Abstract
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome represents a severe adverse drug reaction driven by eosinophilia. Treatment is focused on withdrawal of medication, supportive care and immunosuppression such as high-dose corticosteroid therapy. Here we report a 56-year-old male patient who initially presented with breathlessness and eosinophilia, subsequent development of respiratory failure and admission to ITU for non-invasive ventilation. The patient continued to deteriorate despite high-dose prednisolone and methylprednisolone. Other causes of hypereosinophilia were normal. He was diagnosed with DRESS syndrome secondary to pregabalin and was treated with subcutaneous mepolizumab. We observed the rapid resolution of eosinophilia and clinical improvement; the patient was discharged home within a month of administration. This represents the successful use of mepolizumab in the acute setting of pulmonary failure secondary to DRESS. A similar approach could be adopted in other acute conditions with refractory eosinophilic inflammation where standard steroid therapy has failed.
Keywords: immunology, adult intensive care, pharmacology and therapeutics
Background
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome represents a severe adverse drug reaction which can occur up to 8 weeks after starting medication.1 The term was first used in 1996 by Bocquet et al following a review of 24 patients by Callot et al.2 3 The underlying pathophysiology is unclear but is likely to be multifactorial, including abnormal metabolism of drug metabolites, human leucocyte antigen-specific interactions and possible viral interactions.4 This initial trigger(s) led to complete or partial activation of the eosinophil inflammatory pathway. This activates T-helper 2 cells and a cytokine cascade including interleukin (IL)-4 and IL-13.5 6 These lead to the production of IL-5 and eotaxin (CCL11) which act on the bone marrow to stimulate the release of mature eosinophils. Chemotaxis via IL-4 and IL-13 to the initial site of injury and cause their degranulation. Locally produced IL-5 may have autocrine effects to promote eosinophil survival.7
There has been documentation of DRESS syndrome triggered by anticonvulsant therapy, minocycline, allopurinol, abacavir and nevirapine. Phenytoin and phenobarbital are considered to be the most principal causes of DRESS.8 Diagnosis is mainly based on clinical presentation. Although diagnostic criteria have been suggested, none have been validated. The RegiSCAR criteria have been widely used which were first described by Kardaun et al.9–11 It is based on a scoring system evaluating the presence of fever ≥38.5°C, lymphadenopathy, eosinophilia, skin involvement, other organ involvement and evaluation of other potential causes. An alternative set of criteria from Shiohara et al has also been documented, which include HHV-6 activation.12
DRESS is associated with a reported mortality rate of 10%.10 Management has been mainly based on drug withdrawal, supportive care with immunosuppressive use (eg, topical and systemic corticosteroids and ciclosporin). The monoclonal antibody mepolizumab targets IL-5 signalling as a key regulator or eosinophilic upregulation and has been recently been approved for treatment of severe refractory eosinophilic asthma by the National Institute of Clinical Excellence, UK (NICE UK).13 Mepolizumab is a humanised monoclonal antibody and prevents the association between IL-5 and IL-5 receptor alpha chain. Mepolizumab use has mainly been centred towards eosinophilic asthma, with trials beginning with Leckie et al at the turn of the century showing biochemical improvement in response to a histamine challenge.14 Although initially promising, larger-scale trials did not confer any clinical improvement in patients until trials selecting for patients demonstrating eosinophilia was established.15–17 The mepolizumab as adjunctive therapy in patients with severe asthma (MENSA) trial showed favourable outcomes with regards to frequency of exacerbations and subjective clinical scoring (Asthma Control Questionnaire-5, St George’s Respiratory Questionnaire).17 Further trials have shown the ability of mepolizumab to reduce the long-term glucocorticoid burden of patients with refractory asthma and hypereosinophilic syndrome (HES).
Importantly, the use of mepolizumab has focused on clinically stable, glucocorticoid refractory patients with eosinophilic conditions (asthma, chronic obstructive pulmonary disease and HES).17–19 The successful use of mepolizumab in treatment of DRESS secondary to co-trimoxazole has been documented by Ange et al in with a background of an autologous stem cell transplant secondary to acute myeloid leukaemia.20 We describe the acute use of mepolizumab in a patient admitted with severe hypereosinophilic pneumonitis secondary to DRESS syndrome requiring non-invasive ventilation, refractory to high-dose steroid therapy and broad-spectrum antibiotics.
Case presentation
A 56-year-old man of white European origin presented with breathlessness, fever, malaise and raised eosinophils. He had a history of epilepsy, trigeminal neuralgia, chronic pain syndrome, fibromyalgia, coeliac disease and hypertension and had been commenced on Pregabalin 8 weeks prior to presentation. Initially, he was hypoxaemic with no evidence of other organ dysfunction.
Investigations
Admission blood tests and observations are shown in table 1, which show an eosinophilia of 5.4×109/L with a chest radiograph reported as showing bilateral consolidation (figure 1). Antinuclear antibodies (ANA) and perinuclear antineutrophil cytoplasmic antibodies (p-ANCA) negative; total immunoglobulin (Ig)E raised >1500 kU/L; tryptase normal; microbiology cultures, parasite, hepatitis and HIV serology were negative. T-cell immune-phenotypic and genetic testing did not reveal a haematological or clonal cause of eosinophilia. Echocardiogram was normal. CT of the best revealed extensive bilateral confluent ground-glass opacities (figure 2).
Table 1.
Summary table of admission blood tests and observations
| Blood results | 12 June 2017 | Observations | |
| Haemoglobin (g/L) | 105 | Temperature (°C) | 37 |
| White cell count (109/L) | 13.2 | Blood pressure (mm Hg) | 139/63 |
| Platelets (109/L) | 400 | Pulse rate (/min) | 99 |
| Neutrophils (109/L) | 8.3 | Respiratory rate (/min) | 19 |
| Lymphocytes (109/L) | 0.9 | Oxygen saturations (%) | 95 |
| Monocytes (109/L) | 0.8 | Supplemental oxygen (L/min) | 3 |
| Eosinophils (109/L) | 5.4 | ||
| Mean cell volume (fL) | 84 | ||
| C reactive protein (mg/L) | 204 |
Results above the laboratory reference range are shown in red, those below in blue.
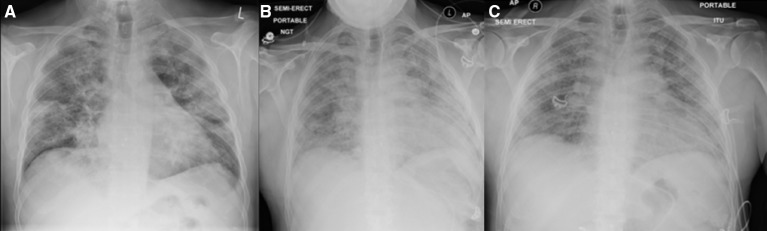
Figure 1.
(A) Patient’s admission PA chest radiograph (12/06/2017). Reported to have bilateral consolidation. (B) AP chest radiograph after deterioration and intensive care admission (28/06/2017). (C) AP chest radiograph prior to discharge from intensive care to respiratory ward, noted persistent fibrotic changes (16/07/2017).
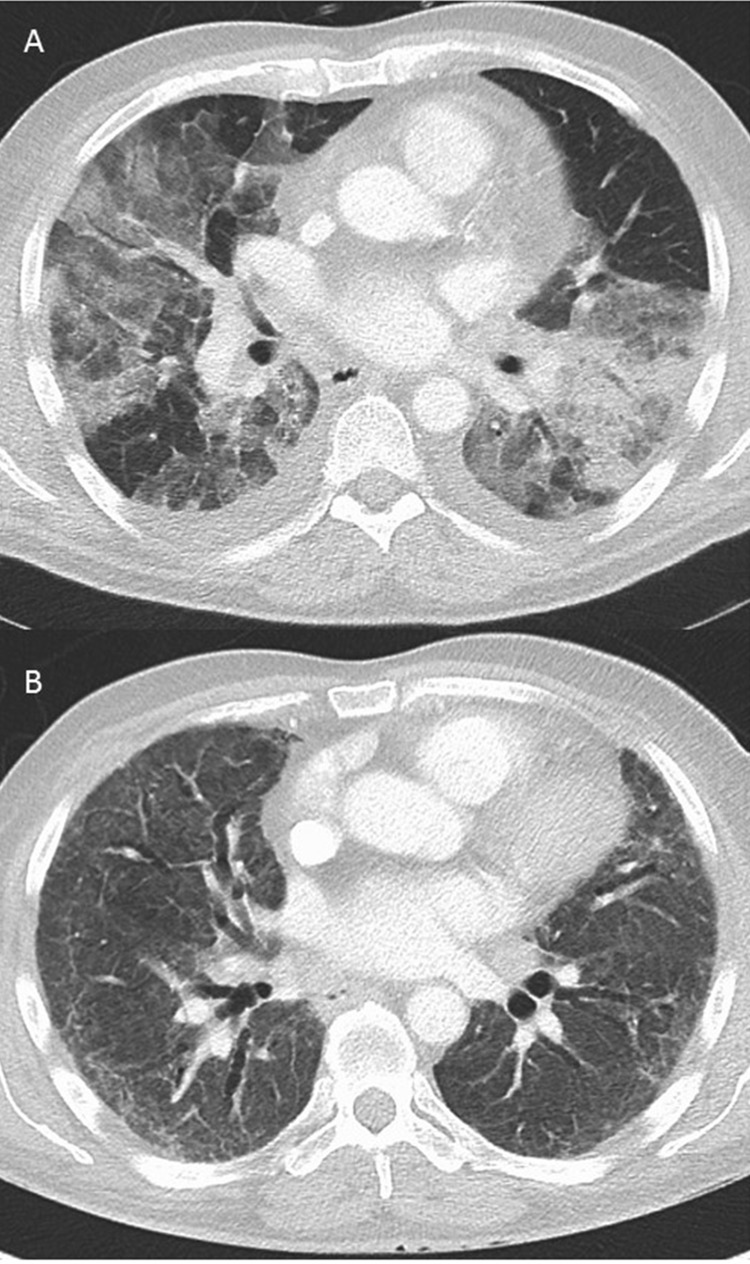
Figure 2.
CT scan of chest at admission and at 3 months follow-up.
Differential diagnosis
Idiopathic HES.
Community-acquired pneumonia.
Connective tissue disease.
Eosinophilic granulomatosis with polyangiitis.
Treatment
On admission, he was treated for community-acquired pneumonia with intravenous co-amoxiclav and oral clarithromycin. Following minimal response, this was escalated to intravenous piperacillin/tazobactam 3 days later with the addition of prednisolone 40 mg daily. However, there was ongoing clinical deterioration by day 7 and the patient was transferred to the intensive care unit. The patient was started on non-invasive ventilation due to worsening respiratory function and oxygen requirements. The rash resolved with steroid prior to a planned biopsy. Bronchoscopy and lung biopsy were deemed too high risk. A diagnosis of drug reaction with eosinophilia and systemic symptoms (DRESS) was made based on RegiSCAR scoring. Intravenous methylprednisolone was commenced (1 g daily for 6 days), followed with prednisolone 60 mg daily. A diagnosis of eosinophilic granulomatosis with polyangiitis (formerly Churg-Strauss vasculitis) was considered but serum ANCAs were equivocal and myeloperoxidase/PR3 negative. There were negative parasite serology and stool samples with negative genetic testing for a clonal or haematological cause. There was a transient response but by day 14 of admission in view of progressive life-threatening respiratory failure (PaO2:FiO2 ratio <150 mm Hg), evidence of liver involvement (rising transaminases) and persistent hypereosinophilia (7.1×109/L) with raised serum IL-5 (22.4 pg/mL; normal, <4.4 pg/mL) despite high-dose glucocorticoids, we chose to try rescue therapy with mepolizumab. Following drug approval by our local medicines management group, a trial of mepolizumab was given after an initial dose of 600 mg subcutaneously split to 300 mg on consecutive days.
After 24 hours, detectable serum IL-5 levels had fallen <4.4 pg/mL. Oxygenation and eosinophilia improved steadily over the next 13 days and he was discharged from critical care. Subsequently, after a phase of recovery he was discharged home. Mepolizumab, 300 mg subcutaneously 4 weekly, has subsequently maintained eosinophils <0.3×109/L and prednisolone weaned.
He was discharged on 3 L/min of long-term home oxygen 2 months after admission, which was further weaned in the community to 2 L/min.
Outcome and follow-up
Unfortunately, the patient has required further inpatient admissions due to recurring chest pain and breathlessness; likely due to a combination of fibrotic sequelae, chronic pain syndrome and underlying anxiety. There was a significant psychological burden on the patient and his wife after this admission due to his ongoing disability.
Discussion
DRESS syndrome has no universally accepted diagnostic criteria. Even though the use of the RegiSCAR criteria has been widely documented, clinical suspicion has to also take priority. Our patient developed unexplained eosinophilia after commencing pregabalin, with severe respiratory involvement. As our patient only partially fulfilled the RegiSCAR diagnostic criteria for a DRESS syndrome reaction to pregabalin, we considered the possibility that this case may represent a drug-induced HES. Clinically, this would not alter his management. DRESS induced by gabapentin has been previously reported but was not treated with mepolizumab.21 The previously described case involving DRESS secondary to co-trimoxazole demonstrated complete resolution after the use of mepolizumab, without ongoing therapy.20 This is the first case to demonstrate its effectiveness in the critical care setting of life-threatening respiratory failure and ensuing multiorgan involvement secondary to DRESS.
For DRESS syndrome in addition to withdrawal of the offending drug, therapy with glucocorticoids such as pulsed methylprednisolone has shown some efficacy in the past, but can also have serious side effects.10 22–24 Our patient continued to deteriorate despite these therapies with a high therapeutic oxygen demand and intensive care admission. This disease progression refractory to conventional treatment is similar to the case described by Yun et al. There is a significant similarity with this case to HES, which can also cause life-threatening disease but also long-term biological and psychological consequences. Treatment of HES is also based on glucocorticoids, with patients with chronic HES having a heavy long-term steroid burden. Mepolizumab has been trialled in this population as an alternative treatment strategy to reduce long-term steroid use. A trial by Rothenberg et al demonstrated a reduction in corticosteroid treatment in patients with HES.19 However, in contrast to our patient, these patients were previously diagnosed and demonstrated a stable clinical picture; corticosteroid therapy was required to maintain clinical remission. Similarly, in trials using mepolizumab in asthmatic patients focus on preventative and maintenance strategies. Previous studies have investigated response to therapy over several days to weeks. They demonstrate an absolute fall in eosinophil count at the endpoint and chronic change.14 19 Beyond the previous case report, there are little data to show the acute effect of mepolizumab administration and correlation to clinical improvement.
Rothenberg et al demonstrated that a dose of 750 mg intravenous mepolizumab in patients with stable HES reduced glucocorticoid maintenance therapy.19 Similarly, this dose was used by GlaxoSmithKline (GSK) in their extension trial (NCT00097370) primarily evaluating safety. However, in a further extension trial (NCT03306043) to characterise long-term safety and dosing a dose of 300 mg subcutaneous mepolizumab was used instead.25 26 Subcutaneous delivery has been previously shown to have increased response compared with intravenous mepolizumab in the MENSA Study (mepolizumab as adjunctive therapy in patients with severe asthma).17 Treatment intervals of 4 weeks have widely been used across multiple trials. NICE UK has approved a dose of 100 mg subcutaneously 4 weekly for the treatment of eosinophilic asthma.13 Given the current trial dosing strategy and previously documented benefit of subcutaneous administration compared with intravenous we opted for subcutaneous mepolizumab. The rationale behind the 600 mg split dosing was derived from data from the initial GSK trial using 750 mg intravenous mepolizumab, although we were unable to obtain this particular dose. Split dosing was used to avoid potential localised skin reactions from an increased dose. Consequent dosing to maintain remission was given at 300 mg subcutaneously in line with the current GSK trial (NCT03306043). In comparison, mepolizumab dosing in the case described by Yun et al was based on that for eosinophilic asthma (100 mg).
Subsequent follow-up and readmission showed disease recurrence despite pregabalin exclusion since the initial admission. Interestingly, a trial off mepolizumab over 2 months showed a steady incline in eosinophilia and worsening of symptoms. At which point, 4 weekly 100 mg dosing was reinstated. Serial axial lung CT imaging demonstrated persistent fibrotic changes bilaterally, likely caused by the initial DRESS syndrome. As with all monoclonal antibody therapy, there is potential for an immunogenicity reaction to have occurred and it would be interesting to further evaluate the development of antibodies to mepolizumab.
The clinical improvement we have documented in our patient with acute pneumonitis secondary to DRESS demonstrates a novel use of mepolizumab. This case, in conjunction with the single previous case of mepolizumab in the treatment of acute DRESS supports further study of mepolizumab in acute DRESS syndrome and HES to induce remission. Given the differences in dosing strategy, further investigation into appropriate doses is required. The rapid effect on eosinophil count and resolution of pulmonary symptoms in these patients may warrant an investigation into using mepolizumab as rescue therapy in the acutely deteriorating eosinophilic asthmatic patient.
Learning points.
Consider other differential diagnoses in those patients whose symptoms do not resolve with initial treatment.
Investigation of hypereosinophilia is important as causes can indicate an obscure diagnosis.
Using a personalised approach to target the specific driver of inflammation rather than global immunosuppression should be how patients with acute pneumonitis should be managed in the era of monoclonal immunotherapy.
Acknowledgments
The authors acknowledge Ms Sharon Rees, Respiratory Clinical Nurse Specialist.
Footnotes
Contributors: OST: literature review, patient consent, manuscript writing. BS: treatment of patient, literature review. DRT: literature review. DP: management and treatment of patient, literature review, manuscript review.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Rzany B, Correia O, Kelly JP, et al. Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis during first weeks of antiepileptic therapy: a case-control study. Study Group of the International Case Control Study on Severe Cutaneous Adverse Reactions. Lancet 1999;353:2190–4. 10.1016/s0140-6736(98)05418-x [DOI] [PubMed] [Google Scholar]
- 2. Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS). Semin Cutan Med Surg 1996;15:250–7. 10.1016/S1085-5629(96)80038-1 [DOI] [PubMed] [Google Scholar]
- 3. Callot V, Roujeau JC, Bagot M, et al. Drug-induced pseudolymphoma and hypersensitivity syndrome. Two different clinical entities. Arch Dermatol 1996;132:1315–21. [PubMed] [Google Scholar]
- 4. Choudhary S, McLeod M, Torchia D, et al. Drug reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome. J Clin Aesthet Dermatol 2013;6:31–7. [PMC free article] [PubMed] [Google Scholar]
- 5. Busse WW, Sedgwick JB. Eosinophils in asthma. Ann Allergy 1992;68:286–90. [PubMed] [Google Scholar]
- 6. Wardlaw AJ. Molecular basis for selective eosinophil trafficking in asthma: A multistep paradigm. J Allergy Clin Immunol 1999;104:917–26. 10.1016/S0091-6749(99)70069-2 [DOI] [PubMed] [Google Scholar]
- 7. Huang CD, Wang CH, Liu CY, et al. Eosinophils from asthmatics release IL-5 in an autocrine fashion to prevent apoptosis through upregulation of Bcl-2 expression. J Asthma 2005;42:395–403. 10.1081/JAS-200063001 [DOI] [PubMed] [Google Scholar]
- 8. Tennis P, Stern RS. Risk of serious cutaneous disorders after initiation of use of phenytoin, carbamazepine, or sodium valproate: a record linkage study. Neurology 1997;49:542–6. 10.1212/WNL.49.2.542 [DOI] [PubMed] [Google Scholar]
- 9. Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol 2007;156:609–11. 10.1111/j.1365-2133.2006.07704.x [DOI] [PubMed] [Google Scholar]
- 10. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med 2011;124:588–97. 10.1016/j.amjmed.2011.01.017 [DOI] [PubMed] [Google Scholar]
- 11. Eshki M, Allanore L, Musette P, et al. Twelve-year analysis of severe cases of drug reaction with eosinophilia and systemic symptoms: a cause of unpredictable multiorgan failure. Arch Dermatol 2009;145:67–72. 10.1001/archderm.145.1.67 [DOI] [PubMed] [Google Scholar]
- 12. Shiohara T, Iijima M, Ikezawa Z, et al. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br J Dermatol 2007;156:1083–4. 10.1111/j.1365-2133.2007.07807.x [DOI] [PubMed] [Google Scholar]
- 13. UK N. Mepolizumab for treating severe refractory eosinophilic asthma. 2017. www.nice.org.uk/guidance/ta431 (cited 2017 14/12/2017).
- 14. Leckie MJ, ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 2000;356:2144–8. 10.1016/S0140-6736(00)03496-6 [DOI] [PubMed] [Google Scholar]
- 15. Flood-Page P, Swenson C, Faiferman I, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med 2007;176:1062–71. 10.1164/rccm.200701-085OC [DOI] [PubMed] [Google Scholar]
- 16. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med 2014;371:1189–97. 10.1056/NEJMoa1403291 [DOI] [PubMed] [Google Scholar]
- 17. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014;371:1198–207. 10.1056/NEJMoa1403290 [DOI] [PubMed] [Google Scholar]
- 18. Pavord ID, Chanez P, Criner GJ, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med 2017;377:1613–29. 10.1056/NEJMoa1708208 [DOI] [PubMed] [Google Scholar]
- 19. Rothenberg ME, Klion AD, Roufosse FE, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med 2008;358:1215–28. 10.1056/NEJMoa070812 [DOI] [PubMed] [Google Scholar]
- 20. Ange N, Alley S, Fernando SL, et al. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome successfully treated with mepolizumab. J Allergy Clin Immunol Pract 2018;6:1059–60. 10.1016/j.jaip.2017.10.020 [DOI] [PubMed] [Google Scholar]
- 21. Valleti M, Kodandaraman T, Lakshmi P, et al. A case report on gabapentin induced DRESS syndrome. World J Pharm Med Res 2017;3:164–6. [Google Scholar]
- 22. Kocaoglu C, Cilasun C, Solak ES, et al. Successful Treatment of Antiepileptic Drug-Induced DRESS Syndrome with Pulse Methylprednisolone. Case Rep Pediatr 2013;2013:1–4. 10.1155/2013/928910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part II. Management and therapeutics. J Am Acad Dermatol 2013;68:709 e1–9. 10.1016/j.jaad.2013.01.032 [DOI] [PubMed] [Google Scholar]
- 24. Walsh SA, Creamer D. Drug reaction with eosinophilia and systemic symptoms (DRESS): a clinical update and review of current thinking. Clin Exp Dermatol 2011;36:6–11. 10.1111/j.1365-2230.2010.03967.x [DOI] [PubMed] [Google Scholar]
- 25. GlaxoSmithKline. Open-Label Extension Of Intravenous Mepolizumab In Patients With Hypereosinophilic Syndrome. 2004. Clinical Trial NCT00097370] Available: https://clinicaltrials.gov/ct2/show/results/NCT00097370?term=mepolizumab&draw=2&rank=19 [cited 2017 14/12/2017].
- 26. GlaxoSmithKline. A Multi-center, Open-label Extension, Safety Study of Mepolizumab in Subjects With Hypereosinophilic Syndrome (HES) From Study 200622. 2017. Clinical Trial NCT03306043]. Available: https://clinicaltrials.gov/ct2/show/NCT03306043?term=mepolizumab&draw=1&rank=8 [cited 2017 14/12/2017].