Abstract
Background
Refractory septic shock is a serious disorder with high mortality. There is currently limited evidence to support the use of extracorporeal membrane oxygenation (ECMO) in adult septic shock. We describe the outcome of patients with refractory septic shock in our hospital and try to identify prognostic factors.
Methods
We studied a total of 23 (14 males and 9 females) refractory septic shock patients treated with venoarterial (VA) ECMO in our hospital. Clinical parameters of survival and death groups, laboratory parameters before and after ECMO placement were analyzed.
Results
Eight patients were successfully weaned off ECMO and five patients were discharged. The sepsis-related organ failure assessment (SOFA) score and shock-to-ECMO interval before ECMO placement in the survival group were significantly lower than those in the death group (12.0 vs. 15.0, P=0.007; 23.5 vs. 42.2 h, P=0.037). The number of cases who had the normal range of ScvO2% between the survival group and the death group at 12 h (4 vs. 4, P=0.033), 18 h (5 vs. 7, P=0.016) and 24 h (5 vs. 9, P=0.043) during ECMO was significantly different. In univariate logistic regression analysis, the case of patients with normal central venous oxygen saturation (ScvO2) % at 12 h during ECMO [odds ratio (OR) 14.0, 95% confidence interval (CI): 1.200–163.367, P=0.035] was significantly associated with risk of the prognosis of patients.
Conclusions
In adult refractory septic shock patients, ScvO2% at 12 h during ECMO may be a risk factor for patient prognosis.
Keywords: Extracorporeal membrane oxygenation (ECMO), refractory septic shock, risk factors
Introduction
Sepsis is defined as a life-threatening organ dysfunction due to dysregulated response to infection. Septic shock is defined as a branch of sepsis in which potential circulation and cellular metabolism are severely abnormal so that the mortality is significantly increased (1). Severe sepsis or septic shock is one of the major causes of pediatric death. It accounted for about 8% pediatric intensive care unit (PICU) admission and the proportion varied across regions from 6.2% to 23.1% (1). The overall mortality rate was 24%, ranging from 21% in North America and 40% in Africa. After 1990, strict anticoagulation management and improvements in extracorporeal membrane oxygenation (ECMO) management have reduced ECMO-related complications (2,3). In successively published guidelines (4,5), ECMO can be considered for septic shock patients who do not respond to fluid resuscitation and positive inotropic drugs. However, the role of venoarterial (VA) ECMO in adult septic shock remains controversial, and the analysis of prognostic factors in these patients is limited with clinical outcomes being inconsistent. Some studies (6) reported 85% of hospital mortality, while others reported 29% of hospital mortality (4/14) (7-9). Therefore, we sought to investigate the clinical outcomes of adult patients supported by VA ECMO during refractory septic shock from an ECMO registry at our ECMO center.
Methods
Patients
We conducted a retrospective study of patients with refractory septic shock (10) who received ECMO from January 2007 to December 2017. The total number of VA ECMO patients during the observation time was 112, among which 23 (20.54%) patients received ECMO for refractory septic shock and they were selected for the main study cohort. A total of 23 patients, 14 males (60.87%), 9 females (39.13%), had a median age of 53 years. All patients were treated with continuous renal replacement therapy (CRRT), and 2 of them received plasma exchange (Figure 1).
Figure 1.
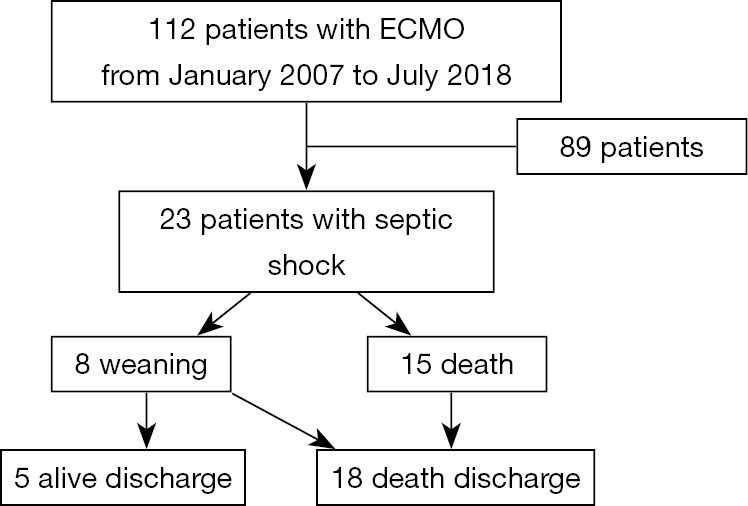
Patient flow chart.
ECMO indications
Inclusion criteria were patients with persistent circulatory failure or worsened refractory septic shock despite treatment with fluids resuscitation, adequate antibiotics and vasoactive drugs. Specific indications: organ hypoperfusion (extensive skin mottling, progressive lactic acidosis, oliguria or altered mental status), despite adequate intravascular volume and the inability to maintain mean arterial pressure >65 mmHg, despite infusion of very high-dose catecholamines (norepinephrine >1 µg/kg/min, dopamine >20 µg/kg/min or epinephrine >1 µg/kg/min with dobutamine >20 µg/kg/min) (10). There is no standard vasopressin in China, so we have little experience in this area. We only use dopamine occasionally.
Exclusion criteria were patients with an advanced malignant tumor, cardiopulmonary resuscitation (CPR) >60 min, irreversible neuropathy, such as a large number of intracranial hemorrhage.
ECMO implantation and management
ECMO catheterization was operated by an experienced ECMO team and was often carried out at the bedside. All patients underwent peripheral cannulation: the arterial catheter was placed into the femoral artery, and the venous catheter was placed into the femoral vein or internal jugular vein. Femoral artery cannula size was 17–19 Fr, femoral vein cannula size was 19–21 Fr. After the arterial cannulation, the distal branch was inserted to perform lower limb perfusion.
The ECMO centrifugal pump and membrane lung were from Maquet (Fairfield, NJ, USA). The intubation was performed using a surgical incision catheter. Because the patient’s hemodynamics were often unstable, all catheters were placed by a trained cardiovascular surgeon at the bedside. The initial flow rate was 4–6 L/min of blood flow, maintaining the ACT at 160–220 sec. Vasoactive drugs were properly used to maintain an average arterial pressure of 60–70 mmHg, a hematocrit of 30–35%, and a platelet count of ≥50,000/mm3. Echocardiography was performed daily to monitor cardiac function. The ECMO weaning test was gradually performed according to the patient’s systemic hemodynamics and tissue perfusion improvement. Successful weaning was defined as maintaining stable condition within 24 hours of ECMO weaning.
Data collection
The primary data of this study was survival rate after discharge. The following detailed data were obtained through a medical review at 24 hours prior to ECMO implantation: age, body mass index (BMI), mechanical assistant time [ECMO, CRRT, mechanical ventilation (MV)], ICU length of stay, hospital length of stay. The clinical biochemical indexes were assessed, including blood routine examination, arterial blood gas analysis, liver function, renal function, coagulation function, shock-to-ECMO interval and the severity of the disease of the sepsis-related organ failure assessment (SOFA) score.
Statistical analysis
All analyses were performed with commercially available statistical software (SPSS v22.0). Patients were categorized into two groups, survivors and non-survivors. The values are presented as the mean ± standard deviation (SD). Laboratory findings were compared between the two groups using the Student’s t-test for normally distributed variables or the Mann-Whitney U-test for continuous data. The proportions of patients were compared using Fisher’s exact test. Univariate logistic regressions were applied to perform analysis for predicting the most significant factors associated with mortality. P<0.05 was considered statistically significant.
Results
Baseline characteristics
Between January 2007 and December 2017, 112 patients underwent VA ECMO due to cardiogenic shock. There were 23 patients with septic shock after 89 patients were excluded according to the exclusion criteria. Fifteen patients (65.22%) were unable to withdraw from ECMO; 8 patients (34.78%) had successful weaning of ECMO, and 3 patients died after weaning because of primary infection (2 patients with extensive burns). Five patients with successful weaning survived until discharge (Figure 1).
Although the BMI, ECMO time, CRRT time, mechanical ventilation time, and ICU time in the death group were longer than those in the survival group, and the hospital stay was shorter than the survival group, the difference was not statistically significant. The SOFA score and shock-to-ECMO interval before ECMO placement in the survival group were significantly lower than those in the death group (12.0 vs. 15.0, P=0.007; 23.5 vs. 42.2 h, P=0.037).
There was significant difference in the number of cases who had the normal range of central venous oxygen saturation (ScvO2) % between the survival group and the death group at 12 h (4 vs. 4, P=0.033), 18 h (5 vs. 7, P=0.016) and 24 h (5 vs. 9, P=0.043) during ECMO (Table 1, Figure 2).
Table 1. Comparison of clinical data between survival and death groups.
| Factors | Survival group (n=5) | Death group (n=18) | P value |
|---|---|---|---|
| Age | 45 [20–62] | 54 [48–61] | 0.279 |
| BMI | 20.9 (19.3–22.9) | 22.6 (20.3–23.7) | 0.297 |
| ECMO duration (h) | 146.0 (125.5–167.5) | 159.0 (142.5-205.3) | 0.331 |
| CRRT duration (h) | 134.0 (80.5–144.5) | 125.0 (111.3–206.5) | 0.412 |
| MV duration (days) | 9.0 (8.5–11.0) | 10.5 (9.0–14.3) | 0.259 |
| ICU length (days) | 12.0 (8.5–17.5) | 16.5 (13.0–19.3) | 0.124 |
| Hospital length (days) | 19.0 (17.5–21.0) | 16.5 (13.0–21.0) | 0.312 |
| ScvO2% at 24 h during ECMO | |||
| ECMO initiation | 1 (20.00%) | 2 (11.11%) | 0.602 |
| 6 h during ECMO | 2 (40.00%) | 4 (22.22%) | 0.259 |
| 12 h during ECMO | 4 (80.00%) | 4 (22.22%) | 0.033* |
| 18 h during ECMO | 5 (100.00%) | 7 (38.89%) | 0.016* |
| 24 h during ECMO | 5 (100.00%) | 9 (50.00%) | 0.043* |
| Shock-to-ECMO interval (h) | 23.5 (14.7–26.9) | 42.2 (24.3–80.9) | 0.037* |
| WBC | 12.9 (9.8–16.5) | 12.2 (10.4–14.0) | 0.801 |
| pH | 7.14 (6.96–7.32) | 7.17 (7.03–7.28) | 0.971 |
| Lac | 4.4 (2.2–7.4) | 6.8 (5.5–8.9) | 0.067 |
| SOFA | 12.0 (10.0–13.0) | 15.0 (13.0–18.3) | 0.007* |
| ALT | 123.0 (88.0–184.0) | 105.5 (88.5–124.8) | 0.446 |
| TBIL | 33.6 (29.5–42.0) | 35.5 (30.8–41.3) | 0.971 |
| Cr | 178.0 (102.5–223.5) | 173.0 (117.5–236.8) | 0.801 |
| PCT | 12.7 (12. 5–15.6) | 16.1 (14.4–17.4) | 0.199 |
| Hb | 98.0 (90.5–116.5) | 100.5 (85.8–105.3) | 0.638 |
| PT | 21.0 (16.5–27.5) | 21.5 (18.8–27.3) | 0.801 |
| APTT | 53.0 (46.5–60.0) | 55.5 (49.3–64.3) | 0.446 |
Values are median (25th to 75th percentile). *, P<0.05. BMI, body mass index; ECMO, extracorporeal membrane oxygenation; CRRT, continuous renal replacement therapy; MV, mechanical ventilation; ICU, intensive care unit; ScvO2, central venous oxygen saturation; WBC, white blood cell; SOFA, sepsis-related organ failure assessment; ALT, alanine aminotransferase; TBIL, total bilirubin; Cr, creatinine; PCT, procalcitonin; Hb, hemoglobin; PT, prothrombin time; APTT, activated partial thromboplastin time.
Figure 2.
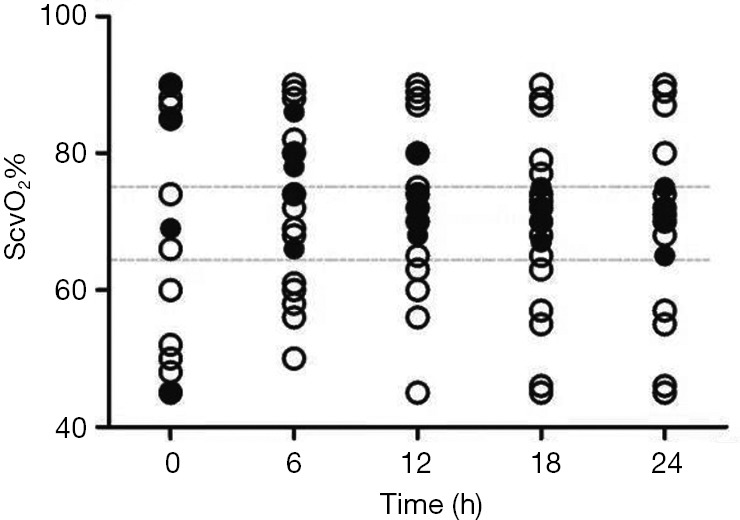
Comparison of ScvO2% in 24 h during ECMO between survivors (solid polt) and non-survivors (hollow polt). ScvO2, central venous oxygen saturation; ECMO, extracorporeal membrane oxygenation.
Causal pathogens
Of the 23 patients, 11 (47.83%) patients had infection, 9 of which were bacteremia, 1 was viral infection, and 1 was fungal infection. Of the 9 bacterial infections, 3 (13.1%) had Staphylococcus epidermidis in the blood, 2 (8.7%) had Enterococcus in ascites, and 2 (8.7%) had Staphylococcus aureus in skin secretion, 2 patients had Escherichia coli and Acinetobacter baumannii in urinary tract and lung, respectively. In addition, 1 patient had intestinal infection with Coxsackie and 1 patient had skin infection of Candida albicans (Table 2).
Table 2. Infection characteristics of the 23 septic shock patients with VA ECMO.
| Pathogens | Microorganism | Source of infection | Diagnostic method | N (%) |
|---|---|---|---|---|
| Bacteria | Enterococcus | Abdomen | Ascites culture | 2 (8.7) |
| Staphylococcus epidermidis | Blood | Blood culture | 3 (13.1) | |
| Staphylococcus aureus | Skin burn wound | Skin secretions culture | 2 (8.7) | |
| Escherichia coli | Urinary tract | Urine culture | 1 (4.3) | |
| Acinetobacter baumannii | Lung | Sputum culture | 1 (4.3) | |
| Virus | Coxsackie | Intestinal tract | PCR | 1 (4.3) |
| Fungi | Candida albicans | Skin burn wound | Secretory culture | 1 (4.3) |
VA ECMO, venoarterial extracorporeal membrane oxygenation; PCR, polymerase chain reaction.
Clinical outcomes of VA ECMO in refractory septic shock
In the univariate analysis, there was no significant difference in age, BMI, ECMO duration, CRRT duration, Mechanical ventilation, ICU length of stay, hospital length of stay, SOFA, various biochemical parameters and shock-to-ECMO interval between the survival group and death group (Table 3).
Table 3. Univariate analysis of risk factors of clinical data between survival and death groups.
| Factors | Survival group (n=5) | Death group (n=18) | Walds | P value | OR (95% CI) |
|---|---|---|---|---|---|
| Age | 45 [20–62] | 54 [48–61] | 2.659 | 0.103 | 1.065 (0.987–1.149) |
| BMI | 20.9 (19.3–22.9) | 22.6 (20.3–23.7) | 1.119 | 0.290 | 1.303 (0.798–2.128) |
| ECMO duration (h) | 146.0 (125.5–167.5) | 159.0 (142.5–205.3) | 1.033 | 0.310 | 1.014 (0.988–1.040) |
| CRRT duration (h) | 134.0 (80.5–144.5) | 125.0 (111.3–206.5) | 1.516 | 0.218 | 1.019 (0.989–1.051) |
| MV duration (days) | 9.0 (8.5–11) | 10.5 (9.0–14.3) | 1.164 | 0.281 | 1.244 (0.837–1.848) |
| ICU length (days) | 12.0 (8.5–17.5) | 16.5 (13.0–19.3) | 2.835 | 0.092 | 1.331 (0.954–1.856) |
| hospital length (days) | 19.0 (17.5-21) | 16.5 (13.0–21.0) | 0.931 | 0.335 | 0.876 (0.670–1.146) |
| WBC | 12.9 (9.8–16.5) | 12.2 (10.4–14.0) | 0.133 | 0.715 | 0.939 (0.669–1.318) |
| pH | 7.14 (6.96–7.32) | 7.17 (7.03–7.28) | 0.000 | 0.995 | 1.021 (0.001–873.333) |
| Lac | 4.4 (2.2–7.4) | 6.8 (5.5–8.9) | 3.054 | 0.081 | 1.662 (0.940–2.940) |
| SOFA | 12.0 (10.0-13.0) | 15.0 (13.0–18.3) | 3.818 | 0.051 | 2.162 (0.998–4.685) |
| ALT | 123.0 (88.0–184.0) | 105.5 (88.5–124.8) | 0.511 | 0.475 | 0.993 (0.973–1.013) |
| TBIL | 33.6 (29.5–42.0) | 35.5 (30.8–41.3) | 0.081 | 0.776 | 1.023 (0.873–1.200) |
| Cr | 178.0 (102.5–223.5) | 173.0 (117.5–236.8) | 0.104 | 0.747 | 1.003 (0.986–1.019) |
| PCT | 12.7 (12. 5–15.6) | 16.1 (14.4–17.4) | 1.551 | 0.213 | 1.284 (0.866–1.903) |
| Hb | 98.0 (90.5–116.5) | 100.5 (85.8–105.3) | 0.630 | 0.427 | 0.966 (0.888–1.052) |
| PT | 21.0 (16.5–27.5) | 21.5 (18.8–27.3) | 0.076 | 0.782 | 1.029 (0.841–1.259) |
| APTT | 53.0 (46.5–60.0) | 55.5 (49.3–64.3) | 0.467 | 0.494 | 1.044 (0.923–1.181) |
| Shock-to-ECMO interval (h) | 23.5 (14.7–26.9) | 42.2 (24.3–80.9) | 2.218 | 0.136 | 1.143 (0.959–1.363) |
Values are median (25th to 75th percentile). OR, odd ratio; CI, confidence interval; BMI, body mass index; ECMO, extracorporeal membrane oxygenation; CRRT, continuous renal replacement therapy; MV, mechanical ventilation; ICU, intensive care unit; ScvO2, central venous oxygen saturation; WBC, white blood cell; SOFA, sepsis-related organ failure assessment; ALT, alanine aminotransferase; TBIL, total bilirubin; Cr, creatinine; PCT, procalcitonin; Hb, hemoglobin; PT, prothrombin time; APTT, activated partial thromboplastin time.
ScvO2% at 24 h during ECMO between survival and death group
The changes of ScvO2% in the two groups were observed dynamically within 24 h (Figure 3). Univariate logistic regression analysis revealed that the case of patients with normal ScvO2% at 12 h during ECMO was the risk factors for the prognosis of patients [odds ratio (OR) 14.0, 95% confidence interval (CI): 1.200–163.367, P=0.035] (Table 4).
Figure 3.
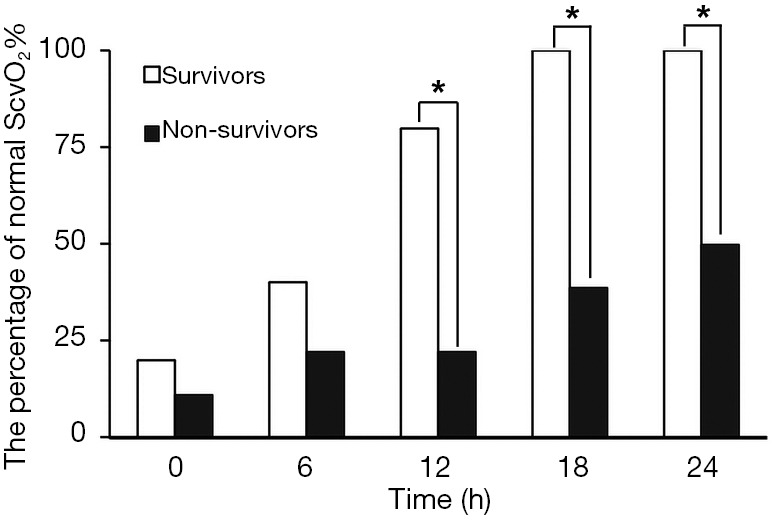
The percentage of patients with normal ScvO2% between survivors and non-survivors at 24 h during ECMO. ScvO2, central venous oxygen saturation; ECMO, extracorporeal membrane oxygenation. *, P<0.05.
Table 4. Univariate analysis of risk factors of ScvO2% at 24 h during ECMO between survival and death group.
| Time | Case of patients with normal ScvO2% | Walds | P value | OR (95% CI) | |
|---|---|---|---|---|---|
| Survival group (n=5) | Death group (n=18) | ||||
| ECMO initiation | 1 (20%) | 2 (11.11%) | 0.265 | 0.607 | 2.000 (0.143–27.990) |
| 6 h during ECMO | 2 (40%) | 4 (22.22%) | 0.622 | 0.430 | 2.333 (0.284–19.172) |
| 12 h during ECMO | 4 (80%) | 4 (22.22%) | 4.432 | 0.035* | 14.000 (1.200–163.367) |
| 18 h during ECMO | 5 (100%) | 7 (38.89%) | – | 0.999 | – |
| 24 h during ECMO | 5 (100%) | 9 (50.00%) | – | 0.999 | – |
*, P<0.05. ScvO2, central venous oxygen saturation; ECMO, extracorporeal membrane oxygenation; OR, odd ratio; CI, confidence interval.
Discussion
Sepsis is the most common cause of death in the ICU with a mortality rate of more than 40% (11). If sepsis progresses to refractory septic shock, even if it is actively treated with conventional treatment, its mortality rate is still as high as 90–100% (12). Refractory septic shock can occur during ECMO bypass. It usually occurs in patients with long bypass time, such as those waiting for cardiopulmonary transplantation (13), and in patients with severe infection risk of primary diseases, such as large area burns (14) and infectious endocarditis (15), which have a high mortality rate. The shock-to-ECMO interval refers to the interval between the start of the use of vasoactive drugs and the initiation of ECMO. Whether the length of shock-to-ECMO interval is related to the prognosis of patients is not clear. Taek et al. treated 32 refractory septic shock patients with VA ECMO (survival rate 21.9%). The shock-to-ECMO interval was 21.1 h in 7 patients in the survival group and 24.9 h in 25 patients in the death group. There was no significant difference between the two groups (P=0.45). However, none of the patients with >30.5 h shock-to-ECMO interval survived (8). Choi et al. performed a retrospective analysis of 28 patients with septic shock [21 in VA, 4 in venovenous (VV), 3 in venoarterial-venous] (mortality 35.7%). The shock-to-ECMO interval was 3.3 h in 10 patients in the survival group and 6.4 h in 18 patients in the death group. There was no significant difference between the two groups (P=0.436) (16). Ro et al. performed a retrospective analysis of 71 septic shock patients treated with ECMO (survival rate was only 7%). The shock-to-ECMO interval was 4 h in 5 patients in the survival group and 18 h in 66 patients in the death group. There was no significant difference between the two groups (P=0.052) (17). Our results showed that the shock-to-ECMO interval was 5 h in 5 patients in the survival group and 18 h in 18 patients in the death group. There was a statistically significant difference between the two groups (P=0.037), but the univariate analysis showed that the shock-to-ECMO interval was not associated with patient discharge survival (P=0.136).
Choi et al. analyzed 28 septic shock patients (35.7% discharge rate) treated with VA, VV, and VAV ECMO. Although univariate analysis showed that SOFA score was associated with patient discharge survival, this correlation was not found in multivariate analysis (16). Chang et al. found that among 55 children with sepsis who received ECMO support (31% discharge survival rate), there was a significant difference in SOFA between the survival and death group within 7 days of ECMO treatment (P<0.05), but there was no significant difference in SOFA after 1 week of ECMO support (18). Ro et al. found that, among 71 septic shock patients treated with VA ECMO (7% discharge survival rate), although the average SOFA score was slightly lower in the survival group than that in the death group, the difference was not statistically significant (17). Ferreira et al. found that, among 32 patients with refractory septic shock (21 males) who received ECMO support therapy, 7 patients (21.9%) survived until discharge. When ECMO was initiated, the simplified acute physiology score (SAPS) 3 and SOFA scores of the two groups of patients were similar. However, the third day SOFA score (15 vs. 18, P=0.01) and subsequent SOFA score (1 vs. 4, P=0.04) were significantly different between the survival and death groups. The trend of SOFA score over time can reflect the patient’s response to ECMO support and objectively evaluate the response to treatment (19). This change can facilitate decision-making regarding the appropriateness of organ support (8). In our group of patients, the SOFA score (12.0) in the survival group were statistically lower than that in the death groups (15.0, P=0.007). The univariate analysis showed that the P value was close to 0.05 (P=0.051), which maybe need to further expand the sample size to clarify.
Systemic hypoxia is a common and important complication of sepsis and may lead to multiple organ dysfunction syndrome. ScvO2% (oxygen saturation in superior vena cava) indicates the level of venous oxygenation in the brain and upper body, with normal values between 73% and 82%. An important feature of venous oxygen saturation is that when it is too high or too low, it represents a pathological state. In a recent cohort of large sepsis patients in the emergency department, it was found that patients with ScvO2% <70% had a mortality rate of 40%, while patients with ScvO2% >90% also had a mortality rate as high as 34% (20). Patients with a high initial ScvO2% value may also lead to adverse outcomes (21), which may be due to microcirculatory failure, resulting in reduced oxygen uptake by the tissue. Park et al. studied 169 patients with severe sepsis or septic shock in emergency department, and calculated their oxygen extraction rate (OER) [OER = ScvO2%/arterial oxygen saturation]. The results showed that the initial low OER (<0.2) was associated with severe organ dysfunction and can result in a high mortality rate in patients with severe sepsis and septic shock. When the patient's initial ScvO2% was >70%, but had abnormally low OER, the hospitalization mortality was higher than those with normal OER (0.2–0.3), suggesting that OER should be considered when ScvO2% was used to predict the prognosis of patients with sepsis (22). Lee et al. (23) analyzed 363 patients with sepsis and found that ScvO2% at 6 hours after shock resuscitation was a prognostic factor for severe sepsis or septic shock. Shin et al. (24) analyzed 880 patients with septic shock or severe sepsis, and divided these patients into 4 groups: group 1 (high ScvO2%; low lactate), group 2 (low ScvO2%; low lactate), group 3 (high ScvO2%; high lactate) and group 4 (low ScvO2%; high lactate). The results showed that initial ScvO2% and lactate levels were significantly associated with 28-day mortality. Patients with ScvO2% ≥70%/low lactate level had the highest 28-day survival rate. In a prospective, multicenter study, Boulain et al. found that, in 111 patients (total 363 patients) with the initial value of ScvO2% being less than 70%, ScvO2% <70% in the 1st and 6th hour of ICU admission is associated with 28-day mortality (25).
ECMO patients often die during the procedure because the tissues and organs are not fully perfused. The circulation condition during ECMO can be determined by the flow provided by the ECMO, but the perfusion of the microcirculation is difficult to determine. Yeh et al. recorded sublingual microcirculation images using an incident dark field microscope at 12, 24, 48, 72, and 96 hours after VA ECMO placement. If the patient could be weaned of VA ECMO, the sublingual microcirculation image was recorded before and after VA ECMO removal. The authors found that there was no significant difference in heart rate, mean arterial pressure, positive inotropic drug score, and lactate level between the death group and the survival group at 12 hours after ECMO. However, the perfusion small vessel density (PSVD) and proportion of perfused vessels (PPV) ratio in the death group was significantly lower than those in the survival group, suggesting that patients with stable large circulation may have microcirculation disturbances at this time. The authors therefore believed that MAP may be less suitable to assess if patients with cardiogenic shock should receive VA ECMO-assisted circulation. In contrast, microcirculatory indicators can better reflect the perfusion of tissues and organs, and can better predict the prognosis of patients (26). In our group of patients, the survival group’s ScvO2% reached normal level within 18 hours after ECMO, while only 50% of the patients in the death group reached normal value of ScvO2% at 24 hours after ECMO. After 12 hours of ECMO, there was 1 case with ScvO2% >75% and 4 cases with normal ScvO2% in the survival group. In contrast, in the death group, 6 cases had ScvO2% >75% (3 cases had successful withdraw of ECMO) and 7 cases had ScvO2% <65% (1 cases had successful withdraw of ECMO). In the univariate analysis, we have found that the case of patients with normal ScvO2% at 12 h during ECMO was the risk factors to the prognosis of patients.
However, there are some limitations to this study. We have included all patients who meet the inclusion and exclusion criteria during the study period for analysis. However, the small patient population and the retrospective of the study do not allow us to draw any conclusion about the effectiveness of septic shock VA ECMO treatment. To overcome this limitation, larger series from multi-center RCT experiments are needed to be done.
In summary, our results suggest that SOFA score before ECMO placement, shock-to-ECMO interval and the number of cases who had the normal range of ScvO2% at 12, 18 and 24 h during ECMO between the survival group and the death group were significantly different. Further univariate logistic regression analysis showed that ScvO2% at 12 h during ECMO may be risk factor for patient prognosis. Making ScvO2% of patients reach the normal range within 12 hours as far as possible may be helpful to improve the prognosis of patients.
Acknowledgments
Funding: This study was supported by the Hospital foundation (SWH2017ZDCX2002, SWH2016ZDCX2007, SWH2016JSTSYB-51 and SWH2017ZDCX-4202).
Ethical Statement: The authors are responsible for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethical Committee of Southwest Hospital (the number/ID of the approval is KY 201847).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315:775-87. 10.1001/jama.2016.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martucci G, Panarello G, Occhipinti G, et al. Anticoagulation and transfusions management in veno-venous extracorporeal membrane oxygenation for acute respiratory distress syndrome: assessment of factors associated with transfusion requirements and mortality. J Intensive Care Med 2019;34:630-9. 10.1177/0885066617706339 [DOI] [PubMed] [Google Scholar]
- 3.Martucci G, Panarello G, Occhipinti G, et al. Impact of cannula design on packed red blood cell transfusions: technical advancement to improve outcomes in extracorporeal membrane oxygenation. J Thorac Dis 2018;10:5813-21. 10.21037/jtd.2018.09.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med 2009;37:666-88. 10.1097/CCM.0b013e31819323c6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580-637. 10.1097/CCM.0b013e31827e83af [DOI] [PubMed] [Google Scholar]
- 6.Riera J, Argudo E, Ruiz-Rodríguez JC, et al. Extracorporeal membrane oxygenation for adults with refractory septic shock. ASAIO J 2018. [Epub ahead of print]. 10.1097/MAT.0000000000000905 [DOI] [PubMed] [Google Scholar]
- 7.Huang CT, Tsai YJ, Tsai PR, et al. Extracorporeal membrane oxygenation resuscitation in adult patients with refractory septic shock. J Thorac Cardiovasc Surg 2013;146:1041-6. 10.1016/j.jtcvs.2012.08.022 [DOI] [PubMed] [Google Scholar]
- 8.Park TK, Yang JH, Jeon K, et al. Extracorporeal membrane oxygenation for refractory septic shock in adults. Eur J Cardiothorac Surg 2015;47:e68-74. 10.1093/ejcts/ezu462 [DOI] [PubMed] [Google Scholar]
- 9.Extracorporeal Life Support Organization (ELSO): guidelines. Available online: https://www. elso.org/ Resources/Guidelines.aspx
- 10.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165-228. 10.1007/s00134-012-2769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Napolitano LM. Sepsis 2018: definitions and guideline changes. Surg Infect (Larchmt) 2018;19:117-25. 10.1089/sur.2017.278 [DOI] [PubMed] [Google Scholar]
- 12.Friesecke S, Stecher SS, Gross S, et al. Extracorporeal cytokine elimination as rescue therapy in refractory septic shock: a prospective single-center study. J Artif Organs 2017;20:252-9. 10.1007/s10047-017-0967-4 [DOI] [PubMed] [Google Scholar]
- 13.Xia W, Xu H, Mao W, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2018;30:1167-72. [DOI] [PubMed] [Google Scholar]
- 14.Zhang YH, Guo GH, Shen GL, et al. Analysis on treatment of extremely severe burn patients with severe inhalation injury in August 2nd Kunshan factory aluminum dust explosion accident. Zhonghua Shao Shang Za Zhi 2018;34:455-8. [DOI] [PubMed] [Google Scholar]
- 15.Nemeth E, Szigeti S, Varga T, et al. Continuous cytokine haemoadsorption incorporated into a venoarterial ECMO circuit for the management of postcardiotomy cardiogenic and septic shock—a case report. Perfusion 2018;33:593-6. 10.1177/0267659118777442 [DOI] [PubMed] [Google Scholar]
- 16.Choi MJ, Ha SO, Kim HS, et al. The simplified acute physiology score II as a predictor of mortality in patients who underwent extracorporeal membrane oxygenation for septic shock. Ann Thorac Surg 2017;103:1246-53. 10.1016/j.athoracsur.2016.07.069 [DOI] [PubMed] [Google Scholar]
- 17.Ro SK, Kim WK, Lim JY, et al. Extracorporeal life support for adults with refractory septic shock. J Thorac Cardiovasc Surg 2018;156:1104-9.e1. 10.1016/j.jtcvs.2018.03.123 [DOI] [PubMed] [Google Scholar]
- 18.Chang TH, Wu ET, Lu CY, et al. Pathogens and outcomes in pediatric septic shock patients supported by extracorporeal membrane oxygenation. J Microbiol Immunol Infect 2018;51:385-91. 10.1016/j.jmii.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 19.Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001;286:1754-8. 10.1001/jama.286.14.1754 [DOI] [PubMed] [Google Scholar]
- 20.Pope JV, Jones AE, Gaieski DF, et al. Multicenter study of central venous oxygen saturation (ScvO2) as a predictor of mortality in patients with sepsis. Ann Emerg Med 2010;55:40-6.e1. 10.1016/j.annemergmed.2009.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perz S, Uhlig T, Kohl M, et al. Low and “supranormal” central venous oxygen saturation and markers of tissue hypoxia in cardiac surgery patients: a prospective observational study. Intensive Care Med 2011;37:52-9. 10.1007/s00134-010-1980-8 [DOI] [PubMed] [Google Scholar]
- 22.Park JS, Kim SJ, Lee SW, et al. Initial low oxygen extraction ratio is related to severe organ dysfunction and high in-hospital mortality in severe sepsis and septic shock patients. J Emerg Med 2015;49:261-7. 10.1016/j.jemermed.2015.02.038 [DOI] [PubMed] [Google Scholar]
- 23.Lee YK, Hwang SY, Shin TG, et al. Prognostic value of lactate and central venous oxygen saturation after early resuscitation in sepsis patients. PLoS One 2016;11:e0153305. 10.1371/journal.pone.0153305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin TG, Jo IJ, Hwang SY, et al. Comprehensive interpretation of central venous oxygen saturation and blood lactate levels during resuscitation of patients with severe sepsis and septic shock in the emergency department. Shock 2016;45:4-9. 10.1097/SHK.0000000000000466 [DOI] [PubMed] [Google Scholar]
- 25.Boulain T, Garot D, Vignon P, et al. Prevalence of low central venous oxygen saturation in the first hours of intensive care unit admission and associated mortality in septic shock patients: a prospective multicentre study. Crit Care 2014;18:609. 10.1186/s13054-014-0609-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeh YC, Lee CT, Wang CH, et al. Investigation of microcirculation in patients with venoarterial extracorporeal membrane oxygenation life support. Crit Care 2018;22:200. 10.1186/s13054-018-2081-2 [DOI] [PMC free article] [PubMed] [Google Scholar]


