Abstract
Purpose
Recent studies confirm the utility of speech-language intervention in primary progressive aphasia (PPA); however, long-term outcomes, ideal dosage parameters, and relative benefits of intervention across clinical variants warrant additional investigation. The purpose of this study was to determine whether naming treatment affords significant, lasting, and generalized improvement for individuals with semantic and logopenic PPA and whether dosage manipulations significantly affect treatment outcomes.
Method
Eighteen individuals with PPA (9 semantic and 9 logopenic variant) underwent lexical retrieval treatment designed to leverage spared cognitive–linguistic domains and develop self-cueing strategies to promote naming. One group (n = 10) underwent once-weekly treatment sessions, and the other group (n = 8) received the same treatment with 2 sessions per week and an additional “booster” treatment phase at 3 months post-treatment. Performance on trained and untrained targets/tasks was measured immediately after treatment and at 3, 6, and 12 months post-treatment.
Results
Outcomes from the full cohort of individuals with PPA showed significantly improved naming of trained items immediately post-treatment and at all follow-up assessments through 1 year. Generalized improvement on untrained items was significant up to 6 months post-treatment. The positive response to treatment was comparable regardless of session frequency or inclusion of a booster phase. Outcomes were comparable across PPA subtypes, as was maintenance of gains over the post-treatment period.
Conclusion
This study documents positive naming treatment outcomes for a group of individuals with PPA, demonstrating strong direct treatment effects, maintenance of gains up to 1 year post-treatment, and generalization to untrained items. Lexical retrieval treatment, in conjunction with daily home practice, had a strong positive effect that did not require more than 1 clinician-directed treatment session per week. Findings confirm that strategic training designed to capitalize on spared cognitive–linguistic abilities results in significant and lasting improvement, despite ongoing disease progression, in PPA.
Primary progressive aphasia (PPA) is a neurodegenerative disorder in which speech and language abilities deteriorate over time. A diagnosis of PPA stipulates that communication deficits are the earliest and most prominent features affecting activities of daily living; however, additional cognitive and motoric impairments emerge with disease progression (Gorno-Tempini et al., 2011; Harciarek, Sitek, & Kertesz, 2014; Mesulam, 2001). There are currently no pharmacological interventions that serve to ameliorate or protect against declining function for individuals with PPA (Tippett, Hillis, & Tsapkini, 2015). Furthermore, individuals with PPA are less likely to be referred to speech-language pathologists than individuals with aphasia caused by stroke (Taylor, Kingma, Croot, & Nickels, 2009). As is the case in Alzheimer's and other dementias, this is likely due to pessimism regarding the efficacy of behavioral intervention in neurodegenerative disease as well as a lack of understanding of the disorder by referring and treating clinicians (Paul & Mehrhoff, 2015; Swan et al., 2018). Nonetheless, a growing number of studies confirm that speech-language treatment can significantly improve communication function in PPA. The literature, which comprises single-subject and small group studies (for reviews, see Croot, Nickels, Laurence, & Manning, 2009; Jokel, Graham, Rochon, & Leonard, 2014; Kortte & Rogalski, 2013; Rising, 2014; Tippett et al., 2015), documents significant improvement for trained behaviors, with some studies showing generalized improvement for untrained items, exemplars, and tasks (Beales, Cartwright, Whitworth, & Panegyres, 2016; Beeson et al., 2011; Henry et al., 2018; Henry, Meese, et al., 2013; Henry, Rising, et al., 2013; Jokel & Anderson, 2012; Jokel et al., 2014; Jokel, Rochon, & Anderson, 2010; Meyer, Tippett, & Friedman, 2018; Newhart et al., 2009). Limited information is available regarding maintenance of gains after cessation of treatment, with a few studies documenting maintenance for 6–8 months post-treatment (Dressel et al., 2010; Henry, Meese, et al., 2013; Henry, Rising, et al., 2013; Heredia, Sage, Lambon Ralph, & Berthier, 2009; Jokel & Anderson, 2012; Jokel et al., 2010; Snowden & Neary, 2002), two studies showing stability of trained behaviors through 1 year post-treatment (Henry et al., 2018; Henry, Meese, et al., 2013), and one study documenting maintenance of items treated in a prophylactic manner (in order to prevent decline) at 15 months post-treatment (Meyer, Tippett, Turner, & Friedman, 2018).
To bolster the evidence base supporting behavioral intervention in PPA, there is a need for additional group-level studies that investigate not only treatment benefits for targeted behaviors but also generalization of treatment effects and maintenance of gains beyond the immediate post-treatment period. In addition, issues related to dosage parameters for intervention (e.g., frequency and duration of treatment) have yet to be systematically addressed in the PPA literature. As has been highlighted in the dementia treatment literature (Bourgeois et al., 2003; Cherry, Hawley, Jackson, & Boudreaux, 2009; Hopper, Drefs, Bayles, Tomoeda, & Dinu, 2010), the traditional model of a single-treatment phase may not be appropriate for individuals with cognitive–linguistic deficits caused by neurodegenerative disease, given the inevitable progression of symptoms. Furthermore, given that treatment needs and response to treatment may differ across PPA variants, studies comparing response to treatment across clinical variants are warranted.
Clinical Variants of PPA
Consensus criteria developed by an international panel of experts delineate three clinical variants of PPA: nonfluent/agrammatic variant (nfvPPA), semantic variant (svPPA), and logopenic variant (lvPPA). Each variant is associated with a unique clinical presentation, distribution of underlying brain atrophy, and neuropathological profile (Gorno-Tempini et al., 2004, 2011; Mesulam, 2001; Spinelli et al., 2017). NfvPPA is characterized by agrammatism and/or motor speech impairment, specifically apraxia of speech and sometimes dysarthria (Caso et al., 2014; Gorno-Tempini et al., 2004, 2011; Mesulam, Wieneke, Thompson, Rogalski, & Weintraub, 2012; Ogar, Dronkers, Brambati, Miller, & Gorno-Tempini, 2007; Rohrer, Rossor, & Warren, 2010; Turner, Kenyon, Trojanowski, Gonatas, & Grossman, 1996). Additional cognitive features may include mild impairments of executive function and, in some patients, episodic memory (Bettcher & Sturm, 2014; Libon et al., 2007; Rohrer, Rossor, et al., 2010), with relative sparing of semantic memory. Brain atrophy is most apparent in left frontoinsular regions and the supplementary motor area (Gorno-Tempini et al., 2011; Mandelli et al., 2016; Wilson, Dronkers, et al., 2010). The syndrome is part of the frontotemporal dementia spectrum of disorders and is most frequently associated with tau pathology at autopsy (Spinelli et al., 2017).
The semantic variant is characterized by marked word-finding deficits, single-word comprehension impairment, and loss of object knowledge, reflecting a gradual deterioration of semantic knowledge (Gorno-Tempini et al., 2011; Hodges & Patterson, 2007; Kertesz, Jesso, Harciarek, Blair, & McMonagle, 2010). Individuals with svPPA present with well-articulated, fluent speech (Hodges & Patterson, 2007; Wilson, Henry, et al., 2010) and often have relatively spared nonverbal episodic and working memory, particularly in earlier stages of disease (Eikelboom et al., 2018; Graham, Simons, Pratt, Patterson, & Hodges, 2000; Kramer et al., 2003; Pengas et al., 2010; Simons, Graham, Galton, Patterson, & Hodges, 2001). SvPPA is associated with bilateral anterior temporal lobe atrophy, which is more prominent in the left hemisphere (Gorno-Tempini et al., 2011; Hodges & Patterson, 2007). As with nfvPPA, the syndrome is part of the frontotemporal dementia spectrum, most commonly associated with TDP-43 proteinopathy at autopsy (Spinelli et al., 2017).
lvPPA is characterized by word-finding difficulties, speech sound errors without frank distortions (i.e., phonemic paraphasias), impaired repetition of phrases and sentences, and intact single-word comprehension, a presentation that is consistent with an underlying phonological impairment (Gorno-Tempini et al., 2008; Henry & Gorno-Tempini, 2010; Henry et al., 2016; Rohrer, Ridgway, et al., 2010). In contrast to nfvPPA and svPPA, respectively, individuals with lvPPA have relatively spared motor speech and semantic processing (Gorno-Tempini et al., 2008; Henry & Gorno-Tempini, 2010). Neuropsychological testing in lvPPA has revealed impairments of executive function, visuospatial skills, and episodic as well as working memory (Butts et al., 2015; Eikelboom et al., 2018). Of note, verbal recognition memory has shown relative sparing when compared to recall, the latter of which is correlated with lexical retrieval ability (Win et al., 2017). This pattern suggests a relationship between anomia and verbal memory deficits in lvPPA. Cortical atrophy in lvPPA is prominent in left temporoparietal regions, and Alzheimer's disease is the most common underlying pathology (Gorno-Tempini et al., 2011; Henry & Gorno-Tempini, 2010; Rohrer et al., 2013; Spinelli et al., 2017).
Lexical Retrieval Impairment in PPA
As with stroke-induced aphasia, naming difficulty is a pervasive feature in PPA (Grossman & Ash, 2004; Henry, Rising, et al., 2013; Mesulam, 2001; Westbury & Bub, 1997); however, lexical retrieval deficits are considered core features in svPPA and lvPPA only. Naming impairment in svPPA results from a gradual loss of conceptual knowledge. Anomia is an early and prominent feature, with single-word comprehension deficits and loss of object knowledge emerging with progression (Hodges & Patterson, 2007). Spontaneous speech in svPPA may contain semantic paraphasias, often with substitution of a more common word for the target (e.g., horse for zebra), and there is a strong frequency/familiarity effect (Bird, Lambon Ralph, Patterson, & Hodges, 2000; Hodges & Patterson, 2007).
In contrast, naming impairment in lvPPA is associated with poor phonological selection, retention, and assembly, resulting in frequent pauses during connected speech and phonological paraphasias (Gorno-Tempini et al., 2011; Henry & Gorno-Tempini, 2010; Rohrer, Ridgway, et al., 2010; Wilson, Henry, et al., 2010). In contrast to Alzheimer's dementia, which has a shared pathological substrate with lvPPA (Lehmann et al., 2013; Leyton et al., 2011; Rabinovici et al., 2008; Santos-Santos et al., 2018), the naming impairment in lvPPA is more severe (Leyton, Hodges, Piguet, & Ballard, 2017; Whitwell et al., 2015). Although Alzheimer's dementia also presents with anomia as a frequent and early sign, error profiles indicate potential differences in underlying cognitive–linguistic factors (phonological and superordinate errors are more common in lvPPA, and circumlocutions are more common in Alzheimer's dementia; Leyton et al., 2017). Thus, although there are shared features between Alzheimer's dementia and lvPPA, lvPPA presents with a distinct behavioral profile.
Treatment for Lexical Retrieval in svPPA and lvPPA
Given the prominence of naming impairment in two of the three clinical subtypes of PPA, it is not surprising that the bulk of PPA treatment research to date has focused on improving word retrieval (for reviews, see Carthery-Goulart et al., 2013; Rising, 2014; Tippett et al., 2015). Much of the literature has addressed lexical retrieval difficulties in svPPA, whereas fewer studies have implemented lexical retrieval training for lvPPA.
Most treatment approaches for svPPA have trained picture and word form pairs, seeking to strengthen semantic and phonological or orthographic representations (Graham, Patterson, Pratt, & Hodges, 1999, 2001; Heredia et al., 2009; Jokel, Rochon, & Leonard, 2006; Mayberry, Sage, Ehsan, & Lambon Ralph, 2011; Meyer, Tippett, & Friedman, 2018; Savage, Ballard, Piguet, & Hodges, 2013; Savage, Piguet, & Hodges, 2015; Snowden & Neary, 2002). Whereas these treatments typically resulted in improved naming for trained items, the gains often did not generalize to untrained items. Therefore, benefits of most interventions were found to be item and context specific, with limited maintenance post-treatment. Other studies implemented more elaborate training hierarchies to encourage the use of residual semantic, phonological, orthographic, and, in some cases, episodic/autobiographical information to promote word retrieval (Dressel et al., 2010; Henry, Rising, et al., 2013; Jokel & Anderson, 2012; Jokel et al., 2010; Newhart et al., 2009). These approaches have the potential to facilitate relearning of target vocabulary and promote generalized improvement in naming by engaging spared cognitive–linguistic systems and compensatory mechanisms. Consistent with this premise, several of these studies (all single-subject designs) documented improved naming of both trained and untrained items or tasks (Henry, Rising, et al., 2013; Jokel & Anderson, 2012; Jokel et al., 2010) as well as maintenance of gains for treated items at follow-up assessments at 2 months (Dressel et al., 2010), 3 months (Jokel et al., 2010), and 4 months post-treatment (Henry, Rising, et al., 2013).
Lexical retrieval treatment studies in lvPPA, although more limited in number, have yielded promising results (Beales et al., 2016; Beeson et al., 2011; Croot et al., 2015; Henry, Rising, et al., 2013; M. Kim, 2017; Meyer, Getz, Brennan, Hu, & Friedman, 2016; Meyer, Snider, Eckmann, & Friedman, 2015; Meyer, Tippett, & Friedman, 2018; Newhart et al., 2009). Methods for promoting word retrieval in lvPPA, like svPPA, have included semantic, phonological, and/or orthographic cueing and stimulation, with significant gains for trained items and several studies reporting generalization to untrained items (Beales et al., 2016; Beeson et al., 2011; Henry, Rising, et al., 2013; Newhart et al., 2009). Two studies (Henry, Rising, et al., 2013; Newhart et al., 2009) examined response to a naming intervention in both svPPA and lvPPA, allowing comparison of outcomes across clinical variants. Findings from these studies were comparable, documenting improvement on trained and untrained items and maintenance of gains 4–6 months following treatment for participants with both lvPPA and svPPA.
Dosage Parameters in 1 PPA Treatment
The effects of treatment dosage manipulations are currently a topic of interest in rehabilitation of individuals with chronic aphasia due to stroke (Baker, 2012; Cherney, 2012; Kleim & Jones, 2008; Warren, Fey, & Yoder, 2007). Dosage parameters, such as treatment frequency and intervention duration, can have significant effects on behavioral outcomes (for a review, see Warren et al., 2007). Recent findings suggest that dosage parameters, including session frequency, number of teaching episodes per session, and total duration of intervention, are important factors in treatment outcomes (Basso & Caporali, 2001; Dignam et al., 2015; Harnish et al., 2014; Hinckley & Craig, 1998; Lee, Kaye, & Cherney, 2009; Off, Griffin, Spencer, & Rogers, 2016; Pulvermüller et al., 2001; Purdy & Wallace, 2016). To date, treatment dosage parameters have been variable across studies, and there are no formal guidelines for optimal dosage for specific aphasia treatments and associated home practice regimens (Cherney, 2012). Questions regarding treatment dosage parameters are equally or perhaps more important in individuals with progressive aphasia. Given that PPA continues to erode the language abilities of patients over time, dosage considerations may be of critical importance, and the single treatment phase that is typical in management of stroke-induced aphasia may be inappropriate. In svPPA, revisiting treated items after an initial period of treatment has proven beneficial for longer term retention of gains (Graham et al., 2001; Savage et al., 2013), and additional “booster” doses of treatment have shown potential for enhancing long-term treatment outcomes in dementia patients who have undergone spaced retrieval training (Cherry et al., 2009; Hopper et al., 2013). However, variations in treatment delivery paradigms that may enhance long-term outcomes, including increased intensity and prolonged or phased intervention over the course of the disease, remain underexplored in PPA.
Current Study
In the current study, we examined the potential benefit of a lexical retrieval treatment designed to capitalize on spared cognitive–linguistic domains and to develop self-cueing strategies in both svPPA and lvPPA. Given the limited evidence addressing generalization and maintenance of treatment effects in PPA, we assessed performance on trained and untrained items as well as standardized tests before and immediately following treatment as well as at 3, 6, and 12 months post-treatment. We also sought to investigate the effect of treatment session frequency and multiple phases of treatment by comparing performance across two studies with different dosage paradigms: An initial study was designed to examine the feasibility and utility of the intervention approach in lvPPA and svPPA. In this first study, individuals received once-weekly intervention in a single-treatment phase. In a follow-up study, designed to investigate an optimized dosage protocol, participants received two treatment sessions per week, with a stricter criterion for advancement, and a “booster” phase of treatment after 3 months. Lastly, we aimed to determine whether treatment was equally efficacious across clinical variants by comparing treatment effects in lvPPA versus svPPA. Based on findings from our previous study (Henry, Rising, et al., 2013), we expected that treatment would result in improved naming performance for trained items in both lvPPA and svPPA. Our previous work indicates that maintenance of gains following this intervention is possible up to 6 months post-treatment (Beeson et al., 2011; Henry, Rising, et al., 2013), and with the option to continue home practice after the end of formal intervention, we predicted maintenance of gains up to 1 year post-treatment (as observed in nfvPPA; Henry et al., 2018). In addition, we predicted that increasing the number of sessions per week would enhance performance at post-treatment and that providing an additional “booster” phase of treatment following cessation of intervention would lead to better maintenance of gains at follow-up evaluations.
Method
Participant Characteristics
Participants were recruited via the University of California, San Francisco (UCSF) Memory and Aging Center's ongoing frontotemporal dementia research projects. Eighteen individuals with PPA (nine with svPPA and nine with lvPPA) were included in the study (see Table 1 for demographic information). All individuals were White/Caucasian. The data from one individual with lvPPA (lv6) were reported in a previous study through the 6-month follow-up (Henry, Rising, et al., 2013). All participants gave written informed consent, and study procedures were approved by the UCSF Committee on Human Research. Only individuals who met current diagnostic criteria for svPPA or lvPPA were included in the study (Gorno-Tempini et al., 2011). Following current criteria for PPA (Mesulam, 2001), individuals were excluded if other psychiatric, neurological, or medical diagnoses could account for their symptoms or in cases of prominent, initial nonlanguage cognitive, perceptual, or behavioral disturbance. Participants underwent neurological and neuropsychological assessment (Gorno-Tempini et al., 2004; Kramer et al., 2003) as well as magnetic resonance imaging. Diagnosis of clinical variant was reached via consensus by a multidisciplinary team comprising neurologists, neuropsychologists, speech-language pathologists, and nurses. To be considered eligible for participation in this study, individuals were required to obtain a minimum score of 15 on the Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975). Additional eligibility criteria included adequate hearing abilities to allow for accurate perception of speech (in person or via computer). When possible, an audiometric hearing screening was conducted. For participants who were seen for remote assessment and treatment, audiologic exam reports and an in-house minimal pairs discrimination task (Henry et al., 2016; performance criterion of 90% correct) were used. If hearing loss could not be accommodated using a personal amplification device or by increasing loudness via headphones (if seen via teleconference), a participant was excluded. Uncorrected visual acuity impairment that affected accurate perception of picture stimuli was also an exclusionary criterion. Lastly, individuals had to be primary speakers of English (two individuals were bilingual, but English was their dominant language). In order to include individuals who were geographically remote from or unable to travel to the research site, testing and treatment sessions were adapted to be conducted online, via HIPAA-compliant videoconferencing software (Fuze or Adobe Connect). Six participants (three svPPA and three lvPPA) completed treatment via teleconference (see Appendix A).
Table 1.
Demographic characteristics and pre-treatment performance (means and standard deviations) on cognitive and linguistic assessments.
| Demographic characteristic/assessment | All PPA |
svPPA |
lvPPA |
|---|---|---|---|
| n = 18 | n = 9 | n = 9 | |
| Sex (male/female) | 7/11 | 4/5 | 3/6 |
| Age | 65.28 (8.32) | 67.33 (8.72) | 63.22 (7.84) |
| Education (years) | 17.67 (2.74) | 18.44 (1.94) | 16.89 (3.30) |
| Reported time postonset of symptoms (years) | 4.63 (2.75) | 4.39 (2.62) | 4.86 (3.02) |
| Handedness (right/left/ambi) | 12/4/2 | 7/1/1 | 5/3/1 |
| Mini-Mental State Examination (30) | 23.67 (4.30) | 25.78 (2.22) | 21.56 (4.93) |
| CVLT Total (36) a | 17.22 (7.64) | 17.78 (7.40) | 16.67 (8.28) |
| CVLT 10-min Recall (9) a | 3.17 (2.36) | 2.56 (2.13) | 3.78 (2.54) |
| Complex Figure Copy (17) a | 14.39 (3.76) | 15.22 (1.30) | 13.56 (5.17) |
| Complex Figure Recall (17) a | 8.22 (3.34) | 9.56 (3.28) | 6.89 (2.98) |
| Complex Figure Recognition (1) a | 0.94 (0.24) | 1 (0) | 0.89 (0.33) |
| Calculations (5) a , b | 3.67 (1.24) | 4.44 (0.73) | 2.89 (1.17) |
| Digit Span Forward a , b | 5.33 (1.53) | 6.44 (0.88) | 4.22 (1.20) |
| Digits Span Backward a , b | 3.76 (1.39) | 4.55 (1.42) | 2.88 (0.64) |
| PPVT Short (16) a , b | 11.33 (4.98) | 8.78 (5.78) | 13.90 (2.15) |
| Phonemic Fluency (Letters) | 6.78 (2.76) | 8 (3.12) | 5.56 (1.74) |
| Category Fluency (Animals) | 7.11 (3.79) | 7.22 (4.18) | 7 (3.61) |
| Western Aphasia Battery AQ (100) | 81.36 (9.55) | 84.84 (8.85) | 77.88 (9.38) |
| Motor Speech Eval: AOS (0–7) | 0.17 (0.71) | 0 (0) | 0.33 (1) |
| Motor Speech Eval: Dysarthria (0–7) | 0 (0) | 0 (0) | 0 (0) |
| Pyramids and Palm Trees Test (52) | 47.79 (6.00) | 45.33 (7.60) | 50.25 (2.25) |
| Boston Naming Test (%) | 41.07 (25.66) | 28.25 (21.12) | 53.89 (21.20) |
| UCSF Syntax Comprehension Test (%) b | 93.37 (7.38) | 97.32 (2.64) | 89.43 (8.58) |
| Arizona Phonological Battery (%) | 58.32 (23.98) | 67.36 (23.71) | 46.28 (20.09) |
Note. PPA = primary progressive aphasia; svPPA = semantic variant primary progressive aphasia; lvPPA = logopenic variant primary progressive aphasia; ambi = ambidextrous; CVLT = California Verbal Learning Test; PPVT = Peabody Picture Vocabulary Test; AQ = Aphasia Quotient; Motor Speech Eval = Motor Speech Evaluation (Wertz et al., 1984; 0 = no impairment, 7 = profound impairment); AOS = apraxia of speech; Pyramids and Palm Trees Test (Howard & Patterson, 1992); University of California, San Francisco (UCSF) Syntax Comprehension Test (Wilson, Dronkers, et al., 2010); Arizona Phonological Battery (Beeson et al., 2010).
Indicates assessments from neuropsychological battery described in Kramer et al. (2003), Knopman et al. (2008), and Staffaroni et al. (2019).
Significant differences (p < 0.05) between the diagnostic groups following independent-samples permutation tests.
Pre-treatment evaluations comprised cognitive and linguistic assessments (see Table 1). The svPPA group demonstrated poor confrontation naming and impaired single-word comprehension, with spared repetition, motor speech, and grammar. Those diagnosed with lvPPA demonstrated impaired naming in spontaneous speech and confrontation naming, production of phonemic paraphasias, and impaired phonological working memory (digit span and repetition). Motor speech, grammatical abilities, single-word comprehension, and object knowledge were relatively spared. Independent-samples permutation tests were used to compare baseline performance across clinical groups. These tests revealed that participants with svPPA performed better on calculations, forward and backward digit span, and syntax comprehension relative to those with a diagnosis of lvPPA. The lvPPA group performed significantly better on single-word comprehension than the group with svPPA.
Neuroimaging
Structural magnetic resonance images were collected at pre-treatment for 17 participants (nine svPPA and eight lvPPA). Voxel-based morphometry (see Henry et al., 2016, for methodological details) was used to determine regions of significant atrophy in each group relative to 30 age-matched healthy controls (see Figure 1). This analysis revealed atrophy patterns consistent with those reported in the literature for each diagnostic group (Gorno-Tempini et al., 2004, 2011; Mesulam et al., 2012; Rohrer, Rossor, & Warren, 2012; Teichmann et al., 2013). Specifically, prominent anterior temporal lobe atrophy (left hemisphere greater than right) was revealed in the svPPA group. By contrast, the lvPPA group demonstrated significant atrophy in the left temporoparietal cortex, with extension into the anterior and inferior temporal lobe.
Figure 1.
Voxel-based morphometry analysis comparing gray and white matter volumes in nine participants with semantic primary progressive aphasia (A) and eight participants with logopenic primary progressive aphasia (B) relative to 30 healthy controls (p < 0.001, uncorrected; covariates include age, sex, and total intracranial volume) Color bars represent t values.
Treatment Procedures
The lexical retrieval training approach was consistent across all participants in the study, but two different training schedules were utilized in order to investigate the effect of dosage modifications on treatment outcomes. The treatment approach will be outlined first, followed by a description of each dosage condition. The intervention, initially developed at the University of Arizona (Lexical Retrieval Cascade Treatment—here, shortened to Lexical Retrieval Treatment, or LRT), utilized a hierarchy of tasks designed to encourage strategic recruitment of spared semantic, orthographic, and phonological knowledge as well as episodic/autobiographical information in order to facilitate word retrieval via self-cueing (Henry, Rising, et al., 2013; see Table 2). The training hierarchy incorporates elements of semantic feature analysis (Boyle, 2004; Boyle & Coelho, 1995; Coelho, McHugh, & Boyle, 2000) as well as phonemic/orthographic cueing techniques (Best, Herbert, Hickin, Osborne, & Howard, 2010; Best, Howard, Bruce, & Gatehouse, 2008; Bruce & Howard, 1987) and other elements of traditional cueing hierarchies utilized in stroke aphasia (Maher & Raymer, 2004; Nickels & Best, 1996). During a treatment session, each of five items in the current set was addressed, in turn, using the following hierarchy of steps, and completion of one iteration of the hierarchy for a single item was considered a single naming trial (see Table 2).
Table 2.
Lexical retrieval training hierarchy (“Lexical Retrieval Cascade Treatment”; Henry, Rising, et al., 2013).
| Treatment step | Procedure | Clinician instructions |
|---|---|---|
| 1. Semantic self-cue | Picture presented; clinician prompts semantic description and/or episodic/autobiographical information | What can you tell me about it? (Where do you find it? What is it used for? What is it made of?) |
| 2. Orthographic self-cue | Clinician prompts written form of the word (or the first letter) | Can you write the word? Can you write the first letter? |
| 3. Phonemic self-cue | Clinician prompts initial phoneme | What sound does that letter make? What is the first sound of the word? |
| 4. Oral reading | If the item is not yet named, clinician provides written word form and participant reads aloud | Here is the word. Can you read it? |
| 5. Written and spoken repetition | Participant writes and says the word three times | Now write and say the word (three times). |
| 6. Semantic plausibility judgment | Clinician asks five semantic plausibility questions | E.g., Is it spicy? Do you buy it at the farmer's market? |
| 7. Recall | Participant provides two semantic features and writes and says the word | Now tell me two important things about this item. What is this called? Can you write it? |
For each item currently in training, a picture of the target was presented for spoken naming, and the participant was guided through generation of semantic/episodic, orthographic, and phonological information, with modeling from the clinician, as needed. Initially, a semantic feature chart was used to introduce the idea of semantic circumlocution, and use of the diagram was gradually faded out as participants became more adept at providing conceptual information for targets. Individuals were instructed to provide typical semantic features in response to standard questions, (e.g., Where do you find it? What is it used for? What is it made of?) but were also encouraged to provide any relevant episodic or autobiographical information that might aid in retrieval of the target (e.g., “I put this in my smoothie every morning”). Following semantic feature analysis, retrieval of residual orthographic (whole word if possible; if not, first letter) and phonological (initial sound) information was requested. If the individual was unable to generate any orthographic or phonological information, the first letter of the target item was provided, and the participant was asked to generate the associated sound. When necessary, the clinician provided a model of the initial phoneme. If the individual was still unable to retrieve the target word, then a written model of the word was provided for oral reading. If the participant was unable to read the target word aloud, then a spoken model was provided. Following these steps, the spoken and written words were produced three times to prompt multimodal rehearsal of the target. Five (yes/no) semantic plausibility judgment questions were then posed (e.g., “Do you keep this in a refrigerator?”). The questions were intended to provide additional semantic stimulation and to create a delay before the individual was prompted to name the item again. Finally, two salient semantic features were elicited, and the spoken and written names of the target were recalled. If the individual was able to name the target at any step in the hierarchy of tasks, they continued with the remaining steps (excluding semantic judgment questions).
For daily homework, participants were provided additional opportunities for spoken and written rehearsal of target lexical items using Copy and Recall Treatment (CART; Beeson & Egnor, 2006). The copy and recall task was structured so that participants viewed a picture of the target and its associated written form. They were instructed to copy the written word and generate the spoken word aloud 10 times. The final step involved recall of the spoken and written words from memory. Homework was typically completed in 15 minutes or less. To assure understanding, the clinician observed the participant completing one page of CART homework before the first homework set was assigned. In subsequent sessions, written homework pages were reviewed for accuracy of completion. Each individual was provided with a homework log to document completion of CART homework.
Dosage Paradigms
Details of the two dosage paradigms are outlined in Table 3. Ten individuals (five svPPA, five lvPPA) were enrolled for naming treatment that was implemented during once-a-week treatment sessions with the clinician (hereafter referred to as Lexical Retrieval Training 1 [LRT1]). The treatment protocol was directed toward naming of 20 of 25 items that were quasirandomly assigned to five sets of five items (four trained sets and one untrained set). Participants met with the clinician once weekly for a 1 hour session (live or via videoconferencing), and all sets were probed at the beginning of each session. Each set was trained until an 80% (four of five) criterion was met, with a maximum of two sessions per set. Total intervention duration lasted between four and eight sessions (weeks), and treatment was completed, on average, in 4–5 weeks (M = 4.7).
Table 3.
Dosage and other treatment parameters for participants who received less intensive training and no booster phase (LRT1 protocol) and those who received more intensive training and a booster phase (LRT2 protocol).
| Protocol | Treatment targets | Dose (teaching episodes per session) | Dose form | Criterion for advancement to new set of items | Session duration/frequency | Homework | Phase 1 intervention duration (varied by participant) | Booster phase (Phase 2) | Average total hours of intervention (varied by participant; Phase 1 + 2) |
|---|---|---|---|---|---|---|---|---|---|
| LRT1 (n = 10) | 4 sets of 5 words = 20 | Approximately one trial a per word per session (5 words total) | Lexical retrieval cascade | 80% correct on current set | One 1-hr session per week | CART: approximately 15 min per day = 1.75 hr/week | M = 4.7 sessions/weeks | None | Phase 1: M = 4.7 hr + 8.2 hr homework Phase 2: N/A |
| LRT2 (n = 8) | 8 sets of 5 words = 40 | Approximately one trial per word per session (5 words total) | Lexical retrieval cascade | 100% correct on current set | Two 1-hr sessions per week | CART: Approximately 15 min per day = 1.75 hr/week | M = 11.4 sessions over 5.6 weeks | At 3 months post-treatment: 2 hr/week for 4 weeks = 8 hr | Phase 1: M = 11.4 hr of treatment + 9.8 hr homework Phase 2: M = 8 hr of treatment + 7 hr homework |
Note. LRT = Lexical Retrieval Training; CART = Copy and Recall Treatment; N/A = not applicable.
Note that one trial involves completion of the full hierarchy described in Table 2, which provides multiple opportunities to produce/rehearse the target word form.
A similar treatment protocol was implemented with eight other individuals (four svPPA, four lvPPA). The clinician-directed treatment sessions (1 hr each) were scheduled twice per week, rather than once; as such, the protocol is referred to as LRT2. Because previously collected data using the LRT1 protocol (e.g., Henry, Rising, et al., 2013) confirmed that participants with both lvPPA and svPPA could consistently reach criterion performance for 20 items with one treatment session per week, we doubled the number of treatment items for LRT2 (40 items in the training set and 10 matched, untrained items), with the prediction that twice the number of items would be attainable with the increase in treatment frequency. Items were quasirandomly assigned to 10 sets of five items (eight trained and two untrained sets). Half of all trained and untrained items, including the current set in training, were probed at each session. Each set was trained until a 100% criterion was met, with a maximum of two sessions per set. Treatment duration was between 4 and 8 weeks (or 8–16 sessions), and the average number of sessions was between 11 and 12 sessions (M = 11.4) over an average of 5.6 weeks. Therefore, the total duration of the first phase of the two dosing paradigms (LRT1 and LRT2) was similar. Homework requirements were also identical across protocols.
In addition, the group who received the LRT2 protocol completed a second phase of treatment following the 3-month follow-up visit. During this “booster” phase, the original set of trained target items was reviewed using the same training hierarchy, with one set trained per session (two sessions per week, for a total of eight additional 1-hr training sessions for the original treatment items) and daily CART homework.
One clinician implemented all LRT1 treatment (M. H.), and another clinician implemented all LRT2 treatment (H. H.). Independent-samples permutation tests comparing those who received the LRT1 training schedule to those who received the LRT2 training schedule revealed no pre-treatment differences in age, education, Boston Naming Test score (BNT; Kaplan, Goodglass, & Weintraub, 2001), or MMSE score (p > 0.05).
Treatment Target Selection
The goal of treatment was to train words that were functionally and personally relevant to each participant while affording practice with word-finding strategies that are broadly beneficial. Initially, participants were asked to generate a set of items of functional importance that were consistently difficult to name, and photographs of those items were collected. When possible, pictures of patients' own personal objects were used rather than generic exemplars. If the individual was unable to generate a sufficient number of items for treatment, the set was supplemented by the clinician. Spoken naming performance was probed over three testing sessions before treatment began. If the person was unable to name a picture on at least two of three occasions, the item was eligible for training. Potential treatment targets were quasirandomized into to-be-trained and untrained sets of items. When possible, items that were orthographically/phonologically similar or semantically related (e.g., from same superordinate category) were not placed in the same set. Sets were balanced for word length (number of syllables and number of letters), frequency, familiarity, and imageability. Psycholinguistic parameters were obtained from the Medical Research Council Psycholinguistic Database (Coltheart, 1981; Wilson, 1988) and the Corpus of Contemporary American English (Davies, 2009).
Self-Assessment of Change Following Treatment
A 20-item post-treatment survey (similar to that used in Henry, Rising, et al., 2013) was administered to LRT2 participants in order to assess individuals' perceptions regarding their communication status at post-treatment relative to pre-treatment. They rated perceived change relative to pre-treatment status on a Likert-type scale, with responses ranging from “a lot worse” to “a lot better.” Questions addressed a number of parameters, including ability to name trained items, ability to detect and correct naming errors in conversation, and perceived stress level during conversation. Individuals enrolled in the study were of mild–moderate impairment, and the survey questions were designed to be easily comprehended by persons with aphasia. When administering the survey, the first question was administered as an example, and options for responding were explained. After that example, the clinician left the room/exited the online meeting, and the participant was instructed to fill out the remaining questions independently. Individuals were told that the clinician would go over any challenging questions at the end of the survey. In general, this was not required.
Follow-Up Assessments
In addition to pre- and post-treatment assessments, follow-up testing was conducted at 3, 6, and 12 months post-treatment when possible. All 18 participants completed pre-treatment and post-treatment evaluations, and the majority completed 3-month (n = 16), 6-month (n = 16), and 12-month (n = 17) follow-up evaluations. Two individuals from the LRT1 group could not be tested at 3 months post-treatment, as one participant passed away and one was unavailable. The latter was able to complete additional testing at 6 and 12 months post-treatment. One person in the LRT2 cohort was unavailable for 6-month follow-up testing. After formal treatment ended, participants were allowed to keep their training materials. Ongoing practice with trained items was encouraged, and those in the LRT2 group were asked to track their practice via a written log, which was collected at each follow-up assessment.
Outcome Measures and Statistical Analysis
Primary outcome measures for statistical analysis at the group level were naming of trained and untrained targets, which was assessed via probes collected during baseline, treatment, and follow-up phases for each individual. Secondary outcome measures included the Western Aphasia Battery (WAB; LRT1 group; Kertesz, 1982) or the WAB–Revised (LRT2 group; Kertesz, 2006) to examine stability of general language abilities and the BNT (Kaplan et al., 2001) to examine overall spoken naming ability. The full BNT was administered at pre- and post-treatment, and equivalent short (15-item or 30-item) versions were administered at follow-up testing sessions, with overlapping subsets of items used for statistical comparisons. There was no overlap between trained or untrained items from the treatment protocol and BNT items.
The distributions of variables did not meet the assumption of normality, so nonparametric permutation tests were conducted with the exactRankTests package (Hothorn & Hornik, 2006) in R (Version 3.3.1, 2016) using a complete enumeration of all possible permutations. T values are reported as well as exact significance levels determined by permutation. Bonferroni correction was used to control for familywise error. Each variable was considered a family of tests for comparisons to pre-treatment performance and a separate family of tests for comparisons to post-treatment performance, and correction was applied across time points within each variable of interest.
In order to evaluate direct treatment effects at the group level, pre-treatment performance (average of three probes immediately prior to treatment) was compared to post-treatment (average of two probes immediately after training on all sets was completed) and all follow-up time points (one probe obtained at each assessment) using paired-samples permutation tests. One-tailed tests were used to examine naming of trained sets at post-treatment and all follow-up assessments (3, 6, and 12 months post-treatment) relative to pre-treatment, as we predicted significant and lasting improvement for these items. Two-tailed tests were used to compare pre- to post-treatment performance for untrained sets and other secondary outcome measures (BNT score and WAB Aphasia Quotient [AQ]), as generalization was less predictable. In addition, maintenance of treatment effects was examined by comparing post-treatment performance to each follow-up assessment using two-tailed paired-samples permutation tests in order to determine stability of treatment effects over time. These analyses were initially conducted for the entire treated group (including both diagnostic subtypes and individuals receiving both dosage paradigms). Additionally, to evaluate changes in cognitive status over the course of the study, we compared MMSE scores at 3-, 6-, and 12-month follow-ups to pre- and post-treatment in the full group of participants using two-tailed paired samples permutation tests.
Subsequently, we examined the effect of dosage paradigm (LRT1 vs. LRT2 training schedules) on treatment outcomes. To directly compare the magnitude of the treatment effect across dosage paradigms, change scores were compared between the two dosage groups. We predicted greater gains with increased treatment intensity at post-treatment and better maintenance of trained items at 3 months post-treatment and following the additional booster phase at the 6- and 12-month follow-ups. As such, one-tailed independent-samples permutation tests were used for all comparisons examining trained item change scores. Two-tailed tests were used for all remaining contrasts. Change scores were calculated by subtracting average pre-treatment performance from average performance at each post-treatment assessment. Maintenance change scores were also calculated by subtracting post-treatment performance from performance at subsequent time points. In order to determine whether response to treatment differed in clinical subgroups, we compared change scores from pre- and post-treatment to each subsequent time point across the two diagnostic groups (lvPPA and svPPA) using two-tailed independent-samples permutation tests.
In order to estimate a standard index of change in response to treatment at the individual level, effect sizes were calculated using a weighted (by number of observations) average of d-statistics from trained sets and untrained sets (see Beeson & Robey, 2006, for calculation details). For this study, the pre-treatment mean included three pre-treatment probes, and the post-treatment mean included all probes collected after training on a given set was completed.
Finally, Pearson correlation analyses were conducted to determine whether change scores representing an individual's performance at each follow-up time point relative to post-treatment were related to amount of reported practice (for LRT2 participants).
Results
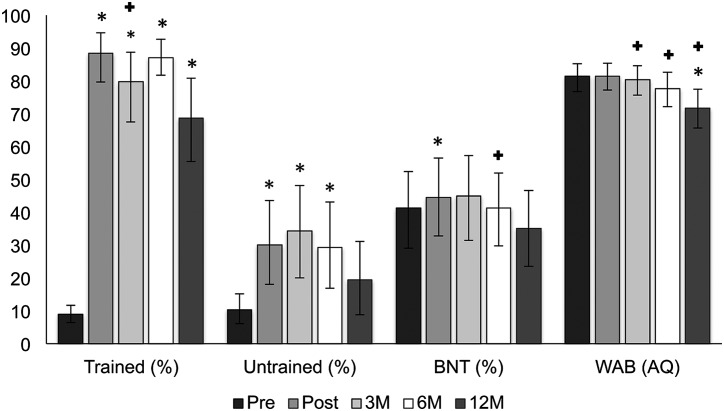
Outcomes From the Full Cohort of Individuals With PPA
Performance on primary and secondary outcome measures over time in the full group of participants with PPA is shown in Figure 2. Permutation tests confirmed significant improvement from pre- to post-treatment for trained items, untrained items, and BNT (see statistical details in Table 4). No significant change was observed for WAB AQ scores from pre- to post-treatment. To assess maintenance, performance at each follow-up time point was compared to pre-treatment and immediately post-treatment scores using additional permutation tests. Naming of trained items remained significantly better than pre-treatment at all follow-up assessments. Relative to post-treatment, a significant decline in naming of trained items was observed at 3 months post-treatment.
Figure 2.
Performance on trained and untrained sets of words as well as standardized tests over time in the full group of participants with primary progressive aphasia. 95% confidence intervals around the mean were derived using n = 1,000 bootstrapped samples. Significant permutation test, with Bonferroni correction, denoted: * = significant difference relative to pre-treatment; + = significant difference relative to post-treatment. Trained = trained items; Untrained = untrained items; BNT = Boston Naming Test; WAB = Western Aphasia Battery; AQ = Aphasia Quotient; Pre = pre-treatment; Post = post-treatment; 3M = 3 months post-treatment; 6M = 6 months post-treatment; 12M = 12 months post-treatment.
Table 4.
Results of paired permutation tests comparing treatment outcome measures and standardized tests at each time point relative to pre-treatment and post-treatment for all participants (both clinical variants and both treatment dosage groups).
| Pre-treatment performance vs. |
Post-treatment performance vs. |
|||||||
|---|---|---|---|---|---|---|---|---|
| Post-treatment | 3-month follow-up | 6-month follow-up | 12-month follow-up | 3-month follow-up | 6-month follow-up | 12-month follow-up | ||
| Trained | n | 18 | 16 | 16 | 17 | 16 | 16 | 17 |
| t | −17.8 | −11.5 | −24.9 | −8.4 | 2.7 | 2.2 | 3.6 | |
| p | < 0.001* | < 0.001* | < 0.001* | < 0.001* | 0.013* | 0.714 | 0.031 | |
| Untrained | n | 18 | 16 | 16 | 17 | 16 | 16 | 17 |
| t | −3.1 | −3.6 | −2.6 | −1.3 | −0.1 | 0.4 | 1.6 | |
| p | 0.003* | < 0.001* | < 0.001* | 0.448 | 0.967 | 0.723 | 0.129 | |
| BNT | n | 18 | 15 | 14 | 14 | 16 | 14 | 15 |
| t | −1.9 | 0.8 | 2.4 | 3.0 | 1.5 | 4.2 | 3.7 | |
| p | 0.003* | 0.427 | 0.053 | 0.053 | 0.714 | 0.012* | 0.027 | |
| WAB | n | 18 | 16 | 16 | 15 | 16 | 16 | 15 |
| t | −0.1 | 2.4 | 3.2 | 5.1 | 3.7 | 3.7 | 5.5 | |
| p | 0.724 | 0.061 | 0.061 | < 0.001* | 0.013* | 0.003* | < 0.001* | |
| MMSE | n | 18 | 15 | 16 | 15 | 15 | 16 | 15 |
| t | −0.2 | −0.2 | 1.9 | 3.0 | 0.9 | 2.8 | 4.0 | |
| p | 0.868 | 0.934 | 0.090 | 0.004* | 0.408 | 0.008* | < 0.001* | |
Note. One-tailed tests were used for trained items for all comparisons to pre-treatment performance. All other comparisons utilized two-tailed tests. Trained = trained items; Untrained = untrained items; BNT= Boston Naming Test; WAB = Western Aphasia Battery.
Bonferroni-corrected thresholds for significance: p < 0.013 for comparisons to pre-treatment performance and p < 0.017 for comparisons to post-treatment performance.
Performance on untrained items remained significantly better than pre-treatment through the 6-month follow-up, and there were no significant differences for untrained items relative to post-treatment at any follow-up. A direct comparison of trained versus untrained sets at each time point revealed equivalent performance at pre-treatment and significantly better performance on trained sets at all other time points (see Table 5). Spoken naming performance on the BNT showed relative stability compared to pre-treatment at the 3-, 6-, and 12-month follow-ups. Compared to post-treatment, BNT scores showed a significant decline at the 6-month follow-up only. Relative to pre-treatment, performance on the WAB showed a significant decline at 12 months post-treatment. Compared to post-treatment, WAB scores showed a significant decline at each follow-up. MMSE scores did not change from pre- to post-treatment but did gradually decline at each follow-up thereafter. This decline was significant relative to pre-treatment at one year post-treatment. MMSE decline relative to post-treatment was significant at six months and one year post-treatment (Table 4).
Table 5.
Results of permutation tests for paired data comparing performance between trained and untrained sets at each time point.
| Pre-treatment | Post-treatment | 3-month follow-up | 6-month follow-up | 12-month follow-up | |
|---|---|---|---|---|---|
| n | 18 | 18 | 16 | 16 | 17 |
| t | −0.7 | 7.4 | 5.5 | 7.5 | 6.3 |
| p | 0.724 | < 0.001* | < 0.001* | < 0.001* | < 0.001* |
Note. Two-tailed tests were used.
Bonferroni-corrected thresholds for significance: p < 0.01.
Comparison of LRT1 Versus LRT2: Effects of Treatment Intensity and Multiple Treatment Phases
Performance on primary and secondary outcome measures in each dosage group is presented in Figure 3. Performance within each of the dosage groups followed a similar pattern as that of the full group of participants (statistical details for within–dosage group comparisons are reported in Appendix B). Findings include significant improvement, as well as maintenance of gains relative to pre-treatment for trained items up to 1 year post-treatment. Some degree of generalized improvement was noted for each protocol (BNT at post-treatment in the group that received less frequent training; LRT1 protocol) and untrained items at 3 months post-treatment in the group that received more frequent training (LRT2 protocol). Our primary interest for this study, however, was the comparison of outcomes between dosage groups. Permutation tests comparing change scores between the two dosage paradigms are presented in Table 6. There were no significant differences in change scores between the two groups at any time point with correction for multiple comparisons. 2
Figure 3.
Performance on trained and untrained sets of words as well as standardized tests over time in participants from each dosage protocol. 95% confidence intervals around the mean were derived using n = 1,000 bootstrapped samples. Significant permutation test, with Bonferroni correction, denoted: for within-group comparisons, * = significant difference relative to pre-treatment; + = significant difference relative to post-treatment. No significant between-groups differences in change scores. LRT1 = Lexical Retrieval Treatment Group 1; LRT2 = Lexical Retrieval Treatment Group 2; Trained = trained items; Untrained = untrained items; BNT= Boston Naming Test; WAB = Western Aphasia Battery; AQ = Aphasia Quotient; Pre = pre-treatment; Post = post-treatment; 3M = 3 months post-treatment; 6M = 6 months post-treatment; 12M = 12 months post-treatment.
Table 6.
Results of permutation tests comparing change scores between the two dosing paradigms (LRT1 vs. LRT2).
| Change score from pre-treatment to |
Change score from post-treatment to |
|||||||
|---|---|---|---|---|---|---|---|---|
| Post-treatment | 3-month follow-up | 6-month follow-up | 12-month follow-up | 3-month follow-up | 6-month follow-up | 12-month follow-up | ||
| Trained | n | 18 | 16 | 16 | 17 | 16 | 16 | 17 |
| t | 1.5 | 1.5 | 0.9 | 1.4 | 0.3 | −0.3 | 0.4 | |
| p | 0.950 | 0.920 | 0.806 | 0.922 | 0.606 | 0.411 | 0.634 | |
| Untrained | n | 18 | 16 | 16 | 17 | 16 | 16 | 17 |
| t | −2.3 | −0.8 | −1.4 | −0.5 | 0.8 | 0.7 | 1.3 | |
| p | 0.037 | 0.505 | 0.179 | 0.663 | 0.504 | 0.577 | 0.231 | |
| BNT | n | 16 | 14 | 13 | 13 | 14 | 12 | 12 |
| t | 1.1 | −0.6 | 0.7 | −1.4 | 0.0 | 0.3 | −0.4 | |
| p | 0.283 | 0.685 | 0.532 | 0.255 | 1.000 | 1.000 | 0.691 | |
| WAB | n | 18 | 16 | 16 | 15 | 16 | 16 | 15 |
| t | −2.3 | −0.9 | −1.4 | −0.6 | 0.9 | −0.1 | 0.0 | |
| p | 0.035 | 0.429 | 0.172 | 0.729 | 0.382 | 0.870 | 0.961 | |
Note. One-tailed tests were utilized in comparing trained item change scores. All other comparisons utilized two-tailed tests. Bonferroni-corrected thresholds for significance: p < 0.013 for comparisons to pre-treatment performance and p < 0.017 for comparisons to post-treatment performance. Differences in n for Boston Naming Test (BNT) due to comparing an equivalent, frequency-matched set of items across LRT1 and LRT2. Because an equivalent item set could not be derived, two participants (sv6, who received LRT1, and sv1, who received LRT2) were not included in between-subjects comparisons. Trained = trained items; Untrained = untrained items; LRT = Lexical Retrieval Training; WAB = Western Aphasia Battery.
Outcomes by Clinical Variant
Performance on primary and secondary outcome measures in each diagnostic group (svPPA and lvPPA) is presented in Figure 4. Performance within each group followed the pattern of the overall group, with significant improvement and maintenance of gains for trained items relative to pre-treatment up to 1 year post-treatment (statistical details for within–clinical variant group comparisons are reported in Appendix C). Of primary interest for this study, however, was the comparison of outcomes between clinical variants. Permutation tests for independent data comparing change scores between the two clinical variants did not reveal any significant differences (see Table 7).
Figure 4.
Performance on trained and untrained sets of words as well as standardized tests over time in each clinical subtype. 95% confidence intervals around the mean were derived using n = 1,000 bootstrapped samples. Significant permutation test, with Bonferroni correction, denoted: for within-group comparisons, * = significant difference relative to pre-treatment; + = significant difference relative to post-treatment. No significant between-groups differences in change scores. SvPPA = semantic variant primary progressive aphasia; lvPPA = logopenic variant primary progressive aphasia; Trained = trained items; Untrained = untrained items; BNT= Boston Naming Test; WAB = Western Aphasia Battery; AQ = Aphasia Quotient; Pre = pre-treatment; Post = post-treatment; 3M = 3 months post-treatment; 6M = 6 months post-treatment; 12M = 12 months post-treatment.
Table 7.
Results of permutation tests comparing change scores between the semantic (n = 9) and logopenic (n = 9) variants of primary progressive aphasia.
| pre-treatment performance vs. |
post-treatment performance vs. |
|||||||
|---|---|---|---|---|---|---|---|---|
| post-treatment | 3-month follow-up | 6-month follow-up | 12-month follow-up | 3-month follow-up | 6-month follow-up | 12-month follow-up | ||
| Trained | n | 18 | 16 | 16 | 17 | 16 | 16 | 17 |
| t | −0.2 | 0.0 | 1.1 | 1.2 | 0.1 | −0.2 | 1.8 | |
| p | 0.952 | 0.959 | 0.291 | 0.238 | 0.956 | 0.916 | 0.122 | |
| Untrained | n | 18 | 16 | 16 | 17 | 16 | 16 | 17 |
| t | 0.4 | 0.6 | 1.3 | 1.9 | 0.4 | 0.6 | 1.0 | |
| p | 0.773 | 0.636 | 0.179 | 0.088 | 0.787 | 0.603 | 0.378 | |
| BNT | n | 16 | 14 | 13 | 13 | 14 | 12 | 12 |
| t | −1.1 | −1.0 | −1.6 | 0.9 | −0.5 | −1.2 | 1.0 | |
| p | 0.350 | 0.495 | 0.175 | 0.502 | 0.691 | 0.317 | 0.369 | |
| WAB | n | 18 | 16 | 16 | 15 | 16 | 16 | 15 |
| t | −1.1 | 0.5 | 1.8 | 1.4 | 2.0 | 2.3 | 1.9 | |
| p | 0.280 | 0.694 | 0.120 | 0.162 | 0.070 | 0.035 | 0.077 | |
Note. All comparisons utilized two-tailed tests for independent data sets. Bonferroni-corrected thresholds for significance: p < 0.013 for comparisons to pre-treatment performance and p < 0.017 for comparisons to post-treatment performance. Trained = trained items; Untrained = untrained items; BNT= Boston Naming Test; WAB = Western Aphasia Battery.
Individual Response to Treatment
All 10 individuals who received the LRT1 protocol met the established criterion of 80% correct for each trained set. Seven of 10 participants who received the LRT2 protocol met the criterion of 100% correct for all trained sets. Effect sizes confirmed that individual response to treatment (see Appendix A) was generally quite robust for trained sets (mean d = 7.22, SD = 3.80, range: 1.52–19.36). Effect sizes for untrained items were small or negative (mean d = 1.06, SD = 1.36, range: −0.58 to 4.55).
Relation Between Ongoing Practice Post-treatment and Stability of Scores of Over Time
We sought to examine the relation between continued home practice and treatment effects over time (in the LRT2 group; follow-up practice data were not systematically collected in the LRT1 group). Specifically, Pearson correlation analysis was used to examine the relation between change score (relative to post-treatment) and number of practice sessions reported at each follow-up assessment (see Appendix A for cumulative practice data). Correlations were not significant for trained or untrained items at 3 months post-treatment (trained items r = −0.03, p = 0.95; untrained items r = −0.02, p = 0.96), 6 months post-treatment (trained items r = −0.33, p = 0.47; untrained items r = −0.24, p = 0.60), or 1 year post-treatment (trained items r = −0.57, p = 0.14; untrained items r = −0.45, p = 0.26).
Self-Assessment of Change
The results of the self-assessment survey in the LRT2 group were tallied such that a report of “a lot worse” was assigned a value of −3 and a report of “a lot better” was assigned +3 (see Figure 5). A score of 0 represented a self-report of “no change.” The mean participant rating across all items was 0.86, which corresponds to a rating between “unchanged” and “somewhat better.” The mean response in the svPPA group was 0.52, corresponding to a report between “unchanged” and “somewhat better.” The lvPPA group showed a higher mean response (1.2), corresponding to a rating between “somewhat better” and “better.”
Figure 5.
Mean response for post-treatment self-assessment survey (LRT2 participants). Scale: −3 = a lot worse; −2 = worse; −1 = somewhat worse; 0 = unchanged; 1 = somewhat better; 2 = better; 3 = a lot better. lvPPA = logopenic variant primary progressive aphasia; svPPA = semantic variant primary progressive aphasia.
Discussion
Findings from this study contribute to the growing evidence base supporting the utility of speech-language intervention for individuals with PPA. Lexical retrieval treatment was found to improve naming for individuals with svPPA and lvPPA, with evidence for maintenance of gains and generalization to untrained items. Relative to pre-treatment, performance on treated items was significantly improved at post-treatment and remained so following the cessation of formal treatment (over a 12-month period in the group who received LRT1; over a 9-month period following the booster phase in the group who received LRT2). Not only does this indicate that lexical retrieval treatment is beneficial for individuals with PPA, but it also suggests that learning can be maintained, with ongoing practice, despite disease progression.
Effects of Dosage Paradigm
At present, there are no guidelines for treatment dosage parameters in PPA. Whereas our initial cohort of 10 individuals demonstrated significant improvement as a result of intervention, we sought to determine the effects of dosage modifications intended to enhance treatment outcomes in a second group of eight participants. Specifically, we varied treatment intensity by manipulating session frequency as well as total number of sessions/hours and cumulative intervention duration, with other dosage parameters (e.g., dose, dose form, and session duration) held constant across protocols (see Table 3). It is noteworthy that, despite the difference in treatment frequency for clinician-moderated practice (one session per week vs. two sessions per week), the intensity of home practice was constant across the two protocols in the initial study phase. The fact that we did not detect greater improvement for trained items with more frequent treatment sessions may indicate that the additional treatment session did not confer additional potency above and beyond the daily practice that was afforded by CART homework, which was implemented in both protocols.
Furthermore, the apparent lack of additional benefit from increased treatment provided in LRT2 versus LRT1 prompts additional consideration of the strategic nature of the treatment. All participants were trained to implement the self-cueing procedures during instances of lexical retrieval difficulty. Ideally, use of the strategies becomes habitual across a broad range of communication contexts, not simply during structured treatment tasks. This scenario would afford considerable stimulation and practice well beyond the clinician-directed sessions and the structured homework. If the once-weekly sessions with daily homework of LRT1 were adequate to establish use of the lexical retrieval strategies and to prompt generalization to untrained items, then it is possible that there was little need for additional training offered in LRT2. Indeed, generalization effects were evident on linguistically matched, untrained items and on the BNT, which probes a broad range of word frequencies. It is not possible to determine with certainty whether the strategic nature of the training, in conjunction with daily homework, served to equalize intervention potency across the two protocols, but it is a potential mechanism that warrants further consideration.
Individuals in the LRT2 group received a booster phase of treatment after the 3-month post-treatment testing session, wherein previously trained vocabulary and naming strategies were revisited. This additional stimulation did not affect outcomes relative to the LRT1 group at subsequent follow-ups, with between-groups comparisons (at 6 months and 1 year post-treatment), revealing no significant differences following the second phase of treatment. It is surprising that additional training did not result in better maintenance of gains relative to the LRT1 group, but this does not necessarily confirm that further training and practice are not beneficial following an initial treatment episode in PPA. It is possible that additional training does confer some benefits but that our study lacks sufficient power to detect an effect. Furthermore, it may be the case that continued practice with mastered items in the second phase of treatment was not as beneficial as training of new items may have been. Introduction of novel or more difficult items (i.e., items that could not consistently be named) in the second phase of treatment may have led to improved performance at subsequent follow-ups. Increased cognitive engagement mandated by training of difficult items may be a critical element in producing the robust treatment effects and generalized improvement in naming that were observed following the first phase of treatment. The impact of varying dosage parameters and potential benefits of including previously trained versus untrained sets in a treatment “booster” warrant additional investigation.
Direct Treatment Effects in svPPA Versus lvPPA
In a previous study, we documented positive response to this lexical retrieval training paradigm in two patients, one with lvPPA and the other with svPPA (Henry, Rising, et al., 2013). The “Lexical Retrieval Cascade Treatment” (here, lexical retrieval treatment) was designed to engage each of the central components of language processing, leveraging spared cognitive–linguistic functions to promote self-cueing and word retrieval, with the potential to benefit participants with naming deficits caused by different underlying mechanisms. Consistent with the original two cases, there was no difference in treatment response in svPPA versus lvPPA at any time point for the larger group of participants reported here. Our findings confirm that this intervention is broadly beneficial for word retrieval deficits in PPA, whether caused by semantic or phonological impairment. Although there are clear differences in clinical presentation in lvPPA versus svPPA, there is also heterogeneity within each of the clinical variants, relating not only to stage of disease but also to the specific topography of neurodegeneration in each individual PPA patient. Effect sizes across participants in this study were generally robust, indicating that lexical retrieval treatment has the potential to benefit most lvPPA and svPPA patients in the mild-to-moderate (MMSE > 15) range of disease severity that was investigated here. Again, this speaks to the broad applicability of this treatment approach and its potential to benefit patients with varying clinical profiles. Future research should explore cognitive–linguistic and neural predictors of treatment outcomes for this and other treatment approaches in order to optimize treatment candidacy decision making in PPA.
Maintenance of Treatment Gains
We report maintenance of gains for trained targets up to 1 year post-treatment and up to 6 months post-treatment for untrained targets. We allowed ongoing practice with trained items in the post-treatment period, with cumulative reported practice at 1 year post-treatment varying considerably across individuals (LRT2; see Appendix A). Untrained targets were not made available for home practice. To date, the majority of studies investigating naming treatment for PPA do not report maintenance data beyond the immediate post-treatment period. Furthermore, of those that do report longer-term maintenance (Beeson et al., 2011; Dressel et al., 2010; Henry, Rising, et al., 2013; Heredia et al., 2009; Jokel & Anderson, 2012; Jokel et al., 2010; Meyer et al., 2015; Meyer, Tippett, Turner, et al., 2018; Snowden & Neary, 2002), only two studies (Heredia et al., 2009; Meyer, Tippett, Turner, et al., 2018) indicate whether ongoing practice with trained items occurred, with both studies reporting that it did not. We did not find a significant relation between post-treatment practice frequency and naming performance over time; however, we only had records of post-treatment practice frequency for eight of our 18 participants; moreover, practice frequency was obtained via self-report, which may not accurately reflect the frequency of practice. Future studies should systematically collect data regarding frequency and duration of home practice during the maintenance phase in order to address the role of ongoing practice in maintenance of treatment gains subsequent to structured intervention with a clinician.
Consistent with the neurodegenerative nature of PPA, we observed a decline in MMSE score over time through the 1-year follow-up period. Thus, the maintenance of treatment gains that we observed is set against a backdrop of ongoing cognitive decline. Our findings confirm that, despite the relentless progression of cognitive and language symptoms, the benefits of skilled, individually tailored intervention are robust and lasting.
Generalization of Treatment Gains
Generalization of acquired communication skills beyond the specific tasks and targets addressed in treatment is an important component of any speech-language intervention. In this study, generalized improvement in naming was observed for untrained targets and also on the BNT at post-treatment. We attribute the generalized improvement of naming in our participants with PPA to the strategic nature of the intervention, which encourages self-cueing via systematic retrieval of residual linguistic knowledge (semantic, phonological, and orthographic information), and episodic/autobiographical information to promote word retrieval. Importantly, performance on untrained items (relative to post-treatment) was maintained through the 12-month follow-up assessment in the full PPA group.
Of note is the observation that the svPPA group showed improvement on untrained items (although not statistically significant with multiple comparisons correction) and maintained performance on these items through the 1-year follow-up. This is a significant finding, given the variability in generalization documented in previous studies of this clinical group. A number of studies have reported improved naming in svPPA for trained items following simple name-to-picture or name-to-definition rehearsal; however, results did not typically generalize to untrained items or exemplars (Graham et al., 1999, 2001; Heredia et al., 2009; Jokel, Rochon, & Leonard, 2002; Jokel et al., 2006; Mayberry et al., 2011; Savage et al., 2013; Snowden & Neary, 2002). This has led some authors to suggest that relearning of vocabulary in svPPA is reliant on episodic memory, a compensatory mechanism thought to facilitate only item-specific, context-bound gains (Croot et al., 2009; Mayberry et al., 2011; Savage et al., 2013; Snowden & Neary, 2002).
In contrast, several studies utilized more elaborated cueing hierarchies that encouraged retrieval of residual semantic, orthographic, phonological, and episodic/autobiographical information. These studies, despite reporting data from participants with svPPA with a similar degree of naming impairment relative to the above studies, documented generalized improvement in naming (Henry, Rising, et al., 2013; Jokel & Anderson, 2012; Jokel et al., 2010; Newhart et al., 2009). In these studies, episodic memory, in conjunction with residual linguistic knowledge, may have served as a vehicle for improved lexical retrieval that is neither item specific nor context bound. Recalling episodic/autobiographical information may serve to improve naming and generalization by shifting reliance from the integration of specific semantic features of a concept to use of personalized contextual information as a substrate for word learning. Multicomponent cueing hierarchies like the one used here and in other studies documenting generalized improvement in naming in svPPA may successfully link episodic detail with residual orthographic and phonological information to compensate for a damaged semantic system. Findings from these studies support the notion that strategic recall of episodic information, in conjunction with spared linguistic knowledge, may facilitate the naming and retention of both trained and untrained vocabulary in svPPA.
In the current study, the use of episodic/autobiographical information was further promoted by targeting naming of individually tailored, functional item sets, utilizing pictures of participants' own objects when possible. In single cases, Snowden et al. (Snowden, Griffiths, & Neary, 1994; Snowden & Neary, 2002) found that participants with svPPA were better able to relearn names of meaningful words relative to less personally meaningful items. Autobiographical experience has also been shown to improve performance on object use and manipulation tasks (Bozeat, Lambon, Ralph, Patterson, & Hodges, 2002;Bozeat, Patterson, Hodges, & Unit, 2004). Furthermore, several previous naming treatment studies that employed personally meaningful targets (Henry, Rising, et al., 2013; Jokel et al., 2002, 2006) noted a reliance on personally relevant cues or autobiographical information to recall words. These findings, in conjunction with the robust outcomes from the current study, suggest that use of personalized stimuli with enhanced autobiographical significance may promote strategy usage and word retrieval for both trained and untrained items.
Improved naming of untrained items was also noted in the lvPPA group (although not statistically significant with multiple comparisons correction). Generalized improvement of naming ability in lvPPA may result from engaging spared semantic associations for targets (Henry, Rising, et al., 2013; Newhart et al., 2009), in conjunction with residual orthographic/phonological information, a strategy that may benefit both trained and untrained vocabulary. In addition, guided practice with semantic circumlocution may improve the specificity and efficiency with which semantic information is provided during circumlocution. As a result, circumlocutory attempts for untrained items may become more precise and informative, enhancing their communicative value. In this study, use of personally relevant items for both trained and untrained conditions ensured that participants had robust conceptual representations from which to draw, enhancing the potency of practice and the potential for carryover.
Additional Considerations
Restitutive interventions such as the lexical retrieval treatment intervention described here may not be appropriate beyond the mild-to-moderate range of severity that was targeted in the current study. Future intervention studies should continue to investigate interventions appropriate across the full spectrum of clinical phenotypes and stages of disease progression in PPA, incorporating multimodality communication techniques, augmentative and alternative communication, and communication partner training.
Assessment and treatment data for six of our 18 participants were collected via a telemedicine platform. The application of “teletherapy” allowed us to recruit and treat a larger cohort of participants than would otherwise have been possible. Furthermore, teletherapy allowed participants with geographical constraints or mobility limitations to participate in treatment in the comfort of their own homes. In a recent study, we report a comparison of immediate and long-term treatment outcomes from in-person treatment compared to treatment administered via teletherapy (Dial et al., 2019). The participants' data that we report here were part of that larger data set, which comprised individuals with each of the variants of PPA who either underwent naming treatment (lvPPA and svPPA) or script training (nfvPPA; Henry et al., 2018). Findings from that study indicate comparable outcomes immediately post-treatment and up to 1 year post-treatment for individuals receiving teletherapy compared to those who received in-person treatment. We contend that telemedicine represents a critical means to broaden the reach of clinicians offering services to individuals with PPA and other communication disorders.
We acknowledge several limitations of the current study. First, although our full group of 18 individuals with PPA represents one of the largest treatment cohorts in the literature, the subgroups that were compared for between-groups (clinical cohort and dosage parameter) comparisons were relatively small. Future studies should attempt to recruit and report findings from larger samples in order to amass generalizable evidence regarding the utility and feasibility of language treatment in PPA broadly and across clinical variants. Second, we acknowledge that we did not collect treatment fidelity data for the interventions that were implemented. Only two clinicians were involved in treatment implementation, both of whom underwent extensive training regarding accurate delivery of the treatment; however, future studies should collect and report fidelity data so that outcomes may be considered in the context of factors affecting overall study quality. Third, although we attribute our large treatment effect sizes, in part, to the combined benefit of in-session practice with the clinician as well as daily home practice, we did not systematically collect information regarding frequency and duration of home practice during the training period. Future studies should attempt to capture frequency and duration of home practice using digital means in order to examine the relation between amount of home practice and immediate and long-term treatment outcomes. Lastly, although we collected full neuropsychological assessment data at pre-treatment, only MMSE was conducted at subsequent assessments. We observed that MMSE did not change from pre- to post-treatment but did gradually decline at each follow-up assessment thereafter. We acknowledge, however, that the MMSE is a language-biased instrument and that it is a gross screening tool, rather than a detailed assessment of relevant cognitive functions. As such, we are unable to determine the extent to which changes in cognitive function more broadly may have contributed to long-term outcomes in our participants. Future PPA treatment studies that address maintenance should collect data on cognitive status (particularly, memory and executive function) at follow-up assessments in order to examine how nonlinguistic cognitive functions relate to long-term treatment outcomes.
Conclusion
Systematic efforts to strengthen the evidence regarding the utility of behavioral rehabilitation for progressive disorders of cognition and communication have resulted in great strides over the last two decades. A significant and growing body of research has documented the benefits of targeted, skilled interventions in individuals with Alzheimer's and related dementias and has also addressed important considerations with regard to treatment optimization, including dosage and modality of treatment delivery (Bayles et al., 2006; Mahendra et al., 2006; Hopper et al., 2005, 2013; E. S. Kim et al., 2006; Mahendra et al., 2005; Zientz, Rackley, Chapman, Hopper, Mahendra, & Cleary, 2007; Zientz, Rackley, Chapman, Hopper, Mahendra, Kim, et al., 2007). This work has paved the way for similar investigations of treatments designed to promote and maintain communicative function in PPA.
Individuals with PPA, their caregivers, and third-party reimbursers seek efficacious intervention options that offer more than item-specific effects or temporary gains. In this study, we document significantly improved word retrieval, with generalization of treatment gains and maintenance (relative to pre-treatment status) up to 1 year post-treatment in individuals with svPPA and lvPPA. These results, in conjunction with qualitative reports of improved communication in our participants, strengthen the evidence base supporting speech-language treatment in PPA by confirming that treatment in this population can result in robust, generalized, and lasting improvement, despite ongoing neurodegeneration and worsening of aphasic symptoms. In light of these findings and those of previous intervention studies in progressive aphasia, behavioral intervention for speech and language should be viewed by the clinical and research communities as a necessary, rather than optional, component of care for individuals with PPA. Future studies should continue to develop and optimize approaches to intervention, including additional investigation of dosage manipulations, in order to maximize outcomes and promote communicative well-being for individuals with this debilitating condition.
Acknowledgments
This work was funded by the National Institute on Deafness and Other Communication Disorders (NIDCD) Grants F32DC010945, R01 DC016291 and R03 DC013403 (awarded to M. H.), National Institute of Neurological Disorders and Stroke Grant R01 NS050915 and NIDCD Grant K24 DC015544 (awarded to M. G. T.), National Institute on Aging Grants P01 AG019724 and P50 AG023501 (awarded to B. M.), NIDCD Grant R01 DC007647 (awarded to P. B.) and the Darrell K Royal Research Fund for Alzheimer's Disease (awarded to M. H.). We thank the members of the University of Texas, Austin Aphasia Research and Treatment Lab and the University of California, San Francisco Memory and Aging Center who contributed to all aspects of patient care. Special thanks to Mithra Sathishkumar, who contributed to magnetic resonance imaging analysis; Lindsey Wineholt and Miranda Babiak, who aided with patient assessment and data management; Holly Hinshelwood and Ariane Welch, who contributed to data management; and Greg Hixon for statistical consultation. Finally, we wish to thank all of our participants with primary progressive aphasia for the time and effort that they have devoted to our research.
Appendix A
Individual Participants' Performance on Trained and Untrained Word Sets and Standardized Assessments at Each Study Time Point
| Participant | Score | Pre-treatment | Post-treatment | d-stat | 3 months | 6 months | 12 months | Total no. of practice sessions reported during maintenance (LRT2 participants only) |
|---|---|---|---|---|---|---|---|---|
| sv1 LRT2 |
Trained | 5.83 | 92.50 | 7.16 | 57.50 | 95.00 | 90.00 | 17.00 |
| Untrained | 13.33 | 35.00 | 0.98 | 20.00 | 60.00 | 40.00 | ||
| BNT | 63.41 | 65.85 | . | 48.33 | 50.00 | 36.67 | ||
| WAB | 91.50 | 91.80 | . | 93.80 | 94.40 | 85.20 | ||
| MMSE | 27.00 | 28.00 | . | 26.00 | 29.00 | 25.00 | ||
| sv2 LRT2 TT |
Trained | 3.33 | 96.25 | 10.56 | 95.00 | 87.50 | 80.00 | 15.00 |
| Untrained | 3.33 | 90.00 | 4.55 | 80.00 | 80.00 | 60.00 | ||
| BNT | 10.00 | 13.33 | . | 16.67 | 3.33 | 10.00 | ||
| WAB | 76.00 | 80.50 | . | 76.20 | 72.30 | 70.50 | ||
| MMSE | 24.00 | 25.00 | . | 22.00 | 24.00 | 20.00 | ||
| sv3 LRT2 |
Trained | 10.00 | 76.25 | 4.27 | 75.00 | 82.50 | 82.50 | 58.00 |
| Untrained | 16.67 | 40.00 | 1.81 | 70.00 | 30.00 | 10.00 | ||
| BNT | 56.67 | 53.33 | . | 46.67 | 26.67 | 43.33 | ||
| WAB | 89.00 | 88.30 | . | 88.50 | 90.30 | 84.00 | ||
| MMSE | 26.00 | 26.00 | . | 27.00 | 28.00 | 26.00 | ||
| sv4 LRT2 |
Trained | 11.67 | 25.00 | 1.52 | 15.00 | N/C | 2.50 | 5.00 |
| Untrained | 10.00 | 20.00 | 1.15 | 10.00 | N/C | 10.00 | ||
| BNT | 18.33 | 15.00 | . | 10.00 | N/C | 0.00 | ||
| WAB | 82.20 | 79.70 | . | 74.60 | N/C | 63.50 | ||
| MMSE | 21.00 | 20.00 | . | 22.00 | N/C | 18.00 | ||
| sv5 LRT1 |
Trained | 8.33 | 80.00 | 5.54 | N/C | N/C | N/C | N/C |
| Untrained | 6.67 | 0.00 | −0.58 | N/C | N/C | N/C | ||
| BNT | 5.00 | 3.30 | . | N/C | N/C | N/C | ||
| WAB | 65.90 | 60.70 | . | N/C | N/C | N/C | ||
| MMSE | 25.00 | 17.00 | . | N/C | N/C | N/C | ||
| sv6 LRT1 TT |
Trained | 0.00 | 100.00 | 19.36 | 100.00 | 100.00 | 100.00 | N/C |
| Untrained | 0.00 | 0.00 | 0.00 | 80.00 | 80.00 | 80.00 | ||
| BNT | 32.50 | 32.50 | . | 40.00 | 60.00 | 60.00 | ||
| WAB | 88.40 | 87.70 | . | 84.90 | 84.50 | 73.40 | ||
| MMSE | 28.00 | 27.00 | . | 28.00 | 27.00 | 25.00 | ||
| sv7 LRT1 |
Trained | 5.00 | 100.00 | 8.95 | 80.00 | 100.00 | 100.00 | N/C |
| Untrained | 0.00 | 50.00 | 1.18 | 0.00 | 0.00 | 0.00 | ||
| BNT | 40.00 | 45.00 | . | 53.30 | 33.30 | 40.00 | ||
| WAB | 94.00 | 95.00 | . | 92.00 | 93.20 | 91.50 | ||
| MMSE | 27.00 | 29.00 | . | 30.00 | 28.00 | 28.00 | ||
| sv8 LRT1 |
Trained | 3.33 | 97.50 | 10.02 | 100.00 | 80.00 | 65.00 | N/C |
| Untrained | 6.67 | 0.00 | −0.58 | 0.00 | 0.00 | 0.00 | ||
| BNT | 16.67 | 21.67 | . | 13.30 | 20.00 | 13.30 | ||
| WAB | 87.70 | 86.00 | . | 86.80 | 83.00 | 82.40 | ||
| MMSE | 26.00 | 30.00 | . | 30.00 | 24.00 | 21.00 | ||
| sv9 LRT1 TT |
Trained | 6.67 | 92.50 | 8.77 | 90.00 | 65.00 | 75.00 | N/C |
| Untrained | 0.00 | 20.00 | 2.19 | 20.00 | 0.00 | 20.00 | ||
| BNT | 11.70 | 15.00 | . | 13.30 | 6.70 | 13.30 | ||
| WAB | 88.90 | 86.60 | . | 87.80 | 83.10 | 80.30 | ||
| MMSE | 28.00 | 30.00 | . | 27.00 | 26.00 | 23.00 | ||
| lv1 LRT2 |
Trained | 2.50 | 83.75 | 7.17 | 95.00 | 82.50 | 35.00 | 79.00 |
| Untrained | 6.67 | 5.00 | −0.01 | 20.00 | 10.00 | 0.00 | ||
| BNT | 3.33 | 15.00 | . | 5.00 | 10.00 | N/C | ||
| WAB | 68.10 | 74.90 | . | 71.10 | 70.20 | 62.40 | ||
| MMSE | 16.00 | 21.00 | . | 21.00 | 19.00 | 18.00 | ||
| lv2 LRT2 TT |
Trained | 23.33 | 96.25 | 3.63 | 85.00 | 100.00 | 75.00 | 92.00 |
| Untrained | 20.00 | 70.00 | 1.84 | 50.00 | 30.00 | 60.00 | ||
| BNT | 78.33 | 83.33 | . | 86.67 | 70.00 | 73.33 | ||
| WAB | 83.60 | 89.60 | . | 84.50 | 83.70 | 63.80 | ||
| MMSE | 26.00 | 28.00 | . | 21.00 | 22.00 | 10.00 | ||
| lv3 LRT2 TT |
Trained | 11.67 | 95.00 | 8.04 | 75.00 | 80.00 | 50.00 | 169.00 |
| Untrained | 33.33 | 80.00 | 2.63 | 70.00 | 70.00 | 30.00 | ||
| BNT | 71.67 | 78.33 | . | 80.00 | 66.67 | 46.67 | ||
| WAB | 87.40 | 85.90 | . | 83.70 | 83.30 | 78.80 | ||
| MMSE | 19.00 | 23.00 | . | 23.00 | 23.00 | 19.00 | ||
| lv4 LRT2 |
Trained | 15.00 | 91.25 | 5.18 | 82.50 | 70.00 | 62.50 | 51.00 |
| Untrained | 30.00 | 70.00 | 1.75 | 50.00 | 50.00 | 20.00 | ||
| BNT | 71.67 | 58.33 | . | 66.67 | 53.33 | 56.67 | ||
| WAB | 75.80 | 79.60 | . | 70.90 | 61.00 | N/C | ||
| MMSE | 18.00 | 22.00 | . | 15.00 | 10.00 | N/C | ||
| lv5 LRT1 |
Trained | 10.00 | 97.50 | 6.62 | N/C | 100.00 | 80.00 | N/C |
| Untrained | 20.00 | 0.00 | 0.10 | N/C | 0.00 | 0.00 | ||
| BNT | 76.70 | 73.30 | . | N/C | 46.70 | 6.70 | ||
| WAB | 80.40 | 77.50 | . | N/C | 67.10 | 49.40 | ||
| MMSE | 27.00 | 25.00 | . | N/C | 22.00 | 22.00 | ||
| lv6 LRT1 TT |
Trained | 6.67 | 90.00 | 7.09 | 95.00 | 85.00 | 60.00 | N/C |
| Untrained | 0.00 | 20.00 | 2.21 | 40.00 | 20.00 | 0.00 | ||
| BNT | 45.00 | 50.00 | . | 80.00 | 53.30 | 40.00 | ||
| WAB | 69.40 | 72.60 | . | 66.70 | 57.50 | 49.10 | ||
| MMSE | 20.00 | 9.00 | . | 15.00 | 10.00 | 4.00 | ||
| lv7 LRT1 |
Trained | 10.00 | 100.00 | 5.45 | 95.00 | 90.00 | 20.00 | N/C |
| Untrained | 6.67 | 0.00 | −0.58 | 0.00 | 0.00 | 0.00 | ||
| BNT | 45.00 | 51.70 | . | 40.00 | N/C | N/C | ||
| WAB | 80.00 | 75.20 | . | 73.90 | 68.80 | N/C | ||
| MMSE | 15.00 | 15.00 | . | N/C | 13.00 | N/C | ||
| lv8 LRT1 |
Trained | 11.67 | 85.00 | 5.84 | 75.00 | 95.00 | 95.00 | N/C |
| Untrained | 0.00 | 0.00 | 0.00 | 0.00 | 20.00 | 0.00 | ||
| BNT | 53.30 | 73.30 | . | 73.30 | 66.70 | 66.70 | ||
| WAB | 64.00 | 66.60 | . | 66.00 | 66.60 | 63.60 | ||
| MMSE | 26.00 | 28.00 | . | 27.00 | 25.00 | 26.00 | ||
| lv9 LRT1 |
Trained | 16.67 | 92.50 | 4.75 | 60.00 | 80.00 | 95.00 | N/C |
| Untrained | 13.33 | 40.00 | 0.44 | 40.00 | 20.00 | 0.00 | ||
| BNT | 40.00 | 46.70 | . | 46.70 | 53.30 | 20.00 | ||
| WAB | 92.20 | 88.40 | . | 84.00 | 82.30 | 76.60 | ||
| MMSE | 27.00 | 27.00 | 27.00 | 13.00 | 13.00 |
Note. sv = semantic variant; lv = logopenic variant; LRT2 = Lexical Retrieval Training Protocol 2; LRT1 = Lexical Retrieval Training Protocol 1; Trained = percent correct for trained items; Untrained = percent correct for untrained items; BNT = percent correct on Boston Naming Test (Full BNT was administered at pre- and post-treatment and equivalent short [15-item or 30-item] versions were administered at follow-up testing sessions. Only equivalent subsets of items were used for statistical comparisons across time points and groups.); WAB = Western Aphasia Battery (AQ out of 100); MMSE = Mini-Mental State Examination (out of 30); TT = received treatment via teletherapy; N/C = not collected.
Appendix B
Statistical Details for Within-Dosage Group Comparisons
Table B1.
Results of paired permutation tests comparing outcome measures and standardized tests at each time point relative to pre-treatment and post-treatment for Lexical Retrieval Training Protocol 1.
| Pre-treatment performance vs. |
Post-treatment performance vs. |
|||||||
|---|---|---|---|---|---|---|---|---|
| Post-treatment | 3-month follow-up | 6-month follow-up | 12-month follow-up | 3-month follow-up | 6-month follow-up | 12-month follow-up | ||
| Trained | n | 10 | 8 | 9 | 9 | 8 | 9 | 9 |
| t | −28.1 | −12.1 | −17.7 | −7.7 | 1.7 | 1.8 | 2.0 | |
| p | < 0.001* | 0.004* | 0.002* | 0.002* | 0.672 | 1.000 | 1.000 | |
| Untrained | n | 10 | 8 | 9 | 9 | 8 | 9 | 9 |
| t | −1.2 | −1.8 | −1.1 | −0.6 | −0.5 | −0.1 | 0.3 | |
| p | 0.184 | 0.047 | 0.086 | 0.359 | 0.750 | 1.000 | 0.875 | |
| BNT | n | 10 | 8 | 8 | 8 | 8 | 8 | 8 |
| t | −2.4 | 0.7 | 1.7 | 2.0 | 1.3 | 4.0 | 2.7 | |
| p | 0.012* | 0.672 | 0.172 | 0.672 | 0.672 | 0.047 | 0.172 | |
| WAB | n | 10 | 8 | 9 | 8 | 8 | 9 | 8 |
| t | 1.6 | 2.5 | 3.6 | 3.4 | 2.3 | 3.5 | 3.5 | |
| p | 0.184 | 0.047 | 0.023 | 0.016 | 0.172 | 0.023 | 0.016* | |
Note. One-tailed tests were used for trained items for all comparisons to pre-treatment performance. All other comparisons utilized two-tailed tests. Trained = trained items; Untrained = untrained items; BNT = Boston Naming Test; WAB = Western Aphasia Battery.
Bonferroni-corrected thresholds for significance: p < 0.013 for comparisons to pre-treatment performance and p < 0.017 for comparisons to post-treatment performance.
Table B2.
Results of paired permutation tests comparing outcome measures and standardized tests at each time point relative to pre-treatment and post-treatment for Lexical Retrieval Training Protocol 2.
| Pre-treatment performance vs. |
Post-treatment performance vs. |
|||||||
|---|---|---|---|---|---|---|---|---|
| Post-treatment | 3-month follow-up | 6-month follow-up | 12-month follow-up | 3-month follow-up | 6-month follow-up | 12-month follow-up | ||
| Trained | n | 8 | 8 | 7 | 8 | 8 | 7 | 8 |
| t | −8.1 | −6.3 | −17.6 | −4.6 | 2.0 | 1.2 | 3.3 | |
| p | 0.004* | 0.004* | 0.008* | 0.008* | 0.047 | 1.00 | 0.047 | |
| Untrained | n | 8 | 8 | 7 | 8 | 8 | 7 | 8 |
| t | −3.5 | −3.3 | −3.0 | −1.3 | 0.8 | 1.1 | 3.1 | |
| p | 0.016 | 0.008* | 0.016 | 0.672 | 0.508 | 0.344 | 0.023 | |
| BNT | n | 8 | 7 | 6 | 6 | 8 | 6 | 7 |
| t | −0.4 | 0.4 | 1.6 | 3.3 | 0.7 | 2.1 | 2.4 | |
| p | 0.172 | 0.344 | 0.688 | 0.188 | 0.672 | 0.688 | 0.344 | |
| WAB | n | 8 | 8 | 7 | 7 | 8 | 7 | 7 |
| t | −1.6 | 1.0 | 1.0 | 4.1 | 2.9 | 1.9 | 4.2 | |
| p | 0.172 | 0.672 | 0.344 | 0.031 | 0.172 | 0.344 | 0.031 | |
Note. One-tailed tests were used for trained items for all comparisons to pre-treatment performance. All other comparisons utilized two-tailed tests. Trained = trained items; Untrained = untrained items; BNT = Boston Naming Test; WAB = Western Aphasia Battery.
Bonferroni-corrected thresholds for significance: p < 0.013 for comparisons to pre-treatment performance and p < 0.017 for comparisons to post-treatment performance.
Appendix C
Statistical Details for Within-Clinical Variant Group Comparisons
Table C1.
Results of paired permutation tests comparing outcome measures and standardized tests at each time point relative to pre-treatment and post-treatment for semantic primary progressive aphasia.
| Pre-treatment performance vs. |
Post-treatment performance vs. |
|||||||
|---|---|---|---|---|---|---|---|---|
| Post-treatment | 3-month follow-up | 6-month follow-up | 12-month follow-up | 3-month follow-up | 6-month follow-up | 12-month follow-up | ||
| Trained | n | 9 | 8 | 7 | 8 | 8 | 7 | 8 |
| t | −8.8 | −6.3 | −15.2 | −5.7 | 1.9 | 1.4 | 2.2 | |
| p | 0.002* | 0.004* | 0.008* | 0.008* | 0.172 | 0.344 | 0.672 | |
| Untrained | n | 9 | 8 | 7 | 8 | 8 | 7 | 8 |
| t | −2.2 | −2.3 | −2.1 | −1.9 | −0.2 | −0.1 | 0.3 | |
| p | 0.023 | 0.016 | .031 | 0.047 | 0.813 | 0.938 | 0.813 | |
| BNT | n | 9 | 7 | 6 | 7 | 8 | 6 | 8 |
| t | −1.2 | 1.7 | 2.3 | 2.4 | 2.3 | 2.0 | 3.1 | |
| p | 0.086 | 1.000 | 0.188 | 1.000 | 0.672 | 0.688 | 0.672 | |
| WAB | n | 9 | 8 | 7 | 8 | 8 | 7 | 8 |
| t | 0.9 | 1.6 | 1.7 | 4.2 | 1.4 | 1.6 | 4.7 | |
| p | 0.359 | 0.172 | 0.344 | 0.016 | 0.672 | 0.344 | 0.016* | |
Note. One-tailed tests were used for trained items for all comparisons to pre-treatment performance. All other comparisons utilized two-tailed tests. Trained = trained items; Untrained = untrained items; BNT = Boston Naming Test; WAB = Western Aphasia Battery.
Bonferroni-corrected thresholds for significance: p < 0.013 for comparisons to pre-treatment performance and p < 0.017 for comparisons to post-treatment performance.
Table C2.
Results of paired permutation tests comparing outcome measures and standardized tests at each time point relative to pre-treatment and post-treatment for logopenic primary progressive aphasia.
| Pre-treatment performance vs. |
Post-treatment performance vs. |
|||||||
|---|---|---|---|---|---|---|---|---|
| Post-treatment | 3-month follow-up | 6-month follow-up | 12-month follow-up | 3-month follow-up | 6-month follow-up | 12-month follow-up | ||
| Trained | n | 9 | 8 | 9 | 9 | 8 | 9 | 9 |
| t | −39.1 | −12.0 | −20.8 | −6.7 | 1.9 | 1.6 | 3.2 | |
| p | 0.002* | 0.004* | 0.002* | 0.002* | 0.172 | 0.359 | 0.086 | |
| Untrained | n | 9 | 8 | 9 | 9 | 8 | 9 | 9 |
| t | −2.0 | −3.4 | −1.8 | 0.4 | 0.4 | 1.2 | 2.7 | |
| p | 0.086 | 0.016 | 0.023 | 0.359 | 0.813 | 0.313 | 0.031 | |
| BNT | n | 9 | 8 | 8 | 7 | 8 | 8 | 7 |
| t | −1.6 | 0.0 | 1.1 | 2.4 | 0.4 | 5.2 | 2.9 | |
| p | 0.023 | 0.172 | 0.672 | 0.094 | 0.672 | 0.047 | 0.094 | |
| WAB | n | 9 | 8 | 9 | 7 | 8 | 9 | 7 |
| t | −0.7 | 1.7 | 2.9 | 3.7 | 4.3 | 3.9 | 4.3 | |
| p | 0.359 | 0.672 | 0.359 | 0.031 | 0.016* | 0.023 | 0.031 | |
Note. One-tailed tests were used for trained items for all comparisons to pre-treatment performance. All other comparisons utilized two-tailed tests. Trained = trained items; Untrained = untrained items; BNT = Boston Naming Test; WAB = Western Aphasia Battery.
Bonferroni-corrected thresholds for significance: p < 0.013 for comparisons to pre-treatment performance and p < 0.017 for comparisons to post-treatment performance.
Funding Statement
This work was funded by the National Institute on Deafness and Other Communication Disorders (NIDCD) Grants F32DC010945, R01 DC016291 and R03 DC013403 (awarded to M. H.), National Institute of Neurological Disorders and Stroke Grant R01 NS050915 and NIDCD Grant K24 DC015544 (awarded to M. G. T.), National Institute on Aging Grants P01 AG019724 and P50 AG023501 (awarded to B. M.), NIDCD Grant R01 DC007647 (awarded to P. B.) and the Darrell K Royal Research Fund for Alzheimer's Disease (awarded to M. H.).
Footnotes
By “dosage parameters,” we refer to multiple parameters that contribute to overall treatment intensity, as discussed by Cherney (2012) and Warren et al. (2007). Dosage parameters include factors such as dose (number of teaching episodes per session), dose form, session duration and frequency, total number of sessions, total intervention duration, and cumulative intervention intensity. In this study, session frequency, total number of sessions, and cumulative intervention duration were specific parameters of interest.
The two dosage protocols differed in total number of items trained (20 items for LRT1 and 40 items for LRT2). In order to compare treatment gains while controlling for both baseline performance and differences in total number of items trained, we conducted a post hoc analysis using a derived metric referred to as “proportion of potential maximal gain” (Lambon Ralph, Snell, Fillingham, Conroy, & Sage, 2010). This metric was calculated as (post-treatment naming accuracy − baseline naming accuracy) / (number of items trained − baseline naming). Independent-samples permutation tests comparing proportion of potential maximal gain in LRT1 versus LRT2 at post-treatment and each follow-up yielded identical results relative to the analysis of change scores derived from percentages (all p values greater than the Bonferroni-corrected threshold of p < 0.0125). Thus, comparisons across the two training protocols when controlling for number of items trained confirmed no differences in treatment gains.
References
- Baker E. (2012). Optimal intervention intensity. International Journal of Speech-Language Pathology, 14(5), 401–409. https://doi.org/10.3109/17549507.2012.700323 [DOI] [PubMed] [Google Scholar]
- Basso A., & Caporali A. (2001). Aphasia therapy or the importance of being earnest. Aphasiology, 15(4), 307–332. https://doi.org/10.1080/02687040042000304 [Google Scholar]
- Bayles K. A., Kim E., Chapman S. B., Zientz J., Rackley A., Mahendra N., … Cleary S. J. (2006). Evidence-based practice recommendations for working with individuals with dementia: Simulated presence therapy. Journal of Medical Speech-Language Pathology, 14(3), xiii–xxi. [Google Scholar]
- Beales A., Cartwright J., Whitworth A., & Panegyres P. K. (2016). Exploring generalisation processes following lexical retrieval intervention in primary progressive aphasia. International Journal of Speech-Language Pathology, 18(3), 299–314. https://doi.org/10.3109/17549507.2016.1151936 [DOI] [PubMed] [Google Scholar]
- Beeson P. M., & Egnor H. (2006). Combining treatment for written and spoken naming. Journal of the International Neuropsychological Society, 12(6), 816–827. https://doi.org/10.1017/S1355617706061005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson P. M., King R. M., Bonakdarpour B., Henry M. L., Cho H., & Rapcsak S. Z. (2011). Positive effects of language treatment for the logopenic variant of primary progressive aphasia. Journal of Molecular Neuroscience, 45(3), 724–736. https://doi.org/10.1007/s12031-011-9579-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson P. M., Rising K., Kim E. S., & Rapcsak S. Z. (2010). A treatment sequence for phonological alexia/agraphia. Journal of Speech, Language, and Hearing Research, 53(2), 450–468. https://doi.org/10.1044/1092-4388(2009/08-0229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson P. M., & Robey R. R. (2006). Evaluating single-subject treatment research: Lessons learned from the aphasia literature. Neuropsychology Review, 16(4), 161–169. https://doi.org/10.1007/s11065-006-9013-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best W., Herbert R., Hickin J., Osborne F., & Howard D. (2010). Phonological and orthographic facilitation of word-retrieval in aphasia: Immediate and delayed effects. Aphasiology, 16(1–2), 151–168. https://doi.org/10.1080/02687040143000483 [Google Scholar]
- Best W., Howard D., Bruce C., & Gatehouse C. (2008). A treatment for anomia combining semantics, phonology and orthography. In Language disorders in children and adults: Psycholinguistic approaches to therapy (pp. 102–129). London, England: Whurr Publishers; https://doi.org/10.1002/9780470699157.ch7 [Google Scholar]
- Bettcher B. M., & Sturm V. E. (2014). Neuropsychological assessment of primary progressive aphasia (PPA). SIG 2 Perspectives on Neurophysiology and Neurogenic Speech and Language Disorders, 24(4), 128–136. https://doi.org/10.1044/nnsld24.4.128 [Google Scholar]
- Bird H., Lambon Ralph M. A., Patterson K., & Hodges J. R. (2000). The rise and fall of frequency and imageability: Noun and verb production in semantic dementia. Brain and Language, 73(1), 17–49. https://doi.org/10.1006/brln.2000.2293 [DOI] [PubMed] [Google Scholar]
- Bourgeois M. S., Camp C., Rose M., White B., Malone M., Carr J., & Rovine M. (2003). A comparison of training strategies to enhance use of external aids by persons with dementia. Journal of Communication Disorders, 36(5), 361–378. https://doi.org/10.1016/S0021-9924(03)00051-0 [DOI] [PubMed] [Google Scholar]
- Boyle M. (2004). Semantic feature analysis treatment for anomia in two fluent aphasia syndromes. American Journal of Speech-Language Pathology, 13(3), 236–249. https://doi.org/10.1044/1058-0360(2004/025) [DOI] [PubMed] [Google Scholar]
- Boyle M., & Coelho C. A. (1995). Application of semantic feature analysis as a treatment for aphasic dysnomia. American Journal of Speech-Language Pathology, 4(4), 94–98. https://doi.org/10.1044/1058-0360.0404.94 [Google Scholar]
- Bozeat S., Lambon Ralph M. A., Patterson K., & Hodges J. R. (2002). The influence of personal familiarity and context on object use in semantic dementia. Neurocase, 8(1), 127–134. https://doi.org/10.1093/neucas/8.1.127 [DOI] [PubMed] [Google Scholar]
- Bozeat S., Patterson K., Hodges J., & Unit B. S. (2004). Relearning object use in semantic dementia. Neuropsychological Rehabilitation, 14(3), 351–363. https://doi.org/10.1080/09602010343000264 [Google Scholar]
- Bruce C., & Howard D. (1987). Computer-generated phonemic cues: An effective aid for naming in aphasia. International Journal of Language & Communication Disorders, 22(3), 191–201. https://doi.org/10.3109/13682828709019862 [DOI] [PubMed] [Google Scholar]
- Butts A. M., Machulda M. M., Duffy J. R., Strand E. A., Whitwell J. L., & Josephs K. A. (2015). Neuropsychological profiles differ among the three variants of primary progressive aphasia. Journal of the International Neuropsychological Society, 21(6), 429–435. https://doi.org/10.1017/S1355617715000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthery-Goulart M. T., da Silveira A. D. C., Machado T. H., Mansur L. L., Parente M. A. M. P., Senaha M. L. H., … Nitrini R. (2013). Nonpharmacological interventions for cognitive impairments following primary progressive aphasia. Dementia & Neuropsychologia, 7(1), 121–131. https://doi.org/10.1590/S1980-57642013DN70100018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caso F., Mandelli M. L., Henry M., Gesierich B., Bettcher B. M., Ogar J., … Gorno-Tempini M. L. (2014). In vivo signatures of nonfluent/agrammatic primary progressive aphasia caused by FTLD pathology. Neurology, 82(3), 239–247. https://doi.org/10.1212/WNL.0000000000000031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherney L. R. (2012). Aphasia treatment: Intensity, dose parameters, and script training. International Journal of Speech-Language Pathology, 14(5), 424–431. https://doi.org/10.3109/17549507.2012.686629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry K. E., Hawley K. S., Jackson E. M., & Boudreaux E. O. (2009). Booster sessions enhance the long-term effectiveness of spaced retrieval in older adults with probable Alzheimer's disease. Behavior Modification, 33(3), 295–313. https://doi.org/10.1177/0145445509333432 [DOI] [PubMed] [Google Scholar]
- Coelho C. A., McHugh R. E., & Boyle M. (2000). Semantic feature analysis as a treatment for aphasic dysnomia: A replication. Aphasiology, 14(2), 133–142. https://doi.org/10.1080/026870300401513 [Google Scholar]
- Coltheart M. (1981). The MRC Psycholinguitic database. The Quarterly Journal of Experimental Psychology: Human Experimental Psychology, 33(A), 497–505. [Google Scholar]
- Croot K., Nickels L., Laurence F., & Manning M. (2009). Impairment- and activity/participation-directed interventions in progressive language impairment: Clinical and theoretical issues. Aphasiology, 23(2), 125–160. https://doi.org/10.1080/02687030801943179 [Google Scholar]
- Croot K., Taylor C., Abel S., Jones K., Krein L., Hameister I., … Nickels L. (2015). Measuring gains in connected speech following treatment for word retrieval: A study with two participants with primary progressive aphasia. Aphasiology, 29(11), 1265–1288. https://doi.org/10.1080/02687038.2014.975181 [Google Scholar]
- Davies M. (2009). The 385 million word Corpus of Contemporary American English (1990–2008): Design, architecture, and linguistic insights. International Journal of Corpus Linguistics, 14(2), 159–190. [Google Scholar]
- Dial H., Hinshelwood H., Grasso S., Hubbard H. I., Gorno-Tempini M. L., & Henry M. L. (2019). Investigating the utility of teleteraphy in individuals with primary progressive aphasia. Clinical Interventions in Aging, 14, 453–471. https://doi.org/10.2147/CIA.S178878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J., Copland D., McKinnon E., Burfein P., O'Brien K., Farrell A., & Rodriguez A. D. (2015). Intensive versus distributed aphasia therapy: A nonrandomized, parallel-group, dosage-controlled study. Stroke, 46(8), 2206–2211. https://doi.org/10.1161/STROKEAHA.115.009522 [DOI] [PubMed] [Google Scholar]
- Dressel K., Huber W., Frings L., Kümmerer D., Saur D., Mader I., … Abel S. (2010). Model-oriented naming therapy in semantic dementia: A single-case fMRI study. Aphasiology, 24(12), 1537–1558. https://doi.org/10.1080/02687038.2010.500567 [Google Scholar]
- Eikelboom W. S., Janssen N., Jiskoot L. C., van den Berg E., Roelofs A., & Kessels R. P. C. (2018). Episodic and working memory function in primary progressive aphasia: A meta-analysis. Neuroscience & Biobehavioral Reviews, 92, 243–254. https://doi.org/10.1016/j.neubiorev.2018.06.015 [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., & McHugh P. R. (1975). “Mini-Mental State.” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. https://doi.org/10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini M. L., Brambati S. M., Ginex V., Ogar J., Dronkers N. F., Marcone A., … Miller B. L. (2008). The logopenic/phonological variant of primary progressive aphasia. Neurology, 71(16), 1227–1234. https://doi.org/10.1212/01.wnl.0000320506.79811.da [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M. L., Dronkers N. F., Rankin K. P., Ogar J. M., Phengrasamy L., Rosen H. J., … Miller B. L. (2004). Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology, 55(3), 335–346. https://doi.org/10.1002/ana.10825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M. L., Hillis A. E., Weintraub S., Kertesz A., Mendez M., Cappa S. F., … Grossman M. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. https://doi.org/10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham K. S., Patterson K., Pratt K. H., & Hodges J. R. (1999). Relearning and subsequent forgetting of semantic category exemplars in a case of semantic dementia. Neuropsychology, 13(3), 359–380. https://doi.org/10.1037/0894-4105.13.3.359 [DOI] [PubMed] [Google Scholar]
- Graham K. S., Patterson K., Pratt K. H., & Hodges J. R. (2001). Can repeated exposure to “forgotten” vocabulary help alleviate word-finding difficulties in semantic dementia? An illustrative case study. Neuropsychological Rehabilitation, 11(3–4), 429–454. https://doi.org/10.1080/09602010042000060 [Google Scholar]
- Graham K. S., Simons J. S., Pratt K. H., Patterson K., & Hodges J. R. (2000). Insights from semantic dementia on the relationship between episodic and semantic memory. Neuropsychologia, 38(3), 313–324. https://doi.org/10.1016/S0028-3932(99)00073-1 [DOI] [PubMed] [Google Scholar]
- Grossman M., & Ash S. (2004). Primary progressive aphasia: A review. Neurocase, 10(1), 3–18. https://doi.org/10.1080/13554790490960440 [DOI] [PubMed] [Google Scholar]
- Harciarek M., Sitek E. J., & Kertesz A. (2014). The patterns of progression in primary progressive aphasia—Implications for assessment and management. Aphasiology, 28(8–9), 964–980. https://doi.org/10.1080/02687038.2014.904498 [Google Scholar]
- Harnish S. M., Morgan J., Lundine J. P., Bauer A., Singletary F., Benjamin M. L., … Crosson B. (2014). Dosing of a cued picture-naming treatment for anomia. American Journal of Speech-Language Pathology, 23(2), 285–299. https://doi.org/10.1044/2014_AJSLP-13-0081 [DOI] [PubMed] [Google Scholar]
- Henry M. L., & Gorno-Tempini M. L. (2010). The logopenic variant of primary progressive aphasia. Current Opinion in Neurology, 23(6), 633–637. https://doi.org/10.1097/WCO.0b013e32833fb93e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. L., Hubbard H. I., Grasso S. M., Mandelli M. L., Wilson S. M., Sathishkumar M. T., … Gorno-Tempini M. L. (2018). Retraining speech production and fluency in non-fluent/agrammatic primary progressive aphasia. Brain, 141(6), 1799–1814. https://doi.org/10.1093/brain/awy101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. L., Meese M. V., Truong S., Babiak M. C., Miller B. L., & Gorno-Tempini M. L. (2013). Treatment for apraxia of speech in nonfluent variant primary progressive aphasia. Behavioural Neurology, 26(1–2), 77–88. https://doi.org/10.3233/BEN-2012-120260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. L., Rising K., DeMarco A. T., Miller B. L., Gorno-Tempini M. L., & Beeson P. M. (2013). Examining the value of lexical retrieval treatment in primary progressive aphasia: Two positive cases. Brain and Language, 127(2), 145–156. https://doi.org/10.1016/j.bandl.2013.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. L., Wilson S. M., Babiak M. C., Mandelli M. L., Beeson P. M., Miller Z. A., … Gorno-Tempini M. L. (2016). Phonological processing in primary progressive aphasia. Journal of Cognitive Neuroscience, 28(2), 210–222. https://doi.org/10.1162/jocn_a_00901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia C. G., Sage K., Lambon Ralph M. A., & Berthier M. L. (2009). Relearning and retention of verbal labels in a case of semantic dementia. Aphasiology, 23(2), 192–209. https://doi.org/10.1080/02687030801942999 [Google Scholar]
- Hinckley J. J., & Craig H. K. (1998). Influence of rate of treatment on the naming abilities of adults with chronic aphasia. Aphasiology, 12(11), 989–1006. https://doi.org/10.1080/02687039808249465 [Google Scholar]
- Hodges J. R., & Patterson K. (2007). Semantic dementia: A unique clinicopathological syndrome. Lancet Neurology, 6(11), 1004–1014. https://doi.org/10.1016/S1474-4422(07)70266-1 [DOI] [PubMed] [Google Scholar]
- Hopper T., Bourgeois M. S., Pimentel J., Qualls C. D., Hickey E. M., Frymark T., … Schooling T. (2013). An evidence based systematic review on cognitive interventions for individuals with dementia. American Journal of Speech-Language Pathology, 22(1), 126–145. https://doi.org/10.1044/1058-0360(2012/11-0137) [DOI] [PubMed] [Google Scholar]
- Hopper T., Drefs S. J., Bayles K. A., Tomoeda C. K., & Dinu I. (2010). The effects of modified spaced-retrieval training on learning and retention of face–name associations by individuals with dementia. Neuropsychological Rehabilitation, 20(1), 81–102. https://doi.org/10.1080/09602010902937590 [DOI] [PubMed] [Google Scholar]
- Hopper T., Mahendra N., Kim E., Azuma T., Bayles K. A., Cleary S. J., & Tomoeda C. K. (2005). Evidence-based practice recommendations for working with individuals with dementia: Spaced-retrieval training. Journal of Medical Speech-Language Pathology, 13(4), 27–34. [Google Scholar]
- Hothorn T., & Hornik K. (2006). exactRankTests: Exact distributions for rank and permutation tests. Retrieved from https://cran.r-project.org/package=exactRankTests
- Howard D., & Patterson K. (1992). Pyramids and palm trees: A test of semantic access from pictures and words. Bury St. Edmunds, United Kingdoms: Thames Valley Test Company. [Google Scholar]
- Jokel R., & Anderson N. D. (2012). Quest for the best: Effects of errorless and active encoding on word re-learning in semantic dementia. Neuropsychological Rehabilitation, 22(2), 187–214. https://doi.org/10.1080/09602011.2011.639626 [DOI] [PubMed] [Google Scholar]
- Jokel R., Graham N. L., Rochon E., & Leonard C. (2014). Word retrieval therapies in primary progressive aphasia. Aphasiology, 28(8–9), 1038–1068. https://doi.org/10.1080/02687038.2014.899306 [Google Scholar]
- Jokel R., Rochon E., & Anderson N. D. (2010). Errorless learning of computer-generated words in a patient with semantic dementia. Neuropsychological Rehabilitation, 20(1), 16–41. https://doi.org/10.1080/09602010902879859 [DOI] [PubMed] [Google Scholar]
- Jokel R., Rochon E., & Leonard C. (2002). Therapy for anomia in semantic dementia. Brain and Cognition, 49(2), 241–244. [PubMed] [Google Scholar]
- Jokel R., Rochon E., & Leonard C. (2006). Treating anomia in semantic dementia: Improvement, maintenance, or both? Neuropsychological Rehabilitation, 16(3), 241–256. https://doi.org/10.1080/09602010500176757 [DOI] [PubMed] [Google Scholar]
- Kaplan E., Goodglass H., & Weintraub S. (2001). Boston Naming Test. Philadelphia, PA: Lippincott, Williams & Wilkins. [Google Scholar]
- Kertesz A. (1982). The Western Aphasia Battery. New York, NY: Grune & Stratton. [Google Scholar]
- Kertesz A. (2006). Western Aphasia Battery–RevisedTM (WAB-RTM). San Antonio, TX: Pearson; Retrieved from https://www.pearsonclinical.ca/en/products/product-master/item-176.html [Google Scholar]
- Kertesz A., Jesso S., Harciarek M., Blair M., & McMonagle P. (2010). What is semantic dementia?: A cohort study of diagnostic features and clinical boundaries. Archives of Neurology, 67(4), 483–489. https://doi.org/10.1001/archneurol.2010.55 [DOI] [PubMed] [Google Scholar]
- Kim E. S., Cleary S. J., Hopper T., Bayles K. A., Mahendra N., Azuma T., & Rackley A. (2006). Evidence-based practice recommendations for working with individuals with dementia: Group reminiscence therapy. Journal of Medical Speech-Language Pathology, 14(3), xxiii–xxiii. [Google Scholar]
- Kim M. (2017). Effect of lexical retrieval cascade treatment on naming and discourse of individuals with logopenic variant of primary progressive aphasia (lvPPA). Clinical Archives of Communication Disorders, 2(3), 197–208. https://doi.org/10.21849/cacd.2017.00171 [Google Scholar]
- Kleim J. A., & Jones T. A. (2008). Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. Journal of Speech, Language, and Hearing Research, 51(1), S225–S239. https://doi.org/10.1044/1092-4388(2008/018) [DOI] [PubMed] [Google Scholar]
- Knopman D. S., Kramer J. H., Boeve B. F., Caselli R. J., Graff-Radford N. R., Mendez M. F., … Mercaldo N. (2008). Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain, 131(11), 2957–2968. https://doi.org/10.1093/brain/awn234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortte K. B., & Rogalski E. J. (2013). Behavioural interventions for enhancing life participation in behavioural variant frontotemporal dementia and primary progressive aphasia. International Review of Psychiatry, 25(2), 237–245. https://doi.org/10.3109/09540261.2012.751017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. H., Jurik J., Sha S. J., Rankin K. P., Rosen H. J., Johnson J. K., & Miller B. L. (2003). Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cognitive and Behavioral Neurology, 16(4), 211–218. https://doi.org/10.1097/00146965-200312000-00002 [DOI] [PubMed] [Google Scholar]
- Lambon Ralph M. A., Snell C., Fillingham J. K., Conroy P., & Sage K. (2010). Predicting the outcome of anomia therapy for people with aphasia post CVA: Both language and cognitive status are key predictors. Neuropsychological Rehabilitation, 20(2), 289–305. https://doi.org/10.1080/09602010903237875 [DOI] [PubMed] [Google Scholar]
- Lee J. B., Kaye R. C., & Cherney L. R. (2009). Conversational script performance in adults with non-fluent aphasia: Treatment intensity and aphasia severity. Aphasiology, 23(7–8), 885–897. https://doi.org/10.1080/02687030802669534 [Google Scholar]
- Lehmann M., Ghosh P. M., Madison C., Laforce R. Jr. Corbetta-Rastelli C., Weiner M. W., … Rabinovici G. D. (2013). Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer's disease. Brain, 136(3), 844–858. https://doi.org/10.1093/brain/aws327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton C. E., Hodges J. R., Piguet O., & Ballard K. J. (2017). Common and divergent neural correlates of anomia in amnestic and logopenic presentations of Alzheimer's disease. Cortex, 86, 45–54. https://doi.org/10.1016/j.cortex.2016.10.019 [DOI] [PubMed] [Google Scholar]
- Leyton C. E., Villemagne V. L., Savage S., Pike K. E., Ballard K. J., Piguet O., … Hodges J. R. (2011). Subtypes of progressive aphasia: Application of the international consensus criteria and validation using β-amyloid imaging. Brain, 134(10), 3030–3043. https://doi.org/10.1093/brain/awr216 [DOI] [PubMed] [Google Scholar]
- Libon D. J., Xie S. X., Moore P., Farmer J., Antani S., McCawley G., … Grossman M. (2007). Patterns of neuropsychological impairment in frontotemporal dementia. Neurology, 68(5), 369–375. https://doi.org/10.1212/01.wnl.0000252820.81313.9b [DOI] [PubMed] [Google Scholar]
- Mahendra N., Hopper T., Bayles K., Azuma T., Cleary S., & Kim E. (2006). Evidence-based practice recommendations for working with individuals with dementia: Montessori-based interventions. Journal of Medical Speech-Language Pathology, 14(1), xv–xxv. [Google Scholar]
- Mahendra N., Kim E., Bayles K., Hopper T., Cleary S., & Azuma T. (2005). Evidence-based practice recommendations for working with individuals with dementia: Computer-assisted cognitive interventions (CACIs). Journal of Medical Speech-Language Pathology, 13(4), xxxv–xxxv. [Google Scholar]
- Maher L. M., & Raymer A. M. (2004). Management of anomia. Topics in Stroke Rehabilitation, 11(1), 10–21. https://doi.org/10.1310/318R-RMD5-055J-PQ40 [DOI] [PubMed] [Google Scholar]
- Mandelli M. L., Vilaplana E., Brown J. A., Hubbard H. I., Binney R. J., Attygalle S., … Gorno-Tempini M. L. (2016). Healthy brain connectivity predicts atrophy progression in nonfluent variant of primary progressive aphasia. Brain, 139(10), 2778–2791. https://doi.org/10.1093/brain/aww195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberry E. J., Sage K., Ehsan S., & Lambon Ralph M. A. (2011). Relearning in semantic dementia reflects contributions from both medial temporal lobe episodic and degraded neocortical semantic systems: Evidence in support of the complementary learning systems theory. Neuropsychologia, 49, 3591–3598. https://doi.org/10.1016/j.neuropsychologia.2011.09.010 [DOI] [PubMed] [Google Scholar]
- Mesulam M. M. (2001). Primary progressive aphasia. Annals of Neurology, 49(4), 425–432. https://doi.org/10.1002/ana.91 [PubMed] [Google Scholar]
- Mesulam M. M., Wieneke C., Thompson C., Rogalski E., & Weintraub S. (2012). Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain, 135(5), 1537–1553. https://doi.org/10.1093/brain/aws080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. M., Getz H. R., Brennan D. M., Hu T. M., & Friedman R. B. (2016). Telerehabilitation of anomia in primary progressive aphasia. Aphasiology, 30(4), 483–507. https://doi.org/10.1080/02687038.2015.1081142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. M., Snider S. F., Eckmann C. B., & Friedman R. B. (2015). Prophylactic treatments for anomia in the logopenic variant of primary progressive aphasia: Cross-language transfer. Aphasiology, 29(9), 1062–1081. https://doi.org/10.1080/02687038.2015.1028327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. M., Tippett D. C., & Friedman R. B. (2018). Prophylaxis and remediation of anomia in the semantic and logopenic variants of primary progressive aphasia. Neuropsychological Rehabilitation, 28(3), 352–368. https://doi.org/10.1080/09602011.2016.1148619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. M., Tippett D. C., Turner R. S., & Friedman R. B. (2018). Long-term maintenance of anomia treatment effects in primary progressive aphasia. Neuropsychological Rehabilitation, 1–25. https://doi.org/10.1080/09602011.2018.1425146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhart M., Davis C., Kannan V., Heidler-Gary J., Cloutman L., & Hillis A. E. (2009). Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology, 23(7–8), 823–834. https://doi.org/10.1080/02687030802661762 [Google Scholar]
- Nickels L., & Best W. (1996). Therapy for naming disorders (Part I): Principles, puzzles and progress. Aphasiology, 10(1), 21–47. https://doi.org/10.1080/02687039608248397 [Google Scholar]
- Off C. A., Griffin J. R., Spencer K. A., & Rogers M. A. (2016). The impact of dose on naming accuracy with persons with aphasia. Aphasiology, 30(9), 983–1011. https://doi.org/10.1080/02687038.2015.1100705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogar J. M., Dronkers N. F., Brambati S. M., Miller B. L., & Gorno-Tempini M. L. (2007). Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Disease and Associated Disorders, 21(4), S23–S30. https://doi.org/10.1097/WAD.0b013e31815d19fe [DOI] [PubMed] [Google Scholar]
- Paul N., & Mehrhoff J. (2015). Descriptive analysis: Survey of direct and indirect interventions for persons with dementia-based communication disorders. SIG 2 Perspectives on Neurophysiology and Neurogenic Speech and Language Disorders, 25, 125–141. https://doi.org/10.1044/nnsld25.4.125 [Google Scholar]
- Pengas G., Patterson K., Arnold R. J., Bird C. M., Burgess N., & Nestor P. J. (2010). Lost and found: Bespoke memory testing for Alzheimer's disease and semantic dementia. Journal of Alzheimer's Disease, 21(4), 1347–1365. https://doi.org/10.3233/JAD-2010-100654 [DOI] [PubMed] [Google Scholar]
- Pulvermüller F., Neininger B., Elbert T., Mohr B., Rockstroh B., Koebbel P., & Taub E. (2001). Constraint-induced therapy of chronic aphasia after stroke. Stroke, 32(7), 1621–1626. https://doi.org/10.1161/01.STR.32.7.1621 [DOI] [PubMed] [Google Scholar]
- Purdy M., & Wallace S. E. (2016). Intensive multimodal communication treatment for people with chronic aphasia. Aphasiology, 30(10), 1071–1093. https://doi.org/10.1080/02687038.2015.1102855 [Google Scholar]
- Rabinovici G. D., Jagust W. J., Furst A. J., Ogar J. M., Racine C. A., Mormino E. C., … Gorno-Tempini M. L. (2008). Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Annals of Neurology, 64(4), 388–401. https://doi.org/10.1002/ana.21451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rising K. (2014). Treatment for lexical retrieval in primary progressive aphasia. SIG 2 Perspectives on Neurophysiology and Neurogenic Speech and Language Disorders, 24(4), 137–144. https://doi.org/10.1044/nnsld24.4.137 [Google Scholar]
- Rohrer J. D., Caso F., Mahoney C., Henry M., Rosen H. J., Rabinovici G., … Gorno-Tempini M. L. (2013). Patterns of longitudinal brain atrophy in the logopenic variant of primary progressive aphasia. Brain and Language, 127(2), 121–126. https://doi.org/10.1016/j.bandl.2012.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J. D., Ridgway G. R., Crutch S. J., Hailstone J., Goll J. C., Clarkson M. J., … Warren J. D. (2010). Progressive logopenic/phonological aphasia: Erosion of the language network. NeuroImage, 49(1), 984–993. https://doi.org/10.1016/j.neuroimage.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J. D., Rossor M. N., & Warren J. D. (2010). Syndromes of nonfluent primary progressive aphasia: A clinical and neurolinguistic analysis. Neurology, 75(7), 603–610. https://doi.org/10.1212/WNL.0b013e3181ed9c6b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J. D., Rossor M. N., & Warren J. D. (2012). Alzheimer's pathology in primary progressive aphasia. Neurobiology of Aging, 33(4), 744–752. https://doi.org/10.1016/j.neurobiolaging.2010.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Santos M. A., Rabinovici G. D., Iaccarino L., Ayakta N., Tammewar G., Lobach I., … Gorno-Tempini M. L. (2018). Rates of amyloid imaging positivity in patients with primary progressive aphasia. JAMA Neurology, 75(3), 342–352. https://doi.org/10.1001/jamaneurol.2017.4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage S. A., Ballard K. J., Piguet O., & Hodges J. R. (2013). Bringing words back to mind—Improving word production in semantic dementia. Cortex, 49(7), 1823–1832. https://doi.org/10.1016/j.cortex.2012.09.014 [DOI] [PubMed] [Google Scholar]
- Savage S. A., Piguet O., & Hodges J. R. (2015). Cognitive intervention in semantic dementia maintaining words over time. Alzheimer Disease and Associated Disorders, 29(1), 55–62. https://doi.org/10.1097/WAD.0000000000000053 [DOI] [PubMed] [Google Scholar]
- Simons J. S., Graham K. S., Galton C. J., Patterson K., & Hodges J. R. (2001). Semantic knowledge and episodic memory for faces in semantic dementia. Neuropsychology, 15(1), 101–114. https://doi.org/10.1037/0894-4105.15.1.101 [PubMed] [Google Scholar]
- Snowden J. S., Griffiths H., & Neary D. (1994). Semantic dementia: Autobiographical contribution to preservation of meaning. Cognitive Neuropsychology, 11(3), 265–288. https://doi.org/10.1080/02643299408251976 [Google Scholar]
- Snowden J. S., & Neary D. (2002). Relearning of verbal labels in semantic dementia. Neuropsychologia, 40(10), 1715–1728. https://doi.org/10.1016/S0028-3932(02)00031-3 [DOI] [PubMed] [Google Scholar]
- Spinelli E. G., Mandelli M. L., Miller Z. A., Santos-Santos M. A., Wilson S. M., Agosta F., … Gorno-Tempini M. L. (2017). Typical and atypical pathology in primary progressive aphasia variants. Annals of Neurology, 81(3), 430–443. https://doi.org/10.1002/ana.24885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffaroni A. M., Ljubenkov P. A., Kornak J., Cobigo Y., Datta S., Marx G., … Rosen H. J. (2019). Longitudinal multimodal imaging and clinical endpoints for frontotemporal dementia clinical trials. Brain, 142(2), 443–459. https://doi.org/10.1093/brain/awy319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan K., Hopper M., Wenke R., Jackson C., Till T., & Conway E. (2018). Speech-language pathologist interventions for communication in moderate–severe dementia: A systematic review. American Journal of Speech-Language Pathology, 27(2), 836–852. https://doi.org/10.1044/2017_AJSLP-17-0043 [DOI] [PubMed] [Google Scholar]
- Taylor C., Kingma R. M., Croot K., & Nickels L. (2009). Speech pathology services for primary progressive aphasia: Exploring an emerging area of practice. Aphasiology, 23(2), 161–174. https://doi.org/10.1080/02687030801943039 [Google Scholar]
- Teichmann M., Kas A., Boutet C., Ferrieux S., Nogues M., Samri D., … Migliaccio R. (2013). Deciphering logopenic primary progressive aphasia: A clinical, imaging and biomarker investigation. Brain, 136(11), 3474–3488. https://doi.org/10.1093/brain/awt266 [DOI] [PubMed] [Google Scholar]
- Tippett D. C., Hillis A. E., & Tsapkini K. (2015). Treatment of primary progressive aphasia. Current Treatment Options in Neurology, 17(8), 362 https://doi.org/10.1007/s11940-015-0362-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. S., Kenyon L. C., Trojanowski J. Q., Gonatas N., & Grossman M. (1996). Clinical, neuroimaging, and pathologic features of progressive nonfluent aphasia. Annals of Neurology, 39(2), 166–173. https://doi.org/10.1002/ana.410390205 [DOI] [PubMed] [Google Scholar]
- Warren S. F., Fey M. E., & Yoder P. J. (2007). Differential treatment intensity research: A missing link to creating optimally effective communication interventions. Mental Retardation and Developmental Disabilities Research Reviews, 13(1), 70–77. https://doi.org/10.1002/mrdd.20139 [DOI] [PubMed] [Google Scholar]
- Wertz R. T., LaPointe L. L., & Rosenbek J. C. (1984). Apraxia of speech in adults: The disorder and its management. New York, NY: Grune & Stratton. [Google Scholar]
- Westbury C., & Bub D. (1997). Primary progressive aphasia: A review of 112 cases. Brain and Language, 60(3), 381–406. https://doi.org/10.1006/brln.1997.1840 [DOI] [PubMed] [Google Scholar]
- Whitwell J. L., Jones D. T., Duffy J. R., Strand E. A., Machulda M. M., Przybelski S. A., … Josephs K. A. (2015). Working memory and language network dysfunctions in logopenic aphasia: A task-free fMRI comparison with Alzheimer's dementia. Neurobiology of Aging, 36(3), 1245–1252. https://doi.org/10.1016/j.neurobiolaging.2014.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. (1988). MRC psycholinguistic database: Machine-usable dictionary, Version 2.00. Behavior Research Methods, Instruments, & Computers, 20(1), 6–10. https://doi.org/10.3758/BF03202594 [Google Scholar]
- Wilson S. M., Dronkers N. F., Ogar J. M., Jang J., Growdon M. E., Agosta F., … Gorno-Tempini M. L. (2010). Neural correlates of syntactic processing in the nonfluent variant of primary progressive aphasia. Journal of Neuroscience, 30(50), 16845–16854. https://doi.org/10.1523/JNEUROSCI.2547-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. M., Henry M. L., Besbris M., Ogar J. M., Dronkers N. F., Jarrold W., … Gorno-Tempini M. L. (2010). Connected speech production in three variants of primary progressive aphasia. Brain, 133(7), 2069–2088. https://doi.org/10.1093/brain/awq129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win K. T., Pluta J., Yushkevich P., Irwin D. J., McMillan C. T., Rascovsky K., … Grossman M. (2017). Neural correlates of verbal episodic memory and lexical retrieval in logopenic variant primary progressive aphasia. Frontiers in Neuroscience, 11, 330 https://doi.org/10.3389/fnins.2017.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zientz J., Rackley A., Chapman S. B., Hopper T., Mahendra N., & Cleary S. (2007). Evidence-based practice recommendations: Caregiver-administered active cognitive stimulation for individuals with Alzheimer's disease. Journal of Medical Speech-Language Pathology, 15(3), xxvii–xxxiv. [Google Scholar]
- Zientz J., Rackley A., Chapman S. B., Hopper T., Mahendra N., Kim E. S., & Cleary S. (2007). Evidence-based practice recommendations for dementia: Educating caregivers on Alzheimer's disease and training communication strategies. Journal of Medical Speech-Language Pathology, 15(1), liii–liii. [Google Scholar]