Abstract
Purpose
The observed sexual dimorphism of velopharyngeal structures among adult populations has not been observed in the young child (4- to 9-year-old) population. The purpose of this study was to examine the age at which sexual dimorphism of velopharyngeal structures become apparent and to examine how growth trends vary between boys and girls.
Method
Static 3-dimensional magnetic resonance imaging velopharyngeal data were collected among 202 participants ranging from 4 to 21 years of age. Participants were divided into 3 groups based on age, including Group 1: 4–10 years of age, Group 2: 11–17 years of age, and Group 3: 18–21 years of age. Nine velopharyngeal measures were obtained and compared between groups.
Results
Significant sex effects were evident for levator length (p = .011), origin to origin (p = .018), and velopharyngeal ratio (p = .036) for those in Group 2 (11–17 years of age). Sex effects became increasingly apparent with age, with 7 of 9 variables becoming significantly different between male and female participants in Group 3. Boys, in general, displayed a delayed growth peak in velopharyngeal growth compared to girls.
Conclusion
Results from this study demonstrate the growth of velopharyngeal anatomy with sexual dimorphism becoming apparent predominantly after 18 years of age. However, velopharyngeal variables displayed variable growth trends with some variables presenting sexual dimorphism at an earlier age compared to other velopharyngeal variables.
Numerous studies have examined sex effects among velopharyngeal structures, particularly in the adult population. Perry, Kuehn, Sutton, Gamage, and Fang (2016) reported significant differences in velopharyngeal muscles and structures among 88 adults between 18 and 36 years of age. The authors found men demonstrated a significantly longer levator veli palatini (levator) muscle compared to women. The distance between the points where levator muscle inserts into the velar body (termed velar insertion distance) and distance between levator muscle origins was also significantly greater in men compared to women. Men were found to have a thicker and longer velum compared to women. Bae, Kuehn, Sutton, Conway, and Perry (2011) similarly reported among 10 adult participants (five men and five women) a significantly longer levator muscle among men compared to women. Perry, Kuehn, Sutton, and Gamage (2014) reported among 30 adult participants (15 men and 15 women) a significantly greater levator muscle length, greater distance between velar insertion points, and greater angles of origins.
Literature supports the notion, however, that such sexual dimorphism is not found among children and may be associated with growth changes in late adolescence. Kollara, Perry, and Hudson (2016) examined 32 children between 4 and 8 years of age and did not observe sex differences in most velopharyngeal structures. Velar insertion distance was the only velopharyngeal variable found to vary based on sex, with boys demonstrating larger values than girls. A large-scale study including 85 children between 4 and 9 years of age observed no sex effect for velopharyngeal measures, except for levator muscle length, which was significantly longer in boys (Perry, Kollara, Kuehn, Sutton, & Fang, 2018). This study, however, did not measure the velar insertion distance, as done by Kollara et al. (2016). These differences in the observation of levator length between studies (Kollara et al., 2016; Perry et al., 2018) may be related to the differences in the sample sizes used between studies.
Perry et al. (2018) also examined the impact of age, after controlling for sex and race effects, on velopharyngeal variables. As expected, an observed gradual increase with growth across variables was observed, with the exception of cranial base angles and distance from the posterior nasal spine (PNS) to the insertion of the levator muscle, which both remained relatively constant across the age span. The sagittal angle of origin (steepness of the levator muscle) and adenoid size showed a decrease in values (inverse relationship to growth) associated with growth. Although most velopharyngeal variables displayed a steady increase between 4 and 9 years of age, the levator muscle and palate width displayed a marked growth spurt between 7 and 9 years of age. Although sex effects were not observed for the group of 4- to 9-year-old children as a whole, it is possible the growth spurts around the ages of 7–9 years of age marked the beginning of early sexual dimorphism that would become apparent as the child progresses toward later adolescence. Jeans, Fernando, Maw, and Leighton (1981) observed the nasopharynx and velum undergoing sexual dimorphism after 13 years of age. Specifically, at the age of 13 years, the nasopharyngeal airway becomes significantly larger and the velum significantly becomes longer in boys compared to girls (Jeans et al., 1981). Because no study has examined these growth variations across a wide age span, it is not known at what age the sex effects for additional velopharyngeal variables (e.g., velopharyngeal muscles and velopharyngeal portal measures) are observed for individuals.
Studies have emphasized the value of using presurgical anatomy that is sex and race specific to guide proper surgical treatment in cleft palate surgeries (Chung & Kau, 1985; Chung, Runck, Bilben, & Kau, 1986; Inouye, Pelland, Lin, Borowitz, & Blemker, 2015; Mason, Perry, Riski, & Fang, 2016; Mason, Riski, & Perry, 2018; Perry, Kuehn, et al., 2014). However, our understanding of the timing in which sex effects are evident is limited to young child and adult age groups and is not reflective of data across the age span. Establishing sex-specific growth variations provides a source of comparison to determine the extent and progression of any velopharyngeal abnormalities observed in the cleft palate population across the age span. Specifically, it is not known how variable cleft palate surgeries impact growth trajectories and how those effects are variable between males and females.
The purpose of this study is to examine the age at which sexual dimorphism of velopharyngeal structures become apparent and to examine how growth trends vary between boys and girls. Comparing findings from studies among adult and child populations support the development of the following research hypotheses: (a) Sexual dimorphism of velopharyngeal structures and muscles occurs at different rates for boys and girls and (b) significant differences by sex occurs after the age of 9 years and with differences being fully apparent by 18 years of age. We hypothesized there would be no sex effect apparent for young children (Group 1) and differences in boys and girls may become apparent during the transition ages between young childhood and young adulthood (Group 2). We hypothesized that significant differences, however, would only be apparent during the older age group. Consistent with findings by Jeans et al. (1981), we expected velar length may show an earlier growth effect than other variables.
Method
Participants
In accordance with the institutional review board, healthy participants between 4 and 21 years of age were recruited to participate in the study using flyers posted throughout the local community. Participants were being recruited as part of three separate studies; however, inclusion/exclusion criteria, recruitment and enrollment processes, and study methods were consistent between sites. All participants were judged to have normal resonance by a single speech-language pathologist with over 15 years of experience in resonance assessments using a 5-point rating scale (0 = normal resonance) at the conversational level. Structural oral abnormalities were evaluated by use of an oral mechanism exam. Participants with a body mass index (BMI) over 30 were excluded to reduce the risk of claustrophobia and because of the involvement of fat deposits known to increase velar thickness among obese individuals (Davies, Ali, & Stradling, 1992; Horner et al., 1989). Inclusion criteria included a negative reported history of swallowing, neurological, hearing, craniofacial, or musculoskeletal disorders.
To increase enrollment, 43 participants were also selected from the National Institute of Mental Health (NIMH) Data Archive. Specifically, 18 participants were part of the National Database for Autism Research, and 25 participants were part of the Adolescent Brain Cognitive Development (ABCD; https://abcdstudy.org) studies. Participants from these archives that were enrolled in this study included 38 children listed as normal control participants and five children with a diagnosis of autism spectrum disorder.
A total of 202 participants were enrolled in the study. Participants were divided into three groups: Group 1 including 103 young children (ages 4–10 years), Group 2 including 53 children (ages 11–17 years), and Group 3 including 46 young adults (ages 18–21 years). Age groupings were determined based on several criteria. First, we aimed to establish groups that were predominantly young children, adolescents, and young adults. In addition, the young age was chosen to be reflective of the typical age at which children with cleft palate are being considered for secondary surgical management for hypernasal speech and have resolution of speech issues related to the cleft palate (Gustafsson, Heliövaara, Leikola, & Rautio, 2018; Nyberg, Peterson, & Lohmander, 2014). Although this study examined children without cleft palate, by using this age range for Group 1, comparisons in future clinical populations of similar age groups may be established and of clinical relevance. Because we had variable subject counts across the age span, we aimed to balance the numbers per group as best possible to optimize comparisons across groups. The selected age ranges to be included for Groups 1 and 2 were defined such that both groups had the same age span of 7 years represented. However, the cutoff of 21 years of age for Group 3 was determined to be adequate with a representation of only a 4-year age span. More specifically, individuals above 21 years of age were not included in this study because inspection of the full data, including individuals up to 36 years of age, highlighted a plateau in growth at 20–21 years of age.
As seen in Table 1, groups included both male and female participants and enrollments reflected racial groups of non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, and other (i.e., individuals reporting non-Hispanic ethnicity with two or more races). Race was determined by self-report, which is considered in epidemiology research to be the gold standard for classification of racial groups in research (Kaufman & Cooper, 2001). Race effects, however, were not the primary aim of this study because of unequal enrollments across racial groups. This is particularly true in Group 2 in which participants included racial classifications of 48 White, one Black, one Asian, and one biracial (and two with an unidentified race). For this reason, statistical analysis for race was not performed for this group. However, comparisons by race were included in the analyses for Groups 1 and 3.
Table 1.
Subject demographics within each group with mean and standard deviation for groups in parentheses.
| Subject group | No. of subjects | Male/female | Age, M (SD) | Race (White/Black/Asian/biracial) |
|---|---|---|---|---|
| 4 years | 11 | 6/5 | 8/3/0/0 | |
| 5 years | 11 | 9/2 | 6/5/0/0 | |
| 6 years | 14 | 4/10 | 8/6/0/0 | |
| 7 years | 9 | 5/4 | 5/4/0/0 | |
| 8 years | 26 | 13/13 | 12/10/4/0 | |
| 9 years | 22 | 9/13 | 15/4/2/1 | |
| 10 years | 10 | 4/6 | 8/0/2/0 | |
| Overall Group 1 | 103 | 50/53 | 7.3 (1.8) | 62/32/8/1 |
| 11 years | 12 | 5/7 | 12/0/0/0 | |
| 12 years | 5 | 4/1 | 5/0/0/0 | |
| 13 years | 9 | 5/4 | 8/0/0/1 | |
| 14 years | 11 | 8/3 | 8/1/0/0 a | |
| 15 years | 5 | 1/4 | 4/0/1/0 | |
| 16 years | 9 | 5/4 | 9/0/0/0 | |
| 17 years | 2 | 1/1 | 2/0/0/0 | |
| Overall Group 2 | 53 | 29/24 | 13.5 (1.9) | 48/1/1/1 a |
| 18 years | 1 | 0/1 | 1/0/0/0 | |
| 19 years | 7 | 4/3 | 1/2/4/0 | |
| 20 years | 14 | 8/6 | 6/5/3/0 | |
| 21 years | 24 | 5/19 | 6/10/8/0 | |
| Overall Group 3 | 46 | 17/28 | 20.3 (0.8) | 14/17/15 |
Indicates missing data for race.
Magnetic Resonance Imaging and Image Analyses
All participants were scanned without the use of sedation or contrast medium. Procedures for magnetic resonance imaging (MRI) methods have been described previously (Perry, Sutton, Kuehn, & Gamage, 2014). Participants were imaged across different MRI sites using three-dimensional MRI sequences with comparable imaging sequence parameters. The participant was in the supine position; the velum was in a relaxed and lowered position during the scan, and images and/or participants that were blurring or appeared to be in an elevated velar position (e.g., such as during a swallow) were excluded from the study.
Variables used in this study were selected based on three comparable MRI studies examining sexual dimorphism among adult (Perry et al., 2016) and child participants (Kollara et al., 2016; Perry et al., 2018). Definitions and methods for measures used in this study were consistent with studies of velopharyngeal analyses using MRI (Ettema, Kuehn, Perlman, & Alperin, 2002; Perry et al., 2016; Perry, Sutton, et al., 2014; Tian & Redett, 2009). Images were manually selected from the MRI data, and midsagittal images were chosen based on the following criteria: contains the corpus callosum in its entire anteroposterior dimension, shows visibility of fourth ventricle, and represents velum at the midline (Ettema et al., 2002). Because of the likelihood of septal defects, the vomer bone was not used to identify midline structures. The oblique coronal images were selected to represent the image that depicts the entire levator muscle from the origin to the insertion. Using these landmarks allows for repeatable measures to be consistently obtained across participants and ensures that image selection is along the same plane across participants.
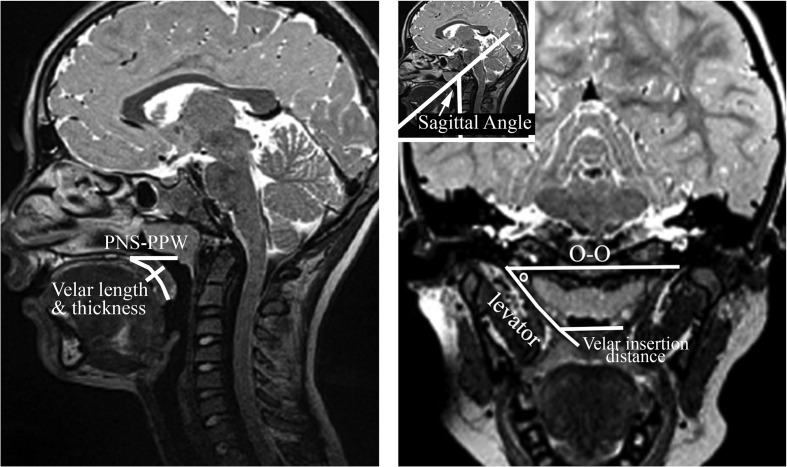
Magnetic resonance images were transferred into Amira 4 Visualization Volume Modeling software (Visage Imaging GmbH), which has a built-in native DICOM (Digital Imaging and Communication in Medicine) support program. The DICOM support system enables the data to preserve original geometry. Measures were obtained while the participant was at rest during nasal breathing. Velopharyngeal measures included levator muscle length, distance between levator muscle origins, angles of origin, sagittal angle (representing steepness of the muscle as it descends from the cranial base), velar insertion distance (representing velar width), velar length, velar thickness, distance from the PNS to posterior pharyngeal wall (PPW), and the velopharyngeal ratio (VP ratio) represented as the depth (PNS to PPW) divided by the velar length. Measures are described in Table 2 and displayed in Figure 1.
Table 2.
Description of velopharyngeal measures.
| Measure | Definition |
|---|---|
| Levator length | Length of the levator veli palatini from its origin to its insertion at the velum |
| O–O | Distance between the right and left points of origin of the levator veli palatini muscle |
| Levator angle of origin | Angle created between a reference line connecting the two origins of the levator muscle and the line drawn to measure the levator muscle length |
| Sagittal angle | Angle created by drawing a line along the anterior boundaries of Vertebrae 3 and 4 and a line coursing along the sagittal plane of the levator muscle. This angle represents the steepness of the levator muscle as it converges toward the velum from the muscle origin. |
| Velar insertion distance | Distance between where the levator veli palatini muscle inserts into the body of the velum on the right and left sides |
| Velar length | Length of the velum from the posterior nasal spine to the tip of the uvula |
| Velar thickness | Distance from the velar knee to the velar dimple |
| PNS–PPW | Distance from the posterior nasal spine to the posterior pharyngeal wall |
| VP ratio | Ratio calculated as the depth (PNS–PPW) / velar length |
Note. O = origin; PNS = posterior nasal spine; PPW = posterior pharyngeal wall; VP = velopharyngeal.
Figure 1.
Demonstration of measures obtained across velopharyngeal variables including levator length, origin to origin (O–O), levator angle of origin (noted as degree symbol), sagittal angle, velar insertion distance, velar length, velar thickness, and PNS to PPW distance. VP ratio is not shown because it is reflective of PNS–PPW/velar length. PNS = posterior nasal spine; PPW = posterior pharyngeal wall; VP ratio = velopharyngeal ratio.
Statistical Treatment
A two-way analysis of covariance using the general linear model procedure was used following a 2 × 3 factorial between subject design to determine the effect of race and the interactions between race and sex across variables (IBM SPSS Version 21.0, 2012). This statistical model allows testing for both sex and race effects on muscle measures simultaneously (with the exception of Group 2, which compared only sex effects). Although race was not the focus of this study, race was included as a covariate for analyses of Groups 1 and 3 to provide insight into the cause of any observed differences between groups. As previously mentioned, race was not included in Group 2 because of the limited number of participants across the racial groups. Levene's test was used to verify if the equal variance assumption is true. Pairwise comparisons with a Bonferroni correction were used to examine the male–male, male–female, and female–female differences across age groups. Anderson–Darling and Shapiro–Wilk tests (calculated using residual errors) were used to check the normality assumption. The null hypotheses for these tests were that the variances can be assumed to be equal or the distribution can be assumed to be normal. Results demonstrated all p values greater than .05; therefore, we did not reject all the null hypotheses, that is, both equal variance and normality assumptions are justified. Data were plotted using an interpolation spline to identify growth trends and sex differences.
A primary and secondary rater with experience in MRI data analyses randomly selected and remeasured all measures from 49 participants (24% of the participants) 5 months after the first measures were obtained. The Pearson product–moment correlation was used to examine if values obtained by both raters and within the primary rater were positively correlated for the velopharyngeal measures. Interrater and intrarater reliability ranged from r = .82 to r = .94 for the velopharyngeal measures. A two-way intraclass correlation coefficient mixed-effects model was used to determine the amount of agreement between raters. Intraclass correlation was .987 (p < .001), demonstrating excellent correlation between repeated measures.
Results
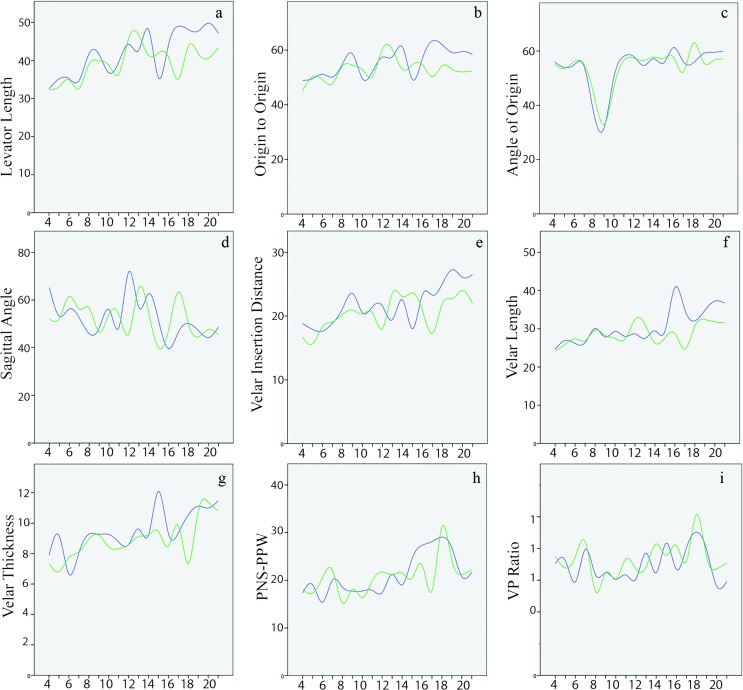
A summary of the group and sex means and standard deviations across the three groups are presented in Table 3. There was a statistically significant difference (p < .001) between groups as determined by one-way analysis of variance for all variables, with the exception of VP ratio, which showed no significant grouping effect (p = .167). A split group linear regression analysis revealed a strong sex effect on velopharyngeal measures, particularly evident at the young adult age group (Group 3), in which seven of the nine variables showed a sex effect. Table 4 provides the analysis of covariance results by group across the dependent variables, and Figures 2a– 2i demonstrate the growth plots across age for each variable. The levator muscle demonstrated similar values between sexes for Group 1. Specifically, boys in Group 1 had a mean levator muscle length of 37.8 mm compared to girls at 37.0 mm. During the 11- to 17-year age range (Group 2), boys had a mean length of 43.7 mm compared to girls at 40.7 mm, which showed a significant (p = .011) sex effect. Significant sex effects were increasingly apparent among the young adult age group (Group 3; p < .001), in which men showed a significantly longer (M = 48.6 mm, SD = 0.9 mm) levator muscle bundles compared to women (M = 42.6 mm, SD = 0.5 mm). The main effect of sex was qualified by an interaction with participant race (p = .006) only for those in the young adult group (Group 3). As evident by the plots, girls showed a peak in growth of the muscle length around the age of 13 years, whereas boys demonstrated a growth peak around 14 years of age followed by a steeper peak at the age of 17 years. Pairwise comparisons using a Bonferroni correction demonstrated a significant difference between the levator lengths for male participants across each group (p < .05). Although there was a significant difference between levator length among female participants in Groups 1 and 2 (p < .01), those in Group 2 were not significantly different from those in Group 3 (p = .294 after Bonferroni correction). Comparisons between groups (male and female participants combined) showed a statistically significant (p < .05) difference. Comparisons of levator length between male and female participants across different groups showed a statistically significant difference (p < .05) between male participants in Group 1 compared to female participants in Group 3 (female participants in Group 3 with longer muscle length compared to male participants Group 1) and male participants in Group 3 compared to female participants in Group 2 (male participants with longer muscle).
Table 3.
Group means and standard deviations (in parentheses) for all variables and under each group mean is the mean and standard deviation for male and female participants (boys/men | girls/women).
| Variable | Group 1 (4- to 10-year-olds) |
Group 2 (11- to 17-year-olds) |
Group 3 (18- to 21-year-olds) |
|---|---|---|---|
| Levator length | 37.4 (4.5) 37.8 (0.6) | 37.0 (0.6) |
42.6 (4.8) 43.7 (0.9) | 40.7 (0.9) |
44.8 (4.2) 48.6 (0.9) | 42.6 (0.5) |
| O–O | 52.2 (5.1) 52.7 (0.7) | 51.6 (0.7) |
55.9 (5.0) 57.0 (0.9) | 54.1 (0.9) |
54.8 (5.5) 59.1 (1.4) | 52.3 (0.7) |
| Levator angle of origin | 45.7 (12.9) 45.5 (1.8) | 45.7 (1.8) |
57.3 (2.8) 57.5 (0.6) | 56.8 (0.4) |
57.9 (3.6) 59.5 (0.8) | 57.0 (0.7) |
| Sagittal angle | 53.2 (10.9) 52.2 (1.3) | 54.2 (1.7) |
53.8 (11.3) 55.1 (2.3) | 52.5 (2.1) |
46.0 (8.7) 46.2 (1.7) | 45.9 (1.8) |
| Velar insertion distance | 19.8 (3.7) 20.2 (0.5) | 19.5 (0.5) |
21.7 (3.2) 21.6 (0.6) | 21.7 (0.7) |
23.9 (3.1) 26.5 (0.6) | 22.4 (0.5) |
| Velar length | 27.8 (4.1) 27.7 (0.7) | 27.7 (0.6) |
29.7 (5.2) 31.0 (1.1) | 28.2 (0.9) |
33.5 (4.2) 36.5 (1.1) | 31.7 (0.6) |
| Velar thickness | 8.6 (1.5) 8.7 (0.2) | 8.5 (0.2) |
9.1 (1.2) 9.3 (0.3) | 8.9 (0.2) |
10.9 (1.6) 11.2 (0.4) | 10.8 (0.3) |
| PNS–PPW | 18.1 (4.1) 18.2 (0.5) | 17.8 (0.6) |
20.9 (4.7) 21.2 (1.1) | 20.9 (0.6) |
22.5 (3.7) 22.5 (1.1) | 21.2 (0.6) |
| VP ratio | 0.66 (0.2) 0.67 (0.02) | 0.65 (0.03) |
0.71 (0.1) 0.68 (0.03) | 0.75 (0.02) |
0.68 (0.1) 0.62 (0.04) | 0.71 (0.02) |
Note. Values are noted in millimeters, with the exception of the angle measures (in degrees). O = origin; PNS = posterior nasal spine; PPW = posterior pharyngeal wall; VP = velopharyngeal.
Table 4.
Effects of race (Groups 1 and 3 only) and sex on dependent variables across the three study groups.
| Variables | Group 1 (4- to 10-year-olds) |
Group 2 (11- to 17-year-olds) |
Group 3 (18- to 21-year-olds) |
|---|---|---|---|
| Levator length | Sex (p = .673) Race (p = .747) |
Sex (p = .011)* | Sex (p < .001)** Race (p = .352) Sex * Race (p = .006)* |
| O–O | Sex (p = .273) Race (p = .283) |
Sex (p = .018)* | Sex (p < .001)** Race (p = .823) |
| Levator angle origin | Sex (p = .736) Race (p = .021)* |
Sex (p = .349) | Sex (p = .027)* Race (p = .098) |
| Sagittal angle | Sex (p = .372) Race (p = .845) |
Sex (p = .419) | Sex (p = .958) Race (p = .442) |
| Velar insertion distance | Sex (p = .328) Race (p = .224) |
Sex (p = .990) | Sex (p < .001)** Race (p = .832) |
| Velar length | Sex (p = .935) Race (p = .001)** |
Sex (p = .064) | Sex (p < .001)** Race (p = .098) |
| Velar thickness | Sex (p = .215) Race (p = .006)* |
Sex (p = .252) | Sex (p = .404) Race (p = .203) |
| PNS–PPW | Sex (p = .758) Race (p = .868) |
Sex (p = .974) | Sex (p = .009)* Race (p = .002)** Sex * Race (p = .004)* |
| VP ratio | Sex (p = .791) Race (p = .05)* |
Sex (p = .036)* | Sex (p < .001)** Race (p < .001)** Sex * Race (p = .002)* |
Note. O = origin; PNS = posterior nasal spine; PPW = posterior pharyngeal wall; VP = velopharyngeal. Asterisks indicate no significant correlations at *p < .05 and **p ≤ .001.
Figure 2.
(a–i) Line plots for dependent variables to display growth trends across age, noted on the x-axis in years, for boys/men (blue line) and girls/women (green line). Units noted on the y-axis are in mean values reported in millimeters, with the exception of angle measures noted as degrees. PNS = posterior nasal spine; PPW = posterior pharyngeal wall; VP = velopharyngeal ratio.
Group mean values for origin-to-origin measure showed an increase with age across the three age groups, with sex effects (male participants showing greater distance between origins) becoming significant for those in Group 2 (p = .018) and remaining apparent for those in Group 3 (p < .001). From visual inspection of growth plots (see Figure 2b), although significant sex effect were apparent in 11- to 17-year-old children, these differences visually become most apparent in the upper age limits of this group, that is, around 15–17 years of age. Pairwise comparisons for origin to origin showed significant differences (p < .05) between female participants in Group 1 compared to male participants in Groups 2 and 3 and between female participants in Group 2 compared to male participants in Group 3 (male participants with larger distance between origins compared to female participants in all cases). Female participants in Group 3 also displayed a significantly (p = .002) smaller distance between origins compared to male participants in Group 2. Comparisons of male participants across the three age groups showed a significant difference for those in Group 1 compared to those in Group 2 and Group 3 (p < .001). There was no significant difference between male participants in Groups 2 and 3 (p = .855). Female participants showed no statistical difference across the groups (p > .05).
The levator angle of origins became more obtuse with increasing age and showing significant (p = .027) sex effects only in the young adult age group (Group 3), with men showing greater angles of origin (M = 60.6°, SD = 1.4°) compared to women (M = 57.6°, SD = 1.4°). The levator angle of origin for boys and girls showed a marked decrease in angle (becoming more acute) at the age of 10 years, followed by a marked increase by the age of 13 years where it remained relatively unchanged through later adolescence and early adulthood. Pairwise comparisons showed female participants in Group 1 had an angle measure that was significantly (p < .001) more acute compared to male participants in Groups 2 and 3. Female participants in Groups 2 and 3 displayed significantly (p < .001) more obtuse angles compared to male participants in Group 1. Significant differences were observed between female participants in Group 1 compared to female participants in Groups 2 and 3 (p < .001). Similarly, male participants in Group 1 displayed differences in angle of origin measures that were significantly more acute (p < .001) compared to male participants in Groups 2 and 3.
The sagittal angle, indicating the steepness of the levator muscle as it descends from the base of the skull, showed a modest trend of becoming more acute (steeper) with increasing age (see Figure 2d). However, there were no statistically significant differences between male and female participants at the three age groups. Pairwise comparisons showed statistically significant differences (p < .05) only for comparisons of male participants in Groups 1 and 3. This observation was also found when comparing female participants across groups. Female participants in Group 3 presented with significantly more acute sagittal angles compared to male participants in Group 2 (p = .022).
Velar insertion distance was similar between boys and girls in childhood age groups (Groups 1 and 2) based on mean values (see Table 3). Significant (p < .001) differences between sexes became evident only during young adult ages (Group 3), with men showing a greater distance (M = 26.5 mm, SD = .6 mm) compared to women (M = 22.4 mm, SD = .5 mm). Pairwise comparisons showed significant differences between male participants in Groups 1 and 3 (p < .001), female participants in Groups 1 compared to those in Group 2 (p = .027) and Group 3 (p < .001), and between male participants in Group 3 and female participants in Group 1 (p < .001), Group 2 (p < .001), and Group 3 (p = .002). Velar length differences became significant (p < .001) for Group 3 (18–21 years) with a longer velum among male participants compared to female participants. As seen in the growth plot (see Figure 2f), girls displayed a steady and minimal increase in velar length with age. In contrast, boys displayed a peak in growth at 16 years of age. Pairwise comparisons were significant for male participants in Group 1 compared to male participants in Group 2 (p = .016) and Group 3 (p < .001) and when comparing male participants in Group 2 to Group 3 (p < .001). Female participants in Group 1 showed a significant difference in means compared only to those in Group 3 (p = .004). Male participants in Group 1 displayed a significantly shorter velar length compared to female participants in Group 3 (p < .001), and male participants in Group 2 had a significantly (p = .031) longer velum compared to female participants in Group 1. Male participants in Group 3 presented with a significantly longer (p < .001) velum compared to female participants in all groups.
Velar thickness values were similar between boys and girls within each age group with a gradual increase observed with age (see Figure 2g). The gradual increase was observed to be at the same rate between boys and girls, thus producing no significant sex differences at any time point. Pairwise comparisons showed significant differences for male participants in Group 1 compared to male participants in Group 3 (p < .001) and female participants in Group 1 compared to female participants in Group 3 (p < .001). Comparisons between male and female participants across groups showed female participants in Group 3 displayed a significantly thicker velum compared to male participants in Groups 1 and 2 (p < .001); similarly, male participants in Group 3 displayed a significantly thicker velum compared to female participants in Groups 1 and 2 (p < .001).
Sex differences were observed for PNS to PPW for those in Group 3 (p = .009). Race effects were significant in Group 3 (p < .002), and there was also an interaction effect between race and sex and an interaction effect (p = .004). Pairwise comparisons showed male participants in Group 1 had significant differences in the PNS to PPW compared to male participants in Group 2 (p = .029) and compared to male participants in Group 3 (p = .003). Female participants in Group 1 displayed a significant difference in mean values compared to female participants in Group 2 (p = .008) and Group 3 (p < .001). There was also a significant difference between female and male participants across the group. Specifically, female participants in Group 3 presented with greater distance from PNS to PPW compared to male participants in Group 1 (p < .001), and male participants in Group 2 (p = .037) and Group 3 (p = .002) displayed significantly larger values compared to female participants in Group 1. The VP ratio maintained a similar ratio across the three age groups with group mean values ranging from 0.62 to 0.75 across the three age groups. In the young adult age group, men displayed a significantly smaller VP ratio (M = 0.62, SD = 0.04) compared to women (M = 0.71, SD = 0.02). There were no significant differences (p > .05) for pairwise comparisons for male–male, female–female, and male–female across the age groups.
Discussion
This study examined the age and sex effect in the development of velopharyngeal structures from 4 to 21 years of age. Numerous studies have demonstrated no significant difference in oral and pharyngeal portions of the vocal tract between boys and girls (Fitch & Giedd, 1999; Roche & Barkla, 1965; Vorperian et al., 2005, 2009). Vorperian et al. (2009) examined 605 individuals at birth to 19 years of age. Results demonstrate a significant sex difference among individuals between 15 and 19 years of age for the oral and pharyngeal segments of the vocal tract. The authors observed earlier sex differences among children 5–9 years of age for anterior to posterior measures of the oral cavity and vocal tract (from lips to PPW). This suggests some vocal tract variables undergo sexual dimorphism earlier than others.
Similarly, we hypothesized there would be sex effects across the age groups in the development of velopharyngeal structures, and we expected growth patterns for boys and girls to vary, particularly during the age ranges typically associated with puberty. As hypothesized, no sex differences for velopharyngeal variables were found in the youngest age group (Group 1). Unlike Kollara and Perry (2014) and Perry et al. (2018), who examined children 4–8 and 4–9 years of age, respectively, we did not find a significant difference by sex for measures of velar insertion distance or levator muscle length in our 4- to 10-year-old group. Ten-year-old children were included in Group 1 for this study to keep equal age spans (7 years) across the childhood age range from 4 to 17 years of age. Future studies should more discretely study each age group to include small age spans, likely covering only two or three ages in each group. Variations in findings of this study to these earlier studies may be related to differences between study sample sizes, age ranges, and differences in racial and ethnic distributions.
However, sexual dimorphism was observed among Group 2 participants (11- to 17-year-old children) for levator muscle length, origin-to-origin distance, and VP ratio. Sexual dimorphism became most evident among participants in Group 3, with seven of the nine variables displaying a significant sex effect. For these seven variables, differences in growth trends based on visual inspection of the growth plots appear to begin around 16–17 years of age, with differences being maintained through young adulthood (21 years of age). Sexual dimorphism for velopharyngeal variables in this study during young adult ages (Group 3) is consistent with a comparison study of adults from 18 to 36 years of age (Perry et al., 2016). This is expected given participants in this study were also part of the comparison study. However, the only exception is that velar thickness differences by sex were not observed in this study among young adults but were evident in the comparison adult study (Perry et al., 2016). This study examined only the young adults (n = 45) from the larger subset of adult participants (n = 88), which included adults up to 36 years of age (Perry et al., 2016). Average BMI has been reported to significantly increase from 23.7 years of age to 25.4 years of age, with male participants showing a greater increase in weight compared to female participants (Reas, Nygård, Svennson, Sørensen, & Sandanger, 2007). Therefore, it is possible that the inclusion of adults up to 36 years of age represented BMI values that were more different between male and female participants than that observed in this study. This is further supported by calculating BMI values from reported height and weight in Table 1 for the adult study by Perry et al. (2016), in which male participants have an average BMI that is nearly 10 points higher than female participants. Although not stated, it is possible differences in BMI between male and female participants become more apparent in the older ages above the age cutoff of 21 years used in this study (Reas et al., 2007). This may explain why the differences in velar thickness were not apparent in this study but may be more related to weight differences that are evident with increasing age, that is, above the age of 21 years.
Jeans et al. (1981) observed a growth spurt in velar length to occur around 13 years of age. Our findings suggest a later growth spurt and add new insights into differences between growth patterns among boys and girls. Specifically, we observed girls displayed a steady but modest increase in velar length with age, whereas boys displayed a peak in growth at 16 years of age. Although Group 2 did not display significant sex differences, these growth patterns are visibly evident and would likely become significant around 16 years of age in future studies using smaller more discrete age ranges for groupings. Accelerated or peaks in velar growth, particularly in the later adolescent age groups noted for boys in this study, may be related to the faster growth of the nasopharyngeal portions noted in prior studies (Castelli, Ramirez, & Nasjleti, 1973; Vorperian et al., 2009). Vorperian et al. (2009) reported sexual dimorphism of the nasopharyngeal height becomes evident among the 10–14 years of age cohort. In such, as the nasopharyngeal cavity lengthens, the velum continues to show a rapid growth to essentially fill the nasopharyngeal space.
Variations in growth patterns between boys and girls were also apparent in the levator muscle length in which girls displayed a peak in growth at 13 years of age and boys showed a delayed peak around 16 years of age. Similarly, boys had a delayed peak in growth for measures of origin to origin, velar insertion distance, and PNS to PPW. Of interest, velar length and PNS to PPW growth patterns for boys and girls suggest a possible coordination of growth. For example, velar length and PNS to PPW both show a peak in growth among boys at 16 years of age. The coordinated growth of velar length and pharyngeal depth (PNS to PPW) plus the changes in adenoidal size across the age range may be of critical importance in maintaining normal VP ratios (i.e., VP ratio variable) to create normal velopharyngeal function for speech. Mean values for VP ratio in this study are similar to those reported by Subtelny (1957), who followed longitudinally 30 children from birth to 18 years of age with normal velopharyngeal anatomy.
An interesting observation on the growth trend plots is the drastic decrease in the angle of levator origin between 7 and 13 years of age that was observed for both male and female participants. This explains the large standard deviation in the levator angle of origins observed within the youngest age group and can be visually appreciated on the growth plots. Because of the insertion at the cranial base, this may be related to changes in the base of the skull and might be more fully understood by examining the coordination of velopharyngeal, vocal tract, and cranial growth trends. In addition, this may reflect a velar position that was not fully lowered. Assessment using dynamic MRI data may be valuable to define positions of velar elevation, velar lowering, and a full rest position of the velum. Without dynamic data, it is difficult to determine the time point at which scans were obtained.
It is well known that rapid and substantial changes occur in the laryngeal anatomy over the course of puberty, especially in boys (e.g., Kahane, 1982). Little is known about the possible changes in velopharyngeal anatomy during puberty in boys and girls. In this study, ages can be loosely categorized as those prepubertal (Group 1), peripubertal (Group 2), and postpubertal (Group 3). Although it is certainly clear that those in the lower limits of Group 1 (e.g., 4- and 5-year-olds) are prepubescent, those within the likely pubertal transition ages are probably less delineated using a categorical approach alone. Thus, it cannot be stated with confidence that these grouping categories are reflective of pubertal time points. Pubertal signs or markers related to pubertal stages were not collected for participants of the current study. In future research, it will be important to record such signs, along with the measures of velopharyngeal structures such as those in the current study. Especially important will be measuring changes in the size of adenoidal tissue along with co-occurring changes in growth in the palatal and pharyngeal regions.
Clinical Implications
The current data provide a valuable first step in understanding the development of velopharyngeal structures. Findings from this study are particularly relevant to understand the normal development of velopharyngeal anatomy and the emergence of sexual dimorphism that has been published to be seen in the adult population (Perry et al., 2016). This line of research should be extended to understand disordered velopharyngeal anatomy, as in cleft palate or syndromic cases. Ma, Shi, Li, and Zheng (2013) examined 66 patients with nonsyndromic cleft palate with or without cleft lip using lateral cephalograms. Patients were separated into three groups: (a) those under 12 years of age, (b) those 12–20 years of age, and (c) 20 years of age and older. Results demonstrated that as age increases among patients with a history of cleft palate, velar length showed a minimal nonsignificant increase in growth. Because participants were of Asian origin, known craniofacial and velopharyngeal structural variations by race (Perry et al., 2016) may be a factor that limits the translation of these insights to other populations. The authors noted a substantial increase in pharyngeal depth. During sustained phonation tasks, children between 12 and 20 years of age showed less mobility and flexibility of the velum compared to other age groups. These findings, combined with observations of this study, suggest differences in the growth and development of the bony and soft tissue velopharyngeal structures among individuals born with cleft palate. Results from this study suggest timing features that may not be common in individuals with cleft palate. In addition, it is not clear how the timing of surgery during velopharyngeal growth impacts the growth trajectories and/or comparisons by race and sex. However, this study did not compare results to a normative age-matched group, and individuals with cleft palate had late primary palate repairs (repairs done only after 5 years of age). Therefore, results may not relate to those who receive early single-stage palate repairs.
Changes in the velopharyngeal closure pattern (circular, sagittal, coronal, or circular with Passavant's ridge) during puberty have been reported to occur in 60% of children without cleft palate compared to only 30% in subjects with cleft palate (Siegel-Sadewitz & Shprintzen, 1986). These changes were attributed to pubertal growth effect in the pharyngeal cavity due to vocal tract growth and adenoid involution. These findings may suggest children with cleft palate display a less flexible velopharyngeal system that does not readily adapt to the morphological changes in the oropharyngeal regions. Results from this study show variation in the growth of the velopharyngeal structures; however, the VP ratio is maintained throughout the ages within the normal ranges despite alterations to surrounding velopharyngeal structures. It is possible that children with cleft palate display asynchrony in the growth of key features that control the velopharyngeal function for speech. Understanding the timing variations and observed age of sex effects provides a source of comparison between cleft and noncleft studies. Future research is needed to examine the relationship of findings from this study to a disordered population, such as the cleft palate.
A limitation of this study is the unequal sample sizes across the three age groups. The use of only three groups across such a large age span is also a noted limitation of this study. Future studies should establish comparisons using age groupings that reflect participants with similar values across velopharyngeal variables. An additional limitation was the use of participants with a diagnosis of autism spectrum disorder. Although this only accounted for five of the 202 participants, it is possible that velopharyngeal variables may be different in those with such diagnosis (although such findings have not been suggested or reported). Every attempt was made to only enroll children without such diagnosis; however, five were selected only when other control participants for the needed ages were not present in the database. An additional limitation is that this study represents cross-sectional growth patterns and may or may not be consistent with longitudinal data analysis. Subtelny (1957) examined children longitudinally and did not observe a significant sex effect at any of the ages (birth through 18 years of age). However, the samples size (15 male and 15 female participants) may have limited statistical power to identify such differences. However, the difference between observations of this study and that of Subtelny (1957) highlights the need for comparison between longitudinally sampled data and cross-sectional data to confirm that growth observations can be accurately assessed using a cross-sectional database.
Although attempts were made to create equal distribution among groups, race and ethnicity were not controlled in this study. Caution should be taken in drawing conclusions from this study about the impact of race and ethnicity on velopharyngeal growth differences. Future studies should examine the dynamics of the velopharyngeal system as a function of puberty. It is well established that young children typically have an adenoid to velar contact during speech and swallowing. Studies have demonstrated differences between male and female adults in velar height during speech (Kuehn & Moon, 1998; McKerns & Bzoch, 1970). Studies have not examined at what age these dynamic changes become apparent. The effects of growth on the velopharyngeal port are seldom considered as factors of interest during surgical planning for pharyngoplasties that involve augmentations to the pharyngeal space. The paucity of these data may thus negatively affect surgeries that involve the mediolateral axis, such as those effecting the cranial vault or pharyngeal cavity. The effects of growth, sex, and race on velopharyngeal measures should be explored in future research.
Conclusions
Results from this study demonstrate a significant age effect for velopharyngeal anatomy. Specifically, sexual dimorphism became apparent for levator muscle length, origin to origin, and VP ratio for Group 2 (11- to 17-year-olds). Sexual dimorphism became most evident, however, during the young adult age group (Group 3) in which seven of the nine variables showed a significant sex effect. Growth trends for boys and girls were observed to show different patterns, with boys displaying, in general, a delayed growth peak in velopharyngeal variables compared to girls.
Acknowledgments
This study was funded by the National Institute on Deafness and Other Communication Disorders Grant 1R03DC009676-01A1 awarded to Perry (principal investigator), Sutton, and Kuehn. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH). In addition, data and/or research tools used in the preparation of this article were obtained, in part, from the NIH-supported National Database for Autism Research (NDAR). The NDAR is a collaborative informatics system created by the NIH to provide a national resource to support and accelerate research in autism. Data set identifier(s): [NIMH Data Archive Collection ID(s) or NIMH Data Archive Digital Object Identifier (DOI)]. This article reflects the views of the authors and may not reflect the opinions or views of the NIH or of the submitters submitting original data to the NDAR. Data used in the preparation of this article were also obtained, in part, from the ABCD Study (https://abcdstudy.org), held in the NIMH Data Archive. This is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9–10 years and follow them over 10 years into early adulthood. The ABCD Study is supported by the NIH and additional federal partners under Awards U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/nih-collaborators. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators.html. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This article reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators.
Funding Statement
This study was funded by the National Institute on Deafness and Other Communication Disorders Grant 1R03DC009676-01A1 awarded to Perry (principal investigator), Sutton, and Kuehn. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH). In addition, data and/or research tools used in the preparation of this article were obtained, in part, from the NIH-supported National Database for Autism Research (NDAR). The NDAR is a collaborative informatics system created by the NIH to provide a national resource to support and accelerate research in autism. Data set identifier(s): [NIMH Data Archive Collection ID(s) or NIMH Data Archive Digital Object Identifier (DOI)]. This article reflects the views of the authors and may not reflect the opinions or views of the NIH or of the submitters submitting original data to the NDAR. Data used in the preparation of this article were also obtained, in part, from the ABCD Study (https://abcdstudy.org), held in the NIMH Data Archive. This is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9–10 years and follow them over 10 years into early adulthood. The ABCD Study is supported by the NIH and additional federal partners under Awards U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/nih-collaborators.
References
- Bae Y., Kuehn D. P., Sutton B. P., Conway C. A., & Perry J. L. (2011). Three-dimensional magnetic resonance imaging of velopharyngeal structures. Journal of Speech, Language, and Hearing Research, 54, 1538–1545. [DOI] [PubMed] [Google Scholar]
- Castelli W. A., Ramirez P. C., & Nasjleti C. E. (1973). Linear growth study of the pharyngeal cavity. Journal of Dental Research, 52(6), 1245–1248. [DOI] [PubMed] [Google Scholar]
- Chung C. S., & Kau M. C. (1985). Racial differences in cephalometric measurements and incidence of cleft lip with or without cleft palate. Journal of Craniofacial Genetics and Developmental Biology, 5, 341–349. [PubMed] [Google Scholar]
- Chung C. S., Runck D. W., Bilben S. E., & Kau M. C. (1986). Effects of interracial crosses on cephalometric measurements. American Journal of Physical Anthropology, 69(4), 465–472. [DOI] [PubMed] [Google Scholar]
- Davies R. J., Ali N. J., & Stradling J. R. (1992). Neck circumference and other clinical features of the obstructive sleep apnea syndrome. Thorax, 47, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettema S. L., Kuehn D. P., Perlman A. L., & Alperin N. (2002). Magnetic resonance imaging of the levator veli palatini muscle during speech. The Cleft Palate–Craniofacial Journal, 39(2), 130–144. [DOI] [PubMed] [Google Scholar]
- Fitch W. T., & Giedd J. (1999). Morphology and development of the human vocal tract: A study using magnetic resonance imaging. The Journal of the Acoustical Society of America, 106, 1511–1522. [DOI] [PubMed] [Google Scholar]
- Gustafsson C., Heliövaara A., Leikola J., & Rautio J. (2018). Incidence of speech-correcting surgery in children with isolated cleft palate. The Cleft Palate–Craniofacial Journal. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Horner R. L., Mohiaddin R. H., Lowell D. G., Shea S. A., Burnman E. D., Longmore D. B., & Guz A. (1989). Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnea and weight matched controls. European Respiratory Journal, 2, 613–622. [PubMed] [Google Scholar]
- Inouye J. M., Pelland C. M., Lin K. Y., Borowitz K. C., & Blemker S. S. (2015). A computational model of velopharyngeal closure for simulating cleft palate repair. Journal of Craniofacial Surgery, 26, 658–662. [DOI] [PubMed] [Google Scholar]
- Jeans W. D., Fernando D. C., Maw A. R., & Leighton B. C. (1981). A longitudinal study of the growth of the nasopharynx and its contents in normal children. British Journal Radiology, 54(638), 117–121. [DOI] [PubMed] [Google Scholar]
- Kahane J. C. (1982). Growth of the human prepubertal and pubertal larynx. Journal of Speech and Hearing Research, 25, 446–455. [DOI] [PubMed] [Google Scholar]
- Kaufman J. S., & Cooper R. S. (2001). Commentary: Considerations for use of racial/ethnic classification in etiologic research. American Journal of Epidemiology, 154, 291–298. [DOI] [PubMed] [Google Scholar]
- Kollara L., & Perry J. L. (2014). Effects of gravity on the velopharyngeal structures in children using upright magnetic resonance imaging. The Cleft Palate–Craniofacial Journal, 51(6), 669–676. [DOI] [PubMed] [Google Scholar]
- Kollara L., Perry J. L., & Hudson S. (2016). Racial variations in velopharyngeal and craniometric morphology in children: An imaging study. Journal of Speech, Language, and Hearing Research, 59(1), 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn D. P., & Moon J. B. (1998). Velopharyngeal closure force and levator veli palatine activation levels in varying phonetic contexts. Journal of Speech, Language, Hearing Research, 41, 51–62. [DOI] [PubMed] [Google Scholar]
- Ma L., Shi B., Li Y., & Zheng Q. (2013). A preliminary study on the characteristics of the velopharyngeal structures in different-age patients with cleft palate. Journal of Craniofacial Surgery, 24(4), 1235–1238. [DOI] [PubMed] [Google Scholar]
- Mason K. N., Perry J. L., Riski J. E., & Fang X. (2016). Age related changes between the level of velopharyngeal closure and the cervical spine. Journal of Craniofacial Surgery, 27, 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason K. N., Riski J. E., & Perry J. L. (2018). Changes in the height of velopharyngeal closure relative to the cervical spine from infancy through adolescence in patients with cleft palate. The Cleft Palate–Craniofacial Journal, 55(4), 508–516. [DOI] [PubMed] [Google Scholar]
- McKerns D., & Bzoch K. R. (1970). Variations in velopharyngeal valving: The factor of sex. Cleft Palate Journal, 7, 652–662. [PubMed] [Google Scholar]
- Nyberg J., Peterson P., & Lohmander A. (2014). Speech outcomes at age 5 and 10 years in unilateral cleft lip and palate after one-stage palatal repair with minimal incision technique—A longitudinal perspective. International Journal of Pediatric Otorhinolaryngology, 78, 1662–1670. [DOI] [PubMed] [Google Scholar]
- Perry J. L., Kollara L., Kuehn D. P., Sutton B. P., & Fang X. (2018). Examining sexual and racial dimorphism among a child population using magnetic resonance imaging. The Cleft Palate–Craniofacial Journal, 55, 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J. L., Kuehn D. P., Sutton B. P., & Gamage J. K. (2014). Sexual dimorphism of the levator veli palatini muscle: An imaging study. The Cleft Palate–Craniofacial Journal, 51(5), 544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J. L., Kuehn D. P., Sutton B. P., Gamage J. K., & Fang X. (2016). Anthropometric analysis of the velopharynx and related craniometric dimensions in three adult populations using MRI. The Cleft Palate–Craniofacial Journal, 53(1), e1–e13. https://doi.org/10.1597/14-015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J. L., Sutton B. P., Kuehn D. P., & Gamage J. K. (2014). Using MRI for assessing velopharyngeal structures and function. The Cleft Palate–Craniofacial Journal, 51(4), 476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reas D. L., Nygård J. F., Svennson E., Sørensen T., & Sandanger I. (2007). Changes in body mass index by age, gender, and socio-economic status among a cohort of Norwegian men and women (1990–2001). BMC Public Health, 7, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche A. F., & Barkla D. H. (1965). The level of the larynx during childhood. Annals of Otology, Rhinology & Laryngology, 74, 645–654. [DOI] [PubMed] [Google Scholar]
- Siegel-Sadewitz V. L., & Shprintzen R. J. (1986). Changes in velopharyngeal valving with age. International Journal of Pediatric Otorhinolaryngology, 11(2), 171–182. [DOI] [PubMed] [Google Scholar]
- Subtelny J. D. (1957). A cephalometric study of the growth of the soft palate. Plastic and Reconstructive Surgery, 19(1), 49–62. [DOI] [PubMed] [Google Scholar]
- Tian W., & Redett R. J. (2009). New velopharyngeal measurements at rest and during speech: Implications and applications. Journal of Craniofacial Surgery, 20(2), 532–539. [DOI] [PubMed] [Google Scholar]
- Vorperian H. K., Kent R. D., Lindstrom M. J., Kalina C. M., Gentry L. R., & Yandell B. S. (2005). Development of vocal tract length during early childhood: A magnetic resonance imaging study. The Journal of the Acoustical Society of America, 117(1), 338–350. [DOI] [PubMed] [Google Scholar]
- Vorperian H. K., Wang S., Chung M. K., Schimek E. M., Durtschi R. B., Kent R. D., … Gentry L. R. (2009). Anatomic development of the oral and pharyngeal portions of the vocal tract: An imaging study. The Journal of the Acoustical Society America, 125(3), 1666–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]