Abstract
Purpose
The purpose of this study was to investigate the information-processing strategies of early-implanted, prelingually deaf cochlear implant (CI) users with the California Verbal Learning Test–Second Edition (CVLT-II; Delis, Kramer, Kaplan, & Ober, 2000), a well-established normed measure of verbal learning and memory used in neuropsychological assessments of memory loss.
Method
Verbal learning and memory skills were compared in 20 older adolescent and young adult prelingually deaf long-term early-implanted CI users and their 24 normal hearing (NH) peers using the CVLT-II, a widely used multitrial free recall test of verbal learning and memory.
Results
On average, CI users recalled fewer words than their NH peers across the immediate, delayed, and cued recall trials of the CVLT-II but were comparable to their NH peers on yes/no recognition memory. CI users showed little evidence of semantic clustering of words during free recall but greater serial clustering compared to their NH peers, suggesting fundamental disturbances in automatic semantic activation of words from long-term memory. No differences were found in verbal memory between CI users and their NH peers on measures of retroactive interference and encoding/retrieval interactions. Performance on the 2nd word list of the CVLT-II (List B) and amount of semantic clustering of words during recall were correlated with sentence recognition in the CI group.
Conclusion
Study findings demonstrate significant differences in free recall performance and information-processing strategies that early-implanted, prelingually deaf CI users use to encode, organize, store, and retrieve spoken words in conventional verbal list learning paradigms, compared to their NH peers. Because verbal learning and memory are core foundational processes routinely used in daily functioning for a wide range of neurocognitive and language processing operations, these findings suggest potential domains for assessment and novel interventions to promote the development of optimal outcomes in prelingually deaf early-implanted long-term CI users.
Cochlear implants (CIs) are surgically implanted devices that are designed to restore the sense of hearing in profoundly deaf individuals who have severe-to-profound sensorineural hearing loss. CIs are now routinely used as a medical intervention to treat children and adults with profound sensorineural hearing loss and are considered “one of the great success stories of modern medicine” (Wilson, Dorman, Woldorff, & Tucci, 2011). However, the coarsely coded sensory representations of sound transmitted by CIs to the auditory nerve result in only partial hearing restoration, providing listeners with compromised and degraded acoustic information about the signal. After implantation, almost all patients need a period of perceptual adaptation and auditory rehabilitation to hear (i.e., detect and discriminate) and process (i.e., recognize, identify, and categorize) underspecified acoustic–phonetic information encoded in the degraded signal. Following adaptation and a period of auditory rehabilitation, many deaf and hard-of-hearing patients are able to achieve substantial benefits from their implants to perceive speech, understand spoken language, and recognize natural environmental sounds.
Although CIs often work very well for many patients, CIs do not provide equivalent benefits for all candidates who receive them, and enormous individual differences and variability in outcomes and benefit following implantation are routinely reported in the literature (Niparko et al., 2010; Pisoni, Cleary, Geers, & Tobey, 1999). Conventional demographic, hearing history, and device factors such as the age of implantation, audiologic variables, and device parameters have been extensively studied in the past and account for some of this variability (Niparko et al., 2012; Young & Kirk, 2016). Recently, cognitive processes involving sequence learning, short-term memory capacity, and working memory dynamics have been found to account for a significant portion of the additional unexplained variance (Conway, Pisoni, & Kronenberger, 2009; Heydebrand, Hale, Potts, Gotter, & Skinner, 2007; Holden et al., 2013; Pisoni & Cleary, 2003; Pisoni & Geers, 2000). These findings suggest that it is not just the ability to encode and recognize auditory signals but also downstream neurocognitive processing operations that involve organization, learning, and retrieval of verbal information from both short- and long-term memory that influence speech and language outcomes.
An extensively investigated subdomain of neurocognitive functioning in prelingually deaf adolescents and young adults with CIs has been verbal short-term and working memory (Nittrouer, Caldwell-Tarr, & Lowenstein, 2013; Pisoni, Kronenberger, Roman, & Geers, 2011). Verbal short-term memory consists of immediate memory for words, which is held in a phonological loop (Baddeley, 2012), whereas verbal working memory reflects maintenance of information in memory while other cognitive processing demands are present (Engle, Tuholski, Laughlin, & Conway, 1999). The presence of concurrent processing demands requires that the individual to actively allocate cognitive resources using executive functioning (EF; Diamond, 2013). Processing the degraded, underspecified input of speech signals provided by a CI presents additional processing demands beyond those necessary for normal hearing (NH). Thus, for CI users, verbal short-term memory tasks include a significant working memory component. Studies of verbal working memory in CI users have found deficits relative to their NH peers (Kronenberger, Colson, Henning, & Pisoni, 2014; Nittrouer et al., 2013; Pisoni et al., 2011). Although most of the current research on verbal memory in CI users has used working memory span tasks, other components of verbal memory have not been investigated. One such paradigm for investigating other components of verbal memory and learning processes in clinical populations is the multitrial free recall paradigm (Delis, Kramer, Kaplan, & Ober, 1994, 2000).
In a multitrial free recall paradigm, the participant is presented with repeated exposures to a study word list and then asked to recall words from the list in any order after each trial (Delis et al., 2000). Subsequent trials introduce a new word list to assess proactive interference (PI; effects of earlier learned information interfering with memory of later information), followed by recall of the original word list to evaluate retroactive interference (RI; effects of later learned information interfering with earlier memory). Measures of cued recall, delayed recall, and recognition memory are also used to investigate long-term memory, storage, and retrieval and to assess the benefits of retrieval cues that facilitate recall. Verbal learning and memory skills are critically important in everyday daily functioning because they are routinely used in a wide range of spoken language processing tasks involving both receptive and expressive skills.
At the present time, there are no studies that have investigated verbal learning and memory in prelingually deaf, long-term adolescent and young adult CI users who experienced a period of deafness prior to cochlear implantation at an early age in childhood. These prelingually deaf, CI users acquired their first language using compromised and degraded auditory input from a CI, after a period of early sensory auditory deprivation and reduced exposure to spoken language. In this study, we used the California Verbal Learning Test–Second Edition (CVLT-II; Delis et al., 2000), a normed, standardized neuropsychological test of multitrial verbal learning and memory, to study the effects of early auditory deprivation, delayed language acquisition, and sparsely coded auditory input from a CI on the verbal learning and memory processes of early-implanted, prelingually deaf adolescents and young adults that used CIs for at least 13 years (and an average of 17.79 years; see Table 1). In order to better understand the association between speech perception outcomes and verbal learning and memory, we also investigated associations between measures of verbal learning and memory obtained from the CVLT-II and sentence recognition skills in CI users.
Table 1.
Sample characteristics.
| Study measures | CI sample (n = 20) |
NH sample (n = 24) |
t | p | ||||
|---|---|---|---|---|---|---|---|---|
| M | (SD) | Range | M | (SD) | Range | |||
| Demographics and hearing history | ||||||||
| Chronological age a | 21.94 | (3.77) | 16.74–29.97 | 22.49 | (2.88) | 17.4–29.28 | −0.54 | .59 |
| Age at implantation b | 49.68 | (20.94) | 18.66–75.76 | n/a | — | — | — | — |
| Duration of CI use a | 17.8 | (3.14) | 13.8–24.5 | n/a | — | — | — | — |
| Age of onset of deafness b | 6.85 | (11.15) | 0–36 | n/a | — | — | — | — |
| Preimplant PTA c | 109.45 | (10.15) | 85–118.43 | n/a | — | — | — | — |
| Communication mode d | 4.75 | (0.79) | — | n/a (NH) | — | — | — | — |
| Income level e | 6.39 | (2.75) | 1–10 | 6.83 | (2.85) | 1–10 | −0.65 | .52 |
| (2 Unknown) | ||||||||
| Nonverbal intelligence f | 100.35 | (11.82) | 85–120 | 106.25 | −9.65 | 78–120 | −1.79 | .08 |
| Sex (female/male) | 9/11 | — | — | 8/16 | — | — | — | — |
| Sentence recognition | ||||||||
| Harvard-S | 53.7 | (30.28) | 0–91 | 97.88 | (1.54) | 95–100 | −6.52 | < .01 |
| Harvard-A | 32.9 | (22.25) | 0–72 | 91.88 | (3.86) | 84–100 | −11.71 | < .01 |
| PRESTO | 39.3 | (27.35) | 0–78 | 97.75 | (1.59) | 94–100 | −9.54 | < .01 |
| PRESTO-FAE | 24.55 | (18) | 0–57 | 84.42 | (5.05) | 72–93 | −14.41 | < .01 |
Note. CI = cochlear implant; NH = normal hearing; Harvard-S = Harvard Standard Sentences Test; Harvard-A = Harvard Anomalous Sentences Test; PRESTO = Perceptually Robust English Sentence Test Open-Set; PRESTO-FAE = PRESTO–Foreign-Accented English.
In years.
In months.
PTA = preimplant unaided pure-tone average for frequencies 500, 1000, and 2000 Hz in dB HL.
Communication mode coded mostly sign (1) to auditory-verbal (6), (Geers & Brenner, 2003).
On a 1 (under $5,500) to 10 ($95,000+) scale (Kronenberger, Pisoni, Henning, & Colson, 2013).
Comprehensive Test of Nonverbal Intelligence–Second Edition Geometric Nonverbal IQ Composite Index (normed standard score).
Heydebrand et al. (2007) administered a nonstandard version of the CVLT-II with simultaneous visual (printed words) and live voice auditory presentation of the test lists before implantation to a group of 33 postlingually deaf adults who were candidates for cochlear implantation. They found that verbal learning, as measured by a composite free recall score based on four CVLT-II subscores, was a strong predictor (42% of variance) of monosyllabic word recognition scores postimplantation after controlling for speech recognition before implantation. Their findings suggest that several core components of verbal learning and memory may play a central role in speech and language outcomes because they share common variance with other information-processing tasks used to measure speech recognition and spoken language understanding. In a second follow-up study with a larger sample, Holden et al. (2013) studied 114 postlingually deaf adult CI users and also found correlations between a CVLT-II composite free recall score and monosyllabic word recognition measured at various intervals postimplantation.
Other than these two studies on postlingually deaf adults with CIs by Heydebrand et al. (2007) and Holden et al. (2013) using the CVLT-II, all of the prior memory research with prelingually deaf, early-implanted long-term CI users has been carried out on short-term memory capacity and working memory dynamics (i.e., processes that involve scanning and retrieving the contents of verbal short-term memory, verbal rehearsal speed, and encoding speed) using measures of immediate memory span. The initial interest in short-term memory and working memory is based on the hypothesis that verbal processing of coarsely coded underspecified phonological and lexical representations of words in long-term memory taxes controlled attention and working memory capacity and that CI users are at risk for delays and disturbances in subdomains of EF, including controlled attention (Pisoni & Cleary, 2004).
In an early study, Pisoni and Geers (2000) reported that the auditory digit spans of pediatric CI users were significantly shorter than age-matched NH controls and were strongly correlated with several behavioral tasks that measured spoken word recognition, speech production, spoken language comprehension, and reading. These initial findings were replicated and extended by Pisoni and Cleary (2003) using a much larger sample of pediatric CI users. Even after controlling for confounding demographic variables, Pisoni and Cleary found that pediatric CI users consistently performed worse on both forward and backward digit span tasks than age-matched NH controls. Further converging support for these initial proposals about the role of verbal short-term and working memory dynamics was reported by Burkholder and Pisoni (2003), who also found shorter digit spans along with slower articulation rates in CI users. They attributed these additional findings to reduced speed of subvocal verbal rehearsal and slower memory scanning (i.e., retrieval) of spoken digits in verbal short-term memory due to the absence of early auditory experience before implantation (see also Cowan et al., 1998).
The earlier studies carried out by Heydebrand et al. (2007) and Holden et al. (2013) provided new information about verbal learning and memory in adult postlingually deaf CI users, adding to previous findings showing delayed verbal short-term memory and working memory in prelingually deaf, early-implanted child CI users. Findings linking measures of verbal learning and memory with speech perception following implantation further established the critical importance of these core foundational cognitive domains for understanding differences in outcomes after implantation. However, both of these studies of verbal learning and memory used postlingually deaf adults who were implanted late in adulthood and the authors reported only a global composite measure of CVLT-II performance, as opposed to specific subscores from the CVLT-II that could be a rich source of additional information about the core processes underlying verbal learning and memory in this clinical population. The CVLT-II incorporates several foundational principles from cognitive psychology and cognitive science to measure different memory and learning processes during verbal list learning (Delis et al., 2000). Examples of the kinds of measures that the CVLT-II can provide include not only global measures of immediate, short-term, and long-term delayed and cued free recall but also measures of recognition memory, interference effects, and learning strategies (i.e., semantic vs. serial clustering), as well as several methods to assess the interaction of encoding versus retrieval processes in this well-defined information-processing task (Atkinson & Shiffrin, 1968).
One of the strongest predictors of speech and language outcomes following implantation is whether hearing loss and implantation occurred before or after language was acquired (Dawson et al., 1992; Niparko et al., 2012; Zwolan, Kileny, & Telian, 1996). Unlike prior CVLT-II research, which studied postlingually deaf adults with CIs, the focus of the current study was on the verbal learning and memory skills of prelingually deaf adolescent and young adult CI users compared to their NH peers who did not differ on nonverbal IQ. Based on earlier studies of CI users, we expected to see reduced overall number of words recalled on the CVLT-II.
Furthermore, it is important to understand factors that might explain individual differences in verbal learning and memory skills in CI users. Prior research has demonstrated associations between speech perception skills and component processes of verbal working memory in CI users (Kronenberger, Colson, et al., 2014). These associations occur because speech recognition processes are assumed to make use of the same elementary processes of encoding, storage, and retrieval that are involved in free recall tasks. We therefore expected to find associations in the sample of CI users between several of the CVLT-II measures of verbal learning and memory and speech perception skills, as measured by sentence recognition tests.
Method
Participants
Participants were 20 prelingually deaf adolescent and young adult CI users (16 with unilateral implants and four with bilateral implants) and their 24 NH peers who met the following inclusion criteria.
Inclusionary criteria for the CI sample were as follows:
severe-to-profound hearing loss (> 70 dB HL) prior to 3 years of age;
cochlear implantation prior to 7 years of age;
use of CI for 7 years or more;
use of a modern, multichannel CI system; and
enrolled in a rehabilitative program (either currently or in the past) that encourages the development of listening and spoken language skills.
Inclusionary criteria for both the CI and NH samples were as follows:
age of 16 years or older,
English-speaking household,
no other neurological or neurodevelopmental disorders or delays documented in chart or reported by parents, and
nonverbal IQ of > 70 as measured by the Comprehensive Test of Nonverbal Intelligence–Second Edition (Hammill, Pearson, & Lee Wiederholt, 2009) Geometric Scale (nonverbal IQ composite).
The demographic information for both groups is presented in Table 1. The CI and NH samples did not differ on age or nonverbal IQ as measured by the Comprehensive Test of Nonverbal Intelligence–Second Edition (Hammill et al., 2009). In the CI sample, etiology of hearing loss was as follows: unknown, n = 14; meningitis, n = 5; familial/genetic (where at least one other family member had hearing loss, of unknown etiology), n = 1. Participants were paid $20/hr. All participants were native speakers of English and had no other developmental or cognitive problems at the time of testing.
Measures
CVLT-II
The CVLT-II is a well-known neuropsychological test of multitrial verbal free and cued recall and learning using categorized word lists (Delis et al., 2000). The CVLT-II provides a number of scores that assess recall and recognition of two lists of words. List A consists of 16 unique words, with four words selected from each of four semantic categories (furniture, vegetables, ways of traveling, animals). The second list, List B, also has 16 unique words that do not overlap with any of the words on List A. The words on List B were also selected from four semantic categories (musical instruments, animals, vegetables, and rooms of a house). Two of the semantic categories overlapped with the categories used for List A (animals and vegetables), and two of the semantic categories were novel (musical instruments and rooms of a house).
The first five trials in the CVLT-II protocol followed a multitrial free recall paradigm: On each trial, the words on List A are read aloud to subjects in the same order by an examiner. Immediately following each presentation of List A, subjects are asked to verbally report as many items from List A as they can without any constraint on the order in which they recall the study items. The examiner records all responses on a response sheet, including intrusions of items from outside the study list and any repetitions of previously recalled words. In this article, we refer to the first five trials as the “List A learning trials.” The sixth trial on the CVLT-II is the “interference trial,” which uses a new set of words on List B. There is only one presentation of List B. Immediately following the presentation of List B, subjects are asked to freely recall items from this list. After the interference trial, there is a free recall trial of List A items. Subjects are not presented with List A again on this trial but are instead asked to freely recall from memory all of the items on List A that they were presented with during the five learning trials. This recall trial is referred to as the short-delay free recall (SDFR) trial because it is used to measure recall of items from List A after a short delay period during which List B was presented. The SDFR trial is then followed by four cued recall trials, which form the short-delay cued recall (SDCR) phase. On each cued recall trial, one of the four semantic categories of List A is provided as a retrieval cue for recall of items on List A from that category. There is then a 20-min long delay period during which subjects were actively engaged in a nonverbal task in this study. After the 20-min delay, the previous two recall phases are repeated again in the form of the long-delay free recall (LDFR) trial and long-delay cued recall (LDCR) trial of List A. Finally, a yes/no recognition memory test is conducted with the 16 List A items serving as targets. The 32 foils in the recognition test consist of the 16 List B items and 16 novel items of which eight share semantic categories with List A and eight do not. We also added a final auditory identification task for all the items from Lists A and B in which subjects simply repeat each of the test words after the examiner said them aloud to ensure that all of the test words on the lists were recognized and correctly identified.
The CVLT-II protocol allows us to calculate a variety of measures of verbal learning and memory based on the data gathered from the tasks outlined above. These measures included not only the raw scores indicating the performance on each of the recall trials and the recognition memory component of the task but also secondary measures that assessed the extent to which participants organize a list of words serially or semantically. The CVLT-II measures are described briefly in Table 2 and also elaborated on in greater detail in the Results section. The raw and norm-based scores (based on a nationally representative normative sample) of the measures summarized in Table 2 were obtained from the CVLT-II software provided by the test developer or were calculated as described in the manual (Delis et al., 2000). We used raw scores from the CVLT-II measures for all the group comparisons and correlations; the only exception was the “contrast measure” that was used to compare performance on recognition and recall performance, which was based on normed z scores.
Table 2.
California Verbal Learning Test–Second Edition (CVLT-II) scores and descriptions.
| Score | Description |
|---|---|
| List A Trial N | Number of words correctly recalled on free recall trials following the Nth exposure to List A, where N = 1–5 |
| List A Trials 1–5 | Total number of words recalled across all five List A learning trials |
| List B | Number of words correctly recalled in free recall following one exposure to List B, the “interference list” |
| Short-delay free recall (SDFR) | Immediately after List B recall, number of List A words recalled (List A is not read again for this trial or for the remainder of the test) |
| Short-delay cued recall (SDCR) | Immediately after SDFR, number of List A words recalled when the subject is provided with each of the four semantic categories (furniture, vegetables, ways of traveling, animals) for List A as retrieval cues |
| Long-delay free recall (LDFR) | Following a 20-min delay after SDCR, number of List A words recalled |
| Long-delay cued recall (LDCR) | Immediately after LDFR, number of List A words recalled when the subject is provided with each of the four semantic categories (furniture, vegetables, ways of traveling, animals) for List A as retrieval cues |
| Long-delay recognition | Immediately after LDCR, percentage of words identified accurately as List A words from a list of 48 words (16 List A words, 16 List B words, and 16 distractor words) |
| Primacy recall | Percentage of the first four words on the list that are recalled on a trial or set of trials (note that this measure differs from the method of calculating primacy recall in the CVLT-II manual) |
| Recency recall | Percentage of the last four words on the list that are recalled on a trial or set of trials (note that this differs from the method of calculating recency recall in the CVLT-II manual) |
| Serial clustering | For each trial, the chance adjusted bidirectional serial clustering index is obtained by taking the difference between observed number of word pairs recalled that were presented together (i.e., in either forward or backward direction) on the trial and the number of word pairs that would be recalled in the same order as presented (i.e., in either forward or backward direction) due to chance alone; the overall chance-adjusted semantic clustering index for a participant is the mean of the clustering indices computed over List A Trials 1–5 |
| Semantic clustering | Chance-adjusted score for recall clustering based on semantic category is obtained for each trial by taking the difference between observed number of words from the same semantic category that were recalled by a participant in serial contiguity and the expected number of words from the same semantic category that would occur in serial contiguity due to chance alone |
| Intrusions | Words recalled by the subject that were not part of the study list |
| Recall consistency | Percentage of words recalled correctly on any of the List A Trials 2–5 that were also correctly recalled on the previous trial |
Sentence Recognition Measures
Four sentence recognition tests were completed by subjects in order to obtain measures of speech perception under challenging listening conditions. For the Harvard Standard Sentences Test (Harvard-S), participants repeated 28 semantically complex, meaningful sentences selected from the Institute of Electrical and Electronics Engineers (IEEE) corpus, a standard database of sentences used for speech intelligibility testing (Egan, 1948; IEEE, 1969). Sentences were produced in the quiet by one male talker and consisted of six to 10 words with five key words per sentence (e.g., “The boy was there when the sun rose”). The Harvard Anomalous Sentences Test (Harvard-A) is similar to the Harvard-S, except that the sentences lacked semantic coherence (see Herman & Pisoni, 2003; Loebach & Pisoni, 2008). Participants repeated 28 semantically meaningless but grammatically correct sentences modified from the IEEE corpus. Sentences were produced in the quiet by one male talker and consisted of five to 10 words with five key words per sentence (e.g., “These dice bend in a hot desk”). The Perceptually Robust English Sentence Test Open-Set (PRESTO) consists of 18 sentences selected from the well-established Texas Instruments–Massachusetts Institute of Technology speech recognition database (Garofolo, Lamel, Fisher, Fiscus, & Pallett, 1993). Each sentence was spoken by a different male or female talker from one of six regional U.S. dialects (Clopper & Pisoni, 2005). The PRESTO requires listeners to rapidly adapt to changes in the vocal sound source (Gilbert, Tamati, & Pisoni, 2013; Tamati, Gilbert, & Pisoni, 2013). Finally, the PRESTO–Foreign-Accented English (PRESTO-FAE) is a new version of PRESTO in which all of the test sentences were read by nonnative English speakers who differed in degree of foreign accent (Tamati & Pisoni, 2015). The 14 sentences selected for the PRESTO-FAE were drawn from the Speech Perception in Noise Test (SPIN) developed by Kalikow, Stevens, and Elliott (1977). Because perception of sentences spoken by talkers with unfamiliar nonnative accents can be very challenging even for NH native English speakers, it was anticipated that PRESTO-FAE would not show any ceiling effects in the NH group, allowing for adequate variability in sentence recognition scores in that group (Tamati & Pisoni, 2015). For all four sentence recognition tests, scores were number of key words correctly repeated from each sentence.
Procedure
Study procedures were approved by the university institutional review board. Written consent and assent were obtained from all participants prior to initiating study procedures. Participants were tested in a single session and were administered the CVLT-II and sentence recognition tests (Harvard-S, Harvard-A, PRESTO, and PRESTO-FAE) using standard verbal instructions. Sentence recognition tests were presented to all subjects in the quiet at 65 decibels using a high-quality loudspeaker located approximately 3 ft from the subject. CVLT-II words were presented live voice by the examiner as described in the administration manual (Delis et al., 2000). All tests were administered by two licensed and highly experienced speech-language pathologists who had worked extensively with deaf and hard-of-hearing children and young adults.
Results
List Learning (Immediate Free Recall of List A Trials 1–5)
Figure 1 and Table 3 present the total number of items correctly recalled on each trial. In Figure 1, recall performance is shown by the five pairs of vertical bars, labeled A1, A2, …, A5 in the figure. The results for prelingually deaf CI users for each trial are shown in light gray–colored bars, whereas the NH controls are represented by the dark gray bars. NH controls recalled more items correctly than CI users on each of the five learning trials of the CVLT-II. Both groups also showed evidence of repetition-based learning, which is reflected by the progressive increase in the number of words correctly recalled on successive study trials. Statistical assessment of the free recall performance across the five learning trials was done using a 2 × 5 mixed analysis of variance (ANOVA) with group (CI vs. NH) as a between-subjects factor and study trials (Trials A1–A5) as a within-subject factor. The number of words recalled per trial was the dependent variable. Significant main effects were found for group, F(1, 42) = 8.65, p < .01, and study trials, F(4, 168) = 175.67, p < .001; the interaction of Group × Study Trials was not significant, F(4, 168) = 0.153, p = .96. These initial findings indicate that NH subjects recalled more words than CI users after each study trial, that both groups recalled more words for later study trials, and that the rate of learning for the NH controls and CI users was comparable. Results from the t tests for each of these trials are reported in Table 3.
Figure 1.
Number of items recalled across all the California Verbal Learning Test–Second Edition recall trials (five immediate free recall trials on List A, one immediate free recall trial on List B, short-delay free and cued recall trials, long-delay free and cued recall trials). CI = cochlear implant.
Table 3.
California Verbal Learning Test–Second Edition (CVLT-II) scores by sample.
| Measures | CI sample, M (SD) | NH sample, M (SD) | t a | df | p |
|---|---|---|---|---|---|
| List A Trial 1 | 5.55 (1.47) | 7.04 (1.60) | −3.22 | 41.58 | .002** |
| List A Trial 2 | 8.40 (2.35) | 10.08 (2.29) | −2.40 | 40.14 | .021** |
| List A Trial 3 | 10.55 (2.65) | 12.46 (2.25) | −2.55 | 37.5 | .015** |
| List A Trial 4 | 12.05 (2.72) | 13.63 (1.86) | −2.19 | 32.58 | .035** |
| List A Trial 5 | 11.85 (2.72) | 13.42 (2.06) | −2.12 | 34.94 | .041** |
| List A Trials (1–5) | 48.4 (10.26) | 56.63 (8.29) | −2.89 | 36.4 | .007** |
| List B free recall | 5.25 (1.83 ) | 6.63 (2.02) | −2.37 | 41.66 | .023** |
| List A short-delay free recallb | 9.7 (12.04) | 12.04 (2.46) | −2.67 | 35.14 | .011** |
| List A short-delay cued recallb | 10.6 (2.26) | 12.21 (2.65) | −2.14 | 42 | .038** |
| List A long-delay free recall b | 10.75 (3.31) | 11.96 (2.93) | −1.27 | 38.39 | .211 |
| List A long-delay cued recall b | 10.80 (2.46) | 12.67 (2.75) | −2.38 | 41.75 | .022** |
| List A long-delay recognition | 3.26 (0.81) | 3.42 (0.68) | −0.73 | 37.06 | .472 |
| Primacy, List A Trials 1–5 | 31.60 (3.95) | 27.58 (3.15) | 3.68 | 36.07 | < .001 |
| Recency, List A Trials 1–5 | 24.95 (6.46) | 28.21 (5.69 ) | −1.76 | 38.28 | .087 |
| Semantic cluster ratio c | −0.14 (0.78) | 1.40 (1.56) | −4.24 | 35.05 | < .001 |
| Serial cluster ratio c | 1.25 (1.25) | 0.94 (0.81) | −4.24 | 35.05 | < .001 |
| Hearing validity scales | |||||
| List A auditory accuracy d | 15.85 (0.67) | 16 | −1 | 19 | .33 |
| List B auditory accuracy d | 15.55 (1.35) | 16 | −1.48 | 19 | .15 |
Note. df for t tests: *p < .05, **p < .01, ***p < .001. CI = cochlear implant; NH = normal hearing.
Welch t statistic (Delacre, Lakens, & Leys, 2017).
Raw scores, based on number of items recalled.
Difference between observed and expected chance contiguous recall clustering based on shared semantic category or serial position of word in the list.
Number of words from the list accurately repeated immediately after spoken by examiner (max = 16).
List B (PI) and List A Short-Delay Recall (RI)
On the immediate free recall of List B items shown in Figure 1, CI users also performed more poorly in comparison to the NH controls (t = −2.367, p = .023). Similar findings were also observed for the SDFR (t = −2.672, p = .011) and SDCR (t = −2.14, p = .038) trials. The free recall scores obtained from List B and SDFR of List A items are used to compute measures of PI and RI, respectively, as follows.
PI (List B)
PI is the tendency of previously learned words to interfere with subsequent learning of new words. In the CVLT-II, this measure is reflected by the detrimental effects of the five List A learning trials on List B free recall. PI is calculated by comparing the number of items recalled during List B with the number of items recalled from List A Trial 1. We tested this difference by first conducting two 1-sample t tests on the difference scores. Neither the CI group (mean difference = −0.3, t = −0.63, p = .53) nor the NH group (mean difference = −0.41, t = −0.816, p = .423) showed any evidence of PI by this method. Second, we performed a t test between the PI scores as reported by the CVLT-II scoring program (calculated as percent reduction on recall of List B compared to List A Trial 1) for the two groups, which also showed no significant differences (t = 0.417, p = .680).
A third analysis method involved the comparison of recall for words from List A and List B that were from the same semantic category with recall of words that were from different semantic categories. For example, words such as spinach and cucumber both come from the shared category (i.e., vegetables), and words such as patio and saxophone come from different nonshared semantic categories. In typically developing individuals, PI is more likely to affect recall of items from shared semantic categories because of the buildup of semantic interference, whereas PI is less likely to affect recall of items from nonshared semantic categories; this latter effect is called “release from PI” (Kramer & Delis, 1991). Figure 2 displays the number of shared and nonshared category words recalled from List A Trial 1 and List B. Inspection of this figure shows that NH participants displayed the expected decline in words recalled from List B that are from shared categories with List A (PI) and the expected absence of decline (i.e., release from PI) for words recalled from List B that are from categories not shared with List A. In contrast, CI users showed little change in the number of List B words recalled compared to List A words recalled from shared or nonshared semantic categories.
Figure 2.
Proactive interference assessment: number of words from shared and nonshared categories that were recalled on List A Trial 1 and List B. Eight of the presented words on List B were from shared semantic categories, whereas the other eight words were from nonshared semantic categories. Cochlear implant users are shown in the left panel, and normal hearing controls are shown in the right panel.
For each group, we performed a 2 (shared vs. nonshared semantic categories) × 2 (List A Trial 1 vs. List B) repeated-measures ANOVA using list and shared categories as within factors and the number of words recalled as the dependent variable. For the NH group, we observed a significant interaction of Shared Categories × List, F(1, 23) = 5.662, p = .026. For the CI group, however, none of the main effects or interactions reached significance. The NH group performed worse on shared items in List B due to a buildup of PI and better on the nonshared items due to release from PI, whereas the CI group did not show any effects due to shared semantic categories of words on List B.
RI (SDFR)
RI refers to the presence of forgetting that occurs when the learning of new words reduces the ability to recall previously learned words. To assess the presence of RI in the CVLT-II, performance on recall of items from the List A SDFR task was compared to recall of items from List A Trial 5 to evaluate the interfering effects of List B on items that were recalled on List A Trial 5. Results of a 2 (CI vs. NH) × 2 (List A Trial 5 vs. List A SDFR) × 2 (shared vs. nonshared categories—e.g., whether the List A category was one that was shared with List B or not) mixed ANOVA with words recalled as the dependent variable revealed that both groups displayed the expected RI decline (List A Trial 5 vs. List A SDFR effect), F(1, 42) = 19.56, p < .001. However, there was no significant Group × Trial, F(1, 42) = 0.739, p < .001, or Group × Trial × Shared Semantic Category interaction, F(1, 42) = 1.89, p = .176, indicating no differences in the amount of retroactive interaction obtained between the two groups. A t test between the RI scores, as reported by the CVLT-II (calculated as percent reduction on recall of SDFR compared to List A Trial 5) for the two groups, also showed no significant differences between the groups (t = −0.582, p = .566). We also performed separate 2 (List A Trial 5 vs. List A SDFR) × 2 (shared vs. nonshared) repeated-measures ANOVAs for the CI and NH groups to test for differences between recall of shared and nonshared items. We found a main effect of trial, CI: F(1, 19) = 7.488, p = .013; NH: F(1, 23) = 16.828, p < .001, but no significant interaction between trial and shared versus nonshared categories, CI: F(1, 19) = 0.826, p = .375; NH: F(1, 23) = 0.189, p = .668. Hence, RI resulted in a decline in words recalled on the short-delay trial (caused by the intervening List B), but this decline did not differ by group or shared versus nonshared semantic category.
Long-Delay Recall Trials
There was no significant difference between CI and NH samples in the number of words recalled on the List A LDFR trial (t = −1.271, p = .211), but there was a difference in the LDCR trial (t = −2.376, p = .022) between the CI and NH groups. A 2 × 2 mixed ANOVA with group (CI vs. NH) as the between factor and trial (SDFR vs. LDFR) as the within factor was used to compare performance on LDFR and SDFR trials to evaluate the effects of delay on memory. We found a significant interaction in this analysis, indicating that the difference in performance between the LDFR and SDFR trials was different for the two groups, F(1, 42) = 4.19, p = .047, and between trials, F(1, 42) = 4.17, p = .048, with a Group × Trial interaction, F(1, 42) = 5.73, p = .021. CI users improved between SDFR and LDFR trials more than their NH peers, who showed little change following the retention interval.
Recognition Memory
Performance on the CVLT-II yes/no recognition memory task was assessed by examining the number of hits (trials on which the participants correctly identified a target item from List A) and false alarms (trials on which the participants incorrectly identified a nontarget item foil as being from List A). These observed scores were used to calculate the discriminability index, d′, which measures the ability of participants to discriminate the target words from foils. The measure of d′ is comparable to a z score that reflects the difference between the participants' hit rate and false-positive rate in standard deviation units (Egan, 1958; Green & Swets, 1966; Pollack, 1959). A larger d′ indicates that the target words were easier for a participant to discriminate from the distractors. Both the CI users and their NH peers performed very well on recognition memory (as shown in Table 3). There were no significant differences in total d′ between the CI users and NH controls on the recognition memory test (3.42 for the NH controls, 3.25 for CI users; t = −0.727, p = .472).
Auditory Word Identification Task
On the final auditory word identification task that was administered after the recognition memory task, the CI and NH participants were near ceiling in performance. Although NH controls achieved 100% accuracy on List A and List B word identification, the CI users achieved 99.06% (t = −1, p = .33) and 97.1% (t = −1.48, p = .15) accuracy on both lists, respectively (see Table 3).
Serial Position Effects
Figure 3 displays the mean probability of free recall across the five learning trials for the CI users and their NH peers split across three serial positions based on item location in the study list (i.e., percentage of items correctly recalled from each serial position region). The first four items in the study list by presentation order are represented in the figure by the “primacy” subcomponent, the middle eight items reflect “prerecency,” and the last four items constitute the “recency” region. This three-way partitioning of subcomponents of the serial position curve corresponds to Salthouse's (1980) characterization of the average size of these list regions and is consistent with the terminology and analysis methods used in the CVLT-II manual. The serial position effect in free recall is the finding that participants tend to recall more items from the primacy and recency regions of the serial position curve compared to recall of items from the middle prerecency region (Glanzer & Cunitz, 1966; Murdock, 1962; Postman & Phillips, 1965).
Figure 3.
Probability of free recall for primacy, prerecency, and recency regions of List A over five successive repetition learning trials. CI = cochlear implant.
List A Trial 1 is the only trial for which primacy and recency recall can be measured without being influenced by prior exposure to the study words from previous learning trials. A 2 (group: CI vs. NH) × 3 (serial position region: primacy vs. prerecency vs. recency) ANOVA on the List A Trial 1 data showed significant main effects for group, F(1, 126) = 0.015, and serial position region (primacy, prerecency, recency), F(2, 126) < 0.001, but no significant interaction was present between group and serial position region, F(2, 126) = 0.176.
In order to examine changes in each serial position region by study trial, we also conducted three separate 2 (group) × 5 (trial) ANOVAs, one for each serial position subcomponent (primacy, prerecency, recency). For the primacy region, there was only a significant main effect of study trial, F(4, 168) = 27.6, p < .01. The main effect for group and the interaction were not significant (see Figure 3, left). For the prerecency region (see Figure 3, middle), there were main effects for both study trial, F(4, 168) = 98.97, p < .001, and group, F(1, 42) = 9.57, p < .01, although the interaction between these two factors was not significant. Finally, for the recency region (see Figure 3, right), we observed main effects of study trials, F(4, 168) = 17.58, p < .01, and group, F(1, 42) = 8.8, p < .01, but no interaction. Hence, differences in the number of words recalled by CI users compared to their NH peers can be attributed to the failure to recall items from prerecency and recency subcomponents, not the primacy subcomponent, of the serial position curve.
The CVLT-II scoring program calculates the primacy and recency measures using a slightly different formula from the approach used here, taking the percentage of total List A Study Trials 1–5 words recalled from each serial position region of the list. By this analysis method, the CI users have higher primacy scores and trend toward a lower recency score than their NH peers (see Table 3). This analysis also demonstrates that CI users recalled more of their words from the primacy region of List A, which is consistent with the ANOVAs reported above, showing that the deficits in free recall on List A Trials 1–5 shown by the CI sample relative to their NH peers are localized in the prerecency and recency regions of the serial position curve and not the primacy region of the curve.
Learning and Self-Generated Organizational Strategies in Free Recall
Based on response output order and the sequence in which participants recall words from memory, two primary strategies for organizing recall can be inferred: (a) a serial clustering strategy, in which the participant recalls words in the same sequential/temporal order in which the words were presented on the study list, and (b) a semantic clustering strategy, in which the participant recalls words based on shared semantic categories (see Delis et al., 2000).
Semantic Clustering
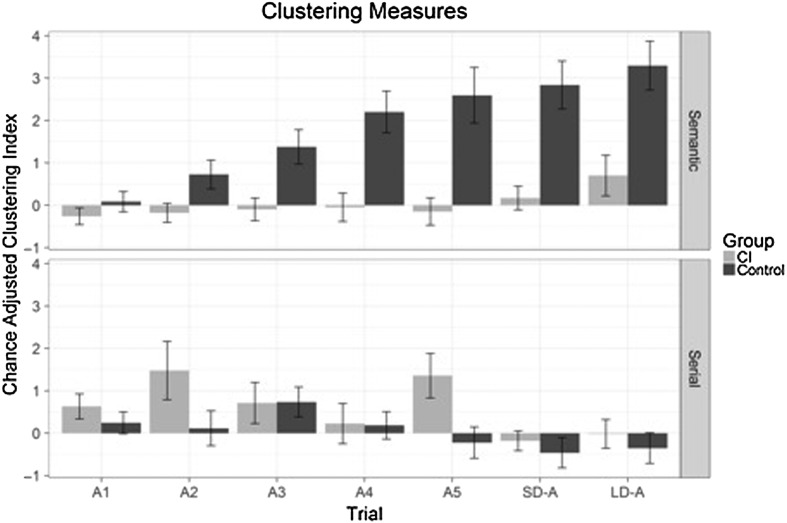
The tendency to use self-generated organizational strategies to form semantic clusters during each free recall trial of the CVLT-II can be quantified by using the chance-adjusted semantic clustering index (Stricker, Brown, Wixted, Baldo, & Delis, 2002) as described in Table 2. The results for the chance-adjusted semantic clustering index for each of the five List A immediate free recall trials, the SDFR, and the LDFR were calculated for both groups of participants and are shown in the top panel of Figure 4. Inspection of this figure reveals that the CI group showed little, if any, evidence of using self-generated semantic clustering organizational strategies in their output responses across the five study trials of List A. The semantic clustering scores for the CI users were dramatically lower than the semantic clustering scores obtained for the NH controls which increased over successive study trials. A 2 × 7 mixed ANOVA, with study trials as a within factor and group as a between factor, revealed highly significant main effects for group, F(1, 42) = 18.4, p < .001, and study trials, F(6, 252) = 9.90, p < .001, and the interaction of Group × Study Trials, F(6, 252) = 4.34, p < .001. The NH group showed strong evidence of robust semantic clustering effects, whereas the CI users showed no evidence of semantic clustering prior to LDFR. It is important to note here that participants were only provided with the semantic cues for retrieval for the first time on the SDCR trial after the SDFR trial and before the LDFR trial. Hence, CI users displayed above-chance semantic clustering only after the semantic categories were explicitly provided to them on the SDCR trials.
Figure 4.
Clustering measures. (a) Top: the chance-adjusted semantic clustering index for each free recall trial on List A. (b) Bottom: chance-adjusted bidirectional serial clustering index for each free recall trial on List A. CI = cochlear implant; SD-A = short delay free recall of List A; LD-A = long delay free recall of List A.
Serial Clustering
The use of a serial clustering strategy in free recall suggests that the organizational cue for the participant is the serial position of items in the study list and their relative temporal positions with each other. As with semantic clustering, serial clustering is computed by deriving an observed score, a chance-expected score, and a chance-adjusted score (Delis et al., 2000). The observed score is calculated by awarding 1 point each time the participant recalls two words in the same or opposite order as the items were presented on the study list. The chance-expected bidirectional serial clustering score is the number of observed bidirectional clusters that would have been expected by chance for the observed recall length. The difference between the two forms is the chance-adjusted bidirectional serial clustering score. The chance-adjusted bidirectional serial clustering index for each free recall trial is shown in the bottom panel of Figure 4 for CI and NH groups. A 2 × 7 mixed ANOVA showed a main effect of study trial, F(1, 252) = 2.75, p = .013, and a main effect of group that approached statistical significance, F(1, 42) = 3.84, p = .057. The CI sample exhibited slightly more serial clustering than the NH group.
Recall Consistency
During each of the five learning trials of List A (A1–A5), a given item from the study list may either be recalled or not recalled by a participant. If a recalled word tends to be successfully recalled again on the following trials, this is said to reflect a “consistent recall” pattern. The CVLT-II defines a simple measure of such response consistency in free recall called recall consistency, as the percentage of words recalled correctly on any of the List A Trials 2–5 that were also correctly recalled on the previous trial. Using this measure of recall consistency, CI users scored a mean of 80.7% and NH controls scored a mean of 86.2% (t = −1.54, p = .132). We also analyzed response consistency across the five List A study trials because of the relevance of response consistency in free recall to encoding and retrieval interactions, which is the topic of the next section. Response consistency also provides information about the underlying response dynamics in item recall in a way that the total number of words recalled or the extent of semantic clustering was not captured completely.
One way to visualize the response dynamics of successive correct recalls or failures in item recall during the CVLT-II learning trials is by plotting the sequence in which words are recalled or not recalled. Figure 5 displays the free recall patterns of participants in both groups over the five List A learning trials in a manner similar to inputs to multinomial processing tree models of free recall developed by Riefer and Batchelder (1988). On any given learning trial, there are two possible states of recall for an item from the presented study list (List A): The item was either recalled (represented by a “1”) or not recalled by the participant (represented by a “0”). This means that, over the five learning trials of List A, there are 25 (32) possible ways that an item can be recalled. These 32 possibilities are shown on the abscissa of Figure 5 and denoted by the 32 unique 5-bit binary strings, where each bit represents a successful recall or failure to recall an item on a learning trial: Using this classification system, a “00000” denotes that the item was not recalled on any of the five learning trials; a “00111” denotes that an item was not recalled on Trials 1 and 2 but was correctly recalled on Trials 3, 4, and 5; a “11111” denotes that an item was recalled on every trial; and so on. This recall consistency measure can be obtained for each subject by expressing as a percentage, the ratio between the number of consistently recalled pairs (the number of “11”s embedded in these events) and the number of inconsistently recalled pairs (the number of “10”s embedded in these events).
Figure 5.
Recall consistency patterns for the five List A learning trials. CI = cochlear implant.
The main reason for including Figure 5 here is that it reveals several additional differences in the dynamics of encoding and free recall in the CVLT-II for the two groups of participants. First, we found that an item is more likely to be in a state of never being recalled (event “00000”) for CI users when compared to NH controls (t = 2.93, p = .007). When we did an item analysis of the individual study words on List A, we did not observe that any particular word or set {of words accounted for this trend, suggesting that different participants made different errors on different words. The events “00000” were equally distributed across all study items and likely reflect a failure in encoding an item. Any item had a 7.5% probability of never being recalled when it was recalled by the CI group, as opposed to 1.3% chance of being recalled when it was recalled by the NH group (t = 2.93, p = .007). We also observed that the events where the first recall happened later in the list were more common for CI users than for NH controls. For instance, event 00011 is more likely for CI users (p = .021), whereas events 11111 (p = .044) and 01111 (p = .13) were more likely for their NH peers. This differential pattern of free recall consistency suggests that CI users often required more study trials before they could successfully recall items consistently. Such results may reflect the inherent differences in early auditory processing and registration or ease of sensory encoding of the study items, which is the topic of the next section.
Encoding and Retrieval Interactions
One benefit of the different types of memory subtests incorporated in the CVLT-II is that they allow inferences to be made about “hidden” underlying variables, such as the extent to which any failure to recall by the participant is due to a failure in encoding and the extent to which retrieval issues can be implicated as a possible cause of forgetting in free recall on the CVLT-II. In this section, we present results suggesting that retrieval processes may also be an additional locus of differences in recall between CI users and NH participants.
The first method we used to quantify encoding and retrieval interactions was to compare performance on recognition memory with free recall. The CVLT-II provides a way to quantify the recognition versus recall performance dichotomy by comparing the z scores (based on CVLT-II population norms) of LDFR (recall performance) with the total d' scores obtained from the yes/no recognition memory test (recognition performance). Using this contrast score, we found that CI users did not differ from NH controls. Although the CI users performed better in recognition memory than in free recall compared to NH controls, these differences were not statistically significant (p = .47), which is consistent with the finding of no differences between the groups on either the LDFR trial or the recognition trial (see Table 3).
However, CI users may have benefitted on the LDFR trial from being explicitly exposed to the semantic categories on the earlier SDCR trial; evidence for the effects of retrieval cues can be found in the increase in semantic clustering strategies in the CI sample on the LDFR trial relative to the SDCR or earlier List A free recall trials (see Figure 4). Because we found equivalent performance by CI and NH on recognition memory and auditory accuracy but also found significant differences in almost all of the free recall measures, we looked further into other indicators of retrieval differences between the two groups of subjects. These additional measures were suggested by Wilde, Boake, and Sherer (1995) in their study on retrieval deficits in patients with brain damage. One indicator of potential retrieval issues in CI users is that, compared to their NH peers, the CI group disproportionately benefitted on the LDFR and SDCR trials after exposure to semantic cues on the SDCR trials, which helped them recover any words that they had successfully encoded but did not retrieve.
Considering all three of these trends related to encoding retrieval interactions together, we found that CI users, on average, performed worse than their NH peers, suggesting that there may also be subtle retrieval deficits in recall of spoken words in prelingually deaf CI users in addition to problems in encoding and sensory registration of the study items. However, the marginal p values obtained in the analyses suggest that these differences should be examined further with other tests that are more sensitive to encoding/retrieval deficits. A larger sample size would also be able to provide more statistical power and possibly reveal more robust differences that are present in the results but are only trending toward conventional levels of significance.
Correlations of CVLT-II Scores and Measures of Sentence Recognition
A second goal of our study was to evaluate the extent to which performance on the CVLT-II was associated with speech recognition scores, using correlational analyses to examine the relationship between CVLT-II scores and sentence recognition (Harvard-S, Harvard-A, PRESTO, and PRESTO-FAE) scores (see Table 4). List B free recall in the CI sample was significantly associated with performance on the three of the four sentence recognition measures. For the NH sample, only LDCR and recognition were associated with PRESTO-FAE. However, because of limited sample sizes, some associations of medium or larger effect size approached but did not reach statistical significance, such as the moderately sized correlations between semantic clustering and sentence recognition in the CI sample (see Table 4).
Table 4.
California Verbal Learning Test–Second Edition (CVLT-II) correlations with sentence recognition key words correct scores.
| CVLT measures | Group | Harvard-S | Harvard-A | PRESTO | PRESTO-FAE |
|---|---|---|---|---|---|
| List A Trial 1 a | CI | .041 | .097 | .072 | .098 |
| NH | .055 | .022 | .106 | .326 | |
| List A Trial 5 a | CI | .318 | .355 | .266 | .335 |
| NH | .236 | −.037 | −.007 | .116 | |
| List A Trials 1–5 a | CI | .226 | .326 | .237 | .305 |
| NH | .034 | −.046 | .048 | .338 | |
| List B free recall a | CI | .523* | .46* | .467* | .387 |
| NH | .222 | .267 | −.003 | .02 | |
| List A short-delay free recall a | CI | −.009 | .056 | .029 | .163 |
| NH | −.033 | −.064 | .003 | .44 | |
| List A short-delay cued recall | CI | .278 | .23 | .221 | .191 |
| NH | −.057 | −.027 | −.018 | .519 | |
| List A long-delay free recall a | CI | .03 | .027 | .029 | .105 |
| NH | −.001 | −.02 | −.086 | .337 | |
| List A long-delay cued recall a | CI | .243 | .191 | .194 | .212 |
| NH | .113 | .029 | 0 | .475* | |
| List A recognition (Total d′) | CI | .102 | .054 | .013 | .091 |
| NH | .111 | −.081 | .291 | .507* | |
| Primacy, List A (Trials 1–5) | CI | −.334 | −.187 | −.282 | −.156 |
| NH | −.092 | −.173 | −.004 | −.388 | |
| Recency, List A (Trials 1–5) | CI | .125 | .142 | .137 | .138 |
| NH | .078 | .049 | .049 | −.203 | |
| Semantic cluster ratio b | CI | .362 | .423 | .387 | .43 |
| NH | .052 | −.109 | .231 | .153 | |
| Serial cluster ratio b | CI | −.288 | −.23 | −.231 | −.162 |
| NH | .292 | .4 | −.143 | −.281 | |
| List A discriminability (d′) | CI | .1 | .052 | .013 | .092 |
| NH | .117 | −.078 | .283 | .497 | |
| List A recall consistency | CI | .086 | .215 | .08 | .24 |
| NH | −.007 | −.201 | .005 | .244 |
Note. Values are Pearson correlation coefficients. Top row correlations are for the CI sample; second row correlations are for the NH sample. Harvard-S = Harvard Standard Sentences Test; Harvard-A = Harvard Anomalous Sentences Test; PRESTO = Perceptually Robust English Sentence Test Open-Set; PRESTO-FAE = PRESTO–Foreign-Accented English; CI = cochlear implant; NH = normal hearing.
Raw scores, based on the number of items recalled.
Difference between of observed and expected (chance-adjusted) clustering index based on contiguous recall clustering of shared semantic category or serial position of word in the list.
*p < .05.
Discussion
In this study, we used the CVLT-II administered in its conventional live voice clinical format to investigate differences in verbal learning and memory between early-implanted, prelingually deaf, long-term, adolescent and adult CI users and a group of NH controls and to assess associations between verbal learning and memory and speech recognition abilities. Development of verbal learning and memory skills following cochlear implantation has received very little research attention in this clinical population even though verbal learning and memory are core foundational components of spoken language processing operations in daily life. Our findings using the CVLT-II provide several new insights into the locus and nature of information-processing differences in verbal learning and memory between the two groups of participants.
We began this report by considering several reasons to anticipate differences in verbal learning and memory between CI users and NH controls. Previous research suggested important contributions of downstream cognitive processing (i.e., information processing following initial perception and encoding) as one of the factors underlying the enormous variability and individual differences observed in speech and language outcomes after implantation. However, except for a few exceptions (Heydebrand et al., 2007; Holden et al., 2013; Pisoni et al., 2018), almost all previous studies of verbal memory in this clinical population have been primarily focused on short-term and working memory using rote memory span measures. Other core aspects of memory such as verbal learning, self-generated organizational strategies, encoding and retrieval interactions, and measures of retention in long-term memory have not been studied by researchers working with this clinical population.
In this study, we compared long-term prelingually deaf CI users with NH controls who were comparable in age and nonverbal IQ. Our sample of CI users differs from the earlier samples studied by Heydebrand et al. (2007), Holden et al. (2013), and Pisoni et al. (2018), which consisted of postlingually deaf adult CI users who had an early period of typical development when they had sufficient hearing to acquire spoken language normally. In addition, Heydebrand et al. and Holden et al. used a global aggregate measure of verbal memory derived from the CVLT-II, whereas the current study focused on specific CVLT-II measures in order to understand the differential contributions of specific component subprocesses of verbal learning and memory.
The current study yielded several important novel findings about verbal learning and memory skills in prelingually deaf, early-implanted, adolescent and young adult long-term CI users. First, CI users recalled fewer words than their NH peers on all immediate memory and short-term free recall trials (List A Trials 1–5, List B, and SDFR and SDCR). Second, across these trials, CI users, unlike their NH peers, did not use self-generated semantic clustering as an organization strategy. CI users and their NH peers performed equally well on LDFR and recognition memory trials, which followed the explicit cuing of semantic categories in the SDCR trial. Third, contrast measure of recognition memory with the measures of free recall (with the notable exception of LDFR) and the reduced consistency in recall suggests that retrieval differences may also exist in the CI group compared to the NH group even though long-term storage differences were minimal. Specifically, the recognition task places very little demand on retrieval because the word is presented to the subject for evaluation (whether or not it was on the target list); in contrast, the recognition task emphasizes storage, because the primary challenge in recognition is indicating whether a word presented by the examiner is on the target list. On the other hand, free recall and (to a lesser extent) cued recall tasks place larger demands on retrieval processes because the subject must recover the word from memory without the word being presented by the examiner. Hence, CI users showed no difference from their NH peers on the recognition task that emphasized storage but displayed greater differences from their NH peers on the recall tasks that required retrieval processes without the presentation of explicit retrieval cues.
CI users also showed little evidence of using self-generated semantic organizational strategies in their recall of words on free recall trials compared to their NH peers, and they did not differ from chance levels in spontaneous use of semantic strategies to facilitate free recall. In contrast, the NH controls showed much more robust semantic clustering in their output responses beginning on List A Trial 2 after only one exposure to the words on the study list. This novel finding indicates that the storage of spoken words in memory by the CI users may be less efficient and more poorly organized because they did not use more efficient semantic organization strategies to promote both encoding and storage of test items used on the CVLT-II. Use of semantic organizational strategies in free recall tasks not only requires attention to the study items on the CVLT-II but also requires engagement of active, effortful mental resources and processing operations to simultaneously process both the words and their semantic attributes, actively extracting semantic categories from the 16 presented words. The processing demands of the basic free recall memory task used in the CVLT-II may be more taxing for CI users because they may not have sufficient additional processing resources available to engage in the simultaneous processing of semantic organization. This finding is consistent with prior research that has shown that prelingually deaf, long-term CI users are at a significant risk for delays in executive functions that could be used to support active effortful processing of multiple tasks during language processing (Kronenberger, Beer, Castellanos, Pisoni, & Miyamoto, 2014); hence, EF delays could explain the absence of self-generated semantic organizational strategies during verbal learning and memory by the CI group. In addition, other converging evidence suggests that the organization of spoken words in the mental lexicon of CI users is less efficient (Kirk, Pisoni, & Osberger, 1995; Luce & Pisoni, 1998), which may reduce the ability of CI users to automatically make use of semantic organization strategies during verbal learning and memory. In contrast, NH controls in this study showed extensive use of self-generated organizational strategies and increasing reliance on semantic clustering over the five successive learning trials of List A.
Because CI users made very little use of semantic organization strategies during free recall, they did not take optimal advantage of one of the most efficient methods for retrieving words from memory storage (Tulving, 1962, 1972). This process may also explain their poor performance on words recalled on all free recall trials, except the LDFR trial. Interestingly, CI users were able to make use of semantic organization strategies at greater than chance levels, but this pattern only occurred for the LDFR trial. This was the only free recall trial on the CVLT-II protocol that they did not differ significantly from their NH peers and was also the only free recall trial on the CVLT-II that followed an explicit declaration of the semantic categories by the examiner (on the SDCR trial).
In addition to differences in semantic clustering, the CI and NH groups showed large and consistent differences in primacy and recency effects in recall of studied items during verbal learning. The CI group showed poorer free recall from the recency and prerecency regions of the list, but no differences from their NH peers in recall of items from the primacy region. In current theories of memory, recall of items from the primacy region is attributed to multiple rehearsals that items in the initial positions of the study lists receive and are considered to be recalled from long-term memory (Atkinson & Shiffrin, 1968; Murdock, 1962; Rundus & Atkinson, 1970). On the other hand, recall of items from the recency region is said to be due to “dumping” from short-term memory, reflecting of short-term memory capacity limitation (Shiffrin & Atkinson, 1969). This interpretation is consistent with several earlier studies suggesting that CI users have deficits in short-term and working memory capacity (Burkholder & Pisoni, 2004; Kronenberger, Pisoni, Henning, Colson, & Hazzard, 2011; Pisoni & Cleary, 2004).
Another significant finding of this study was the difference in performance on recall of List B items between CI users and their NH peers. As with the other free recall trials, CI users recalled fewer words than their NH peers on List B. However, a closer inspection of List B free recall relative to List A Trial 1 free recall showed that the typical buildup of PI (for items from shared semantic categories) versus release from PI (for items from nonshared categories) profile was present only in the NH sample. In contrast, the CI users showed approximately equivalent levels of performance for both shared and nonshared semantic category words on List B compared to List A Trial 1. One explanation for this difference may be the lack of automatic self-generated semantic organization strategies in word encoding and storage in the CI sample. If semantic categories were minimally used by the CI sample, then semantic organization strategies would be less likely to affect PI or show evidence of release from PI on the nonshared trials of List B.
The findings from this study also showed more inconsistent recall patterns for repeatedly presented words for the CI sample compared to the NH sample. Inconsistency in recall suggests a failure to consistently use effective organization strategies across study trials as well as the failure to engage consistent, active, effortful attention-demanding processes in order to encode and store the same words across trials. In NH populations, verbal learning is a cumulative process that results in greater consistency of information stored over repeated exposures to the same set of words. The failure of CI users to show the same degree of consistency in verbal learning may ultimately impact the efficiency of their speech recognition skills and their strategies for learning and processing spoken language in other information-processing tasks that measure retrieval fluency and comprehension.
A final important set of findings from this study is related to the associations found between several CVLT-II scores and performance on measures of sentence recognition based on key words correct. The sentence recognition measures used in this study were specifically designed to provide a robust assessment of critical speech perception skills under demanding conditions that are ecologically valid for real-world functioning, including recognition of sentences produced by multiple talkers with different foreign/nonnative accents and regional dialects and access to different amounts of top-down contextual information. Consistent relationships between CVLT-II scores and sentence recognition scores were found for List B free recall in the CI sample. In addition, correlations between semantic clustering and sentence recognition in the CI sample were large but did not reach significance because of limited power. In contrast, List B and semantic cluster ratio were unrelated to sentence recognition scores in the NH sample possibly because of ceiling effects and restricted variance in speech recognition scores. Importantly, List B reflects verbal memory under challenging conditions of PI, and performance on List B has been found to be one of the best predictors of speech recognition in postlingually implanted adult CI users (Pisoni et al., 2018). Although the causal direction of this association cannot be definitively identified at this time due to the correlational nature of results, this particular finding suggests that verbal memory performance that is taxed by PI may be specifically associated with speech recognition outcomes in CI users. This suggests that CI users with poorer speech perception skills may have more poorly specified phonological and lexical representations of spoken words in long-term memory, rendering them more vulnerable to difficulty with verbal learning and memory when PI is present from previously presented verbal materials (Pisoni, Kronenberger, Chandramouli, & Conway, 2016).
The findings obtained in this study have several important clinical implications. First, delays in verbal learning and memory of CI users should be a target of clinical evaluation because of the importance and centrality of verbal learning and memory in language processing tasks and the suggestion from the present results that verbal learning and memory may be a core foundational domain of information processing that is at risk for delay in CI users. The finding of an absence of self-generated semantic organization skills in this study indicates that mnemonic processing strategies such as cuing, recognition, and explicit instruction in the active use of semantic learning strategies may promote better verbal learning and memory in CI users who are at risk for delays and therefore may produce more robust downstream effects on speech recognition and spoken language comprehension. Finally, relationships between verbal learning and memory and sentence recognition should be a target of evaluation and personalized interventions for low-functioning CI users who display poor outcomes after several years of CI use (Moberly, Bates, Harris, & Pisoni, 2016).
Limitations of This Study
Although this study examined several different aspects of verbal learning and memory in this unique clinical population of early-implanted, prelingually deaf adolescents and adults, the study is limited by the amount of data that can be gathered during a short clinical test, such as the CVLT-II, and the relatively small sample sizes. Thus, study results (especially those investigating relations between speech recognition and verbal learning and memory) should be considered to be preliminary and in need of replication. On the other hand, the present results provide new evidence of significant delays and disturbances in verbal learning and memory in CI users as well as differences in the underlying mnemonic processes (e.g., semantic clustering) that may underlie those delays and may be related to other elementary information-processing operations involving spoken language understanding outside the laboratory and audiology clinic.
In this study, we used the standard clinical presentation of the CVLT-II, which involves live voice presentation using spoken language. Although the use of auditory presentation of the CVLT-II items places demands on speech perception and may explain some of the challenges that CI users experienced with the CVLT-II task, with multiple repetitions of the list, one would expect that improved familiarity would reduce cognitive demand on basic components such as speech perception. Consistent with this, our CI sample did show robust repetition learning effects. In addition, the CI and NH samples did not differ on several key measures of CVLT words recalled (such as the number of words recalled from the primacy portion of the list as well as LDFR and long-delay recognition memory), indicating that a global effect of speech perception and audibility did not affect results. Furthermore, many of the CVLT-II process measures are not dependent on the volume of words recalled and therefore would not reflect speech perception deficits alone.
We did not use any corrections for multiple statistical tests as a result of the small sample size and because of the novel and exploratory nature of this research. In addition, the correlations obtained for the verbal learning and memory measures and sentence recognition scores for the NH sample may have been affected by ceiling effects in the NH group (although by using a set of challenging perceptually robust sentence recognition tests, this effect was minimized). Furthermore, we found only four significant correlations out of multiple correlations calculated between verbal learning and memory measures and sentence recognition scores (see Table 4), which could have been a result of Type 1 errors. Therefore, our correlational analyses should be regarded with caution pending direct replication with larger samples and conceptual replication using different methods.
Because we did not want to change the CVLT-II task protocol from the way it is routinely clinically administered, we used the conventional live voice presentation format. However, elementary modality-specific auditory and early sensory encoding factors could have influenced the present results. We attempted to address questions about audibility and early sensory encoding of the test words and the accuracy of auditory perception by having subjects identify all of the test words from List A and List B following the CVLT-II administration. Results showed that, on this metric, both groups performed extremely well: NH controls identified 100% of the items, whereas CI users identified 98.13% of the presented words (see Table 3 for List A and List B accuracy on this test). Nevertheless, encoding, storage, and processing the auditory test signals likely taxed the effortful cognitive processing resources of the CI group of participants, and results using visual presentation of the test words might have been different. We are currently using a visual presentation format of the CVLT-II to address this question in older postlingually deaf adults with CIs (Pisoni et al., 2018). In addition, the samples varied in age from adolescent to young adult, and age or CI cohort factors (e.g., quality of the CI at time of implantation) could have influenced results. It should be noted that these study results may not apply to postlingually deaf CI users or to a group of younger cohorts.
Summary and Conclusions
In this study, we investigated verbal learning and memory in prelingually deaf, early-implanted adolescent and young adult long-term CI users using the CVLT-II, a well-established, normed clinical neuropsychological test designed to assess verbal learning and memory. CI users recalled fewer words than their NH peers during free recall trials, did not consistently use self-generated semantic clustering strategies, and demonstrated atypical PI effects. On the other hand, CI users performed at the same level of their NH peers on cued recall and recognition memory trials, and their List B free recall scores were correlated with sentence recognition scores. Study results such as these have important clinical implications for the evaluation, accommodations, and training of verbal learning and memory in CI users who show poor outcomes after implantation. In addition, the knowledge of results for different types of CI populations on a test of verbal learning and memory, such as the CVLT-II, can be useful in understanding fundamental differences in cognition and information processing that are brought about by different types of developmental trajectories and etiologies of hearing loss.
Acknowledgments
The research reported in this article was supported by National Institute on Deafness and Other Communication Disorders Research Grants R01 DC-000111 (to D. B. P.), R01 DC-015257 (to D. B. P. and W. G. K.), and R01 DC-009581 (to D. B. P.) and by an IU Grant Linking University-Wide Expertise Award (GLUE Grant) to D. B. P. and W. G. K. The authors would like to express their special thanks to Shirley Henning for collecting data from participants, Luis Hernandez for his technical advice, and Terren Green for her organization and support during the preparation of the final draft of this manuscript. The authors also wish to thank all of the patients with cochlear implants and normal hearing controls who served as subjects in this study.
Funding Statement
The research reported in this article was supported by National Institute on Deafness and Other Communication Disorders Research Grants R01 DC-000111 (to D. B. P.), R01 DC-015257 (to D. B. P. and W. G. K.), and R01 DC-009581 (to D. B. P.) and by an IU Grant Linking University-Wide Expertise Award (GLUE Grant) to D. B. P. and W. G. K.
References
- Atkinson R. C., & Shiffrin R. M. (1968). Human memory: A proposed system and its control processes. Psychology of Learning and Motivation, 2, 89–195. [Google Scholar]
- Baddeley A. A. (2012). Working memory: Theories, models, and controversies. Annual Review of Psychology, 63, 1–29. [DOI] [PubMed] [Google Scholar]
- Burkholder R. A., & Pisoni D. B. (2003). Speech timing and working memory in profoundly deaf children after cochlear implantation. Journal of Experimental Child Psychology, 85(1), 63–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder R. A., & Pisoni D. B. (2004). Digit span recall error analysis in pediatric cochlear implant users. International Congress Series, 1273, 312–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clopper C. G., & Pisoni D. B. (2005). Perception of dialect variation. In Pisoni D. B. & Remez R. E. (Eds.), Handook of speech perception (pp. 313–337). Oxford: Blackwell. [Google Scholar]
- Conway C. M., Pisoni D. B., & Kronenberger W. G. (2009). The importance of sound for cognitive sequencing abilities: The auditory scaffolding hypothesis. Current Directions in Psychological Science, 18(5), 275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N., Wood N. L., Wood P. K., Keller T. A., Nugent L. D., & Keller C. V. (1998). Two separate verbal processing rates contributing to short-term memory span. Journal of Experimental Psychology, 127, 141–160. [DOI] [PubMed] [Google Scholar]
- Dawson P. W., Blamey P. J., Rowland L. C., Dettman S. J., Clark G. M., Busby P. A., … Rickards F. W. (1992). Cochlear implants in children, adolescents, and prelinguistically deafened adults: Speech perception. Journal of Speech and Hearing Research, 35(2), 401–417. [DOI] [PubMed] [Google Scholar]
- Delacre M., Lakens D., & Leys C. (2017). Why psychologists should by default use Welch's t-test instead of Student's t-test. International Review of Social Psychology, 30(1), 92–101. [Google Scholar]
- Delis D. C., Kramer J. H., Kaplan E., & Ober B. A. (1994). California Verbal Learning Test–Children's Version. San Antonio, TX: Pearson. [Google Scholar]
- Delis D. C., Kramer J. H., Kaplan E., & Ober B. A. (2000). California Verbal Learning Test–Second Edition (CVLT-II). San Antonio, TX: Pearson. [Google Scholar]
- Diamond A. (2013). Executive functions. Annual Review of Psychology, 64, 135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan J. P. (1948). Articulation Testing Methods. The Laryngoscope, 58(9), 955–991. [DOI] [PubMed] [Google Scholar]
- Egan J. P. (1958). Recognition memory and the operating characteristic. USAF Operational Applications Laboratory Technical Note, 32(ii). [Google Scholar]
- Engle R. W., Tuholski S. W., Laughlin J. E., & Conway A. R. A. (1999). Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. Journal of Experimental Psychology: General, 128(3), 309–331. [DOI] [PubMed] [Google Scholar]
- Garofolo J. S., Lamel L. F., Fisher W. M., Fiscus J. G., & Pallett D. S. (1993). DARPA TIMIT acoustic–phonetic continuous speech corpus CD-ROM, NIST speech disc 1-1.1. NASA STI/Recon Technical Report No. 93.
- Geers A. E., & Brenner C. (2003). Background and educational characteristics of prelingually deaf children implanted by five years of age. Ear and Hearing, 24(Suppl. 1), 2S–14S. [DOI] [PubMed] [Google Scholar]
- Gilbert J. L., Tamati T. N., & Pisoni D. B. (2013). Development, reliability, and validity of PRESTO: A new high-variability sentence recognition test. Journal of the American Academy of Audiology, 24(1), 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanzer M., & Cunitz A. R. (1966). Two storage mechanisms in free recall. Journal of Verbal Learning and Verbal Behavior, 5(4), 351–360. [Google Scholar]
- Green D. M., & Swets J. A. (1966). Signal detection theory and psychophysics. New York, NY: Wiley. [Google Scholar]
- Hammill D. D., Pearson N. A., & Lee Wiederholt J. (2009). Comprehensive Test of Nonverbal Intelligence–Second Edition (CTONI-2). Austin, TX: Pro-Ed. [Google Scholar]
- Herman R., & Pisoni D. B. (2003). Perception of “elliptical speech” following cochlear implantation: use of broad phonetic categories in speech perception. The Volta Review, 102(4), 321–347. [PMC free article] [PubMed] [Google Scholar]
- Heydebrand G., Hale S., Potts L., Gotter B., & Skinner M. (2007). Cognitive predictors of improvements in adults' spoken word recognition six months after cochlear implant activation. Audiology and Neuro-Otology, 12(4), 254–264. [DOI] [PubMed] [Google Scholar]
- Holden L. K., Finley C. C., Firszt J. B., Holden T. A., Brenner C., Potts L. G., … Skinner M. W. (2013). Factors affecting open-set word recognition in adults with cochlear implants. Ear and Hearing, 34(3), 342–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Electrical and Electronics Engineers. (1969). IEEE recommended practice for speech quality measurements. New York, NY: Author. [Google Scholar]
- Kalikow D. N., Stevens K. N., & Elliott L. L. (1977). Development of a test of speech intelligibility in noise using sentence materials with controlled word predictability. The Journal of the Acoustical Society of America, 61(5), 1337–1351. [DOI] [PubMed] [Google Scholar]
- Kirk K. I., Pisoni D. B., & Osberger M. J. (1995). Lexical effects on spoken word recognition by pediatric cochlear implant users. Ear and Hearing, 16(5), 470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. H., & Delis D. C. (1991). Interference effects on the California Verbal Learning Test: A construct validation study. Psychological Assessment: A Journal of Consulting and Clinical Psychology, 3(2), 299–302. [DOI] [PubMed] [Google Scholar]
- Kronenberger W. G., Beer J., Castellanos I., Pisoni D. B., & Miyamoto R. T. (2014). Neurocognitive risk in children with cochlear implants. JAMA Otolaryngology—Head & Neck Surgery, 140(7), 608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberger W. G., Colson B. G., Henning S. C., & Pisoni D. B. (2014). Executive functioning and speech-language skills following long-term use of cochlear implants. Journal of Deaf Studies and Deaf Education, 19(4), 456–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberger W. G., Pisoni D. B., Henning S. C., & Colson B. G. (2013). Executive functioning skills in long-term users of cochlear implants: A case control study. Journal of Pediatric Psychology, 38(8), 902–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberger W. G., Pisoni D. B., Henning S. C., Colson B. G., & Hazzard L. M. (2011). Working memory training for children with cochlear implants: A pilot study. Journal of Speech, Language, and Hearing Research, 54(4), 1182–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebach J. L., & Pisoni D. B. (2008). Perceptual learning of spectrally degraded speech and environmental sounds. The Journal of the Acoustical Society of America, 123(2), 1126–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luce P. A., & Pisoni D. B. (1998). Recognizing spoken words: The neighborhood activation model. Ear and Hearing, 19(1), 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberly A. C., Bates C., Harris M. S., & Pisoni D. B. (2016). The enigma of poor performance by adults with cochlear implants. Otology & Neurotology, 37(10), 1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock B. B., Jr. (1962). The serial position effect of free recall. Journal of Experimental Psychology, 64(5), 482–488. [Google Scholar]
- Niparko J. K., Kirk K. I., Robbins A. M. C., Mellon N. K., Tucci D. L., & Wilson B. S. (2012). Cochlear implants: Principles and practices (2nd ed.). Wolters Kluwer Health. [Google Scholar]
- Niparko J. K., Tobey E. A., Thal D. J., Eisenberg L. S., Wang N. Y., Quittner A. L., … CDaCI Investigative Team. (2010). Spoken language development in children following cochlear implantation. Journal of the American Medical Association, 303(15), 1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nittrouer S., Caldwell-Tarr A., & Lowenstein J. H. (2013). Working memory in children with cochlear implants: Problems are in storage, not processing. International Journal of Pediatric Otorhinolaryngology, 77(11), 1886–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni D. B., Broadstock A., Wucinich T., Safdar N., Miller K., Hernandez L. R., … Moberly A. C. (2018). Verbal learning and memory after cochlear implantation in postlingually deaf adults: Some new findings with the CVLT-II. Ear and Hearing, 39(4), 720–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni D. B., & Cleary M. (2003). Measures of working memory span and verbal rehearsal speed in deaf children after cochlear implantation. Ear and Hearing, 24(Suppl. 1), 106S–120S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni D. B., & Cleary M. (2004). Learning, memory and cognitive processes in deaf children following cochlear implantation. In Zeng F. G., Popper A. N., & Fay R. R. (Eds.), Cochlear implants: Auditory prostheses and electric hearing. Springer Handbook of Auditory Research (Vol. 20, pp. 377–426). New York, NY: Springer. [Google Scholar]
- Pisoni D. B., Cleary M., Geers A. E., & Tobey E. A. (1999). Individual differences in effectiveness of cochlear implants in children who are prelingually deaf: New process measures of performance. The Volta Review, 101(3), 111–164. [PMC free article] [PubMed] [Google Scholar]
- Pisoni D. B., & Geers A. E. (2000). Working memory in deaf children with cochlear implants: Correlations between digit span and measures of spoken language processing. Annals of Otology, Rhinology & Laryngology. Supplement, 185, 92–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni D. B., Kronenberger W. G., Chandramouli S. H., & Conway C. M. (2016). Learning and memory processes following cochlear implantation: The missing piece of the puzzle. Frontiers in Psychology, 7, 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni D. B., Kronenberger W. G., Roman A. S., & Geers A. E. (2011). Measures of digit span and verbal rehearsal speed in deaf children after more than 10 years of cochlear implantation. Ear and Hearing, 32(Suppl. 1), 60S–74S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack I. (1959). Identification of elementary auditory displays and the method of recognition memory. The Journal of the Acoustical Society of America, 31, 1126–1128. [Google Scholar]
- Postman L., & Phillips L. W. (1965). Short-term temporal changes in free recall. The Quarterly Journal of Experimental Psychology, 17(2), 132–138. [Google Scholar]
- Riefer D. M., & Batchelder W. H. (1988). Multinomial modeling and the measurement of cognitive processes. Psychological Review, 95(3), 318–339. [Google Scholar]
- Rundus D., & Atkinson R. C. (1970). Rehearsal processes in free recall: A procedure for direct observation. Journal of Verbal Learning and Verbal Behavior, 9(1), 99–105. [Google Scholar]
- Salthouse T. A. (1980). Age and memory: Strategies for localizing the loss. In Poon L. W., Fozard J. L., Cermak L., Arenberg D., & Thompson L. W. (Eds.), New directions in memory and aging (pp. 47–65). Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- Shiffrin R. M., & Atkinson R. C. (1969). Storage and retrieval processes in long-term memory. Psychological Review, 76(2), 179–193. [Google Scholar]
- Stricker J. L., Brown G. G., Wixted J., Baldo J. V., & Delis D. C. (2002). New semantic and serial clustering indices for the California Verbal Learning Test–Second Edition: Background, rationale, and formulae. Journal of the International Neuropsychological Society, 8(3), 425–435. [DOI] [PubMed] [Google Scholar]
- Tamati T. N., Gilbert J. L., & Pisoni D. B. (2013). Some factors underlying individual differences in speech recognition on PRESTO: A first report. Journal of the American Academy of Audiology, 24(7), 616–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamati T. N., & Pisoni D. B. (2015). The perception of foreign-accented speech by cochlear implant users. Paper presented at the 18th International Congress of Phonetic Sciences Retrieved from https://www.internationalphoneticassociation.org/icphs-proceedings/ICPhS2015/Papers/ICPHS0669.pdf [Google Scholar]
- Tulving E. (1962). Subjective organisation in free recall of “unrelated” words. Psychological Review, 69, 344–354. [DOI] [PubMed] [Google Scholar]
- Tulving E. (1972). Episodic and semantic memory: 1. Organization of memory. London, United Kingdom: Academic Press. [Google Scholar]
- Wilde M. C., Boake C., & Sherer M. (1995). Do recognition-free recall discrepancies detect retrieval deficits in closed-head injury? An exploratory analysis with the California Verbal Learning Test. Journal of the American Academy of Audiology, 17(6), 849–855. [DOI] [PubMed] [Google Scholar]
- Wilson B. S., Dorman M. F., Woldorff M. G., & Tucci D. L. (2011). Cochlear implants matching the prosthesis to the brain and facilitating desired plastic changes in brain function. Progress in Brain Research, 194, 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N., & Kirk K. I. (Eds.). (2016). Cochlear implants in children: Learning and the brain. New York, NY: Springer. [Google Scholar]
- Zwolan T. A., Kileny P. R., & Telian S. A. (1996). Self-report of cochlear implant use and satisfaction by prelingually deafened adults. Ear and Hearing, 17(3), 198–210. [DOI] [PubMed] [Google Scholar]