Abstract
Erythromelalgia (EM) is a rare disorder of small nerve fibres that leads to painful flushing and burning paresthesisas of the distal extremities and is typically associated with heat or physical activity; relief is found using cooling measures. Its effects are often debilitating in the general population, but this patient had an excellent response to specific treatment options and continues to maintain employment, something many individuals suffering from EM are unable to do. His presentation was also unique in that he had isolated, proximal involvement as his condition progressed whereas typically only the distal extremities are affected. Routine electromyography and nerve conduction studies were normal, whereas nerve biopsy demonstrated findings of small fibre neuropathy. Ultimately, his condition was managed with carbamazepine and his symptoms have almost entirely resolved to date.
Keywords: pain (neurology), neuro genetics, neurology
Background
Erythromelalgia (EM) is a unique and rare condition with an estimated age-adjusted and sex-adjusted incidence rate of 1.3 per 100 000 people per year. Typically, it affects the distal extremities, whereas this individual had isolated, proximal involvement, suggesting an atypical presentation. There is much uncertainty regarding the pathophysiology and treatment of this rare condition. It is subdivided into primary, typically familial, and secondary, which is usually related to myeloproliferative disease, autoimmune or neuropathic causes.1 Among the many cases of primary EM, most are related to mutations in the SCN9A gene that codes for the Nav1.7 sodium channel and are typically diagnosed in children and adolescents.2 A mutation has not been identified in sporadic adult-onset cases, and no curative therapy has yet been elucidated. In terms of pathophysiology, it is also thought to result from vasomotor abnormalities or dysfunction in the normal constriction and dilation of the calibre of certain blood vessels, leading to abnormalities of blood flow to the extremities. Further research is needed for a more complete understanding of this disease and its basis. This will help in terms of its management, prognosis and ultimately, patient quality of life, which can greatly suffer due to their symptomatology.3
Case presentation
This is the case of a 31-year-old white male (WM) patient diagnosed with small fibre neuropathy 10 years ago. His history of neuropathy included infrequent episodes of feeling numbness and burning in his lower extremities bilaterally. It was not until recently over the last few years where he would have episodes of ‘extreme sunburn’ as he described them. These were characterised by swollen, red patches with a burning sensation.
He presented to the neurology clinic with painful erythema and a sensation of warmth and burning affecting the chest, ears and feet, which progressed to his hands over the past 3 years. He also had isolated, erythematous patches of skin on his knees and trunk (figure 1). He reported that warm environments, exercise or heat precipitates episodes. Episodes were also associated with positional orientation of his upper extremities, which was relieved with arm-raising. His symptoms were not associated with any relevant family history and secondary causes of small nerve damage or myeloproliferative disorders have been negative to date. With his history of small fibre neuropathy and lack of other associated medical or familial conditions, this was the primary working theory.
Figure 1.
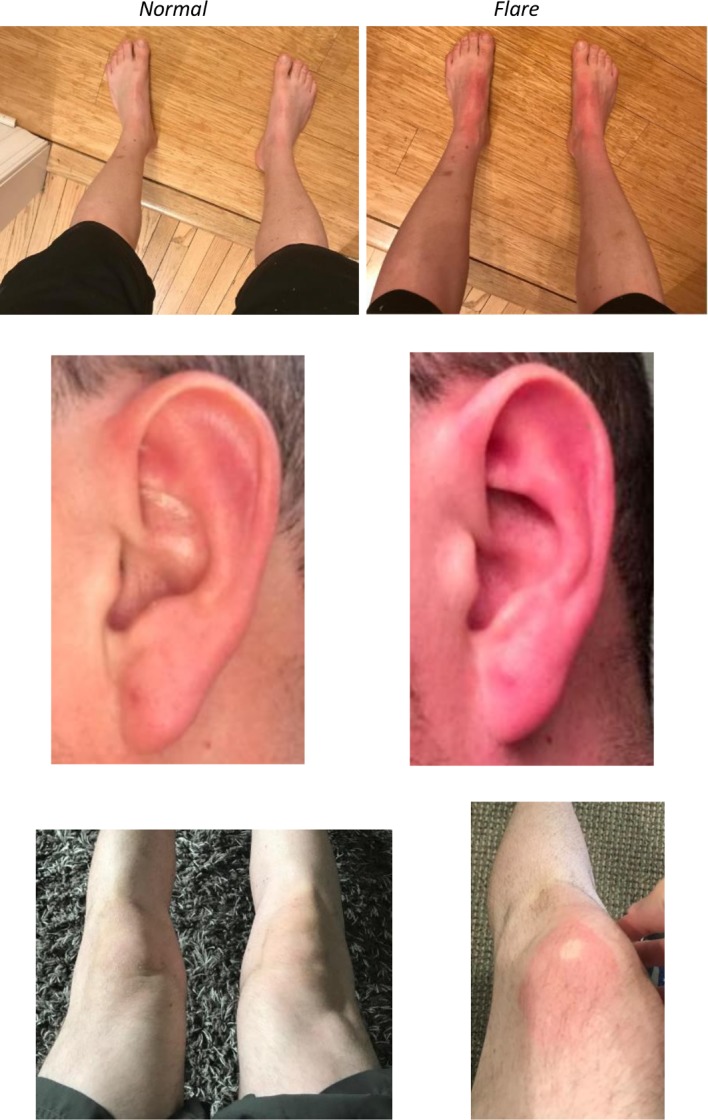
Patient images depicting normal skin (left) versus a flare-up (right) in three different distributions.
Differential diagnosis
Important to the management of patients with EM, and in particular this patient, multiple work-ups from a variety of specialists (haematology, oncology, dermatology, rheumatology) effectively ruled out haematological, autoimmune and oncological disorders.
The differential diagnosis for a patient presenting with clinical findings of erythema and burning paresthesisas of the extremities can be quite broad. Fabry disease, Raynaud’s, thrombotic thrombocytopenic purpura, infectious mononucleosis, systemic lupus erythematosus (SLE) and EM can all present in a similar fashion. Laboratory testing ruled out these conditions. In addition to laboratory tests, in terms of Raynaud’s disease, this condition is exacerbated by the cold and not the heat as in this patient and can be distinguished clinically (table 1).
Table 1.
Differential diagnosis of erythromelalgia
| Condition | Cutaneous Findings |
| Fabry disease | Angiokeratomas, anhidrosis, neuropathy/burning extremity pain |
| Raynaud’s syndrome | Pain within the affected extremities, discolouration (paleness) and sensations of cold and/or numbness when exposed to cold temperatures |
| Thrombotic thrombocytopenic purpura | Jaundice or paleness, bruising/purpura, bleeding, petechiae |
| Infectious mononucleosis | Macular or papular rash, erythema nodosum/multiforme |
| Systemic lupus erythematosus | Thick, red scaly patches, malar rash, ulcers |
In order to further investigate our suspicion that small fibre neuropathy was possibly responsible for the patient’s condition, he underwent routine electromyography and nerve conduction studies. These tests are helpful, but are unable to detect small fibre neuropathy, which limits their utility, but does help narrow the differential in the sense that it rules out conditions related to large fibre neuropathy or another specific neuromuscular disease. Further diagnostic studies involving nerve biopsy had to be performed in order to evaluate for small fibre neuropathy, which in concordance demonstrated findings consistent with decreased nerve fibre density This is something important to keep in mind when ruling out secondary causes of this condition, especially in view of the limitations and mimics of clinical examination.4 5
Treatment
The patient’s medical record and personal account noted that he had trialled and failed multiple medical therapies including aspirin, oral Benadryl, antihistamines, Neurontin, Elavil and Coreg for treatment of his presenting symptoms over the past 10 years. Continued follow-up revealed that his episodes have primarily been managed with topical 5% Lidoderm patches and submersion in cool water. Ultimately, he found relief with the use of carbamazepine (CBZ) (daily) in addition to topical Lidoderm patches (as needed).
Outcome and follow-up
After presentation to the neurology clinic, the patient was started on CBZ 200 mg two times per day and found significant relief within a few hours of starting this new medication. He was also counselled on the danger of excessive cooling as that has led to hypothermia and skin necrosis in some EM sufferers.3 Whereas many patients are unable to continue working due to significant morbidity associated with triggers in the workplace, thus far, he has been able to maintain employment because of successful treatment of his symptoms.
He presented to our clinic for follow-up once following the initiation of this new medication and it was noted that he had near complete resolution of his symptoms without a significant flare-up. He has not followed-up in the clinic since then, but he remains in communication via phone calls and email, and states that he is doing well. He elected to forego genetic testing which would have solidified the suspected mutation in the SCN9A gene. This was offered after the diagnosis of EM was clinically made, but secondary causes were ruled out. In some individuals, this can offer peace of mind as to why they are experiencing the symptoms associated with this condition, but he was simply happy to achieve relief from the pain.
In the long run, the plan for the patient is to continue with CBZ 200 mg two times per day. Similar to treating someone with maintenance medication for seizures, he will continue with this dosing for the foreseeable future. Fortunately, he is on a low dose of CBZ and the side effects in the long term should be minimal. Common side effects include nausea/vomiting, dizziness and loss of balance or coordination. Nonetheless, he will periodically be monitored with laboratory work including a complete blood count and liver function tests. His symptoms have been very well controlled and he has gone almost a year without any flare-ups of his condition. There have been some reports of chemical sympathectomy to help patients achieve long-term remission, but that may be difficult with this patient as he has diffuse and patchy involvement of his condition.6
Discussion
EM is a rare condition typically characterised by warmth, burning discomfort and erythema of the distal extremities including the hands and feet. Although there are many proposed aetiologies regarding the pathophysiology, there is no definitive answer. This condition is thought to be of various origins including vascular factors, genetics and neuropathic conditions. With all the uncertainty, there are a couple working theories in terms of the pathophysiology of this condition: (1) mutations of the Nav1.7 sodium channel, a channel responsible for the transmission of pain, can produce unregulated signal transmission; and (2) dysfunction of the normal dilation and constriction of blood vessels can lead to the painful flushing and erythema. The symptoms are exacerbated by minor trauma, exercise or heat and have been found to be relieved by cooling and elevation of the affected limb. While there is no cure for EM, there are multiple interventions that are aimed at improving quality of life and reducing the number and severity of symptoms.3 Despite the multiple interventions, many patients still find relief elusive because of the trial and error nature of treatment, various aetiologies of the condition and the multitude of exacerbating factors. Approaching EM with agents that would regulate the function of mutated sodium channels is considered one of the optimal ways of treating this condition. Several types of pharmacological agents, including anaesthetics, antiarrhythmic drugs and anticonvulsants, can play a role in systemic regulation of sodium channels and improvement in patient symptoms.4
In further discussion of the relationship between small fibre neuropathy and EM’s clinical presentation, it is suspected that EM causes damage to nociceptors resulting in chronic pain. It is also thought that A-Delta afferent and C efferent sympathetic fibres could be damaged. These small fibres are part of the autonomic neural system that help to control sweat, blood pressure and heart rate. Alteration of the alpha subunit of the Nav1.7 sodium channel due to genetic mutation, which is a hallmark of familial and sporadic EM, can decrease the threshold for activation of C fibres and increase spontaneous activity. Because C fibres are also active in vasodilation, the aforementioned chain of events ultimately results in the burning, warmth and redness these patients experience. EM is also thought to cause dysfunction of the microcirculation in the peripheral vascular system. This dysfunction results in increased perfusion to the affected area, but paradoxically causes tissue ischaemia and subsequently hypoxic tissue pain due to abnormal distribution of blood flow.4
The patient presented with a unique form of this condition in that his involvement was more proximal rather than distal. In addition, his symptoms responded to Tegretol, but not Mexiletine, suggesting that he has a specific mutation on his sodium channels. The idea behind this is that the Nav1.7 sodium channel plays a large role in pain signalling. Patients with EM have been found to have unusually high activity of these channels, thus causing their exaggerated pain responses, even to minor stimuli such as mild warmth. By blocking these channels, the goal is to reduce the firing of pain signals and the subsequent pain response.
In this male patient’s case, he responded to CBZ. Some studies performed have identified mutant Nav1.7 channels with CBZ-responsive inherited EM.1 They demonstrate a normalising effect of this medication, in terms of the activation and inactivation, on these channels. A recent study evaluated a family suffering from this condition found that they have CBZ-responsive Nav1.7 channels. Traditionally, CBZ has been used to reduce neuronal excitability in terms of epilepsy and trigeminal neuralgia. In this case, it was suspected that it normalises the voltage dependence of activation and inactivation of this mutant channel.1 Detection of gene mutations can play a major role in the management of EM in the future, especially when it comes to targeted therapy.
In addition to the possibility of one having a family history of this condition, there are numerous conditions EM can be associated with including SLE, Raynaud’s disease, infectious mononucleosis and thrombotic thrombocytopenic purpura, just to name a few. The patient does not have a family history of this condition and secondary causes of his condition have been ruled out. This suggests that our patient suffers from a primary form of EM rather than familial or one due to another cause.7
While the symptomatology of this disease is important, it is also vital to consider the debilitating effects it has on patients who suffer from it. Because of exacerbations in hot temperatures, many of these individuals will refrain from leaving the house and lose social interaction.8 In addition, many individuals will be unable to maintain employment and their overall quality of life can suffer leading to depression.3
This rare condition suffers from much uncertainty in terms of its pathophysiology and, subsequently, its treatment. Further research is needed for a more complete understanding of this disease and its basis. This will help in terms of its management, prognosis and ultimately, patient quality of life.9 10
Patient’s perspective.
I was planting flowers for my wife when my hands began to feel like they had an extreme sunburn. They felt like they were burning and were red and swollen. I immediately sought after a water source to cool them down and soon this would become a regular occurrence. As erythromelalgia flare ups became more regular and spread to other body parts (feet, ears, knees, elbows) I knew something was wrong. After 5 doctor visits, some of which had witnessed my flares, I still got no answers or treatments. Everywhere I went I needed a emergency bag packed with ice packs, cold water and lotions to help my symptoms. Only through my own research did I find a possible diagnosis and a specialty that could help. I brought this information to a neurologist who was able to confirm my diagnosis of erythromelalgia.
Looking back I had symptom's for years, but initially they were so mild and intermittent that I did not associate them with any significant disease process. Eventually, the pain started to interfere with every aspect of my life and began to take a mental toll on me. Unfortunately, because there is no standard treatment for EM I had tried over 30 different medications, most with minimal to no relief. I initially found some modest relief from mexilitine and midodrine, but ultimately it was a combination of Tegretol and lidocaine patches that brought me the most relief.
I am very thankful to still be working and caring for my family. In addition to the medications I am taking, many lifestyle modifications have helped reduce flares. All things considered, I am doing well. I’m still working, staying fit, and fully independent. Many people are not this lucky. I can only hope that it does not progress and that I can remain active and free for as long as possible. I also hope for new treatments and even more effective medications in the future, but for now, just stay cool.
Learning points.
Erythromelalgia (EM) is a rare and debilitating condition if not diagnosed and treated appropriately.
A comprehensive and multidisciplinary approach is necessary when it comes to the diagnosis due to the multitude of mimics and limitations of testing.
Certain individuals have a mutant channel that is responsive to carbamazepine, but not other sodium channel blockers and should be considered after multiple failed therapies.
Nerve biopsy and genetic testing may ultimately be needed to help hone in on a diagnosis due to limitation of clinical examination, and needle electromyography/nerve conduction.
Footnotes
Contributors: All contributing authors provided substantial contributions to the creation of this work including drafting, revising and final approval of the submission. All authors are accountable for all aspects of the work. PP was the lead author on writing this manuscript. YZ, LHU and CE all assisted with writing of this submission and all provided critical feedback and helped shape the analysis, discussion and manuscript. CE assisted with research and data acquisition regarding this condition. YZ and LHU helped supervise the project.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Han C, Dib-Hajj SD, Lin Z, et al. Early- and late-onset inherited erythromelalgia: genotype-phenotype correlation. Brain 2009;132:1711–22. 10.1093/brain/awp078 [DOI] [PubMed] [Google Scholar]
- 2. Fischer TZ, Gilmore ES, Estacion M, et al. A novel Nav1.7 mutation producing carbamazepine-responsive erythromelalgia. Ann Neurol 2009;65:733–41. 10.1002/ana.21678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davis MDP, O'Fallon WM, Rogers III RS, et al. Natural history of erythromelalgia. Arch Dermatol 2000;136:330–6. 10.1001/archderm.136.3.330 [DOI] [PubMed] [Google Scholar]
- 4. Leroux MB. Erythromelalgia: a cutaneous manifestation of neuropathy? An Bras Dermatol 2018;93:86–94. 10.1590/abd1806-4841.20187535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dabby R. Pain disorders and erythromelalgia caused by voltage-gated sodium channel mutations. Curr Neurol Neurosci Rep 2012;12:76–83. 10.1007/s11910-011-0233-8 [DOI] [PubMed] [Google Scholar]
- 6. Cerci FB, Kapural L, Yosipovitch G. Intractable erythromelalgia of the lower extremities successfully treated with lumbar sympathetic block. J Am Acad Dermatol 2013;69:e270–2. 10.1016/j.jaad.2013.06.047 [DOI] [PubMed] [Google Scholar]
- 7. Davis MDP, Sandroni P, Rooke TW, et al. Erythromelalgia: vasculopathy, neuropathy. or Both? Archives of Dermatology 2003;139. [DOI] [PubMed] [Google Scholar]
- 8. Friberg D, Chen T, Tarr G, et al. Erythromelalgia? a clinical study of people who experience red, hot, painful feet in the community. Int J Vasc Med 2013;2013:1–7. 10.1155/2013/864961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mann N, King T, Murphy R. Review of primary and secondary erythromelalgia. Clin Exp Dermatol 2019;44:477–82. 10.1111/ced.13891 [DOI] [PubMed] [Google Scholar]
- 10. Parker LK, Ponte C, Howell KJ, et al. Clinical features and management of erythromelalgia: long term follow-up of 46 cases. Clin Exp Rheumatol 2017;35:80–4. [PubMed] [Google Scholar]


