Abstract
Apart from floral morphology and colours perceived by the human eye, ultraviolet (UV) reflectance acts as an important visual advertisement of numerous flowering plant species for pollinators. However, the effect of UV signalling on attracting pollinators of particular plant species is still insufficiently studied, especially in the Afrotropics. Therefore, we studied the pollination system of Hypoxis camerooniana in montane grasslands of Mount Cameroon, West/Central Africa. We focused mainly on the effects of the flowers’ UV reflectance on its visitors. We experimentally removed UV reflection from petals either completely or partially. Thereafter, flower visitors were recorded and pistils were collected post-flowering to quantify germinated pollen tubes per treatments. The most important visitors were bees, followed by flies. Due to their contacts with reproductive organs bees are considered as the primary pollinators. Visitation rates were lower when UV reflectance was completely removed, whereas the decrease of frequency on half-treated flowers did not differ significantly from control treatments. The complete removal of UV also affected bees’ landing behaviour, but not that of flies. We showed that the presence of UV reflectance is more important than UV pattern for bees visiting flowers of H. camerooniana. We hypothesize that exploiting all flowers irrespective of their pattern can be more efficient for pollinators in the open grasslands of high altitudes to spot these relatively scarce flowers by their UV reflectance. Furthermore, we highlight the necessity of both experimental and natural controls in similar studies to control for additional effects of the used UV manipulations.
Many plants advertise their flowers with UV reflectance visible to their insect visitors. By manipulating the UV reflectance and pattern of Hypoxis camerooniana in the Afromontane grasslands of Mount Cameroon, we have shown how crucial it is for the predominant visitor, bees. Both bees' preferences for flowers and their behaviour during visits are influenced by changes in UV reflectance. However, the presence of some UV signal is more important than the specific pattern. Especially in montane grasslands with higher UV irradiation, the UV floral colours are important for recognition of flowers by potential pollinators.
Keywords: Afromontane grasslands, floral traits, foraging behaviour, Mount Cameroon National Park, pollination interactions, UV manipulation
Introduction
Unlike humans, many insect pollinators are sensitive to the ultraviolet (UV) part of the electromagnetic light spectrum in addition to the visible spectrum (Briscoe and Chittka 2001). Ultraviolet light is reflected by flowers of ~25 % of angiosperms, with the highest reflectance found in plant species with yellow flowers (Chittka et al. 1994; Papiorek et al. 2016). Consequently, the UV vision helps floral visitors in recognition of individual flowers of such plants which differ in their UV colouration from other plants in the community (Johnson and Andersson 2002). To increase distinction by certain groups of pollinators, some flowers create a contrasting pattern of UV absorbance and reflectance on the surface of their petals, whereas others contrast petals and reproductive parts by an inverse pattern of absorbance and reflectance of UV light. Floral guides (Penny 1983; Dinkel and Lunau 2001; Lunau 2006; Papiorek et al. 2016) and the so-called bullseye patterns (Lunau 1992a; Koski and Ashman 2014, 2015a, b), which has reflecting apices and absorbing bases of petals, are among the most commonly known examples of this phenomenon. These UV patterns are believed to improve the identification of the landing and/or foraging parts of flowers, or mimic such parts to the pollinator (Lunau et al. 2017). Their importance was shown in numerous studies revealing the influence of UV patterns on pollinator visitation preferences (e.g. Burr et al. 1995; Campbell et al. 2010; Horth et al. 2014; Koski and Ashman 2014; Peterson et al. 2015) and behaviour (Hansen et al. 2012).
The specific colour vision, which includes UV, of some insects and spectral properties of flowers have evolved into mutualistic relationships between plants and their pollinators. One of the best understood systems of vision is that of bees (Dyer et al. 2015). von Helversen (1972) measured the capability of honeybees to distinguish colours and showed that bees best discriminate wavelengths at ~400 and 500 nm where the spectral sensitivity curves of UV, blue and green photoreceptors overlap. Peitsch et al. (1992) quantified this further, showing the trichromatic vision of 43 different taxa of hymenopterans with the sensitivity peak of UV light at 340 nm, blue light at 430 nm and yellow light at 535 nm. Some specific colour patterns of flowers, such as floral guides, are of such importance for bee flower recognition that they were included into melittophily, i.e. the pollination syndrome related to bees (Faegri and van der Pijl 1979; Lunau 1992a; Fenster et al. 2004; Willmer 2011). However, the utility of these syndromes has been questioned recently (Ollerton et al. 2009), and some authors started using more precise systems, such as looking at single explanatory traits (e.g. Stang et al. 2009) or buzz pollination (e.g. De Luca and Vallejo-Marín 2013). The importance of UV patterns for bees was confirmed by a decreased frequency of flower visitation by different bee species after elimination of the UV reflection from petals of various plant species (Johnson and Andersson 2002; Peter and Johnson 2008; Welsford and Johnson 2012; Rae and Vamosi 2013; Brock et al. 2016).
Nevertheless, floral colour evolution has been influenced by numerous other factors (Grimaldi 1999; Friis et al. 2011; Song et al. 2015), such as floral defence against solar radiation (Robberecht and Caldwell 1978; Koski and Ashman 2015a). It was shown that the absorbance of UV-A by plant tissues can be related to plant protection against harmful UV-B radiation (Robberecht and Caldwell 1978; Caldwell et al. 1983). The importance of UV colour reflectance and absorbance can be manifested along the gradient of UV irradiation, e.g. towards high altitudes and the equator (Johnson et al. 1976; Beckmann et al. 2014; Koski and Ashman 2015a). It was shown that UV irradiance as the selection agent affects the size of the UV-absorbing floral centre (bullseye), with increases towards the equatorial ecosystems and along altitudinal gradients as well (Koski and Ashman 2015a, b). Despite long-term research on floral UV signalling many questions remain unanswered. For example, it is unclear if the ability of pollinators to recognize the flower is caused by any UV-reflecting area on the flower or if it is related to specific UV patterns. Additionally, we only have limited knowledge on how the common experimental manipulation using UV-absorbing creams (e.g. Johnson and Andersson 2002; Peter and Johnson 2008; Welsford and Johnson 2012; Rae and Vamosi 2013) generally affects the natural (i.e. unmanipulated) pollination system of the studied plant species.
To study the role of UV reflection in pollination we selected Hypoxis camerooniana (Hypoxidaceae) as model species. Hypoxis camerooniana is endemic to the mountains of south-western Nigeria and western Cameroon (Hutchinson et al. 1968) and therefore, better understanding its pollination system can help to better focus potential conservation efforts for both plant and its pollinators. Due to growing at low latitudes and high altitudes (above 2000 m a.s.l. on Mount Cameroon), H. camerooniana is exposed to intensive UV irradiance (Johnson et al. 1976; Beckmann et al. 2014). It has yellow UV-reflecting petals (Fig. 1) and yellow UV-absorbing anthers, consequently creating a contrasting central pattern in the flower. Firstly, we aimed to gain insights in the unknown pollination system of this endemic plant. Secondly, to study the role of UV colour on the visitation frequency, behaviour and pollination success of H. camerooniana, we used the same approach as Johnson and Andersson (2002) by manipulating flowers with an UV-absorbing cream either by complete removal of the UV reflectance, or by maintaining the UV reflectance on half of petals, i.e. changing the UV pattern (Fig. 1). Our study extends the previous work of, e.g., Johnson and Andersson (2002) by including a natural unmanipulated control to test the influence of the experimental treatments on the flower visitation frequency.
Figure 1.
A flower of H. camerooniana: (A) a normal photograph, (B) a UV photograph of a non-manipulated flower, (C) a UV photograph with half absorbent cream treatment (UV50 %), (D) a UV photograph with full absorbent cream (UV100 %) treatment.
Materials and Methods
Study locality
This study was carried out at the montane grasslands above Mann’s Spring (~2250 m a.s.l.) on Mount Cameroon, the highest mountain in western and central sub-Saharan Africa (4095 m a.s.l.; Cable and Cheek 1998). It is situated in the Southwest region of Cameroon (4.203°N and 9.170°E), offering a wide range of habitats (see Cable and Cheek 1998) and hosting a great biodiversity including endemics with exceptional ecological features (Bergl et al. 2007). Especially ecotones along the timberline, found also at Mann’s Spring, harbour many species which are not found elsewhere on the mountain
Study plant
The genus Hypoxis contains an estimated 90 species in Africa, North and South America, South-Eastern Asia and Australia, with the centre of diversity in Southern Africa (Singh 1999). These plants are characterized by their bright yellow flowers, lanceolate and densely hairy leaves. They are weak competitors and thus grow mostly in places with low vegetation cover. Hypoxis camerooniana (Hypoxidaceae) is a perennial pyrophytic herb, restricted to high elevations of the Cameroonian Volcanic Line (Cable and Cheek 1998; GBIF Secretariat 2019). Its leaves are tristichous, 50 cm long and 0.5–2 cm wide, covered with golden hairs, recurved and ± prostrate to erect. On a scape up to 25 cm tall 5–7 flowers can be found (African Plant Database 2019). On Mount Cameroon, we always observed only 1 or 2 active flowers per plant. The flowers have a short lifespan, they open at daybreak and usually last 1 day or less.
Hypoxis species are used across Africa as traditional medicine and were reported to have a wide spectrum of pharmacological properties (Ncube et al. 2013). Hypoxis hemerocallidea has already been known for its strong UV reflectance and due to its relatively robust flowers was used for manipulative experiments with UV-absorbing cream (Johnson and Andersson 2002). Outside of the two sweet-smelling species H. fischerii var. zernyi and H. goetzei East African Hypoxis flowers appeared to be without scent (Wiland-Szymańska 2009). So far, no nectar has been found in any Hypoxis species (Johnson and Andersson 2002; Rudall 2002; Ren et al. 2019).
Some studied Hypoxis species are pollinated by solitary bees and honeybees (Singh 1999; Johnson and Andersson 2002), while the autogamous H. decumbens attracts ‘generalist insects, like dipterans’ (Raimúndez and Ramirez 1998). Furthermore, pollen- and tepal-feeding beetles were observed in Southern and Eastern Africa (Steiner 1998; Wiland-Szymańska 2009). No data on pollination and visitation of H. camerooniana exist.
UV manipulation
The study was carried out in October and November 2016. To manipulate the UV patterns on flowers, we followed the approach of Johnson and Andersson (2002). The studied specimens of H. camerooniana were randomly selected in the study area. At daybreak, just after opening of selected buds, four different treatments were applied: (i) UV100 %treatment, i.e. complete removal of UV reflectance from the flower using UV cream on all petals of the flower; (ii) FAT100 %treatment, i.e. a control for UV100 %, all petals were covered with duck preen gland fat, a non-UV-absorbing cream compound; (iii) UV50 %treatment, i.e. the UV cream was used on three out of six petals, covering every second petal; and (iv) FAT50 %treatment, i.e. a control for UV50 %, every second petal was treated with the non-UV-absorbing compound. Both UV cream and non-UV-absorbing cream were applied carefully using cotton swabs. Besides these four treatments, natural (Natural control) non-manipulated flowers were studied to control for the effect of any treatment on flowers. The UV-absorbing chemicals were equal amounts of Parsol 1789® (butyl methoxydibenzoylmethane) and Parsol MCX® (ethylhexyl methoxycinnamate) dissolved in the duck preen gland fat as a solvent (at 40:60 w/w) by gentle heating (Andersson and Amundsen 1997; Johnson and Andersson 2002). On each day of the experiment, 10 plant specimens were selected in the grasslands and randomly treated, two replicates of each treatment per day, resulting in a total of 50 experimental plants. When two flowers were found on a single experimental plant, we applied the same treatment for both. Each experimental flower was recorded by a security camera (VIVOTEK IB8367-T with IR night vision) for 24 h following Mertens et al. (2018); however, most of the flowers were short-lived and closed at the beginning of the night after ~12 h of recording. Due to the short lifespan of flowers, all their visitors were certainly observed. Afterwards the recordings were watched, and all floral visitors were noted. Besides arrival of visitors we also identified them to the most detailed taxon level as possible, and we noted their behaviour (both landing behaviour and activity after landing, e.g. feeding on pollen) and touches to reproductive organs, which allows us to better distinguish between visitors and potential pollinators.
After camera removal, stigmas of the recorded flowers were collected and stored in ethyl alcohol. Germinated pollen tubes were stained and counted later in the lab using fluorescence microscopy following the methods described by Dafni et al. (2005) to see how changes in visitation frequency potentially caused by the experimental treatment affect the plant’s pollination success.
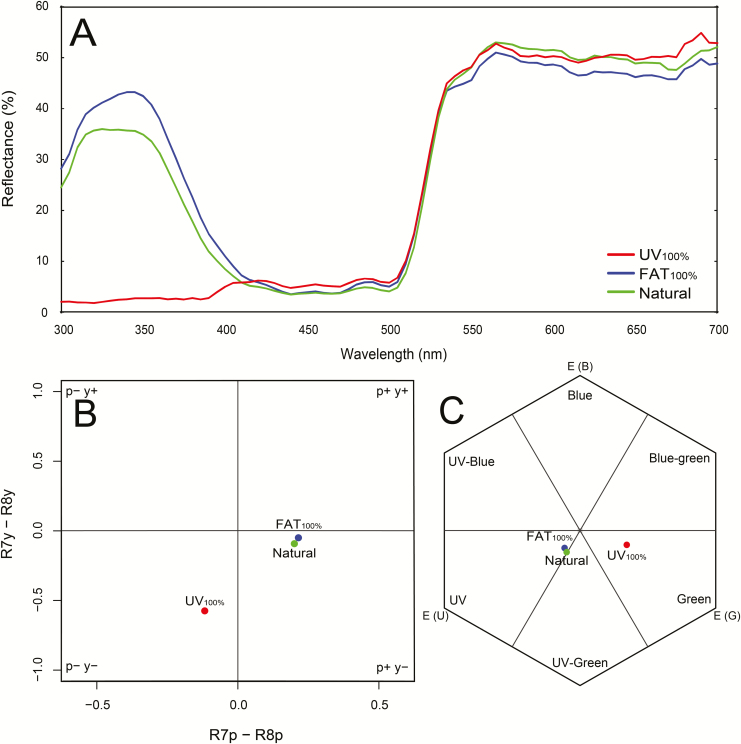
To check the floral UV pattern, UV photographs were taken using a Canon EOS 80D DSLR camera with a Helios 44-2 lens; UV conversion (i.e. replacing the internal hot mirror filter by a custom UV band pass filter) was done by LifePixel (Mukilteo, USA). During the picture taking, a 5-W UV flashlight was used for lighting (Fig. 1). To demonstrate the effect of experimental treatment, we measured reflectance of three flowers per treatment type (FAT100 %, Natural control and UV100 %) with 10 repeated measures per flower, using an Ocean Optics (Largo, USA) Jaz spectrometer. The graph depicts the mean of the repeated measures per treatment for the range of 300–700 nm (Fig. 2A). The bee and fly colour visual system was then mapped using the Troje model for flies (Troje 1993; Fig. 2B) and the colour hexagon for bees (Chittka 1992; Fig. 2C).
Figure 2.
(A) reflectance of natural H. camerooniana flowers and those with experimental treatment and control (see Materials and Methods for more details). (B) Fly colour visual system displayed using the Troje model. (C) Bee colour visual system displayed using the colour hexagon model.
Statistical analyses
To standardize the sampling effort (differences in flower longevity, as well as in the case of one or two flowers per experimental plant), all visits were transformed to visitation frequencies (no. of visits per hour and flower). This visitation frequency data (no. of visits per hour and flower) did not show normal distribution due to an overabundance of null values. In consequence, we used non-parametrical tests, being a permutational analogue of ANOVA and MANOVA in PRIMER 6 v. 6.1.13 and Permanova+ v. 1.0.3 (Anderson et al. 2008). Post hoc tests were used to compare the frequencies between the different treatments, with the recording day treated as random effect. Similarly, the effects of treatments on insect behaviour were tested by a permutational MANOVA. To check differences in amount of morphospecies and pollen tubes (i.e. the non-frequency data) we tried to implement generalized mixed-effect models, specifically Poisson, quasi-Poisson and zero-inflated distributions. However, due to a combination of the high overabundance of zero values and the negative values of the maximum likelihood estimations of the models, we were not able to apply these parametric methods and therefore, the non-parametrical tests (permutational analogue of ANOVA) were applied as well. The dependency of the number of pollen tubes on visitation frequency was tested by linear regression in STATISTICA (Statsoft, Inc 2011).
Results
Visitors of H. camerooniana
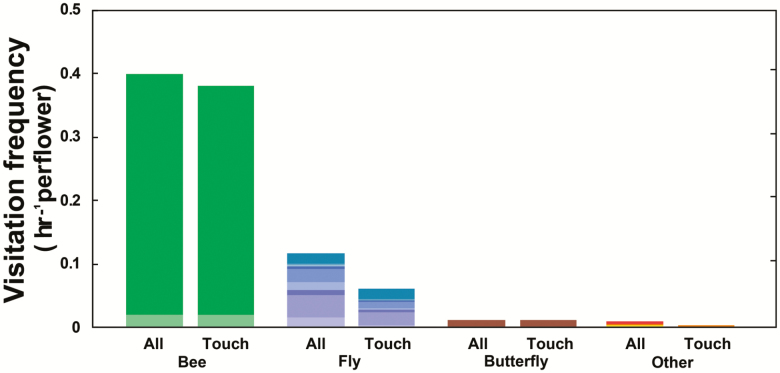
Considering all 50 observed plants, a total of 281 visitors were recorded. During daytime the flowers were mostly visited by bees (192 visits) and flies (59 visits), the only other considerable group of visitors were skipper butterflies (four visits). All other visitors (five visits) were evaluated as accidental and thus merged (Fig. 3). All bee visitors were composed of a single abundant morphospecies of solitary bee (187 visits) and the substantially rarer honeybee (Apis mellifera; five visits). The less abundant flies were considerably more taxonomically diverse, compared to bees, with nine recognized morphospecies. Bees visited the studied flowers mainly during morning hours, whereas fly visitation was distributed throughout the day [seeSupporting Information—Figs S2 and S3]. Contrastingly, night visitors were rare (21 visits by 10 morphospecies) and consequently, with much lower visitation frequencies [seeSupporting Information—Fig. S1]. Based on contacts with reproductive organs (Fig. 3; Table 1) bees may be considered as the main pollinator.
Figure 3.
Frequency of all H. camerooniana flower visitors and visitors in contact with reproductive organs during daytime. Different colours indicate different morphospecies.
Table 1.
Proportion of visits during which bees and flies touched reproductive organs of H. camerooniana. See Materials and Methods for the description of treatments.
| Bees | Flies | |||
|---|---|---|---|---|
| Treatment | Stigma | Anthers | Stigma | Anthers |
| UV 100 % | 85.7 | 92.9 | 0.0 | 57.1 |
| F 100 % | 76.7 | 95.3 | 15.4 | 23.1 |
| UV 50 % | 59.4 | 90.6 | 28.6 | 57.1 |
| F 50 % | 84.8 | 97.0 | 47.6 | 57.1 |
| Natural | 74.3 | 95.7 | 45.5 | 63.6 |
Effects of UV pattern on visitors
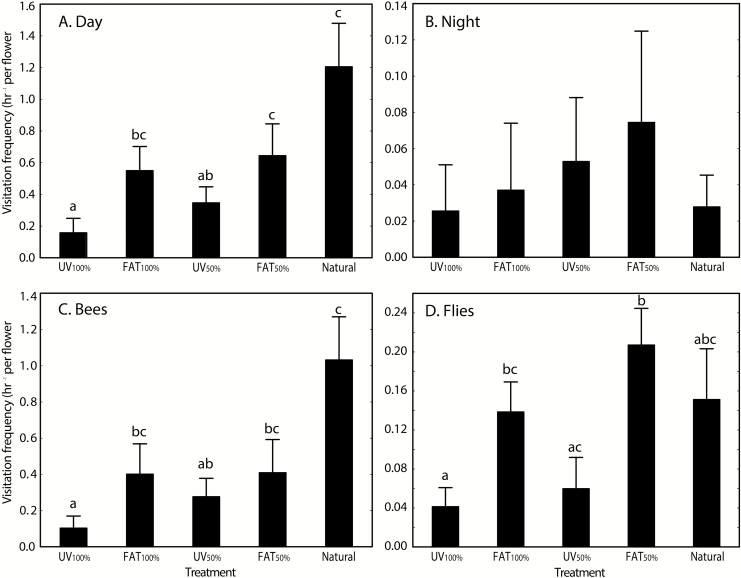
Individual treatments significantly affected visitation frequency during the day (Fps = 6.71; df = 4; Pperm < 0.001). UV100 % was significantly lower than all other treatments except UV50 %, which differed from FAT50 % and Natural control. The highest visitation frequency was observed on untreated plants, but these did not significantly differ from the other two control treatments (FAT100 %, FAT50 %; Fig. 4). During the night, there was no significant treatment effect on frequency of flower visitors (Fps = 0.36; df = 4; Pperm = 0.851). Visitation frequency was significantly affected by the treatment for both bees (Fps = 6.13; df = 4; Pperm < 0.001) and flies (Fps = 3.92; df = 4; Pperm = 0.009). In both visiting groups, FAT100 % and FAT50 % treatment has a significantly higher frequency than UV100 %, but UV50 % was significantly lower than FAT50 % for flies only (Fig. 4). The non-treated control (Natural control) significantly differed from UV-manipulated plants for bees only (Fig. 4). There was no significant effect of treatment on the number of morphospecies observed on the flowers (Fps = 2.08; df = 4; Pperm = 0.103).
Figure 4.
Effect of UV pattern manipulation on visitation frequencies of H. camerooniana for (A) day; (B) night; (C) bee; and (D) fly visitation during the day separately. Note: scaling of the Y-axis is not standardized due to the substantially lower number of visits between day and night, and between bees and flies. Means (bars) and SE (whiskers) are shown. The same letters above the columns indicate non-significant differences in the pairwise post hoc tests. See Materials and Methods for the description of treatments.
Effects of treatments on visitor behaviour
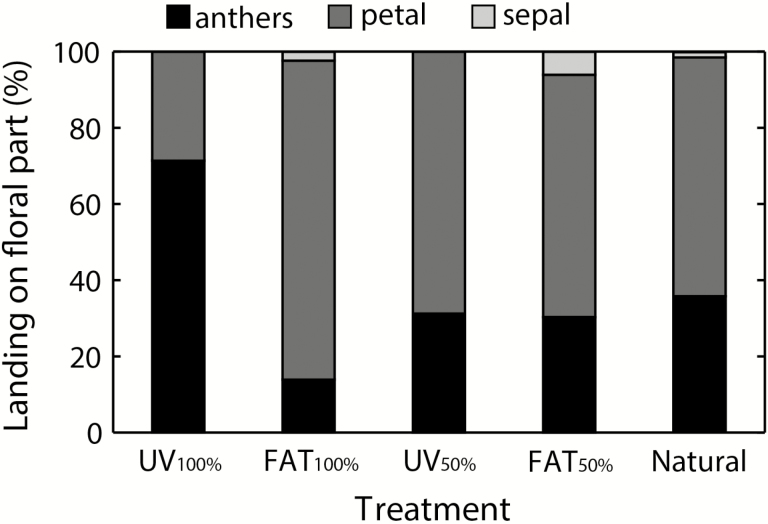
We found a significant effect of treatment on bee landing behaviour (Fps = 5.04; df = 4; Pperm = 0.004), but not on fly landing (Fps = 1.08; df = 4; Pperm = 0.373). On UV100 %-treated flowers, bees landed mostly on anthers, whereas in other treatments bees usually landed on the petals before moving to anthers and stigma (Fig. 5). When collecting pollen, bees usually touched both anthers and stigmas, whereas flies had considerably fewer contacts with the reproductive organs during their visits (Table 1). There was no significant effect of treatment on bees (Fps = 0.49; df = 4; Pperm = 0.770) and flies (Fps = 0.44; df = 4; Pperm = 0.903) behaviour after landing. Bees spent 95 % of the flower visit duration by collecting pollen, while flies spent most time (68 %) crawling, sitting and flying between individual floral parts [seeSupporting Information—Fig. S4].
Figure 5.
Effect of UV pattern manipulation on bee landing behaviour of H. camerooniana flowers. See Materials and Methods for the description of treatments.
Effect of treatment on the plant
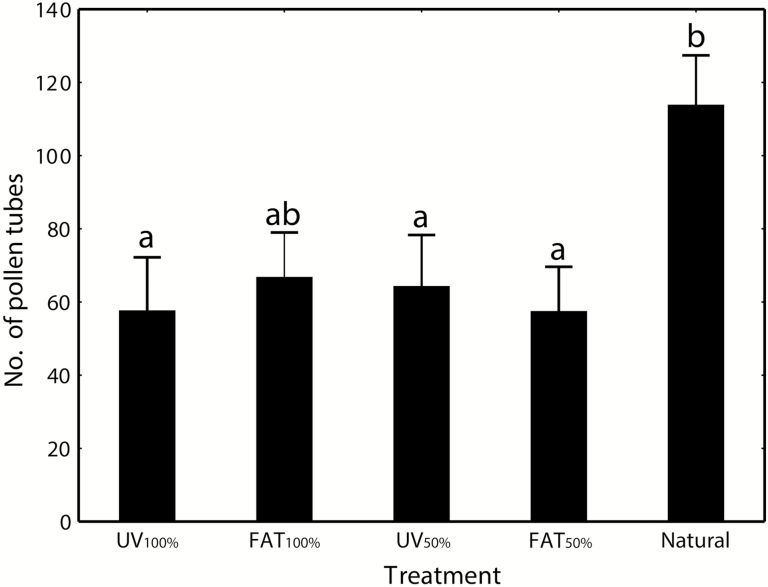
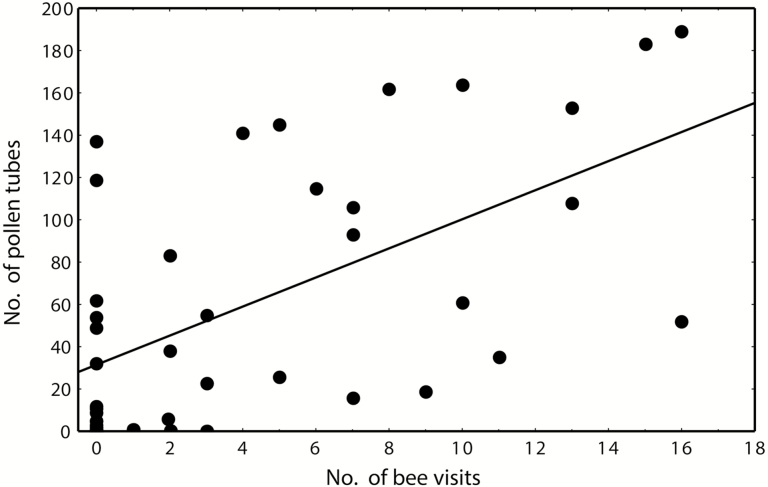
The number of germinated pollen tubes significantly differed among treatments (Fps = 3.66; df = 4; Pperm = 0.010), mainly due to a significantly higher number of pollen tubes germinated in non-manipulated flowers (Fig. 6). The pollen tube count increases with number of visits by bees (r = 0.57, P < 0.001; Fig. 7), but not of flies (r = −0.0073, P = 0.962).
Figure 6.
Effect of UV pattern manipulation on the number of germinated pollen tubes in stigmas of H. camerooniana. Means (bars) and SE (whiskers) are shown. The same letters above the columns indicate non-significant differences in the pairwise post hoc tests.
Figure 7.
Effect of number of bee visits on number of germinated pollen tubes in stigmas of H. camerooniana.
Discussion
Our study demonstrated that H. camerooniana is mainly pollinated by bees, confirming previous studies on pollination of Hypoxis plants (Singh 1999; Johnson and Andersson 2002), with the notable exception of H. decumbens, pollinated by flies in Venezuela (Raimúndez and Ramirez 1998). In addition to the previous studies of the genus based on visitation rates, we have confirmed that bees are the most efficient pollinators of H. camerooniana since: (i) bees were the most common visitors; (ii) in a large percentage of visits they are in contact with reproductive organs, which is expectable for these voracious pollen feeders; and (iii) their visits significantly increased numbers of germinated pollen tubes in stigmas. Contrary to Johnson and Andersson’s (2002) observations on H. hemerocallidea in South Africa, there were only few visits of honeybees compared to the abundant visits by a single morphospecies of small solitary bees. Moreover, honeybees seemed to be mostly searching for nectar, although we did not observe any nectar in flowers of H. camerooniana, consistent with other Hypoxis species (Johnson and Andersson 2002; Rudall 2002; Ren et al. 2019).
UV and visitor frequency
The UV signal of flowers influenced the particular visitor frequency in different ways. Bees visited flowers more often when at least half of the petals reflected UV. However, although not significant, even the control flowers treated with non-absorbing cream differed in bee (but not fly) visitation frequencies from untreated flowers. The drop in visitation frequency between the treatments and their respective controls is consistent with the previous study of H. hemerocallidea (Johnson and Andersson 2002) in which fewer honeybees (A. mellifera) visited flowers with the floral UV reflectance obscured. While in alpine communities of New Zealand, Campbell et al. (2010) found experimentally manipulated flower colour, and not UV reflectance, to be more important for the visitation rates, other studies on UV pollinator visitation preferences showed similar results to ours (Peter and Johnson 2008; Rae and Vamosi 2013; Horth et al. 2014; Koski and Ashman 2014). Ultraviolet reflectance was revealed as highly important for bees pollinating Eulophia zeyheriana (Orchidaceae; Peter and Johnson 2008), whereas the general visitation rates of various visitors declined after manipulation of UV reflectance in two Rudbeckia species (Horth et al. 2014), Mimulus guttatus (Phrymaceae; Rae and Vamosi 2013), and Argentina anserine (Rosaceae; Koski and Ashman 2014). Therefore, UV reflectance plays an important role in pollinator attraction, but it can differ among flowering species, since other floral traits, such as scent, shape and colour, could be equally important.
Additionally, we have shown that having at least some UV reflectance is more important for selection of H. camerooniana flowers by bees than its UV pattern (sensuKoski and Ashman 2014), as flowers with fully covered petals by the UV-absorbing cream differed in bee visitation from those with all petals fully reflecting UV. Flies, however, although also showing a higher visitation frequency when the flower is fully or partially reflecting UV, did not significantly differentiate between UV100 % and the Natural control. These results are discordant with the previous study of yellow UV-reflecting flowers of A. anserine (Koski and Ashman 2014) which demonstrated that the presence of UV pattern increased visitation by both bees and syrphid flies relative to both fully UV reflective or absorptive flowers. Nevertheless, bees were repeatedly described to prefer flowers with some colour pattern above the unicoloured ones (e.g. Waser and Price 1985; Papiorek et al. 2016).
It must be stipulated that UV reflectance is just one channel of communication of plants with insects (Chittka et al. 1994; Johnson and Andersson 2002). Additionally, other traits or factors need to be considered when looking at the pollination system. For example, the community context (Peter and Johnson 2008; Campbell et al. 2010), positioning of flowers and inflorescences (Lunau 1992b; Johnson et al. 2003a, b; Rae and Vamosi 2013; van der Kooi 2016), other optical principles of flower colouration (e.g. van der Kooi et al. 2014, 2016) and learning ability of visitors (Giurfa et al. 1995; Hammer 1997; Dyer et al. 2015). The learning ability of visitors is difficult to include into such field experimental studies. Supplementary controlled studies with naïve bees would be greatly beneficial to further disentangle the factors important in shaping the studied pollination system.
UV and bee behaviour
The significant difference in the bee landing behaviour implies that the floral UV pattern can play an important role in orientation of bees on visited flowers. On the flowers completely covered with the UV-absorbing cream, bees mostly landed directly on the anthers and immediately started to collect pollen, whilst they landed mostly on petals of the flowers that at least partly reflected UV (i.e. all other treatments). This has proven that a disturbance of the UV pattern may change bees’ behaviour. Likewise, other colour patterns, such as floral guides or bullseye patterns, are considered to increase the plants’ reproductive success by helping pollinators to orientate to the flower centre (Waser and Price 1985; Dinkel and Lunau 2001; Leonard and Papaj 2011; Papiorek et al. 2016). However, bees actually make their first antennal contact preferably at the UV-absorbing floral area, irrespective of its spatial position within a flower (Papiorek et al. 2016). Therefore, one would expect bee visitors of H. camerooniana to prefer the centre of flowers with the UV-absorbing anthers, which is not the case. We thus hypothesize that in H. camerooniana, the UV-reflecting petals probably act as a landing platform, making flowers more visible for potential pollinators in its typical habitat of burnt montane grasslands, since the general UV reflection of similar grasslands vegetation is low (<5 %; Caldwell et al. 1983).
Methodological biases of UV manipulation
When Johnson and Andersson (2002) used the genus Hypoxis for the experimental manipulation of floral UV reflectance to study the response of insect pollinators, they did not include the experimentally untreated plants (Natural control). They thus did not control for the effect of experimental manipulation on natural insect behaviour. In our experiment, which based the methodology largely on the referred study, we demonstrated that such experimental setting is useful to investigate the effect of floral UV signalling on visitors. But at the same time, we discovered that experimental controls (i.e. flowers covered by the non-UV-absorbing cream compound; FAT100 %, FAT50 %) can differ from the untreated natural flowers. The experimental controls showed lower (but not statistically significant) visitation rates than the natural control for bees. Furthermore, the numbers of germinated pollen tubes on stigmas of natural control flowers of H. camerooniana were significantly higher compared to all treated flowers, apart from the control with fully covered non-UV-absorbing cream. These lower visitation rates and lower number of germinated pollen tubes could be explained by several factors, e.g. less evaporation of scents or changes in the glossiness of the flower. It also proved that we did not cause pollination during handling of experimental flowers.
Additionally, we showed that this effect can be visitor-specific. Flies, generally a more olfactory-oriented group than bees (Roy and Raguso 1997), were not affected by the experimental controls at all. For this group, the UV manipulation treatments (UV100 %, UV50 %) did not significantly differ from the Natural control. Although, they were visited by significantly less flies than the controls (FAT100 %, FAT50 %). We thus speculate that the effect of UV manipulation is at least partly compensated by the potential attraction of Muscidae and Sarcophagidae flies (which created most of the diversity of recorded fly visitors) to the duck preen gland fat. Bees, as generally more visually oriented insects, expressed higher, but still non-significant, differences among natural and experimental controls. Furthermore, the thickness of the cream layer or amount of cream in the treatments has not been considered in our study but might play a role in the attraction of flies.
In summary, although the duck preen gland fat is a commonly used vector for the UV-manipulating agents (e.g. Johnson and Andersson 2002; Peter and Johnson 2008; Welsford and Johnson 2012; Rae and Vamosi 2013), it affects natural insect behaviour. Consequently, we strongly encourage ‘calibration’ of results by controlling for the chemical vector’s (duck preen gland fat in our case) effects in similar experimental studies.
Conclusion
The primary pollinators of H. camerooniana in the Afromontane grasslands of Mount Cameroon were bees. When UV reflectance was completely removed visitation rates of bees decreased, whereas the decrease of frequency on half-treated flowers was not significant (although it decreased as well when considering all daytime visitors). The complete UV reflectance removal changed the landing behaviour of bees as well, confirming that altering the natural UV patterns affects both visitation rates and behaviour.
Furthermore, based on our results we also encourage the inclusion of a natural control in the experimental designs of similar manipulative studies to control for the substances used in floral manipulation.
Supporting Information
The following additional information is available in the online version of this article—
Figure S1. Frequency of nocturnal visits of Hypoxis camerooniana flowers.
Figure S2. Diurnal changes in bee visitation frequencies on flowers of Hypoxis camerooniana after manipulation of their ultraviolet (UV) reflectance. Means (bars) and SE (whiskers) are shown.
Figure S3. Diurnal changes in fly visitation frequencies on flowers of Hypoxis camerooniana after manipulation of their ultraviolet (UV) reflectance. Means (bars) and SE (whiskers) are shown.
Figure S4. Bee (A) and fly (B) behaviour on flowers of Hypoxis camerooniana after manipulation of their ultraviolet (UV) reflectance. There was no significant effect of treatment on both bee (Fps = 0.49; df = 4; Pperm = 0.770) and fly (Fps = 0.44; df = 4; Pperm = 0.903) behaviour after landing.
Data
An excel file with the data used for analyses and graphs is available in the online version of this article.
Sources of Funding
This project was funded by the Czech Science Foundation (16‐11164Y), the Grant Agency of the Charles University (GAUK No. 356217) and the Charles University (PRIMUS/17/SCI/8 and UNCE204069).
Contributions by the authors
All authors helped design the experiment, Y.K., R.D.K., M.B., J.E.J.M. and S.J. were involved in data collection; R.D.K., S.J., R.T. and Y.K. were in charge of data analyses and writing first drafts of the manuscripts. All authors contributed to writing and editing of the manuscript.
Conflict of Interest
None declared.
Acknowledgements
The authors are grateful to V. Maicher, F. E. Luma, H. Andukwa and our local field assistants for help in the field. Furthermore, we would like to thank the staff of the Mount Cameroon National Park for their support. This study was performed with the authorizations of the Republic of Cameroon Ministries for Forestry and Wildlife and for Scientific Research and Innovation.
Citation: Klomberg Y, Dywou Kouede R, Bartoš M, Mertens JEJ, Tropek R, Fokam EB, Janeček Š. 2019. The role of ultraviolet reflectance and pattern in the pollination system of Hypoxis camerooniana (Hypoxidaceae). AoB PLANTS11: plz057; doi: 10.1093/aobpla/plz057
Literature Cited
- African Plant Database 2019. Conservatoire et Jardin botaniques de la Ville de Genève and South African National Biodiversity Institute, Pretoria. Version 3.4.0.http://www.ville-ge.ch/musinfo/bd/cjb/africa/. (30 January 2019).
- Andersson S, Amundsen T. 1997. Ultraviolet colour vision and ornamentation in bluethroats. Proceedings of the Royal Society B: Biological Sciences 264:1587–1591. [Google Scholar]
- Anderson MJ, Gorley RN, Clarke KR. 2008. PERMANOVA+ for PRIMER: guide to software and statistical methods. Plymouth, UK: PRIMER-E [Google Scholar]
- Beckmann M, Václavík T, Manceur AM, Šprtová L, von Wehrden H, Welk E, Cord AF. 2014. glUV: a global UV-B radiation data set for macroecological studies. Methods in Ecology and Evolution 5:372–383. [Google Scholar]
- Bergl RA, Oates JF, Fotso R. 2007. Distribution and protected area coverage of endemic taxa in West Africa’s Biafran forests and highlands. Biological Conservation 134:195–208. [Google Scholar]
- Briscoe AD, Chittka L. 2001. The evolution of color vision in insects. Annual Review of Entomology 46:471–510. [DOI] [PubMed] [Google Scholar]
- Brock MT, Lucas LK, Anderson NA, Rubin MJ, Markelz RJ, Covington MF, Devisetty UK, Chapple C, Maloof JN, Weinig C. 2016. Genetic architecture, biochemical underpinnings and ecological impact of floral UV patterning. Molecular Ecology 25:1122–1140. [DOI] [PubMed] [Google Scholar]
- Burr B, Rosen D, Barthlott W. 1995. Untersuchungen zur Ultraviolett reflexion von Angiospermenblüten. III. Dilleniidae und Asteridae. Tropische und Subtropische Pflanzenwelt 93:1–185. [Google Scholar]
- Cable S, Cheek M. 1998. The plants of Mount Cameroon, a conservation checklist. Kew, UK: Royal Botanic Gardens, Kew. [Google Scholar]
- Caldwell MM, Robberecht R, Flint SD. 1983. Internal filters: prospects for UV‐acclimation in higher plants. Physiologia Plantarum 58:445–450. [Google Scholar]
- Campbell DR, Bischoff M, Lord JM, Robertson AW. 2010. Flower color influences insect visitation in alpine New Zealand. Ecology 91:2638–2649. [DOI] [PubMed] [Google Scholar]
- Chittka L. 1992. The colour hexagon: a chromaticity diagram based on photoreceptor excitations as a generalized representation of colour opponency. Journal of Comparative Physiology A 170:533–543. [Google Scholar]
- Chittka L, Shmida A, Troje N, Menzel R. 1994. Ultraviolet as a component of flower reflections, and the colour perception of Hymenoptera. Vision Research 34:1489–1508. [DOI] [PubMed] [Google Scholar]
- Dafni A, Pacini E, Nepi M. 2005. Pollen and stigma biology. In: Dafni A, Kevan PG, Husband BC, eds. Practical pollination biology. Cambridge, ON, Canada: Enviroquest Ltd, 83–146. [Google Scholar]
- De Luca PA, Vallejo-Marín M. 2013. What’s the “buzz” about? The ecology and evolutionary significance of buzz-pollination. Current Opinion in Plant Biology 16:429–435. [DOI] [PubMed] [Google Scholar]
- Dinkel T, Lunau K. 2001. How drone flies (Eristalis tenax L., Syrphidae, Diptera) use floral guides to locate food sources. Journal of Insect Physiology 47:1111–1118. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Garcia JE, Shrestha M, Lunau K. 2015. Seeing in colour: a hundred years of studies on bee vision since the work of the Nobel laureate Karl Von Frisch. Proceedings of the Royal Society of Victoria 127:66–72. [Google Scholar]
- Faegri K, van der Pijl L. 1979. The principles of pollination ecology. Pergamon Press. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. 2004. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution, and Systematics 35:375–403. [Google Scholar]
- Friis EM, Crane PR, Pedersen KR. 2011. Early flowers and angiosperm evolution. Cambridge, UK: Cambridge University Press. [Google Scholar]
- GBIF Secretariat 2019. Hypoxis Camerooniana Baker, GBIF Backbone Taxonomy. https://www.gbif.org/species/2852906(11 January 2019).
- Giurfa M, Núñez J, Chittka L, Menzel R. 1995. Colour preferences of flower-naive honeybees. Journal of Comparative Physiology A 177:247–259. [Google Scholar]
- Grimaldi D. 1999. The co-radiations of pollinating insects and angiosperms in the cretaceous. Annals of the Missouri Botanical Garden 86:373. [Google Scholar]
- Hammer M. 1997. The neural basis of associative reward learning in honeybees. Trends in Neurosciences 20:245–252. [DOI] [PubMed] [Google Scholar]
- Hansen DM, van der Niet T, Johnson SD. 2012. Floral signposts: testing the significance of visual “nectar guides” for pollinator behaviour and plant fitness. Proceedings of the Royal Society B: Biological Sciences 279:634–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horth L, Campbell L, Bray R. 2014. Wild bees preferentially visit Rudbeckia flower heads with exaggerated ultraviolet absorbing floral guides. Biology Open 3:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson J, Dalziel JM, Keay RWJ. 1968. The flora of West Tropical Africa. London, UK: Crown Agents for Overseas Governments and Administrations. [Google Scholar]
- Johnson SD, Alexandersson R, Linder HP. 2003a. Experimental and phylogenetic evidence for floral mimicry in a guild of fly-pollinated plants. Biological Journal of the Linnean Society 80:289–304. [Google Scholar]
- Johnson SD, Andersson S. 2002. A simple field method for manipulating ultraviolet reflectance of flowers. Canadian Journal of Botany 80:1325–1328. [Google Scholar]
- Johnson FS, Mo T, Green AE. 1976. Average latitudinal variation in ultraviolet radiation at the earth’s surface. Photochemistry and Photobiology 23:179–188. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Peter CI, Nilsson LA, Ågren J. 2003b. Pollination success in a deceptive orchid is enhanced by co-occurring rewarding magnet plants. Ecology 84:2919–2927. [Google Scholar]
- Koski MH, Ashman TL. 2014. Dissecting pollinator responses to a ubiquitous ultraviolet floral pattern in the wild. Functional Ecology 28:868–877. [Google Scholar]
- Koski MH, Ashman TL. 2015a. Floral pigmentation patterns provide an example of Gloger’s rule in plants. Nature Plants 1:14007. [DOI] [PubMed] [Google Scholar]
- Koski MH, Ashman TL. 2015b. An altitudinal cline in UV floral pattern corresponds with a behavioral change of a generalist pollinator assemblage. Ecology 96:3343–3353. [DOI] [PubMed] [Google Scholar]
- Leonard AS, Papaj DR. 2011. “X” marks the spot: the possible benefits of nectar guides to bees and plants. Functional Ecology 25:1293–1301. [Google Scholar]
- Lunau K. 1992a. Innate recognition of flowers by bumble bees: orientation of antennae to visual stamen signals. Canadian Journal of Zoology 70:2139–2144. [Google Scholar]
- Lunau K. 1992b. A new interpretation of flower guide colouration: absorption of ultraviolet light enhances colour saturation. Plant Systematics and Evolution 183:51–65. [Google Scholar]
- Lunau K. 2006. Stamens and mimic stamens as components of floral colour patterns. Botanische Jahrbücher 127:13–41. [Google Scholar]
- Lunau K, Konzmann S, Winter L, Kamphausen V, Ren ZX. 2017. Pollen and stamen mimicry: the alpine flora as a case study. Arthropod-Plant Interactions 11:427–447. [Google Scholar]
- Mertens JEJ, Tropek R, Dzekashu FF, Maicher V, Fokam EB, Janeček Š. 2018. Communities of flower visitors of Uvariopsis dioica (Annonaceae) in lowland forests of Mt. Cameroon, with notes on its potential pollinators. African Journal of Ecology 56:146–152. [Google Scholar]
- Ncube B, Ndhlala AR, Okem A, Van Staden J. 2013. Hypoxis (Hypoxidaceae) in African traditional medicine. Journal of Ethnopharmacology 150:818–827. [DOI] [PubMed] [Google Scholar]
- Ollerton J, Alarcón R, Waser NM, Price MV, Watts S, Cranmer L, Hingston A, Peter CI, Rotenberry J. 2009. A global test of the pollination syndrome hypothesis. Annals of Botany 103:1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papiorek S, Junker RR, Alves-Dos-Santos I, Melo GA, Amaral-Neto LP, Sazima M, Wolowski M, Freitas L, Lunau K. 2016. Bees, birds and yellow flowers: pollinator-dependent convergent evolution of UV patterns. Plant Biology 18:46–55. [DOI] [PubMed] [Google Scholar]
- Peitsch D, Fietz A, Hertel H, de Souza J, Ventura DF, Menzel R. 1992. The spectral input systems of hymenopteran insects and their receptor-based colour vision. Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology 170:23–40. [DOI] [PubMed] [Google Scholar]
- Penny JHJ. 1983. Nectar guide colour contrast: a possible relationship with pollination strategy. The New Phytologist 95:707–721. [Google Scholar]
- Peter CI, Johnson SD. 2008. Mimics and magnets: the importance of color and ecological facilitation in floral deception. Ecology 89:1583–1595. [DOI] [PubMed] [Google Scholar]
- Peterson ML, Miller TJ, Kay KM. 2015. An ultraviolet floral polymorphism associated with life history drives pollinator discrimination in Mimulus guttatus. American Journal of Botany 102:396–406. [DOI] [PubMed] [Google Scholar]
- Rae JM, Vamosi JC. 2013. Ultraviolet reflectance mediates pollinator visitation in Mimulus guttatus. Plant Species Biology 28:177–184. [Google Scholar]
- Raimúndez E, Ramirez N. 1998. Estrategia reproductiva de una hierba perenne: Hypoxis decumbens (Hypoxidaceae). Revista de Biologia Tropical 46:555–565. [Google Scholar]
- Ren ZX, Bernhardt P, Edens-Meier R, Zweck J, Arduser M, Li HD, Wang H. 2019. Comparative pollen–pistil interactions and insect pollination in two Hypoxis species (Hypoxidaceae) in China and North America. Plant Systematics and Evolution 305:115–126. [Google Scholar]
- Robberecht R, Caldwell MM. 1978. Leaf epidermal transmittance of ultraviolet radiation and its implications for plant sensitivity to ulraviolet-radiation induced injury. Oecologia 32:277–287. [DOI] [PubMed] [Google Scholar]
- Roy BA, Raguso RA. 1997. Olfactory versus visual cues in a floral mimicry system. Oecologia 109:414–426. [DOI] [PubMed] [Google Scholar]
- Rudall PJ. 2002. Unique floral structures and iterative evolutionary themes in Asparagales: insights from a morphological cladistic analysis. The Botanical Review 68:488–509. [Google Scholar]
- Singh Y. 1999. Hypoxis: yellow stars of horticulture, folk remedies and conventional medicine. Veld & Flora 9:123–125. [Google Scholar]
- Song B, Niu Y, Stöcklin J, Chen G, Peng DL, Gao YQ, Sun H. 2015. Pollinator attraction in Cornus capitata (Cornaceae): the relative role of visual and olfactory cues. Journal of Plant Ecology 8:173–181. [Google Scholar]
- Stang M, Klinkhamer PGL, Waser NM, Stang I, Van Der Meijden E. 2009. Size-specific interaction patterns and size matching in a plant-pollinator interaction web. Annals of Botany 103:1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statsoft, Inc 2011. STATISTICA (data analysis software system), version 10. www.statsoft.com. [Google Scholar]
- Steiner KE. 1998. The evolution of beetle pollination in a South African orchid. American Journal of Botany 85:1180–1193. [PubMed] [Google Scholar]
- Troje N. 1993. Spectral categories in the learning behaviour of blowflies. Zeitschrift fur Naturforschung 48c:96–104. [Google Scholar]
- van der Kooi CJ. 2016. Plant biology: flower orientation, temperature regulation and pollinator attraction. Current Biology 26:R1143–R1145. [DOI] [PubMed] [Google Scholar]
- van der Kooi CJ, Elzenga JTM, Staal M, Stavenga DG. 2016. How to colour a flower: on the optical principles of flower coloration. Proceedings of the Royal Society B: Biological Sciences 283:20160429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooi CJ, Wilts BD, Leertouwer HL, Staal M, Elzenga JT, Stavenga DG. 2014. Iridescent flowers? Contribution of surface structures to optical signaling. The New Phytologist 203:667–673. [DOI] [PubMed] [Google Scholar]
- von Helversen O. 1972. Zur spektralen Unterschiedsempfindlichkeit der Honigbiene. Journal of Comparative Physiology 80:439–472. [Google Scholar]
- Waser NM, Price MV. 1985. The effect of nectar guides on pollinator preference: experimental studies with a montane herb. Oecologia 67:121–126. [DOI] [PubMed] [Google Scholar]
- Welsford MR, Johnson SD. 2012. Solitary and social bees as pollinators of Wahlenbergia (Campanulaceae): single-visit effectiveness, overnight sheltering and responses to flower colour. Arthropod-Plant Interactions 6:1–14. [Google Scholar]
- Wiland-Szymańska J. 2009. The genus Hypoxis L. (Hypoxidaceae) in the East Tropical Africa: variability, distribution and conservation status. Biodiversity: Research and Conservation 14:1–129. [Google Scholar]
- Willmer P. 2011. Pollination and floral ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.