Abstract
Objective:
To examine the rate and time to relapse for remitters and responders to ketamine in treatment-resistant depression (TRD).
Methods:
Subjects with TRD were randomized to a single infusion of one of several doses of intravenous ketamine, or midazolam. Using Kaplan-Meier survival function, the current report examines the rate and time to relapse, defined as MADRS ≥ 22, over a period of 30 days, in subjects who achieved remission (MADRS ≤ 10) or response (≥ 50% reduction in MADRS) on day three post-infusion of intravenous ketamine 0.1, 0.5, or 1.0 mg/kg.
Results:
Of the 60 randomized participants who received a single ketamine (0.1, 0.5, or 1.0 mg/kg) infusion, 19 (34%) met criteria for remission and 27 (48%) for response, on day 3 post-infusion. A numerical dose-response relationship was observed, with remitters/responders on ketamine 1.0 mg/kg having the lowest relapse rate, followed by ketamine 0.5 mg/kg and 0.1 mg/kg, respectively (% of remitters who relapsed by day 14: 38% with 1.0 mg/kg, 50% with 0.5 mg/kg, 100% with 0.1 mg/kg; % of responders who relapsed by day 14: 30% with 1.0 mg/kg, 50% with 0.5 mg/kg, 80% with 0.1 mg/kg).
Limitations:
The sample size was small. No MADRS measurements at day one post-infusion. The study was not powered to assess differences in relapse prevention between different doses of ketamine.
Conclusion:
Time to relapse after successful treatment with a single infusion of ketamine appears to follow a dose-response relationship, where higher dosage leads to increased time to relapse.
Keywords: Major Depressive Disorder, Treatment Resistant Depression, ketamine, remission, relapse
INTRODUCTION
In the past decades, a number of pharmacological treatments for major depression have proven successful, in a substantial proportion of patients, to significantly reduce depressive symptomatology, albeit through a common pathway, namely the modulation of the monoaminergic system (Cipriani et al., 2018). And while there is today a number of these medications marketed as effective in treating patients with major depressive disorder (MDD), a large proportion of depressed patients fail to respond to available antidepressant therapies (Nierenberg, Katz, & Fava, 2007; Trivedi et al., 2006). These patients are considered to have treatment-resistant depression (TRD). TRD is associated with a reduced quality of life, social and occupational impairment, high rates of medico-psychiatric co-morbidities, higher likelihood of prior suicide attempt, and substantially increased resource utilization (Gaspersz et al., 2017; Greden, 2001; Kautzky et al., 2017; Nelsen & Dunner, 1995; Russell et al., 2004).
Ketamine is an N-methyl-D-aspartate (NMDA) receptor antagonist and glutamatergic modulator, that has been in use since the 1960s as a dissociative anesthetic (Corssen & Domino, 1966). It has also garnered considerable attention in the past two decades as a rapidly acting therapy in TRD (Sanacora et al., 2017), following two independent reports demonstrating its fast and substantial efficacy in patients with TRD (Berman et al., 2000; Zarate et al., 2006). Since then, there have been several published randomized controlled trials (RCT) confirming the acute and robust antidepressant effect of a single infusion of ketamine (Ionescu & Papakostas, 2016, 2017; Molero et al., 2018). Patients in these trials are primarily monitored for the next few days post-infusion for response or remission, with some trials following patients for up to 14 days. However, it is still unclear how many and at what point patients who respond to a single infusion of ketamine experience depressive relapse. One study thus far has examined this question for ketamine monotherapy (Murrough et al., 2013), but none yet for ketamine augmentation. Therefore, further investigation is warranted in order to shed light on this important research question, and inform dosing and frequency of ketamine administration in future trials.
An NIMH-funded network, Rapidly-Acting Treatments for Treatment-Resistant Depression (RAPID) (“Rapidly-Acting Treatments for Treatment-Resistant Depression (RAPID). https://www.nimh.nih.gov/research-priorities/research-initiatives/rapidly-acting-treatments-for-treatment-resistant-depression-rapid.shtml,”), recently conducted a multi-site, randomized, double-blind, active placebo-controlled trial of intravenous ketamine in patients with unipolar treatment-resistant depression (TRD), and demonstrated that responses to ketamine at doses of 0.1mg/kg, 0.5 mg/kg and 1.0 mg/kg (but not 0.2mg/kg) were found to be superior to midazolam (active placebo) at day one post-infusion (Fava et al., 2018). The primary aim was assessment of short-term efficacy, although data were collected to assess longer-term outcomes. This current report investigates the rate and time to relapse for remitters and responders to ketamine over a follow-up period of one month for doses found to be more effective than midazolam one day post-infusion (0.1, 0.5 and 1.0 mg/kg).
METHODS
Patient Selection
Both men and women were selected between the ages of 18 and 70 years, with a primary psychiatric diagnosis of MDD and experiencing a major depressive episode (MDE) of at least eight weeks in duration prior to screening as defined by the Diagnosis and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria (Association AP. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition: DSM-IV-TR®: American Psychiatric Association; 2000). Additionally, participants were experiencing TRD during the current MDE, defined as a failure to achieve a satisfactory response (<50% response) to at least two, but not more than seven, adequate treatment courses of ADT with a minimal dose approved for the treatment of MDD and of at least eight weeks’ duration. Patients were also required to be on stable doses of antidepressants for at least four weeks prior to screening. Patients were screened between 7 and 28 days, during which eligibility was determined by site staff as well as remote raters, and prohibited medications were discontinued. For a more detailed description of patient selection, please refer to the main publication of results (Fava et al., 2018).
Study Overview and Design
A detailed description of the original trial design and results have been previously published (Fava et al., 2018). In brief, this was a multi-site, randomized, double-blind, active placebo-controlled trial of the acute efficacy of intravenous ketamine compared to intravenous midazolam added to ongoing, stable, and adequate antidepressant therapy (ADT) in the treatment of adults with TRD. This work was conducted as part of a collaborative effort between the MGH Clinical Trials Network and Institute (CTNI), multiple academic sites, and the National Institute of Mental Health (NIMH). All study participants signed written informed consent approved by the respective Institutional Review Board (IRB) and NIMH Data Safety and Monitoring Board.
All enrolled participants were male and female outpatients between the ages of 18–70 years old with a diagnosis of MDD in a current depressive episode of at least eight week-duration (as defined by the DSM-IV-TR™), had TRD, defined as failure to achieve a subjective satisfactory response (e.g., less than 50% improvement of depression symptoms) to at least two adequate treatment courses during the current depressive episode (including the current antidepressant therapy), and had a Montgomery Asberg Depression Rating Scale17 (MADRS) score >20 at both the screen and baseline visits. Participants were stratified by body mass index (BMI) (≤ 30 and >30), and randomized into one of the five study arms, through a block randomization model. A total of N=99 participants were randomly assigned to one of these five arms in a 1:1:1:1:1 fashion: a single dose of ketamine 0.1 mg/kg (n=18), a single dose of ketamine 0.2 mg/kg (n=20), a single dose of ketamine 0.5 mg/kg (n=22), a single dose of ketamine 1.0 mg/kg (n=20), or a single dose of midazolam 0.045 mg/kg (n=19). To note, the current report’s sample size is 60, with ketamine 0.2 mg/kg and midazolam groups excluded (rationale below). At the baseline visit (Day 0), randomized participants received their assigned study drug by continuous intravenous infusion via an electronic syringe infusion pump, over a period of 40 minutes. Participants were continuously monitored throughout the process, with blood pressure and heart rate measured at time 0 (right before starting the infusion), and at 15–20-min intervals for 120 minutes following the infusion. Subsequently, participants were followed up for 30 days and study assessments were performed at Days 0, 1, 3, 5, 7, 14, and 30 to assess the safety and efficacy of all doses of ketamine compared to midazolam. For a full report of primary and secondary efficacy and safety measures used in the study, please refer to the original report (Fava et al., 2018). This report focuses on the follow-up phase of the study (days 3 through 30) and the time to relapse for participants that met response or remission criteria as defined below. The MADRS (Montgomery & Asberg, 1979) was used to define remission, response, and relapse. Participants were considered to have remitted if they had a MADRS score of 10 or lower on day 3 post-ketamine infusion. Response was defined as a 50% or greater reduction in MADRS score from baseline to day 3 post-ketamine infusion. Relapse was defined as a MADRS score of 22 or higher on any subsequent visit.
Statistical Analyses
Data analyses for this paper were generated using SAS software, Version 9.4 of the SAS System for Windows 7.
Descriptive statistics
We examined the data in a descriptive fashion by first creating a subset of the group to include participants who achieved remission, defined as a MADRS score of 10 or lower, on Day 3 post infusion. We then generated a bar graph to show the percentage of these participants who remained in remission status by each day of assessment (Day 3, 5, 7, 14, 30).
Survival Analysis Plots
We created a series of Kaplan-Meier curves to examine the data in four different ways. First, we selected a starting group of those at risk that included participants who achieved remission (MADRS ≤ 10) on Day 3. In the first survival plot, failure was defined as relapsing (MADRS ≥ 22) and we tracked the number of participants who remained at risk, avoiding relapse, from Day 3 through 27 days of follow-up using the lifetest procedure. We graphed survival probability by group according to ketamine dose (0.1 mg/kg, 0.5 mg/kg, and 1.0 mg/kg). In the absence of re-randomization at day 3, and because the dose of 0.2 mg/kg did not differ significantly from the midazolam arm on the primary outcome, as evidenced in the original study report (Fava et al., 2018), participants in the 0.2 mg/kg group who remitted or responded at day 3 would not be comparable with participants who achieved response or remission in the other ketamine groups, justifying the exclusion of these participants from the present analyses. For the same reason, participants in the midazolam were also excluded. The next survival curve was similar to the first but combines all participants who received ketamine 0.1 mg/kg, 0.5 mg/kg, and 1.0 mg/kg into one group. The 0.2 mg/kg dose as well as the midazolam arm were, again, excluded.
In the second set of survival curves, the starting group of participants at risk was defined as “responders” or those who had seen a 50% or greater reduction in their MADRS scores between baseline and Day 3 of the trial. Similar to the first two survival curves, failure was defined as relapsing (MADRS ≥ 22) and we tracked the number of participants who remained at risk, avoiding relapse, from Day 3 through 27 days of follow-up using the lifetest procedure. We generated one curve that compares the participants by dose of ketamine received and one that combines the participants who received ketamine, regardless of dose. Once again we excluded the 0.2 mg/kg dose and midazolam groups from these curves.
RESULTS
Of the 60 randomized participants who received a single ketamine (0.1, 0.5, or 1.0 mg/kg) infusion, 56 had a MADRS performed on day 3, out of which 19 (34%) met criteria for remission and 27 (48%) for response. Demographic and clinical features of remitters are presented in table 1.
Table 1:
Demographics and clinical variables of patients who remitted three days after ketamine infusion
| Ketamine 0.1 mg/kg N=3 | Ketamine 0.5 mg/kg N=8 | Ketamine 1.0 mg/kg N=8 | ||||
|---|---|---|---|---|---|---|
| Mean/% | (SD) | Mean/% | (SD) | Mean/% | (SD) | |
| Demographics | ||||||
| Age | 47.0 | 8.1 | 45.5 | 11.9 | 45.3 | 9.6 |
| Gender (% female) | 33.3 | 37.5 | 62.5 | |||
| Hispanic (% yes) | 0.0 | 0.0 | 0.0 | |||
| Race | ||||||
| White | 100.0 | 100.0 | 87.5 | |||
| Asian | 0.0 | 0.0 | 0.0 | |||
| Black | 0.0 | 0.0 | 12.5 | |||
| Other | 0.0 | 0.0 | 0.0 | |||
| BMI | 26.9 | 4.8 | 26.5 | 6.0 | 26.4 | 5.3 |
| Concomitant Medications (% used) | ||||||
| Benzo | 66.7 | 25.0 | 50.0 | |||
| Non-benzo hypnotic | 33.3 | 0.0 | 37.5 | |||
| SSRI | 66.7 | 50.0 | 37.5 | |||
| SNRI | 33.3 | 37.5 | 25.0 | |||
| TCA | 0.0 | 12.5 | 0.0 | |||
| Other antidep | 0.0 | 62.5 | 25.0 | |||
| Clinical Severity at Baseline | ||||||
| MADRS | 33.3 | 6.1 | 31.0 | 3.5 | 29.9 | 4.3 |
BMI: body mass index; Benzo: benzodiazepine; SSRI: selective serotonin reuptake inhibitor; SNRI: serotonin norepinephrine reuptake inhibitor; TCA: tricyclic antidepressant; MADRS: Montgomery–Åsberg Depression Rating Scale
Remission rates on day 3 were 8/20 (40%), 8/21 (38%), and 3/15 (20%), on ketamine 1.0 mg/kg, 0.5 mg/kg, and 0.1 mg/kg, respectively. Response rates on day 3 were 10/20 (50%), 12/21 (57%), and 6/19 (33%), on ketamine 1.0 mg/kg, 0.5 mg/kg, and 0.1 mg/kg, respectively. Fifty-two (87%) of the 60 randomized participants (to ketamine 0.1, 0.5, and 1.0 mg/kg doses) were retained at day 30 of the study. Dropout rates were as follows: 3 with ketamine 1.0 mg/kg, 1 with ketamine 0.5 mg/kg, and 4 with ketamine 0.1 mg/kg. Figure 1 shows the percentage of patients on ketamine who remain in remission after achieving remission at day 3 following a single ketamine infusion. At day 7, 53% (10/19) of those patients continue to remain in remission, with 26% (5/19) and 21% (4/19) remaining in remission at days 14 and 30, respectively.
Figure 1:
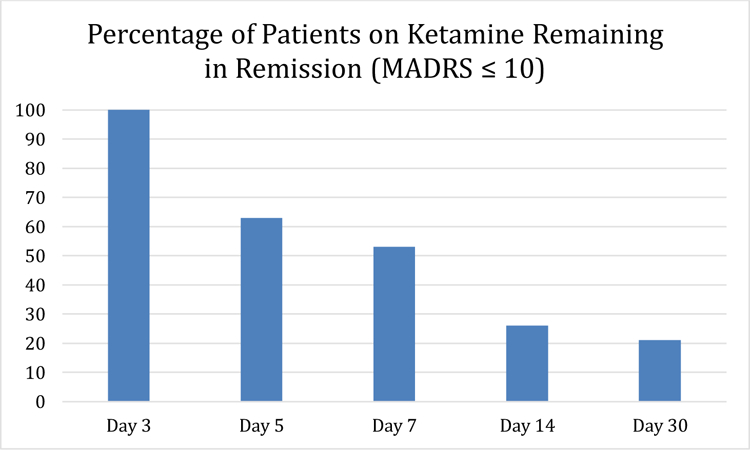
Remission rates in the follow-up period (days 3 to day 30 post-ketamine infusion) for patients who achieved remission (MADRS ≤10) on day 3 post-infusion (ketamine doses 0.1 mg/kg, 0.5 mg/kg, and 1.0 mg/kg) (N=19)
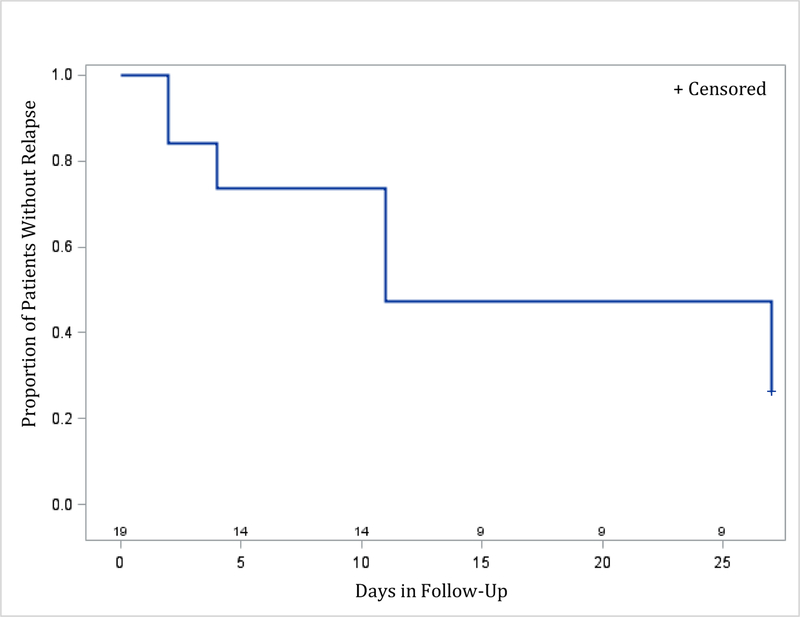
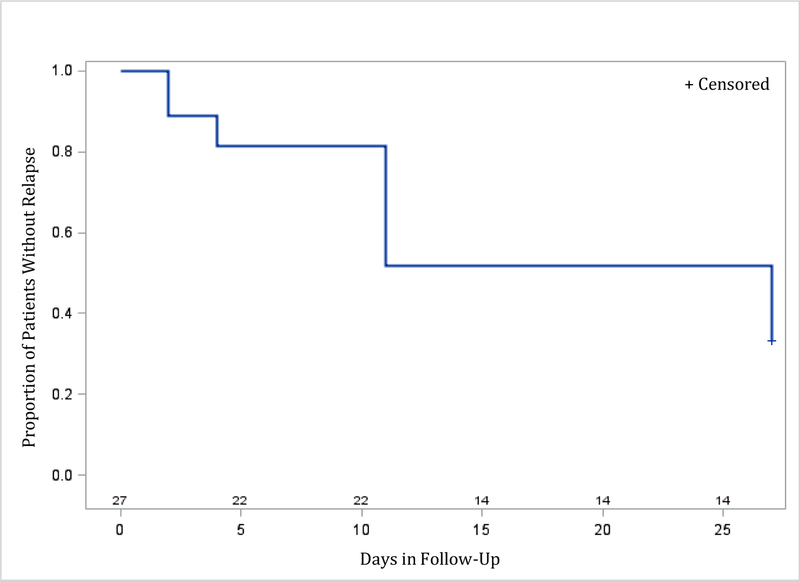
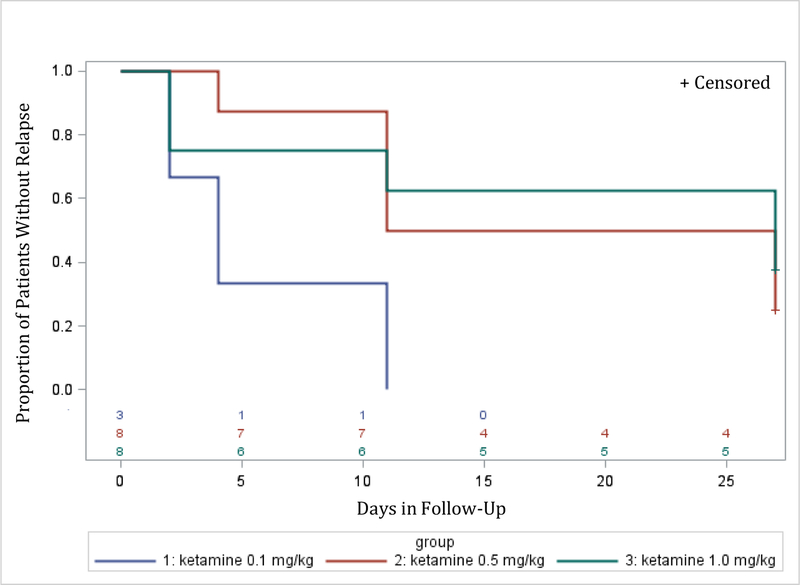
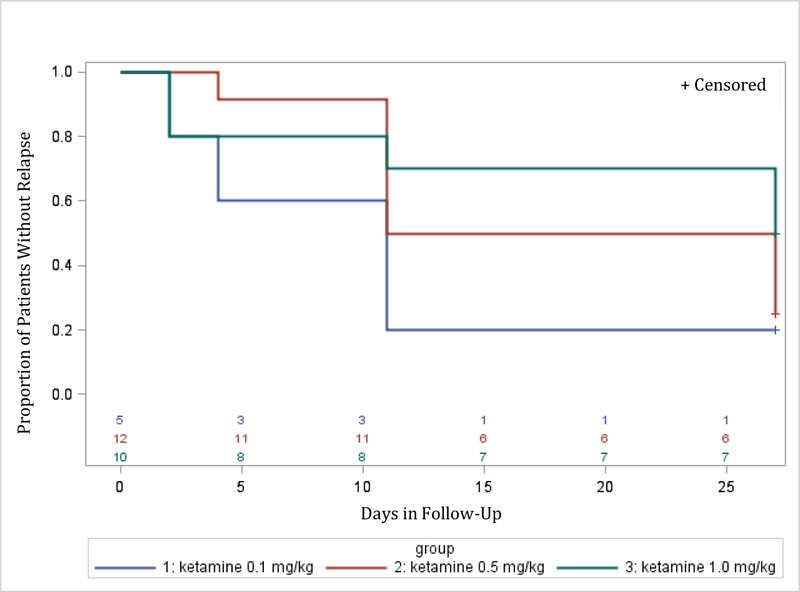
Time to relapse was examined for both remitters and responders. The Kaplan-Meier survival function was computed for the ketamine groups combined (for remitters: figure 2; for responders: figure 4), and for the different ketamine dosages groups (remitters: figure 3; responders: figure 5). Among the 19 remitters 3 days post-infusion, 26% relapsed by day 7, 53% by day 14, and 74% by day 30 (figure 2). Similarly, among the 27 responders 3 days post-infusion, 19% relapsed by day 7, 48% by day 14, and 67% by day 30 (figure 4). When looking at the different ketamine doses, a numerical dose-response relationship is observed, with remitters/responders on ketamine 1.0 mg/kg having the lowest relapse rate, followed by ketamine 0.5 mg/kg and 0.1 mg/kg, respectively (% of remitters who relapsed by day 14: 38% with 1.0 mg/kg, 50% with 0.5 mg/kg, 100% with 0.1 mg/kg; % of responders who relapsed by day 14: 30% with 1.0 mg/kg, 50% with 0.5 mg/kg, 80% with 0.1 mg/kg; Figures 3 and 5, respectively). A similar trend is seen when examining MADRS score change for remitters at day 3 over the 30 day follow-up period, whereby remitters on ketamine 0.1 mg/kg had the highest increase in MADRS score over the 30 days following the injection, following by remitters on ketamine 0.5 and 1.0 mg/kg (Figure 6).
Figure 2:
Relapse during follow-up period (days 3 to day 30 post-ketamine infusion) for patients (ketamine doses 0.1 mg/kg, 0.5 mg/kg, and 1.0 mg/kg, combined) who achieved remission (MADRS ≤10) on day 3 post-infusion (N=19)
Day 0 on the x-axis corresponds to day 3 post-ketamine infusion
Figure 4:
Relapse during follow-up period (days 3 to day 30 post-ketamine infusion) for patients (ketamine doses 0.1 mg/kg, 0.5 mg/kg, and 1.0 mg/kg, combined) who achieved response (50% or greater reduction in MADRS score from baseline to day 3 post-infusion) (N=27)
Day 0 on the x-axis corresponds to day 3 post-ketamine infusion
Figure 3:
Relapse during follow-up period (days 3 to day 30 post-ketamine infusion) by treatment arm (ketamine doses 0.1 mg/kg, 0.5 mg/kg, and 1.0 mg/kg) for patients who achieved remission (MADRS ≤10) on day 3 post-infusion (N=19)
Day 0 on the x-axis corresponds to day 3 post-ketamine infusion
Figure 5:
Relapse during follow-up period (days 3 to day 30 post-ketamine infusion) by treatment arm (ketamine doses 0.1 mg/kg, 0.5 mg/kg, and 1.0 mg/kg) for patients who achieved response (50% or greater reduction in MADRS score from baseline to day 3 post-infusion) (N=27).
Day 0 on the x-axis corresponds to day 3 post-ketamine infusion
Figure 6:
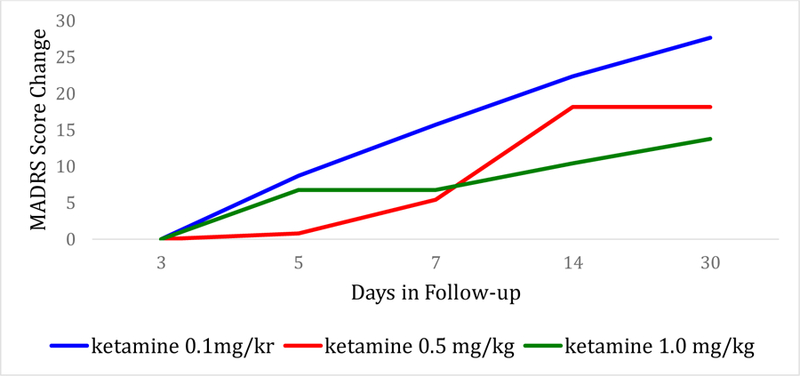
MADRS Score Change over the follow-up period (days 3 to day 30 post-ketamine infusion) by treatment arm (ketamine doses 0.1 mg/kg, 0.5 mg/kg, and 1.0 mg/kg) for patients who achieved remission (MADRS ≤10) on day 3 post-infusion
DISCUSSION
This study examines the longer-term antidepressant effects following varying doses of a single administration of intravenous ketamine treatment in patients with MDD (TRD). Preclinical data from rodent studies suggest the cellular and antidepressant-like effects of ketamine may last for a week or more following a single exposure. A single administration of ketamine was shown to increase spine density and excitatory postsynaptic currents in the medial prefrontal cortex (mPFC) pyramidal neurons of rats, which are associated with sustained antidepressant-like responses persisting for up to 1 week in the forced-swim test (Li et al., 2010; Liu et al., 2013). Mice injected one time with a subanesthetic dose of ketamine showed increased rates of dendritic spine formation and higher spine density in areas of the mPFC lasting for up to 2 weeks (Liu et al., 2013). When subanesthetic ketamine was repeatedly administered to mice on a daily basis for 5 days, the spine formation rate was found to be significantly elevated at 3 and 5 days after the first ketamine administration (Pryazhnikov et al., 2018). These data, while very limited, suggest the cellular effects of ketamine in areas of the mPFC could be sustained and last for extended periods beyond the time of the actual drug administration. In the present study, while many subjects maintained symptom improvement after a single infusion for several weeks, a considerable subset also experienced a return of symptoms. Specifically, nearly a quarter of patients who were in remission 72 hours post-ketamine infusion relapsed 7 days post-treatment, with another quarter relapsing by the end of the second week. Similar figures were seen with ketamine responders. Specifically, nearly one fifth of patients who were in clinical response 72 hours post-ketamine infusion relapsed 7 days post-treatment, and half by the end of the second week.
Only one other randomized, midazolam-controlled trial examined time to relapse following response to intravenous ketamine in TRD (Murrough et al., 2013). In this study, 21 subjects who were clinical responders one week post-infusion were assessed with MADRS over an additional 4 weeks. Similarly to the current study, nearly 25% of those relapsed in the first follow-up week, with 60% relapsing within 2.5 weeks. Of note, ketamine 0.5 mg/kg dosage was the only dose used in that study. Corresponding figures for remitters were not reported.
While underpowered for outcomes after day 3, we found that time to relapse after successful ketamine treatment appears to follow a dose-response relationship, where higher dosage leads to increased time to relapse. Specifically, more than 60% of patients who remitted 72 hours after ketamine 1.0 mg/kg infusion remained in remission 2 weeks post-infusion. In contrast, none of the patients who remitted 72 hours after ketamine 0.1 mg/kg infusion, experienced sustained remission two weeks post-infusion. While this is the only study comparing response and remission rates of various doses of intravenous ketamine over the course of several weeks, our results appear similar to those recently reported with repeat-dose (twice-weekly) administration of intranasal esketamine (28mg, 56mg, or 84 mg) (Daly et al., 2018). In that study, a significant ascending dose-response relationship was found (p<0.001) at the end of week 2, with remission rates of 13% [1 of 8], 27%[3 of 11], and 40%[4 of 10] in the 28-mg, 56-mg, and 84-mg groups, while the authors note that “efficacy appeared to be better sustained between drug administrations with the two higher doses”. Therefore, the finding of a more durable antidepressant effect may pertain to ketamine delivered via various routes of administration.
If our observations are confirmed in future adequately powered studies, these results may have several clinical implications. First, it may inform the optimal dose frequency of the administration of ketamine infusions in TRD in order to maintain euthymia, while minimizing patients’ visit burden. A RCT testing both the twice and thrice weekly intravenous ketamine frequency over 2 weeks found both schedules to be significantly more efficacious than placebo on day 15 (twice-weekly: mean change in MADRS score at day 15 was −18.4 (SD=12.0) for ketamine and −5.7 (SD=10.2) for placebo, p<0.001; thrice-weekly: −17.7 (SD=7.3) for ketamine and −3.1 (SD=5.7) for placebo, p<0.001) with no apparent difference in efficacy or tolerability between the two frequencies tested (Singh et al., 2016). As a result, the authors favored the twice-weekly regimen pointing to the comparable efficacy and tolerability to the thrice-weekly regimen, but with reduced patient and clinic burden and costs. However, whether the same results can be achieved with fewer infusions, particularly with higher doses, is also of interest. Our results show that less than a quarter of patients remitting on ketamine 0.5 or 1.0 mg/kg, relapse one week post-infusion. This finding offers a rationale for testing higher doses of ketamine at a once-a-week frequency for patients who remit on ketamine, in order to enhance chances of remission maintenance while increasing the treatment’s feasibility (i.e. fewer clinic visits) and reducing its cost (clinicians currently employ two to three infusions per week (Wilkinson et al., 2017)). Furthermore, clinicians should consider increasing the ketamine dosage in patients who respond/remit to lower doses, but experience frequent worsening during the time lag between subsequent doses. Additionally, in light of these findings, and the results from the original report (Fava et al., 2018), the clinician’s decision to start on a lower versus higher dose of ketamine will be informed by multiple considerations, including acute efficacy and safety of the dose in question, efficacy of treatment maintenance, feasibility of treatment schedule, and patient preference. Finally, the maximum dose of ketamine studied in this report was 1.0 mg/kg. It remains unknown to date whether a higher dose of ketamine with potentially superior efficacy can be safely administered. Poor tolerability was not observed in our study at the 1.0 mg/kg dose.
One methodological strength of this study was the use of a randomized, active placebo-controlled design in the original trial. In addition, remote ratings were conducted by research psychiatrists and psychologists who were blinded to patient group assignment. However, the current report also has several limitations worth noting. First, the study was not powered to assess differences in relapse prevention between different doses of ketamine, therefore limiting our ability to conduct comparative statistical analyses of the treatment arms. Second, the response and remission definitions at day three post-infusion significantly reduced the sample size. Therefore, results must be interpreted with caution. Third, the lack of MADRS measurements at day one post-infusion prevented us from analyzing patients who remitted or responded at this time point. Therefore, the current report applies to a subset of patients who achieved remission within 3 days post-infusion and maintained remission at day 3 post-infusion. Future studies should address rapidly relapsing remitters (i.e. loss of remission within 3 days post-infusion).
In conclusion, results from the present study demonstrate a dose-response relationship for relapse prevention after the successful treatment with a single add-on IV ketamine infusion in patients with TRD. These findings, although still exploratory, may have direct clinical implications on the choice of treatment dosage and frequency. As previously stated, future larger and adequately powered studies are warranted. In addition, studies that examine patient characteristics of those who experience more sustained antidepressant benefits of ketamine are warranted in order to tailor treatment regimens for individual patients.
Highlights.
A quarter of patients in remission post-ketamine infusion relapsed 7 days post-treatment
Time to relapse after successful ketamine treatment follows a dose-response relationship
Higher dosage leads to increased time to relapse
Acknowledgments:
This project was funded by the National Institute of Mental Health (NIMH) under Contract Rapidly-Acting Treatments for Treatment-Resistant Depression (RAPID) Number: HHSN271201100006I, to the Massachusetts General Hospital (Maurizio Fava, MD and George Papakostas, co-principal investigators). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. We would like to thank Drs. Mi Hillefors, Steven Zalcman, Adam Haim, and Galia Siegel from NIMH, for their support, which was absolutely critical to both the planning and the implementation of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statements:
Naji C. Salloum: As part of his clinical translational fellowship at MGH, Dr. Salloum also works in the Pfizer digital medicine group. His work at Pfizer presents no financial or non-financial conflict of interest with the current manuscript.
Maurizio Fava: Research Support: Abbott Laboratories; Acadia Pharmaceuticals; Alkermes, Inc.; American Cyanamid;Aspect Medical Systems; AstraZeneca; Avanir Pharmaceuticals; AXSOME Therapeutics; Biohaven; BioResearch; BrainCells Inc.; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon; Cerecor; Clintara, LLC; Covance; Covidien; Eli Lilly and Company;EnVivo Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Forest Pharmaceuticals, Inc.; FORUM Pharmaceuticals; Ganeden Biotech, Inc.; GlaxoSmithKline; Harvard Clinical Research Institute; Hoffman-LaRoche; Icon Clinical Research; i3 Innovus/Ingenix; Janssen R&D, LLC; Jed Foundation; Johnson & Johnson Pharmaceutical Research & Development; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; Lundbeck Inc.; Marinus Pharmaceuticals; MedAvante; Methylation Sciences Inc; National Alliance for Research on Schizophrenia & Depression (NARSAD); National Center for Complementary and Alternative Medicine (NCCAM);National Coordinating Center for Integrated Medicine (NiiCM); National Institute of Drug Abuse (NIDA); National Institute of Mental Health (NIMH); Neuralstem, Inc.; NeuroRx; Novartis AG; Organon Pharmaceuticals; Otsuka Pharmaceutical Development, Inc.; PamLab, LLC.; Pfizer Inc.; Pharmacia-Upjohn; Pharmaceutical Research Associates., Inc.; Pharmavite® LLC; PharmoRx Therapeutics; Photothera; Reckitt Benckiser; Roche Pharmaceuticals; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Sanofi-Aventis US LLC; Shire; Solvay Pharmaceuticals, Inc.; Stanley Medical Research Institute (SMRI); Synthelabo; Taisho Pharmaceuticals; Takeda Pharmaceuticals; Tal Medical; VistaGen; Wyeth-Ayerst Laboratories; Advisory Board/ Consultant: Abbott Laboratories; Acadia; Affectis Pharmaceuticals AG; Alkermes, Inc.; Amarin Pharma Inc.; Aspect Medical Systems; AstraZeneca; Auspex Pharmaceuticals; Avanir Pharmaceuticals; AXSOME Therapeutics; Bayer AG; Best Practice Project Management, Inc.; Biogen; BioMarin Pharmaceuticals, Inc.; Biovail Corporation; BrainCells Inc; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon, Inc.; Cerecor; CNS Response, Inc.; Compellis Pharmaceuticals; Cypress Pharmaceutical, Inc.; DiagnoSearch Life Sciences (P) Ltd.; Dinippon Sumitomo Pharma Co. Inc.; Dov Pharmaceuticals, Inc.; Edgemont Pharmaceuticals, Inc.; Eisai Inc.; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; ePharmaSolutions; EPIX Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals, Inc.; Forum Pharmaceuticals; GenOmind, LLC; GlaxoSmithKline; Grunenthal GmbH; Indivior; i3 Innovus/Ingenis; Intracellular; Janssen Pharmaceutica; Jazz Pharmaceuticals, Inc.; Johnson & Johnson Pharmaceutical Research & Development, LLC; Knoll Pharmaceuticals Corp.; Labopharm Inc.; Lorex Pharmaceuticals; Lundbeck Inc.; Marinus Pharmaceuticals; MedAvante, Inc.; Merck & Co., Inc.; MSI Methylation Sciences, Inc.; Naurex, Inc.; Navitor Pharmaceuticals, Inc.; Nestle Health Sciences; Neuralstem, Inc.; Neuronetics, Inc.; NextWave Pharmaceuticals; Novartis AG; Nutrition 21; Orexigen Therapeutics, Inc.; Organon Pharmaceuticals; Osmotica; Otsuka Pharmaceuticals; Pamlab, LLC.; Pfizer Inc.; PharmaStar; Pharmavite® LLC.; PharmoRx Therapeutics; Precision Human Biolaboratory; Prexa Pharmaceuticals, Inc.; PPD; Purdue Pharma; Puretech Ventures; PsychoGenics; Psylin Neurosciences, Inc.; RCT Logic, LLC ( formerly Clinical Trials Solutions, LLC); Relmada Therapeutics, Inc.; Rexahn Pharmaceuticals, Inc.; Ridge Diagnostics, Inc.; Roche; Sanofi-Aventis US LLC.; Sepracor Inc.; Servier Laboratories; Schering-Plough Corporation; Shenox Pharmaceuticals; Solvay Pharmaceuticals, Inc.; Somaxon Pharmaceuticals, Inc.; Somerset Pharmaceuticals, Inc.; Sunovion Pharmaceuticals; Supernus Pharmaceuticals, Inc.; Synthelabo; Taisho Pharmaceuticals; Takeda Pharmaceutical Company Limited; Tal Medical, Inc.; Tetragenex; Teva Pharmaceuticals; TransForm Pharmaceuticals, Inc.; Transcept Pharmaceuticals, Inc.; Usona Institute,Inc.; Vanda Pharmaceuticals, Inc.; Versant Venture Management, LLC; VistaGen; Speaking/Publishing: Adamed, Co; Advanced Meeting Partners; American Psychiatric Association; American Society of Clinical Psychopharmacology; AstraZeneca; Belvoir Media Group; Boehringer Ingelheim GmbH; Bristol-Myers Squibb; Cephalon, Inc.; CME Institute/Physicians Postgraduate Press, Inc.; Eli Lilly and Company; Forest Pharmaceuticals, Inc.; GlaxoSmithKline; Imedex, LLC; MGH Psychiatry Academy/Primedia; MGH Psychiatry Academy/Reed Elsevier; Novartis AG; Organon Pharmaceuticals; Pfizer Inc.; PharmaStar; United BioSource,Corp.; Wyeth-Ayerst Laboratories; Equity Holdings: Compellis; PsyBrain, Inc.; Royalty/patent, other income:Patents for Sequential Parallel Comparison Design (SPCD), licensed by MGH to Pharmaceutical Product Development, LLC (PPD) (US_7840419, US_7647235, US_7983936, US_8145504, US_8145505); and patent application for a combination of Ketamine plus Scopolamine in Major Depressive Disorder (MDD), licensed by MGH to Biohaven. Patents for pharmacogenomics of Depression Treatment with Folate (US_9546401, US_9540691); Copyright: for the MGH Cognitive & Physical Functioning Questionnaire (CPFQ), Sexual Functioning Inventory (SFI), Antidepressant Treatment Response Questionnaire (ATRQ), Discontinuation-Emergent Signs & Symptoms (DESS), Symptoms of Depression Questionnaire (SDQ), and SAFER; Lippincott, Williams & Wilkins; Wolkers Kluwer; World Scientific Publishing Co. Pte.Ltd.
Rebecca S. Hock: no conflict of interest to disclose.
Marlene P. Freeman: (past 36 months): Investigator Initiated Trials (Research): Takeda, JayMac, Sage; Advisory boards: Janssen, Sage; JDS therapeutics; Sunovion; Independent Data Safety and Monitoring Committee: Janssen (Johnson& Johnson); Medical Editing: GOED newsletter. Dr. Freeman is an employee of Massachusetts General Hospital, and works with the MGH National Pregnancy Registry [Current Registry Sponsors: Teva (2018- present), Alkermes, Inc. (2016-Present); Otsuka America Pharmaceutical, Inc. (2008-Present); Forest/Actavis (2016-Present), Sunovion Pharmaceuticals, Inc. (2011-Present)]. As an employee of MGH, Dr. Freeman works with the MGH CTNI, which has had research funding from multiple pharmaceutical companies and NIMH.
Martina Flynn: no conflict of interest to disclose.
Bettina Hoeppner: no conflict of interest to disclose.
Cristina Cusin: Research: from NIMH (R01MH102279); consulting fees: from Janssen Pharmaceuticals, Takeda, Boehringer, Lundbeck. She has also participated in research funded by Janssen, Medtronic, Otsuka, Takeda.
Dan V. Iosifescu: Over the last 12 months Dr. Iosifescu reports consulting fees from Axsome, Alkermes, Centers of Psychiatric Excellence, Jazz, and Lundbeck, and research grants from LiteCure and Neosync.
Madhukar H. Trivedi: Past 24 months - Consulting/Advisory Board: ACADIA Pharmaceuticals Inc., AcademyHealth, Alkeremes Inc., Akili Interactive, Allergan Pharmaceuticals, American Society of Clinical Psychopharmacology (ASCP), Axsome Therapeutics, BlackThorn, Brain Institute Canada (CAN-BIND), Brintellix Global, Global Medical Education, Healthcare Global Village, Health Research Associates, Jazz Pharmaceuticals, Lundbeck Research USA, Medscape LLC, MSI Methylation Sciences Inc., Navitor Nestle Health Science – Pamlab Inc., Naurex Inc., One Carbon Therapeutics, Otsuka America Pharmaceutical Inc., Saatchi, Takeda Global Research; Speaking Fees: Darmouth College, Global Medical Education, University of Illinois Chicago, University of Ottawa, University of Texas Health Science Center at San Antonio; Reimbursed or Sponsored Travel: AcademyHealth, Alkermes Inc., Allergan Pharmaceuticals, American College of Clinical Psychopharmacology (ASCP), Brintellix Global, Dartmouth College, Global Medical Education, Health Research Associates, Lundbeck Research USA, Medscape LLC, Navitor, One Carbon Therapeutics, University of Illinois Chicago, University of Ottawa, University of Texas Health Science Center at San Antonio; Editorial Compensation: Healthcare Global Village Inc.; Publications: Janssen Asia Pacific, Oxford University Press; Research Activities: NIMH, NIDA, J&J, Janssen Research and Development LLC; Royalties: Janssen Research and Development LLC, Oxford University Press
Gerard Sanacora (past 36 months): Consulting: Allergan, Alkermes, AstraZeneca, Avanier Pharmaceuticals, Axsome Therapeutics Biohaven Pharmaceuticals, Boehringer Ingelheim International GmbH, Bristol-Myers Squibb, Hoffman La-Roche, Intra-Cellular Therapies, Janssen, Merck, Naurex, Navitor Pharmaceuticals, Novartis, Noven Pharmaceuticals, Otsuka, Praxis Therapeutics, Sage Pharmaceuticals, Servier Pharmaceuticals, Taisho Pharmaceuticals, Teva, Valeant, and Vistagen therapeutics; Research Contracts: AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Hoffman La-Roche, Merck, Naurex, and Servier. Equity: Biohaven pharmaceuticals; holds patent: US8778979 B2 with royalties paid from Biohaven Pharmaceuticals.
Sanjay J. Mathew: Consulting: Clexio Biosciences, Alkermes Inc., Fortress Biotech, SAGE Therapeutics; Research support: NeuroRx; Janssen.
Charles Debattista: Dr. DeBattista has received grant support from Janssen, Neuronetics, St. Jude, and Biolite. He has served on the Advisory Board of Alkermes.
Dawn F. Ionescu: Presently an employee of Janssen Pharmaceuticals Research & Development, La Jolla, CA. Research grants: from NARSAD, AFSP.
George I. Papakostas: Consulting: Abbott Laboratories, Acadia Pharmaceuticals, Inc*, AstraZeneca PLC, Avanir Pharmaceuticals, Axsome Therapeutics*, Brainsway Ltd, Bristol-Myers Squibb Company, Cephalon Inc., Dey Pharma, L.P., Eli Lilly Co., Genentech, Inc*, Genomind, Inc*, GlaxoSmithKline, Evotec AG, H. Lundbeck A/S, Inflabloc Pharmaceuticals, Janssen Global Services LLC*, Jazz Pharmaceuticals, Johnson & Johnson Companies*, Methylation Sciences Inc, Mylan Inc*, Novartis Pharma AG, One Carbon Therapeutics, Inc*, Osmotica Pharmaceutical Corp.*, Otsuka Pharmaceuticals, PAMLAB LLC, Pfizer Inc., Pierre Fabre Laboratories, Ridge Diagnostics (formerly known as Precision Human Biolaboratories), Shire Pharmaceuticals, Sunovion Pharmaceuticals, Taisho Pharmaceutical Co, Ltd, Takeda Pharmaceutical Company LTD, Theracos, Inc., and Wyeth, Inc; Received honoraria (for lectures or consultancy) from: Abbott Laboratories, Acadia Pharmaceuticals, Inc, Asopharma America Cntral Y Caribe, Astra Zeneca PLC, Avanir Pharmaceuticals, Bristol-Myers Squibb Company, Brainsway Ltd, Cephalon Inc., Dey Pharma, L.P., Eli Lilly Co., Evotec AG, Forest Pharmaceuticals, GlaxoSmithKline, Inflabloc Pharmaceuticals, Grunbiotics Pty LTD, Jazz Pharmaceuticals, H. Lundbeck A/S, Medichem Pharmaceuticals, Inc, Meiji Seika Pharma Co. Ltd, Novartis Pharma AG, Otsuka Pharmaceuticals, PAMLAB LLC, Pfizer, Pharma Trade SAS, Pierre Fabre Laboratories, Ridge Diagnostics, Shire Pharmaceuticals, Sunovion Pharmaceuticals, Takeda Pharmaceutical Company LTD, Theracos, Inc., Titan Pharmaceuticals, and Wyeth Inc; Research support (paid to hospital): AstraZeneca PLC, Bristol-Myers Squibb Company, Forest Pharmaceuticals, the National Institute of Mental Health, Neuralstem, Inc*, PAMLAB LLC, Pfizer Inc., Ridge Diagnostics (formerly known as Precision Human Biolaboratories), Sunovion Pharmaceuticals, Tal Medical, and Theracos, Inc.; Served (not currently) on the speaker’s bureau for BristolMyersSquibb Co and Pfizer, Inc. * Asterisk denotes activity undertaken on behalf of Massachusetts General Hospital.
REFERENCES
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, & Krystal JH (2000). Antidepressant effects of ketamine in depressed patients. Biol Psychiatry, 47(4), 351–354. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, … Geddes JR (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet, 391(10128), 1357–1366. doi: 10.1016/S0140-6736(17)32802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corssen G, & Domino EF (1966). Dissociative anesthesia: further pharmacologic studies and first clinical experience with the phencyclidine derivative CI-581. Anesth Analg, 45(1), 29–40. [PubMed] [Google Scholar]
- Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, … Drevets WC (2018). Efficacy and Safety of Intranasal Esketamine Adjunctive to Oral Antidepressant Therapy in Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry, 75(2), 139–148. doi: 10.1001/jamapsychiatry.2017.3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, … Papakostas GI (2018). Double-Blind, Placebo-Controlled, Dose-Ranging Trial of Intravenous Ketamine as Adjunctive Therapy in Treatment-Resistant Depression (TRD). Mol Psychiatry doi: 10.1038/s41380-018-0256-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspersz R, Lamers F, Kent JM, Beekman AT, Smit JH, van Hemert AM, … Penninx BW (2017). Longitudinal Predictive Validity of the DSM-5 Anxious Distress Specifier for Clinical Outcomes in a Large Cohort of Patients With Major Depressive Disorder. J Clin Psychiatry, 78(2), 207–213. doi: 10.4088/JCP.15m10221 [DOI] [PubMed] [Google Scholar]
- Greden JF (2001). The burden of disease for treatment-resistant depression. J Clin Psychiatry, 62 Suppl 16, 26–31. [PubMed] [Google Scholar]
- Ionescu DF, & Papakostas GI (2016). Current Trends in Identifying Rapidly Acting Treatments for Depression. Curr Behav Neurosci Rep, 3(2), 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu DF, & Papakostas GI (2017). Experimental medication treatment approaches for depression. Transl Psychiatry, 7(3), e1068. doi: 10.1038/tp.2017.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautzky A, Dold M, Bartova L, Spies M, Vanicek T, Souery D, … Kasper S (2017). Refining Prediction in Treatment-Resistant Depression: Results of Machine Learning Analyses in the TRD III Sample. J Clin Psychiatry, 79(1). doi: 10.4088/JCP.16m11385 [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, … Duman RS (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science, 329(5994), 959–964. doi: 10.1126/science.1190287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, & Aghajanian GK (2013). GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology, 38(11), 2268–2277. doi: 10.1038/npp.2013.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molero P, Ramos-Quiroga JA, Martin-Santos R, Calvo-Sánchez E, Gutiérrez-Rojas L, & Meana JJ (2018). Antidepressant Efficacy and Tolerability of Ketamine and Esketamine: A Critical Review. CNS Drugs doi: 10.1007/s40263-018-0519-3 [DOI] [PubMed] [Google Scholar]
- Montgomery SA, & Asberg M (1979). A new depression scale designed to be sensitive to change. Br J Psychiatry, 134, 382–389. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, … Mathew SJ (2013). Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry, 170(10), 1134–1142. doi: 10.1176/appi.ajp.2013.13030392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen MR, & Dunner DL (1995). Clinical and differential diagnostic aspects of treatment-resistant depression. J Psychiatr Res, 29(1), 43–50. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Katz J, & Fava M (2007). A critical overview of the pharmacologic management of treatment-resistant depression. Psychiatr Clin North Am, 30(1), 13–29. doi: 10.1016/j.psc.2007.01.001 [DOI] [PubMed] [Google Scholar]
- Pryazhnikov E, Mugantseva E, Casarotto P, Kolikova J, Fred SM, Toptunov D, … Khiroug L (2018). Longitudinal two-photon imaging in somatosensory cortex of behaving mice reveals dendritic spine formation enhancement by subchronic administration of low-dose ketamine. Sci Rep, 8(1), 6464. doi: 10.1038/s41598-018-24933-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapidly-Acting Treatments for Treatment-Resistant Depression (RAPID). https://www.nimh.nih.gov/research-priorities/research-initiatives/rapidly-acting-treatments-for-treatment-resistant-depression-rapid.shtml. In.
- Russell JM, Hawkins K, Ozminkowski RJ, Orsini L, Crown WH, Kennedy S, … Rush AJ (2004). The cost consequences of treatment-resistant depression. J Clin Psychiatry, 65(3), 341–347. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, … Treatments, A. P. A. A. C. o. R. T. F. o. N. B. a. (2017). A Consensus Statement on the Use of Ketamine in the Treatment of Mood Disorders. JAMA Psychiatry, 74(4), 399–405. doi: 10.1001/jamapsychiatry.2017.0080 [DOI] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, … Van Nueten L (2016). A Double-Blind, Randomized, Placebo-Controlled, Dose-Frequency Study of Intravenous Ketamine in Patients With Treatment-Resistant Depression. Am J Psychiatry, 173(8), 816–826. doi: 10.1176/appi.ajp.2016.16010037 [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, … Team, S. D. S. (2006). Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry, 163(1), 28–40. doi: 10.1176/appi.ajp.163.1.28 [DOI] [PubMed] [Google Scholar]
- Wilkinson ST, Toprak M, Turner MS, Levine SP, Katz RB, & Sanacora G (2017). A Survey of the Clinical, Off-Label Use of Ketamine as a Treatment for Psychiatric Disorders. Am J Psychiatry, 174(7), 695–696. doi: 10.1176/appi.ajp.2017.17020239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, … Manji HK (2006). A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry, 63(8), 856–864. doi: 10.1001/archpsyc.63.8.856 [DOI] [PubMed] [Google Scholar]