Abstract
Background
Disturbed sleep is a core symptom of major depressive disorder (MDD), with nearly 90% of those with MDD reporting disturbed sleep. However, combining insomnia and hypersomnia into a single diagnostic domain ignores distinct biological differences between those symptom presentations. To better understand depression it may be necessary to explore these symptoms independently, beginning with the more prevalent insomnia.
Method
The present study evaluated global insomnia symptom severity in a broad sample of MDD outpatients from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, excluding patients who reported hypersomnia symptoms. The three insomnia-related symptoms from the 16-item Quick Inventory of Depressive Symptomatology- clinician rated (QIDS-C) were combined to create a global insomnia score to classify baseline insomnia severity. A modified depression severity score was then used to assess depression severity (mQIDS-C), excluding sleep-related items.
Results
A repeated measures ANCOVA revealed a significant improvement in insomnia score over the acute phase treatment (F = 33.1, d.f. = 6, 9897, p < 0.0001). Improvement in insomnia score over the acute phase treatment remained statistically significant even after controlling for change in depression severity (p = 0.0004). Participants with one point higher insomnia score at baseline were significantly less likely to remit at study exit (odds ratio = 0.88, 95% confidence interval = 0.85, 0.92, p < 0.0001) even after controlling for baseline depression severity.
Limitations
Objective confirmation of sleep profiles was not available.
Conclusion
Greater severity of insomnia reduces likelihood of MDD remission, and insomnia symptoms improved independent of depression remission.
Keywords: Depression, insomnia, sleep, remission
1. Introduction
Insomnia is thought to be a disorder of hyperarousal which interferes with induction, maintenance, and/or emergence from sleep (Levenson et al., 2015). Previous research on comorbid major depressive disorder (MDD) and insomnia has attempted to use individual symptoms as top-down characteristics to differentiate between separate neurobiological insomnia subtypes, e.g., initial or middle or terminal insomnia (Yokoyama et al., 2010). However, subjective insomnia symptom reports are of questionable temporal stability (Hohagen et al., 1994), often incongruent with polysomnography (Rezaie et al., 2018), and a landmark study involving 4322 participants has shown that subjective insomnia symptoms are not sufficient to characterize biologically distinct subtypes (Blanken et al., 2019). Furthermore, both the Diagnostic and Statistical Manual of Mental Disorders-5 (American Psychiatric Association, 2013) and the International Classification of Sleep Disorders-3 (ICSD-3) (American Academy of Sleep Medicine, 2014) have replaced their respective earlier nosology of characterizing insomnia by subjective symptoms in lieu of considering insomnia as a syndrome, which conceptualizes these dysfunctions as a spectrum, rather than as these distinct symptom domains of insomnia. Specifically, the ICSD-3 characterizes chronic, short-term, and other insomnia disorders as including include difficulty initiating sleep, maintaining sleep, or waking up too early (American Academy of Sleep Medicine, 2014). Thus, attempting to disentangle the heterogeneity of sleep dysfunction in depression solely by analyzing these subjective insomnia subtypes is unlikely to be fruitful. However, the use of global insomnia symptom severity scores has been well validated (Bastien et al., 2001) and has been linked to biophysiological changes in the context of MDD (Rethorst et al., 2015).
The prevailing neurobiological mechanism thought to underlie insomnia is increased activity of the ascending reticular activating system (Riemann et al., 2010). It is unclear if hypersomnia, conceptualized as excessive sleep time, is a continuation of the same spectrum of hyperarousal dysfunction through decreased activity of the ascending reticular activating system (Jang et al., 2018), or driven by a different neurobiological mechanism altogether (Urade, 2017). As others have done before, utilizing a technique which differentiates this divergent biology may lead to a more clear understanding of the underlying pathology when comorbid with MDD (Chaste et al., 2015).
Assessing the relationship between insomnia symptoms and depression in a population which does not include mixed presentations of biologically divergent sleep symptoms presents an opportunity for progress (Fried et al., 2014). The goal of the present study was to investigate the role of global insomnia symptom severity in MDD in a broad sample of outpatients in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, excluding patients who present with or develop hypersomnia symptoms. In particular, we explored the interaction between insomnia severity and depression remittance in level 1 of STAR*D, and baseline insomnia severity as a moderator of depression remittance.
2. Methods
2.1. Study design and participants
Funded by the National Institutes of Mental Health, the STAR*D study was a multicenter, prospective, randomized multistep clinical trial to assess the efficacy of several antidepressants and cognitive therapy for individuals with treatment resistant depression. The details and rationale of the study are provided elsewhere (Fava et al., 2003; Rush et al., 2004). Briefly, the study enrolled 4041 outpatients aged 18 to 75 who had a diagnosis of nonpsychotic MDD meeting DSM-IV guidelines. Participants were eligible for the study if their symptom severity was moderate or higher, with a baseline 17-item Hamilton Rating Scale for Depression (HRSD17) score ≥ 14 (Hamilton, 1960). Enrollment for STAR*D was from 2000 to 2004.
Broad inclusion criteria and minimal exclusion criteria allowed for patients with a variety of comorbid psychiatric and medical diagnoses who were undergoing treatment for those conditions. Concomitant medications were permitted throughout the duration of the study, including anxiolytics (except alprazolam) and sedative hypnotics. During level 1 of STAR*D, participants received treatment with citalopram for 12 weeks (or 14 weeks if response or remission was only achieved at week 12). Citalopram was administered at 20 mg/day and could be increased up to 60 mg/day by the prescribing physician. In order to reduce the contribution of medications to the variability of clinical symptoms, only level 1 data was utilized for this analysis.
2.2. Measures
The 16-item Quick Inventory of Depressive Symptomatology- clinician rated (QIDS-C) was administered at weeks 0,2,4,6,9,12 and 14 to assess depression symptom severity of the prior 7 days (Rush et al., 2003; Trivedi et al., 2004). Each item on the QIDS is rated on a 0–3 Likert-type scale, with higher scores representing a greater severity of symptoms. A global insomnia score ranging from 0–9 was created by adding the three insomnia-related symptoms from the QIDS-C (sleep onset insomnia, mid-nocturnal insomnia, and early morning insomnia). These insomnia related items have shown high correlation with mean weekly values of time to sleep onset, time awake after sleep onset, and time awake prior to the planned wake-up derived from sleep diaries (Manber et al., 2005). The QIDS-C also contains an item on hypersomnia. For the purposes of this analysis, any participant that presented with or developed hypersomnia symptoms over the course of the trial as measured by the QIDS-C was excluded from the sample. A modified depression severity score defined by excluding the sleep-related items from the QIDS-C total score was used to assess depression severity (mQIDS-C). Remission was defined as 16-item Quick Inventory of Depressive Symptomatology- Self Report (QIDS-SR) ≤ 5 at study exit.
General medical conditions were measured by clinicians or specifically trained staff with the 14-item Cumulative Illness Rating Scale which assesses the severity/morbidity of medical illness relevant to different organ systems(Linn et al., 1968). Each organ system was rated from 0 (no problem) to 4 (extremely severe, immediate treatment required, end organ failure, or severe impairment in function). For the purposes of this analysis, the CIRS sum score was calculated with each organ system multiplied by the severity index (the average severity of the categories endorsed).
2.3. Statistical analysis
Continuous data were summarized as mean ± standard deviation (sd) or median and interquartile range (IQR) while categorical data were summarized as frequency and percentages. A repeated measures analysis of variance model was used to assess if the insomnia scores changes over time and if the depression severity assessed by mQIDS-C over the acute phase accounts for changes in insomnia score during the same study period. A logistic regression analysis was used to assess if baseline insomnia score adjusted for baseline mQIDS-C predicts remission at study exit. All analyses were done using SAS 9.4 (SAS Inc., Cary, NC). Statistical significance was assessed at p < 0.05.
3. Results
A total of 4,041 participants were enrolled in STAR*D study. Of these, 370 had no postbaseline assessments and were excluded. Additionally, 883 participants reported hypersomnia symptoms during level 1 and were excluded from the study. Thus, n = 2788 were included in the present analysis. The participants were mostly white (78.1%), and female (61.0%). The mean age of participants were 41.2. Details are in Table 1.
Table 1:
Sociodemographic characteristics of the STAR*D participants who only reported insomnia symptoms throughout level 1.
| Variable | Mean (standard deviation) |
|---|---|
| Age | 41.2 (13.2) |
| mQIDS-C at study entry | 13.8 (3.3) |
| School years | 13.5 (3.2) |
| CIRS Score | 4.2 (3.7) |
| n (%) | |
| Female Gender | 1709 (61.3) |
| White Race | 2178 (78.1) |
| Hispanic | 352 (12.6) |
| Marital Status | |
| Never Married | 731 (26.3) |
| Married/Cohabitating | 1217 (43.7) |
| Divorced/Separated | 744 (26.7) |
| Widowed | 92 (3.3) |
| Employment Status | |
| Unemployed | 961 (34.5) |
| Employed | 1656 (59.5) |
| Retired | 166 (6.0) |
| Use of Hypnotic Medication | 702 (25.2) |
CIRS = Cumulative Illness Rating Scale
mQIDS-C= Modified Quick Inventory of Depressive Symptomatology- clinician rated
A repeated measures ANCOVA revealed a significant improvement in clinician evaluated insomnia score over the acute phase treatment (F = 33.1, d.f. = 6, 9897, p < 0.0001). Participants had an average insomnia score of 6.2 that gradually reduced to 3.7 by week 9 before increasing slightly to 4.4 at the end of acute phase (up to week 14). The analysis was adjusted for age, use of hypnotic medication, medication dose, and general medical comorbidity score.
To assess if improvement in depression severity accounts for improvement in insomnia score, a repeated measures analysis of covariance (ANCOVA) of insomnia score over level 1 was used with mQIDS-C over the same study period as a covariate was used. Improvement in insomnia score over the acute phase treatment remained statistically significant even after controlling for change in depression severity over the same study period (F = 4.1, d.f. = 6, 9898, p = 0.0004). The analysis was also adjusted for age, use of hypnotic medication, and general medical comorbidity score.
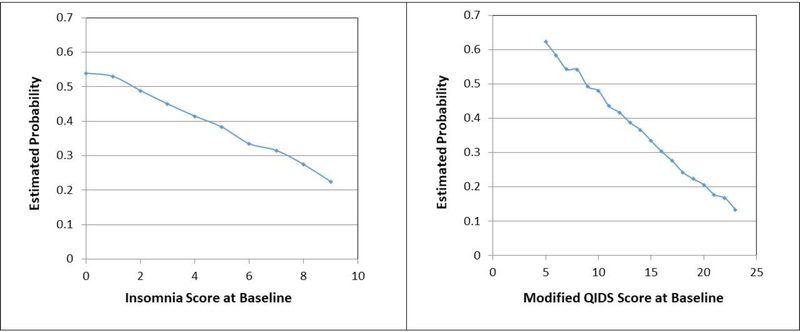
A logistic regression analysis with baseline insomnia score was used to assess if higher insomnia score at baseline predicted remission at exit from level 1. Baseline depression severity measured by mQIDS-C, age, use of hypnotic medication, and general medical comorbidity score were also included in the model. Participants with one point higher insomnia score at baseline were significantly less likely to remit at study exit (odds ratio = 0.88, 95% confidence interval = 0.85, 0.92, p < 0.0001) even after controlling for baseline depression severity measured by mQIDS-C as well as other variables in the model. The maximal to minimal baseline insomnia score difference reduced the likelihood of remission by 31% (Figure 1). Participants with higher mQIDS-C at baseline were also less likely to achieve remission at study exit (odds ratio = 0.90, 95% confidence interval = 0.88, 0.93, p < 0.0001). The maximal to minimal baseline mQIDS-C total score reduced the likelihood of remission by 50% (Figure 1).
Figure 1:
Estimated average likelihood of remission by insomnia score and mQIDSTOT at baseline.
Depression severity measured by mQIDS-C at baseline predicts remission, even after controlling for insomnia score and other clinical characteristics. However, the insomnia score at baseline reduces the likelihood by about 31% (from low to high insomnia score) even after controlling for depression severity at baseline and the same clinical characteristics.
4. Discussion
Greater severity of baseline insomnia symptoms was associated with reduced likelihood of remission of MDD. This result is consistent with the previous literature, including a recent cohort study of 230,801 patients that found insomnia at baseline being associated (risk ratio = 1.63) with treatment resistant depression (Cepeda et al., 2018). The contributions of insomnia to the reduced chance of disease remission were not explained by the severity of depression and all participants included in this analysis were prescribed the same antidepressant medication. Thus greater insomnia severity itself reduced the likelihood of MDD remission.
Approximately 25% of the cohort used some type of sedative hypnotic (Table 1). The use of sedative-hypnotics was statistically controlled for in each of the analyses due to the known impact of these medications on sleep (Matheson and Hainer, 2017). We have shown here that although insomnia score is not the greatest predictor of depression remission, it still has a considerable effect on the likelihood of achieving remission (Figure 1). A meta-analysis of the effect of insomnia treatments in comorbid MDD supported the concept that treating insomnia will have moderate to large effect size improvements in MDD symptomatology (Gebara et al., 2018). Combined with those findings, it is worthwhile to consider insomnia as significant barrier to MDD remission.
The improvements in insomnia symptoms were independent of depression remission. This finding provides further support that insomnia and MDD could be instead separate, and comorbid, disorders (Pigeon and Perlis, 2007) which if true, should shift the manner in which we conceptualize and treat them. Treatment-emergent insomnia is a side-effect of antidepressants (Wichniak et al., 2017), and insomnia is a well-known residual symptom after depression remission (Iovieno et al., 2011; McClintock et al., 2011). This may support the conclusion that insomnia itself is a separate disease process from depression. The result that citalopram improved insomnia symptoms independent of MDD remission further validates the sedative effects of this antidepressant medication (Wichniak et al., 2017).
Current assessment of hypersomnia in commonly used reports, such as those detailed here, make the accurate diagnosis of hypersomnia much harder to assess. Compensatory sleep, oversleeping, or using sleep as an avoidance tactic, are not clearly distinguished. Thus, we were unable to utilize these data to compare hypersomnia changes to insomnia changes and test our hypotheses that these different biological dysfunctions may require different clinical decision making.
Some additional limitations include that the STAR*D study was not designed to assess insomnia and only three items from the QIDS-C were used as a sleep measure. We appreciate that the utilization of multiple items detailing this particular symptom domain does enhance the ability to detect some contribution of insomnia to depression symptom change. Collapsing the discrete responses from the QIDS-C into a total score also poses some challenges however as the items are not continuous in nature However, it is important that the totality of the sleep dysfunction severity be examined in the context of response to treatment. Using sum scores as a method to interpret severity has been extensively used in depression research and does pose significant merit. Ideally, a more objective measurement of subjective insomnia, such as polysomnography, would be used to clarify self-reporting of sleep issues.
Importantly, combining hypersomnia and insomnia symptoms into a single construct for assessment likely impairs adequate monitoring of symptom improvement, particular if the symptoms switch between insomnia and hypersomnia over time. Furthermore, this introduces variability which makes treatment optimization that could help improve symptom severity harder to achieve (Jindal and Thase, 2004). By more clearly distinguishing insomnia from hypersomnia and evaluating these dysfunctions independently, we may better characterize this underlying physiology and have a clearer sense of which pharmacotherapies may be better targeted in a personalized fashion. These data highlight the need for a more distinct focus on insomnia as an important disease process related to depression, and may suggest a shift in thinking about how disturbed sleep is included as a core symptom domain for MDD.
Highlights.
Severity of global insomnia contributes to decreased likelihood of MDD remission.
Improvement of insomnia symptoms was independent from MDD remission.
Insomnia symptoms of MDD require nuanced clinical attention and may be a separate but comorbid disorder.
Acknowledgments
The authors would like to acknowledge the STAR*D Team for their work on the original trial and the efforts, energy, and dedication of Bruce D. Grannemann, M.A., which were invaluable for this research.
Role of funding source
The STAR*D trial (NCT00021528) was funded by the National Institute of Mental Health (NIMH) under contract N01 MH-90003 to the University of Texas Southwestern Medical Center at Dallas, and in part by the Hersh Foundation. NIMH had no role in the drafting or review of the manuscript or in the collection or analysis of the data.
Footnotes
Conflict of Interest
B.L.M., A.D., and A.M. have no conflicts of interest to declare. M.H.T. reports within the last 3 years: consulting/advisory board for AcademyHealth, ACADIA Pharmaceuticals, Akili Interactive, Alkermes Inc, Allergan, Axsome Therapeutics, American Society of Clinical Psychopharmacology (Speaking Fees & Reimbursement), American Psychiatric Association (Deputy Editor for American Journal of Psychiatry), Boegringer Ingelheim, Janssen Pharmaceutical, Jazz Pharmaceutical, Lundbeck Research USA, Medscape, Navitor, One Carbon Therapeutics, Otsuka America Pharmaceutical Inc, Oxford Pharmagenesis, SAGE Therapeutics, Takeda; research activities with NIMH, NIDA, Patient-Centered Outcomes Research Institute (PCORI), Cancer Prevention Research Institute of Texas (CPRIT), J&J, Janssen Research and Development LLC and editorial compensation with Healthcare Global Village, Engage Health Media, Oxford University Press.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Academy of Sleep Medicine, 2014. International Classification of Sleep Disorders, 3rd ed, Darien, IL. [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders 5th ed, Washington, DC. [Google Scholar]
- Bastien CH, Vallieres A, Morin CM, 2001. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep medicine 2, 297–307. [DOI] [PubMed] [Google Scholar]
- Blanken TF, Benjamins JS, Borsboom D, Vermunt JK, Paquola C, Ramautar J, Dekker K, Stoffers D, Wassing R, Wei Y, Van Someren EJW, 2019. Insomnia disorder subtypes derived from life history and traits of affect and personality. The lancet. Psychiatry 6, 151–163. [DOI] [PubMed] [Google Scholar]
- Cepeda MS, Reps J, Ryan P, 2018. Finding factors that predict treatment-resistant depression: Results of a cohort study. Depression and anxiety 35, 668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaste P, Klei L, Sanders SJ, Hus V, Murtha MT, Lowe JK, Willsey AJ, Moreno-De-Luca D, Yu TW, Fombonne E, Geschwind D, Grice DE, Ledbetter DH, Mane SM, Martin DM, Morrow EM, Walsh CA, Sutcliffe JS, Lese Martin C, Beaudet AL, Lord C, State MW, Cook EH Jr., Devlin B, 2015. A genome-wide association study of autism using the Simons Simplex Collection: Does reducing phenotypic heterogeneity in autism increase genetic homogeneity? Biological psychiatry 77, 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Trivedi MH, Nierenberg AA, Thase ME, Sackeim HA, Quitkin FM, Wisniewski S, Lavori PW, Rosenbaum JF, Kupfer DJ, 2003. Background and rationale for the sequenced treatment alternatives to relieve depression (STAR*D) study. The Psychiatric clinics of North America 26, 457–494, x. [DOI] [PubMed] [Google Scholar]
- Fried EI, Nesse RM, Zivin K, Guille C, Sen S, 2014. Depression is more than the sum score of its parts: individual DSM symptoms have different risk factors. Psychological medicine 44, 2067–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebara MA, Siripong N, DiNapoli EA, Maree RD, Germain A, Reynolds CF, Kasckow JW, Weiss PM, Karp JF, 2018. Effect of insomnia treatments on depression: A systematic review and meta-analysis. Depression and anxiety 35, 717–731. [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohagen F, Kappler C, Schramm E, Riemann D, Weyerer S, Berger M, 1994. Sleep onset insomnia, sleep maintaining insomnia and insomnia with early morning awakening--temporal stability of subtypes in a longitudinal study on general practice attenders. Sleep 17, 551–554. [PubMed] [Google Scholar]
- Iovieno N, van Nieuwenhuizen A, Clain A, Baer L, Nierenberg AA, 2011. Residual symptoms after remission of major depressive disorder with fluoxetine and risk of relapse. Depression and anxiety 28, 137–144. [DOI] [PubMed] [Google Scholar]
- Jang SH, Kim SH, Kwon YH, 2018. Excessive Daytime Sleepiness and Injury of the Ascending Reticular Activating System Following Whiplash Injury. Frontiers in neuroscience 12, 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal RD, Thase ME, 2004. Treatment of insomnia associated with clinical depression. Sleep medicine reviews 8, 19–30. [DOI] [PubMed] [Google Scholar]
- Levenson JC, Kay DB, Buysse DJ, 2015. The pathophysiology of insomnia. Chest 147, 1179–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn BS, Linn MW, Gurel L, 1968. Cumulative illness rating scale. Journal of the American Geriatrics Society 16, 622–626. [DOI] [PubMed] [Google Scholar]
- Manber R, Blasey C, Arnow B, Markowitz JC, Thase ME, Rush AJ, Dowling F, Koscis J, Trivedi M, Keller MB, 2005. Assessing insomnia severity in depression: comparison of depression rating scales and sleep diaries. Journal of psychiatric research 39, 481–488. [DOI] [PubMed] [Google Scholar]
- Matheson E, Hainer BL, 2017. Insomnia: Pharmacologic Therapy. American family physician 96, 29–35. [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Wisniewski SR, Nierenberg AA, Stewart JW, Trivedi MH, Cook I, Morris D, Warden D, Rush AJ, 2011. Residual symptoms in depressed outpatients who respond by 50% but do not remit to antidepressant medication. Journal of clinical psychopharmacology 31, 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigeon W, Perlis M, 2007. Insomnia and Depression: Birds of a Feather? Int J Sleep Disorders 1, 82–91. [Google Scholar]
- Rethorst CD, Greer TL, Toups MS, Bernstein I, Carmody TJ, Trivedi MH, 2015. IL-1beta and BDNF are associated with improvement in hypersomnia but not insomnia following exercise in major depressive disorder. Translational psychiatry 5, e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie L, Fobian AD, McCall WV, Khazaie H, 2018. Paradoxical insomnia and subjective–objective sleep discrepancy: A review. Sleep medicine reviews 40, 196–202. [DOI] [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, Nissen C, 2010. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep medicine reviews 14, 19–31. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Fava M, Wisniewski SR, Lavori PW, Trivedi MH, Sackeim HA, Thase ME, Nierenberg AA, Quitkin FM, Kashner TM, Kupfer DJ, Rosenbaum JF, Alpert J, Stewart JW, McGrath PJ, Biggs MM, Shores-Wilson K, Lebowitz BD, Ritz L, Niederehe G, 2004. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Controlled clinical trials 25, 119–142. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB, 2003. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological psychiatry 54, 573–583. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, Crismon ML, Shores-Wilson K, Toprac MG, Dennehy EB, Witte B, Kashner TM, 2004. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychological medicine 34, 73–82. [DOI] [PubMed] [Google Scholar]
- Urade Y, 2017. Neurobiological Basis of Hypersomnia. Sleep medicine clinics 12, 265–277. [DOI] [PubMed] [Google Scholar]
- Wichniak A, Wierzbicka A, Walęcka M, Jernajczyk W, 2017. Effects of Antidepressants on Sleep. Current psychiatry reports 19, 63–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama E, Kaneita Y, Saito Y, Uchiyama M, Matsuzaki Y, Tamaki T, Munezawa T, Ohida T, 2010. Association between depression and insomnia subtypes: a longitudinal study on the elderly in Japan. Sleep 33, 1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]