Abstract
Purpose
Recovery from aphasia after stroke has a decelerating trajectory, with the greatest gains taking place early and the slope of change decreasing over time. Despite its importance, little is known regarding evolution of language function in the early postonset period. The goal of this study was to characterize the dynamics and nature of recovery of language function in the acute and early subacute phases of stroke.
Method
Twenty-one patients with aphasia were evaluated every 2–3 days for the first 15 days after onset of acute ischemic or hemorrhagic stroke. Language function was assessed at each time point with the Quick Aphasia Battery (Wilson, Eriksson, Schneck, & Lucanie, 2018), which yields an overall summary score and a multidimensional profile of 7 different language domains.
Results
On a 10-point scale, overall language function improved by a mean of 1.07 points per week, confidence interval [0.46, 1.71], with 19 of 21 patients showing positive changes. The trajectory of recovery was approximately linear over this time period. There was significant variability across patients, and patients with more impaired language function at Day 2 poststroke experienced greater improvements over the subsequent 2 weeks. Patterns of recovery differed across language domains, with consistent improvements in word finding, grammatical construction, repetition, and reading, but less consistent improvements in word comprehension and sentence comprehension.
Conclusion
Overall language function typically improves substantially and steadily during the first 2 weeks after stroke, driven mostly by recovery of expressive language. Information on the trajectory of early recovery will increase the accuracy of prognoses and establish baseline expectations against which to evaluate the efficacy of interventions.
Supplemental Material
Aphasia is one of the most common and debilitating consequences of stroke affecting the dominant hemisphere. Most individuals with aphasia experience some degree of recovery of language function after a stroke. In the early acute period (first ~48 hr), reperfusion of the ischemic penumbra can sometimes lead to rapid resolution of aphasic symptoms (Hillis et al., 2002). In the longer term, the trajectory of recovery is decelerating, with the greatest gains taking place early and the slope of change decreasing over time (Basso, 1992; Culton, 1969; Demeurisse et al., 1980; Kertesz & McCabe, 1977; Laska, Hellblom, Murray, Kahan, & von Arbin, 2001; Lendrem & Lincoln, 1985; Nicholas, Helm-Estabrooks, Ward-Lonergan, & Morgan, 1993; Porch, 1981; Shewan & Kertesz, 1984; Swinburn, Porter, & Howard, 2004; Wertz et al., 1981).
Most studies of recovery from aphasia after stroke have initially recruited patients at least 2 weeks to 1 month postonset. Therefore, little is known regarding the evolution of language function prior to that, within the first 2 weeks. This is an important gap because the few studies that have reported data on this phase indicate that many patients make substantial gains during this period. Three detailed longitudinal studies of one to three cases have documented daily recovery of language function in the first 2 weeks (Mohr, 1973; Holland et al., 1985; Yagata et al. 2017); however, the small number of cases makes it difficult to generalize these findings. A number of cohort studies have included two or more time points within the first 2 weeks (Cloutman, Newhart, Davis, Heidler-Gary, & Hillis, 2009; Denier et al., 2016, 2015; El Hachioui et al., 2013; El Hachioui, van de Sandt-Koenderman, Dippel, Koudstaal, & Visch-Brink, 2011; Furlanis et al., 2018; Hartman, 1981; Hillis & Heidler, 2002; Hillis et al., 2006, 2002; Maas et al., 2012; Mattioli et al., 2014; Pashek & Holland, 1988; Pedersen, Jørgensen, Nakayama, Raaschou, & Olsen, 1995). Although all of these studies documented varying extents of recovery from aphasia in the early postonset phase, only a few studies included more than two early time points (Denier et al., 2016, 2015; Furlanis et al., 2018; Pashek & Holland, 1988), and only a few studies evaluated aphasia in sufficient detail to be able to report patterns of recovery separately across different language domains (El Hachioui et al., 2013, 2011; Furlanis et al., 2018; Mattioli et al., 2014).
In this study, we recruited acute stroke patients in the first few days after ischemic stroke or primary intracerebral hemorrhage and assessed their language function every 2–3 days for the first 2 weeks, typically at the bedside. We aimed to address five specific questions about the dynamics and nature of language recovery over this late acute/early subacute time period: (a) What is the rate of recovery of language function? (b) Does recovery already show a decelerating curve during this time period? (c) How variable are patients in terms of rate of recovery? (d) Is initial severity (at 2 days) predictive of the subsequent rate of recovery? (e) How similar are the dynamics of recovery across different language domains (word finding, grammatical construction, etc.)? A better understanding of the dynamics of early language recovery has the potential to improve the accuracy of prognoses and to establish baseline expectations against which to evaluate the efficacy of interventions.
Method
Participants
Patients who were seen by the stroke services at two certified primary stroke centers—The University of Arizona Medical Center between late December 2014 and the end of February 2016 and Tucson Medical Center between mid-October 2015 and February 2016—were screened for eligibility. The study was approved by the institutional review board at The University of Arizona, and all participants were compensated for their time.
The inclusion criteria were as follows: (a) acute ischemic stroke or primary intracerebral hemorrhage confirmed on magnetic resonance imaging (MRI) or computed tomography (CT); (b) stroke localized to left hemisphere supratentorial regions (i.e., cortex, cortical white matter, basal ganglia, thalamus) with no involvement of the right hemisphere, brainstem, or cerebellum; (c) aged 18–90 years; and (d) fluent and literate in English premorbidly. The exclusion criteria were as follows: (a) previous stroke, except for asymptomatic lacunar strokes or microhemorrhages; (b) dementia or impaired cognitive or language function at baseline for any other reason; and (c) major psychiatric disorders.
Ninety-one patients met all criteria. Of those, 13 were too medically unstable or severely impaired to be testable at any point during their hospitalization, and 11 were unable to be approached for situational reasons (usually rapid discharge due to minimal symptoms). The remaining 67 patients were approached at their bedside by a speech-language pathologist. After learning about the study, 58 patients (or their authorized representatives) provided written informed consent to participate, whereas nine declined.
Of the 58 participants, 28 presented with aphasia on initial evaluation, and two or more data points were obtained for 21 of the 28; these patients were included in the analyses reported here. For the remaining seven, follow-up data were not obtained for situational reasons, so these patients were not included in the study. For the 30 patients without aphasia, a second evaluation was performed whenever practical, 2 weeks or more after stroke onset; 17 of these 30 patients completed this second evaluation and were included in our analyses.
Demographic and medical history information was obtained from interviews with patients and/or their caregivers, as well as from medical records. Clinical information about the stroke was obtained from medical records (see Table 1).
Table 1.
Demographic, medical history, and stroke information.
| Aphasia | No aphasia | ||
|---|---|---|---|
| No. of patients | 21 | 17 | |
| Age (years) | 65.2 ± 18.4 (32–87) | 63.8 ± 13.6 (35–85) | |
| Sex (M/F) | 16/5 | 11/6 | |
| Handedness (R/L/ambi) | 20/1/0 | 14/2/1 | |
| Education (years) | 14.0 ± 2.4 (9–18) | 16.5 ± 1.8 (12–19) | *(p = .001) |
| Native English speaker | 17 (81.0%) | 14 (82.4%) | |
| Hypertension | 15 (71.4%) | 11 (64.7%) | |
| Diabetes mellitus | 7 (33.3%) | 4 (23.5%) | |
| Hyperlipidemia | 7 (33.3%) | 6 (35.3%) | |
| Coronary artery disease | 7 (33.3%) | 1 (5.9%) | |
| Cardiac arrhythmia | 2 (9.5%) | 2 (11.8%) | |
| Atrial fibrillation | 5 (23.8%) | 2 (11.8%) | |
| Myocardial infarction | 7 (33.3%) | 1 (5.9%) | |
| Tobacco use | 3 (14.3%) | 4 (23.5%) | |
| Antiplatelet medications | 10 (47.6%) | 7 (41.2%) | |
| Anticoagulant medications | 1 (4.8%) | 1 (5.9%) | |
| NIH Stroke Scale | 9.5 ± 7.3 (1–26) | 2.5 ± 2.4 (0–8) | *(p = .001) |
| Stroke subtype | *(p = .011) | ||
| Ischemic | |||
| Large artery atherosclerosis | 2 (9.5%) | 0 (0.0%) | |
| Cardioembolism | 6 (28.6%) | 1 (5.9%) | |
| Small vessel occlusion | 0 (0.0%) | 4 (23.5%) | |
| Cryptogenic/multiple | 6 (28.6%) | 10 (58.8%) | |
| Hemorrhagic | 7 (33.3%) | 2 (11.8%) | |
| TPA | 6 (28.6%) | 3 (17.6%) | |
| Endovascular therapy | 3 (14.3%) | 1 (5.9%) | |
| Hemorrhagic transformation | 3 (14.3%) | 0 (0.0%) |
Note. Values shown are counts, percentages, or mean ± standard deviation (range). Groups were compared with t tests, Fisher's exact test, or chi-square test as appropriate. M = male; F = female; R = right; L = left; ambi = ambidextrous; NIH = National Institutes of Health; TPA = tissue plasminogen activator.
p < .05.
Language Evaluation
All language evaluations were performed by a speech-language pathologist. Patients were approached as soon as practical after admission, but at least 1 day after stroke. For the 21 included patients with aphasia, the initial approach was on mean Day 2.7 ± 1.3 (standard deviation) after stroke (three patients on Day 1, eight on Day 2, five on Day 3, three on Day 4, one on Day 5, one on Day 6). The first subtest of the Quick Aphasia Battery (QAB; Wilson, Eriksson, Schneck, & Lucanie, 2018) was administered, which aims to determine whether patients are testable, defined as medically stable and able to stay awake, maintain attention, and attempt to follow commands. At initial approach, 16 of 21 patients were testable, so the remainder of the QAB was administered. When patients were not testable, we visited them again every 2–3 days until they were testable. After the first language evaluation, we attempted to evaluate patients every 2–3 days until Day 15 poststroke. Across patients, the mean number of evaluations where patients were testable within the first 15 days was 4.0 ± 1.4 (five patients had two evaluations, two had three, five had four, seven had five, two had six). Most patients were discharged during this time, and for 13 of the 21 patients, one or more of the final evaluations were obtained in inpatient rehabilitation facilities. The total number of testable evaluations per day for the first 15 days was one, six, five, eight, eight, five, 10, four, eight, four, six, six, four, five, and three. On the stroke units, all patients were seen most days by speech-language pathologists. The priorities at that time were generally administering swallowing evaluations; making changes to diet if needed; providing compensatory strategies and techniques with regard to dysphagia; and administering speech, language, and cognitive evaluations and reevaluations relevant to determining rehabilitation needs. Some patients were provided with communication boards, but patients did not generally receive significant speech-language therapy. Most of the patients who went to inpatient rehabilitation facilities began to receive daily speech-language therapy at that time.
For the 17 included patients without aphasia, the initial data point was obtained 2.0 ± 1.1 days (range: 1–4 days) after stroke. One follow-up evaluation was obtained for each patient, at mean Day 23.4 ± 10.3 (range: 14–50 days).
Language function was evaluated with the QAB, which provides a reliable and multidimensional assessment of language function in less than 20 min in most stroke patients (Wilson et al., 2018). The QAB is an impairment-based aphasia battery designed for the research context of acute stroke recovery. It is made up of eight subtests, each comprising sets of items that probe different language domains, vary in difficulty, and are scored with a graded system to maximize the informativeness of each item. From the eight subtests, eight summary measures are derived (including an overall summary measure), which constitute a multidimensional profile of language function, quantifying strengths and weaknesses across core language domains. In a validation study (Wilson et al., 2018), all measures showed good or excellent test–retest reliability in a cohort of individuals with chronic poststroke aphasia (overall summary measure: intraclass correlation coefficient = .98) and excellent interrater reliability (overall summary measure: intraclass correlation coefficient = .99). Sensitivity and specificity for diagnosis of aphasia were .91 and .95, respectively. Concurrent validity was established with respect to the widely used Western Aphasia Battery (Kertesz, 2007).
Each language evaluation was transcribed and scored offline by one of four speech-language pathologist researchers (who had not administered the evaluation). The longitudinal series of evaluations for each patient were always scored by the same researcher.
Neuroimaging
Acute stroke lesions were manually delineated on standard clinical MRI or CT scans. Ischemic infarcts were drawn on diffusion-weighted scans, whereas hemorrhages were generally drawn on gradient echo sequences; CT scans were used when MRI was unavailable. Anatomical and lesion images were normalized to Montreal Neurological Institute (MNI) space using the clinical toolbox in SPM12 (Rorden, Bonilha, Fridriksson, Bender, & Karnath, 2012). The normalized lesion images were then overlaid.
Statistical Analysis
To characterize the trajectory of recovery of overall language function, a mixed model was fit using the lme4 package (Bates, Mächler, Bolker, & Walker, 2015) in R (R Core Team, 2018). Only the patients with aphasia and only Days 2 through 15 were included in the model. The dependent measure was the QAB summary measure of overall language function. Time postonset was specified as a fixed effect. Random intercepts and slopes were included for each patient, and a correlation was modeled between intercepts and slopes. This model was compared to (a) a model without a fixed effect of time postonset to determine whether there was a positive trajectory of recovery, (b) a model including a second-degree polynomial term to determine whether the slope of recovery was linear, (c) a model without random slopes to determine whether there was meaningful variability among patients, and (d) a model without a correlation between intercepts and slopes to determine whether initial severity was predictive of recovery rate. p Values were obtained by likelihood ratio tests (LRTs) comparing pairs of models. Null distributions for the LRT statistic 2(l F–l R), where l F is the log likelihood of the full model and l R is the log likelihood of the reduced model, were derived using a parametric bootstrap approach (Faraway, 2016).
These same analyses were then repeated for each of the seven language domains. Recovery slopes were compared between receptive and expressive domains and between each pair of domains; these contrasts were corrected for multiple comparisons based on false discovery rate.
Results
The 21 patients with aphasia all had lesions in perisylvian language regions or the white matter and/or subcortical structures underlying them (see Figure 1). The 17 patients without aphasia mostly had very small lesions, and the few lesions that were larger were not localized to perisylvian cortex (see Figure 1). Lesion volumes were greater in patients with aphasia (M = 44.1 ± 50.3 cm3, range: 1.7–228.0 cm3) than patients without aphasia (2.8 ± 5.0 cm3, range: 0.1–18.1 cm3; t test, p = .002).
Figure 1.
Lesion overlay maps for patients with aphasia (n = 21) and patients without aphasia (n = 17).
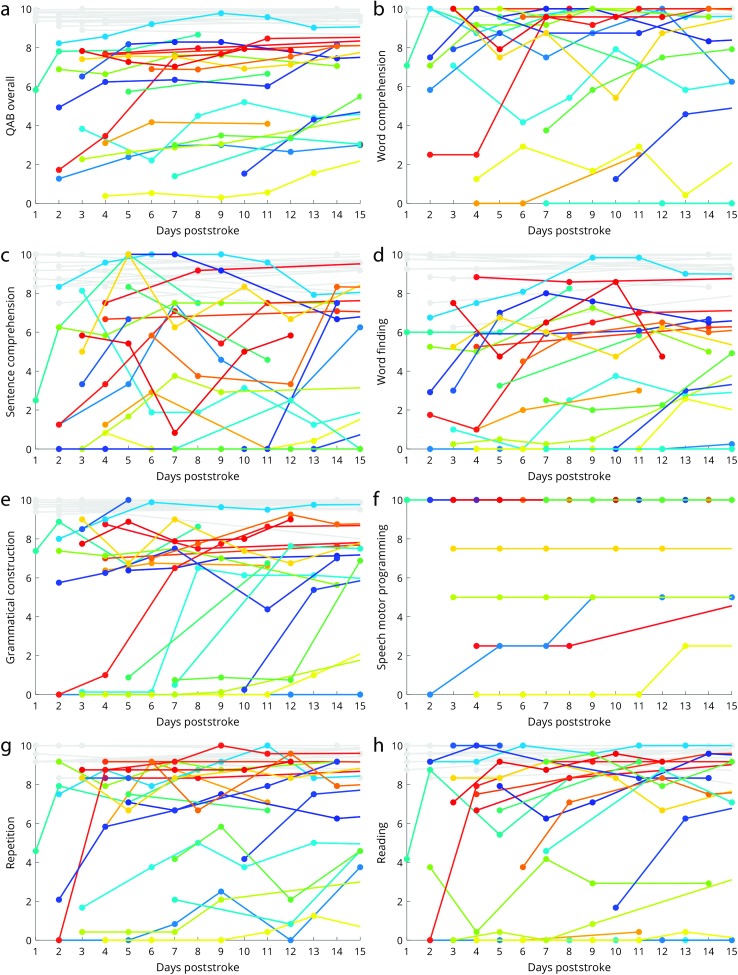
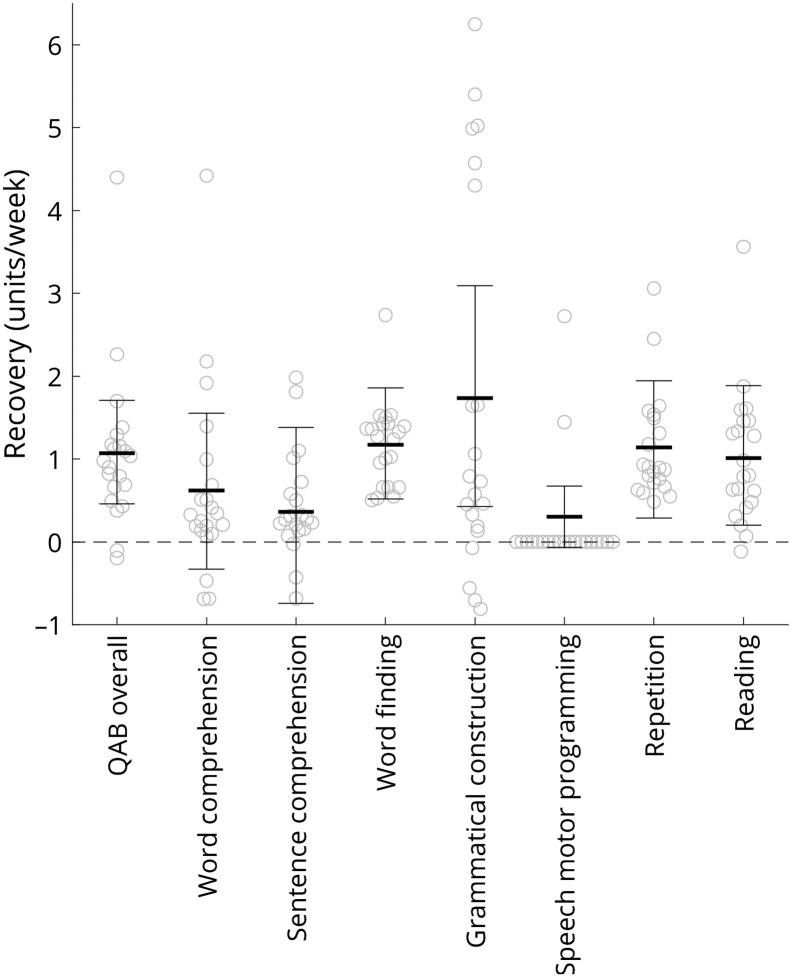
Trajectories of early recovery for overall language function and seven language domains are shown in Figure 2. The fitted slopes are reported in Table 2 and depicted in Figure 3, associated statistics are shown in Table 2, and the complete data set is provided in Supplemental Material S1. On a 10-point scale, overall language function improved a mean of 1.07 points per week, confidence interval [0.46, 1.71], LRT = 10.150; p = .007, with 19 of 21 patients with aphasia showing positive trajectories.
Figure 2.
Trajectories of early recovery for overall language function and seven language domains. All scores range between 0 (no function) and 10 (normal function). Each patient with aphasia is indicated with a unique arbitrary color, whereas the patients without aphasia are all shown in light gray. Filled circles indicate language evaluations, which are connected by solid lines. For some patients, additional language evaluations were obtained after 15 days, in which case lines are shown so that the subsequent trajectory can be observed. Untestable data points are not shown, so when evaluations begin after the first few days, this implies that the patient was untestable prior to that point. (a) Quick Aphasia Battery (QAB) overall score. (b) Word comprehension. (c) Sentence comprehension. (d) Word finding. (e) Grammatical construction. (f) Speech motor programming. (g) Repetition. (h) Reading.
Table 2.
Slope of recovery in each language domain.
| QAB measure | Recovery (units/week) | LRT | p |
|---|---|---|---|
| QAB overall | 1.071, CI [0.458, 1.709] | 10.150 | .007* |
| Word comprehension | 0.620, CI [–0.328, 1.556] | 1.747 | .22 |
| Sentence comprehension | 0.362, CI [–0.741, 1.380] | 0.471 | .52 |
| Word finding | 1.174, CI [0.521, 1.861] | 10.530 | .006* |
| Grammatical construction | 1.734, CI [0.425, 3.091] | 6.329 | .022* |
| Speech motor programming | 2/5 patients with impairment improved | a | a |
| Repetition | 1.138, CI [0.285, 1.942] | 6.351 | .022* |
| Reading | 1.014, CI [0.200, 1.886] | 5.736 | .024* |
Note. p Values reflect comparisons between the main models and reduced models with no fixed effect of time postonset. QAB = Quick Aphasia Battery; LRT = likelihood ratio test statistic; CI = confidence interval.
Improvements in speech motor programming could not be analyzed statistically.
p < .05.
Figure 3.
The fitted rate of recovery (units/week) for overall language function and seven language domains. Thick horizontal lines show the mean, error bars show 95% confidence intervals, and circles show individual data points. Fitted slopes were derived from mixed models (fixed effect of time postonset, random intercepts and slopes for each patient, correlation between intercepts and slopes), except for speech motor programming where the mixed model was not appropriate, so slopes were fit individually to the two patients who showed improvements in this domain. QAB = Quick Aphasia Battery.
Significant improvements were observed in four expressive language domains: word finding, grammatical construction, repetition, and reading. In two of these—word finding and repetition—all 21 patients improved. In contrast, improvements in word comprehension and sentence comprehension were less consistent and did not reach statistical significance. Speech motor programming was never impaired in 16 patients (i.e., they had no apraxia of speech), whereas three patients showed speech motor programming deficits that did not change over the 2- to 15-day period, and two patients showed speech motor programming deficits that improved. Because of the small number of patients with speech motor programming deficits, recovery of speech motor programming was not analyzed statistically. Note that 11 patients presented with dysarthria. The severity of dysarthria did not decrease appreciably over the study period, except in one patient. Dysarthria does not contribute to the QAB overall score.
Post hoc tests showed that there was more improvement in the mean of the four production measures (not including speech motor programming) than the mean of the two comprehension measures (p < .001); specifically, word finding, repetition, and grammatical construction all improved significantly more than sentence comprehension (p = .037, p = .039, and p < .001, respectively), and grammatical construction improved more than word comprehension (p = .002).
For overall language function and all language domains, models incorporating a polynomial term were not significantly better than models with only linear slope terms, indicating that all trajectories of recovery were approximately linear (see Table 3). Note that the polynomial coefficients for all models were in the direction of deceleration, and this potential deceleration approached significance for the domain of word finding (p = .070).
Table 3.
Details of recovery trajectories in each language domain.
| QAB measure | Polynomial p | Random slopes p | Intercept slope p |
|---|---|---|---|
| QAB overall | .167 | .002* | .046* |
| Word comprehension | .160 | .026* | .123 |
| Sentence comprehension | .412 | .259 | .308 |
| Word finding | .070 | .234 | .613 |
| Grammatical construction | .849 | < .001* | < .001* |
| Speech motor programming | a | a | a |
| Repetition | .166 | .172 | .248 |
| Reading | .548 | .070 | .814 |
Note. p Values reflect model comparisons between the main models and models with polynomial terms (polynomial p), without random slopes (random slopes p), and without correlations between random intercepts and slopes (intercept slope p). QAB = Quick Aphasia Battery.
Improvements in speech motor programming could not be analyzed statistically.
p < .05.
Variability across patients in slopes of recovery can be observed in Figure 3. This variability was statistically significant for overall language function, as well as the domains of word comprehension and grammatical construction (see Table 3). Slopes in other language domains were no more variable than would be expected by chance (see Table 3).
The variability of recovery slopes for overall language function and grammatical construction was partially explained by the degree of impairment at Day 2 poststroke, with patients who were more impaired at Day 2 showing steeper recovery slopes (overall language function: p = .046; grammatical construction: p < .001; see Table 3). For the other domains, there was no association between Day 2 scores and subsequent slopes (see Table 3).
In the 17 patients without aphasia, overall scores and domain-specific scores were high and remained stable at the time of the follow-up assessment (see Figure 2), supporting the specificity of the QAB for detecting changes in language function.
Discussion
In this study, we addressed five questions about the dynamics and nature of language recovery over the late acute/early subacute poststroke period. First, what is the rate of recovery of language function? We found that between Day 2 and Day 15 after stroke, individuals with aphasia improved in overall language function by about 1 point per week on a 10-point scale. This rate of recovery can be compared to reported rates in four prior studies by scaling reported rates to a common metric. To do so, we define scaled points per week (SPPW) as the rate of recovery per week scaled to a 10-point scale, making the necessary simplifying assumption that each measurement instrument spans a continuum from no function to normal function. Hartman (1981) reported that 14 patients tested within 1 week of stroke, and then again 1 week later, improved by a mean of 1.8 Porch Index of Communicative Ability (Porch, 1981) points (a 16-point scale), which corresponds to 1.13 SPPW. El Hachioui et al. (2011) tested 15 patients on mean Day 3 poststroke and mean Day 10. On the Aphasia Severity Recovery Scale (Goodglass & Kaplan, 1972), patients improved from 1.77 (out of 5) to 2.31 points, corresponding to 1.08 SPPW. On the Token Test (De Renzi & Faglioni, 1978), patients improved from 11.89 (out of 36) to 15.81, corresponding to 1.09 SPPW. On the ScreeLing total score, patients improved from 34.31 (out of 72) to 43.15, corresponding to 1.23 SPPW. In a larger follow-up study, El Hachioui et al. (2013) tested over 130 patients on mean Day 4 and mean Day 12 and reported that the Token Test improved by 3.9 points (out of 36) in ~8 days, corresponding to 0.95 SPPW. Mattioli et al. (2014) reported a mean improvement of 5.1 points (out of 50) on the Token Test between Day 2 and Day 16, corresponding to 0.51 SPPW, a lower rate than the other studies, which may reflect one of the inclusion criteria of the study, which was that comprehension had to be no more than mildly impaired. In summary, the rate of recovery during this period is remarkably consistent across this study and previous comparable studies. One caveat to this conclusion is that, although some researchers have interpreted the Token Test as a measure of aphasia severity (e.g., El Hachioui et al., 2013, 2011), it is a receptive measure that might alternatively be compared to our two receptive measures, which showed a slower rate of recovery.
Second, we asked whether recovery already shows a decelerating curve during this time period. We did not observe any statistically significant deceleration of the rate of recovery in the first 2 weeks. This may reflect lack of power, but it does appear to be the case that many patients make greater gains in the second week than the first. Pashek and Holland (1988) found that aphasia type evolved (generally from a more severe type to a less severe type) in 59% of the patients they followed. Of the patients whose aphasia type evolved, only 20% did so within the first week, whereas 65% did so by the end of the second week, emphasizing the substantial and clinically meaningful changes that take place during the second week after stroke.
After the second week, it is clear that recovery slows dramatically. El Hachioui et al. (2013) found that from ~12 to ~43 days, Token Test scores improved by 4.4 points (out of 36) in ~31 days, corresponding to 0.28 SPPW, that is, less than a third of the rate of change between Days 4 and 12. Hartman (1981) reported that, over a 4-week period with the second assessment at 27–30 days, patients improved by 2.96 Porch Index of Communicative Ability points in 28 days, corresponding to 0.46 SPPW, which, given the much greater rate of recovery reported within the first 2 weeks, implies that recovery must decelerate rapidly in the second half of the first month. Pedersen et al. (1995) showed that 95% of patients with mild aphasia on admission had reached stable language function after 2 weeks, and the greatest gains for patients with moderate to severe aphasia were also observed in the first 2 weeks, though these patients did not reach stable function until 6 and 10 weeks, respectively. After 1 month, recovery slows further still. For example, Swinburn et al. (2004) reported that patients' mean aphasia severity assessed with the Comprehensive Aphasia Test improved from 44.78 (out of 100) at 1 month to 48.88 at 3 months, which corresponds to just 0.05 SPPW.
Next, we asked how variable patients are in terms of rate of recovery and whether initial severity is predictive of the subsequent rate of recovery. We observed significant variability between patients in recovery of overall language function. The striking variability in trajectories of aphasia recovery after stroke is well known (Kertesz & McCabe, 1977; Laska et al., 2001; Pedersen, Vinter, & Olsen, 2004), but the determinants of this variability are still not well understood. Initial scores are one clear predictor: We found that patients with more severe aphasia at Day 2 made greater subsequent gains in overall language function. This is consistent with a previous report that patients with poststroke aphasia regain about 70% of their lost language function between an initial assessment within the first 3 days after stroke and a follow-up assessment at 90 days (Lazar et al., 2010). This reflects in part the fact that patients with greater deficits have more room to improve.
Finally, we compared the dynamics of recovery across different language domains. We found that patterns of recovery differed across domains, with consistent improvements in word finding, grammatical construction, repetition, and reading, but less consistent improvements in word comprehension and sentence comprehension. Several of these differences were statistically significant, including a composite contrast between expressive and receptive language domains. It is noteworthy that the test–retest reliability of the receptive measures on the QAB is not as good as the reliability of the expressive measures (Wilson et al., 2018). However, although this would increase the variability in the estimates of recovery slopes, it would not account for the significantly lower slopes relative to expressive measures. It is also noteworthy that chance performance on receptive measures scales to 0 on the QAB, based on the assumption that chance performance reflects complete impairment. If raw scores were used as in some other aphasia batteries, the slopes for recovery of receptive functions would be even shallower. We are aware of only a few previous studies that have reported early recovery rates separately for different language domains (El Hachioui et al., 2013, 2011; Furlanis et al., 2018; Mattioli et al., 2014); however, none of these studies statistically compared rates across domains. In longer term recovery, trajectories of recovery in different language domains have generally been reported to be quite similar to one another (e.g., Demeurisse et al., 1980; Swinburn et al., 2004).
Our study had several noteworthy limitations. First, there were only 21 patients with aphasia included in this study. This relatively small sample size limits the precision with which we were able to estimate trajectories of recovery and means that all nonsignificant findings should be treated with caution because some patterns may be significant in a larger sample. Second, although we attempted to evaluate language function every 2–3 days between Poststroke Days 2 through 15, our ability to do so was limited by situational factors, so the number of data points acquired and their timing differed across patients. However, the mixed models that we employed allowed us to draw inferences where possible despite the unbalanced nature of the data (Bates et al., 2015). Third, our analyses were limited only to time points at which patients were testable, which was defined as medically stable and able to stay awake, maintain attention, and attempt to follow commands. Therefore, our findings must be interpreted only as applying to patients who meet these criteria, leaving open the question of the natural history of patients who are more severely impaired. Fourth, we assessed recovery in terms of reductions in language impairment. However, from a clinical standpoint, it will be important in future studies to investigate recovery of communicative efficacy, which can be expected to be partly, but not wholly, determined by reductions in impairment of language function. Fifth, most of the patients received at least some speech-language therapy; therefore, we cannot determine to what extent the patterns of recovery we documented were mediated by treatment. It is likely, however, that changes associated with physiological restitution during the first 2 weeks account for the great majority of the observed recovery, given that behavioral intervention was necessarily limited. This assumption is in line with findings from several recent clinical trials that did not report any significant differences between patients receiving early speech-language therapy and those experiencing natural recovery alone (Bowen et al., 2012; Laska, Kahan, Hellblom, Murray, & von Arbin, 2011; Nouwens et al., 2017). Sixth, we did not systematically study patients after the first 2 weeks, so we were unable to establish any relationships between early patterns of recovery and longer term outcomes. Future research is warranted to track patients from the acute through to the subacute and chronic phases (Lazar et al., 2010; Maas et al., 2012). Finally, we included patients with ischemic stroke and primary intracerebral hemorrhage. The underlying pathophysiology of tissue injury and time course of recovery likely differs in these two populations; however, because of the small sample size, we were not able to compare the two groups.
Our findings have several implications for clinical practice. Most important, our results provide a clear basis for prognosis of language recovery within the late acute and early subacute period, which is highly relevant information to share with patients and their caregivers, for whom questions such as “How long will this last?” and “When can I expect this to get better?” typically loom large. The observed rate of recovery of 1 point per week on a 10-point scale during the first 2 weeks represents a clinically meaningful rate of improvement, given that 1 point on the QAB corresponds to about 10 points on the Western Aphasia Battery–Aphasia Quotient (Wilson et al., 2018), and even a 5-point change in aphasia quotient is generally considered clinically meaningful (Hula, Donovan, Kendall, & Gonzalez-Rothi, 2010). It should also be helpful to educate patients and their caregivers that gains will typically slow down after the first 2 weeks and that comprehension deficits may resolve less rapidly than expressive deficits, minimizing potential discouragement if the pace of early recovery is not sustained. However, clinicians should never imply that the potential for meaningful recovery ceases at any particular point because longer term recovery is certainly possible (Holland, Fromm, Forbes, & MacWhinney, 2017), especially in the context of intensive speech-language therapy (Breitenstein et al., 2017). Our findings also establish baseline expectations against which to evaluate the efficacy of interventions. It is noteworthy that, of the three largest clinical trials to date of early speech-language therapy, Laska et al. (2011) initiated therapy within 2 days of stroke onset, whereas Bowen et al. (2012) and Nouwens et al. (2017) initiated therapy about 2 weeks after stroke onset. Although our data do not bear on the question of whether it is better to try to ride the coattails of rapid early recovery or to begin therapy after the pace of recovery has begun to slow, our findings do suggest that these phases are different enough that the potential efficacy of speech-language therapy should probably be investigated independently in each phase.
Supplementary Material
Acknowledgments
This research was supported in part by National Institute on Deafness and Other Communication Disorders Grant R01 DC013270 (awarded to Stephen M. Wilson). We thank Alexa Bautista, Ashley Chavez, Angelica McCarron, Hannah Payne, and Matt Stib for contributing to data collection; Andrew DeMarco, Kendra Drake, Julia Fisher, Wayneho Kam, Rihan Khan, Kambiz Nael, and Steven Rapcsak for helpful discussions; the many health care professionals who facilitated patient recruitment; and the patients and their caregivers who participated in the study.
Funding Statement
This research was supported in part by National Institute on Deafness and Other Communication Disorders Grant R01 DC013270 (awarded to Stephen M. Wilson).
References
- Basso A. (1992). Prognostic factors in aphasia. Aphasiology, 6, 337–348. [Google Scholar]
- Bates D., Mächler M., Bolker B. M., & Walker S. C. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67, 1–48. [Google Scholar]
- Bowen A., Hesketh A., Patchick E., Young A., Davies L., Vail A., … Tyrrell P. (2012). Effectiveness of enhanced communication therapy in the first four months after stroke for aphasia and dysarthria: A randomised controlled trial. British Medical Journal, 345, e4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenstein C., Grewe T., Flöel A., Ziegler W., Springer L., Martus P., … FCET2EC Study Group. (2017). Intensive speech and language therapy in patients with chronic aphasia after stroke: A randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. The Lancet, 389, 1528–1538. [DOI] [PubMed] [Google Scholar]
- Cloutman L., Newhart M., Davis C., Heidler-Gary J., & Hillis A. E. (2009). Acute recovery of oral word production following stroke: Patterns of performance as predictors of recovery. Behavioural Neurology, 21, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culton G. L. (1969). Spontaneous recovery from aphasia. Journal of Speech and Hearing Research, 12, 825–832. [DOI] [PubMed] [Google Scholar]
- Demeurisse G., Demol O., Derouck M., de Beuckelaer R., Coekaerts M. J., & Capon A. (1980). Quantitative study of the rate of recovery from aphasia due to ischemic stroke. Stroke, 11, 455–458. [DOI] [PubMed] [Google Scholar]
- Denier C., Chassin O., Vandendries C., Bayon de la Tour L., Cauquil C., Sarov M., … Flamand-Roze C. (2016). Thrombolysis in stroke patients with isolated aphasia. Cerebrovascular Diseases, 41, 163–169. [DOI] [PubMed] [Google Scholar]
- Denier C., Flamand-Roze C., Dib F., Yeung J., Solignac M., Bayon de la Tour L., … Pico F. (2015). Aphasia in stroke patients: Early outcome following thrombolysis. Aphasiology, 29, 442–456. [Google Scholar]
- De Renzi E., & Faglioni P. (1978). Normative data and screening power of a shortened version of the Token Test. Cortex, 14, 41–49. [DOI] [PubMed] [Google Scholar]
- El Hachioui H., Lingsma H. F., van de Sandt-Koenderman M. E., Dippel D. W., Koudstaal P. J., & Visch-Brink E. G. (2013). Recovery of aphasia after stroke: A 1-year follow-up study. Journal of Neurology, 260, 166–171. [DOI] [PubMed] [Google Scholar]
- El Hachioui H., van de Sandt-Koenderman M. W., Dippel D. W., Koudstaal P. J., & Visch-Brink E. G. (2011). A 3-year evolution of linguistic disorders in aphasia after stroke. International Journal of Rehabilitation Research, 34, 215–221. [DOI] [PubMed] [Google Scholar]
- Faraway J. J. (2016). Extending the linear model with R: Generalized linear, mixed effects and nonparametric regression models (2nd ed.). Boca Raton, FL: CRC Press. [Google Scholar]
- Furlanis G., Ridolfi M., Polverino P., Menichelli A., Caruso P., Naccarato M., … Manganotti P. (2018). Early recovery of aphasia through thrombolysis: The significance of spontaneous speech. Journal of Stroke and Cerebrovascular Diseases, 27, 1937–1948. [DOI] [PubMed] [Google Scholar]
- Goodglass H., & Kaplan E. (1972). The assessment of aphasia and related disorders. Philadelphia, PA: Lea and Febiger. [Google Scholar]
- Hartman J. (1981). Measurement of early spontaneous recovery from aphasia with stroke. Annals of Neurology, 9, 89–91. [DOI] [PubMed] [Google Scholar]
- Hillis A. E., & Heidler J. (2002). Mechanisms of early aphasia recovery. Aphasiology, 16, 885–895. [Google Scholar]
- Hillis A. E., Kleinman J. T., Newhart M., Heidler-Gary J., Gottesman R., Barker P. B., … Chaudhry P. (2006). Restoring cerebral blood flow reveals neural regions critical for naming. Journal of Neuroscience, 26, 8069–8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis A. E., Wityk R. J., Barker P. B., Beauchamp N. J., Gailloud P., Murphy K., … Metter E. J. (2002). Subcortical aphasia and neglect in acute stroke: The role of cortical hypoperfusion. Brain, 125, 1094–1104. [DOI] [PubMed] [Google Scholar]
- Holland A. L., Fromm D., Forbes M., & MacWhinney B. (2017). Long-term recovery in stroke accompanied by aphasia: A reconsideration. Aphasiology, 31, 152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland A. L., Miller J., Reinmuth O. M., Bartlett C., Fromm D., Pashek G., … Swindell C. (1985). Rapid recovery from aphasia: A detailed language analysis. Brain and Language, 24, 156–173. [DOI] [PubMed] [Google Scholar]
- Hula W., Donovan N. J., Kendall D. L., & Gonzalez-Rothi L. J. (2010). Item response theory analysis of the Western Aphasia Battery. Aphasiology, 24, 1326–1341. [Google Scholar]
- Kertesz A. (2007). Western Aphasia Battery–Revised. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Kertesz A., & McCabe P. (1977). Recovery patterns and prognosis in aphasia. Brain, 100, 1–18. [DOI] [PubMed] [Google Scholar]
- Laska A. C., Hellblom A., Murray V., Kahan T., & von Arbin M. (2001). Aphasia in acute stroke and relation to outcome. Journal of Internal Medicine, 249, 413–422. [DOI] [PubMed] [Google Scholar]
- Laska A. C., Kahan T., Hellblom A., Murray V., & von Arbin M. (2011). A randomized controlled trial on very early speech and language therapy in acute stroke patients with aphasia. Cerebrovascular Diseases Extra, 1, 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar R. M., Minzer B., Antoniello D., Festa J. R., Krakauer J. W., & Marshall R. S. (2010). Improvement in aphasia scores after stroke is well predicted by initial severity. Stroke, 41, 1485–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendrem W., & Lincoln N. B. (1985). Spontaneous recovery of language in patients with aphasia between 4 and 34 weeks after stroke. Journal of Neurology Neurosurgery and Psychiatry, 48, 743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas M. B., Lev M. H., Ay H., Singhal A. B., Greer D. M., Smith W. S., … Furie K. L. (2012). The prognosis for aphasia in stroke. Journal of Stroke and Cerebrovascular Diseases, 21, 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli F., Ambrosi C., Mascaro L., Scarpazza C., Pasquali P., Frugoni M., … Gasparotti R. (2014). Early aphasia rehabilitation is associated with functional reactivation of the left inferior frontal gyrus: A pilot study. Stroke, 45, 545–552. [DOI] [PubMed] [Google Scholar]
- Mohr J. P. (1973). Rapid amelioration of motor aphasia. Archives of Neurology, 28, 77–82. [DOI] [PubMed] [Google Scholar]
- Nicholas M. L., Helm-Estabrooks N., Ward-Lonergan J., & Morgan A. R. (1993). Evolution of severe aphasia in the first two years post onset. Archives of Physical Medicine and Rehabilitation, 74, 830–836. [DOI] [PubMed] [Google Scholar]
- Nouwens F., de Lau L. M. L., Visch-Brink E. G., van de Sandt-Koenderman W. M. E., Lingsma H. F., Goosen S., … Dippel D. W. (2017). Efficacy of early cognitive-linguistic treatment for aphasia due to stroke: A randomised controlled trial (Rotterdam Aphasia Therapy Study–3). European Stroke Journal, 2, 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashek G. V., & Holland A. L. (1988). Evolution of aphasia in the first year post-onset. Cortex, 24, 411–423. [DOI] [PubMed] [Google Scholar]
- Pedersen P. M., Jørgensen H. S., Nakayama H., Raaschou H. O., & Olsen T. S. (1995). Aphasia in acute stroke: Incidence, determinants, and recovery. Annals of Neurology, 38, 659–666. [DOI] [PubMed] [Google Scholar]
- Pedersen P. M., Vinter K., & Olsen T. S. (2004). Aphasia after stroke: Type, severity and prognosis. The Copenhagen Aphasia Study. Cerebrovascular Diseases, 17, 35–43. [DOI] [PubMed] [Google Scholar]
- Porch B. E. (1981). Porch Index of Communicative Ability–Third Edition. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- R Core Team. (2018). R: A language and environment for statistical computing. Retrieved from https://www.r-project.org
- Rorden C., Bonilha L., Fridriksson J., Bender B., & Karnath H. O. (2012). Age-specific CT and MRI templates for spatial normalization. NeuroImage, 61, 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewan C. M., & Kertesz A. (1984). Effects of speech and language treatment on recovery from aphasia. Brain and Language, 23, 272–299. [DOI] [PubMed] [Google Scholar]
- Swinburn K., Porter G., & Howard D. (2004). Comprehensive Aphasia Test. Hove, United Kingdom: Psychology Press. [Google Scholar]
- Wertz R. T., Collins M. J., Weiss D., Kurtzke J. F., Friden T., Brookshire R. H., … Resurreccion E. (1981). Veterans administration cooperative study on aphasia: A comparison of individual and group treatment. Journal of Speech and Hearing Research, 24, 580–594. [DOI] [PubMed] [Google Scholar]
- Wilson S. M., Eriksson D. K., Schneck S. M., & Lucanie J. M. (2018). A Quick Aphasia Battery for efficient, reliable, and multidimensional assessment of language function. PLoS One, 13, e0192773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagata S. A., Yen M., McCarron A., Bautista A., Lamair-Orosco G., & Wilson S. M. (2017). Rapid recovery from aphasia after infarction of Wernicke's area. Aphasiology, 31, 951–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.