Abstract
Background
Excessive adipose tissue, particularly with a central distribution, consists of visceral fat, which is metabolically active and could impinge upon central nervous system functioning. The aim of the current study was to examine levels of visceral adiposity in relation to key cerebral metabolite ratios localized in the occipitoparietal grey matter.
Methods
Seventy-three adults, aged between 40–60 years, underwent structural magnetic resonance imaging and single voxel 1H Magnetic Resonance Spectroscopy (1H MRS). Visceral fat was assessed using Dual Energy X Ray Absorptiometry (DXA).
Results
Individuals with higher visceral fat mass and volume had significantly lower ratios of N-acetyl-aspartate to total creatine (phosphocreatine+creatine, PCr+Cr) (NAA/PCr+Cr) (β = −0.29, p=0.03, β = −0.28, p=0.04). They also had significantly higher ratios of myo-inositol to total creatine (mI/PCr+Cr ) (β = 0.36, p=0.01, β = 0.36, p=0.01). Visceral fat mass and volume were not significantly related to ratios of glutamate to total creatine (Glu/PCr+Cr).
Conclusions
Visceral fat was associated with lower NAA/PCr+Cr ratio and higher mI/PCr+Cr ratios. While future studies are necessary, these results indicate central adiposity is associated with metabolic changes that could impinge upon the central nervous system in middle age.
Keywords: Obesity, visceral fat, 1H-MRS, neurochemistry
Introduction
The prevalence of obesity has increased exponentially (World Health Organization, 2009), rendering it the fifth leading cause of mortality worldwide (World Health Organization, 2009). Furthermore, obesity is implicated in a host of negative outcomes including increased risk of stroke, gall bladder disease, as well as cancer (Kopelman et al, 2000). However, the association between obesity and physical health is not straightforward. In what is known as the obesity paradox, obese individuals with chronic conditions such as heart failure have lower mortality rates (Curtis et al, 2005). Published research has also highlighted a similarly complex relationship between obesity and brain health. Obesity at midlife has been linked to increased risk for Alzheimer’s Disease (Gustafson et al, 2003), diminished working memory related functional activation (Gonzales et al, 2010) and altered ratios of crucial cerebral metabolites (Haley et al, 2013). However, despite these established and well regarded findings, others have found a potential protective effect of high body mass index (BMI) and subsequent incident dementia (Qizilibash et al, 2015). The relationship between being overweight/obese in midlife and brain health is thus complicated, and warrants further investigation. Given that neurodegeneration is irreversible, it is imperative to elucidate the mechanisms that drive these changes early in life, where targeted preventative efforts may be launched.
Adipose tissue comprises of metabolically active cells that could have deleterious effects on central nervous system functioning through metabolic or hormonal pathways (Gustafson et al., 2010). However, the distribution of adipose tissue throughout the body appears to selectively predict cognitive ability (Cereda et al., 2007). Centrally distributed adipose tissue is indicative of visceral fat, which is metabolically active and a more salient predictor of cognitive decline and dementia compared to body mass index (BMI) (Cereda et al., 2007, Kerwin et al., 2011). We have previously described the deleterious effects of various proxies of visceral fat (waist circumference and BMI) on working memory related functional brain activation (Gonzales et al., 2014) and cerebral neurochemical profiles (Gonzales et al., 2012) in middle aged adults. However, to our best knowledge, very little research has been done utilizing direct measures of visceral fat and its effects on brain integrity in middle age. As more direct measures of abdominal obesity have increased utility in predicting neurodegeneration in elderly populations (Isaac et al., 2011), it is imperative to directly examine the impact of actual visceral fat mass and volume on brain integrity. We recently reported on the effects of visceral fat mass and volume on the thickness of the cortical mantle using an exploratory whole brain approach (Kaur et al., 2015). Here, we aim to expand on this work by exploring the relationship between visceral fat and neuronal viability measured by Magnetic Resonance Spectroscopy (MRS).
MRS allows for the identification and quantitation of several neurochemicals of neurobiological significance such as N-acetyl-aspartate (NAA), myo-inositol (ml) and total creatine including phosphocreatine+creatine (PCr+Cr) among others. NAA is exclusively found in the adult central nervous system (Urenjak et al., 1992) and has been directly linked to changes in cognitive functioning in patients with Alzheimer’s Disease (Jessen et al., 2001) and neurologically intact younger adults (Grachev et al., 2001). It is widely regarded as a marker of neuronal density. However, as changes in NAA are not always irreversible, it would be more accurately termed a marker of neuronal viability. Cortical NAA levels have also been reported to be reduced in older adults with higher BMI (Gazdzinski et al., 2010). However, very little work has been done examining the association between direct measures of visceral fat and these neurometabolites. Changes in levels of NAA have been associated with poorer performance on executive function tests in healthy elderly (Ross et al., 2005). Furthermore, baseline cortical NAA is a useful marker for predicting post stroke cognitive decline (Ross et al., 2006). Myo-inositol (mI), on the other hand, is an organic osmolyte and hypothesized glial marker (Brand et al., 1993). MI is elevated in neurodegenerative disorders such as multiple sclerosis (Fernando et al., 2004) and prodromal Alzheimer’s Disease (Kantarci et al., 2000) as well as middle-aged adults with higher BMI, indirectly impacting their memory performance (Gonzales et al., 2012). Elevated levels of mI precede cognitive decline in patients with Alzheimer’s Disease (Huang et al., 1999), Human Immunodeficiency Virus (HIV)(Cloak et al., 2004) and multiple sclerosis (Fernando et al., 2004). Other neurochemical markers such as total choline are associated with white matter integrity in individuals with fragile X syndrome (Filley et al., 2015). Spectroscopic markers are thus potent predictors of brain integrity and were chosen as primary outcomes for this study. As visceral fat is a more salient predictor of cognitive outcome and brain integrity than BMI, it is critical to tease out the unique contribution of high visceral fat to alterations in cerebral neurochemical profiles. Visceral adipose tissue mass and volume were directly measured using dual energy X-ray absorptiometry (DXA) (Xia et al., 2014).
Materials and methods
Participants
Adults between the ages of 40–60 years were recruited from the community through electronic and print advertisements. Individuals with self-reported history of coronary artery disease, angina pectoris, myocardial infarctions, heart failure and cardiac surgery were excluded. Additional exclusionary criteria comprised of: self-reported history of neurological illness (e.g., Parkinson’s disease, neurodegeneration, clinically significant traumatic brain injury), major psychiatric disorder (schizophrenia, anxiety), substance abuse (i.e., diagnosed abuse and/or previous hospitalization for substance abuse), metabolic disorder (thyroid disorder), smoking (within the last 2 years) or MRI contraindications. These were assessed through a telephone screening by trained research assistants. Participants who passed the initial screen were enrolled in the study after providing written consent. Data from individuals with uncontrolled hypertension (systolic blood pressure > 160 mm Hg), diabetes (fasting blood glucose > 126 mg/dl) and acute inflammation (fasting blood C-Reactive Protein > 10 mg/dl) were also excluded from this study.
Procedures
The study was approved by the University of Texas Institutional Review Board and was completed in accordance with the declaration of Helsinki, 1975. Participants underwent two separate study visits, a general health assessment where DXA data were collected and a neuropsychological/brain imaging assessment.
General health assessment
After an 8-hour fast, blood samples were collected from the antecubital vein by venipuncture. Fasting glucose and total cholesterol level were assessed using standard enzymatic techniques. Brachial systolic and diastolic blood pressure was assessed using a semi-automated device (VP-2000, Omron Healthcare, Bannockburn, IL, USA) after a 15-minute period of rest. Visceral fat mass and volume were estimated non-invasively via dual-energy X-ray absorptiometry (DXA) using a Lunar Dual Energy X-Ray Absorptiometry DPX (General Electric Medical Systems, Fairfield, Connecticut). This procedure requires that the subject lay down on a padded table that emits energy for approximately five minutes while an arm passed overhead and involves a small amount of radiation, which is equivalent to less than 1/20 of a chest X-ray. While DXA was traditionally used to measure bone density, it has been well validated as a sensitive, relatively inexpensive tool for visceral fat measurement (Kaul et al., 2012, Xia et al., 2014) with results comparable to that of computed tomography (CT) (Kaul et al., 2012, Xia et al., 2014). This is noteworthy as CT is the gold standard for measuring visceral fat. As visceral fat is associated with chronic systemic inflammation (de Luca et al., 2008), peripheral levels of C-Reactive Protein, a marker of chronic inflammation, was measured using commercially available high sensitivity Enzyme Linked Immunosorbent Assays (ELISA) (Alpha Diagnostics, San Antonio, TX) with a minimum detectable concentration of 0.35 ng/mL.
Neuropsychological assessment
Details of the neuropsychological assessment battery administered have been described elsewhere (Gonzales et al., 2014). Briefly, participants underwent a 1.5-hour battery of standard clinical instruments with established reliability and validity including the Wechsler Abbreviated Scales of Intelligence II (WASI-II). Trained research assistants administered all assessments with standard administration and scoring criteria.
Neuroimaging
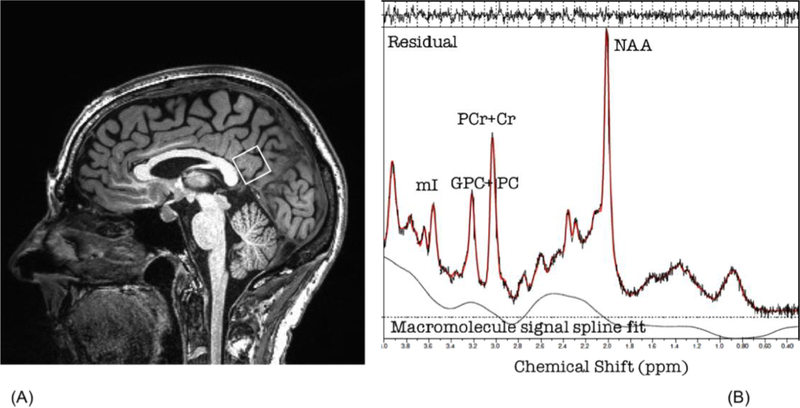
Magnetic resonance imaging was conducted using a 3T Siemens Skyra scanner equipped with a standard head coil. Anatomical scans of the entire brain were collected using high-resolution Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) sequences (256 × 256 matrix, flip angle = 7°, field of view (FOV) = 24 × 24 cm2, 1 mm slice thickness, 0 gap, voxel size = 1.0 × 1.0 × 1.0 mm3, TR = 2530.0 ms) for voxel localization. Cerebral metabolite ratios were obtained with Point-RESolved Spectroscopy (PRESS) sequence (svs_se_30) with the following parameters: TE/TR = 30/3000 ms, 80 excitations, 2000 Hz spectral width, volume ~6 cm3 localized in occipitoparietal grey matter including the posterior cingulate gyrus. We concentrated on the occipitoparietal grey matter because spectroscopically detectible changes in this region correspond to severity of cognitive impairment in clinical populations (Kantarci et al., 2000). The voxel placement is depicted in Figure 1a. The commercially available LCModel software was used to quantify and identify metabolite resonances. In accordance with standard clinical quantification techniques, the concentrations of N-acetyl-aspartate (NAA), myo-inositol (mI), total choline including glycerophosphocholine and phosphocholine (GPC+PC) and glutamate (Glu) were calculated as ratios to total creatine including phosphocreatine + creatine (PCr+Cr) (Kantarci et al., 2000). An example spectrum with these ratios is included in Figure 1b.
Figures 1:
Voxel placement (A) and example fitted spectrum (B) NAA=N-acetyl-aspartate; Cr=Creatine and phosphocreatine; Cho=choline and phosphocholine; mI=myo-Inositol
Data analysis
All variable distributions and regression residuals were examined using the Shapiro-Wilk test for normality. The effect of visceral fat mass and volume on NAA and mI ratios were assessed using simple linear regression models. Clinically relevant covariates (age, gender) were chosen a priori based on published relationships with NAA and mI (Fayed et al., 2014, Zhang et al., 2013). As we have previously documented an association between ml and peripheral CRP levels (Eagan et al., 2012), CRP was also included as a covariate in order to better estimate the unique contribution of visceral fat to any changes in cerebral neurochemical profiles. All statistical analyses were carried out using SPSS version 22.0 (IBM SPSS Inc, Chicago, IL, USA).
Results
Descriptive statistics
Seventy-three participants were included in this study. Participants had a mean age of 49.55 years (S.D=6.08 years) and a mean BMI of 27.88 (S.D=5.12). Age range of the participants ranged from 40–60 years. Forty-eight participants identified as Caucasian, 16 identified as Hispanic/Latin American, 3 identified as African American and 6 identified as Asian/Other. Participants had a mean visceral fat mass of 1022.47g (S.D=745.93g) and a mean visceral fat volume of 1083.90 cm3 (S.D=790.67cm3). Visceral fat mass ranged from 11g- 2941g, while visceral fat volume ranged from 12 cm3 – 3117cm3. This was a relatively well educated (mean education = 16.47 years, S.D.=2.82) and cognitively intact (mean full scale intelligence quotient = 116.33, S.D= 12.78). Further information on the demographic and physiologic characteristics of this sample is presented in table 1.
Table 1:
Selected demographic and physiological characteristics
| Characteristic | Mean ± SD |
|---|---|
| N, (men & women) | 73 (36 & 37) |
| Age, years | 49.55 ± 6.08 |
| Education, years | 16.47 ± 2.82 |
| BMI, kg/m2 | 27.88 ± 5.12 |
| Systolic blood pressure, mm Hg | 118.15 ± 11.38 |
| Diastolic blood pressure, mm Hg | 71.45 ± 8.55 |
| Blood glucose, mg/dl | 92.69 ± 9.45 |
| Total cholesterol, mg/dl | 201.15 ± 38.92 |
| Visceral fat mass, g | 1022.47 ± 745.93 |
| Visceral fat volume, cm3 | 1083.90 ± 790.67 |
Visceral fat and cerebral neurochemical profiles
In order to control for non-normality of visceral fat data (Shapiro-Wilk’s statistic = 0.936, p <0.001 for visceral fat mass; 0.936, p <0.001 for visceral fat volume), a square root transformation was carried out.
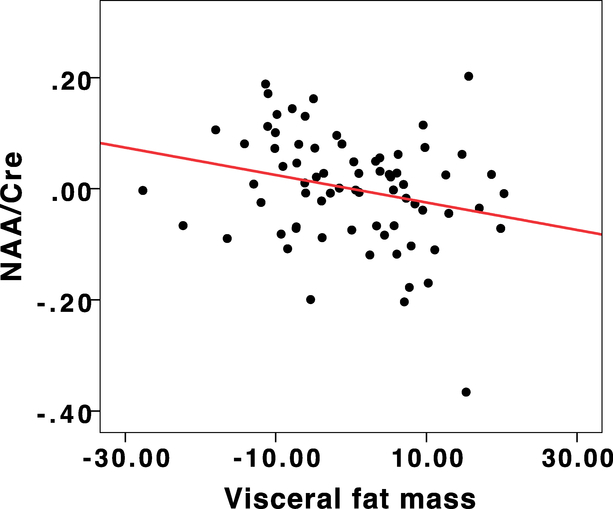
Higher visceral fat mass and volume were both significantly associated with reduced ratios of NAA/PCr+Cr in the occipitoparietal junction, controlling for age, gender and chronic inflammation (β = −0.29, p=0.03, R2 = 0.22, adjusted R2 = 0.18, C.I = −0.005−−0.001 for visceral fat mass and β = −0.28, p=0.04, R2 = 0.22, adjusted R2 = 0.18, C.I.= −0.005- −0.001 for visceral fat volume). The residuals plot from this analysis is depicted in Figure 2.
Figure 2:
Regression residuals depicting the relationship between NAA/Cre and Visceral fat mass
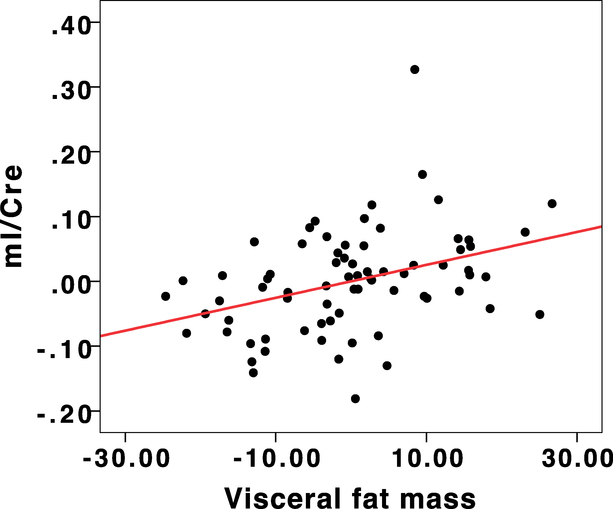
After controlling for the effects of age, gender and chronic inflammation, visceral fat mass and volume were also related to significant increases in the mI/PCr+Cr ratio in the occipitoparietal junction (β = 0.36, p=0.01, R2 = 0.15, adjusted R2 = 0.10, C.I.= 0.001–0.004 for visceral fat mass and β = 0.36, p=0.01, R2 = 0.15 adjusted R2 = 0.10, C.I.= 0.001–0.004 for visceral fat volume). The residuals plot depicting the relationship between mI/PCr+Cr and visceral fat mass is depicted in Figure 3.
Figure 3:
Regression residuals depicting the relationship between mI/Cre and Visceral fat mass
Visceral fat mass and volume were not related to GPC+PC/PCr+Cr or Glu/PCr+Cr after controlling for age, gender, and chronic inflammation (ps>0.05).
Discussion
To our knowledge, this is the first study to utilize a direct measure of visceral fat to examine the effects of visceral adipose tissue on brain integrity as measured by cerebral neurochemical profiles. Our results point to ratios of NAA/PCr+Cr and mI/PCr+Cr in the posterior cingulate gyrus/occipitoparietal junction as particularly vulnerable to the effects of high visceral fat in middle aged adults. Visceral fat mass and volume was associated with lower levels of NAA/PCr+Cr and elevated levels of mI/PCr+Cr in the posterior cingulate gyrus. These effects remained significant after controlling for age, gender and systemic inflammation.
A particularly interesting finding was the relationship between NAA/PCr+Cr levels and high visceral fat. Mouse models of Alzheimer’s disease suggest that elevations in cerebral concentrations of mI could precede reductions in concentrations of NAA (Chen et al., 2010). It is however, possible that NAA ratios in our sample are moderated by other factors associated with obesity. For example, NAA levels could be modulated by levels of cerebral Brain Derived Neurotrophic Factor, a key neurotrophin responsible for synaptic plasticity and neuronal regeneration that is diminished in mouse models of obesity (Kernie et al, 2000). Recently published work has revealed an association between genetic risk for low BDNF and lower hippocampal NAA levels (Stern et al., 2008). Furthermore, examination of the animal literature have revealed simultaneous increases in cortical NAA levels and reductions in cortical mI levels after intraventricular BDNF infusion (Zhang et al., 2013). The BDNF pathway is thus a mechanism that warrants further investigation. Direct examination of a possible visceral fat-BDNF-neurochemistry relationship is, however, beyond the scope of the current study.
It is also possible that decreases in NAA/PCr+Cr could be driven by obesity mediated decreases in energy metabolism. Published research on traumatic brain injury indicates that decreased NAA levels and levels of ATP are temporally correlated suggesting that NAA levels are related to energetic impairments (Gasparovic et al, 2001; Signoretti et al., 2001, Signoretti et al., 2004). The mouse model for Huntingdon’s disease has also documented decreases in NAA in the absence of any cell death (Jenkins et al., 2000). Furthermore, dietary creatine supplementation has significantly improved survival and delayed decreases in NAA (Andreassen et al. 2001; Ferrante et al., 2000). However, while these studies favor a link between NAA synthesis and energy metabolism, there has so far not been any published work establishing a direct link between NAA synthesis and ATP. It is possible that the two are indirectly linked, for example NAA synthesis is an energy dependent process, however, more research is needed to confirm this.
We also demonstrate an association between higher ratios of mI/PCr+Cr and high visceral fat. Previously, we have highlighted an indirect effect of elevated BMI on memory performance through elevations in mI/PCr+Cr (Gonzales et al., 2012). This is consistent with other published work on mI and cognition in multiple disease states (Cloak et al., 2004, Fernando et al., 2004, Huang et al., 1999). It has been hypothesized that changes in mI/PCr+Cr occur in the context of systemic inflammation (Eagan et al., 2012). Our results show an effect of visceral fat on mI/PCr+Cr ratios over and above that of inflammation, however, that warrants further investigation.
We hypothesize that visceral fat could affect cerebral mI/PCr+Cr concentrations through increasing blood brain barrier (BBB) permeability. The BBB consists primarily of endothelial cells that are joined by endothelial tight junctions (Pan et al., 2007). Adiponectin, an adipokine that is markedly reduced in individuals with high visceral fat (Warren et al., 2012), has been shown to stimulate production of nitric oxide (NO), a critical vasodilator in endothelial cells. Furthermore, adiponectin modifies the deleterious effects of pro-inflammatory cytokines on endothelial cells that comprise the BBB (Spranger et al., 2006). Direct examination of these relationships was, however beyond the scope of the current study.
It is noteworthy that we did not find significant relationships between visceral fat and other neurochemical markers (Glu/PCr+Cr and GPC+PC/PCr+Cr). This is unsurprising, given the stronger body of research highlighting the effects of NAA/PCr+Cr and mI/PCr+Cr on cognition across disease states. Other published work has demonstrated that GPC+PC/PCr+Cr and Glu/PCr+Cr are reduced in individuals with Fragile X Syndrome (Bruno et al., 2013, Filley et al., 2015). It is thus possible that levels of GPC+PC/PCr+Cr and Glu/PCr+Cr are influenced by genes unrelated to obesity.
The main limitation of this study lies in the cross-sectional design, which prevents the determination of causality. However, we demonstrate a relationship between measures of visceral fat and neurochemical markers that have been highlighted in the literature as early risk factors for cognitive decline and Alzheimer’s Disease. Our sample size (n =73), while larger than other published MRS research (Gazdzinski et al., 2010), was modest and thus limited the number of analyses that were feasible. Furthermore, while every effort was made to position the voxel mostly in the occipitoparietal grey matter, we cannot rule out the possibility that our volume of interest may have included a small percentage of white matter. Finally, as metabolite ratios were used, it is possible that the denominator (PCr+Cr) could have been driving the results. While this is unlikely considering that other ratios involving PCr+ Cr were not significantly altered by visceral fat, we cannot rule out this possibility. Future research should include mediation/moderation models that highlight possible interactive effects of neurochemistry, as well as other potential risk factors known to affect cognitive decline such as genetic risk, stress, diet and exercise.
Despite the above limitations, the current study is a useful early step in teasing out the mechanisms behind the complex relationship between obesity and cognition. As the sample comprised of middle aged, cognitively intact individuals, it provides crucial information on preclinical effects of visceral fat that precede neurodegeneration.
Acknowledgements
This work was made possible by funding provided by the National Institute of Neurological Disorders and Stroke (R01 NS075565, APH).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Brand A, Richter-Landsburg C; Leibfritz D (1993). Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Developmental Neuroscience 15, 289–298. [DOI] [PubMed] [Google Scholar]
- 2.Bruno JL, Shelly EW; Quintin E-M; Rostami M; Patnaik S; Spielman D; et al. (2013). Aberrant basal ganglia metabolism in fragile X syndrome: a magnetic resonance spectroscopy study. Journal of Neurodevelopmental Disorders 5, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cereda E, Sansone V, Meola G & Malavazos AE (2007). Increased visceral adipose tissue rather than BMI as a risk factor for dementia. Age Ageing 36, 488–91. [DOI] [PubMed] [Google Scholar]
- 4.Chen SQ, Wang PJ, Ten GJ, Zhan W; Li MH, et al. (2010). Role of Myo-Inositol by Magnetic Resonance Spectroscopy in Early Diagnosis of Alzheimer’s Disease in APP/PS1 Transgenic Mice. Dementia and Geriatric Cognitive Disorders 28, 558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cloak CC; Chang L; Ernst T (2004). Increased frontal white matter diffusion is associated with glial metabolites and psychomotor slowing in HIV. Journal of neuroimmunology 157, 147–152. [DOI] [PubMed] [Google Scholar]
- 6.Curtis JP; Selter JG; Wang Y; Rathore SS; Jovin IS; Jadbabaie F; Kosiborod M,; Portnay EL; Sokol SI; Bader F; Krumholz HM (2005). The obesity paradox: Body Mass Index and Outcomes in Patients with Heart Failure. Journal of The American Medical Association Internal Medicine 165, 55–61. [DOI] [PubMed] [Google Scholar]
- 7.de Luca C, Olefsky JM (2008). Inflammation and insulin resistance. Federation of European Biochemical Societies Letters 582, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eagan DE, Gonzales MM, Tarumi T, Tanaka H, Stautberg S, Haley AP (2012). Elevated serum C-Reactive Protein relates to increased cerebral myoinositol levels in middle aged adults. Cardiovascular Psychiatry and Neurology 2012, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fayed N; Andres E; Viguera L; Modrego PJ; Garcia-Campayo J (2014). Higher glutamate + glutamine and reduction of N-Acetylaspartate in Posterior Cingulate according to age range in patients with cognitive impairment and/or pain. Academic Radiology 21, 1211–1217. [DOI] [PubMed] [Google Scholar]
- 10.Fernando KT; Mclean MA; Chard DT; MacManus DG; Dalton CM; Miszkiel MA; et al. (2004). Elevated white matter myo-inositol in clinically isolated syndromes suggestive of multiple sclerosis. Brain 127, 1361–1369. [DOI] [PubMed] [Google Scholar]
- 11.Filley CM, Brown MS; Onderko K; Ray M; Bennett RE; Berry-Kravis E; et al. (2015). White matter disease and cognitive impairment in FMR1 premutation carriers. Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gazdzinski S; Millin R; Kaiser LG; Durazzo TC; Mueller SG; Weiner MW; et al. (2010). BMI and neuronal integrity in healthy, cognitively normal elderly: A proton magnetic resonance spectroscopy study. Obesity (Silver Spring) 18, 743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzales MM, Kaur S, Eagan DE, Goudarzi K, Pasha E, Doan DC, Tanaka H & Haley AP (2014). Central adiposity and the functional magnetic resonance imaging response to cognitive challenge. International Journal of Obesity (Lond). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzales MM, Takashi T, Eagan DE, Tanaka H, Vaghasia M, Haley AP (2012). Indirect Effects of Elevated Body Mass Index on Memory Performance Through Altered Cerebral Metabolite Concentrations. Psychosomatic Medicine 74, 691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grachev ID; Kumar R; Ramachandran TS; Szevereny NM (2001). Cognitive interference is associated with neuronal marker N-acetyl aspartate in the anterior cingulate cortex: an in vivo (1)H-MRS study of the Stroop Color-Word task. Molecular Psychiatry 6, 529–539. [DOI] [PubMed] [Google Scholar]
- 16.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I (2003). An 18-year follow up of overweight and risk of Alzheimer Disease. Archives of Internal Medicine 163, 1524–1528. [DOI] [PubMed] [Google Scholar]
- 17.Gustafson DR (2010). Adiposity hormones and dementia. Journal of Neurological Science 299, 30–4. [DOI] [PubMed] [Google Scholar]
- 18.Haley AP; Gonzales MM; Tarumi T; Tanaka H (2013). Dyslipidemia links obesity to early cerebral neurochemical alterations. Obesity 21, 2007–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hattingen E; Raab P; Franz K; Zanella FE; Lanfermann H; Pilatus U (2007). Myo-Inositol: a marker of reactive astrogliosis in glial tumors. NMR in biomedicine 21, 233–241. [DOI] [PubMed] [Google Scholar]
- 20.Huang W, Alexander GE; Daly EM; Shetty HU; Krasuski JS; Rapopport SI et al. (1999). High brain myo-inositol levels in the predementia phase of Alzheimer’s disease in adults with Down’s syndrome: a 1H MRS study. American Journal of Psychiatry 156, 1876–1886. [DOI] [PubMed] [Google Scholar]
- 21.Isaac V, Sim Sam, Zheng Hui, Zagorodnov V, Tai ES, Chee M (2011). Adverse associations between visceral adiposity, brain structure and cognitive performance in healthy elderly. Frontiers in Aging Neuroscience 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jessen F; Block W; Traber F; Keller E; Flacke S; et al. (2001). Decrease of N-acetylaspartate in the MTL correlates with cognitive decline of AD patients. Neurology 57, 930–932. [DOI] [PubMed] [Google Scholar]
- 23.Kantarci K; Jack CR; Campeau NG; O’Brien PC; Smith GE; Ivnik RJ; Boeve BF; Kokmen E; Tangalos EG et al. (2000). Regional metabolic patterns in mild cognitive impairment and Alzheimer’s Disease: a H MRS study. Neurology 55, 210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur S, Gonzales M, Strasser B, Pasha E, McNeely J, Tanaka H, Haley AP (2015) Central adiposity & cortical thickness in midlife, Psychosomatic Medicine 77, 671–678 [DOI] [PubMed] [Google Scholar]
- 25.Kernie SG, Leibl D, Parada lf (2000). BDNF regulates eating behavior and locomotor activity in mice. EMBO J 19, 1290–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerwin DR, Gaussoin SA, Chlebowski RT, Kuller LH, Vitolins M, Coker LH et al. (2011). Interaction between body mass index and central adiposity and risk of incident cognitive impairment and dementia: results from the Women’s Health Initiative Memory Study. Journal of the American Geriatric Society 59, 107–12. [DOI] [PubMed] [Google Scholar]
- 27.Kopelman P (2000). Obesity as a medical problem. Nature 404, 635–643. [DOI] [PubMed] [Google Scholar]
- 28.Pan W; Kastin AJ (2007). Adipokines and the blood-brain barrier. Peptides 28, 1317–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Provencher SW (1993). Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic Resonance in Medicine 30, 672–679. [DOI] [PubMed] [Google Scholar]
- 30.Qizilibash N; Gregson J; Johnson ME; Pearce N,; Douglas I; Wing K; et al. (2015). BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinology. [DOI] [PubMed] [Google Scholar]
- 31.Ross AJ; Sachdev PS; Wen W; Brodaty H; Joscelyn A; Lorentz LMm (2006). Prediction of cognitive decline after stroke using proton magnetic resonance spectroscopy. Journal of the Neurological Sciences 251, 62–69. [DOI] [PubMed] [Google Scholar]
- 32.Ross AJ; Sachdev PS; Wen W; Valenzuela MJ, Brodaty H (2005). Cognitive correlates of H MRS measures in the healthy elderly brain. Brain Research Bulletin 66, 9–16. [DOI] [PubMed] [Google Scholar]
- 33.Serrano-Pozo AMM, Gomez-Isla T, Betensky RA, Growdon JH, Frosch MP, Hyman BT (2011). Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s Disease. The American Journal of Pathology 179, 1373–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spranger J, Verma S, Gurling I, Bobbert T, Selfert J, Sindler AL, et al. (2006). Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes 55, 141–147. [PubMed] [Google Scholar]
- 35.Stern AJ; Savostyanova AA; Goldman A; Barnett AS; van der Veen JWC; Callicott JH et al. (2008). Impact of the Brain-Derived Neurotrophic Factor Val66Met Polymorphism on levels of hippocampal N-Acetyl-Aspartate assessed by magnetic resonance spectroscopic imaging at 3 Tesla. Biological Psychiatry 64, 856–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urenjak J; Williams SR; Gadian DG; Noble M (1992). Specific expression of N-acetyl aspartate in neurons, oligodendrocyte-type-2 astrocyte progenitors and immature oligodendrocytes in vitro. Journal of Neurochemistry 59, 55–61. [DOI] [PubMed] [Google Scholar]
- 37.Warren MW, Hynan LS & Weiner MF (2012). Lipids and adipokines as risk factors for Alzheimer’s disease. Journal of Alzheimers Disease 29, 151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization (2009). Global Health Risks: Mortality and burden of disease attributable to selected major risks. World Health Organization: Geneva, Switzerland. [Google Scholar]
- 39.Xia Y, Ergun DL, Wacker WK, Wang X, Davis CE, Kaul S (2014). Relationship between Dual-Energy X-Ray Apsorptiometry Volumetric Assessment and X-ray Computed Tomography-Derived Single Slice Measurement of Visceral Fat. Journal of Clinical Densitometry: Assessment & Management of Musculoskeletal Health 17. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W; Wang PJ; Li MH; Wang GL; Li P; Gao ZL (2013). 1H-MRS Assessment of the therapeutic effect of bilateral intraventricular BDNF infusion into APP/PS1 double transgenic mice. Journal of Molecular Neuroscience 50, 434–442. [DOI] [PubMed] [Google Scholar]