Abstract
Introduction:
DSM421, a dihydroorotate dehydrogenase inhibitor, was in preclinical development as a potential treatment option for malaria. When tested in a core battery of safety pharmacology assays, DSM421 did not produce any effects at oral doses up to 750 mg/kg in an Irwin test in rats, but a respiratory study in rats using head-out plethysmography resulted in substantial changes in respiratory function as well as moribundity and mortality at that and lower doses. An investigation was performed to determine the source of this discrepancy.
Methods:
Potential testing errors, differences in types of plethysmography testing chambers, effects on stress indicators, and off-target activity were investigated.
Results:
Respiratory changes and toxicity (resulting in euthanasia in extremis) were confirmed in a repeat, head-out plethysmography test, but the effects of DSM421 were much less severe overall when the rats were tested in whole-body chambers. Additionally, at the end of the 5-hour post-dosing respiratory monitoring periods, levels of stress-related hormones (particularly corticosterone) were higher overall in the head-out, than in the whole- body, tested rats. Furthermore, DSM421 was found to produce changes in cardiovascular function in unrestrained rats, and it was shown to have off-target binding affinity at the adenosine A3 receptor (which is associated with bronchoconstriction).
Discussion:
The generalized stress inherent to head-out plethysmography testing exacerbated the respiratory effects of DSM421 and was possibly compounded by DSM421’s cardiovascular effects, thus artifactually resulting in moribundity and mortality in rats. Care should be taken when choosing whether to use head-out versus whole-body plethysmography chambers during respiratory function testing in animals.
Keywords: Adenosine A3 Receptor, Cardiovascular, DSM421, Methods, Plethysmography, Rat, Respiratory, Restraint, Safety Pharmacology, Stress
1. Introduction
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guidance document entitled “S7A Safety Pharmacology Studies for Human Pharmaceuticals” recommends testing the effects of pharmaceutical compounds on vital functions in laboratory animals prior to first-in-human studies (ICH, 2001). The cardiovascular, respiratory, and central nervous systems are considered to be the three vital organ systems that should be examined in a “core battery” of safety pharmacology assays. For assessing potential effects on respiratory function, plethysmography is the most common assay performed during the evaluation of drug candidates, with some laboratories performing such testing in rodents using a whole-body technique and other laboratories using a head-out procedure (Harris et al., 2005; Nirogi et al., 2012). Both plethysmography methods measure chamber pressure to determine changes in ventilatory function, which most often includes the parameters of respiratory rate, tidal volume and minute volume, but can also include other outputs such as inspiratory/expiratory time and peak inspiratory/expiratory flow. With the whole-body plethysmography method, the animals are free to move about the test chamber, and the changes in chamber pressure occur due to the respired air having been warmed and humidified in the animals’ lungs. With the head-out method, the animals are restrained due to a sealed neck collar, and the changes in chamber pressure occur due to thoracic cavity movement.
DSM421 is a plasmodial dihydroorotate dehydrogenase inhibitor that was being investigated for the prevention and treatment of malaria (Phillips et al., 2016). As part of its nonclinical development plan, a core battery of safety pharmacology assays was conducted under Good Laboratory Practice (GLP) regulations, all at the same laboratory. Within this core package, a combination of cardiovascular and respiratory assessment in telemetry- instrumented dogs dosed with 30 and 100 mg/kg, p.o. (estimated maximum concentrations [Cmax] of DSM421 in plasma of 29 and 69 μg/mL, respectively), found no effects on cardiovascular function (heart rate, blood pressure, and ECG) or the respiratory parameter of tidal volume at either dose, but the data showed increases in respiratory rate and minute volume of approximately 4- to 5-fold as well as emesis at 100 mg/kg. The lower dose of 30 mg/kg was determined to be the no-observed-effect level (NOEL) in dogs. To assess central nervous system (CNS) function, an Irwin test was performed in rats at oral doses of 200, 500 and 750 mg/kg (estimated Cmax of 49, 73 and 97 μg/mL, respectively) and no effects were observed. Respiratory function was also tested in rats, at these same doses and with the same dosing parameters, using head-out plethysmography. Changes in respiratory function were observed (significant increases in respiratory rate, tidal volume and minute volume of approximately 30 – 130%), with effects present even at the lowest dose of 200 mg/kg. In addition, in all three of the DSM421 dosing groups in this respiratory study (n=8 rats/group), 2 to 3 rats per group were either found dead or needed to be euthanized in extremis within 3 to 5 hours after administration.
An investigation was conducted to determine why moribundity and mortality were observed during the plethysmograph testing, but not during the Irwin testing, in rats. The investigation focused on employing the same doses to conduct a new respiratory study in rats using whole-body plethysmography and subsequently using the rats to repeat the head-out plethysmography study. Potential experimental errors, plethysmography methods (head-out versus whole-body), stress indicators (cardiovascular function in telemetry-instrumented rats; hormone levels), and off-target activity were examined.
2. Methods
2.1. DSM421 synthesis and administration
DSM421 (2-(1,1-difluoroethyl)-5-methyl-N-[6-trifluromethyl-3-pyridyl]- [1,2,4]triazolo[1,5-a]pyrimidin-7-amine) was synthesized by Manchester Organics (United Kingdom). Its purity, as assessed by high-performance liquid chromatography (HPLC), was 99.8% by area (98.8% by weight/weight). For the in vitro assays, DSM421 was dissolved in dimethyl sulfoxide (DMSO) to produce 10 or 33 mM stock solutions, which were then diluted 1000-fold or 330-fold to test concentrations of 10 or 100 μM (respectively) using aqueous buffered solutions. Therefore, test concentrations in all of the in vitro assays contained 0.1 – 0.3% DMSO. For the in vivo assays, DSM421 was suspended in 0.2% (for the cardiovascular study) or 0.5% (for all other studies) hydroxypropyl methylcellulose (HPMC) in water and was administered as a single dose, by oral gavage, at a dose volume of 10 mL/kg.
2.2. In vitro assays (off-target activity)
A battery of in vitro binding, enzyme, and uptake assays was performed on 112 receptors, enzymes, ion channels and transporters to examine potential off-target activity of DSM421. The initial screening concentration was 10 μM and follow-up testing was at 100 μM. In addition, in vitro functional assays for the agonist and antagonist effects of DSM421 on the transient receptor potential cation channel subfamily V member 1 (TRPV1) were performed at 100 μM. For the binding assays, the results were expressed as the percent inhibition of control specific binding obtained in the presence of DSM421. For the enzyme and uptake assays, the results were expressed as the percent inhibition of control values or the percent stimulation relative to controls. For the functional assays, the results were expressed as the percent of control agonist response (for agonist function) or as the percent inhibition of control agonist response (for antagonist function).
2.3. In vivo assays
2.3.1. Test system
Male Sprague Dawley rats (approximately 9 – 12 weeks of age [290 – 470 grams of body weight] at the time of testing) were obtained from Charles River Laboratories Inc. (Raleigh, North Carolina, U.S.A.) and were housed in a temperature- and humidity-regulated environment with fluorescent lighting provided via an automatic timer for 12 hours per day (approximately from 06:00 to 18:00 hours). All animals were allowed a minimum of 7 days for acclimation from the date of their arrival at the testing facilities until first testing. Food and water were available ad libitum (except during the respiratory monitoring periods) and the animals were not fasted prior to dosing. Dosing groups for all of the assays consisted of four to eight rats. All animal studies were conducted in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC), and all protocols were approved by the Institutional Animal Care and Use Committee (IACUC).
The same set of rats was used for pharmacokinetics and respiratory testing, with the pharmacokinetics testing occurring first and then, after a 15-day washout period, the first respiratory testing. A separate set of rats was used for cardiovascular function testing.
2.3.2. Pharmacokinetics
Rats were dosed orally with 0 (vehicle), 200, 500 or 750 mg/kg DSM421 (n=4 rats/group), and blood samples were collected at 2, 6, 12, 24, 48 and 144 hours after dosing for the determination of DSM421 plasma levels. Blood samples of approximately 0.2 mL of volume were obtained from the sublingual vein and were collected into tubes containing K3-EDTA as the anticoagulant. The blood samples were centrifuged and the resultant plasma samples were analyzed using a validated liquid chromatography (LC)-mass spectrometry (MS)/MS method.
2.3.3. Respiratory function
Two GLP respiratory function studies were conducted, both at the same laboratory, which was different from the laboratory that conducted the original head-out plethysmography testing and the Irwin testing that was described in the Introduction section above. For the currently described studies, the whole-body plethysmography study was conducted first, and then the head-out plethysmography study was conducted in the same set of rats with a 9-day washout period between studies. Rats were dosed orally with 0 (vehicle), 200, 500 or 750 mg/kg DSM421 with n = 8 rats/group, except in the head-out plethysmography study for the high-dose group that was limited to n = 4 rats due to the severe toxicity observed in all four of the animals tested. Respiratory rate and tidal volume were measured, and minute volume was calculated from these values, using Ponemah Physiology Platform (Data Sciences International [DSI], Saint Paul, Minnesota, U.S.A.).
2.3.3.1. Whole-body plethysmography
Respiratory monitoring was performed using whole-body plethysmograph chambers (Buxco, DSI, Saint Paul, Minnesota, U.S.A.) that allowed the rats free movement within the testing chambers. Each animal was singly placed into a chamber for at least 2.5 hours prior to dosing to allow for sufficient acclimation (no other acclimation had been performed prior to this period) and to collect pre-dosing respiratory data, after which time they were temporarily removed for dosing. Immediately after dosing, the animals were returned to the plethysmograph chambers and respiration was continuously monitored for a period of approximately 5 hours. Although the time to maximum plasma concentration (Tmax) was subsequently determined to be greater than 5 hours at all doses tested (see Section 3.3), the plasma concentrations at 5 hours post-dosing were estimated to have been within approximately 80% of those at Tmax.
2.3.3.2. Head-out plethysmography
During the 9-day washout period after the whole-body plethysmography testing had been conducted, all animals were conditioned to head-out, neck-sealed, plethysmograph chambers (Buxco, DSI, Saint Paul, Minnesota, U.S.A.) during 3 acclimation sessions of extended durations, with a restraint of 5 hours minimum during the last session. On the day of dosing, each animal was singly placed into a head-out plethysmograph chamber for at least 1.5 hours prior to dosing to allow for sufficient acclimation and to collect pre-dosing respiratory data, after which time they were temporarily removed for dosing. Immediately after dosing, the animals were returned to the plethysmograph chambers and respiration was continuously monitored for a period of approximately 5 hours.
2.3.4. Stress-related hormones
During the whole-body and head-out plethysmography testing described above, blood samples were collected from the rats pre-dosing (i.e., prior to the whole-body study at approximately 10:00 hours) and at approximately 5 hours post-dosing (i.e., at the end of 5- hour plethysmography testing sessions for both methods at approximately 15:00 hours) to measure levels of stress-related hormones. Due to circadian variation in stress hormone levels, with minimum blood concentrations at the beginning of the lights-on period and maximum blood concentrations just prior to the start of the lights-off period (Atkinson and Waddell, 1997), natural levels of these stress hormones should have been relatively low at the pre-dosing sampling time point and relatively high at the 5 hours post-dosing sampling time point. Therefore, care was taken to keep the scheduling of all rats similar such that each testing procedure occurred at approximately the same time of the day for each rat.
At the time points described above, approximately 0.5 mL of blood was collected from the sublingual vein for each of the three hormone analytes. Adrenocorticotropic hormone (ACTH) concentrations were determined in plasma using a qualified Immulite solid-phase chemiluminescent immunoassay with a lower limit of quantification (LLOQ) of 10.0 pg/mL. Aldosterone and corticosterone concentrations were determined in serum using qualified solid phase enzyme-linked immunosorbent assays (ELISAs) with LLOQs of 15.0 pg/mL and 16.1 ng/mL, respectively. For data summary purposes, samples with corticosterone levels below its LLOQ were artificially assigned a value of 16.0 ng/mL. None of the values for the other two hormones were below their LLOQs in any of the samples tested.
2.3.5. Cardiovascular function
Rats were anesthetized with sevoflurane (AbbVie, Inc.; North Chicago, Illinois, U.S.A.), placed on a heating pad, and sterilely implanted with dual-pressure catheter telemetry transmitters (model HD-S21; DSI, St. Paul, Minnesota, U.S.A.). One catheter was inserted into the abdominal aorta to measure peripheral blood pressure; the other was inserted into the left ventricle of the heart to record left ventricular pressure. The body of the transmitter was located to the intraperitoneal cavity. The abdominal wall was then sutured and the skin was closed by using sterile wound clips. Buprenorphine (0.1 mg/kg, s.c.; Buprenex, Phoenix Pharmaceuticals, Belmont, California, U.S.A.) was administered over the next 2 days for postoperative analgesia. The wound clips were removed 7 to 10 days post-implantation. The rats were allowed a 2-week post-surgical recovery period prior to testing. These conscious, telemetry-instrumented rats were then dosed orally with 0 (vehicle), 75, 200 or 750 mg/kg DSM421 (n = 6 rats/group), and blood pressure, heart rate, left ventricular cardiac contractility at 50 mmHg (dP/dt50), and core body temperature were continuously measured and reported in 1-hour time bins using DSI software (DSI, St. Paul, Minnesota, U.S.A.).
2.3.6. Data analysis and statistics
For the respiratory studies, Levene’s test was used to assess homogeneity of group variances for each of the respiratory parameters and for each collection interval. If Levene’s test was not significant (p ≥ 0.01), a pooled estimate of the variance (Mean Square Error or MSE) was computed from a one-way analysis of variance (ANOVA) and utilized by a Dunnett’s comparison of each treatment group with the control group. If Levene’s test was significant (p < 0.01), comparisons with the control group were made using Welch’s t-test with a Bonferroni correction. Results of all pair-wise comparisons are reported at the 0.05 significance levels. The step-down Sidak method was applied to previously obtained p-values to adjust for multiple testing. All endpoints were analyzed using two-tailed tests.
For the cardiovascular study, the endpoints were analyzed by ANOVAs with the repeated measure of time and with Bonferroni’s post hoc test. With hormone levels, ANOVAs with Bonferroni’s post hoc test were performed. Results of all comparisons are reported at the 0.05 significance levels.
3. Results
3.1. Review of original testing
As discussed above, when DSM421 was previously tested in rats, no changes were observed in the Irwin test at oral doses of 200, 500 and 750 mg/kg DSM421, but increases in all of the respiratory parameters tested as well as moribundity and mortality were found at these same doses in the (head-out) plethysmography test. Despite careful reviews of the study records from these two experiments that were performed using GLP conditions at the same laboratory, no errors were discovered that could explain this discrepancy between the results. Most importantly, the scientists performing the studies were well qualified to run the assays and the same formulations of DSM421 were used for dosing in both studies, so these were likely not contributing factors in the disparate results. Additionally, in the plethysmography study, the animals went through several days of acclimation to the head-out plethysmography chambers prior to testing (in this original study as well as in the repeat study) and had not turned around in the chambers during the assay, so these were also not likely the reasons why severe effects were observed during the plethysmography testing but not during the Irwin testing. During unscheduled clinical observations at approximately 4 hours after dosing in the plethysmography study, gasping/labored respiration and increased salivation were noted in a number of the animals dosed with DSM421, including those that were found dead or were sacrificed in extremis. Such observations were not present throughout the Irwin testing, which had the monitoring time points of 1, 2, 3 and 5 hours after dosing. This all suggested that something specific to the head-out plethysmography testing chambers, and not just the test compound DSM421, was resulting in severe effects.
3.2. Off-target activity
In the battery of in vitro binding, enzyme and uptake assays, a 10 μM concentration of DSM421 had minimal to no effects (≤ 19%) at all of the receptors, enzymes, ion channels and transporters being tested for off-target activity, except at the adenosine A3 receptor where 40% inhibition of control specific binding occurred. At 100 μM (36 μg/mL), there was 91% inhibition at the adenosine A3 receptor, 73% at phosphodiesterase (PDE) type 10A2 (PDE10A2), 72% at PDE6, and 59% at PDE5. Additionally, when tested at the 100 μM test concentration, DSM421 was found to be a weak agonist at TRPV1 (26%), where it had no effect as an antagonist at this concentration.
3.3. Pharmacokinetics
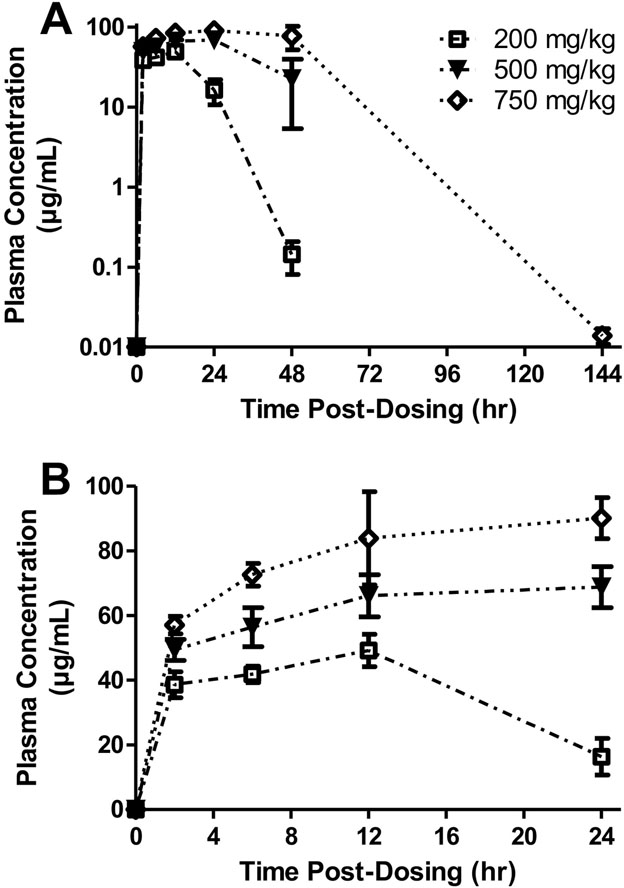
Rats administered DSM421 over the dose range of 200 to 750 mg/kg exhibited a dose-dependent increase in plasma concentrations (Fig. 1), although the Cmax and area under the curve from 0 to 24 hours post-dosing (AUC0–24hr) values were less than proportional to dose. Plasma Cmax values (mean ± S.E.M.) were 49 ± 5.0, 73 ± 6.6 and 97 ± 11 μg/mL at the doses of 200, 500 and 750 mg/kg, respectively. Tmax values (mean ± S.E.M.) at these respective doses were 12 ± 0, 18 ± 3.5 and 27 ± 7.5 hours. By 6 days after dosing, plasma levels at the low and middle doses were below the LLOQ (i.e., < 0.005 μg/mL) and only slightly greater than that at the high dose, suggesting that there would be little to no carryover of plasma concentrations between the whole-body and head-out plethysmography tests that were performed in the same group of rats with a 9-day washout period between the two studies.
Fig. 1.
Plasma concentrations of DSM421 in rats dosed orally with 200, 500 or 750 mg/kg DSM421 graphed over 144 hours post-dosing (A) and over 24 hours post-dosing (B). Mean ± S.E.M. (n = 4 animals/dosing group).
3.4. Respiratory function
Prior to dosing, differences in baseline values were notable between the whole-body and head-out plethysmography studies, despite using the same set of rats for both studies. Mean baseline respiratory rates ranged from 106 – 121 breaths/minute in the whole-body study compared to 180 – 206 breaths/minute in the head-out study indicating that, prior to dosing, the rats in the head-out chambers were breathing more rapidly overall than when they were in the whole-body chambers (Figs. 2A and 3A). For tidal volume, the mean baseline values ranged from 1.60 – 1.65 mL in the whole-body study compared to 1.00 – 1.20 mL in the head-out study signifying that, prior to dosing, the rats in the head-out chambers were breathing more shallowly than when they were in the whole-body chambers (Figs. 2B and 3B). For minute volume, the mean baseline values ranged from 169 – 189 mL/minute in the whole-body study compared to 198 – 224 mL/minute in the head-out study indicating that, prior to dosing, the overall ventilatory capacity was greater in rats when they were in the head-out chambers (Figs. 2C and 3C).
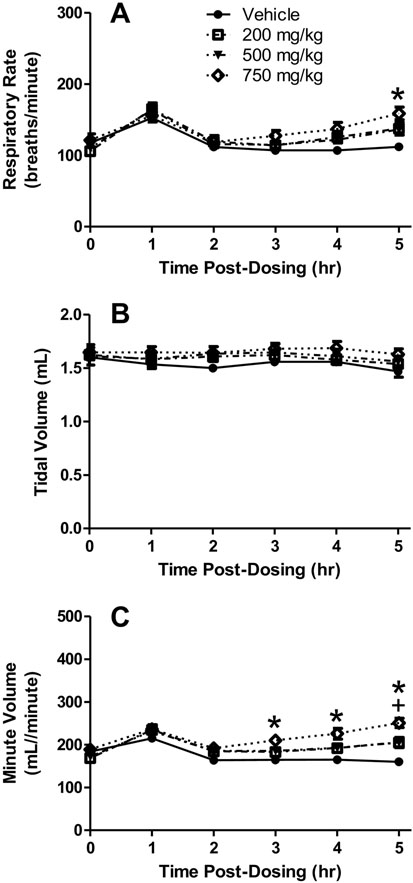
Fig. 2.
Whole-body plethysmography chamber results. Respiratory rate (A), tidal volume (B), and minute volume (C) in rats dosed orally with 200, 500 or 750 mg/kg DSM421. Mean ± S.E.M. (n = 8 animals/dosing group). +P<0.05 for 500 mg/kg versus vehicle. *P<0.05 for 750 mg/kg versus vehicle.
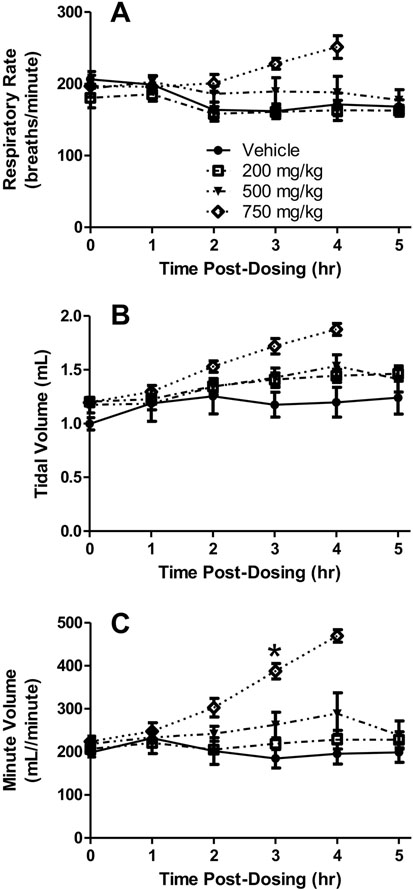
Fig. 3.
Head-out plethysmography chamber results. Respiratory rate (A), tidal volume (B), and minute volume (C) in rats dosed orally with 200, 500 or 750 mg/kg DSM421. Mean ± S.E.M. (n = 4–8 animals/dosing group, except at the 4 and 5 hours post-dosing time points due to severe behavioral signs that resulted in removal of some of the animals from the testing chambers prior to planned study completion). *P<0.05 for 750 mg/kg versus vehicle.
3.4.1. Whole-body plethysmography
During the whole-body plethysmography phase of the study, no DSM421-related clinical signs were noted throughout the 5-hour post-dosing period. However, the plethysmography results revealed gradual, dose-dependent increases in respiratory rate beginning at 3 hours post-dosing and reaching peak levels at the end of the 5-hour post-dosing monitoring period (Fig. 2A). The mean respiratory rate increases reached approximately 22–23% for the 200 and 500 mg/kg dosing groups and approximately 42% for the 750 mg/kg dosing group, as compared to the vehicle control dosing group. The respiratory rate increases in the 750 mg/kg dosing group reached statistical significance at 5 hours post-dosing. In addition, slight, dose dependent increases in tidal volume were observed throughout the 5-hour post-dosing monitoring period, generally maintaining increases of approximately 5, 6 and 11% for the 200, 500 and 750 mg/kg groups, respectively (Fig. 2B). None of the increases in tidal volume reached statistical significance. Reflective of the increases observed in both respiratory rate and tidal volume, dose-dependent increases in minute volume were found during the 5-hour post-dosing monitoring period, reaching peak levels at the end of the 5-hour post-dosing monitoring period (Fig. 2C). The mean minute volume increases reached approximately 28% for the 200 and 500 mg/kg dosing groups and approximately 57% for the 750 mg/kg dosing group. The minute volume increases for the 500 mg/kg dosing group reached statistical significance at 5 hours post dosing and those for the 750 mg/kg dosing at 3–5 hours post dosing.
3.4.2. Head-out plethysmography
During the head-out plethysmography phase of the study, no DSM421-related clinical signs were noted for the 200 mg/kg dosing group throughout the plethysmography testing and at the end of the 5-hour post-dosing monitoring period. However, in the 500 mg/kg dosing group, 2 of the eight animals tested exhibited signs of hypoactivity (i.e., reduction in typical amounts of movement including struggling behavior), salivation, dyspnea and/or increased body temperature (i.e., skin felt warm when touched) between approximately 2.5 to 4 hours post-dosing. As a result, both of these rats were removed from the plethysmograph chambers prior to the end of the monitoring session and one, which did not recover, was euthanized in extremis. In the 750 mg/kg dosing group, all four animals tested exhibited clinical signs similar to those in the 500 mg/kg dosing group. As a result, these four rats were removed from the plethysmograph chambers prior to the end of the monitoring session and two, which did not recover, were euthanized in extremis.
When the plethysmography results were analyzed, no changes in respiratory rate were noted for the 200 mg/kg dosing group, but dose-dependent increases in respiratory rate were observed starting at 2 hours after dosing with 500 and 750 mg/kg of DSM421 (Fig. 3A). The increases in respiratory rate found at 500 mg/kg appeared to remain relatively constant throughout the majority of the monitoring period, with increases of 5–17% above controls; however, the individual animal data indicate that the respiratory rate values did increase over time, and to a greater degree, especially in the two animals that exhibited the most pronounced clinical signs. For the 750 mg/kg dosing group, the values reached peak levels of approximately 41–47% above controls at the end of the monitoring periods for these animals (i.e., 3–4 hours post-dosing, due to the need to remove all of these animals from the monitoring chambers because of the severity of their clinical signs). None of the increases in respiratory rate reached statistical significance, likely due to the limited sample size (in particular for 750 mg/kg dosing group where only four out of 8 animals were dosed).
The plethysmography results also revealed dose-dependent increases in tidal volume for all three DSM421 dosing groups beginning at 2 hours following treatment (Fig. 3B). The increases in tidal volume observed at 200 and 500 mg/kg appeared to remain relatively constant throughout the majority of the monitoring period, with increases of 14–28% above controls; however, the individual animal data indicate that the tidal volume values did increase between 3 and 4 hours post-dosing for the two animals that exhibited the most pronounced clinical signs (44 and 51% above controls at 4 hours after dosing with 500 mg/kg). For the 750 mg/kg dosing group, the values reached peak levels of approximately 46–57% above controls at the end of the monitoring periods for these animals (i.e., 3–4 hours post-dosing). None of the increases in tidal volume reached statistical significance, likely due to the reason mentioned above.
Reflective of the increases observed in both respiratory rate and tidal volume, substantial dose-dependent increases in minute volume were observed for all three DSM421 treatments (Fig. 3C). At 200 and 500 mg/kg, the mean minute volume values reached approximately 17 and 48% above the control group, respectively. At 750 mg/kg, mean minute volume values reached peak levels of 141% above controls at the end of the monitoring periods for these animals (i.e., 3–4 hours post-dosing). The minute volume increases for the 750 mg/kg group reached statistical significance at 3 hours post-dosing.
3.5. Stress-related hormones
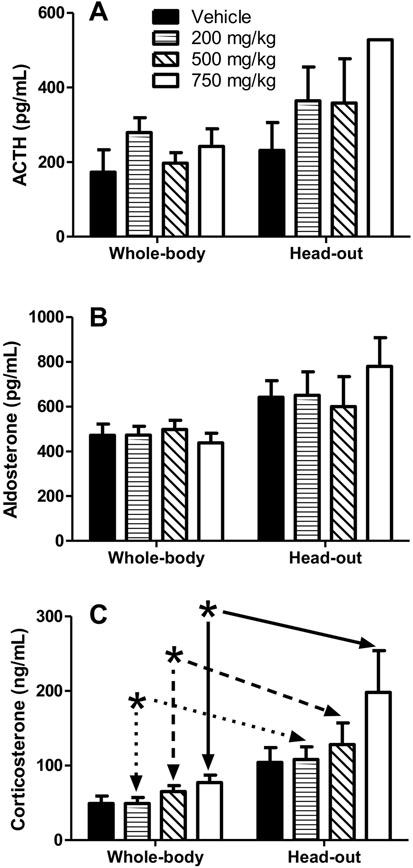
Prior to any plethysmography testing (i.e., before the rats had ever been introduced to the plethysmography chambers), blood sampled and assayed for ACTH, aldosterone and corticosterone concentrations showed no significant differences between the dosing groups in terms of the levels of these three stress-related hormones. At approximately 5 hours post-dosing in the plethysmography studies (i.e., at the end of the testing periods, at approximately 15:00 hours, for all rats during both methods of respiratory testing), levels of all 3 hormones were greater in the head-out phase of the study, as demonstrated by a statistically significant difference (for each of the three hormones) in overall hormone levels between the whole-body and head-out testing procedures (Fig. 4). However, for ACTH and aldosterone, there was no overall effect for dose, and post hoc analysis found no differences between the two plethysmography methods when compared for each dosing group. For corticosterone, in addition to its difference in overall hormone levels between the two plethysmography testing methods, there was also an overall effect for dose, and post hoc analysis found differences between the two plethysmography methods at the doses of 200, 500 and 750 mg/kg, but not for the vehicle control.
Fig. 4.
Blood levels of ACTH (A), aldosterone (B) and corticosterone (C) in rats approximately 5 hours after oral dosing with 200, 500 or 750 mg/kg DSM421. Immediately prior to blood sampling, the rats had either been tested in whole-body or head-out plethysmography chambers (see Figs. 2 and 3). Mean ± S.E.M. (n = 4–8 animals/dosing group, but number of samples/group reduced for the 750 mg/kg dosing group in the head-out plethysmography study due to euthanasia in extremis of 2 of the 4 animals prior to planned study completion). *P<0.05 for whole-body versus head-out data matched to dose.
3.6. Cardiovascular function
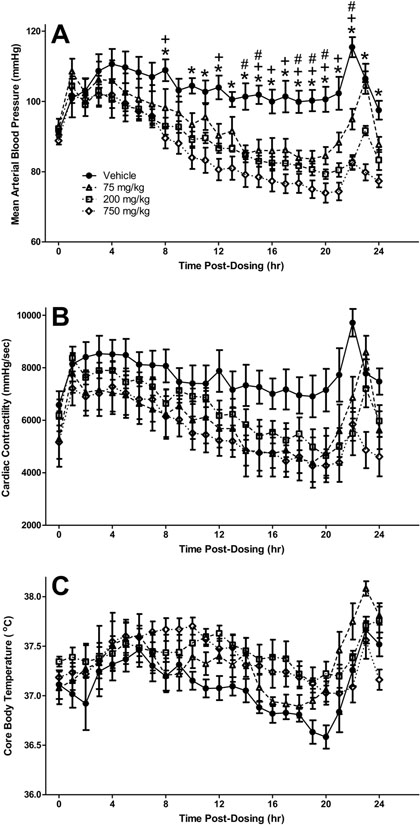
In the telemetry-instrumented conscious rats administered DSM421, a dose-related decrease in mean arterial blood pressure was observed, as compared to the vehicle control-dosed rats, starting at approximately 3 hours after dosing and with statistically-significant differences occurring during the 8 to 24 hours post-dosing period (Fig. 5A). Maximal effects were approximately −15, −20 and −25 mmHg for the doses of 75, 200 and 750 mg/kg, respectively. There were also similar trends toward decreases in left ventricular cardiac contractility at all three doses tested (maximal effects approximately −2000 to −2500 mmHg/sec [approximately −25 to −35%]; Fig. 5B) and increases in core body temperature mainly at the highest dose tested (approximately ≤ 0.5°C; Fig. 5C); however, there were no statistically-significant overall effects for dose for either of these two parameters. No statistically- or biologically-significant effects were observed for heart rate over the 24-hour post-dosing monitoring period.
Fig. 5.
Mean arterial blood pressure (A), cardiac contractility (B), and core body temperature in conscious, unrestrained rats dosed orally with 75, 200 or 750 mg/kg DSM421. Mean ± S.E.M. (n = 6 animals/dosing group). #P<0.05 for 75 mg/kg versus vehicle. +P<0.05 for 200 mg/kg versus vehicle. *P<0.05 for 750 mg/kg versus vehicle.
4. Discussion
In rats dosed orally with DSM421, it was perplexing why no effects were observed in the Irwin study but the initial respiratory study resulted in moribundity and mortality when performed at the same laboratory with rats treated at the same doses and using exactly the same formulations of DSM421. Because these studies were both conducted under GLP, comprehensive study records were available for review to try to identify any events that may have accounted for the substantial difference between the two studies. After carefully reviewing the study records and consulting with the personnel that had performed and overseen the studies, no events were identified that could have accounted for this large difference. Therefore, the respiratory study was repeated at a different laboratory to see if the effects could be confirmed. At the second laboratory, the findings of increased respiratory rate, tidal volume and minute volume were repeated using a similar head-out plethysmography testing procedure (Fig. 3), and the same severe effects of moribundity were also observed. The discrepant outcomes between the Irwin study and the head-out plethysmography studies suggested that some factor other than just the DSM421 was producing the severe effects. Indeed, in a previous study in which dogs had been dosed to comparable plasma concentrations of DSM421 (see the Introduction section), respiratory effects were observed (i.e., increases in both respiratory rate and minute volume, with no changes in tidal volume), but no moribundity and/or mortality was seen. Because these dogs had been pre-instrumented with radiotelemetry transmitters to monitor their respiratory (and cardiovascular) function, they were minimally restrained (i.e., free to move about their cages) throughout the testing.
Prior to conducting the repeat head-out plethysmography study in rats, the respiratory effects of DSM421 were also tested using the same dosing parameters and same set of rats but in whole-body plethysmography chambers to determine whether this less restraining method would make a difference. While DSM421 produced changes in respiratory function that paralleled those from the head-out study, the respiratory effects in the whole-body study were less severe (Fig. 2) and moribundity did not occur. Thus, the effects of DSM421 in the whole-body plethysmography study were more in line with the results in the Irwin test than in either of the two head-out plethysmography studies. It should be noted that, in all four of these rat studies, no blood gas assessment was performed such as the hemoglobin oxygen saturation measurement that is one of the two accompaniments to respiratory rate assessment suggested in the ICH’s S7A guidance (ICH, 2001). The guidance’s alternative suggested accompaniment is tidal volume measurement, which was performed during all of the respiratory studies. It is unknown how useful such blood gas data would have been for characterizing the respiratory changes and other adverse findings in these studies.
Interestingly, even prior to dosing with DSM421, the rats while in the head-out chambers had greater respiratory rates and minute volumes and lesser tidal volumes than while in whole-body chambers, even though the same animals were being tested just a little more than one week apart (see the 0 hour post-dosing time points in Figs. 2 and 3). Differences in baseline respiratory measures between these two types of testing chambers have previously been reported (Nirogi et al., 2012), suggesting that the head-out procedure is more stressful than the whole-body procedure because of the restraint used with the former method. Indeed, in the current study, blood levels of three stress-related hormones (ACTH, aldosterone and corticosterone) were all found to be elevated after testing in the head-out chambers as compared to after testing in the whole-body chambers (Fig. 4). As outlined in the Methods section, care was taken to test all rats at approximately the same time of the day, so diurnal variation was likely not a confounding factor in the differences observed in stress hormones levels between the more (head-out) and less (whole-body) restrained rats. Restraint, such as that necessary for head-out plethysmography testing, has previously been shown to elevate blood levels of ACTH and corticosterone, and appropriate acclimation to the testing chambers has been found to reduce such signs of stress and result in quality respiratory data even during 6 hours of continuous restraint (Ewart et al., 2010; Grissom et al., 2008; Harris et al., 2005). Despite the rats in the current studies being allowed to acclimate to their plethysmography testing chambers prior to dosing, the animals still showed elevated levels of these stress indicators due to their being restrained in the head-out testing chambers.
Once the rats in the respiratory studies were dosed with DSM421, changes in respiratory function were observed in both types of testing chambers, but with the greater effects in the head-out chambers (Figs. 2 and 3). Although dose-related, the increases in respiratory rates, tidal volumes and minute volumes did not appear to be dose-proportional, and this was in agreement with the non-linear pharmacokinetics of DSM421 (Fig. 1). There has been one metabolite of DSM421 identified in the plasma of rats (DSM565, which is the product of hydroxylation and is present at approximately 4-fold lower concentrations than those of DSM421), and this metabolite also has non-linear pharmacokinetics. It is unlikely that this metabolite is responsible for the difference in severity of effects found between the head-out and whole-body plethysmography studies because there is no plausible reason to expect plasma concentrations of DSM565 (as well as DSM421 itself) to be significantly different based on the method of restraint.
Differences in respiratory results between head-out versus whole-body plethysmography testing have previously been reported for reference compounds but, as in the current study, the effects were still generally in the same directions between the two testing methods (Nirogi et al., 2012). To the best of our knowledge, no reports have been published demonstrating more extreme differences between these two testing methods such as the moribundity and mortality that were observed in rats in the head-out chambers of the current study, suggesting that an interaction was occurring between the testing method and the DSM421. Indeed, trends toward such an interaction were observed for blood levels of stress hormones in the current study, in particular for serum levels of corticosterone, although these interactions were not statistically significant (Fig. 4).
As alternate biomarkers of pharmacologically-induced stress, the effects of DSM421 on cardiovascular function and body temperature were monitored in conscious, freely-moving (i.e., non-restrained) rats. Although there was a trend towards an increase in body temperature (≤0.5°C; Fig. 5C), which would correlate with an increase in stress, both blood pressure and cardiac contractility were decreased (Figs. 5A and 5B), which is in the opposite direction of a stress response. Therefore it appears that DSM421 administration alone is not producing much of a stress response in the freely-moving rats and, consequently, stress- related cardiovascular changes by DSM421 are not likely responsible for the moribundity and mortality that were observed during the head-out plethysmography testing. Instead, the decreases in blood pressure and cardiac contractility that were observed in the unrestrained rats dosed with DSM421 may have been reflected, at least in part, in the hypoactivity that was noted in the restrained, dosed rats in the head-out chambers, and these hypotensive and negative inotropic cardiovascular effects of DSM421 may have ultimately also contributed to the moribundity that was observed during the head-out testing.
To further determine why DSM421 could be producing severe adverse effects during the head-out plethysmography testing but not in our other in vivo assays, we investigated its off- target activity and found that its most potent off-target binding affinity was at the adenosine A3 receptor (91% at 100 μM [36 μg/mL]). For comparison, mean plasma Cmax values were 49, 73 and 97 μg/mL in the rats orally dosed with 200, 500 and 750 mg/kg of DSM421, respectively. Adenosine A3 receptor ligands, among their other effects, have been associated with inflammatory responses including bronchoconstriction/relaxation (Fishman et al., 2012; Mikus et al., 2013). Specifically, activation of the adenosine A3 receptor can result in increased bronchoconstriction (Mikus et al., 2013). Bronchoconstriction produces changes to the normal respiratory pattern, and those changes can vary both in terms of their severity and direction with various respiratory parameters, potentially due to differences in the species and/or bronchoconstrictor tested (Authier et al., 2009; Harris et al., 2005). In the current study, respiratory rate and tidal and minute volumes were all increased in the rats dosed with DSM421, and the same direction of changes to these respiratory parameters was previously reported in asthma patients that had been challenged with the bronchoconstrictor methacholine (Lavorini et al., 2013). Any bronchoconstrictive effects related to DSM421 due to its off-target activity at adenosine A3 receptor could have had additivity or synergy with any bronchoconstrictive effect produced by the physical seal around the sensitive neck area of the test animals in the head-out testing chambers, in particular in any of the rats that were struggling more than normally. This, in turn, may have led to a vicious cycle that eventually produced the dyspnea and other severe adverse effects of DSM421 occurring during the head- out plethysmography testing.
DSM421 also had some relatively weaker binding affinity at PDE5, PDE6 and PDE10A2 (59 – 72% at 100 μM). PDE, in general, has been associated with changes in cardiovascular and respiratory function. For example, PDE5 inhibitors are employed in the treatment of pulmonary arterial hypertension in patients, and they have been demonstrated to reduce airway inflammation in animal studies (Anderson, 2018; Mokry et al., 2017). Therefore, there is also the potential that some weak effects of DSM421 on any one, or a combination, of these three PDE subtypes could have resulted in cardiovascular and/or respiratory changes that were intensified by the stress induced by the head-out plethysmography chambers.
DSM421 was also found to be a weak pharmacological agonist at TRPV1 (26% at 100 μM [36 μg/mL]), which has been associated with changes in body temperature. In general, TRPV1 agonists produce hypothermia while antagonists produce the opposite effect, hyperthermia (Fosgerau et al., 2010; Gomtsyan, 2015). However, there have been recent reports demonstrating that some TRPV1 antagonists can produce hypothermia or have even no effect on body temperature (Garami et al., 2018; Gomtsyan et al., 2015). Therefore, there is the possibility that the small hyperthermic effect produced by DSM421 in the rat telemetry study (Fig. 5C), that was also observed in the head-out plethysmography study, was due to its off-target activity at TRPV1. Although the hyperthermic effect of DSM421 was relatively small (≤ 0.5°C) in the freely-moving rats in the telemetry study, we do not know if body temperature was further increased in the rats in the head-out plethysmography chambers, where body temperature was only qualitatively assessed by how warm the rats’ skin felt when touched. Therefore it is unknown how much, if any, the hyperthermic response to DSM421 contributed to the severe signs that were observed in rats during the head-out plethysmography testing, or even whether TRPV1 was involved.
In conclusion, although DSM421 administration produced some changes in respiratory and cardiovascular function in rats, it did not result in severe clinical signs per se. However, when the rats were tested in head-out plethysmography chambers, the restraint inherent to such testing resulted in a stress response as well as an exaggeration of the respiratory effects in the dosed animals. In addition, any bronchoconstrictive effect that DSM421 may have had due to off-target activity at the adenosine A3 receptor could have further exacerbated these respiratory changes, thus resulting in the moribundity and mortality that were observed with DSM421 only when the rats were restrained in head-out chambers. This severe, potential amplification found with head-out plethysmography testing should be considered when choosing which method to perform for respiratory safety pharmacology assessment and when interpreting the resultant findings.
Acknowledgements
Charles River Laboratories (Ashland, Ohio, U.S.A.), MPI Research (Mattawan, Michigan, U.S.A.), Eurofins Cerep (Celle-L’Evescault, France), Antech Diagnostics (Morrisville, North Carolina, U.S.A.), and AbbVie Inc. conducted the testing, and CapEval Pharma (Archamps, France) drafted initial versions of this manuscript. This work was funded in part under National Institute of Allergy and Infectious Diseases (NIAID) Contract No. HHSN2722011000221 to SRI International.
Disclosures
J.J.L., T.R.V.V., K.C.M., J.A.S., M.R. and S.W.M. are employees of AbbVie. Parts of the design, study conduct and financial support for this research were provided by AbbVie. AbbVie participated in the interpretation of the data, review, and approval of the publication.
J.J.M. is an employee of Medicines for Malaria Venture (MMV), a nonprofit research organization, and E.R. was an employee of MMV at the time that this research was undertaken. Parts of the design, overall project leadership and financial support were provided by MMV. MMV participated in the interpretation of the data, review, and approval of the publication.
T.P. and J.M. are employees of SRI International, a nonprofit research institute, who designed and conducted the initial Irwin and plethysmography studies. SRI staff participated in the interpretation of the data, review, and approval of the publication.
S.E.O. is an employee of Takeda Pharmaceutical International Company. Parts of the design and some financial support for this research were provided by Takeda. Takeda participated in parts of the interpretation of the data, review, and approval of the publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson K-E (2018). PDE5 inhibitors – pharmacology and clinical applications 20 years after sildenafil discovery. British Journal of Pharmacology, 175, 2554–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson HC, & Waddell BJ (1997). Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: Sexual dimorphism and changes across the estrous cycle. Endocrinology, 138, 3842–3848. [DOI] [PubMed] [Google Scholar]
- Authier S, Legaspi M, Gauvin D, & Troncy E (2009). Respiratory safety pharmacology: Positive control drug responses in Sprague-Dawley rats, Beagle dogs and cynomolgus monkeys. Regulatory Toxicology and Pharmacology, 55, 229–235. [DOI] [PubMed] [Google Scholar]
- Ewart LC, Haley M, Bickerton S, Bright J, Elliott K, McCarthy A, Williams L, Ricketts S-A, Holland T, & Valentin J-P (2010). Pharmacological validation of a telemetric model for the measurement of bronchoconstriction in conscious rats. Journal of Pharmacological and Toxicological Methods, 61, 219–229. [DOI] [PubMed] [Google Scholar]
- Fishman P, Bar-Yehuda S, Liang BT, & Jacobson KA (2012). Pharmacological and therapeutic effects of A3 adenosine receptor agonists. Drug Discovery Today, 17, 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosgerau K, Weber UJ, Gotfredsen JW, Jayatissa M, Buus C, Kristensen NB, Verstergaard M, Teschendorf P, Schneider A, Hansen P, Raunsø J, Køber L, Torp-Pedersen C, & Videbaek C (2010). Drug-induced mild therapeutic hypothermia obtained by administration of a transient receptor potential vanilloid type 1 agonist. BMC Cardiovascular Disorders, 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garami A, Pakai E, McDonald HA, Reilly RM, Gomtsyan A, Corrigan JJ, Pinter E, Zhu DXD, Lehto SG, Gavva NR, Kym PR, & Romanovsky AA (2018). TRPV1 antagonists that cause hypothermia, instead of hyperthermia, in rodents: Compounds’ pharmacological profiles, in vivo targets, thermoeffectors recruited and implications for drug development. Acta Physiologica, 223, e13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomtsyan A, McDonald HA, Schmidt RG, Daanen JF, Voight EA, Segreti JA, Puttfarcken PS, Reilly RM, Kort ME, Dart MJ, & Kym PR (2015). TRPV1 ligands with hyperthermic, hypothermic and no temperature effects in rats. Temperature, 1, 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom N, Kerr W, & Bhatnagar S (2008). Struggling behavior during restraint is regulated by stress experience. Behavioural Brain Research, 191, 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D, Graham M, Price J, Munro F, Templeton A, Young R, Paterson K, Anderson L, Gillies S, McKendrick S, Low G, Patmore L, Bodine R, Kallman MJ, Hoffman WP, Lee C, & Wolff RK (2005). Respiratory function in rats restrained for extended periods: Assessment of the effects of bethanecol. Journal of Pharmacological and Toxicological Methods, 52, 83–89. [DOI] [PubMed] [Google Scholar]
- ICH (2001). Guidance on S7A safety pharmacology studies for human pharmaceuticals. Federal Register, 66, 36791–36792. [PubMed] [Google Scholar]
- Lavorini F, Magni C, Chellini E, Camiciottoli G, Pistolesi M, & Fontana GA (2013). Different respiratory behaviors disclosed by induced bronchoconstriction in mild asthma patients. Respiratory Physiology & Neurobiology, 189, 521–529. [DOI] [PubMed] [Google Scholar]
- Mikus EG, Szeredi J, Boer K, Tímári G, Finet M, Aranyi P, & Galzin A-M (2013).Evaluation of SSR161421, a novel orally active adenosine A3 receptor antagonist on pharmacology models. European Journal of Pharmacology, 699, 172–179. [DOI] [PubMed] [Google Scholar]
- Mokry J, Urbanova A, Medvedova I, Kertys M, Mikolka B, Kosutova P, & Mokra D (2017). Effects of tadalafil (PDE5 inhibitor) and roflumilast (PDE4 inhibitor) on airway reactivity and markers of inflammation in ovalbumin-induced airway hyperresponsiveness in guinea pigs. Journal of Physiology and Pharmacology, 68, 721–730. [PubMed] [Google Scholar]
- Nirogi R, Shanmuganathan D, Jayarajan P, Abraham R, & Kancharla B (2012). Comparison of whole body and head out plethysmography using respiratory stimulant and depressant in conscious rats. Journal of Pharmacological and Toxicological Methods, 65, 37–43. [DOI] [PubMed] [Google Scholar]
- Phillips MA, White KL, Kokkonda S, Deng X, White J, El Mazouni F, … Charman SA (2016). A triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor (DSM421) with improved drug-like properties for treatment and prevention of malaria. ACS Infectious Diseases, 2, 945–957. [DOI] [PMC free article] [PubMed] [Google Scholar]