in the model because they matched the reference pulse rates tested in the perceptual experiment. The SNRs at 40- and 20-Hz rates were therefore excluded.
The random slope of rate was then added to the model, which allowed the effect of rate on relative JNDs to vary among participants. This model converged, and thus cross-level interactions were added to the model. The two-way interactions of age group with rate, values from the three SNR conditions with rate, and three-way interactions of interest were all added to the model. Backward elimination was then used on this fully fitted model to determine a “final model” that contained fixed effects composed only of significant predictors, significant interactions, and any nonsignificant predictors that were part of a significant interaction. This final model is presented in Table 3.
Table 3.
Final generalized linear mixed-effects model for relative just noticeable differences including electrophysiological predictors.
| Fixed effects | Coefficient | SE | t | p | Odds ratio |
|---|---|---|---|---|---|
| Intercept | −1.73 | 0.18 | −9.53 | < .001 | 0.18 |
| 80 Hz (compared with 400 Hz) | 0.31 | 0.11 | 2.87 | .004 | 1.37 |
| 200 Hz (compared with 400 Hz) | 0.20 | 0.10 | 1.93 | .055 | 1.22 |
| SNR at 80 Hz | 0.03 | 0.01 | 5.22 | < .001 | 1.03 |
| SNR at 400 Hz | −0.09 | 0.02 | −4.53 | < .001 | 0.91 |
| 80 Hz × SNR at 400 Hz | 0.07 | 0.01 | 5.90 | < .001 | 1.08 |
| 200 Hz × SNR at 400 Hz |
0.06 |
0.01 |
4.35 |
< .001 |
1.06 |
|
Random effects
|
Variance
|
SE
|
p
|
||
| Subject (intercept) | 0.51 | 0.15 | .001 | ||
| Subject (Hz) | 0.06 | 0.03 | .028 | ||
| Residual | 0.24 | 0.03 | < .001 |
Note. SNR = signal-to-noise ratio.
Coefficients for the final model are interpreted by raising e to the power of the coefficient in question. Because the 400-Hz rate was the referent category for the rate predictor and the SNR predictors were centered at their medians, the intercept coefficient represented the relative JND logit value of a participant in the 400-Hz rate condition, who has median SNRs at 400 and 80 Hz (B = −1.73, p < .001). The relative JND values were significantly larger at the 80-Hz rate (B = 0.31, p = .004) compared with the 400-Hz rate for these median-SNR participants, but were not significantly different at the 200-Hz rate (B = 0.20, p = .055). Thus, for participants with median SNRs for the 400- and 80-Hz rates, relative JNDs were similar across the 400- and 200-Hz rates and then increased for the 80-Hz rate.
The significant interaction of rate and SNR at the 400-Hz rate indicated that, for participants who had SNRs above the median at the 400-Hz rate, the impact of rate on relative JNDs was significantly different than for those participants who had median SNRs at the 400-Hz rate. First, the significant effect of SNR at the 400-Hz rate indicated that, for each 1-dB increase in SNR above the median, participants' relative JNDs at the 400-Hz rate decreased multiplicatively by −0.09 (p < .001). In contrast, each 1-dB increase in SNR above the median at the 400-Hz rate was associated with relative JNDs at the 80-Hz rate increasing multiplicatively by 0.07 (p < .001) and with relative JNDs at the 200-Hz rate increasing multiplicatively by 0.06 (p < .001). To summarize, above-median SNRs at the 400-Hz rate significantly improved (i.e., decreased) relative JNDs at the 400-Hz rate specifically, but did not appear to improve (i.e., decrease) relative JNDs in conditions with different rates.
Cognitive
The YNH participants had higher performance on tests of speed of processing than the ONH participants. Independent-samples t tests (assumed equal variance) indicated significant differences on the raw scores of the Digit Symbol Coding subtest (YNH: M ± SD = 44.3 ± 7.7; ONH: M ± SD = 28.6 ± 5.2), t(1, 24) = 6.1, p < .001, and on the raw scores of the Digit Symbol Search subtest (YNH: M ± SD = 92.9 ± 15.3; ONH: M ± SD = 61.5 ± 13.9), t(1, 24) = 5.4, p < .001.
Perceptual–Neural–Cognitive Relationships
Pearson's correlations were conducted to assess relationships among the 4-kHz threshold, perceptual (400-Hz relative JNDs), neural (80-, 200-, and 400-Hz rates of SNRs), and speed of processing (Digit Symbol Coding and Digit Symbol Search raw scores) variables and are displayed in Table 4. Note that log-transformed values were used for both JNDs and SNRs due to highly skewed data. The processing speed scores and 4-kHz thresholds were normally distributed. The false discovery rate was applied to control for multiple comparisons, and the corrected α level was .026. The 400-Hz relative JND was negatively correlated with speed of processing (Digit Symbol Coding; r = −.45, p = .023) and positively correlated with the 80-Hz rate of SNR (r = .45, p = .013). Refer to Table 4 for correlation values among the remaining variables.
Table 4.
Intercorrelations among relative 400-Hz just noticeable difference (JND), 4-kHz hearing threshold, speed of processing (Digit Symbol Coding [DSC] and Digit Symbol Search [DSS]), and auditory steady-state response (ASSR) signal-to-noise ratios.
| Variables | 400-Hz JND% | 4 kHz | DSC | DSS | 80-Hz ASSR | 200-Hz ASSR | 400-Hz ASSR |
|---|---|---|---|---|---|---|---|
| 400-Hz JND% | 1 | ||||||
| 4 kHz | .25 | 1 | |||||
| DSC | −.45* | −.55** | 1 | ||||
| DSS | −.36 | −.58** | .92*** | 1 | |||
| 80-Hz ASSR | .45* | −.13 | .00 | .03 | 1 | ||
| 200-Hz ASSR | −.11 | −.24 | .52* | .47* | .02 | 1 | |
| 400-Hz ASSR | −.28 | −.49* | .62** | .62 | .08 | .51* | 1 |
p < .05.
p < .01.
p < .001.
(False discovery rate corrected values.)
Results of the hierarchical linear regression indicated that both speed of processing and neural measures significantly contributed to the variance in the 400-Hz rate of relative JNDs. The model (Digit Symbol Coding entered on the first step and 80- and 400-Hz rates of SNRs entered on the second step) was a good fit for the data, F(2, 33) = 8.63, p < .001, with an R 2 value of .54. Both the Symbol Coding score and the SNR values significantly contributed to the variance (p = .023 and p = .011, respectively). Table 5 displays standardized (β) coefficients and levels of significance for the independent variables for the two models—(a) processing speed and (b) processing speed and SNR values—that were created using the hierarchical regression procedure.
Table 5.
Summary of “enter” hierarchical regression analysis for variables.
| Variable | R 2 change | β | p |
|---|---|---|---|
| Model 1 | .21 | .023 | |
| Digit Symbol Coding | −.45 | .023 | |
| Model 2 | .50 | .002 | |
| Digit Symbol Coding | −.29 | .155 | |
| 80-Hz SNR | .52 | .003 | |
| 400-Hz SNR | −.29 | .163 |
Note. SNR = signal-to-noise ratio.
Although the older adults had clinically normal hearing, their thresholds were worse at all frequencies tested. For this reason, a “stepwise” linear regression was conducted to avoid the bias of entry order, and we included the 4-kHz threshold (see Table 6). Two models were generated. The first model, including only the 80-Hz ASSR variable, was a good fit for the data, F(1, 23) = 6.78, p = .016, with an R 2 value of .23. The second model, including both the 80-Hz ASSR and Digit Symbol Coding variables, was also a good fit for the data, F(1, 22) = 8.77, p = .007, with an R 2 value of .45. The remaining variables (4-kHz threshold and 400-Hz rate of SNR) were excluded from the models.
Table 6.
Summary of “stepwise” hierarchical regression analysis for variables.
| Variable | R 2 change | β | p |
|---|---|---|---|
| Model 1 | .23 | .016 | |
| 80-Hz SNR | .48 | .016 | |
| Model 2 | .22 | .007 | |
| 80-Hz SNR | .23 | .49 | .005 |
| Digit Symbol Coding | .22 | −.47 | .007 |
Note. SNR = signal-to-noise ratio.
Discussion
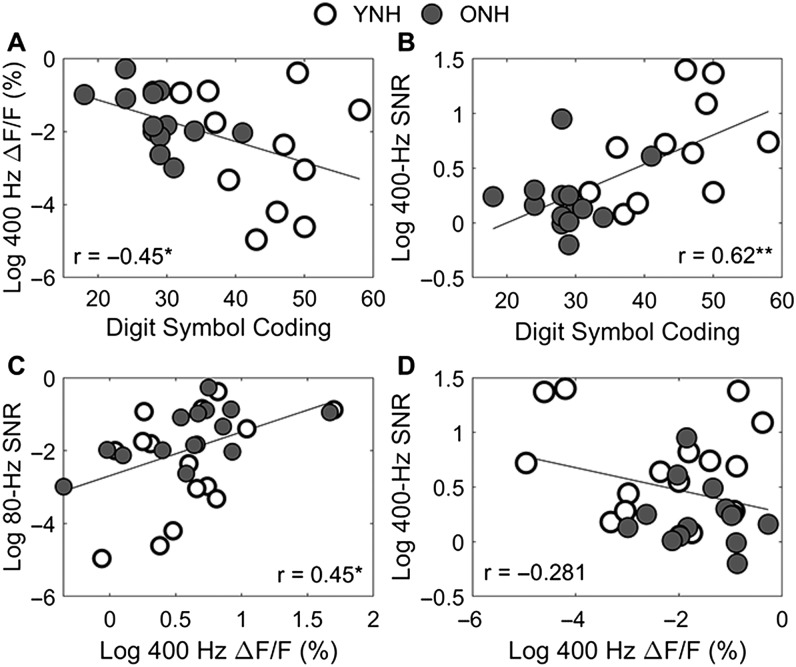
The purpose of the study was to investigate aging effects on encoding of rapid acoustic pulse trains and to determine if neural encoding relates to perceptual performance. The results of this study provide support for some, but not all, of the posited hypotheses. Across participants, there was a decline in perceptual rate discrimination performance when comparing 80- to 400-Hz absolute JNDs. There was also a decrease in ASSR SNRs with increasing rate, but only in the ONH participants. There were no group differences in perceptual results (see Figure 4). However, the YNH participants had higher overall ASSR SNRs than the ONH participants (see Figures 2 and 3), and this group effect was driven by a significant difference at the 400-Hz rate. There was a significant Group × Rate interaction, driven by a steeper decline in SNRs with increasing rate in the older compared with the younger participants. We also found that speed of processing and ASSR SNR values accounted for significant variance in the 400-Hz relative JND (see Figure 5).
Figure 5.
Scatter plots demonstrating correlations among speed of processing, perceptual, and neural variables in younger normal-hearing (YNH; white) and older normal-hearing (ONH; gray) participants. (A) Digit Symbol Coding raw score and 400-Hz relative just noticeable difference (JND). (B) Digit Symbol Coding raw score and auditory steady-state response (ASSR) 400-Hz signal-to-noise ratio (SNR). (C) 400-Hz relative JND and ASSR 80-Hz SNR. (D) 400-Hz relative JND and ASSR 400-Hz SNR. Note that the JND and ASSR values have been log transformed because of skewed data. Note that Panels A and B display an N of 25, and Panels C and D display an N of 30. *p < .05, **p < .01.
Effects of Rate
Figure 4 displays perceptual performance in YNH and ONH participants. Absolute JNDs in Hertz for all participants were higher for faster rates. The increase in JNDs suggests that both participant groups require greater absolute frequency differences between the target and reference rates to identify the change in rate—particularly for faster pulse rate conditions. When observing the relative frequency differences, JNDs decreased with increasing rate, which may be a result of resolved harmonics for the 400-Hz pulse trains (Carlyon & Deeks, 2002). Including more intermediate rates may help clarify this issue. Carlyon and Deeks (2002) also tested rate discrimination using pulse trains at a higher center frequency of approximately 9000 Hz, because the broader filters for this higher frequency would result in resolved harmonics at higher rates. However, the use of higher frequencies in an aging study is problematic, because of the elevation of high-frequency thresholds associated with presbycusis. The finding of increased absolute JNDs with increasing rate, especially at 400 Hz, is consistent with expectations; however, the decreased relative JNDs with increasing rate may be confounded by resolved harmonics in the participants of this study; therefore, we cannot speak directly to age-related differences in rate limitations. In the future, testing age-related changes in rate limitations with CI participants would clarify this issue, because one does not need to consider the physical filtering of stimuli with electrical stimulation.
Similar to what was observed for absolute JNDs, a significant effect of rate is also apparent in Figure 2, which shows the ASSR spectral energy for YNH and ONH participants as a function of rate. Both groups had relatively robust spectral energy at lower pulse rates of 20 and 40 Hz, with the greatest energy at 40 Hz as expected given previous findings of maximum amplitudes for the 40-Hz rate compared with other rates (Picton et al., 2003; Stapells, Linden, Suffield, Hamel, & Picton, 1984). Although spectral energy is apparent at higher rates, there is a decrease of energy at higher rates, especially at 400 Hz. These results parallel the decrease in rate discrimination for participants at faster pulse rates. The declines are similar in the two groups, but there is a steeper decline in energy in ONH participants. These effects are consistent with the results of Rees et al. (1986), who found that response amplitude to AM stimuli decreased with increasing rates from 0.5 to 400 Hz. Figure 3 summarizes the rate effects on SNR, and in this figure, the SNR decreases are only seen in the ONH group. The decreasing amplitudes would be expected given that the higher rates originate from more peripheral sources at greater distances from the recording electrodes.
Effects of Age
Contrary to our hypothesis, there was neither a main effect of Age nor an Age × Rate interaction in the perceptual rate discrimination experiment. Although we noted no group differences in rate discrimination, we observed larger variability in perceptual performance for the 200- and 400-Hz rates than for the 80-Hz rate (see Figure 4). Therefore, although the means were lower in the YNH group than in the ONH group, especially at the higher rates, the large variability at these rates would necessitate a larger number of participants than were recruited to demonstrate group differences. It is also worth noting that many of the ONH participants had extensive experience participating in psychoacoustic perceptual tasks, compared with a relatively more naive group of YNH participants, which may have contributed to the lack of group differences and interactions observed. We did observe that the group differences gradually increased with rate; although the interaction was not significant, we may have seen differences emerge at higher frequencies above 400 Hz.
Despite the apparent differences in spectral energy on Figure 2, especially at relatively high rates, significant group differences in ASSR spectral energy were not found at any rate. However, there were higher overall levels of neural activity in the older group, especially at the 400-Hz rate, in the time regions corresponding to no stimulation. Therefore, these higher levels of activity raised baseline activity in the older adults, thus reducing group differences in spectral energy. This increase in baseline activity may reflect age-related reductions in inhibitory neurotransmission that have been noted in rodent aging studies (Caspary, Milbrandt, & Helfert, 1995; Caspary, Schatteman, & Hughes, 2005). In particular, Caspary and colleagues (2005) have found a decrease in the number of glycinergic markers in the dorsal cochlear nuclei of older rats compared with younger rats, resulting in higher maximum discharge rates in the fusiform cells in the older rats. The SNR is therefore a more sensitive measure of group differences in the auditory system's ability to lock onto rates of stimulus presentation than ASSR spectral energy.
The Group × Rate interaction for the ASSR SNR was driven by an accelerated decrease in spectral energy with rate in the older compared with the younger group. To some extent, the use of different rates permits localization of the source of neural energy. Higher modulation rates have more peripheral sources than lower rates, although there may be cortical contributions even at higher rates (Herdman et al., 2002). To our knowledge, only one study has measured the ASSR at rates as high as 400 Hz, and previous source localization studies have not evaluated rates higher than 80–100 Hz (Herdman et al., 2002; Weisz & Lithari, 2017). The current study was not designed to explicitly assess source localization; however, general inferences may be made regarding neural sources based on response latency. Examination of the data revealed latencies of approximately 4.0–5.0 ms to the 400-Hz rate, 7.5–8.5 ms to the 200-Hz rate, 20–25 ms to the 80-Hz rate, 25–35 ms to the 40-Hz rate, and 40–70 ms to the 20-Hz rate. The only previous study that recorded ASSRs to the 400- and 200-Hz rates did not report latency values (Rees et al., 1986), but the values that were obtained for the rates from 20 to 80 Hz are consistent with the findings of Herdman et al. (2002), suggesting increased cortical contributions with lower rates. The latency values of 4.0–5.0 ms suggest a lower brainstem source for the 400-Hz rate (cochlear nucleus/superior olivary complex), whereas the values of 7.5–8.5 ms would suggest a more midbrain source for the 200-Hz rate. Therefore, the group effect that was specific to the higher rate (400 Hz) may reflect a relatively peripheral/lower brainstem origin. The lack of group differences at the higher rates is consistent with the results of previous aging ASSR studies (Goossens et al., 2016; Grose et al., 2009) and may suggest central compensation, which has been noted in previous studies (Chambers et al., 2016; Presacco, Simon, & Anderson, 2016). Finally, the lack of behavioral differences at 400 Hz in the presence of significant group differences for the 400-Hz ASSR SNR suggests that neural measures may be more sensitive to age-related decreases in temporal processing than behavioral measures. Behavioral performance can be affected by many factors including level of effort and attention. Listening effort studies have demonstrated that older adults may expend more effort to achieve equivalent perceptual performance to younger adults (Kuchinsky et al., 2016; Ward, Shen, Souza, & Grieco-Calub, 2017; Zekveld, Kramer, & Festen, 2011).
Relationships Among Neural, Perceptual, and Cognitive Variables
We found that speed of processing and neural responses each predicted a significant amount of variance in perceptual performance on the 400-Hz rate. Although there were age-related deficits in neural representation to the 400-Hz rate, older adults' rate discrimination, even at higher rates, may match rate discrimination in younger adults if their performance is aided by higher speed of processing. We also found that speed of processing positively correlated with the strength of neural responses to the higher rates of 200 and 400 Hz. We expected that cognitive processing speed may partially explain the perceptual performance, as a previous study found that processing speed relates to another example of temporal processing—detection of gaps in noise (Harris, Eckert, Ahlstrom, & Dubno, 2010). In that study, higher scores on their processing speed measure related to better gap detection thresholds across a group of younger and older adults. In a follow-up study, Harris, Wilson, Eckert, and Dubno (2012) found that attention modulation of cortical-evoked responses to gaps in noise related to behavioral gap detection thresholds and measures of processing speed. Although processing speed related to temporal encoding at earlier levels of the auditory system in our study compared with the Harris et al. (2012) study, perhaps, deficits in early temporal coding have downstream consequences that may affect other nonauditory functions. This finding would lend support for the general idea that decreases in the quality of sensory information flowing from the periphery to the cortex can result in cognitive declines (Smith et al., 2009).
Summary and Conclusion
We investigated the effects of aging and stimulus pulse-train rate on perceptual performance and neural representation. We found age-related deficits in the 400-Hz ASSR, but perceptual performance was equivalent between groups. We also found an Age × Rate interaction in the ASSR, driven by a steep decline in ASSR SNRs with rate in the ONH participants that was not seen in the YNH participants. Although we did not see age-related group differences on our rate discrimination task, it is likely that the neural systems in older adults have a decreased ability to synchronize to fast rates. This decreased ability may contribute to deficits on tasks that rely on the ability to respond to rapid changes in acoustic stimuli, including speech, especially when cognitive resources are inadequate to compensate for these deficits. Finally, the deficits in the neural but not behavioral data suggest that behavioral measures obtained during an audiometric examination may not capture the extent of age-related deficits that may become apparent in difficult listening situations.
Acknowledgments
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award R01AG051603 (M. J. G.) and the National Institute on Deafness and Other Communication Disorders under Award R21 DC015843 (S. A.). This work was also supported by a Tier IV seed grant from the Office of the Vice President for Research at the University of Maryland, College Park. We would like to thank Erin Walter for help with data collection. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding Statement
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award R01AG051603 (M. J. G.) and the National Institute on Deafness and Other Communication Disorders under Award R21 DC015843 (S. A.). This work was also supported by a Tier IV seed grant from the Office of the Vice President for Research at the University of Maryland, College Park.
References
- American National Standards Institute. (2010). Specification for audiometers (Standard No. S3.6-2010). Retrieved from https://webstore.ansi.org/RecordDetail.aspx?sku=ANSI%2FASA+S3.6-2010#
- Anderson S., Parbery-Clark A., White-Schwoch T., & Kraus N. (2012). Aging affects neural precision of speech encoding. The Journal of Neuroscience, 32(41), 14156–14164. https://doi.org/10.1523/jneurosci.2176-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein L. R., & Trahiotis C. (2002). Enhancing sensitivity to interaural delays at high frequencies by using “transposed stimuli.” The Journal of the Acoustical Society of America, 112(3, Pt. 1), 1026–1036. https://doi.org/10.1121/1.1497620 [DOI] [PubMed] [Google Scholar]
- Brown A. D., & Stecker G. C. (2010). Temporal weighting of interaural time and level differences in high-rate click trains. The Journal of the Acoustical Society of America, 128(1), 332–341. https://doi.org/10.1121/1.3436540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyon R. P., & Deeks J. M. (2002). Limitations on rate discrimination. The Journal of the Acoustical Society of America, 112(3, Pt. 1), 1009–1025. https://doi.org/10.1121/1.1496766 [DOI] [PubMed] [Google Scholar]
- Carlyon R. P., Long C. J., & Deeks J. M. (2008). Pulse-rate discrimination by cochlear-implant and normal-hearing listeners with and without binaural cues. The Journal of the Acoustical Society of America, 123(4), 2276–2286. https://doi.org/10.1121/1.2874796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary D. M., Milbrandt J. C., & Helfert R. H. (1995). Central auditory aging: GABA changes in the inferior colliculus. Experimental Gerontology, 30(3–4), 349–360. https://doi.org/10.1016/0531-5565(94)00052-5 [DOI] [PubMed] [Google Scholar]
- Caspary D. M., Schatteman T. A., & Hughes L. F. (2005). Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: Role of inhibitory inputs. The Journal of Neuroscience, 25(47), 10952–10959. https://doi.org/10.1523/jneurosci.2451-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHABA (Committee on Hearing, Bioacoustics, and Biomechanics). (1988). Speech understanding and aging. The Journal of the Acoustical Society of America, 83(3), 859–895. https://doi.org/10.1121/1.395965 [PubMed] [Google Scholar]
- Chambers A. R., Resnik J., Yuan Y., Whitton J. P., Edge A. S., Liberman M. C., & Polley D. B. (2016). Central gain restores auditory processing following near-complete cochlear denervation. Neuron, 89(4), 867–879. https://doi.org/10.1016/j.neuron.2015.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary I. J., Johnson W., & Starr J. M. (2010). Are processing speed tasks biomarkers of cognitive aging? Psychology and Aging, 25(1), 219–228. https://doi.org/10.1037/a0017750 [DOI] [PubMed] [Google Scholar]
- Dupuis K., Pichora-Fuller M. K., Chasteen A. L., Marchuk V., Singh G., & Smith S. L. (2015). Effects of hearing and vision impairments on the Montreal Cognitive Assessment. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 22(4), 413–437. https://doi.org/10.1080/13825585.2014.968084 [DOI] [PubMed] [Google Scholar]
- Goossens T., Vercammen C., Wouters J., & van Wieringen A. (2016). Aging affects neural synchronization to speech-related acoustic modulations. Frontiers in Aging Neuroscience, 8, 133 https://doi.org/10.3389/fnagi.2016.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Salant S., & Fitzgibbons P. J. (1993). Temporal factors and speech recognition performance in young and elderly listeners. Journal of Speech and Hearing Research, 36(6), 1276–1285. https://doi.org/10.1044/jshr.3606.1276 [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S., Yeni-Komshian G., & Fitzgibbons P. (2008). The role of temporal cues in word identification by younger and older adults: Effects of sentence context. The Journal of the Acoustical Society of America, 124(5), 3249–3260. https://doi.org/10.1121/1.2982409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Salant S., Yeni-Komshian G. H., Fitzgibbons P. J., & Barrett J. (2006). Age-related differences in identification and discrimination of temporal cues in speech segments. The Journal of the Acoustical Society of America, 119(4), 2455–2466. https://doi.org/10.1121/1.2171527 [DOI] [PubMed] [Google Scholar]
- Goupell M. J., Laback B., & Majdak P. (2009). Enhancing sensitivity to interaural time differences at high modulation rates by introducing temporal jitter. The Journal of the Acoustical Society of America, 126(5), 2511–2521. https://doi.org/10.1121/1.3206584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimault N., Micheyl C., Carlyon R. P., Bacon S. P., & Collet L. (2003). Learning in discrimination of frequency or modulation rate: Generalization to fundamental frequency discrimination. Hearing Research, 184(1–2), 41–50. https://doi.org/10.1016/S0378-5955(03)00214-4 [DOI] [PubMed] [Google Scholar]
- Grose J. H., Mamo S. K., & Hall J. W. III (2009). Age effects in temporal envelope processing: Speech unmasking and auditory steady state responses. Ear and Hearing, 30(5), 568–575. https://doi.org/10.1097/AUD.0b013e3181ac128f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafter E. R., & Buell T. N. (1990). Restarting the adapted binaural system. The Journal of the Acoustical Society of America, 88(2), 806–812. https://doi.org/10.1121/1.399730 [DOI] [PubMed] [Google Scholar]
- Hafter E. R., Dye R. H. Jr., & Wenzel E. (1983). Detection of interaural differences of intensity in trains of high-frequency clicks as a function of interclick interval and number. The Journal of the Acoustical Society of America, 73(5), 1708–1713. https://doi.org/10.1121/1.389394 [DOI] [PubMed] [Google Scholar]
- Harris K. C., Eckert M. A., Ahlstrom J. B., & Dubno J. R. (2010). Age-related differences in gap detection: Effects of task difficulty and cognitive ability. Hearing Research, 264(1–2), 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K. C., Wilson S., Eckert M. A., & Dubno J. R. (2012). Human evoked cortical activity to silent gaps in noise: Effects of age, attention, and cortical processing speed. Ear and Hearing, 33(3), 330–339. https://doi.org/10.1097/AUD.0b013e31823fb585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdman A. T., Lins O., Van Roon P., Stapells D. R., Scherg M., & Picton T. W. (2002). Intracerebral sources of human auditory steady-state responses. Brain Topography, 15(2), 69–86. https://doi-org.proxy-um.researchport.umd.edu/10.1023/A:1021470822922 [DOI] [PubMed] [Google Scholar]
- Hox J. J., Moerbeek M., & Van de Schoot R. (2017). Multilevel analysis: Techniques and applications (3rd ed.). New York, NY: Routledge. [Google Scholar]
- Kong Y. Y., Cruz R., Jones J. A., & Zeng F. G. (2004). Music perception with temporal cues in acoustic and electric hearing. Ear and Hearing, 25(2), 173–185. https://doi.org/10.1097/01.AUD.0000120365.97792.2F [DOI] [PubMed] [Google Scholar]
- Kuchinsky S. E., Vaden K. I. Jr., Ahlstrom J. B., Cute S. L., Humes L. E., Dubno J. R., & Eckert M. A. (2016). Task-related vigilance during word recognition in noise for older adults with hearing loss. Experimental Aging Research, 42(1), 50–66. https://doi.org/10.1080/0361073x.2016.1108712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laback B., Dietz M., & Joris P. (2017). Temporal effects in interaural and sequential level difference perception. The Journal of the Acoustical Society of America, 142(5), 3267 https://doi.org/10.1121/1.5009563 [DOI] [PubMed] [Google Scholar]
- Macherey O., & Carlyon R. P. (2014). Re-examining the upper limit of temporal pitch. The Journal of the Acoustical Society of America, 136(6), 3186 https://doi.org/10.1121/1.4900917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. L., & Kingston R. A. (2014). Age-related decline in emotional prosody discrimination: Acoustic correlates. Experimental Psychology, 61(3), 215–223. https://doi.org/10.1027/1618-3169/a000241 [DOI] [PubMed] [Google Scholar]
- Nasreddine Z. S., Phillips N. A., Bédirian V., Charbonneau S., Whitehead V., Collin I., … Chertkow H. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. http://doi.org/10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Picton T. W., John M. S., Dimitrijevic A., & Purcell D. (2003). Human auditory steady-state responses. International Journal of Audiology, 42(4), 177–219. https://doi.org/10.3109/14992020309101316 [DOI] [PubMed] [Google Scholar]
- Presacco A., Bohórquez J., Yavuz E., & Özdamar Ö. (2010). Auditory steady-state responses to 40-Hz click trains: Relationship to middle latency, gamma band and beta band responses studied with deconvolution. Clinical Neurophysiology, 121(9), 1540–1550. http://doi.org/10.1016/j.clinph.2010.03.020 [DOI] [PubMed] [Google Scholar]
- Presacco A., Simon J. Z., & Anderson S. (2016). Evidence of degraded representation of speech in noise, in the aging midbrain and cortex. Journal of Neurophysiology, 116(5), 2346–2355. https://doi.org/10.1152/jn.00372.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees A., Green G. G., & Kay R. H. (1986). Steady-state evoked responses to sinusoidally amplitude-modulated sounds recorded in man. Hearing Research, 23(2), 123–133. https://doi.org/10.1016/0378-5955(86)90009-2 [DOI] [PubMed] [Google Scholar]
- Smith G. E., Housen P., Yaffe K., Ruff R., Kennison R. F., Mahncke H. W., & Zelinski E. M. (2009). A cognitive training program based on principles of brain plasticity: Results from the improvement in memory with plasticity-based adaptive cognitive training (IMPACT) study. Journal of the American Geriatrics Society, 57(4), 594–603. https://doi.org/10.1111/j.1532-5415.2008.02167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapells D. R., Linden D., Suffield J. B., Hamel G., & Picton T. W. (1984). Human auditory steady state potentials. Ear and Hearing, 5(2), 105–113. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C., Bertrand O., Delpuech C., & Pernier J. (1996). Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. The Journal of Neuroscience, 16(13), 4240–4249. https://doi.org/10.1523/JNEUROSCI.16-13-04240.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward K. M., Shen J., Souza P. E., & Grieco-Calub T. M. (2017). Age-related differences in listening effort during degraded speech recognition. Ear and Hearing, 38(1), 74–84. https://doi.org/10.1097/aud.0000000000000355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (1997). Wechsler Adult Intelligence Scale–Third Edition (WAIS-III). San Antonio, TX: Pearson Assessment. [Google Scholar]
- Weisz N., & Lithari C. (2017). Amplitude modulation rate dependent topographic organization of the auditory steady-state response in human auditory cortex. Hearing Research, 354, 102–108. https://doi.org/10.1016/j.heares.2017.09.003 [DOI] [PubMed] [Google Scholar]
- Zekveld A. A., Kramer S. E., & Festen J. M. (2011). Cognitive load during speech perception in noise: The influence of age, hearing loss, and cognition on the pupil response. Ear and Hearing, 32(4), 498–510. https://doi.org/10.1097/AUD.0b013e31820512bb [DOI] [PubMed] [Google Scholar]