Key messages.
What is already known about this subject?
Intermetatarsal bursitis (IMB) is frequent in patients with rheumatoid arthritis (RA), but there are large discrepancies in the described prevalence and the most frequent localisations.
What does this study add?
We found that one in five patients with established RA had IMB, and that most of the bursitis were located in the spaces between metatarsophalangeal (MTP) joint 2 and 3 as well as between MTP 3 and 4. The presence of IMB was not associated with the total ultrasound scores of a high number of joints/tendons, but with the ultrasound scores of inflammation in the MTP joints. In addition, presence of IMB was associated with presence of anti-cyclic citrullinated peptide and rheumatoid factor antibodies.
How might this impact on clinical practice?
Clinicians should explore for IMB as a cause of forefoot pain especially in patients with seropositive rheumatoid arthritis.
Introduction
Ultrasound is sensitive for detection of inflammatory changes in patients with rheumatoid arthritis (RA).1 Intermetatarsal bursitis (IMB) is located on the dorsal side of the deep intermetatarsal ligament and may easily be detected by use of longitudinal and transverse dorsal scans between the metatarsophalangeal (MTP) joints.2 In a longitudinal scan, they are relatively large and have usually a round shape, caused by hypoechoic synovitis and they may contain fluid. They are often power Doppler (PD) positive. In the transverse plane, an IMB is detected as a hypoechoic structure between the metatarsal heads where the upper border is rounded. There are few studies on IMB,3–6 and the objective of this study was to explore the prevalence of IMB and its associations with subjective, clinical and laboratory assessments in established RA patients.
Methods
This post hoc analysis of 209 patients with RA (mean (SD) age 53 (13) years, disease duration 10 (9) years, 81% women, 79% anti-cyclic citrullinted peptide (anti-CCP) positive, 69% rheumatoid factor (RF) positive) initiating biological disease-modifying antirheumatic drugs (bDMARDs)7 included assessment of patient’s global disease activity VAS, clinical examination (assessor’s disease activity VAS, tender and swollen joint counts (of 28) performed by a study nurse, with additional MTP 1-5 assessed combined as one joint bilaterally for tenderness) and laboratory variables (Erythrocyte Sedimentation Rate (ESR), C-Reactive Protein (CRP), anti-CCP and RF). Composite clinical scores (Disease Activity Score of 28 joints (DAS28,ESR), Clinical Disease Activity Index (CDAI) and Simplified Disease Activity Index (SDAI) were calculated. The presence of patient-reported joint pain (PRJP) was scored 0–3 at joint level for pain the last 24 hours by use of a manikin (including bilateral wrist, MCP1–5, PIP2–3, elbow, knee, ankle, MTP 1–5), and the same joints were examined by ultrasound. A semi-quantitative score (0–3) using grey scale (GS) and PD were performed by one rheumatologist with high intra-reader reliability8 by use of Siemens Acuson Antares, excellence version, 5–13 MHz probe and in accordance with the Norwegian ultrasound atlas.8 In addition, both feet were at each examination assessed by dorsal longitudinal scan (and transverse when indicated), of all spaces between the MTP joints for IMB. The presence of MTP synovitis (defined as GS score ≥2) was explored for the two MTP joints neighbouring the IMB. Baseline sum scores of GS, PD and PRJP of all joints as well as of only MTP 1–5 bilaterally were calculated. Associations were explored by use of Mann-Whitney test, ORs and binary logistic regression analysis.
Results
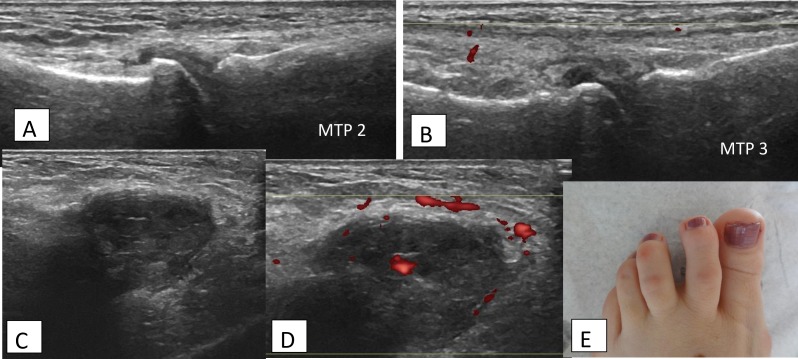
Forty-three patients (20.6%) had a total of 69 IMB, either unilaterally or bilaterally (27.9% right side, 37.2% left side and 30.2% bilaterally, while in 4.7% there was no description of side). The patients had up to 4 IMB (n=1 in 65.1%, n=2 in 18.6%, n=3 in 7.0% and n=4 in 9.3%). Figure 1 shows the typical clinical and longitudinal GS/PD ultrasound scanning of IMB. IMB was found in the following intermetatarsal spaces: MTP 1–2; 4.3%, MTP 2–3; 33.3%, MTP 3–4; 56.5% and MTP 4–5; 5.8%. When IMB was found, there was no synovitis in neighbouring MTPs in 33.3%, synovitis in one of the MTPs in 29.0% and synovitis in both MTPs in 37.7%. However, if synovitis was present, it was mainly moderate (mostly GS score of 2 (in 95.7%)). There were no differences in the presence of anti-CCP/RF in patients with vs without synovitis in the neighbouring MTPs.
Figure 1.
RA patient with intermetatarsal bursitis between MTP2 and MTP3. (A) Longitudinal, dorsal scan of normal MTP2. (B) Longitudinal, dorsal scan of normal MTP3. (C) Longitudinal dorsal grey scale scan of intermetatarsal bursitis between MTP2 and MTP3. (D) Similar to (C), with power Doppler. (E) Photo of RA patient with intermetatarsal bursitis between MTP2 and MTP3. RA, rheumatoid arthritis; MTP, metatarsal phalangeal joint.
Presence of IMB was not associated with baseline total sum scores of GS, PD or PRJP, or with the clinical or composite scores assessments. However, patients with IMB had significantly higher MTP 1–5 sum scores of GS and PD (p=0.05 and p=0.002, respectively), but no difference in sum score PRJP or tenderness of MTPs. Logistic regression analysis with either anti-CCP or RF positivity as the dependent variable showed that presence of IMB significantly explained seropositivity, while this was not found for GS and PD sum scores of MTP 1–5 as independent variables. In addition, IMB was associated with anti-CCP (OR (95% CI) 4.1 (1.2 to 14.0)) and RF (3.7 (1.4 to 10.1)), with 93% of patients with IMB being anti-CCP positive and 87% RF positive.
Discussion
The present frequency of IMB was as described in the study by Iagnocco et al.3 However, we found much lower percentage of patients with IMB than Bowen et al 5 who described IMB in 90.8% of RA patients. In addition, in contrast to our study, Bowen et al found most of the IMB located in the MTP 4–5 space and least in the MTP 2–3 space. In the study by Bowen et al, only plantar longitudinal and transverse scans were used, while we assessed the IMB by use of dorsal longitudinal and transverse scanning. A recent retrospective study including conventional MRI and ultrasound used dorsal scans for the ultrasound examination of IMB.6 However, MRI found the IMB to be big enough to be easily detectable by both dorsal and plantar scanning.
All patients in the present study were treated with bDMARDs, and this could have reduced the presence of IMB. However, a previous study has addressed the change of forefoot bursal hypertrophy after 12 weeks of bDMARD treatment.9 They found no significant change, only a trend towards reduction. Thus, it seems that despite effective anti-inflammatory treatment, these bursae may take time to normalise.
We found no associations between the presence of IMB and clinical or patient-reported outcomes. This is supported by a previous study exploring the long-term associations between changes in forefoot bursae (including both IMB and plantar bursae) and changes in DAS28 or ESR,10 where no significant associations were found over 3 years. On the other hand, presence of forefoot bursae was associated with patient-reported foot-related disability scores. This is supported by the present findings, where ultrasound sum scores of the MTP joints were higher in patients having IMB.
Conclusion
Our study shows that IMB is a frequent finding in patients with established RA and primarily in the MTP 2–3 and MTP 3–4 spaces. IMB was associated with higher levels of ultrasound synovitis in MTPs as well as with presence of anti-CCP and RF. Thus, ultrasound examination of forefeet should also focus on this pathology which may be typical for seropositive RA and indicate more severe disease of the feet.
Acknowledgments
We want to thank Anne Katrine Kongtorp and Britt Birketvedt who were study nurses and performed important assessments including the clinical examinations in the study.
Footnotes
Contributors: HBH has made a substantial contributions to the conception and design of the work; the acquisition of data, all the analysis, interpretation of data for the work; and drafted the manuscript as well as revising it critically for important intellectual content; and given a final approval of the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. TKK and LT have given substantial contributions to the design of the manuscript as well as the interpretation of data for the work; and revised the manuscript critically for important intellectual content; and given a final approval of the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This work was supported by AbbVie, Pfizer and Roche in form of study grants to the Department of Rheumatology, Diakonhjemmet Hospital, Oslo/ Hilde Berner Hammer.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the Norwegian Regional Committee for Medical and Health Research Ethics South East (reference number 2009/1254) and the patients gave their written informed consent according to the Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon request.
References
- 1. Tan YK, Ostergaard M, Bird P, et al. . Ultrasound versus high field magnetic resonance imaging in rheumatoid arthritis. Clin Exp Rheumatol 2014;32:S99–105. [PubMed] [Google Scholar]
- 2. Bianchi S. Practical us of the forefoot. J Ultrasound 2014;17:151–64. 10.1007/s40477-014-0078-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iagnocco A, Coari G, Palombi G, et al. . Sonography in the study of metatarsalgia. J Rheumatol 2001;28:1338–40. [PubMed] [Google Scholar]
- 4. Endo Y, Koga T, Eguchi M, et al. . Utility of power Doppler ultrasonography for detecting forefoot bursae in early rheumatoid arthritis. Medicine 2018;97:e13295 10.1097/MD.0000000000013295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bowen CJ, Hooper L, Culliford D, et al. . Assessment of the natural history of forefoot bursae using ultrasonography in patients with rheumatoid arthritis: a twelve-month investigation. Arthritis Care Res 2010;62:1756–62. 10.1002/acr.20326 [DOI] [PubMed] [Google Scholar]
- 6. Albtoush OM, Xenitidis T, Horger M. Intermetatarsal bursitis as first disease manifestation in different rheumatological disorders and related MR-imaging findings. Rheumatol Int 2019;25. doi: 10.1007/s00296-019-04381-x. [Epub ahead of print: 18 Jul 2019]. [DOI] [PubMed] [Google Scholar]
- 7. Hammer HB, Uhlig T, Kvien TK, et al. . Pain catastrophizing, subjective outcomes, and inflammatory assessments including ultrasound: results from a longitudinal study of rheumatoid arthritis patients. Arthritis Care Res 2018;70:703–12. 10.1002/acr.23339 [DOI] [PubMed] [Google Scholar]
- 8. Hammer HB, Bolton-King P, Bakkeheim V, et al. . Examination of intra and interrater reliability with a new ultrasonographic reference atlas for scoring of synovitis in patients with rheumatoid arthritis. Ann Rheum Dis 2011;70:1995–8. 10.1136/ard.2011.152926 [DOI] [PubMed] [Google Scholar]
- 9. Bowen CJ, Edwards CJ, Hooper L, et al. . Improvement in symptoms and signs in the forefoot of patients with rheumatoid arthritis treated with anti-TNF therapy. J Foot Ankle Res 2010;3:10 10.1186/1757-1146-3-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hooper L, Bowen CJ, Gates L, et al. . Prognostic indicators of foot-related disability in patients with rheumatoid arthritis: results of a prospective three-year study. Arthritis Care Res 2012;64:1116–24. [DOI] [PubMed] [Google Scholar]