Abstract
BCR-ABL1 tyrosine kinase inhibitors (TKIs) are the cornerstone of treatment in chronic myeloid leukemia. Although there are now four TKIs approved for use in the front-line setting, acquired TKI resistance via secondary kinase domain mutations remains a problem for patients. K706 is a novel BCR-ABL1 TKI currently under clinical investigation with structural elements that bear similarity to ponatinib and dasatinib. In this brief report, we functionally characterize the anti-leukemic activity of K706 using cell proliferation assays in conjunction with drug resistance screening. We provide details from molecular modeling to support our in vitro findings and additionally describe our limited clinical experience with this drug in two patients treated on trial. We demonstrate that while K706 retains efficacy against a large spectrum of clinically relevant mutations, it does not appear to have activity against BCR-ABL1T315I. Early trial experience suggests excellent tolerability, which may positively impact the place of K0706 within the ever-expanding CML treatment paradigm.
Keywords: chronic myeloid leukemia, tyrosine kinase inhibitor, drug resistance, clinical trial
Introduction
Patients with chronic phase CML (CP-CML) are effectively managed with BCR-ABL1 tyrosine kinase inhibitors (TKIs) and their survival approaches that of age-matched controls [1]. K0706 was designed as a BCR-ABL1 TKI to provide an option for patients experiencing resistance or intolerance to the first-line TKIs imatinib, nilotinib, dasatinib, and bosutinib [2, 3]. Structural elements that overlap with dasatinib and ponatinib are noted (Figure 1A).
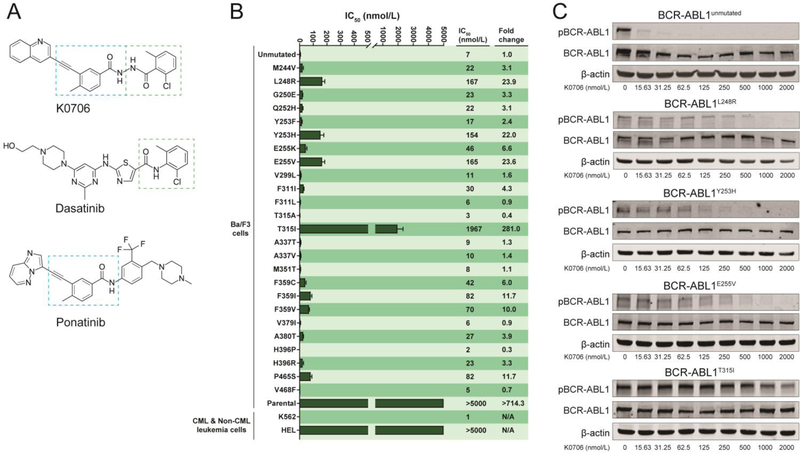
Figure 1. K0706 inhibits native and mutant BCR-ABL1.
(A) Structure of K0706. The boxed areas indicate sub-structure elements shared with ponatinib (blue box) and dasatinib (green box), which are shown for comparison. (B) Cellular IC50 values for K0706 in BCR-ABL1-positive and -negative cell lines. Note: Table S1: Comparison of K0706 to the BCR-ABL1 TKIs bosutinib, nilotinib, dasatinib, and ponatinib. (C) Immunoblot analysis of phosphorylation of BCR-ABL1 (pBCR-ABL1) in Ba/F3 cells expressing BCR-ABL1, BCR-ABL1L248V, BCR-ABL1Y253H, BCR-ABL1E255V, or BCR-ABLT315I following exposure to K0706.
Materials and Methods
Results and Discussion
Cellular proliferation assays established that K0706 is a potent inhibitor of BCR-ABL1 (IC50: 7 nM) and exhibits activity against most clinically important BCR-ABL1 point mutants. The only BCR-ABL1 point mutants with an IC50 above 100 nM were: BCR-ABL1L248R (IC50: 167 nM), BCR-ABL1Y253H (IC50: 154 nM), BCR-ABL1E255V (IC50: 165 nM) and BCR-ABL1T315I (IC50: 1967 nM) (Figure 1B). K0706 was compared to the BCR-ABL1 TKIs bosutinib, dasatinib, nilotinib, and ponatinib across a comprehensive panel of cell lines expressing TKI resistant mutants of BCR-ABL1 (Figure S1). Immunoblot analysis demonstrated direct, potent inhibition of BCR-ABL1 tyrosine autophosphorylation by K0706 as well as inhibition of BCR-ABL1L248R, BCR-ABL1Y253H and BCR-ABL1E255V at high concentrations of inhibitor. BCR-ABL1T315I was not inhibited to a level reaching the IC50 at any of the tested concentrations of K0706 (Figure 1C).
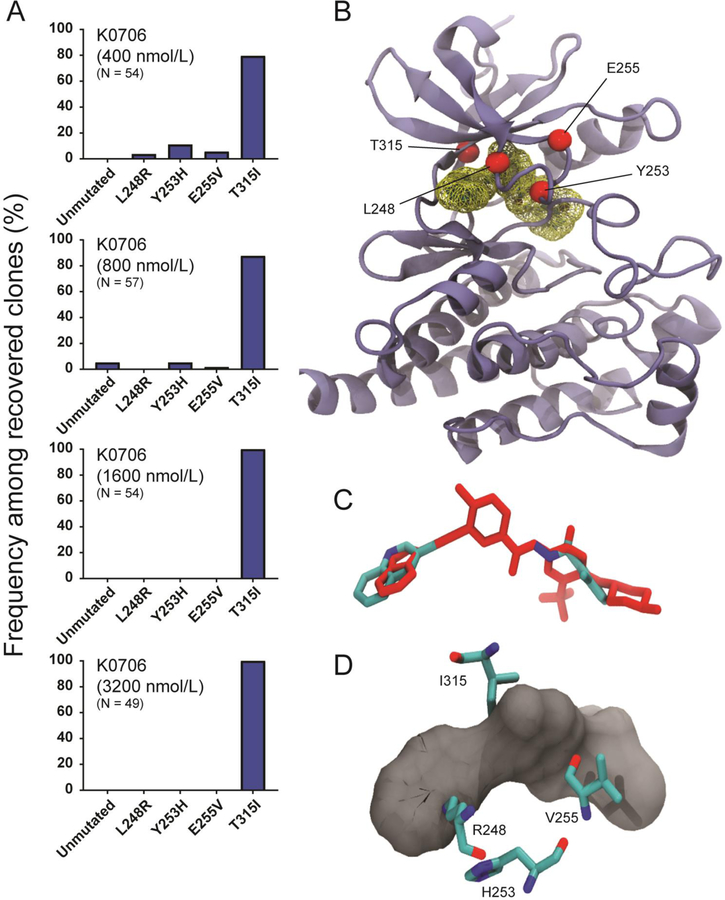
To establish the point mutation-based resistance profile of K0706, an ENU-based resistance screen was performed using Ba/F3 BCR-ABL1 cells. K0706 exhibited a narrow scope of BCR-ABL1 mutations (Figure 2A and Table S1), and the resistance profile narrowed exclusively to BCR-ABL1T315I at concentrations above 800 nM. A dosing finding study is current ongoing and achievable plasma drug concentrations are not yet known. Based on our in vitro results we speculate that BCR-ABL1T315I is beyond the reach of K0706 and that three additional mutants (BCR-ABL1L248R; BCR-ABL1Y253H; BCR-ABL1E255V) confer resistance to this investigational TKI. All other tested BCR-ABL1 point mutants are expected to be sensitive to K0706. Molecular modeling provides a structural basis for the reduced potency of K0706 against BCR-ABL1L248R, BCR-ABL1Y253H, BCR-ABL1E255V, and BCR-ABL1T315I. The binding mode is predicted to be similar to the type II, inactive binding mode accessed by ponatinib (Figure 2B) and the two structurally related inhibitors bind in a strikingly similar conformation (Figure 2C). As in the case of ponatinib, the Y253H and E255V mutations cause local re-orientations within the phosphate-binding loop that result in lowered inhibitor affinity (Figure 2D) [4, 5]. The resistance profile of K0706 demonstrated high-level resistance to the L248R mutant, in contrast to the resistance profile of ponatinib. BCR-ABL1L248R has been reported clinically and was found to be highly resistant against imatinib, bosutinib, dasatinib, and nilotinib, and intermediately resistant against ponatinib (IC50: 13 nM) [6] In our experiments, the IC50 values were 2.8 nM (ponatinib) and 167 nM (K0706). P-loop residues interact favorably with the aromatic ring of the type II inhibitor and any mutation in this region can cause local perturbation and potential steric clashes with the inhibitor, thereby altering its binding affinity significantly. Leu248 (which is part of the P-loop) interacts with the quinoline ring of K0706, which is larger than the imidazopyridazine ring system of ponatinib. Based on the structural alignment, we hypothesize that upon introduction of the K to R substitution at residue 248, a steric clash with K0706 significantly reduces binding affinity (Figure 2D). While ponatinib binding is also adversely affected, the magnitude is not as large. The most important mutational liability for K0706 is BCR-ABL1T315I. Minor differences in the alignment of K0706 as compared to ponatinib within the binding pocket bring the inhibitor into direct contact with the sidechain of I315 and carbonyl group of K0706, an interaction that is incompatible with high-potency binding (Figure 2D).
Figure 2. K0706 exhibits few BCR-ABL1 mutational vulnerabilities.
(A) BCR-ABL1 mutants recovered from cell-based resistance screens for K0706, starting from Ba/F3 BCR-ABL1 cells. ENU-mutagenized cells were plated with inhibitor, monitored for outgrowth, expanded, and sequenced for mutations. Bars represent frequency of a given mutant among all recovered clones at a given inhibitor concentration; percent of wells demonstrating outgrowth and number of clones sequenced is indicated. (B) K0706 in ABL1 binding site, with residues identified in the resistance screen highlighted. (C) K0706 (cyan) superimposed on ponatinib (red) based on x-ray structure. (D) Close-in view of K0706 in ABL1 binding site, with mutated residues identified in the resistance screen shown in ball-and-stick representation.
K0706 is currently in clinical evaluation for several indications. A multicenter phase 1/2 trial of K0706 in adult patients with chronic, accelerated or blast-phase CML or Ph+ acute lymphoblastic leukemia refractory to or intolerant of at least three TKIs is enrolling patients in its two-part dose escalation and open label arms (SPARC: ). K0706 is also currently being studied in a multisite, phase 2 randomized, placebo-controlled trial (PROSEEK: ) in patients with early Parkinson’s disease. The basis for this study is preclinical data showing that c-ABL inhibition reverses neurodegeneration related to α-synuclein accumulation and early clinical data suggesting cognitive improvement with nilotinib treatment [7, 8]. A concomitant Sun Pharma sponsored study is enrolling healthy volunteers to evaluate the pharmacokinetics of K0706 in cerebrospinal fluid ().
Two CML patients at our institution have received K0706 in the clinical trial setting (Part B: ). Patient 1 is a 29 year-old man diagnosed with chronic-phase CML in 2002 and initially treated with imatinib (400 mg daily) who lost molecular and cytogenetic response in 2006. Mutation analysis following imatinib failure did not reveal a BCR-ABL1 kinase domain mutation and he was switched to dasatinib (140 mg daily), which was subsequently dose-reduced to 50 mg due to joint pain. He maintained deep molecular response until March 2016, when his BCR-ABL1 transcript levels rose to 0.92% on the international scale (IS). The patient was switched to ponatinib (15 mg daily) but tolerated it poorly due to gastrointestinal toxicity, grade 4 lipase elevation and headaches. Subsequently, bosutinib was prescribed and although the patient had grade 4 lipase elevation requiring dose reduction, he achieved sustained molecular response approaching major molecular response (MMR) on substandard doses until January 2018, when his BCR-ABL1 transcript levels rose to 7% IS. Mutation analysis was negative and the patient was switched back to ponatinib (15 mg every other day), on which he experienced a rise in BCR-ABL1 transcript level to 34% IS in July 2018 and was found to have accelerated-phase CML based on clonal evolution (karyotype 46,XY,inv(3)(q21q26.2),t(9;22)(q34;q11.2)[17]/46,XY[3]). He enrolled in the SPARC study and began treatment with K0706 in July 2018 (90 mg daily). Cytogenetics following 3 months of K0706 demonstrated complete cytogenetic response and the patient achieved MMR at 5 months with maintenance of MMR ongoing through March 2019. This dose has been well tolerated with grade 1 fatigue and hyperbilirubinemia and grade 2 lipase elevation that resolved without treatment interruption. Of note, use of approved second- and third-generation BCR-ABL1 TKIs to treat this patient was majorly limited by toxicity and inability to achieve appropriate dose intensity.
Patient 2 is a 63 year-old woman diagnosed with chronic-phase CML in 2010 initially treated with imatinib (400 mg daily) until 2013, when she was switched to nilotinib due to peripheral edema and suboptimal molecular response. She developed pancreatitis on nilotinib and was switched to bosutinib in 2015, on which she achieved MMR in November 2016. However, in May 2017 her BCR-ABL1 transcript level increased to 7% IS. Mutation analysis showed E255V mutation and she was switched to dasatinib (140 mg daily) in August 2017, on which her BCR-ABL1 transcript level remained between 5–6% IS. Mutation analysis in December 2017 showed E255V at 100% allele frequency. She continued on dasatinib (140 mg daily) until December 2018 and started K0706 (174 mg daily) in January 2019. BCR-ABL1 transcript percentage in January prior to initiation of K0706 was 6% IS with bone marrow cytogenetics demonstrating Ph+ in 29% (6/21) metaphases. Following 3 months of K0706 (174 mg daily), her BCR-ABL1 PCR increased to 32% IS with 3 month bone marrow cytogenetics showing an increase to 80% (16/20) Ph+ metaphases. Drug tolerance is excellent, with grade 1 fatigue.
Our pre-clinical evaluation of K0706 and associated molecular modeling provide a mechanistic basis for the divergent responses in the two patients. In Patient 1 BCR-ABL1 kinase domain sequencing of trial entry and longitudinal on-treatment samples demonstrated exclusively native BCR-ABL1. In contrast, Patient 2 carried BCR-ABL1E255V from the outset, explaining the limited effectiveness of K0706.
K0706 has potential to be an important, well-tolerated new addition to the group of effective BCR-ABL1 TKIs. The spectrum of BCR-ABL1 mutants sensitive to K0706 compares favorably to all approved BCR-ABL1 TKIs except ponatinib and the allosteric inhibitor asciminib, both of which inhibit the BCR-ABL1T315I gatekeeper mutant. Despite structural similarity to dasatinib, K0706 exhibits limited activity against dasatinib-sensitive mutants Y253H and E255V, corresponding to the lack of clinical response observed in Patient 2. Ongoing dose escalation studies should further clarify the role of K0706 against these mutants. Despite early predictions, K0706 is not active against BCR-ABL1T315I [9]. Another investigational BCR-ABL1 TKI with close structural similarity to ponatinib, PF-114, is currently in phase 1 clinical evaluation in patients with CML with failure of prior TKI therapy (), including failures due to BCR-ABL1T315I [10]. Early results suggest PF-114 has clinical activity, but that skin toxicity may be dose limiting. Another BCR-ABL1 inhibitor, HQP1351, has demonstrated significant clinical activity in a phase 1 study of patients with TKI resistant CML that included patients with the T315I mutation. There was a 64% rate of grade 3 or grade 4 toxicity, including 16% non-hematologic toxicity [11]. A phase 2 study of HQP1351 in CML patients with theT315I mutation is recruiting (). Given that active second or third generation TKIs are approved, new additions to this armamentarium will have to match available TKIs with respect to activity, tolerability and cost.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (NIH) National Cancer Institute grant R01CA178397 (M.W.D. and T.O.). A.B.P. is supported by an American Society of Hematology Research Training Award for Fellows.
Disclosure of Conflicts of Interest
M.W.D. is on the advisory board and is a consultant for Incyte, Novartis and Pfizer, and serves on the advisory board for Takeda, Blueprint and Galena BioPharma. His laboratory receives research funding from Novartis and Pfizer.
References
- [1].Hochhaus A, Larson RA, Guilhot F, et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. The New England journal of medicine 2017;376:917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib Versus Imatinib for Newly Diagnosed Chronic Myeloid Leukemia: Results From the Randomized BFORE Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018;36:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Saglio G, Jabbour E. First-line therapy for chronic phase CML: selecting the optimal BCR-ABL1-targeted TKI. Leukemia & lymphoma 2018;59:1523–1538. [DOI] [PubMed] [Google Scholar]
- [4].O’Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell 2009;16:401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zabriskie MS, Eide CA, Tantravahi SK, et al. BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in Ph chromosome-positive leukemia. Cancer Cell 2014;26:428–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Redaelli S, Mologni L, Rostagno R, et al. Three novel patient-derived BCR/ABL mutants show different sensitivity to second and third generation tyrosine kinase inhibitors. Am J Hematol 2012;87:E125–128. [DOI] [PubMed] [Google Scholar]
- [7].Hebron ML, Lonskaya I, Moussa CEH. Nilotinib reverses loss of dopamine neurons and improves motor behavior via autophagic degradation of -synuclein in Parkinsons disease models. Hum Mol Genet 2013;22:3315–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pagan F, Hebron M, Valadez EH, et al. Nilotinib Effects in Parkinson’s disease and Dementia with Lewy bodies. J Parkinsons Dis 2016;6:503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carella AM, Saglio G, Mahon XF, Mauro MJ. Present results and future perspectives in optimizing chronic myeloid leukemia therapy. Haematologica 2018;103:928–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mian AA, Rafiei A, Haberbosch I, et al. PF-114, a potent and selective inhibitor of native and mutated BCR/ABL is active against Philadelphia chromosome-positive (Ph+) leukemias harboring the T315I mutation. Leukemia 2015;29:1104–1114. [DOI] [PubMed] [Google Scholar]
- [11].Jiang Q, Huang XJ, Chen Z, et al. Safety and efficacy of HQP1351, a 3rd generation oral BCR-ABL inhibitor in patients with tyrosine kinase inhibitor-resistant chronic myelogenous leukemia: Preliminary results of phase 1 study. Blood 2018;132:791.29991556 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.