Abstract
We report a case of prolonged survival in a patient with known cervical intramedullary H3K27M-mutant diffuse midline glioma. A 39-year-old man presented for evaluation with several months of progressive upper extremity pain and weakness. MRI of the cervical spine revealed an intramedullary ring-enhancing lesion centred at C3-C4. Following subtotal surgical resection, a diagnosis of glioblastoma (GBM) was confirmed. Subsequent testing at a later date revealed an H3K27M mutation. He was initially treated with radiation and concomitant and adjuvant temozolomide. He had multiply recurrent disease and was treated with various regimens, including the histone deacetylase inhibitor valproic acid. The patient passed away 31 months (~2.5 years) after diagnosis. Our case is one of few reported adult spinal cord GBMs possessing the H3K27M mutation, and one with the longest reported overall survival in the literature to date.
Keywords: cancer intervention, genetics, neuro-oncology, spinal cord, CNS cancer
Background
Primary spinal cord glioblastoma (GBM) is a rare entity, accounting for 7.5% of intramedullary spinal cord tumours and only 1.5% of all spinal cord tumours.1 2 The low incidence of primary spinal cord GBM has led to a poor understanding of the clinical characteristics and treatment options for this devastating and ultimately fatal disease. Less than 200 cases of spinal cord GBM have been presented in the literature, even fewer of which have been found to carry the histone H3K27M mutation.1–3
Diffuse midline glioma with histone H3K27M mutation is a newly described entity in the revised 2016 WHO and corresponds to a grade IV diagnosis, regardless of histological grade.4 H3K27M mutation was originally described in paediatric diffuse intrinsic pontine gliomas (DIPG) but has since been reported in adult midline gliomas, including adult spinal cord tumours.5 The literature reviewed contains fewer than 100 cases of H3K27M-mutant tumours in the spinal cord of adults.5–7 The H3K27M mutation has been found to be associated with a worse prognosis in several studies.7 8
Here we present an adult H3K27M spinal cord diffuse midline glioma with initial GBM histology, one of just a few reported in the literature thus far. Our patient’s overall survival was approximately 2.5 years, 10–14 months longer than reports to date.6 7 9
Case presentation
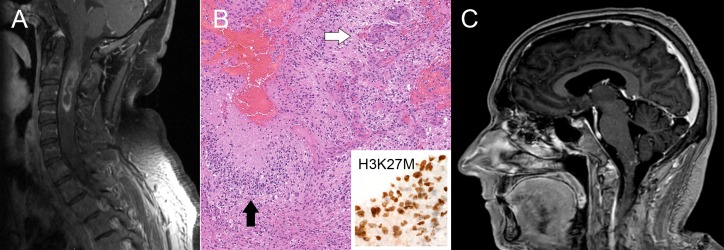
A 39-year-old previously healthy man initially developed numbness and pain in his left upper extremity. This progressed over the next 3–4 months to left upper extremity weakness, and spread to involve the right upper extremity as well. MRI of the cervical spine demonstrated an intramedullary ring-enhancing lesion centred between C3 and C4 (figure 1A). He underwent C1–C4 laminectomy with subtotal resection of the mass. Histological examination revealed classic features of GBM with positive staining for H3K27M and retained ATRX expression (figure 1B). The tumour was submitted for comprehensive molecular profiling through the Michigan Oncology Sequencing Project, which confirmed the H3K27M mutation via a lysine to methionine mutation of the 27 codon (K27M) in the H3F3A gene.10 PCR for MGMT promoter methylation was negative.
Figure 1.
(A) Initial MRI at diagnosis demonstrating heterogeneously enhancing mass with central areas of non-enhancement within the cervical spinal cord from approximately C2 to C4. (B) Histopathology demonstrating characteristic features of glioblastoma (GBM), including microvascular proliferation (white arrow) and pseudopalisading necrosis (black arrow). The tumour showed nuclear immunoreactivity for H3K27M (inset). (C) Sagittal MRI brain demonstrates new areas of nodular enhancement in the dorsal aspect of the medulla oblongata, likely contributing to the patient’s new-onset intractable nausea and vomiting.
The patient completed 54 Gy of external beam radiation therapy with concomitant temozolomide. He then completed 12 cycles of adjuvant temozolomide 5/28 regimen. Five months later (20 months after diagnosis), the patient developed intermittent but persistent nausea. Repeat MRI was obtained and demonstrated new enhancement in the medulla (figure 1C). The patient was started on lomustine, but this was stopped after three cycles due to progression of disease on MRI. Twenty-five months after diagnosis, the patient had further disease progression and was initiated on valproic acid, a histone deacetylase (HDAC) inhibitor, with the aim of targeting the H3 mutation.11
The patient continued to decline over the next 2 months, with further evidence of cord oedema on MRI and progression into the brainstem and supratentorial white matter. The patient was admitted to the hospital 30 months after initial diagnosis for worsening shortness of breath and weakness. He was given a single dose of bevacizumab, but experienced little clinical benefit. The patient then suffered an aspiration event requiring admission to the intensive care unit. He was started on antibiotics due to concern for aspiration pneumonia. Despite adequate treatment of pneumonia, the patient’s respiratory status continued to decline. This was likely due, in part, to worsening cervical spine tumour infiltration noted on repeated MRI, causing dysfunction of diaphragmatic innervation.
Outcome and follow-up
The patient and his family ultimately decided to transition to comfort care. He died shortly thereafter, approximately 31 months after his resection.
Discussion
Primary GBM of the spinal cord is a rare entity, representing only 1%–3% of all spinal cord tumours.1 2 Fewer than 200 cases of spinal GBM have been reported in the literature.3 Despite continued advances, prognosis remains poor with overall survival ranging from 6 to 18 months12 with a median of 12 months.3
Recent developments in paediatric brain tumours have identified a somatic missense mutation in the H3F3A gene (H3K27M), resulting in a lysine to methionine substitution on position 27 of histone H3 (H3K27me3) that alters downstream gene expression.13 14 The patient we present is one of only a few spinal GBMs reported in the literature that also harbours the H3K27M mutation.
Originally identified in paediatric DIPGs,15 the H3K27M mutation has since been identified in other midline structures including the thalamus, pons and spinal cord.5 In the recently revised 2016 WHO classification, diffuse midline glioma with histone H3K27M mutation is now a recognised diagnostic entity and corresponds to a grade IV glioma.4 A meta-analysis of studies comparing the overall survival of those with and without the H3K27M mutation in paediatric DIPG, with tumour locations including the cortex, basal ganglia, brainstem and spinal cord, demonstrated significantly worse overall survival by approximately 2.3 years in those harbouring the H3K27M mutation.16 However, given its rarity in adults, it is still uncertain if this mutation confers the same poor prognosis as observed in children.17
Diffuse midline gliomas may be more common in the adult population than previously thought. In a recent study by Schreck et al, approximately 15% of midline tumours identified in the parasagittal cortex, basal ganglia, cerebellum and spinal cord harboured the H3K27M mutation. This study also found slightly improved survival in those with the H3K27M mutation (17.6 months’ survival) versus those without (11.3 months).17 However, another study of patients with rare thalamic gliomas demonstrated significantly worse survival in those with the H3K27M mutation.18
Although several studies have reported the presence of H3K27M mutation in adult midline tumours, the number of reported cases occurring in the spinal cord is notably less than 100.5–9 19 Interestingly, further review of these studies reveals that even fewer of these H3K27M mutants were grade IV by histopathology. For example, Yi et al reported on 25 spinal tumours with H3K27 mutation; however, only 15 of these were grade IV gliomas by histological grade.20 Despite an often lower histological grade, tumours that harbour the H3K27 mutation are clinically aggressive and often behave as high-grade gliomas regardless of histology.21 22
The overall survival reported of those diagnosed with H3K27M-mutant spinal cord diffuse glioma is dismal, with a median survival of 6–16 months reported across the literature,1–3 with the longest reported survival of 20 months.9 20 The low incidence of spinal cord GBM overall has resulted in a lack of clinical trials to establish the best course of treatment and management for this devastating disease. Although most patients are treated similar to those with intracranial GBM, efficacy in this approach has not been established.12
Our patient was treated with radiotherapy and concurrent and adjuvant temozolomide but tumour progression was noted after approximately 20 months. Given the poor prognosis associated with standard therapy, researchers have begun to target the epigenetic regulators that drive oncogenesis as new potential therapies for this disease.
The H3K27M mutation drives tumorigenesis by globally reducing dimethylation and trimethylation of H3K27, leading to an altered transcription programme and reduced differentiation.23 As understanding of epigenetic changes in tumour cells has improved, researchers have developed new potential treatments to target these changes. For example, HDAC inhibitors, like panobinostat, attempt to correct acetylation of aberrant histones. Grasso et al screened 83 drugs and found that panobinostat increased H3K27me3, increased apoptosis and lowered cell viability in K27M-DIPG cell lines.14 24 Valproic acid is an antiepileptic drug with HDAC inhibitor activity.25 While Grasso et al did not show sensitivity of DIPG- K27M cell lines to valproate,24 others have reported that valproate can induce cell cycle arrest in GBM-derived cell lines26 and sensitise GBM cells to radiotherapy and temozolomide therapy.25 Further studies are needed to demonstrate whether there is truly benefit of treatment with valproic acid for patients with H3K27M mutations.
Review of case series presented in the literature has shown conflicting reports on overall survival in those diagnosed with H3K27M-mutant spinal cord glioma. Wang et al reported a median overall survival of 13.1 months for H3.3 and H3.1 mutants (in thalamus, pons, spinal cord) versus 23.1 months for H3 wild-type patients.8 For the spinal cord alone, H3K27M mutation has been associated with a significantly worse prognosis compared with wild-type tumours.8 However, Yi et al reported improved survival with H3K27M tumours compared with wild type. Despite this, the median survival was reported to be 20.2 months for those with grade IV histopathology.20 Regardless, the average overall survival for patients in both of these studies ranged from 13.2 to 20.2 months.8 20 The patient presented here lived for 31 months after his surgery, making him one of the longest living patients presented in the literature with a histological GBM and a known H3K27M mutation.
Several other cases in the literature have reported prolonged survival with H3K27M mutation and non-diffuse histology. Orillac et al reported on two patients, one with a midline grade I pilocytic astrocytoma and one with grade III glioneuronal tumour, each harbouring the H3K27M mutation. These patients survived 14+ and 22+ months, respectively, and both were still alive at the time the reports were published. The improved survival seen in these patients with non-diffuse histology raises the question of whether or not the H3K27M mutation should always signify a WHO grade IV diagnosis.27 In addition, the H3K27M mutation has been identified in non-midline gliomas and the tumour was reported to have a more indolent course.28 In tumours harbouring the H3K27M mutation, those with low-grade circumscribed pathology had better overall survival than those with diffuse high-grade pathology.29 Given the conflicting reports in the literature, further studies are necessary to define the prognostic implications of this marker.
In summary we present a rare adult spinal cord H3K27M-mutant GBM. Per our review of the literature, our patient’s overall survival was upwards of 10 months longer than any other median survival reported to date. In addition, he was also the first patient to receive valproic acid as monotherapy for his H3K27M mutation. Further reporting of patients with spinal cord GBM is needed to better understand the clinical characteristics, treatment and prognosis for those with this disease. It remains unclear if the H3K27M mutation is universally associated with a poorer prognosis than wild-type tumours. Other factors, including tumour location and histological grade, may need to be considered when discussing prognosis.
Learning points.
Adult H3K27M spinal cord diffuse glioma is rare and has a poor prognosis.
There is no good standard-of-care treatment for this disease.
Histone deacetylase inhibitors represent a potential targeted therapy.
Acknowledgements.
We thank Drs Larry Junck, Michelle K Kim and Daniel A Orringer for their care of the patient and help with this manuscript. We thank Bernard Marini for his help with clinical pharmacology and with the preparation of this manuscript. We thank Dr Douglas Quint for his help with the figures.
Footnotes
Twitter: @DeniseLeungMD
Contributors: KP: substantial contributions to the design of the work, drafting the work. CK, DP: substantial contributions to the analysis and interpretation of data, revising it critically for important intellectual content. DL: substantial contributions to the conception or design of the work, the acquisition, analysis and interpretation of data, drafting the work, revising it critically for important intellectual content. All authors gave final approval of the version published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Morais N, Mascarenhas L, Soares-Fernandes JP, et al. Primary spinal glioblastoma: a case report and review of the literature. Oncol Lett 2013;5:992–6. 10.3892/ol.2012.1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shen C-X, Wu J-F, Zhao W, et al. Primary spinal glioblastoma multiforme. Medicine 2017;96:e6634 10.1097/MD.0000000000006634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Timmons JJ, Zhang K, Fong J, et al. Literature review of spinal cord glioblastoma. Am J Clin Oncol 2018;41:1281–7. 10.1097/COC.0000000000000434 [DOI] [PubMed] [Google Scholar]
- 4. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 2016;131:803–20. 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 5. Solomon DA, Wood MD, Tihan T, et al. Diffuse midline gliomas with histone H3-K27M mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol 2016;26:569–80. 10.1111/bpa.12336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gessi M, Gielen GH, Dreschmann V, et al. High frequency of H3F3A (K27M) mutations characterizes pediatric and adult high-grade gliomas of the spinal cord. Acta Neuropathol 2015;130:435–7. 10.1007/s00401-015-1463-7 [DOI] [PubMed] [Google Scholar]
- 7. Kleinschmidt-DeMasters BK, Mulcahy Levy JM, Levy JMM. H3 K27M-mutant gliomas in adults vs. children share similar histological features and adverse prognosis. Clin Neuropathol 2018;37 (2018:53–63. 10.5414/NP301085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang L, Li Z, Zhang M, et al. H3 K27M-mutant diffuse midline gliomas in different anatomical locations. Hum Pathol 2018;78:89–96. 10.1016/j.humpath.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 9. Meyronet D, Esteban-Mader M, Bonnet C, et al. Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol 2017;19:1127–34. 10.1093/neuonc/now274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roychowdhury S, Iyer MK, Robinson DR, et al. Personalized oncology through integrative high-throughput sequencing: a pilot study. Sci Transl Med 2011;3:111ra121 10.1126/scitranslmed.3003161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Killick-Cole CL, Singleton WGB, Bienemann AS, et al. Repurposing the anti-epileptic drug sodium valproate as an adjuvant treatment for diffuse intrinsic pontine glioma. PLoS One 2017;12:e0176855 10.1371/journal.pone.0176855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tseng H-M, Kuo L-T, Lien H-C, et al. Prolonged survival of a patient with cervical intramedullary glioblastoma multiforme treated with total resection, radiation therapy, and temozolomide. Anticancer Drugs 2010;21:963–7. 10.1097/CAD.0b013e32833f2a09 [DOI] [PubMed] [Google Scholar]
- 13. Chan K-M, Fang D, Gan H, et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev 2013;27:985–90. 10.1101/gad.217778.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wan YCE, Liu J, Chan KM. Histone H3 mutations in cancer. Curr Pharmacol Rep 2018;4:292–300. 10.1007/s40495-018-0141-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu G, Broniscer A, McEachron TA, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet 2012;44:251–3. 10.1038/ng.1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu VM, Alvi MA, McDonald KL, et al. Impact of the H3K27M mutation on survival in pediatric high-grade glioma: a systematic review and meta-analysis. J Neurosurg Pediatr 2018;23:308–16. 10.3171/2018.9.PEDS18419 [DOI] [PubMed] [Google Scholar]
- 17. Schreck KC, Ranjan S, Skorupan N, et al. Incidence and clinicopathologic features of H3 K27M mutations in adults with radiographically-determined midline gliomas. J Neurooncol 2019;143:87–93. 10.1007/s11060-019-03134-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Y, Zhang Y, Hua W, et al. Clinical and molecular characteristics of thalamic gliomas: retrospective report of 26 cases. World Neurosurg 2019;126:e1169–82. 10.1016/j.wneu.2019.03.061 [DOI] [PubMed] [Google Scholar]
- 19. Gao Y, Feng Y-Y, Yu J-H, et al. Diffuse midline gliomas with histone H3-K27M mutation: a rare case with PNET-like appearance and neuropil-like islands. Neuropathology 2018;38:165–70. 10.1111/neup.12413 [DOI] [PubMed] [Google Scholar]
- 20. Yi S, Choi S, Shin DA, et al. Impact of H3.3 K27M mutation on prognosis and survival of grade IV spinal cord glioma on the basis of new 2016 World Health organization classification of the central nervous system. Neurosurgery 2019;84:1072–81. 10.1093/neuros/nyy150 [DOI] [PubMed] [Google Scholar]
- 21. Daoud EV, Rajaram V, Cai C, et al. Adult brainstem gliomas with H3K27M mutation: radiology, pathology, and prognosis. J Neuropathol Exp Neurol 2018;77:302–11. 10.1093/jnen/nly006 [DOI] [PubMed] [Google Scholar]
- 22. Buczkowicz P, Bartels U, Bouffet E, et al. Histopathological spectrum of paediatric diffuse intrinsic pontine glioma: diagnostic and therapeutic implications. Acta Neuropathol 2014;128:573–81. 10.1007/s00401-014-1319-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williams MJ, Singleton WGB, Lowis SP, et al. Therapeutic targeting of histone modifications in adult and pediatric high-grade glioma. Front Oncol 2017;7 10.3389/fonc.2017.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grasso CS, Tang Y, Truffaux N, et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med 2015;21:555–9. 10.1038/nm.3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weller M, Gorlia T, Cairncross JG, et al. Prolonged survival with valproic acid use in the EORTC/NCIC temozolomide trial for glioblastoma. Neurology 2011;77:1156–64. 10.1212/WNL.0b013e31822f02e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tseng J-H, Chen C-Y, Chen P-C, et al. Valproic acid inhibits glioblastoma multiforme cell growth via paraoxonase 2 expression. Oncotarget 2017;8 10.18632/oncotarget.14716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Orillac C, Thomas C, Dastagirzada Y, et al. Pilocytic astrocytoma and glioneuronal tumor with histone H3 K27M mutation. Acta Neuropathol Commun 2016;4 10.1186/s40478-016-0361-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. López G, Oberheim Bush NA, Berger MS, et al. Diffuse non-midline glioma with H3F3A K27M mutation: a prognostic and treatment dilemma. Acta Neuropathologica Communications 2017;5 10.1186/s40478-017-0440-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pratt D, Natarajan SK, Banda A, et al. Circumscribed/non-diffuse histology confers a better prognosis in H3K27M-mutant gliomas. Acta Neuropathol 2018;135:299–301. 10.1007/s00401-018-1805-3 [DOI] [PMC free article] [PubMed] [Google Scholar]