Abstract
Secreted IgM (sIgM) is a multifunctional evolutionary conserved antibody that is critical for the maintenance of tissue homeostasis as well as the development of fully protective humoral responses to pathogens. Constitutive secretion of self- and poly-reactive natural IgM, produced mainly by B-1 cells, provides a circulating antibody that engages with autoantigens as well as invading pathogens, removing apoptotic and other cell debris and initiating strong immune responses. Pathogen-induced IgM production by B-1 and conventional B-2 cells strengthens this early, passive layer of IgM-mediated immune defense and regulates subsequent IgG production. The varied effects of secreted IgM on immune homeostasis and immune defense are facilitated through its binding to numerous different cell types via different receptors. Recent studies identified a novel function for pentameric IgM, namely as a transporter for the effector protein ″apoptosis-inhibitor of macrophages″ (AIM/CD5L). This review aims to provide a summary of the known functions and effects of sIgM on immune homeostasis and immune defense, and its interaction with its various receptors, and to highlight the many critical immune regulatory functions of this ancient and fascinating immunoglobulin.
Graphical Abstract
Introduction:
The humoral immune system provides an intricate and complex response to protect the host from infections and pathological alterations of cells and/or tissues. B cells, activated in response to such alterations, begin to secrete immunoglobulins that can then bind to and inactivate or eliminate the threat. The evolutionary highly conserved immunoglobulin isotype M (IgM), forms the cell surface antigen receptor (BCR) of all developing B cells of jawed vertebrates, is the first antibody to be generated in ontogeny, and the first to be secreted during an immune response. Most secreted (s)IgM is generated as a pentameric Ig molecule, displaying ten antigen-binding sites, which greatly enhances the avidity with which it binds cognate antigen (Schroeder & Cavacini, 2010).
As recently shown, IgM is an asymmetric pentamer that also forms a pocket for an effector protein, termed “apoptosis-inhibitor of macrophages” (AIM/CD5L), whose serum half-life is greatly enhanced through binding to IgM (Hiramoto et al., 2018). In addition to IgM’s direct effector functions and its AIM transport function, studies conducted for over 30 years have shown direct immunoregulatory functions for sIgM. Particularly intriguing are the immune-enhancing effects of sIgM for inducing maximal IgG responses and the immune-protective effects against the development of antibody-mediated autoimmune disease (N. Baumgarth et al., 2000; Boes et al., 1998; Heyman, Pilstrom, & Shulman, 1988) (T. T. T. Nguyen, Graf, Randall, & Baumgarth, 2017). The mechanisms by which sIgM regulates these processes remain poorly understood, but recent studies have implicated the newly discovered FcμR in facilitating direct interaction of sIgM with cells of the immune system, specifically B and T cells. Mice lacking the FcμR also show reduced IgG responses following immunization and infection and develop increased circulating autoantibody titers (S. C. Choi et al., 2013; T. T. Nguyen et al., 2017; T. T. T. Nguyen et al., 2017). In this article, we review the initial discovery of IgM, its structure and functions, and summarize the known interactions with various IgM-binding receptors, focusing in particular on the FcμR. Although IgM was identified >75 years ago, much remains to be learned about this evolutionarily highly conserved immunoglobulin, and the receptors that support its effector and regulatory functions.
The discovery of IgM:
The study of the humoral immune system was sparked In 1888 when the bacteriologist George Nuttal demonstrated antibacterial properties in the serum of animals inoculated previously with anthrax bacilli (Black, 1997; Crist & Tauber, 1997; Rowley & Wardlaw, 1958). In a landmark paper published in 1890, Emil von Behring with Shibasaburo Kitasato reported that blood taken from rabbits vaccinated with tetanus bacteria and then injected into mice, protected the mice against lethal doses of Clostridium tetani (Behring, 1890a). Shortly thereafter, Von Behring published similar results using Corynebacterium diphtheria infection of guinea pigs (Behring, 1890b; Hooper, 2015; Kantha, 1991). In 1894, Richard Pfeiffer performed in vitro experiments with Vibrio cholera showing that the plasma of previously immunized guinea pigs could lyse the bacteria, terming this the “Pfeiffer’s phenomenon” (Crist & Tauber, 1997), and thus providing a mechanism for the earlier discovered protective capacity of serum. In 1930, Tiselius developed a technique, later adopted by Landsteiner, to separate and visualize proteins by electrophoresis (Black, 1997). Using this technique, Tiselius and Kabat In 1939 identified a major band of serum proteins (γ-globulins), which we now understand to contain most immunoglobulins (Black, 1997), (Hooper, 2015) (Tiselius & Kabat, 1939).
The first inference of IgM was made in 1937 in horses immunized with pneumococcus polysaccharide. The vaccine-induced anticarbohydrate antibodies were shown to be 3–4 times larger than γ-globulins (Heidelberger & Pedersen, 1937). Immunoglobulin (M) (IgM) was first characterized in 1944 via immunoelectrophoresis and ultracentrifugation by Waldenström, Pederson, and Kunkel from patients with B cell lymphoma (WALDENSTRÖM, 1944) (Wallenius, Trautman, Kunkel, & Franklin, 1957). In these experiments, highly elevated concentrations of IgM were visualized. Originally named macroglobulin, it was not until the 1960’s when the nomenclature for Ig-isotypes was developed, that macroglobulin became the “M” in IgM (Black, 1997; Cohen, 1965; R Ceppellini, 1964).
Expression of IgM:
All jawed vertebrates express IgM, including amphibians, reptiles, shark, fish, and mammals (Wang, Coligan, & Morse, 2016) (Boyden, 1966), while jawless vertebrates, such as hagfish and lamprey, lack IgM (Flajnik, 2002). IgM production can be detected very early, in humans as early as week 20 of gestation (Furth, 1965). The fact that both antigen-free and germfree mice have serum IgM levels similar to that of SPF or conventional-housed mice (300–800 μg/ml (Wang et al., 2016), suggested that foreign antigen exposure does not control serum IgM concentrations (Ehrenstein & Notley, 2010), (Haury et al., 1997). However, whether it may affect the specificity of the circulating IgM has not been analyzed. The half-life of sIgM is very short, with reports ranging from 8h – 2 days (Hughey, Brewer, Colosia, Rosse, & Corley, 1998) in mice and 5–8 days in humans (Brekke & Sandlie, 2003). In contrast, the half-life of IgG is closer to 3 weeks. Obvious sexual dimorphism exists in the levels of circulating IgM, with female mice showing significantly higher serum IgM concentrations than male mice. Recent studies have linked this difference to the regulation of IgM-secreting cells by estrogen. With that, they also linked the presence of higher levels of oligosaccharide-specific serum IgM to a selective survival advantage of females compared to males following bacterial infection (Zeng et al., 2018).
Due to its large size, ~970 kDa, pentameric IgM does not easily extravasate into tissues (Casali, 1998; Plomp, Bondt, de Haan, Rombouts, & Wuhrer, 2016)). Similar to dimeric IgA, pentameric IgM can bind to the polymeric Ig receptor via its J (joining) chain, which facilitates the transport of IgM across epithelial layers into the lumen of mucosal tissues (see below; (Moh, Lin, Thaysen-Andersen, & Packer, 2016)). The lamina propria of the human but not the mouse gastrointestinal tract contains significant frequencies of IgM plasma cells. Secreted IgM, together with secretory IgA, seems to affect the gut microbial diversity by binding to commensals and anchoring them in the mucus layer (Magri et al., 2017). Further work is required to reveal the distinct functions of IgM and IgA binding to the microbiota and to understand the apparent species-specific differences of these processes. IgA deficiency in both humans and mice has surprisingly subtle effects on their health status, given the large daily production of IgA and its presence on mucosal surfaces. The observed compensatory increases of sIgM in IgA-deficiency may explain, at least in part, these findings (Yel, 2010).
A newly discovered structure for a very old molecule
IgM is structurally distinct from the other immunoglobulin subtypes. This is not only because of its usually pentameric form (Schroeder & Cavacini, 2010),(Hiramoto et al., 2018) but also because IgM lacks a flexible hinge region (Casali, 1998). Secreted and membrane forms of IgM are generated by alternative splicing (Sitia et al., 1990), with membrane-bound IgM expressing a hydrophobic transmembrane domain of ~25 amino acids at the hydroxy terminus of the Ig-heavy chain and secreted IgM carrying a hydrophilic secretory tail at its carboxy terminus. Cysteine residues at that tail prevent premature secretion and support the assembly of the mature secreted form in the ER (Anelli & van Anken, 2013). In the absence of the joining chain (J-chain), IgM usually aggregates into hexamers, which seems to bind complement more efficiently (Petrusic et al., 2011), (Hughey et al., 1998). Increased hexameric IgM in humans is present in disorders such as macroglobulinemia, while for example, in Xenopus hexameric IgM is the predominant form of IgM (Parkhouse, Askonas, & Dourmashkin, 1970). Very little information exists about the hexameric form and its potential physiologic functions.
Pentameric and monomeric IgM has been notoriously difficult to crystallize. The first inference of the structure of IgM stems from negative stain electron microscopy (Czajkowsky & Shao, 2009) (Hiramoto et al., 2018) (Feinstein & Munn, 1969). Without antigen, IgM exhibited a planar, star-shaped complex. However, when bound to its cognate antigen (flagella from Salmonella sp.), a conformational change was visualized, with IgM adopting a table-like conformation (Czajkowsky & Shao, 2009). More recently, through cryo-atomic force microscopy, modeling of the antigen-free IgM pentamer was revised showing that IgM adopts a non-planar, mushroom-shaped structure. Furthemore, the table-like conformation following antigen-binding was shown to expose the C1q binding site on the heavy chain constant region (Czajkowsky & Shao, 2009). Most recently, using single-particle electron microscopy it was revealed that the IgM pentamer does not form a symmetric pentameric star, as always assumed, but rather takes the form of an asymmetric pentagon with one large gap at a 50° angle between two of the five IgM monomers (Hiramoto et al., 2018). Surprisingly, this gap contained a serum protein, the “apoptosis inhibitor expressed by macrophages” (AIM/CD5L). Thus, the data identified secreted IgM not only as an effector protein but also as a transporter for another effector protein (Figure 1).
Figure 1: A new structure for sIgM.
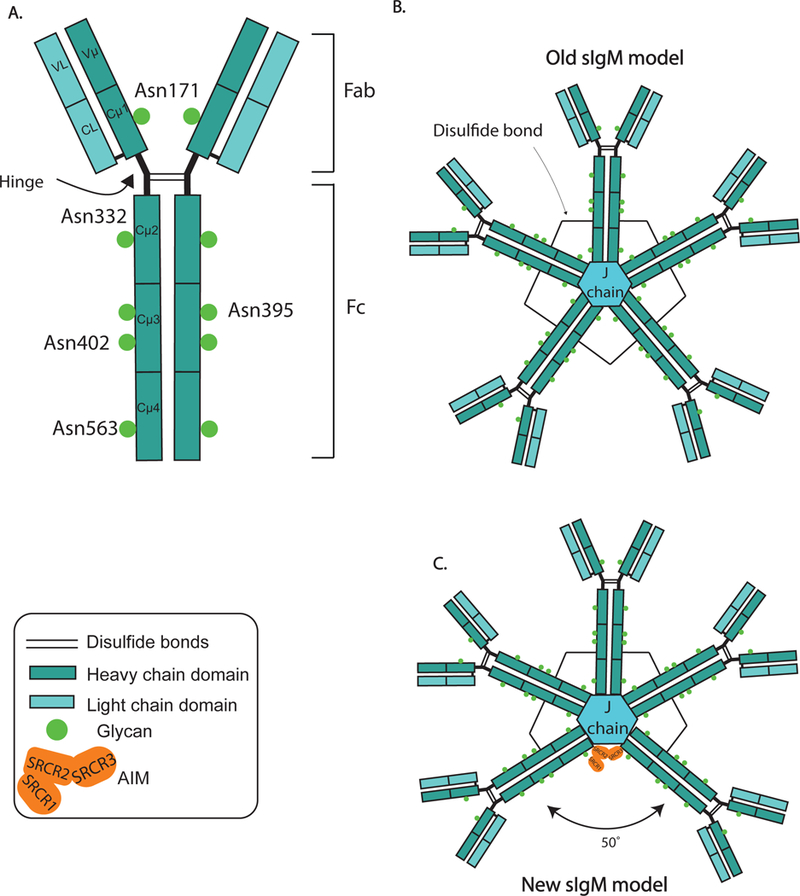
(A) Schematic of an IgM monomer possessing two immunoglobulin heavy and light chains each. The Fab region (fragment antibody binding) encodes the antigen binding sites, and the Fc region (fragment constant) regulates its function. The heavy chain contains five glycosylation sites, while there are no glycosylation sites encoded on the light chains. (B) Secreted IgM was thought to form a symmetric pentamer in which five monomers are joined together by a J chain and disulfide bonds. (C) New data now show that pentameric IgM is asymmetrical with a 50-degree groove that allows for one AIM (apoptosis inhibitor of macrophage/CD5L) molecules to bind, stabilizing their serum half-life.
AIM/CD5L/SP-α is a member of the scavenger-receptor cysteine-like domain superfamily and is produced by macrophages (T. Miyazaki, Yamazaki, Sugisawa, Gershwin, & Arai, 2018). AIM is present in the serum, where its half-life is greatly enhanced by binding to IgM (T. Miyazaki et al., 2018)((Arai et al., 2005; Koyama et al., 2018; Tissot et al., 2002). In vitro and in vivo studies with gene-targeted mice suggested that AIM is involved in the regulation of apoptosis of developing CD4/CD8 double-positive thymocytes. However, its high expression by tissue macrophages, including macrophages in the liver, peritoneal cavity, and splenic red pulp, and its binding to low-density lipoproteins, among other cholesterol-containing antigens, suggests that this molecule has additional functions that require further study (Toru Miyazaki, Hirokami, Matsuhashi, Takatsuka, & Naito, 1999). Specifically, it will be important to reexamine whether any of the many defects observed in mice lacking sIgM may be explained in fact by the lack of-or an altered availability of AIM.
Each human IgM heavy chain carries five N-linked glycosylation sites and an additional site on the J-chain (Colucci et al., 2015) (Figure 1), accounting for 12–14% of its molecular weight and making it the most heavily N-glycosylated antibody of humans (Arnold, Wormald, Sim, Rudd, & Dwek, 2007). IgM’s function is significantly affected by glycosylation. For example, sialylation was reported to support the internalization of IgM by T cells, leading to inhibition of T cell responses, an effect diminished by the absence of sialic acid (Colucci et al., 2015). Glycosylation of IgM at position 46 (N46) was shown to be required for pre-BCR function (Ubelhart et al., 2010), and mutation of Asn-402 disables complement protein C1-binding (Wright, Shulman, Isenman, & Painter, 1990). In contrast to IgG, whose interactions with the various FcμR were shown to be critically affected by its glycosylation (Arnold et al., 2007; Lloyd, Wang, Urban, Czajkowsky, & Pleass, 2017), interaction of IgM with the hFCMR appears to be glycan independent (Lloyd et al., 2017).
Natural IgM production: Two sites to a coin
Principally two types of secreted IgM have been distinguished in the literature based on their cellular origins, regulation of their production, and overall function: natural IgM and immune/induced IgM. Whereas the former is thought to be produced constitutively without stimulation by a foreign antigen, induced IgM production is the direct outcome of a tissue insult and/or infection. The two types of IgM have distinct modes of induction and distinct repertoires, fulfilling a myriad of effector functions that we will outline below (Figure 2).
Figure 2: Functions of secreted IgM.
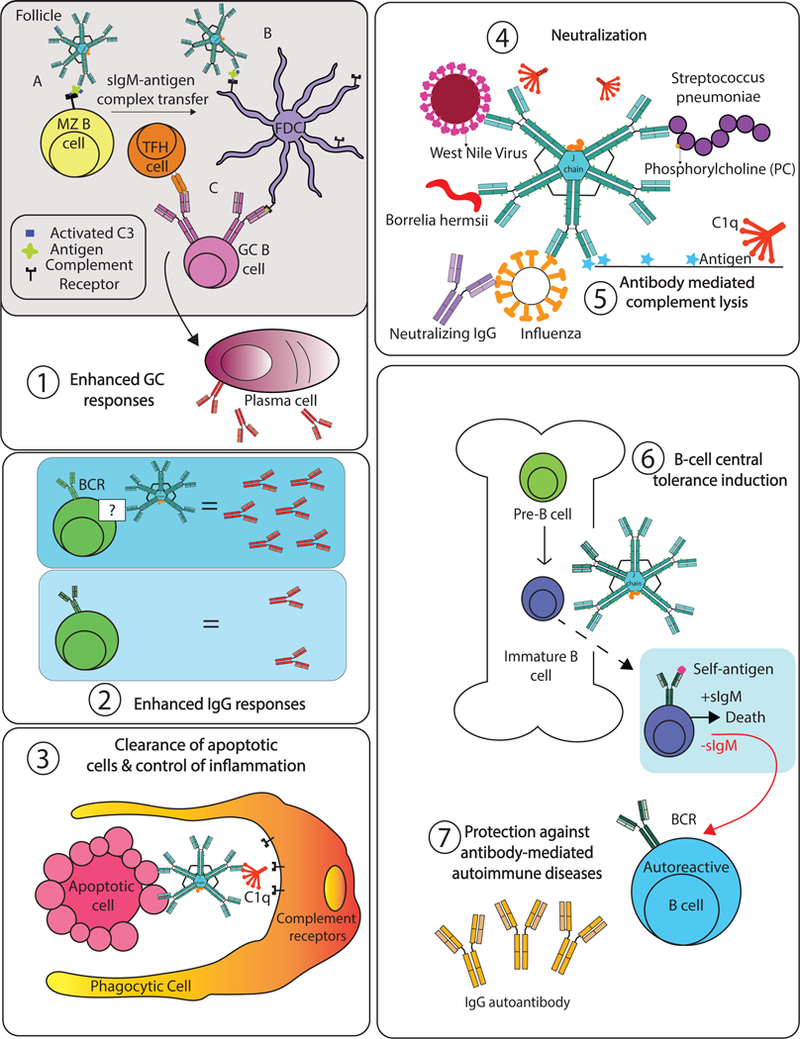
(1A). Marginal zone splenic B cells capture sIgM-antigen/complement C3 complexes and (B) migrates into the B cell follicle where it transfers the complexes onto CR1/2 expressed by follicular dendritic cells (FDCs), where (C) the FDC presents antigen to germinal cell B cells in support of T-dependent antibody production. (2) sIgM strongly supports IgG response development by additional but poorly understood processes. (3) Antigen opsonization by sIgM via complement C1q-mediated uptake by macrophages. This process can also be mediated by mannose-binding lectin (not shown). (4) Pathogen neutralization to blocking pathogen entry or inducing pathogen aggregations. Natural IgM is polyreactive and recognizes conserved antigens, such as phosphorylcholine (PC) present in the cell walls of Streptococcus pneumoniae as well as on dead or dying mammalian host cells. (5) sIgM can recruit complement components to initiate the classical pathway of complement activation, eventually leading to the formation of the Membrane Attack Complex (MAC) that can result in lysis of the pathogen. (6) Although the mechanisms are incompletely resolved, sIgM prevents autoimmune antibody formation through multiple mechanisms, including effecting central tolerance induction in the bone marrow and through the removal of DAMPS, such as dead and dying cell debris. (7) The absence of sIgM causes changes in V-gene usage encoding increased self-reactivity.
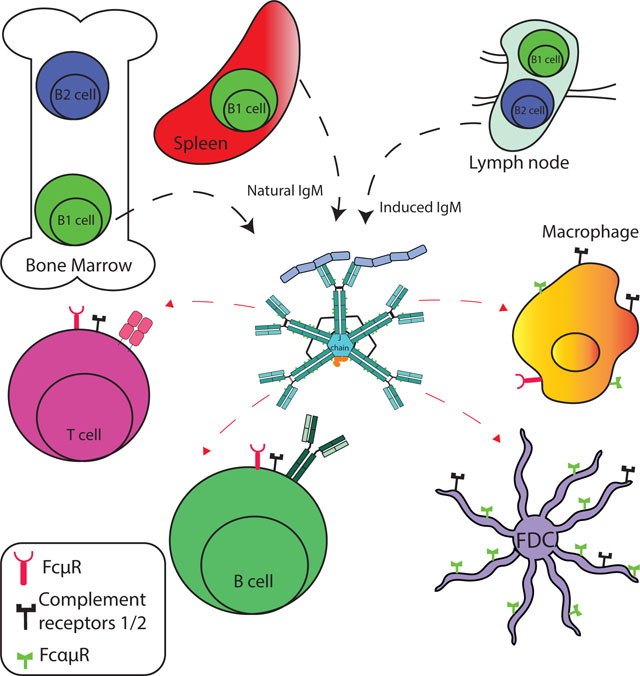
In mice, natural IgM production has been linked to a small subset of B cells, termed B-1 cells. These cells are of fetal/neonatal origin and are the initial source of IgM after birth. Early adoptive transfer studies showed that much of the circulating IgM was produced by these cells (Forster & Rajewsky, 1987) and subsequent studies using transfer of B-1 cells into neonatal B cell-depleted mice showed that amount to be > 90% (Nicole Baumgarth, Jager, Herman, Herzenberg, & Herzenberg, 2000; Lalor, Herzenberg, Adams, & Stall, 1989). Others reported that marginal zone (MZ) B-cells also contribute to natural antibody production in both humans and mice (Appelgren, Eriksson, Ernerudh, & Segelmark, 2018; Ichikawa et al., 2015).
Serum concentrations of natural IgM are similar between mice held under standard housing conditions, and those kept free of microbiota or even solid food antigens (Hooijkaas, Benner, Pleasants, & Wostmann, 1984). The CD5+ B-1 cells in spleen and body cavities of germfree, as well as SPF-housed, mice also showed very similar Ig-VH gene repertoires, supporting the long-held concept of “natural” IgM production, i.e., production of IgM that is independent of foreign antigen (Y. Yang et al., 2015). This was further supported by findings that depletion or genetic ablation of T cells had little effect on serum IgM levels, while most IgG subtypes were strongly reduced, suggesting that B-1 cells do not require T cell-mediated activation in order to secrete IgM (Fehr et al., 1998; Kushnir et al., 2001).
Natural or “spontaneous” IgM production occurs mainly in the spleen and the bone marrow (Y. S. Choi, Dieter, Rothaeusler, Luo, & Baumgarth, 2012; Haaijman, Slingerland-Teunissen, Benner, & Van Oudenaren, 1979; Hooijkaas et al., 1984; Savage et al., 2017). Two cell populations producing these antibodies have recently been identified: “classical” non-terminally differentiated CD19+ Blimp-1neg CD43+ B-1 cells and B-1-derived CD19lo/- CD43+ Blimp-1pos plasma cells (Y. S. Choi et al., 2012; Reynolds, Kuraoka, & Kelsoe, 2015; Savage et al., 2017). Our studies showed that the Blimp-1neg IgM-secreting cells did not upregulate Blimp-1 expression and continued to secrete IgM in the absence of Blimp-1 in conditional, B cell-specific Blimp-1 deficient mice (Savage et al., 2017). This is significant, as it suggests that only some B-1 cells are activated to terminally differentiate to plasma cells to secrete IgM. It raises the question of what are the stimuli inducing the differentiation of B-1 cells forming B-1PC versus those inducing IgM secretion without terminal differentiation. It may also indicate that the antigen-specificity of the IgM secreted by those distinct activation events differ. There have been no reports of B-1 cells participating in germinal center responses, thus it is unlikely that the plasma cells have emerged from such response. Their recent extensive RNA sequencing experiments also provided no evidence that B-1 cells undergo extensive somatic hypermutation, with significant components being germline encoded. Although an analysis of only IgM-secreting B-1 cells was not conducted (Prohaska et al., 2018; Y. Yang et al., 2015)).
With regards to the specificity of IgM, current evidence suggests that the pool of natural IgM-secreting B-1 cells is positively selected upon the recognition of self-antigens during development (Hayakawa et al., 1999). This explains the highly skewed, self-reactive repertoire of natural IgM and the fact that the antigen-specificity of B-1 cells is tightly linked to their unique functions (Graf et al., 2019). Given the similarity in the B-1a cell repertoire of gnotobiotic and conventional-housed mice, it then appears that self-antigen recognition shapes the B-1 cell repertoire through activation and clonal expansion, which explains the strong changes in VH-usage over the first few months of life (Prohaska et al., 2018; Y. Yang et al., 2015). How B-1 cells can be selected and then differentially activated by self-antigens to secrete self-reactive IgM, without this process resulting in autoimmune disease development is a further important but unresolved question.
One possible explanation might be the unique structure and the size of natural IgM, which ensures that IgM remains circulating in the blood without extravasation unless tissue destruction or extensive inflammation leads to endothelial cell damage/leakage. In the circulation, IgM has been associated with protection from systemic infections, i.e., bacteremia and viremia, such as after infection with Borrelia hermsii (Alugupalli et al., 2004) enteropathogenic Escherichia coli (Zeng et al., 2018) or during more general sepsis (Boes et al., 1998). It has also been shown to be protective in atherosclerosis, plaque formation in the blood vessel wall, where natural IgM is believed to help remove modified LDL and apoptotic and necrotic cell debris, thereby reducing triggers of inflammation (Christoph J. Binder & Silverman, 2005; Kyaw, Tipping, Bobik, & Toh, 2012). A potential clinical application for natural IgM was indicated by recent reports that enhancing the number of IgM-secreting B-1 cells via treatment of mice with anti-TIM-1 attenuated atherosclerosis development, providing a promising potential therapeutic approach that requires exploration in humans (Hamid Hosseini et al., 2015; H. Hosseini et al., 2018). In contrast, when IgM extravasates into tissues, such as during endothelial damage and leakage created by a lack of blood perfusion and subsequent reperfusion of an organ, as occurs for example following surgery, the presence of IgM in these tissues causes excessive activation of complement and resultant tissue and organ damage (Zhang et al., 2004) (Zhang et al., 2006).
The ability of IgM to contribute to immune homeostasis by binding to and helping to remove a large number of “altered” self-antigens, coupled with the ability to also bind to shared molecules on pathogens is a hallmark of natural IgM. The classic example is the simultaneous recognition of both Streptococcus pneumoniae and oxidized LDLs by a particular IgM natural antibody (C. J. Binder et al., 2003). These unique polyreactive properties of IgM may provide a powerful evolutionary advantage, explaining why IgM, but not other immunoglobulins are shared among all jawed vertebrates (N. Baumgarth, Tung, & Herzenberg, 2005; Lobo, 2016; Zhou, Tzioufas, & Notkins, 2007). The polyreactive nature of natural IgM has long been recognized {reviewed in (Gunti & Notkins, 2015). A possible mechanistic explanation for its polyreactivity is that natural IgM is encoded by V regions containing higher frequencies of tyrosine and serine residues, which contain hydroxylated side chains that enable IgM greater binding flexibility ((reviewed in (Wang et al., 2016)). With regards to the specificity of IgM, in addition to the antigens mentioned above, natural IgM also binds to various epitopes on phosphorylcholine and other phospholipids, carbohydrates such as phosphatidylcholine, ssDNA and dsDNA, oxidized LDL, and certain PAMPS (Shaw et al., 2000),(Gronwall & Silverman, 2014),(N. Baumgarth, 2011),(Gronwall, Vas, & Silverman, 2012). Although their polyreactivity is overall characterized by low-affinity antigen interactions, the ten binding sites of IgM still enable overall higher avidity binding (D. D. Jones, DeIulio, & Winslow, 2012).
Taken together, natural IgM is a self-reactive, serum protein with unique structural characteristics that enable its effectiveness in binding to self-antigens and thereby contributing to immune homeostasis by reducing the threat of inflammation and tissue damage (Figure 2). In situations of chronic or extreme inflammation, however, this otherwise highly protective immune effector molecule can gain access to tissues otherwise inaccessible, where it can promote inflammation and therefore often also tissue damage and disease.
Immune/Induced IgM:
Remarkably little attention has been paid to the initial and transient induction of IgM secretion, which is one of the first contributions of the adaptive immune system to immune defense. Induced IgM is generated by either the activation of B-1 cells and/or by activation of conventional B (B-2) cells. It is generally assumed that early IgM production occurs because all B cells first express IgM and that the generation of other Ig-isotypes requires time for class-switch recombination to occur, while IgM secretion occurs rapidly through alternative RNA splicing, excluding the membrane-domain usually tethering the IgM (B cell receptor) onto the surface of developing B cells. However, we now understand that a sizable population of memory B cells remains non-class switched, while having undergone activation events that drove them towards memory development, even hyper-affinity maturation in germinal centers (Pape et al., 2018; Pape, Taylor, Maul, Gearhart, & Jenkins, 2011), suggesting that the production of IgM is about more than simply “being there early”. Effector functions of IgM, such as effective activation of complement might be critical for early immune defense (Jayasekera, Moseman, & Carroll, 2007), or the ability to bind to various IgM-binding receptors (see below). The presence of early IgM was also shown to be important for maximal IgG response induction (Table 1). Finally, the lack of easy diffusion may ensure that locally-produced IgM remains mainly at the site of production, i.e., the secondary lymphoid tissues, where it could support the filter function of lymph nodes. In support, we showed previously that B-1 cell-derived IgM production in the respiratory tract regional lymph nodes following influenza infection, measured by ELISPOT, was not reflected in the serum, where B-1 derived IgM titers were unchanged, while conventional B cell-derived serum IgM did increase transiently in response to the infection. The tissue location of the IgM-secreting conventional B cells giving rise to this systemically-produced IgM was more difficult to discern, however, as conventional, but not B-1 cell-derived increased IgM production also occurs in the spleen after influenza infection and is not restricted to the draining lymph nodes of infected mice (Nicole Baumgarth et al., 2000; J. G. Choi et al., 2008) (P. D. Jones & Ada, 1986). Splenic IgM-producing cells have been found mainly in the marginal zone and the red pulp, both areas with immediate access to the circulating blood.
Table 1:
Effects of IgM-deficiency on humoral immunity in mice
| Biological Process | Effect of IgM deficiency | Reference |
|---|---|---|
| B cell subset development | Increased numbers CD5+ B-1 cells in PerC and spleen | Boes et al. 1998 |
| Increased numbers CD5+ CD21lo CD23− CD43- anergic B cells in PerC and spleen with enhanced turnover | Nguyen et al. 2015 | |
| Reduced pre-B cell development and bone marrow B cell output | Nguyen et al. 2015 | |
| Abnormal spleen development with increased marginal zone B cells Increased BCR signaling |
Boes et al. 1998 Nguyen et al. 2015 Tsiantoulas D. et al. 2017 |
|
| BCR-repertoire | Altered VH usage in peripheral B cells | Nguyen et al. 2015 |
| Increased auto-antibody production: anti-dsDNA; anti-histone; ANA. | Boes et al. 2000; Ehrenstein et al. 2000 Nguyen et al. 2015 |
|
| Enhanced development of glomerulonephritis and other signs of antibody-mediated autoimmune disease | Boes et al. 2000 Ehrenstein et al. 2000 |
|
| T-independent humoral immunity | No effect on total IgG, but enhanced IgG2a and reduced IgG2b responses to NP-Ficoll (1, 10 and 100 μg) immunization. Enhanced IgG 1, 2a, 2b and 3 responses to NP-Ficoll (5μg) to all IgG subclasses |
Boes et al. 1998 Ehrenstein et al. 2000 |
| T-dependent humoral immunity | Reduced IgG responses to low (1μg) but not higher (10 and 100 μg) NP-KLH immunization. Reduced IgG1 primary and secondary responses to NP-KLH (50μg). Delayed responses to NP-CG and phOx-CG immunization. Reduced affinity maturation. Normal secondary responses |
Boes et al. 1998 Ehrenstein et al. 2000 |
| Reduced IgG1 and IgG2a responses to influenza infection Reduced IgG2a and IgG2b responses to influenza infection |
Baumgarth et al. 2000; Nguyen et al. 2017 Kopf M et al. 2002 |
Both, B-1 and conventional B-2 cells generate secreted IgM in the draining lymph nodes early after influenza infection, and both contributed to immune defense (J. G. Choi et al., 2008) (Waffarn et al., 2015). Whether B-1 cells are activated via antigen-specific or innate signals remains an open question. They are exquisitely responsive to TLR-mediated stimulation but unable to respond to BCR-mediated signaling with clonal expansion. Yet, observations of antigen-specific B-1 cells responses have been made in response to numerous pathogens, including B. hermsii, Francisella tularensis, Salmonella typhimurium and S. pneumoniae, (Alugupalli et al., 2004; Gil-Cruz et al., 2009; Haas, Poe, Steeber, & Tedder, 2005; D. D. Jones et al., 2012; Yang Yang et al., 2012), suggesting that B-1 cells in vivo can respond to BCR-mediated signals and thus act as part of the adaptive rather than the innate response. We refer to our recent in-depth discussion on this topic elsewhere (N. Baumgarth, 2016).
The antigen-specific, T-dependent and T-independent activation of conventional B cells results in a transient induction of sIgM that lasts for 1–2 weeks after the insult. This temporary induction, measurable also in the blood, has been exploited clinically for distinguishing early from longer-term infections by determining the ratio of antigen-specific IgM and IgG levels in repeated blood draws. The transient production of IgM coupled with its very short half-life explains its only brief presence in most acute infections. Given the early effectiveness of IgM in suppressing infections (N. Baumgarth et al., 2000; Boes et al., 1998; Ochsenbein et al., 1999), it is unclear what benefit the rapid curtailment of IgM production may have; an intriguing yet unexplored question. However, some chronic infections do induce continued IgM production, for example following infection with Ehrlichia muris, where a protective, T-independent and BAFF-dependent IgM response was shown to be induced long-term by plasmablasts in spleen and bone marrow (D. D. Jones et al., 2013; Racine, Chatterjee, & Winslow, 2008; Racine et al., 2011). We have made similar observations during chronic infection of mice with Borrelia burgdorferi (Hastey CJ, Elsner, RA, Olsen, K., and Baumgarth, N. in preparation). Interestingly, in humans infected with Borrelia burgdorferi, patients may remain seropositive for IgM or IgG for up to 20 years (Halperin, Baker, & Wormser, 2013; Hammers-Berggren, Hansen, Lebech, & Karlsson, 1993; Kalish et al., 2001). In addition, humans infected with West Nile virus have shown persistent IgM levels from one up to 8 years post-infection (Murray, Garcia, Yan, & Gorchakov, 2013). Continued and in fact enhanced IgM production was also reported after recovery from Plasmodium vivax infection. However, the time point of analysis was only 30 days after completion of treatment (Patgaonkar et al., 2018).
Effects of IgM-deficiency:
Mice that lack secreted but not membrane-bound IgM developed antibody-mediated autoimmune disease, suggesting that sIgM plays a regulatory role in the development of self- and foreign-antigen induced humoral immunity (Table 1). Similar findings have been made in humans with a primary IgM-deficiency (Gupta & Gupta, 2017). At least three distinct, non-mutually exclusive mechanisms for autoimmune antibody-development have been suggested: First, natural IgM usually rapidly removes self-antigens, such as cellular debris, from the body (Ogden, Kowalewski, Peng, Montenegro, & Elkon, 2005). In IgM-deficiency such cellular content could act as damage-associated molecular patterns (DAMPS) and stimulate inflammatory responses, enhancing the risk of autoimmune-disease development (Kawano & Nagata, 2018; Lleo, Selmi, Invernizzi, Podda, & Gershwin, 2008). Furthermore, natural IgM recognition of such antigens also appeared to drive IL-10 production by B and T cells, further reducing the risk of inflammation-induced tissue damage (Notley, Brown, Wright, & Ehrenstein, 2011) (Boes et al. 1998; Ehrenstein 2000). Second, Tsiantoulas et al. reported that sIgM−/− mice showed increased BCR-signaling, a process that was reversed with treatment with a Btk-inhibitor at low doses. The authors suggested that sIgM may act as a “decoy receptor” competing for binding between sIgM and membrane-IgM, reducing the likelihood by which B cells respond to self-antigens (Tsiantoulas et al., 2017). Third, we observed that bone marrow B cell development and peripheral B cell repertoires were significantly altered in sIgM−/− mice, concomitant with the appearance of anergic CD5+ B cells in the periphery. The data suggested that the lack of central tolerance induction during B cell development in the absence of sIgM may explain the increased production of autoreactive IgG (Nguyen et al. 2015).
Deletion of sIgM also significantly reduced immune protection against numerous infectious agents, as outlined above. This strong effect of sIgM on protection might be due, at least in part, to the enhancing effects of sIgM in the development of maximal IgG responses (Table 1). Although the phenomenon of IgM-enhanced IgG production has been reported for many years and appears to be strongest during immune induction to limiting amounts of antigen and following infections (N. Baumgarth et al., 2000; Boes et al., 1998; Henry & Jerne, 1968; Heyman et al., 1988), we still do not fully understand the mechanism underlying these effects. We will return to this topic below in the context of sIgM interaction with its receptors.
In summary, sIgM has many functions both in maintaining homeostasis and system health, as well as in the immediate protection from infectious and other noxious insults. Its production is tightly controlled in time, and its functions are closely associated with its unique structure. The apparent immune regulatory functions of sIgM require further investigation, to obtain a better molecular understanding of how sIgM might be exploited therapeutically for enhancing immunity and reducing metabolic and autoimmune diseases.
Receptors that bind IgM:
Multiple receptors expressed by a variety of cell types can bind IgM. These include the complement receptors (CR1/CR2), FcR, polymeric Ig receptor, and the FcR (Figure 3). The latter is the only bona fide Fc-receptor for IgM and the most recently identified receptor (Kubagawa et al., 2009). Below is a brief review of these receptors and how they may support the various functions of sIgM.
Figure 3: Receptors that bind sIgM.
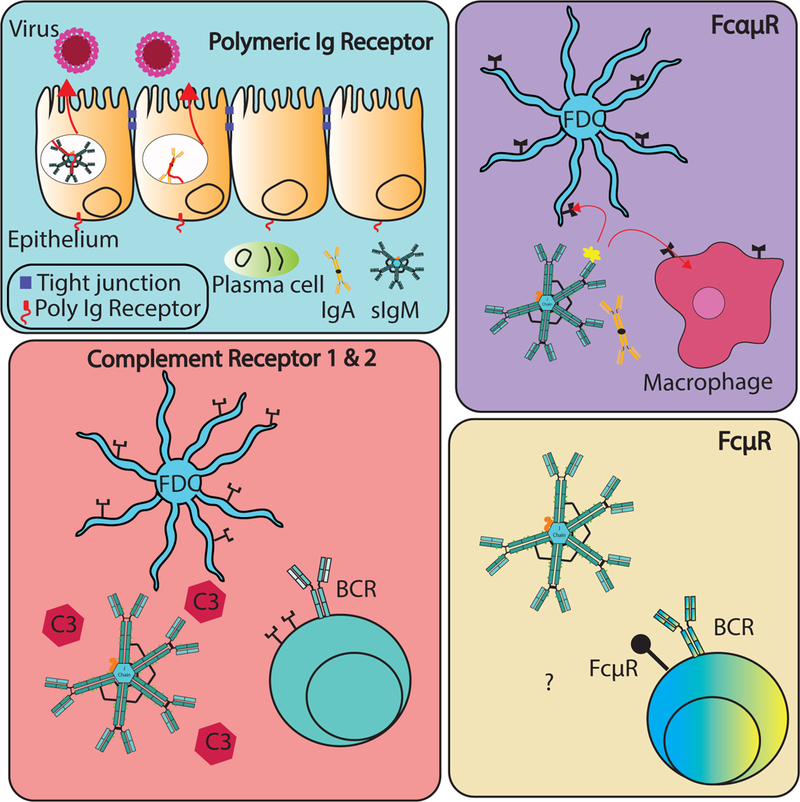
The polymeric Ig receptor can bind pentameric IgM as well as dimeric IgA via binding to the J-chain, resulting in transcytosis through epithelial cells located on mucosal surfaces and luminal excretion. FcR: This receptor is expressed predominantly by macrophages and follicular dendritic cells, where it binds to IgA and IgM with moderate and high affinity, respectively, to mediate endocytosis of IgA/IgM-antigen complexes. Complement Receptors: Complement receptor 1 is the C3b/C4b receptor is expressed on a variety of immune cells, including B cells and follicular dendritic cells (FDC), where it anchors sIgM-antigen-C3 complexes onto FDCs. Complement receptor 2 binds to iC3b (inactive derivative of C3b), C3dg and/or C3b. This receptor is expressed on B cells as well as FDCs. FcR: The FcR is highly expressed on B cells and binds sIgM selectively, which is rapidly internalized. The functional consequences of FcμR/sIgM interaction remain to be fully resolved.
Complement Receptors Complement receptors 1 and 2 (CD35/CD21) are primarily expressed on B cells and on follicular dendritic cells (FDCs) (C. Rutemark et al., 2012) (T. T. Nguyen & Baumgarth, 2016). Complement receptor engagement has been shown to be critically involved in the induction of maximal antibody responses following immunization (reviewed in (Sorman, Zhang, Ding, & Heyman, 2014)). Secreted IgM is an effective activator of complement, and complement itself was shown to support maximal IgG response induction. However, a mouse in which the complement binding site on the CH3 domain of the IgM heavy chain was inactivated, showed no effect on IgG responses during primary or secondary responses following immunization with sheep red blood cells, KLH, and NP-KLH (Christian Rutemark et al., 2011). The conclusion that IgM-mediated enhancement of IgG responses can occur without complement activation is supported by studies on influenza-infection in mice, where removal of complement via injection of cobra venom factor did not significantly affect the overall IgG response, while the lack of sIgM and the lack of FcμR expression on B cells showed significant and continuing IgG reductions (T. T. T. Nguyen et al., 2017). In apparent contrast to those studies, when antigen-specific IgM was co-administered with antigen, the ensuing IgG response was greatly enhanced, while the same experiment conducted with sIgM unable to bind C1q did not provide such enhancement. The complement-binding dependent IgM-enhancing effects on IgG responses required the presence of CR1/2 on both B cells and FDC (Donius, Handy, Weis, & Weis, 2013; Kranich & Krautler, 2016).
The data suggest a fundamental difference in the kinetics and functions of IgM and complement under those two very distinct situations. In one case, sIgM was co-administered and thus already bound to antigen, thus forming a large antigen-antibody complex, while in the other case, sIgM must have first been produced and then engage with antigen. It has been concluded from those data that pre-existing natural IgM may not act via complement-mediated immune response enhancement, or that natural sIgM may not have been present at the site of antigen-deposition, as immediate IgG enhancement was not seen when antigen was injected without first being mixed with sIgM (Sorman et al., 2014). This hypothesis appears less likely to us, however, as at least during an infection both, pre-existing natural and antigen-induced IgM, were shown to be necessary for maximal IgG responses (N. Baumgarth et al., 2000) and both types of IgM were present in the regional lymph nodes of influenza-infected mice, the main tissue source of anti-influenza IgG (J. G. Choi et al., 2008; Waffarn et al., 2015). An intriguing alternative possibility is that the observed differences in the effects of sIgM on the IgG response, and the role of the complement receptors, are due to the distinct pathways soluble and sIgM-complexed antigen would take traveling to and through the secondary lymphoid tissues, due to their large differences in size.
At the molecular level, IgM-CR1/2 interactions may enhance IgG responses via enhanced BCR-signaling through co-ligation of antigen with CR1/2 and the BCR. It has also been shown that sIgM-CR1/2-mediated binding to splenic marginal zone (MZ) B cells supported the shuffling of antigen from the splenic marginal zone to the follicle via migration of the MZ B cells in and out of the follicle (Cinamon, Zachariah, Lam, Foss, & Cyster, 2008). IgM-complement receptor-mediated enhanced presentation of antigen to B cells can also occur via tethering of sIgM-antigen complexes onto the FDC in germinal centers. Indeed, expression of CR1/2 is particularly high on those cells. A recent review article by Heyman and colleagues provides a more in-depth discussion on that topic (Sorman et al., 2014).
Fcα/μR (CD351):
The Fcα/μR is a transmembrane protein that binds both IgA and IgM. Interestingly, binding of sIgM to the Fcα/μR was greatly reduced when pentameric sIgM was complexed with AIM (Arai et al., 2013). The Fcα/μR appears to be expressed predominantly on antigen-presenting cells, including macrophages, and to be particularly abundant on FDC, while it is not expressed on granulocytes, T cells or NK cells (Honda et al., 2009) (Sakamoto et al., 2001; Shibuya et al., 2000) (Kubagawa, Oka, et al., 2014).
The presence of the Fcα/μR appeared to be largely dispensable for mediating the immune protective role of sIgM following B. hermsii infection, and therefore opsonization of antigen for macrophage uptake (Colombo, Abraham, Shibuya, & Alugupalli, 2011). Instead, the receptor has been shown to mediate the endocytosis of IgM-coated bacteria by B cells (Shibuya et al., 2000) (Sakamoto et al., 2001) and the binding and internalization of sIgM-antigen complexes by FDC. Thus, the receptor can promote antigen processing and presentation to CD4 helper T cells via binding to sIgM and IgA-antigen complexes (Shibuya et al., 2000), while reducing the availability of antigen-tethering on the surface of FDC, and thus antigen-presentation to B cells (Arai et al., 2013). The data suggest that sIgM binds to at least two distinct types of receptors on the FDC: the complement receptors and the Fcα/μR. Given that AIM complexing affected binding of sIgM to the Fcα/μR but not to complement receptors, the antigen-presenting function of FDC is greatly affected by the nature of the sIgM-antigen complexes. Secreted IgM-antigen complexes containing AIM mainly were tethered onto the FDC surface via binding to the complement receptors, while those lacking AIM bound to the Fcα/μR and were internalized for processing and presentation on MHCII.
Despite these notable effects of the Fcα/μR, concentrations of virus-specific serum IgG in Fcamr−/− mice infected with influenza virus were similar to those of wild type mice, indicating that regulation B cell responses by sIgM were independent of the Fcα/μR (T. T. T. Nguyen et al., 2017). This finding was supported by studies in AIM−/− mice that showed no difference in germinal center formation following immunization. Instead, studies in these mice suggested that by removing natural IgM-autoantigen complexes, the Fcα/μR facilitates the previously-observed sIgM-mediated suppressive effects on IgG autoantibody generation and autoimmune disease, a process that is enhanced in the absence of AIM (Arai et al., 2013). Taken together, current evidence suggests an important role for sIgM-Fcα/μR interaction in the regulation of immune homeostasis by the FDC.
Polymeric immunoglobulin receptor (pIgR):
The pIgR is a highly conserved glycosylated receptor of about 81 kDa that is expressed principally by mucosal epithelial cells. The receptor binds both, dimeric IgA as well as pentameric sIgM via recognition of the J-chain (Asano & Komiyama, 2011) (Klimovich, 2011; T. T. Nguyen & Baumgarth, 2016; Shimada et al., 1999; Turula & Wobus, 2018). The main function of the pIgR appears to be linked to the transepithelial transport of dimeric IgA, generated by plasma cells in the mucosal lamina propria, across the epithelial cell layer. This is followed by exocytosis into the lumen of mucosal tissues following cleavage of the pIgR, causing the retention of a small piece of the pIgR as “secretory component” on the sIgA. Although sIgM can bind to and be transported by the pIgR, mucosal tissues of mice usually contain much higher frequencies of IgA- compared to IgM-secreting plasma cells and thus effects of pIgR deficiencies measured in mice are mostly on homeostasis of IgA rather than sIgM (Shimada et al., 1999) (Tjarnlund et al., 2006). However, as outlined above, sIgM-secreting plasma cells appear to be more numerous in humans compared to mice and thus it is possible that the pIgR plays a larger role in the transport of sIgM onto the mucosal surfaces than indicated by studies on mice.
FcμR (FAIM3/TOSO):
The FcμR, a 60 kDa transmembrane protein, was originally identified as “Fas apoptosis inhibitory molecule 3” (FAIM3 or TOSO), as its expression in Jurkat T cells prevented Fas (CD95)-mediated apoptosis induced by an anti-Fas antibody (Hitoshi et al., 1998; Song & Jacob, 2005). Later studies identified the same surface protein as a sIgM-binding receptor on the surface of human and mouse B cells. While the receptor’s ability to bind sIgM has been repeatedly demonstrated, the originally reported anti-apoptotic function of this molecule appeared to have been due to the use of an anti-Fas antibody of the IgM isotype, as the anti-apoptotic activity was not seen with an IgG anti-Fas antibody (Honjo et al. 2012; Kubagawa 2009). Although further studies are required to resolve some of the apparent discordant data, these findings have since led to the proposal to rename this gene “FCMR” (Kubagawa et al., 2015; Kubagawa et al., 2009).
In humans, the FCMR is located on chromosome 1q32.2, adjacent to the PIGR and the FCAMR (Kubagawa et al., 2009; Kubagawa, Oka, et al., 2014). The FCMR shares sequence homology with those receptors, further supporting its function as an Ig-binding protein, although it is more distantly related than the PIGR and FCAMR are related to each other (Kubagawa et al., 2009). A splice variant of the FcμR lacking the transmembrane exon seems to encode a soluble form of the receptor. It has been proposed that the soluble receptor serves as a “decoy” for sIgM-interaction with cell (Kubagawa, Kubagawa, et al., 2014; Kubagawa et al., 2009), however, more follow-up studies are required to confirm these findings and their biological significance.
The FcμR is a high-affinity sIgM receptor, binding to IgM mainly via the Cμ4 domain and independent of the J-chain. This explains why the FcμR can bind both, monomeric IgM and pentameric sIgM, although binding to pentameric IgM occurs with higher affinity (Lloyd et al., 2017) (Kubagawa et al., 2009) (Honda et al., 2009; Kubagawa et al., 2017; Shima et al., 2010). In both, humans and mice, expression of the FcμR is strongest on B cells, where it can be readily visualized by flow cytometry. In addition, low expression has also been observed on granulocytes, macrophages, and dendritic cells in mice. In humans, the receptor was shown to be expressed on T and NK cells, but not on myeloid cells (Shima et al., 2010) (S. C. Choi et al., 2013; Honjo et al., 2012; Kubagawa, Oka, et al., 2014; Kubagawa et al., 2017; Murakami et al., 2012; T. T. Nguyen et al., 2017; Ouchida et al., 2012; Vire, David, & Wiestner, 2011; Wang et al., 2016). Expression on human T cells was higher on α/β T cells than γ/δ T cells, and higher on CD4+ than on CD8+ T cells (Kubagawa, Oka, et al., 2014). It will be important to understand the functional significance of these expression differences between mice and humans.
On murine B cells, Fcmr expression is induced first after the pre-B cell stage in the bone marrow (S. C. Choi et al., 2013; Honjo et al., 2012; T. T. Nguyen et al., 2017; Ouchida et al., 2012). The FcμR is rapidly upregulated in immature B cells, thus at the time the fully rearranged IgM is first being generated and cells are undergoing negative selection. Here the receptor is found at high levels co-localized with IgM in the Golgi transport network and to a lesser extent on the cell surface (S. C. Choi et al., 2013; T. T. Nguyen et al., 2017). The FcμR was shown to inhibit the transport and expression of the IgM-BCR onto the cell surface, such that B cells lacking the FcμR express about 30% higher levels of surface IgM-BCR, while IgD-BCR levels were unchanged (T. T. Nguyen et al., 2017). This explains why in Chronic Lymphocytic Leukemia (CLL), where the FcμR is highly expressed, surface expression of IgM-BCR is low (Frenzel et al., 2010; Kubagawa, Oka, et al., 2014; Vire et al., 2011). While the process that inhibits BCR-IgM-transport to the cell surface remains to be fully revealed, the outcome of this enhanced IgM-BCR expression was linked to changes in B cell selection (T. T. Nguyen et al., 2017). The data provide a potential mechanistic explanation for the increases in autoantibody production seen by multiple investigators in FcμR knock out mice (S. C. Choi et al., 2013; Ouchida et al., 2012) (Honjo et al., 2014). Furthermore, they explain the increased activation and terminal differentiation of B-1 cells, resulting in significantly increased frequencies of B-1 derived IgM-plasma cells in spleen and bone marrow with concomitant increases in serum sIgM levels in these mice (T. T. Nguyen et al., 2017).
On resting peripheral B cells, the FcμR is expressed mainly on the cell surface, at least some co-localized with IgM-BCR (Honjo et al., 2012; T. T. Nguyen et al., 2017; Ouchida et al., 2012). Some modest gene expression differences were observed between B-1, MZ B and Follicular B cells (T. T. Nguyen et al., 2017) (S. C. Choi et al., 2013), but the functional significance of these differences is unclear. As stated above, in CLL the FcμR is strongly expressed, possibly linked to the chronic activation of these cells through BCR-mediated signaling, which was shown to increase FcμR expression (Frenzel et al., 2010). Stimulation through TLR, CD40L and IL-4 strongly inhibited FcµR expression in normal human B cells, as well as in CLL (Frenzel et al., 2010; Kubagawa et al., 2009; Pallasch et al., 2008; Vire et al., 2011), consistent with the loss of FcμR expression among germinal center B cells (S. C. Choi et al., 2013). The FcμR appears to be re-expressed on more differentiated cells, however, as it was found on IgG+ and IgA+ memory B cells and CD138+ plasma cells in spleen and lymph nodes, as well as on a subset of CD138+ bone marrow plasma cells (Honjo et al., 2012; Kubagawa, Oka, et al., 2014).
This dynamic up and down-regulation of FcμR surface expression suggests differentiation-stage specific functional consequences following FcμR engagement. Using Ig-allotype-disparate B cells, we were able to differentiate sIgM-binding from IgM-BCR surface expression and showed constitutive binding of sIgM onto the cell surface of B cells in vivo, which was reduced in the absence of the FcμR (T. T. Nguyen et al., 2017). Studies in transfected HeLa cells, as well as primary mouse B cells, showed that sIgM-binding to surface-expressed FcµR caused the rapid internalization and degradation of the complex through the endocytic pathway (Murakami et al., 2012; T. T. Nguyen & Baumgarth, 2016; Vire et al., 2011), a process that in CCL was shown to depend on the state of FcμR glycosylation (Vire et al., 2011). Interestingly, some sIgM uptake was shown to occur also in the absence of the FcμR, suggesting that not all sIgM internalization is mediated by the FcμR (Murakami et al., 2012; T. T. Nguyen & Baumgarth, 2016). Recent studies showed rapid FcμR-mediated sIgM internalization by human CD4 T cells (Meryk et al., 2019). The internalization triggered a positive feed-forward mechanism resulting in increased FcμR expression as well as upregulation of the TCR and co-stimulatory molecules. This, in turn, triggered increased T cell responses after low-, but not high-dose stimulation (Meryk et al., 2019). The data provide an intriguing mechanisms by which early production of antigen-specific sIgM in secondary lymphoid tissues could facilitate uptake by T cells, enhancing their responses. Presumably, such enhancement could not replace T cell priming, but rather enhance already primed and activated T cells, independent of their specificity. It is unclear how the uptake of IgM and/or antigen may affect T cell responses, other than through signaling through the FcμR. In the steady-state, however, most if not all sIgM is natural, self-reactive. Its continued uptake presumably contributes to immune quiescence and enforcement of T cell tolerance rather than immune activation. Studies showing that the lack of the FcμR causes increased auto-antibody formation over time, similar to the changes seen in mice lacking sIgM (Boes et al., 1998; Ehrenstein, Cook, & Neuberger, 2000; T. T. Nguyen, Elsner, & Baumgarth, 2015) may suggest additional mechanisms by which FcμR /sIgM interactions contribute to immune homeostasis.
Thus, the FcμR seems to serve multiple functions in the steady-state: maintaining appropriate IgM-BCR-expression levels in development, preventing the overshooting activation of self-reactive B cells, specifically B-1, and preventing the development of autoimmune antibody-mediated diseases. Following foreign antigen exposure, depending on the antigen-dose, the FcμR may modify the strength of the B cell response directly by binding to B cells and, at least in humans, also indirectly by enhancing T cell responses. The fact that both, enhancing and suppressive effects of FcμR expression have been reported on the humoral response to T-independent and T-dependent antigens (S. C. Choi et al., 2013; Honda et al., 2009; T. T. T. Nguyen et al., 2017; Ouchida et al., 2012), suggests the potential of additional mechanisms of regulation that are antigen-type, dose, and cell subset specific. The dysregulation of surface IgM-BCR expression in FcμR −/− mice remains to be fully considered for its potential effects, however, as it may contribute to the observed effects on immune response development in the absence of the FcμR.
Concluding Remarks:
The identification of the FcμR as a bona fide receptor for sIgM and the identification of sIgM as a transport molecule for AIM has increased our ability to further resolve the complex functions of sIgM, a structurally and functionally unique, yet highly evolutionary conserved immunoglobulin that support immune homeostasis and immune defense. The functions of sIgM are intimately linked to the functions of natural IgM-secreting B-1 cells and perhaps distinguishable from the early burst of sIgM produced in response to an insult or infection by B-1 and B-2 cells. Further clarity may come from beginning to distinguish the source of the sIgM as much as the cell types with which the molecule interacts in a given situation. The limited tissue-penetrability of sIgM and its ability to act as a trojan horse carrying and increasing the half-life and thus availability of an effector protein in the blood offer some potential exciting future therapeutic intervention strategies.
Acknowledgement
Recent work from our laboratory discussed in this review was supported by funding from the National Institutes of Health/NIAID R01 AI17890 and U19 AI148652.
References
- Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, & Gerstein RM (2004). B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity, 21(3), 379–390. doi: 10.1016/j.immuni.2004.06.019 [DOI] [PubMed] [Google Scholar]
- Anelli T, & van Anken E (2013). Missing links in antibody assembly control. Int J Cell Biol, 2013, 606703. doi: 10.1155/2013/606703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelgren D, Eriksson P, Ernerudh J, & Segelmark M (2018). Marginal-Zone B-Cells Are Main Producers of IgM in Humans, and Are Reduced in Patients With Autoimmune Vasculitis. Front Immunol, 9, 2242. doi: 10.3389/fimmu.2018.02242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S, Maehara N, Iwamura Y, Honda S, Nakashima K, Kai T, … Miyazaki T. (2013). Obesity-associated autoantibody production requires AIM to retain the immunoglobulin M immune complex on follicular dendritic cells. Cell Rep, 3(4), 1187–1198. doi: 10.1016/j.celrep.2013.03.006 [DOI] [PubMed] [Google Scholar]
- Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL, … Miyazaki T (2005). A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab, 1(3), 201–213. doi: 10.1016/j.cmet.2005.02.002 [DOI] [PubMed] [Google Scholar]
- Arnold JN, Wormald MR, Sim RB, Rudd PM, & Dwek RA (2007). The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol, 25, 21–50. doi: 10.1146/annurev.immunol.25.022106.141702 [DOI] [PubMed] [Google Scholar]
- Asano M, & Komiyama K (2011). Polymeric immunoglobulin receptor. J Oral Sci, 53(2), 147–156. [DOI] [PubMed] [Google Scholar]
- Baumgarth N (2011). The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol, 11(1), 34–46. doi: 10.1038/nri2901 [DOI] [PubMed] [Google Scholar]
- Baumgarth N (2016). B-1 Cell Heterogeneity and the Regulation of Natural and Antigen-Induced IgM Production. Front Immunol, 7, 324. doi: 10.3389/fimmu.2016.00324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, & Chen J (2000). B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med, 192(2), 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarth N, Jager GC, Herman OC, Herzenberg LA, & Herzenberg LA (2000). CD4+ T cells derived from B cell-deficient mice inhibit the establishment of peripheral B cell pools. Proceedings of the National Academy of Sciences, 97(9), 4766–4771. doi: 10.1073/pnas.97.9.4766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarth N, Tung JW, & Herzenberg LA (2005). Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol, 26(4), 347–362. doi: 10.1007/s00281-004-0182-2 [DOI] [PubMed] [Google Scholar]
- Behring E. v. (1890a). Über das zustandekommen der diphtherie-immunität und der tetanus-immunität bei thieren. [PubMed] [Google Scholar]
- Behring E. v. (1890b). Untersuchungen über das Zustandekommen der Diphtherie-Immunität bei Thieren. [Google Scholar]
- Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, … Silverman GJ. (2003). Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med, 9(6), 736–743. doi: 10.1038/nm876 [DOI] [PubMed] [Google Scholar]
- Binder CJ, & Silverman GJ (2005). Natural antibodies and the autoimmunity of atherosclerosis. Springer Seminars in Immunopathology, 26(4), 385–404. doi: 10.1007/s00281-004-0185-z [DOI] [PubMed] [Google Scholar]
- Black CA (1997). A brief history of the discovery of the immunoglobulins and the origin of the modern immunoglobulin nomenclature. Immunol Cell Biol, 75(1), 65–68. doi: 10.1038/icb.1997.10 [DOI] [PubMed] [Google Scholar]
- Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, & Chen J (1998). Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol, 160(10), 4776–4787. [PubMed] [Google Scholar]
- Boyden SV (1966). Natural antibodies and the immune response. Adv Immunol, 5, 1–28. [DOI] [PubMed] [Google Scholar]
- Brekke OH, & Sandlie I (2003). Therapeutic antibodies for human diseases at the dawn of the twenty-first century. Nature Reviews Drug Discovery, 2, 52. doi: 10.1038/nrd984 [DOI] [PubMed] [Google Scholar]
- Casali P (1998). IgM In Delves PJ (Ed.), Encyclopedia of Immunology (Second Edition) (pp. 1212–1217). Oxford: Elsevier. [Google Scholar]
- Choi JG, Lee YJ, Kim YJ, Lee EK, Jeong OM, Sung HW, … Kwon JH. (2008). An inactivated vaccine to control the current H9N2 low pathogenic avian influenza in Korea. J Vet Sci, 9(1), 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SC, Wang H, Tian L, Murakami Y, Shin DM, Borrego F, … Coligan JE. (2013). Mouse IgM Fc receptor, FCMR, promotes B cell development and modulates antigen-driven immune responses. J Immunol, 190(3), 987–996. doi: 10.4049/jimmunol.1202227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Dieter JA, Rothaeusler K, Luo Z, & Baumgarth N (2012). B-1 cells in the bone marrow are a significant source of natural IgM. Eur J Immunol, 42(1), 120–129. doi: 10.1002/eji.201141890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinamon G, Zachariah MA, Lam OM, Foss FW Jr., & Cyster JG (2008). Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol, 9(1), 54–62. doi: 10.1038/ni1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S (1965). Nomenclature of Human Immunoglobulins. Immunology, 8, 1–5. [PMC free article] [PubMed] [Google Scholar]
- Colombo MJ, Abraham D, Shibuya A, & Alugupalli KR (2011). B1b lymphocyte-derived antibodies control Borrelia hermsii independent of Fcalpha/mu receptor and in the absence of host cell contact. Immunol Res, 51(2–3), 249–256. doi: 10.1007/s12026-011-8260-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci M, Stockmann H, Butera A, Masotti A, Baldassarre A, Giorda E, … Vivarelli M (2015). Sialylation of N-linked glycans influences the immunomodulatory effects of IgM on T cells. J Immunol, 194(1), 151–157. doi: 10.4049/jimmunol.1402025 [DOI] [PubMed] [Google Scholar]
- Crist E, & Tauber AI (1997). Debating humoral immunity and epistemology: the rivalry of the immunochemists Jules Bordet and Paul Ehrlich. J Hist Biol, 30(3), 321–356. [DOI] [PubMed] [Google Scholar]
- Czajkowsky DM, & Shao Z (2009). The human IgM pentamer is a mushroom-shaped molecule with a flexural bias. Proc Natl Acad Sci U S A, 106(35), 14960–14965. doi: 10.1073/pnas.0903805106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donius LR, Handy JM, Weis JJ, & Weis JH (2013). Optimal germinal center B cell activation and T-dependent antibody responses require expression of the mouse complement receptor Cr1. J Immunol, 191(1), 434–447. doi: 10.4049/jimmunol.1203176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein MR, Cook HT, & Neuberger MS (2000). Deficiency in serum immunoglobulin (Ig)M predisposes to development of IgG autoantibodies. J Exp Med, 191(7), 1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein MR, & Notley CA (2010). The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol, 10(11), 778–786. doi: 10.1038/nri2849 [DOI] [PubMed] [Google Scholar]
- Fehr T, Naim HY, Bachmann MF, Ochsenbein AF, Spielhofer P, Bucher E, … Zinkernagel RM. (1998). T-cell independent IgM and enduring protective IgG antibodies induced by chimeric measles viruses. Nat Med, 4(8), 945–948. [DOI] [PubMed] [Google Scholar]
- Feinstein A, & Munn EA (1969). Conformation of the free and antigen-bound IgM antibody molecules. Nature, 224(5226), 1307–1309. [DOI] [PubMed] [Google Scholar]
- Flajnik MF (2002). Comparative analyses of immunoglobulin genes: surprises and portents. Nat Rev Immunol, 2(9), 688–698. doi: 10.1038/nri889 [DOI] [PubMed] [Google Scholar]
- Forster I, & Rajewsky K (1987). Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur J Immunol, 17(4), 521–528. doi: 10.1002/eji.1830170414 [DOI] [PubMed] [Google Scholar]
- Frenzel A, Labi V, Chmelewskij W, Ploner C, Geley S, Fiegl H, … Villunger A. (2010). Suppression of B-cell lymphomagenesis by the BH3-only proteins Bmf and Bad. Blood, 115(5), 995–1005. doi: 10.1182/blood-2009-03-212670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth R (1965). THE IMMUNOLOGICAL DEVELOPMENT OF THE HUMAN FETUS (Vol. 122). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Cruz C, Bobat S, Marshall JL, Kingsley RA, Ross EA, Henderson IR, … Cunningham AF. (2009). The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proceedings of the National Academy of Sciences, 106(24), 9803–9808. doi: 10.1073/pnas.0812431106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf R, Seagal J, Otipoby KL, Lam KP, Ayoub S, Zhang B, … Rajewsky K. (2019). BCR-dependent lineage plasticity in mature B cells. Science, 363(6428), 748–753. doi: 10.1126/science.aau8475 [DOI] [PubMed] [Google Scholar]
- Gronwall C, & Silverman GJ (2014). Natural IgM: beneficial autoantibodies for the control of inflammatory and autoimmune disease. J Clin Immunol, 34 Suppl 1, S12–21. doi: 10.1007/s10875-014-0025-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronwall C, Vas J, & Silverman GJ (2012). Protective Roles of Natural IgM Antibodies. Front Immunol, 3, 66. doi: 10.3389/fimmu.2012.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunti S, & Notkins AL (2015). Polyreactive Antibodies: Function and Quantification. J Infect Dis, 212 Suppl 1, S42–46. doi: 10.1093/infdis/jiu512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, & Gupta A (2017). Selective IgM Deficiency-An Underestimated Primary Immunodeficiency. Front Immunol, 8, 1056. doi: 10.3389/fimmu.2017.01056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaijman JJ, Slingerland-Teunissen J, Benner R, & Van Oudenaren A (1979). The distribution of cytoplasmic immunoglobulin containing cells over various lymphoid organs of congenitally athymic (nude) mice as a function of age. Immunology, 36(2), 271–278. [PMC free article] [PubMed] [Google Scholar]
- Haas KM, Poe JC, Steeber DA, & Tedder TF (2005). B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity, 23(1), 7–18. doi: 10.1016/j.immuni.2005.04.011 [DOI] [PubMed] [Google Scholar]
- Halperin JJ, Baker P, & Wormser GP (2013). Common misconceptions about Lyme disease. Am J Med, 126(3), 264 e261–267. doi: 10.1016/j.amjmed.2012.10.008 [DOI] [PubMed] [Google Scholar]
- Hammers-Berggren S, Hansen K, Lebech AM, & Karlsson M (1993). Borrelia burgdorferi-specific intrathecal antibody production in neuroborreliosis: a follow-up study. Neurology, 43(1), 169–175. doi: 10.1212/wnl.43.1_part_1.169 [DOI] [PubMed] [Google Scholar]
- Haury M, Sundblad A, Grandien A, Barreau C, Coutinho A, & Nobrega A (1997). The repertoire of serum IgM in normal mice is largely independent of external antigenic contact. Eur J Immunol, 27(6), 1557–1563. doi: 10.1002/eji.1830270635 [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Asano M, Shinton SA, Gui M, Allman D, Stewart CL, … Hardy RR. (1999). Positive selection of natural autoreactive B cells. Science, 285(5424), 113–116. [DOI] [PubMed] [Google Scholar]
- Heidelberger M, & Pedersen KO (1937). The Molecular Weight of Antibodies. J Exp Med, 65(3), 393–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C, & Jerne NK (1968). Competition of 19S and 7S antigen receptors in the regulation of the primary immune response. J Exp Med, 128(1), 133–152. doi: 10.1084/jem.128.1.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman B, Pilstrom L, & Shulman MJ (1988). Complement activation is required for IgM-mediated enhancement of the antibody response. J Exp Med, 167(6), 1999–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramoto E, Tsutsumi A, Suzuki R, Matsuoka S, Arai S, Kikkawa M, & Miyazaki T (2018). The IgM pentamer is an asymmetric pentagon with an open groove that binds the AIM protein. Sci Adv, 4(10), eaau1199. doi: 10.1126/sciadv.aau1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitoshi Y, Lorens J, Kitada SI, Fisher J, LaBarge M, Ring HZ, … Nolan GP. (1998). Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity, 8(4), 461–471. [DOI] [PubMed] [Google Scholar]
- Honda S, Kurita N, Miyamoto A, Cho Y, Usui K, Takeshita K, … Shibuya A (2009). Enhanced humoral immune responses against T-independent antigens in Fc alpha/muR-deficient mice. Proc Natl Acad Sci U S A, 106(27), 11230–11235. doi: 10.1073/pnas.0809917106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo K, Kubagawa Y, Jones DM, Dizon B, Zhu Z, Ohno H, … Kubagawa H. (2012). Altered Ig levels and antibody responses in mice deficient for the Fc receptor for IgM (FcmuR). Proc Natl Acad Sci U S A, 109(39), 15882–15887. doi: 10.1073/pnas.1206567109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo K, Kubagawa Y, Suzuki Y, Takagi M, Ohno H, Bucy RP, … Kubagawa H (2014). Enhanced auto-antibody production and Mott cell formation in FcmuR-deficient autoimmune mice. Int Immunol, 26(12), 659–672. doi: 10.1093/intimm/dxu070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooijkaas H, Benner R, Pleasants JR, & Wostmann BS (1984). Isotypes and specificities of immunoglobulins produced by germ-free mice fed chemically defined ultrafiltered “antigen-free” diet. Eur J Immunol, 14(12), 1127–1130. doi: 10.1002/eji.1830141212 [DOI] [PubMed] [Google Scholar]
- Hooper JA (2015). The history and evolution of immunoglobulin products and their clinical indications. LymphoSign Journal, 2(4), 181–194. doi: 10.14785/lpsn-2014-0025 [DOI] [Google Scholar]
- Hosseini H, Li Y, Kanellakis P, Tay C, Cao A, Tipping P, … Kyaw T. (2015). Phosphatidylserine liposomes mimic apoptotic cells to attenuate atherosclerosis by expanding polyreactive IgM producing B1a lymphocytes. Cardiovascular Research, 106(3), 443–452. doi: 10.1093/cvr/cvv037 [DOI] [PubMed] [Google Scholar]
- Hosseini H, Yi L, Kanellakis P, Cao A, Tay C, Peter K, … Kyaw T. (2018). Anti-TIM-1 Monoclonal Antibody (RMT1–10) Attenuates Atherosclerosis By Expanding IgM-producing B1a Cells. J Am Heart Assoc, 7(13). doi: 10.1161/JAHA.117.008447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughey CT, Brewer JW, Colosia AD, Rosse WF, & Corley RB (1998). Production of IgM hexamers by normal and autoimmune B cells: implications for the physiologic role of hexameric IgM. J Immunol, 161(8), 4091–4097. [PubMed] [Google Scholar]
- Ichikawa D, Asano M, Shinton SA, Brill-Dashoff J, Formica AM, Velcich A, … Hayakawa K. (2015). Natural anti-intestinal goblet cell autoantibody production from marginal zone B cells. J Immunol, 194(2), 606–614. doi: 10.4049/jimmunol.1402383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasekera JP, Moseman EA, & Carroll MC (2007). Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol, 81(7), 3487–3494. doi: 10.1128/JVI.02128-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DD, DeIulio GA, & Winslow GM (2012). Antigen-driven induction of polyreactive IgM during intracellular bacterial infection. J Immunol, 189(3), 1440–1447. doi: 10.4049/jimmunol.1200878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DD, Jones M, DeIulio GA, Racine R, MacNamara KC, & Winslow GM (2013). B cell activating factor inhibition impairs bacterial immunity by reducing T cell-independent IgM secretion. Infect Immun, 81(12), 4490–4497. doi: 10.1128/IAI.00998-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PD, & Ada GL (1986). Influenza virus-specific antibody-secreting cells in the murine lung during primary influenza virus infection. J Virol, 60(2), 614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalish RA, McHugh G, Granquist J, Shea B, Ruthazer R, & Steere AC (2001). Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10–20 years after active Lyme disease. Clin Infect Dis, 33(6), 780–785. doi: 10.1086/322669 [DOI] [PubMed] [Google Scholar]
- Kantha SS (1991). A centennial review; the 1890 tetanus antitoxin paper of von Behring and Kitasato and the related developments. Keio J Med, 40(1), 35–39. [DOI] [PubMed] [Google Scholar]
- Kawano M, & Nagata S (2018). Efferocytosis and autoimmune disease. Int Immunol, 30(12), 551–558. doi: 10.1093/intimm/dxy055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimovich VB (2011). IgM and its receptors: structural and functional aspects. Biochemistry (Mosc), 76(5), 534–549. doi: 10.1134/S0006297911050038 [DOI] [PubMed] [Google Scholar]
- Koyama N, Yamazaki T, Kanetsuki Y, Hirota J, Asai T, Mitsumoto Y, … Okanoue T. (2018). Activation of apoptosis inhibitor of macrophage is a sensitive diagnostic marker for NASH-associated hepatocellular carcinoma. J Gastroenterol, 53(6), 770–779. doi: 10.1007/s00535-017-1398-y [DOI] [PubMed] [Google Scholar]
- Kranich J, & Krautler NJ (2016). How Follicular Dendritic Cells Shape the B-Cell Antigenome. Frontiers in Immunology, 7(225). doi: 10.3389/fimmu.2016.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubagawa H, Carroll MC, Jacob CO, Lang KS, Lee KH, Mak T, … Coligan JE. (2015). Nomenclature of Toso, Fas apoptosis inhibitory molecule 3, and IgM FcR. J Immunol, 194(9), 4055–4057. doi: 10.4049/jimmunol.1500222 [DOI] [PubMed] [Google Scholar]
- Kubagawa H, Kubagawa Y, Jones D, Nasti TH, Walter MR, & Honjo K (2014). The old but new IgM Fc receptor (FcmuR). Curr Top Microbiol Immunol, 382, 3–28. doi: 10.1007/978-3-319-07911-0_1 [DOI] [PubMed] [Google Scholar]
- Kubagawa H, Oka S, Kubagawa Y, Torii I, Takayama E, Kang DW, … Wang JY. (2009). Identity of the elusive IgM Fc receptor (FcmuR) in humans. J Exp Med, 206(12), 2779–2793. doi: 10.1084/jem.20091107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubagawa H, Oka S, Kubagawa Y, Torii I, Takayama E, Kang DW, … Honjo K. (2014). The long elusive IgM Fc receptor, FcmuR. J Clin Immunol, 34 Suppl 1, S35–45. doi: 10.1007/s10875-014-0022-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubagawa H, Skopnik CM, Zimmermann J, Durek P, Chang HD, Yoo E, … Radbruch A. (2017). Authentic IgM Fc Receptor (FcmuR). Curr Top Microbiol Immunol, 408, 25–45. doi: 10.1007/82_2017_23 [DOI] [PubMed] [Google Scholar]
- Kushnir N, Bos NA, Zuercher AW, Coffin SE, Moser CA, Offit PA, & Cebra JJ (2001). B2 but Not B1 Cells Can Contribute to CD4+ T-Cell-Mediated Clearance of Rotavirus in SCID Mice. Journal of Virology, 75(12), 5482–5490. doi: 10.1128/jvi.75.12.5482-5490.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyaw T, Tipping P, Bobik A, & Toh B-H (2012). Protective role of natural IgM-producing B1a cells in atherosclerosis. Trends in cardiovascular medicine, 22(2), 48–53. [DOI] [PubMed] [Google Scholar]
- Lalor PA, Herzenberg LA, Adams S, & Stall AM (1989). Feedback regulation of murine Ly-1 B cell development. European Journal of Immunology, 19(3), 507–513. doi: 10.1002/eji.1830190315 [DOI] [PubMed] [Google Scholar]
- Lleo A, Selmi C, Invernizzi P, Podda M, & Gershwin ME (2008). The consequences of apoptosis in autoimmunity. J Autoimmun, 31(3), 257–262. doi: 10.1016/j.jaut.2008.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd KA, Wang J, Urban BC, Czajkowsky DM, & Pleass RJ (2017). Glycan-independent binding and internalization of human IgM to FCMR, its cognate cellular receptor. Sci Rep, 7, 42989. doi: 10.1038/srep42989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo PI (2016). Role of Natural Autoantibodies and Natural IgM Anti-Leucocyte Autoantibodies in Health and Disease. Front Immunol, 7, 198. doi: 10.3389/fimmu.2016.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri G, Comerma L, Pybus M, Sintes J, Lligé D, Segura-Garzón D, … Cerutti A. (2017). Human Secretory IgM Emerges from Plasma Cells Clonally Related to Gut Memory B Cells and Targets Highly Diverse Commensals. Immunity, 47(1), 118–134.e118. doi: 10.1016/j.immuni.2017.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meryk A, Pangrazzi L, Hagen M, Hatzmann F, Jenewein B, Jakic B, … Grubeck-Loebenstein B. (2019). Fcmu receptor as a Costimulatory Molecule for T Cells. Cell Rep, 26(10), 2681–2691 e2685. doi: 10.1016/j.celrep.2019.02.024 [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Hirokami Y, Matsuhashi N, Takatsuka H, & Naito M (1999). Increased Susceptibility of Thymocytes to Apoptosis in Mice Lacking AIM, a Novel Murine Macrophage-derived Soluble Factor Belonging to the Scavenger Receptor Cysteine-rich Domain Superfamily. The Journal of Experimental Medicine, 189(2), 413–422. doi: 10.1084/jem.189.2.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Yamazaki T, Sugisawa R, Gershwin ME, & Arai S (2018). AIM associated with the IgM pentamer: attackers on stand-by at aircraft carrier. Cell Mol Immunol, 15(6), 563–574. doi: 10.1038/cmi.2017.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moh ES, Lin CH, Thaysen-Andersen M, & Packer NH (2016). Site-Specific N-Glycosylation of Recombinant Pentameric and Hexameric Human IgM. J Am Soc Mass Spectrom, 27(7), 1143–1155. doi: 10.1007/s13361-016-1378-0 [DOI] [PubMed] [Google Scholar]
- Murakami Y, Narayanan S, Su S, Childs R, Krzewski K, Borrego F, … Coligan JE (2012). Toso, a functional IgM receptor, is regulated by IL-2 in T and NK cells. J Immunol, 189(2), 587–597. doi: 10.4049/jimmunol.1200840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KO, Garcia MN, Yan C, & Gorchakov R (2013). Persistence of detectable immunoglobulin M antibodies up to 8 years after infection with West Nile virus. Am J Trop Med Hyg, 89(5), 996–1000. doi: 10.4269/ajtmh.13-0232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, & Baumgarth N (2016). Natural IgM and the Development of B Cell-Mediated Autoimmune Diseases. Crit Rev Immunol, 36(2), 163–177. doi: 10.1615/CritRevImmunol.2016018175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Elsner RA, & Baumgarth N (2015). Natural IgM prevents autoimmunity by enforcing B cell central tolerance induction. J Immunol, 194(4), 1489–1502. doi: 10.4049/jimmunol.1401880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Klasener K, Zurn C, Castillo PA, Brust-Mascher I, Imai DM, … Baumgarth N. (2017). The IgM receptor FcmuR limits tonic BCR signaling by regulating expression of the IgM BCR. Nat Immunol, 18(3), 321–333. doi: 10.1038/ni.3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TTT, Graf BA, Randall TD, & Baumgarth N (2017). sIgM-FcmuR Interactions Regulate Early B Cell Activation and Plasma Cell Development after Influenza Virus Infection. J Immunol, 199(5), 1635–1646. doi: 10.4049/jimmunol.1700560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notley CA, Brown MA, Wright GP, & Ehrenstein MR (2011). Natural IgM is required for suppression of inflammatory arthritis by apoptotic cells. J Immunol, 186(8), 4967–4972. doi: 10.4049/jimmunol.1003021 [DOI] [PubMed] [Google Scholar]
- Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, & Zinkernagel RM (1999). Control of early viral and bacterial distribution and disease by natural antibodies. Science, 286(5447), 2156–2159. [DOI] [PubMed] [Google Scholar]
- Ogden CA, Kowalewski R, Peng Y, Montenegro V, & Elkon KB (2005). IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity, 38(4), 259–264. [DOI] [PubMed] [Google Scholar]
- Ouchida R, Mori H, Hase K, Takatsu H, Kurosaki T, Tokuhisa T, … Wang JY. (2012). Critical role of the IgM Fc receptor in IgM homeostasis, B-cell survival, and humoral immune responses. Proc Natl Acad Sci U S A, 109(40), E2699–2706. doi: 10.1073/pnas.1210706109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallasch CP, Schulz A, Kutsch N, Schwamb J, Hagist S, Kashkar H, … Wendtner CM. (2008). Overexpression of TOSO in CLL is triggered by B-cell receptor signaling and associated with progressive disease. Blood, 112(10), 4213–4219. doi: 10.1182/blood-2008-05-157255 [DOI] [PubMed] [Google Scholar]
- Pape KA, Maul RW, Dileepan T, Paustian AS, Gearhart PJ, & Jenkins MK (2018). Naive B Cells with High-Avidity Germline-Encoded Antigen Receptors Produce Persistent IgM(+) and Transient IgG(+) Memory B Cells. Immunity, 48(6), 1135–1143 e1134. doi: 10.1016/j.immuni.2018.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape KA, Taylor JJ, Maul RW, Gearhart PJ, & Jenkins MK (2011). Different B cell populations mediate early and late memory during an endogenous immune response. Science, 331(6021), 1203–1207. doi: 10.1126/science.1201730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhouse RM, Askonas BA, & Dourmashkin RR (1970). Electron microscopic studies of mouse immunoglobulin M; structure and reconstitution following reduction. Immunology, 18(4), 575–584. [PMC free article] [PubMed] [Google Scholar]
- Patgaonkar M, Herbert F, Powale K, Gandhe P, Gogtay N, Thatte U, … Pathak S. (2018). Vivax infection alters peripheral B-cell profile and induces persistent serum IgM. Parasite Immunol, 40(10), e12580. doi: 10.1111/pim.12580 [DOI] [PubMed] [Google Scholar]
- Petrusic V, Zivkovic I, Stojanovic M, Stojicevic I, Marinkovic E, & Dimitrijevic L (2011). Hexameric immunoglobulin M in humans: desired or unwanted? Med Hypotheses, 77(6), 959–961. doi: 10.1016/j.mehy.2011.08.018 [DOI] [PubMed] [Google Scholar]
- Plomp R, Bondt A, de Haan N, Rombouts Y, & Wuhrer M (2016). Recent Advances in Clinical Glycoproteomics of Immunoglobulins (Igs). Mol Cell Proteomics, 15(7), 2217–2228. doi: 10.1074/mcp.O116.058503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohaska TA, Que X, Diehl CJ, Hendrikx S, Chang MW, Jepsen K, … Witztum JL. (2018). Massively Parallel Sequencing of Peritoneal and Splenic B Cell Repertoires Highlights Unique Properties of B-1 Cell Antibodies. The Journal of Immunology, 200(5), 1702–1717. doi: 10.4049/jimmunol.1700568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceppellini R, D S., Edelman G, Fahey J, Franěk F, Franklin E, Goodman HC, Grabar P, Gurvich AE, Heremans JF, Isliker H, Karush F, Press E, Trnka Z. (1964). Nomenclature for human immunoglobulins. Immunochemistry, 1(3), 145–149.14252223 [Google Scholar]
- Racine R, Chatterjee M, & Winslow GM (2008). CD11c Expression Identifies a Population of Extrafollicular Antigen-Specific Splenic Plasmablasts Responsible for CD4 T-Independent Antibody Responses during Intracellular Bacterial Infection. The Journal of Immunology, 181(2), 1375–1385. doi: 10.4049/jimmunol.181.2.1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine R, McLaughlin M, Jones DD, Wittmer ST, MacNamara KC, Woodland DL, & Winslow GM (2011). IgM Production by Bone Marrow Plasmablasts Contributes to Long-Term Protection against Intracellular Bacterial Infection. The Journal of Immunology, 186(2), 1011–1021. doi: 10.4049/jimmunol.1002836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AE, Kuraoka M, & Kelsoe G (2015). Natural IgM is produced by CD5- plasma cells that occupy a distinct survival niche in bone marrow. J Immunol, 194(1), 231–242. doi: 10.4049/jimmunol.1401203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D, & Wardlaw AC (1958). Lysis of gram-negative bacteria by serum. J Gen Microbiol, 18(2), 529–533. doi: 10.1099/00221287-18-2-529 [DOI] [PubMed] [Google Scholar]
- Rutemark C, Alicot E, Bergman A, Ma M, Getahun A, Ellmerich S, … Heyman B (2011). Requirement for complement in antibody responses is not explained by the classic pathway activator IgM. Proceedings of the National Academy of Sciences, 108(43), 17589–17590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutemark C, Bergman A, Getahun A, Hallgren J, Henningsson F, & Heyman B (2012). Complement receptors 1 and 2 in murine antibody responses to IgM-complexed and uncomplexed sheep erythrocytes. PLoS One, 7(7), e41968. doi: 10.1371/journal.pone.0041968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto N, Shibuya K, Shimizu Y, Yotsumoto K, Miyabayashi T, Sakano S, … Shibuya A. (2001). A novel Fc receptor for IgA and IgM is expressed on both hematopoietic and non-hematopoietic tissues. Eur J Immunol, 31(5), 1310–1316. doi: [DOI] [PubMed] [Google Scholar]
- Savage HP, Yenson VM, Sawhney SS, Mousseau BJ, Lund FE, & Baumgarth N (2017). Blimp-1-dependent and -independent natural antibody production by B-1 and B-1-derived plasma cells. J Exp Med, 214(9), 2777–2794. doi: 10.1084/jem.20161122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder HW Jr., & Cavacini L (2010). Structure and function of immunoglobulins. J Allergy Clin Immunol, 125(2 Suppl 2), S41–52. doi: 10.1016/j.jaci.2009.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PX, Horkko S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, & Witztum JL (2000). Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest, 105(12), 1731–1740. doi: 10.1172/JCI8472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya A, Sakamoto N, Shimizu Y, Shibuya K, Osawa M, Hiroyama T, … Nakauchi H. (2000). Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat Immunol, 1(5), 441–446. doi: 10.1038/80886 [DOI] [PubMed] [Google Scholar]
- Shima H, Takatsu H, Fukuda S, Ohmae M, Hase K, Kubagawa H, … Ohno H (2010). Identification of TOSO/FAIM3 as an Fc receptor for IgM. Int Immunol, 22(3), 149–156. doi: 10.1093/intimm/dxp121 [DOI] [PubMed] [Google Scholar]
- Shimada S, Kawaguchi-Miyashita M, Kushiro A, Sato T, Nanno M, Sako T, … Ohwaki M. (1999). Generation of polymeric immunoglobulin receptor-deficient mouse with marked reduction of secretory IgA. J Immunol, 163(10), 5367–5373. [PubMed] [Google Scholar]
- Sitia R, Neuberger M, Alberini C, Bet P, Fra A, Valetti C, … Milstein C. (1990). Developmental regulation of IgM secretion: the role of the carboxy-terminal cysteine. Cell, 60(5), 781–790. [DOI] [PubMed] [Google Scholar]
- Song Y, & Jacob CO (2005). The mouse cell surface protein TOSO regulates Fas/Fas ligand-induced apoptosis through its binding to Fas-associated death domain. J Biol Chem, 280(10), 9618–9626. doi: 10.1074/jbc.M413609200 [DOI] [PubMed] [Google Scholar]
- Sorman A, Zhang L, Ding Z, & Heyman B (2014). How antibodies use complement to regulate antibody responses. Mol Immunol, 61(2), 79–88. doi: 10.1016/j.molimm.2014.06.010 [DOI] [PubMed] [Google Scholar]
- Tiselius A, & Kabat EA (1939). An Electrophoretic Study of Immune Sera and Purified Antibody Preparations. J Exp Med, 69(1), 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissot JD, Sanchez JC, Vuadens F, Scherl A, Schifferli JA, Hochstrasser DF, … Duchosal MA (2002). IgM are associated to Sp alpha (CD5 antigen-like). Electrophoresis, 23(7–8), 1203–1206. doi: [DOI] [PubMed] [Google Scholar]
- Tjarnlund A, Rodriguez A, Cardona PJ, Guirado E, Ivanyi J, Singh M, … Fernandez C. (2006). Polymeric IgR knockout mice are more susceptible to mycobacterial infections in the respiratory tract than wild-type mice. Int Immunol, 18(5), 807–816. doi: 10.1093/intimm/dxl017 [DOI] [PubMed] [Google Scholar]
- Tsiantoulas D, Kiss M, Bartolini-Gritti B, Bergthaler A, Mallat Z, Jumaa H, & Binder CJ (2017). Secreted IgM deficiency leads to increased BCR signaling that results in abnormal splenic B cell development. Sci Rep, 7(1), 3540. doi: 10.1038/s41598-017-03688-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turula H, & Wobus CE (2018). The Role of the Polymeric Immunoglobulin Receptor and Secretory Immunoglobulins during Mucosal Infection and Immunity. Viruses, 10(5). doi: 10.3390/v10050237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubelhart R, Bach MP, Eschbach C, Wossning T, Reth M, & Jumaa H (2010). N-linked glycosylation selectively regulates autonomous precursor BCR function. Nat Immunol, 11(8), 759–765. doi: 10.1038/ni.1903 [DOI] [PubMed] [Google Scholar]
- Vire B, David A, & Wiestner A (2011). TOSO, the Fcmicro receptor, is highly expressed on chronic lymphocytic leukemia B cells, internalizes upon IgM binding, shuttles to the lysosome, and is downregulated in response to TLR activation. J Immunol, 187(8), 4040–4050. doi: 10.4049/jimmunol.1100532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waffarn EE, Hastey CJ, Dixit N, Soo Choi Y, Cherry S, Kalinke U, … Baumgarth N. (2015). Infection-induced type I interferons activate CD11b on B-1 cells for subsequent lymph node accumulation. Nat Commun, 6, 8991. doi: 10.1038/ncomms9991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALDENSTRÖM J (1944). Incipient myelomatosis or «essential« hyperglobulinemia with fibrinogenopenia — a new syndrome? Acta Medica Scandinavica, 117(3‐4), 216–247. doi: 10.1111/j.0954-6820.1944.tb03955.x [DOI] [Google Scholar]
- Wallenius G, Trautman R, Kunkel HG, & Franklin EC (1957). Ultracentrifugal studies of major non-lipide electrophoretic components of normal human serum. J Biol Chem, 225(1), 253–267. [PubMed] [Google Scholar]
- Wang H, Coligan JE, & Morse HC 3rd. (2016). Emerging Functions of Natural IgM and Its Fc Receptor FCMR in Immune Homeostasis. Front Immunol, 7, 99. doi: 10.3389/fimmu.2016.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JF, Shulman MJ, Isenman DE, & Painter RH (1990). C1 binding by mouse IgM. The effect of abnormal glycosylation at position 402 resulting from a serine to asparagine exchange at residue 406 of the mu-chain. J Biol Chem, 265(18), 10506–10513. [PubMed] [Google Scholar]
- Yang Y, Ghosn EEB, Cole LE, Obukhanych TV, Sadate-Ngatchou P, Vogel SN, … Herzenberg LA. (2012). Antigen-specific memory in B-1a and its relationship to natural immunity. Proceedings of the National Academy of Sciences, 109(14), 5388–5393. doi: 10.1073/pnas.1121627109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang C, Yang Q, Kantor AB, Chu H, Ghosn EE, … Herzenberg LA (2015). Distinct mechanisms define murine B cell lineage immunoglobulin heavy chain (IgH) repertoires. Elife, 4, e09083. doi: 10.7554/eLife.09083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yel L (2010). Selective IgA deficiency. J Clin Immunol, 30(1), 10–16. doi: 10.1007/s10875-009-9357-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z, Surewaard BGJ, Wong CHY, Guettler C, Petri B, Burkhard R, … Kubes P. (2018). Sex-hormone-driven innate antibodies protect females and infants against EPEC infection. Nat Immunol, 19(10), 1100–1111. doi: 10.1038/s41590-018-0211-2 [DOI] [PubMed] [Google Scholar]
- Zhang M, Austen WG, Chiu I, Alicot EM, Hung R, Ma M, … Carroll MC (2004). Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proceedings of the National Academy of Sciences of the United States of America, 101(11), 3886–3891. doi: 10.1073/pnas.0400347101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Takahashi K, Alicot EM, Vorup-Jensen T, Kessler B, Thiel S, … Carroll MC. (2006). Activation of the Lectin Pathway by Natural IgM in a Model of Ischemia/Reperfusion Injury. The Journal of Immunology, 177(7), 4727–4734. doi: 10.4049/jimmunol.177.7.4727 [DOI] [PubMed] [Google Scholar]
- Zhou ZH, Tzioufas AG, & Notkins AL (2007). Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J Autoimmun, 29(4), 219–228. doi: 10.1016/j.jaut.2007.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]


