Abstract
Background:
ASPS, a rare vascular sarcoma with clinically indolent course, frequently presents with metastases. Vascular endothelial growth factor (VEGF) is a promising therapeutic target. In a phase II trial of the VEGF receptor inhibitor cediranib for adults with ASPS, the partial response (PR) rate (RECIST v1.0) was 35% (15/43;95% CI:21–51%). We evaluated cediranib in the pediatric population.
Procedure:
Patients (pts) <16 years old (yo) with metastatic, unresectable ASPS received cediranib at the pediatric maximum tolerated dose of 12 mg/m2 (≈70% of the fixed adult phase II dose orally daily). Tumor response was assessed every 2 cycles (cy) (RECIST v1.0). A Simon two-stage optimal design (target response rate 35%, rule out 5%) was used.
Results:
7 pts (4 female), median age 13 yo, (range 9–15), enrolled on stage 1. The most frequent grade 2 or 3 adverse events were neutropenia, diarrhea, hypertension, fatigue, and proteinuria. Best response was stable disease (SD) (median cy number=34). 3 pts were removed from study treatment for disease progression (cy 4, 5, and 36). 5 of 7 pts had SD for ≥14 months. 2 pts with SD remain on study (34–57+ cy).
Conclusions:
Cediranib did not reach the target response rate in this small pediatric cohort, in contrast to the adult 35% PR rate. Pediatric dosing was 30% lower compared to adult dosing, which may contribute to response differences. Prolonged SD was observed in 5 pts, but given the indolent nature of ASPS, SD cannot be clearly attributed to cediranib. Cediranib has an acceptable safety profile.
Keywords: cediranib, alveolar soft part sarcoma, pediatric, phase II study
INTRODUCTION
Alveolar soft part sarcoma (ASPS) is a rare, highly vascular tumor accounting for less than 1% of soft tissue sarcomas, predominantly affecting adolescents and young adults 1. It is associated with a characteristic unbalanced t(X,17)(p11;q25) translocation, which results in the ASPL-TFE3 fusion protein, associated with enhanced MET-related signal transduction. While ASPS often has an indolent clinical course compared to aggressive sarcomas such as Ewing sarcoma, it is characterized by a high frequency of metastases to the lungs, brain, and bones, with a 5-year survival rate of 20% in patients with unresectable metastatic disease 2,3. ASPS has proven to be resistant to conventional cytotoxic chemotherapy, with radical surgery, when feasible, as the only recognized effective treatment 4.
Relatively little is known with regards to relevant molecular markers as potential therapeutic targets for ASPS 5–7. Results from a gene expression profiling study performed at the NCI identified several transcripts associated with angiogenesis, cell proliferation, metastasis, and myogenic differentiation 8.
Cediranib (AZD2171, AstraZeneca Pharmaceuticals, Wilmington, DE) is an orally bioavailable, small-molecule inhibitor of all three vascular endothelial growth factor receptor (VEGFR-1, −2, and −3) tyrosine kinases, which mediate angiogenesis and lymphangiogenesis 9,10. At the time of this trial’s initiation, first open only to an adult patient cohort, cediranib had recently demonstrated antitumor activity in early phase clinical trials for the refractory solid tumor population 11,12. A pediatric phase I study of cediranib for children and adolescents with refractory solid tumors defined the maximum tolerated monotherapy dose of 12 mg/m2/dose administered orally, once daily, continuously 11. Based on the results from the pediatric phase I study of cediranib, the adult clinical trial was amended to include a pediatric ASPS cohort starting in 2012. At that time, there was substantial single-agent activity being observed in the adult cohort of the trial, with accrual ongoing. Considering the vascularity of ASPS in conjunction with the preliminary evidence of cediranib’s anti-tumor activity, we initiated an open-label, single-arm, phase II trial of cediranib to evaluate the objective response rate (ORR) in pediatric patients with metastatic ASPS. We describe in this report the results of the pediatric cohort of this single site, CTEP-sponsored, phase II trial of cediranib in pediatric patients with ASPS. Data from the adult cohort has been separately published previously, with a partial response (PR) rate (Response evaluation criteria in solid tumors (RECIST) v1.0) of 35% (15/43;95% confidence interval (CI):21–51%) 13.
METHODS
Patient eligibility
At study enrollment, patients with measurable, histologically or cytologically confirmed metastatic ASPS not curable by surgery were eligible to participate. Measurable disease, defined as at least one lesion measured in at least one dimension as ≥20mm with conventional techniques or as ≥10mm with spiral CT scan, was required. Patients were required to be <16 years of age, with BSA ≥ 1.04 m2. Standard organ function 13 and Lansky/Karnofsky performance status ≥ 50% were required, in addition to normal blood pressure (≤ the 95th percentile for age, height, and gender) without antihypertensive agents. Prior treatment, including other anti-angiogenic treatments, was allowed. Pediatric patients were required to have a normal left ventricular function with ejection fraction > 55% or shortening fraction ≥ 27%. More detailed information regarding general eligibility and exclusion criteria are described in a previous publication focused on the adult cohort 13.
The trial was approved by the National Cancer Institute Institutional Review Board. All patients or their legal guardians signed a document of informed consent and assent was obtained as appropriate according to institutional guidelines.
Drug Administration
Cediranib (AZD2171) was supplied as 15- and 20-mg tablets by the Cancer Therapy Evaluation Program (NCI, Bethesda, MD) under a collaborative agreement with AstraZeneca (Wilmington, DE). All pediatric patients received 12 mg/m2/dose administered orally, once daily, continuously for 28-day cycles using a dosing nomogram provided in the protocol.
Cycles were repeated without interruption, without any maximum number of cycles, if the patient had at least stable disease (SD) and continued to meet the hematologic and organ function criteria required at enrollment. A maximum of two dose reductions (approximately 30% decrease per dose reduction) were allowed for subjects who experienced a reversible dose modifying toxicity (DMT).
Study Design
A Simon two-stage optimal design was used to evaluate cediranib in the pediatric population in order to rule out an unacceptably low 5% overall response rate (p0=0.05) in favor of a higher response rate of 35% (p1=0.35), with alpha=0.10 (probability of accepting a poor treatment=0.10) and beta = 0.10 (probability of rejecting a good treatment=0.10). At the first stage, 6 patients were to be enrolled. If no patient experienced an objective response, cediranib would be considered inactive and no further patients would be accrued. If 1 or more of the first 6 evaluable patients achieved a partial response or complete response by RECIST v1.0, then accrual would continue until a total of 12 evaluable patients were enrolled. If ≥ 2 of 12 (16.7% or more) patients experienced a partial or complete response, this would be sufficiently interesting to warrant further study in later trials. Under the null hypothesis (5% response rate), the probability of early termination was 74%.
All eligible patients who received at least one cycle of cediranib and had their disease re-evaluated were considered evaluable for response. In addition, any eligible patient who exhibited disease progression prior to the end of cycle 1 was also considered evaluable for response.
Response evaluation
Tumor disease evaluations including standard anatomic imaging for measurable disease with CT and/or magnetic resonance imaging (MRI) scans were conducted at baseline and repeated every 2 cycles during first 18 cycles; after 18 and 36 cycles, repeat imaging was performed every 3 or 4 cycles, respectively. RECIST version 1.0 was used to categorize objective response, stable disease and progression of disease.
Toxicity evaluation
Each cycle of cediranib was considered in the analysis of toxicity. History and physical examination, complete blood counts (CBCs), and serum chemistries were performed at baseline, with laboratory studies performed weekly for the first cycle, every two weeks for the second and third cycles, and then every 4 weeks thereafter. Blood pressure was measured weekly during the first two cycles and then every 2 weeks by a health care provider, with patients required to maintain a study diary with home blood pressure monitoring twice daily. To monitor for potential skeletal toxicity of cediranib, all patients <16 years old had a lower extremity radiograph for growth plate assessment performed at baseline, with non-contrast MRI of the right knee to measure the volume of the distal femoral growth plate required for patients with open growth plates. For these patients, MRIs were obtained after cycles 2, 4, 8, 12, and then every 4 cycles for as long as the growth plate remained open. For growth plate evaluation, we used a semiautomated method of volumetric growth plate measurement as previously described 14. Growth plate expansion greater than 2 times the volume from baseline to interval measurement was considered dose limiting, at which point study drug would be discontinued.
Adverse events (AEs) were graded according to NCI Common Terminology Criteria for Adverse Events version 4.0. Each AE was categorized as hematologic or non-hematologic. Hematologic dose-modifying toxicity (DMT) was defined as grade 4 neutropenia or thrombocytopenia. Non-hematologic DMT was defined as any Grade 3 or 4 non-hematological toxicity with the exception of the following Grade 3 or 4 toxicities: electrolyte abnormalities correctable within 48 hours and tumor pain. Any patient who had a non-hematologic DMT that did not return to baseline within 14 days while holding cediranib was removed from study treatment. Grade 2 toxicity that persisted for ≥7 days and was considered sufficiently medically significant or sufficiently intolerable by the patient that it required treatment interruption was also a DMT. Dose-modifying hypertension was defined as any grade ≥4 hypertension, confirmed systolic or diastolic blood pressure ≥ 25mmHg above the 95th percentile for age, height, and gender 15, or an elevated blood pressure not controlled by anti-hypertensive medication within 14 days according to a previously described algorithm 11.
All patients were considered evaluable for toxicity from the time of their first dose of cediranib.
RESULTS
Patient Characteristics
This study (ClinicalTrials.gov identifier: ) enrolled seven pediatric patients between May 30, 2012 and June 01, 2016. Data as of January 01, 2019 were included in this analysis. All subjects were eligible and evaluable for the primary study endpoint. Patient demographics and disease characteristics are described in Table 1. One patient had received prior VEGF-targeted therapy. At the time of data cut-off, two patients continued protocol therapy, neither of whom had previously received prior VEGF-targeted therapy.
Table 1.
Patient demographics and disease characteristics
| Characteristic | No. of Patients (N=7) |
|---|---|
| Age (years) | |
| Median (Range) | 13 (9–15) |
| Sex | |
| Male | 3 |
| Female | 4 |
| Race | |
| White | 3 |
| Asian | 0 |
| American Indian or Alaska Native | 0 |
| Black or African American | 3 |
| Multiracial | 1 |
| Lansky/Karnofsky | |
| 100% | 1 |
| 90% | 4 |
| 80% | 2 |
| Prior systemic therapy | 4 |
| Prior resection | 3 |
| Prior radiation | 2 |
| Primary site of disease | |
| Lower extremity | 3 |
| Upper extremity | 1 |
| Pelvis/gluteal area | 1 |
| Chest/chest wall | 1 |
| Axilla | 1 |
| Metastatic site of disease | |
| Pulmonary | 7 |
| Brain | 2 |
Antitumor Activity
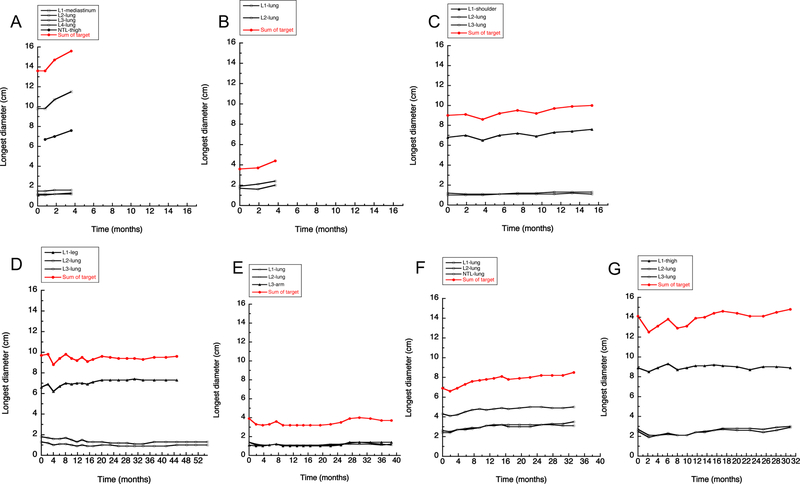
No objective responses were observed in the 7 patients enrolled, with stable disease (SD) as the best observed response (Figure 1). The median number of treatment cycles for all evaluable patients was 34 (range 4–57+). Five of 7 patients had SD for ≥14 months.
Figure 1: Tumor response data in 7 evaluable patients.
Individual response data for patients #45–53 (Panel A: #45, Panel B: #48, Panel C: #49, Panel D: #50, Panel E: #51, Panel F: #52, Panel G: #53). For the individual patient, each graph displays longest diameter of individual target lesions (primary site and lung), along with total sum of target lesions (by longest diameter). If there was a measurable non-target lesion (NTL), this data is also displayed (see panels A [NTL-thigh] &F [NTL-lung]). Four patients (#50–53) remained on study drug for an extended period of time.
According to the protocol design, cediranib was not considered to have sufficient efficacy for further development. According to the design, 6 patients were to be enrolled in stage 1. In total, there was enrollment of 7 patients, with unintentional over-enrollment by 1 patient.
Toxicity Evaluation
The grade 2 or higher AEs considered possibly, probably or definitively related to cediranib are displayed in Table 2. The most frequent grade 2 or 3 AEs were neutropenia, diarrhea, hypertension, fatigue, and proteinuria. The most frequent grade 2 or 3 AEs occurring in later cycles (after Cycle 6) were neutropenia, diarrhea, fatigue, and weight loss. There were no cediranib-related grade 4 or 5 toxicities. One patient demonstrated DMT during the first cycle of therapy, which was grade 3 alanine aminotransferase (ALT) elevation. All other DMTs were grade 2 or 3 events in subsequent cycles considered possibly or probably related to cediranib, including hypertension (n=1), fatigue (n=2), and proteinuria (n=1). The patient with cycle 1 DMT required a second dose reduction due to grade 2 proteinuria in cycle 38. The other 3 patients who developed a DMT required only one dose reduction, with 2 of these patients experiencing a DMT due to intolerable grade 2 fatigue, and 1 patient experiencing a DMT due to grade 3 hypertension. Two patients requiring dose reduction remain on study drug to date with prolonged SD, with the two other patients now off study, one due to patient preference and the other due to progressive disease on cycle 36 restaging, after a prolonged SD. Four patients required treatment for drug-associated toxicity of Grade II-III hypertension (n=4) and Grade II hypothyroidism (n=2). There was no growth plate toxicity detected on MRI for any enrolled patient, with no growth plate expansion greater than 2-fold from baseline observed. Height in all patients developed along expected growth curves.
Table 2.
Grade 2 and Higher Toxicities Possibly, Probably, or Likely Related to Cediranib (CTCAE version 4.0)
| Maximum grade of toxicity per patient | ||
|---|---|---|
| Toxicity Type | Grade 2 | Grade 3 |
| Hematologic | ||
| Lymphocyte count decreased | 1 | |
| Neutrophil count decreased | 1 | 2 |
| White blood cell count decreased | 3 | |
| Constitutional | ||
| Fatigue | 3 | |
| Weight loss | 3 | |
| Dermatologic | ||
| Palmar-plantar erythrodysesthesia | 1 | |
| Paronychia | 1 | |
| Eye | ||
| Papilledema^ | 1 | |
| Gastrointestinal | ||
| Abdominal pain | 1 | |
| Anorexia | 1 | |
| Diarrhea | 2 | 1 |
| Nausea | 1 | |
| Endocrine | ||
| Hypothyroidism | 2 | |
| Metabolic/Laboratory | ||
| Alanine aminotransferase increased | 1 | |
| Blood bilirubin increased | 1 | |
| Creatine phosphokinase increased | 1 | |
| Dehydration | 1 | |
| Hypercalcemia | 1 | |
| Hypophosphatemia | 1 | |
| Nervous System | ||
| Headache | 1 | |
| Renal and Urinary | ||
| Proteinuria | 3 | |
| Reproductive System | ||
| Vaginal pain | 1 | |
| Respiratory Disorders | ||
| Epistaxis | 1 | |
| Vascular Disorders | ||
| Hypertension | 3 | 1 |
On follow-up evaluation of this patient, Ophthalmology identified that the patient had pseudo-papilledema rather than papilledema.
DISCUSSION
To date there are no FDA-approved therapies for ASPS. Given the preclinical rationale and reported clinical activity in adults with ASPS with a PR rate of 35%, our goal was to evaluate activity in children with ASPS. With prolonged SD as the best observed response in stage I, the target response rate was not achieved in this pediatric cohort with single-agent cediranib. As such, no additional patients were enrolled. There was a prolonged period of disease stability observed in the majority of pediatric patients. However, considering the indolent nature of ASPS, the prolonged SD observed cannot be clearly attributed to cediranib. In contrast, in the adult cohort of this study, cediranib demonstrated substantial single-agent activity, with an objective response rate of 35% (15/43; 95% CI: 21–51%) and a disease control rate at 6 months of 84% (36/43; 95% CI: 69–93%) 13. Given the striking response rate in the adult cohort, a multicenter phase II trial in which adult patients were randomized to receive either open-label cediranib or sunitinib, with cross over at disease progression, was subsequently initiated (ClinicalTrials.gov identifier: ). This study may provide additional information on the activity of cediranib in this population. The pediatric dosing in our study was approximately 30% lower compared to adult dosing, which may have contributed to the lack of response observed in children. With the application of the dosing nomogram used, the median starting dose received by the pediatric cohort was 11.0 mg/m2/dose, with a range of 10.5 to 14.2 mg/m2/dose. The pediatric dosing of 12 mg/m2/dose once daily was based on the established maximum tolerated dose in a pediatric phase I study of cediranib 11. The adult fixed dose equivalent of this pediatric dose is 20 mg once daily. Patients enrolled in the adult cohort received a dose of 30 mg daily, which is lower than the adult MTD of 45 mg established in a phase I trial 16, which was found to be poorly tolerated in subsequent trials. It is unlikely that the pediatric patients would have tolerated dosing at any higher levels, considering that all patients on study drug for an extended period required at least one dose reduction due to toxicity. Furthermore, the differential response observed among the pediatric and adult cohorts could result from underlying differences in tumor biology. Further studies are required to determine and characterize such possible differences. Of note, only one pediatric patient had received prior VEGF-targeted therapy.
The role of cediranib in the treatment of children and adolescents with ASPS is uncertain. In this present study, cediranib as a single agent was found to be inactive in ASPS. There were objective responses observed in pediatric patients with Ewing sarcoma, synovial sarcoma, and osteosarcoma in a prior phase I study 11. It is likely that combination therapy will be needed for an effective treatment strategy of pediatric ASPS.
Cediranib administered continuously at the recommended solid tumor pediatric phase 2 dose of 12 mg/m2/dose once daily was adequately tolerated. However, all 4 patients who received prolonged administration of cediranib required at least one dose reduction, with one patient requiring two dose reductions (in cycles 1 and 38). The most frequent grade 2 or 3 AEs observed in the pediatric cohort were somewhat distinct from those observed in the adult cohort, with diarrhea, hypertension, and proteinuria as frequent toxicities across both pediatric and adult cohorts. Neutropenia and fatigue, frequently observed in the pediatric cohort, were not frequent AEs in the adult population, and the frequent AE of transaminitis, hypothyroidism, and tumor pain in adults were not frequently observed in the pediatric population.
With regard to monitoring for growth plate toxicity, while there is no normative data to compare with our findings, we can exclude major growth plate expansion. Height in all patients developed along expected growth curves suggesting that growth complications due to VEGF inhibitor therapy are unlikely with cediranib.
In summary, cediranib has an acceptable safety profile in the pediatric population, with no growth plate toxicity observed by volumetric MRI assessment. Single agent cediranib was determined inactive. The lack of objective responses in this study suggests that combination studies may be warranted. A recent retrospective review of targeted therapies in 69 children and young adults with ASPS concluded that cediranib, with its inhibition of all three VEGFRs, may be a reasonable first choice for patients with metastatic disease, given the promising adult experience with documented responses 17. It is apparent that further studies are needed to comprehensively evaluate the role of cediranib, along with other targeted agents, in the treatment of metastatic ASPS.
ACKNOWLEDGEMENTS
ClinicalTrials.gov Identifier:
Research reported in this publication was supported in part by the Center for Cancer Research, National Cancer Institute (NCI) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviation
- ASPS
Alveolar soft part sarcoma
- VEGF
Vascular endothelial growth factor
- RECIST
Response evaluation criteria in solid tumors
- CI
Confidence interval
- PR
Partial response
- SD
Stable disease
- ORR
Objective response rate
- MTD
Maximum tolerated dose
- BSA
Body surface area
- DMT
Dose modifying toxicity
- AE
Adverse event
- CBC
Complete blood count
- ALT
Alanine aminotransferase
- MRI
Magnetic resonance imaging
- Pts
Patients
- Yo
Years old
- Cy
Cycles
- CI
Confidence interval
Footnotes
Presented in part at the 2018 Annual Meeting of the American Society of Clinical Oncology (ASCO), June 1–5, 2018, Chicago, IL. “Cediranib Phase II Study in Children with Metastatic Alveolar Soft Part Sarcoma (ASPS).”
CONFLICT OF INTEREST STATEMENT
Nothing to declare
DATA AVAILABILITY STATEMENT
The data that support the findings of this study will be openly available at ClinicalTrials.gov, reference number .
REFERENCES
- 1.Zarrin-Khameh N, Kaye KS. Alveolar soft part sarcoma. Arch Pathol Lab Med 2007;131(3):488–491. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman PH, Brennan MF, Kimmel M, Erlandson RA, Garin-Chesa P, Flehinger BY. Alveolar soft-part sarcoma. A clinico-pathologic study of half a century. Cancer 1989;63(1):1–13. [DOI] [PubMed] [Google Scholar]
- 3.Portera CA Jr., Ho V, Patel SR, et al. Alveolar soft part sarcoma: clinical course and patterns of metastasis in 70 patients treated at a single institution. Cancer 2001;91(3):585–591. [DOI] [PubMed] [Google Scholar]
- 4.Reichardt P, Lindner T, Pink D, Thuss-Patience PC, Kretzschmar A, Dorken B. Chemotherapy in alveolar soft part sarcomas. What do we know? Eur J Cancer 2003;39(11):1511–1516. [DOI] [PubMed] [Google Scholar]
- 5.Argani P, Antonescu CR, Illei PB, et al. Primary renal neoplasms with the ASPL-TFE3 gene fusion of alveolar soft part sarcoma: a distinctive tumor entity previously included among renal cell carcinomas of children and adolescents. Am J Pathol 2001;159(1):179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ladanyi M, Lui MY, Antonescu CR, et al. The der(17)t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene 2001;20(1):48–57. [DOI] [PubMed] [Google Scholar]
- 7.Tsuda M, Davis IJ, Argani P, et al. TFE3 fusions activate MET signaling by transcriptional up-regulation, defining another class of tumors as candidates for therapeutic MET inhibition. Cancer Res 2007;67(3):919–929. [DOI] [PubMed] [Google Scholar]
- 8.Stockwin LH, Vistica DT, Kenney S, et al. Gene expression profiling of alveolar soft-part sarcoma (ASPS). BMC Cancer 2009;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith NR, James NH, Oakley I, et al. Acute pharmacodynamic and antivascular effects of the vascular endothelial growth factor signaling inhibitor AZD2171 in Calu-6 human lung tumor xenografts. Mol Cancer Ther 2007;6(8):2198–2208. [DOI] [PubMed] [Google Scholar]
- 10.Wedge SR, Kendrew J, Hennequin LF, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res 2005;65(10):4389–4400. [DOI] [PubMed] [Google Scholar]
- 11.Fox E, Aplenc R, Bagatell R, et al. A phase 1 trial and pharmacokinetic study of cediranib, an orally bioavailable pan-vascular endothelial growth factor receptor inhibitor, in children and adolescents with refractory solid tumors. J Clin Oncol 2010;28(35):5174–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardner K, Judson I, Leahy M, et al. Activity of cediranib, a highly potent and selective VEGF signaling inhibitor, in alveolar soft part sarcoma. Journal of Clinical Oncology 2009;27(15_suppl):10523–10523. [Google Scholar]
- 13.Kummar S, Allen D, Monks A, et al. Cediranib for metastatic alveolar soft part sarcoma. J Clin Oncol 2013;31(18):2296–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim A, Dombi E, Solomon J, Fox E, Balis FM, Widemann BC. Automated volumetric growth plate measurement using magnetic resonance imaging for monitoring skeletal toxicity in children treated on investigational drug trials. Clin Cancer Res 2011;17(18):5982–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National High Blood Pressure Education Program Working Group on High Blood Pressure in C, Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114(2 Suppl 4th Report):555–576. [PubMed] [Google Scholar]
- 16.Drevs J, Siegert P, Medinger M, et al. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol 2007;25(21):3045–3054. [DOI] [PubMed] [Google Scholar]
- 17.Flores RJ, Harrison DJ, Federman NC, et al. Alveolar soft part sarcoma in children and young adults: A report of 69 cases. Pediatr Blood Cancer 2018;65(5):e26953. [DOI] [PubMed] [Google Scholar]