Abstract
Background
Tyrosine kinase inhibitors (TKIs) and checkpoint inhibitors have been established as effective treatment for metastatic renal cell carcinoma (mRCC), but only a minority of patients achieve complete response. Additional strategies are necessary to improve these agents’ efficacy.
Methods
Patients with stable disease for at least 4 months on TKI or checkpoint inhibitors were included. Stereotactic body radiotherapy (SBRT) was delivered to an organ with comparable lesions, where one lesion was in the treatment target and the other one was intentionally left untreated (control lesion). Response in both lesions was scored using the Response Evaluation Criteria in Solid Tumors V.1.1 criteria 2 months after completion of SBRT. The primary endpoint was the rate of SBRT adverse events, and the secondary endpoints included the rate of reduction in target lesion size.
Results
17 patients were enrolled (14 men and 3 women, median age: 54.5 years old). SBRT was delivered to the lungs (n=5), bones (n=4), lymph nodes (n=4), liver (n=1), primary renal cell carcinoma (RCC) (n=1) and locally recurrent RCC (n=2). The equivalent dose in 2 Gy with an alpha to beta ratio of 2.6 was 114 Gy. With a median follow-up of 8 months, the cumulative rate of SBRT-related toxicity (grade 1) was 12% (n=2), consisting of oesophagitis and skin erythema. No grade 2 or higher toxicity was detected. Radiographic response in the target lesion was seen in 13 patients (76%), with complete response in 5 (29%) patients and partial response in 8 (47%), including abscopal effect in 1 patient. Control lesions remained stable in 16 patients. The difference between response in the target and control lesions as judged by the mean sizes of these lesions before and at 2 months after SBRT was statistically significant (p<0.01). Fraction size of 10 Gy or greater was associated with complete response (p<0.01).
Conclusion
Extracranial SBRT in patients with mRCC treated with TKI or checkpoint inhibitors is well tolerated and could be effective.
Trial registration number
Keywords: metastatic renal cell carcinoma, stereotactic body radiotherapy, targeted therapy, immunotherapy
Key questions.
What is already known about this subject?
RCC was considered one of the most radiation-resistant malignancies.
SBRT was shown to be highly effective in controlling intracranial RCC metastases, however, data on SBRT effectiveness in RCC affecting other organs are limited.
Only a minority of patients achieve complete response on TKIs and checkpoint inhibitors.
What does this study add?
Extracranial SBRT in patients with mRCC treated with TKI or checkpoint inhibitors is well tolerated, with no grade 2 or higher toxicity detected, and could be effective.
Radiographic response in the target lesion was seen in 76% of patients with complete response in 29% of patients and partial response in 47% of patients.
Fraction size of equal to or greater than 10 Gy was associated with complete response in the target lesion (p<0.01).
Abscopal effect was registered in one out of 17 patients after fractionated RT to one of the lung lesions.
How might this impact on clinical practice?
As far as SBRT to extracranial metastases of mRCC appears to be well tolerated when combined with targeted or immunotherapy and leads to partial and complete response in treated lesions in the majority of patients, it can be more widely used for the treatment of patients with multiple metastases who receive modern effective systemic therapy, with benefit expected from the addition of local therapies.
Introduction
Renal cell carcinoma (RCC) is one of the ten most common malignancies in the world, with incidence rates steadily rising. It predominantly affects patients 60 years of age and older.1 Surgery is the standard treatment for primary RCC; however, local recurrences after surgery occur in over 30% of patients, and distant metastases develop in another 30%.2 Historically, RCC was considered one of the most radiation-resistant malignancies. Two mechanisms are believed to contribute to its relative resistance to conventionally fractionated radiation therapy (RT): the inherent characteristics of the RCC tumour cells and their microenvironment.3 4 Cells with a low alpha to beta ratio, such as RCC, do not show great response to low doses of conventionally fractionated RT due to their inherent ability to repair sublethal DNA damage. A published characterisation of molecular and genetic profile of RCC has revealed a dramatic lack of mutations in genes that are responsible for DNA repair.5 This, in part, may explain RCC resistance to both conventionally fractionated RT and systemic therapy. At the same time, early in vitro cell culture studies revealed that ablative doses of radiation—in which high-dose RT is delivered over very few fractions—can effectively eradicate RCC cells.
Stereotactic body radiotherapy (SBRT) has become an attractive treatment modality because of its ability to deliver highly conformal large radiation doses to a well-localised treatment volume in the course of a limited number of fractions. SBRT as a treatment modality was a logical extension of cranial stereotactic radiosurgery for brain metastases from various primary sites, including RCC, with excellent local control rates reaching 90%.6 In several clinical studies, SBRT was shown to be highly effective in controlling extracranial RCC metastases, primarily affecting the bones and the lungs.7 8 Unfortunately, there are limited data on SBRT effectiveness in RCC affecting other organs, which prevents clinicians from using this modality in patients with RCC metastases in the lymph nodes, liver and other sites.
New treatment options such as tyrosine kinase inhibitors (TKIs) and checkpoint inhibitors have been established as effective therapy for metastatic renal cell carcinoma (mRCC), but only a minority of patients achieve complete response. Additional strategies are necessary to improve the treatment efficacy. One strategy relies on combination of TKI and checkpoint inhibitors with RT, thus leading to increased sensitivity of RCC to the effect of ionising radiation due to the synergy between these modalities.9 This synergy could lead to the development of abscopal effect, whereby tumour regression is observed in non-irradiated areas, which is postulated to be driven by immune system-mediated cascade of events.
The safety of combination of SBRT with TKI agents and checkpoint inhibitors is largely unknown and must be established on prospective clinical trials. We have designed and launched the prospective phase Ib study ‘Volga’ to determine the safety and effectiveness of SBRT for extracranial mRCC metastases in combination with TKI or checkpoint inhibitors.
Methods and materials
Subjects
The study was open to adult patients (over 18 years of age) with metastatic histologically or cytologically proven clear-cell RCC (mRCC) who received standard TKI or checkpoint inhibitors therapy without dose reduction and achieved stable disease for the duration of at least 4 months. Subjects underwent CT with contrast in order to identify two measurable and radiographically stable metastatic lesions for at least 4 months located in the same organ. Allowed sites of disease included the lungs, liver, lymph nodes, kidney, kidney fossa and bones. Other sites of metastatic disease were excluded. One lesion in the organ was identified as ‘control’ and was not included in the SBRT treatment field, whereas the ‘target’ lesion underwent treatment with SBRT. The allowed size of each lesion was ≥5 mm and ≤50 mm. The difference between the volume of control and target lesions was ≤20%.
Study design
We conducted a prospective multicentre phase Ib clinical study, with five centres involved and three of them located in the Volga region of Russia. Eligible patients received SBRT to the target lesion, while the control lesion was excluded from the radiation treatment field. The total prescribed radiation dose and the number of fractions were determined based on the target localisation and proximity of critical organs. Normal tissue dose constraints followed established QUANTEC (Quantitative Analysis of Normal Tissue Effects in the Clinic) recommendations.10 The goal of the study was to determine the safety and efficacy of SBRT in patients with extracranial RCC metastases receiving standard systemic therapy with TKI or checkpoint inhibitors.
The primary endpoint was the rate of any adverse events related to SBRT. All patients were assessed for safety according to Common Terminology Criteria for Adverse Events (V.4.0). Safety assessments consisted of monitoring and recording of all adverse events, regular monitoring of haematology and clinical chemistry measurements, regular measurement of vital signs, performance of physical examinations, and recording of all concomitant medications and therapies.
The secondary endpoints included the rate of treatment response and time to progression of the target lesion in comparison with the control lesion. Three-dimensional radiographic evaluation of both target and control lesions with contrast-enhanced CT was performed 2 months after completion of SBRT. Standard clinical evaluation of a patient’s response to systemic therapy was also performed using Response Evaluation Criteria in Solid Tumors (V.1.1). All subsequent imaging studies were performed in 2-month intervals.
Statistical analyses
Based on the results of the CheckMate 025 study demonstrating an objective response rate of nivolumab of 26% in patients with mRCC,11 we aimed to test the null hypothesis—response rate of target lesion=26%—versus an alternative hypothesis—response rate of target lesion=65%. Setting α=0.05, 17 patients will be required to achieve 90% power using a two-sided log-rank test.
Summary statistics (mean, median and proportion) were used to describe baseline patient characteristics and treatment patterns. All statistical analyses were carried out using IBM SPSS Statistics Base V.22.0.
Results
Seventeen subjects were enrolled from November 2016 until April 2018, including 14 men and 3 women, with a median age of 54.5 years old (range 32–72). The characteristics of the subjects are listed in table 1. Six patients presented with newly diagnosed mRCC, and the remaining 11 patients subsequently developed mRCC after having undergone initial radical nephrectomy for a localised RCC, with interval between initial nephrectomy and mRCC diagnosis ranging between 6 months and 5 years. Twelve patients received TKI therapy and five received nivolumab. SBRT was delivered to the target lesions in the lungs (n=5), bones (n=4), lymph nodes (n=4), liver (n=1), primary kidney (n=1) and local recurrence in the kidney fossa (n=2). With an alpha to beta ratio of 2.6 for RCC, the mean equivalent dose in 2 Gy (EQD2) was 114 Gy (range, 40–276 Gy). SBRT was given on the same days with the systemic treatment in the majority of patients (n=15) or sandwiched in between two consequent cycles of systemic therapy (n=2).
Table 1.
Baseline demographic and clinical characteristics
| Age (years), mean (SD) | 54.5 (±27.5) |
| Gender, n (%) | |
| Male | 14 (82) |
| Female | 3 (18) |
| Karnofsky performance status ≥80, n (%) | 17 (100) |
| Metastatic sites, n (%) | |
| ≤1 | 6 (35) |
| ≥2 | 11 (65) |
| Site of metastasis, n (%) | |
| Lung | 12 (71) |
| Lymph nodes | 9 (53) |
| Liver | 5 (29) |
| Bone | 5 (29) |
| Locally recurrent RCC | 3 (18) |
| Size of lesions (cm), median | |
| Target | 3 |
| Control | 2.3 |
| Difference (p value) | 0.67 |
| Previous surgery, n (%) | |
| Radical nephrectomy | 12 (71) |
| Cytoreductive nephrectomy | 4 (24) |
| SBRT, n (%) | |
| EQD2 ≥100 Gy | 11 (65) |
| EQD2 <100 Gy | 6 (35) |
| Systemic therapy, n (%) | |
| Sunitinib | 6 (35) |
| Nivolumab | 5 (29) |
| Everolimus | 3 (18) |
| Lenvatinib + everolimus | 1 (6) |
| Temsirolimus | 1 (6) |
| Sorafenib | 1 (6) |
EQD2, equivalent dose in 2 Gy; RCC, renal cell carcinoma; SBRT, stereotactic body radiotherapy.
Subjects were followed for a mean duration of 8 months (range 3–18 months). All subjects (100%) received the prescribed course of SBRT with no dose reduction or treatment plan modification. No subjects required dose reduction or interruption in systemic therapy.
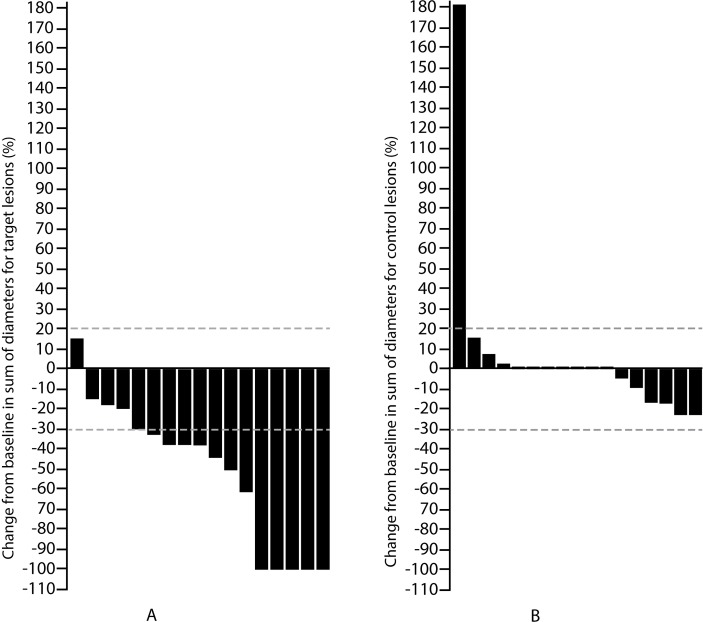
The rate of grade 1 toxicity was 12% and included oesophagitis (n=1) and skin erythema (n=1). No grade 2 or higher toxicity was observed. The response rate in the target lesions was 76%, with complete response in the target lesions registered in 5 out of 17 subjects (29%). Partial response was observed in 8 out of 17 subjects (47%), including 1 patient (6%) with abscopal effect. There was no difference in treatment response among patients on TKI versus checkpoint inhibitors (p=0.8). The median duration of response was not reached.
The response rate in the control lesions was 0%; they remained stable in 16 out of 17 patients (94%). One patient had control lesion progression, while the target lesion showed partial response.
The response rate in the target lesions was significantly higher than in the control lesions (p<0.001; figure 1). The change in the sum of the three diameters was greater in the target versus the control lesions (p=0.003).
Figure 1.
Response rate in target (A) and control (B) lesions after stereotactic body radiotherapy.
There was no association between the response rate and organ receiving radiation; however, a fraction size of 10 Gy or higher (EQD2 dose of 100 Gy or higher) most often led to complete response (p<0.01).
Clinical history of select subjects
Subject A
A 48-year-old man underwent right radical nephrectomy in 2013 and was found to have a pT3apN0M0 RCC. In March 2014 he developed multiple metastases in the lungs, mediastinal lymph nodes and ribs. The patient was started on targeted therapy and received pazopanib (from May until August 2014) and sorafenib (from September 2014 until January 2015). Due to lack of treatment response to prior therapies, he was switched to everolimus in March 2015 and had stable disease over the following 2.5 years. The patient was enrolled in the protocol in October 2017. He received SBRT (50 Gy in 5 fractions) to the metastatic lesion on the right upper lung. No radiation-related side effects were reported. First post-treatment CT of the chest revealed stability in both the target and control lesions. The next imaging study in March 2018 revealed partial response in the target lesion and all visible lesions in the lungs and mediastinum, with up to 50% regression in some lesions. This effect of RT on the non-treated lesions was deemed abscopal.
Subject B
A 49-year-old man underwent right radical nephrectomy in 2004 and was diagnosed with pT2pN0M0 RCC. In September 2005 he developed multiple lung metastases. He underwent upper lobectomy and radiofrequency ablation of one of the lesions and received systemic therapy with interferon-alpha and bevacizumab. Since March 2012 he was started on everolimus with clinical stability thereafter. He was enrolled on the study in January 2017 and received 50 Gy in 5 fractions to the right lower lobe metastatic lesion (figure 2). Thoracic CT in 2.5 months after SBRT revealed fibrotic changes with no evidence of malignancy (figure 3), which was scored as a complete response. The control lesion remained stable with no evidence of growth.
Figure 2.
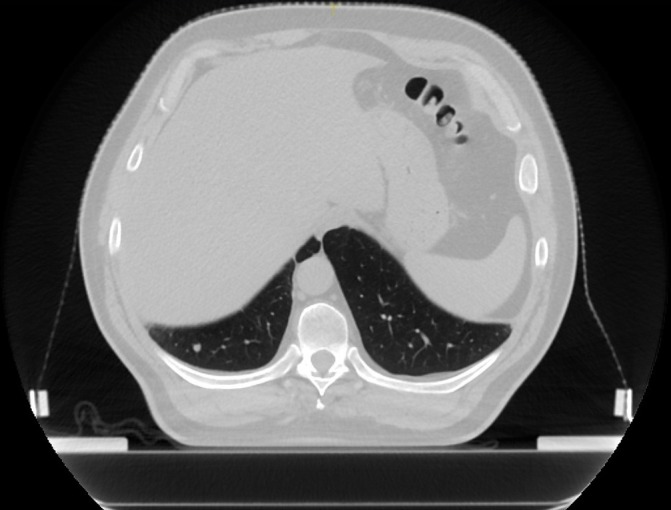
Subject B, before stereotactic body radiotherapy. Metastatic lesion on the right lower lobe.
Figure 3.
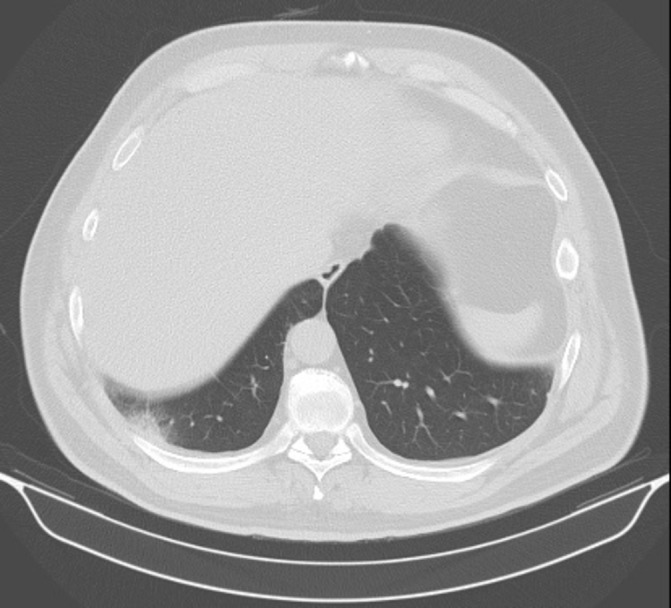
Subject B, 2.5 months after stereotactic body radiotherapy (50 Gy in 5 fractions). Localised fibrotic changes in the lung tissue. Lesion was not visualised.
Discussion
The topic of differential radiosensitivities among different malignant histologies appeared in the literature in the early 1950s. Deacon et al 12 classified RCC as the most radioresistant, along with melanoma, sarcoma and glioblastoma. However, preclinical studies revealed that ablative doses of radiation could effectively eradicate RCC cells. The two most common cell lines—Caki-1 and A498—demonstrated a low alpha to beta ratio, suggesting a higher sensitivity to large fractional doses.13 Survival curves in these cell lines exhibited a small decrease in survival with radiation doses between 0 and 6 Gy, yet an exponential decrease in survival ensued at doses over 6 Gy. In this context, SBRT technology, which allows delivery of high doses to small target volumes, appears most promising for treatment of both primary and metastatic RCC lesions. Furthermore, ablative irradiation with SBRT induces microvascular damage in the tumour microenvironment, which further increases the cytotoxic effect of RT. RCC is a highly vascularised malignancy and the angiogenesis is central to its development and progression.14 15 Therefore direct damage of the RCC vasculature and functional disruption of the tumour vascular endothelium, leading to increased penetration of systemic agents, further support SBRT as a potentially effective treatment modality for patients with RCC. Biological mechanisms other than mitotic catastrophe following double-strand DNA breaks are likely responsible for the increased sensitivity of RCC to large fractional doses. One proposed mechanism is through the production of proapoptotic second messenger ceramide molecules that stimulate endothelial cell apoptosis when a large fraction of 15–20 Gy is administered.16
The principle of combination of RT, and SBRT in particular, with targeted and immunotherapy has been previously described by several experts.9 17 Sunitinib, one of the most studied TKIs in RCC, is capable of potentiating the RT effect by normalising the tumour microenvironment and decreasing the levels of myeloid-derived-supressor cells (MDSCs) and regulatory T cells (T-regs). Sorafenib, despite being immunosuppressive, also decreases the number of T-regs cells and suppresses the inhibition of killer T cells, thus stimulating the immune effect. Pazopanib, one of the newest TKIs, in combination with RT, exhibits similar action to sunitinib. mammalian Target Of Rapamycin (mTOR) inhibitors, such as temsirolimus and everolimus, increase CD8+ T cell activation, and in combination with RT further stimulate immune response.
Combination of RT with immunotherapy appears to be most promising in RCC due to the inherent immunogenicity of this malignancy. Clinical response in lesions outside of the radiation field—known as abscopal effect—is of significant interest and has been documented by many authors.18 19 Preclinical and early clinical data support the immune-mediated nature of abscopal effect. At the same time, there are very limited data supporting the safety and efficacy of a combination of SBRT with targeted and immunotherapy in clinical setting.
The analysis of our prospective clinical study reveals that the combination of SBRT with targeted and immunotherapy is safe. The treatment-related toxicity rate was low, affecting only 2 out of 17 subjects, and was limited to grade 1. This suggests that SBRT can safely be administered to patients with mRCC receiving TKI or checkpoint inhibitors, as long as standard normal tissue constraints are strictly followed.10
The addition of SBRT to systemic therapy led to a rapid regression of the target lesions in the majority of patients, with 13 out of 17 subjects experiencing complete or partial response, while the control lesions remained stable in the context of continued systemic therapy. We did not observe a clear impact of the total RT dose on the treatment response; however, complete response was documented more often (in four out of five subjects) when fractional dose was ≥10 Gy, achieving EQD2 ≥100 Gy. Given that even with smaller fractional doses the majority of patients in our study achieved target lesion regression or stability with tendency towards regression over a longer period of time, ablative doses (≥10 Gy) may not be necessary in clinical practice. There is evidence that in combination with immunotherapy smaller fractional doses may lead to desired clinical outcomes. An in vivo analysis published in 2017 by Vanpouille-Box et al 20 revealed that expression of endonuclease Trex1, which plays a crucial role in the elimination of DNA fragments in cytosol, is induced by high fractional doses (≥12 Gy). This, in turn, leads to decreased immunogenicity of these cells. When the cells are irradiated with doses under the threshold of Trex1 induction, the production of interferon-gamma increases, which leads to enlarged presence and activation of dendritic cells, which are important for simulation of CD8+ T cells, which in turn potentiates the abscopal effect, especially in combination with checkpoint inhibitors. The authors concluded that Trex1 is one of the principal regulators of radiation-induced immune response, and future studies are needed to fine-tune the dosing and fractionation of RT necessary to achieve abscopal effect, when combined with checkpoint inhibitors.
In our study, SBRT to extracranial metastases of mRCC is well tolerated when combined with targeted or immunotherapy and leads to partial and complete response in treated lesions in the majority of patients. One patient experienced regression of multiple lung and mediastinal lymph node metastases, which were previously stable over the course of 2.5 years, following fractionated RT to one of the lung lesions, which we interpret as a clinical manifestation of abscopal effect. It appears that even patients with multiple RCC metastases receiving modern effective systemic therapy may benefit from addition of local therapies, getting probably one more chance for prolonged life. This treatment paradigm needs to be further investigated in a larger cohort of patients.
Footnotes
Presented at: This study was previously presented as an abstract as part of the 2019 Research Workshop: Treatment of Oligometastatic Disease - Closer to the Cure, and published in the International Journal of Radiation Oncology.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The trial was approved by RUSSCO (Russian Society of Clinical Oncology) ethics committee, institutional review board and ethics committees at every centre, and complied with the Good Clinical Practice guidelines, the Declaration of Helsinki and local laws. All patients provided written informed consent before any trial procedure.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open access repository.
References
- 1. Tsimafeyeu I, Zolotareva T, Varlamov S, et al. Five-Year survival of patients with metastatic renal cell carcinoma in the Russian Federation: results from the RENSUR5 registry. Clin Genitourin Cancer 2017;15:e1069–72. 10.1016/j.clgc.2017.07.017 [DOI] [PubMed] [Google Scholar]
- 2. Eggener SE, Yossepowitch O, Pettus JA, et al. Renal cell carcinoma recurrence after nephrectomy for localized disease: predicting survival from time of recurrence. J Clin Oncol 2006;24:3101–6. 10.1200/JCO.2005.04.8280 [DOI] [PubMed] [Google Scholar]
- 3. Alongi F, Arcangeli S, Triggiani L, et al. Stereotactic ablative radiation therapy in renal cell carcinoma: from oligometastatic to localized disease. Crit Rev Oncol Hematol 2017;117:48–56. 10.1016/j.critrevonc.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 4. Dengina N, Tsimafeyeu I, Mitin T. Current role of radiotherapy for renal-cell carcinoma: review. Clin Genitourin Cancer 2017;15:183–7. 10.1016/j.clgc.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 5. Cancer Genome Atlas Research Network Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013;499:43–9. 10.1038/nature12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim WH, Kim DG, Han JH, et al. Early significant tumor volume reduction after radiosurgery in brain metastases from renal cell carcinoma results in long-term survival. Int J Radiat Oncol Biol Phys 2012;82:1749–55. 10.1016/j.ijrobp.2011.03.044 [DOI] [PubMed] [Google Scholar]
- 7. Amini A, Altoos B, Bourlon MT, et al. Local control rates of metastatic renal cell carcinoma (RCC) to the bone using stereotactic body radiation therapy: is RCC truly radioresistant? Pract Radiat Oncol 2015;5:e589–96. 10.1016/j.prro.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ranck MC, Golden DW, Corbin KS, et al. Stereotactic body radiotherapy for the treatment of oligometastatic renal cell carcinoma. Am J Clin Oncol 2013;36:589–95. 10.1097/COC.0b013e31825d52b2 [DOI] [PubMed] [Google Scholar]
- 9. De Wolf K, Vermaelen K, De Meerleer G, et al. The potential of radiotherapy to enhance the efficacy of renal cell carcinoma therapy. Oncoimmunology 2015;4:e1042198 10.1080/2162402X.2015.1042198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Folkert MR, Timmerman RD. Stereotactic ablative body radiosurgery (SABR) or stereotactic body radiation therapy (SBRT). Adv Drug Deliv Rev 2017;109:3–14. 10.1016/j.addr.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 11. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–13. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deacon J, Peckham MJ, Steel GG. The radioresponsiveness of human tumours and the initial slope of the cell survival curve. Radiother Oncol 1984;2:317–23. 10.1016/S0167-8140(84)80074-2 [DOI] [PubMed] [Google Scholar]
- 13. Ning S, Trisler K, Wessels BW, et al. Radiobiologic studies of radioimmunotherapy and external beam radiotherapy in vitro and in vivo in human renal cell carcinoma xenografts. Cancer 1997;80:2519–28. [DOI] [PubMed] [Google Scholar]
- 14. Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003;300:1155–9. 10.1126/science.1082504 [DOI] [PubMed] [Google Scholar]
- 15. Qian C-N, Huang D, Wondergem B, et al. Complexity of tumor vasculature in clear cell renal cell carcinoma. Cancer 2009;115(10 Suppl):2282–9. 10.1002/cncr.24238 [DOI] [PubMed] [Google Scholar]
- 16. De Meerleer G, Khoo V, Escudier B, et al. Radiotherapy for renal-cell carcinoma. Lancet Oncol 2014;15:e170–7. 10.1016/S1470-2045(13)70569-2 [DOI] [PubMed] [Google Scholar]
- 17. Park S, Kim KH, Rhee WJ, et al. Treatment outcome of radiation therapy and concurrent targeted molecular therapy in spinal metastasis from renal cell carcinoma. Radiat Oncol J 2016;34:128–34. 10.3857/roj.2016.01718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ishiyama H, Teh BS, Ren H, et al. Spontaneous regression of thoracic metastases while progression of brain metastases after stereotactic radiosurgery and stereotactic body radiotherapy for metastatic renal cell carcinoma: abscopal effect prevented by the blood-brain barrier? Clin Genitourin Cancer 2012;10:196–8. 10.1016/j.clgc.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 19. Wersäll PJ, Blomgren H, Pisa P, et al. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol 2006;45:493–7. 10.1080/02841860600604611 [DOI] [PubMed] [Google Scholar]
- 20. Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. Dna exonuclease TREX1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 2017;8:15618 10.1038/ncomms15618 [DOI] [PMC free article] [PubMed] [Google Scholar]