Abstract
Purpose
This study evaluated the accuracy of respiratory calibration methods for estimating lung volume during speech breathing.
Method
Respiratory kinematic data were acquired via inductance plethysmography in 32 young adults, 22 older adults, and 13 older adults with Parkinson's disease (PD). Raw rib cage (RC) and abdomen (AB) signals (V) were calibrated to liters using 4 correction methods: (a) isovolume maneuvers, (b) a constant 2:1 RC-to-AB ratio, (c) least squares method with RC correction only (LsqRC), and (d) least squares method with both RC and AB corrections (LsqRC/AB). Mean percent error, the absolute difference between estimated and actual lung volumes then normalized to each speaker's vital capacity, was calculated for each method.
Results
For young adults, the LsqRC/AB method significantly reduced mean percent error compared to all other methods. Although LsqRC/AB also resulted in smaller errors for older adults and adults with PD, LsqRC/AB and LsqRC were not significantly different from one another in these groups.
Conclusion
The LsqRC/AB method reduces errors across all cohorts, but older adults and adults with PD also have reduced errors when using LsqRC. Further research should investigate both least squares methods across larger age and disease severity ranges.
Respiratory inductance plethysmography captures circumferential changes of the rib cage (RC) and abdomen (AB) during breathing. The signals acquired from the RC and AB are recorded as raw voltage changes that require calibration to liters for further interpretation. Although a number of respiratory kinematic calibration methods have been proposed, few studies have directly compared these methods to one another. This has resulted in ambiguity as to which method reduces error between estimated and actual lung volumes and, furthermore, whether specific populations require different calibration methods due to known differences in respiratory function. Accordingly, the purpose of this study was to compare respiratory kinematic calibration methods across participants of different age, sex, and health status, in order to determine which method results in the lowest overall measurement error. The goal of this work was to provide further information to researchers and clinicians seeking to quantify lung volumes using respiratory kinematic methods.
Respiratory Kinematic Correction Methods
The respiratory system is modeled with 2 df, wherein the RC and AB differentially contribute to the overall lung volume displaced during breathing for vital function and for speech purposes (Konno & Mead, 1967). In order to convert the raw voltages captured during breathing to a more physiologically functional unit of measure, such as liters, a series of calibration procedures have been developed (Banzett, Mahan, Garner, Brughera, & Loring, 1995; Chadha et al., 1982; Cohn, Watson, Weisshaut, Stott, & Sackner, 1977; Stagg, Goldman, & Davis, 1978). These calibration procedures calculate separate correction factors for the RC and AB signals. When the RC and AB voltage signals are weighted by the correction factors and subsequently summed, an estimate of lung volume in liters can then be reported and analyzed.
Some of the first analyses of respiratory kinematics for speech breathing were completed before specialized computer software was available to assist in the calculations of the RC and AB correction factors. As such, the isovolume method was developed as a means to calibrate the contributions of the RC and AB at the time of data acquisition (Konno & Mead, 1967). Isovolume maneuvers are based on the assumption that, when the respiratory system is closed (i.e., when there is no air escaping out of the larynx), there is a constant lung volume with just 1 df. During the isovolume maneuver, the unchanging lung volume can be transferred between the RC and AB compartments of the system resulting in different thoracic configurations with the same lung volume. As such, graphical representations of the AB and RC voltages (x-axis and y-axis values, respectively) at different configurations can be adjusted so that each x–y pair sums to an equal lung volume (thereby forcing the slope of the regression between these values to be equal to −1). Previous studies have implemented this technique to adjust the RC and AB voltages and estimate lung volumes during breathing and speech tasks (Banzett et al., 1995; Chadha et al., 1982; Hixon, 1973; Solomon & Hixon, 1993; Winkworth & Davis, 1997). However, the isovolume method is limited because it requires the speaker to accurately complete the maneuver and, furthermore, is not able to incorporate actual lung volume in liters during analysis. Rather, the correction factors are later checked against actual volumes during other breathing tasks.
Another correction method, frequently referred to as the least squares method, determines calibration factors to the RC and AB via direct comparisons to actual lung volumes acquired during the same task (Chadha et al., 1982). The actual volumes can be obtained by breathing in and out of a bag of known amount (e.g., spirobag) or via a spirometer that transduces oral airflow (liters per second). Determining the correction factors for breathing a constant volume compared to breathing different volumes requires slightly different calculations but is based on the same principles. When breathing in and out a constant volume, multiple positions are needed (e.g., sitting, standing, supine) in order to acquire different RC and AB contributions at the same lung volume. Much like the isovolume method, the correction factors for the RC and AB are acquired from the different thoracic configurations, as the sum of contributions in each position should ultimately equal the same known volume. A least squares regression line is fit to values from different positions (once again, AB voltages as the x values and RC voltages as the y values) to a consistent slope equal to −1. Generally, this technique incorporates the voltages only at the greatest point of inspiration (inspiratory peak) and the lowest points of expiration (expiratory trough) into its calculations, thereby limiting the number of data points used for estimation of correction factors.
With the advent of digital spirometers and advanced algorithmic processing, the tedious task of breathing in and out a set amount at different positions can be circumvented. The newest version of the least squares technique employs a more ecologically valid set of breathing tasks at natural lung volumes in a consistent postural position (Huber, Chandrasekaran, & Wolstencroft, 2005). Resulting RC and AB voltages are adjusted to reduce the error between estimated and actual lung volumes obtained over multiple tasks and thousands of data points, which include the inspiratory peaks, expiratory troughs, and all points in between.
The aforementioned calibration methods still require individuals to complete different breathing tasks and maneuvers and/or to change positions. It follows that a fixed correction factor would be advantageous for individuals who are unable to complete specific tasks due to physical or motor impairments (e.g., spinal injury, Parkinson's disease [PD]). As such, Banzett et al. (1995) proposed a 2:1 standard ratio in which the RC is multiplied by a factor of 2 and the AB is multiplied by a factor of 1 (i.e., the AB is held constant). The authors determined that the 2:1 ratio resulted in similar lung volume estimates to those obtained from RC correction with isovolume maneuvers.
Despite the myriad of correction methods described above, few studies have sought to compare correction methods for speech breathing purposes. Moreover, the evaluation of methods is limited due to the consistent enrollment of young healthy adults instead of those with different age and health status. For example, Chadha et al. (1982) examined several correction methods (including methods for correction to magnetometers instead of plethysmography; Stagg et al., 1978) but enrolled 10 young healthy subjects with a mean age of 25 years. Likewise, Banzett et al. (1995) proposed the standard 2:1 correction ratio based on data from 11 healthy participants, aged 22–46 years (M = 31.9), and Strömberg, Dahlbäck, and Gustafsson (1993) evaluated three different correction methods in 10 healthy adults (average age of 33 years). We are not aware of any study that has evaluated the impact of age, sex, or medical diagnosis on estimation accuracy.
Nevertheless, these correction methods—which were developed and validated in young healthy adults—have been consistently applied to patient populations with the assumption that resulting values will be of the same estimation accuracy. Although there is no gold standard respiratory kinematic calibration method, the least squares method is the most common mathematical technique used to calculate the RC and AB correction factors across different populations, including those with PD (Darling-White & Huber, 2017; Huber & Darling, 2011; Stathopoulos et al., 2014), postlaryngectomy patients with and without chronic obstructive pulmonary disease (Bohnenkamp, Forrest, Klaben, & Stager, 2011, 2012), healthy young adults during modulations of vocal effort and breathiness (Heller Murray, Michener, Enflo, Cler, & Stepp, 2018; McKenna, Llico, Mehta, Perkell, & Stepp, 2017), and healthy adults of all ages during fatigue paradigms (Herndon, Sundarrajan, Sivasankar, & Huber, 2017; Sundarrajan, Huber, & Sivasankar, 2017). Because these studies are critical for identifying physiological targets for speech breathing intervention, the accuracy of the calibration becomes essential to the interpretation of the results and the implications for treatment. It is possible that the aforementioned methods used for respiratory kinematic calibration may be sufficient for use across different populations; however, to date, no studies have demonstrated this. As such, it is crucial that these calibration methods be compared against one another in healthy and disordered populations to assess overall estimation accuracy.
Age- and Disease-Related Changes to the Respiratory System
A number of studies have established age-related changes to the respiratory system (Frank, Mead, & Ferris, 1957; Hoit & Hixon, 1987; Mittman, Edelman, Norris, & Shock, 1965; Niewoehner, Kleinerman, & Liotta, 1975; Turner, Mead, & Wohl, 1968). Older adults (aged > 50 years) have larger residual lung volumes and reduced vital capacity. These findings are hypothesized to be due to loss of pulmonary recoil pressure with aging. Furthermore, there is evidence for sarcopenia of the inspiratory and expiratory muscles (Enright, Kronmal, Manolio, Schenker, & Hyatt, 1994; Watsford, Murphy, & Pine, 2007). The impact of aging on respiratory kinematic measures for speech breathing includes greater lung volume excursions (Hoit & Hixon, 1987; Huber, 2008) and greater RC volume initiations during extraneous speech (Hoit & Hixon, 1987). Because the findings of these studies suggest a reliance on the RC movement, many follow-up investigations solely consider the correction of the RC voltages, holding the AB value constant (e.g., Banzett et al., 1995).
Older adults with PD exhibit additional changes to respiratory function beyond those associated with aging. For example, PD is characterized by increased chest wall rigidity, which is thought to contribute to reduced RC expansion at speech initiation as compared to healthy older adults (Solomon & Hixon, 1993). Subsequently, greater displacement and larger AB volumes have been noted to occur compared to healthy older adults, indicative of poor abdominal control during speech breathing (Solomon & Hixon, 1993; Stathopoulos et al., 2014). As PD progresses, lung volume at speech initiation and overall lung volume used for speech decrease (Huber & Darling-White, 2017). Older adults with PD can also demonstrate reduced coordination between the RC and AB; specifically, Solomon and Hixon (1993) reported that a subset of participants with PD exhibited paradoxical movements of the RC and AB, wherein the RC and AB moved in opposition instead of synergistically. AB signals have been described as unstable and inconsistent (e.g., Murdoch, Chenery, Bowler, & Ingram, 1989), further driving the trend of RC-only adjustments during calibration procedures (Darling-White & Huber, 2017; Stathopoulos et al., 2014). Yet, despite these findings, there has been no study to date that has compared the accuracy of adjusting for the AB versus holding it constant in young adults, older adults, or older adults with PD.
Research Purpose and Hypotheses
The purpose of this study was to examine the accuracy of respiratory kinematic calibration methods for lung volume estimates during speech breathing. In order to examine the accuracy of methods across patient populations, we evaluated three cohorts of adults that varied in age, sex, and health status. The following hypotheses were proposed:
Respiratory calibration method would significantly impact lung volume estimation error. We had no hypothesis regarding which method would be most effective in reducing error nor how that error might vary across cohorts.
There would be no significant differences between the least squares method that corrects for both the RC and AB versus the least squares calculation of RC correction only for older adults and adults with PD. Our hypothesis is based on evidence of age-related changes to the respiratory system and inconsistent AB signals in people with PD.
Materials and Method
Participants
Data were collected from 2003 to 2005 at the Purdue University Motor Speech Laboratory (please see additional publications on participants: Huber, 2007, 2008; Huber et al., 2005; Huber & Darling, 2011; Huber & Darling-White, 2017; Huber & Spruill, 2008). Participants included 32 healthy young adults aged 19–34 years (M = 22.2 years, SD = 2.8; 16 women, 16 men) and 22 healthy older adults aged 65–88 years (M = 71.7 years, SD = 5.9; 12 women, 10 men). All healthy adults did not have any history of speech, language, or hearing problems and reported no neurological or pulmonary disease (e.g., asthma, chronic obstructive pulmonary disease). All participants were free from colds or infection on the day of testing and were nonsmoking for at least 5 years before the study. Furthermore, all healthy adults had typical vital capacities and forced expiratory volume in 1 s for their age, sex, height, weight, and ethnicity.
Fifteen participants with PD were identified for inclusion in the study; however, two participants (one man, one woman) were unable to complete isovolume maneuvers and were therefore excluded from the study. The remaining 13 participants had an age range of 68–90 years (M = 74.8 years, SD = 6.6; four women, nine men). The sex distribution of the participants with PD is consistent with the current estimates of the incidence of PD in men and women (Gillies, Pienaar, Vohra, & Qamhawi, 2014). Participants with PD were diagnosed by a neurologist, with a range of time from diagnosis of 1–11 years (M = 5.41 years). Ten of the 13 adults with PD self-reported speech problems. Consistent with previous literature, the participants with PD had lower vital capacities than the healthy older adults. Like all other participants enrolled, participants with PD reported no pulmonary disease (e.g., asthma, chronic obstructive pulmonary disease) and no neurological disease other than PD, were free from colds or infections on the day of testing, and were nonsmoking for at least 5 years before the study. Please see Table A1 in the Appendix for more demographic information on participants with PD. Consent was obtained from all participants prior to participation in accordance with the guidelines set by the institutional review board at Purdue University.
Procedure
Participants were asked to wear clothing that was fitted or to change into well-fitting scrubs before data collection. They were then fit with two flexible bands with inset wires (“respibands”) to capture chest wall and abdominal movements during respiration. The wires in the respibands transduce changes in impedance that result from thoracic expansion and contraction during breathing. One respiband was placed around the RC (across the chest directly inferior to the axilla), and the second was placed around the AB, near the umbilicus and just inferior to the floating rib. Participants were in a supported seated position for the duration of respiratory kinematic data acquisition. Respibands were taped to the participant's clothing at the start of the study and monitored for any changes in positioning because changes to position affect the validity of a calibration.
A series of calibration procedures were completed. For these tasks, participants wore a nose plug (to ensure no air escapes from the nasal cavity) and placed a spirometer between the lips. The spirometers provided the actual lung volumes during respiratory kinematic calibration calculations. The following tasks were completed:
Rest breathing: Participants were instructed to relax during the recording, with limited instruction so as not to impact their typical breathing patterns. Rest breathing was recorded for 30–45 s, for three times.
Speechlike breathing: Reich and McHenry (1990) reported reduced error in calibrations for speech breathing that included a speech task. As such, participants were instructed to breathe in and silently say to themselves “Buy Bobby a puppy now if he wants one” once during each exhalation. This sentence was chosen because it is the length of a typical communication utterance (Huber et al., 2005). These instructions created a speechlike respiratory waveform that generally results in a larger total lung excursion and longer exhalation period compared to rest breathing. We were able to monitor compliance to the task by visualizing breathing patterns. Figure 1 provides an example of rest breaths and speechlike breaths. Speechlike breathing was recorded at least two times for 45 s.
Figure 1.
Example of four rest breaths and two speechlike breaths for rib cage and abdomen signals (V). Note the larger amplitude for speechlike breathing compared to rest breathing.
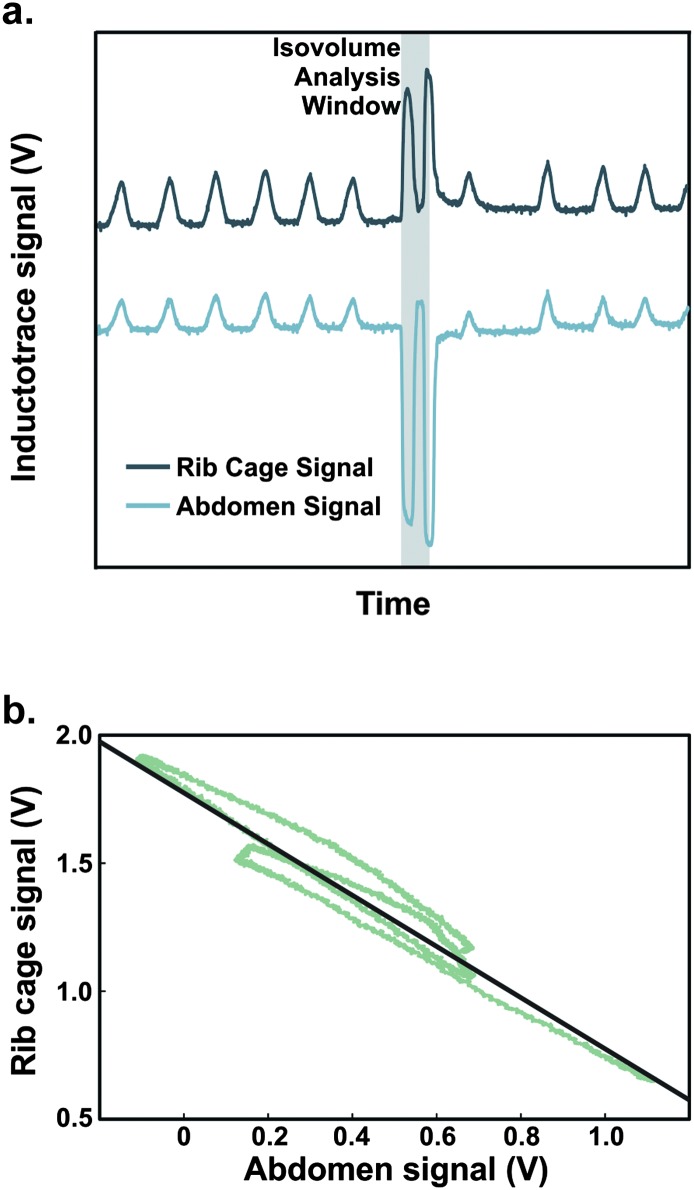
After these tasks, the spirometer was removed, while the nose plug stayed in place for completion of the isovolume maneuver. This maneuver requires the participant to move inspired air between the RC and AB compartments while maintaining a held breath. To accurately complete this task, the participant must maintain a tight seal at the lips during the maneuver so that the lung volume remains the same; any air escape can reduce the accuracy of the maneuver and impact the resultant correction factor. Participants were instructed to complete isovolume maneuvers with the following instructions: “I want you to hold your breath, but do not take a breath in, just stop breathing. Then, while holding your breath, I will ask you to suck your belly in and let it flop out. Then I will have you suck your belly in again and let it flop again.” All isovolume maneuvers were cued to be completed at an end expiratory level (i.e., the end of a tidal expiration before the initiation of an inspiration). Lip seal was visually monitored, and waveforms were monitored throughout the acquisition process in order to ensure that breath holding was complete during the maneuver. Participants completed isovolume maneuvers at least three times. Figure 2a provides an example of RC and AB voltage signals during an isovolume maneuver.
Figure 2.
(a) Schematic of an isovolume maneuver with rest breaths before and after the maneuver. The analysis window represents the segments in which the participant pulled the belly inward, let it relax out, and brought it inward once more. (b) Example of an isovolume maneuver plotted as abdomen voltages to rib cage voltages. The slope of the line-of-best fit has been adjusted to be equal to −1, via determination of a correction factor for rib cage values only. Forcing the slope to −1 assumes that the lung volume has remained constant throughout the entire isovolume maneuver.
Data Acquisition and Processing
Respiratory kinematic data were acquired with Inductotrace (Ambulatory Monitoring, Inc.), set on the DC setting. Data were digitized with the Optotrak system (Northern Digital) at a sampling rate of 2 kHz and 16 bits. All data were processed via a semiautomated algorithm in MATLAB 9.2. Prior to analysis, rest breathing and speechlike breathing waveforms were first normalized to the end expiratory level of rest breathing. First, end expiratory levels were identified from three rest breaths at the beginning of each recording. Next, the end expiratory level was averaged for the RC signal and the AB signal separately and then subtracted from the entire recording (Stathopoulos & Sapienza, 1997). Because there was a tendency for the offset of the spirometer signal to drift during the recordings (a known acquisition problem; Stathopoulos et al., 2014), waveforms were visually inspected, and the repetitions toward the beginning of the recording (i.e., those not impacted by the spirometer drift) were used for analysis. On average, participants had a total of 13.06 breaths (SD = 3.65) available for processing. This average number of breaths is consistent with previous analyses of rest breathing and speech breathing tasks (e.g., 15 breaths; Solomon & Hixon, 1993).
Calculation of Correction Factors
Four calibration methods were completed to convert raw voltages acquired from the RC and AB respibands to actual lung volumes acquired with the spirometer. These included the original isovolume maneuver described by Konno and Mead (1967), the Banzett method that does not require any respiratory maneuvers, and the most commonly used technique employed in research today: the least squares method (with correction to the RC only [LsqRC] and correction to both the RC and the AB [LsqRC/AB]). These specific methods were chosen for comparison to encapsulate the original calibration method, a standard method that would circumvent breathing maneuvers in the motor-impaired population of individuals with PD and the techniques used in the most recent research literature. The following correction methods were used to determine correction factors for both the RC and AB signals.
Isovolume method. The RC and AB signals were acquired over each isovolume maneuver. The isovolume maneuver segment, defined as the portion of the signal in which the participant pulled the belly inward, let it relax out, and pulled the belly inward once more (see highlighted maneuver segment in Figure 2a), was extracted from the recording. Next, the RC and AB signals of the isolated maneuver were plotted against one another (e.g., see Figure 2b). A least squares regression line was determined for the data points for each maneuver. Then, in accordance with previous work (see Hixon, 1973), a customized MATLAB algorithm calculated an RC adjustment factor to force a line of best fit to a slope of −1, which assumes a constant lung volume during the transfer of air between the RC and AB compartments. The AB signal was kept constant (i.e., multiplied by 1).
Banzett method. The Banzett method is a simple 2:1 correction in which the RC voltages are multiplied by a value of 2 and the AB is multiplied by a value of 1. The correction factors are the same for every participant and do not require any mathematical determinations or special breathing maneuvers.
LsqRC. The raw RC and AB voltages as well as actual lung volumes from the spirometer were acquired during rest breathing and speechlike breathing. These two tasks create variation in total lung excursion, which is required for determination of a least squares regression line between the summed RC and AB and the actual lung volumes. The Moore–Penrose pseudoinverse function (see Equation 1) solves for the unknown RC correction factor (k 1) that reduces the error between all lung excursion estimates and actual volumes.
| (1) |
LsqRC/AB. The same pseudoinverse function can be used to solve for both the RC and AB correction factors (k 1 and k 2) during the same rest breathing and speechlike breathing tasks described above. The new formula can be found in Equation 2, which results in two correction factors.
| (2) |
The correction factors for the correction methods were individually applied to the RC and AB voltages from a set of rest breathing and speechlike breathing repetitions. The corrected RC and AB signals were then summed together to get an estimated lung volume (see Equation 3). An absolute mean error between the estimated lung volumes and the actual lung volumes acquired with the spirometer was calculated. The absolute mean error (L) was then converted to a percent error via normalization to each speaker's vital capacity (see Equation 4). The normalization allowed for direct comparison across individuals with different lung capacities.
| (3) |
| (4) |
Statistical Analysis
Three separate analysis of variance (ANOVA) models were calculated to analyze percent error across the three cohorts: young adults, older adults, and older adults with PD. The main effect variables were correction method (four levels), sex (two levels), and their interaction (Correction Method × Sex). The dependent measure was the mean percent error. For all ANOVAs, significance was set a priori to p < .05, and effect size was calculated as ηp 2 (Witte & Witte, 2007). When indicated, a Tukey's post hoc analysis was completed with significance set to p < .05. Effect size was calculated for each subsequent comparison using Cohen's d. All statistical analyses were completed in Minitab Statistical Software (Version 18).
Results
Descriptive Statistics
For all cohorts, RC correction factors were larger for the isovolume, Banzett, and LsqRC methods compared to those determined from the LsqRC/AB method (see Table 1 for descriptive statistics). Young adults also tended to have smaller correction factors when compared to older adults and adults with PD. Figure 3 provides the mean and 95% confidence interval for each correction method and cohort; note that the dotted line in the figure represents the Banzett correction method, in which all RC voltages are corrected by a factor of 2.
Table 1.
Descriptive statistics for rib cage (RC) and abdomen (AB) correction factors.
| Cohort | Descriptive metric | RC correction |
AB correction |
||
|---|---|---|---|---|---|
| Isovolume | LsqRC | LsqRC/AB | LsqRC/AB | ||
| Young adults | M (SD) | 2.36 (1.07) | 1.46 (0.33) | 0.92 (0.64) | 0.38 (0.50) |
| Median | 2.10 | 1.43 | 0.90 | 0.41 | |
| Maximum | 6.10 | 2.28 | 2.30 | 1.74 | |
| Minimum | 0.50 | 0.80 | −0.97 | −0.79 | |
| Older adults | M (SD) | 2.55 (1.32) | 2.41 (1.07) | 1.35 (1.01) | 1.32 (1.75) |
| Median | 2.19 | 2.15 | 1.27 | 0.85 | |
| Maximum | 5.92 | 5.84 | 3.27 | 5.93 | |
| Minimum | 0.80 | 1.25 | −1.25 | −1.08 | |
| Adults with PD | M (SD) | 1.93 (0.80) | 2.07 (0.72) | 1.32 (0.96) | 1.01 (1.81) |
| Median | 1.86 | 1.76 | 1.37 | 0.46 | |
| Maximum | 3.47 | 4.18 | 2.92 | 6.75 | |
| Minimum | 0.42 | 1.48 | −0.82 | −0.55 | |
Note. The Banzett method is not included because it employs constant correction factors that do not change, and only the AB correction factors for the least square method are included because all other methods hold AB constant. LsqRC = least squares method with RC correction only; LsqRC/AB = least squares method with both RC and AB corrections; PD = Parkinson's disease.
Figure 3.
Mean and 95% confidence interval for rib cage correction factors by cohort (YA = young adult, OA = older adult, and PD = Parkinson's disease) and correction method. The dotted line at 2.0 on the y-axis represents the Banzett correction method, which uses a factor of 2 to correct rib cage voltages. LsqRC = least squares method with rib cage correction only; LsqRC/AB = least squares method with both rib cage and abdomen corrections.
Resulting mean percent errors ranged from 1% to 27% of vital capacity across all participants. On average, the smallest percent errors were observed for the LsqRC/AB correction method, and the largest errors were determined from the isovolume method. The mean and standard deviations can be found in Table 2, and the overall group means and 95% confidence interval for each cohort and each method can be found in Figure 4.
Table 2.
Mean and standard deviation of mean percent error (%) by cohort, sex, and correction method.
| Correction method | Cohort |
|||||
|---|---|---|---|---|---|---|
| Young adults |
Older adults |
Adults with PD |
||||
| F n = 16 |
M n = 16 |
F n = 12 |
M n = 10 |
F n = 4 |
M n = 9 |
|
| Isovolume | 12.10 (6.38) | 11.97 (5.49) | 9.51 (4.98) | 7.91 (5.96) | 9.33 (2.96) | 10.13 (3.55) |
| Banzett | 13.18 (5.31) | 8.52 (3.21) | 9.18 (4.22) | 3.61 (1.08) | 9.03 (2.45) | 9.07 (3.68) |
| LsqRC | 8.01 (3.03) | 6.23 (1.73) | 6.79 (1.85) | 4.22 (1.28) | 8.46 (1.99) | 7.83 (3.13) |
| LsqRC/AB | 2.48 (0.68) | 1.66 (0.45) | 3.62 (1.26) | 2.41 (0.65) | 5.64 (1.70) | 4.15 (1.60) |
Note. PD = Parkinson's disease; F = female; M = male; LsqRC = least square method with correction to rib cage (RC) while holding abdomen (AB) constant; LsqRC/AB = least square method with correction to both RC and AB voltages.
Figure 4.
Mean and 95% confidence interval for mean percent error for each correction method for each cohort: young adult, older adult, and adult with Parkinson's disease (PD). Note that mean percent error is normalized to vital capacity. LsqRC = least squares method with rib cage correction only; LsqRC/AB = least squares method with both rib cage and abdomen corrections.
ANOVA and Post Hoc Comparisons
All data met the assumptions of each planned analysis, including normality and homogeneity of variance. The results of each ANOVA can be found in Table 3. For all cohorts, there was a significant main effect of correction method with large effect sizes of ηp 2 = .26–.49 (Tabachnick & Fidell, 2007). For the young adult and older adult cohorts, the main effect of sex was also significant, with female participants showing significantly greater mean percent errors than male participants. There was no significant difference found between male and female participants with PD (p = .735). No significant interaction effects were found for any model.
Table 3.
Results of each analysis of variance for each cohort.
| Cohort | Effect | df | F | p | Effect size (ηp 2) | Effect size interpretation |
|---|---|---|---|---|---|---|
| Young adults | Correction method | 3 | 38.99 | < .001 | .49 | Large |
| Sex | 1 | 7.20 | .008 | .06 | Small–medium | |
| Correction × Sex | 3 | 2.21 | .091 | — | — | |
| Older adults | Correction method | 3 | 10.08 | < .001 | .27 | Large |
| Sex | 1 | 13.66 | < .001 | .15 | Medium | |
| Correction × Sex | 3 | 1.77 | .159 | — | — | |
| Adults with PD | Correction method | 3 | 5.16 | .004 | .26 | Large |
| Sex | 1 | 0.12 | .735 | — | — | |
| Correction × Sex | 3 | 0.27 | .849 | — | — |
Note. Effect sizes and interpretations are presented for significant findings (p < .05). PD = Parkinson's disease.
Post hoc comparisons were performed for the main effect of correction method for each cohort (see Table 4). All comparisons, except for isovolume method versus Banzett method, were significantly different from one another for young adults. Effect sizes of the paired significant differences were deemed medium to very large in size with values of d = 0.63–1.74 (Cohen, 1988). Older adults exhibited similar trends as younger adults with significant differences between LsqRC/AB and the Banzett and isovolume methods; however, there was no significant difference between the LsqRC/AB and LsqRC methods (p = .09) for the older adults.
Table 4.
Results of post hoc pairwise comparisons for each cohort.
| Pairwise comparisons | Cohort |
||
|---|---|---|---|
| Young adult (n = 32) |
Older adults (n = 22) |
Adults with PD (n = 13) |
|
| Isovolume vs. Banzett | .562 (d = 0.23) |
.130 (d = 0.47) |
.956 (d = 0.14) |
| Isovolume vs. LsqRC | < .001*
(d = 0.86) |
.016*
(d = 0.65) |
.636 (d = 0.33) |
| Isovolume vs. LsqRC/AB | < .001*
(d = 1.74) |
< .001*
(d =1.16) |
.004*
(d = 1.01) |
| Banzett vs. LsqRC | .003*
(d = 0.63) |
.833 (d = 0.18) |
.905 (d = 0.19) |
| Banzett vs. LsqRC/AB | < .001*
(d = 1.51) |
.010*
(d = 0.69) |
.016*
(d = 0.86) |
| LsqRC/AB vs. LsqRC | < .001*
(d = 0.88) |
.090 (d = 0.51) |
.085 (d = 0.68) |
Note. Adjusted p value and effect size (Cohen's d) are reported for each comparison. PD = Parkinson's disease; LsqRC = least squares method with correction to rib cage (RC) while holding abdomen (AB) constant; LsqRC/AB = least squares method with correction to both RC and AB inputs.
p < .05.
The PD cohort had the fewest number of significant paired comparisons. Specifically, only the LsqRC/AB method was found to be significantly different from the Banzett and isovolume methods. Both of these comparisons had large effect sizes of d = 0.86 and 1.01, respectively. Similar to older adults, there was no significant difference between the LsqRC/AB and LsqRC methods (p = .85).
Discussion
This study sought to determine the accuracy of a variety of respiratory kinematic correction methods with respect to actual lung volume. We evaluated four different correction methods across three adult cohorts. Consistent with our first hypothesis, mean percent errors were affected by correction method across all cohorts, with large effect sizes.
On average, the LsqRC/AB resulted in the lowest mean percent errors for each group at 2.1% for young adults, 3.1% for older adults, and 4.6% for adults with PD. Because we normalized percent errors to each participant's vital capacity, we have no other study to directly compare our results to. The decision to normalize to vital capacity allows us to interpret our results and compare our errors with other kinematic measures of speech breathing (e.g., lung volume initiation, lung volume termination, total lung excursion), because all of these measures are in terms of percent vital capacity normalized to end expiratory level. Still, the magnitude of the errors reported in this study is similar to those previously reported by Strömberg et al. (1993), which indicated approximately 6% absolute error (not normalized to vital capacity) for young adults.
Our findings for the young adults unequivocally show that the LsqRC/AB significantly reduces error compared to all other methods. These results are consistent with prior work by Chadha et al. (1982) and Strömberg et al. (1993), which indicated that LsqRC/AB reduced mean errors in young healthy adults when directly compared to other methods (e.g., isovolume method, nonlinear equations). The superiority of the LsqRC/AB method could be for two reasons: (a) The LsqRC/AB uses natural speech tasks (rest breathing, speechlike breathing) to obtain correction factors across thousands of data points, and (b) the method corrects for both RC and AB voltages, which is consistent with knowledge of anatomical and physiological function of the respiratory system in young healthy adults.
The interpretation of the results for the other cohorts is not quite as clear. We hypothesized that older adults and adults with PD would not require correction to their AB voltages due to age-related changes in respiratory function (for reviews, see Janssens, Pache, & Nicod, 1999; Kim & Sapienza, 2005) and reduced muscle strength and coordination reported in adults with PD (Hovestadt, Bogaard, Meerwaldt, van der Meché, & Stigt, 1989). Our second hypothesis was supported when there were no significant differences between LsqRC and LsqRC/AB for either cohort. Yet, the small sample size of older adults (n = 22) and adults with PD (n = 13) is a limiting factor to the interpretation and application of the present findings. Reviewing the effect sizes of the paired comparisons revealed that a large effect size had to be present for a significant p value. Our results showed only medium effect sizes (d = 0.51 and 0.68) between the LsqRC and LsqRC/AB methods, yielding nonsignificant p values at the < .05 level. Based on these findings, a cohort of 34 individuals would be needed to report a significant medium effect, per sample size calculation of p < .05, medium effect size of d = 0.50, and power of 0.80 (G*Power v.3.1.9.2; Faul, Erdfelder, Buchner, & Lang, 2009).
To investigate whether the error differences between the LsqRC and LsqRC/AB methods may be clinically meaningful, we calculated the average difference between mean percent errors for the two methods across older adults and adults with PD. The mean percent error increased by 2.5% (3.1% for LsqRC/AB vs. 5.6% for LsqRC) in older adults and 3.4% in adults with PD (4.6% for LsqRC/AB vs. 8.0% for LsqRC). Prior work has shown that errors in the range of 2%–5% may be large enough to impact statistical comparisons and study findings; for example, results from a mixed group of young and older adults showed that there is an increase in total lung excursion by approximately 2% from speaking at a normal vocal volume to speaking at a louder vocal volume (1.9% difference; Huber, 2008). Moreover, a comparison of healthy older adults and adults with PD revealed a 5.2% difference in total lung excursions between the two groups (Huber & Darling, 2011). As such, the elevated error associated with the LsqRC method has the potential to impact comparisons between cohorts of speakers as well as comparisons of different speaking conditions, especially if one group uses the LsqRC method while the other uses the LsqRC/AB method. For that reason, we suggest that the same calibration method be used for both cohorts in studies that seek to compare the two populations. Furthermore, we recommend that the LsqRC and the LsqRC/AB be evaluated in a larger cohort of speakers to determine if the trends seen here are maintained in larger samples and a greater range of disease severity.
It is unsurprising that the isovolume method resulted in the greatest error in adults with PD due to known motor deficits in this population. Studies have reported that participants with PD have exhibited difficulty in completing respiratory maneuvers for calibration, specifically isolated abdominal movements (Hoit & Hixon, 1987). Subjectively, participants with PD enrolled in this study appeared to have more difficulty in completing the isovolume maneuver with more frequent instances of observed air escape during the maneuver (it is important to note that these instances were not used in the calibration calculations). Two adults with PD were excluded from the study due to their inability to complete isovolume maneuvers, further limiting the current application of this method as a viable means to determine correction values. Yet, a review of the resultant correction factors and overall variability for those participants who were able to complete the maneuver was comparable to the other cohorts.
To date, few studies have reported RC correction factors across different cohorts. The study by Banzett et al. (1995) reported correction factors for the LsqRC method and isovolume maneuvers for young adults. The LsqRC correction factors ranged from −3.2 to 3.8; the range was much larger than this study's factors of 0.80–2.28 (M = 1.46). Conversely, the study by Banzett et al. reported a smaller range for isovolume factors of 0.5–3.6 than the current study, which yielded the same minimum (0.50) but a considerably larger maximum of 6.10. Banzett et al. stated that the standard ratio of 2:1 resulted in similar lung volume estimates obtained from the isovolume maneuver. Based on our statistical findings, the isovolume method and the Banzett method were not significantly different from one another across any cohort, including the young adults. This supports the methods employed by Banzett et al. when establishing the standard ratio; however, based on the evidence here, it is likely that a new standard ratio tested against the least squares method would be best for reducing overall lung volume estimation errors.
We are not aware of any study that has reported AB correction factors for any cohort, other than those that held the AB constant. In this study, descriptively, young adults had lower AB correction factors and a reduced overall range of values (M = 0.38, range: −0.79 to 1.74) compared to the other two cohorts. Older adults and adults with PD exhibited an average value much closer to that of the proposed constant of 1 with averages of 1.32 and 1.01, respectively. Yet, the two cohorts had large maximum AB correction factors of 5.93 for older adults and 6.75 for adults with PD. These results highlight the variability of AB contributions during speech breathing. It further indicates that a consistent AB correction factor of 1 would be appropriate for some older individuals, but not all. An investigation into characteristics that might predict greater reliance on the AB during speech breathing (e.g., age, PD severity) is a recommended next step.
A rather unexpected finding in this study was the main effect of sex on mean percent errors. We decided to incorporate sex as a factor in our analysis because previous work comparing calibration methods have disregarded this analysis. Our results showed young female and older female participants yielded greater mean percent errors when directly compared to male participants in their same cohorts. Although there is evidence that recoil pressure is lower in women compared to men (due to differences in lung size; Colebatch, Greaves, & Ng, 1979), it is not readily apparent how a biological difference like this would manifest in greater mean percent errors. Importantly, the lack of an interaction effect in any model shows that the error differences between sexes were not different across the methods employed. Thus, sex does not need to be considered when determining which calibration method to use. Although sex was not a significant main effect in the PD cohort, it is possible that this was due to the small, uneven sample size of four women and nine men. Still, review of the averages (see Table 2 for review) showed that men and women with PD had similar mean percent errors across each correction method.
Limitations and Future Directions
The present work is limited because it examined preexisting calibration methods and standard ratios. It is quite possible that there is an alternative calibration strategy that has not been proposed yet that could be tailored to specific patient populations. For example, there may be a different ratio other than the one proposed by Banzett et al. (1995) that may be appropriate for individuals with PD when they are unable to successfully complete traditional calibration methods. Darling-White and Huber (2017) reported an RC correction of the LsqRC of 1.68 for 12 adults with PD using the LsqRC method. That average factor is quite similar to the outcome of this study, which reports an average RC correction factor of 2.07 and a median of 1.76. An in-depth analysis across a larger group may yield a more accurate standard correction factor.
Further work is needed to generalize the present results in patient populations in which airway obstruction, airway restriction, reduced lung compliance, and increased/decreased elasticity are primary factors (e.g., asthma, chronic obstructive pulmonary disease). From the present findings, it is likely that the LsqRC/AB would be the best method to continue calibrating respiratory kinematics, but to date, no study has compared calibration methods in these populations.
Furthermore, there is an assumption that a consistent linear solution exists for RC and AB factor calculations across all patient populations. However, elastic recoil of the lungs is not the same at large and small lung volumes, and moreover, individuals with PD have increased chest wall rigidity and reduced AB control that alter passive forces. Some nonlinear approaches to calibration have been discussed, each of which imply an interaction between the RC and AB (Revow, England, Stogryn, & Wilkes, 1987; Strömberg et al., 1993). Strömberg et al. (1993) determined that linear equations were “simple and robust” in young healthy adults but indicated that nonlinear equations would be more appropriate for situations in which the relationship between the RC and AB is affected. To date, a thorough vetting of nonlinear approaches in patient populations that do not have the same underlying physiology as young healthy adults has not been attempted. One suggested change to the current protocols is to include maximum inspiratory and expiratory movements as a cross-check for calibration accuracy. Currently, studies do not report calibration error ranges, nor do they assess accuracy across a variety of lung volumes. We recommend that future studies report some measure of error with respect to actual volume when reporting respiratory kinematic data. Furthermore, we suggest continued development of more accurate methods of estimating lung volume during speech.
Finally, correction methods should be evaluated in adults with PD at different stages of the disease. Motor function deteriorates with time, ultimately affecting coordination for speech. It is unknown at this time how respiratory and motor changes in advanced stages might impact the accuracy of speech breathing calibration methods. We recommend enrollment of a large group of individuals with PD at various stages to elucidate how calibration may be affected across the disease.
Conclusion
Across all cohorts, the LsqRC/AB method resulted in, on average, the lowest error between estimated and actual lung volumes for speech breathing. However, older adults and older adults with PD showed no significant differences in mean percent errors between LsqRC/AB and LsqRC, meaning that adjustment for the AB signal did not significantly change lung volume estimation accuracy. This finding is consistent with prior work that has opted not to correct the AB voltage signal at all due to known age- and disease-related respiratory changes. This study was completed in a relatively small cohort of participants (22 older adults and 13 adults with PD) in which a medium effect size metric did not yield a significant p value, thereby limiting the full interpretation of these nonsignificant findings. We suggest that future work specifically investigate whether corrections to both the RC and AB are necessary in older adults with and without PD in order to make recommendations for individuals across different ages and disease severity ranges.
Acknowledgments
This work was supported by National Institute on Deafness and Other Communication Disorders Grant T32DC000030, awarded to E. A. S. The data in this study were acquired with National Institute on Deafness and Other Communication Disorders Grant R03DC005731, awarded to J. E. H.
Appendix
Table A1.
Descriptive Information for Participants Diagnosed with Parkinson's Disease.
| Participant | Sex | Age (years) | Time since Dx (years) | Vital capacity (L) | Reported speech problem | Clinical rating of speech/voice severity |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reduced loudness | Monotonicity | Breathiness | Articulatory precision | Speech rate | Hoarseness | ||||||
| PD01 | F | 72 | 1 | 2.43 | Yes | Mild | Mild | Mild–moderate | WNL | Minimal | Minimal |
| PD02 | F | 69 | 9 | 2.4 | Yes | Mild | WNL | WNL | WNL | WNL | WNL |
| PD03 | F | 74 | 5 | 2.54 | No | WNL | WNL | WNL | WNL | WNL | WNL |
| PD04 | F | 79 | 6 | 1.74 | No | WNL | Mild | Mild–moderate | WNL | WNL | Mild |
| PD05 | M | 83 | 4.92 | 2.69 | Yes | Severe | Severe | Moderate | Mild | Moderate | Moderate |
| PD06 | M | 76 | 5 | 3.56 | Yes | Moderate | Moderate–severe | Moderate–severe | Moderate | Moderate–severe | Moderate–severe |
| PD07 | M | 73 | 11 | 2.71 | Yes | Moderate | Severe | Moderate | Moderate | Mild | Mild |
| PD08 | M | 68 | 3.5 | 5.08 | Yes | Mild | Mild | WNL | WNL | WNL | WNL |
| PD09 | M | 90 | 3 | 2.46 | Yes | Mild | Moderate | Moderate | Mild | Severe | Severe |
| PD10 | M | 69 | 4.92 | 4.22 | Yes | Mild | Mild | WNL | Mild | WNL | WNL |
| PD11 | M | 68 | 9 | 4.44 | Yes | Mild | Mild–moderate | WNL | Minimal | WNL | WNL |
| PD12 | M | 70 | 4.33 | 2.98 | Yes | Minimal | Mild–moderate | Mild–moderate | WNL | WNL | WNL |
| PD13 | M | 82 | 3.67 | 3.31 | No | Mild | Mild | Moderate | Minimal | Minimal | Minimal |
Note. Order of speech/voice severity is minimal, mild, moderate, and severe. Dx = diagnosis; F = female; WNL = within normal limits; M = male.
Funding Statement
This work was supported by National Institute on Deafness and Other Communication Disorders Grant T32DC000030, awarded to E. A. S. The data in this study were acquired with National Institute on Deafness and Other Communication Disorders Grant R03DC005731, awarded to J. E. H.
References
- Banzett R. B., Mahan S. T., Garner D. M., Brughera A., & Loring S. H. (1995). A simple and reliable method to calibrate respiratory magnetometers and Respitrace. Journal of Applied Physiology, 79(6), 2169–2176. https://doi.org/10.1152/jappl.1995.79.6.2169 [DOI] [PubMed] [Google Scholar]
- Bohnenkamp T. A., Forrest K. M., Klaben B. K., & Stager J. M. (2011). Lung volumes used during speech breathing in tracheoesophageal speakers. Annals of Otology, Rhinology & Laryngology, 120(8), 550–558. https://doi.org/10.1177/000348941112000811 [DOI] [PubMed] [Google Scholar]
- Bohnenkamp T. A., Forrest K. M., Klaben B. K., & Stager J. M. (2012). Chest wall kinematics during speech breathing in tracheoesophageal speakers. Annals of Otology, Rhinology & Laryngology, 121(1), 28–37. https://doi.org/10.1177/000348941212100106 [DOI] [PubMed] [Google Scholar]
- Chadha T. S., Watson H., Birch S., Jenouri G. A., Schneider A. W., Cohn M. A., & Sackner M. A. (1982). Validation of respiratory inductive plethysmography using different calibration procedures. The American Review of Respiratory Disease, 125(6), 644–649. [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Cohn M. A., Watson H., Weisshaut R., Stott F., & Sackner M. A. (1977). A transducer of noninvasive monitoring of respiration. In Proceedings of the Second International Symposium on Ambulatory Monitoring (pp. 119–128). London, United Kingdom: Academic Press. [Google Scholar]
- Colebatch H. J., Greaves I. A., & Ng C. K. (1979). Exponential analysis of elastic recoil and aging in healthy males and females. Journal of Applied Physiology, 47(4), 683–691. https://doi.org/10.1152/jappl.1979.47.4.683 [DOI] [PubMed] [Google Scholar]
- Darling-White M., & Huber J. E. (2017). The impact of expiratory muscle strength training on speech breathing in individuals with Parkinson's disease: A preliminary study. American Journal of Speech-Language Pathology, 26(4), 1159–1166. https://doi.org/10.1044/2017_AJSLP-16-0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright P. L., Kronmal R. A., Manolio T. A., Schenker M. B., & Hyatt R. E. (1994). Respiratory muscle strength in the elderly. Correlates and reference values. cardiovascular health study research group. American Journal of Respiratory and Critical Care Medicine, 149(2, Pt. 1), 430–438. https://doi.org/10.1164/ajrccm.149.2.8306041 [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Buchner A., & Lang A.-G. (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160. https://doi.org/10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- Frank N. R., Mead J., & Ferris B. G. Jr. (1957). The mechanical behavior of the lungs in healthy elderly persons. The Journal of Clinical Investigation, 36(12), 1680–1687. https://doi.org/10.1172/JCI103569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies G. E., Pienaar I. S., Vohra S., & Qamhawi Z. (2014). Sex differences in Parkinson's disease. Frontiers in Neuroendocrinology, 35(3), 370–384. https://doi.org/10.1016/j.yfrne.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller Murray E. S., Michener C. M., Enflo L., Cler G. J., & Stepp C. E. (2018). The impact of glottal configuration on speech breathing. Journal of Voice, 32(4), 420–427. https://doi.org/10.1016/j.jvoice.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon N. E., Sundarrajan A., Sivasankar M. P., & Huber J. E. (2017). Respiratory and laryngeal function in teachers: Pre- and postvocal loading challenge. Journal of Voice, 33(3), 302–309. https://doi.org/10.1016/j.jvoice.2017.11.015 [DOI] [PubMed] [Google Scholar]
- Hixon T. J. (1973). Kinematics of the chest wall during speech production: Volume displacements of the rib cage, abdomen, and lung. Journal of Speech and Hearing Disorders, 16(1), 78–115. [DOI] [PubMed] [Google Scholar]
- Hoit J. D., & Hixon T. J. (1987). Age and speech breathing. Journal of Speech and Hearing Disorders, 30(3), 351–366. [DOI] [PubMed] [Google Scholar]
- Hovestadt A., Bogaard J. M., Meerwaldt J. D., van der Meché F. G., & Stigt J. (1989). Pulmonary function in Parkinson's disease. Journal of Neurology, Neurosurgery, & Psychiatry, 52(3), 329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J. E. (2007). Effect of cues to increase sound pressure level on respiratory kinematic patterns during connected speech. Journal of Speech, Language, and Hearing Research, 50(3), 621–634. https://doi.org/10.1044/1092-4388(2007/044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J. E. (2008). Effects of utterance length and vocal loudness on speech breathing in older adults. Respiratory Physiology & Neurobiology, 164(3), 323–330. https://doi.org/10.1016/j.resp.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J. E., Chandrasekaran B., & Wolstencroft J. J. (2005). Changes to respiratory mechanisms during speech as a result of different cues to increase loudness. Journal of Applied Physiology, 98(6), 2177–2184. https://doi.org/10.1152/japplphysiol.01239.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J. E., & Darling M. (2011). Effect of Parkinson's disease on the production of structured and unstructured speaking tasks: Respiratory physiologic and linguistic considerations. Journal of Speech, Language, and Hearing Research, 54(1), 33–46. https://doi.org/10.1044/1092-4388(2010/09-0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J. E., & Darling-White M. (2017). Longitudinal changes in speech breathing in older adults with and without Parkinson's disease. Seminars in Speech and Language, 38(3), 200–209. https://doi.org/10.1055/s-0037-1602839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J. E., & Spruill J. (2008). Age-related changes to speech breathing with increased vocal loudness. Journal of Speech, Language, and Hearing Research, 51(3), 651–668. https://doi.org/10.1044/1092-4388(2008/047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens J. P., Pache J. C., & Nicod L. P. (1999). Physiological changes in respiratory function associated with ageing. The European Respiratory Journal, 13(1), 197–205. [DOI] [PubMed] [Google Scholar]
- Kim J., & Sapienza C. M. (2005). Implications of expiratory muscle strength training for rehabilitation of the elderly: Tutorial. The Journal of Rehabilitation Research and Development, 42(2), 211–224. https://doi.org/10.1682/JRRD.2004.07.0077 [DOI] [PubMed] [Google Scholar]
- Konno K., & Mead J. (1967). Measurement of the separate volume changes of rib cage and abdomen during breathing. Journal of Applied Physiology, 22(3), 407–422. https://doi.org/10.1152/jappl.1967.22.3.407 [DOI] [PubMed] [Google Scholar]
- McKenna V. S., Llico A. F., Mehta D. D., Perkell J. S., & Stepp C. E. (2017). Magnitude of neck-surface vibration as an estimate of subglottal pressure during modulations of vocal effort and intensity in healthy speakers. Journal of Speech, Language, and Hearing Research, 60(12), 3404–3416. https://doi.org/10.1044/2017_JSLHR-S-17-0180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittman C., Edelman N. H., Norris A. H., & Shock N. W. (1965). Relationship between chest wall and pulmonary compliance and age. Journal of Applied Physiology, 20(6), 1211–1216. https://doi.org/10.1152/jappl.1965.20.6.1211 [Google Scholar]
- Murdoch B. E., Chenery H. J., Bowler S., & Ingram J. C. (1989). Respiratory function in Parkinson's subjects exhibiting a perceptible speech deficit: A kinematic and spirometric analysis. Journal of Speech and Hearing Disorders, 54(4), 610–626. [DOI] [PubMed] [Google Scholar]
- Niewoehner D. E., Kleinerman J., & Liotta L. (1975). Elastic behavior of postmortem human lungs: Effects of aging and mild emphysema. Journal of Applied Physiology, 39(6), 943–949. https://doi.org/10.1152/jappl.1975.39.6.943 [DOI] [PubMed] [Google Scholar]
- Reich A. R., & McHenry M. A. (1990). Estimating respiratory volumes from rib cage and abdominal displacements during ventilatory and speech activities. Journal of Speech and Hearing Disorders, 33(3), 467–475. [DOI] [PubMed] [Google Scholar]
- Revow M. D., England S. J., Stogryn H. A., & Wilkes D. L. (1987). Comparison of calibration methods for respiratory inductive plethysmography in infants. Journal of Applied Physiology, 63(5), 1853–1861. https://doi.org/10.1152/jappl.1987.63.5.1853 [DOI] [PubMed] [Google Scholar]
- Solomon N. P., & Hixon T. J. (1993). Speech breathing in Parkinson's disease. Journal of Speech and Hearing Research, 36(2), 294–310. [DOI] [PubMed] [Google Scholar]
- Stagg D., Goldman M., & Davis J. N. (1978). Computer-aided measurement of breath volume and time components using magnetometers. Journal of Applied Physiology, 44(4), 623–633. https://doi.org/10.1152/jappl.1978.44.4.623 [DOI] [PubMed] [Google Scholar]
- Stathopoulos E. T., Huber J. E., Richardson K., Kamphaus J., DeCicco D., Darling M., … Sussman J. E. (2014). Increased vocal intensity due to the Lombard effect in speakers with Parkinson's disease: Simultaneous laryngeal and respiratory strategies. Journal of Communication Disorders, 48, 1–17. https://doi.org/10.1016/j.jcomdis.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos E. T., & Sapienza C. M. (1997). Developmental changes in laryngeal and respiratory function with variations in sound pressure level. Journal of Speech, Language, and Hearing Research, 40(3), 595–614. https://doi.org/10.1044/jslhr.4003.595 [DOI] [PubMed] [Google Scholar]
- Strömberg N. O., Dahlbäck G. O., & Gustafsson P. M. (1993). Evaluation of various models for respiratory inductance plethysmography calibration. Journal of Applied Physiology, 74(3), 1206–1211. https://doi.org/10.1152/jappl.1993.74.3.1206 [DOI] [PubMed] [Google Scholar]
- Sundarrajan A., Huber J. E., & Sivasankar M. P. (2017). Respiratory and laryngeal changes with vocal loading in younger and older individuals. Journal of Speech, Language, and Hearing Research, 60(9), 2551–2556. https://doi.org/10.1044/2017_JSLHR-S-17-0106 [DOI] [PubMed] [Google Scholar]
- Tabachnick B. G., & Fidell L. S. (2007). Using multivariate statistics (5th ed.). Boston, MA: Allyn & Bacon/Pearson Education. [Google Scholar]
- Turner J. M., Mead J., & Wohl M. E. (1968). Elasticity of human lungs in relation to age. Journal of Applied Physiology, 25(6), 664–671. https://doi.org/10.1152/jappl.1968.25.6.664 [DOI] [PubMed] [Google Scholar]
- Watsford M. L., Murphy A. J., & Pine M. J. (2007). The effects of ageing on respiratory muscle function and performance in older adults. Journal of Science and Medicine in Sport, 10(1), 36–44. https://doi.org/10.1016/j.jsams.2006.05.002 [DOI] [PubMed] [Google Scholar]
- Winkworth A. L., & Davis P. J. (1997). Speech breathing and the Lombard effect. Journal of Speech, Language, and Hearing Research, 40(1), 159–169. https://doi.org/10.1044/jslhr.4001.159 [DOI] [PubMed] [Google Scholar]
- Witte R. S., & Witte J. S. (2007). Statistics (8th ed.). Hoboken, NJ: Wiley. [Google Scholar]