Abstract
Manganese is an essential metal ion that bacterial pathogens need to acquire from the vertebrate host during infection. In the mammalian nutritional immunity strategy to combat bacterial infection, the host restricts bacterial access to Mn(II) by sequestering this metal nutrient using the protein calprotectin (CP). The role of murine calprotectin (mCP) in Mn(II) sequestration has been demonstrated in vivo, but the molecular basis of this function has not been evaluated. Herein, biochemical assays and electron paramagnetic resonance (EPR) spectroscopy are employed to characterize the Mn(II)-binding properties of mCP. We report that mCP has one high-affinity Mn(II)-binding site. This site is a His6 site composed of His17 and His27 of mS100A8 and His92, His97, His105 and His107 of mS100A9. Similar to the human orthologue, Ca(II) binding to the EF-hand domains of mCP enhances the Mn(II) affinity of the protein; however, this effect requires ~10-fold more Ca(II) than what was previously observed for hCP. Mn(II) coordination to the His6 site also promotes self-association of two mCP heterodimers to form a heterotetramer. Low-temperature X-band EPR spectroscopy revealed a nearly octahedral Mn(II) coordination sphere for the Mn(II)-His6 site characterized by zero-field splitting (ZFS) parameters D = 525 MHz and E/D = 0.3. Further electron-nuclear double resonance (ENDOR) studies with globally 15N-labeled mCP provided hyperfine couplings from the coordinating ε-nitrogen atoms of the His ligands (aiso = 4.3 MHz) as well as the distal δ-nitrogen atoms (aiso = 0.25 MHz). Mn(II)-competition assays between mCP and two bacterial Mn(II) solute-binding proteins, staphylococcal MntC and streptococcal PsaA showed that mCP outcompetes both proteins for Mn(II) under conditions of excess Ca(II). In total, this work provides the first coordination chemistry study of mCP and reveals striking similarities in the Mn(II) coordination sphere as well as notable differences in Ca(II) sensitivity and oligomerization behavior between hCP and mCP.
Graphical Abstract
Biochemical and EPR spectroscopic evaluation show that murine calprotectin (mCP) sequesters Mn(II) at a hexahistidine site and displays Ca(II)-dependent Mn(II) affinity. The protein can outcompete MntC and PsaA for Mn(II), two solute-binding proteins used by bacterial pathogens to acquire this important nutrient in the mammalian host.
Introduction
Transition metal ions are essential for many cellular processes including regulation, catalysis, and signaling.1 During bacterial infection, the invading pathogen must obtain metal nutrients from the host. As a result, the competition for nutrient metal ions between host and pathogen is an important facet of the host-pathogen interaction.1 In a process termed “nutritional immunity,” the host innate immune system deploys metal-sequestering proteins to lower the levels of available metal ion at infection sites in an effort to starve the invading pathogen.1–2
Mn(II) sequestration is an important component of this host response to infection because many bacterial pathogens utilize manganese-containing enzymes when colonizing the host.3–5 Manganese-containing superoxide dismutases are expressed and utilized by bacterial pathogens to curb reactive oxygen species-mediated cell destruction.6–8 Manganese is an important cofactor for enzymes involved in central metabolism, including class Ib ribonucleotide reductase, which produces deoxyribonucleotides from ribonucelotides.9–10 Additional metabolic enzymes, such as Salmonella typhimurium propionate kinase and Escherichia coli lactonase UlaG, have Mn(II)-dependent activity.11–12 The Mn(II)-dependent enzyme fructose 1,6-bisphosphatase II, involved in gluconeogenesis, is essential for the virulence of the intracellular pathogen Francisella tularensis.13–15 Manganese enzymes also contribute to antibiotic resistance. In particular, the fosfomycin-inactivating enzyme FosB in Staphylococcus aureus and the ATP-binding cassette (ABC)-F ATPase protein involved in antibiotic resistance in Mycobacterium tuberculosis utilize Mn(II) as cofactors.16–17 In order to obtain Mn(II), bacteria express ABC transporters and natural resistance associated macrophage protein (NRAMP) transporters that allow import of this nutrient.5 For instance, the importance of staphylococcal Mn(II) import systems MntABC and MntH have been highlighted in murine models of S. aureus infection.18–19 PsaABC, the Mn(II) import system in Streptococcus pneumoniae, is critical for infection in multiple model systems.20 Mn(II) import systems are important for virulence in a number of other pathogens, including Salmonella enterica serovar Typhimurium, 8 Bacillus anthracis,21 and Enterococcus faecalis.22
To date, the only known Mn(II)-sequestering host-defense protein is calprotectin (CP, S100A8/S100A9 oligomer, MRP8/MRP14 oligomer).23 The role of CP in biology and infectious disease has been illuminated though many studies involving mouse models in which S100A9−/− (CP-deficient) mice are utilized.8, 24–29 In 2008, murine CP (mCP) was shown to reduce Mn(II) levels and limit S. aureus growth in murine abscesses.25 This seminal work provided the first evidence for the ability of CP to sequester Mn(II) and inspired biochemical and biophysical investigations into the Mn(II)-binding properties of the protein.7, 30–34 Nevertheless, current understanding of the metal-binding properties of CP is almost exclusively based on evaluation of hCP, the human form of the protein.23
hCP is a heterooligomer of the S100 proteins S100A8 (10.8 kDa) and S100A9 (13.2 kDa). Each subunit contains two Ca(II)-binding sites: one C-terminal canonical EF-hand and one N-terminal non-canonical EF-hand.35 In the absence of Ca(II) ions, hCP exists as a heterodimer.36 Ca(II) binding to the EF-hand domains causes the self-association of two heterodimers to form a heterotetramer.37–39 Each hCP heterodimer contains two distinct transition-metal-binding sites that form at the S100A8/S100A9 interface: a His3Asp site (site 1) and a His6 site (site 2).23 In the current working model, hCP is released from immune cells or epithelial cells at infection sites, where it encounters high (~2 mM) extracellular Ca(II) levels and forms a heterotetramer.23, 39–40 Tetramerization enhances the transition-metal-ion affinities and protease stability of hCP.23, 39, 41 Biochemical, structural, and spectroscopic studies of Mn(II) coordination by hCP has provided a comprehensive molecular description of how the protein sequesters Mn(II).30–34 Importantly, hCP employs a biologically unprecedented hexahistidine (His6) motif to sequester Mn(II) and the presence of excess Ca(II) ions lowers the apparent dissociation constant (Kd,Mn(II)) by at least three orders of magnitude.30–34 On the basis of all available data, Ca(II)-bound hCP coordinates Mn(II) with sub-nanomolar affinity at the His6 site.34 The His3Asp site, in contrast, has relatively low affinity for Mn(II) in the absence and presence of Ca(II) ions (micromolar Kd assigned by room-temperature EPR spectroscopy in the presence of excess Ca(II) ions).30 Thus, the His3Asp site binds Mn(II), but lacks the ability to sequester it.
Given the importance and broad application of murine models in studying the biological function of CP, understanding the metal-binding properties of mCP is necessary. Amino acid sequence alignment reveals similarities and differences between the human and murine S100A8 and S100A9 subunits. The alignment indicates that the residues that compose sites 1 and 2 are conserved (Figure 1A), which suggests that hCP and mCP exhibit similar metal-binding properties and provides a working hypothesis that mCP employs a high-affinity His6 motif to sequester divalent transition metal ions, including Mn(II). Nevertheless, hCP and mCP share only ~56% amino acid identity, a lower value compared to the average of 85% amino acid identity between human and murine polypeptides.42 This relatively large variability in primary structures suggests there may be some important structural and functional differences between the human and murine orthologues that warrant careful examination. Towards addressing the current knowledge gap about mCP, we recently reported initial biochemical characterization of the protein, which demonstrated that, similar to hCP, apo mCP exists as a mS100A8/mS100A9 heterodimer, and Ca(II) binding results in self-association of two heterodimers to form a heterotetramer.43 However, ~10-fold more Ca(II) equivalents were required to completely tetramerize mCP compared to hCP even though the two proteins exhibit similar Ca(II)-binding regions (Figure 1A). To the best of our knowledge, this observation provided the first experimental indication that the biophysical properties of hCP and mCP differ, and we reason that additional studies are warranted to further evaluate this possibility and elucidate whether there are functional consequences. Pertinent to the current study, our preliminary evaluation of the metal-binding ability of mCP demonstrated that mCP coordinates a number of transition metal ions, including manganese, present in a complex bacterial growth medium.43
Figure 1.

Overview of the primary structure and His6 metal-binding site of mCP and crystal structure of human calprotectin. (A) Amino acid sequence alignment of human (h) and murine (m) S100A8 and S100A9 subunits of CP. The Ca(II)-binding regions are indicated, and the conserved transition-metalbinding residues are shown in red. Cysteine residues are shown in blue, and the portions of the S100A9 C-terminal tails containing His residues are underlined. The secondary structural elements represented are for the human subunits. (B) Predicted and experimentally determined His6 site of mCP based on sequence alignment of the human and murine S100A8 and S100A9 subunits. A coordinated Mn(II) ion is shown. (C) Crystal structure of the Mn(II)-, Ca(II)-, and Na(I)-bound human calprotectin heterotetramer (PDB: 4XJK).32 One heterodimer shows the S100A8 subunit in green and the S100A9 subunit in blue, and the other heterodimer is colored gray. Metal ions are shown as spheres: Mn(II) is magenta, Ca(II) is yellow, and Na(I) is purple.
In this work, we investigate the Mn(II)-binding properties of mCP. We report that mCP coordinates Mn(II) at a high-affinity His6 site, displays Ca(II)-dependent Mn(II) affinity at this site, and that Mn(II) binding at the His6 site confers tetramerization in the absence of Ca(II) ions. We also demonstrate that mCP can outcompete the solute-binding proteins staphylococcal MntC and streptococcal PsaA for Mn(II) under conditions of high Ca(II). These Mn(II)-binding studies are consistent with a role of mCP in Mn(II) sequestration in the host-pathogen interaction, as expected based on prior work that involved animal models of infection. Lastly, the current work reveals an important similarity in the Mn(II) binding capability between hCP and mCP – the His6 site – that is a significant contributor during the battle between host and pathogen for nutrient metal ions as well as additional differences in Ca(II)-dependent behavior.
Results and Discussion
Preparation and Biochemical Characterization of mCP Metal-binding Site Variants
We first designed protein variants to probe the amino acid residues in mS100A8 and mS100A9 predicted to be important for Mn(II) binding. Because the metal-binding-site residues are conserved in hCP and mCP, we prepared and characterized mCP variants with mutations that disrupted either the putative His3Asp site (∆His3Asp; mS100A8(H83A)(H87A)/mS100A9(H21A)(D31A)) or four residues of the putative His6 site (∆His4; mS100A8(H17A)(H27A)/mS100A9(H92A)(H97A)) (Figure 1, Table S1). The C-terminal tail of mS100A9 contains an HXHXH motif spanning residues 103–107. To evaluate whether these His residues contribute to Mn(II) binding, we prepared variants with one or two His→Ala mutations in this region (Tables 1, S1, S2). Following overexpression of each subunit in E. coli and reconstitution of the mCP heterodimers according to a reported protocol,43 the variants were characterized by inductively-coupled plasma mass spectrometry (ICP-MS), sodium docecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), liquid chromatography-mass spectrometry (LC-MS), circular dichroism (CD) spectroscopy, and analytical size exclusion chromatography (SEC) (Tables S3–S6, Figures S1–S3). ICP-MS and SDS-PAGE analyses indicated that the proteins were isolated in the apo form (Tables S4, S5) and in high purity (Figure S1), respectively. LC-MS analysis demonstrated that the mS100A8 subunits displayed partial loss of the initiator methionine, whereas mS100A9 subunits lacked this residue (Table S3). Cleavage of Met1 is a common post-translational modification observed during heterologous overexpression in E. coli.44 All variants afforded CD spectra consistent with an α-helical fold in the presence and absence of Ca(II) (Figure S2). Lastly, analytical SEC established that the purified proteins were isolated as heterodimers, as indicated by elution volumes of 12.0–12.1 mL (Table S6, Figure S3). In the presence of excess Ca(II) ions in the running buffer, the peak for each variant displayed a shift to a lower elution volume (11.3–11.4 mL), indicating Ca(II)-induced tetramerization (Table S6, Figure S3). This behavior is consistent with our prior characterization of mCP.43
Table 1.
mS100A9 C-terminal tail variants.
| mCP variants | tail residues (101–109) |
|---|---|
| mCP | RGHGHSHGK |
| (H103A) | RGAGHSHGK |
| (H105A) | RGHGASHGK |
| (H107A) | RGHGHSAGK |
| (H103A)(H105A) | RGAGASHGK |
| (H103A)(H107A) | RGAGHSAGK |
| (H105A)(H107A) | RGHGASAGK |
Analytical Size-Exclusion Chromatography Uncovers the His6 Site and Mn(II)-induced Tetramerization of mCP
Guided by our initial studies of Mn(II) binding to hCP,30–31 we first incubated mCP (100 µM) with 10 equiv. Mn(II) and analyzed the sample by SEC at pH 7.0. In the absence of Mn(II), mCP exhibits a single peak that elutes at 12.1 min. The presence of Mn(II) caused the peak to broaden and exhibit two local maxima at 11.5 and 11.8 min (Figure 2A,B). Quantitation of protein and Mn(II) concentrations in the fractions by optical absorption spectroscopy (A280) and ICP-MS, respectively, revealed negligible Mn(II) in the apo sample, as expected. In contrast, ~1 equiv of Mn(II) was retained over the course of elution of the +Mn(II) sample. Thus, in the absence of Ca(II) ions, mCP coordinates Mn(II) with sufficiently high affinity to retain the metal during elution. The Mn(II)-induced peak shift to lower elution volumes also indicates that Mn(II) binding to mCP induces tetramerization, behavior previously observed for hCP.30–31
Figure 2.
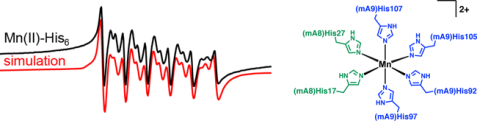
Representative data demonstrating Mn(II) retention by mCP and variants following SEC. Eachv plot shows a representative SEC chromatogram (black trace) and the quantification of protein and Mn(II) concentrations in the collected fractions (bars). For experiments in the absence of added Ca(II), the protein concentration was either 100 μM (A-D) or 500 μM (G-H). For experiments in the presence of 25 mM Ca(II), the protein concentration was 100 μM (E-F). The samples were incubated without (A) or with Mn(II) (B-H). For samples containing Mn(II), the Mn(II) concentration was 1 mM (B-D), 100 μM (D-E, +Ca(II) samples), or 5 mM (G-H). The elution buffer was 75 mM HEPES, 100 mM NaCl, 200 μM TCEP, pH 7.0 and T= 4 °C.
We extended this experiment to examine the ability of the ∆His3Asp and ∆His4 variants to retain Mn(II). When we employed 100 µM protein and 10 equiv. Mn(II), both proteins displayed peak elution volumes consistent with heterodimers, and neither protein retained Mn(II) (Figure 2C,D). The result for ∆His4 was consistent with our working hypothesis that mCP uses a high-affinity His6 site to coordinate Mn(II). In contrast, the results with ∆His3Asp were unexpected. Because Ca(II) ions enhanced the Mn(II) affinity of hCP, we questioned whether ∆His3Asp retains Mn(II) when excess Ca(II) is present. Thus, we examined samples of mCP and ∆His3Asp (100 µM) containing 1 equiv. Mn(II) using an elution buffer supplemented with 25 mM Ca(II) (Figure 2E,F). Under these conditions, the peaks for both mCP and ∆His3Asp displayed a shift to a lower elution volume, corresponding to tetramerization, and both proteins retained sub-stoichiometric Mn(II). Next, we repeated the SEC experiments with mCP and ∆His3Asp in the absence of Ca(II) ions using a 5-fold higher protein concentration (500 µM) and 10 equiv. Mn(II) (Figure 2G,H). Under these conditions, both proteins displayed comparable peak elution volume shifts consistent with nearly complete tetramerization, and both proteins retained Mn(II). Taken together, these results indicate that (i) Mn(II)-induced tetramerization of mCP exhibits protein-concentration dependence, (ii) mutation of the His3Asp residues compromises the ability of the protein to retain Mn(II) at site 2 and display Mn(II)-dependent self-association, and (iii) the Mn(II) affinity is enhanced by Ca(II)-binding to the EF-hand domains. We also note that ∆His3Asp displays a greater increase in α-helicity upon Ca(II) addition compared to mCP and the other variants (Figure S2), further suggesting that the ∆His3Asp mutations impact the protein structure.
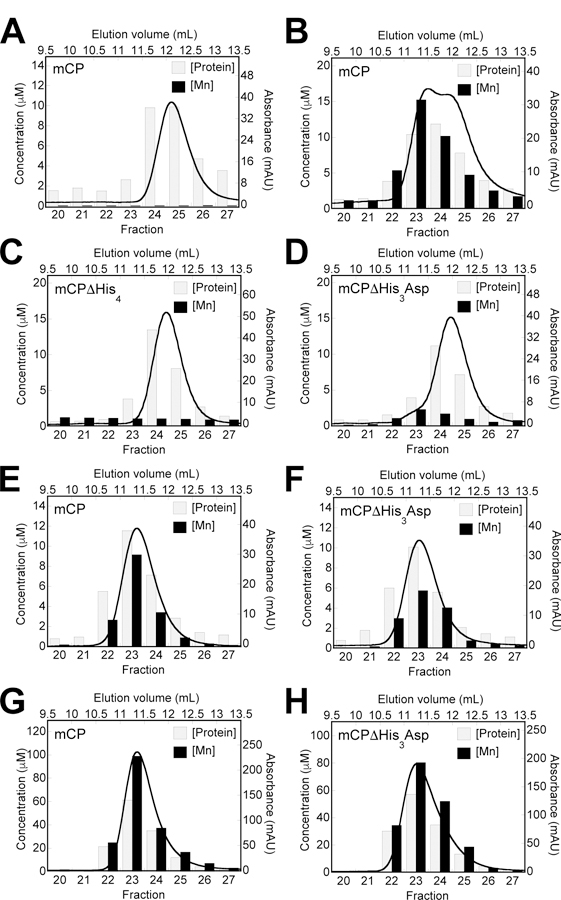
Because the four residues of the putative His6 site are essential for Mn(II) retention during elution from the SEC column, we next examined the contribution of H103, H105, and H107 in the mS100A9 C-terminal tail (Figure 3). For mCP(H103A), we observed a similar behavior as mCP with Mn(II) retention and partial tetramerization when the protein (100 µM) was incubated with 10 equiv. Mn(II) prior to elution from the SEC column (Figure 3A). In contrast, under these same experimental conditions, mCP(H105A) and mCP(H107A) eluted as heterodimers with negligible Mn(II) retained (Figure 3B,C). These results indicate that H105A and H107A are involved in Mn(II) coordination and that H103 does not participate in Mn(II) binding. Thus, these initial SEC experiments indicate that mCP employs a His6 site composed of His17 and His27 of mS100A8 and His92, His97, His105 and His107 of mS100A9 to coordinate Mn(II). These residues are conserved in mCP and hCP. Moreover, this site is the high-affinity site and appears to display Ca(II)-dependent Mn(II) affinity.
Figure 3.
Representative data demonstrating Mn(II) retention by mCP C-terminal tail variants following SEC. Each plot shows a representative SEC chromatogram (black trace) and the quantification of protein and Mn(II) concentrations in the collected fractions (bars). The variants (100 μM) H103A (A), H105A (B), and H107A (C) were incubated with Mn(II) (1 mM) in the absence of added Ca(II). The elution buffer was 75 mM HEPES, 100 mM NaCl, 200 μM TCEP, pH 7.0 and T= 4 °C.
Mn(II) Competition Studies Demonstrate that Ca(II) Ions Enhance the Mn(II) affinity of mCP
On the basis of our prior studies of hCP which demonstrated that Ca(II) ions enhance the transition-metal affinities of the His3Asp and His6 sites,39 and our preliminary biochemical evaluation of mCP,43 we hypothesized that mCP displays similar Ca(II)-dependent behavior. In this prior work on hCP, we performed Zinpyr-1 (ZP1) competition assays to examine Ca(II)-dependent Mn(II) binding to the protein.30–31 ZP1 is a Ca(II)-independent fluorescent sensor with two di(2-picolyl)amine-based metal-binding sites that exhibits fluorescence quenching upon Mn(II) binding (Kd1 = 550 nM).45 When we combined ZP1 (1 µM), mCP (4 µM), and Mn(II) (5 µM), the fluorescence from ZP1 was quenched, indicating that Mn(II) bound to the sensor and not mCP (Figure 4). This behavior is consistent with that of hCP.30 Upon titration of Ca(II) into this solution, the fluorescence of ZP1 increased and reached its maximum emission once >200 equiv. Ca(II) relative to mCP were added. In agreement with previous reports, maximum ZP1 emission occurred following addition of ~20 equiv. Ca(II) to mixtures of ZP1, hCP-Ser (hS100A8(C42S)/hS100A9(C3S) variant), and Mn(II) (Figure 4).30 These results show that the Mn(II) affinity of mCP is Ca(II)-dependent, and that mCP is able to outcompete ZP1 for Mn(II) when excess Ca(II) ions are added, indicating that mCP binds Mn(II) with Kd1 < 550 nM under conditions of high Ca(II). The requirement of >200 equiv. Ca(II) to fully enhance the Mn(II) affinity is consistent with our prior report on mCP that showed that >200 equiv. Ca(II) were necessary for complete tetramerization.43 We also note that the sigmoidal titration curve for mCP indicates cooperativity is at play, the origin and details of which are a subject for future work. Lastly, the Ca(II) responses of hCP and mCP clearly differ, which may have implications for the working model of mCP action. Along these lines, the total extracellular Ca(II) concentration is ~2 mM and mCP subunits are reported to reach concentrations on the order of hundreds of micromolar at infection sites.40, 46 Taken together with the current data, these [Ca(II)] / [mCP] ratios present the possibility that the speciation of mCP in the extracellular space may include mixtures of heterodimers and -tetramers. Nevertheless, it is also possible that the 2 mM value for total extracellular Ca(II) does not accurately estimate the Ca(II) concentrations mCP encounters at infection sites. Recent laser-ablation mass spectrometry studies of infected murine tissues revealed that Ca localizes to neutrophils and mCP, and although not quantified, the Ca concentration at these locales is higher than that in the surrounding tissues.28
Figure 4.
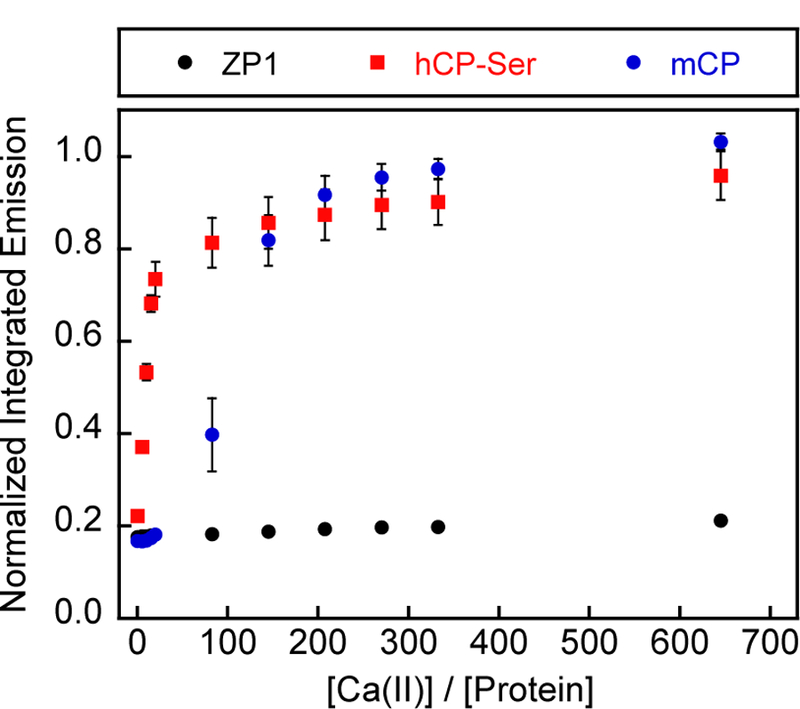
Titration of Ca(II) into a mixture of ZP1 (1 μM), hCP-Ser or mCP (4 μM), and Mn(II) (5 μM) monitored by fluorescence spectroscopy. The integrated emission was normalized to the integrated emission from the apo sensor (average ± SEM, n = 3). The buffer was 75 mM HEPES, 100 mM NaCl, 200 μM TCEP, pH 7.0 and T= 25 °C.
Mn(II) Competition Studies Further Define the His6 Site
We next evaluated how many equivalents of Mn(II) mCP binds with high affinity. We performed Mn(II) competition titrations between ZP1 (1 µM) and mCP or variant (4 µM) in the presence of 300 equiv. Ca(II) (1.2 mM). Titration of Mn(II) into the ZP1/mCP/Ca(II) mixture resulted in no change of ZP1 fluorescence until a ~1:1 Mn(II):protein ratio was achieved, further demonstrating that mCP binds 1 equiv. Mn(II) with greater affinity than ZP1 (Figure 5A). The ∆His3Asp variant also outcompeted ZP1 for ~1 equiv. Mn(II), whereas the ∆His4 variant was unable to compete with ZP1 for this metal ion. Moreover, the mS100A9 C-terminal tail variants H105A and H107A afforded titration curves indicative of Mn(II) competition and reduced Mn(II) affinity compared to mCP and ∆His3Asp (Figure 5B). The H103A variant, in contrast, outcompeted ZP1. Taken together, these results are in agreement with the observations from the SEC experiments and show that mCP coordinates one equivalent of Mn(II) with high affinity at the His6 site that is completed by residues H105 and H107 of the mS100A9 C-terminal tail.
Figure 5.

Titration of Mn(II) into a mixture of ZP1 (1 μM), mCP or variant (4 μM), and Ca(II) (1.2 mM) monitored by fluorescence spectroscopy. Data for mCP, DHis3Asp and DHis4 (A) and mS100A9 Cterminal tail variants (B). The integrated emission was normalized to the integrated emission from the apo sensor (average ± SEM, n ≥ 3). The buffer was 75 mM HEPES, 100 mM NaCl, 200 μM TCEP, pH 7.0 and T= 25 °C. In the legend, “ZP1” refers to a sample containing ZP1 and no protein.
EPR Spectroscopy of Mn(II)-mCP
To further characterize the Mn(II)-His6 site of mCP and describe its electronic structure, we applied multi-frequency EPR spectroscopic methods. First, we examined the X-band EPR spectra of Mn(II)-bound mCP and several variants (∆His3Asp, H103A, H105A, and H107A) analyzed in the biochemical experiments described above. We also studied mCP-Ser, an all Cys→Ser variant that we previously reported (Figure S4).43 Guided by our prior EPR spectroscopic evaluation of the human orthologue30–32 and the Mn(II)-binding studies of mCP presented in this work, we prepared EPR samples containing 200 µM protein, 0.9 equiv. Mn(II), and 300 equiv. Ca(II). Under these high Ca(II) conditions, we expect that all of the Mn(II) will be bound to the His6 site with negligible free Mn(II) in solution.
The CW X-band EPR spectrum of Mn(II) bound to the His6 site of mCP is typical for the high-spin d5 Mn(II) ion (Figure 6). The isotropic g-value of 2.0008 is typical for the spherical symmetry of the electron distribution. The magnitude of the g-value is remarkably similar to the value of 2.001 that was determined for Mn(II)- and Ca(II)-bound hCP-Ser.32 The sextet features centered around g = 2.0008 results from hyperfine interaction with the 55Mn nucleus (I = 5/2, 100% abundance). This observed sextet arises from ms transitions in the “inner” ms = ±½ Kramer’s doublet. Hyperfine splitting in the “outer” Kramer’s doublet (ms = ±½ ↔ ±3/2 ↔ ±5/2) is rarely resolved in frozen spectra due to orientation-dependent broadening.47–48 The magnitude of this hyperfine interaction, 248 MHz, is nearly identical to the 247 MHz measured for Mn(II)- and Ca(II)-bound hCP-Ser and consistent with the decrease in 55Mn hyperfine observed upon increasing imidazole concentration. 32, 49 In between each sextet pair are two additional transitions. These transitions arise from formally forbidden Δms ±1 and ΔmI ±1 transitions. At higher frequencies, the intensity of these transitions diminish, which further confirms their identities as forbidden transitions (Figures S5, S6). The intensity and positions of these transitions are indicative of the magnitude of the zero-field splitting (ZFS) parameters D and E/D. Simulations of the X-band EPR spectrum of Mn(II)- and Ca(II)-bound mCP utilizing EasySpin50 afford values of 525 MHz and 0.30 for D and E/D, respectively (Figure 6, red trace). The low ZFS is indicative of a highly symmetric, octahedral coordination sphere. The values are remarkably similar to the simulated D and E/D values of 485 MHz and 0.30 determined for Mn(II)- and Ca(II)-bound hCP-Ser.32
Figure 6.

X-band EPR spectra (black) and simulations (red) for Mn(II)- and Ca(II)-bound mCP and ΔHis3Asp. The buffer was 75 mM HEPES, 100 mM NaCl, pH 7.5.
We employed the same protein/Mn(II)/Ca(II) ratio to prepare samples of the mCP variants and found that Mn(II)- and Ca(II)-bound ∆His3Asp exhibits the same EPR spectrum as mCP (Figure 6) and can be simulated using identical parameters (Figure 6, red trace), supporting the conclusion that substoichiometric Mn(II) is only bound at the His6 site. Moreover, the EPR spectrum of Mn(II)- and Ca(II)-bound mCP-Ser could also be simulated with the same parameters (Figure S4), indicating that the Cys→Ser mutations have negligible effect on Mn(II) binding at the His6 site. We attempted to make EPR samples of the ∆His4 variant, but precipitation occurred during sample preparation so we did not analyze the samples. The mCP variants with mutations in the mS100A9 C-terminal tail afforded EPR spectra consistent with H105 and H107 completing the Mn(II)-His6 coordination sphere, but not H103 (Figure 7). H103A showed no change in the EPR spectrum compared to mCP (Figure 6) and can be simulated using parameters identical to mCP (Figure 7, red trace). In contrast, H105A and H107A show markedly perturbed EPR spectra compared to the spectra for mCP and H103A (Figures 6 and 7). The large increase in the intensity of the forbidden transitions relative to the allowed transitions indicates that the symmetry of the Mn(II) site is lowered. Along these lines, our prior EPR spectroscopic study of Mn(II)-bound variants of hCP-Ser revealed that analogous mutations in the C-terminal tail resulted in a coordination sphere that included solvent-derived ligands, which reduced the symmetry,32 and we reason a similar situation occurs with the H105A and H107A variants of mCP.
Figure 7.

X-band EPR spectra (black) of Mn(II)- and Ca(II)-bound mCP variants H103A, H105A and H107A. The red trace for H103A is a simulation using the parameters determined for Mn(II)- and Ca(II)-bound mCP. The peak marked by * is a background quartz radical. The buffer was 75 mM HEPES, 100 mM NaCl, pH 7.5.
To more quantitatively measure the interaction of the Mn(II) ion with the histidine residues of the His6 site of mCP, we prepared globally 15N-labeled protein (15N-mCP) for electron nuclear double resonance (ENDOR) spectroscopy.51-53 This sample contained 15N-mCP (200 µM), 0.9 equiv Mn(II), and 300 equiv. Ca(II). The Mims ENDOR spectrum of Mn(II)- and Ca(II)-bound 15N-mCP displays two sets of resonances centered at the 15N Larmor frequency (Figure 8). The broader doublet (labeled A in Figure 8) is assigned to the proximal nitrogen atoms of the histidine imidazoles (Nε2, based on structures of Mn(II)-bound hCP-Ser).32–33 The splitting of the peaks is caused by a τ‒dependent blind spot confirmed by collecting the spectrum with different τ values (Figure S7). This doublet can be simulated with a hyperfine tensor of [3.3 3.6 6.0] MHz and aiso of 4.3 MHz. The isotropic hyperfine coupling (aiso) is nearly identical to the 4.36 MHz measured for the Nε2 nitrogen of hCP-Ser.32 The second, narrower doublet (labeled B in Figure 8) is assigned to the distal nitrogens of the histidine imidazoles (Nδ1). This doublet can be simulated with an isotropic hyperfine coupling constant of 0.25 MHz. Consistent with the CW results comparing Mn(II)- and Ca(II)-bound mCP with hCP-Ser, this value is nearly identical to the 0.24 MHz measured for hCP-Ser.32 The approximately 18x difference in the isotropic nitrogen hyperfine value of the ε and δ nitrogens is consistent with the observed trend for other histidine-coordinated metal ions.51–53
Figure 8.

15N-Mims ENDOR spectrum (black) of Mn(II)- and Ca(II)-bound to globally-labeled 15N-mCP. The red trace is a simulation. The τ value is 700 ns. Spectra collected with additional τ values are presented in Figure S7. A and B indicate the two sets of resonances centered at the 15N Larmor frequency. The inverted triangle indicates the 15N Larmor frequency. The buffer was 75 mM HEPES, 100 mM NaCl, pH 7.5. Experimental settings: MW frequency = 34.0 GHz, Field = 1216.6 mT, T = 10 K.
In summary, a comparison of these EPR spectroscopic data for Mn(II)- and Ca(II)-bound mCP with those previously analyzed for the human orthologue reveals that the electronic structure of the Mn(II) ion coordinated to the His6 site is remarkably similar in both proteins. In particular, for samples prepared such that only the His6 site is occupied with Mn(II), the 55Mn hyperfine parameters, ZFS values, and g-values for Mn(II)- and Ca(II)-bound mCP are nearly identical to the parameters previously reported for Mn(II)- and Ca(II)-bound hCP-Ser.32
mCP Can Outcompete MntC and PsaA for Mn(II)
The bacterial pathogens S. aureus and S. pneumoniae employ the ABC transporters MntABC and PsaABC, respectively, to import Mn(II).54–55 MntC and PsaA are the solute-binding proteins (SBPs) responsible for capturing extracellular Mn(II) and delivering it to MntB and PsaC, respectively. We recently demonstrated that hCP-Ser can outcompete MntC and PsaA for Mn(II) under conditions of high Ca(II).34 To evaluate whether mCP exhibits similar behavior, we prepared samples containing a 1:1:1 ratio of mCP, SBP, and Mn(II) (500 µM each) in the absence and presence of 120 equiv. Ca(II) and evaluated the Mn(II) speciation in the samples by X-band EPR spectroscopy. As described previously, the EPR spectroscopic signatures of Mn(II)-bound hCP-Ser and the Mn(II)-bound SBPs are distinct.34 In contrast to the six-line feature centered at ca. g = 2 for Mn(II)- and Ca(II)-bound hCP-Ser, the Mn(II)-bound SBPs presents a broad, relatively featureless spectrum at ca. g = 2 and sharp spectral features in the g ~ 4.5 region.34 Similar to our observations for hCP-Ser, this Mn(II) speciation assay revealed that mCP outcompetes MntC and PsaA for Mn(II) under conditions of excess Ca(II) but not in the absence of this cation (Figure 9). Based on the reported Kd,Mn(II) values of MntC and PsaA (Kd,Mn(II) ~ 4 nM56 and ≤10 nM57, respectively) these observations indicate that Ca(II)-bound mCP binds Mn(II) with a sub-nanomolar Kd value. We also note that these results complement prior biological work. In a murine model of infection, deletion of MntABC was detrimental to the ability of S. aureus to cause infection in mice expressing mCP, which suggested that mCP competes with MntABC for Mn(II).18
Figure 9.
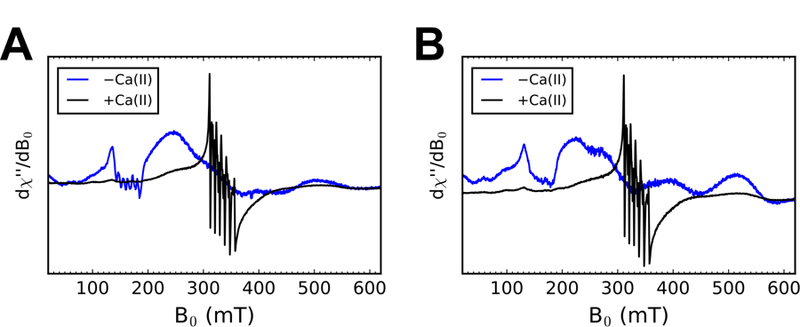
X-band EPR spectra of 500 μM mCP, 500 μM Mn(II), and 500 μM MntC (A) or PsaA (B) without or with 60 mM Ca(II) following a 14-h incubation at room temperature. The buffer was 75 mM HEPES, 100 mM NaCl, pH 7.5
Comparisons of hCP and mCP
The current studies examine the molecular basis of Mn(II) sequestration by mCP. This work is important because CP is the only known Mn(II)-sequestering host protein, and the vast majority of biochemical and biophysical characterization reported to date has focused on the human orthologue, whereas the understanding of the biological function of this protein is largely derived from murine infection models.23 This current biochemical and EPR spectroscopic investigation demonstrates that the Mn(II)-binding properties of mCP are remarkably similar to those of hCP. In particular, both proteins bind one equivalent of Mn(II) with high affinity at a His6 site that is formed by four His residues that reside at the S100A8/S100A9 dimer interface and two His residues in an HXH motif of the S100A9 C-terminal tail. The EPR spectroscopic analyses reveal that Mn(II) binding to mCP occurs in a highly symmetric coordination sphere with very similar parameters to those determined previously for hCP. Moreover, both proteins show enhanced Mn(II) affinities in the presence of excess Ca(II) ions, Mn(II)-induced tetramerization in the absence of Ca(II), and the ability to outcompete two bacterial SBPs for this metal ion.
Despite these similarities, there are also notable differences in the Mn(II)-binding properties of hCP and mCP. In particular, how Ca(II) modulates the biophysical properties of the two proteins differs; mCP requires ~10-fold more Ca(II) than hCP to exhibit complete conversion to the heterotetramer and fully enhanced Mn(II) affinity at the His6 site. Ca(II) ions are an important component of the working model for the extracellular function of CP in metal-withholding because Ca(II) binding induces tetramerization, enhances the transition metal affinities and antibacterial activity, and protects the protein from proteolytic degradation.23 Further biophysical and biological work is needed to illuminate both the origins and impacts of the differing Ca(II) sensitivities of hCP and mCP. The higher Ca(II) requirement of mCP suggests that it may exhibit different speciation in the extracellular space where the Ca(II) concentration is ~2 mM,40 possibly with a higher proportion of heterodimers compared to hCP. Along similar lines, we also observed reduced tetramerization of mCP in the presence of excess Mn(II) compared to prior studies of hCP, which further indicates that the self-association properties of the hCP and mCP heterodimers vary. Taken together, these observations suggest that local and/or global differences in protein structures and/or dynamics exist, which warrant further investigation.
Conclusion
In closing, this work provides a foundation for understanding the molecular basis for Mn(II) sequestration by mCP in murine models of infection as well as further examining the coordination chemistry and function of the His6 site of this protein. Whether the conserved His6 metal-binding motif that both mice and humans utilize in the metal-withholding innate immune response is a feature present in a wider range of CP orthologues remains to be investigated. Ultimately, this work helps to bridge the gap between molecular and physiological studies of CP, and emphasizes the importance of investigating and appreciating both the similarities and differences in human and murine host-defense strategies.
Experimental Section
General Materials and Methods
All chemicals were purchased from commercial suppliers and used as received. Milli-Q (18.2 MΩ cm) water was used in the preparation of all buffers and metal ion solutions. Buffers used for biochemical and spectroscopic experiments were prepared from ULTROL grade HEPES (CalBiochem) and TraceSelect NaCl (MilliporeSigma) to limit metal ion contamination from buffer components and stored in polypropylene containers. Calcium-containing buffers for analytical SEC were prepared with CaCl2•2H2O (>99.0%, MilliporeSigma). All buffers were filtered (0.2 µm) before use. Stock solutions of Ca(II) (1 M, 100 mL) were prepared from CaCl2•2H2O (>99.0%, MilliporeSigma) in acid-washed volumetric glassware and stored in polypropylene tubes. Stock solutions of Mn(II) (1 M, 100 mL) were prepared from MnCl2•4H2O (99.999%, Alfa Aesar) in acid-washed volumetric glassware and stored in polypropylene tubes. Working solutions of metal ions were prepared daily by dilution into Milli-Q water and stored in polypropylene tubes. Protein aliquots were stored at −80 °C and thawed only once prior to use. All protein concentrations were determined using calculated extinction coefficients (https://web.expasy.org/protparam/). All CP concentrations are for the heterodimer form of mCP (mS100A8/mS100A9, ε280 = 5,960 M−1 cm−1) or hCP-Ser ( hS100A8/hS100A9 ε280 = 18,450 M−1 cm−1). Reported equivalents of Mn(II) and Ca(II) are relative to the CP heterodimer. MntC (ε280 = 35,870 M−1 cm−1) and PsaA (ε280 = 35,870 M−1 cm−1) are monomers and concentrations of these proteins are for the monomer.
Design of Synthetic Genes
The synthetic genes containing the nucleotide sequences of mS100A8 and mS100A9 and all variants were ligated into the NdeI and XhoI restriction sites of the pET41a vector, as described previously.43 Synthetic genes for mS100A8(H17A)(H27A), mS100A8(H83A)(H87A), mS100A9(H21A)(D31A), and mS100A9(H92A)(H97A) were codon-optimized for Escherichia coli expression, synthesized, and ligated into the NdeI and XhoI restriction sites of pET41a vectors by ATUM (formerly DNA2.0). The nucleotide sequences are provided as Supporting Information. Each plasmid was transformed into chemically-competent E. coli TOP10 cells (ThermoFisher), isolated using a miniprep kit (Qiagen), and analyzed by DNA sequencing (Quintara Biosciences). The pET41a vectors containing mS100A8(H103A), mS100A9(H105A), mS1009(H107A), mS100A9(H103A)(H105A), mS100A9(H103A)(H107A), and mS100A9(H105A)(H107A) genes were prepared by site-directed mutagenesis as described below.
Site-directed Mutagenesis
The His→Ala point mutations in the mS100A9 gene were prepared by site-directed mutagenesis using the primer pairs and templates listed in Table S1. The oligonucleotide primers were synthesized by Integrated DNA Technologies (Coralville, IA). A modified Quick-Change site-directed mutagenesis protocol was employed to generate the variants using Pfu Turbo DNA polymerase(Agilent). The PCR protocol was 95 °C for 30 s; 95 °C for 30 s, 60–66 °C for 1 min (Table S1), 68 °C for 12 min (25x); 4 °C hold temperature. DpnI (New England Biolabs) was used to degrade the template plasmid. A 0.75 µL aliquot was added to the sample and incubated at 37 °C for 3 h. At 1.5 h into the incubation, a supplemental 0.75 µL aliquot of DpnI was added. The remaining plasmid DNA was transformed into chemically-competent E. coli TOP10 cells and the resulting plasmids isolated using miniprep kit (Qiagen) and analyzed by DNA sequencing (Quintara Biosciences).
Protein Overexpression and Purification
mCP and variants were overexpressed and purified as described previously.43 MntC and PsaA were overexpressed and purified as described previously.34 hCP-Ser was also overexpressed and purified as described previously.39 The globally 15N-labeled mS100A8 and mS100A9 subunits were overexpressed using reported procedures for the hCP-Ser subunits.32 In brief, M9 minimal medium (2 L) for overexpression was prepared with 15NH4Cl as the nitrogen source. Immediately before use, 1 mL 1,000x vitamin mix (400 mg choline chloride, 500 mg folic acid, 1.1 g pantothenic acid, 500 mg nicotinamide, 500 mg myo-inositol, 500 mg pyridoxal·HCl, 500 mg thiamine·HCl, and 50 mg riboflavin in 15 mL of Milli-Q water) was added to 2 L shaker flasks containing 1 L of the M9 minimal medium along with a few flakes each of FeCl3 (MilliporeSigma), biotin (Alfa Aesar), and thiamine-HCl (MilliporeSigma). Next, kanamycin was added to 50 µg/mL. A 20 mL aliquot of a saturated culture of BL21(DE3) cells containing either pET41a-mS100A8 or pET41a-mS100A9 was pelleted and resuspended in M9 minimal medium (25 mL). The centrifugation and resuspension steps were repeated and the resulting cell suspensions were used to inoculate the culture media for overexpression of each subunit. The overexpression was performed as described previously for mCP, with induction at OD600 = 0.6.43 The 15N-labeled protein was reconstituted and purified as described previously for mCP.43 Protein yields ranged from ≈14–60 mg / 2 L culture for mCP and all variants, including 15N-labeled protein.
Analytical Size Exclusion Chromatography (SEC)
An ÄKTA Purifier FPLC system housed in a cold room (4 ºC) and outfitted with a Superdex 75 10/300 GL SEC column was used for all analytical SEC. The elution buffer was 75 mM HEPES, pH 7.0, 100 mM NaCl, 200 µM TCEP ± 25 mM Ca(II). Mn(II) (500 µM or 5 mM) was added to samples from a 1 M stock solution and the samples were incubated at room temperature for at least 15 min prior to SEC analysis. The column was calibrated with blue dextran and a low-molecular-weight protein mixture (GE Healthcare Life Sciences) consisting of ribonuclease A (13.7 kDa), carbonic anhydrase (29 kDa), ovalbumin (44 kDa), and conalbumin (75 kDa) prior to use (Table S6). Proteins were buffer-exchanged (3x) into the running buffer using an Amicon spin filter (0.5 mL, 10 kDa MWCO). Samples (100 µM, 300 µL) were prepared and loaded into a 100 µL injection loop, and the FLPC system was programmed to load 500 µL of solution through the injection loop (sample + elution buffer) onto the column. The samples were eluted over one column volume (24 mL) at a flow rate of 0.5 mL/min. At least two independent replicates were performed for each experiment, and representative data from one experiment are shown. The protein concentration of each collected fraction was determined by absorbance at 280 nm in acid-washed quartz cuvettes using a Beckman DU 800 UV-Vis spectrophotometer equipped with a Peltier thermostat. To prepare ICP-MS samples for total Mn analyses, an aliquot (50 µL) of each fraction was diluted to 2 mL with 5% nitric acid (TraceSELECT, Fluka) and supplemented with 2 ppb Tb internal standard.
Liquid Chromatography-Mass Spectrometry (LC-MS)
An Agilent 6545 series Q-TOF LC-MS system with an Agilent Eclipse plus C18 with 2.1 × 50 mm and 1.8 µm particle size was used for all analyses. Each protein was diluted in Milli-Q water to a final concentration of ≈10 µM. A 5 or 10 µL protein aliquot was injected onto the column, and each sample was eluted using a gradient of 25–75% B over 10 min with a flow rate of 0.3 mL/min (solvent A: 0.1% formic acid in water; solvent B: 0.1% formic acid in acetonitrile). The spectra were deconvoluted using the maximum entropy algorithm in the MassHunter Bioconfirm software (Agilent).
Inductively-coupled Plasma Mass Spectrometry (ICP-MS)
The metal content of protein samples was determined using an Agilent 7900 ICP-MS with an autosampler housed in the Center for Environmental Health Sciences Bioanalytical Core Facility at MIT. The instrument was used in helium mode. Calibration standards were prepared from a serial dilution of the Environmental Standard calibration mixture (Agilent, part #5183–4688). Standards and samples were spiked with 2 ppb Tb (100 ppb stock in 5% nitric acid) internal standard (Agilent, part # 5190–8590). Sample volumes of either 1.7 or 2.0 mL were employed.
Circular Dichroism Spectroscopy
Circular dichroism (CD) spectra were collected using a Jasco J-500 spectrometer housed in the Biophysical Instrumentation Facility at MIT. Proteins were buffer-exchanged into CD buffer (1 mM Tris-HCl, pH 7.5, 1 mM DTT) prior to analysis. For samples containing Ca(II), an aliquot was added to the sample from a 1 M Ca(II) stock solution to afford 3 mM Ca(II) in the sample. Samples (10 µM, 300 µL) were transferred to a Hellma quartz cuvette (1 mm pathlength) for analysis. Spectra were collected from 195 to 260 nm using continuous scan mode (50 nm/min) and a 1 nm bandwidth. Reported spectra are averages of triplicate baseline-subtracted scans.
Fluorescence Titrations
Zinpyr-1 (ZP1) was synthesized as described previously58 and kindly provided by Dr. Jacob Goldberg and Prof. Stephen J. Lippard. Stock solutions of ZP1 (~3 mM) were prepared in anhydrous DMSO, aliquoted, and stored at −20 °C. Each aliquot was thawed only once. Titrations were performed in 75 mM HEPES, 100 mM NaCl, pH 7.0. Mn(II) competition titrations between ZP1 and mCP (or variant) were performed as described previously for hCP.30 In brief, aliquots of protein were thawed and subsequently buffer-exchanged into 75 mM HEPES, 100 mM NaCl, pH 7.0 using 0.5 mL Amicon spin filters (10 kDa MWCO). For each titration, a 2 mL solution of titration buffer containing ZP1 (1 µM) and protein (4 µM) was prepared in a 1 cm path length nitric acid-washed quartz cuvette. Ca(II) (1.2 mM) was added from a 1 M stock solution (2.4 µL). The mixture was titrated with Mn(II) (2 or 4 µL of a 500 µM Mn(II) solution). After each Mn(II) addition, the solution was gently mixed and incubated for ~10 min at room temperature and in the dark prior to recording the fluorescence emission spectrum. For the Ca(II) titration, protein (4 µM) and Mn(II) (4 µM) were pre-incubated for ~10 min prior to titration of Ca(II) (2 µL additions of either 20 or 250 mM Ca(II)) into the sample.
Emission spectra were collected on a Photon Technologies International QuantaMaster 40 fluorimeter outfitted with a continuous xenon source for excitation, autocalibrated QuadraScopic monochromators, a multimode PMT detector, and a circulating water bath maintained at 25 °C. This instrument was controlled by the FelixGX software package. The excitation wavelength was 490 nm. The emission spectra were collected and integrated over 500–650 nm.
EPR Spectroscopy
To prepare mCP samples for EPR spectroscopy, all protein aliquots were buffer-exchanged into 75 mM HEPES, 100 mM NaCl, pH 7.5. For X-band EPR spectroscopy, 200 µM protein was prepared with 180 µM Mn(II) (0.9 equiv) in the absence or presence of 60 mM Ca(II) (300 equiv). Samples (500 µL) were incubated for at least 15 min prior to being frozen in liquid nitrogen. Most protein samples were shipped in a liquid nitrogen dewar to the CalEPR facility at University of California, Davis for analysis. For 388 GHz EPR spectroscopy, a sample (500 µL) of mCP (500 µM) was incubated with Ca(II) (50 mM) and Mn(II) (450 µM) for at least 15 min, transferred to a 1 mL LDPE vial (Fisher Scientific), frozen in liquid nitrogen, and shipped on dry ice to the National High Magnetic Field Laboratory (Tallahassee, FL) for analysis. To evaluate the competition for Mn(II) between mCP and SBPs, mixed samples of SBP and mCP were prepared as described previously,34 with the exception that the +Ca(II) samples contained 60 mM Ca(II). A 1:1 Mn(II):SBP sample (1 mM each) was prepared along with an mCP sample (1 mM) that contained Ca(II) (120 mM). Following ~15 min incubation, aliquots of each solution were mixed in a 1:1 ratio and incubated for ~14 h at room temperature. The samples (170 µL) were subsequently frozen in liquid nitrogen and stored at −80 °C prior to analysis at MIT using instrumentation in the Department of Chemistry Instrumentation Facility.
At CalEPR, continuous wave (CW) X-band (ca. 9.38 GHz) EPR spectra were collected on a Bruker ELEXSYS E500 CW-EPR spectrometer with a super high QE (SHQE) resonator and an Oxford Instruments ESR900 liquid helium cryostat. Spectra were collected under slow-passage conditions so as not to distort the line shape. The spectrometer conversion time was set to 80 ms and a data point collected every ca. 0.3 mT with 0.5 mT modulation amplitude at 100 kHz. Spectra were collected at a temperature of 10 K with 0.2 mW of microwave power.
At CalEPR, Q-band (ca. 34.1 GHz) spectra were collected on a Bruker ELEXSYS E580 X/Q-band pulse EPR spectrometer equipped with an Oxford Instrument CF935 liquid helium cryostat and a home-built probe.59 Field sweeps were acquired using a 2-pulse Hahn echo sequence (π/2 – τ – π – τ ‒ echo) with a 2-step phase cycling program and data were collected every 0.3 mT. 15N electron nuclear double resonance (ENDOR) spectra were collected using the Mims ENDOR sequence (π/2 ‒ τ ‒ π/2 ‒ πRF ‒ π/2 ‒ τ – echo).60 The 50 µs long πRF pulse was amplified using an ENI A1000 amplifier providing 1 kW peak power. The amplified RF pulse was utilized without attenuation before the sample. The shot repetition time was 1 ms at 10 K for all pulse sequences.
At the National High Magnetic Field Lab, the transmission spectrometer was utilized to obtain a 388 GHz field sweep of Mn(II)-mCP in the presence of excess Ca(II).61 The temperature was 30 K with a modulation amplitude of 0.5 mT at 50 kHz, and downfield sweep rate 0.50 mT/s.
At MIT, X-band EPR spectra investigating the Mn(II) competition between mCP and SBPs was performed on a Bruker EMXplus spectrometer equipped with an ER4119HS resonator and a ColdEdge Technologies 4K waveguide cryogen-free cryostat. The spectrometer conversion time was set to 7.2 ms and a data point collected every ca. 0.1 mT with 0.5 mT modulation amplitude at 100 kHz. Spectra were collected at 10 K.
EPR Data Analysis
All spectra were simulated in Matlab R2017a (the Mathworks, Inc.) using the freely available EasySpin toolbox.50 The field-swept EPR spectrum can be interpreted using the phenomenological Spin Hamiltonian below.65–66
| (1) |
The terms in the Hamiltonian are the electron Zeeman interaction with an applied static magnetic field B, the isotropic hyperfine interaction (aiso) of the unpaired electrons with the 55Mn nucleus, the nuclear Zeeman interaction, the axial zero-field-splitting (ZFS) tensor D, and the rhombic ZFS term E for the high-spin Mn(II) ion (S = 5/2). βe is the Bohr magneton, g is the electron g factor, respectively, and h is the Planck constant. Example code for the simulation of the field sweeps and expanded discussion on how the simulations were performed are provided as Supporting Information. The simulation procedure was adapted from prior work.32
The 15N ENDOR spectra can be interpreted with a simplified phenomenological spin Hamiltonian.62–63
| (2) |
The zero-field splitting and 55Mn hyperfine are ignored and the system is treated as a pseudo S = ½ spin system. Here, Ai corresponds to the electron–nuclear hyperfine interaction tensor for atom i; βn is the nuclear magneton; gn,i is nuclear g factor for atom i; other terms are as defined above for equation 1. An expanded discussion of the simulation is provided as Supporting Information. The approximation used for the simulations was adapted from prior work.32
Supplementary Material
Acknowledgements
We gratefully acknowledge NIH grants R01GM118695 (E.M.N.) and R35GM126961 (R.D.B) for financial support. The ICP-MS is housed in the MIT Center for Environmental Health Sciences Bioanalytical Core, which is supported by NIH grant P30-ES002109. The MIT Biophysical Instrumentation Facility for the Study of Complex Macromolecular Systems is supported by NSF grant 0070319. A portion of this work was performed at the National High Magnetic Field Laboratory, which is supported by the National Science Foundation Cooperative Agreement No. DMR-1644779 and the State of Florida. The Q-TOF mass spectrometer is housed in the MIT Department of Chemistry Instrumentation Facility.
Footnotes
Supporting Information
Design of the synthetic genes, Tables S1–S6, and Figures S1–S7. This information is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hood MI; Skaar EP Nutritional immunity: transition metals at the pathogen–host interface. Nat. Rev. Microbiol 2012, 10 (8), 525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberg ED, Nutritional immunity: Host’s attempt to withhold iron from microbial invaders. J. Am. Med. Assoc 1975, 231 (1), 39–41. [DOI] [PubMed] [Google Scholar]
- 3.Juttukonda LJ; Skaar EP, Manganese homeostasis and utilization in pathogenic bacteria. Mol. Microbiol 2015, 97 (2), 216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brophy MB; Nolan EM, Manganese and microbial pathogenesis: sequestration by the mammalian immune system and utilization by microorganisms. ACS Chem. Biol 2015, 10 (3), 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papp-Wallace KM; Maguire ME, Manganese transport and the role of manganese in virulence. Annu. Rev. Microbiol 2006, 60, 187–209. [DOI] [PubMed] [Google Scholar]
- 6.Garcia YM; Barwinska-Sendra A; Tarrant E; Skaar EP; Waldron KJ; Kehl-Fie TE, A superoxide dismutase capable of functioning with iron or manganese promotes the resistance of Staphylococcus aureus to calprotectin and nutritional immunity. PLoS Pathog 2017, 13 (1), e1006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kehl-Fie TE; Chitayat S; Hood MI; Damo S; Restrepo N; Garcia C; Munro KA; Chazin WJ; Skaar EP, Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe 2011, 10 (2), 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz-Ochoa VE; Lam D; Lee CS; Klaus S; Behnsen J; Liu JZ; Chim N; Nuccio S-P; Rathi SG; Mastroianni JR; Edwards RA; Jacobo CM; Cerasi M; Battistoni A; Ouellette AJ; Goulding CW; Chazin WJ; Skaar EP; Raffatellu M, Salmonella mitigates oxidative stress and thrives in the inflamed gut by evading calprotectin-mediated manganese sequestration. Cell Host Microbe 2016, 19 (6), 814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makhlynets O; Boal AK; Rhodes DV; Kitten T; Rosenzweig AC; Stubbe J, Streptococcus sanguinis class Ib ribonucleotide reductase: high activity with both iron and manganese cofactors and structural insights. J. Biol. Chem 2014, 289 (9), 6259–6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhodes DV; Crump KE; Makhlynets O; Snyder M; Ge X; Xu P; Stubbe J; Kitten T, Genetic characterization and role in virulence of the ribonucleotide reductases of Streptococcus sanguinis. J. Biol. Chem 2014, 289 (9), 6273–6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chittori S; Simanshu DK; Banerjee S; Murthy AMV; Mathivanan S; Savithri HS; Murthy MRN, Mechanistic features of Salmonella typhimurium propionate kinase (TdcD): insights from kinetic and crystallographic studies. Biochim. Biophys. Acta 2013, 1834 (10), 2036–2044. [DOI] [PubMed] [Google Scholar]
- 12.Garces F; Fernández FJ; Montellà C; Penya-Soler E; Prohens R; Aguilar J; Baldomà L; Coll M; Badia J; Vega MC, Molecular architecture of the Mn2+-dependent lactonase UlaG reveals an RNase-like metallo-beta-lactamase fold and a novel quaternary structure. J. Mol. Biol 2010, 398 (5), 715–729. [DOI] [PubMed] [Google Scholar]
- 13.Brissac T; Ziveri J; Ramond E; Tros F; Kock S; Dupuis M; Brillet M; Barel M; Peyriga L; Cahoreau E; Charbit A, Gluconeogenesis, an essential metabolic pathway for pathogenic Francisella. Mol. Microbiol 2015, 98 (3), 518–534. [DOI] [PubMed] [Google Scholar]
- 14.Gutka HJ; Wolf NM; Bondoc JMG; Movahedzadeh F, Enzymatic characterization of fructose 1,6-bisphosphatase II from Francisella tularensis, an essential enzyme for pathogenesis. Appl. Biochem. Biotechnol 2017, 183 (4), 1439–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadzhaev K; Zingmark C; Golovliov I; Bolanowski M; Shen H; Conlan W; Sjöstedt A, Identification of genes contributing to the virulence of Francisella tularensis SCHU S4 in a mouse intradermal infection model. PLoS One 2009, 4 (5), e5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson MK; Keithly ME; Goodman MC; Hammer ND; Cook PD; Jagessar KL; Harp J; Skaar EP; Armstrong RN, Structure and function of the genomically encoded fosfomycin resistance enzyme, FosB, from Staphylococcus aureus. Biochemistry 2014, 53 (4), 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniel J; Abraham L; Martin A; Pablo X; Reyes S, Rv2477c is an antibiotic-sensitive manganese-dependent ABC-F ATPase in Mycobacterium tuberculosis. Biochem. Biophys. Res. Commun 2018, 495 (1), 35–40. [DOI] [PubMed] [Google Scholar]
- 18.Kehl-Fie TE; Zhang Y; Moore JL; Farrand AJ; Hood MI; Rathi S; Chazin WJ; Caprioli RM; Skaar EP, MntABC and MntH contribute to systemic Staphylococcus aureus infection by competing with calprotectin for nutrient manganese. Infect. Immun 2013, 81 (9), 3395–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Handke LD; Gribenko AV; Timofeyeva Y; Scully IL; Anderson AS, MntC-dependent manganese transport Is essential for Staphylococcus aureus oxidative stress resistance and virulence. mSphere 2018, 3 (4), e0036–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marra A; Lawson S; Asundi JS; Brigham D; Hromockyj AE, In vivo characterization of the psa genes from Streptococcus pneumoniae in multiple models of infection. Microbiology 2002, 148, 1483–1491. [DOI] [PubMed] [Google Scholar]
- 21.Gat O; Mendelson I; Chitlaru T; Ariel N; Altboum Z; Levy H; Weiss S; Grosfeld H; Cohen S; Shafferman A, The solute-binding component of a putative Mn(II) ABC transporter (MntA) is a novel Bacillus anthracis virulence determinant. Mol. Microbiol 2005, 58 (2), 533–551. [DOI] [PubMed] [Google Scholar]
- 22.Colomer-Winter C; Flores-Mireles AL; Baker SP; Frank KL; Lynch AJL; Hultgren SJ; Kitten T; Lemos JA, Manganese acquisition is essential for virulence of Enterococcus faecalis. PLoS Pathog 2018, 14 (9), e1007102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zygiel EM; Nolan EM, Transition metal sequestration by the host-defense protein calprotectin. Ann. Rev. Biochem 2018, 87, 621–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hobbs JAR; May R; Tanousis K; McNeill E; Mathies M; Gebhardt C; Henderson R; Robinson MJ; Hogg N, Myeloid cell function in MRP-14 (S100A9) null mice. Mol. Cell. Biol 2003, 23 (7), 2564–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corbin BD; Seeley EH; Raab A; Feldmann J; Miller MR; Torres VJ; Anderson KL; Dattilo BM; Dunman PM; Gerads R; Caprioli RM; Nacken W; Chazin WJ; Skaar EP, Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 2008, 319, 962–965. [DOI] [PubMed] [Google Scholar]
- 26.Liu JZ; Jellbauer S; Poe AJ; Ton V; Pesciaroli M; Kehl-Fie TE; Restrepo NA; Hosking MP; Edwards RA; Battistoni A; Pasquali P; Lane TE; Chazin WJ; Vogl T; Roth J; Skaar EP; Raffatellu M, Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe 2012, 11 (3), 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hood MI; Mortensen BL; Moore JL; Zhang Y; Kehl-Fie TE; Sugitani N; Chazin WJ; Caprioli RM; Skaar EP, Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog 2012, 8 (12), e1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juttukonda LJ; Berends ETM; Zackular JP; Moore JL; Stier MT; Zhang Y; Schmitz JE; Beavers WN; Wijers CD; Gilston BA; Kehl-Fie TE; Atkinson J; Washington MK; Peebles RS; Chazin WJ; Torres VJ; Caprioli RM; Skaar EP, Dietary manganese promotes staphylococcal infection of the heart. Cell Host Microbe 2017, 22 (4), 531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Besold AN; Gilston BA; Radin JN; Ramsoomair C; Culbertson EM; Li CX; Cormack BP; Chazin WJ; Kehl-Fie TE; Culotta VC, The role of calprotectin in withholding zinc and copper from Candida albicans. Infect. Immun 2017, 86 (2), e00779–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayden JA; Brophy MB; Cunden LS; Nolan EM, High-affinity manganese coordination by human calprotectin is calcium-dependent and requires the histidine-rich site formed at the dimer interface. J. Am. Chem. Soc 2013, 135 (2), 775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brophy MB; Nakashige TG; Gaillard A; Nolan EM, Contributions of the S100A9 C-terminal tail to high-affinity Mn(II) chelation by the host-defense protein human calprotectin. J. Am. Chem. Soc 2013, 135 (47), 17804–17817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gagnon DM; Brophy MB; Bowman SEJ; Stich TA; Drennan CL; Britt RD; Nolan EM, Manganese binding properties of human calprotectin under conditions of high and low calcium: X-ray crystallographic and advanced electron paramagnetic resonance spectroscopic analysis. J. Am. Chem. Soc 2015, 137 (8), 3004–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damo SM; Kehl-Fie TE; Sugitani N; Holt ME; Rathi S; Murphy WJ; Zhang Y; Betz C; Hench L; Fritz G; Skaar EP; Chazin WJ, Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc. Natl. Acad. Sci 2013, 110 (10), 3841–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hadley RC; Gagnon DM; Brophy MB; Gu Y; Nakashige TG; Britt RD; Nolan EM, Biochemical and spectroscopic observation of Mn(II) sequestration from bacterial Mn(II) transport machinery by calprotectin. J. Am. Chem. Soc 2018, 140 (1), 110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gifford JL; Walsh MP; Vogel HJ, Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem. J 2007, 405 (2), 199–221. [DOI] [PubMed] [Google Scholar]
- 36.Hunter MJ; Chazin WJ, High level expression and dimer characterization of the S100 EF-hand proteins, migration inhibitory factor-related proteins 8 and 14. J. Biol. Chem 1998, 273 (20), 12427–12435. [DOI] [PubMed] [Google Scholar]
- 37.Vogl T; Roth J; Sorg C; Hillenkamp F; Strupat K, Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 detected by ultraviolet matrix-assisted laser desorption/ionization mass spectrometry. J. Am. Soc. Mas Spectrom 1999, 10 (11), 1124–1130. [DOI] [PubMed] [Google Scholar]
- 38.Korndörfer IP; Brueckner F; Skerra A, The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting J. Mol. Biol 2007, 370 (5), 887–898. [DOI] [PubMed] [Google Scholar]
- 39.Brophy MB; Hayden JA; Nolan EM, Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin. J. Am. Chem. Soc 2012, 134 (43), 18089–18100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brini M; Ottolini D; Cali T; Carafoli E, Calcium in health and disease. Met. Ions Life Sci 2013, 13, 81–137. [DOI] [PubMed] [Google Scholar]
- 41.Stephan JR; Nolan EM, Calcium-induced tetramerization and zinc chelation shield human calprotectin from degradation by host and bacterial extracellular proteases. Chem. Sci 2016, 7 (3), 1962–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makałowski W; Zhang J; Boguski MS, Comparative analysis of 1196 orthologous mouse and human full-length mRNA and protein sequences. Genome Res 1996, 6 (9), 846–857. [DOI] [PubMed] [Google Scholar]
- 43.Hadley RC; Gu Y; Nolan EM, Initial biochemical and functional evaluation of murine calprotectin reveals Ca(II)-dependence and its ability to chelate multiple nutrient transition metal ions. Biochemistry 2018, 57 (19), 2846–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bradshaw RA; Brickey WW; Walker KW, N-Terminal processing: the methionine aminopeptidase and Nα-acetyl transferase families. Trends Biochem. Sci 1998, 23 (7), 263–267. [DOI] [PubMed] [Google Scholar]
- 45.You Y; Tomat E; Hwang K; Atanasijevic T; Nam W; Jasanoff AP; Lippard SJ, Manganese displacement from Zinpyr-1 allows zinc detection by fluorescence microscopy and magnetic resonance imaging. Chem. Commun 2010, 46 (23), 4139–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kocher M; Kenny PA; Farram E; Abdul Majid KB; Finlay-Jones JJ; Geczy CL, Functional chemotactic factor CP-10 and MRP-14 are abundant in murine abscesses. Infect. Immun 1996, 64 (4), 1342–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duboc C; Phoeung T; Zein S; Pécaut J; Collomb M-N; Neese F, Origin of the zero-field splitting in mononuclear octahedral dihalide MnII complexes: an investigation by multifrequency high-field electron paramagnetic resonance and density functional theory. Inorg. Chem 2007, 46 (12), 4905–4916. [DOI] [PubMed] [Google Scholar]
- 48.Duboc C; Collomb M-N; Neese F, Understanding the zero-field splitting of mononuclear manganese(II) complexes from combined EPR spectroscopy and quantum chemistry. Appl. Magn. Reson 2010, 37, 229–245. [Google Scholar]
- 49.Un S, Structure and nature of manganese(II) imidazole complexes in frozen aqueous solutions. Inorg. Chem 2013, 52 (7), 3803–3813. [DOI] [PubMed] [Google Scholar]
- 50.Stoll S; Schweiger A, EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson 2006, 178 (1), 42–55. [DOI] [PubMed] [Google Scholar]
- 51.Mims WB; Peisach J, The nuclear modulation effect in electron spin echoes for complexes of Cu2+ and imidazole with 14N and 15N. J. Chem. Phys 1978, 69 (11), 4921–4930. [Google Scholar]
- 52.Zweier J; Aisen P; Peisach J; Mims WB, Pulsed electron paramagnetic resonance studies of the copper complexes of transferrin. J. Biol. Chem 1979, 254 (9), 3512–3515. [PubMed] [Google Scholar]
- 53.Deligiannakis Y; Louloudi M; Hadjiliadis N, Electron spin echo envelope modulation (ESEEM) spectroscopy as a tool to investigate the coordination environment of metal centers. Coord. Chem. Rev 2000, 204, 1–112. [Google Scholar]
- 54.Horsburgh MJ; Wharton SJ; Cox AG; Ingham E; Peacock S; Foster SJ, MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol. Microbiol 2002, 44 (5), 1269–1286. [DOI] [PubMed] [Google Scholar]
- 55.Dintilhac A; Alloing G; Granadel C; Claverys J-P, Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol. Microbiol 1997, 25 (4), 727–739. [DOI] [PubMed] [Google Scholar]
- 56.Gribenko A; Mosyak L; Ghosh S; Parris K; Svenson K; Moran J; Chu L; Li S; Liu T; Woods VL Jr.; Jansen KU; Green BA; Anderson AS; Matsuka YV, Three-dimensional structure and biophysical characterization of Staphylococcus aureus cell surface antigen–manganese transporter MntC. J. Mol. Biol 2013, 425 (18), 3429–3445. [DOI] [PubMed] [Google Scholar]
- 57.McDevitt CA; Ogunniyi AD; Valkov E; Lawrence MC; Kobe B; McEwan AG; Paton JC, A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog 2011, 7 (11), e1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walkup GK; Burdette SC; Lippard SJ; Tsien RY, A new cell-permeable fluorescent probe for Zn2+. J. Am. Chem. Soc 2000, 122 (23), 5644–5645. [Google Scholar]
- 59.Forrer J; García-Rubio I; Schuhmam R; Tschaggelar R; Harmer J, Cryogenic Q-band (35 GHz) probehead featuring large excitation microwave fields for pulse and continuous wave electron paramagnetic resonance spectroscopy: performance and applications. J. Magn. Reson 2008, 190 (2), 280–291. [DOI] [PubMed] [Google Scholar]
- 60.Mims WB, Pulsed endor experiments. Proc. R. Soc. Math. Phys. Eng. Sci 1965, 283 (1395), 452–457. [Google Scholar]
- 61.Hassan A; Pardi L; Krzystek J; Sienkiewicz A; Goy P; Rohrer M; Brunel L-C, Ultrawide band multifrequency high-frequency EMR technique: a methodology for increasing spectroscopic information. J. Magn. Reson 2000, (142), 300–312. [DOI] [PubMed] [Google Scholar]
- 62.Stich TA; Lahiri S; Yeagle G; Dicus M; Brynda M; Gunn A; Aznar C; Derose VJ; Britt RD, Multifrequency pulsed EPR studies of biologically relevant manganese(II) complexes. Appl. Magn. Reson 2007, 31, 321–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Well JA; Bolton JR, Electron Paramagnetic Resonance: Elementary Theory and Practical Applications 2007, 2nd ed.; Wiley-Interscience: Hoboken, N. J. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


