Abstract
Purpose
The current study adopts a systematic approach to the examination of working memory components in pediatric cochlear implant (CI) users by separately assessing contributions of encoding, storage, and retrieval.
Method
Forty-nine long-term CI users and 56 typically hearing controls completed forward and backward span tasks with 3 stimulus sets: visually presented digits, pictures of concrete nouns, and novel symbols. In addition, measures associated with each memory stage were collected: Rapid digit naming provided an estimate of phonological recoding speed, nonword repetition assessed the robustness of representations within phonological storage, and vocabulary knowledge (as measured by the Peabody Picture Vocabulary Test; Dunn & Dunn, 1997) estimated redintegration abilities during retrieval.
Results
Linear mixed modeling revealed that digit naming speed and vocabulary knowledge were consistently related to short-term and working memory span in both CI users and typically hearing controls. However, nonword repetition only contributed to the model for short-term memory.
Conclusions
Nonword repetition, an index of phonological storage, explained little of the individual variability inworking memory differences between CI users and typically hearing peers. On the other hand, individual differences in encoding and retrieval explained a significant amount of outcome variability in both short-term and working memory tasks. Differences between CI users and typically hearing peers in working memory therefore appear to reflect process components of encoding and retrieval and not simply differences in memory storage.
Supplemental Material
Many pediatric cochlear implant (CI) users struggle on short-term and working memory tasks even years after implantation (AuBuchon, Pisoni, & Kronenberger, 2015a; Dawson, Busby, McKay, & Clark, 2002). Short-term and working memory abilities are related to higher level cognitive skills, such as intelligence, reading, and math (e.g., Archibald & Harder Griebeling, 2016; Unsworth, Redick, Heitz, Broadway, & Engle, 2009). Identifying the precise nature of pediatric CI users' short-term and working memory deficits could lead to a better understanding of factors related to long-term speech, language, and literacy outcomes, which show considerable variability and individual differences in the CI population (Geers, Strube, Tobey, Pisoni, & Moog, 2011; Niparko et al., 2010). One line of research examining CI users' short-term and working memory deficits has focused on phonological storage (e.g., Nittrouer, Caldwell-Tarr, Low, & Lowenstein, 2017). Storage represents only the second of three stages of memory: encoding, storage, and retrieval. Other research has shown that, when assessed independently, processing measures associated with all three stages—specifically encoding speed, verbal rehearsal speed during storage, and scanning of retrievable items in short-term memory—each predict individual variance in pediatric CI users' short-term and working memory performance (AuBuchon, Pisoni, & Kronenberger, 2015b; Burkholder & Pisoni, 2003; Pisoni & Cleary, 2003). However, each stage does not occur in isolation; therefore, it is important to obtain estimates of the relative contribution of phonological storage simultaneously with processes associated with each memory stage.
We will provide a brief explanation of each memory stage, as well as the possible ramifications of hearing with a CI on the ability to encode, store, and retrieve information from short-term and working memory. Then, we will present data examining the variance of short-term and working memory related to each stage. The multicomponent model of working memory—the standard reference within speech and hearing sciences—does not conceptualize the flow of information as it progresses from encoding to storage to retrieval but, instead, emphasizes a fundamental modularity between processing and domain-specific storage. Therefore, the present results will be discussed in terms of current models of working memory, specifically Cowan's (2001) embedded processes model and the time-based resource sharing (TBRS) model (Barrouillet & Camos, 2012). Both of these models emphasize shifts of controlled attentional processes as information is transformed within the working memory system.
Short-Term and Working Memory
Human memory is routinely divided into three subsystems: sensory memory, short-term/working memory, and long-term memory (Atkinson & Shiffrin, 1968). Within each memory system, information progresses through three stages: encoding, storage, and retrieval. The three memory systems are differentiated, in part, by the duration of the storage stage. Short-term memory tasks measure the temporary maintenance of information (e.g., recalling a sequence of words in the order it was presented). Working memory tasks additionally require processing or manipulation of the information maintained in short-term memory (e.g., reversing the order of the words before recalling them; Unsworth & Engle, 2007). Thus, short-term memory (i.e., temporary storage) can operate on its own or as part of the larger working memory system (i.e., temporary storage plus processing). A general rule of thumb states that the duration of short-term memory is under 30 s (Atkinson & Shiffrin, 1968). In contrast, storage in sensory memory is very brief, lasting on the order of milliseconds (Sperling, 1960), whereas storage in long-term memory is presumed to be indefinite (Atkinson & Shiffrin, 1968).
CI users' short-term and working memory deficits are particularly pronounced when the to-be-remembered information is verbal or phonological in nature; however, their performance is comparable to typically hearing peers when information is visual–spatial (Dawson et al., 2002; Kronenberger, Colson, Henning, & Pisoni, 2014; Lyxell et al., 2008). Notably, peripheral contributions of audibility and speech motor output do not drive these differences as CI users' deficits persist even when stimuli are presented visually and no vocal output is required (AuBuchon et al., 2015a). Therefore, basic information-processing mechanisms within the working memory system must be explored as possible sources to pediatric CI users' short-term and working memory disturbances beyond sensory or speech motor explanations.
The Multicomponent Model of Working Memory
Within speech and hearing sciences, the most commonly referenced working memory model is Baddeley's multicomponent model of working memory (e.g., Bielski & Lansing, 2012). According to this model, storage and processing represent distinct, separable components of working memory; storage is further subdivided into “phonological loop” and “visuospatial sketchpad,” which store domain-specific material (Baddeley & Hitch, 1974). The phonological loop is a specialized short-term memory store for information in the verbal–phonological domain and is responsible for a domain-specific verbal maintenance strategy known as verbal rehearsal. Verbal rehearsal is the silent repetition and recycling of a to-be-remembered item's phonological code. The visuospatial sketchpad is a specialized short-term memory store for information in the visual–spatial domain (Baddeley, 1996). These two memory stores are controlled by the “central executive,” which directs processing resources within the working memory system and is independent of the short-term stores (Baddeley, 1996).
The strongest support for short-term and working memory delays in pediatric CI users has been found for the verbal domain. Nittrouer, Caldwell-Tarr, and Lowenstein (2013) proposed that CI users' short-term and working memory problems lie in storing phonological representations within the phonological loop. According to Nittrouer et al., the degraded nature of auditory input through a CI leads to increased phonological overlap within the phonological loop. As phonological overlap increases, items become confusable or overwritten (Conrad & Hull, 1964). The phonological loop is regularly implicated as an explanatory mechanism for short-term and working memory deficits in other populations with atypical language development—although the source of impairment varies across populations. For example, children with developmental language disorder appear to have smaller phonological loops than their typically developing peers (Archibald & Harder Griebeling, 2016), and children with Down syndrome have difficulty in maintaining item order within the phonological loop (Jarrold, Cowan, Hewes, & Riby, 2004).
Three Stages of Memory
Although sensory, short-term, and long-term memory systems are often differentiated based on the duration of storage, storage alone is not sufficient for remembering to occur. Within each system, information must first be encoded into the relevant store and eventually retrieved in order to respond.
Encoding. Encoding occurs when information enters the memory system and forms a memory trace. A newly created short-term memory trace comprises a signal registered via a sensory process. However, short-term memory is the intermediary between the physical world and long-term memory (Cowan, 2001). Therefore, it is worth noting that a memory trace can also enter short-term memory by being activated from long-term memory (Cantor & Engle, 1993). If an incoming sensory signal is associated with a long-term memory trace in a different sensory modality, the short-term trace may be recoded into its associated form. As they are being encoded, visual representations often activate associated verbal/phonological forms from long-term lexical memory (Conrad & Hull, 1964). If this occurs, the original sensory signal is replaced or recoded into the associated form. For example, you might “hear” your “inner voice” as you read and remember this text—recoding the visual orthographic representation into associated phonological and lexical forms.
As children display faster, more efficient recoding abilities, they also display a corresponding increase in memory span (Case, Midian Kurland, & Goldberg, 1982). Recoding speed is determined by the time it takes to register the encoded visual signal, access an associated long-term lexical representation, and apply a verbal label—such as naming digits, letters, or colors (Wolf, Bowers, & Biddle, 2000). Although verbal recoding of visual stimuli supports short-term memory performance, it also alters the memory trace, such that fine-grained episodic detail of the visual representation is lost (Carmichael, Hogan, & Walter, 1932).
Pediatric CI users have slower recoding speeds than their typically hearing peers, even when measured with highly familiar stimuli such as the digits 1, 2, and 3; moreover, for both groups, recoding speeds are strongly correlated with performance on executive function tasks (AuBuchon et al., 2015b). This finding suggests that, like their typically hearing peers, pediatric CI users transform visually presented information into a verbal–phonological form for processing. Furthermore, pediatric CI users' slower recoding speeds are related to poorer executive function skills, at least before taking into account other working memory mechanisms (AuBuchon et al., 2015b).
Storage. During storage, information is maintained within a memory system over time. Distinctiveness of the representation is important within the short-term store. In particular, the ability to maintain order information about the items is impaired when the to-be-stored items share phonological features (e.g., mat, man, bat, pat, pan). This phenomenon is known as the phonological similarity effect (Conrad & Hull, 1964). The importance of robust, distinctive phonological codes underlies the proposal that less efficient encoding of fine acoustic–phonetic detail leads to problems in phonological storage and drives CI users' memory deficits (Nittrouer et al., 2013; Nittrouer, Caldwell-Tarr, Sansom, Twersky, & Lowenstein, 2014).
This interpretation of pediatric CI users' disruption in short-term memory is broadly consistent with interference models of short-term memory (e.g., Cowan, 2001; Oberauer & Kliegl, 2006). According to these models, features that are shared among items—such as similar phonemes—overwrite one another, making individual items less distinctive and, therefore, more difficult to retrieve. Unlike Nittrouer and colleagues' proposal, these feature overwriting models do not specify a separate phonological store. Instead, overwriting models note that items are more likely to share features—thus, cause interference—when the items are from the same domain. Because a CI combines acoustic information across frequency bands, otherwise distinctive features converge during encoding and the number of shared features among memory traces increases. Thus, even items that can be discriminated will nonetheless interfere with one another once they are simultaneously held in the short-term store.
Phonological storage is often assessed with a nonword repetition test during which the participant hears and repeats a novel sequence of phonemes. This sequence follows English phonotactic rules but does not represent a known word (Gathercole & Baddeley, 1996). Nonword repetition depends on both phonological encoding skills and phonological storage; sequences can be as long as five syllables, requiring the initial phonemes to be maintained in short-term memory for the entire duration of the nonword's presentation. Consequently, nonword repetition reflects the quality of the memory trace as it enters and is maintained in short-term storage. Because nonwords do not have preexisting lexical representations in long-term memory, storage of nonwords occurs with minimal downstream support from long-term memory (Hulme, Maughan, & Brown, 1991; though see also Gathercole, 1995). Despite retaining the prosodic properties (i.e., number of syllables and syllable stress) of these novel nonwords, pediatric CI users struggle to retain the fine-grained acoustic–phonetic content—suggesting impairments in phonological storage (Dillon, Cleary, Pisoni, & Carter, 2004; Nittrouer et al., 2014).
Retrieval. Retrieval is the stage of remembering in which memory is scanned and a memory trace is selected for recall. Memory scanning appears to be an amodal process because scan speed is not affected by stimulus qualities such as word length or even lexical status (Cowan et al., 1994). However, memory scanning does become faster with development, and this increase in scan speed is related to improvements in short-term memory span (Cowan et al., 1994).
Once an item is selected for retrieval, the decaying (i.e., fading) memory trace must be restored. This retrieval-based restoration process is known as redentigration, and it occurs for both visual and phonological information (Bower & Glass, 1976; Schweikert, 1993). If the decaying short-term memory trace has a representation in long-term memory, then the long-term memory representation can be used as a template to restore and repair any missing segments lost to decay. Computational models of short-term memory demonstrate that larger vocabularies support redintegration processes by providing fast access to many possible lexical templates (Gupta & Tisdale, 2009). Indeed, pediatric CI users' vocabulary knowledge has been found to predict their performance on a word span task (Nittrouer et al., 2017). Redintegration processes also depend on stimulus characteristics. For example, redintegration is most effective for high-frequency items and lists with high interitem associations; nonwords, which have no long-term lexical entries (thus are of low frequency and have no interitem associations) do not benefit from redintegration (Hulme, 2003).
Previous Investigations Into Pediatric CI Users' Short-Term and Working Memory
The primary goal of early investigations into pediatric CI users' working memory was to describe their performance relative to typically hearing controls. These studies identified the presence of working memory disruptions and determined the magnitude of individual variation within this patient population (e.g., Pisoni & Geers, 2000). The authors of these studies acknowledged the potential role of central information-processing mechanisms during encoding, storage, and retrieval in CI users' short-term and working memory. For example, Pisoni and colleagues (Pisoni & Cleary, 2003; Pisoni, Kronenberger, Roman, & Geers, 2011) observed that articulation rate predicted both forward and backward digit span, implicating slow, inefficient verbal rehearsal during storage—a conclusion based on decades of literature in working memory (e.g., Baddeley, Thomson, & Buchanan, 1975; Hulme, Thomson, Muir, & Lawrence, 1984). During recall, young CI users also displayed a slower response rate than typically hearing peers (Burkholder & Pisoni, 2003). Slow response rates, when driven by long pause durations between items during recall—as was the case for CI users—had been associated with slow scanning speeds during retrieval (Cowan et al., 1994). Unfortunately, many of these early studies utilized auditory stimuli and spoken responses, making it impossible to rule out effects of auditory sensory encoding and speech motor planning.
The emphasis on phonological storage followed a study reported by Nittrouer et al. (2013) in which they modified a standard test of phonological similarity. In Nittrouer et al.'s version of the task, children were presented with pictures and auditory labels of either phonologically similar nouns or phonologically distinct nouns. At recall, they were given an array of all of the pictures in the set and were required to point to the to-be-remembered pictures in their order of presentation. Notably, the locations of pictures within the array were randomized on each trial. The premise of the study was that any decrements in CI users' recall accuracy should reflect storage problems within the phonological loop whereas any slowing of CI users' recall response speed should reflect processing problems of the central executive. Nittrouer et al. (2013) observed lower recall accuracy in the pediatric CI users relative to their typically hearing peers. However, speed of responding was similar for both groups, suggesting that earlier reports regarding scanning speed may have been influenced by the need for speech motor planning.
One limitation of Nittrouer et al.'s approach is that the modified test of phonological similarity has not been validated against more well-known and conventional tests of short-term and working memory. Moreover, the study's conclusion that processing played no role in CI users' short-term memory deficits appears to generalize beyond scanning speed to other components and stages of processing. However, the processing measured by the task occurs only once the participant is ready to respond—after encoding, maintenance in storage, and redintegration. Searching for and selecting items for recall therefore reflect only a subset of all possible processing operations, some of which may differ between pediatric CI users and their typically hearing peers. Hence, questions persist about the relative contributions of storage and processing in short-term verbal memory in CI users, which raises the need for additional research on the role of specific domains of processing such as encoding and retrieval.
Instead of broadly dividing the working memory system into storage and processing, it may be more useful to consider pediatric CI users' short-term and working memory disruptions in reference to the three stages of memory formation: encoding, storage, and retrieval. Conceptually, this framework allows a differentiation among the multiple processing operations that might occur during a given task. From a clinical point of view, this framework should stimulate the development of interventions to address specific weaknesses at each stage of memory. Such targeted interventions will likely improve the efficacy of language and memory interventions for children with CIs.
Current Study
We previously demonstrated that pediatric CI users' short-term memory impairments persist even when demands on audibility and verbal output are completely eliminated by comparing CI users and typically hearing peers on two versions of forward and backward digit span (AuBuchon et al., 2015a): (a) Auditory Digit Span Tasks from the Wechsler Intelligence Scale for Children–Third Edition (Wechsler, 1991), which use auditory presentation and require spoken responses, and (b) computerized digit span tasks in which the digits were presented visually and responses were made by pointing to items on a touchscreen response grid. Not only did CI users continue to perform poorly when the demands of audibility and speech motor planning were eliminated, but their performance on the two versions was highly correlated (AuBuchon et al., 2015a). These findings support the general claim that pediatric CI users' short-term memory deficits are due to cognitive mechanisms within the working memory system rather than (a) solely a failure to encode the stimulus via the auditory system or (b) a by-product of listening or speaking demands during the task.
The current study extends the computerized digit span paradigm used in our earlier study to simultaneously assess contributions of encoding, storage, and retrieval. We combined the computerized digit span data with memory span for two additional stimulus sets: pictures of concrete nouns and visual displays of novel symbols that have no prelearned verbal labels (see Figure 1). We expected that all participants—regardless of hearing status—would display a similar pattern of remembering across the three types of stimuli. Both pediatric CI users and their typically hearing peers should display the highest accuracy with digits. These stimuli have well-known, highly familiar verbal labels, which should encourage rapid phonological recoding during the encoding stage; in addition, the set of digits has high-frequency and high-interitem associations to support the redintegration or recovery of fading memory traces during the retrieval stage. In contrast, the novel visual symbols have no prelearned lexical entries; thus, they have neither verbal labels nor interitem associations. In effect, they are visual analogues to written nonwords, except that they impose no demands on reading or phonological awareness. Both groups of participants should recall the fewest lists when remembering these novel visual symbols. Accuracy for pictures of concrete objects should fall between digits and symbols. The visually presented objects have obvious verbal labels; however, their lower interitem associations would be less supportive of redintegration than for digits.
Figure 1.
Stimuli (as seen in their respective response grid layouts).
If encoding and retrieval processes contribute to pediatric CI users' short-term/working memory, then memory performance would be predicted by individual differences in phonological recoding and redintegration, respectively. We obtained measures of rapid digit naming as a proxy for phonological recoding. In rapid digit naming, the participant must rapidly recognize the printed image of a number, recode the number into its verbal label, and produce the verbal label. Although speech production is a component of rapid digit naming, the task is a well-validated measure of phonological recoding; moreover, rapid digit naming corresponds to reading ability (Wolf et al., 2000) as well as executive function skills, including verbal working memory, inhibition concentration, and controlled fluency speed (AuBuchon et al., 2015b).
In addition, we obtained scores on the Peabody Picture Vocabulary Test (PPVT; Dunn & Dunn, 1997) as a proxy for redintegration abilities. Computational models of verbal short-term memory demonstrate that increasing vocabulary knowledge leads to faster and more successful redintegration (Gupta & Tisdale, 2009). In an earlier study, we observed that rapid naming skills were uncorrelated with a broader language composite, which included scores from the PPVT, indicating that rapid digit naming and PPVT should allow us to observe the independent effects of each variable even when both are entered into the model (AuBuchon et al., 2015b).
Nonword repetition was assessed as an estimate of robustness of phonological representations within the phonological store. If phonological storage is sufficient to explain the overall deficits and variability observed in CI users' short-term and working memory, then we would expect only nonword repetition to predict variation in short-term and working memory performance. However, if phonological recoding speed and/or vocabulary knowledge contributes predictive power to the model, explanations focusing solely on storage would be inadequate to fully explain the wide variability in pediatric CI users' short-term and working memory abilities. In this case, phonological recoding, redintegration, and other processes involved in encoding and retrieval should undergo experimental manipulations to better understand the causal role of informational processing operations during development—especially in children with atypical hearing and language development.
Hypotheses
The following hypotheses were tested in this study:
Both pediatric CI users and their typically hearing peers will show a decline in the number of lists recalled as the stimuli change from digits to objects to abstract symbols, suggesting that CI users, like their typically hearing peers, use long-term linguistic knowledge of the stimulus during encoding and retrieval.
Individual variability in performance will be predicted by speeded digit repetition (encoding) and vocabulary (redentigration during retrieval) above and beyond contributions of nonword repetition (storage).
Method
Participants
Forty-nine pediatric CI users (23 female and 26 male users; 20 bilateral) were recruited through a CI clinic and research center, as well as local advertisements, with the following inclusion criteria: (a) onset of severe-to-profound hearing loss (> 70 dB hearing loss in the better hearing ear) before the age of 3 years, (b) cochlear implantation before the age of 7 years, (c) minimum of 7 years of CI use, and (d) consistent use of a multichannel CI system with updates to maps and processors as necessary. One CI user (aged 10 years) was excluded for not completing all of the span tasks. The remaining 48 CI users' hearing history is reported in Table 1.
Table 1.
Summary of pediatric cochlear implant (CI) recipients' hearing history.
| Variable | Count | |
|---|---|---|
| Etiology | ||
| Genetic | 7 | |
| Meningitis | 6 | |
| Other/unknown | 35 | |
| Age at identification of deafness | ||
| Birth | 39 | |
| Under 12 months | 4 | |
| 13–24 months | 3 | |
| 25–36 months | 2 | |
| Age of fit of CI (months) | ||
| 8–12 | 1 | |
| 13–24 | 16 | |
| 25–36 | 11 | |
| 37–76 | 20 | |
| M (SD) | Range | |
| Duration of CI use (years) | 14 (4.3) | 7–24.5 |
| Preimplant residual hearing (PTA) | 106 (11.9) | 85–118 |
Note. Preimplant residual hearing is expressed as mean unaided pure-tone average (PTA) for the frequencies 500, 1000, and 2000 Hz in dB HL. Other/unknown etiology for hearing loss includes auditory neuropathy (n = 3), large vestibular aqueduct (n = 1), Mondini malformation (n = 2), ototoxicity (n = 1), and unknown (n = 28).
In addition, 56 typically hearing participants (36 female and 20 male participants) were recruited from advertisements in the same clinics and local sites as the CI sample. All typically hearing controls passed a hearing screening (0.5–4 kHz at 20 dB bilaterally). CI users and typically hearing participants had no other comorbid developmental or neurocognitive delays or disabilities, and all participants lived in a home where spoken English was the primary language.
Procedure
All procedures were approved by the local institutional review board. All testing was completed during two visits that occurred 1 month to 4 years apart. On the first visit, participants' vocabulary knowledge (PPVT) and rapid digit naming speeds were measured as part of a larger battery of neurocognitive, speech, and language tests. On the second visit, participants completed the nonverbal intelligence (Comprehensive Test of Nonverbal Intelligence–Second Edition [CTONI-2]; Hammill, Pearson, & Wiederholt, 2009), nonword repetition, and computerized memory span tests. Prerecorded audio stimuli for the nonword repetition test were presented to subjects in a quiet room at 65 dB using a high-quality loudspeaker located approximately 3 ft from the subject. All other tests were administered using standardized directions that were identical for the CI users and typically hearing peers. The CI users listened with their CI processor set to everyday settings, and they were all tested by one of two certified speech-language pathologists who were experienced in testing CI users.
Measures
Computerized Memory Span Tasks
All participants completed six computerized memory span tasks: symbol span (forward and backward), object span (forward and backward), and digit span (forward and backward). The span tasks differed only by the nature of the stimulus set (see Figure 1) or the direction of recall (forward or backward); otherwise, the procedure for each span task was identical. Before each of the two tasks with a given stimulus set, participants were familiarized with the items from that set. An item from the stimulus set was presented in the center of the computer screen for 1 s, after which a 3 × 3 response grid (450 × 450 pixels) containing all nine items from that stimulus set appeared. The participant was instructed to select the just-seen item by touching that item on the display screen. This process was repeated until the participant correctly identified all nine items. To minimize visual search demands, items remained in the same grid location throughout the study.
The stimulus familiarization phase was followed by the corresponding forward and backward span tasks, each of which was preceded by two practice trials: one at List Length 2 and one at List Length 3. For the actual memory span task, two lists were presented at each list length, beginning with List Length 2. Regardless of list length, each item appeared for 1 s, one at a time, in the center of the screen. The corresponding response grid appeared 250 ms after the offset of the final list item. Participants were instructed to reproduce the list of items by using the touchscreen monitor (12.1 in., 800 × 600 pixels, Model Keytec L1201S) to select items in either forward or backward order. If the participant correctly reproduced at least one list at a given list length, then list length increased by one item; if the participant failed to reproduce at least one list at a given length, testing was terminated. Scores were calculated as the total number of lists correctly reproduced.
Digit Naming
Digit naming was calculated using the numeral naming baseline control condition of the counting interference task (Hummer et al., 2011). Participants rapidly named a series of randomly presented digits (1, 2, or 3) from a stimulus page for 45 s; digit naming scores were the number of digits read prior to the time limit; thus, higher scores correspond to faster naming.
Nonword Repetition
Nonword repetition was adopted from the Children's Test of Nonword Repetition (Gathercole & Baddeley 1996), which requires the participant to repeat two- to five-syllable nonwords. Recordings of the nonwords were made by a native speaker of American English. Repetitions were transcribed and scored by a certified speech-language pathologist. Scores represent the percentage of nonwords correctly recalled.
PPVT
PPVT (Dunn & Dunn, 1997) is a one-word receptive vocabulary test in which the examiner says a word aloud and the participant must point to one of four pictures that correctly depict that word. Scores on the PPVT were used to index lexical retrieval from long-term memory during redentigration.
The Geometric Scale of the CTONI-2
The CTONI-2 is a measure of nonverbal reasoning and concept formation. The Geometric Scale is calculated as the norm-based standard score from the subtests using abstract visual design stimuli. This standard score is reflective of nonverbal (fluid) IQ. The CTONI-2 provides an estimate of nonverbal intelligence with reduced language input; it has excellent reliability and validity and has been used with populations with hearing loss and/or language delays (Hammill et al., 2009).
Statistical Analyses
Statistical analyses were performed using R Version 3.4.2 (R Core Team, 2017), the packages ggplot2 (Wickham, 2009) and lmerTest (Kuznetsova, Brockhoff, & Christensen, 2015), and the lmer function in the lme4 package (Bates, Maechler, Bolker, &Walker, 2014). We utilized linear mixed modeling to test our hypothesis that changing the stimuli would affect span and determine which predictors contribute to individual differences in span. Unlike standard regression, which assumes independence, linear mixed modeling takes into account that participants' three computerized memory span scores are correlated. 1 By including random intercepts for each participant, linear mixed models account for participants' baseline differences in span performance. Furthermore, linear mixed models are robust to sample size differences between groups and violations of normality in the data—although visual inspection did not suggest any violations of normality.
Because forward span tasks are thought to rely only on short-term memory in typically hearing populations whereas backward span tasks are thought to tap the entire working memory system, all analyses were conducted separately for forward and backward span scores. In order to examine our first prediction that spans for both CI users and typically hearing peers would be influenced by stimulus characteristics, we created a model that included random intercepts for subjects, the control variables of age and nonverbal intelligence (CTONI-2), and fixed effects of stimulus type (digits, objects, symbols), as well as all four predictor variables of interest: hearing status (CI, typically hearing), phonological recoding speed (digit naming), phonological storage (nonword repetition), and vocabulary knowledge (PPVT raw score).
In order to test our second prediction that measures associated with encoding and retrieval skills—above and beyond phonological storage—would predict individual variation in performance, we compared models using a χ2 likelihood ratio test (Winter, 2013). The objective was to achieve the best fit with the simplest model (i.e., fewest parameters) with the exception that the control variables of age and nonverbal intelligence would remain in the model regardless of their individual contributions. One limitation in this study was the collection of data over two testing visits. On average, the lag between Visit 1 and Visit 2 was longer for CI users (M = 2.2 years, SD = 1.1) than for typically hearing peers (M = 1.5 years, SD = .8), t(103) = 19.5, p < .001. However, because of our targeted recruitment efforts, 2 the two groups had similar age distributions at each visit (see Table 2). Because performance on the span task is our primary outcome variable, we opted to use age at Visit 2 (when span was measured) as a fixed effect in models. We also included CTONI-2 standard score as a fixed effect to account for individual differences in nonverbal intelligence. Therefore, we can examine the contributions of hearing status (CI or typically hearing), phonological recoding speed (digit naming), vocabulary knowledge (PPVT raw score), and phonological storage (nonword repetition) after taking into account two known correlates of working memory—age and IQ.
Table 2.
Means, standard deviation, and range of demographic variables for the cochlear implant (CI) users and typically hearing controls.
| Variable | CI sample (n = 49) |
Control sample (n = 56) |
t | ||
|---|---|---|---|---|---|
| M (SD) | Range | M (SD) | Range | ||
| Age at Visit 1 (years) | 14.9 (4.8) | 7.8−26.7 | 15.8 (5.3) | 7.1−29.0 | 0.9 |
| Age at Visit 2 (years) | 17.1 (5.2) | 9.3−30.0 | 17.4 (5.1) | 9.9−29.3 | 0.2 |
| Time between testing (years) | 2.2 (1.1) | 0.1−4.0 | 1.5 (0.8) | 0.1−3.3 | 3.7* |
| CTONI-2 standard score | 103.0 (14.2) | 70−130 | 106.1 (11.7) | 78−127 | −1.2 |
Note. CTONI-2 = Comprehensive Test of Nonverbal Intelligence–Second Edition.
p < .05.
Results
Forward Span
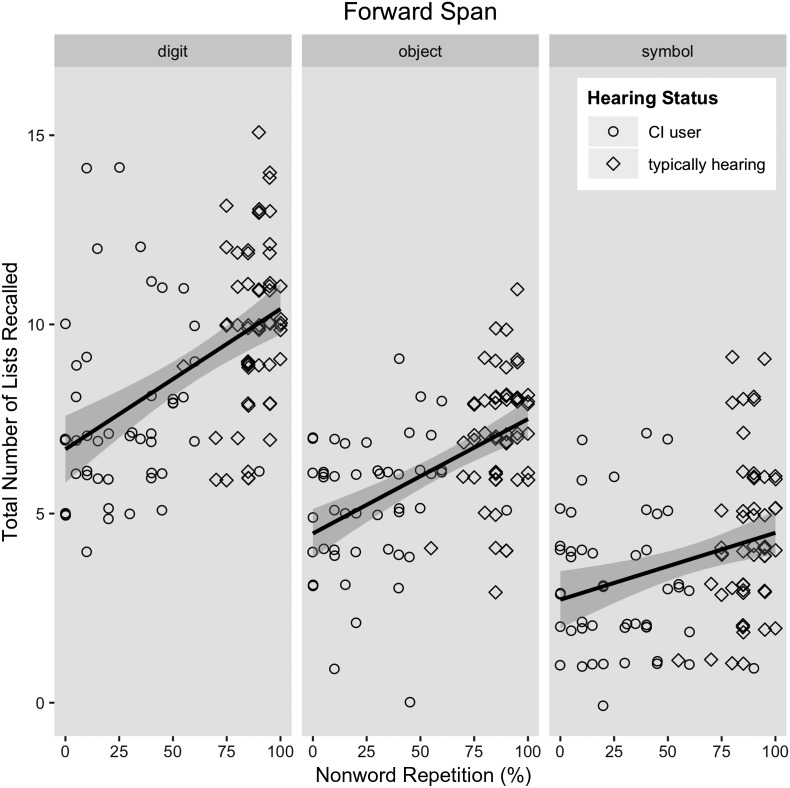
Prediction 1: More lists will be recalled for digits, then objects, and then symbols. As predicted, more lists were recalled when the stimuli were digits rather than objects, B = −2.63, t(208) = −12.88, p < .00l, or symbols, B = −5.13, t(208) = −25.10, p < .00l, and more lists of objects were recalled than lists of symbols, B = −2.50, t(208) = −12.22, p < .001. Separate analyses for the two groups confirmed that both groups showed the overall decline from digits to objects to symbols (all ts > 8.0, all ps < .001; see means in Table 3).
Table 3.
Mean accuracy and standard deviation on forward span tasks.
| Stimulus type | CI sample | Control sample |
|---|---|---|
| Digit | 7.6 (2.4) | 10.1 (2.2) |
| Object | 5.2 (1.8) | 7.2 (1.6) |
| Symbol | 3.1 (1.8) | 4.4 (2.0) |
Note. Accuracy refers to the total number of correctly recalled lists. CI = cochlear implant.
Prediction 2: Individual differences related to encoding and retrieval will predict memory performance above and beyond phonological storage. The model of main effects described above led to an unexpected finding regarding our four predictors of interest. In this model, neither hearing status, t(104) = 1.0, p = .33, nor nonword repetition (as an estimate of phonological storage), t(104) = 0.86, p = .39, uniquely contributed to performance on the forward span tasks. Successively eliminating each of these variables demonstrated that the model with these variables accounted for no more variance than either reduced model. Notably, when hearing status was removed, a main effect of nonword repetition emerged, B = 0.01, t(104) = 3.12, p = .002. Likewise, when nonword repetition was removed, a main effect of hearing status emerged, B = 0.92, t(104) = 3.16, p = .002. As shown in Figure 2, the redundancy of nonword repetition and hearing status cannot be due to a lack of variability on the nonword repetition task within each group. Because hearing status and nonword repetition accounted for the same variance, only one was necessary for further analysis. Because the purpose of the study is to simultaneously examine the contributions of encoding, storage, and retrieval, we opted to retain nonword repetition as an indicator of phonological storage. However, the results were identical when hearing status, rather than nonword repetition, was retained, suggesting that the variability in nonword repetition performance afforded no more predictive value than the categorical nature of hearing status (see Supplemental Material S2).
Figure 2.
Scatter plots of nonword repetition and accuracy (number of lists recalled). Groups separated for display only, as hearing status did not interact with any other variable.
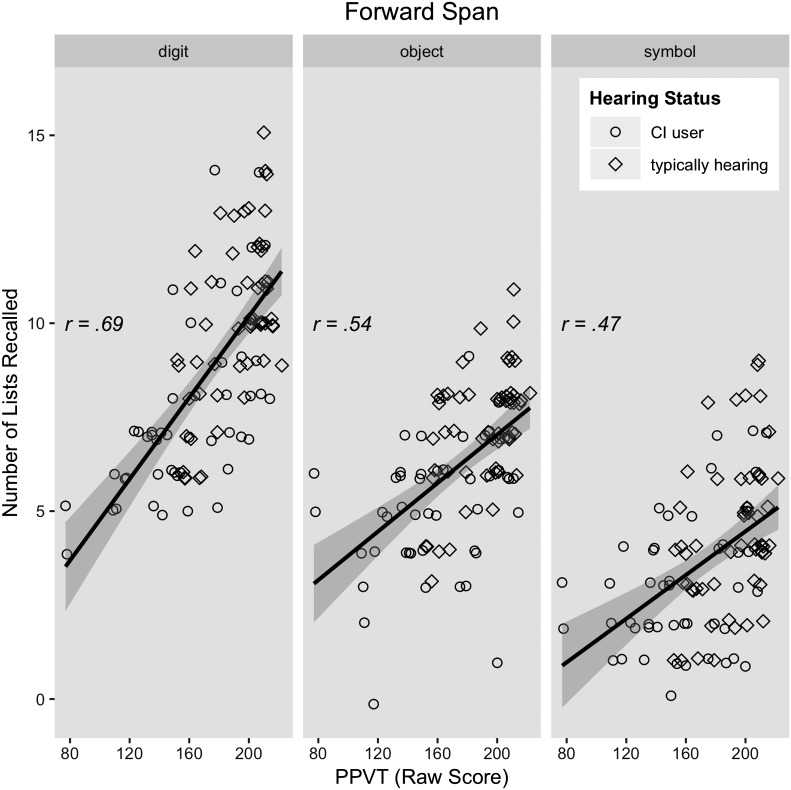
The best model of forward span included random intercepts for subject, control variables of age and nonverbal intelligence, as well as significant effects of digit naming, nonword repetition, vocabulary knowledge, and a two-way interaction of vocabulary knowledge and stimulus type (see Table 4). Vocabulary knowledge was a better predictor of digit span than of object or symbol span, which did not differ (see Figure 3). Fitted model data were strongly correlated with our observed data (r = .91, p < .001), and visual inspection of the model residuals suggested a reasonable fit.
Table 4.
Linear mixed models.
| Span direction | Random intercept variance | Predictors | t | p |
|---|---|---|---|---|
| Forward | 0.77, SD = 0.88 | Age | 1.2 | .25 |
| CTONI-2 | 2.7 | .01 | ||
| Stimulus (digits−objects) | 1.1 | .25 | ||
| Stimulus (digits−symbols) | −0.7 | .52 | ||
| Stimulus (objects−symbols) | −1.7 | .07 | ||
| Rapid digit naming | 2.2 | .03 | ||
| Nonword repetition | 3.1 | < .01 | ||
| PPVT | 4.9 | < .01 | ||
| PPVT:stimulus (digits−objects) | −3.6 | < .01 | ||
| PPVT:stimulus (digits−symbols) | −4.2 | < .01 | ||
| PPVT:stimulus (objects−symbols) | −0.53 | .59 | ||
| Backward | 1.08, SD = 1.04 | Age | 1.0 | .33 |
| CTONI-2 | 3.4 | .01 | ||
| Stimulus (digits−objects) | 1.9 | .06 | ||
| Stimulus (digits−symbols) | 3.0 | < .01 | ||
| Stimulus (objects−symbols) | 1.1 | .27 | ||
| Rapid digit naming | 4.3 | < .01 | ||
| PPVT | 5.7 | < .01 | ||
| PPVT:stimulus (digits−objects) | −4.0 | < .01 | ||
| PPVT:stimulus (digits−symbols) | −6.1 | < .01 | ||
| PPVT:stimulus (objects−symbols) | −2.1 | .03 |
Note. Significant contributors to the model are denoted in bold. A minus sign (−) denotes the effect of changing levels of the variable stimulus—for example, the variance due to shifting stimuli from digits to objects. A colon (:) denotes an interaction term. CTONI-2 = Comprehensive Test of Nonverbal Intelligence–Second Edition; PPVT = Peabody Picture Vocabulary Test.
Figure 3.
Scatter plots depicting the two-way interaction of stimulus type and Peabody Picture Vocabulary Test (PPVT; raw score) on forward span accuracy. Groups separated for display only, as hearing status did not interact with any other variable.
Backward Span
All analyses were repeated, but with backward span as the outcome variable. This analysis was designed to assess whether the mechanisms uncovered for forward span would also underlie group differences and individual variability when an additional processing operation is required to complete the task.
Prediction 1: More lists will be recalled for digits, then objects, and then symbols. As was the case for forward span, a model with all variables confirmed that accuracy for digits was higher than accuracy for both objects, t(208) = −10.00, p < .001, and symbols, t(208) = −15.13, p < .001, and accuracy for objects was higher than that for symbols, t(208) = −5.13, p < .001. Separate analyses for the two groups confirmed that both groups showed the overall decline from digits to objects to symbols (all ts > 2.0, all ps < .05; see means in Table 5).
Table 5.
Mean accuracy and standard deviation on backward span tasks.
| Stimulus type | CI sample | Control sample |
|---|---|---|
| Digit | 5.4 (2.4) | 8.0 (3.2) |
| Object | 3.8 (1.9) | 5.1 (2.0) |
| Symbol | 3.0 (1.6) | 3.6 (1.9) |
Note. Accuracy refers to the total number of correctly recalled lists. CI = cochlear implant.
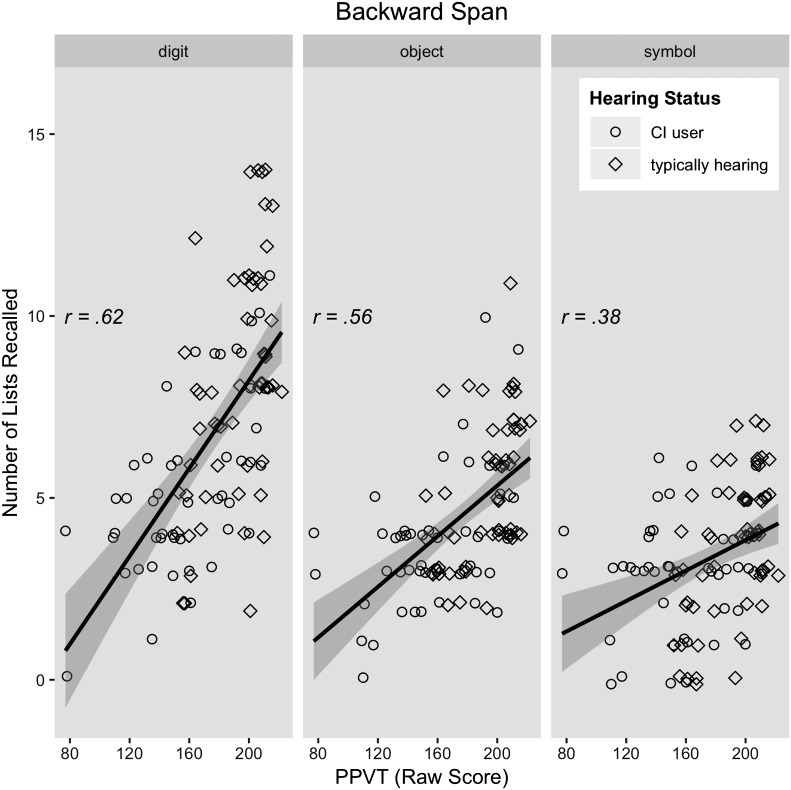
Prediction 2: Individual differences related to encoding and retrieval will predict span above and beyond phonological storage. The best model of backward span included random intercepts for subject, control variables of age and nonverbal intelligence, as well as significant effects of stimulus type, rapid digit naming, vocabulary knowledge, and a two-way interaction of vocabulary knowledge and stimulus type (see Table 4). Vocabulary knowledge was a better predictor of digit span than of object span and symbol span, as well as a better predictor of object span than symbol span (see Figure 4). The best fitting model for backward span included neither hearing status nor nonword repetition. Fitted model data were strongly correlated with our observed data (r = .91, p < .001), and visual inspection of the model residuals suggested a reasonable fit.
Figure 4.
Depiction of the two-way interaction between Peabody Picture Vocabulary Test (PPVT; raw score) and stimulus type for backward span accuracy. Groups separated for display only, as hearing status did not interact with any other variable.
Role of Hearing History
The associations of hearing history and demographics with span memory were further explored within the group of pediatric CI users only, as opposed to the prior analyses that included both CI users and typically hearing peers. This analysis allowed for testing the hearing history variables that were present only in the CI sample. Additional models were built for the forward and backward spans, which included fixed effects of nonverbal IQ 3 and stimulus type, as well as age at identification of deafness, preimplant better ear pure-tone average, age of first CI, duration of use, and bilateral/unilateral implantation. None of these hearing history or demographic variables explained accuracy on forward span, and only duration of CI use—likely as a proxy for age at testing or language ability—was related to backward span (see Supplemental Material S3). Given our inclusion criteria, many of these variables could be considered to have restricted ranges. Therefore, no further interpretation will be made from these results.
Discussion
The results obtained in this study provide converging evidence that phonological storage alone does not provide a full explanation for the high variability observed in pediatric CI users' short-term and working memory for digits, objects, and symbols. Specifically, we proposed that individual differences in the efficiency of long-term lexical access during encoding and retrieval processes, as indexed by rapid digit naming and vocabulary knowledge, respectively, should also be considered. Our first prediction—that the stimulus type would influence memory span—was based on prior research demonstrating that an item's lexical status and familiarity impact both encoding and redintegration processes, which support short-term and working memory performance. This prediction was supported. List recall on both forward and backward spans was highest for visually presented digits, which have highly familiar verbal labels to support encoding and high interitem associations to support redintegration. Performance was lowest for novel visual symbols, which both are difficult to verbally label and have no long-term lexical representations to support redintegration. Performance for visual images of concrete nouns fell between these extremes. Importantly, the overall pattern of stimulus type held for both groups, suggesting that pediatric CI users' sensitivity to verbal labels and interitem associations mirrors that of their typically hearing peers.
Our primary interest in this study was the underlying information-processing operations that contribute to individual variation in short-term and working memory performance. Some findings have suggested that pediatric CI users' disruptions are due to degraded phonological storage (Nittrouer et al., 2013), whereas other results have shown that rapid naming speed is correlated with executive functions in both pediatric CI users and their typically hearing peers (AuBuchon et al., 2015b). The latter findings suggested that encoding may contribute to pediatric CI users' individual differences in short-term and working memory, supplementing an earlier discovery that processing operations during storage were related to span (Pisoni & Cleary, 2003). Additional findings implicating CI users' vocabulary knowledge as the primary predictor for word span also suggested a role for retrieval mechanisms (Nittrouer et al., 2017). These previous results led us to test whether individual variation in memory span performance was better accounted for by measures related to processing operations during encoding and retrieval—such as recoding speed and vocabulary knowledge—or measures related to storage, such as nonword repetition.
Individual Differences in Short-Term Memory Performance
Phonological storage, as measured by nonword repetition, was predictive of forward span performance. Specifically, impairments in phonological storage contributed to pediatric CI users' overall impaired performance on verbal short-term memory tasks. However, individual differences in phonological storage provided no more predictive power than simply knowledge of hearing status alone. This redundancy was not due to a lack of sensitivity in the nonword repetition task. Rather, this finding suggests that the role of phonological storage appears to be broad and pervasive in nature. In other words, the phonological degradation due to a CI may have an overall negative impact on short-term storage—driving group differences between CI users and typically hearing peers (though the finding that nonword repetition predicted no variance for backward span indicates an alternative explanation presented below). Nonetheless, it is clear that subtle variation in CI users' quality of phonological detail cannot fully account for individual differences in outcomes.
The finding that variability in nonword repetition did not predict span does not lead us to discount the roles of audibility and/or degraded memory traces. For prelingually implanted CI users, lexical representations in long-term memory would have been learned under acoustic constraints of the CI. Thus, retrieval from long-term memory is likely to be impacted by phonological overlap in the same ways as described earlier; that is, shared features among items lead to slower, more effortful activation of memory traces from lexical long-term memory into short-term memory. Slowed activation from lexical long-term memory would impact both phonological recoding and redintegration.
Indeed, individual variation in short-term memory span is better explained by the number of lexical representations available in long-term memory and the speed with which those representations can be accessed during recoding. Specifically, phonological recoding speed (as measured by rapid digit naming) and vocabulary knowledge (as measured by PPVT) accounted for additional variance in forward span performance above and beyond age, nonverbal intelligence, and phonological storage (as measured by nonword repetition). Contributions of language ability to complex memory span performance are well documented in typical development (Daneman & Carpenter, 1980). Furthermore, other factors in addition to language may contribute to the decay of items in memory, such as mental efficiency, processing speed, organizational strategies, and effort (Kronenberger et al., 2014; Kronenberger & Pisoni, 2016; Pisoni, Kronenberger, Chandramouli, & Conway, 2016; Rönnberg et al., 2013).
We also observed an interaction between stimulus type and vocabulary knowledge. For both groups, vocabulary knowledge was predictive of performance on all three span tasks, and this relationship was strongest for digits. The strong relation between PPVT and the forward (r = .69) and backward (r = .62) digit span tasks may seem counterintuitive if we consider PPVT merely as a reflection of vocabulary achievement. The youngest participant was over 9 years of age when the span tasks were administered, so one might expect digit span to impose few linguistic demands on any of our participants. Yet, digit span was sensitive enough to capture individual differences in linguistic ability. Such a relation is consistent with an interpretation in which PPVT represents a long-term semantic network that can fortify individual representations against decay and promote redintegration (see also Jones & Macken, 2015).
We were more surprised that vocabulary knowledge was predictive of short-term memory for novel visual symbols. Not only were these symbols novel to the participants, but they were also not designed to have a specific referent. Therefore, we anticipated that these symbols would not have universally agreed-upon verbal labels. Anecdotally, however, many participants were observed to be overtly labeling these items (e.g., “tornado,” “basket,” “seashell”). This observation is consistent with our hypothesis that individual differences in the efficiency of long-term lexical access are related to short-term memory performance. In other words, the relation between vocabulary knowledge and performance on novel visual symbols may reflect the usefulness of verbal labeling even for ambiguous, never-before-encountered images. For example, it is possible that those participants with a larger vocabulary were better able to access and assign consistent verbal labels for items that differentiated the target item from the remaining items in the set. Unfortunately, it may be difficult to find truly nonverbal stimuli to address this limitation while maintaining a paradigm that requires binding of item and temporal order—a task inherent within spoken language processing. An alternative explanation is that all tests—including symbol span and PPVT—are influenced by nonspecific test-taking factors (e.g., factors influencing performance on all types of tests), such as comfort with test taking, decision-making ability, and reasoning skills, which could be an additional variable driving the observed correlation rather than verbal labeling.
Individual Differences in Working Memory Performance
A crucial finding was that neither hearing status nor nonword repetition was predictive of performance on the backward span (i.e., working memory) tasks when rapid digit naming and vocabulary knowledge were included in the model. Watson, Titterington, Henry, and Toner (2007) observed poorer performance on backward digit span in 7- to 13-year-old pediatric CI users than in age-matched controls. In a longitudinal study of early-implanted, long-term CI users, Pisoni et al. (2011) found that, in elementary school, 23% of pediatric CI users scored less than 1 SD lower than norm; by the time they reached high school, 38% of the group had fallen below the 1-SD cutoff. In a prior study with an overlapping sample of the present cohort, pediatric CI users had smaller average Wechsler Intelligence Scale for Children Backward Digit Spans than typically hearing controls (AuBuchon et al., 2015a). More importantly, however, the use of linear mixed model analyses has moved beyond confirming that group differences exist. The current analyses allow us to make more detailed inferences regarding the underlying information-processing mechanisms, which may contribute to these group differences. Specifically, variability in the availability and retrieval of long-term lexical representations appears to drive both group and individual differences on working memory span.
Unfortunately, the correlational nature of the present design restrains this interpretation; experimental research is needed to establish a causal relationship and specify the mechanisms of action proposed here. Such experimentation will likely require moving beyond simple forward and backward span tasks—or even the standardized complex working memory span tasks often found in commercial cognitive testing batteries. Instead, we might look to experimental research in cognitive science for paradigms that can better isolate specific process components within the working memory system (e.g., Camos, Lagner, & Barrouillet, 2009; Ecker, Lewandowsky, Oberauer, & Chee, 2010; for a review, see Unsworth & Engle, 2007).
Most earlier work on prelingually implanted CI users has conceptualized language as a long-term outcome predicted by short-term and working memory abilities (e.g., Geers et al., 2011), but the current study emphasizes the role of language skill as a support for short-term and working memory performance. The relationship between working memory and language is likely bidirectional, with an intact working memory system being a necessary prerequisite for language learning (Baddeley et al., 1998) and previously learned language knowledge feeding back to support current working memory functions (Gathercole, 1995). Although Baddeley and his colleagues reference the impact of long-term lexical knowledge in supporting short-term and working memory, the potential role of controlled attentional processes as a mediator between short- and long-term activation is more thoroughly elaborated in the contemporary models of working memory currently being developed within cognitive science.
Active Attention-Based Models of Working Memory
Contemporary models of working memory place less emphasis on modular, domain-specific storage and processing components in order to account for differences in performance across verbal and spatial tasks. 4 Instead, the embedded processes model of working memory, developed by Cowan (2001), and the TBRS model of working memory, developed by Barrouillet and Camos (2012), both emphasize a single, modality-general attentional mechanism that can be used either as a store, which protects information from decay, or to process information. Items protected by attention represent a limited subset of short-term memory. The remainder of short-term memory can hold an unlimited number of traces, but these traces are susceptible to decay. If resources are directed toward a secondary processing task—as is the case in traditional working memory tasks, such as backward digit span, reading span, and listening span—then to-be-remembered information is susceptible to decay, meaning the representation fades over time.
According to the embedded processes and TBRS models, attention's capacity limit can be overcome by using coding and processing strategies. Activated memory traces in the short-term store are quickly and easily accessible to attention. Therefore, items can be temporarily reactivated via attentional refreshing—an active control strategy in which attention rapidly shifts between its storage and processing roles (Camos et al., 2009). Unlike verbal rehearsal, attentional refreshing can be applied to any type of information, regardless of its domain. Attentional refreshing is considered an “active” strategy because it requires conscious attentional resources and effort. In addition, information that is part of a robust long-term memory network appears to be fortified against decay—even when it is being held outside attention (Cowan, Rouder, Blume, & Saults, 2012). Thus, it is common to see performance as high as seven—or even nine—items on a short-term memory task that uses highly familiar stimuli and does not impose a secondary processing task to limit strategy use.
Typical development leads to an asymmetry in which short-term memory for verbal materials tends to be prioritized over memory for visual–spatial materials (Atkinson & Shiffrin, 1968). Morey, Morey, van der Reijden, and Holweg (2013) have demonstrated that at least part of this visual–verbal asymmetry stems from attention-demanding maintenance strategies—such as attentional refreshing—for visual materials, but attention-free use of rehearsal processes for verbal materials. Crucially, the independence of refreshing and verbal rehearsal processes may develop over time. Young children appear to recruit attentional resources to engage verbal rehearsal (Oftinger & Camos, 2016). Thus, although young children may be able to optionally select to either refresh or rehearse stored items, their ability to simultaneously use both strategies is limited. We suspect that verbally mediated strategies that occur during other stages of memory may also require attentional resources early in development but become automatic with linguistic experience and actively using spoken language.
Even short delays in exposure to language can alter pediatric CI users' developmental trajectory (Levine, Strother-Garcia, Golinkoff, & Hirsh-Pasek, 2016). Consequently, even if these CI users “catch up” to their peers on standardized language tests, they may still need more attentional resources to reach similar levels of performance (Nicholas & Geers, 2007). Therefore, we suggest that many pediatric CI users may not have developed the robust lexical long-term memory structure that supports automatic engagement of linguistic strategies across these three stages of memory. Similar assumptions are shared by the ease of language understanding model (Rönnberg et al., 2013). Generally, the ease of language understanding model specifies that, in order for language processing to proceed from poorly detailed acoustic information (as is the case for CI users), the working memory system must include modality-general mechanisms such as attention and retrieval from long-term memory.
Although our findings indicate that attention, encoding, and retrieval are crucially important processes explaining individual and group differences in working memory for both pediatric CI users and typically hearing samples, memory storage (in general, and phonological storage, in particular) is also clearly important in working memory (and especially the short-term storage component of working memory) outcomes. Cowan (2001) posits that long-term memory functions to fortify short-term memory traces from decay. Without a robust lexical long-term memory network and distinctive lexical long-term memory representations, CI users' phonological and lexical traces are fragile and easily compromised.
We suggest that the distinctiveness and quality of the lexical long-term representations do not merely create a problem of passive short-term storage but also constrain attentional resources, limiting strategic processing operations. Indeed, Cantor and Engle (1993) observed direct relationships between the efficiency of lexical long-term memory activation and working memory capacity. As observed here and in many prior studies, pediatric CI users have smaller vocabularies and slower phonological recoding speeds than typically hearing peers, suggesting that they also have more effortful, limited access to lexical long-term memory. Moreover, pediatric CI users also have slower verbal rehearsal speeds, and as for their typically hearing peers, rehearsal speed predicts short-term memory performance when the task is auditory (Pisoni et al., 2011). Interestingly, however, pediatric CI users do not show consistent use of verbal rehearsal in visual tasks, which require phonological recoding before rehearsal can occur (AuBuchon et al., 2015b). Our interpretation of these findings is that pediatric CI users deplete their limited attentional processing resources during phonological recoding, leaving fewer resources for rehearsal processes, which may also require active, controlled attention in this group (Hunter & Pisoni, 2018). A similar explanation has been proposed to explain working memory deficits in children with Down syndrome (Jarrold et al., 2004). Despite hearing loss being a common co-occurrence in Down syndrome, verbal rehearsal and language ability—not hearing levels—explained working memory performance in these children.
Examining pediatric CI users' memory delays through the lens of attention also provides a plausible explanation of our finding that hearing status/nonword repetition contributed to the model predicting accuracy on forward, but not backward, span tasks. Differences between the CI users and typically hearing samples in forward span performance might be explained more parsimoniously by differences in controlled attentional processing than by differences in phonological storage. Forward span tasks require few controlled attention resources because of their rote nature and no explicit task demands to simultaneously process information (Engle, Tuholski, Laughlin, & Conway 1999). Perhaps even on the forward span task, pediatric CI users actively deployed attention to complete components of encoding, storage, and retrieval that were rote and automatic for the typically hearing participants. In contrast, backward span requires greater allocation of executive, controlled attention resources and is a more classically “working memory” task (Engle et al., 1999). As a result, active processing in encoding and retrieval processes is required for both groups, and differences in measures of these processes (digit naming speed and vocabulary in this study) sufficiently accounted for differences between CI users and typically hearing samples, such that hearing status was not a predictor in the backward span analyses after digit naming speed and vocabulary were accounted for.
In other words, for the typically hearing participants, forward span and backward span likely represent the traditional division between short-term and working memory (Engle et al., 1999). However, for pediatric CI users, both forward and backward span may act as working memory tasks because CI users must tap attentional resources to recode, store, and retrieve phonological information to carry out the span task successfully; furthermore, the requirement to simultaneously code order (e.g., forward or backward) and content (stimuli) information is more attention demanding for CI users, even for forward order span tasks (Kronenberger & Pisoni, 2018). Therefore, it is likely that forward span taps a qualitatively different engagement of attentional resources in CI versus typically hearing groups, resulting in significant contributions not only of encoding (digit naming speed) and retrieval (vocabulary) processes but also of hearing status (or some proxy such as nonword repetition). In backward span, on the other hand, the additional instructions to reverse the list for recall required both groups to similarly engage attention, resulting in greater influence of encoding and retrieval processes on performance relative to hearing status (or phonological storage). In fact, nonword repetition has been shown to depend on both perceptual discrimination and the structural organization of lexical long-term memory (Coady & Evans, 2008; Montgomery & Evans, 2006). These studies provide converging evidence that phonological storage should not be considered in isolation but, instead, should be examined as part of a larger working memory system that acts as an interface between incoming sensory experiences and existing phonological and lexical representations in long-term memory.
Summary
One recently proposed explanation for pediatric CI users' short-term and working memory deficits, phonological storage (as measured by nonword repetition), did not fully account for individual variability in these CI users' short-term memory performance or for group differences between CI users and typically hearing samples in working memory (e.g., backward span) performance. In fact, it provided no more information to the model of forward span than knowledge of hearing status. Moreover, neither hearing status nor phonological storage predicted working memory (backward span) performance when estimates of long-term lexical access were included. These estimates, based on digit naming speed and vocabulary knowledge, are related to phonological recoding and redintegration within the working memory system. Phonological recoding occurs during the encoding stage, and redintegration occurs during retrieval. These new findings on short-term and working memory span provide additional converging evidence that information processing mechanisms contribute to pediatric CI users' short-term and working memory delays.
One implication of separately considering each stage of working memory—encoding, storage, and retrieval—is a new direction for interventions. A “storage-only” framework leads to two conclusions regarding pediatric CI users' short-term and working memory, especially when storage is strictly defined by the robustness of phonological representations. First, it limits the scope of short-term/working memory challenges (and potential interventions) only to a “broken” phonological loop. Second, it places the burden of intervention on either designing new devices that preserve more acoustic–phonological detail in the auditory signal or improving phonological representations in memory using some other approach.
Empirical results from this study broaden the explanation of deficits in CI users' short-term and working memory from storage alone to additionally including other processes of short-term and working memory. Specifically, we propose that the impact of hearing loss on short-term and working memory occurs not solely from storing the compromised acoustic–phonetic signal. Reframing the locus of the deficit from storage—a fixed characteristic of the working memory system—to information processing operations at each stage of memory moves the focus of intervention into developing more precise strategies, which are under the patient's control. An approach that emphasizes the roles of long-term lexical knowledge and attention will encourage exploration of potential interventions, which can be implemented in the clinic. For example, cognitive science has a large body of literature on recoding and rehearsal processes in adults. A clear first step would be to elucidate how attention is related to these processes in both children with typical hearing and children who use CIs. Then, we may better understand how to modify and improve automatization of these processes for children with CIs who currently struggle on tasks of short-term and working memory.
Supplementary Material
Acknowledgments
This work was supported by National Institute on Deafness and Other Communication Disorders Grants R01DC000111, R01DC009581, R01DC015257, and T32DC000012, awarded to David Pisoni. The authors wish to thank the participants for their time and Bethany Coleson, Shirly Henning, and Lindsay Stone for assisting with data collection.
Funding Statement
This work was supported by National Institute on Deafness and Other Communication Disorders Grants R01DC000111, R01DC009581, R01DC015257, and T32DC000012, awarded to David Pisoni.
Footnotes
Pairwise correlations among all tasks can be found in Supplemental Material S1.
To account for the large age range in our pediatric CI user group, focused recruitment efforts were made to match CI users with normal hearing peers of the same age (at both Visits 1 and 2) and nonverbal intelligence. This resulted in overrecruitment of normal hearing peers and some CI users for whom matches could not be found. In previous articles, we have utilized only those participants with a 1:1 match on age and nonverbal intelligence (AuBuchon et al., 2015a, 2015b; Kronenberger et al., 2013). However, in this article, we have chosen to report data from all participants in order to maximize variability and power in the linear mixed-effects modeling. For a group comparison of the computerized digit span data using only matched participants, see AuBuchon et al. (2015a).
Age was left out of the model including hearing history variables, because age at fit and duration of use, together, perfectly correlate with age at testing.
For a recent review distinguishing current models of working memory on the continuums of modularity and attentional control—and their implications for language processing—see Adams, Nguyen, and Cowan (2018).
References
- Adams E. J., Nguyen A. T., & Cowan N. (2018). Theories of working memory: Differences in definition, degree of modularity, role of attention, and purpose. Language, Speech, and Hearing Services in Schools, 49(3), 340–355. https://doi.org/10.1044/2018_LSHSS-17-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald L. M. D., & Harder Griebeling K. (2016). Rethinking the connection between working memory and language impairment. International Journal of Language & Communication Disorders, 51(3), 252–264. https://doi.org/10.1111/1460-6984.12202 [DOI] [PubMed] [Google Scholar]
- Atkinson R. C., & Shiffrin R. M. (1968). Human memory: A proposed system and its control processes. In Spence K. W. & Spence J. T. (Eds.), The psychology of learning and motivation: Advances in research and theory (pp. 89–195). New York, NY: Academic Press; Retrieved from http://www.sciencedirect.com/science/article/pii/S0079742108604223 [Google Scholar]
- AuBuchon A. M., Pisoni D. B., & Kronenberger W. G. (2015a). Short-term and working memory impairments in early-implanted, long-term cochlear implant users are independent of audibility and speech production. Ear and Hearing, 36(6), 733–737. https://doi.org/10.1097/AUD.0000000000000189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AuBuchon A. M., Pisoni D. B., & Kronenberger W. G. (2015b). Verbal processing speed and executive functioning in long-term cochlear implant users. Journal of Speech, Language, and Hearing Research, 58(1), 151–162. https://doi.org/10.1044/2014_JSLHR-H-13-0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. (1996). The fractionation of working memory. Proceedings of the National Academy of Sciences of the United States of America, 93, 13468–13472. https://doi.org/10.1073/pnas.93.24.13468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. D., Gathercole S. E., & Papagno C. A. (1998). The phonological loop as a language learning device. Psychological Review, 1, 158–173. [DOI] [PubMed] [Google Scholar]
- Baddeley A. D., & Hitch G. (1974). Working memory. In Bower G. H. (Ed.), The psychology of learning and motivation (Vol. 8, pp. 47–89). Cambridge, MA: Academic Press. [Google Scholar]
- Baddeley A. D., Thomson N., & Buchanan M. (1975). Word length and the structure of short-term memory. Journal of Verbal Learning and Verbal Behavior, 14, 575–589. https://doi.org/10.1016/S0022-5371(75)80045-4 [Google Scholar]
- Barrouillet P., & Camos V. (2012). The time-based resource-sharing model of working memory. In Osaka N., Logie R. H., & D'Esposito M. (Eds.), The cognitive neuroscience of working memory (pp. 59–80). Oxford, England: Oxford University Press; https://doi.org/10.1093/acprof:oso/9780198570394.003.0004 [Google Scholar]
- Bates D., Maechler M., Bolker B., & Walker S. (2014). lme4: Linear mixed-effects models using Eigen and S4 (R package Version 1.1-6). Retrieved from https://cran.r-project.org/
- Bielski L. M., & Lansing C. R. (2012). Utility of the Baddeley and Hitch model of short-term working memory to investigate spoken language understanding: A tutorial. SIG 7 Perspectives on Aural Rehabilitation and Its Instrumentation, 19(1), 25–33. https://doi.org/10.1044/arii19.1.25 [Google Scholar]
- Bower G. H., & Glass A. L. (1976). Structural units and the redintegrative power of picture fragments. Journal of Experimental Psychology: Human Learning and Memory, 2(4), 456–466. https://doi.org/10.1037/0278-7393.2.4.456 [PubMed] [Google Scholar]
- Burkholder R. A., & Pisoni D. B. (2003). Speech timing and working memory in profoundly deaf children after cochlear implantation. Journal of Experimental Child Psychology, 85(1), 63–88. https://doi.org/10.1016/S0022-0965(03)00033-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camos V., Lagner P., & Barrouillet P. (2009). Two maintenance mechanisms of verbal information in working memory. Journal of Memory and Language, 61(3), 457–469. https://doi.org/10.1016/j.jml.2009.06.002 [Google Scholar]
- Cantor J., & Engle R. W. (1993). Working-memory capacity as long-term memory activation: An individual-differences approach general capacity model. Journal of Experimental Psychology: Learning, Memory, and Cognition, 19(5), 1101–1314. [DOI] [PubMed] [Google Scholar]
- Carmichael L., Hogan H. P., & Walter A. A. (1932). An experimental study of the effect of language on the reproduction of visually perceived form. Journal of Experimental Psychology, 15(1), 73–86. [Google Scholar]
- Case R., Midian Kurland D., & Goldberg J. (1982). Operational efficiency and the growth of short-term memory span. Journal of Experimental Child Psychology, 33, 386–404. [Google Scholar]
- Coady J., & Evans J. (2008). Uses and interpretations of non-word repetition tasks in children with and without specific language impairments (SLI). International Journal of Language & Communication Disorders, 43, 1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad R., & Hull A. J. (1964). Information, acoustic confusion and memory span. British Journal of Psychology, 55(4), 429–432. https://doi.org/10.1111/j.2044-8295.1964.tb00928.x [DOI] [PubMed] [Google Scholar]
- Cowan N. (2001). The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences, 24, 87–185. [DOI] [PubMed] [Google Scholar]
- Cowan N., Keller T. A., Hulme C., Roodenrys S., McDougall S., & Rack J. (1994). Verbal memory span in children: Speech timing clues to the mechanisms underlying age and word length effects. Journal of Memory and Language, 33, 234–350. [Google Scholar]
- Cowan N., Rouder J. N., Blume C. L., & Saults J. S. (2012). Models of verbal working memory capacity: What does it take to make them work? Psychological Review, 119(3), 480–499. https://doi.org/10.1037/a0027791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman M., & Carpenter P. A. (1980). Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior, 19, 450–466. https://doi.org/10.1016/S0022-5371(80)90312-6 [Google Scholar]
- Dawson P. W., Busby P. A., McKay C. M., & Clark G. M. (2002). Short-term auditory memory in children using cochlear implants and its relevance to receptive language. Journal of Speech, Language, and Hearing Research, 45(4), 789–801. https://doi.org/10.1044/1092-4388(2002/064) [DOI] [PubMed] [Google Scholar]
- Dillon C. M., Cleary M., Pisoni D. B., & Carter A. K. (2004). Imitation of nonwords by hearing-impaired children with cochlear implants: Segmental analyses. Clinical Linguistics and Phonetics, 18(1), 39–55. https://doi.org/10.1080/0269920031000151669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L. M., & Dunn L. M. (1997). Peabody Picture Vocabulary Test–Third Edition. Circle Pines, MN: AGS. [Google Scholar]
- Ecker U. K. H., Lewandowsky S., Oberauer K., & Chee A. E. H. (2010). The components of working memory updating: An experimental decomposition and individual differences. Journal of Experimental Psychology: Learning Memory and Cognition, 36(1), 170–189. https://doi.org/10.1037/a0017891 [DOI] [PubMed] [Google Scholar]
- Engle R. W., Tuholski S. W., Laughlin J. E., & Conway A. R. A. (1999). Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. Journal of Experimental Psychology: General, 3, 309–331. [DOI] [PubMed] [Google Scholar]
- Gathercole S. E. (1995). Is nonword repetition a test of phonological memory or long-term knowledge? It all depends on the nonwords. Memory & Cognition, 23(1), 83–94. [DOI] [PubMed] [Google Scholar]
- Gathercole S. E., & Baddeley A. L. (1996). The Children's Test of Nonword Repetition. London: The Psychological Corporation. [Google Scholar]
- Geers A. E., Strube M. J., Tobey E. A., Pisoni D. B., & Moog J. S. (2011). Epilogue: Factors contributing to long-term outcomes of cochlear implantation in early childhood. Ear and Hearing, 32, 84S–92S. https://doi.org/10.1097/AUD.0b013e3181ffd5b5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P., & Tisdale J. (2009). Does phonological short-term memory causally determine vocabulary learning? Toward a computational resolution of the debate. Journal of Memory and Language, 61, 481–502. https://doi.org/10.1016/j.jml.2009.08.001 [Google Scholar]
- Hammill D. D., Pearson N. A., & Wiederholt J. L. (2009). Comprehensive Test of Nonverbal Intelligence–Second Edition. Austin, TX: Pro-Ed. [Google Scholar]
- Hulme C. (2003). High- and low-frequency words are recalled equally well in alternating lists: Evidence for associative effects in serial recall. Journal of Memory and Language, 49(4), 500–518. https://doi.org/10.1016/S0749-596X(03)00096-2 [Google Scholar]
- Hulme C., Maughan S., & Brown G. D. A. (1991). Memory for familiar and unfamiliar words: Evidence for a long-term memory contribution to short-term memory span. Journal of Memory and Language, 30(6), 685–701. https://doi.org/10.1016/0749-596X(91)90032-F [Google Scholar]
- Hulme C., Thomson N., Muir C., & Lawrence A. (1984). Speech rate and the development of short-term memory span. Journal of Experimental Child Psychology, 38(2), 241–253. https://doi.org/10.1016/0022-0965(84)90124-3 [DOI] [PubMed] [Google Scholar]
- Hummer T. A., Kronenberger W. G., Wang Y., Dunn D. W., Mosier K. M., Kalnin A. J., & Mathews V. P. (2011). Executive functioning characteristics associated with ADHD comorbidity in adolescents with disruptive behavior disorders. Journal of Abnormal Child Psychology, 39(1), 11–19. https://doi.org/10.1007/s10802-010-9449-3 [DOI] [PubMed] [Google Scholar]
- Hunter C. R., & Pisoni D. B. (2018). Extrinsic cognitive load impairs spoken word recognition in high- and low-predictability sentences. Ear and Hearing, 39, 378–389. https://doi.org/10.1097/AUD.0000000000000493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrold C., Cowan N., Hewes A. K., & Riby D. M. (2004). Speech timing and verbal short-term memory: Evidence for contrasting deficits in Down syndrome and Williams syndrome. Journal of Memory and Language, 51(3), 365–380. https://doi.org/10.1016/j.jml.2004.06.007 [Google Scholar]
- Jones G., & Macken B. (2015). Questioning short-term memory and its measurement: Why digit span measures long-term associative learning. Cognition, 144, 1–13. https://doi.org/10.1016/j.cognition.2015.07.009 [DOI] [PubMed] [Google Scholar]
- Kronenberger W. G., Colson B. G., Henning S. C., & Pisoni D. B. (2014). Executive functioning and speech-language skills following long-term use of cochlear implants. Journal of Deaf Studies and Deaf Education, 19(4), 456–470. https://doi.org/10.1093/deafed/enu011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberger W. G., & Pisoni D. B. (2016). Working memory training in deaf children with cochlear implants. In Young N. M. & Kirk K. I. (Eds.), Pediatric cochlear implantation: Learning and the brain (pp. 275–292). New York, NY: Springer. [Google Scholar]
- Kronenberger W. G., & Pisoni D. B. (2018). Neurocognitive functioning in deaf children with cochlear implants. In Knoors H. & Marschark M. (Eds.), Evidence-based practices in deaf education. London, United Kingdom: Oxford. [Google Scholar]
- Kronenberger W. G., Pisoni D. B., Henning S. C., & Colson B. G. (2013). Executive functioning skills in long-term users of cochlear implant: A case control study. Journal of Pediatric Psychology, 38(8), 902–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A., Brockhoff P. B., & Christensen R. H. B. (2015). lmerTest: Tests in linear mixed effects models (R package Version 2.0-29). Retrieved from http://CRAN.R-project.org/
- Levine D., Strother-Garcia K., Golinkoff R. M., & Hirsh-Pasek K. (2016). Language development in the first year of life: What deaf children might be missing before cochlear implantation. Otology and Neurotology, 37(2), 56–62. https://doi.org/10.1097/MAO.0000000000000908 [DOI] [PubMed] [Google Scholar]
- Lyxell B., Sahlén B., Wass M., Ibertsson T., Larsby B., Hällgren M., & Mäki-Torkko E. (2008). Cognitive development in children with cochlear implants: Relations to reading and communication. International Journal of Audiology, 47(Suppl. 2), S47–S52. https://doi.org/10.1080/14992020802307370 [DOI] [PubMed] [Google Scholar]
- Montgomery J., & Evans J. L. (2006). Commentary on “Nonword repetition and word learning: The nature of the relationship” by S. E. Gathercole. Applied Psycholinguistics, 27(4), 573–577. [Google Scholar]
- Morey C. C., Morey R. D., van der Reijden M., & Holweg M. (2013). Asymmetric cross-domain interference between two working memory tasks: Implications for models of working memory. Journal of Memory and Language, 69(3), 324–348. https://doi.org/10.1016/j.jml.2013.04.004 [Google Scholar]
- Nicholas J. G., & Geers A. E. (2007). Will they catch up? The role of age at cochlear implantation in the spoken language development of children with severe to profound hearing loss. Journal of Speech, Language, and Hearing Research, 50(4), 1048–1062. https://doi.org/10.1044/1092-4388(2007/073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niparko J. K., Tobey E. A., Thal D. J., Eisenberg L. S., Wang N. Y., Quittner A. L., … CDaCI Investigative Team. (2010). Spoken language development in children following cochlear implantation. JAMA, 303(15), 1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nittrouer S., Caldwell-Tarr A., Low K. E., & Lowenstein J. H. (2017). Verbal working memory in children with cochlear implants. Journal of Speech, Language, and Hearing Research, 60(11), 3342–3364. https://doi.org/10.1044/2017_JSLHR-H-16-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nittrouer S., Caldwell-Tarr A., & Lowenstein J. H. (2013). Working memory in children with cochlear implants: Problems are in storage, not processing. International Journal of Pediatric Otorhinolaryngology, 77(11), 1886–1898. https://doi.org/10.1016/j.ijporl.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nittrouer S., Caldwell-Tarr A., Sansom E., Twersky J., & Lowenstein J. H. (2014). Nonword repetition in children with cochlear implants: A potential clinical marker of poor language acquisition. American Journal of Speech-Language Pathology, 23(4), 679–695. https://doi.org/10.1044/2014_AJSLP-14-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberauer K., & Kliegl R. (2006). A formal model of capacity limits in working memory. Journal of Memory and Language, 55(4), 601–626. https://doi.org/10.1016/j.jml.2006.08.009 [Google Scholar]
- Oftinger A.-L., & Camos V. (2016). Developmental improvement in strategies to maintain verbal information in working memory. International Journal of Behavioral Development, 42(2), 182–191. https://doi.org/10.1177/0165025416679741 [Google Scholar]
- Pisoni D. B., & Cleary M. (2003). Measures of working memory span and verbal rehearsal speed in deaf children after cochlear implantation. Ear and Hearing, 24(1), 106S–120S. https://doi.org/10.1097/01.AUD.0000051692.05140.8E [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni D. B., & Geers A. E. (2000). Working memory in deaf children with cochlear implants: Correlations between digit span and measures of spoken language processing. Annals of Otology, Rhinology & Laryngology. Supplement, 185, 92–93. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3429114&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni D. B., Kronenberger W. G., Chandramouli S. H., & Conway C. M. (2016). Learning and memory processes following cochlear implantation: The missing piece of the puzzle. Frontiers in Psychology, 7, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni D. B., Kronenberger W. G., Roman A. S., & Geers A. E. (2011). Measures of digit span and verbal rehearsal speed in deaf children after more than 10 years of cochlear implantation. Ear and Hearing, 32, 60–74. https://doi.org/10.1097/AUD.0b013e3181ffd58e [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2017). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from https://www.r-project.org/ [Google Scholar]
- Rönnberg J., Lunner T., Zekveld A., Sörqvist P., Danielsson H., Lyxell B., … Rudner M. (2013). The ease of language understanding (ELU) model: Theoretical, empirical, and clinical advances. Frontiers in Systems Neuroscience, 7, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweikert R. (1993). A multinomial processing tree model for degradation and redintegration in immediate recall. Memory & Cognition, 21, 168–175. [DOI] [PubMed] [Google Scholar]
- Sperling G. (1960). The information available in brief visual presentations. Psychological Monographs: General and Applied, 74(11), 1–29. https://doi.org/10.1037/h0093759 [Google Scholar]
- Unsworth N., & Engle R. W. (2007). On the division of short-term and working memory: An examination of simple and complex span and their relation to higher order abilities. Psychological Bulletin, 133, 1038–1066. https://doi.org/10.1037/0033-2909.133.6.1038 [DOI] [PubMed] [Google Scholar]
- Unsworth N., Redick T. S., Heitz R. P., Broadway J. M., & Engle R. W. (2009). Complex working memory span tasks and higher-order cognition: A latent-variable analysis of the relationship between processing and storage. Memory, 17, 635–654. https://doi.org/10.1080/09658210902998047 [DOI] [PubMed] [Google Scholar]
- Watson D. R., Titterington J., Henry A., & Toner J. G. (2007). Auditory sensory memory and working memory processes in children with normal hearing and cochlear implants. Audiology and Neurotology, 12, 65–76. https://doi.org/10.1159/000097793 [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1991). Manual for Wechsler Intelligence Scale for Children–Third Edition. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wickham H. (2009). ggplot2: Elegant graphics for data analysis. New York, NY: Springer-Verlag. [Google Scholar]
- Winter B. (2013). Linear models and linear mixed effects models in R with linguistic applications. arXiv:1308.5499. http://arxiv.org/pdf/1308.5499.pdf [Google Scholar]
- Wolf M., Bowers P. G., & Biddle K. (2000). Naming-speed processes, timing, and reading. Journal of Learning Disabilities, 33, 387–407. https://doi.org/10.1177/002221940003300409 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.