Abstract
Sex Positive![+] is a two-arm, video-based web intervention aimed at reducing condomless anal sex (CAS) with partners of known and unknown serostatus that was delivered online to a racially and ethnically diverse sample of 830 gay, bisexual, and other men who have sex with men living with HIV. Men in each arm received 6 weekly videos after completing a baseline assessment and 4 weekly booster videos following a 6-month assessment. Follow-up assessments were conducted every 3 months for 1 year. At 3-month follow-up, men in the intervention arm reported significantly reduced risk of having unknown serodiscordant CAS partners than men in the control arm (RR 0.60, 95% CI 0.39–0.92), partially supporting study hypotheses. Aside from this finding, similar reductions in sexual risk behaviors were observed in both arms over the study period. There is much to be learned about video-based web interventions in terms of methodological development and intervention delivery, including frequency and duration of intervention components.
Keywords: eHealth, HIV, MSM, Video, Intervention
Resumen
Sex Positive! [+] es una intervención vía web de dos brazos basado en videos diseñados para reducir el sexo anal sin condón (SASC) con parejas de estado serológico conocido y desconocido. Los videos se distribuyeron vía Internet a una muestra racial y étnicamente diversa de 830 hombres homosexuales, bisexuales y otros hombres que tienen sexo con hombres que viven con VIH. Después de completar una evaluación basal, semanalmente se presentó un video a los hombres de cada brazo durante 6 semanas. Comenzando en el sexto mes, un video de refuerzo se presentó semanalmente durante 4 semanas. Las evaluaciones de seguimiento se realizaron cada tres meses durante un año. A los 3 meses de seguimiento, los hombres en el brazo de intervención reportaron una reducción significativa en el riesgo de tener SASC con parejas de estado serológico desconocido comparados con hombres en el brazo de control (RR = 0,60; IC del 95%: 0,39–0,92), lo cual apoya parcialmente la hipótesis del estudio. Además de este hallazgo, se observaron reducciones similares en las conductas de riesgo sexual en ambos brazos durante el período de estudio. Aún hay mucho que aprender acerca de las intervenciones con videos administradas por medio del Internet, específicamente en términos de desarrollo metodológico y modos de implementación, incluyendo la frecuencia y la duración de los componentes de la intervención.
Introduction
Nationally, white, black, and Hispanic gay, bisexual, and other men who have sex with men (GBMSM) accounted for 66% of new HIV diagnoses reported in 2017 [1]. What is particularly concerning is that approximately 60% of GBMSM known to be HIV-positive are not virally suppressed, which is a proxy measure for use of and adherence to combination antiretroviral therapy (cART) [2]. Compared to white GBMSM, men of color are less likely to be engaged in HIV care, less adherent to their treatment, and more likely to have detectable HIV viremia [3–5]. Preventing transmission from virally unsuppressed or suboptimally cART-adherent men who have condomless anal sex (CAS) with HIV-negative or unknown status partners is a public health imperative.
The Internet and other forms of technology (e.g., global positioning system or GPS) are efficient for sex-seeking purposes in that they can allow seekers to choose partners based on certain characteristics (e.g., HIV status), sexual preferences (e.g., condomless sex), or geography (e.g., same city, same block). Because new HIV infections in GBMSM have been attributed in part to meeting sex partners on sexual networking websites and smartphone applications (apps) [6, 7], it is critical to also engage GBMSM in risk reduction interventions through these venues [8]. Moreover, behavioral health interventions that enable users to participate privately on a computer, tablet, or smartphone, and on their own schedule, may be more appealing than traditional interventions conducted in structured clinical settings [9]. This may be notably so for GBMSM who typically are early adopters of technology and social media [10–12]. The Centers for Disease Control and Prevention (CDC) Compendium includes 65 HIV risk reduction evidence-based behavioral interventions; however, of those, only one intervention targets HIV-positive GBMSM [13, 14] and only one intervention includes Internet or mobile technology for HIV-negative and HIV-positive black bisexual men [15].
Video messages have greater potential to engage learners than conventional text or graphics found in web-based or print materials [16–18]. Theory-based dramatic videos are an effective means of delivering HIV/STI prevention content to at-risk populations [19–22], including GBMSM [23–25]. In a review of video-based interventions, researchers concluded that delivery of health information via video has been effective at modifying certain behaviors including HIV testing, treatment adherence, and cancer screening [20]. We have conducted online studies with HIV-negative and HIV-positive GBMSM that included theory-driven videos. The Morning After, a 9-min scripted video developed in 2005, relied on principles of storytelling [26] to engage viewers and elicit HIV-related critical thinking [24]. The within-group online evaluation of The Morning After [24] found significant decreases in CAS during the most recent sexual encounter. These findings were confirmed in a sample of 3092 GBMSM participating in an online video-based randomized controlled trial (RCT) with 60-day follow-up that included The Morning After [25]; additionally, HIV-positive participants in the video arm reported significantly reduced CAS with HIV-negative and unknown status partners at 60-day follow-up compared to baseline. Participants in the static control arm did not report reduced risk.
Adding an attention control video arm was one of the reasons for conducting the current video-based intervention. Other reasons include that the aforementioned video interventions were developed specifically for HIV-negative men and therefore did not reflect the needs and perspective of men living with HIV. Moreover, the unexpectedly high proportion of HIV-positive men participating in our prior prevention studies (and reporting reductions in risk behavior) [25] signaled the need for developing targeted interventions. Furthermore, the dearth of theory-driven videos addressing sexual risk reduction in GBMSM living with HIV prompted us to design Sex Positive![+], an eHealth video-based intervention tailored specifically for this subgroup. For the intervention, we produced Just A Guy, a six-episode video series adapted from The Morning After. As we have described else where, both the study design and video series were informed by social learning and social cognitive theories (SLT/SCT) in order to promote critical thinking [27, 28]. Specifically, the dramatic video series models HIV serostatus disclosure, safer sex discussions, and medication adherence, all of which are intended to promote critical thinking among study participants. Unlike The Morning After, Just A Guy, the video series developed for Sex Positive![+], features a protagonist named Guy, a gay man living with HIV who, during the course of six episodes, overcomes a “victim” status while developing a sense of empowerment that positively impacts his personal relationships and physical health.
The current paper presents findings from an evaluation of Sex Positive![+] based on data collected from a sample of 830 GBMSM living with HIV who indicated a detectable viral load or suboptimal cART adherence and engaged in CAS with known or unknown serodiscordant male partners. The primary outcome assessed for this one-year RCT was a reduction in the number of serodiscordant CAS partners among participants assigned to the intervention arm compared to those in an attention control arm. We hypothesized that men in the video intervention arm would report significantly fewer known and unknown serodiscordant CAS partners at 3 and 12-month follow-up, compared to men in the video attention control arm.
Methods
Study Design
Sex Positive![+] was a two-arm video-based RCT with a 1:1 allocation. Participants were randomized to either the video intervention or video attention control condition. Intervention participants received a series of 10 theoretically-driven videos that addressed issues of sexual risk, HIV status disclosure, treatment adherence, substance use, and social support. Participants in the attention control condition received a series of 10 healthy living videos (e.g., nutrition, physical exercise) of comparable length (2–4 min each) but not associated with the primary outcome. Regardless of study condition (i.e., intervention videos or attention control videos), 1 video per week for 6 weeks was delivered to participants in each study arm following the baseline assessment; and 4 booster videos were delivered weekly to each study arm following the 6-month assessment. All participants completed screener and baseline assessments, and were invited to complete follow-up assessments at 4 time points (3-, 6-, 9-, and 12-month follow-up). The primary outcome, number of known and unknown serodiscordant CAS partners, was assessed at each survey time point.
Ethical Considerations
The Institutional Review Board at Public Health Solutions approved all study procedures. A Data and Safety Monitoring Board (DSMB), comprised of experts in trial designs, Internet research, web design, and HIV-positive populations met 3 times (approximately once every 6 months) during active study recruitment to discuss issues related to participant safety, study validity, and data integrity. A Certificate of Confidentiality was also obtained from the National Institute of Mental Health (NIMH) to provide additional privacy protections for participants enrolled in this study.
Procedures
Video Content
Just A Guy Intervention videos. Based in part on recommendations made during the formative phase by a community advisory group meeting in June 2014, the study team collaborated with a professional local production team to develop Just A Guy (described above). The story in this six-episode series is intended to engage gay and bisexual men who are living with HIV and to promote critical thinking about HIV status disclosure to sex partners, serodiscordant CAS, sexual decision making while under the influence of drugs or alcohol, adherence to cART, and viral suppression. As we noted earlier, the script, informed by SLT/SCT [27, 28], included a modified storyline (e.g., main character living with HIV, discussion of pre-exposure prophylaxis [PrEP] with an HIV-negative partner, use of GPS-based sexual partnering) from The Morning After [24].
The SLT/SCT framework for Just A Guy was tailored to promote critical thinking regarding sexual decision making, HIV disclosure, and cART adherence. Critical thinking is the willingness to confront one’s misconceptions or insufficient understanding about a topic [29]. The theoretical framework also guided design dimensions embedded within the storyline, script, and video development. Examples of these dimensions include cognitive dissonance [30] and expectation failure [31, 32] that are depicted through the instructional strategy of modeling [27, 28, 33, 34]. Cognitive dissonance is discomfort felt when there is a discrepancy between what an individual already knows or believes and new information encountered. Expectation failure occurs when new information challenges viewers, along with characters in stories. Both cognitive dissonance and expectation failure were embedded within the storyline and script in episode 5 of Just A Guy. At his healthcare provider visit, Guy and a female nurse have an emotionally charged interaction. The nurse asks Guy to define what having an undetectable viral load means and then tells him he is now detectable and wants to know what changed in his life. Guy becomes visibly upset and shouts that every time he takes his medication, he is reminded of the day that he found out he was HIV-positive. The nurse says that he will be reminded of his HIV one way or another—taking a pill or getting really sick. She then shows Guy a picture of her husband and daughter and says that they are both HIV-negative because she takes a pill every day, and that is what reminds her when she takes her medication. In episode 6, a quick string of video clips model cART adherence, showing Guy taking a pill every day.
Following the 6-month follow-up assessment, participants received four booster videos. These included three edited segments from a sequel to The Morning After, titled Ask Me Tell Me [35], a dramatic HIV prevention video series developed in 2011 featuring interconnected vignettes that dramatize HIV-negative GBMSM “asking and telling” STI status in wide-ranging realistic situations, and a short segment about the importance of social support for individuals living with HIV [36]. Ask Me Tell Me segments were chosen as booster videos for several reasons: first, there were few available videos online focusing on the primary outcome with this specific population; second, Ask Me Tell Me was developed using the same theoretical underpinnings and modeling approaches as Just A Guy and thus provided continuity; and third, Ask Me Tell Me included an HIV-positive character in a serodiscordant relationship.
Attention control videos. Because the purpose of the equal attention control arm was to prevent potential attrition differences in the control arm, the study team selected 10 healthy living videos that were similar in length to the intervention videos and addressed relevant topics for GBMSM and HIV-positive populations, including testicular self-exams, nutrition, physical exercise, smoking cessation, homelessness, anti-bullying, and sleep. Although the control videos did not include any content related to the primary outcome (e.g., no information about sexual risk behavior), participants had access to health-related PDF fact sheets including HIV transmission risk (described below). The attention control videos were chosen from a video-sharing website (Vimeo) based on consensus by members of the study team.
Administrative Platform
The study utilized a custom-built administrative platform to deploy and monitor data collection and intervention activities, including screening, informed consent, enrollment, registration, and randomization. In addition, the platform hosted a study dashboard for participants to log-in and complete study activities, update personal information, and redeem incentives [37]. Through the study dashboard, participants could access the baseline and follow-up assessments, which were hosted by SurveyGizmo [38]. SurveyGizmo uses Amazon Web Services to host survey data, which has encrypted, redundant, and geographically dispersed servers that meet standard disaster recovery protocols. The administrative platform also hosted fact sheets on healthy living, including mental health, exercise, nutrition, Hepatitis C, HIV transmission risk, and a suicide prevention hotline link. These fact sheets were available on all participants’ dashboards throughout the duration of the study and could be clicked on to view the information.
Eligibility Screener Survey
Interested individuals were asked to complete an online screener survey to determine their eligibility for Sex Positive![+]. Eligible participants for the online intervention had to: (a) be assigned male sex at birth and identify their current gender as male or genderqueer; (b) be 18 years of age or older; (c) report their race or ethnicity as white, black, or Hispanic; (d) be able to read and respond in English; (e) self-report residing within the United States or U.S. territories (and confirmed via IP address); (f) report being HIV-positive; (g) report a past-year detectable viral load (> 200 copies/ml) or past-month suboptimal cART adherence [39]; (h) report CAS with any HIV-negative or unknown status male partners in the past six months; (i) be willing to participate in an online intervention study for one year; and, (j) have a working e-mail address and cell phone number for intervention follow-up [37]. In recent years, the U.S. population online has begun to reflect similar proportions of white, black, and Hispanic adults [40, 41], with black and Hispanic populations being more likely to use a mobile phone for texting and social networking than white populations [42]. More importantly, white, black, and Hispanic GBMSM comprise the majority of HIV infections in the U.S. [1] and were the target groups for the current study. We excluded men from other racial/ethnic backgrounds in order to have enough statistical power to conduct subgroup analyses.
During the recruitment phase, more than 5000 HIV-positive men had completed the online study screener; however, most of these men were ineligible because they reported taking HIV medication and/or having an undetectable viral load. While this finding demonstrated that GBMSM living with HIV had some level of engagement with the healthcare system, it was a challenge for study enrollment. However, while men were reporting having HIV medication, they were not necessarily adherent. Since cART adherence is strongly related to viral load, we received IRB approval to incorporate the 3-item Wilson measure of HIV medication adherence into the online study screener [39]. Men who self-reported less than 90% adherence over the last 30 days, as determined by the three items, were now considered eligible for the study even if they did not report having a detectable viral load in the past year (assuming all other inclusion criteria were met).
Recruitment and Study Retention
To better reflect the demographic characteristics of the U.S. HIV epidemic, we implemented stratified randomization by age group (18–29 vs. 30 and over) and race/ethnicity (white, black, or Hispanic) prior to online recruitment. To reach younger men and men of color, we conducted online advertising campaigns on websites like BGCLive and by using recruitment language specifying young men. Additionally, men were recruited through social and sexual networking websites, online bulletin boards, and GPS-based smartphone apps that utilize targeted recruitment by city, race and ethnicity, and age. Based on previous research that sought to understand and mitigate lower click-through rates of MSM of color [43], our targeted recruitment strategy included banner advertisements featuring male models that mirrored the racial and ethnic composition of each subgroup [43]. POZ Personals, the online dating site for POZ Magazine, distributed an email blast to a defined national subset of male-identified POZ Personals members (i.e., those who identified as HIV-positive, were at least 18 years of age, and identified as gay or bisexual). POZ also ran banner ads on its websites. Individuals who clicked on the email-embedded link or a study ad were directed to an online screener survey.
Regardless of online recruitment venue, men who screened eligible were then directed to an online consent form, followed by an online study registration process that included validating both an email address and cell phone number via a unique code. Post-study enrollment, participants received automated text message and email notifications on the day that a study activity was scheduled. Study staff called participants who had not completed a scheduled activity after 14 days. If a participant was unreachable by phone, study staff called the participant’s contact person to help reach the participant without indicating what the subject matter was.
Measures
All study participants completed online assessments that included demographic, HIV testing and care, and sexual history questions. Most questions were developed in prior studies or for the current study; where applicable, validated measures are described. Most questions included a “prefer not to answer” option.
Demographic and Socioeconomic Characteristics
The screener survey assessed age, race and ethnicity, current gender identity, sex at birth, and country of residence of participants. U.S. region of residence was determined by participants’ self-reported zip code.
Eligible participants who enrolled in the online intervention were asked during the baseline assessment about their highest level of education, annual income, and current relationship status. The baseline assessment included an item to measure perceived city size where participants reside (e.g., rural area, suburb of a big city, big city).
HIV Testing and Care
Participants reported results from their most recent HIV test as part of the screener survey. Response options included: Positive, Negative, Never obtained results, Indeterminate, Never been tested, and Prefer not to answer. Those who indicated that their most recent HIV test result was Positive received two follow-up items to assess whether or not they had received a viral load test in the past year. Men who reported having a past-year viral load test were then asked to select their most recent results from the following: My viral load was undetectable, OR ≤ 200 copies/ml; My viral load was detectable, OR ≥ 00 copies/ml; I don’t know—but I think I was undetectable; I don’t know—but I think I was detectable. The screener survey assessed current use of antiretroviral medications (Are you currently taking antiretroviral medications to treat your HIV infection? yes, no).
Participants also completed the 3-item Wilson measure of cART adherence (past 30 days) as part of the screener survey [39]. At screener, 6-, and 12-month follow-up, participants were asked: “In the last 30 days, on how many days did you miss at least one dose of any of your HIV medicines?” (0–30 days); “In the last 30 days, how good a job did you do at taking your HIV medicines in the way you were supposed to?” (never, rarely, sometimes, usually, almost always, always); and “In the last 30 days, how often did you take your HIV medicines in the way you were supposed to?” (never, rarely, sometimes, usually, almost always, always). Responses to each question were linearly transformed to a 0–100 scale. Participants with a mean 3-item score less than 90 were classified as having suboptimal HIV medication adherence. We chose the 90% cut-off threshold for the 3-item Wilson adherence measure based on discussions with clinicians, as well as a meta-analysis [44] that assessed different thresholds for optimal cART adherence and subsequent viral suppression; based on the meta-analysis, 90% is considered acceptable, especially with newer cART regimens.
Sexual History and Condomless Anal Sex
The baseline survey assessed lifetime and past-year number of male anal insertive and receptive sex partners. At baseline and follow-up time points, participants were also asked about their number of male anal sex partners for the past 3 months. Pull-down menus listed 0 through 100 partners, 101+ partners, I don’t know, and prefer not to answer. Participants indicating any male anal sex partners were asked additional encounter-level questions for up to the last three partners in the past 3 months. At every time point, encounter-level CAS was assessed by several items which asked the participant about insertive and receptive anal sex with their partner (i.e., “Was your penis in Partner 1’s anus” and “Was Partner 1’s penis in your anus?”), and if yes, whether or not a condom was used (i.e., “While your penis was in Partner 1’s anus did you wear a condom?” and “While his penis was in your anus did he wear a condom?”). Items were combined and reported as insertive or receptive CAS with up to three most recent anal sex partners in the past 3 months.
Serodiscordant Condomless Anal Sex
Based on the CAS variables described above, known and unknown serodiscordant CAS variables were constructed for up to 3 partners at every time point. Participants reporting any anal sex with another man in the past 3 months received follow-up questions focusing on the last encounter with each of their three most recent male anal sex partners, their partner’s relationship type, and their partner’s serostatus at the last encounter (i.e., “did you know his HIV status?” with response options “Yes, Positive; Yes, Negative; No, I assume Positive; No, I assume Negative; I didn’t think about it; and Prefer not to answer”). We defined known serodiscordant CAS as having HIV-negative CAS partners. Unknown sero-discordant CAS partners included those who did not disclose to the participant (e.g., assumed status).
Statistical Analysis
Data analyses were performed with IBM SPSS version 22 [45] for the primary outcome (known and unknown) serodiscordant CAS partners at 3-and 12-month follow-up. Known and unknown serodiscordant CAS partners were analyzed separately, but were not mutually exclusive, as some participants reported both known and unknown serostatus CAS partners; missing or refusal data on partner serostatus was excluded from analyses. Stratified randomization by race/ethnicity and age was conducted to ensure balanced arms and 20% representation of GBMSM aged 18–29. Table 1 presents sociodemographic and behavioral characteristics of randomized participants. Table 2 presents differences in the primary outcome between the intervention and control arms, from baseline to 3-and 12-month follow-up. Risk differences and risk ratios were estimated to compare change in the primary outcome for known and unknown serodiscordant CAS partners [46]. In Table 3, separate linear regression models estimated the treatment effect (vs. attention control) of the intervention videos on the main outcome, specifically the change in known and unknown serodiscordant CAS partners at 3-and 12-month follow-up; regression models adjusted for baseline number of known and unknown serodiscordant CAS partners. The analyses performed for Tables 2 and 3 are based on a continuous encounter-level serodiscordant CAS variable, with values ranging from − 3 to 3 (reflecting a change in the number of SDCAS partners between time points). Of note, analyses in Tables 2 and 3 were constructed in a similar format to those reported for an online digital media intervention for GBMSM [8].
Table 1.
Sociodemographic and behavioral characteristics of participants at study screening/baseline (n =830)
| Intervention (n = 413) |
Control (n = 417) |
χ2 | Control vs Intervention |
|
|---|---|---|---|---|
| n (%) | n (%) | p value | ||
| Age (n = 829) | 3.14 | 0.53 | ||
| 18–24 | 32 (7.8) | 33 (7.9) | ||
| 25–29 | 78 (18.9) | 65 (15.6) | ||
| 30–39 | 131 (31.8) | 123 (29.5) | ||
| 40–49 | 100 (24.3) | 116 (27.8) | ||
| 50+ | 71 (17.2) | 80 (19.2) | ||
| Race/Ethnicity (n = 830) | 0.07 | 0.97 | ||
| White | 206 (49.9) | 209 (50.1) | ||
| Black | 112 (27.1) | 110 (26.4) | ||
| Hispanic | 95 (23.0) | 98 (23.5) | ||
| Residence (n = 830) | 10.6 | 0.10 | ||
| North Easta | 82 (19.9) | 68 (16.3) | ||
| South Atlanticb | 100 (24.2) | 104 (24.9) | ||
| North Centralc | 52 (12.6) | 59 (14.1) | ||
| South Centrald | 93 (22.5) | 71 (17.0) | ||
| Mountaine | 24 (5.8) | 24 (5.8) | ||
| Pacificf | 61 (14.8) | 88 (21.1) | ||
| Puerto Rico | 1 (0.2) | 3 (0.7) | ||
| Education (n = 829) | 3.29 | 0.19 | ||
| High School or less | 48 (11.6) | 45 (10.8) | ||
| Some college or enrolled | 166 (40.2) | 193 (46.4) | ||
| College degree or more | 199 (48.2) | 178 (42.8) | ||
| Income (n = 800) | 1.94 | 0.86 | ||
| Less than $10,000 | 69 (17.4) | 69 (17.1) | ||
| $10,000-$ 19,999 | 72 (18.2) | 75 (18.6) | ||
| $20,000-$39,999 | 104 (26.3) | 110 (27.2) | ||
| $40,000-$59,999 | 64 (16.2) | 60 (14.9) | ||
| $60,000-$99,999 | 53 (13.4) | 63 (15.6) | ||
| $100,000 or more | 34 (8.6) | 27 (6.7) | ||
| City Size (n = 828) | 7.24 | 0.20 | ||
| Rural area | 15 (3.6) | 15 (3.6) | ||
| Small town | 37 (9.0) | 22 (5.3) | ||
| Suburb of a smaller urban area | 18 (4.4) | 19 (4.6) | ||
| Smaller urban area | 31 (7.5) | 46 (11.1) | ||
| Suburb of a big city | 82 (19.9) | 90 (21.7) | ||
| Big city | 230 (55.7) | 223 (53.7) | ||
| Single (n = 830) | 0.01 | 0.92 | ||
| Yes | 267 (64.6) | 271 (65.0) | ||
| No | 146 (35.4) | 146 (35.0) | ||
| Recruitment Source (n = 827) | 6.72 | 0.15 | ||
| Bareback website/app | 195 (47.4) | 216 (51.9) | ||
| Sexual networking website for Black and Latino GBMSM | 46 (11.2) | 41 (9.9) | ||
| Sexual networking mobile apps | 124 (30.2) | 112 (26.9) | ||
| Other sexual networking websitesg | 25 (6.1) | 15 (3.6) | ||
| Non-sexual sourceh | 21 (5.1) | 32 (7.7) | ||
| Past 6 month viral load (n = 745) | 0.00 | 0.96 | ||
| Undetectable | 284 (77.0) | 290 (77.1) | ||
| Detectable | 85 (23.0) | 86 (22.9) | ||
| Adherencei (n=588) | 1.86 | 0.17 | ||
| ≥ 90% adherence | 63 (22.0) | 53 (17.5) | ||
| < 90% adherence | 223 (78.0) | 249 (82.5) | ||
| Male anal sex partners | ||||
| Lifetime (n = 828) | 1.00 | 0.91 | ||
| 1–10 | 22 (5.3) | 25 (6.0) | ||
| 11–50 | 84 (20.4) | 76 (18.3) | ||
| 51–100 | 30 (7.3) | 27 (6.5) | ||
| More than 100 | 201 (48.8) | 211 (50.7) | ||
| I don’t know | 75 (18.2) | 77 (18.5) | ||
| Past 12 months (n = 828) | 0.81 | 0.94 | ||
| 1–10 | 198 (48.2) | 193 (46.3) | ||
| 11–50 | 136 (33.1) | 136 (32.6) | ||
| 51–100 | 18 (4.4) | 20 (4.8) | ||
| More than 100 | 18 (4.4) | 22 (5.3) | ||
| I don’t know | 41 (10.0) | 46 (11.0) | ||
| Past 3 months (n = 828) | 3.35 | 0.65 | ||
| 0 | 17 (4.1) | 16 (3.8) | ||
| 1–5 | 218 (52.9) | 222 (53.4) | ||
| 6–10 | 80 (19.4) | 68 (16.3) | ||
| 11–50 | 74 (18.0) | 82 (19.7) | ||
| More than 50 | 4 (1.0) | 9 (2.2) | ||
| I don’t know | 19 (4.6) | 19 (4.6) |
Some variables have missing data; Regions are defined by the U.S. Census Bureau
North East residence includes NY, NJ, CT, PA, MA, RI, NH, ME, and VT
South Atlantic residence includes DE, DC, MD, VA, WV, NC, SC, GA, and FL
North Central residence includes IN, MI, IA, OH, WI, MN, SD, ND, IL, MO, KS, and NE
South Central residence includes AL, TN, MS, KY, LA, AR, OK, and TX
Mountain residence includes MT, CO, WY, ID, UT, AZ, NM, and NV
Pacific residence includes CA, HI, OR, WA, and AK
Other Sexual Networking Websites include dating and hookup websites and mobile apps
Non-Sexual Source includes social networking websites, magazines, friends or family members, and other
Based on Wilson score; Missing 133 cases who enrolled before the implementation of this screening measure
Table 2.
Change in number of known and unknown serodiscordant condomless anal sex (CAS) partners from baseline to 3-and 12-month follow-up by treatment arm
| Intervention %(n=173) |
Control % (n = 171) | Risk difference | Risk ratioa | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|---|---|---|
| Change in known serodiscordant CAS partners between baseline and 3-months | ||||||
| One or more fewer partners | 33 (19.1) | 39 (22.8) | −3.7 | 0.84 | 0.55 | 1.26 |
| No change in partners | 105 (60.7) | 87 (50.9) | 9.8 | 1.19 | 0.99 | 1.44 |
| One or more additional partners | 35 (20.2) | 45 (26.3) | −6.1 | 0.77 | 0.52 | 1.13 |
| Intervention %(n=132) |
Control % (n= 149) | Risk difference | Risk ratioa | Lower 95% CI | Upper 95% CI | |
| Change in known serodiscordant CAS partners between baseline and 12-months | ||||||
| One or more fewer partners | 31 (23.5) | 46 (30.9) | −7.4 | 0.76 | 0.51 | 1.12 |
| No change in partners | 60 (45.5) | 69 (46.3) | −0.8 | 0.98 | 0.76 | 1.27 |
| One or more additional partners | 41 (31.1) | 34 (22.8) | 8.3 | 1.36 | 0.92 | 2.01 |
| Intervention %(n=180) |
Control % (n= 196) | Risk difference | Risk ratioa | Lower 95% CI | Upper 95% CI | |
| Change in unknown serodiscordant CAS partners between baseline and 3-months | ||||||
| One or more fewer partners | 65 (36.1) | 59 (30.1) | 6.0 | 1.20 | 0.90 | 1.60 |
| No change in partners | 88 (48.9) | 88 (44.9) | 4.0 | 1.09 | 0.88 | 1.35 |
| One or more additional partners | 27 (15.0) | 49 (25.0) | −10.0 | 0.60* | 0.39 | 0.92 |
| Intervention %(n=136) |
Control % (n=163) | Risk difference | Risk ratioa | Lower 95% CI | Upper 95% CI | |
| Change in unknown serodiscordant CAS partners between baseline and 12-months | ||||||
| One or more fewer partners | 48 (35.3) | 63 (38.7) | −3.4 | 0.91 | 0.68 | 1.23 |
| No change in partners | 53 (39.0) | 67 (41.1) | −2.1 | 0.95 | 0.72 | 1.25 |
| One or more additional partners | 35 (25.7) | 33 (20.2) | 5.5 | 1.27 | 0.84 | 1.93 |
n (%) and 95% confidence intervals (CIs) presented. Risk difference = intervention% − control%
Risk ratios (intervention/control) all p >.05 unless otherwise indicated.
p <.05
Table 3.
Summary of treatment effect estimates from linear regression models on changein known and unknown serodiscordant condomless anal sex (CAS) with male partners at 3-month and 12-month follow-up
| R2 | B | SE B | β | Lower 95% CI | Upper 95% CI | P | |
|---|---|---|---|---|---|---|---|
| Change in known serodiscordant cas partners | |||||||
| 3-month outcome | |||||||
| Unadjusted | 0.000 | −0.006 | 0.094 | −0.003 | −0.189 | 0.178 | 0.95 |
| Adjusteda | 0.232 | 0.005 | 0.088 | 0.003 | −0.168 | 0.178 | 0.96 |
| 12-month outcome | |||||||
| Unadjusted | 0.007 | −0.156 | 0.115 | −0.081 | −0.382 | 0.070 | 0.17 |
| Adjusteda | 0.374 | −0.141 | 0.097 | −0.073 | −0.332 | 0.051 | 0.15 |
| Change in Unknown Serodiscordant CAS Partners | |||||||
| 3-month outcome | |||||||
| Unadjusted | 0.006 | 0.159 | 0.106 | 0.077 | −0.049 | 0.366 | 0.13 |
| Adjusteda | 0.319 | 0.146 | 0.099 | 0.071 | −0.050 | 0.341 | 0.14 |
| 12-month outcome | |||||||
| Unadjusted | 0.006 | −0.162 | 0.122 | −0.076 | −0.402 | 0.079 | 0.19 |
| Adjusteda | 0.334 | −0.177 | 0.113 | −0.084 | −0.399 | 0.045 | 0.12 |
Unstandardized beta coefficient (B), standard error of unstandardized beta coefficient (SE B), standardized beta (β), 95% confidence intervals (CIs), and significance value (p) reported
Adjusted for baseline number of known and unknown serodiscordant CAS partners, up to three
Results
Study Flow
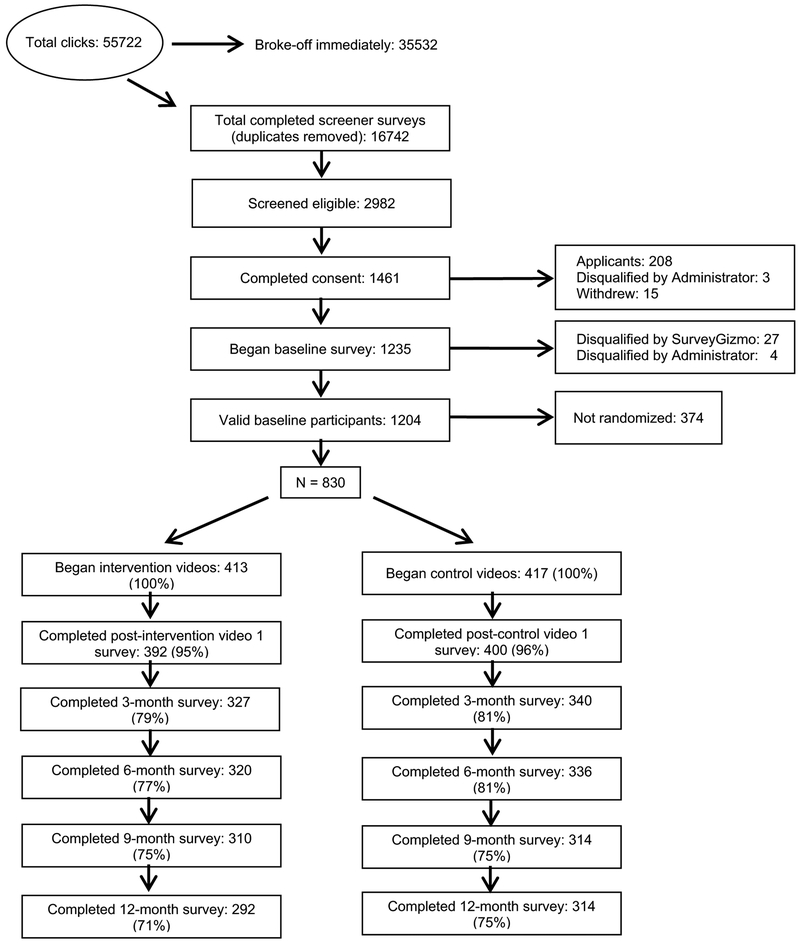
Between June 1, 2015 and December 31, 2015, there were 55,722 visits to the study screener survey, which was accessed by clicking on an online study banner ad or invitation link that advertised the study for men living with HIV. Of those, 35,532 left the webpage without completing any survey items and 3718 were identified as duplicates based on IP address. Of the 16,472 completed, unique screener surveys, 2982 were considered eligible to participate in the intervention and a total of 1461 individuals consented and enrolled (Fig. 1). Of those, 631 participants were excluded from analyses: 3 were disqualified after completing the screener survey and 4 were disqualified after completing the baseline survey for providing fraudulent data; 208 consented and enrolled but did not complete any study activities and were considered participant applicants by the DSMB; 15 withdrew from the study; 27 were disqualified by survey software (e.g., failed survey trap questions, reported being HIV-negative); and 374 were not properly randomized due to an ad campaign launched by a community partner that caused a large and sudden influx of several hundred potential participants simultaneously attempting to complete the study screener and enroll, leaving an eligible sample of 830 participants (Fig. 1). Eligible participants were randomized to either the video intervention arm (n = 413) or the video equal attention control arm (n = 417).
Fig. 1.
Sex Positive![+] retention flow of randomized participants
Baseline Characteristics
Most participants (94%) were recruited from sexual networking websites or GPS-based smartphone apps. Among the 830 men in the baseline sample, half were white with low income and high education (Table 1). The mean age was 39 years (range 19 to 77). Participants resided in 43 U.S. states and Puerto Rico. Most participants reported at least some college or more, though a majority of men in both arms reported earning less than $40,000 a year. Approximately two-thirds of participants in each study arm reported that they were single at the time of enrollment. Importantly, randomization yielded groups that were equivalent on all key characteristics, including age, race/ethnicity, geographic region, socioeconomic status, relationship status, recruitment source, and number of male anal sex partners (past 3 months, past 12 months, and lifetime) between intervention and control participants. Relationship status of participants’ male sex partners were similar across study arms, with 6.6% reporting having a main partner only, 23.0% reporting both main and non-main partners, and 70.1% reporting only non-main partners. Sexual behavior data with female partners was uncommon; a small proportion of men in the sample reported vaginal or anal sex with a woman in the past 12 months (3.6%, 30/830) or past 3 months (0.007%, 6/830).
Male Anal Sex Partners
At each study time point, sexual risk behavior was reported for the past 3 months; men who reported at least 1 male anal sex partner were asked to report behaviors with their most recent (up to 3) one-on-one partner(s). At baseline, 91.2% (n = 757) reported having male anal sex partners in the past 3 months; the median number of male anal sex partners was 4 (IQR 2–10). Among these men, 57.3% reported 3 male anal sex partners, 23.5% reported 2 male anal sex partners, and 18.0% reported 1 male anal sex partner. At 3-month follow-up, 87.2% (n = 582/667) reported having any male anal sex partners in the past 3 months. The median number of male anal sex partners was 3 (IQR 1–8), with 54.8% reporting 3 partners, 24.1% reporting 2 partners, and 18.9% reporting 1 partner. At 12-month follow-up, 82.2% (n = 498/606) reported having at least one male anal sex partner in the past 3 months and the median number of male anal sex partners was 2 (IQR 1–5).
Primary Outcome Analyses
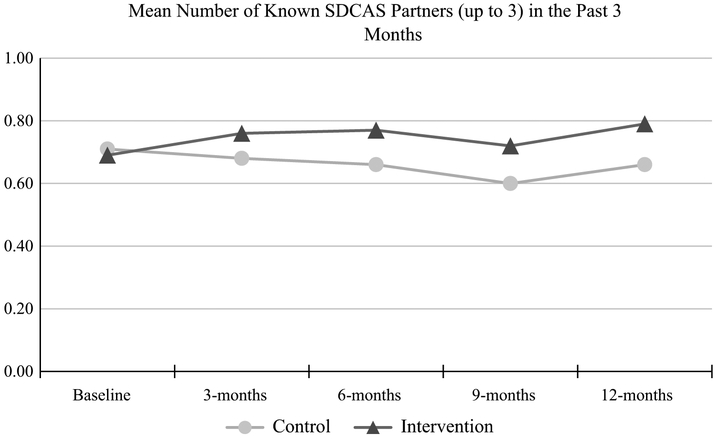
The primary outcome was number of known and unknown serodiscordant CAS partners. We hypothesized that men in the video intervention arm would report significantly fewer known and unknown serodiscordant CAS partners than men in the video attention control arm at 3-and 12-month follow-up. We focused on these two time points for the primary outcome analyses for two reasons. First, intervention and attention control videos were delivered between baseline and 3-month follow-up. Second, the study assessed behavior change over the 12-month period. Table 2 presents risk ratios for 3-and 12-month change from baseline, in number of known and unknown serodiscordant CAS partners by study arm for the categories one or more additional partners, no change in the number of partners, and one or fewer partners. For known serodiscordant partners, at 3-month follow-up, the likelihood of having no change in the number of known serodiscordant CAS partners among men in the intervention arm approached significance (RR 1.19, 95% CI 0.99–1.44) compared to men in the control arm. No differences were found between men in the intervention and control arms for reporting one or more additional known serodiscordant CAS partners (RR 0.77, 95% CI 0.52–1.13) or reporting fewer known serodiscordant CAS partners (RR 0.84, 95% CI 0.55–1.26). At 12-month follow-up, no differences were found between men in both arms regarding no change in known serodiscordant CAS partners (RR 0.98, 95% CI 0.76–1.27) from baseline. Finally, no differences were found between study arms for reporting one or more additional known serodiscordant CAS partners (RR 1.36, 95% CI 0.92–2.01) or fewer known serodiscordant CAS partners (RR 0.76, 95% CI: 0.51–1.12). Figure 2 depicts mean differences of known serodiscordant CAS partners (up to 3) by treatment arm and time point over the study period.
Fig. 2.
Mean number of known serodiscordant insertive or receptive condomless anal sex partners, up to three, by time point and treatment arm
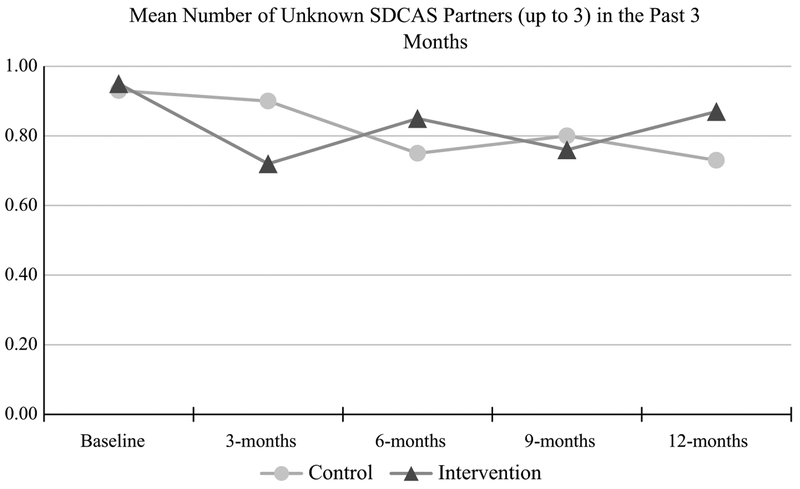
Regarding 3-month change in unknown serodiscordant CAS partners from baseline, no differences were seen between study arms for reporting no change in number of unknown serodiscordant CAS partners (RR 1.09, 95% CI 0.88–1.35) or fewer unknown serodiscordant CAS partners (RR 1.20, 95% CI 0.90–1.60). However, intervention arm participants were significantly less likely (RR 0.60, 95% CI 0.39–0.92) to report one or more additional unknown serodiscordant CAS partners compared to men in the control arm, partially supporting the study hypothesis. This significant finding had a medium effect size (McNemar Chi square = 5.80, p = 0.016; Cohen’s d = 0.57, power = 74%). Nevertheless, by 12-month follow-up, no differences were found between study arms for reporting no change in number of unknown serodiscordant CAS partners (RR 0.95, 95% CI 0.72–1.25), fewer unknown serodiscordant CAS partners (RR 0.91, 95% CI 0.68–1.23), or one or more additional unknown serodiscordant CAS partners (RR 1.27, 95% CI 0.84–1.93). Figure 3 depicts mean differences of unknown serodiscordant CAS partners (up to 3) by treatment arm and time point over the study period.
Fig. 3.
Mean number of unknown serodiscordant insertive or receptive condomless anal sex partners, up to three, by time point and treatment arm
Table 3 presents results from separate linear regression models (unadjusted and adjusted for baseline number of known serodiscordant CAS partners, up to three), which estimated the effect of the intervention on the primary study outcome (i.e., reduced known serodiscordant CAS). Study arm did not significantly predict change in the primary study outcome at 3-month (β = 0.003, 95% CI [− 0.157, 0.166], p = 0.96, R2 = 0.232) or 12-month follow-up (β = − 0.074, 95% CI [− 0.323, 0.039], p = 0.12, R2 = 0.366). Thus, our hypothesis was not supported by the data.
Retention Sample Characteristics
No attrition differences were found by study arm or by number of missing follow-up time points; only 24% of participants completed any study activities without the need for reminder phone calls. Overall retention at 12-month follow-up was 73% (71% for the intervention arm and 75% for the control arm) (Fig. 1). Compared to men who completed all follow-up time points (n = 506, 61%), men who did not complete any of the four follow-up time points (n = 87, 10.5%) were significantly more likely to be black (OR 2.37, 95% CI 1.38–4.06) or Hispanic (OR 2.08, 95% CI 1.17–3.72) than white, and have less than a four-year degree compared to men with a college or graduate degree (Up to a high school diploma: OR 2.46, 95% CI 1.16–5.21; Some college: OR 2.40, 95% CI 1.44–4.00). The mean age for men who did not complete any follow-up activities was significantly lower than those who completed all activities (M 36.6 [SD 10.3] vs. 39.9 [SD 11.3], p < 0.01). Likewise, men not completing any follow-up activities reported significantly lower likelihood of having a current cART regimen to treat HIV (OR 0.35, 95% CI 0.20–0.61). Conversely, men who completed all study time points reported a higher median number of known serodiscordant CAS partners than men who did not complete all study time points (Z = − 1.96, p = 0.05). Finally, men who watched all 6 of the intervention or control videos (78.6%) were significantly more likely to complete 12-month follow-up than men who did not watch all of the videos (p < 0.001).
Discussion
HIV eHealth interventions have demonstrated efficacy among GBMSM, including increases in HIV disclosure, HIV testing, and cART adherence and decreases in CAS and serodiscordant CAS [24, 25, 47–49]. Sex Positive![+], a 2-arm randomized controlled trial with a theoretically-based dramatic video series (Just A Guy), was designed to reduce sexual HIV transmission risk over a 12-month period in a national online sample of GBMSM living with HIV. Efficacy of this video-based intervention on men’s risk behavior was assessed at 3-and 12-month follow-up. The primary study outcome was reduction in the number of known and unknown serodiscordant CAS partners. We focused on change at 3-and 12-month follow-up from base-line to examine the immediate and long-term effects of the intervention in reducing serodiscordant CAS. At 3-month follow-up, compared to men in the control arm, men in the intervention arm reported a 40% reduced risk of having additional unknown serodiscordant CAS partners (RR 0.60, 95% CI 0.39–0.92). This finding signals a short-term impact of the Just A Guy video series that addressed serodiscordant CAS with negative and unknown status partners. This finding is not surprising, given that two of our prior online video interventions demonstrated short-term reductions in risk among both HIV-negative and HIV-positive GBMSM [24, 25]. Indeed, similarities between these interventions include engaging GBMSM with online content and having a brief follow-up period (3 months or less). However, aside from this finding, similar reductions were observed in both groups over time in past 3-month sexual risk behaviors including anal sex, CAS, and known and unknown serodiscordant CAS with male partners.
Null Findings and Possible Explanations
With the exception of a significant group difference in the number of unknown serodiscordant CAS partners at 3-month follow-up, findings from this report indicate similar reductions in risk across study arms. There are possible explanations for this phenomenon. First, both arms received 5 identical survey assessments, which included detailed questions on sexual behavior, healthcare, medication, and cART adherence. Second, being involved in a study may have had an effect on control participants (e.g., Hawthorne effect) and contributed to their behavior change [50]. Third, regression to the mean is another phenomenon that has been reported in intervention studies and may have occurred in this study; men reporting serodiscordant CAS were selected at baseline, so it is possible that some men were experiencing a relative peak in risk behavior and naturally declined in risk during the follow-up periods [51]. It is also important to note that men reported risk behavior in the past 6 months on the screener survey but then were asked to report risk in the past 3 months on the baseline survey, which was completed the same day or soon after completing the screener survey; thus, a subset of men did not report serodiscordant CAS in the past 3 months and were lower risk than other participants. Fourth, with regard to equal attention control interventions such as videos, there is limited information regarding the actual impact of watching a video (e.g., time spent viewing, dramatic versus other video style, actors, topic matter, number of videos received, presence or absence of post-video questions to elicit critical thinking, dosing of videos versus binge watching).
Receiving videos, even if not related to study outcomes, may have an intervention effect, though effect size ranges are unknown. In a prior online RCT, participants who received videos, and even those who received a static webpage with HIV prevention content, reported a significant reduction in CAS partners, while no reduction in CAS was reported in the no-content control group [25]. Finally, the relatively low rate of study attrition (27%) across study arms by 12-month follow-up may explain the lack of difference on the primary outcome; the same intensive level of retention efforts for both study arms could have created an intervention effect. Indeed, remaining in the study and completing risk-related survey assessments over time in both groups may have elicited self-reflection about risk, contributing to risk reduction over time. Men completed surveys, watched videos, and were repeatedly engaged by project staff for the purpose of study retention. Notably, 76% of participants required at least one telephone call as part of retention efforts.
The phenomenon of null findings in HIV prevention research is relatively common [52–55] and signals the need for implementing more scientifically appropriate control arms. Attention control arms, such as the one used in the current study, likely do not reflect the usual level of engagement experienced by GBMSM online or in real-world settings, making it difficult to parse specific intervention effects from attention control design issues (i.e., participation frequency, survey and video content, and duration of participant activities) [56]. Methodological considerations for control groups include (1) having a matched frequency control (e.g., receive the same number of videos but of shorter duration than the intervention) rather than an attention control; and (2) providing fewer and shorter survey assessments in order to reduce the likelihood of an intervention effect. Alternately, newer types of study designs may address control group issues such as conducting an online stepped wedge design, where all participants eventually receive intervention content.
Limitations
Our data have limitations that deserve mention. First, it is likely that the video intervention component only provided short-term effects at reducing serodiscordant CAS in the intervention arm compared to the video attention control arm. It is possible that the survey assessments influenced behavior change in both study arms or that participants who are retained in a study reduce risk over time. Findings from this online video-based intervention may not be generalizable to all HIV-positive GBMSM who access personals websites or apps, to HIV-positive GBMSM who do not identify as gay or bisexual, to men who were exposed to a study email or banner advertisement but did not click on it, or to men who do not identify as black, white or Hispanic. GBMSM were recruited primarily through online sex-seeking and dating websites, which facilitate meeting partners by location and specific characteristics (e.g., body type, preferred sexual positions, age, race/ethnicity, HIV status). In addition, certain websites/apps cater to men seeking particular sexual experiences (e.g., CAS, BDSM, group sex). Thus, findings from this study may not generalize to HIV-positive GBMSM who do not seek partners on these particular websites/apps. The magnitude of change based on the intervention was likely diminished due to the very high risk barebacking online venue from which half of the sample was recruited. When recruiting from multiple online sources, it is important for researchers to assess and document whether participants from distinct recruitment sources report a similar range of outcome behaviors that are central to the study (e.g., number of recent CAS partners).
Another limitation that is common in other HIV prevention studies is that intervention studies tend to lose more men of color and younger men at higher rates than white men and older men (e.g., [25, 57]); although young men and men of color living with HIV completed the study screener, consented, registered and completed the baseline survey they were less likely to remain engaged in the year-long study. Research is needed to understand how to maintain study engagement with younger GBMSM and GBMSM of color living with HIV. Utilizing social media platforms, such as Facebook, to deliver peer-delivered HIV information have shown a higher likelihood of discussions of sexual behaviors online and HIV testing among mostly black and Hispanic GBMSM [58, 59]. These studies indicate that responsive web design approaches to HIV prevention are promising, especially for black and Hispanic GBMSM; however, there continues to be a gap in extending these approaches to address the needs of men living with HIV [60]. These gaps may be addressed through closer coordination of efforts between researchers and community based organizations or health practitioners to develop and test web-and app-based interventions or programs to address risk reduction strategies among GBMSM living with HIV. Another potential limitation is that we only assessed the last 3 sexual partners (at each time point) regarding the primary study outcome. Furthermore, even with a responsive web design, study participants still required human interaction. This finding alone signals that scaling-up eHealth and mHealth interventions must include human facilitation and address the needs of younger GBMSM, and GBMSM of color, living with HIV who may be at greater risk of study attrition. Finally, we did not collect information on whether sex partners were taking PrEP.
Implications for Future Implementation
Findings from this study and from other eHealth interventions provides support for designing and testing modules of short-term interventions with video or other digital media components (e.g., a new Just A Guy season to be viewed every 3 months over a 12-month period) to improve long-term impact. Hence, there is a need in the HIV prevention field to determine component-level effects of surveys and digital media, such as videos, in order to improve an intervention’s impact and deliver the most efficacious component(s). Conducting a factorial design would shed light on specific study components that influence behavior change [61]. Implementing an online factorial design by videos and surveys with the current study materials could be a next step to address the relative lack of differences between study arms; this design would help to accurately test effect sizes based on video content and the number of surveys received (e.g., baseline versus no baseline, video dosing versus binging). If the intervention videos are deemed efficacious in factorial analyses, this eHealth intervention could be scaled-up to help reduce HIV transmission risk in a cost-efficient manner [62]. Additionally, healthcare providers could use some or all of the video vignettes in group treatment settings to encourage discussion on HIV risk reduction techniques based on the characters and behavioral modeling.
Conclusions
This eHealth intervention demonstrated our ability to engage high-risk, racially and ethnically diverse GBMSM living with HIV, and findings indicate support for short-term risk reduction interventions. However, the similar reductions in sexual risk across arms in the current study indicates that there is still much to be learned about video-based web interventions in terms of methodological development and intervention delivery. To this end, the prioritization of funding mechanisms by federal and state agencies to support web-and app-based risk reduction approaches to prevention among high-risk GBMSM is needed to develop and rigorously evaluate eHealth interventions for GBMSM living with HIV, particularly those that target black and Hispanic GBMSM, and to address critical questions that remain about these approaches [63, 64]. Failing to provide such support will result in a notable missed opportunity to improve the health and well-being of those living with HIV through eHealth and mHealth communication channels, as well as to reduce overall high rates of HIV infection among this population [65].
Funding
This study was supported by a grant from the National Institute of Mental Health (R01 MH100973, PI: Hirshfield). A Certificate of Confidentiality was also obtained from the National Institute of Mental Health to provide additional privacy protections for participants enrolled in this study Clinicaltrials.gov ).
Footnotes
Conflict of interest All authors declare that they have no conflict of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Centers for Disease Control and Prevention. HIV surveillance report, 2017 November 2018. [Google Scholar]
- 2.Singh S, Mitsch A, Wu B. HIV care outcomes among men who have sex with men with diagnosed HIV infection—United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(37):969–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gant Z, Bradley H, Hu X, et al. Hispanics or Latinos living with diagnosed HIV: progress along the continuum of HIV care—United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(40):886–90. [PMC free article] [PubMed] [Google Scholar]
- 4.Whiteside YO, Cohen SM, Bradley H, et al. Progress along the continuum of HIV care among blacks with diagnosed HIV—United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(5):85–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Hall HI, Holtgrave DR, Tang T, Rhodes P. HIV transmission in the United States: considerations of viral load, risk behavior, and health disparities. AIDS Behav. 2013;17(5):1632–6. [DOI] [PubMed] [Google Scholar]
- 6.Beymer MR, Weiss RE, Bolan RK, et al. Sex on demand: geo-social networking phone apps and risk of sexually transmitted infections among a cross-sectional sample of men who have sex with men in Los Angeles County. Sex Transm Infect. 2014;90(7):567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rendina HJ, Jimenez RH, Grov C, Ventuneac A, Parsons JT. Patterns of lifetime and recent HIV testing among men who have sex with men in New York city who use grindr. AIDS Behav. 2014;18(1):41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosser BR, Oakes JM, Konstan J, et al. Reducing HIV risk behavior of men who have sex with men through persuasive computing: results of the Men’s INTernet Study-II. AIDS. 2010;24(13):2099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolitski RJ, Gomez CA, Parsons JT. Effects of a peer-led behavioral intervention to reduce HIV transmission and promote serostatus disclosure among HIV-seropositive gay and bisexual men. AIDS. 2005;19(Suppl 1):S99–109. [DOI] [PubMed] [Google Scholar]
- 10.Grov C, Breslow AS, Newcomb ME, Rosenberger JG, Bauermeister JA. Gay and bisexual men’s use of the Internet: research from the 1990s through 2013. Annu Rev Sex Res. 2014;51(4):390–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnan A, Ferro EG, Weikum D, et al. Communication technology use and mHealth acceptance among HIV-infected men who have sex with men in Peru: implications for HIV prevention and treatment. AIDS Care. 2015;27(3):273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seidenberg AB, Jo CL, Ribisl KM, et al. A national study of social media, television, radio, and internet usage of adults by sexual orientation and smoking status: implications for campaign design. Int J Environ Res Public Health. 2017;14(4):E450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKirnan DJ, Tolou-Shams M, Courtenay-Quirk C. The Treatment Advocacy Program: a randomized controlled trial of a peer-led safer sex intervention for HIV-infected men who have sex with men. J Consult Clin Psychol. 2010;78(6):952–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Complete Listing of Risk Reduction Evidence-based Behavioral Interventions. 2019; http://www.cdc.gov/hiv/resea rch/inter venti onres earch/compe ndium/rr/compl ete.html. Accessed 13 March 2019.
- 15.Fernandez MI, Hosek SG, Hotton AL, et al. A randomized Controlled trial of POWER: an internet-based HIV prevention intervention for black bisexual men. AIDS Behav. 2016;20(9):1951–60. [DOI] [PubMed] [Google Scholar]
- 16.Bransford J, Sherwood R, Hasselbring T, Kinzer C, Williams S. Anchored instruction: why we need it and how technology can help. In: Nix D, Spiro R, editors. Cognition, education, and multimedia: exploring ideas in high technology. Hillsdale: Lawrence Erlbaum Associates; 1990. p. 115–41. [Google Scholar]
- 17.Collins A Design issues for learning environments. In: Vosniadou S, De Corte E, Glaser R, Mandl H, editors. International perspectives on the design of technology-supported learning environments. Mahwah: Lawrence Erlbaum Associates; 1996. [Google Scholar]
- 18.Jonassen D, Howland J, Moore J, Marra R. Learning to solve problems with technology: a constructivist perspective. 2nd ed Columbus: Merrill/Prentice-Hall; 2003. [Google Scholar]
- 19.Rivera AV, DeCuir J, Crawford ND, Amesty S, Harripersaud K, Lewis CF. Factors associated with HIV stigma and the impact of a nonrandomized multi-component video aimed at reducing HIV stigma among a high-risk population in New York City. AIDS Care. 2015;27(6):772–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuong W, Larsen ER, Armstrong AW. Videos to influence: a systematic review of effectiveness of video-based education in modifying health behaviors. J Behav Med. 2014;37(2):218–33. [DOI] [PubMed] [Google Scholar]
- 21.Calderon Y, Cowan E, Leu CS, et al. A human immunodeficiency virus posttest video to increase condom use among adolescent emergency department patients. J Adolesc Health. 2013;53(1):79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warner L, Klausner JD, Rietmeijer CA, et al. Effect of a brief video intervention on incident infection among patients attending sexually transmitted disease clinics. PLoS Med. 2008;5(6):e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blas MM, Alva IE, Carcamo CP, et al. Effect of an online video based intervention to increase HIV testing in men who have sex with men in Peru. PLoS ONE. 2010;5(5):e10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiasson MA, Shaw FS, Humberstone M, Hirshfield S, Hartel D. Increased HIV disclosure three months after an online video intervention for men who have sex with men (MSM). AIDS Care. 2009;21(9):1081–9 [DOI] [PubMed] [Google Scholar]
- 25.Hirshfield S, Chiasson MA, Joseph H, et al. An online randomized controlled trial evaluating HIV prevention digital media interventions for men who have sex with men. PLoS ONE. 2012;7:e46252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schank R, Abelson R. Knowledge and memory: the real story. In: Wyer R, editor. Advances in social cognition, vol. 8 Hillsdale: Lawrence Erlbaum Associates; 1995. p. 1–85. [Google Scholar]
- 27.Bandura A Social learning theory. Englewood Cliffs: Prentice-Hall; 1977. [Google Scholar]
- 28.Bandura A Social foundations of thought and action: a social cognitive theory. Englewood Cliffs: Prentice Hall; 1986. [Google Scholar]
- 29.Brookfield S Developing critical thinkers: challenging adults to explore alternative ways of thinking and acting. San Francisco: Jossey-Bass; 1987. [Google Scholar]
- 30.Freijy T, Kothe EJ. Dissonance-based interventions for health behaviour change: a systematic review. Br J Health Psychol. 2013;18(2):310–37. [DOI] [PubMed] [Google Scholar]
- 31.Schank R, Berman T. The pervasive role of stories in knowledge and action In: Green M, Strange JJ, Brock TC, editors. Narrative impact: social and cognitive foundations. Mahway: Lawrence Erlbaum Associates; 2002. p. 287–313. [Google Scholar]
- 32.Schank R Dynamic memory: a theory of reminding and learning in computers and people. New York: Cambridge: University Press; 1982. [Google Scholar]
- 33.Bandura A Psychological modeling: conflicting theories. New York: Lieber-Atherton; 1974. [Google Scholar]
- 34.Bandura A Analysis of modeling processes, vol. 1–62. New York: Lieber-Atherton; 1974. [Google Scholar]
- 35.Chiasson MA. Ask me, tell me. New York: Public Health Solutions; 2011. [Google Scholar]
- 36.Fred says. Chicago: Love Lion Studio; 2013 [Google Scholar]
- 37.Hirshfield S, Downing MJ Jr, Parsons JT, et al. Developing a video-based eHealth intervention for HIV-positive gay, bisexual, and other men who have sex with men: study protocol for a randomized controlled trial. JMIR Res Protoc. 2016;5(2):e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.SurveyGizmo [computer program]. Boulder, CO: Widgix LLC dba SurveyGizmo; 2019. [Google Scholar]
- 39.Wilson IB, Lee Y, Michaud J, Fowler FJ Jr, Rogers WH. Validation of a new three-item self-report measure for medication adherence. AIDS Behav. 2016;20(11):2700–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith A Technology trends among people of color [Report]. Washington: Pew Internet & American Life Project; 2010. [Google Scholar]
- 41.PewInternet.org. Spring tracking survey. Pew Internet & American Life Project. 2011; http://pewinternet.org/Trend-Data/Whos-Onlin e.aspx. Accessed 8 November 2011 [Google Scholar]
- 42.Smith A Mobile access report 2010. Washington: Pew Internet & American Life Project; 2010. [Google Scholar]
- 43.Sullivan PS, Khosropour CM, Luisi N, et al. Bias in online recruitment and retention of racial and ethnic minority men who have sex with men. J Med Internet Res. 2011;13(2):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bezabhe WM, Chalmers L, Bereznicki LR, Peterson GM. Adherence to antiretroviral therapy and virologic failure: a meta-analysis. Medicine. 2016;95(15):e3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.IBM SPSS Statistics for Windows, Version 22.0 [computer program]. Armonk, NY: IBM Corp; 2013. [Google Scholar]
- 46.Kline RB. Beyond significance testing: statistics reform in the behavioral sciences. 2nd ed Washington: American Psychological Association; 2013. [Google Scholar]
- 47.Horvath KJ, Oakes JM, Rosser BS, et al. Feasibility, acceptability and preliminary efficacy of an online peer-to-peer social support ART adherence intervention. AIDS Behav. 2013;17(6):2031–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mustanski B, Garofalo R, Monahan C, Gratzer B, Andrews R. Feasibility, acceptability, and preliminary efficacy of an online HIV prevention program for diverse young men who have sex with men: the keep it up! intervention. AIDS Behav. 2013;17(9):2999–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bauermeister JA, Pingel ES, Jadwin-Cakmak L, et al. Acceptability and preliminary efficacy of a tailored online HIV/STI testing intervention for young men who have sex with men: the Get Connected! program. AIDS Behav. 2015;19(10):1860–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen LF, Vander Weg MW, Hofmann DA, Reisinger HS. The Hawthorne effect in infection prevention and epidemiology. Infect Control Hosp Epidemiol. 2015;36(12):1444–50. [DOI] [PubMed] [Google Scholar]
- 51.Morton V, Torgerson DJ. Regression to the mean: treatment effect without the intervention. J Eval Clin Pract. 2005;11(1):59–65. [DOI] [PubMed] [Google Scholar]
- 52.Jones R, Hoover DR, Lacroix LJ. A randomized controlled trial of soap opera videos streamed to smartphones to reduce risk of sexually transmitted human immunodeficiency virus (HIV) in young urban African American women. Nurs Outlook. 2013;61(4):205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koblin BA, Bonner S, Powell B, et al. A randomized trial of a behavioral intervention for black MSM: the DiSH study. AIDS. 2012;26(4):483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mansergh G, Koblin BA, McKirnan DJ, et al. An intervention to reduce HIV risk behavior of substance-using men who have sex with men: a two-group randomized trial with a nonrandomized third group. PLoS Med. 2010;7(8):e1000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castor D, Pilowsky DJ, Hadden B, et al. Sexual risk reduction among non-injection drug users: report of a randomized controlled trial. AIDS Care. 2010;22(1):62–70. [DOI] [PubMed] [Google Scholar]
- 56.Padian NS, McCoy SI, Balkus JE, Wasserheit JN. Weighing the gold in the gold standard: challenges in HIV prevention research. AIDS. 2010;24(5):621–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du Bois SN, Johnson SE, Mustanski B. Examining racial and ethnic minority differences among YMSM during recruitment for an online HIV prevention intervention study. AIDS Behav. 2012;16(6):1430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang E, Marlin RW, Young SD, Medline A, Klausner JD. Using grindr, a smartphone social-networking application, to increase HIV self-testing among black and Latino men who have sex with men in Los Angeles, 2014. AIDS Educ Prev. 2016;28(4):341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young SD, Holloway I, Jaganath D, Rice E, Westmoreland D, Coates T. Project HOPE: online social network changes in an HIV prevention randomized controlled trial for African American and Latino men who have sex with men. Am J Public Health. 2014;104(9):1707–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noar SM, Willoughby JF. eHealth interventions for HIV prevention. AIDS Care. 2012;24(8):945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bazazi AR, Wickersham JA, Wegman MP, et al. Design and implementation of a factorial randomized controlled trial of methadone maintenance therapy and an evidence-based behavioral intervention for incarcerated people living with HIV and opioid dependence in Malaysia. Contemp Clin Trials. 2017;59:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De P, Downing MJ Jr, Hirshfield S. Cost analysis of implementing a video-based eHealth intervention for HIV-positive gay, bisexual, and other men who have sex with men. AIDS Educ Prev. 2018;30(4):301–8. [DOI] [PubMed] [Google Scholar]
- 63.Noar SM. Computer technology-based interventions in HIV prevention: state of the evidence and future directions for research. AIDS Care. 2011;23(5):525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sullivan PS, Jones J, Kishore N, Stephenson R. The roles of technology in primary HIV prevention for men who have sex with men. Curr HIV/AIDS Rep. 2015;12(4):481–8. [DOI] [PubMed] [Google Scholar]
- 65.Simoni JM, Kutner BA, Horvath KJ. Opportunities and challenges of digital technology for hiv treatment and prevention. Curr HIV/AIDS Rep. 2015;12(4):437–40. [DOI] [PMC free article] [PubMed] [Google Scholar]