There are ongoing efforts to increase the efficacy of influenza vaccines and to promote production strategies that can rapidly respond to newly emerging viruses. It is important to understand if current alternative seasonal vaccines, such as Flublok and Flucelvax, that use alternate production strategies can induce protective influenza-specific antibodies and to evaluate what type of epitopes are targeted by distinct vaccine formulations.
KEYWORDS: Flublok, Flucelvax, antibody responses, influenza vaccines
ABSTRACT
Vaccination is the best measure of protection against influenza virus infection. Vaccine-induced antibody responses target mainly the hemagglutinin (HA) surface glycoprotein, composed of the head and the stalk domains. Recently two novel vaccine platforms have been developed for seasonal influenza vaccination: a recombinant HA vaccine produced in insect cells (Flublok) and Flucelvax, prepared from virions produced in mammalian cells. In order to compare the fine specificity of the antibodies induced by these two novel vaccine platforms, we characterized 42 Flublok-induced monoclonal antibodies (MAbs) and 38 Flucelvax-induced MAbs for avidity, cross-reactivity, and any selectivity toward the head versus the stalk domain. These studies revealed that Flublok induced a greater proportion of MAbs targeting epitopes near the receptor-binding domain on HA head (hemagglutinin inhibition-positive MAbs) than Flucelvax, while the two vaccines induced similar low frequencies of stalk-reactive MAbs. Finally, mice immunized with Flublok and Flucelvax also induced similar frequencies of stalk-reactive antibody-secreting cells, showing that HA head immunodominance is independent of immune memory bias. Collectively, our results suggest that these vaccine formulations are similarly immunogenic but differ in the preferences of the elicited antibodies toward the receptor-binding domain on the HA head.
IMPORTANCE There are ongoing efforts to increase the efficacy of influenza vaccines and to promote production strategies that can rapidly respond to newly emerging viruses. It is important to understand if current alternative seasonal vaccines, such as Flublok and Flucelvax, that use alternate production strategies can induce protective influenza-specific antibodies and to evaluate what type of epitopes are targeted by distinct vaccine formulations.
INTRODUCTION
Seasonal influenza virus infection causes major mortality and morbidity globally every year. Vaccines can be effective but offer protection mostly to viruses that were included in the vaccine and have to be reformulated annually due to antigenic drift (1). Antibody responses induced by vaccination target predominantly the hemagglutinin (HA) glycoprotein and more specifically the immunodominant head domain of HA. The head is highly plastic and allows the virus to escape immune pressure, causing antigenic drift (2). Antibodies targeting the head domain are common but are often strain specific. Conversely, antibodies targeting the conserved stalk region are broadly cross-reactive, neutralizing a wide range of influenza viruses, but are more rarely induced by vaccination (3–7).
Traditional influenza virus vaccines are egg grown, but the manufacturing process is slow and mutations occurring in eggs are an important issue, as they affect antigenicity (8). Cell-based vaccine approaches have been FDA approved and are currently in use. These include Flublok, a recombinant HA (rHA) vaccine produced in insect cell culture using a baculovirus expression system, and Flucelvax, a subunit inactivated vaccine prepared from virus propagated in Madin-Darby canine kidney (MDCK) cells (9, 10). Flublok contains three times more HA than other influenza virus vaccines, and the protein is purified after extraction from the cells with a detergent (11). Flucelvax is produced in a fashion similar to that of traditional egg-grown vaccines where the viruses are purified and inactivated, with the exception that the viruses are grown in a mammalian cell line (12). Both vaccines were well tolerated and have demonstrated protective efficacy in human trials (13–15).
The concept of B cell immunodominance is of great interest in the context of vaccine development. Antigens activate naive B cells in the secondary lymphoid organs where germinal centers (GC) form (16). In the GCs, B cells with enough affinity for the immunogen proliferate and experience somatic hypermutation (SHM) of their B cell receptors (BCR) as well as class-switch recombination (CSR) (17). The entire surface of an immunogen, if accessible, has the theoretical potential to be recognized by B cell precursors expressing specific BCRs. Interestingly, however, antibody responses to influenza virus or other pathogens seem to be focused on certain immunodominant epitopes (18). Early studies using mouse monoclonal antibodies defined the HA major antigenic sites, all situated in the HA head domain (19–21). Altman et al. immunized lampreys and found that the immunodominance hierarchy was nearly identical between lampreys and mice in response to influenza A viruses. Eighty percent of the response was focused on HA and mainly through recognition of the major antigenic sites in the HA head domain (22). A recent study in mice demonstrated that HA head- and stalk-reactive precursor frequencies are similar and that the head immunodominance was the result of naive B cell competition for full-length HA (23). Results in humans also suggested that the stalk domain is less accessible than the head domain on virions, explaining why stalk-reactive antibodies are rare (24).
HA displays N-glycosylation sites both on the head and the stalk domains, and these can interfere with antibody recognition (8, 25). Sites on the HA stalk domain are more conserved across groups than the ones on the HA head (26). Importantly, recent studies suggested that the complexity of HA glycan structures impacts the breadth of the immune response (27, 28). The nature of the glycans and, as a consequence, their complexity depends on host cells. Mammalian cell-derived HA will have more complex glycans than insect cell-derived HA (29). Thus, vaccines using insect cells supposedly could induce a larger breadth of immune responses than mammalian or egg-based vaccines (30). Additionally, larger glycan structures can mask epitopes on HA and, as a result, induce the targeting of other epitopes that might have been immunosubdominant (31).
In this study, we compared antibody responses at the monoclonal antibody level of individuals immunized in 2015 to 2016 with Flublok (n = 6), a rHA protein vaccine produced in insect cells, or Flucelvax (n = 5), a subunit virus vaccine produced in mammalian cells. We hypothesized that the two vaccines display different epitopes on the head and stalk domain and induce different breadths of immune responses.
RESULTS
Antibody-secreting cell (ASC) responses to Flublok and Flucelvax immunization.
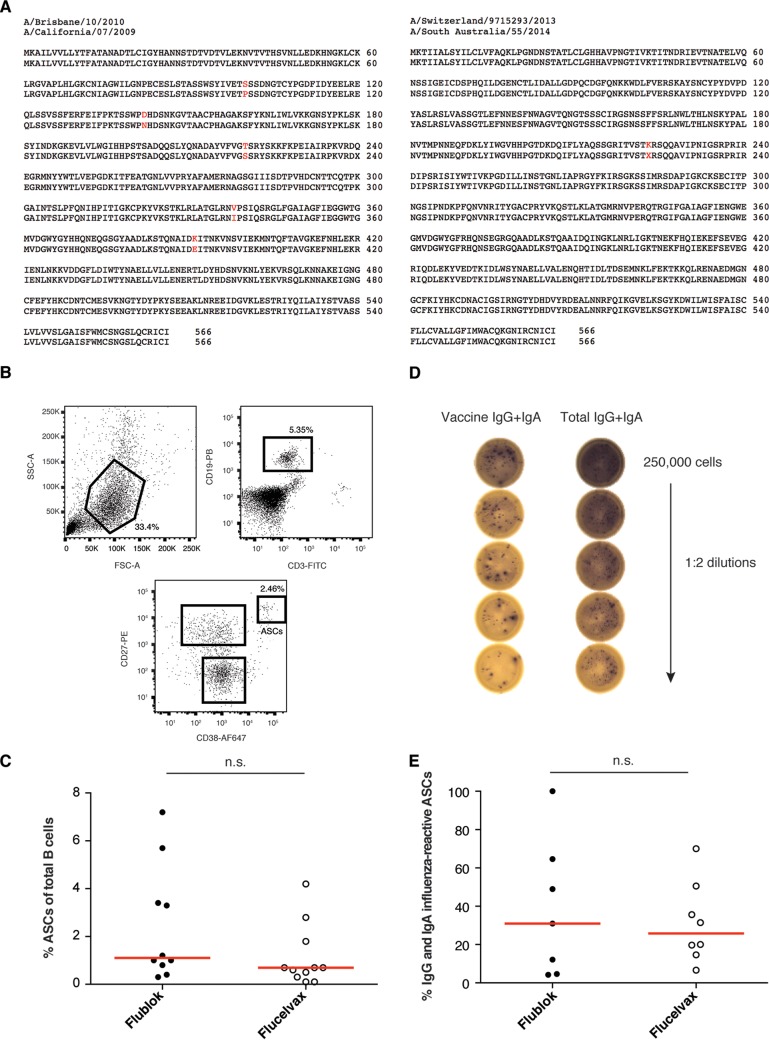
Ten healthy individuals were immunized with the 2015–2016 Flublok trivalent influenza virus vaccine (TIV) (Protein Sciences) and eleven with the 2015–2016 Flucelvax TIV (Seqirus). The rHAs present in the Flublok vaccine were from the strains A/California/07/2009 (H1N1), A/Switzerland/9715293/2013 (H3N2), and B/Phuket/3073/2013. The virus strains present in the Flucelvax vaccine were A/Brisbane/10/2010 (A/California/07/2009 [H1N1] pdm09-like virus), A/South Australia/55/2014 (A/Switzerland/9715293/2013 [H3N2]-like virus), and B/Utah/9/2014 (B/Phuket/3073/2013-like virus). Amino acid differences between the H1N1 and H3N2 strains are shown in Fig. 1A. The B/Utah/9/2014 and B/Phuket/3073/2013 viruses share the exact same amino acid sequence (data not shown).
FIG 1.
Induction of ASCs after Flublok or Flucelvax immunization. (A) Amino acid sequence comparison between A/California/07/2009 and A/Brisbane/10/2010 (H1N1) as well as A/Switzerland/3073/2013 and A/South Australia/55/2014 (H3N2) strains. Sequences were available on GISAID. (B and C) Characterization of the ASC population at day 7 postimmunization by flow cytometry. (B) Gating strategy. ASCs are gated as CD3− CD19+ CD27++ CD38++. SSC, side scatter; FSC, forward scatter. (C) Percent ASCs given as a percentage of the total CD3− CD19+ B cells at day 7 after Flublok (n = 10) or Flucelvax (n = 11) immunization. Each dot represents an individual subject. Statistical significance was determined by Mann-Whitney test. n.s., not significant. (D and E) ELISpot assay using PBMCs at day 7 postimmunization. The experiment was done once for each subject. (D) Example of ELISpot assay results for one vaccinated subject. A total of 250,000 PBMCs were added to the top wells, and 1:2 dilutions down the plate were performed. One row per subject was coated with the vaccine (Flublok or Flucelvax), and one row per subject was coated with total IgG-IgA. (E) Percent IgG and IgA influenza vaccine-reactive ASCs given as a percentage of total IgG and IgA ASCs at day 7 after Flublok (n = 7) or Flucelvax (n = 8) immunization. Spots were counted for multiple dilutions and then averaged. Each dot represents an individual subject. Statistical significance was determined by Mann-Whitney test. n.s., not significant.
ASCs (CD3− CD19+ CD27hi CD38hi) were isolated at day 7 (d7) postimmunization and single cell sorted (Fig. 1B). ASCs, also called plasmablasts, are a transient population of B cells activated upon antigen exposure and reflect the ongoing immune response (32). Percentages of ASCs of total B cells were compared by subject. There were similar frequencies of ASCs induced by Flublok and Flucelvax (Fig. 1C). Enzyme-linked immunospot (ELISpot) assays were performed at day 7 (Flublok, 7 out of 10 individuals; Flucelvax, 8 out of 11) to assess the percentage of IgG and IgA vaccine-reactive ASCs (Fig. 1D). On average, the proportion of ASCs from Flublok-vaccinated subjects that were vaccine reactive was similar to the proportion of ASCs from Flucelvax-vaccinated subjects (median, 30.95% and 25.76%, respectively) (Fig. 1E). Thus, at day 7, ASC responses did not differ between Flublok and Flucelvax vaccines.
We then cloned monoclonal antibodies (MAbs) from single cell-sorted ASCs. To obtain greater numbers of influenza-reactive antibodies, we prioritized individuals with greater ASC percentages at d7 and/or higher vaccine-reactive ASCs by ELISpot assay. The cohorts were aged matched, and information about the subjects is available in Table 1. We were able to clone 42 influenza-reactive MAbs from six individuals immunized with Flublok and 38 influenza-reactive MAbs from five individuals immunized with Flucelvax.
TABLE 1.
Information about the subjects included in the monoclonal antibody study (age, gender, and vaccine received)
| Subject ID | Age (yr) | Genderb | Vaccine | HAI titersa |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A/California/2009 H1N1 |
A/Switzerland/2013 H3N2 |
B/Phuket/2013 |
||||||||||
| d0 | d28 | Fold change | d0 | d28 | Fold change | d0 | d28 | Fold change | ||||
| 150055-003 | 21 | F | Flublok | 1,280 | 1,280 | 1 | 80 | 1,280 | 16 | 320 | 640 | 2 |
| 150055-008 | 47 | F | Flublok | 10 | 640 | 64 | 10 | 640 | 64 | 80 | 320 | 4 |
| 150055-010 | 20 | F | Flublok | 320 | 2,560 | 8 | 20 | 2,560 | 128 | 20 | 40 | 2 |
| 150055-023 | 30 | M | Flublok | |||||||||
| 150055-032 | 19 | M | Flublok | 20 | 2,560 | 128 | 40 | 10,240 | 256 | 40 | 160 | 4 |
| 150055-037 | 20 | F | Flublok | 40 | 2,560 | 64 | 40 | 1,280 | 32 | 40 | 1,280 | 32 |
| 150055-001 | 30 | M | Flucelvax | 20 | 320 | 16 | 10 | 20 | 2 | 40 | 80 | 2 |
| 150055-005 | 19 | F | Flucelvax | 10 | 160 | 16 | 10 | 1,280 | 128 | 160 | 640 | 4 |
| 150055-011 | 29 | F | Flucelvax | 320 | 640 | 2 | 640 | 1,280 | 2 | 40 | 160 | 4 |
| 150055-015 | 31 | M | Flucelvax | 20 | 2,560 | 128 | 320 | 1,280 | 4 | 10 | 640 | 64 |
| 150055-029 | 42 | M | Flucelvax | 320 | 2,560 | 8 | 80 | 320 | 4 | 640 | 640 | 1 |
Absolute serum HAI titers at d0 and d28 as well as fold changes are presented for each virus strain (H1N1, H3N2, and B strain). Titers could not be determined for one individual (150055-023).
F, female; M, male.
Characterization of human monoclonal antibodies after Flublok and Flucelvax immunization.
The MAbs were first tested for virus and rHA reactivity by enzyme-linked immunosorbent assay (ELISA) against strains present in the vaccines. All 42 Flublok-reactive MAbs bound to HA and virus by ELISA. All 38 Flucelvax-reactive MAbs bound to virus by ELISA, and 34 out of 38 of these MAbs bound to HA (Fig. 2; see also Tables S1 and S2 in the supplemental material).
FIG 2.
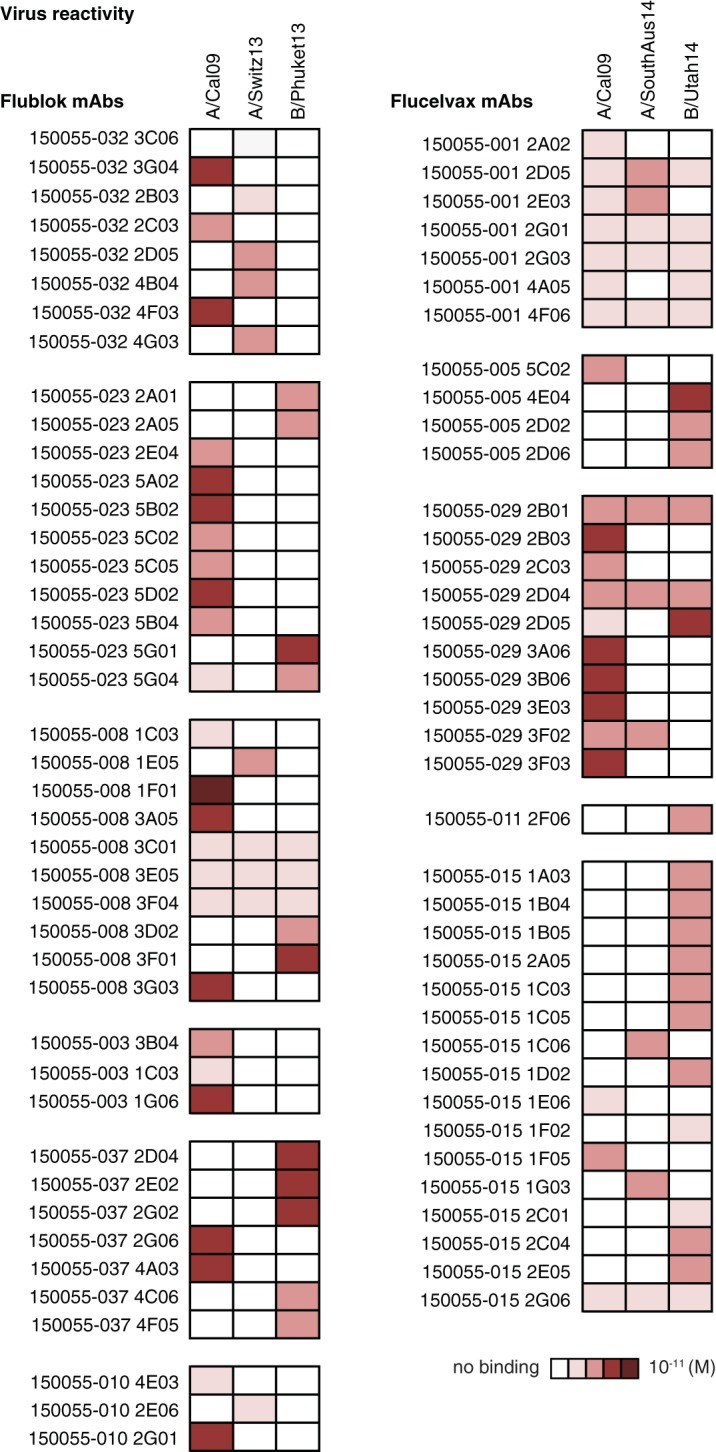
Virus cross-reactivity and epitopes targeted by MAbs induced by Flublok or Flucelvax immunization. Cross-reactivity to influenza group 1 (A/California/07/2009 H1N1 virus), group 2 (A/Switzerland/9715293/2013 H3N2 virus or A/South Australia/55/2014), and B strain (B/Phuket/3073/2013 virus or B/Utah/9/2014) as tested by ELISA. Assays were performed in duplicate three times for each antibody. ELISA binding affinities represented by KD (M) were plotted as a heatmap. Flublok-induced MAbs (n = 42) are compared to Flucelvax-induced MAbs (n = 38). MAbs were named based on the study name (150055), the subject identifier (032, for example) and the individual clone’s name. KD (M) values can also be found in Table S1 in the supplemental material.
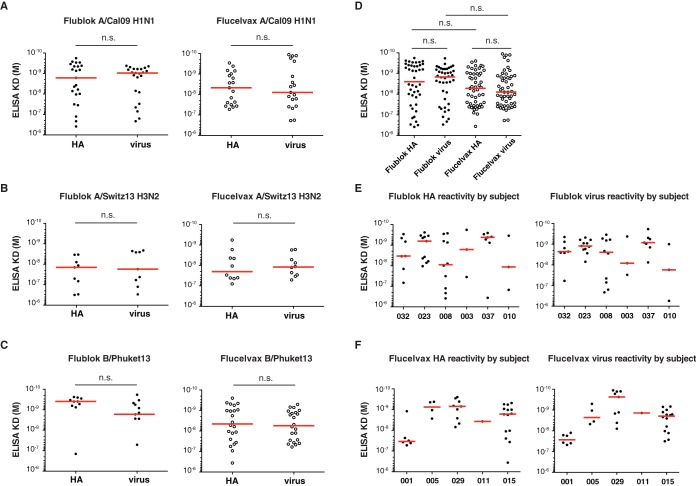
Because Flublok is a rHA vaccine produced in insect cells and Flucelvax is a subunit virus vaccine produced in mammalian cells, we suspected the accessibility of antibody epitopes might differ, either because of glycosylation or differences in availability in stalk domain epitopes. To address this, we compared the ability of all HA-reactive MAbs to bind by ELISA to rHA and whole virus (A/California/07/2009 for H1N1, A/Switzerland/9715293/2013 for H3N2, and B/Phuket/3073/2013 for B strain). Independently of the strain of virus analyzed, no differences between Flublok and Flucelvax were observed in the binding avidity of the derived MAb to rHA or to whole virus (Fig. 3A to C). Notably, although not statistically significant, there was almost a log difference between the Flucelvax-induced MAb avidity to whole virus and Flublok-induced MAb avidity for the same virus strains (Fig. 3D). We then analyzed differences by subject and noticed that MAbs from one subject in the Flucelvax cohort had reduced binding avidity compared to that of MAbs from the other subjects (Fig. 3E and F). Thus, we conclude that the lower avidity could not be generalized to all MAbs induced by Flucelvax.
FIG 3.
Affinity of MAbs induced by Flublok or Flucelvax immunization. (A to F) Approximated KD values were determined for each MAb following binding to various rHAs and virus strains by ELISA. Each dot represents one MAb. Comparison of MAbs induced by Flublok or Flucelvax for binding against A/California/07/2009 H1N1 HA and virus, Flublok (n = 23) and Flucelvax (n = 19) (A); A/Switzerland/9715293/2013 H3N2 HA and virus, Flublok (n = 9) and Flucelvax (n = 10) (B); and B/Phuket/3073/2013 HA and virus, Flublok (n = 10) and Flucelvax (n = 22) (C). Cross-reactive MAbs that bound multiple strains were included in multiple comparisons. Statistical significance was determined by paired Wilcoxon test. n.s., not significant. (D) Comparison of all MAbs induced by Flublok or Flucelvax for binding against HA and virus. Statistical significance was determined by Mann-Whitney test for unpaired comparisons and Wilcoxon test for paired comparison. n.s., not significant. (E and F) Comparison of all MAbs induced by Flublok or Flucelvax for binding against HA and virus by subject.
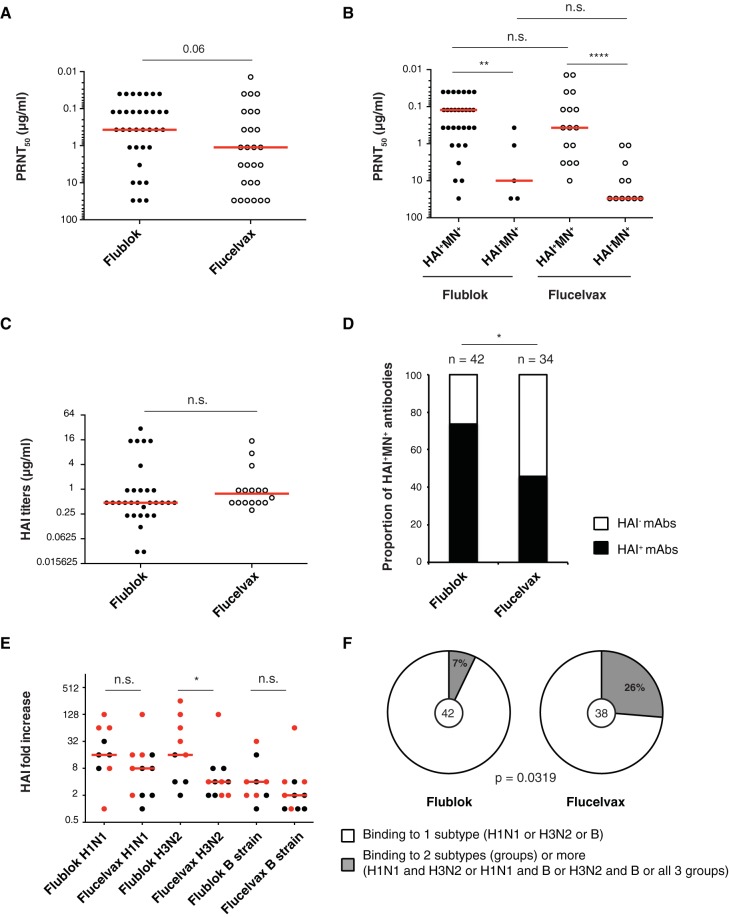
MAbs were then tested for hemagglutination inhibition (HAI) assay as well as in vitro neutralization by plaque assay. The concentrations of MAbs that reduced the number of plaques by 50% (PRNT50) were determined and compared between the two vaccines. Although not statistically significant, there was a tendency for MAbs induced by Flublok to be of higher potency (lower PRNT50) than those induced by Flucelvax (Flublok median, 0.37; Flucelvax median, 1.11) (Fig. 4A). When separated by HAI status, both Flublok- and Flucelvax-induced HAI+ MN+ (MN+ for neutralizing) MAbs had higher in vitro neutralization potency than HAI− MN+ MAbs (Fig. 4B). The HAI titers did not vary between Flublok- and Flucelvax-induced HAI+ MN+ MAbs (Flublok median, 0.47; Flucelvax median, 0.78) (Fig. 4C). In conclusion, MAbs induced by Flublok and Flucelvax vaccines are similar in terms of affinity and in vitro neutralization potency.
FIG 4.
Characterization of MAbs induced by Flublok or Flucelvax immunization. (A to C) Hemagglutination inhibition assay (HAI) and in vitro neutralization by plaque reduction neutralization assay. Each dot represents one antibody. Statistical significance was determined by Mann-Whitney test. The assays were performed 2 to 3 times for each antibody. (A) PRNT50 (μg/ml) for all neutralizing Flublok (n = 35)- and Flucelvax (n = 27)-induced MAbs. (B) PRNT50 (μg/ml) for HAI+ MN+ versus HAI− MN+ neutralizing Flublok- and Flucelvax-induced MAbs. (C) HAI titers (μg/ml) for Flublok (n = 31)- and Flucelvax (n = 16)-induced MAbs. (D) Proportion of HAI+ MN+ MAbs out of the total number of HA-reactive MAbs induced by Flublok (n = 42) or Flucelvax (n = 34). Statistical significance was determined by Fisher’s exact test. (E) Serum antibody HAI titers in Flublok (n = 9)- or Flucelvax (n = 11)-vaccinated individuals. Each dot represents the fold change increase between d0 and d28 in an individual subject. Red dots represent the subjects from which MAbs were cloned. Medians are represented in red. Testing for statistical significance was performed using a Mann-Whitney U test. HAI titer absolute values can be found in Table 1. (F) Percentage of virus-reactive MAbs induced by Flublok or Flucelvax binding to only one subtype (H1N1, H3N2, or B strain). Statistical significance was determined by Fisher’s exact test. The number in the middle of the pie charts represents the total number of virus-reactive MAbs tested.
Epitopes targeted by human monoclonal antibodies after Flublok and Flucelvax immunization.
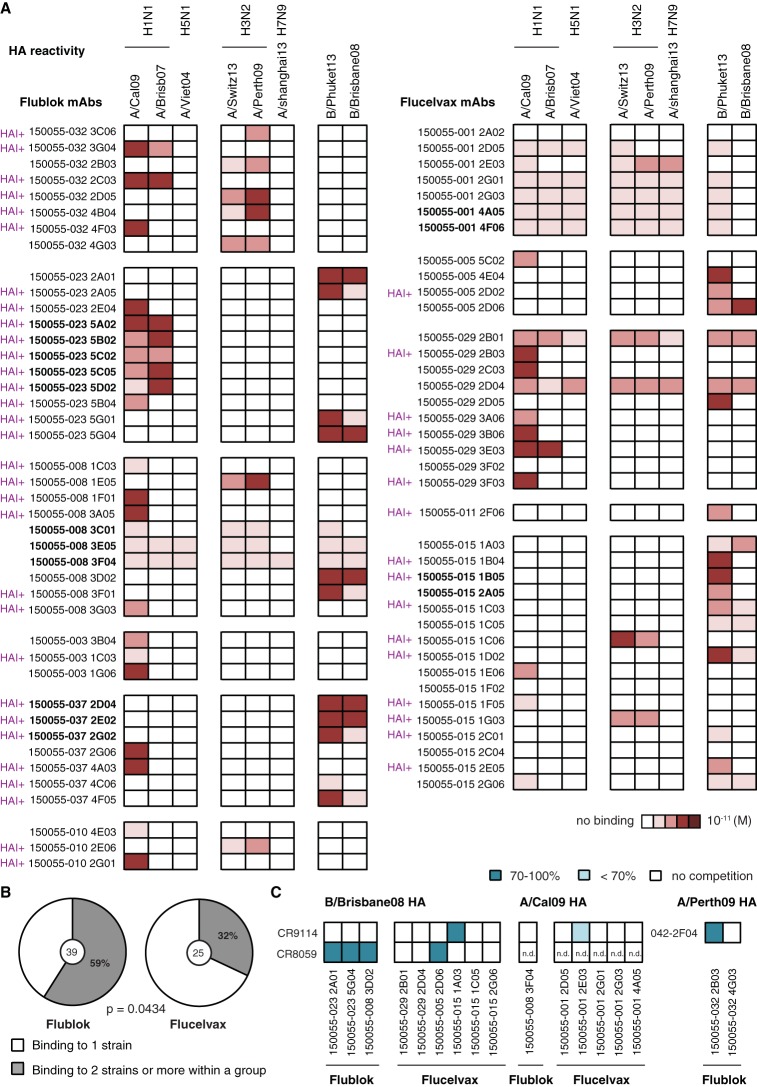
Because HAI+ MAbs target the head domain, we first calculated the proportion of HAI+ MAbs out of the total number of HA-reactive MAbs and found that Flublok induced more HAI+ MAbs (targeting the head domain around the receptor-binding domain) than Flucelvax (71% versus 47%, respectively) (Fig. 4D). This was reflected by a tendency toward higher HAI fold increases between d28 and d0 in the serum of Flublok-vaccinated individuals (Fig. 4E and Table 1). Additionally, the proportion of broadly cross-reactive MAbs that bound 2 or more virus strains across groups (H1N1 for group 1, H3N2 for group 2, and B strain) was higher in the Flucelvax group than the Flublok one (26% versus 7%, respectively) (Fig. 4F). We also tested the MAbs for HA reactivity against various strains within the different subtypes and groups: two H1N1 strains and one H5N1 strain for group 1 HAs, two H3N2 strains and one H7N9 strain for group 2 HAs, and two B strains (one from each distinct lineage) (Fig. 5A). Interestingly, when we restricted our analysis to homosubtypic MAbs (that bound only within one group: group 1, group 2, or B strain), MAbs induced by Flublok bound more frequently to 2 or more virus strains than did Flucelvax-induced MAbs (59% versus 32%, respectively) (Fig. 5B).
FIG 5.
HA cross-reactivity and epitopes targeted by MAbs induced by Flublok or Flucelvax immunization. (A and B) Cross-reactivity to influenza group 1 (H1N1 and H5N1), group 2 (H3N2 and H7N9), and B strain rHAs tested by ELISA. Assays were performed in duplicate three times for each antibody. (A) ELISA binding affinities represented by KD (M) were plotted as a heatmap. MAbs were named based on the study name (150055), the subject identifier (032 for example), and the individual clone’s name. MAbs in boldface from the same subject are part of a clonal expansion. (B) Proportion of MAbs binding to 2 or more rHAs within the same group out of the total number of homosubtypic MAbs (bound to group 1 or group 2 or B strain HAs). Statistical significance was determined by Fisher’s exact test. The number in the middle of the pie charts represents the total number of homosubtypic HA-reactive MAbs for Flublok (n = 39) and Flucelvax (n = 25). (C) Competition for conserved epitopes on HA. Biotinylated MAbs CR9114 (stalk reactive, A and B strains, MN+), CR8059 (head, B strain only, HAI− MN+), and DY-2F04 (stalk reactive, group 2, MN+) were tested for binding to rHA protein by ELISA with or without the presence of a competitor MAb. HAs from B/Brisbane/60/2008, A/California/07/2009 H1N1, and A/Perth/16/2009 H3N2 influenza strains were used depending on the specificities of the MAbs. Only HAI− HA-reactive MAbs were used as competitors. The experiment was done in duplicate 2 to 3 times. The average percentage of competition was calculated. Represented here are different degrees of inhibition (100%, 70% to 100%, or <70%). An inhibition greater than 50% was considered a positive competition. n.d., not determined.
To determine the proportion of stalk-reactive antibodies, we performed competition ELISAs between all HAI− MAbs and known stalk broadly cross-reactive MAbs (CR9114 for H1N1 and B strains [6] and 042-2F04 for H3N2 strains [3]). CR9914 is a typical neutralizing stalk-reactive antibody. Other antibodies that target slightly different epitopes on the stalk domain have been described and have overlapping/competing epitopes with CR9114, including 042-2F04 (3). Although the majority of stalk-reactive antibodies will compete with these two antibodies in our assay, there might be antibodies targeting other unknown epitopes on the stalk domain. Using our competition assay, we observed that 1 out of 42 (2.4%) HA-reactive MAbs induced by Flublok targeted the stalk domain compared to 2 out of 38 (5.3%) HA-reactive MAbs induced by Flucelvax (Fig. 5C). We additionally performed competition with the broadly cross-reactive MAb CR8059, which binds a conserved epitope at the base of the head on B strains (6). We observed that 3 out of 42 (7.1%) HA-reactive MAbs induced by Flublok target the conserved epitope between the head and the stalk compared to 1 out of 38 (2.6%) HA-reactive MAbs induced by Flucelvax (Fig. 5C).
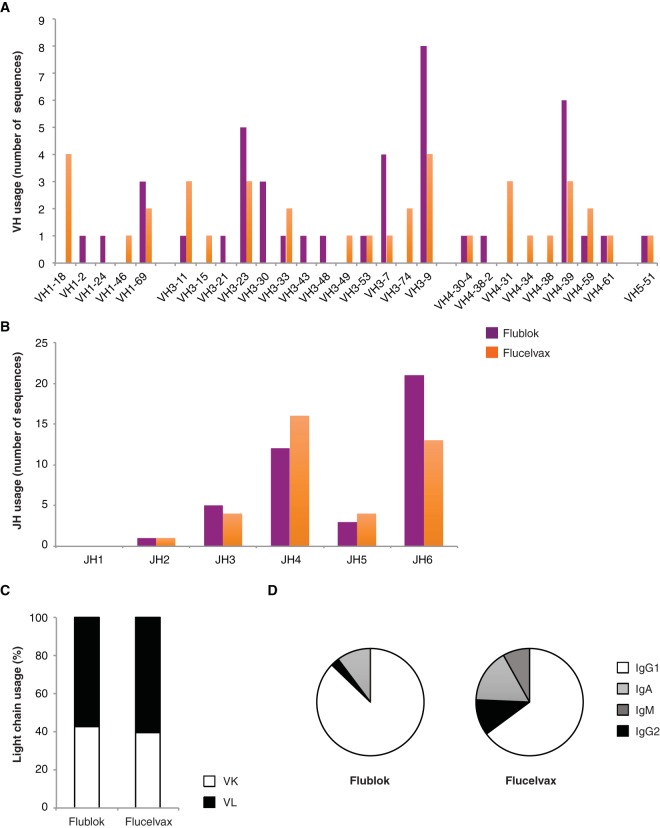
We then analyzed the immunoglobulin repertoire and the antibody isotype (Fig. 6). The few biases observed in the heavy-chain variable region (VH) usage were due to specific clonal expansions in some of the individuals and cannot be generalized (Fig. 6A and B). The variable light-chain usage (kappa [VK] versus lambda [VL]) was not different between the two vaccine cohorts (Fig. 6C). Finally, while the majority of MAbs from both cohorts were IgG1, the proportion of IgG2 and IgM influenza-reactive MAbs was higher in the Flucelvax cohort than in the Flublok cohort (Fig. 6D).
FIG 6.
Repertoire of MAbs induced by Flublok or Flucelvax immunization. (A to D) Molecular characteristics of the immunoglobulin genes coding for all influenza virus-reactive MAbs (Flublok, n = 42; Flucelvax, n = 38). Ig genes coding for each antibody were analyzed using VGenes and IMGT to determine the variable heavy chain (VH) usage (A), joining heavy chain (JH) usage (B), and light-chain usage (C). (D) Proportion of each isotype (IgG1, IgA [including IgA1 and IgA2], IgM, and IgG2) out of the total number of VH sequences with a determinable isotype for MAbs induced by Flublok (n = 39) or Flucelvax (n = 37). Additional information about antibody features is available in Table S2.
We conclude from these experiments that Flublok and Flucelvax induce similar frequencies of stalk-reactive MAbs in humans.
Flublok and Flucelvax induce similar frequencies of stalk-reactive antibodies in naive mice.
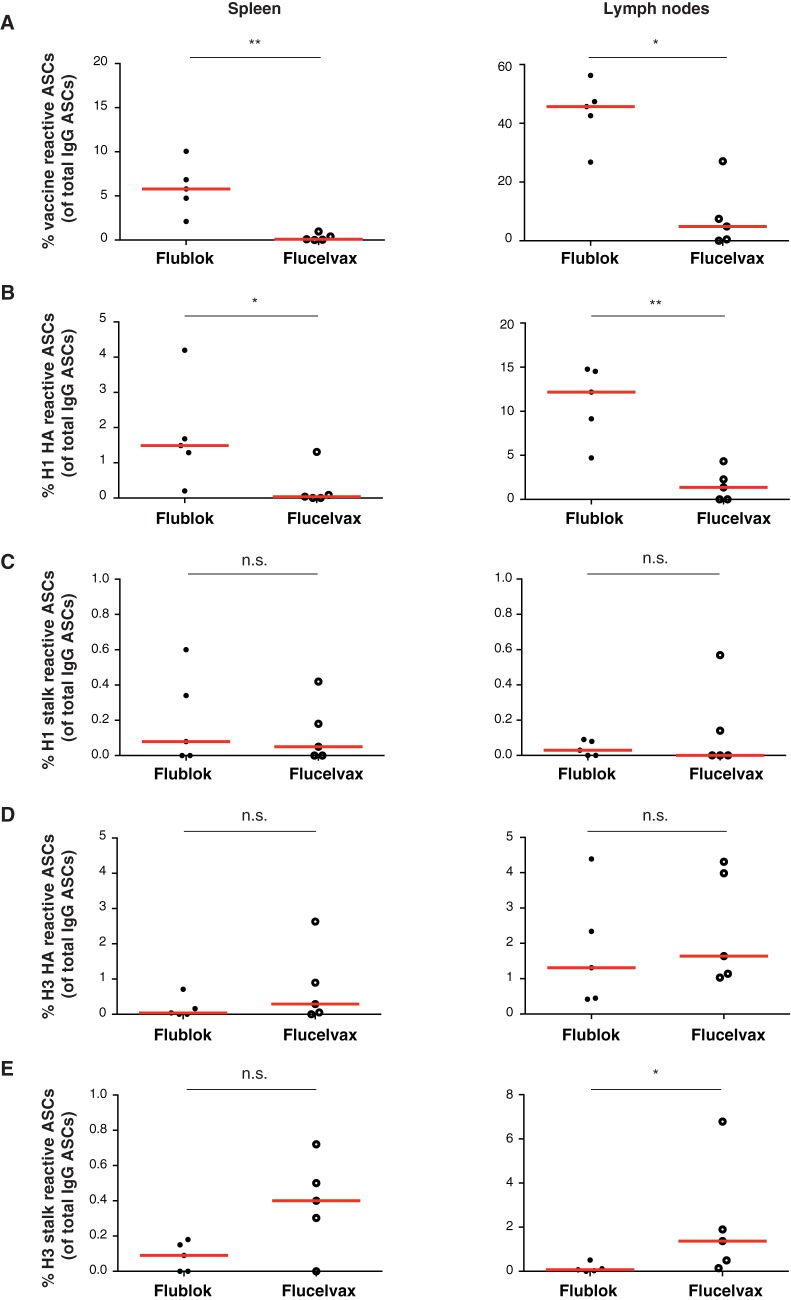
Because humans are not naive to influenza viruses and preexisting immunity as well as immune history will likely play a role in the induction of broadly reactive antibodies, we immunized mice with either Flublok or Flucelvax. Female BALB/c mice were immunized subcutaneously with 50 μl of vaccine adjuvanted with 10 μg poly(I·C). Ten days later, the draining lymph nodes and spleens were collected and ELISpot assays were performed. We observed that the mice immunized with Flublok had higher proportions of vaccine-reactive ASCs than mice immunized with Flucelvax both in the spleen and lymph nodes (Fig. 7A). This could be explained by the fact that Flublok contains 3 times more HA. Using chimeric HA proteins (combining the H1 or H3 stalk from the vaccine with avian heads not present in the vaccine) as well as full-length H1N1 and H3N2 HAs, we assessed the induction of stalk-reactive ASCs. While Flublok induced more H1N1-reactive ASCs in the spleen and lymph nodes than Flucelvax (Fig. 7B), the proportion of group 1 stalk-reactive ASCs were similar for both vaccines (Fig. 7C). In addition, both vaccines induced similar H3N2-reactive ASCs against the full-length HA and Flucelvax induced slightly more group 2 stalk-reactive ASCs (Fig. 7D and E). These results recapitulated what we observed in our human vaccinated cohorts with regard to the focus of the antibodies elicited by the two vaccines.
FIG 7.
Frequency of stalk-reactive ASCs in mice immunized with Flublok or Flucelvax. (A to E) Seven- to 8-week-old female BALB/cJ mice were immunized subcutaneously with 50 μl of vaccine (Flublok [n = 5] or Flucelvax [n = 5]) plus 10 μg poly(I·C) in PBS. Mice were sacrificed at day 10 postimmunization, spleen and draining lymph nodes were collected, and ELISpot assays were performed. Results are expressed as a percentage of total IgG ASCs in the given organ. (A) Percent IgG vaccine-reactive ASCs in the spleen and lymph nodes. (B) Percent IgG H1N1 full-length HA-reactive ASCs in the spleen and lymph nodes. (C) Percent IgG H1N1 HA stalk-reactive ASCs in the spleen and lymph nodes. (D) Percent IgG H3N2 full-length HA-reactive ASCs in the spleen and lymph nodes. (E) Percent IgG H3N2 HA stalk-reactive ASCs in the spleen and lymph nodes.
DISCUSSION
In the present study, we report the characterization of MAbs isolated from ASCs induced by Flublok and Flucelvax. Both vaccines are highly purified vaccines produced in cell lines, unlike more traditional inactivated virus vaccines, which are manufactured in eggs. First, we observed similar frequencies of stalk-reactive MAbs induced by Flublok and Flucelvax in vaccinated individuals. A recent study focusing on stalk-reactive serum antibodies and age demonstrated that young adults have the highest induction of stalk-reactive antibodies following Flublok vaccination, but the titers of stalk-reactive antibodies observed were in general moderate (33). Unfortunately there was no direct comparison with other influenza virus vaccine formulations. The rHAs present in Flublok are extracted from insect cells with a detergent. When the detergent is later removed, the hydrophobic regions of the trimerization domain cluster together again and form rosettes (9, 34, 35). As a consequence, epitopes on the stalk might be partially hidden (compared to soluble HA trimers) but are comparable to subunit vaccines where rosettes will form as well. Epitopes on the stalk domain might be differentially exposed in split-virion or whole-virus vaccines compared to exposure in highly purified HA vaccines (recombinant and subunit). Further studies would have to be performed to assess such differences.
While in this study we did not directly compare our results to those for antibodies induced by traditional egg-grown vaccines, we have previously observed similar frequencies of stalk-reactive antibodies induced by seasonal vaccination with egg-grown vaccines (3). We found in the past that 3.6% of H3N2-reactive MAbs from d7 plasmablasts were stalk reactive (competed with CR9114). Additionally, a recent study comparing all three vaccine platforms demonstrated that fold change in HAI as well as antibody binding to the HA1 domain (head) tended to be higher in Flublok than in Flucelvax and Flublok (36). Importantly, the first study comparing recombinant and inactivated (egg-grown) quadrivalent influenza vaccines in young adults reported comparable immunogenicity for both vaccines (37).
Because humans are not naive to influenza, their immune history, i.e., the imprint left by past exposures to certain influenza strains, will likely affect the targeting of specific epitopes on HA (38, 39). One caveat of our study is the low number of individuals (Flublok, n = 6; Flucelvax, n = 5) that we cloned MAbs from. Individual variation as well as immune history could bias our conclusions. Thus, we verified our results by immunizing antigen-naive mice with either Flublok or Flucelvax and then measured the frequency of HA (full-length)-reactive as well as stalk-reactive B cells induced by each vaccine. We observed a similar induction of head- versus stalk-reactive ASCs between both vaccines, suggesting that previous immune history or imprinting was not a major bias in our cohort.
Angeletti and colleagues have shown that the stalk on its own is immunogenic and that full-length HA fails to induce stalk-reactive antibodies in mice. The head immunodominance depends on the physical attachment of the head to the stalk domain, and stalk immunogenicity was enhanced by immunizing with “stalk-only” constructs or by increasing local HA concentrations in the draining lymph nodes (23). Interestingly, Flublok contains 3 times more HA than traditional influenza virus vaccines. However, despite observing a higher proportion of vaccine-reactive ASCs in mice immunized with Flublok than in mice immunized with Flucelvax, we did not see a higher proportion of stalk-reactive ASCs in this group. This discrepancy could be explained by the fact that the full-length HA and stem construct used in this paper were soluble proteins (40). In addition, proteins prepared in insect cells are glycosylated with less complex sugars than with mammalian cells; insect cells will add shorter N-glycans, with little sialylation, than mammalian cells (41). Thus, we could expect that rHA protein produced from insect cells in the Flublok vaccine would be better at inducing cross-reactive antibodies. We indeed observed that homosubtypic head-reactive MAbs induced by Flublok are more cross-reactive within the same group than homosubtypic MAbs induced by Flucelvax. We also observed a higher proportion of HAI+ MAbs, i.e., antibodies that target epitopes near the receptor-binding domain. Interestingly, a recent study demonstrated that Flublok elicited more protective antibody responses in older adults than egg-based vaccines in 2014 to 2015 (42). In addition, it has been demonstrated that a high-dose trivalent inactivated influenza virus vaccine induced higher serum HAI titers and seroprotection rates in elderly individuals (>65 years old) than a normal-dose vaccine (43).
Altogether, these results support a model in which a higher dose of antigen (HA) induces a greater proportion of antibodies targeting the immunodominant head domain around the receptor-binding domain. Both vaccine platforms induced low frequencies of stalk-reactive antibodies, likely due to the fact that the stalk is part of a full-length HA in a nonsoluble form (rosettes). Thus, alternative vaccine strategies using recombinant soluble proteins or headless HAs or chimeric HAs with avian exotic heads will be of interest in the quest for a universal influenza virus vaccine based on broadly cross-reactive antibodies (44, 45).
MATERIALS AND METHODS
Human cohorts.
Healthy adults (18 to 49 years old) enrolled in a vaccine comparison study in 2015 to 2016 (Flublok [Protein Sciences] versus Flucelvax [Seqirus] versus Fluzone [Sanofi]) were included in this analysis (ClinicalTrials.gov registration no. NCT03068949). The institutional review boards of The University of Rochester and the University of Chicago approved the study. Informed consent was obtained from all participants. Blood samples were collected from each participant at d0, d7, and d28 postvaccination, and plasmablasts were isolated from d7 blood samples.
Cells, viruses, and recombinant proteins.
Human embryonic kidney HEK293T mice (female; CRL-11268) were purchased and authenticated by the American Type Culture Collection (ATCC). Mice of the MDCK London line (female; IRR number FR-58) were purchased and authenticated by IRR. All cell lines were maintained under a humidified atmosphere of 5% CO2 at 37°C. HEK293T cells were maintained in advanced DMEM (Dulbecco’s modified Eagle medium) (Invitrogen) supplemented with 2% ultralow IgG fetal bovine serum (FBS) (Invitrogen), 1% l-glutamine (Invitrogen), and 1% antibiotic-antimycotic (Invitrogen). MDCK cells were maintained in DMEM supplemented with 10% FBS (Invitrogen), 1% l-glutamine (Invitrogen), and 1% penicillin-streptomycin (Invitrogen). All cell lines were used at low passages. All influenza virus stocks used for the assays were freshly grown in specific-pathogen-free eggs and purified, and titers were determined. Recombinant HA proteins derived from influenza virus strains A/Brisbane/59/2007 (H1N1), A/Perth/16/2009 (H3N2), and B/Brisbane/60/2008 were obtained from BEI resources. All other HA proteins were expressed in the baculovirus expression system as described before (46). Amino acid sequences were obtained from the GISAID: A/California/7/2009 (GenBank accession no. EPI177294), A/Brisbane/10/2010 (EPI745532), A/Switzerland/9715293/2013 (EPI814528), A/South Australia/55/2014 (EPI539694), B/Phuket/3073/2013 (EPI544267), and B/Utah/9/2014 (EPI538119).
Monoclonal antibody production.
Monoclonal antibodies were generated as previously described (47), in accordance with the protocols of the University of Chicago Institutional Review Board. Briefly, plasmablasts were single-cell sorted in 96-well plates using the following antibodies: CD3-fluorescein isothiocyanate (MHCD0301; Life Technologies), CD19-PB (clone HIB19; BioLegend), CD27-phycoerythrin (clone O323; BioLegend), and CD38-AF647 (clone HIT2; BioLegend). The BCR heavy- and light-chain variable regions were amplified and cloned into expression vectors. HEK293T cells were cotransfected with the plasmids using polyethylenimine (PEI; Polysciences), and supernatant containing the secreted antibody was collected 4 to 5 days after transfection and purified using protein A beads (Pierce).
Human PBMC ELISpot assays.
Filter plates (96-well; Millipore) were incubated overnight at 4°C with anti-human IgG, IgA, and IgM (KPL) and 50 μl/ml influenza vaccine (Flublok or Flucelvax). After a blocking step with RPMI medium–10% fetal calf serum (FCS; Invitrogen) for 2 h at 37°C, freshly isolated peripheral blood mononuclear cells (PBMCs) were washed three times and resuspended in medium (RPMI medium [Invitrogen] supplemented with 1% l-glutamine, 1% HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] [Invitrogen], 1% penicillin-streptomycin, 10% FCS), added to each plate, and serially diluted 2-fold down the plate. After an overnight incubation at 37°C, the plate was washed extensively with phosphate-buffered saline (PBS) and PBS–0.05% Tween 20, and ASCs were detected with biotinylated anti-human IgG or IgA (Southern Biotech) followed by streptavidin-alkaline phosphatase (Southern Biotech) and developed with nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (NBT/BCIP; Thermo Scientific). The plates were imaged (Cellular Technologies) and spots were manually counted to determine the number of ASCs.
ELISA.
Plates (96-well Costar; Corning) were coated with recombinant proteins at various concentrations depending on the protein (from 0.5 to 2 μg/ml) in PBS (pH 7.4) overnight at 4°C. For viruses, plates were coated with eight hemagglutination units (HAU) of virus in carbonate buffer overnight at 4°C. After blocking, antibodies were incubated (starting concentration, 10 μg/ml) for 1 h at 37°C. Horseradish peroxidase (HRP)-conjugated goat anti-human IgG antibody (Jackson ImmunoResearch) was used to detect binding of the MAbs, followed by development with Super Aquablue ELISA substrate (eBiosciences). Absorbance was measured at 405 nm on a microplate spectrophotometer (Bio-Rad). To standardize the assays, antibodies with known binding characteristics were included on each plate, and the plates were developed when the absorbance of these controls reached 3.0 ± 0.1 optical density (OD) units. An antibody was considered positive (strain reactive) if the OD at 405 nm was >0.5 after background subtraction when the positive control had reached an OD of 3. Equilibrium dissociation constant (KD) values of antibody binding were determined by Scatchard analysis using nonlinear regression (one-site binding model) on GraphPad Prism 6 software.
Competition ELISA.
Competition ELISAs were performed by inhibition of binding of each biotinylated antibody of interest at the half-maximal binding concentration with a 10-fold molar excess of competitor antibody. HRP-conjugated streptavidin (Southern Biotech) was used for detection. The absorbance value of each antibody against itself is scored at 100% inhibition, and comparison of different antibodies was done as a percentage of this 100% inhibition.
HAI assay.
Viruses were diluted to eight HA units/50 μl, and 25 μl was combined in duplicate wells with an equal volume of antibody serially diluted in PBS and incubated at room temperature (RT) for 45 min. Fifty microliters of 0.5% turkey red blood cells (Lampire Biological) was then added and incubated for 1 h at RT. Minimum effective concentrations were read based on the final dilution for which hemagglutination was observed.
Plaque assay and PRNT50 assay.
Plaque assays were performed as previously described (48), with the exception that the MDCK cells were incubated for 48 h with the agar overlay supplemented with MAbs. Plaques were counted, and the final concentration of antibody that reduced plaque numbers to 50% (PRNT50) was determined using GraphPad Prism 6 software.
Mouse immunization and ELISpot assay.
Seven- to 8-week-old female BALB/cJ mice (Jackson Laboratory) were immunized subcutaneously with 50 μl of seasonal influenza virus vaccine [Flublok (n = 5) or Flucelvax (n = 5) plus 10 μg poly(I·C); InvivoGen] in 50 μl PBS for a total injection volume of 100 μl. The mice were sacrificed at day 10 postimmunization, and the spleen and draining lymph nodes were collected. Single-cell suspensions were prepared in complete medium (RPMI supplemented with 100 U/ml penicillin/streptomycin, 4 mM l-glutamine, 50 μM β-mercaptoethanol [Sigma], and 10% FBS) and used for ELISpot assay. Ninety-six-well ELISpot plates (Thermo Fisher Scientific) were coated overnight at 4°C with vaccine at 50 μl/ml (Flublok or Flucelvax), recombinant protein at 2 μg/ml (full-length A/California/4/2009 H1N1 HA, chimeric H8/1 with H8 head from A/mallard/Sweden/24/02 [H8N4] combined with the A/California/4/2009 stalk, full-length A/Switzerland/9715293/2014 H3N2 HA, and chimeric H14/3 with H14 head from A/mallard/Gurjev/263/82 [H14N5] combined with H3 A/Perth/16/09 stalk), or goat-anti mouse Igκ (Southern Biotech) at 2 μg/ml, all diluted in PBS. The plates were washed in PBS and blocked with complete medium before a 2-fold serial dilution of cell suspension was added, starting at 0.1 × 106 to 2.0 × 106 cells. After incubation overnight at 37°C, 5% CO2, the plates were washed in PBS with 0.05% Tween 20, and IgG-secreting cells were detected by biotinylated goat-anti mouse IgG (Southern Biotech) followed by streptavidin-alkaline phosphatase (Southern Biotech) and NBT/BCIP substrate. The plates were imaged (Cellular Technologies) and spots manually counted. This experiment was approved by the University of Chicago Institutional Animal Care and Use Committee.
Statistical analysis.
All statistical analyses were performed using GraphPad Prism 6. Statistical significance was usually determined using two-tailed nonparametric unpaired Mann-Whitney U test, Fisher’s exact test, or paired Wilcoxon test, as detailed in the figure legends. Nonparametric tests were used because of nonnormal distribution for the majority of the data, as determined by GraphPad Prism using the D’Agostino and Pearson omnibus normality test. P values equal to or lower than 0.05 were considered significant.
Data availability.
The antibody sequences have been deposited in GenBank under accession numbers MN267205 to MN267364.
Supplementary Material
ACKNOWLEDGMENTS
We thank Parnavi Desai, Fatima Amanat, and Shirin Strohmeier for technical assistance. We thank Karla Thatcher Rojas, Donna Neu, and the University of Rochester Medical Center clinical team for subject recruitment and blood sample collection.
P.C.W. and C.H. are supported by National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, CEIRS contract HHSN272201400005C. P.C.W. is also supported by National Institute of Allergy and Infectious Diseases grant numbers U19AI082724, P01AI097092, U19AI109946, and U19AI057266. Work in the Krammer laboratory was supported by CEIRS contract HHSN272201400008C.
We have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.01150-19.
REFERENCES
- 1.Weir JP, Gruber MF. 2016. An overview of the regulation of influenza vaccines in the United States. Influenza Other Respir Viruses 10:354–360. doi: 10.1111/irv.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heaton NS, Sachs D, Chen CJ, Hai R, Palese P. 2013. Genome-wide mutagenesis of influenza virus reveals unique plasticity of the hemagglutinin and NS1 proteins. Proc Natl Acad Sci U S A 110:20248–20253. doi: 10.1073/pnas.1320524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry Dunand CJ, Leon PE, Kaur K, Tan GS, Zheng NY, Andrews S, Huang M, Qu X, Huang Y, Salgado-Ferrer M, Ho IY, Taylor W, Hai R, Wrammert J, Ahmed R, Garcia-Sastre A, Palese P, Krammer F, Wilson PC. 2015. Preexisting human antibodies neutralize recently emerged H7N9 influenza strains. J Clin Investig 125:1255–1268. doi: 10.1172/JCI74374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moody MA, Zhang R, Walter EB, Woods CW, Ginsburg GS, McClain MT, Denny TN, Chen X, Munshaw S, Marshall DJ, Whitesides JF, Drinker MS, Amos JD, Gurley TC, Eudailey JA, Foulger A, DeRosa KR, Parks R, Meyerhoff RR, Yu JS, Kozink DM, Barefoot BE, Ramsburg EA, Khurana S, Golding H, Vandergrift NA, Alam SM, Tomaras GD, Kepler TB, Kelsoe G, Liao HX, Haynes BF. 2011. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS One 6:e25797. doi: 10.1371/journal.pone.0025797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margine I, Hai R, Albrecht RA, Obermoser G, Harrod AC, Banchereau J, Palucka K, Garcia-Sastre A, Palese P, Treanor JJ, Krammer F. 2013. H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J Virol 87:4728–4737. doi: 10.1128/JVI.03509-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dreyfus C, Laursen NS, Kwaks T, Zuijdgeest D, Khayat R, Ekiert DC, Lee JH, Metlagel Z, Bujny MV, Jongeneelen M, van der Vlugt R, Lamrani M, Korse HJ, Geelen E, Sahin O, Sieuwerts M, Brakenhoff JP, Vogels R, Li OT, Poon LL, Peiris M, Koudstaal W, Ward AB, Wilson IA, Goudsmit J, Friesen RH. 2012. Highly conserved protective epitopes on influenza B viruses. Science 337:1343–1348. doi: 10.1126/science.1222908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekiert DC, Friesen RH, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJ, Brandenburg B, Vogels R, Brakenhoff JP, Kompier R, Koldijk MH, Cornelissen LA, Poon LL, Peiris M, Koudstaal W, Wilson IA, Goudsmit J. 2011. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 333:843–850. doi: 10.1126/science.1204839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zost SJ, Parkhouse K, Gumina ME, Kim K, Diaz Perez S, Wilson PC, Treanor JJ, Sant AJ, Cobey S, Hensley SE. 2017. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A 114:12578–12583. doi: 10.1073/pnas.1712377114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox MM, Hollister JR. 2009. FluBlok, a next generation influenza vaccine manufactured in insect cells. Biologicals 37:182–189. doi: 10.1016/j.biologicals.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Moro PL, Winiecki S, Lewis P, Shimabukuro TT, Cano M. 2015. Surveillance of adverse events after the first trivalent inactivated influenza vaccine produced in mammalian cell culture (Flucelvax) reported to the Vaccine Adverse Event Reporting System (VAERS), United States, 2013-2015. Vaccine 33:6684–6688. doi: 10.1016/j.vaccine.2015.10.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox MM, Patriarca PA, Treanor J. 2008. FluBlok, a recombinant hemagglutinin influenza vaccine. Influenza Other Respir Viruses 2:211–219. doi: 10.1111/j.1750-2659.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milian E, Kamen AA. 2015. Current and emerging cell culture manufacturing technologies for influenza vaccines. Biomed Res Int 2015:504831. doi: 10.1155/2015/504831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treanor JJ, Schiff GM, Hayden FG, Brady RC, Hay CM, Meyer AL, Holden-Wiltse J, Liang H, Gilbert A, Cox M. 2007. Safety and immunogenicity of a baculovirus-expressed hemagglutinin influenza vaccine: a randomized controlled trial. JAMA 297:1577–1582. doi: 10.1001/jama.297.14.1577. [DOI] [PubMed] [Google Scholar]
- 14.Cox MM, Karl Anderson D. 2007. Production of a novel influenza vaccine using insect cells: protection against drifted strains. Influenza Other Respir Viruses 1:35–40. doi: 10.1111/j.1750-2659.2006.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frey S, Vesikari T, Szymczakiewicz-Multanowska A, Lattanzi M, Izu A, Groth N, Holmes S. 2010. Clinical efficacy of cell culture-derived and egg-derived inactivated subunit influenza vaccines in healthy adults. Clin Infect Dis 51:997–1004. doi: 10.1086/656578. [DOI] [PubMed] [Google Scholar]
- 16.Allen CD, Okada T, Cyster JG. 2007. Germinal-center organization and cellular dynamics. Immunity 27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z, Woo CJ, Iglesias-Ussel MD, Ronai D, Scharff MD. 2004. The generation of antibody diversity through somatic hypermutation and class switch recombination. Genes Dev 18:1–11. doi: 10.1101/gad.1161904. [DOI] [PubMed] [Google Scholar]
- 18.Angeletti D, Yewdell JW. 2018. Understanding and manipulating viral immunity: antibody immunodominance enters center stage. Trends Immunol 39:549–561. doi: 10.1016/j.it.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Caton AJ, Brownlee GG, Yewdell JW, Gerhard W. 1982. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell 31:417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- 20.Yewdell JW, Webster RG, Gerhard WU. 1979. Antigenic variation in three distinct determinants of an influenza type A haemagglutinin molecule. Nature 279:246–248. doi: 10.1038/279246a0. [DOI] [PubMed] [Google Scholar]
- 21.Gerhard W, Yewdell J, Frankel ME, Webster R. 1981. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature 290:713–717. doi: 10.1038/290713a0. [DOI] [PubMed] [Google Scholar]
- 22.Altman MO, Bennink JR, Yewdell JW, Herrin BR. 2015. Lamprey VLRB response to influenza virus supports universal rules of immunogenicity and antigenicity. Elife 4:e07467. doi: 10.7554/eLife.07467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angeletti D, Kosik I, Yewdell WT, Boudreau CM, Mallajosyula VV, Chambers M, Prabhakaran M, Hickman HD, McDermott AB, Alter G, Chaudhuri J, Yewdell JW. 2018. Outflanking immunodominance to target subdominant broadly neutralizing epitopes. bioRxiv 10.1101/346437. [DOI] [PMC free article] [PubMed]
- 24.Andrews SF, Huang Y, Kaur K, Popova LI, Ho IY, Pauli NT, Henry Dunand CJ, Taylor WM, Lim S, Huang M, Qu X, Lee JH, Salgado-Ferrer M, Krammer F, Palese P, Wrammert J, Ahmed R, Wilson PC. 2015. Immune history profoundly affects broadly protective B cell responses to influenza. Sci Transl Med 7:316ra192. doi: 10.1126/scitranslmed.aad0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skehel JJ, Stevens DJ, Daniels RS, Douglas AR, Knossow M, Wilson IA, Wiley DC. 1984. A carbohydrate side chain on hemagglutinins of Hong Kong influenza viruses inhibits recognition by a monoclonal antibody. Proc Natl Acad Sci U S A 81:1779–1783. doi: 10.1073/pnas.81.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tate MD, Job ER, Deng YM, Gunalan V, Maurer-Stroh S, Reading PC. 2014. Playing hide and seek: how glycosylation of the influenza virus hemagglutinin can modulate the immune response to infection. Viruses 6:1294–1316. doi: 10.3390/v6031294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang CC, Chen JR, Tseng YC, Hsu CH, Hung YF, Chen SW, Chen CM, Khoo KH, Cheng TJ, Cheng YS, Jan JT, Wu CY, Ma C, Wong CH. 2009. Glycans on influenza hemagglutinin affect receptor binding and immune response. Proc Natl Acad Sci U S A 106:18137–18142. doi: 10.1073/pnas.0909696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen JR, Yu YH, Tseng YC, Chiang WL, Chiang MF, Ko YA, Chiu YK, Ma HH, Wu CY, Jan JT, Lin KI, Ma C, Wong CH. 2014. Vaccination of monoglycosylated hemagglutinin induces cross-strain protection against influenza virus infections. Proc Natl Acad Sci U S A 111:2476–2481. doi: 10.1073/pnas.1323954111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.An Y, Rininger JA, Jarvis DL, Jing X, Ye Z, Aumiller JJ, Eichelberger M, Cipollo JF. 2013. Comparative glycomics analysis of influenza hemagglutinin (H5N1) produced in vaccine relevant cell platforms. J Proteome Res 12:3707–3720. doi: 10.1021/pr400329k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krammer F, Palese P. 2015. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov 14:167–182. doi: 10.1038/nrd4529. [DOI] [PubMed] [Google Scholar]
- 31.Eggink D, Goff PH, Palese P. 2014. Guiding the immune response against influenza virus hemagglutinin toward the conserved stalk domain by hyperglycosylation of the globular head domain. J Virol 88:699–704. doi: 10.1128/JVI.02608-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. 2008. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nachbagauer R, Choi A, Izikson R, Cox MM, Palese P, Krammer F. 2016. Age dependence and isotype specificity of influenza virus hemagglutinin stalk-reactive antibodies in humans. mBio 7:e01996. doi: 10.1128/mBio.01996-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox M. 2013. Recombinant influenza vaccine. In Buckland B, Aunins J, Alves P, Jansen K (ed), Proceedings of Vaccine Technology IV. Engineering Conferences International, New York, NY. [Google Scholar]
- 35.McCraw DM, Gallagher JR, Harris AK. 2016. Characterization of influenza vaccine hemagglutinin complexes by cryo-electron microscopy and image analyses reveals structural polymorphisms. Clin Vaccine Immunol 23:483–495. doi: 10.1128/CVI.00085-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khurana S, Hahn M, Coyle EM, King LR, Lin TL, Treanor J, Sant A, Golding H. 2019. Repeat vaccination reduces antibody affinity maturation across different influenza vaccine platforms in humans. Nat Commun 10:3338. doi: 10.1038/s41467-019-11296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunkle LM, Izikson R, Patriarca PA, Goldenthal KL, Muse D, Cox M. 2017. Randomized comparison of immunogenicity and safety of quadrivalent recombinant versus inactivated influenza vaccine in healthy adults 18–49 years of age. J Infect Dis 216:1219–1226. doi: 10.1093/infdis/jix478. [DOI] [PubMed] [Google Scholar]
- 38.Guthmiller JJ, Wilson PC. 2018. Harnessing immune history to combat influenza viruses. Curr Opin Immunol 53:187–195. doi: 10.1016/j.coi.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Henry C, Palm AE, Krammer F, Wilson PC. 2018. From original antigenic sin to the universal influenza virus vaccine. Trends Immunol 39:70–79. doi: 10.1016/j.it.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mallajosyula VV, Citron M, Ferrara F, Lu X, Callahan C, Heidecker GJ, Sarma SP, Flynn JA, Temperton NJ, Liang X, Varadarajan R. 2014. Influenza hemagglutinin stem-fragment immunogen elicits broadly neutralizing antibodies and confers heterologous protection. Proc Natl Acad Sci U S A 111:E2514–E2523. doi: 10.1073/pnas.1402766111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi X, Jarvis DL. 2007. Protein N-glycosylation in the baculovirus-insect cell system. Curr Drug Targets 8:1116–1125. doi: 10.2174/138945007782151360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunkle LM, Izikson R, Patriarca P, Goldenthal KL, Muse D, Callahan J, Cox MMJ, PSC12 Study Team. 2017. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 376:2427–2436. doi: 10.1056/NEJMoa1608862. [DOI] [PubMed] [Google Scholar]
- 43.DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, Pollak R, Christoff J, Earl J, Landolfi V, Martin E, Gurunathan S, Nathan R, Greenberg DP, Tornieporth NG, Decker MD, Talbot HK. 2014. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 371:635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 44.Krammer F, Palese P. 2019. Universal influenza virus vaccines that target the conserved hemagglutinin stalk and conserved sites in the head domain. J Infect Dis 219:S62–S67. doi: 10.1093/infdis/jiy711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krammer F, Garcia-Sastre A, Palese P. 2018. Is it possible to develop a “universal” influenza virus vaccine? Potential target antigens and critical aspects for a universal influenza vaccine. Cold Spring Harb Perspect Biol 10:a028845. doi: 10.1101/cshperspect.a028845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Margine I, Palese P, Krammer F. 2013. Expression of functional recombinant hemagglutinin and neuraminidase proteins from the novel H7N9 influenza virus using the baculovirus expression system. J Vis Exp 81:51112. doi: 10.3791/51112:e51112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith K, Garman L, Wrammert J, Zheng NY, Capra JD, Ahmed R, Wilson PC. 2009. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat Protoc 4:372–384. doi: 10.1038/nprot.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wrammert J, Koutsonanos D, Li G-M, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, Mehta A, Razavi B, Del Rio C, Zheng N-Y, Lee J-H, Huang M, Ali Z, Kaur K, Andrews S, Amara RR, Wang Y, Das SR, O'Donnell CD, Yewdell JW, Subbarao K, Marasco WA, Mulligan MJ, Compans R, Ahmed R, Wilson PC. 2011. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med 208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The antibody sequences have been deposited in GenBank under accession numbers MN267205 to MN267364.