Abstract
Anticipation of a painful experience can influence brain activity and increase sensitivity to experimental somatosensory stimuli in healthy adults, but this response is poorly understood among individuals with chronic musculoskeletal pain (CMP). Studies of brain and perceptual responses to somatosensory stimuli are used to make inferences about central nervous system dysfunction as a potential mechanism of symptoms. As such, we sought to (i) determine the influence of pain anticipation on pain-relevant brain regions and pain perception and (ii) characterize potential differences in these responses between Gulf War Veterans with CMP and matched healthy control veterans (CO). CMP (N = 30) and CO Veterans (N = 31) were randomized to conditions designed to generate expectations that either painful (‘pain’) or non painful (‘no pain’) stimuli would be administered. Brain responses to five non painful thermal stimuli were measured during functional magnetic resonance imaging and each stimulus was rated for pain intensity and unpleasantness. In the ‘pain’ condition, an incremental linear decrease in activity across stimuli was observed in the posterior cingulate cortex, cingulate cortex, and middle temporal gyrus. Further, in the ‘pain’ condition, differential responses were observed between CMP and CO in the middle temporal gyrus. These findings indicate that brain responses to non painful thermal stimuli in Veterans with CMP are sensitive to pain anticipation and we recommend accounting for the influence of pain anticipation in future investigations of central nervous system dysfunction in CMP.
Keywords: BOLD, Central nervous system, Chronic pain, fibromyalgia, MRI, Nocebo
1. Introduction
Following deployment to the Persian Gulf War, approximately 15–38% of Veterans report unresolved chronic musculoskeletal pain (CMP) and these symptoms also appear to be elevated in Veterans of more recent conflicts in Iraq and Afghanistan (Kang, Mahan, Lee, Magee, & Murphy, 2000; T. C. Smith et al., 2014). Currently, there is no widely accepted medical explanation for reports of CMP and other debilitating symptoms in Gulf War Veterans (Dursa, Barth, Schneiderman, & Bossarte, 2016; Iversen, Chalder, & Wessely, 2007). Although the pathophysiology of medically unexplained CMP is also unknown, functional magnetic resonance imaging (fMRI) and behavioral studies involving symptomatic Gulf War Veterans (Cook, Stegner, & Ellingson, 2010; Gopinath et al., 2012) and civilians (Cook et al., 2004; Ellingson, Stegner, Schwabacher, Koltyn, & Cook, 2016; McLoughlin, Stegner, & Cook, 2011) suggest central nervous system dysregulation as a potential mechanism. By examining brain and perceptual responses to painful and non painful thermal stimuli, these studies have begun to identify specific brain regions involved in pain encoding and processing that may contribute to the augmentation and maintenance of medically unexplained CMP symptoms.
Prior work involving healthy adults has repeatedly demonstrated that participant expectations are a powerful psychological mechanism that can influence neural and perceptual responses to painful and non painful stimuli. For instance, the presence or absence of negative expectations, such as the anticipation of pain or bodily harm, affects which brain regions show significant responses to near pain threshold stimuli and the likelihood those stimuli are rated as painful (Wiech et al., 2010). The degree of expected pain also appears to be an important factor in brain and perceptual responses to experimental pain, as expectations for higher pain intensity augment brain and perceptual responses to painful stimuli to a greater extent than expectations for lower pain intensity (Keltner, 2006). In further support, self-reported expectations for pain predict pain perception (Corsi & Colloca, 2017) and brain responses (Atlas, Bolger, Lindquist, & Wager, 2010) to experimental pain stimuli. Investigations that measure responses to non painful somatosensory stimuli are less common, but at least one fMRI study and one behavioral study have provided evidence suggesting that pain anticipation can also affect brain and perceptual responses to non painful stimuli (Colloca et al., 2008; Sawamoto et al., 2000).
This collective body of literature characterizing the influence of negative expectations on perceptual responses to painful and non painful stimuli can be described as the study of nocebo- hyperalgesia and –allodynia, respectively (Colloca et al., 2008; Corsi & Colloca, 2017; Reicherts, Gerdes, Pauli, & Wieser, 2016). During stimulus administration, differences in brain activity between conditions in which expectations for pain have or have not been experimentally manipulated could indicate brain regions that are involved in the top-down regulation of nocebo effects (Colloca & Grillon, 2014). For analytical reasons, it is important to highlight that pain anticipation is the psychological mechanism that precedes the nocebo effect. Thus, an fMRI analysis aimed toward examining brain activity related to pain anticipation should focus on the time period in between the presentation of a visual or auditory cue that alerts a participant to an upcoming stimulus and the presentation of that stimulus (see Palermo, Benedetti, Costa, & Amanzio, 2015 for a review). On the other hand, an analysis aimed toward examining brain activity related to nocebo effects should focus on the time period during the presentation of the stimulus. For instance, a study by Burgmer and colleagues examined brain activity prior to the presentation of moderate, medium, or severe pressure pain stimuli in fibromyalgia patients and healthy controls (Burgmer et al., 2011). Thus, the investigators examined brain activity related to pain anticipation rather than nocebo effects because they did not evaluate neural responses during the presentation of pressure stimuli or how experimentally manipulating pain anticipation may have affected those responses.
Habituation of brain activity and perceptual responses to somatosensory stimuli is another important consideration that may help clarify the role of central nervous system dysregulation in medically unexplained CMP. Decreases in neural responses across repeated stimulus exposures may reflect an attentive capability of the brain to recognize and filter redundant and irrelevant incoming information (Montoya et al., 2006). However, in fibromyalgia patients neuroimaging and behavioral data suggest a compromised habituation response to repeated stimulus exposures. For instance, an electroencephalography study by Montoya and colleagues demonstrated that repeated exposure to non painful somatosensory stimuli reduced event-related potentials in healthy adults but not fibromyalgia patients (Montoya et al., 2006). Moreover, when habituation is measured by heat pain thresholds, fibromyalgia patients show decreased rates of habituation across repeated exposure to thermal stimuli compared to healthy controls (B. W. Smith et al., 2008). This reduced ability to habituate to somatosensory stimuli may be related to the development of chronic pain in patients with medically unexplained pain (B. W. Smith et al., 2008) and testing for habituation effects in the context of pain anticipation may provide further insight into this hypothesis. To date, the effect of pain anticipation on neural and perceptual habituation responses to somatosensory stimuli remains untested in patients with medically unexplained pain.
Because studies of central nervous system dysfunction in medically unexplained CMP use measures that are clearly affected by pain anticipation, it is critical to understand the role of pain anticipation in this context and determine whether it may have a differential impact on CMP patients compared to healthy controls. Although brain (Gopinath et al., 2012) and perceptual (Cook et al., 2010) responses to experimental thermal stimuli have been studied in Gulf War Veterans with CMP, no prior work has considered the impact of pain anticipation on these responses in this population. Here, we address this gap with an experiment that tested the effect of anticipation on brain and perceptual responses to thermal stimuli in Gulf War Veterans with widespread CMP and whether those responses differed from healthy control (CO) Veterans. To that end, participants were randomly assigned to one of two conditions wherein an expectancy manipulation procedure was used to generate and reinforce expectations that either painful (‘pain’) or non painful (‘no pain’) thermal stimuli would be received during an fMRI scan. We then examined the effect of the experimental manipulation on brain and perceptual responses to a series of non painful thermal stimuli and tested whether pain anticipation differentially affected CMP and CO Veterans in terms of their neural and perceptual responses to those stimuli.
Based on prior research that used an expectancy manipulation to augment the expectation of pain, we hypothesized that changes in activity in pain-relevant brain regions (Reicherts et al., 2017; Wiech et al., 2010) and pain ratings (Colloca et al., 2008; Wiech et al., 2010) would be greater in the ‘pain’ condition than the ‘no pain’ condition. Because patients with medically unexplained CMP show differential responses during pain anticipation (Burgmer et al., 2011) and elevated processing of painful stimuli (Cook et al., 2004; Gracely, Petzke, Wolf, & Clauw, 2002), we hypothesized that, in the ‘pain’ condition, there would be a differential blood oxygen level dependent (BOLD) response between CMP and CO Veterans and that CMP Veterans would perceive thermal stimuli as more painful than CO Veterans.
2. Design and Method
The procedures and methods described below detail a randomized-controlled experiment that tested the influence of pain anticipation on pain processing and perception as part of a larger investigation examining brain structure (Van Riper et al., 2017) and functional responses to somatosensory stimuli in Gulf War Veterans with CMP.
2.1. Participants
Sixty-one Veterans (CMP = 30; CO = 31) deployed to the Persian Gulf region (e.g., Iraq, Kuwait, Saudi Arabia) during the Persian Gulf War or more recent Iraq War completed the study protocol. Groups were matched on age, sex, education, and deployment status. CMP was operationally defined as moderate pain lasting at least 6 months and occurring in three or more quadrants of the body. Additionally, reported pain could not be explained by an acute injury or other known chronic pain condition (e.g., rheumatoid arthritis). Presence or absence of CMP was confirmed via a clinical assessment and medical chart review by a local Department of Veterans Affairs (VA) rheumatologist. Veterans who did not endorse the presence of a chronic illness or pain were designated as CO. All participants gave their informed written consent and received financial compensation for taking part in the experiment. The study was approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board and Madison VA Research and Development Committee.
2.1.1. Exclusion criteria
Potential participants were excluded for MRI contraindications (e.g., claustrophobia, ferrous metal in the body, planned or actual pregnancy). Individuals weighing greater than 136 kilograms were excluded due to inherent weight limitations of the MRI scanning bed. To reduce variability in brain structure/function and to control for potential confounds, individuals were also excluded if they had one or more of the following: bipolar disorder, schizophrenia, major depressive disorder, post-traumatic stress disorder, medical or neurologic disorders, diabetes, an episode of unconsciousness lasting longer than 5 minutes, current use of illegal drugs, substance abuse or dependence (within 2 years), or current use of muscle relaxants, anti-convulsant medications, or any prescribed narcotic pain medications. Absence of exclusionary criteria was confirmed via medical chart review and urine screening.
2.1.2. Recruitment
With VA and IRB approval, a letter of invitation to participate was sent to Veterans in the VA Great Lakes Health Care System patient database meeting our most basic inclusion criteria, that is, age (30–55) and deployment (Persian Gulf region, 1990–92, 2003–2011). In addition, participants were recruited through postings of study information at local Veteran organizations, on approved Veteran websites and newspaper advertisements. Recruitment began August 2009 and ended October 2014.
2.2. Procedures
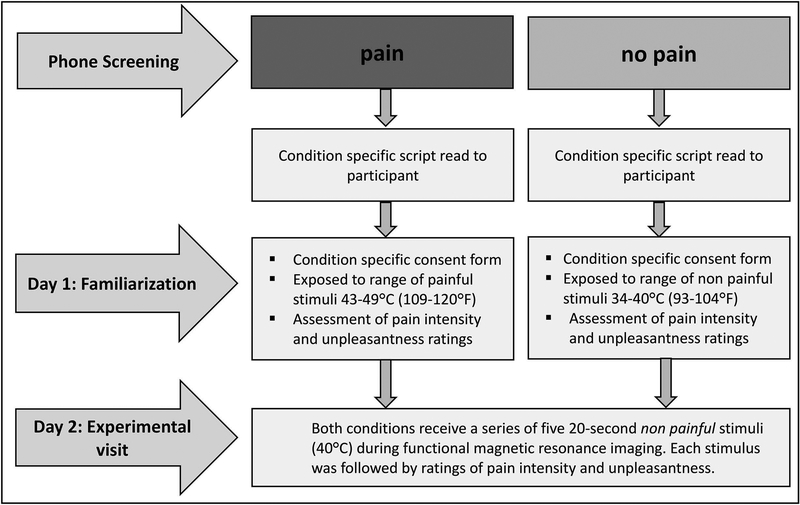
Procedures included an initial phone screening and two separate visits to the laboratory, separated by approximately one week. A flow chart of the study progression is provided in Figure 1.
Figure 1. Flow chart of study progression.
Participants were randomly assigned to a ‘pain’ condition in which they were led to believe that they would receive painful thermal stimuli or ‘no pain’ condition in which they were reassured that they would receive non painful thermal stimuli during a functional magnetic resonance imaging scan. Anticipation of receiving painful or non painful stimuli was reinforced with a psychophysical expectancy manipulation procedure that using exposure to painful (pain condition) or non painful (no pain condition) stimuli on Day 1 and was supplemented with explicit information communicated through informed consent and interactions with study personnel.
2.2.1. Phone screening
After confirmation of eligibility, participants were randomly assigned to either an (i) experimental ‘pain’ condition in which they were instructed (warned) that they would receive a painful thermal stimulus or (ii) control ‘no pain’ condition in which they were instructed (reassured) that they would never receive a painful thermal stimulus during an fMRI scan study visit. Participants were then read a condition specific script with explicit information about the temperature of the stimuli that they would be exposed to during the laboratory visits on Days 1 and 2 (see Supplementary File 1 for specific study script).
2.2.2. Day 1 familiarization visit
On Day 1, participants were provided with a condition specific consent form with similar information that was conveyed during the screening phase. Following a physical exam, medical chart review, and urine screening, participants completed a set of questionnaires (described below) and underwent a familiarization trial in a simulated MRI environment designed to mimic the fMRI testing environment on Day 2. Prior to the simulation, a condition specific summary statement was read to prime participants for the psychophysical expectancy manipulation. Participants in the ‘pain’ condition were informed that they would receive a range of painful temperatures and that some of these stimuli could be considered to be extremely painful whereas participants in the ‘no pain’ condition were reassured that only non painful stimuli would be delivered. Next, anticipation of pain or no pain was reinforced by exposing participants to a range of painful (43–49°C) or non painful (34–40°C) thermal stimuli, respectively.
Each temperature within the assigned range for the ‘pain’ or ‘no pain’ conditions was presented twice in a random order for a total of 14 stimulus presentations, with an inter-stimulus interval of 1 min. Participants also practiced rating pain intensity and unpleasantness following each stimulus. As participants became more familiar with the scales, they were encouraged to provide their ratings within the 10-second time period that would be allotted during the Day 2 scan. Following stimulus administration, participants in the ‘pain’ condition were informed that those same stimuli would be administered during the Day 2 experimental visit. Participants in the ‘no pain’ condition were given the same instructions unless they rated a given thermal stimulus higher than 0, in which case they were informed that only stimuli they rated as 0 (i.e., “No pain sensation”) would be administered during the Day 2 experimental visit.
2.2.3. Day 2 experimental visit
On Day 2, participants arrived to the laboratory and were given an overview of the study procedures for the experimental scan. Participants in the ‘pain’ condition were reminded that the same painful temperatures from Day 1 would be used during scanning and some of these stimuli could be considered to be “extremely painful”. In contrast, participants in the ‘no pain’ condition were reassured that only non painful stimuli would be delivered and that the investigators were solely interested in their brain responses to warm stimuli. They were also reminded that the same non painful temperatures administered on Day 1 would be used during the MRI scan.
To determine the influence of pain anticipation on BOLD activity and perceptual ratings, participants in both conditions received a series of five 40°C thermal stimuli during an initial functional brain imaging scan. Thus, despite the fact that participants in the ‘pain’ condition were led to believe they would receive the same painful stimuli that were administered during Day 1, participants in both conditions received a stimulus temperature that was high enough to be perceived, but would have a low likelihood of being perceived as painful. Compared to higher temperatures that more reliably elicit pain responses, administering a 40°C stimulus in both conditions permitted the ability to isolate the influence of pain anticipation on brain activity and perceptual ratings in the absence of the somatosensory experience of pain (Colloca et al., 2008).
2.3. Instruments
2.3.1. Questionnaires
Several questionnaires were administered on Day 1 to characterize demographic information and self-reported depression, anxiety, general physical and mental health, pain catastrophizing, and physical activity behavior of the participants, including: the Beck Depression Inventory (Beck, Steer, & Brown, 1996), the International Physical Activity Questionnaire (Craig et al., 2003), the Pain Catastrophizing Scale (Sullivan, Bishop, & Pivik, 1995), the 36-Item Short Form Health Survey (Ware & Sherbourne, 1992), and Spielberger’s State-Trait Anxiety Inventory (Spielberger, 1983).
Participants also completed an investigator-created pain expectancy questionnaire immediately prior to entering the MRI on Day 2. This questionnaire was used as part of a manipulation check to quantify participant expectations about the intensity of pain they would experience from the thermal stimuli during the MRI scan. Participants were instructed to rate how much pain they expected on a scale of 0–10, where ‘0’ corresponded to “No pain at all” and ‘10’ corresponded to “Extremely intense pain.”
2.3.2. Pain rating scales
On both study visits, thermal stimuli were rated with the Gracely Pain Scale, a self-report measure that uses two separate category-ratio scales designed to measure sensory and affective components of pain (Gracely, McGrath, & Dubner, 1978). The Gracely Pain Scale has (i) established psychometric properties (Gracely & Dubner, 1987), (ii) been used to evaluate the degree of perceived pain or absence of pain following the administration of a painful or non painful thermal stimulus (Kong et al., 2006), and (iii) been previously used to assess subjective pain ratings in fMRI studies of treatment expectancy in chronic pain patients (Gollub et al., 2018; Kong et al., 2018) and healthy adults (Kong et al., 2008).
The pain intensity (sensory) scale was always presented first and was followed by the pain unpleasantness (affective) scale for each stimulus administration. Each scale contained numbers from 0 to 20 with verbal anchors placed alongside to assist participants in selecting a number that best represented the sensation they felt in response to the thermal stimulus. Participants were instructed to provide a 0 rating on the pain intensity and unpleasantness scales if they did not experience any sensory or affective pain, respectively. All participants were given identical, detailed instructions for the use of the scales on Day 1 and those directions were reiterated before the experimental MRI scan on Day 2.
During the experimental scan on Day 2, the rating scales were viewed with a set of MRI-compatible goggles (Avotec, Inc., Stuart, FL) and presented with the use of E-Prime software (Psychology Software Tools, Pittsburg, PA). Each participant made their ratings on the presented scales within 10 seconds after each thermal stimulus using a scanner-compatible button press response unit (Current Designs, Philadelphia, PA), which was operated by the right hand. All participants, regardless of condition assignment, were instructed to use the scales only to rate pain experienced from the thermal stimuli and avoid rating other pain sensations they might be feeling (e.g., musculoskeletal pain symptoms).
2.3.3. Thermal stimulus administration
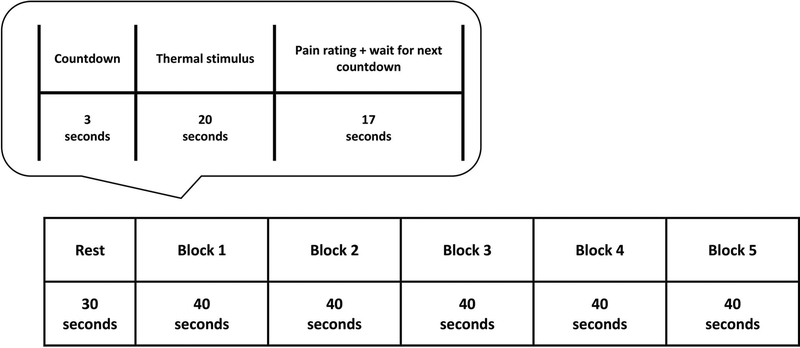
Thermal stimuli were administered to the thenar eminence of the left hand via a Pathway Pain & Sensory Evaluation System with a 900 mm2 Peltier thermode (Medoc Advanced Medical Systems, Ramat Yishai, Israel). Baseline temperature for the functional MRI scan was maintained at 32°C and increased to the stimulus target temperature at a rate of 8°C/second. During the experimental scan on Day 2, a series of five non painful warm stimuli (40°C) were administered to participants in both conditions. Following a 3-second visual countdown to alert the participant of stimulus onset, each stimulus was administered for 20 seconds followed by a 10-second period during which participants provided perceptual ratings (see Figure 2). The initial countdown was preceded by a rest period during which a fixation cross was visible to participants. Following the completion of each perceptual rating period, the fixation cross would reappear until the next visual countdown began. Total run duration was 230 seconds.
Figure 2. Schematic of thermal stimulus and pain rating administration during experimental functional magnetic resonance imaging on Day 2.
On Day 2, we used a block design to measure brain responses to five non painful warm stimuli (40°C) during a functional magnetic resonance imaging (fMRI) scan. The sequence of events in each block was: (i) 3-second countdown, (ii) 20-second thermal stimulus, (iii) 10-second pain rating period, and (iv) 7-second waiting period. For participants who completed pain intensity and unpleasantness ratings before the 10-second time limit, the remaining time was added to the 7-second waiting period to account for this difference.
2.3.4. fMRI acquisition and processing
All anatomical and functional magnetic resonance images were collected on a 3-Tesla GE SIGNA MRI scanner (GE Health Systems, Waukesha, WI) with a whole-head transmit-receive coil. A vacuum pillow and/or foam padding was used to limit head motion within the coil. Participants were fitted with MRI-compatible headphones for communications to and from the experimenter and to minimize scanner noise during acquisition.
Anatomical acquisitions were collected using 3D IR-prepped fast-gradient echo-pulse sequence, which consisted of 124 (1–2mm thick), T1-weighted (repetition time 9000ms, echo time 93ms, field of view 24cm, flip angle 30/90°), axial images with a matrix of 256 × 256 × 64. High-resolution functional images were obtained using echoplanar imaging (EPI) with a gradient echo EPI sequence (repetition time 2000ms, echo time 30ms, flip angle 90°) and consisted of 30 4-mm thick (1-mm gap) sagittal slices. The acquisition matrix was 64 × 64 mm and the field of view 24 cm, delivering an in-plane voxel resolution of 3.75 × 3.75 × 4 mm. Data processing was conducted using Statistical Parametric Mapping version 12 (SPM 12; Wellcome Department of Imaging Neuroscience, London, UK) and MATLAB (MathWorks, Natick, MA, USA) software.
Functional MRI images were motion corrected, field-map corrected, normalized, smoothed with an 8-mm Gaussian filter, and registered to the Montreal Neurological Institutes 152 template using an affine transformation. To avoid saturation effects, the first three volumes collected were removed from the functional data analyses.
2.4. Behavioral data analysis
Distributions of questionnaire and pain rating data were checked for normality with the Kolmogorov-Smirnov test. Comparisons between groups (CMP, CO) or conditions (Pain, No Pain) for normally distributed data were conducted using independent samples t-tests in SPSS version 25.0 (IBM SPSS Statistics) with Hedges’ g (g) effect sizes and 95% confidence intervals (CI) (Cumming & Finch, 2001; Fritz, Morris, & Richler, 2012). Hedges’ g values of 0.2, 0.5, and 0.8 were considered to be small, medium and large, respectively (Cohen, 1992).
For non normally distributed data, we used the WRS2 package in R (R Core Team, 2013) to conduct Yuen’s modified t-test for independent trimmed means. This test is considered to be more robust to violations of normality and homoscedasticity than the independent samples t-test (Field & Wilcox, 2017; Mair & Wilcox, 2019; R. Wilcox, 2017). We followed Yuen’s modified t-test with Wilcox and Tian’s explanatory measure of effect size (ξ) with percentile bootstrapped 95% CI because it is more robust to violations of homoscedasticity than parametric effect sizes (R. R. Wilcox & Tian, 2011). Wilcox and Tian’s ξ values of 0.10, 0.30, and 0.50 were considered to be small, medium and large, respectively (Mair & Wilcox, 2019).
2.5. fMRI data analysis
Data from two participants were excluded from fMRI analyses either because of technical difficulties with the thermal stimulus administration equipment or poor signal-to-noise ratio from motion artifact. Thus, a total of 59 (CMP/pain = 13; CMP/no pain = 17; CO/pain = 13; CO/no pain = 16) participants were included in the analysis.
2.5.1. First level analysis: Neural responses to non painful thermal stimuli
Using a block design, fMRI data were applied to the Generalized Linear Model in SPM 12. First level design matrix regressors included the five thermal stimuli and their corresponding countdown and rating periods. Positive and negative t-contrasts characterized BOLD activity increases and decreases in response to each individual thermal stimulus as well as the countdown and rating periods. We then created a linear contrast that included all five thermal stimuli and was weighted in the order of stimulus administration to test whether BOLD activity decreased (2, 1, 0, −1, −2) or increased (−2, −1, 0, 1, 2) in an incremental linear fashion across repeated stimulus exposures. These contrasts allowed us to test for potential habituation (linear decrease in BOLD signal) and sensitization (linear increase in BOLD signal) responses to non painful stimuli.
2.5.2. First level analysis: Neural responses during perceptual ratings of non painful thermal stimuli
We also included a positive and a negative contrast to explore potential increases or decreases in average BOLD activity during the perceptual rating period that followed each thermal stimulus administration. This analysis was performed for descriptive purposes to illustrate the effect of the cognitive evaluation of pain intensity and unpleasantness on brain activity. The amount of time (sec) it took each participant to provide their pain intensity and unpleasantness ratings was included in each first-level model to account for potential differences between participants who took more or less time to provide a perceptual rating.
2.5.3. Second level analysis
Our primary analysis tested whether linear increases or decreases in BOLD responses across the five thermal stimuli differed between conditions (i.e., main effect of condition) and whether those responses in the pain condition differed between CMP and CO participants (i.e., group-by-condition interaction). Thus, linear contrasts were submitted to separate full factorial ANOVAs with group (CMP, CO) and condition (pain, no pain) as between-subjects factors. For descriptive purposes, we also tested for increases and decreases in activity during pain ratings (Kong et al., 2006). This was performed with a separate full factorial ANOVA that analyzed the average brain response across the five perceptual rating periods that followed thermal stimulus administration.
Because this study was focused on examining the effect of pain anticipation on activity in brain regions that are involved in pain encoding and processing, we performed a region of interest analysis with a study specific mask (Ellingson, Shields, Stegner, & Cook, 2012; Ellingson et al., 2016) that was based on prior literature of experimental pain in healthy participants and those with fibromyalgia (Tracey & Mantyh, 2007). The following regions were included in the mask: pre and post central gyri, superior parietal lobule, cingulate cortices, brainstem, frontal medial cortex, frontal and parietal opercula, frontal pole, insula, thalamus, and middle frontal and orbital frontal gyri.
To account for multiple comparisons, the statistical map was thresholded at a voxelwise p < .005 and clusters of activity of less than 98 contiguous voxels ( > 320mm3) were ignored. The cluster threshold was calculated using 3dClustSim, an Analysis of Functional NeuroImages program (Cox, 2010; http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html), with the uncorrected per-voxel p value set at .005, the corrected cluster alpha value set at .05 and the full width at half maximum Gaussian filter value set at 5.6 for only those voxels included in the region of interest mask.
3. Results
3.1. Behavioral data
Normally distributed data are presented as means (SD) and non normal data are presented as 20% trimmed means (20% Winsorized SD) (Field & Wilcox, 2017; Mair & Wilcox, 2019; R. Wilcox, 2017).
3.1.1. Participant characteristics
Comparisons between CMP and CO Veterans with independent samples t-tests and Yuen’s modified t-test for independent trimmed means revealed several significant differences for self-reported depression, anxiety, pain catastrophizing, and mental and physical health (Table 1). A descriptive group-by-condition breakdown of participant characteristics is also provided in Supplementary File 2.
Table 1.
Statistical comparison of mean (SD) questionnaire data between Gulf War Veterans with chronic musculoskeletal pain (CMP) and healthy control Gulf War Veterans (CO).
| CMP (N = 30) |
CO (N = 29) |
p | Effect size | 95% CId | |
|---|---|---|---|---|---|
| Age (years)a | 46.33 (4.77) | 45.32 (5.24) | .64 | 0.09 | 0, 0.45 |
| Sex (Male/Female)b | 27/3 | 25/4 | .71 | n/a | n/a |
| Body Mass Index (kg/m2)a | 28.49 (2.25) | 26.62 (2.92) | .10 | 0.30 | 0, 0.61 |
| Beck Depression Inventory (total score)a | 5.64 (2.08) | 0.53 (0.93) | < .05 | 0.86 | 0.73, 0.98 |
| State Trait Anxiety Inventory (form Y-2 score) | 31.4 (8.3)c | 26.5 (4.5) | .01 | 0.73 | 0.20, 1.27 |
| Pain Catastrophizing Scale (total score)a | 7.44 (4.3) | 3.63 (3.42) | .01 | 0.45 | 0.07, 0.73 |
| Medical Outcomes Survey Short-Form (physical health score)a | 40.51 (4.06) | 57.07 (1.59) | < .05 | 0.93 | 0.86, 0.96 |
| Medical Outcomes Survey Short-Form (mental health score) | 53.4 (7.9) | 56.8 (3.9) | .008 | −0.57 | −1.09, −0.05 |
| International Physical Activity Questionnaire (MET mins/week)a | 5328.89 (3001.44) | 4948.37 (3157.44) | .78 | 0.05 | 0, 0.49 |
Note. Between-group comparisons for continuous outcomes were made using independent-samples t-tests or Yuen’s modified t-test for independent trimmed means. Hedges’ g and Wilcox and Tian’s explanatory measure of effect size (ξ) effect sizes are used for independent-samples t-tests and Yuen’s modified t-test for independent trimmed means, respectively.
n/a = not applicable
Yuen’s modified t-test for independent trimmed means performed because of non normally distributed data in Chronic Pain or Healthy Control Group. These data are reported as 20% trimmed means and 20% Winsorized standard deviations rather than mean (SD).
Between-group difference examined with Fisher’s Exact Test
data missing for N = 1 participant.
95% confidence intervals for Hedges’ g and Wilcox and Tian’s ξ effect sizes are estimated based on recommendations from Cummings & Finch (2001) and Wilcox and Mair (2019), respectively.
3.1.2. Manipulation check
For thermal stimuli that were administered to participants in the ‘pain’ condition during the Day 1 expectancy manipulation, trimmed mean (Winsorized SD) pain ratings ranged from 2.28 (2.8) to 12.44 (1.86) for intensity and from 1.22 (1.94) to 9.16 (2.83) for unpleasantness (Supplementary File 3). Trimmed mean (Winsorized SD) intensity and unpleasantness ratings were all 0 for thermal stimuli that were administered to participants in the ‘no pain’ condition (Supplementary File 3). This confirmed that the amount of pain experienced during the expectancy manipulation was greater in the ‘pain’ condition relative to the ‘no pain’ condition.
The trimmed mean (Winsorized SD) ratings on the pain expectancy questionnaire were 4.34 (1.65) and 0.57 (0.82) for the ‘pain’ and ‘no pain’ conditions, respectively. Yuen’s modified t-test for independent trimmed means revealed that participants in the ‘pain’ condition anticipated experiencing significantly more pain compared to participants in the ‘no pain’ condition. The magnitude of the effect size indicated that the expectancy manipulation had a large effect on pain anticipation for participants in the ‘pain’ condition relative to the ‘no pain’ condition (ξ = 0.91, 95% CI: 0.72, 0.99).
3.1.3. Day 2 perceptual responses to non painful thermal stimuli
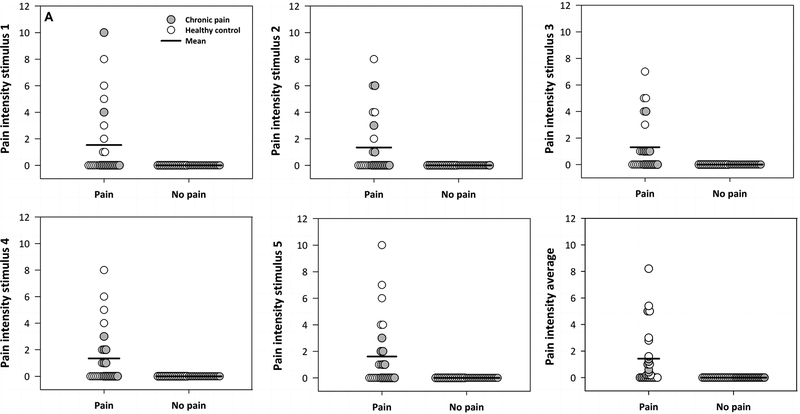
For participants in the ‘pain’ condition, trimmed mean (Winsorized SD) pain ratings across all five non painful thermal stimuli ranged from 0.44 (1.29) to 0.69 (1.26) for intensity and from 0.19 (0.47) to 0.25 (0.49) for unpleasantness (Table 2). Trimmed mean (Winsorized SD) intensity and unpleasantness ratings were all 0 for participants in the ‘no pain’ condition (Table 2). On average across all five non painful stimuli, 38.5% and 100% of participants provided a pain intensity rating of 0 in the ‘pain’ and ‘no pain’ groups, respectively. For unpleasantness ratings, 50% and 97% of participants in the ‘pain’ and ‘no pain’ conditions provided a rating of 0, respectively. Individual intensity and unpleasantness ratings for each stimulus and average ratings across all five stimuli are illustrated in Figure 3. A series of Yuen’s modified t-tests for independent trimmed means revealed that the amount of time (sec) used to provide pain ratings for each stimulus did not significantly differ between conditions (all p > .11).
Table 2.
Trimmed mean (Winsorized SD) pain ratings and rating durations in ‘no pain’ (control) and ‘pain’ (experimental) groups for five non painful thermal stimuli administered during Day 2 experimental functional magnetic resonance imaging scan.
| Stimulus | 1 | 2 | 3 | 4 | 5 | Average |
|---|---|---|---|---|---|---|
| No pain condition (N = 33) | ||||||
| Intensity | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Unpleasantness | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rating period (sec) | 8.1 (1.41) | 7.54 (1.41) | 7.72 (1.14) | 7.31 (1.28) | 7.08 (1.29) | 7.41 (1.2) |
| Pain condition (N = 26) | ||||||
| Intensity | 0.44 (1.29) | 0.44 (1.29) | 0.56 (1.23) | 0.56 (0.92) | 0.69 (1.26) | 0.66 (1.14) |
| Unpleasantness | 0.19 (0.47) | 0.19 (0.86) | 0.25 (0.49) | 0.19 (0.47) | 0.19 (0.47) | 0.41 (0.69) |
| Rating period (sec) | 9.01 (1.23) | 7.56 (1.42) | 7.59 (1.57) | 7.39 (1.34) | 7.61 (1.42) | 7.6 (0.85) |
Note. Trimmed means for pain intensity and unpleasantness ratings were all higher in the ‘pain’ condition compared to the ‘no pain’ condition for each 40°C stimulus administration and when ratings were averaged across all five stimuli. A series of Yuen’s modified t-tests for independent trimmed means revealed that the amount of time (sec) used to provide pain ratings did not significantly differ between conditions (all p > .11).
Figure 3. Between-condition comparison of pain ratings across five sequential non painful stimuli (40°C).
Panel A depicts pain intensity ratings and Panel B depicts pain unpleasantness ratings for participants in the ‘pain’ and ‘no pain’ conditions across five repeated exposures to non painful thermal stimuli. These figures illustrate that there was a greater amount of variability in the ‘pain’ condition compared to the ‘no pain’ condition in terms of perceptual responses to the non painful thermal stimuli.
3.2. Neural responses to non painful thermal stimuli
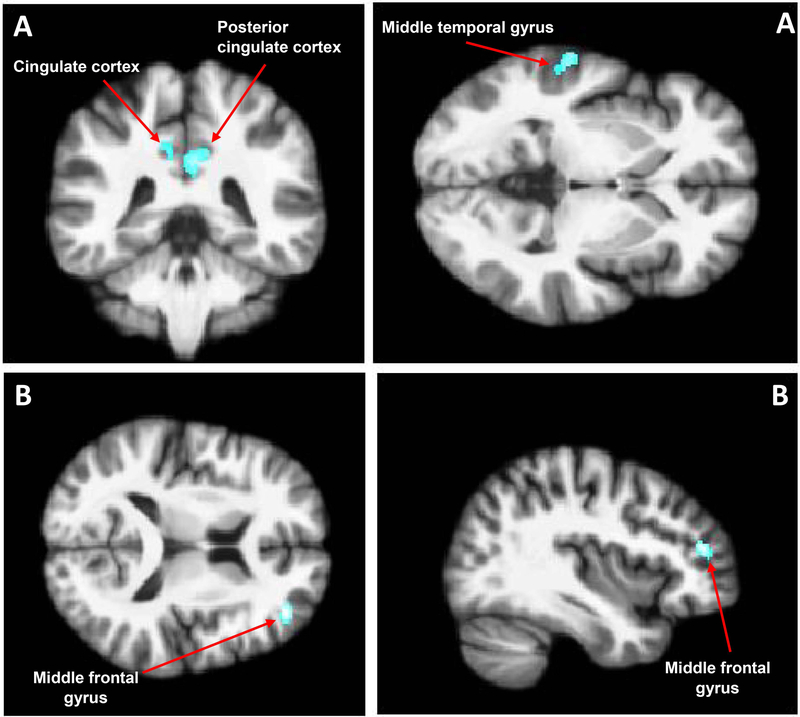
Factorial ANOVA for the linear decrease contrast revealed a significant main effect of condition in the right posterior cingulate cortex (p < .001), left cingulate cortex (p < .001), and left middle temporal gyrus (p = .001) in the ‘pain’ condition (Figure 4A), indicating that in these regions, a model of a linear decrease in activity across thermal stimuli better fit the responses of individuals in the ‘pain’ condition compared to the ‘no pain’ condition. Further, there was a significant group-by-condition interaction in the right middle frontal gyrus (p < .001) (Figure 4B). This interaction appeared to be driven by CMP Veterans in the ‘pain’ condition as they were the only group to exhibit an incremental linear decrease in brain activity across the thermal stimuli in this region. No main or interaction effects were found for the linear increase contrast. These results are detailed in Table 3.
Figure 4. Neural responses across five sequential non painful stimuli (40°C).
A two-way full factorial ANOVA of linear trends in blood oxygen level dependent (BOLD) responses across five non painful thermal stimuli revealed a significant main effect of condition and a group-by-condition interaction on (p < .005; cluster threshold > 320mm3). Panel A shows that participants in the experimental ‘pain’ condition displayed a significant incremental linear decrease in the right posterior cingulate cortex, left cingulate cortex, and left middle temporal gyrus compared to participants in the ‘no pain’ control condition. Panel B shows that, in the ‘pain condition’, participants with chronic musculoskeletal pain displayed a significant incremental linear decrease in the middle frontal gyrus compared healthy control participants.
Table 3.
Results from full factorial Group (CMP, CO) by Condition (pain, no pain) analysis of incremental linear decreases in brain activity across five non painful thermal stimuli
| Region in focus point | Hemisphere | Peak MNI coordinates | Number of voxels in cluster | T | p | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Main effect: Condition (no pain < pain) | |||||||
| Posterior Cingulate Cortex | Right | 4 | −54 | 30 | 1382 | 3.77 | < .001 |
| Cingulate Cortex | Left | −9 | −46 | 28 | 3.66 | < .001 | |
| Posterior Cingulate Cortex | Right | 4 | −45 | 28 | 3.58 | < .001 | |
| Middle Temporal Gyrus | Left | −62 | −30 | 4 | 207 | 3.37 | .001 |
| Interaction effect | |||||||
| Middle Frontal Gyrus | Right | 38 | 42 | 16 | 147 | 3.50 | < .001 |
Note: CMP: chronic musculoskeletal pain; CO: healthy control; no pain: participants who were reassured that they would only receive warm, non painful stimuli during scanning; pain: participants who were led to believe that they would receive painful thermal stimuli during scanning.
3.3. Neural responses during perceptual ratings of non painful thermal stimuli
Analysis of the average brain response during pain ratings that followed each non painful thermal stimulus revealed a significant main effect of condition and group-by-condition interaction. These results are detailed in Supplementary File 4.
4. Discussion
This study examined the effect of pain anticipation on neural and perceptual responses to non painful stimuli in Gulf War Veterans with medically unexplained CMP. By experimentally manipulating expectations through a psychophysical procedure that was supplemented with exposure to real world cues during study participation (i.e., explicit language used in informed consent documents, interactions with study personnel), we showed that neural responses to non painful thermal stimuli were affected by whether a participant was led to expect having a painful or non painful experience during an fMRI scan. We also observed that, in participants who were led to believe that they would have a painful experience, brain responses to non painful stimuli differed between CMP patients and healthy participants. Although there was not enough variability in perceptual responses to the non painful stimuli to conduct a formal statistical analysis of differences between conditions or groups, Figure 3 clearly illustrates that pain ratings for some individual participants were affected by the experimental manipulation (Figure 3).
The methodological approach used here may limit comparability to prior studies of healthy adult samples that used more rigorous nocebo conditioning models to examine the effect of pain anticipation on brain activity (Atlas et al., 2010; Keltner, 2006; Reicherts et al., 2016, 2017; Wiech et al., 2010). However, our findings do generally agree with the wider body of research showing that both neural and perceptual responses to somatosensory stimuli are affected by expectations. Below, we interpret these results in the context of prior experimental pain studies that manipulated expectations and discuss their broader implications for studies of central nervous system dysfunction in medically unexplained CMP.
4.1. Pain anticipation altered brain activity
Compared to the ‘no pain’ control condition, participants in the experimental ‘pain’ condition showed a significant incremental linear decrease in brain activity across thermal stimuli (Table 3). One explanation for why brain activity decreased rather than increased over repeated stimulus administrations is that expectations for pain were highest prior to the initial stimulus and then subsequently decreased as participants learned that the thermal stimuli were not painful. Thus, is it possible that the observed pattern of decreased brain activity was closely related to decreases in expectations for pain across the five thermal stimuli. This interpretation is supported by prior work showing that expectations can change across repeated exposures to painful stimuli (Corsi & Colloca, 2017) and that brain responses to painful stimuli can depend on the magnitude of expected pain (Keltner, 2006; Koyama, McHaffie, Laurienti, & Coghill, 2005). Unlike previous studies, we are unable to test for decreases in expectations across stimulus exposures because we did not measure expectations at multiple time-points or experimentally manipulate the level of expectations. Conforming with recent expert recommendations on measuring expectations (Kirsch, 2018), we agree that performing measures at multiple time-points may help account for the dynamic nature of expectations and clarify the extent to which they change over the course of a given experiment.
4.2. Anticipation differentially affected brain activity for Veterans with chronic pain
A novel feature of this study is that we explored the effect of negative expectations on brain activity in Veterans with medically unexplained CMP. We found that, in the ‘pain’ condition, a model of a linear decrease in activity in the middle frontal gyrus across thermal stimuli better fit the responses of CMP Veterans relative to CO Veterans. Prior experimental pain studies have observed altered activity in this brain region during painful stimulation but not during non painful stimulation or pain anticipation (i.e., immediately prior to stimulus administration). A study of healthy adults by Kong et al. (2010) showed increases in middle frontal gyrus activity during low (~5 on the 0–20 Gracely intensity scale) and high (~15 on the 0–20 Gracely intensity scale) experimental pain stimulation (Kong et al., 2010). Conversely, Cook and colleagues did not observe differential middle frontal gyrus activity between fibromyalgia patients and healthy controls during the presentation of non painful thermal stimuli (Cook et al., 2004). Differences in middle frontal gyrus activity between fibromyalgia patients and healthy controls were also not observed during the anticipatory phase of experimental pain (Burgmer et al., 2011), but activity in this region during painful stimulation has been positively associated with pain catastrophizing in fibromyalgia patients (Gracely, 2004). Based on these prior experimental pain studies and other work showing middle frontal gyrus activity differences between chronic back pain patients and healthy controls during an attention task (Tagliazucchi, Balenzuela, Fraiman, & Chialvo, 2010), we speculate that middle frontal gyrus activity may be especially prominent in medically unexplained pain patients when attention toward pain is heightened by the psychological state of the participant. To help account for this possibility in future experimental pain studies involving CMP patients, investigators may consider measuring participant expectations in addition to other psychological factors related to attention towards pain (Morton, Jones, & Sandhu, 2016).
Interestingly, the linear decrease in BOLD signal that was observed for CMP patients could indicate that these Veterans had a stronger habituation response than CO Veterans. This finding is somewhat contrary to a prior electroencephalography study by Montoya and colleagues which instead reported a decrease in brain activity across repeated non painful stimuli in healthy adults rather than fibromyalgia patients (Montoya et al., 2006). Thus, it is possible that pain habituation is not similarly compromised in Gulf War Veterans with medically unexplained CMP and fibromyalgia patients. However, there were a number of methodological differences that limit comparability between the present study and the study by Montoya and colleagues, including the number of stimulus exposures, type of stimulus that was administered, and neuroimaging technology that was used to measure brain responses. Moreover, our study manipulated pain anticipation whereas the study by Montoya and colleagues did not. Therefore, the study design used by Montoya and colleagues may have allowed for a more precise test of habituation because brain responses to non painful stimuli were influenced to a lesser extent by variability related to pain anticipation. A future study that directly compares habituation and sensitization responses to repeated stimuli between Gulf War Veterans with medically unexplained CMP and fibromyalgia patients may help clarify whether the discrepancy between our study and Montoya and colleagues is representative of a true difference between patient populations or more so related to differences in study design.
4.3. Pain anticipation introduced variability in perceptual ratings of non painful thermal stimuli
As part of our a priori hypothesis, we predicted that pain ratings of non painful stimuli would be higher in the ‘pain’ condition. Furthermore, we expected that in the ‘pain’ condition, Veterans with CMP would show elevated pain ratings relative to CO Veterans. Although the experimental manipulation clearly had an effect on inter-individual variability in the pain group (Figure 3), the high frequency of participants in the ‘pain’ and ‘no pain’ conditions rated the thermal stimuli as 0 limited our ability to conduct a formal statistical analysis to test these hypotheses. Thus, for future investigators who attempt to test the effect of pain anticipation on perceptual responses, the trade-off between administering a stimulus intensity that is low enough to isolate the effect of expectations on pain ratings (Colloca et al., 2008) but high enough to elicit variability in perceptual responses is a worthwhile consideration.
In light of prior work showing differences in pain ratings between CMP and CO Veterans in response to thermal stimuli ranging between 44–50°C (Cook et al., 2010), it is possible that higher stimulus intensities are needed before the impact of pain anticipation on perceptual differences between CMP and CO Veterans can be observed. Despite showing that perceptual responses to non painful stimuli can be affected by pain anticipation, the present study did not directly test the influence of pain anticipation on painful stimuli. Addressing this gap may be of value for drawing interpretations from experimental pain fMRI studies involving medically unexplained CMP patients, which have more commonly measured responses to higher stimulus temperatures than temperatures that are non painful (Cook et al., 2004; Ellingson et al., 2016; Gopinath et al., 2012).
4.4. Limitations
The findings should be interpreted in the context of several potential limitations. First, the study sample included Veterans of the 1991 Gulf War and more recent 2003 Iraq War. However, we controlled for this potential confound by matching the CMP and CO groups on deployment status. Nonetheless, caution should be taken when generalizing the study results to the at-large population of 1991 Gulf War Veterans with chronic pain. Second, we excluded a number of medical conditions that are comorbid with CMP which may also reduce the generalizability to other chronic pain populations with one or more comorbid chronic conditions. Ruling out other chronic conditions is a significant challenge when studying patients with medically unexplained CMP, but the strict exclusionary strategy used for our participant sample was designed to reduce heterogeneity and control for conditions that are known to influence brain structure and function. Third, a majority of the study sample was male, which may preclude comparisons to other medically unexplained CMP conditions with a higher prevalence in females such as fibromyalgia (Jones et al., 2015). Finally, these results should be confirmed in a larger sample before more definitive conclusions about the effect of negative expectations on brain activity and pain perception can be made in Gulf War Veterans with CMP.
5. Conclusion
The present study provides a direct example of how participant expectations could potentially influence the results of a pathophysiological study of medically unexplained CMP. The finding that pain anticipation elicited differential brain responses between CMP and CO Veterans suggests that negative expectations are a potential source of variability in studies of central nervous system dysfunction in Veterans with medically unexplained CMP. Future investigations of chronic pain conditions with unknown pathophysiology may consider how best to design their own studies to reduce or account for the impact of participant expectations.
Supplementary Material
Acknowledgements
The contents do not represent the views of the Department of Veterans Affairs or the United States Government. The authors would like to thank the participants for volunteering for the study. We would also like to thank Neda Almassi and Jake Ninneman for their assistance with editing the manuscript content. Finally, we would like to thank the reviewers for their thoughtful comments throughout the peer review process.
Funding
This study was supported by Dept. of Veterans Affairs grant: 561–00436 and NIAM/NIH AR50969 (D.B. Cook, PI). Jacob Lindheimer was supported by Career Development Award Number IK2 CX001679 from the United States (U.S.) Department of Veterans Affairs Clinical Sciences R&D (CSR&D) Service. Ryan J. Dougherty was supported by a National Research Service Award from the National Institute on Aging of the National Institutes of Health under Award Number F31AG062009.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Atlas LY, Bolger N, Lindquist MA, & Wager TD (2010). Brain mediators of predictive cue effects on perceived pain. Journal of Neuroscience, 30(39), 12964–12977. 10.1523/JNEUROSCI.0057-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Beck depression inventory manual. San Antonio, TX: The Psychological Corp. [Google Scholar]
- Burgmer M, Petzke F, Giesecke T, Gaubitz M, Heuft G, & Pfleiderer B (2011). Cerebral activation and catastrophizing during pain anticipation in patients with fibromyalgia. Psychosomatic Medicine, 73(9), 751–759. 10.1097/PSY.0b013e318236588a [DOI] [PubMed] [Google Scholar]
- Cohen J (1992). A power primer. Psychological Bulletin, 112(1), 155–159. 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- Colloca L, & Grillon C (2014). Understanding placebo and nocebo responses for pain management. Current Pain and Headache Reports, 18(6), 419 10.1007/s11916-014-0419-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, Sigaudo M, & Benedetti F (2008). The role of learning in nocebo and placebo effects: Pain, 136(1), 211–218. 10.1016/j.pain.2008.02.006 [DOI] [PubMed] [Google Scholar]
- Cook DB, Lange G, Ciccone DS, Liu W-C, Steffener J, & Natelson BH (2004). Functional imaging of pain in patients with primary fibromyalgia. The Journal of Rheumatology, 31(2), 364–378. [PubMed] [Google Scholar]
- Cook DB, Stegner AJ, & Ellingson LD (2010). Exercise alters pain sensitivity in gulf war veterans with chronic musculoskeletal pain. The Journal of Pain, 11(8), 764–772. 10.1016/j.jpain.2009.11.010 [DOI] [PubMed] [Google Scholar]
- Corsi N, & Colloca L (2017). Placebo and nocebo effects: The advantage of measuring expectations and psychological factors. Frontiers in Psychology, 8 10.3389/fpsyg.2017.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, … Oja P (2003). International physical activity questionnaire: 12-country reliability and validity. Medicine & Science in Sports & Exercise, 35(8), 1381–1395. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- Cumming G, & Finch S (2001). A primer on the understanding, use, and calculation of confidence intervals that are based on central and noncentral distributions. Educational and Psychological Measurement, 61(4), 532–574. 10.1177/0013164401614002 [DOI] [Google Scholar]
- Dursa EK, Barth SK, Schneiderman AI, & Bossarte RM (2016). Physical and mental health status of gulf war and gulf era veterans: Results from a large population-based epidemiological study. Journal of Occupational and Environmental Medicine, 58(1), 41–46. 10.1097/JOM.0000000000000627 [DOI] [PubMed] [Google Scholar]
- Ellingson LD, Shields MR, Stegner AJ, & Cook DB (2012). Physical activity, sustained sedentary behavior, and pain modulation in women with fibromyalgia. J Pain, 13(2), 195–206. 10.1016/j.jpain.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson LD, Stegner A, Schwabacher I, Koltyn K, & Cook D (2016). Exercise strengthens central nervous system modulation of pain in Fibromyalgia. Brain Sciences, 6(1), 8 10.3390/brainsci6010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field AP, & Wilcox R (2017). Robust statistical methods: A primer for clinical psychology and experimental psychopathology researchers. Behaviour Research and Therapy, 98, 19–38. 10.1016/j.brat.2017.05.013 [DOI] [PubMed] [Google Scholar]
- Fritz CO, Morris PE, & Richler JJ (2012). Effect size estimates: Current use, calculations, and interpretation. Journal of Experimental Psychology: General, 141(1), 2–18. 10.1037/a0024338 [DOI] [PubMed] [Google Scholar]
- Gollub RL, Kirsch I, Maleki N, Wasan AD, Edwards RR, Tu Y, … Kong J (2018). A functional neuroimaging study of expectancy effects on pain response in patients with knee osteoarthritis. The Journal of Pain, 19(5), 515–527. 10.1016/j.jpain.2017.12.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath K, Gandhi P, Goyal A, Jiang L, Fang Y, Ouyang L, … Haley R (2012). FMRI reveals abnormal central processing of sensory and pain stimuli in ill Gulf War veterans. NeuroToxicology, 33(3), 261–271. 10.1016/j.neuro.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracely RH (2004). Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain, 127(4), 835–843. 10.1093/brain/awh098 [DOI] [PubMed] [Google Scholar]
- Gracely RH, & Dubner R (1987). Reliability and validity of verbal descriptor scales of painfulness. Pain, 29(2), 175–185. 10.1016/0304-3959(87)91034-7 [DOI] [PubMed] [Google Scholar]
- Gracely RH, McGrath P, & Dubner R (1978). Ratio scales of sensory and affective verbal pain descriptors. Pain, 5(1), 5–18. 10.1016/0304-3959(78)90020-9 [DOI] [PubMed] [Google Scholar]
- Gracely RH, Petzke F, Wolf JM, & Clauw DJ (2002). Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis & Rheumatism, 46(5), 1333–1343. 10.1002/art.10225 [DOI] [PubMed] [Google Scholar]
- Iversen A, Chalder T, & Wessely S (2007). Gulf War Illness: Lessons from medically unexplained symptoms. Clinical Psychology Review, 27(7), 842–854. 10.1016/j.cpr.2007.07.006 [DOI] [PubMed] [Google Scholar]
- Jones GT, Atzeni F, Beasley M, Flüß E, Sarzi-Puttini P, & Macfarlane GJ (2015). The prevalence of fibromyalgia in the general population: a comparison of the american college of rheumatology 1990, 2010, and modified 2010 classification criteria: Prevalence of fibromyalgia. Arthritis & Rheumatology, 67(2), 568–575. 10.1002/art.38905 [DOI] [PubMed] [Google Scholar]
- Kang HK, Mahan CM, Lee KY, Magee CA, & Murphy FM (2000). Illnesses among united states veterans of the gulf war: a population-based survey of 30,000 veterans. Journal of Occupational and Environmental Medicine, 42(5), 491–501. 10.1097/00043764-200005000-00006 [DOI] [PubMed] [Google Scholar]
- Keltner JR (2006). Isolating the modulatory effect of expectation on pain transmission: A functional magnetic resonance imaging study. Journal of Neuroscience, 26(16), 4437–4443. 10.1523/JNEUROSCI.4463-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I (2018). Response expectancy and the placebo effect. International Review of Neurobiology, 138, 81–93. https://doi.org/DOI: 10.1016/bs.irn.2018.01.003 [DOI] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Polich G, Kirsch I, LaViolette P, Vangel M, … Kaptchuk TJ (2008). A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. Journal of Neuroscience, 28(49), 13354–13362. 10.1523/JNEUROSCI.2944-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Loggia ML, Zyloney C, Tu P, LaViolette P, & Gollub RL (2010). Exploring the brain in pain: Activations, deactivations and their relation: Pain, 148(2), 257–267. 10.1016/j.pain.2009.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Wang Z, Leiser J, Minicucci D, Edwards R, Kirsch I, … Gollub RL (2018). Enhancing treatment of osteoarthritis knee pain by boosting expectancy: A functional neuroimaging study. NeuroImage: Clinical, 18, 325–334. 10.1016/j.nicl.2018.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, & Gollub RL (2006). Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Human Brain Mapping, 27(9), 715–721. 10.1002/hbm.20213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, McHaffie JG, Laurienti PJ, & Coghill RC (2005). The subjective experience of pain: Where expectations become reality. Proceedings of the National Academy of Sciences, 102(36), 12950–12955. 10.1073/pnas.0408576102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair P, & Wilcox R (2019). Robust statistical methods in R using the WRS2 package. Behavior Research Methods 10.3758/s13428-019-01246-w [DOI] [PubMed] [Google Scholar]
- McLoughlin MJ, Stegner AJ, & Cook DB (2011). The relationship between physical activity and brain responses to pain in fibromyalgia. The Journal of Pain, 12(6), 640–651. 10.1016/j.jpain.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya P, Sitges C, García-Herrera M, Rodríguez-Cotes A, Izquierdo R, Truyols M, & Collado D (2006). Reduced brain habituation to somatosensory stimulation in patients with fibromyalgia. Arthritis & Rheumatism, 54(6), 1995–2003. 10.1002/art.21910 [DOI] [PubMed] [Google Scholar]
- Morton D, Jones A, & Sandhu J (2016). Brain imaging of pain: State of the art. Journal of Pain Research, Volume 9, 613–624. 10.2147/JPR.S60433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo S, Benedetti F, Costa T, & Amanzio M (2015). Pain anticipation: An activation likelihood estimation meta-analysis of brain imaging studies. Human Brain Mapping, 36(5), 1648–1661. 10.1002/hbm.22727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2013). R: A language and environment for statistical computing. Retrieved from http://www.R-project.org/ [Google Scholar]
- Reicherts P, Gerdes ABM, Pauli P, & Wieser MJ (2016). Psychological placebo and nocebo effects on pain rely on expectation and previous experience. The Journal of Pain, 17(2), 203–214. 10.1016/j.jpain.2015.10.010 [DOI] [PubMed] [Google Scholar]
- Reicherts P, Wiemer J, Gerdes ABM, Schulz SM, Pauli P, & Wieser MJ (2017). Anxious anticipation and pain: The influence of instructed vs conditioned threat on pain. Social Cognitive and Affective Neuroscience, 12(4), 544–554. 10.1093/scan/nsw181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto N, Honda M, Okada T, Hanakawa T, Kanda M, Fukuyama H, … Shibasaki H (2000). Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: An event-related functional magnetic resonance imaging study. Journal of Neuroscience, 20(19), 7438–7445. 10.1523/JNEUROSCI.20-19-07438.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BW, Tooley EM, Montague EQ, Robinson AE, Cosper CJ, & Mullins PG (2008). Habituation and sensitization to heat and cold pain in women with fibromyalgia and healthy controls: Pain, 140(3), 420–428. 10.1016/j.pain.2008.09.018 [DOI] [PubMed] [Google Scholar]
- Smith TC, Powell TM, Jacobson IG, Smith B, Hooper TI, Boyko EJ, & Gackstetter GD (2014). Chronic multisymptom illness: A comparison of iraq and afghanistan deployers with veterans of the 1991 gulf war. American Journal of Epidemiology, 180(12), 1176–1187. 10.1093/aje/kwu240 [DOI] [PubMed] [Google Scholar]
- Spielberger CD (1983). Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Sullivan MJ, Bishop SR, & Pivik J (1995). The pain catastrophizing scale: Development and validation. Psychological Assessment, 7(4), 524–532. 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- Tagliazucchi E, Balenzuela P, Fraiman D, & Chialvo DR (2010). Brain resting state is disrupted in chronic back pain patients. Neuroscience Letters, 485(1), 26–31. 10.1016/j.neulet.2010.08.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I, & Mantyh PW (2007). The cerebral signature for pain perception and its modulation. Neuron, 55(3), 377–391. 10.1016/j.neuron.2007.07.012 [DOI] [PubMed] [Google Scholar]
- Van Riper SM, Alexander AL, Koltyn KF, Stegner AJ, Ellingson LD, Destiche DJ, … Cook DB (2017). Cerebral white matter structure is disrupted in gulf war veterans with chronic musculoskeletal pain. Pain, 158(12), 2364–2375. 10.1097/j.pain.0000000000001038 [DOI] [PubMed] [Google Scholar]
- Ware JE, & Sherbourne CD (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care, 30(6), 473–483. [PubMed] [Google Scholar]
- Wiech K, Lin C.-s., Brodersen KH, Bingel U, Ploner M, & Tracey I (2010). Anterior insula integrates information about salience into perceptual decisions about pain. Journal of Neuroscience, 30(48), 16324–16331. 10.1523/JNEUROSCI.2087-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox R (2017). Introduction to robust estimation and hypothesis testing (4th ed.). Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- Wilcox RR, & Tian TS (2011). Measuring effect size: A robust heteroscedastic approach for two or more groups. Journal of Applied Statistics, 38(7), 1359–1368. 10.1080/02664763.2010.498507 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.