Abstract
Carbon nanotubes (CNTs) are nanomaterials with unique physicochemical properties that are targets of great interest for industrial and commercial applications. Notwithstanding, some characteristics of CNTs are associated with adverse outcomes from exposure to pathogenic particulates, raising concerns over health risks in exposed workers and consumers. Indeed, certain forms of CNTs induce a range of harmful effects in laboratory animals, among which inflammation, fibrosis, and cancer are consistently observed for some CNTs. Inflammation, fibrosis, and malignancy are complex pathological processes that, in summation, underlie a major portion of human disease. Moreover, the functional interrelationship among them in disease pathogenesis has been increasingly recognized. The CNT-induced adverse effects resemble certain human disease conditions, such as pneumoconiosis, idiopathic pulmonary fibrosis (IPF), and mesothelioma, to some extent. Progress has been made in understanding CNT-induced pathologic conditions in recent years, demonstrating a close interconnection among inflammation, fibrosis, and cancer. Mechanistically, a number of mediators, signaling pathways, and cellular processes are identified as major mechanisms that underlie the interplay among inflammation, fibrosis, and malignancy, and serve as pathogenic bases for these disease conditions in CNT-exposed animals. These studies indicate that CNT-induced pathological effects, in particular, inflammation, fibrosis, and cancer, are mechanistic-ally, and in some cases, causatively, interrelated. These findings generate new insights into CNT adverse effects and pathogenesis and provide new targets for exposure monitoring and drug development against inflammation, fibrosis, and cancer caused by inhaled nanomaterials.
Keywords: Carbon nanotube, inflammation, fibrosis, tumorigenesis, immune mechanism
Introduction
The past two decades have witnessed rapid growth and development in nanotechnology and the commercialization of products and devices containing engineered nanomaterials, which could help address global issues concerning energy, transportation, pollution, health, and food (Drexler 1992; NSF 2011). Carbon nanotubes (CNTs) are new nanomaterials with potentials for a broad range of applications (De Volder et al. 2013; Zhang et al. 2013). CNTs, both single-walled and multi-walled CNTs (SWCNTs and MWCNTs, respectively), are made of one-atom-thick graphene sheets that roll into seamless cylinder-like structures. These nanotubes vary greatly in dimension and shape, but commonly exhibit certain unique properties that are of interest for industrial and commercial utility. These attributes indicate substantial mechanical strength, excellent electrical, optical, and thermal conducting capabilities, nano-scaled size, and large surface area, which are useful for products in the fields of electronics, energy production, construction, drug delivery, and health care. The annual productions of CNTs and CNT-containing materials and products have increased markedly in recent years (Abdalla et al. 2015; De Volder et al. 2013; Sharma et al. 2016; Zhang et al. 2013). As such, exposure to CNTs is expected to increase substantially in human populations, including workers producing CNTs and CNT-containing materials, and patients taking CNT-carried drugs or using CNT-containing medical devices (Fatkhutdinova et al. 2016; Schulte et al. 2012; Vlaanderen et al. 2017).
Certain properties of CNTs, such as high respirability, low solubility, and biopersistence are known to be associated with inflammatory, fibrotic, and tumorigenic effects of inhaled pathogenic particles and fibers, such as silica and asbestos. In fact, pulmonary exposure to certain forms of CNTs produces a range of adverse effects in laboratory animals. In addition to inhalation, exposures to CNTs may occur via skin absorption, ingestion, or use of CNT-containing medicine and medical devices. These findings raise concerns over possible health risks of CNT exposure in humans from occupational, environmental, and commercial sources (Donaldson et al. 2006; Dong and Ma 2015; Johnston et al. 2010).
A plethora of toxicological and pathological studies have been conducted to characterize the biological effects of CNTs in the recent decade. CNTs can cause a range of adverse effects in experimental animals, cultured mammalian cells, and certain human populations, including cytotoxicity, inflammation, fibrosis, genotoxicity, tumorigenesis, and immunotoxicity. At the mechanistic level, CNTs stimulate the activation of certain molecular, cellular, and systemic processes and signaling pathways that may propel the development of these effects in exposure-, time-, and context-dependent manners. From this prospect, some CNTs represent a new type of fiber-like materials with a propensity to cause toxic effects. CNT-induced pathological effects resemble certain human diseases, such as organ fibrosis and malignancy, with regard to pathological features. Some specific aspects of these findings have been summarized and discussed in several recent reviews (Donaldson and Poland 2012; Dong and Ma 2015; Dong and Ma 2016b; Dong and Ma 2018b; Duke and Bonner 2018; Ema, Gamo, and Honda 2016; Kuempel et al. 2017; Luanpitpong, Wang, and Rojanasakul 2014; Møller et al. 2014; Vietti Lison and van den Brule 2016; Zhao and Liu 2012). A better understanding of the underlying mechanisms that trigger and enhance the responses to CNT exposure at molecular, cellular, and organismal levels is needed for the toxicological evaluation, risk assessment, and safe design of nanomaterials. Such analysis is also informative to the understanding and treatment of human fibrotic disease and cancer.
A number of adverse effects are commonly observed in different model systems exposed to different CNTs and CNT preparations, despite large variations in CNT physicochemical properties. Among these effects, chronic inflammation, fibrosis, and malignancy in the lung and the pleura are the most concerned due to their severe outcomes. Increasing evidence indicates that there exist close interactions among inflammation, fibrosis, and cancer both phenotypically and mechanistically. These interactions appear to be intrinsic and critical to the development of pathological outcomes. Moreover, the interrelations elicited by CNTs are reminiscent of the relationships among chronic inflammation, fibrosis, and cancer identified in many human diseases. In these diseases, chronic inflammation, fibrosis, and cancer form a mechanistic triad, through which they interact with each other to determine the development, outcomes, and therapeutic responses of disease. How CNT-activated signaling pathways and mechanisms cross-interact with each other to bring about CNT adverse effects remains unclear. Given the importance of such interactions noted in human diseases, elucidation of these interactions in CNT-exposed individuals could shed new lights into the mechanism by which CNTs elicit pathological outcomes.
In this review, we discuss the evidence supporting the interplays among CNT-induced mediators, pathways, and events that modulate the adverse effects of CNTs, with focus on the development of inflammation, fibrosis, and cancer. These analyses would reveal new aspects of the pathogenesis and outcomes of CNT nanotoxicity. Such investigations may also suggest new targets and strategies for intervention against chronic inflammation, fibrosis, and malignancy in humans.
Inflammation, fibrosis, and malignancy in human disease: a mechanistic triad
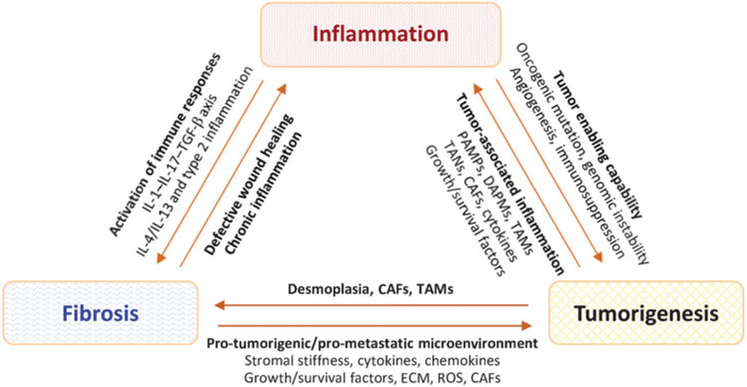
Inflammation, fibrosis, and malignancy are phenotypically distinct, but functionally related, complex biological processes that, in summation, underlie a major portion of human disease. The functional relationships among inflammation, fibrosis, and cancer were noted early in the history of modern medicine. The Virchow’s triad of arterial or endothelial injury, stasis of blood flow, and hypercoagulability/thrombosis observed by Rudolf Virchow in the 19th century first described a close association among vascular injury, clot formation, and inflammation (Bagot and Arya 2008; Lucas 2012). This association is now recognized as a pivotal, driving mechanism for the development of wound healing, which, at a fundamental level, provides a biological basis for the development of fibrosis and tumorigenesis. In this connection, fibrosis resembles exacerbated wound healing to result in the persistent buildup of fibrotic extracellular matrix (ECM) and scarring of involved tissue (Duffield et al. 2013). On the other hand, tumors act as wounds that fail to heal, wherein proliferating cells that sustain DNA damage and/or mutagenic assault continue to proliferate in a microenvironment that is rich in inflammatory cells, growth factors, and neovasculature, which support their growth (Dvorak 1986). A more direct association between cancer and inflammation/immune responses was also suggested by Virchow who, upon observation of the presence of leukocytes within tumors, postulated that the origin of cancer is at the site of chronic inflammation (Balkwill and Mantovani 2001; Virchow 1863). These and other early observations on inflammation, wound healing, and tumorigenesis by Virchow and many others provided a foundation for understanding disease pathogenesis and development involving inflammation, fibrosis, and cancer. Nonetheless, it is not until the recent two decades that a better understanding of the interrelations among inflammation, fibrosis, and cancer was obtained and some underlying molecular mechanisms delineated, which illustrate a triad of mutual interaction and regulation among them to underlie the development of many human diseases (Figure 1).
Figure 1.
Illustration of interactions among inflammation, fibrosis, and cancer in human disease. Inflammation, fibrosis, and cancer, in summation, underlie a major portion of human disease. Importantly, these pathologic processes cross-interact with each other at multiple levels and by multiple means, which are recognized as the causes for the development of many diseases, as well as the important host factors to influence the effectiveness of drug therapy. The IL-1—IL-17—TGF-β axis is activated by bacterial or viral infection, biliary obstruction, and toxins, such as bleomycin, to stimulate organ fibrosis, whereas the IL-4/IL-13 signaling and type 2 inflammation predominate the fibrotic response to allergen exposure, parasitic infection, and fungal infection. CAF: cancer-associated fibroblast; DAMP: danger-associated molecular pattern; ECM: extracellular matrix; IL: interleukin; PAMP: pathogen-associated molecular pattern; ROS: reactive oxygen species; TAM: tumor-associated macrophage; TAN: tumor-associated neutrophil; TGF: transforming growth factor.
Inflammation
Upon exposure or injury, inflammation is first observed, whereas fibrosis and cancer are typically chronic manifestations. Inflammation is defined as the tissue response to harmful stimuli, such as injury, pathogens, and irritants. The purpose of this response is to eliminate insults, mitigate lesions, and repair damaged tissue, which is accomplished mainly through the actions of innate immune cells, blood vessels, and molecular mediators. The initial event of an inflammatory response to a harmful stimulus is characterized by the increased movement of plasma and leukocytes (especially granulocytes) from the blood into injured tissue. This acute innate immune response to injury is highly conserved through evolution and is reminiscent of the immediate rapid massing of inflammatory cells in starfish upon stimulation by a splinter observed by Ilya Ilyich Mechnikov in the late 19th century (Kaufmann 2008). Acute inflammation propagates through cascades of events involving the local vasculature, immune functions, and various cells within the injured tissue. If injury or infection becomes persistent or the lesion is overwhelming and exceeds the capacity of tissue repair, the wound fails to heal and inflammation propagates to a chronic state. During chronic inflammation, there is a progressive shift in the type of cells present at the site of inflammation, often marked by increasing mononuclear cells, and the simultaneous destruction and healing of the tissue from the inflammatory process. In this regard, chronic inflammation may reflect a homeostatic imbalance of physiological systems and functions resulting from the malfunction of tissue, rather than a direct response to classical instigators of inflammation, such as injury and infection. Importantly, chronic inflammation is causatively associated with or is an integral component of many chronic diseases, exemplified by chronic infection, autoimmune dysfunction, atherosclerosis, neurodegeneration, and, pertinent to this review, fibrosis, and malignancy.
Inflammation and fibrosis
Fibrosis is a common pathologic outcome of many chronic inflammatory diseases. In these scenarios, inflammatory and fibrogenic signals interact with each other to stimulate an inflammatory wound healing process, which goes awry and evolves into a progressive and irreversible fibrotic process, if the injury is severe or repetitive, or when the repair response becomes dysregulated. Diseases in which fibrosis is a major cause of morbidity and mortality encompass both organ-specific and multi-systemic illnesses. These include chronic renal disease from infection and diabetes; liver fibrosis and cirrhosis from viral and parasitic infection, alcoholic and non-alcoholic hepatitis, and drug-induced liver injury; pulmonary fibrosis from idiopathic pulmonary fibrosis (IPF), chronic obstructive pulmonary disease (COPD), and inhalation of fibrogenic particles; myocardial infarction; and systemic autoimmune diseases, such as lupus and systemic sclerosis. Despite this vastly diverse etiology and clinical presentation, fibrosis in many diseases appears to follow a common path of development, wherein fibrogenic pathways converge to boost the activation and migration of fibroblasts, and the differentiation of fibroblasts to myofibroblasts (Dong and Ma 2016b; Duffield et al. 2013). Myofibroblasts are rich in cellular machineries for protein synthesis and secretion, which enables cells to produce copious amounts of collagens in fibrosing tissues. Myofibroblasts also synthesize α-smooth muscle actin (α-SMA) that incorporates into the contractile stress fibers to strengthen contraction by myofibroblasts during scar formation (Dong and Ma 2016b; Tomasek et al. 2002).
The influence of inflammation on fibrosis is multifold and is inducer-, time-, and context-dependent (Dong and Ma 2018b; Gieseck, Wilson, and Wynn 2018; Wynn and Ramalingam 2012). Upon infection and wounding, inflammatory cells and injured tissue cells secrete soluble mediators, among which transforming growth factor (TGF)-β1 appears to serve as a common mediator of fibro-blast activation and transformation. In the presence of bacterial or viral infection, toxins, or biliary obstruction, type 1 inflammation is elicited where macrophages, typically, classically activated (M1) macrophages, and neutrophils secrete interleukin(IL)-1 and IL-6, which in turn, induce IL-17. IL-17 signaling then stimulates the production and activation of TGF-β1, forming an IL-1—IL-17—TGF-β1 axis to stimulate sustained tissue repair or organ fibrosis. On the other hand, type 2 inflammation ensues upon exposure to allergens, helminths, fungi, or fibrogenic particles and fibers. In these scenarios, alarmins, such as IL-25, IL-33, and thymic stromal lymphopoietin (TSLP), are produced and released by injured structural cells, such as epithelial cells. Alarmins recruit type 2 inflammatory cells, such as basophils, eosinophils, mast cells, and type 2 innate lymphoid cells (ILC2s), which provide the early production of type 2 cytokines, IL-4 and IL-13. IL-4 and IL-13 induce the formation of T helper (Th) 2 cells that amplify type 2 inflammation by producing type 2 cytokines. IL-13 appears to serve as a major signal to stimulate fibroblast activation and the fibroblast-to-myofibroblast transformation to result in fibrosis. In all cases, fibrosis and granuloma formation reflect the maladaptation of tissue to chronic injury and infection, the purpose of which is to preserve tissue integrity and limit invading pathogens or foreign bodies to a local environment, which is, albeit, at a considerable expense of tissue functions that are lost due to tissue destruction and distortion from fibrosis.
Inflammation and cancer
Tumor-promoting inflammation has been recognized as one of the enabling characteristics of tumor development (Hanahan and Weinberg 2011). Chronic inflammation associated with infection or prolonged exposure to environmental insults often precedes and contributes to tumor development by several means, such as oncogenic mutations, genomic instability, and angiogenesis; tumor progression and metastasis; and immunosuppression (Coussens and Werb 2002; Grivennikov, Greten, and Karin 2010; Shalapour and Karin 2015). It is estimated that approximately 25% of the cancers are associated with chronic inflammation caused by infection or physicochemical insults (Balkwill and Mantovani 2012). For example, patients suffering from chronic inflammatory bowel diseases, such as ulcerative colitis and Crohn’s disease, have a 10-fold increased risk of colon cancer (Coussens and Werb 2002). Persistent gastritis caused by Helicobacter pylori increases the risk of gastric cancer by 75% (Eiro and Vizoso 2012), whereas hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are strongly associated with the formation of hepatocellular carcinoma (Ringelhan, McKeating, and Protzer 2017).
At cellular and molecular levels, pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) are known to be released from microbes and dying cancer or tissue cells, respectively. These initial signals activate myeloid cells that are recruited to the site of the tumor through the action of chemokines, resulting in local inflammation. On the other hand, inflammation promotes tumor initiation by increasing the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) from inflammatory cells to damage DNA, proteins, and organelles, and by inducing epigenetic changes, both of which favor tumorigenesis. Inflammation also stimulates tumor promotion through multiple means. For instance, tumor-associated macrophages (TAMs) and neutrophils (TANs) secrete cytokines, such as tumor necrosis factor (TNF)-α, IL-1, and IL-6. These pro-inflammatory cytokines evoke inflammatory responses, and act directly on tumor cells leading to the activation of NF-κB (nuclear factor-jB), STAT3 (signal transducer and activator of transcription 3), YAP (Yes-associated protein), and Notch signaling pathways, thereby promoting tumor cell survival and proliferation (Shalapour and Karin 2015). TNF, as its name implies, also kills tumor cells, highlighting a complex interrelation between inflammatory cytokines and tumor cells (Carswell et al. 1975).
Rapidly growing tumors create a hypoxic condition that activates cancer-associated fibroblasts (CAFs) through a hypoxia-inducible factor (HIF)-1-induced TGF-β signaling axis. In this scenario, TAMs may convert from type 1 (TAM1, pro-inflammatory) to type 2 macrophages (TAM2, anti-inflammatory and pro-fibrotic). TAM2 cells produce vascular endothelial growth factor (VEGF) to foster neoangiogenesis both surrounding and within the tumor tissue to support rapid tumor growth. Lymphocytes also play roles in tumor formation. CAFs secrete TGF-β and CXCL13 that recruit lymphotoxin-producing B2 lymphocytes to further support tumor growth, whereas chemokines produced in the tumor recruit tumor-promoting Th17 cells and immunosuppressive regulatory T cells (Tregs). Finally, tumor-infiltrating B cells may undergo class-switch recombination to induce an exhausted or anergic phenotype in cytotoxic T cells (Shalapour and Karin 2015). On the other hand, acute inflammatory reactions often stimulate dendritic cell maturation and antigen presentation that boost anti-tumor immunity, which may be targeted for immunotherapy against cancer. These findings indicate that chronic inflammation influences tumorigenesis and tumor progression and metastasis at several levels via multiple means.
Fibrosis and cancer
Accumulating evidence supports a close relationship between fibrosis and cancers. It has long been established that certain tumors can arise where scars are formed, giving rise to the term scar carcinoma. Scar carcinoma is most prominent in patients with pneumoconiosis including asbestosis, silicosis, and coal worker’s lung disease (Davis and Cowie 1990; Doll 1955). In other examples, certain fibrotic diseases are associated with increased risks of certain cancers. For instance, patients suffering from cystic fibrosis have an odds ratio of 6.5 for developing digestive tract cancers compared with the general population in North America (Neglia et al. 1995). Nonetheless, a causal relationship between fibrosis and cancer has long been debated, in particular, with regard to whether desmoplasia, which denotes the growth of fibrous connective tissue characteristically associated with malignant neoplasms, precedes, accompanies, or succeeds tumor initiation, progression, and metastasis.
In many tumors, the tumor-associated ECM is strikingly different from the ECM of normal tissues, which, together with infiltrated inflammatory cells and structural epithelial, endothelial, and mesenchymal cells, forms the tumor microenvironment that modulates cancer initiation, progression, and metastasis (Cox and Erler 2016). The initial desmoplastic response to primary tumors by the host tissue is believed to be a defensive one and represents a balance between tumor-promoting and tumor-suppressing cues. This balance eventually tips toward a pro-tumorigenic and pro-metastatic environment. In this scenario, activation of myofibroblasts leads to excessive production of collagen and increased bio-mechanical stiffness, whereas the release of growth factors and cytokines stimulates angiogenesis and cell growth, both of which allow tumor cells to grow unchecked and to invade and metastasize freely. By similar means, fibrotic remodeling at remote sites creates a pro-metastatic microenvironment or niche that favors and supports the colonization of circulating tumor cells or activation of dormant resident tumor cells. These interactions between tumor cells and their stromal microenvironment also affect the response to anti-tumor therapy and, therefore, represent important targets for therapeutic intervention against cancer.
Untransformed human cells would undergo cell cycle arrest with reduced proliferation and mitogenic signaling, and cessation of cell movement if cell density reaches a certain level, a phenomenon known as contact inhibition of proliferation and locomotion of cells that contact each other. This cell contact inhibition is essential to embryonic development, morphogenesis, and tissue repair. In these scenarios, cell-cell contact is essential for contact inhibition but is not sufficient for inhibition of mitotic division of contacting cells. The contact-inhibited cells must also be forced to reduce their cell area under the mechanical stress and constraints for mitotic inhibition to occur. These mechanical constraints are largely imposed by surrounding cells and the ECM (Shraiman 2005). In cancers, transformed cells typically lose contact inhibition and, therefore, would divide and grow over each other in an uncontrolled manner, even when in contact with neighboring cells. This lack of contact inhibition in transformed or cancerous cells is necessary for tumorigenesis, invasion of tumor cells into surrounding tissues, and metastasis of tumor cells to distant organs. The mechanism by which cancerous cells become insensitive to contact inhibition remains largely unclear (Ribatti 2017). Both biochemical and physical mechanisms are involved, including interactions of tumor cells with the matrix. How the tumor microenvironment, chronic inflammation, and fibrosis contribute to the loss of contact inhibition in tumor cells for both mitotic division and locomotion is an intriguing question. In this regard, increased stiffness of the matrix is found to serve as a scaffold to promote tumor cell migration through a process called duro-taxis (Lo et al. 2000), which boosts tumor invasion and metastasis in stiff tissue (Friedl and Wolf 2010).
Inflammation, fibrosis, and cancer are major adverse effects caused by CNTs
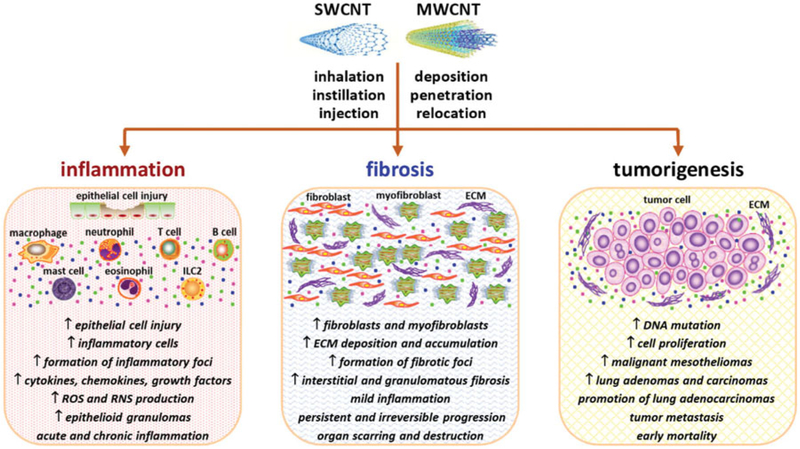
Exposure to CNTs elicits a range of pathologic outcomes in laboratory animals. Among them, inflammation, fibrosis, and tumorigenesis appear to predominate and have received particular attention because these pathologic conditions frequently adopt a chronic and progressive course, and lead to severe outcomes, such as organ failure and mortality (Figure 2). This notion raises concerns about the health risk of exposure to CNTs in human populations and, therefore, demands a better understanding.
Figure 2.
Pathological outcomes induced by CNTs. The fiber-like shape and nano-scaled size enable CNTs to cross biological barriers, penetrate into the cell, enter the circulation, and translocate to distant organs, resulting in cytotoxic and pathologic effects. The predominant pathologic consequences induced by CNT exposure include inflammation, fibrosis, and tumorigenesis. For each effect, the representative features are listed. The main target organs of inhaled CNTs are the lung, the pleura, and the liver. Phenotypic and mechanistic analyses demonstrate that the CNT-induced effects possess a high similarity to those observed in certain human diseases and animal disease models triggered by other agents, suggesting the potential of using CNT-exposed animals as a disease model.
Inflammation
Pulmonary inflammation
Lung inflammation is the pulmonary response to inhaled CNTs that, under a physiological condition, is to remove CNT deposits from the lung and repair damaged lung tissue. Clearance of CNTs from the lung is largely mediated through phagocytosis by macrophages in the airway, alveolar, and interstitial spaces where CNTs deposit. Macrophages degrade engulfed pathogens and materials by digestion, but CNTs generally cannot be digested, or are degraded only slowly and to a limited extent. Most engulfed CNTs are transported to conducting airways for clearance via the mucociliary system, and to the draining lymph nodes where they enter the lymphatic drainage and ultimately the blood circulation. Transportation by engulfing macrophages is believed to be a major mechanism by which CNTs relocate in the lung. On the other hand, needlelike CNTs that are rigid and relatively thick and short in morphology tend to penetrate through cell membranes and barrier structures in the lung, thereby translocating across barriers and reaching remote sites, such as the pleural space (Mercer et al. 2013). At the chronic stage, a majority of CNTs that retain in the lung are found within granulomas.
When CNTs persist in the lung or the exposure continues and overwhelms the lung capacity for clearance, inflammation becomes chronic and CNTs are enclaved in granulomas that are packed with fibrous matrix and macrophages containing engulfed CNTs. Both acute and chronic inflammation causes tissue damage through inflammatory cells and cytotoxic factors, such as ROS, RNS, cytotoxic cytokines, and digestive enzymes, which are produced and released from phagocytic cells and dying tissue cells. While CNTs entering the blood may cause off-site effects in distant organs, most inhaled CNTs deposit in the interstitial space of the lung, leading to airway and alveolar inflammation and injury. These inflammatory events create a milieu with excessive cytokines, chemokines, and growth factors that foster the progression of disease processes, such as fibrosis and tumorigenesis.
Acute inflammation is the first response in CNT-exposed lungs that emerges rapidly within 1 day and reaching an apex on day 7 post-exposure in mice (Table 1). CNTs induce the recruitment and infiltration of inflammatory cells, dominated by neutrophils and macrophages, in the interstitial, perivascular, and peribronchial regions of the lung (Aiso et al. 2010; Dong and Ma 2016c; Dong et al. 2015; Lam et al. 2004; Park et al. 2011; Porter et al. 2013; Porter et al. 2010; Reddy et al. 2012; Rydman et al. 2015; Ryman-Rasmussen et al. 2009; Shvedova et al. 2008; Shvedova et al. 2005; Taylor et al. 2014). CNTs also stimulate significant enrichment of T and B lymphocytes in the lung during the acute phase response (Dong and Ma 2016a; Dong and Ma 2016c; Rydman et al. 2015). Activation of the pro-inflammatory functions of these immune cells leads to increased expression and secretion of pro-inflammatory cytokines and chemokines, typified by TNF-α, IL-1α, IL-1β, IL-6, and monocyte chemotactic protein-1 (MCP-1) (Dong and Ma 2016b; Dong et al. 2015; Vietti, Lison, and van den Brule 2016). During the chronic phase response to CNT exposure, the interstitial, perivascular, and peribronchial inflammation is reduced to a relatively mild level, but granulomatous inflammation, characterized by the local accumulation of activated macrophages, intermingled with fibrotic collagen fiber bundles, CNT deposit clusters, fibroblasts, and myofibroblasts, gradually becomes the prominent inflammatory phenotype in the lung (Huizar et al. 2011; Lam et al. 2004; Muller et al. 2005; Shvedova et al. 2005). Pulmonary lesions caused by CNTs may differ, depending on the dimensions, shapes, and other physicochemical properties of the testing CNTs. For instance, a single intratracheal instillation of either long (8.6 μm) or short (0.55 μm) SWCNTs for a long-term (up to 104 weeks) caused inflammatory lesions in the lung. The lesions caused by the two types of SWCNTs exhibited certain regional specificity, because most long SWCNTs preferentially deposited at the terminal bronchioles, whereas a large number of short SWCNTs reached the alveolar space, giving rise to chronic inflammation in the corresponding lung space (Honda et al. 2017).
Table 1.
CNT-induced pathologic effects in rodent lungs.
| Effect | CNT (length, diameter) | Animal model (exposure) | Pathological phenotype | References |
|---|---|---|---|---|
| Inflammation and fibrosis | Three SWCNTs with varying contents of iron | B6C3F1, mice, male (i.t.i., once, 0.1 or 0.5 mg for 7 or 90 d) | All SWCNTs caused dose-dependent epithelioid granuloma and interstitial inflammation at 7 d, persisting to 90 d | Lam et al. 2004 |
| SWCNT(1–4nm) | C57BL/6 mice, female (i.t.i., once, 10, 20, or 40 μm for 1, 3, 7, 28, or 60 d) | Inflammatory infiltration; ↑ pro-inflammatory cytokines; rapid progressive and diffusive interstitial fibrosis with granulomas | Shvedova et al. 2005 | |
| MWCNT (5.9 ± 0.05 μm, 5.2 ± 1.5 nm) | SD rats (i.t.i., once, 0.5, 2, or 5 mg for 2 m) | Granulocyte accumulation; fibrosis with increased collagen deposition and granuloma formation; elevated level of TNF-α | Muller et al. 2005 | |
| SWCNT (100–1000 nm, 0.8–1.2 nm) | C57BL/6 mice, female (t.b.i., 5 h/d for 4 d, 5 mg/m3, examined after 1, 7, or 28 d; i.t.i, once, 5, 10, or 20 μm for 1, 7, or 28 d) | Inflammatory infiltration; ↑ pro-inflammatory cytokines; multifocal granulomatous pneumonia; interstitial fibrosis | Shvedova et al. 2008 | |
| MWCNT (5–9 μm, 110–170 nm, Sigma #659258) | ICR mice, male, (i.t.i., once, 5, 20, or 50 mg/kg for 1, 3, 7, or 14 d) | Dose and time-dependent increase in neutrophils, type 1 and type 2 cytokines, and granulomas, as well as B cells and IgE production | Park et al. 2009 | |
| MWCNT (0.3–50 μm, 30–50 nm) | C57BL/6 mice, male (nose-only inhalation at 100 mg/m3 for 6 h, alveolar deposition at ~12mg/kg and tracheobronchial deposition at ~4 mg/kg) | MWCNT caused airway fibrosis in ovalbumin-sensitized mice. A role of PDGF and TGF-β1 was implicated | Ryman-Rasmussen et al. 2009 | |
| MWNT-7 (lot #061220, 5 μm, 88 nm) | Fisher 344 rats, male (i.t.i., once, 40 or 160 μm for 1, 7, 28, or 91 d) | Dose and time-dependent pulmonary inflammation, type II cell hyperplasia, microgranulomas, and fibrosis | Aiso et al. 2010 | |
| MWCNT | Rats, male (i.t.i., once, 0.2, 1, or 5 mg/kg for 24 h, 1 w, 1 m, and 3 m) | Dose-dependent lung inflammation, fibrosis, and lesions | Reddy et al. 2012 | |
| SWCNT (5–15 mm, <2 nm) | C57BL/6 mice, female (i.t.i., once, 80 μm for 1, 7, 14, 28, 42, or 56 d) | Pulmonary epithelial and mesenchymal injury, granuloma, and fibrotic change. Epithelial-mesenchymal transition is implicated | Chang et al. 2012 | |
| MWNT-7 (lot #061220–31, MMAD of 1.5 μm) | C57BL/6 mice, male (t.b.i., 5 h/d for 2, 4, 8, or 12 d, 10 mg/m3) | Bronchiolocentric inflammation with ↑ inflammatory cells; fibrosis with inflammation, expanded interstitium, and MWCNT deposition | Porter et al. 2013 | |
| MWNT-7 (lot #061220–31) | C57BL/6J mice, male (t.b.i., 5 mg/m3 for 5 h/d for 12 d; 4 times/w for 3 w; mice examined at 1, 14, 84, 168, or 336 d post-exposure) | Lung inflammation and fibrosis; average thickness of connective tissue in the alveolar region increased by 70% at 336 d post-exposure, indicating progressive and persistent fibrotic response to MWCNT | Mercer et al. 2013 | |
| SWCNT (1–3 μm, ~65 nm, single tube: 1–4 nm) | C57BL/6 mice, female (t.b.i., 5 h/d for 4 d, 5 mg/m3, examined after 1 y) | Chronic bronchopneumonia and lymphadenitis; granulomatous bronchointerstitial pneumonia; fibrosis | Shvedova et al. 2014 | |
| MWCNT (0.5–40 μm in length) | C57BL/6J mice (o.p.a., once, 4 mg/kg for 1 or 28 d) | Pristine MWCNT caused pulmonary inflammation by d 1 and fibrosis by d 28 | Taylor et al. 2014 | |
| MWNT-7 (lot #05072001 k28) | C57BL/6 mice, male (o.p.a., once, 5, 20, or 40 ng for 1, 3, 7, or 14 d) | Inflammatory infiltration; ↑ pro-inflammatory and pro-fibrotic factors; fibrosis and t fibrotic marker protein expression | Dong et al. 2015 | |
| MWCNT (5.53–6.19 μm, 94.1–98.0 nm) | F344 rats (t.b.i., 6 h/d, 5 d/w for 13 w, 0.2, 1, or 5 mg/m3) | Increased lymphocytes and neutrophils; fibrosis with granuloma formation and ↑ collagen deposition | Kasai et al. 2015 | |
| Malignancy | MWNT-7 (lot #061220–31, 3.68 μm, 49 nm) | B6C3F1 mice, male (two stage tumorigenesis: MCA, i.p., once, 10 μg/kg b.w.; MWNT-7, t.b.i., 5 mg/m3, 5 h/d, 5 d/w, total of 15 d; examined after 17 m) | MWNT-7 following MCA treatment significantly ↑ incidences of bronchoalveolar carcinomas and adenocarcinomas compared with controls, demonstrating tumor promotion by MWNT-7; hyperplasia and macrophage infiltration in the lung | Sargent et al. 2014 |
| MWNT-7 (5.2 μm, 83.8 nm; 5.7 μm, 90.7 nm) | F344 rats, male and female (t.b.i. 0.02, 0.2, or 2 mg/m3; 6 h/day, 5 d/w, 104 w) | Significantly ↑ lung carcinomas in males at 0.2 and 2 mg/m3 and in females at 2 mg/m3. Concentration-dependent increase in lung epithelial hyperplasia, granulomatous change, localized fibrosis, and BALF changes | Kasai et al. 2016 | |
| MWCNT-N (51% 1–4 μm, 47% 5–20 μm, 30–100 nm) | F344/GJ rats (t.i.p.s., 8 times over 2 w, 125 mg each; up to 109 w | Both lung tumors and pleural mesothelioma were induced by MWCNT-N sieve fractions; MWCNT-N deposited in granulomas and macrophages | Suzui et al. 2016 | |
| Long SWCNT (8.5 μm); short SWCNT (0.55 μm) | F344 rats, male (i.t.i., once, 0.2 or 1.0 mg/kg b.w. for 26, 52, or 104 w) | Most long SWCNTs deposited at the terminal bronchioles and a large number of small SWCNTs in the alveolus, causing chronic inflammatory responses, but no apparent genotoxicity in the lungs. Lung adenoma and carcinoma observed with no statistical significance | Honda et al. 2017 |
References are listed chronologically. Abbreviations used: CNT: carbon nanotube; d: day; h: hour; i.p.: intraperitoneal injection; i.p.i.: intrapleural injection; i.t.i.: intratracheal instillation; m: month; MCA: methylcholanthrene; MWCNT: multi-walled CNT; MWNT-7: Mitsui MWNT-7; o.p.a.: oropharyngeal aspiration; SWCNT: single-walled CNT; t.b.i.: total body inhalation; t.i.p.s.: transtracheal intrapulmonary spraying; w: week; y: year.
Extrapulmonary inflammatory effect
Exposure to CNTs induces inflammatory responses in organs other than the lung, such as the pleural and peritoneal mesothelial tissues (Table 2). Inflammation in the pleural space is a particular concern because of its potential to lead to pleural fibrosis and mesothelial malignancy. The pleural cavity is a potential space between the visceral and parietal pleural membranes. How inhaled CNTs gain access to the pleural space is an intriguing, yet unresolved question. Two pathways have been proposed for pleural translocation of CNTs. CNTs reaching peripheral lungs may penetrate through the visceral pleura to enter the pleural space (Mercer et al. 2013). CNTs accumulating at the subpleural space may also cause inflammation in the visceral pleura and leak into the pleural space through inflammatory lesions (Xu et al. 2012). Alternatively, CNTs may reach the parietal pleura via the systemic circulation, a route that has been proposed for pleural translocation of pathogenic asbestos fibers (Miserocchi et al. 2008). In both cases, CNTs are likely to be cleared from the pleural space via lymphatic drain through stomatal openings in the parietal pleura, including those on the diaphragm. The stomatal openings are 3–10 μm in diameter. Short CNTs are readily cleared through the stomas, whereas long CNTs that cannot easily negotiate through the openings retain and persist at the parietal pleura, causing parietal inflammation and persistent lesions like pleural plagues and mesotheliomas (Donaldson et al. 2010; Murphy et al. 2011; Poland et al. 2008; Takagi et al. 2008; Xu et al. 2014). These findings support a fiber length-pathogenicity relationship for CNT-induced pleural lesions, which is analogous to the “fiber pathogenicity paradigm” originally defined for pathogenic asbestos and glass fibers (Donaldson et al. 2010; Kane, Hurt, and Gao 2018). For this reason, pathogenic CNTs are sometimes collectively called high-aspect-ratio nanomaterials or HARNs to emphasize the importance of fiber length in CNT pathogenicity and risk evaluation (Kane, Hurt, and Gao 2018; Kuempel et al. 2017).
Table 2.
CNT-induced pathologic effects in rodent mesothelial tissues
| Effect | CNT (length, diameter) | Animal model (exposure) | Pathologic phenotype | References |
|---|---|---|---|---|
| Inflammation and fibrosis | MWCNT (variable dimensions) | C57BL/6 mice, female (i.p., once, 50 ug for 24 h or 7 d) | Long (10–20 um or >20 um), but not short/tangled (<5 um), MWCNT caused peritoneal inflammation at 24 h and granulomas at 7 d post-exposure | Poland et al. 2008 |
| MWCNT (0.7 μm, 11.3nm) | Wistar rats (i.p., once, 2 or 20 mg for 24 m) | Moderate peritoneal inflammation and granulomas by MWCNT | Muller et al. 2009 | |
| MWCNT (variable dimensions) | C57BL/6 mice, female (i.p.i., once, 5 μm, up to 24 w) | Deposition, acute inflammation, and fibrosis in the parietal pleura by long MWCNT (NTlong2, 84% >15 μm) | Murphy et al. 2011 | |
| MWCNT (12.4 μm, 60 nm) | C57BL/6J mice (i.p., once, 50 ug for 24 h or 7 d) | Peritoneal inflammation and fibrosis by pristine fibers; reduced inflammation and fibrosis by less durable MWCNT | Osmond-McLeod et al. 2011 | |
| MWCNT-N; MWCNT-M (MWNT-7) | F344 rats (t.i.p.s., 5 times in 9 d; 250 μm each) | MWCNT translocated to the pleural cavity; visceral pleural inflammation and mesothelial proliferation | Xu et al. 2012 | |
| MWCNT (tangled CNTs, 15 nm) | F344-Brown Norway F1 hybrids (i.p., once, 10 mg for 3 y) | Absence of mesothelioma or granuloma in the peritoneal cavity | Nagai et al. 2013 | |
| MWCNT-L (8 μm, 150 nm); MWCNT-S (3 μm, 15 nm) | F344 rats (t.i.p.s., once every 2 w, 13 times over 24 w, 125 μm each time) | MWCNT-L translocated to the pleural cavity, deposited in parietal pleura, and induced fibrosis and mesothelial proliferation; MWCNT-S induced stronger inflammation in the lung than MWCNT-L | Xu et al. 2014 | |
| SWCNT (3.83 nm or 4.3 nm) | Wistar rats (i.p., once, 0.1, 0.3, 1, 3, 10 mg for 4 w) | No remarkable peritoneal inflammation or other lesions | Toyokuni et al. 2015 | |
| MWNT-7 [1.81 μm (0.12–21.5)]; SWCNT [0.5 μm (0.05–8.14)] | Wistar rats (i.t.i., once, 0.15 or 1.5mg/kg b.w., 90 d) | Persistent pulmonary inflammation by SWCNT, but greater levels of pleural inflammation by MWNT-7 | Fujita et al. 2016 | |
| CNT-1 (MWNT-7); CNT-2 (MWCNT) | C57BL/6 mice, IL1α/β double KO (i.p.i., once, 50 or 100 μg, 28 d) | Pleural mesothelial hyperplasia, inflammation, and fibrosis; CNT-1 induced stronger response than CNT-2; IL1α/β double KO mice showed ↓ lesions | Arnoldussen et al. 2018 | |
| Malignancy | MWNT-7 (1-<5 μm, 72.5%; 100 nm) | p53+/− mice, male (i.p., once; 3mg, up to 180 d) | Peritoneal fibrous scars and granulomas; atypical hyperplasia; typical mesothelioma; mortality from metastasis and peritoneal adhesion | Takagi et al. 2008 |
| MWNT-7 (same as in Takagi et al. 2008) | F344 rats (i.s., once; 1 mg/kg b.w., up to 52 w) | High incidence of peritoneal mesothelial hyperplasia, ascites, mesothelioma, metastasis, and mortality | Sakamoto et al. 2009 | |
| MWNT-7 (4 μm, 50 nm); MWCNTs (varied dimensions and rigidity) | F344-Brown Norway F1 hybrids (i.p., twice with 1 w interval; total of 1 or 10 mg for 1 y) | MWNT-7 induced frequent and early mesothelioma, and granulomas, fibrosis, iron deposition, mesothelial hyperplasia, and Cdkn2a/2b deletions | Nagai et al. 2011 | |
| MWNT-7 (2 μm, 90 nm) | p53+/− mice, male (i.p., once; 3, 30, or 300 mg for 1 y) | Concentration-dependent inflammation, granuloma, monocytes, hyperplasia, mesothelioma | Takagi et al. 2012 | |
| MWCNT (varied dimensions) | Wistar rats (i.p., once; two doses for each MWCNTs for up to 2 y) | Peritoneal mesothelioma with frequencies inversely correlated with MWCNT curvature; granuloma | Rittinghausen et al. 2014 | |
| MWNT-7 (7.1 μm or 2.8 μm) | Wistar rats (i.p., once; 6 mg for 12 m; 2mg for 30 d) | Long and short MWNT-7 induced mesothelioma, promoted by an early and sustained accumulation of immunosuppressive monocytes | Huaux et al. 2016 | |
| MWCNT-N (51% 1–4 μm, 47% 5–20 μm, 30–100 nm) | F344/Crj rats (t.i.p.s., 8 times over 2 w, 125 mg each time; up to 109 w) | Both pleural mesothelioma and lung tumors induced by MWCNT-N sieve fractions; MWCNT-N deposited in granulomas and macrophages | Suzui et al. 2016 | |
| Long MWCNT (>15 μm, 165 nm); short MWCNT (<15 μm, 125 nm) | C57BL/6 mice, female (i.p.i., variable dose and duration) | Mesothelioma with sustained inflammation and increased proliferation by long MWCNT correlated with epigenetic silencing of the tumor suppressor gene Cdkn2a | Chernova et al. 2017 |
References are listed chronologically. Abbreviations used: b.w.: body weight; CNT: carbon nanotube; d: day; h: hour; i.p.: intraperitoneal injection; i.p.i.: intrapleural injection; i.s.: intrascrotal injection; i.t.i.: intratracheal instillation; KO: knockout; m: month; MWCNT: multi-walled CNT; MWNT-7: Mitsui MWNT-7; SWCNT: single-walled CNT; t.b.i.: total body inhalation; t.i.p.s.: transtracheal intrapulmonary spraying; w: week; y: year.
The pleural pathologic effects of CNT exposure are summarized in Table 2. Some studies employed direct injection to deliver CNTs to the pleural space. Direct injection of long MWCNTs into the pleural cavity induced an acute inflammatory response in the parietal pleura and resulted in significantly increased numbers of total cells and granulocytes in the pleural cavity lavage fluid (PCLF) in mice (Murphy et al. 2011). Intrapleural injection of Mitsui XNRI MWNT-7 (MWNT-7), a well-characterized MWCNT preparation with a mean length of 3.86 μm and a count mean diameter of 49 nm caused an inflammatory response in mouse pleura, showing inflammatory infiltration and formation of granulomas (Arnoldussen et al. 2018). In two studies, pulmonary administration of MWCNTs by transtracheal intrapulmonary spraying (t.i.p.s.) induced strong inflammatory responses in rat visceral (Xu et al. 2012) and parietal (Xu et al. 2014) pleura, resulting in increased numbers of inflammatory cells and elevated levels of inflammatory cytokines, such as RANTES (regulated on activation, normal T-cell expressed and secreted, or CCL5), IL-2, and IL-18 in PCLF (Xu et al. 2014). In a separate study, intratracheal instillation of MWCNTs in rats caused inflammation in both the lung and the pleura, with the pleural changes shown as increased numbers of total nucleated cells and macrophages, and elevated IL-18 and SPP1 (secreted phosphoprotein 1, or osteopontin or OPN) levels in PCLF (Fujita et al. 2016). These findings indicate that the pleura is a frequent target of CNT exposure, either directly via injection into the pleural cavity, or indirectly by way of airways and the lung parenchyma.
The peritoneal mesothelium is also a frequent target of mesothelioma-inducing agents, such as asbestos. Intraperitoneal or intrascrotal injection of CNTs induced inflammation in the mesothelial tissues of the peritoneal cavity (Muller et al. 2009; Nagai et al. 2013; Osmond-McLeod et al. 2011; Poland et al. 2008; Toyokuni et al. 2015). Of note, many studies demonstrate CNT-induced pleural and abdominal mesothelial inflammation occurs together with mesotheliomas, which is, therefore, discussed separately under ‘Tumorigenesis’.
The liver is another organ that can be targeted by CNTs upon pulmonary and systemic exposures. Intratracheal instillation of MWCNTs caused inflammation in mouse liver, manifesting inflammatory cell infiltration at 1-month post-exposure, and lobular and portal inflammation with enrichment of macrophages and activation of inflammatory signaling pathways at 1-year post-exposure (Kim et al. 2015). Intravenous injection of SWCNTs induced inflammation in mouse liver, indicated by increased infiltration of total leukocytes and accumulation of macrophages (Principi et al. 2016). Further study is needed to validate the toxicological implication of the hepatic inflammatory effect from CNT exposure.
Fibrosis
Pulmonary fibrosis
CNT-induced fibrosis has been observed and characterized in a large number of studies in rodents. These studies establish that many CNTs are fibrosis inducers and some are with potencies greater than those of silica and asbestos. CNT fibrogenicity in rodents has been reviewed in recent articles (Dong and Ma 2016b; Vietti, Lison, and van den Brule 2016).
Pulmonary exposure to CNTs stimulates a rapid-onset fibrotic response in the lung, which is detectable as early as day 1, reaches a peak on day 7 post-exposure and then declines and transits to chronic phenotypes (Table 1). Chronic fibrosis is fully developed by day 28 and can persist for at least 1-year post-exposure in mouse lungs (Chang et al. 2012; Kasai et al. 2015; Lam et al. 2004; Mercer et al. 2013; Shvedova et al. 2014). Overall, CNT-induced lung fibrosis illustrates a biphasic course of fibrosis development, ultimately to progressive and irreversible fibrosis. At the pathologic level, CNT-induced fibrotic lesions are characterized by the presence of inflammation marked by the accumulation of macrophages, monocytes, and lymphocytes, sustained enrichment of fibroblasts and myofibroblasts, increased deposition of fibrous ECM, elevated expression of fibrosis marker proteins, thickened alveolar septa, and formation of fibrotic foci and epithelioid granulomas. These features of CNT-induced lung fibrosis resemble the pulmonary response to inhaled microbes, invading parasites, and deposited fibrogenic foreign bodies, exemplified by silica, asbestos, and coal dust that cause pneumoconiosis. CNT-induced lung fibrosis also exhibits certain similarities to IPF, a deadly human disease of unknown etiology characterized by progressive and irreversible pulmonary fibrosis. Patients suffering from IPF exhibit a mean survival time of merely 2–5 years after diagnosis. Notably, mechanistic studies reveal that the mediators, signaling pathways, and cellular and pathological processes involved in CNT-induced lung fibrosis are in agreement with the overall understanding of lung fibrosis derived from human fibrotic lung diseases and experimental animal models to a considerable degree (Dong and Ma 2016a; Dong and Ma 2018a; Dong and Ma 2018b; Dong et al. 2015). Thus, CNT-induced lung fibrosis may serve as an animal disease model for identifying cellular and molecular mechanisms implicated in human fibrotic lung diseases.
Pleural fibrosis
Intrapleural injection of long MWCNTs induced the formation of a fibrotic layer over the parietal pleura over a period of 4–24 weeks post-exposure in mice with increased thickness and a high content of collagen (Murphy et al. 2011). Intrapleural injection of XNRI MWNT-7 also caused fibrosis, indicated by increased collagen fibers, in the visceral pleura in mice on day 28 post-exposure (Arnoldussen et al. 2018). Furthermore, transtracheal intrapulmonary spraying of long needle-like MWCNTs once every 2 weeks for 24 weeks in rats enabled MWCNTs to translocate into the pleural cavity, deposit in the parietal pleura, and induce fibrosis in both the visceral and the parietal pleura with thickened lesions composed of collagen fibers (Xu et al. 2014; Xu et al. 2012). In these cases, pleural fibrotic thickening and granulomatous lesions occur in the presence of inflammatory infiltration and secretion (Table 2). Since pleural fibrosis in humans is often associated with exposure to asbestos, these findings suggest a potential of CNTs as a new type of inducer to cause pleural fibrosis in exposed humans. Since pleural fibrosis may contribute to the development of mesothelioma, the potential of CNTs to induce fibrosis in the pleura raises concern. Indeed, increased fibrotic deposition and granuloma formation were found to co-exist with mesotheliomas in the pleural and/or abdominal mesothelial tissues of mice exposed to CNTs (Table 2). Nevertheless, the mechanism by which CNTs induce pleural and abdominal mesothelial fibrosis at the molecular level remains to be specified.
Liver fibrosis
CNT exposure may also induce fibrotic lesions in the liver. A single intraperitoneal injection of MWNT-7 resulted in fibrosis on the liver surface and a significantly elevated fibrosis index 1-month post-exposure in rats, detected by Masson’s Trichrome staining. On the contrary, the injection of tangled MWCNTs (diameter ~2–20 nm) did not cause such changes (Nagai et al. 2011). In another study, intratracheal instillation of MWCNTs (length:13.0 ± 1.5 μm; diameter: 12.5 ± 2.5 nm) caused perisinusoidal fibrosis in mouse liver 1-year post-exposure, shown by increased collagen fibers with Masson’s Trichrome staining and elevated expression of α-SMA measured by immunoblotting (Kim et al. 2015).
Altogether, these studies provide substantial evidence for apparent fibrogenic activities of CNTs in the lung, the pleura and other organs in animals. Although the evidence for CNT-induced organ fibrosis in humans is currently not available, several recent field studies have demonstrated that the body fluids of workers manufacturing CNTs contain increased levels of fibrotic mediators and biomarkers, supporting a possible fibrogenic outcome from CNT exposure in humans (Fatkhutdinova et al. 2016; Liou et al. 2015; Schulte et al. 2012).
Tumorigenesis
Certain CNTs have the physicochemical properties that resemble those of asbestos, such as high aspect ratio, low solubility, and high biopersistence, suggesting that CNTs induce tumorigenesis similarly to asbestos. A number of animal studies have been carried out to specifically test this possibility in recent years, with particular attention to mesothelioma and lung cancer. In particular, MWNT-7 CNTs have been consistently found to be a strong tumor-inducing agent, both as an initiator and a promoter. As a result, the MWNT-7 CNTs have been classified as a possible human carcinogen (Group 2B) by the International Agency for Research on Cancer (IARC) (Grosse et al. 2014; Kuempel et al. 2017).
Mesothelioma
Mesothelioma occurs in the visceral and parietal epithelial linings of the pleural, abdominal, and other cavities. Malignant mesothelioma is notoriously difficult to diagnose and treat, with most patients succumbing to the disease within one to two years of diagnosis. Mesotheliomas are historically linked to exposures to asbestos from mining, construction, shipbuilding, and several other occupations. Although the industrial production and use of asbestos have ceased in the United States and many Western countries, exposures from environmental and secondary sources, in particular, asbestos-insulated buildings, continue to drive new cases. The latency of mesothelioma from asbestos exposure to clinical diagnosis is extremely long, typically up to 20–40 years. As a result, rates of mesothelioma may continue to rise in countries where asbestos use has long stopped. The notion that new materials with properties like those of asbestos, such as carbon nanotubes, may cause mesothelial malignancy in exposed populations, similarly to asbestos, has raised particular concern for workers that manufacture CNTs and CNT-containing materials and consumers using CNT-containing products. As such, there has been a substantial effort in testing and analyzing the potentials of various CNTs to cause mesotheliomas in animal models.
Some early studies demonstrated the deposition of CNTs and the concurrence of inflammation and fibrosis at the parietal pleura, which is the exclusive site of mesotheliomas in humans, but failed to detect mesothelial tumors (Muller et al. 2009; Murphy et al. 2011; Osmond-McLeod et al. 2011; Poland et al. 2008; Xu et al. 2014; Xu et al. 2012). Early studies that demonstrated the tumorigenic activity of CNTs for mesotheliomas employed direct injection of CNTs into the abdominal, scrotal, and pleural cavities, and the application of large doses and genetically predisposed strains of rodents (Table 2) (Nagai et al. 2011; Sakamoto et al. 2009; Takagi et al. 2012; Takagi et al. 2008). These studies provided the early demonstration of the feasibility and methodology for studying CNT tumorigenicity in mesothelial tissues in vivo.
MWNT-7 is a strong inducer of mesotheliomas in rodents following various routes of exposure. Intraperitoneal injection of MWNT-7 at both high (3 mg/mouse) and low (3, 30, or 300 μg/mouse) doses resulted in peritoneal mesotheliomas in heterozygous p53+/− mice (Takagi et al. 2012; Takagi et al. 2008). A single intrascrotal administration induced abdominal mesotheliomas that were invasive to adjacent organs and tissues, such as the liver and the pleura, leading to early death in rats (Sakamoto et al. 2009). Intraperitoneal injection (twice with 1-week interval) of MWNT-7 or non-aggregated MWNT-7 resulted in the formation of malignant mesotheliomas on the liver surface, which decreased the survival rate in rats at 1-year post-exposure. On the contrary, the injection of tangled MWCNTs (diameter ~2–20 nm) did not cause such tumors (Nagai et al. 2011).
Several other types of CNTs besides MWNT-7 have been shown to induce mesotheliomas in rodents. Intraperitoneal injection (twice with 1-week interval) of MWCNTs with diameters of ~50 nm (morphologically similar to MWNT-7) or MWCNTs with diameters of ~145 nm, at a dose of 10 mg per animal, induced malignant mesotheliomas on the liver surface in rats at 1-year post-exposure (Nagai et al. 2011). Four types of MWCNTs, with diameters in the range of 37–85 nm and lengths in the range of7.91–10.24 μm, were administered to rats through intraperitoneal injection and all of them induced malignant mesotheliomas on the serosal surface of the abdominal cavity. These mesotheliomas invaded peritoneal organs, and straight MWCNTs caused higher incidences of carcinogenic events than tangled MWCNTs (Rittinghausen et al. 2014). MWCNTs (MWCNT-N) with diameters of 30–80 nm and lengths of 4.2 ± 2.9 μm, as well as their filtered fractions, induced pleural malignant mesotheliomas and/or lung bronchioloalveolar adenomas and carcinomas upon pulmonary airway exposure by transtracheal intrapulmonary spraying (Suzui et al. 2016). Long (7.1 μm) and short (2.8 μm) MWCNTs (MWNT-7 with different lengths) injected into the peritoneal cavity caused peritoneal mesotheliomas, which was associated with an early and sustained accumulation of immunosuppressive monocytes, i.e. monocytic myeloid derived suppressor cells (M-MDSC), and inflammation (Huaux et al. 2016). Presumably, accumulated M-MDSC boost immunosuppression, which counteracts immune surveillance of tumor cells, thereby increasing tumor cell survival. Long MWCNTs with diameters of ~50 nm and lengths of larger than 15 μm (85% fibers), which were directly instilled into the pleural cavity, induced mesotheliomas in the pleura of mice, with a similar carcinogenic potential to that of long asbestos; moreover, similarities between long CNT- and asbestos-induced mesotheliomas were noted, including latency, time course of progression, preceding inflammation and oxidative DNA damage, and epigenetic silencing of the tumor suppressor gene Cdkn2a (cyclin-dependent kinase 2a), which encodes p16Ink4a and p19ARF (Chernova et al. 2017). Altogether, the findings from these studies suggest that tumorigenesis by CNTs, including MWNT-7 and some other types of CNTs, is a frequent pathologic outcome of CNT exposure in rodents.
Lung cancer
The first published study on CNT tumorigenic activities in the lung was carried out by using a two-stage tumor initiation/promotion protocol in mice. Inhalation of MWNT-7 nanotubes following tumor initiation with a single injection of methylcholanthrene (MCA) resulted in increased incidences of lung bronchoalveolar adenomas and adenocarcinomas, as well as serosal tumors morphologically consistent with sarcomatoid mesothelioma, in comparison with treatment with MCA alone, thereby establishing MWNT-7 as a strong tumor promoter in the lung (Table 1) (Sargent et al. 2014). In a separate study, whole-body inhalation exposure of MWNT-7 aerosol for 104 weeks induced lung carcinomas, mainly bronchioloalveolar carcinomas and combined carcinomas and adenomas, in both male and female rats, establishing MWNT-7 as a complete carcinogen in rat lungs (Kasai et al. 2016). Another MWCNT preparation (MWCNT-N) was shown to induce lung tumors, in which MWCNT-N were fractionated by passing through a sieve into unfiltered (4.2 μm in length), flow-through (2.6 μm in length), and retained (length not determined) fractions (Suzui et al. 2016). Pulmonary administration of the factions by transtracheal intrapulmonary spraying (total of 1 mg/rat during the initial 2 weeks, observation for up to 109 weeks) caused bronchioloalveolar adenomas and carcinomas in 14 out of 38 rats exposed to MWCNT-N factions, whereas none of the rats in the no treatment and vehicle control groups developed cancers. The sieve fractionation of MWCNT-N did not have a significant effect on tumor incidence. Thus, MWCNT-N nanotubes are another type of MWCNTs demonstrated to be a complete carcinogen in rat lungs. Comparison between long (8.5 μm) and short (0.55 μm) SWCNTs demonstrated that long SWCNTs deposited at the terminal bronchioles and short SWCNTs in the alveolus, with much higher incidences of inflammatory changes in long SWCNT-treated group than in short SWCNT-treated group, in rat lungs. Lung adenomas and carcinomas were observed (Honda et al. 2017).
The interplay among CNT-induced pathological effects
Given that inflammation, fibrosis, and malignancy are persistently observed in animal models of CNT toxicity as major outcomes, it is rational to posit that these CNT-induced pathologic responses are interrelated to each other and these interactions are involved in the pathogenesis of CNTs. This rationale is consistent with the current understanding of the interrelations among inflammation, fibrosis, and cancer in human diseases discussed above, and is supported by evidence obtained from some studies. Here, we discuss the progress on this emerging and promising research direction with regard to CNT pathologic effects and disease outcomes.
Inflammation and fibrosis
Pulmonary exposure to CNTs induces a rapid-onset fibrotic response that takes place alongside acute inflammation, forming prominent inflammatory and fibrotic foci during the early phase response. The lesions are characterized by significantly elevated deposition of collagen fibers and increased cellularity consisting of infiltrated inflammatory cells, including a large number of macrophages, with some containing engulfed CNTs, and activated fibroblasts and myofibroblasts, interspersed with CNT fiber singlets, bundles, and aggregates. As CNTs persist in the lung, acute inflammation is resolved to a large extent, but not completely. Instead, it is converted to chronic inflammatory phenotypes, which are relatively mild in intensity but persist throughout the chronic course, leading to progressive chronic fibrosis, indicated by interstitial thickening and formation of fibrotic foci and granulomas, in most animal models. A similar pathogenic process is believed to take place for the development of inflammation and fibrosis in mesothelial tissues, as both mesothelial inflammation and mesothelial fibrotic lesions are consistently observed in animal models with or without mesotheliomas (Table 2). This co-existence and co-development between inflammation and fibrosis in the lung and mesothelial tissues during both the acute and chronic phases indicate a close interaction between inflammation and fibrosis with respect to pathogenic effect development.
The interplay between inflammation and fibrosis plays an important role in the development of a variety of fibrotic diseases. These include diseases caused by infection of bacteria, viruses, fungi or parasites, lesions from toxic chemical agents, such as bleomycin and paraquat, and deposition of fibrogenic foreign bodies, exemplified by silica and asbestos (Borthwick, Wynn, and Fisher 2013; Eming, Wynn, and Martin 2017; Greenberg, Waksman, and Curtis 2007; Lupher and Gallatin 2006; Meneghin and Hogaboam 2007; Mossman and Churg 1998; Pourgholamhossein et al. 2018; Rom, Travis, and Brody 1991; Wick et al. 2010; Wynn and Ramalingam 2012; Xie et al. 2012). In these scenarios, the inciting agents and lesions recruit innate immune cells and activate inflammatory responses. In these inflammatory responses, excessive amounts of pro-inflammatory and pro-fibrotic cytokines, chemokines, growth factors, and ROS are produced and released. The molecular pathways that are activated and mediate these early inflammatory responses may differ, depending on the nature, time, and context of stimulation. For example, activation of the NLRP3 (nucleotide-binding oligomerization domain-like receptor: pyrin domain-containing 3) inflammasome is commonly observed and is required for the maturation and secretion of several pro-inflammatory cytokines, in particular, the pleiotropic IL-1β and IL-18, during acute inflammation (Borthwick 2016; Coll et al. 2015; Hornung et al. 2008). On the other hand, the chronic progression of inflammation and fibrosis depends on pro-fibrotic mechanisms, such as IL-13 and IL-17 signaling cascades (Gieseck, Wilson, and Wynn 2018). These mechanisms exert pathogenic activities to promote fibrosis initiation and progression via multiple means, such as stimulating fibroblast proliferation and inducing fibro-blast-to-myofibroblast differentiation. Reciprocally, the enriched and activated fibroblasts and myofibroblasts during fibrotic responses can produce large amounts of cytokines, chemokines, and ECM proteins that promote the recruitment and activation of immune cells and, thereby, boost and prolong inflammation leading to chronic inflammation (Buckley et al. 2001; Flavell et al. 2008; Kendall and Feghali-Bostwick 2014; Phan et al. 1999). A number of cellular processes and molecular mediators have been identified to mediate the communication between inflammation and fibrosis in human fibrotic diseases and animal models. Some of these factors are induced by CNT exposure and impact the development of CNT-induced inflammation and fibrosis (Figure 3).
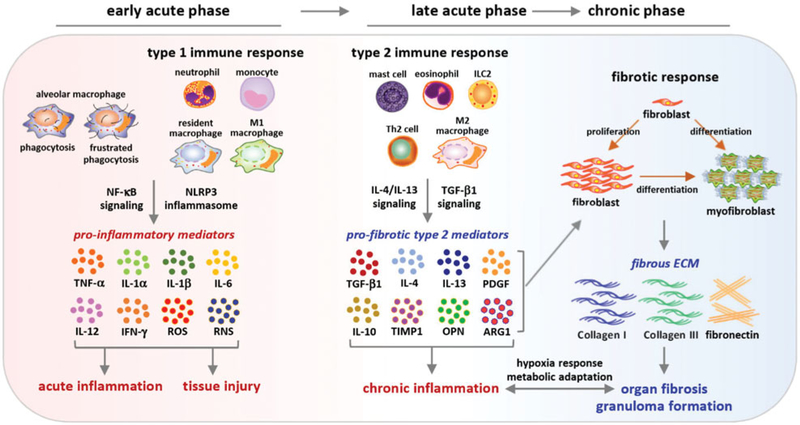
Figure 3.
Pathways implicated in pulmonary inflammation-to-fibrosis progression. Inflammatory cells are recruited and enriched in the lung upon CNT exposure. In the early acute phase, CNTs stimulate the secretion of pro-inflammatory mediators via phagocytosis and type 1 immune response to boost acute inflammation, which requires the activation of the NLRP3 inflammasome. On the other hand, Th2-dependent type 2 immune response is activated in the late acute phase and signals the inhibition and resolution of acute inflammation and the progression to chronic inflammation, interstitial fibrosis, and granuloma through the action of type 2 cells, such as M2 macrophages and type 2 mediators, such as IL-4, IL-13, TGF-β1, TIMP1, and OPN. The type 2 immune response and fibrotic response prolong to the chronic phase. Both inflammation and fibrosis would lead to tissue hypoxia and activate HIF-1α to stimulate angiogenesis and metabolic adaptation. This time-dependent alteration in signaling pathways during pulmonary inflammation-to-fibrosis progression in part reflects the adaptation of innate immune functions to exposure of pathogenic CNTs and tissue injury in the lung.
The migration and infiltration of inflammatory cells occur immediately upon CNT exposure and become pronounced within 1 day post-exposure in the lung (Dong et al. 2015; Porter et al. 2010; Shvedova et al. 2005). Infiltrated macrophages constitute the first line of innate immune defense to inhaled CNTs through phagocytosis. Because of their small sizes, most CNTs can be phagocytosed by macrophages. However, phagocytosed CNTs cannot be degraded or are degraded only to a limited extent in macrophages, due to their graphene structure and sp2 bonds. For elongate CNTs with a fiber length larger than 15 μm, the length of CNTs exceeds the maximal diameter of macrophages and, as such, these CNTs cannot be phagocytosed easily. In either scenario, frustrated phagocytosis ensues and the involving macrophages release inflammatory mediators, which would amplify inflammation. Elevated inflammatory infiltration and the release of associated mediators would increase the defensive activities against CNT deposition; but, at the same time, these intensified inflammatory events would cause damage to the tissue substantially (Rothen-Rutishauser et al. 2010). Studies have shown that exposure to SWCNTs or MWCNTs commonly induces the production and secretion of cytokines, chemokines, growth factors, and ROS from macrophages in vivo and in vitro, which has been highlighted in a few recent reviews (Dong and Ma 2015; Dong and Ma 2016b; Duke and Bonner 2018; Vietti, Lison, and van den Brule 2016).
CNTs induce the recruitment and infiltration of neutrophils, as well as T and B lymphocytes, in exposed lungs (Dong and Ma 2016c; Dong et al. 2015; Rydman et al. 2015). Notably, CNTs activate the NLRP3 inflammasome to regulate the maturation and secretion of IL-1β and IL-18 in mouse lungs to promote acute inflammation (Rydman et al. 2015), whereas Th2 cell-mediated type 2 immune responses are activated to produce type 2 mediators, such as IL-4, IL-5, IL-13, and TGF-β1 (Dong and Ma 2016a; Dong and Ma 2018a; Dong and Ma 2018b). Some of the factors induced by CNTs are pro-fibrotic and play critical roles in the initiation and propagation of fibrosis, providing a mechanism for the interaction between inflammation and fibrosis in CNT-exposed animals. Functionally, NLRP3—IL-1β pathway is clearly required for the acute inflammatory response, as suppression of the pathway by knocking out the gene encoding IL-1 receptor 1 (IL-1R1) or inhibiting IL-1R1 signaling using anakinra attenuated neutrophilia significantly (Nikota et al. 2017; Rydman et al. 2015). Blockade of the NLRP3—IL-1β pathway did not seem to affect the pro-fibrotic IL-4 and IL-13 signaling. On the other hand, knockout (KO) of STAT6 in mice suppressed both the acute inflammation and the fibrotic lesions induced by CNTs in comparison with wild-type mice (Nikota et al. 2017). These findings support the sequential events in the development of fibrosis (Dong and Ma 2018b), which are sometimes summarized as an adverse outcome pathway (AOP) of lung fibrosis induced by CNTs for risk evaluation of CNT pulmonary fibrogenicity (Labib et al. 2016; Nikota et al. 2017; Vietti, Lison, and van den Brule 2016).
TGF-β1 is a major endogenous pro-fibrotic mediator in most human fibrotic diseases and animal models of fibrosis. Many fibrotic signaling pathways, such as the Th2-driven type 2 inflammatory signaling and the IL-1—IL-17 signaling, converge to induce TGF-β1 expression and activate latent TGF-β1 in tissue. As a negative feedback regulation, TGF-β1 inhibits Th responses by inhibiting T cell proliferation. During type 2 immune responses, M2 macrophages are a major source of TGF-β1 production (Murray and Wynn 2011; Wynn and Ramalingam 2012). TGF-β1, in turn, exerts multiple pro-fibrotic effects on fibroblasts and myofibroblasts, which are the major effector cells for fibrotic matrix deposition, remodeling, and contraction. These include promoting fibroblast proliferation, stimulating fibroblast-to-myofibroblast differentiation, and inducing the expression of ECM proteins (Dong and Ma 2016b; Hinz et al. 2012; Leask and Abraham 2004; Tomasek et al. 2002; Wynn and Ramalingam 2012). Upon exposure to CNTs, TGF-β1 is substantially elevated in the lung and in cultured macrophages (Dong and Ma 2016b; Dong and Ma 2017a; Dong and Ma 2018b).
Knockout of TGF-β1 in mice is lethal by the age of 2–4 weeks due to excessive inflammatory responses in organs such as the heart and the lung (Christ et al. 1994; Kulkarni and Karlsson 1993). As such, it is difficult to investigate the effect of TGF-β1 on organ fibrosis by knocking out or knocking down TGF-β1 in animals. Similarly, direct inhibition of TGF-β1 with neutralizing antibodies or chemical inhibitors in vivo may induce side effects, such as inflammation and abnormalities, and, therefore, is not effective (Akhurst and Hata 2012; Mallat et al. 2001). Since TGF-β1 is expressed in multiple cell types, including macrophages, fibroblasts, and epithelial cells, conditional KO of TGF-β1 in a specific cell type may not be sufficient to reduce the overall level of TGF-β1 in vivo and, therefore, is not a preferred approach either. For these reasons, assessing the fibrogenic effect of modulating TGF-β1 expression and activity has been challenging.
The OPN (osteopontin, secreted phosphoprotein 1 or Spp1) KO mouse strain provides a partial solution regarding TGF-β1 for the study of CNT-induced lung fibrosis. OPN is both a cytokine and an ECM protein that regulates a number of important biological processes, including inflammatory cell infiltration, tissue remodeling, and organ fibrosis (Berman et al. 2004; O’Regan 2003; Rittling and Singh 2015; Takahashi et al. 2001). In relation to TGF-β1 functions in fibrosis, OPN was markedly induced by CNTs in the lung and modulated a number of functions via TGF-β1 (Dong and Ma 2017a; Khaliullin et al. 2017; Thompson et al. 2015). On days 7 and 28 post-exposure to MWNT-7, the levels of TGF-β1 protein in lung tissue and lung macrophages were significantly lower in OPN KO mice than those in wild-type (WT) mice (Dong and Ma 2017a). Accordingly, activation of Smad-dependent TGF-β1 signaling was significantly attenuated in lung cells, including fibroblasts and myofibroblasts, in OPN KO lungs, compared with WT. Decreased levels of TGF-β1 protein and impaired TGF-β1 signaling correlated with a reduction in fibrosis in OPN KO lungs exposed to MWCNTs, indicated by reduced fibrotic focus formation, fewer fibroblasts and myofibroblasts, and less ECM deposition, in comparison with WT. This study, therefore, demonstrates a critical role of TGF-β1 in promoting the development of fibrosis induced by MWCNTs, which is regulated by OPN. In a separate study, a two-fold induction of TGF-β1 protein in the bronchoalveolar fluid (BAL) from WT lungs exposed to SWCNTs for 7 days was observed. On the other hand, induction of TGF-β1 protein was undetectable in the BAL from OPN KO lungs exposed to SWCNTs for 7 days, which correlated with decreased deposition of collagen in OPN KO lungs on day 28 post-exposure (Khaliullin et al. 2017). These two studies using OPN KO mice provide evidence supporting TGF-β1 as a signaling mediator to link lung inflammatory responses with fibrosis development induced by CNT exposure.
In a reciprocal manner, fibrotic conditions exhibit a strong propensity to boost and propagate inflammatory responses and promote the conversion of acute inflammation to chronic inflammation characteristic of chronic fibrosis, such as the formation of granulomas. During pathologic fibrosis development, fibroblasts and myofibroblasts are markedly enriched through fibroblast recruitment and proliferation and the fibroblast-to-myofibroblast differentiation. These cells function as the major effector cells for fibrosis development, as they mediate the excessive ECM production and remodeling of injured tissues (Katzenstein and Myers 1998; Tomasek et al. 2002; White, Lazar, and Thannickal 2003; Wynn 2008; Wynn and Ramalingam 2012; Zhang et al. 1994). Nevertheless, the effects of the elevated fibroblastic cell activities go beyond ECM reorganization and tissue contraction. In this respect, activated fibroblasts and myofibroblasts synthesize and secrete cytokines, chemokines, and growth factors, as well as express cell surface receptors, constitutively and through induction. Therefore, these cells exhibit certain features of inflammatory cells and have the capability to respond to immune and ECM signals (Finlay et al. 2000; Heino et al. 1989; Mezzano et al. 2000; Phan et al. 1999; Thannickal, Aldweib, and Fanburg 1998b). Myofibroblasts also produce and release ROS and RNS, leading to oxidative stress (Sambo et al. 2001; Sugiura et al. 2006; Thannickal, Aldweib, and Fanburg 1998a; Thannickal and Fanburg 1995; Wu et al. 2013). These pro-inflammatory mediators and ECM proteins generated by fibroblastic cells during fibrosis can, in turn, create a milieu that fosters persistent inflammation through recruiting and activating immune cells, leading to chronic inflammation in fibrotic tissues.
CNT exposure has been demonstrated to trigger significant increases of fibroblasts and myofibroblasts during the acute and chronic phase responses in the lung, especially in fibrotic foci where CNT fibers deposit and aggregate (Dong and Ma 2016b). Meanwhile, in a few studies performed in cultured fibroblastic cells, the fibroblast-to-myofibroblast transformation was observed when primary lung fibroblasts or fibroblast cell lines were treated with CNTs (Dong and Ma 2016b; Dong and Ma 2017a). Furthermore, certain pro-inflammatory mediators were induced in and secreted by, cultured fibroblastic cells when exposed to CNTs, such as cytokines, ROS, and ECM molecules (Alarifi and Ali 2015; Dong and Ma 2016b; Dong and Ma 2017a). As an example, OPN was induced by MWNT-7 in cultured mouse primary fibroblastic cells and was at high levels in fibrotic and granulomatous foci in mouse lungs during the chronic stage, i.e., on day 28 and day 56, post-exposure to MWNT-7 (Dong and Ma 2017a). In another study, a different type of MWCNTs induced high levels of OPN in granulomatous foci, where increased recruitment of macrophages and CD3+ T cells was concurrent, in mouse lungs on day 60 and day 90 post-exposure (Huizar et al. 2011). Thus, OPN may function as a molecular mediator in fibrosis-promoted chronic inflammation.
Altogether, previous findings support the notion that CNTs stimulate multiple signaling pathways in time and context-dependent manner, which are principally inflammatory in the early phase and fibrotic in the later. These pathways constitute the underlying mechanisms that interconnect inflammation and fibrosis induced by CNT exposure in the lung (Figure 3).
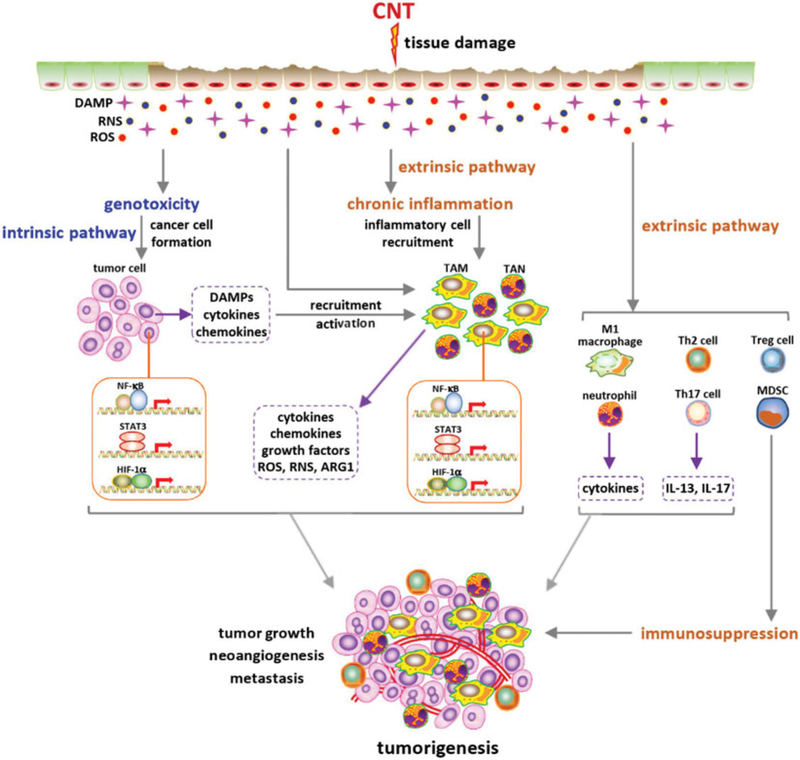
Inflammation and tumorigenesis
A close interplay between inflammation and cancer has been recognized in many types of malignancy (Coussens and Werb 2002; Coussens, Zitvogel, and Palucka 2013; Mantovani et al. 2008). The identified interactions involve both intrinsic (tumor cell-initiated) and extrinsic (non-tumor cell initiated) pathways in tumorigenesis. Under chronic inflammatory conditions, cancer initiation and progression are promoted by extrinsic pathways driven by cytokines, chemokines, and growth factors secreted by immune and inflammatory cells. Conversely, tumor cells cause and boost inflammation through intrinsic pathways activated by genetic and molecular events in tumor cells. Altogether, these events render cancer-associated inflammation to serve as a critical hallmark and an enabling factor for tumorigenesis, tumor promotion, and metastasis. Cancer-related inflammation is characterized by a number of activities, including the activation of transcription factors NF-κB, STAT3, and HIF-1α; infiltration of immune cells, such as macrophages, neutrophils, and mast cells; and production of functional molecules, such as cytokines TNF-α, IL-1β, and IL-6, and chemokines CCL2, CXCL1, and CXCR4. These events are capable of initiating and promoting cancer by enhancing cell proliferation and survival, angiogenesis, and tumor cell invasion and metastasis. In the case of occupational and environmental exposure-induced human diseases, such as asbestosis and silicosis, chronic inflammation is commonly observed and is a critical factor in determining the development of mesothelioma and lung carcinoma (Matsuzaki et al. 2012; Mossman et al. 1990; Mossman and Churg 1998; Otsuki et al. 2016).
In response to CNT exposure, inflammation occurs immediately and prolongs over the entire pathologic stage, as discussed above. It is plausible that inflammation precedes tumorigenesis, and tumorigenesis and inflammation co-occur during the chronic stage after exposure. Indeed, a number of studies have demonstrated the co-existence of inflammation and malignancy in CNT-exposed animals, suggesting an association between inflammation and cancer induced by CNTs (Tables 1 and 2) (Kuempel et al. 2017; Sinis et al. 2018). For instance, when directly instilled into the pleural cavity of mice, long MWCNTs with the length of 85% fibers more than 15 μm, which are similar to long fiber amosite asbestos, resulted in chronic inflammation and mesotheliomas in the pleura (Chernova et al. 2017). This study revealed the induction of several markers of cancer-associated inflammation by long MWCNTs, including elevated mRNA levels of the Il6 and the Stat3 genes in CNT-damaged areas, the increased protein level of phosphorylated STAT3, and augmented cell proliferation and oxidative DNA damage, providing direct evidence supporting an interplay between inflammation and cancer. In vitro studies using mesothelial cells indicated that CNTs have a propensity to induce cancer-associated inflammation through intrinsic pathways. Short-term (up to 24-hour) exposure to SWCNTs induced ROS production, DNA damage, and NF-κB activation in human normal and malignant mesothelial cells in a dose-dependent manner (Pacurari et al. 2008). In another study, 4-month chronic exposure to SWCNTs or MWCNTs resulted in increased proliferation, migration, and invasion of human pleural mesothelial MeT5A cells, and elevated expression and activity of matrix metalloproteinase (MMP)-2 (Lohcharoenkal et al. 2013). These findings indicate the tendency for CNTs to generate tumorigenic effects and promote cancer-associated inflammation. Altogether, the initiation of the multilayered genotoxic and inflammatory responses associates inflammation with tumorigenesis in response to CNT exposure, which governs tumorigenesis by CNTs, including the initiation, promotion, and metastasis of tumors in the lung and the chest pleura (Figure 4).
Figure 4.
Interplay between inflammatory and tumorigenic pathways in tumor development induced by CNTs. Deposition of CNTs and associated lesions in the lung or the pleura cause the local production of a range of DNA-damaging agents, such as ROS and RNS, leading to tumorigenic mutations in epithelial and mesothelial cells and formation of tumor cells. A variety of DAMPs and chemokines are released from live and dead tumor cells (intrinsic pathways) and from tissue lesions (extrinsic pathways), which recruit and activate TAMs and TANs. Cancer-associated innate immune cells secrete certain factors, such as IL-6, IL-1, TNF, NO, ROS, and ARG1, which activate NF-κB, STAT3, YAP, and Notch in tumor cells and boost the survival and growth of tumor cells. CNT-stimulated M1 macrophages and neutrophils also contribute to this positive regulation of tumorigenesis through inflammatory cytokines. In addition, activation of Th2-dependent IL-13 and Th17-dependent IL-17 signaling enhances tumor cell growth. Accumulation of Treg and inhibitory MDSC boosts immunosuppression, which counteracts immune surveillance of tumor cells, thereby increasing tumor cell survival. The rapid growth of tumor cells creates a hypoxic microenvironment that activates HIF signaling in tumor cells and TAMs, leading to further production of TGF-β1 and VEGF, neoangiogenesis, and increased oxygen supply. Together, these events promote tumor cell proliferation, tumor growth, and metastasis.
Fibrosis and tumorigenesis
In response to tissue injury or fibrogenic agents and under many disease conditions, fibroblasts and myofibroblasts are enriched and activated to mediate the initiation and progression of tissue fibrosis. Pathologic fibrosis can also be elicited by tumor cells resulting from genetic insults. A close relationship between fibrosis and cancer has been highlighted in recent publications (Kalluri 2016; Ohlund, Elyada, and Tuveson 2014; Rybinski, Franco-Barraza, and Cukierman 2014; Schafer and Werner 2008; Yazdani, Bansal, and Prakash 2017). On one hand, certain factors generated during fibrosis are pro-tumorigenic and contribute to the development of cancer in fibrotic tissues. On the other hand, certain types of tumors induce the formation of fibrotic tissues, i.e. tumor stroma, in which fibroblasts, myofibroblasts, macrophages, and ECM proteins are predominant. The close association between cancer and stromal cells promotes cancer initiation, progression, and metabolism by multiple means and mediators including ROS, cytokines, chemokines, MMPs, and ECM proteins. In this scenario, myofibroblasts and macrophages are major effector stromal cells that interplay with tumor cells to promote tumorigenesis.
CNT-induced fibrosis occurs in fibrosis-prone organs, specifically, the lung, pleura, and liver, where tumors are also identified at a high frequency, supporting a possible interaction between fibrosis and certain cancers (Tables 1 and 2). For example, a single intraperitoneal injection of MWNT-7 (1 mg) induced fibrosis on the liver surface at 1-month post-exposure, whereas intraperitoneal injection of MWNT-7 at 10 mg for two times with 1-week interval resulted in malignant mesotheliomas on the liver surface at 1-year post-exposure, in rats (Nagai et al. 2011). A number of factors, such as TGF-β, PDGF, ECM, MMPs, and ROS, are known to be produced during fibrosis and are pro-tumorigenic. These factors are induced by CNTs in target organs, providing potential molecular links between fibrotic responses and tumorigenesis in CNT-exposed animals.
The tissue inhibitor of metalloproteinase 1 (TIMP1) was rapidly and highly induced in the lung by MWNT-7 in a time- and dose-dependent manner and high levels of TIMP1 were present in the BAL, serum, and lung tissue of MWCNT-exposed mice (Dong and Ma 2017b). Knockout of Timp1 in mice caused significant decreases in fibroblast accumulation, myofibroblast differentiation, collagen fiber deposition, and fibrotic focus formation in lungs exposed to MWNT-7, compared with WT. Importantly, exposure to MWNT-7 significantly increased the expression of the cell proliferation markers Ki-67 (marker of proliferation) and PCNA (proliferating cell nuclear antigen), as well as a panel of cell cycle-controlling genes, in the lung in a TIMP1-dependent manner. Meanwhile, MWNT-7 elicited a significant induction of CD63 and integrin β1 in lung fibroblasts and the formation of the TIMP1/CD63/integrin β1 complex on the surface of fibroblasts, which triggered the phosphorylation and activation of Erk1/2 that mediates cell proliferation (Dong and Ma 2017b). Notably, TIMP1 was identified as an important mediator of tumor cell proliferation and tumor growth with pro-proliferative activities in mouse lung cancer and glioblastoma multiforme models (Friedmann-Morvinski et al. 2016; Xia et al. 2012). The pro-proliferative role of TIMP1 in mouse lung cancer was shown to involve high-level ERK activation (Xia et al. 2012). These studies suggest a possible role of TIMP1 produced during the fibrotic response to CNTs in promoting CNT-induced lung cancer development.
CNTs of varying types commonly induce the excessive production and deposition of ECM proteins, in particular, collagens and fibronectin, markers of tissue fibrosis (Dong and Ma 2016b; Vietti, Lison, and van den Brule 2016). Increased deposition of ECM components can promote cancer progression in multiple ways, such as enhancing the release of growth factors, regulating the behavior of stromal cells, and promoting angiogenesis (Bonnans, Chou, and Werb 2014; Lu, Weaver, and Werb 2012). As discussed above, fibrosis and cancer co-occur in several organs in CNT-exposed animals, including the lung, pleura, and liver. In these scenarios, the newly synthesized fibrotic ECM from fibrosis creates a tumorigenic microenvironment that boosts tumorigenesis in CNT-deposited tissues, and tumor progression and metastasis in nearby and distal tissues, respectively. Notably, MWCNTs that induce severe fibrosis have a higher capability to induce tumorigenesis than those that cannot, or only slightly, induce fibrosis (Kuempel et al. 2017; Sinis et al. 2018). For instance, intraperitoneal injection of MWNT-7 induced fibrosis on the liver surface in rats 1-month post-exposure, whereas injection of tangled MWCNTs did not. Consistently, intraperitoneal injection of MWNT-7 or non-aggregated MWNT-7 resulted in the formation of malignant mesotheliomas on the liver surface in rats at 1-year post-exposure, but injection of tangled MWCNTs did not (Nagai et al. 2011). This study supports a promoting function of fibrosis in tumor development in CNT-exposed animals to some extent.
Nevertheless, the evidence that directly demonstrates the association between fibrosis and cancer in CNT pathology is currently lacking and awaits future studies, which is in part attributable to a lack of appropriate methodology and models for fibrosis and cancer that have long latencies and require prolonged exposure and development. The identification of the mediators that regulate the interplay between CNT-induced fibrosis and cancer would provide new insights into, and the therapeutic targets for, the prevention and treatment of fibrogenic particle and fiber-induced cancer development.
Implication for human disease
The interactions among inflammation, fibrosis, and cancer in disease development discussed above are largely based on animal studies. Extrapolation of these laboratory findings to human health is necessary, which is particularly true for evaluation of the adverse health effects of nanomaterials including CNTs, as the human effects of nano exposures remain largely to be defined at the present stage. The recent classification of MWNT-7 as ‘possibly carcinogenic to humans (Group 2B)’ and SWCNTs and all other MWCNTs (excluding MWNT-7) as ‘not classifiable as to their carcinogenicity to humans (Group 3)’ by IARC illustrates the utility and importance of this approach for risk evaluation and regulation of nano exposures where animal studies and mechanistic understanding were emphasized (Grosse et al. 2014). Nonetheless, considerable knowledge gaps exist in elucidating the mechanistic steps involved in CNT carcinogenicity, which hinders further risk assessment of tumorigenic CNTs (Kuempel et al. 2017). As has been demonstrated for many human cancers, the close interplay among inflammation, fibrosis, and cancer is an integral component of cancer development induced by CNTs and, therefore, represents a valuable mechanistic knowledge base for the risk assessment of CNT tumorigenesis. Several recent studies have employed end point-targeted pathway analyses, i.e. AOPs, to outline and integrate major steps toward the development of a specific endpoint phenotype (Labib et al. 2015; Nikota et al. 2017; Vietti, Lison, and van den Brule 2016). In these analyses, interactions among inflammation, fibrosis, and cancer were demonstrated as major events in the development of pulmonary fibrosis and cancer induced by CNTs.
Comparison between animal studies and human epidemiology (where available) is another useful and necessary step in translating animal data to human health. Since human studies of nano exposure and health impact are currently lacking, knowledge obtained from human diseases caused by particles and fibers, such as pneumoconiosis, lung cancer, and mesothelioma, is sometimes used as a comparison in the animal-to-human extrapolation of nanotoxicity. This approach is particularly useful for the analysis of the mode of action and disease pathogenesis, which is well illustrated in the demonstration of the fiber length paradigm for some adverse effects of nanotubes. However, a comparison between animal and human studies can be challenging. In part, this is due to the fact that human disease is often heterogeneous in terms of etiology and pathological and clinical phenotypes, and that epidemiological data are generally limited with regard to the size, genetic variability, and demographic distribution of the populations studied, the uncertainty in exposures (exposure agent, mixture, and the dose, duration, and route of exposure), long latency for many disease phenotypes, and numerous confounding factors, such as co-existing disease conditions and medications. Variations in association analysis of human data have been observed for particle- and fiber-induced diseases. A few examples of such variations are discussed below.
While the fiber pathogenicity paradigm has been well demonstrated in animal studies and is commonly supported by epidemiological studies for asbestos-induced mesotheliomas (Donaldson et al. 2010; McDonald et al. 1989; Rogers et al. 1991), some human data indicated otherwise. In one case, mesotheliomas were not found associated with long asbestos fibers as believed but were probably associated with some low-aspect-ratio fibers (Churg and Vedal 1994).
The association between fibrosis and cancer is generally accepted for silica and asbestos-induced diseases, but variations exist in terms of inducer, dose, and latency. There is a general agreement that, for amosite or crocidolite-induced diseases, mesotheliomas and plagues appear at much lower burdens than does asbestosis; but mesotheliomas were shown to appear at higher fiber burdens than do pleural plaques (Churg 1991; Roggli, 1990; Roggli, Pratt, and Brody 1986; Wagner et al. 1988). Chrysotile-induced mesotheliomas appeared at very high burdens of tremolite and chrysotile that are comparable to that of asbestosis or airway fibrosis (Churg 1991; Roggli 1990; Roggli, Pratt, and Brody 1986). In one study, co-existence of pleural plaque and mesothelioma was found to be 70% for plaques (69 in 103 cases) and 83% for mesotheliomas (69 in 83 cases) (Churg and Vedal 1994). In this case, a higher fiber concentration was found for induction of lung cancer than for asbestosis by chrysotile, while the fiber concentration for induction of mesothelioma was the same as that for induction of plague. For amosite, however, mesotheliomas or lung cancer appeared at much lower burdens than that seen with airway fibrosis or asbestosis. Therefore, the relative fiber burdens required for tumorigenesis and fibrogenesis in the lung and the pleura by asbestos fibers can differ greatly, depending on the location and type of the lesions and the type and properties of asbestos fibers deposited.
Due to the long latency and variable pace of development for fibrosis and mesothelioma, the association between fibrosis and mesothelioma by way of latency and slope of dose-response curve can also be difficult and variable in human studies. In a study on lung tissue samples from 819 individuals, which consist of 419 cases of malignant mesothelioma, 340 cases of lung carcinoma, and 206 cases of asbestosis, all associated with asbestos exposures, the samples were divided chronologically into two groups, with those occurring in the first half in group 1 (median exposure duration: 27 years) and those occurring in the second half in group 2 (median exposure duration: 25 years). It was found that patients with asbestosis appeared to have slightly older ages (64 years in group 1 and 69 years in group 2) than patients with mesothelioma (62 years in group 1 and 65 years in group 2) or lung carcinoma (62 years in group 1 and 67 years in group 2) (Roggli and Vollmer 2008). The source of this slight difference in age between lung fibrosis and malignancy caused by asbestos exposures was not identified but possibly reflects a slightly longer latency for clinically recognized fibrosis than for mesothelioma and lung cancer.
Overall, these associations in humans support a mechanistic connection among inflammation, fibrosis, and cancer induced by particles and fibers, and, at the same time, emphasize the importance of taking into considerations of the multiple factors involved and the specific context examined when analyzing the associations in humans and when extrapolating animal findings to human health.
Conclusion
A large number of studies in animals demonstrate that CNTs generate adverse effects that impact multiple fundamental biological processes, such as cell growth, differentiation, and migration, innate and adaptive immune functions, tissue repair, and genomic stability, which in turn lead to the development of prevalent pathologic conditions, including cytotoxicity, genotoxicity, immunotoxicity, and, pertinent to this review, inflammation, fibrosis, and tumorigenesis, in several organ systems. The possibility that exposure to CNTs causes these adverse outcomes in human populations is a major and rational concern, which has unfortunately been supported to a certain degree by a number of studies on CNT-exposed workers (Fatkhutdinova et al. 2016; Schulte et al. 2012; Vlaanderen et al. 2017). These findings demand the safer design, production, and use of CNT-containing materials and commercial products, which must build upon a better and detailed understanding of the adverse effects and their underlying mechanisms at the molecular, cellular, organ, and organismal levels. Among the many unaddressed questions regarding CNT-induced pathologic effects, the cross-interactions among several mechanisms, namely, inflammation, fibrosis, and cancer, attract particular attention, as these interactions appear to be, at least in part, responsible for the initiation and progression of disease conditions associated with CNT exposure. The discussion from this angle represents an emerging, yet promising research area in the studies of disease pathology, which is expected to provide new clues to the understanding of disease initiation and development induced by CNT exposure.
The mechanistic connections among inflammation, fibrosis, and cancer are recognized at several levels. At organ and system levels, chronic or recurrent inflammatory lesions frequently lead to tissue fibrosis and increase the likelihood of cancer development in most organs, which indicates that the mediators and effectors generated during inflammation are critical components in promoting fibrosis and tumorigenesis. Conversely, fibrosing tissues and tumors produce certain factors that stimulate and sustain chronic inflammation. In the context of CNT exposure, mechanistic studies in experimental animals have revealed evidence supporting these connections in the development of pathological responses to CNTs. Importantly, a variety of signaling mediators have been identified to potentially regulate these interactions, including type 1 cytokines, such as IL-1β, IL-6, and TNF-α, and type 2 mediators, such as IL-4, IL-13, TGF-β1, PDGF, and VEGF, in addition to ROS, RNS, and the ECM. At the cellular level, immune cells are recognized to play important roles in the development of inflammation, fibrosis, and cancer; on the other hand, fibroblasts and myofibroblasts are essential for fibrosis and tumorigenesis, whereas epithelial and endothelial cells are target cells of tissue injury that generate DAMPs to initiate and promote inflammation, fibrosis, as well as cancer. In the case of cancer, tumor cells act both as initiator cells (via the intrinsic pathways) and as target cells (via the extrinsic pathways). All these types of cells have been shown to be enriched, activated, or injured in target organs from CNT-exposed mammals. The analysis of the functions of these cells has provided new clues to understanding the communication among the mediators and pathways activated by CNTs. At the molecular level, a number of transcription factors and their upstream signaling pathways have been demonstrated to be induced and activated by CNTs, such as NF-κB, Erk1/2, STAT3, and STAT6. These transcription factors are multifunctional and play critical roles in promoting disease development both directly and indirectly, which render them potential functions in the development of CNT-induced pathological responses. Altogether, accumulating evidence supports the association and integral mechanisms underlying the occurrence of the major pathologic effects induced by CNTs.
It is necessary to point out that the research in this direction regarding CNT pathology is at an early stage, and there are major issues that remain to be addressed. Listed here are a few of them. What are the exact mediators that exert functions in the relations among inflammation, fibrosis, and cancer under CNT exposure? How are these mediators induced by CNTs? Do these mediators cross-interact with each other, and, if so, which ones are the upstream factors controlling the activation of the signaling cascades? What are the modes of action and determinant activities of these functional mediators? These questions can be addressed with the facility of multiple tools, such as genetically engineered mouse strains, neutralizing antibodies, and specific chemical inhibitors. In vitro approaches that integrate specific testing capabilities with customized systems that mimic specific in vivo conditions, such as three-dimensional cultures, as well as scalable, programmable, and standardized procedures and reagents, have been increasingly used in screening and mechanistic analyses of the health effects of numerous nanomaterials and products. The findings may help to identify the molecular mediators and cellular processes that promote and link the underlying pathways for disease development in response to CNT exposure.
CNT fibers are foreign bodies in the body. Due to their unique physicochemical properties, i.e. the nano-scaled size, poor solubility, and biopersistence, CNTs may penetrate cells and tissues or associate with ECM components, leading to sustained deposition and accumulation in target organs. With this feature, CNTs can generate continuous and prolonged exposure that results in the development of chronic inflammation, fibrosis, and cancer. The concurrence of these three prevalent pathologic phenotypes induced by CNTs renders CNT-exposed animals to serve as a disease model for elucidating the integral mechanisms underlying these lesions in humans. Moreover, the identified pathological features and cellular and molecular mechanisms of CNT pathology are in agreement with the overall understanding of similar disease conditions in humans and in animal disease models to a great extent. Therefore, studies in this research direction would provide insights into the mechanistic understanding and clinical treatment of human diseases associated with chronic inflammation, fibrosis, and malignancy, in addition to advancing the knowledge on CNT pathology and toxicology. As an example, the signaling pathways geared toward type 1 and type 2 immune and inflammatory responses to particulates and nanomaterials have been analyzed for risk assessment in nanotoxicology and nanosafety, exemplified by the AOP approach. It is rational to posit that these pathways and approaches can also be explored to identify new targets for intervention against chronic inflammation, fibrosis, and cancer, providing new opportunities for translational research in the near future.
Funding
This work was funded to Q. M. by the Health Effects Laboratory Division and the Nanotechnology Research Center at National Institute for Occupational Safety and Health.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
References
- Abdalla S, Al-Marzouki F, Al-Ghamdi AA, and Abdel-Daiem A. 2015. “Different Technical Applications of Carbon Nanotubes.” Nanoscale Research Letters 10 (1): 358. doi: 10.1186/s11671-015-1056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiso S, Yamazaki K, Umeda Y, Asakura M, Kasai T, Takaya M, Toya T, et al. 2010. “Pulmonary Toxicity of Intratracheally Instilled Multiwall Carbon Nanotubes in Male Fischer 344 rats.” Industrial Health 48 (6): 783–795. doi: 10.2486/indhealth.ms1129. [DOI] [PubMed] [Google Scholar]
- Akhurst RJ, and Hata A. 2012. “Targeting the TGFβ Signalling Pathway in Disease.” Nature Reviews Drug Discovery 11 (10): 790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarifi S, and Ali D. 2015. “Mechanisms of Multi-Walled Carbon Nanotubes-Induced Oxidative Stress and Genotoxicity in Mouse Fibroblast Cells.” International Journal of Toxicology 34 (3): 258–265. doi: 10.1177/1091581815584799. [DOI] [PubMed] [Google Scholar]
- Arnoldussen YJ, Skaug V, Aleksandersen M, Ropstad E, Anmarkrud KH, Einarsdottir E, Chin-Lin F, et al. 2018. “Inflammation in the Pleural Cavity following Injection of Multi-Walled Carbon Nanotubes Is Dependent on Their Characteristics and the Presence of IL-1 Genes.” Nanotoxicology 12 (6): 522–538. doi: 10.1080/17435390.2018.1465139. [DOI] [PubMed] [Google Scholar]
- Bagot CN, and Arya R. 2008. “Virchow and His Triad: A Question of Attribution.” British Journal of Haematology 143 (2): 180–190. doi: 10.1111/j.1365-2141.2008.07323.x. [DOI] [PubMed] [Google Scholar]
- Balkwill F, and Mantovani A. 2001. “Inflammation and Cancer: Back to Virchow?” Lancet (London, England) 357 (9255): 539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Balkwill FR, and Mantovani A. 2012. “Cancer-Related Inflammation: Common Themes and Therapeutic Opportunities.” Seminars in Cancer Biology 22 (1): 33–40. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Berman JS, Serlin D, Li X, Whitley G, Hayes J, Rishikof DC, Ricupero DA, et al. 2004. “Altered Bleomycin-Induced Lung Fibrosis in Osteopontin-Deficient Mice.” American Journal of Physiology, Lung Cellular and Molecular Physiology 286 (6): L1311–1318. doi: 10.1152/ajplung.00394.2003. [DOI] [PubMed] [Google Scholar]
- Bonnans C, Chou J, and Werb Z. 2014. “Remodelling the Extracellular Matrix in Development and Disease.” Nature Reviews Molecular Cell Biology 15 (12): 786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthwick LA, Wynn TA, and Fisher AJ. 2013. “Cytokine Mediated Tissue Fibrosis.” Biochimica et Biophysica Acta (Bba) – Molecular Basis of Disease 1832 (7): 1049–1060. doi: 10.1016/j.bbadis.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthwick LA 2016. “The IL-1 Cytokine Family and Its Role in Inflammation and Fibrosis in the Lung.” Seminars in Immunopathology 38 (4): 517–534. doi: 10.1007/s00281-016-0559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, and Salmon M. 2001. “Fibroblasts Regulate the Switch from Acute Resolving to Chronic Persistent Inflammation.” Trends Immunol 22 (4): 199–204. [DOI] [PubMed] [Google Scholar]
- Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, and Williamson B. 1975. “An Endotoxin-Induced Serum Factor That Causes Necrosis of Tumors.” Proceedings of the National Academy of Sciences of the United States of America 72 (9): 3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Tsai ML, Huang HC, Chen CY, and Dai SX. 2012. “Epithelial-Mesenchymal Transition Contributes to SWCNT-Induced Pulmonary Fibrosis.” Nanotoxicology 6(6): 600–610. doi: 10.3109/17435390.2011.594913. [DOI] [PubMed] [Google Scholar]
- Chernova T, Murphy FA, Galavotti S, Sun X-M, Powley IR, Grosso S, Schinwald A, et al. 2017. “Long-Fiber Carbon Nanotubes Replicate Asbestos-Induced Mesothelioma with Disruption of the Tumor Suppressor Gene Cdkn2a (Ink4a/Arf).” Current Biology 27 (21): 3302–3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ M, McCartney-Francis NL, Kulkarni AB, Ward JM,Mizel DE, Mackall CL, Gress RE, et al. 1994. “Immune Dysregulation in TGF-Beta 1-Deficient Mice.” Journal of Immunology 153 (5): 1936–1946. [PubMed] [Google Scholar]
- Churg A, and Vedal S. 1994. “Fiber Burden and Patterns of Asbestos-Related Disease in Workers with Heavy Mixed Amosite and Chrysotile Exposure.” American Journal of Respiratory and Critical Care Medicine 150 (3): 663–669. doi: 10.1164/ajrccm.150.3.8087335. [DOI] [PubMed] [Google Scholar]
- Churg A 1991. “Analysis of Lung Asbestos Content.” British Journal of Industrial Medicine 48 (10): 649–652. doi: 10.1136/oem.48.10.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll RC, Robertson AAB, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, et al. 2015. “A Small-Molecule Inhibitor of the NLRP3 Inflammasome for the Treatment of Inflammatory Diseases.” Nature Medicine 21 (3): 248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, and Werb Z. 2002. “Inflammation and Cancer.” Nature 420 (6917): 860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Zitvogel L, and Palucka AK. 2013. “Neutralizing Tumor-Promoting Chronic Inflammation: A Magic Bullet?” Science (New York, N.Y.) 339 (6117): 286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TR, and Erler JT. 2016. “Fibrosis and Cancer: Partners in Crime or Opposing Forces?” Trends in Cancer 2 (6): 279–282. doi: 10.1016/j.trecan.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Davis JM, and Cowie HA. 1990. “The Relationship between Fibrosis and Cancer in Experimental Animals Exposed to Asbestos and Other Fibers.” Environmental Health Perspectives 88: 305–309. doi: 10.1289/ehp.9088305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Volder MF, Tawfick SH, Baughman RH, and Hart AJ. 2013. “Carbon Nanotubes: Present and Future Commercial Applications.” Science (New York, N.Y.) 339 (6119): 535–539. doi: 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- Doll R 1955. “Mortality from Lung Cancer in Asbestos Workers.” British Journal of Industrial Medicine 12 (2): 81–86. doi: 10.1136/oem.12.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G, and Alexander A. 2006. “Carbon Nanotubes: A Review of Their Properties in Relation to Pulmonary Toxicology and Workplace Safety.” Toxicological Sciences: An Official Journal of the Society of Toxicology 92 (1): 5–22. doi: 10.1093/toxsci/kfj130. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Murphy FA, Duffin R, and Poland CA. 2010. “Asbestos, Carbon Nanotubes and the Pleural Mesothelium: A Review of the Hypothesis regarding the Role of Long Fibre Retention in the Parietal Pleura, Inflammation and Mesothelioma.” Particle and Fibre Toxicology 7: 5. doi: 10.1186/1743-8977-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K, and Poland CA. 2012. “Inhaled Nanoparticles and Lung Cancer – What we Can Learn from Conventional Particle Toxicology.” Swiss Medical Weekly 142: W 13547. doi: 10.4414/smw.2012.13547. [DOI] [PubMed] [Google Scholar]
- Dong J, and Ma Q. 2015. “Advances in Mechanisms and Signaling Pathways of Carbon Nanotube toxicity.” Nanotoxicology 9 (5): 658–676. doi: 10.3109/17435390.2015.1009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, and Ma Q. 2016a. “In Vivo Activation of a T Helper 2-Driven Innate Immune Response in Lung Fibrosis Induced by Multi-Walled Carbon Nanotubes.” Archives of Toxicology 90 (9): 2231–2248. doi: 10.1007/s00204-016-1711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, and Ma Q. 2016b. “Myofibroblasts and Lung Fibrosis Induced by Carbon Nanotube Exposure.” Particle and Fibre Toxicology 13 (1): 60. doi: 10.1186/s12989-016-0172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, and Ma Q. 2016c. “Suppression of Basal and Carbon Nanotube-Induced Oxidative Stress, Inflammation and Fibrosis in Mouse Lungs by Nrf2.” Nanotoxicology 10 (6): 699–709. doi: 10.3109/17435390.2015.1110758. [DOI] [PubMed] [Google Scholar]
- Dong J, and Ma Q. 2017a. “Osteopontin Enhances Multi-Walled Carbon Nanotube-Triggered Lung Fibrosis by Promoting TGF-beta1 Activation and Myofibroblast Differentiation.” Particle and Fibre Toxicology 14 (1): 18. doi: 10.1186/s12989-017-0198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, and Ma Q. 2017b. “TIMP1 Promotes Multi-Walled Carbon Nanotube-Induced Lung Fibrosis by Stimulating Fibroblast Activation and Proliferation.” Nanotoxicology 11 (1): 41–51. doi: 10.1080/17435390.2016.1262919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, and Ma Q. 2018a. “Macrophage Polarization and Activation at the Interface of Multi-Walled Carbon Nanotube-Induced Pulmonary Inflammation and Fibrosis.” Nanotoxicology 12 (2): 153–168. doi: 10.1080/17435390.2018.1425501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, and Ma Q. 2018b. “Type 2 Immune Mechanisms in Carbon Nanotube-Induced Lung Fibrosis.” Frontiers in Immunology 9: 1120. doi: 10.3389/fimmu.2018.01120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Porter DW, Batteli LA, Wolfarth MG, Richardson DL, and Ma Q. 2015. “Pathologic and Molecular Profiling of rapid-onset fibrosis and inflammation induced by multi-walled carbon nanotubes.” Archives of Toxicology 89 (4): 621–633. doi: 10.1007/s00204-014-1428-y. [DOI] [PubMed] [Google Scholar]
- Drexler KE 1992. Nanosystems: Molecular Machinery, Manufacturing, and Computation. New York: John Wiley and Sons. [Google Scholar]
- Duffield JS, Lupher M, Thannickal VJ, and Wynn TA. 2013. “Host Responses in Tissue Repair and Fibrosis.” Annual Review of Pathology 8: 241–276. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke KS, and Bonner JC. 2018. “Mechanisms of Carbon Nanotube-Induced Pulmonary Fibrosis: A Physicochemical Characteristic Perspective.” Wiley Interdisciplinary Reviews. Nanomedicine and Nanobiotechnology 10 (3): e1498. doi: 10.1002/wnan.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF 1986. “Tumors: Wounds That Do Not Heal. Similarities between Tumor Stroma Generation and Wound Healing.” New England Journal of Medicine 315: 1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Eiro N, and Vizoso FJ. 2012. “Inflammation and Cancer.” World Journal of Gastrointestinal Surgery 4 (3): 62–72. doi: 10.4240/wjgs.v4.i3.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M, Gamo M, and Honda K. 2016. “A Review of Toxicity Studies of Single-Walled Carbon Nanotubes in Laboratory Animals.” Regulatory Toxicology and Pharmacology: RTP 74: 42–63. doi: 10.1016/j.yrtph.2015.11.015. [DOI] [PubMed] [Google Scholar]
- Eming SA., Wynn TA, and Martin P. 2017. “Inflammation and Metabolism in Tissue Repair and Regeneration.” Science (New York, N.Y.) 356 (6342): 1026–1030. doi: 10.1126/science.aam7928. [DOI] [PubMed] [Google Scholar]
- Fatkhutdinova LM, Khaliullin TO, Vasil’yeva OL, Zalyalov RR, Mustafin ISG, Kisin ER, Birch ME, Yanamala N, and Shvedova AA. 2016. “Fibrosis Biomarkers in Workers Exposed to MWCNTs.” Toxicology and Applied Pharmacology 299: 125–131. doi: 10.1016/j.taap.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay GA, Thannickal VJ, Fanburg BL, and Paulson KE. 2000. “Transforming Growth Factor-Beta 1-Induced Activation of the ERK Pathway/Activator Protein-1 in Human Lung Fibroblasts Requires the Autocrine Induction of Basic Fibroblast Growth Factor.” The Journal of Biological Chemistry 275 (36): 27650–27656. doi: 10.1074/jbc.M000893200. [DOI] [PubMed] [Google Scholar]
- Flavell SJ, Hou TZ, Lax S, Filer AD, Salmon M, and Buckley CD. 2008. “Fibroblasts as Novel Therapeutic Targets in Chronic Inflammation.” British Journal of Pharmacology 153 (S1): S241–S246. doi: 10.1038/sj.bjp.0707487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, and Wolf K. 2010. “Plasticity of Cell Migration: A Multiscale Tuning Model.” The Journal of Cell Biology 188(1): 11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann-Morvinski D, Narasimamurthy R, Xia Y, Myskiw C, Soda Y, and Verma IM. 2016. “Targeting NF-κB in glioblastoma: A therapeutic approach.” Science Advances 2 (1): e1501292. doi: 10.1126/sciadv.1501292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K, Fukuda M, Endoh S, Maru J, Kato H, Nakamura A, Shinohara N, Uchino K, and Honda K. 2016. “Pulmonary and Pleural Inflammation after Intratracheal Instillation of Short Single-Walled and Multi-Walled Carbon Nanotubes.” Toxicology Letters 257: 23–37. doi: 10.1016/j.toxlet.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Gieseck RL 3rd, Wilson MS, and Wynn TA. 2018. “Type 2 Immunity in Tissue Repair and Fibrosis.” Nature Reviews Immunology 18 (1): 62–76. doi: 10.1038/nri.2017.90. [DOI] [PubMed] [Google Scholar]
- Greenberg MI, Waksman J, and Curtis J. 2007. “Silicosis: A Review.” Disease-a-Month 53 (8): 394–416. doi: 10.1016/j.disamonth.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, and Karin M. 2010. “Immunity, Inflammation, and Cancer.” Cell 140 (6): 883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse Y, Loomis D, Guyton KZ, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, et al. 2014. “Carcinogenicity of Fluoro-Edenite, Silicon Carbide Fibres and Whiskers, and Carbon Nanotubes.” The Lancet Oncology 15 (13): 1427–1428. doi: 10.1016/S1470-2045(14)71109-X. [DOI] [PubMed] [Google Scholar]
- Hanahan D, and Weinberg RA. 2011. “Hallmarks of Cancer: The Next Generation.” Cell 144 (5): 646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Heino J, Kahari VM, Jaakkola S, and Peltonen J. 1989. “Collagen in the Extracellular Matrix of Cultured Scleroderma Skin Fibroblasts: Changes Related to Ascorbic Acid-Treatment.” Matrix 9 (1): 34–39. [DOI] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, De Wever O, Mareel M, and Gabbiani G. 2012. “Recent Developments in Myofibroblast Biology: Paradigms for Connective Tissue Remodeling.” The American Journal of Pathology 180 (4): 1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Naya M, Takehara H, Kataura H, Fujita K, and Ema M. 2017. “A 104-Week Pulmonary Toxicity Assessment of Long and Short Single-Wall Carbon Nanotubes after a Single Intratracheal Instillation in Rats.” Inhalation Toxicology 29 (11): 471–482. doi: 10.1080/08958378.2017.1394930. [DOI] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, and Latz E. 2008. “Silica Crystals and Aluminum Salts Activate the NALP3 Inflammasome through Phagosomal Destabilization.” Nature Immunology 9 (8): 847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huaux F, d’Ursel de Bousies V, Parent M-A, Orsi M, Uwambayinema F, Devosse R, Ibouraadaten S, et al. 2016. “Mesothelioma Response to Carbon Nanotubes Is Associated with an Early and Selective Accumulation of Immunosuppressive Monocytic Cells.” Particle and Fibre Toxicology 13 (1): 46 doi: 10.1186/s12989-016-0158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizar I, Malur A, Midgette YA, Kukoly C, Chen P, Ke PC, Podila R, et al. 2011. “Novel Murine Model of Chronic Granulomatous Lung Inflammation Elicited by Carbon nanotubes.” American Journal of Respiratory Cell and Molecular Biology 45 (4): 858–866. doi: 10.1165/rcmb.2010-0401OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston HJ, Hutchison GR, Christensen FM, Peters S Hankin S, Aschberger K, and Stone V. 2010. “A Critical Review of the Biological Mechanisms Underlying the in Vivo and in Vitro Toxicity of Carbon Nanotubes: The Contribution of Physico-Chemical Characteristics.” Nanotoxicology 4 (2): 207–246. doi: 10.3109/17435390903569639. [DOI] [PubMed] [Google Scholar]
- Kalluri R 2016. “The Biology and Function of Fibroblasts in Cancer.” Nature Reviews Cancer 16 (9): 582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- Kane AB, Hurt RH , and Gao H. 2018. “The Asbestos-Carbon Nanotube Analogy: An Update.” Toxicology and Applied Pharmacology 361: 68–80. doi: 10.1016/j.taap.2018.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai T, Umeda Y, Ohnishi M, Kondo H, Takeuchi T, Aiso S, Nishizawa T, Matsumoto M, and Fukushima S. 2015. “Thirteen-Week Study of Toxicity of Fiber-like Multi-Walled Carbon Nanotubes with Whole-Body Inhalation Exposure in Rats.” Nanotoxicology 9 (4): 413–422. doi: 10.3109/17435390.2014.933903. [DOI] [PubMed] [Google Scholar]
- Kasai T, Umeda Y, Ohnishi M, Mine T, Kondo H, Takeuchi T, Matsumoto M, and Fukushima S. 2016. “Lung Carcinogenicity of Inhaled Multi-Walled Carbon Nanotube in Rats.” Particle and Fibre Toxicology 13 (1): 53. doi: 10.1186/s12989-016-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenstein AL, and Myers JL. 1998. “Idiopathic Pulmonary Fibrosis: Clinical Relevance of Pathologic Classification.” American Journal of Respiratory and Critical Care Medicine 157 (4 Pt 1): 1301–1315. doi: 10.1164/ajrccm.157.4.9707039. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH 2008. “Immunology’s Foundation: The 100-Year Anniversary of the Nobel Prize to Paul Ehrlich and Elie Metchnikoff.” Nature Immunology 9 (7): 705–712. doi: 10.1038/ni0708-705. [DOI] [PubMed] [Google Scholar]
- Kendall RT, and Feghali-Bostwick CA. 2014. “Fibroblasts in Fibrosis: Novel Roles and Mediators.” Frontiers in Pharmacology 5: 123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliullin TO, Kisin ER, Murray AR, Yanamala N, Shurin MR, Gutkin DW, Fatkhutdinova LM, Kagan VE, and Shvedova AA. 2017. “Mediation of the Single-Walled Carbon Nanotubes Induced Pulmonary Fibrogenic Response by Osteopontin and TGF-β1.” Experimental Lung Research 43 (8): 311–326. doi: 10.1080/01902148.2017.1377783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Lee S, Lee AY, Seo HW, Chae C, and Cho MH. 2015. “Intratracheal Exposure to Multi-Walled Carbon Nanotubes Induces a Nonalcoholic Steatohepatitis-like Phenotype in C57BL/6J Mice.” Nanotoxicology 9 (5): 613–623. doi: 10.3109/17435390.2014.963186. [DOI] [PubMed] [Google Scholar]
- Kuempel ED, Jaurand M-C, Møller P, Morimoto Y, Kobayashi N, Pinkerton KE, Sargent LM, et al. 2017. “Evaluating the Mechanistic Evidence and Key Data Gaps in Assessing the Potential Carcinogenicity of Carbon Nanotubes and Nanofibers in Humans.” Critical Reviews in Toxicology 47 (1): 1–58. doi: 10.1080/10408444.2016.1206061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AB, and Karlsson S. 1993. “Transforming Growth Factor-Beta 1 Knockout Mice. A Mutation in One Cytokine Gene Causes a Dramatic Inflammatory Disease.” American Journal of Pathology 143 (1): 3–9. [PMC free article] [PubMed] [Google Scholar]
- Labib S, Williams A, Yauk CL, Nikota JK, Wallin H, Vogel U, and Halappanavar S. 2016. “Nano-Risk Science: Application of Toxicogenomics in an Adverse Outcome Pathway Framework for Risk Assessment of Multi-Walled Carbon Nanotubes.” Particle and Fibre Toxicology 13 (1): 15. doi: 10.1186/s12989-016-0125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CW, James JT, McCluskey R, and Hunter RL. 2004. “Pulmonary Toxicity of Single-Wall Carbon Nanotubes in Mice 7 and 90 Days after Intratracheal Instillation.” Toxicological Sciences: An Official Journal of the Society of Toxicology 77 (1): 126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- Leask A, and Abraham DJ. 2004. “TGF-Beta Signaling and the Fibrotic Response.” FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 18 (7): 816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- Liou SH, Tsai CS, Pelclova D, Schubauer-Berigan MK, and Schulte PA. 2015. “Assessing the First Wave of Epidemiological Studies of Nanomaterial Workers.” Journal of Nanoparticle Research: An Interdisciplinary Forum for Nanoscale Science and Technology 17: 413. doi: 10.1007/s11051-015-3219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CM, Wang HB, Dembo M, and Wang YL. 2000. “Cell Movement Is Guided by the Rigidity of the Substrate.” Biophysical Journal 79 (1): 144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohcharoenkal W, Wang L, Stueckle TA, Dinu CZ, Castranova V, Liu Y, and Rojanasakul Y. 2013. “Chronic Exposure to Carbon Nanotubes Induces Invasion of Human Mesothelial Cells through Matrix Metalloproteinase-2.” American Chemical Society Nano 7(9): 7711–7723. doi: 10.1021/nn402241b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Weaver VM, and Werb Z. 2012. “The Extracellular Matrix: A Dynamic Niche in Cancer Progression.” The Journal of Cell Biology 196 (4): 395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luanpitpong S, Wang L, and Rojanasakul Y. 2014. “The Effects of Carbon Nanotubes on Lung and Dermal Cellular Behaviors.” Nanomedicine (London, England) 9 (6): 895–912. doi: 10.2217/nnm.14.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas A 2012. “Atherosclerosis, Cancer, Wound Healing, and Inflammation – Shared or Parallel Evolution.” International Journal of Cardiovascular Research 01 (01): 1–2. doi: 10.4172/2324-8602.1000e101. [DOI] [Google Scholar]
- Lupher ML Jr.., and Gallatin WM. 2006. “Regulation of Fibrosis by the Immune System.” Advances in Immunology 89: 245–288. doi: 10.1016/S0065-2776(05)89006-6. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamaté C, Merval R, Fradelizi D, and Tedgui A. 2001. “Inhibition of Transforming Growth Factor-Beta Signaling Accelerates Atherosclerosis and Induces an Unstable Plaque Phenotype in Mice.” Circulation Research 89 (10): 930–934. doi: 10.1161/hh2201.099415. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, and Balkwill F. 2008. “Cancer-Related Inflammation.” Nature 454 (7203): 436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Maeda M, Lee S, Nishimura Y, Kumagai-Takei N, Hayashi H, Yamamoto S, et al. 2012. “Asbestos-Induced Cellular and Molecular Alteration of Immunocompetent Cells and Their Relationship with Chronic Inflammation and Carcinogenesis.” Journal of Biomedicine and Biotechnology 2012: 492608. doi: 10.1155/2012/492608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JC, Armstrong B, Case B, Doell D, McCaughey WT, McDonald AD, and Sébastien P. 1989. “Mesothelioma and Asbestos Fiber Type. Evidence from Lung Tissue analyses.” Cancer 63 (8): 1544–1547. doi: 10.1002/1097-0142(19890415)63:8andlt;1544::aid-cncr2820630815andgt;3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Meneghin A, and Hogaboam CM. 2007. “Infectious Disease, the Innate Immune Response, and Fibrosis.” The Journal of Clinical Investigation 117 (3): 530–538. doi: 10.1172/JCI30595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni JF, Hubbs AF, Battelli LA, McKinney W, Friend S, Wolfarth MG, et al. 2013. “Distribution and Fibrotic Response following Inhalation Exposure to Multi-Walled Carbon Nanotubes.” Particle and Fibre Toxicology 10: 33. doi: 10.1186/1743-8977-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzano SA, Droguett MA, Burgos ME, Ardiles LG, Aros CA, Caorsi I, and Egido J. 2000. “Overexpression of Chemokines, Fibrogenic Cytokines, and Myofibroblasts in Human Membranous nephropathy.” Kidney International 57 (1): 147–158. doi: 10.1046/j.1523-1755.2000.00830.x. [DOI] [PubMed] [Google Scholar]
- Miserocchi G, Sancini G, Mantegazza F, and Chiappino G. 2008. “Translocation Pathways for Inhaled Asbestos Fibers.” Environmental Health: A Global Access Science Source 7: 4. doi: 10.1186/1476-069X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller P, Christophersen DV, Jensen DM, Kermanizadeh A, Roursgaard M, Jacobsen NR, Hemmingsen JG, et al. 2014. “Role of Oxidative Stress in Carbon nanotube-generated health effects.” Archives of Toxicology 88 (11): 1939–1964. doi: 10.1007/s00204-014-1356-x. [DOI] [PubMed] [Google Scholar]
- Mossman BT, Bignon J, Corn M, Seaton A, and Gee JB. 1990. “Asbestos: Scientific Developments and Implications for Public Policy.” Science (New York, N.Y.) 247 (4940): 294–301. doi: 10.1126/science.2153315. [DOI] [PubMed] [Google Scholar]
- Mossman BT, and Churg A. 1998. “Mechanisms in the Pathogenesis of Asbestosis and Silicosis.” American Journal of Respiratory and Critical Care Medicine 157 (5 Pt 1): 1666–1680. doi: 10.1164/ajrccm.157.5.9707141. [DOI] [PubMed] [Google Scholar]
- Muller J, Delos M, Panin N, Rabolli V, Huaux F, and Lison D. 2009. “Absence of Carcinogenic Response to Multiwall Carbon Nanotubes in a 2-Year Bioassay in the Peritoneal Cavity of the Rat.” Toxicological Sciences 110 (2): 442–448. doi: 10.1093/toxsci/kfp100. [DOI] [PubMed] [Google Scholar]
- Muller J, Huaux F, Moreau N, Misson P, Heilier J-F, Delos M, Arras M, et al. 2005. “Respiratory Toxicity of Multi-Wall Carbon Nanotubes.” Toxicology and Applied Pharmacology 207 (3): 221–231. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Murphy FA, Poland CA, Duffin R, Al-Jamal KT, Ali-Boucetta H, Nunes A, Byrne F, et al. 2011. “Length-Dependent Retention of Carbon Nanotubes in the Pleural Space of Mice Initiates Sustained Inflammation and Progressive Fibrosis on the Parietal Pleura.” The American Journal of Pathology 178 (6): 2587–2600. doi: 10.1016/j.ajpath.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, and Wynn TA. 2011. “Protective and Pathogenic Functions of Macrophage Subsets.” Nature Reviews Immunology 11 (11): 723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, Okazaki Y, Chew SH, Misawa N, Miyata Y, Shinohara H, and Toyokuni S. 2013. “Intraperitoneal Administration of Tangled Multiwalled Carbon Nanotubes of 15 nm in Diameter Does Not Induce Mesothelial Carcinogenesis in Rats.” Pathology International 63 (9): 457–462. doi: 10.1111/pin.12093. [DOI] [PubMed] [Google Scholar]
- Nagai H, Okazaki Y, Chew SH, Misawa N, Yamashita Y, Akatsuka S, Ishihara T, et al. 2011. “Diameter and Rigidity of Multiwalled Carbon Nanotubes Are Critical Factors in Mesothelial Injury and Carcinogenesis.” Proceedings of the National Academy of Sciences of the United States of America 108 (49): E1330–1338. doi: 10.1073/pnas.1110013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neglia JP, FitzSimmons SC, Maisonneuve P, Schöni MH, Schöni-Affolter F, Corey M, and Lowenfels AB. 1995. “The Risk of Cancer among Patients with Cystic Fibrosis. Cystic Fibrosis and Cancer Study Group.” New England Journal of Medicine 332 (8): 494–499. doi: 10.1056/NEJM199502233320803. [DOI] [PubMed] [Google Scholar]
- Nikota J, Banville A, Goodwin LR, Wu D, Williams A, Yauk CL, Wallin H, Vogel U, and Halappanavar S. 2017. “Stat-6 Signaling Pathway and Not Interleukin-1 Mediates Multi-Walled Carbon Nanotube-Induced Lung Fibrosis in Mice: Insights from an Adverse Outcome Pathway Framework.” Particle and Fibre Toxicology 14 (1): 37. doi: 10.1186/s12989-017-0218-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NSF. 2011. “Nanotechnology Research Directions for Societal Needs in 2020: Retrospective and Outlook Summary. National Science Foundation” In Science Policy Reports, edited by Roco M, Mirkin C, Hersan M eds. New York: Springer. [Google Scholar]
- Ohlund D, Elyada E, and Tuveson D. 2014. “Fibroblast Heterogeneity in the Cancer Wound.” The Journal of Experimental Medicine 211 (8): 1503–1523. doi: 10.1084/jem.20140692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Regan A 2003. “The Role of Osteopontin in Lung Disease.” Cytokine Growth Factor Rev 14 (6): 479–488. [DOI] [PubMed] [Google Scholar]
- Osmond-McLeod MJ, Poland CA, Murphy F, Waddington L, Morris H, Hawkins SC, Clark S, et al. 2011. “Durability and Inflammogenic Impact of Carbon Nanotubes Compared with Asbestos Fibres.” Particle and Fibre Toxicology 8: 15. doi: 10.1186/1743-8977-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki T, Matsuzaki H, Lee S, Kumagai-Takei N, Yamamoto S, Hatayama T, Yoshitome K, and Nishimura Y. 2016. “Environmental Factors and Human Health: Fibrous and Particulate Substance-Induced Immunological Disorders and Construction of a Health-Promoting Living Environment.” Environmental Health and Preventive Medicine 21 (2): 71–81. doi: 10.1007/s12199-015-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacurari M, Yin XJ, Zhao J, Ding M, Leonard SS, Schwegler-Berry D, Ducatman BS, et al. 2008. “Raw Single-Wall Carbon Nanotubes Induce Oxidative Stress and Activate MAPKs, AP-1, NF-kappaB, and Akt in Normal and Malignant Human Mesothelial Cells.” Environmental Health Perspectives 116 (9): 1211–1217. doi: 10.1289/ehp.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E-J, Cho W-S, Jeong J, Yi J, Choi K, and Park K. 2009. “Pro-inflammatory and potential allergic responses resulting from B cell activation in mice treated with multi-walled carbon nanotubes by intratracheal instillation.” Toxicology 259 (3): 113–121. doi: 10.1016/j.tox.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Park E-J, Roh J, Kim S-N, Kang M-S, Han Y-A, Kim Y, Hong JT, and Choi K. 2011. “A Single Intratracheal Instillation of Single-Walled Carbon Nanotubes Induced Early Lung Fibrosis and Subchronic Tissue Damage in Mice.” Archives of Toxicology 85 (9): 1121–1131. doi: 10.1007/s00204-011-0655-8. [DOI] [PubMed] [Google Scholar]
- Phan SH, Zhang K, Zhang HY, and Gharaee-Kermani M. 1999. “The Myofibroblast as an Inflammatory Cell in Pulmonary Fibrosis.” Ergebnisse Der Pathologie [Current Topics in Pathology] 93: 173–182. [DOI] [PubMed] [Google Scholar]
- Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WAH, Seaton A, Stone V, et al. 2008. “Carbon Nanotubes Introduced into the Abdominal Cavity of Mice Show Asbestos-like Pathogenicity in a Pilot Study.” Nature Nanotechnology 3 (7): 423–428. doi: 10.1038/nnano.2008.111 [DOI] [PubMed] [Google Scholar]
- Porter DW, Hubbs AF, Chen BT, McKinney W, Mercer RR, Wolfarth MG, Battelli L, et al. 2013. “Acute Pulmonary Dose-Responses to Inhaled Multi-Walled Carbon Nanotubes.” Nanotoxicology 7 (7): 1179–1194. doi: 10.3109/17435390.2012.719649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DW, Hubbs AF, Mercer RR, Wu N, Wolfarth MG, Sriram K, Leonard S, et al. 2010. “Mouse Pulmonary Dose- and Time Course-Responses Induced by Exposure to Multi-Walled Carbon Nanotubes.” Toxicology 269 (2–3): 136–147. doi: 10.1016/j.tox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Pourgholamhossein F, Rasooli R, Pournamdari M, Pourgholi L, Samareh-Fekri M, Ghazi-Khansari M, Iranpour M, et al. 2018. “Pirfenidone Protects against Paraquat-Induced Lung Injury and Fibrosis in Mice by Modulation of Inflammation, Oxidative Stress, and Gene Expression.” Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 112: 39–46. doi: 10.1016/j.fct.2017.12.034. [DOI] [PubMed] [Google Scholar]
- Principi E, Girardello R, Bruno A, Manni I, Gini E, Pagani A, Grimaldi A, et al. 2016. “Systemic Distribution of Single-Walled Carbon Nanotubes in a Novel Model: Alteration of Biochemical Parameters, Metabolic Functions, Liver Accumulation, and Inflammation in Vivo.” International Journal of Nanomedicine 11: 4299–4316. doi: 10.2147/IJN.S109950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AR, Reddy YN, Krishna DR, and Himabindu V. 2012. “Pulmonary Toxicity Assessment of Multiwalled Carbon Nanotubes in Rats following Intratracheal Instillation.” Environmental Toxicology 27 (4): 211–219. doi: 10.1002/tox.20632. [DOI] [PubMed] [Google Scholar]
- Ribatti D 2017. “A Revisited Concept: Contact Inhibition of Growth. From Cell Biology to Malignancy.” Experimental Cell Research 359 (1): 17–19. doi: 10.1016/j.yexcr.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Ringelhan M, McKeating JA, and Protzer U. 2017. “Viral Hepatitis and Liver Cancer.” Philosophical Transactions of the Royal Society of London B: Biological Sciences 372 (1732): 20160274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittinghausen S, Hackbarth A, Creutzenberg O, Ernst H, Heinrich U, Leonhardt A, and Schaudien D. 2014. “The Carcinogenic Effect of Various Multi-Walled Carbon Nanotubes (MWCNTs) after Intraperitoneal Injection in Rats.” Particle and Fibre Toxicology 11: 59. doi: 10.1186/s12989-014-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittling SR, and Singh R. 2015. “Osteopontin in Immune-Mediated Diseases.” Journal of Dental Research 94 (12): 1638–1645. doi: 10.1177/0022034515605270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers AJ, Leigh J, Berry G, Ferguson DA, Mulder HB, and Ackad M. 1991. “Relationship Between Lung Asbestos Fiber Type and Concentration and Relative Risk of Mesothelioma. A Case-Control Study.” Cancer 67 (7): 1912–1920. doi: 10.1002/1097-0142(19910401)67:7andlt;1912::aid-cncr2820670716andgt;3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Roggli VL, Pratt PC, and Brody AR. 1986. “Asbestos Content of Lung Tissue in Asbestos Associated Diseases: A Study of 110 Cases.” British Journal of Industrial Medicine 43 (1): 18–28. doi: 10.1136/oem.43.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggli VL, and Vollmer RT. 2008. “Twenty-Five Years of Fiber Analysis: What Have we Learned?” Human Pathology 39 (3): 307–315. doi: 10.1016/j.humpath.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Roggli VL 1990. “Human Disease Consequences of Fiber Exposures: A Review of Human Lung Pathology and Fiber Burden Data.” Environmental Health Perspectives 88: 295–303. doi: 10.1289/ehp.9088295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rom WN, Travis WD, and Brody AR. 1991. “Cellular and Molecular Basis of the Asbestos-Related Diseases.” The American Review of Respiratory Disease 143 (2): 408–422. doi: 10.1164/ajrccm/143.2.408. [DOI] [PubMed] [Google Scholar]
- Rothen-Rutishauser B, Brown DM, Piallier-Boyles M, Kinloch IA, Windle AH, Gehr P, and Stone V. 2010. “Relating the Physicochemical Characteristics and Dispersion of Multiwalled Carbon Nanotubes in Different Suspension Media to Their Oxidative Reactivity in Vitro and Inflammation in Vivo.” Nanotoxicology 4 (3): 331–342. doi: 10.3109/17435390.2010.489161. [DOI] [PubMed] [Google Scholar]
- Rybinski B, Franco-Barraza J, and Cukierman E. 2014. “The Wound Healing, Chronic Fibrosis, and Cancer Progression Triad.” Physiological Genomics 46 (7): 223–244. doi: 10.1152/physiolgenomics.00158.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydman EM, Ilves M, Vanhala E, Vippola M, Lehto M, Kinaret PAS, Pylkkänen L, et al. 2015. “A Single Aspiration of Rod-like Carbon Nanotubes Induces Asbestos-like Pulmonary Inflammation Mediated in Part by the IL-1 Receptor.” Toxicological Sciences: An Official Journal of the Society of Toxicology 147 (1): 140–155. doi: 10.1093/toxsci/kfv112. [DOI] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Tewksbury EW, Moss OR, Cesta MF, Wong BA, and Bonner JC. 2009. “Inhaled Multiwalled Carbon Nanotubes Potentiate Airway Fibrosis in Murine Allergic Asthma.” American Journal of Respiratory Cell and Molecular Biology 40 (3): 349–358. doi: 10.1165/rcmb.2008-0276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto Y, Nakae D, Fukumori N, Tayama K, Maekawa A, Imai K, Hirose A, et al. 2009. “Induction of Mesothelioma by a Single Intrascrotal Administration of Multi-Wall Carbon Nanotube in Intact Male Fischer 344 Rats.” The Journal of Toxicological Sciences 34 (1): 65–76. [DOI] [PubMed] [Google Scholar]
- Sambo P, Baroni SS, Luchetti M, Paroncini P, Dusi S, Orlandini G, and Gabrielli A. 2001. “Oxidative Stress in Scleroderma: Maintenance of Scleroderma Fibroblast Phenotype by the Constitutive up-Regulation of Reactive Oxygen Species Generation through the NADPH Oxidase Complex Pathway.” Arthritis and Rheumatism 44 (11): 2653–2664. doi: 10.1002/1529-0131(200111)44:11andlt;2653::aid-art445andgt;3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Sargent LM, Porter DW, Staska LM, Hubbs AF, Lowry DT, Battelli L, Siegrist KJ, et al. 2014. “Promotion of Lung Adenocarcinoma following Inhalation Exposure to Multi-Walled Carbon Nanotubes.” Particle and Fibre Toxicology 11: 3. doi: 10.1186/1743-8977-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M, and Werner S. 2008. “Cancer as an Overhealing Wound: An Old Hypothesis revisited.” Nature Reviews. Molecular Cell Biology 9 (8): 628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Kuempel ED, Zumwalde RD, Geraci CL, Schubauer-Berigan MK, Castranova V, Hodson L, et al. 2012. “Focused Actions to Protect Carbon Nanotube Workers.” American Journal of Industrial Medicine 55 (5): 395–411. doi: 10.1002/ajim.22028. [DOI] [PubMed] [Google Scholar]
- Shalapour S, and Karin M. 2015. “Immunity, Inflammation, and Cancer: An Eternal Fight between Good and Evil.” The Journal of Clinical Investigation 125 (9): 3347–3355. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Mehra NK, Jain K, and Jain NK. 2016. “Biomedical Applications of Carbon Nanotubes: A Critical Review.” Current Drug Delivery 13 (6): 796–817. [DOI] [PubMed] [Google Scholar]
- Shraiman BI 2005. “Mechanical Feedback as a Possible Regulator of Tissue Growth.” Proceedings of the National Academy of Sciences of the United States of America 102(9): 3318–3323. doi: 10.1073/pnas.0404782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kisin E, Murray AR, Johnson VJ, Gorelik O, Arepalli S, Hubbs AF, et al. 2008. “Inhalation vs. Aspiration of Single-Walled Carbon Nanotubes in C57BL/6 Mice: Inflammation, Fibrosis, Oxidative Stress, and Mutagenesis.” The American Journal of Physiology-Lung Cellular and Molecular Physiology 295 (4): L552–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, Tyurina YY, et al. 2005. “Unusual Inflammatory and Fibrogenic Pulmonary Responses to Single-Walled Carbon Nanotubes in Mice.” American Journal of Physiology Lung Cellular and Molecular Physiology 289 (5): L698–708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Yanamala N, Kisin ER, Tkach AV, Murray AR, Hubbs A, Chirila MM, et al. 2014. “Long-Term Effects of Carbon Containing Engineered Nanomaterials and Asbestos in the Lung: One Year Postexposure Comparisons.” American Journal of Physiology Lung Cellular and Molecular Physiology 306 (2): L170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinis SI, Hatzoglou C, Gourgoulianis KI, and Zarogiannis SG. 2018. “Carbon Nanotubes and Other Engineered Nanoparticles Induced Pathophysiology on Mesothelial Cells and Mesothelial Membranes.” Frontiers in Physiology 9: 295. doi: 10.3389/fphys.2018.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura H, Liu X, Kobayashi T, Togo S, Ertl RF, Kawasaki S, Kamio K, et al. 2006. “Reactive Nitrogen Species Augment fibroblast-mediated collagen gel contraction, mediator production, and chemotaxis.” American Journal of Respiratory Cell and Molecular Biology 34 (5): 592–599. doi: 10.1165/rcmb.2005-0339OC. [DOI] [PubMed] [Google Scholar]
- Suzui M, Futakuchi M, Fukamachi K, Numano T, Abdelgied M, Takahashi S, Ohnishi M, et al. 2016. “Multiwalled Carbon Nanotubes Intratracheally Instilled into the Rat Lung Induce Development of Pleural Malignant Mesothelioma and Lung Tumors.” Cancer Science 107 (7): 924–935. doi: 10.1111/cas.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi A, Hirose A, Futakuchi M, Tsuda H, and Kanno J. 2012. “Dose-Dependent Mesothelioma Induction by Intraperitoneal Administration of Multi-Wall Carbon Nanotubes in p53 Heterozygous Mice.” Cancer Science 103(8): 1440–1444. doi: 10.1111/j.1349-7006.2012.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi A, Hirose A, Nishimura T, Fukumori N, Ogata A, Ohashi N, Kitajima S, and Kanno J. 2008. “Induction of Mesothelioma in p53+/− Mouse by Intraperitoneal Application of Multi-Wall Carbon Nanotube.” The Journal of Toxicological Sciences 33 (1): 105–116. doi: 10.2131/jts.33.105. [DOI] [PubMed] [Google Scholar]
- Takahashi F, Takahashi K, Okazaki T, Maeda K, Ienaga H, Maeda M, Kon S, Uede T, and Fukuchi Y. 2001. “Role of Osteopontin in the Pathogenesis of Bleomycin-Induced Pulmonary Fibrosis.” American Journal of Respiratory Cell and Molecular Biology 24 (3): 264–271. doi: 10.1165/ajrcmb.24.3.4293. [DOI] [PubMed] [Google Scholar]
- Taylor AJ, McClure CD, Shipkowski KA, Thompson EA, Hussain S, Garantziotis S, Parsons GN, and Bonner JC. 2014. “Atomic Layer Deposition Coating of Carbon Nanotubes with Aluminum Oxide Alters Pro-Fibrogenic Cytokine Expression by Human Mononuclear Phagocytes in Vitro and Reduces Lung Fibrosis in Mice in Vivo.” PLOS One 9 (9): e106870. doi: 10.1371/journal.pone.0106870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal VJ, Aldweib KD, and Fanburg BL. 1998a. “Tyrosine Phosphorylation Regulates H2O2 Production in Lung Fibroblasts Stimulated by Transforming Growth Factor Beta1.” Journal of Biological Chemistry 273 (36): 23611–23615. doi: 10.1074/jbc.273.36.23611. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Aldweib KD, Rajan T, and Fanburg BL. 1998b. “Upregulated Expression of Fibroblast Growth Factor (FGF) Receptors by Transforming Growth Factor-beta1 (TGF-beta1) Mediates Enhanced Mitogenic Responses to FGFs in Cultured Human Lung Fibroblasts.” Biochemical and Biophysical Research Communications 251(2): 437–441. doi: 10.1006/bbrc.1998.9443. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, and Fanburg BL. 1995. “Activation of an H2O2-Generating NADH Oxidase in Human Lung Fibroblasts by Transforming Growth Factor Beta 1.” Journal of Biological Chemistry 270 (51): 30334–30338. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- Thompson EA, Sayers BC, Glista-Baker EE, Shipkowski KA, Ihrie MD, Duke KS, Taylor AJ, and Bonner JC. 2015. “Role of Signal Transducer and Activator of Transcription 1 in Murine Allergen-Induced Airway Remodeling and Exacerbation by Carbon Nanotubes.” American Journal of Respiratory Cell and Molecular Biology 53 (5): 625–636. doi: 10.1165/rcmb.2014-0221OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, and Brown RA. 2002. “Myofibroblasts and Mechano-Regulation of Connective Tissue Remodelling.” Nature Reviews. Molecular Cell Biology 3 (5): 349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Toyokuni S, Jiang LI, Kitaura R, and Shinohara H. 2015. “Minimal Inflammogenicity of Pristine Single-Wall Carbon Nanotubes.” Nagoya Journal of Medical Science 77 (1–2): 195–202. [PMC free article] [PubMed] [Google Scholar]
- Vietti G, Lison D, and van den Brule S. 2016. “Mechanisms of Lung Fibrosis Induced by Carbon Nanotubes: Towards an Adverse Outcome Pathway (AOP).” Particle and Fibre Toxicology 13: 11. doi: 10.1186/s12989-016-0123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virchow R 1863. Cellular pathology as based upon physiological and pathological histology. Philadelphia: J. B. Lippincott. [DOI] [PubMed] [Google Scholar]
- Vlaanderen J, Pronk A, Rothman N, Hildesheim A, Silverman D, Hosgood HD, Spaan S, et al. 2017. “A Cross-Sectional Study of Changes in Markers of Immunological Effects and Lung Health due to Exposure to Multi-Walled Carbon Nanotubes.” Nanotoxicology 11 (3): 395–404. doi: 10.1080/17435390.2017.1308031. [DOI] [PubMed] [Google Scholar]
- Wagner JC, Newhouse ML, Corrin B, Rossiter CE, and Griffiths DM. 1988. “Correlation Between Fibre Content of the Lung and Disease in East London Asbestos Factory Workers.” British Journal of Industrial Medicine 45 (5): 305–308. doi: 10.1136/oem.45.5.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White ES, Lazar MH, and Thannickal VJ. 2003. “Pathogenetic Mechanisms in Usual Interstitial Pneumonia/Idiopathic Pulmonary Fibrosis.” The Journal of Pathology 201 (3): 343–354. doi: 10.1002/path.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick G, Backovic A, Rabensteiner E, Plank N, Schwentner C, and Sgonc R. 2010. “The Immunology of Fibrosis: Innate and Adaptive Responses.” Trends in Immunology 31 (3): 110–119. doi: 10.1016/j.it.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Chitapanarux T, Chen Y, Soon RK Jr., and Yee HF Jr.. 2013. “Intestinal Myofibroblasts Produce Nitric Oxide in Response to Combinatorial Cytokine Stimulation.” Journal of Cellular Physiology 228 (3): 572–580. doi: 10.1002/jcp.24164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, and Ramalingam TR. 2012. “Mechanisms of Fibrosis: Therapeutic Translation for Fibrotic Disease.” Nature Medicine 18 (7): 1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA 2008. “Cellular and Molecular Mechanisms of Fibrosis.” The Journal of Pathology 214 (2): 199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Yeddula N, Leblanc M, Ke E, Zhang Y, Oldfield E, Shaw RJ, and Verma IM. 2012. “Reduced Cell Proliferation by IKK2 Depletion in a Mouse Lung-Cancer Model.” Nature Cell Biology 14 (3): 257–265. doi: 10.1038/ncb2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Wang R, Tang X, Xiong Y, Xu R, and Wu X. 2012. “Paraquat-Induced Pulmonary Fibrosis Starts at an Early Stage of Inflammation in Rats.” Immunotherapy 4 (12): 1809–1815. doi: 10.2217/imt.12.122. [DOI] [PubMed] [Google Scholar]
- Xu J, Alexander DB, Futakuchi M, Numano T, Fukamachi K, Suzui M, Omori T, Kanno J, Hirose A, Tsuda H., et al. 2014. “Size- and Shape-Dependent Pleural Translocation, Deposition, Fibrogenesis, and Mesothelial Proliferation by Multiwalled Carbon Nanotubes.” Cancer Science 105 (7): 763–769. doi: 10.1111/cas.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Futakuchi M, Shimizu H, Alexander DB, Yanagihara K, Fukamachi K, Suzui M, et al. 2012. “Multi-Walled Carbon Nanotubes Translocate into the Pleural Cavity and Induce Visceral Mesothelial Proliferation in Rats.” Cancer Science 103 (12): 2045–2050. doi: 10.1111/cas.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani S, Bansal R, and Prakash J. 2017. “Drug Targeting to Myofibroblasts: Implications for Fibrosis and Cancer.” Advanced Drug Delivery Reviews 121: 101–116. doi: 10.1016/j.addr.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Zhang K, Rekhter MD, Gordon D, and Phan SH. 1994. “Myofibroblasts and Their Role in Lung Collagen Gene Expression during Pulmonary Fibrosis. A Combined Immunohistochemical and in Situ Hybridization Study.” American Journal of Pathology 145 (1): 114–125. [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Huang JQ, Qian WZ, Zhang YY, and Wei F. 2013. “The Road for Nanomaterials Industry: A Review of Carbon Nanotube Production, Post-Treatment, and Bulk Applications for Composites and Energy Storage.” Small (Weinheim an Der Bergstrasse, Germany) 9 (8): 1237–1265. doi: 10.1002/smll.201203252. [DOI] [PubMed] [Google Scholar]
- Zhao X, and Liu R. 2012. “Recent Progress and Perspectives on the Toxicity of Carbon Nanotubes at Organism, Organ, Cell, and Biomacromolecule Levels.” Environment International 40: 244–255. doi: 10.1016/j.envint.2011.12.003. [DOI] [PubMed] [Google Scholar]