Abstract
Transcranial infrared laser stimulation (TILS) at 1064 nm, 250 mW/cm2 has been proven safe and effective for increasing neurocognitive functions in young adults in controlled studies using photobiomodulation of the right prefrontal cortex. We hypothesized that TILS may also improve neurocognitive function in older adults with subjective memory complaint at risk for cognitive decline (e.g., increased carotid artery intima-media thickness or mild traumatic brain injury). We investigated the cognitive effects of TILS in older adults (ages 49–90, n=12) using prefrontal cortex measures of attention (psychomotor vigilance task, PVT) and memory (delayed match-to-sample, DMS), carotid artery intima-media thickness (measured by ultrasound), and evaluated the potential neural mechanisms mediating the cognitive effects of TILS using exploratory brain studies of electroencephalography (EEG, n=6) and functional magnetic resonance imaging (fMRI, n=6). Cognitive performance, age and carotid artery intima-media thickness were highly correlated, but all participants improved in all cognitive measures after TILS treatments. Baseline vs. Chronic (5 weekly sessions, 8 minutes each) comparisons of mean cognitive scores all showed improvements, significant for PVT reaction time (p < 0.001), PVT lapses (p < 0.001), and DMS correct responses (p < 0.05). The neural studies also showed for the first time that TILS increases resting-state EEG alpha, beta and gamma power, and promotes more efficient prefrontal BOLD-fMRI response. Importantly, no adverse effects were found. These preliminary findings support the use of TILS for larger randomized clinical trials with this non-invasive approach to augment neurocognitive function in older people to combat aging-related and vascular disease-related cognitive decline.
Keywords: Brain Photobiomodulation, Infrared Laser, Attention, Memory, EEG, fMRI
Introduction
Photobiomodulation involves the absorption of high-energy photons and the subsequent modulation of metabolic processes in cells, including neurons [1, 2]. For red-to-near-infrared light, the major intracellular molecule absorbing photons is cytochrome c oxidase [3], a mitochondrial respiratory enzyme that can be up-regulated in vitro and in vivo [4–7]. Up-regulation of cytochrome c oxidase serves to convert high-energy photons into a source for ATP-based metabolic energy production in the brain [8]. Photobiomodulation of neural functions has been successful at 633–1070 nm wavelengths (for reviews, see [9–11]). For example, Naeser and collaborators [12] pioneered using transcranial photobiomodulation with LED arrays of 633 and 870 nm in case studies showing improvement of cognitive functions in patients with mild traumatic brain injury. In addition, 1064 nm transcranial infrared laser stimulation (TILS) has been proven effective for increasing human cognitive and emotional functions in controlled studies using photobiomodulation aimed at the right prefrontal cortex [13–17]. In particular, TILS of the human prefrontal cortex with a wavelength of 1064 nm increases the levels of oxidized cytochrome c oxidase, the conformation of the enzyme that has the highest activity, which leads to improved cerebral oxygenation [18, 19]. This photonics-bioenergetics in vivo mechanism is important for cognitive brain functions because nerve cells are critically dependent on oxidative energy metabolism [10, 20, 21].
We hypothesized that TILS may also improve cognitive performance in middle-aged and older adults at risk for cognitive decline in general. This risk may be associated with aging-related subjective memory complaint, cardiovascular disease or brain trauma. For example, cerebrovascular disease poses a severe threat to public health, and is only expected to become more widespread as the worldwide population ages. Specifically, atherosclerosis of the carotid artery is a strong predictor of cognitive decline [22]. The carotid artery intima-media thickness (IMT) is also recognized as a risk factor for brain damage in asymptomatic patients [23].
Middle-aged and older adults reporting memory complaint, showing early signs of carotid atherosclerosis (increased IMT values) or with a history of brain trauma, are prime candidates for early interventions aimed to minimize or prevent cognitive decline. One potential non-invasive intervention may be increasing cerebral oxygenation by upregulating mitochondrial respiration using TILS [18, 24]. TILS in the present study was administered using the same 1064-nm laser and stimulation parameters as in our previous cognitive studies with young adults [13–17], but with repeated weekly sessions for five weeks. Our study aimed to investigate the neurocognitive effects of TILS on prefrontal cortex measures of attention and memory in a middle-aged and older population with IMT values measured by carotid ultrasound, and to evaluate the potential neural mechanisms mediating the cognitive effects using exploratory brain studies of electroencephalography (EEG) and functional magnetic resonance imaging (fMRI).
Materials and methods
Participants
A total of 21 participants were recruited mainly through the use of flyers and were not provided compensation. For the cognitive study, there were 12 participants (5 males and 7 postmenopausal females) aged 49–90 with self-reported subjective memory complaint. Three of the participants in the cognitive study also had mild traumatic brain injury (TBI) within a year before the study, and one participant was medically diagnosed with mild cognitive impairment (MCI) prior to the cognitive study. These four participants were referred by their neurologists to our study. Our strategy was to focus on cognitively at-risk adults and exclude participants with already established cardiovascular disease or dementia. Other exclusion criteria were: current pregnancy, prior institutionalization or imprisonment, diagnosis of psychotic disorder, or history of violent behavior. For the neural studies there were also 12 participants (n=6 for EEG and n=6 for fMRI). The participants for the cognitive and neural studies were different except for three participants selected from the cognitive study that were also included in the fMRI study done before and after TILS, and these three participants were matched with three control subjects of the same age, sex, and IMT scores. Males and females are not affected differently by TILS [13–17].
The experimenters obtained informed consent at the beginning of the cognitive study before any experimental session had taken place. The consent form explained the safety procedures used in the operation of the laser throughout the treatment sessions. Separate consent was sought for participants who participated in the neural studies. Once the procedure and rationale of the study was made clear to the participant, each participant was given the chance to opt out of the study without any negative repercussions. The study protocol was approved by the University of Texas at Austin’s Institutional Review Board (IRB).
Instruments and procedures
Laser treatment
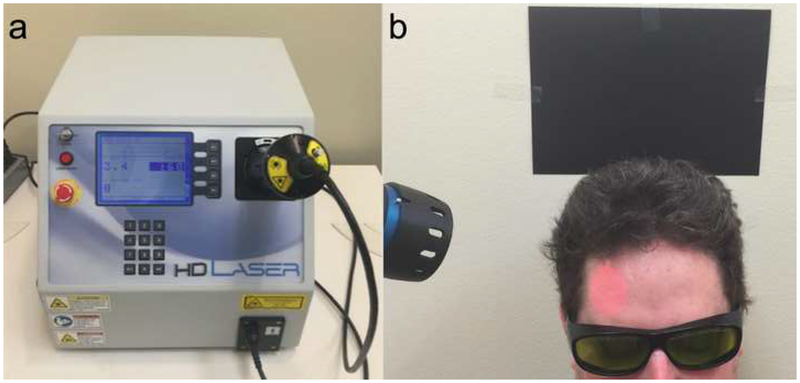
TILS was administered once a week for 5 weeks, following the same stimulation procedure as in our approved IRB protocol and as reported in Barrett and Gonzalez-Lima [13]. Administration of TILS consisted of applying coherent infrared light of a specific wavelength (1064 nm) using a well-collimated laser diode (HD laser, Cell Gen Therapeutics, Dallas, TX; Fig. 1A). This apparatus is FDA-cleared for human use for other indications, such as improving circulation, and has received approval from The University of Texas at Austin Laser Safety Program. Both participants and experimenters wore protective eyewear, the administrators of the TILS were careful not to shine the light in or near the eyes, and participants’ eyes remained closed during laser application.
Fig. 1.
a The FDA-cleared Class 4 laser apparatus (HD Laser, Cell Gen Therapeutics, Dallas, TX) consisted of a control unit (16” × 14” × 13”) with a black hand piece (45-mm diameter laser aperture) connected via a fiber-optic cable. b The laser was aimed at the forehead using an internal red diode aiming light. Since the 1064 nm laser is invisible, the beam area provided visual confirmation to facilitate precise tissue targeting. During laser operation, participants were instructed to keep their eyes closed, and the experimenters and participants both wore dark safety glasses that block the specific infrared wavelength from reaching the eyes
The laser beam area was uniform and measured 13.6 cm2. The laser wave was continuous (CW) and the power output used was 3.4 W. The irradiance (power density) used, 250 mW/cm2 (3400 mW/13.6 cm2 = 250 mW/cm2) as well as the cumulative fluence (energy density) used per site, 60 J/cm2 (0.25 W/cm2 × 240 s = 60 J/cm2) are the same parameters that previously showed psychologically beneficial effects [13–17, 25]. We have verified previously that these TILS parameters effectively enhance cerebral oxygenation [19] and cytochrome c oxidase levels in humans [18]. At the parameters described, the power density at this setting is one quarter of the skin MPE (0.25 W/cm2), exposure to it is not deemed harmful to tissue, and it causes no detectable physical damage and negligible heat. The laser’s power output was automatically calibrated by an internal mechanism every time the user set a power level.
The laser was directed at the right forehead (Fig. 1B), targeting the right prefrontal cortex, the area of the brain with the strongest relationship between carotid IMT and working memory performance in our prior fMRI study [22]. The forehead stimulation location extended 4 cm medial and 4 cm lateral to the FP2 point used in the standard 10–20 EEG placement system. The laser stimulation was alternated every minute between these medial and lateral sites for a total of 4 min per site (3.4 W × 240 s = 816 J/site). The total treatment duration lasted 8 minutes and was repeated weekly for 5 weeks. TILS took place in a locked room in the Gonzalez-Lima lab that has been approved by the University of Texas at Austin Laser Safety Program for housing of the laser apparatus.
Cognitive performance
The sequence of events for the cognitive study is listed in Table 1. Cognitive performance was assessed using two tasks: the psychomotor vigilance task (PVT), a test of sustained attention; and the delayed match-to-sample memory task (DMS), a test of visual working memory. The prefrontal cortex region that the TILS targeted is involved in these cognitive tasks [13]. The first testing session was a pre-treatment baseline evaluation (this was preceded by a brief 1-minute-long introduction to both tasks; these data were not recorded). After the weekly TILS, participants repeated the PVT and DMS tasks for 5 weeks in total. We have previously demonstrated an improvement in performance on both the PVT and DMS tasks after a single TILS in healthy young adults [13, 16], but we have not previously investigated the effects of repeated weekly TILS sessions.
Table 1.
Sequence of events for cognitive study.
| 1. Confirmation of subject screening with exclusionary/inclusionary criteria. |
| 2. Medical questionnaire and informed consent document are filled out. |
| 3. Ultrasound for carotid artery intima media thickness (IMT) measurement (half hour). |
| 4. Brief introduction to the psychomotor vigilance task (PVT) (1 minute). |
| 5. Baseline cognitive testing with PVT (5 minutes). |
| 6. Brief introduction to the delayed match-to-sample task (DMS) (1 minute). |
| 7. Baseline cognitive testing with DMS (5 minutes). |
| 8. First treatment with TILS (8 minutes). |
| 9. Post-treatment cognitive testing with PVT (5 minutes). |
| 10. Post-treatment cognitive testing with DMS (5 minutes). |
| [11–14 below: each subsequent week for 4 additional weeks; session duration, 30 minutes]: |
| 11. Subjects answer the question, “Did you have any side effects from the treatment?” |
| 12. Weekly continuing treatment with TILS (8 minutes). |
| 13. Post-treatment cognitive testing with PVT (5 minutes). |
| 14. Post-treatment cognitive testing with DMS (5 minutes). |
| [One week after final TILS treatment session]: |
| 16. Contacted by email, subjects will answer the side-effects question a final time. |
The PVT is a reaction time test in which participants attend to a small fixation point at the center of a computer screen. At random intervals, a bright millisecond timer appears in the center of the screen. Participants are instructed to respond via button press as rapidly as possible upon detection of the counter stimulus; participant response stops the counter from updating. The final counter value corresponds to the participant’s reaction time and is briefly displayed on-screen, thus providing feedback for that particular trial. If a participant makes a keypress prior to the onset of the timer, the feedback “Too soon” is displayed onscreen. Information about each trial’s reaction time is stored for later analysis. The block of 40 PVT trials is approximately five minutes long; intertrial intervals are pseudorandomly chosen without replacement from between 2 and 10 seconds; thus, the average intertrial interval is around 6 seconds.
The DMS also measures attention and reaction time, but has a short-term memory component as well. Participants view a 6×6 grid of brightly-colored yellow and red squares with a unique pattern. Then, with a key press, that stimulus disappears, and the screen is blank through a delay; then, two stimuli are presented on screen (a “match” and “nonmatch”). The participant indicates which stimulus is the correct “match” with a keypress. Feedback (Correct or Incorrect) is briefly displayed onscreen before the next trial commences. Success/failure and reaction time across 30 trials are measured by the computer and stored for later analysis.
Ultrasound scan
Images of the carotid artery were obtained using a GE LOGIQ e Ultrasound System as previously described [22]. A longitudinal image (B-mode) of the cephalic portion of the common carotid artery was acquired at 90° to the vessels, thus giving clear images of the near and far walls. Subsequently, ultrasound images were analyzed using computerized image-analysis software (Vascular Research Tool Carotid Analyzer, Medical Imaging Applications, Coralville, IA). Carotid IMT is defined as the distance between the leading edge of the lumen-intima interface and the leading edge of the media-adventitia interface of the far wall.
EEG
Standard 10–20 methods were used to record scalp EEG signals using electrodes placed over the frontal, temporal, parietal and occipital lobes of both hemispheres. Since the EEG alpha wave is more obvious than other wave frequencies when eyes are closed, we recorded waves during eyes closed and open conditions. We followed the same protocol for laser stimulation as in our previous study of TILS-induced cerebral oxygenation measured with functional near infrared spectroscopy (fNIRS) [19]. The eyes were open for 5 sec and closed for 55 sec during each recording cycle (1-min epoch). The motions of opening and closing the eyes provided clear EEG signals that served to mark each epoch in order to have consistent measurements. In each of 6 subjects who underwent EEG examinations, EEG was continuously recorded before, during and after TILS in three successive periods: 1) 10 times of 55 sec closed eyes, 5 sec open eyes, without TILS; 2) 10 times of 55 sec closed eyes with TILS, 5 sec of open eyes; 3) 10 times of 55 sec closed eyes, 5 sec open eyes, without TILS. The total EEG recording time was 30 min/subject. With 55 sec of laser exposure per cycle, the laser fluence per cycle was 0.25 W/cm2 × 55 sec = 13.75 J/cm2. The laser energy delivered per cycle was 3.4 W × 55 sec = 187 J.
fMRI
Imaging data for selected participants was acquired pre- and post- TILS on a 3T Siemens Skyra MRI scanner as previously described [26]. Briefly, anatomical scans of the entire brain were collected in the sagittal plane using a high-resolution ultrafast Gradient Echo 3D (MPRAGE) sequence (256 × 256 matrix, flip angle = 7°, field of view (FOV) = 24 × 24 cm2, 1 mm slice thickness, 0 gap). Functional MRI was performed during completion of the 2-back verbal working memory using a whole-brain echo-planar imaging sequence (TR=3000ms, TE=30ms, flip angle=90°, FOV=24×24cm2, 64 × 64 matrix, 42 axial slices, 3 mm slice thickness, 0.3 mm gap). During this task, participants are asked to decide if a letter appearing on a screen is the letter H (0-back control condition), or the same as the letter that appeared one or two stimuli earlier (n-back working memory condition). Each letter is displayed for 500 ms with 2500 ms inter-stimulus interval during which the person can make a response using an MRI-compatible button box. All letters are consonants presented in random order, organized in alternating blocks of 12 (0-back) or 15 (1- and 2-back) consonants. 33% of the letters are targets. Each of the two runs of the task consist of three alternating 0-back, 1-back, and 2-back blocks. Mean accuracy and reaction time for correct trials are used as performance indicators. All EPI images were processed using Analysis of Functional NeuroImages (AFNI) software. Each time series was spatially registered to the sixth volume of the session. The resulting information on displacement and rotation of each volume was later used to correct for head motion. Pre-processing steps also included temporal smoothing and spatial blurring using a 4.5 mm kernel. Averaged task-related activation, to be used as an outcome measure in subsequent analyses, was extracted from a set of a-priori regions of interest (ROIs) where signal intensity changes have been shown to be significantly related to 2-back performance in an independent sample, to avoid circularity [22].
BOLD data were collected pre- and post- TILS treatment for a subset of 6 subjects, carefully selected so that they had varying levels of thickness of the carotid artery wall, as measured by ultrasound. This allowed us to gather proof-of-concept data to determine if there is a relationship between increasing carotid atherosclerosis and neurocognitive fMRI activity, and whether non-invasive transcranial laser treatment can modify this relationship.
Statistical analyses
Data analyses were performed using R, an open source statistical software package. Repeated measures ANOVA were used to compare the baseline cognitive scores with the five weeks of treatment. Planned pairwise comparisons were also done with paired t-tests conducted on Baseline vs. Acute (single session, week 1) and on Baseline vs. Chronic (repeated sessions, weeks 2–5). Pearson product-moment correlations were used to determine the relationships between cognitive performance, carotid IMT and age. EEG power spectral density (PSD) analyses were used to determine TILS effects on resting-state EEG frequencies. Task-related BOLD-fMRI responses were determined using voxel-wise multiple regression analyses including all task trials, separate regressors for each task condition, convolved with a gamma function, and covariates accounting for instruction screens and head motion [26]. The significance level was set at p < 0.05, two-tailed.
Results
Cognitive enhancement after TILS in older adults
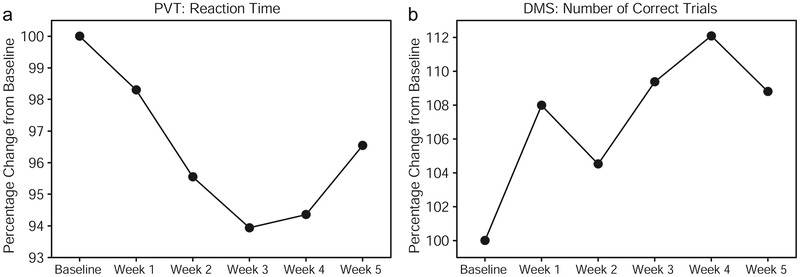
Table 1 shows subject characteristics and complete behavioral data of each of the 12 subjects who participated in the weekly cognitive tests. All participants in this older sample (ages 49–90 with a median age of 56) improved after repeated treatments in all cognitive measures. Reaction time (in msec) and number of lapses (trials in which the subject failed to respond within 500 msec of the stimulus) were recorded for the PVT; number of correct responses were recorded for the DMS. The normalized data using percentage change from baseline demonstrated that all subjects improved over time after repeated treatments (Fig. 2). For a repeated measures ANOVA on the PVT data (Fig. 2a), using six data points (baseline + five weeks of treatment), and using percentage change from baseline as the dependent variable, the effect of the within-subject variable (Session) was significant: F(5,40) = 2.7877, p < 0.03. Also, a repeated measures ANOVA on the DMS data (Fig. 2b), using six data points (baseline + five weeks of treatment), using percentage change from baseline as the dependent variable, showed the effect of the within-subject variable (Session) was significant: F(5,40) = 3.5799, p < 0.01.
Fig. 2.
Normalized mean performance (n=12) on the psychomotor vigilance test (a: PVT) and delayed match-to-sample test (b: DMS) across five weeks of treatment. Reaction time and number of correct trials are expressed as the percentage change from performance at baseline. Subjects showed progressively faster responding in the PVT, and progressively more accurate responses in the DMS, over weeks of laser treatment. The apparently weaker mean effects for week 5 were due to 3 subjects approaching ceiling performance and missing the last session
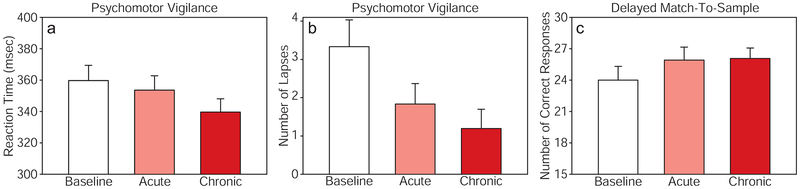
Fig. 3 illustrates mean cognitive scores for Baseline vs. Acute (after a single session at week 1) pairwise comparisons, where paired t-tests indicated significant improvements for PVT lapses (p < 0.001), but not for PVT reaction time (p = 0.12) and borderline for DMS correct responses (p = 0.05). However, paired t-tests conducted on Baseline vs. Chronic (after repeated sessions at weeks 2–5) showed all improvements were significant for PVT reaction time (p < 0.001), PVT lapses (p < 0.001), and DMS correct responses (p < 0.05).
Fig. 3.
Cognitive effects of 1064-nm laser treatment on attention and memory in older adults with subjective memory complaint (n=12). The psychomotor vigilance test (PVT) and delayed match-to-sample test (DMS) were conducted immediately before the first laser treatment (Baseline: week 1), immediately after the first laser treatment (Acute: week 1), and on subsequent weeks after additional laser treatments (Chronic: average of weeks 2, 3, 4, 5). a Reaction time (in msec) and b number of lapses (trials in which the subject failed to respond within 500 msec of the stimulus) were recorded for the PVT; c number of correct responses were recorded for the DMS. Bars show group means plus standard errors
Detailed inspection of Table 1 also indicated that the three participants with TBI and the one diagnosed with MCI had poorer baseline scores, with longer reaction times (mean of 383 msec as compared to 348 msec in the other subjects) and larger number of lapses (mean of 6 as compared to 2 in the others) in the vigilance task. However, each one of these TBI and MCI participants benefited from TILS.
Increasing carotid IMT was related to increasing age and cognitive decline
The three participants with TBI were excluded from the IMT evaluation because their brain injuries could account for their cognitive decline. The mean IMT of the rest of the sample was 0.74 mm and values ranged from 0.55 in the youngest participant (age 49) to 1.04 in the oldest participant (age 90), increasing as a linear function of the age of the participants. Therefore, IMT and age were highly correlated (r = 0.83). As expected, IMT was also related to cognitive performance. Specifically, IMT values were positively correlated with reaction time (r = 0.42) and number of response lapses (r = 0.47), whereas IMT values were negatively correlated with number of correct DMS responses (r = −0.31). Therefore, older participants with larger IMT values seemed to benefit relatively more from the TILS treatments. For example, the number of trials in which these older subjects failed to respond within 500 msec of the stimuli was reduced by 55% after a single laser treatment and by 74% after four additional laser treatments.
TILS increased resting-state EEG alpha, beta and gamma power
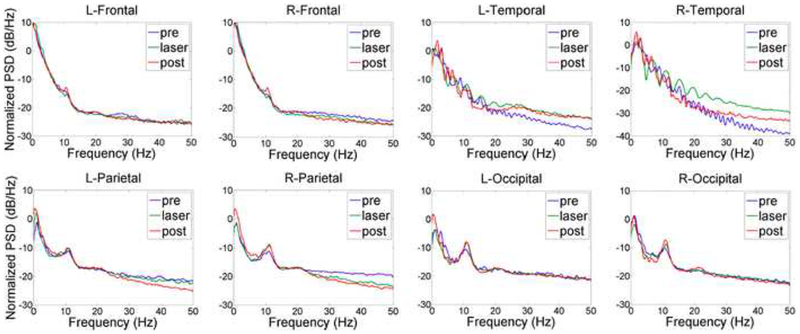
TILS effects on resting-state EEG frequencies occurred in both right and left hemispheres. Fig. 4 shows the EEG power spectral density (PSD) normalized by the power within the bandwidth of 0–50 Hz. The integral of PSD in each bandwidth is the relative power of the signal within that bandwidth. There were clear enhancements of alpha power, both during and after TILS. As illustrated in Fig. 4, the largest peaks were seen in the occipital recordings, visible as peaks around 10 Hz for alpha. Similar but smaller effects were seen in the parietal recordings. Small effects were found in the frontal recordings, and no alpha effects were found in the temporal recordings. Instead, temporal recordings showed enhanced gamma power (32+ Hz) and smaller increases during the laser treatment around 20 Hz for beta bands.
Fig. 4.
Effects of TILS on EEG power spectral density (PSD) normalized by the power within the bandwidth of 0–50 Hz before (pre, blue curve), during (laser, green) and after (post, red) stimulation. The integral of PSD in each bandwidth is the relative power of the signal within that bandwidth. This power times epoch duration time gives the energy of the signal within that bandwidth (10 epochs of 1-min each for pre, laser and post recording periods). There were clear bilateral enhancements of alpha power in the occipital recordings (peaks around 10 Hz for alpha). The alpha power increased progressively from frontal, to parietal, to occipital recordings during laser and post-laser relative to pre-laser. No alpha effects were found in temporal recordings, which instead showed increased gamma power (32+ Hz) and beta power around 20 Hz
TILS reduced task-evoked BOLD-fMRI responses
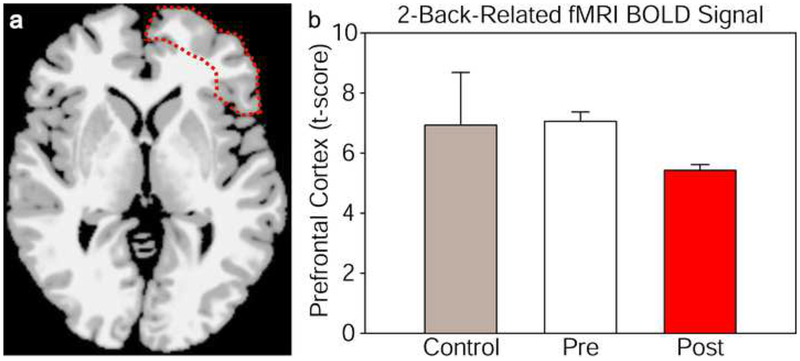
Table 3 lists the regions of interest analyzed and the corresponding stereotaxic coordinates and t-scores from the mean BOLD differences between 0-back vs. 2-back cognitive tasks for each region and condition. The right prefrontal cortex region which showed a BOLD decrease is delineated in Fig. 5a, with mean change in brain intensity signal from this region in Fig. 5b. Cognitive improvement was accompanied by a decrease in BOLD-fMRI signal in right prefrontal cortex areas targeted by TILS, likely indicating an improved cognitive efficiency, though the difference was only a strong trend, likely due to low number of subjects. Comparing t-scores from the right prefrontal cortex (superior, middle and inferior frontal gyri) for pre-TILS vs. post-TILS with uncorrected 2-tailed paired t-tests gave p < 0.03 for each comparison; the same comparisons of pre-TILS vs. controls matched by age, sex, and IMT gave p > 0.3.
Table 3.
Brain regions of interest analyzed by fMRI BOLD responses (mean t-scores) to 0-back vs. 2-back cognitive tasks performed before and after TILS (Pre vs. Post), and in untreated controls matched by age, sex and carotid artery intima-media thickness. Coordinates (Talairach or TAL X, Y, Z), Brodmann area and voxel size are given for each region of interest.
| TAL Coordinates | fMRI BOLD Signal | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Brain Region | Brodmann area | X | Y | Z | Size (mm2) | Pre | Post | Control | ||
| Right superior frontal gyrus | BA6 | 32 | 5 | 55 | 553 | 7.44 | 5.82 | 10.39 | ||
| Right middle frontal gyrus | BA10 | 33 | 48 | 15 | 955 | 6.43 | 5.23 | 5.76 | ||
| Right inferior frontal gyrus | BA44 | 47 | 14 | 3 | 335 | 7.31 | 5.24 | 4.64 | ||
| Left superior frontal gyrus | BA8 | −5 | 19 | 44 | 1651 | 7.74 | 8.18 | 8.34 | ||
| Left middle frontal gyrus | BA6 | −33 | 4 | 56 | 2087 | 6.83 | 8.26 | 6.78 | ||
| Left inferior frontal gyrus | BA46 | −44 | 45 | 13 | 1139 | 3.72 | 3.75 | 3.77 | ||
| Right superior parietal lobule | BA7 | 37 | −63 | 53 | 683 | 6.75 | 4.83 | 10.72 | ||
| Left inferior parietal lobule | BA39 | −49 | −52 | 44 | 1333 | 6.30 | 6.98 | 7.30 | ||
| Right cerebellar hemisphere | 34 | −61 | −24 | 437 | 7.79 | 8.59 | 6.83 | |||
| Left cerebellar hemisphere | −38 | −61 | −45 | 644 | 6.04 | 5.26 | 4.69 | |||
Fig. 5.
Right prefrontal cortex region with lower BOLD-fMRI responses to a 2-back vs. 0-back cognitive task compared between pre- and post-treatment with TILS to the right forehead (delineated area in a). The bar graphs b show the corresponding values of brain intensity signal (t-score) from this region, averaged between 3 treated subjects (pre- and post-treatment) and 3 untreated subjects (control). Bars show means plus standard errors
Discussion
In the present study, we report novel findings on the neurocognitive benefits of repeated TILS on middle-aged and older adults with subjective memory complaint. This pilot study was conducted to investigate the potential of TILS as a therapy aimed to improve cognitive function in high-risk participants. Reaction time was measured using a sustained attention psychomotor vigilance task (PVT), while working memory was measured using a delayed match-to-sample (DMS) memory retrieval task. All participants that took part in this study improved in their reaction time (PVT), number of correct trials (DMS), and in reducing their number of lapses (PVT). Each of these dependent variables was significantly improved after five weeks of treatment; even a single treatment significantly improved the number of lapses, with strong trends for improvement in the other two dependent variables. Both age and number of lapses were positively correlated with carotid IMT values. This should not be surprising, as both vascular health and attentional processes have been shown to decline with growing age [22]. As predicted, cognitive improvements as measured by the DMS and PVT tasks were larger in the older patients with higher IMT values than the younger participants. Importantly, the participants with TBI also benefited from TILS, a finding consistent with previous photobiomodulation studies of TBI [12].
EEG was used to explore resting-state brain activity, because there is evidence showing that cognitive performance is associated with EEG alpha, beta and gamma power levels [27–29]. Our findings indicate that TILS induced power increases in alpha, beta and gamma frequencies relative to pre-stimulation baseline, which is consistent with a brain resting-state implicated in improved cognition. These EEG changes were found both ipsilateral to the stimulation side (right) as well as contralateral (left), indicating that TILS modulated bilateral neural networks in the resting state. In particular, the alpha power changes were greater in occipital, parietal and frontal regions, which are part of the neural networks tested in our PVT and DMS tasks. These EEG changes were found not only during TILS (10 min) but also after TILS (10 min), which is consistent with the duration of TILS-induced increases in cytochrome c oxidase and cerebral oxygenation we have reported in our previous fNIRS studies that used an identical laser stimulation protocol [7, 18, 19]. This suggests that the beneficial bioenergetic and hemodymamic effects of TILS on the brain modulate the resting-state electrophysiological activity of the human brain in a manner that may facilitate cognitive processing.
Furthermore, BOLD-fMRI responses to a working memory 2-back task pre- and post-treatment were also measured in select participants, to determine if subjects treated with TILS would show any alterations in the cerebrovascular physiological responses in vivo. The BOLD-fMRI signal is a combined output of increased cerebral blood flow, cerebral blood volume and reduced oxygen consumption. Since TILS increases oxygen consumption and oxygenated hemoglobin in the prefrontal cortex, as shown in our previous studies [6, 18, 19], a reduced BOLD-fMRI signal is expected. Cognitive improvement was accompanied by a strong trend in decreasing task-evoked BOLD-fMRI signal in stimulated prefrontal brain areas across varying IMT values. Relative to their own baseline, participants who received TILS exhibited a lower BOLD-fMRI response to a 2-back vs. 0-back task post-treatment, while no difference was observed with the control group matched by age, sex and IMT.
Several limitations affect the present pilot study. First, without a placebo group, we cannot rule out the possibility that the cognitive benefits could be due to a placebo or practice effect. We chose to treat with TILS all of the 12 participants with subjective memory complaint because we felt it was important to determine first if there was a potential benefit to these older participants before conducting larger placebo-controlled studies. The functional neuroimaging study included a comparison control group without laser treatment (Table 3). Second, limitations of cost prevented us from using BOLD-fMRI on all participants. Third, in an ideal situation, all age groups would be represented; however, no one in their 70s participated in our study. Fourth, three participants did not attend the final sessions. With additional funding, we could perhaps offer a financial incentive to complete the experiment and minimize attrition of participants.
Conclusion
The results showed for the first time that TILS increases resting-state EEG alpha, beta and gamma power, promotes more efficient prefrontal BOLD-fMRI activity, and facilitates behavioral cognitive processing in middle-aged and older adults at risk for cognitive decline. Importantly, none of the participants reported adverse effects from TILS. Despite the study limitations, TILS showed very promising results in its potential to minimize the effects of vascular-induced cognitive decline in this older population. These preliminary findings support the use of TILS for larger randomized clinical trials to investigate this safe intervention as a new noninvasive therapy to augment neurocognitive function in older people and to combat aging-related and vascular disease-induced cognitive decline.
Table 2.
Characteristics and behavioral test results for each subject at baseline and across five weeks of treatment.
| PVT: Reaction Times (msec) | PVT: Number of Lapses | DMS: Correct Responses | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | Condition | BL | W1 | W2 | W3 | W4 | W5 | BL | W1 | W2 | W3 | W4 | W5 | BL | W1 | W2 | W3 | W4 | W5 |
| 49 | F | SMC | 335±5 | 329±6 | 315±5 | 315±4 | 320±6 | 314±3 | 2 | 0 | 0 | 0 | 0 | 0 | 25 | 28 | 25 | 29 | 30 | 28 |
| 50 | F | SMC | 342±6 | 328±7 | 325±5 | 308±5 | 327±6 | 345±6 | 1 | 0 | 2 | 0 | 1 | 0 | 28 | 30 | 27 | 30 | 29 | 29 |
| 53 | M | SMC | 389±5 | 385±6 | 361±4 | 378±5 | 378±4 | 355±5 | 2 | 0 | 0 | 0 | 0 | 0 | 22 | 26 | 27 | 29 | 30 | 27 |
| 53 | M | SMC | 355±6 | 327±6 | 322±5 | 304±6 | * | * | 1 | 0 | 0 | 0 | * | * | 25 | 28 | 28 | 30 | * | * |
| 55 | M | SMC, TBI | 416±6 | 404±7 | 387±5 | 393±6 | 368±4 | 390±6 | 4 | 3 | 1 | 0 | 0 | 0 | 27 | 30 | 26 | 26 | 29 | 26 |
| 56 | F | SMC | 285±7 | 302±8 | 288±6 | 303±6 | 296±5 | 307±7 | 1 | 0 | 0 | 1 | 0 | 0 | 30 | 28 | 28 | 28 | 27 | 30 |
| 57 | M | SMC, TBI | 356±6 | 356±7 | 334±7 | 318±5 | 320±6 | 306±3 | 5 | 3 | 3 | 0 | 0 | 0 | 27 | 28 | 28 | 29 | 30 | 30 |
| 63 | F | SMC, TBI | 368±10 | 355±10 | 359±9 | 372±9 | 352±9 | 360±10 | 7 | 3 | 3 | 2 | 1 | 3 | 19 | 28 | 23 | 26 | 26 | 27 |
| 68 | F | SMC | 340±7 | 329±4 | 335±8 | 299±6 | * | * | 1 | 1 | 0 | 1 | * | * | 26 | 24 | 25 | 26 | * | * |
| 68 | F | SMC | 363±10 | 373±8 | 366±10 | 330±9 | 310±8 | 373±9 | 4 | 4 | 4 | 6 | 2 | 4 | 23 | 25 | 25 | 22 | 26 | 23 |
| 90 | M | SMC | 377±8 | 362±6 | 340±6 | 337±6 | 348±7 | * | 4 | 3 | 0 | 0 | 1 | * | 23 | 21 | 22 | 19 | 25 | * |
| 90 | F | SMC, MCI | 391±7 | 395±9 | 393±8 | 398±10 | 375±8 | 375±8 | 8 | 5 | 5 | 5 | 4 | 7 | 13 | 15 | 17 | 21 | 17 | 15 |
PVT: psychomotor vigilance task, DMS: delayed match-to-sample, BL: baseline, W: week, SMC: subjective memory complaint, TBI: mild traumatic brain injury, MCI: mild cognitive impairment,
missing data from 3 subjects that did not complete all sessions.
Acknowledgements
The authors thank Stephanie Oleson and Alex Birdill, who were instrumental in collecting the fMRI data; Evan Pasha, the carotid ultrasound data; and Revanth Poondla, Angelymar Fuentes, Nadia Abdo, and Veronica Almendarez, the behavioral data. EV was supported by a student research fellowship and FGL was supported by a faculty research fellowship from the College of Liberals Arts of the University of Texas at Austin. This study was supported in part by grants from the National Institute on Aging (R21 AG055772) and the Darrell K. Royal Research Fund for Alzheimer’s Disease.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Research involving human participants All procedures were approved by the University of Texas at Austin Institutional Review Board (IRB) and were conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Anders JJ, Moges H, Wu X, Erbele ID, Alberico SL, Saidu EK, Smith JT, Pryor BA (2014) In vitro and in vivo optimization of infrared laser treatment for injured peripheral nerves. Lasers Surg Med 46(1):34–45 [DOI] [PubMed] [Google Scholar]
- 2.Anders JJ, Lanzafame RJ, Arany PR (2015) Low-level light/laser therapy versus photobiomodulation therapy. Photomed Laser Surg 33(4):183–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI (2005) Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. J Photochem Photobiol B Biol 81:98–106 [DOI] [PubMed] [Google Scholar]
- 4.Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT (2005) Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem 280:4761–4771 [DOI] [PubMed] [Google Scholar]
- 5.Rojas JC, Lee J, John JM, Gonzalez-Lima F (2008) Neuroprotective effects of near-infrared light in an in vivo model of mitochondrial optic neuropathy. J Neurosci 28(50):13511–13521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rojas JC, Bruchey AK, Gonzalez-Lima F (2012) Low-level light therapy improves cortical metabolic capacity and memory retention. J Alzheimers Dis 32:741–752 [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Tian F, Soni SS, Gonzalez-Lima F, Liu H (2016) Interplay between up-regulation of cytochrome-c oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci Rep 6:30540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mochizuki-Oda N, Kataoka Y, Cui Y, Yamada H, Heya M, Awazu K (2002) Effects of near-infra-red laser irradiation on adenosine triphosphate and adenosine diphosphate contents of rat brain tissue. Neurosci Lett 323(3):207–10 [DOI] [PubMed] [Google Scholar]
- 9.Rojas JC, Gonzalez-Lima F (2013) Neurological and psychological applications of transcranial lasers and LEDs. Biochem Pharmacol 86:447–457 [DOI] [PubMed] [Google Scholar]
- 10.Rojas JC, Gonzalez-Lima F (2017) Transcranial low-level laser light therapy for neurocognitive enhancement In: Hamblin MR, de Sousa MVP, Agrawal T (eds) Handbook of low-level laser therapy, 1st edn Pan Stanford Publishing, Singapore, pp 1057–1076 [Google Scholar]
- 11.Hamblin MR (2016) Shining light on the head: Photobiomodulation for brain disorders. BBA Clin 6:113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naeser MA, Martin PI, Ho MD, Krengel MH, Bogdanova Y, Knight JA, Yee MK, Zafonte R, Frazier J, Hamblin MR, Koo BB (2016) Transcranial, red/near-infrared light-emitting diode therapy to improve cognition in chronic traumatic brain injury. Photomed Laser Surg 34(12):610–626 [DOI] [PubMed] [Google Scholar]
- 13.Barrett DW, Gonzalez-Lima F (2013) Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience 230:13–23 [DOI] [PubMed] [Google Scholar]
- 14.Blanco NJ, Maddox WT, Gonzalez-Lima F (2015) Improving executive function using transcranial infrared laser stimulation. J Neuropsychol May 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanco NJ, Saucedo CL, Gonzalez-Lima F (2017) Transcranial infrared laser stimulation improves rule-based, but not information-integration, category learning in humans. Neurobiol Learn Mem 139:69–75 [DOI] [PubMed] [Google Scholar]
- 16.Hwang J, Castelli DM, Gonzalez-Lima F (2016) Cognitive enhancement by transcranial laser stimulation and acute aerobic exercise. Lasers Med Sci 31(6):1151–60 [DOI] [PubMed] [Google Scholar]
- 17.Disner SG, Beevers CG, Gonzalez-Lima F (2016) Transcranial laser stimulation as neuroenhancement for attention bias modification in adults with elevated depression symptoms. Brain Stimul 9(5):780–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Tian F, Reddy DD, Nalawade SS, Barrett DW, Gonzalez-Lima F, Liu H (2017) Up-regulation of cerebral cytochrome-c-oxidase and hemodynamics by transcranial infrared laser stimulation: A broadband near infrared spectroscopy study. J Cereb Blood Flow Metab (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian F, Hase SN, Gonzalez-Lima F, Liu H (2016) Transcranial laser stimulation improves human cerebral oxygenation. Lasers Surg Med 48(4):343–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rojas JC, Gonzalez-Lima F (2011) Low-level light therapy of eye and brain. Eye Brain 3:49–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Lima F, Barrett DW (2014) Augmentation of cognitive brain functions with transcranial lasers. Front Syst Neurosci 8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haley AP, Sweet LH, Gunstad J, Forman DE, Poppas A, Paul RH, Tate DF, Cohen RA (2007) Verbal working memory and atherosclerosis in patients with cardiovascular disease. An fMRI study. J Neuroimaging 17(3): 227–233 [DOI] [PubMed] [Google Scholar]
- 23.Nguyen-Thanh HT, Benzaquen BS (2009) Screening for subclinical coronary artery disease measuring carotid intima media thickness. Am J Cardiol 104(10):1383–8 [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Lima F, Barksdale BR, Rojas JC (2014) Mitochondrial respiration as a target for neuroprotection and cognitive enhancement. Biochem Pharmacol 88:584–593 [DOI] [PubMed] [Google Scholar]
- 25.Schiffer F, Johnston AL, Ravichandran C, Polcari A, Teicher MH, Webb RH, Hamblin MR (2009) Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behav Brain Funct 5:46–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzales MM, Kaur S, Eagan D, Goudarzi KK, Pasha E, Doan D, Tanaka H, Haley AP (2014) Central adiposity and the functional magnetic resonance imaging response to cognitive challenge. Int J Obes (Lond) 38(9):1193–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palva S, Palva JM (2011) Functional roles of alpha-band phase synchronization in local and large-scale cortical networks. Front Psychol 2:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka M, Shigihara Y, Ishii A, Funakura M, Kanai E, Watanabe Y (2012) Effect of mental fatigue on the nervous system: an electroencephalography study. Behav Brain Funct 8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basar E (2013) A review of gamma oscillations in healthy subjects and in cognitive impairment. Int J Psychophysiol 90(2):99–117 [DOI] [PubMed] [Google Scholar]