Graphical Abstract
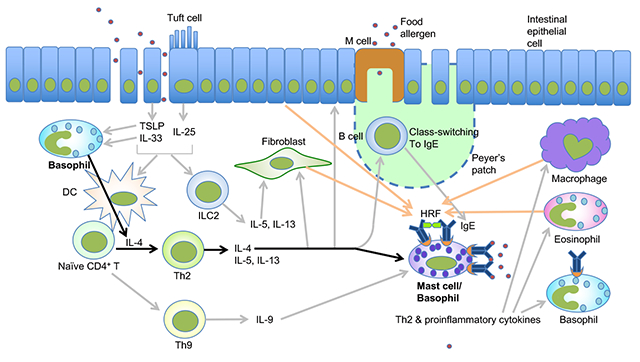
Keywords: food allergy, basophil, mast cell, IL-4, IgE
To the Editor
The prevalence of food allergy has been dramatically increasing for the last decades. Symptoms of food allergy range from itching, hives, and diarrhea to life-threatening anaphylaxis. Understanding of the pathogenesis of food allergy at the cellular and molecular levels is largely dependent on basic studies using animal models. For example, Brandt et al. showed that exposure to repeated doses of intragastric (i.g.) ovalbumin (OVA) induced acute diarrhea in OVA/alum-sensitized mice, associated with increased small intestinal permeability, eosinophilia and mastocytosis 1. This type 2 inflammation and the occurrence of diarrhea depend on mast cells, IgE and high-affinity IgE receptor (FcεRI), which is composed of an IgE-binding α subunit, a signal-amplifying β subunit, and a signal-generating disulfide-bonded dimer of γ subunits. In this ‘classical pathway’ of anaphylaxis, exposure to allergen allows allergen-specific IgE to cross-link FcεRI on the surface of mast cells to activate them, ultimately leading to the release of various anaphylactogenic and inflammatory mediators. The clinical importance of IgE in human food allergy is supported by the efficacy of omalizumab, a humanized anti-IgE monoclonal antibody that blocks IgE binding to FcεRI, in facilitating oral desensitization.
Oral food challenge is the gold standard to diagnose food allergy. However, it is a labor- and resource-intensive procedure with the risk of causing severe adverse effects. The basophil activation test (BAT) has emerged as a new diagnostic test for food allergy, as its results correlate well with oral food challenge results 2. Thus, the role of basophils in food allergy seems to be widely accepted in the community of clinical allergists. Despite this assumption and the fact that basophils express the αβγ2-type FcεRI like mast cells, their role in food allergy is less well characterized than that of mast cells. In previous studies using mice sensitized epicutaneously with OVA, basophil-depleted mice were shown to not produce OVA-specific IgE and to be protected from oral OVA challenge-induced anaphylaxis 3,4. Attenuation of food allergy signs was also observed in basophil-depleted mice in an intradermal OVA+TSLP (thymic stromal lymphopoietin)-sensitization/OVA gavage model 5. However, the role of basophils in the effector phase of food allergy has not been assessed. Our current study addressed this point using basophil-depleted mice in which the specific roles and functions of basophils in food allergy can be better defined. All animal experiments were approved by the Institutional Animal Care and Use Committee of RIKEN Center for Integrative Medical Sciences Sciences and the Hokkaido University animal ethics committee. With the OVA sensitization/OVA challenge model of Brandt et al. 1, we depleted basophils before oral OVA challenges in OVA/alum-sensitized mice. First, basophil depletion was done by intraperitoneal (i.p.) treatment of mice with Ba103 mAb, anti-CD200R3 antibody that depletes basophils 6, on day 27 (i.e., two days before the first OVA challenge), day 34, and day 41 (Fig. 1A). Basophils in the peripheral blood quantified by flow cytometry were increased by immunization and were efficiently reduced by Ba103 treatment (Fig. 1B). Upon basophil depletion, the incidence of diarrhea and clinical scores were significantly reduced (Fig. 1C and Fig. E1A-B). Even more efficient suppression of diarrhea development was observed with Bas-TRECK mice, which have a diphtheria toxin receptor transgene expressible in a basophil-specific manner 7 (Fig. 1D-F). These mice were treated i.p. with diphtheria toxin to deplete basophils before and during OVA challenges (Fig. 1D, E). Diarrhea was seen in only 40% of basophil-depleted Bas-TRECK mice after 9th OVA challenge (Fig. 1F) and clinical scores were strongly suppressed (Fig. 1G-H). Both Ba103 mAb-treated WT mice and diphtheria toxin-treated Bas-TRECK mice had serum levels of total and OVA-specific IgE and IgG1 similar to those in basophil-sufficient mice (Figs. 1I-J, E1C-F, & E2), consistent with our previous study showing that these antibodies were mainly generated during the sensitization phase 8. These results demonstrate that basophils were essential for causing clinical signs including diarrhea in the effector phase in this food allergy model.
Figure 1. Basophil depletion inhibits the development of allergic diarrhea and intestinal inflammation.
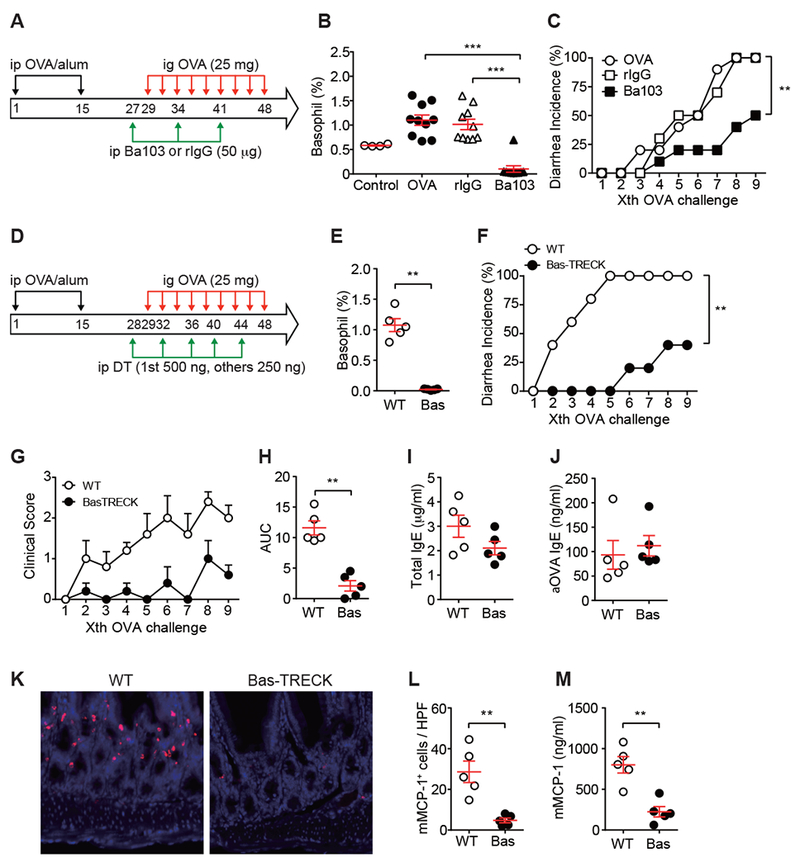
(A-C) BALB/c mice were i.p. immunized with OVA plus alum and i.g. challenged with OVA (25 mg) three times a week. Immunized mice were either non-pretreated (OVA, n=10) or i.p. pretreated with 50 μg of Ba103 mAb (n=10) or rat IgG (rIgG, n=10). Another control (Control, n=4) was non-immunized mice. (A) Procedure scheme. (B) Basophil numbers in blood at day 29 were monitored by flow cytometry. ***, p<0.001 by Student’s t-test. (C) The occurrence of diarrhea. p = 0.0046 for isotype vs. Ba103; p = 0.0024 for OVA vs. Ba103 by Log-rank test. (D-M) WT (n=5) and Bas-TRECK (n=5) BALB/c mice were i.p. immunized with OVA plus alum and i.g. challenged with OVA (25 mg) three times a week. Immunized mice were either i.p. pretreated with diphtheria toxin at days 28, 32, 36, 40 and 44 (1st treatment at 500 ng/mouse, other treatments at 250 ng/mouse). (D) Procedure scheme. (E) Basophil numbers in blood at day 29 (before 1st OVA challenge) were monitored by flow cytometry. Data shown as mean ± SEM. **, p<0.01 by Mann-Whitney test. (F) The occurrence of diarrhea. p = 0.0017 for WT vs. Bas-TRECK by Log-rank test. (G) Clinical scores of OVA-challenged WT and Bas-TRECK mice (n = 5 in each group) (H) AUC (area under the curve) analysis of clinical scores. Data shown as mean ± SEM. **, p<0.001 by Mann-Whitney U test. (I) Total IgE and (J) OVA-specific IgE. (K) Representative immunofluorescence staining of mMCP-1+ cells in jejunum. (L) Quantification of mMCP-1+ cells. HPF, high power field. (M) mMCP-1 levels in blood were measured by ELISA. Data shown as mean ± SEM.
As previous studies showed the crucial role for mast cells in this food allergy model 1, mast cells in the small intestine were quantified by confocal microscopy. mMCP-1+ mast cell numbers recruited upon OVA challenges were reduced in basophil-depleted, OVA-sensitized mice (Figs. 1K-L & E1G-H). Consistent with this, serum levels of mMCP-1, which was secreted by activated mast cells, were reduced in basophil-depleted mice (Figs. 1M & E1I). Basophils are a rich source of IL-4. IL-4 secreted by basophils stimulated by cytokines (e.g., IL-3, IL-18, and IL-33) or TLR ligands (e.g., lipopolysaccharide and peptidoglycans) can initiate or modulate allergic responses by promoting Th2 differentiation leading to IgE production and enhanced survival and activation of mast cells 9. In food allergy models, intestinal mast cell accumulation and activation depend directly on IL-4 10. Therefore, we tested the possibility that IL-4 (probably derived from basophils) stimulates mast cells to amplify the allergic inflammation. Ba103-treated OVA-sensitized mice were treated i.p. with IL-4 before OVA challenges. IL-4 administration caused a higher incidence of diarrhea and higher clinical scores in Ba103-treated mice, compared with PBS administration in Ba103-treated mice (Fig. 2A-D). All Ba103-treated mice had reduced numbers of blood basophils (Fig. 2E). However, jejunal mast cells were increased by IL-4 treatment (Fig. 2F). Complementary to this experiment, OVA-sensitized WT mice were treated with a neutralizing anti-IL-4 mAb one day before and during OVA challenges (Fig. 2G-L). These mice exhibited reduced incidence of diarrhea and clinical scores (Fig. 2G-J). Frequency of blood basophils in anti-IL-4 mAb treated mice after 9th OVA challenge was similar to that in control IgG treated mice (Fig. 2K). By contrast, jejunal mast cells were reduced by anti-IL-4 mAb (Fig. 2L). These results suggest that IL-4 is required for mast cell recruitment and/or proliferation, but not for basophil accumulation.
Figure 2. The basophil-IL-4-mast cell axis regulates the development of allergic diarrhea and intestinal inflammation.
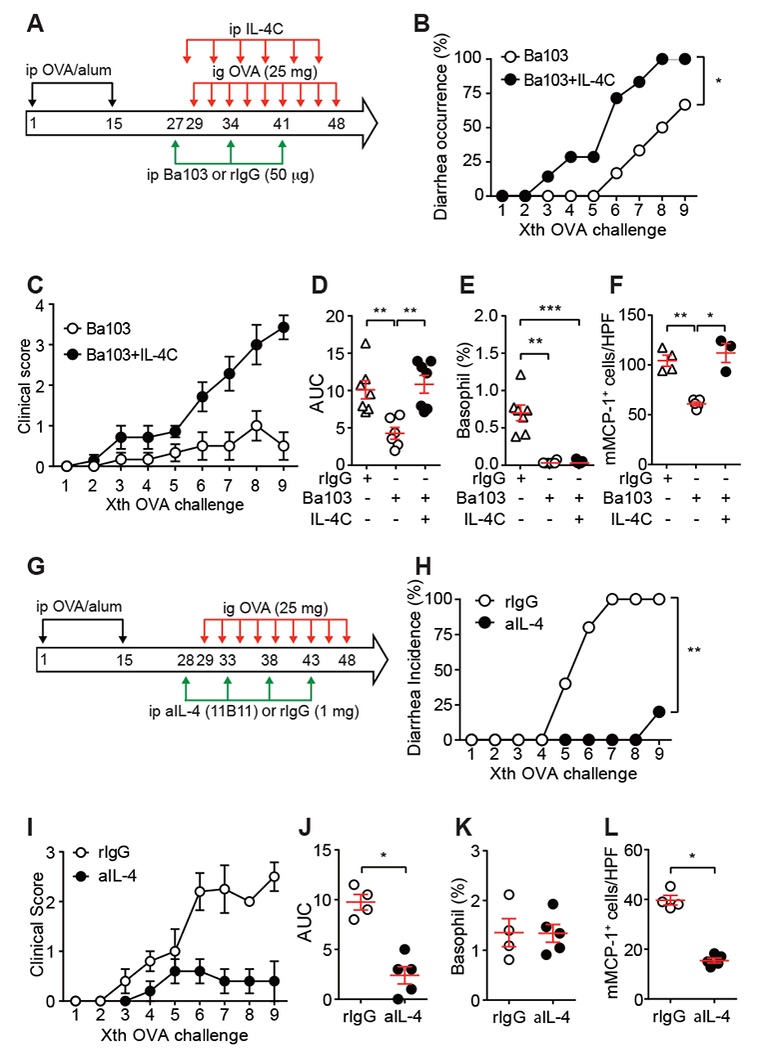
(A-F) BALB/c mice (n=7) were i.p. immunized with OVA plus alum and i.g. challenged with OVA three times a week. Some mice were i.p. injected with 50 μg rat IgG (n=7) or Ba103 mAb (n=6) at days 27, 34, and 41. Some basophil-depleted mice were i.v. injected with IL-4/anti-IL-4 immune complex (IL-4C; 3 μg IL-4 + 15 μg anti-IL-4, n=7) at days 28, 31, 35, 37, 42, and 45. One hour after OVA administration, diarrhea was visually scored. (A) Procedure scheme. (B) The occurrence of diarrhea. p = 0.0172 by Log-rank test. (C) Clinical scores. Data shown as mean ± SEM (n = 6 or 7). (D) AUC analysis of clinical score data. Data shown as mean ± SEM. **, p<0.01 by Mann-Whitney U test. (E) Basophil numbers in blood at day 29 (before 1st OVA challenge) were monitored by flow cytometry. Data shown as mean ± SEM. ***, p<0.001 by Mann-Whitney U test. (F) mMCP-1+ mast cells were quantified. *, p<0.05; **, p<0.01 by Mann-Whitney U test. rat IgG (n=4), Ba103 mAb (n=5), IL-4C (n=3). HPF, high power field. (G-L) BALB/c mice were i.p. immunized with OVA plus alum and i.g. challenged with OVA three times a week. Some mice were i.p. injected with 1 mg of rat IgG (n=5) or anti-IL-4 mAb (clone 11B11, n=5) at days 28, 33, 38 and 43. (G) Procedure scheme. aIL-4, anti-IL-4 mAb. (H) The occurrence of diarrhea. **, p<0.01 by Log-rank test. (I) Clinical scores. Data shown as mean ± SEM (n = 4 or 5). (J) AUC analysis of clinical score data. Data shown as mean ± SEM. *, p<0.05 by Mann-Whitney U test. (K) Basophil numbers in blood at day 29 (before 1st OVA challenge) were monitored by flow cytometry. (L) mMCP-1+ mast cells were quantified. Data shown as mean ± SEM. *, p<0.05 by Mann-Whitney U test. HPF, high power field.
Collectively, our results along with the previous study 10 indicate that the basophil-IL-4-mast cell axis plays a crucial role in the effector phase of the OVA-induced food allergy. This and previous studies 3–5 showed a critical role of basophils in both the sensitization and effector phases of food allergy.
Supplementary Material
FIG E1. Antibody-mediated basophil depletion inhibits the development of intestinal inflammation. Ba103 mAb-mediated basophil depletion and induction of food allergy were done as described in Fig. 1A. After immunization, mice were either non-pretreated (OVA, n=10) or i.p. pretreated with 50 μg of Ba103 mAb (n=10) or rat IgG (rIgG, n=10). (A) Clinical scores of three cohorts of mice. (B) AUC analysis of clinical scores. Data shown as mean ± SEM (n = 4 or 10). **, p<0.001 by Mann-Whitney U test. (C) Total IgE, (D) OVA-specific IgE, (E) total IgG1, and (F) OVA-specific IgG1 were quantified by ELISA. (G) Representative immunofluorescence staining of mMCP-1+ cells in jejunum. (H) Quantification of mMCP-1+ cells. HPF, high power field. (I) mMCP-1 levels in blood were measured by ELISA. **, p<0.01 by Mann-Whitney U test.
FIG E2. IgG1 production in Bas-TRECK mice during food allergy. Food allergy induction in WT and Bas-TRECK BALB/c mice was depicted in Fig. 1 D. (A) Total IgG1 and (B) OVA-specific IgG1 were measured by ELISA.
Funding Sources
This study was supported in part by grants from the US National Institutes of Health (AR064418 and AI124734), the Ministry of Education, Culture, Sports, Science and Technology, Japan (25670483, 26860765, 15K10794), Japan Research Foundation for Clinical Pharmacology, and Nipponham Foundation for the Future of Food.
Footnotes
The authors have declared that no conflict of interest exists.
References
- 1.Brandt EB et al. Mast cells are required for experimental oral allergen-induced diarrhea. The Journal of clinical investigation 112, 1666–1677, doi: 10.1172/JCI200319785112/11/1666 [pii] (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santos AF & Lack G Basophil activation test: food challenge in a test tube or specialist research tool? Clin Transl Allergy 6, 10, doi: 10.1186/s13601-016-0098-7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noti M et al. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. The Journal of allergy and clinical immunology 133, 1390–1399, 1399 e1391–1396, doi: 10.1016/j.jaci.2014.01.021 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muto T et al. The role of basophils and proallergic cytokines, TSLP and IL-33, in cutaneously sensitized food allergy. International immunology 26, 539–549, doi: 10.1093/intimm/dxu058 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Han H, Thelen TD, Comeau MR & Ziegler SF Thymic stromal lymphopoietin-mediated epicutaneous inflammation promotes acute diarrhea and anaphylaxis. The Journal of clinical investigation 124, 5442–5452, doi: 10.1172/JCI77798 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obata K et al. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood 110, 913–920, doi:blood-2007–01-068718 [pii] 10.1182/blood-2007-01-068718 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Sawaguchi M et al. Role of mast cells and basophils in IgE responses and in allergic airway hyperresponsiveness. Journal of immunology 188, 1809–1818, doi: 10.4049/jimmunol.1101746 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Ando T et al. Histamine-releasing factor enhances food allergy. The Journal of clinical investigation 127, 4541–4553, doi: 10.1172/JCI96525 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyake K & Karasuyama H Emerging roles of basophils in allergic inflammation. Allergology international : official journal of the Japanese Society of Allergology 66, 382–391, doi: 10.1016/j.alit.2017.04.007 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Burton OT et al. Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy. Mucosal immunology 6, 740–750, doi: 10.1038/mi.2012.112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIG E1. Antibody-mediated basophil depletion inhibits the development of intestinal inflammation. Ba103 mAb-mediated basophil depletion and induction of food allergy were done as described in Fig. 1A. After immunization, mice were either non-pretreated (OVA, n=10) or i.p. pretreated with 50 μg of Ba103 mAb (n=10) or rat IgG (rIgG, n=10). (A) Clinical scores of three cohorts of mice. (B) AUC analysis of clinical scores. Data shown as mean ± SEM (n = 4 or 10). **, p<0.001 by Mann-Whitney U test. (C) Total IgE, (D) OVA-specific IgE, (E) total IgG1, and (F) OVA-specific IgG1 were quantified by ELISA. (G) Representative immunofluorescence staining of mMCP-1+ cells in jejunum. (H) Quantification of mMCP-1+ cells. HPF, high power field. (I) mMCP-1 levels in blood were measured by ELISA. **, p<0.01 by Mann-Whitney U test.
FIG E2. IgG1 production in Bas-TRECK mice during food allergy. Food allergy induction in WT and Bas-TRECK BALB/c mice was depicted in Fig. 1 D. (A) Total IgG1 and (B) OVA-specific IgG1 were measured by ELISA.


