Due to the limiting coding capacity for members of the Picornaviridae family of positive-strand RNA viruses, their successful replication cycles require complex interactions with host cell functions.
KEYWORDS: RNA binding proteins, RNA metabolism, RNA splicing, RNA virus replication, coxsackievirus, microRNA, picornavirus, poliovirus, rhinovirus
ABSTRACT
Due to the limiting coding capacity for members of the Picornaviridae family of positive-strand RNA viruses, their successful replication cycles require complex interactions with host cell functions. These interactions span from the down-modulation of many aspects of cellular metabolism to the hijacking of specific host functions used during viral translation, RNA replication, and other steps of infection by picornaviruses, such as human rhinovirus, coxsackievirus, poliovirus, foot-and-mouth disease virus, enterovirus D-68, and a wide range of other human and nonhuman viruses. Although picornaviruses replicate exclusively in the cytoplasm of infected cells, they have extensive interactions with host cell nuclei and the proteins and RNAs that normally reside in this compartment of the cell. This review will highlight some of the more recent studies that have revealed how picornavirus infections impact the RNA metabolism of the host cell posttranscriptionally and how they usurp and modify host RNA binding proteins as well as microRNAs to potentiate viral replication.
INTRODUCTION
The production of cellular RNA, from transcription to decay, is complex and tightly regulated. Proper execution of RNA processing is critical for cell homeostasis and, when misregulated, is frequently implicated in disease states, such as tumorigenesis, neurodegeneration, and musculoskeletal pathologies (1–4), to name a few. Therefore, given the complexity of RNA metabolism, viruses are provided many opportunities to exploit this process to their advantage. In this review, we will focus on recent advances in our understanding of how members of the Picornaviridae family modify and usurp different elements of host cell RNA metabolism at the posttranscriptional level.
RNA metabolism can be broadly defined by the following categories: synthesis/transcription, folding, precursor messenger (pre-mRNA) processing, editing/nucleotide modification, transport and nuclear export, and decay. Additionally, all cellular RNAs are not created equal, and noncoding RNAs are being increasingly appreciated as having important roles during picornavirus infection, most notably as having an impact on the outcome of disease caused by these viruses (5, 6). Therefore, in addition to focusing in-depth on the recent developments in picornavirus modification of RNA processing, we will explore picornavirus interactions with noncoding RNA, specifically, microRNA (miRNA).
ALTERATIONS OF mRNA EXPORT PATHWAYS
Viral modulation of cellular nuclear pore complexes.
Picornaviruses, which comprise 29 different genera, are nonenveloped, icosahedral viruses that contain a small, positive-sense RNA genome of ∼7.5 kb. Although picornavirus genomic RNA lacks a 5′ 7-methylguanosine cap, the viral RNA can be immediately translated upon release into the cytoplasm of infected cells. Viral translation occurs using a cap-independent mechanism via an internal ribosome entry site (IRES) located in the 5′ noncoding region (5′ NCR) of viral RNA. The RNA also possesses a virally encoded poly(A) tract and is translated into a single polyprotein, which is then proteolytically processed into functional precursor molecules and mature viral proteins. Picornaviruses complete their replication cycle entirely in the host cytoplasm; however, these viruses have extensive interactions with the host cell nucleus and, in particular, induce the shutoff of mRNA transcription, a subject that has been comprehensively reviewed (7–12). Pertinent to the current microreview are the alterations that picornaviruses make to host RNA nuclear export pathways. Specifically, the 2A proteinase (2Apro) encoded by poliovirus (PV), the prototypical picornavirus, has been demonstrated to cleave components of the nuclear pore complex (NPC), specifically, Nup98, Nup153, and Nup62 (13–15), leading to a bidirectional increase in nuclear envelop permeability (16). Notably, these cleavage events have an impact on aspects of RNA export (13). Castello and colleagues (13) demonstrated that poliovirus 2Apro expression in HeLa cells resulted in Nup cleavage, in addition to a dose-dependent retention of a subset of cellular mRNAs in the nucleus, including those encoding Cox-1, Cox-2, interleukin 6 (IL-6), c-myc, and p53. This retention was also observed for 18S rRNA and U2 small nuclear RNA (snRNA) but, interestingly, not for cellular tRNAs or the constitutively expressed β-actin mRNA. The nuclear retention of this subset of RNAs was reversed by pretreating cells with gamma interferon (IFN-γ) prior to 2Apro expression; however, this reversal was not dependent on IFN-γ antagonism of 2Apro proteolytic activity, as the proteinase remained active in the presence of the cytokine.
A picornavirus in the Cardiovirus genus, Theiler’s murine encephalomyelitis virus (TMEV), was also shown to block mRNA export but through a different mechanism. The 2A peptide of cardioviruses does not possess proteolytic activity. Instead, these viruses express what is known as the L or leader protein, which causes alterations to the NPC via hyperphosphorylation of several Nups (17, 18; for a review, see reference 7), including Nup 98 (19). Using in situ hybridization with an oligo(dT) probe in BALB/3T3 cells expressing the L protein, it was demonstrated that TMEV L induced phosphorylation of Nup 98 and that this resulted in global retention of the main cellular pool of polyadenylated RNA within the nucleus (19). Notably, infection of cells with TMEV expressing the wild-type L protein also prevented the dimerization of interferon regulatory factor 3 (IRF3), a precursor event to IRF3 translocation to the nucleus and transactivation of IFN-α/β and interferon-stimulated gene expression.
Unlike with TMEV, global mRNA export does not appear to be affected during poliovirus infection with a wild-type virus expressing active 2Apro, as demonstrated by Park and colleagues (15) using an in situ hybridization, oligo(dT) experimental strategy. This may suggest that the bulk of cellular RNAs may not be retained in the nucleus during poliovirus infection, but as observed previously (13), the restriction during infection may be limited to certain classes of RNA. Park et al. also showed that degradation of Nup98 alone does not result in mRNA nuclear retention, consistent with multiple Nups playing roles in the nuclear export of RNA (15). Future work in this area will be required to clarify the impact of Nup cleavage during poliovirus infection on cellular mRNA export. Moreover, the mechanism of how interferon antagonizes viral-protein-induced RNA nuclear retention should be further investigated. Finally, it will be important to determine if there are virus species- and/or genus-specific differences that govern alterations to this critical step of RNA processing.
DISRUPTION OF PRE-mRNA SPLICING AND MOONLIGHTING OF HOST RNA SPLICING FACTORS DURING PICORNAVIRUS INFECTION
An all-important element of host RNA metabolism is pre-mRNA splicing. Encompassed in this process is the alternative inclusion and exclusion of exons and the removal of introns to generate multiple isoforms of a given mRNA. Alternative splicing allows for the expansion and diversification of the eukaryotic proteome relative to the number of genes in the DNA genome (20). Pre-mRNA splicing is carried out by an intricate ribonucleoprotein (RNP) complex termed the spliceosome, which is comprised of 5 small nuclear RNPs (snRNPs) (U1, U2, U4/U6, and U5) and over ∼100 auxiliary proteins (21). Furthermore, alternative splicing is governed by splicing regulators, including the serine/arginine-rich (SR) proteins and heterogeneous nuclear RNPs (hnRNPs) (22, 23). It is important to note that both classes of these proteins have diverse functions, including roles in splicing, transcriptional regulation, mRNA stability, translation, telomere function, and mediation of DNA damage responses, among others (23–26). Thus, given their pleiotropic nature, coupled with their ubiquitous and multiple RNA binding domains, it follows that cellular splicing factors are also important additions to the picornavirus arsenal of repurposed cellular proteins.
Functions of the host proteins PCBP1 and PCBP2 in picornavirus translation and the switch to RNA synthesis.
Several host cell hnRNPs have been shown to have indispensable roles in translation and RNA replication for specific picornaviruses, including poliovirus, human rhinoviruses (HRVs), and coxsackievirus. These viruses take advantage of poly(rC) binding proteins 1 and 2 (PCBP1 and PCBP2, also known as hnRNP E1 and hnRNP E2), proteins that function in RNA splicing and transcriptional regulation, among other activities, in uninfected host cells (27). In particular, PCBP2 has recently been shown to be a key regulator of erythropoiesis during embryonic development by promoting the inclusion of exon 6 in the hematopoietic master regulator Runx1 (28). For picornaviruses, it is well established that PCBP1 and PCBP2 act as IRES trans-acting factors (ITAFs) to promote virus translation by forming an RNP complex with a major stem-loop structure, called SL-IV, within the IRES (29, 30). PCBP1 and PCBP2 also bind to C-rich sequences within the first ∼100 nucleotides of the 5′ NCR to promote viral RNA replication (31–34). However, there are differences in the affinities of PCBP1 and -2 for poliovirus SL-IV, with PCBP2 having a 50-fold-higher binding affinity than PCBP1 (35, 36). This differential affinity was shown to be mediated by the linker region between the second and third K-homologous (KH) domains of PCBP2 (35). Moreover, it has been demonstrated that only PCBP2, not PCBP1, can rescue in vitro translation of poliovirus genomic RNA in PCBP-depleted HeLa S10 extracts (36). This result, together with the observations (i) that several other picornavirus IRESs, including those of enterovirus 71 (EV71) (37), coxsackievirus B3 (CVB3) (38), HRV (38), and hepatitis A virus (39), require PCBP2 to drive translation and (ii) that the protein has been shown to bind to the IRES, supports the conclusion that PCBP2 plays a critical role in facilitating type I (and possibly type III) picornavirus IRES translation.
As noted above, PCBP2 (as well as PCBP1) also binds to sequences within the first ∼100 nucleotides of the poliovirus 5′ NCR that form a cloverleaf-like structure (also called stem-loop I or SL-I) (32, 33). A viral proteinase-polymerase precursor protein (3CD) binds to a distinct sequence element within SL-I to form a ternary complex with PCBP1/2 and a shift in the occupancy of PCBP2 from SL-IV to SL-I, which reduces the levels of virus translation and promotes negative-strand RNA synthesis (31, 36, 40–42). This is important to note because a crucial challenge of any positive-stranded RNA virus, including the picornaviruses, is to balance the use of the positive-strand RNA for translation versus negative-strand RNA synthesis, as both processes cannot occur simultaneously on the same RNA template (41, 43). One possible activity that resolves this biological challenge is the cleavage of PCBP2 by the viral 3CD proteinase during poliovirus infection, which has been proposed as a mechanism to mediate the switch between virus translation and RNA replication in this family of viruses (44, 45). Specifically, the protein is cleaved between the KH2 and KH3 domains, producing a truncated form of PCBP2 lacking the KH3 domain. Cleaved PCBP2 does not bind to the viral IRES but retains its ability to form the replication-competent, ternary complex with the 5′ cloverleaf RNA structure, suggesting that removal of this key RNA binding domain facilitates the switch. In addition, the critical interactions that occur between PCBP1/2 at the 5′ end and poly(A) binding protein (PABP) at the 3′ end suggest a possible mechanism in which circularization of the positive-strand RNA is required to facilitate viral translation and, subsequently, negative-strand RNA synthesis (46, 47).
hnRNP C.
Circularization of viral RNA has also been proposed to function in the priming of positive-strand RNA synthesis on the negative-strand template for picornaviruses, albeit via a different repertoire of host cell proteins than for negative-strand RNA synthesis (48–50). A critical factor involved in this mechanism is hnRNP C (51). In the uninfected cell, hnRNP C functions as a splicing regulator, most notably acting to suppress the deleterious inclusion of Alu elements into mature gene transcripts (52). For picornaviruses, hnRNP C has been shown to bind separately to both the 5′- and 3′-terminal sequences of the negative-strand RNA template, and binding may be dependent on multimerization of the protein (48). Accordingly, replication of viral RNA in vitro is enhanced by the addition of recombinant hnRNP C, and depletion of the protein in HeLa cells results in a decrease in positive-strand RNA synthesis (48, 51). Replication kinetics are also delayed for poliovirus in cells expressing levels of endogenous hnRNP C that are lower than those of other cells (53). Collectively, these studies suggest a mechanism by which hnRNP C facilitates interactions between the 5′ and 3′ termini of viral RNAs, which are critical for positive-strand RNA synthesis. While this is a current model, an important caveat to note is that binding of hnRNP C to both termini simultaneously has not yet been demonstrated. The difficulty in observing simultaneous binding of hnRNP C to the ends of viral RNA suggests that the interactions of hnRNP C with the RNA may be transient, are needed only to facilitate the initiation of RNA synthesis, and do not need to be sustained throughout the elongation process. This would make the protein available to initiate the synthesis of multiple positive-strand RNAs on a single negative-strand template, generating the well-characterized picornavirus replicative intermediate. Further studies in this area are needed to characterize the nature and composition of the hnRNP C-mediated protein complex.
hnRNP A1.
For viral ITAFs to bind the IRES and initiate translation, the RNA must fold into the appropriate tertiary structure (54, 55). Recently, the structure of SL-2 in the EV71 IRES was obtained using nuclear magnetic resonance (NMR)–small-angle X-ray scattering techniques (56). It was revealed that a conserved 5′-AUAGC-3′ bulge in SL-2 and the accompanying base-stacking interactions facilitate the binding of the established EV71 ITAF, hnRNP A1 (57–59), providing critical molecular insights into the function of this protein during viral translation. Normally, hnRNP A1 has functions in uninfected mammalian cells that include RNA splicing and telomere maintenance, among a host of others (60). Consistently with its use as a proviral ITAF, hnRNP A1 has also been shown to relocalize from the nucleus to the cytoplasm during EV71 and HRV16 infection (14, 61), and this is likely dependent on the Misshapen/nuclear shuttle protein-interacting kinase (NIK)-related kinase/p38 mitogen-activated kinase phosphorylation pathway (62). Given that hnRNP A1 has also been shown to bind to SL-2 of the CVB16 IRES and that, importantly, binding of hnRNP A1 impacts CVB16 virulence (63), it will be informative to determine if the protein acts as an ITAF for other picornaviruses and to what degree (if any) its binding affects viral pathogenicity.
PTBP1 and PVS/1(RIPO).
Polypyrimidine tract binding protein 1 (PTBP1, also known as hnRNP1) is a nucleus-cytoplasm shuttling protein involved in a wide range of RNA metabolism processes (64) that acts as an additional critical picornavirus ITAF (37, 65–72). It is also a key determinant of viral neurovirulence (73, 74) and neurotropism (75). Particularly, overexpression of PTBP1 in SK-N-MC neuroblastoma cell lines has been shown to enhance the replication of PV1(RIPO) (75). PV1(RIPO) is a chimeric virus consisting of the poliovirus type 1 (Mahoney strain) coding region and the HRV2 IRES that grows to high titers in grade IV malignant glioma-derived cell lines but is attenuated in normal neuronal tissues (76, 77). When injected into mice bearing glioma xenografts, PV1(RIPO) halted tumor progression or eliminated the tumor entirely (78). A variant of this virus, PVS(RIPO), which utilizes the live, attenuated Sabin poliovirus type 1 coding region, has recently demonstrated promise in phase I clinical trials for treatment of patients with recurrent glioblastoma (79).
Despite its success as a treatment, the exact molecular mechanisms governing the difference between the PVS/1(RIPO) growth phenotypes in normal versus malignant brain tissues have only recently begun to be elucidated. The growth-suppressive element of PV1(RIPO) was mapped to SL-V/VI of the HRV2 IRES (77, 80). An RNA affinity chromatography screen using SL-V/VI RNA identified a double-stranded RNA binding protein, DRBP76, as a critical restrictive ITAF for PV1(RIPO) exclusively in nonmalignant neuronal cells (80). Intriguingly, the protein is also present in glioma-derived cells, although the subcellular distribution is distinct (80). These observations suggest that cell type-specific differences control the availability and/or capability of DRBP76 to bind to the HRV2 IRES (80). Since PTBP1 confers the ability of PV1(RIPO) to replicate in cells of neuronal origin and other nonpermissive cell lines (i.e., mouse L20B cells) and is upregulated in several cancers, including glioblastoma (81), it is tempting to speculate that PTBP1 might compete with DRBP76 for binding to the 5′ NCR of the HRV2 IRES. Competitive binding between DRBP76 and PTBP1 may be a potential mechanism for the growth-restricted phenotype observed for the PV1(RIPO) virus.
hnRNP M.
In contrast to the hnRNPs mentioned above, not all hnRNPs have a direct role in the molecular underpinnings of picornavirus replication, and their actions may extend to other facets of infection. An example of this is the recent finding that hnRNP M has a positive regulatory role during poliovirus and CVB3 infection (82). This protein is normally involved in mRNA splicing in uninfected cells (83). Specifically, hnRNP M has been shown to play a role in promoting the epithelial-mesenchymal transition during breast cancer by regulating the production of a mesenchyme-specific CD44 splice variant (84). The protein was subsequently identified as part of a large-scale screen to uncover novel substrates of CVB3 proteinase 3C (82). It was shown that hnRNP M relocalizes from the nucleus to the cytoplasm during infection and that knockdown of the protein reduces both poliovirus and CVB3 virus yields, supporting the idea that it facilitates virus infection. However, depletion of hnRNP M does not interfere with viral IRES-mediated translation or affect RNA stability, and hnRNP M is not directly involved in viral RNA synthesis (although it does partially colocalize with a viral replication protein, 2C, in the cytoplasm of human-rhinovirus-infected cells [85]). This suggests that the full-length protein and/or the cleavage products may act on other steps of virus infection, such as modulating the host innate immune response. Additional studies will be required to characterize the mechanism of action for hnRNP M during a picornavirus infection. It will be of significant interest to determine if there are any conserved trends throughout the virus family in terms of the types of binding interactions that proteolytic cleavage fragments of hnRNP M have with other cellular proteins and/or the viral RNA itself.
SRp20 and TIA1.
SRp20 (also known as SRSF3) is an RNA binding protein that shuttles between the nucleus and the cytoplasm and is involved in cellular mRNA splicing (86). SRp20 has been shown to interact with host protein PCBP2 and may act synergistically to drive poliovirus translation (87). Several follow-up studies revealed that SRp20 is relocalized from the nucleus to the cytoplasm during poliovirus infection and that this relocalization is dependent on the activity of viral 2Apro (88, 89). Interestingly, SRp20 was also demonstrated to interact with the stress granule protein and splicing regulator TIA1 in the cytoplasm of poliovirus-infected cells, although exogenous expression of a wild-type or dominant negative form of TIA1 had no significant effect on virus infection (90).
In a subsequent publication, a putative role for TIA1 during poliovirus infection that involves alternative splicing was suggested (91). Viral 2Apro induces a selective disruption of nucleocytoplasmic trafficking in which several splicing regulators, including TIA1, and essential splicing factors, U2AF35 and U2AF65, are relocalized to the cytoplasm, while human antigen R (HuR) is retained in the nucleus. HuR and TIA1 were demonstrated to have opposing effects on the inclusion of exon 6 in the mRNA encoding the receptor for Fas (a key mediator of apoptosis) in 2Apro-expressing cells. The authors speculated that selective relocalization of splicing regulators during infection might be a previously unrecognized strategy deployed by picornaviruses to regulate alternative splicing and, in turn, cellular gene expression to their advantage (91).
Disruption of pre-mRNA slicing via Prp8.
By modulating key regulators of splicing, picornaviruses may exert global control over this aspect of RNA metabolism and modulate large classes of genes, as opposed to regulating the splicing of only certain mRNAs encoding specific proteins. Another mechanism by which picornaviruses might achieve this level of control is by altering core components of the spliceosome itself, which was observed following EV71 infection. Specifically, Liu and coworkers (92) followed up on the finding that several picornavirus proteins enter the host cell nucleus, including nonstructural protein 3CD and the capsid protein VP1 (93–96), a puzzling observation given that these viruses replicate exclusively in the cytoplasm. They found that the RNA-dependent RNA polymerase (RdRp) of EV71, 3Dpol, translocates to the nucleus during viral infection and interacts with human pre-mRNA splicing factor 8 (Prp8), a central component within the catalytic core of the spliceosome. EV71 3Dpol was shown to interfere with the splicing of a radiolabeled pre-mRNA substrate using in vitro splicing assays. The addition of 3Dpol induced an accumulation of the lariat intermediate form of the pre-mRNA, indicating that the polymerase inhibits the second step of the splicing pathway. Interestingly, this result was also observed for poliovirus 3Dpol but not for RdRps of the closely related picornaviruses CVB3 and HRV. Importantly, EV71 3Dpol-mediated disruption of pre-mRNA splicing was concluded to occur via its interaction with Prp8. Finally, the global nature of this mechanism of splicing control was demonstrated using RNA immunoprecipitation followed by high-throughput sequencing. Over 2,000 Prp8-associated mRNA transcripts were found to be differentially expressed in infected cells compared to in mock-infected controls. These mRNAs were related to cell growth, proliferation, and differentiation. Given that all three of these pathways are involved in picornavirus infections (97–101), additional studies will be required to characterize the precise molecular and cellular changes that result from large-scale, Prp8-mediated disruption of alternative pre-mRNA splicing.
SFPQ, a novel proviral factor.
The localization of 3CD to the nuclei of picornavirus-infected cells was also observed in a recent study aimed at elucidating proteome-wide alterations to nucleocytoplasmic trafficking during human rhinovirus type 16 (HRV16) infection (85). Using quantitative mass spectrometry, a number of candidate nuclear proteins which were relocalized to the cytoplasm during infection, including splicing factor proline and glutamine rich (SFPQ), were identified. SFPQ was shown to be necessary for efficient HRV16 replication in HeLa cells, as small interfering RNA (siRNA)-mediated knockdown of the protein resulted in decreased viral titers, virus translation, and RNA synthesis (85). The protein was also demonstrated to be cleaved by 3CD, and the cleavage fragment had the ability to bind to HRV16 genomic RNA sequences. Notably, SFPQ relocalization of the cleavage product occurred concomitantly with the increase in viral RNA synthesis and after the peak of virus translation. These observations suggest that SFPQ likely plays a role in HRV16 RNA synthesis or virion assembly (85). Furthermore, given that a significant majority of the proteins identified in the screen are involved in RNA splicing, this observation further supports the idea that picornaviruses may use the selective disruption of nucleocytoplasmic trafficking as a mechanism to exert global control over multiple RNA metabolism processes, including RNA splicing.
BRIEF UPDATE OF PICORNAVIRUS INTERACTIONS WITH THE mRNA DECAY MACHINERY
The cellular factors that have been discussed thus far have mostly proviral roles during picornavirus infection. However, there have been several mRNA decay factors described to have antiviral properties. For a recent comprehensive review of this topic, see reference 102. This section will briefly highlight two examples that have emerged since the publication of this review.
XRN1 and the interplay between autophagy and mRNA decay during picornavirus infections.
Within the last decade, there has been a paradigm shift with respect to the notion that picornaviruses are exclusively lytic upon egress from the host cell, a logical extension of their status as nonenveloped viruses. There is a growing body of evidence to suggest that picornaviruses also engage in an alternate host cell exit strategy termed autophagosome-mediated exit without lysis (AWOL) (103–110). During AWOL, the virus is proposed to be secreted from the cell as cargo inside double-membrane, autophagosome-derived vesicles (111). Consistently with the AWOL hypothesis, it was recently demonstrated that a protein associated with mRNA decay and processing bodies, XRN1, functions as a negative regulator of autophagy (112). Importantly, depletion of XRN1 in either poliovirus or CVB3-infected HeLa or human brain microvascular endothelial cells results in a significant increase in titer for both viruses, compared to those of controls (112). These data further implicate the autophagy pathway in picornavirus egress and demonstrate a novel intersection between autophagy and mRNA decay for picornaviruses.
KHSRP.
The mRNA decay protein KH-type splicing-regulatory protein (KHSRP) is also a negative regulator of picornavirus replication, albeit one that acts more directly than the above example. KHSRP had previously been demonstrated to be a negative ITAF for EV71 translation (113), and further insight into the mechanism of its negative regulatory role was recently gained (114). Using isobaric tags for relative and absolute quantitation (iTRAQ) mass spectrometry, Kelch-like protein 12 (KLHL12) was identified as a binding partner of KHSRP in EV71-infected human muscle rhabdomyosarcoma cells. KLHL12 was shown to promote CUL3-mediated ubiquitination of KHSRP, a modification required for the activity of KHSRP to downregulate EV71 translation. Moreover, variants of KHSRP with mutated ubiquitination domains were demonstrated to compete less efficiently with FUBP1 (a positive regulator of EV71 translation) than wild-type KHSRP for binding to the EV71 IRES, suggesting that ubiquitination status affects the ability of KHSRP to negatively regulate EV71 translation through competition with positive ITAFs.
PICORNAVIRUS INTERACTIONS WITH HOST miRNAs
miRNAs and picornavirus pathogenesis.
The role of miRNAs in controlling gene expression is critical. Additionally, it is well appreciated that miRNAs possess tissue specificity and that the same miRNA may have different or even opposing effects based on the cellular context (115, 116). Because of this, several transcriptomic analyses have been carried out to characterize the miRNA expression profile changes that occur during picornavirus infection (5, 117–122) and, critically, how these changes might impact viral pathogenesis. For example, it was recently demonstrated that the miRNA miR-1303 is likely involved in regulating blood-brain barrier permeability during CVA16 infection (120), an important finding given the severe neurological complications caused by the virus. Specifically, these studies demonstrate that miR-1303 is downregulated during either CVA16 or EV71 infection—also a causative agent of central nervous system (CNS) infections—compared to levels in mock-infected controls and that matrix metalloprotease 9 (MMP9) is a target of miR-1303 (120). On the basis of these findings, using in vivo experiments with rhesus monkeys as well as in vitro experiments, Song and coworkers showed that CVA16 upregulates MMP9 during infection, which leads to the decreased expression of cell junction proteins, including claudin5, VE-cadherin, and ZO-1, thereby increasing the permeability of the blood-brain barrier. This result was reversed in vitro by overexpressing miR-1303 (120). Histological analysis also revealed damage to the thalami of infected monkeys compared to those of mock-infected controls. Together, these results suggest that miR-1303 and MMP9 form a regulatory network that CVA16 (and perhaps EV71) exploits to invade the CNS. Other investigators have shown that several other miRNAs, including miR-3473a (123) and miR‑206 (124), may also be involved in modulating CNS injury during EV71 infection via control of focal adhesion assembly and leukocyte migration (miR-3473a) and upregulation of the chemokine CCL2, which promotes inflammation of the CNS (miR‑206).
The role of miRNAs in picornavirus pathogenesis is not limited to neurological disease. For example, coxsackieviruses have also been implicated in the development of type I diabetes (125). There are many prevailing hypotheses about the pathogenic mechanisms of the virus in this disease, and viral interactions with miRNAs have been identified as having potential roles. Engelmann and colleagues showed that persistent CVB4 infection in human pancreatic cells results in the dysregulation of a panel of 81 miRNAs (126). Moreover, using miRNA target prediction software, these researchers identified 49 known type I diabetes risk genes as potential targets of one or more of the dysregulated miRNAs, suggesting a link between miRNA expression profile changes elicited by persistent CVB4 and the risk for developing type I diabetes. In support of this proposed link, Kim et al. reported that acute CVB5 infection results in the dysregulation of 33 miRNAs in infected human pancreatic β islet cells and that 57 putative type I diabetes risk genes were predicted targets for the identified miRNAs (127). Determining the key gene expression changes that are sustained after coxsackievirus infection and/or maintained during persistent infection will be critical for elucidating the mechanisms by which these viruses might contribute to the development of type I diabetes.
Persistent coxsackievirus infections are also a significant cause of viral myocarditis, and miRNA profiling has been carried out in this disease context as well to uncover how the virus damages the heart during infection. Several recent studies have identified miRNAs that are associated with the outcome of virus-induced cardiac injury and involve both the innate and adaptive immune responses. miR-214 was shown to be upregulated in tissues from the hearts of patients with CVB3-induced myocarditis. This miRNA represses the expression of the itchy E3 ubiquitin ligase (ITCH), a repressor of NF-κB (128). miR-214 repression of ITCH was shown to cause the upregulation of several cytokines activated by the NF-κB pathway, including tumor necrosis factor alpha (TNF-α) and IL-6, suggesting that viral modulation of miR-214 might contribute to the inflammatory tissue microenvironment of acute myocarditis. In contrast, Bao and Lin found that miR-155, which is also upregulated in patients with myocarditis caused by CVB3, acts as a negative regulator of the NF-κB pathway through the targeting of RelA, a subunit of NF-κB (129). Interestingly, miR-155 was also shown to have proinflammatory effects during CVB3 myocarditis in mice, resulting in the infiltration of macrophages and T lymphocytes to the infection site (130). Concordantly, knockdown of miR-155 improved the survival and cardiac function of infected mice (130). Moreover, Liu et al. reported that TH-17 cells contribute to the pathology of viral myocarditis in mice and that inhibiting both miR-21 and miR-146b reduces the production of TH-17-associated markers, such as IL-17, IL-6, transforming growth factor β (TGF-β), and retinoic acid receptor-related orphan receptor gamma t (RORγt) (131). Downregulating these two miRNAs reduced cardiac injury and resulted in a decrease in the proportion of TH-17 cells harvested from the spleens of CVB3-infected, miRNA inhibitor-treated mice.
As a key mediator of both innate and adaptive immunity, type I interferon has been shown to have interactions with miRNAs that have significant consequences for the outcomes of picornavirus infections. Ho and colleagues showed that EV71 upregulates miR-146a through the activity of activating protein-1 (AP-1), a dimeric transcription factor consisting of Jun, Fos, or other factors (132), and that this upregulation reduces the expression of the Toll-like receptor signaling molecules IRAK1 and TRAF6 (133). Moreover, Ho et al. demonstrated that treating both EV71-infected mouse embryonic fibroblast cell lines and live mice with a long noncoding RNA (lnc-RNA)-based miR-146a inhibitor (i.e., an antagomir) restored IRAK1 and TRAF6 expression, improved mouse survival, and, importantly, restored IFN-β production. These data suggest a novel mechanism by which miR-146a expression is achieved by EV71 activation of the c-Jun kinase pathway, which consequently downregulates and weakens immune responses to viral infection, allowing for viral innate immune escape. Finally, EV71 has also been demonstrated to induce the expression of miR-141 during infection, which facilitates the shutoff of host translation via targeting of the eukaryotic translation initiation factor eIF4E. Downregulation of eIF4E was shown to contribute to the switch between translation and RNA synthesis during virus infection. Consequently, this influenced the efficiency of virus production and the extent of the cytopathic effect induced by the infection in cultured cells (134), an observation which, if expanded to an in vivo context, may have implications for pathogenesis as well.
Together, the above-described findings underscore the importance of elucidating the myriad gene expression targets of miRNAs and understanding their functions in picornavirus pathogenesis, likely illuminating key intersections between miRNA regulation and the immune system. Critically, the data also highlight the possibility of miRNA and/or antagomir-based antivirals to treat picornavirus infections.
CONCLUDING REMARKS
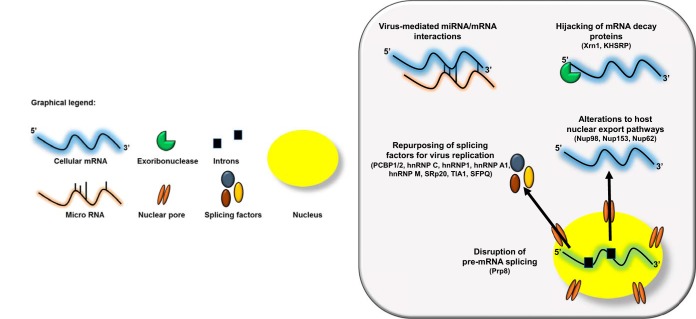
As summarized in Fig. 1, picornavirus alterations to RNA metabolic pathways represent a master class in viral host cell manipulation. The preceding is only a small vignette of the numerous (and clever) ways that picornaviruses usurp RNA processing machinery. Several critical questions remain, including the following.
FIG 1.
Picornaviruses alter host RNA metabolism at multiple steps. The viral-RNA-dependent RNA polymerase 3Dpol of the picornavirus EV71 disrupts host cell pre-mRNA splicing via its interaction with the core splicing machinery protein Prp8. Nuclear cytoplasmic trafficking is also dysregulated during picornavirus infection via either the cleavage of nuclear pore complexes (NPCs) by the enteroviral proteinase 2Apro or hyperphosphorylation of the NPC by cardiovirus L protein. These alterations result in the nuclear retention of certain classes of cellular RNAs, in addition to the cytoplasmic relocalization of a suite of splicing factors required for picornavirus replication. Several picornaviruses hijack mRNA decay proteins, most notably the processing body protein Xrn1, to facilitate their replication cycle as well as an interaction that positively regulates autophagy during picornavirus infection and provides further evidence for the AWOL hypothesis for picornavirus egress. Finally, picornaviruses have been shown to mediate several novel cellular mRNA-miRNA interactions, which have implications for viral pathogenesis.
-
1.
What are the specific gene expression changes that occur as a result of picornavirus alterations to pre-mRNA splicing and nuclear-cytoplasmic relocalization of splicing factors? How do these changes contribute to overall remodeling of the host cell during infection? It is clear that picornaviruses utilize hnRNPs and SR proteins for the mechanics of their replication cycles, but what are the global consequences of siphoning these proteins away from their normal cell functions?
-
2.
What are the fundamental intersections between mRNA decay and autophagy as it relates to picornavirus replication? Can these provide further molecular insights into the use of AWOL as an alternate picornavirus egress strategy?
-
3.
How are gene expression changes sustained after clearance of acute picornavirus infection or maintained throughout persistent virus infection to cause disease? How does the reconfiguration of the miRNA expression profile contribute to these gene expression changes? What role does the immune system play in this process?
By examining these questions in greater detail, we can deepen our understanding of picornavirus biology in tandem with RNA metabolism and, in turn, develop targeted antivirals for these viruses and retool them to our advantage as oncolytic therapeutics, drug delivery systems, and more.
ACKNOWLEDGMENTS
We are indebted to Alexis Bouin for critical comments on the manuscript.
Research described in our lab was supported by U.S. Public Health Service grants AI126879 and AI026765 to B.L.S. A.C.H. was the recipient of the Lorna Carlin Scholar Award from the UC, Irvine, School of Medicine and was supported, in part, by the NIH-IMSD Graduate Program at UC, Irvine (grant R25 GM055246).
REFERENCES
- 1.Chang G, Leu JS, Ma L, Xie K, Huang S. 2019. Methylation of RNA N(6)-methyladenosine in modulation of cytokine responses and tumorigenesis. Cytokine 118:35–41. doi: 10.1016/j.cyto.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Falcone C, Mazzoni C. 2018. RNA stability and metabolism in regulated cell death, aging and diseases. FEMS Yeast Res 18:yoy050. doi: 10.1093/femsyr/foy050. [DOI] [PubMed] [Google Scholar]
- 3.Ravanidis S, Kattan FG, Doxakis E. 2018. Unraveling the pathways to neuronal homeostasis and disease: mechanistic insights into the role of RNA-binding proteins and associated factors. Int J Mol Sci 19:E2280. doi: 10.3390/ijms19082280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Y, Kuek V, Liu Y, Tickner J, Yuan Y, Chen L, Zeng Z, Shao M, He W, Xu J. 2019. MiR-214 is an important regulator of the musculoskeletal metabolism and disease. J Cell Physiol 234:231–245. doi: 10.1002/jcp.26856. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, Song J, Liu L, Li J, Tang B, Wang J, Zhang X, Zhang Y, Wang L, Liao Y, He Z, Li Q. 2016. Different microRNA alterations contribute to diverse outcomes following EV71 and CA16 infections: insights from high-throughput sequencing in rhesus monkey peripheral blood mononuclear cells. Int J Biochem Cell Biol 81:20–31. doi: 10.1016/j.biocel.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Sun L, Sun H, Liu X, Luo X, Li C, Sun D, Li T. 2017. Overexpression of microRNA-133b reduces myocardial injuries in children with viral myocarditis by targeting Rab27B gene. Cell Mol Biol (Noisy-le-grand) 63:80–86. doi: 10.14715/cmb/2017.63.10.13. [DOI] [PubMed] [Google Scholar]
- 7.Flather D, Semler BL. 2015. Picornaviruses and nuclear functions: targeting a cellular compartment distinct from the replication site of a positive-strand RNA virus. Front Microbiol 6:594. doi: 10.3389/fmicb.2015.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gustin KE. 2003. Inhibition of nucleo-cytoplasmic trafficking by RNA viruses: targeting the nuclear pore complex. Virus Res 95:35–44. doi: 10.1016/S0168-1702(03)00165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiscox JA. 2003. The interaction of animal cytoplasmic RNA viruses with the nucleus to facilitate replication. Virus Res 95:13–22. doi: 10.1016/S0168-1702(03)00160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weidman MK, Sharma R, Raychaudhuri S, Kundu P, Tsai W, Dasgupta A. 2003. The interaction of cytoplasmic RNA viruses with the nucleus. Virus Res 95:75–85. doi: 10.1016/S0168-1702(03)00164-3. [DOI] [PubMed] [Google Scholar]
- 11.Yarbrough ML, Mata MA, Sakthivel R, Fontoura BM. 2014. Viral subversion of nucleocytoplasmic trafficking. Traffic 15:127–140. doi: 10.1111/tra.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Younessi P, A Jans D, Ghildyal R. 2012. Modulation of host cell nucleocytoplasmic trafficking during picornavirus infection. Infect Disord Drug Targets 12:59–67. doi: 10.2174/187152612798994993. [DOI] [PubMed] [Google Scholar]
- 13.Castello A, Izquierdo JM, Welnowska E, Carrasco L. 2009. RNA nuclear export is blocked by poliovirus 2A protease and is concomitant with nucleoporin cleavage. J Cell Sci 122:3799–3809. doi: 10.1242/jcs.055988. [DOI] [PubMed] [Google Scholar]
- 14.Gustin KE, Sarnow P. 2001. Effects of poliovirus infection on nucleo-cytoplasmic trafficking and nuclear pore complex composition. EMBO J 20:240–249. doi: 10.1093/emboj/20.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park N, Katikaneni P, Skern T, Gustin KE. 2008. Differential targeting of nuclear pore complex proteins in poliovirus-infected cells. J Virol 82:1647–1655. doi: 10.1128/JVI.01670-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belov GA, Lidsky PV, Mikitas OV, Egger D, Lukyanov KA, Bienz K, Agol VI. 2004. Bidirectional increase in permeability of nuclear envelope upon poliovirus infection and accompanying alterations of nuclear pores. J Virol 78:10166–10177. doi: 10.1128/JVI.78.18.10166-10177.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter FW, Bochkov YA, Albee AJ, Wiese C, Palmenberg AC. 2006. A picornavirus protein interacts with Ran-GTPase and disrupts nucleocytoplasmic transport. Proc Natl Acad Sci U S A 103:12417–12422. doi: 10.1073/pnas.0605375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porter FW, Palmenberg AC. 2009. Leader-induced phosphorylation of nucleoporins correlates with nuclear trafficking inhibition by cardioviruses. J Virol 83:1941–1951. doi: 10.1128/JVI.01752-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricour C, Delhaye S, Hato SV, Olenyik TD, Michel B, van Kuppeveld FJ, Gustin KE, Michiels T. 2009. Inhibition of mRNA export and dimerization of interferon regulatory factor 3 by Theiler’s virus leader protein. J Gen Virol 90:177–186. doi: 10.1099/vir.0.005678-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y, Rio DC. 2015. Mechanisms and regulation of alternative pre-mRNA splicing. Annu Rev Biochem 84:291–323. doi: 10.1146/annurev-biochem-060614-034316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faustino NA, Cooper TA. 2003. Pre-mRNA splicing and human disease. Genes Dev 17:419–437. doi: 10.1101/gad.1048803. [DOI] [PubMed] [Google Scholar]
- 22.Busch A, Hertel KJ. 2012. Evolution of SR protein and hnRNP splicing regulatory factors. Wiley Interdiscip Rev RNA 3:1–12. doi: 10.1002/wrna.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shepard PJ, Hertel KJ. 2009. The SR protein family. Genome Biol 10:242. doi: 10.1186/gb-2009-10-10-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anantha RW, Alcivar AL, Ma J, Cai H, Simhadri S, Ule J, Konig J, Xia B. 2013. Requirement of heterogeneous nuclear ribonucleoprotein C for BRCA gene expression and homologous recombination. PLoS One 8:e61368. doi: 10.1371/journal.pone.0061368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geuens T, Bouhy D, Timmerman V. 2016. The hnRNP family: insights into their role in health and disease. Hum Genet 135:851–867. doi: 10.1007/s00439-016-1683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moumen A, Masterson P, O'Connor MJ, Jackson SP. 2005. hnRNP K: an HDM2 target and transcriptional coactivator of p53 in response to DNA damage. Cell 123:1065–1078. doi: 10.1016/j.cell.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 27.Makeyev AV, Liebhaber SA. 2002. The poly(C)-binding proteins: a multiplicity of functions and a search for mechanisms. RNA 8:265–278. doi: 10.1017/s1355838202024627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghanem LR, Kromer A, Silverman IM, Ji X, Gazzara M, Nguyen N, Aguilar G, Martinelli M, Barash Y, Liebhaber SA. 2018. Poly(C)-binding protein Pcbp2 enables differentiation of definitive erythropoiesis by directing functional splicing of the Runx1 transcript. Mol Cell Biol 38:e00175-18. doi: 10.1128/MCB.00175-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blyn LB, Swiderek KM, Richards O, Stahl DC, Semler BL, Ehrenfeld E. 1996. Poly(rC) binding protein 2 binds to stem-loop IV of the poliovirus RNA 5′ noncoding region: identification by automated liquid chromatography-tandem mass spectrometry. Proc Natl Acad Sci U S A 93:11115–11120. doi: 10.1073/pnas.93.20.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blyn LB, Towner JS, Semler BL, Ehrenfeld E. 1997. Requirement of poly(rC) binding protein 2 for translation of poliovirus RNA. J Virol 71:6243–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gamarnik AV, Andino R. 2000. Interactions of viral protein 3CD and poly(rC) binding protein with the 5′ untranslated region of the poliovirus genome. J Virol 74:2219–2226. doi: 10.1128/JVI.74.5.2219-2226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gamarnik AV, Andino R. 1997. Two functional complexes formed by KH domain containing proteins with the 5′ noncoding region of poliovirus RNA. RNA 3:882–892. [PMC free article] [PubMed] [Google Scholar]
- 33.Parsley TB, Towner JS, Blyn LB, Ehrenfeld E, Semler BL. 1997. Poly (rC) binding protein 2 forms a ternary complex with the 5′-terminal sequences of poliovirus RNA and the viral 3CD proteinase. RNA 3:1124–1134. [PMC free article] [PubMed] [Google Scholar]
- 34.Toyoda H, Franco D, Fujita K, Paul AV, Wimmer E. 2007. Replication of poliovirus requires binding of the poly(rC) binding protein to the cloverleaf as well as to the adjacent C-rich spacer sequence between the cloverleaf and the internal ribosomal entry site. J Virol 81:10017–10028. doi: 10.1128/JVI.00516-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sean P, Nguyen JH, Semler BL. 2008. The linker domain of poly(rC) binding protein 2 is a major determinant in poliovirus cap-independent translation. Virology 378:243–253. doi: 10.1016/j.virol.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walter BL, Parsley TB, Ehrenfeld E, Semler BL. 2002. Distinct poly(rC) binding protein KH domain determinants for poliovirus translation initiation and viral RNA replication. J Virol 76:12008–12022. doi: 10.1128/JVI.76.23.12008-12022.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu Y, Sweeney TR, Kafasla P, Jackson RJ, Pestova TV, Hellen CU. 2011. The mechanism of translation initiation on Aichivirus RNA mediated by a novel type of picornavirus IRES. EMBO J 30:4423–4436. doi: 10.1038/emboj.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walter BL, Nguyen JH, Ehrenfeld E, Semler BL. 1999. Differential utilization of poly(rC) binding protein 2 in translation directed by picornavirus IRES elements. RNA 5:1570–1585. doi: 10.1017/S1355838299991483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang B, Seitz S, Kusov Y, Zell R, Gauss-Müller V. 2007. RNA interaction and cleavage of poly(C)-binding protein 2 by hepatitis A virus protease. Biochem Biophys Res Commun 364:725–730. doi: 10.1016/j.bbrc.2007.09.133. [DOI] [PubMed] [Google Scholar]
- 40.Franco D, Pathak HB, Cameron CE, Rombaut B, Wimmer E, Paul AV. 2005. Stimulation of poliovirus RNA synthesis and virus maturation in a HeLa cell-free in vitro translation-RNA replication system by viral protein 3CDpro. Virol J 2:86. doi: 10.1186/1743-422X-2-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gamarnik AV, Andino R. 1998. Switch from translation to RNA replication in a positive-stranded RNA virus. Genes Dev 12:2293–2304. doi: 10.1101/gad.12.15.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silvera D, Gamarnik AV, Andino R. 1999. The N-terminal K homology domain of the poly(rC)-binding protein is a major determinant for binding to the poliovirus 5′-untranslated region and acts as an inhibitor of viral translation. J Biol Chem 274:38163–38170. doi: 10.1074/jbc.274.53.38163. [DOI] [PubMed] [Google Scholar]
- 43.Barton DJ, Morasco BJ, Flanegan JB. 1999. Translating ribosomes inhibit poliovirus negative-strand RNA synthesis. J Virol 73:10104–10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chase AJ, Daijogo S, Semler BL. 2014. Inhibition of poliovirus-induced cleavage of cellular protein PCBP2 reduces the levels of viral RNA replication. J Virol 88:3192–3201. doi: 10.1128/JVI.02503-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perera R, Daijogo S, Walter BL, Nguyen JH, Semler BL. 2007. Cellular protein modification by poliovirus: the two faces of poly(rC)-binding protein. J Virol 81:8919–8932. doi: 10.1128/JVI.01013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barton DJ, O'Donnell BJ, Flanegan JB. 2001. 5′ cloverleaf in poliovirus RNA is a cis-acting replication element required for negative-strand synthesis. EMBO J 20:1439–1448. doi: 10.1093/emboj/20.6.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herold J, Andino R. 2001. Poliovirus RNA replication requires genome circularization through a protein-protein bridge. Mol Cell 7:581–591. doi: 10.1016/S1097-2765(01)00205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ertel KJ, Brunner JE, Semler BL. 2010. Mechanistic consequences of hnRNP C binding to both RNA termini of poliovirus negative-strand RNA intermediates. J Virol 84:4229–4242. doi: 10.1128/JVI.02198-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roehl HH, Parsley TB, Ho TV, Semler BL. 1997. Processing of a cellular polypeptide by 3CD proteinase is required for poliovirus ribonucleoprotein complex formation. J Virol 71:578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roehl HH, Semler BL. 1995. Poliovirus infection enhances the formation of two ribonucleoprotein complexes at the 3′ end of viral negative-strand RNA. J Virol 69:2954–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brunner JE, Nguyen JH, Roehl HH, Ho TV, Swiderek KM, Semler BL. 2005. Functional interaction of heterogeneous nuclear ribonucleoprotein C with poliovirus RNA synthesis initiation complexes. J Virol 79:3254–3266. doi: 10.1128/JVI.79.6.3254-3266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zarnack K, Konig J, Tajnik M, Martincorena I, Eustermann S, Stevant I, Reyes A, Anders S, Luscombe NM, Ule J. 2013. Direct competition between hnRNP C and U2AF65 protects the transcriptome from the exonization of Alu elements. Cell 152:453–466. doi: 10.1016/j.cell.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brunner JE, Ertel KJ, Rozovics JM, Semler BL. 2010. Delayed kinetics of poliovirus RNA synthesis in a human cell line with reduced levels of hnRNP C proteins. Virology 400:240–247. doi: 10.1016/j.virol.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lozano G, Martinez-Salas E. 2015. Structural insights into viral IRES-dependent translation mechanisms. Curr Opin Virol 12:113–120. doi: 10.1016/j.coviro.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 55.Martinez-Salas E, Francisco-Velilla R, Fernandez-Chamorro J, Lozano G, Diaz-Toledano R. 2015. Picornavirus IRES elements: RNA structure and host protein interactions. Virus Res 206:62–73. doi: 10.1016/j.virusres.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 56.Tolbert M, Morgan CE, Pollum M, Crespo-Hernandez CE, Li ML, Brewer G, Tolbert BS. 2017. HnRNP A1 alters the structure of a conserved enterovirus IRES domain to stimulate viral translation. J Mol Biol 429:2841–2858. doi: 10.1016/j.jmb.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levengood JD, Tolbert M, Li ML, Tolbert BS. 2013. High-affinity interaction of hnRNP A1 with conserved RNA structural elements is required for translation and replication of enterovirus 71. RNA Biol 10:1136–1145. doi: 10.4161/rna.25107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin JY, Shih SR, Pan M, Li C, Lue CF, Stollar V, Li ML. 2009. hnRNP A1 interacts with the 5′ untranslated regions of enterovirus 71 and Sindbis virus RNA and is required for viral replication. J Virol 83:6106–6114. doi: 10.1128/JVI.02476-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shih SR, Stollar V, Li ML. 2011. Host factors in enterovirus 71 replication. J Virol 85:9658–9666. doi: 10.1128/JVI.05063-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jean-Philippe J, Paz S, Caputi M. 2013. hnRNP A1: the Swiss army knife of gene expression. Int J Mol Sci 14:18999–19024. doi: 10.3390/ijms140918999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gustin KE, Sarnow P. 2002. Inhibition of nuclear import and alteration of nuclear pore complex composition by rhinovirus. J Virol 76:8787–8796. doi: 10.1128/jvi.76.17.8787-8796.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leong SY, Ong BK, Chu JJ. 2015. The role of misshapen NCK-related kinase (MINK), a novel Ste20 family kinase, in the IRES-mediated protein translation of human enterovirus 71. PLoS Pathog 11:e1004686. doi: 10.1371/journal.ppat.1004686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Z, Liu X, Wang S, Li J, Hou M, Liu G, Zhang W, Yu XF. 2016. Identification of a nucleotide in 5′ untranslated region contributing to virus replication and virulence of Coxsackievirus A16. Sci Rep 6:20839. doi: 10.1038/srep20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Romanelli MG, Diani E, Lievens PM. 2013. New insights into functional roles of the polypyrimidine tract-binding protein. Int J Mol Sci 14:22906–22932. doi: 10.3390/ijms141122906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borman A, Howell MT, Patton JG, Jackson RJ. 1993. The involvement of a spliceosome component in internal initiation of human rhinovirus RNA translation. J Gen Virol 74:1775–1788. doi: 10.1099/0022-1317-74-9-1775. [DOI] [PubMed] [Google Scholar]
- 66.Borovjagin A, Pestova T, Shatsky I. 1994. Pyrimidine tract binding protein strongly stimulates in vitro encephalomyocarditis virus RNA translation at the level of preinitiation complex formation. FEBS Lett 351:299–302. doi: 10.1016/0014-5793(94)00848-5. [DOI] [PubMed] [Google Scholar]
- 67.Hellen CU, Witherell GW, Schmid M, Shin SH, Pestova TV, Gil A, Wimmer E. 1993. A cytoplasmic 57-kDa protein that is required for translation of picornavirus RNA by internal ribosomal entry is identical to the nuclear pyrimidine tract-binding protein. Proc Natl Acad Sci U S A 90:7642–7646. doi: 10.1073/pnas.90.16.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kafasla P, Lin H, Curry S, Jackson RJ. 2011. Activation of picornaviral IRESs by PTB shows differential dependence on each PTB RNA-binding domain. RNA 17:1120–1131. doi: 10.1261/rna.2549411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaminski A, Jackson RJ. 1998. The polypyrimidine tract binding protein (PTB) requirement for internal initiation of translation of cardiovirus RNAs is conditional rather than absolute. RNA 4:626–638. doi: 10.1017/S1355838298971898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niepmann M, Petersen A, Meyer K, Beck E. 1997. Functional involvement of polypyrimidine tract-binding protein in translation initiation complexes with the internal ribosome entry site of foot-and-mouth disease virus. J Virol 71:8330–8339. doi: 10.1016/0014-5793(96)00509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toyoda H, Koide N, Kamiyama M, Tobita K, Mizumoto K, Imura N. 1994. Host factors required for internal initiation of translation on poliovirus RNA. Arch Virol 138:1–15. doi: 10.1007/BF01310034. [DOI] [PubMed] [Google Scholar]
- 72.Venkatramana M, Ray PS, Chadda A, Das S. 2003. A 25 kDa cleavage product of polypyrimidine tract binding protein (PTB) present in mouse tissues prevents PTB binding to the 5′ untranslated region and inhibits translation of hepatitis A virus RNA. Virus Res 98:141–149. doi: 10.1016/j.virusres.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 73.Guest S, Pilipenko E, Sharma K, Chumakov K, Roos RP. 2004. Molecular mechanisms of attenuation of the Sabin strain of poliovirus type 3. J Virol 78:11097–11107. doi: 10.1128/JVI.78.20.11097-11107.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pilipenko EV, Viktorova EG, Guest ST, Agol VI, Roos RP. 2001. Cell-specific proteins regulate viral RNA translation and virus-induced disease. EMBO J 20:6899–6908. doi: 10.1093/emboj/20.23.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jahan N, Wimmer E, Mueller S. 2013. Polypyrimidine tract binding protein-1 (PTB1) is a determinant of the tissue and host tropism of a human rhinovirus/poliovirus chimera PV1(RIPO). PLoS One 8:e60791. doi: 10.1371/journal.pone.0060791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gromeier M, Alexander L, Wimmer E. 1996. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc Natl Acad Sci U S A 93:2370–2375. doi: 10.1073/pnas.93.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gromeier M, Bossert B, Arita M, Nomoto A, Wimmer E. 1999. Dual stem loops within the poliovirus internal ribosomal entry site control neurovirulence. J Virol 73:958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. 2000. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci U S A 97:6803–6808. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Desjardins A, Gromeier M, Herndon JE II, Beaubier N, Bolognesi DP, Friedman AH, Friedman HS, McSherry F, Muscat AM, Nair S, Peters KB, Randazzo D, Sampson JH, Vlahovic G, Harrison WT, McLendon RE, Ashley D, Bigner DD. 2018. Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med 379:150–161. doi: 10.1056/NEJMoa1716435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Merrill MK, Dobrikova EY, Gromeier M. 2006. Cell-type-specific repression of internal ribosome entry site activity by double-stranded RNA-binding protein 76. J Virol 80:3147–3156. doi: 10.1128/JVI.80.7.3147-3156.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anczukow O, Krainer AR. 2016. Splicing-factor alterations in cancers. RNA 22:1285–1301. doi: 10.1261/rna.057919.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jagdeo JM, Dufour A, Fung G, Luo H, Kleifeld O, Overall CM, Jan E. 2015. Heterogeneous nuclear ribonucleoprotein M facilitates enterovirus infection. J Virol 89:7064–7078. doi: 10.1128/JVI.02977-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Han SP, Tang YH, Smith R. 2010. Functional diversity of the hnRNPs: past, present and perspectives. Biochem J 430:379–392. doi: 10.1042/BJ20100396. [DOI] [PubMed] [Google Scholar]
- 84.Xu Y, Gao XD, Lee JH, Huang H, Tan H, Ahn J, Reinke LM, Peter ME, Feng Y, Gius D, Siziopikou KP, Peng J, Xiao X, Cheng C. 2014. Cell type-restricted activity of hnRNPM promotes breast cancer metastasis via regulating alternative splicing. Genes Dev 28:1191–1203. doi: 10.1101/gad.241968.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Flather D, Nguyen JHC, Semler BL, Gershon PD. 2018. Exploitation of nuclear functions by human rhinovirus, a cytoplasmic RNA virus. PLoS Pathog 14:e1007277. doi: 10.1371/journal.ppat.1007277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caceres JF, Screaton GR, Krainer AR. 1998. A specific subset of SR proteins shuttles continuously between the nucleus and the cytoplasm. Genes Dev 12:55–66. doi: 10.1101/gad.12.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bedard KM, Daijogo S, Semler BL. 2007. A nucleo-cytoplasmic SR protein functions in viral IRES-mediated translation initiation. EMBO J 26:459–467. doi: 10.1038/sj.emboj.7601494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fitzgerald KD, Chase AJ, Cathcart AL, Tran GP, Semler BL. 2013. Viral proteinase requirements for the nucleocytoplasmic relocalization of cellular splicing factor SRp20 during picornavirus infections. J Virol 87:2390–2400. doi: 10.1128/JVI.02396-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fitzgerald KD, Semler BL. 2011. Re-localization of cellular protein SRp20 during poliovirus infection: bridging a viral IRES to the host cell translation apparatus. PLoS Pathog 7:e1002127. doi: 10.1371/journal.ppat.1002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fitzgerald KD, Semler BL. 2013. Poliovirus infection induces the co-localization of cellular protein SRp20 with TIA-1, a cytoplasmic stress granule protein. Virus Res 176:223–231. doi: 10.1016/j.virusres.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alvarez E, Castello A, Carrasco L, Izquierdo JM. 2013. Poliovirus 2A protease triggers a selective nucleo-cytoplasmic redistribution of splicing factors to regulate alternative pre-mRNA splicing. PLoS One 8:e73723. doi: 10.1371/journal.pone.0073723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu YC, Kuo RL, Lin JY, Huang PN, Huang Y, Liu H, Arnold JJ, Chen SJ, Wang RY, Cameron CE, Shih SR. 2014. Cytoplasmic viral RNA-dependent RNA polymerase disrupts the intracellular splicing machinery by entering the nucleus and interfering with Prp8. PLoS Pathog 10:e1004199. doi: 10.1371/journal.ppat.1004199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Amineva SP, Aminev AG, Palmenberg AC, Gern JE. 2004. Rhinovirus 3C protease precursors 3CD and 3CD′ localize to the nuclei of infected cells. J Gen Virol 85:2969–2979. doi: 10.1099/vir.0.80164-0. [DOI] [PubMed] [Google Scholar]
- 94.Sharma R, Raychaudhuri S, Dasgupta A. 2004. Nuclear entry of poliovirus protease-polymerase precursor 3CD: implications for host cell transcription shut-off. Virology 320:195–205. doi: 10.1016/j.virol.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 95.Walker E, Jensen L, Croft S, Wei K, Fulcher AJ, Jans DA, Ghildyal R. 2016. Rhinovirus 16 2A protease affects nuclear localization of 3CD during infection. J Virol 90:11032–11042. doi: 10.1128/JVI.00974-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang T, Yu B, Lin L, Zhai X, Han Y, Qin Y, Guo Z, Wu S, Zhong X, Wang Y, Tong L, Zhang F, Si X, Zhao W, Zhong Z. 2012. A functional nuclear localization sequence in the VP1 capsid protein of coxsackievirus B3. Virology 433:513–521. doi: 10.1016/j.virol.2012.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barlow-Anacker A, Bochkov Y, Gern J, Seroogy CM. 2017. Neonatal immune response to rhinovirus A16 has diminished dendritic cell function and increased B cell activation. PLoS One 12:e0180664. doi: 10.1371/journal.pone.0180664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hewson CA, Haas JJ, Bartlett NW, Message SD, Laza-Stanca V, Kebadze T, Caramori G, Zhu J, Edbrooke MR, Stanciu LA, Kon OM, Papi A, Jeffery PK, Edwards MR, Johnston SL. 2010. Rhinovirus induces MUC5AC in a human infection model and in vitro via NF-kappaB and EGFR pathways. Eur Respir J 36:1425–1435. doi: 10.1183/09031936.00026910. [DOI] [PubMed] [Google Scholar]
- 99.Huttunen M, Turkki P, Maki A, Paavolainen L, Ruusuvuori P, Marjomaki V. 2017. Echovirus 1 internalization negatively regulates epidermal growth factor receptor downregulation. Cell Microbiol 19:e12671. doi: 10.1111/cmi.12671. [DOI] [PubMed] [Google Scholar]
- 100.Kalinowski A, Ueki I, Min-Oo G, Ballon-Landa E, Knoff D, Galen B, Lanier LL, Nadel JA, Koff JL. 2014. EGFR activation suppresses respiratory virus-induced IRF1-dependent CXCL10 production. Am J Physiol Lung Cell Mol Physiol 307:L186–L196. doi: 10.1152/ajplung.00368.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Long Q, Liao YH, Xie Y, Liang W, Cheng X, Yuan J, Yu M. 2016. Coxsackievirus B3 directly induced Th17 cell differentiation by inhibiting Nup98 expression in patients with acute viral myocarditis. Front Cell Infect Microbiol 6:171. doi: 10.3389/fcimb.2016.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ullmer W, Semler BL. 2016. Diverse strategies used by picornaviruses to escape host RNA decay pathways. Viruses 8:335. doi: 10.3390/v8120335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bird SW, Maynard ND, Covert MW, Kirkegaard K. 2014. Nonlytic viral spread enhanced by autophagy components. Proc Natl Acad Sci U S A 111:13081–13086. doi: 10.1073/pnas.1401437111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen YH, Du W, Hagemeijer MC, Takvorian PM, Pau C, Cali A, Brantner CA, Stempinski ES, Connelly PS, Ma HC, Jiang P, Wimmer E, Altan-Bonnet G, Altan-Bonnet N. 2015. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell 160:619–630. doi: 10.1016/j.cell.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jackson WT, Giddings TH Jr, Taylor MP, Mulinyawe S, Rabinovitch M, Kopito RR, Kirkegaard K. 2005. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol 3:e156. doi: 10.1371/journal.pbio.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lai JK, Sam IC, Chan YF. 2016. The autophagic machinery in enterovirus infection. Viruses 8:32. doi: 10.3390/v8020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Richards AL, Jackson WT. 2012. Intracellular vesicle acidification promotes maturation of infectious poliovirus particles. PLoS Pathog 8:e1003046. doi: 10.1371/journal.ppat.1003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Robinson SM, Tsueng G, Sin J, Mangale V, Rahawi S, McIntyre LL, Williams W, Kha N, Cruz C, Hancock BM, Nguyen DP, Sayen MR, Hilton BJ, Doran KS, Segall AM, Wolkowicz R, Cornell CT, Whitton JL, Gottlieb RA, Feuer R. 2014. Coxsackievirus B exits the host cell in shed microvesicles displaying autophagosomal markers. PLoS Pathog 10:e1004045. doi: 10.1371/journal.ppat.1004045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Taylor MP, Burgon TB, Kirkegaard K, Jackson WT. 2009. Role of microtubules in extracellular release of poliovirus. J Virol 83:6599–6609. doi: 10.1128/JVI.01819-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Too IH, Yeo H, Sessions OM, Yan B, Libau EA, Howe JL, Lim ZQ, Suku-Maran S, Ong WY, Chua KB, Wong BS, Chow VT, Alonso S. 2016. Enterovirus 71 infection of motor neuron-like NSC-34 cells undergoes a non-lytic exit pathway. Sci Rep 6:36983. doi: 10.1038/srep36983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Munz C. 2017. The autophagic machinery in viral exocytosis. Front Microbiol 8:269. doi: 10.3389/fmicb.2017.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Delorme-Axford E, Abernathy E, Lennemann NJ, Bernard A, Ariosa A, Coyne CB, Kirkegaard K, Klionsky DJ. 2018. The exoribonuclease Xrn1 is a post-transcriptional negative regulator of autophagy. Autophagy 14:898–912. doi: 10.1080/15548627.2018.1441648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin JY, Li ML, Shih SR. 2009. Far upstream element binding protein 2 interacts with enterovirus 71 internal ribosomal entry site and negatively regulates viral translation. Nucleic Acids Res 37:47–59. doi: 10.1093/nar/gkn901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kung YA, Hung CT, Chien KY, Shih SR. 2017. Control of the negative IRES trans-acting factor KHSRP by ubiquitination. Nucleic Acids Res 45:271–287. doi: 10.1093/nar/gkw1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hammond SM. 2015. An overview of microRNAs. Adv Drug Deliv Rev 87:3–14. doi: 10.1016/j.addr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mohr AM, Mott JL. 2015. Overview of microRNA biology. Semin Liver Dis 35:3–11. doi: 10.1055/s-0034-1397344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Basagoudanavar SH, Hosamani M, Tamil Selvan RP, Sreenivasa BP, Sanyal A, Venkataramanan R. 2018. Host serum microRNA profiling during the early stage of foot-and-mouth disease virus infection. Arch Virol 163:2055–2063. doi: 10.1007/s00705-018-3824-8. [DOI] [PubMed] [Google Scholar]
- 118.Hu Y, Song J, Liu L, Li J, Tang B, Zhang Y, Wang J, Wang L, Fan S, Feng M, Li Q. 2017. Comparison analysis of microRNAs in response to EV71 and CA16 infection in human bronchial epithelial cells by high-throughput sequencing to reveal differential infective mechanisms. Virus Res 228:90–101. doi: 10.1016/j.virusres.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 119.Jin J, Li R, Jiang C, Zhang R, Ge X, Liang F, Sheng X, Dai W, Chen M, Wu J, Xiao J, Su W. 2017. Transcriptome analysis reveals dynamic changes in coxsackievirus A16 infected HEK 293T cells. BMC Genomics 18:933. doi: 10.1186/s12864-016-3253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Song J, Hu Y, Li H, Huang X, Zheng H, Hu Y, Wang J, Jiang X, Li J, Yang Z, Fan H, Guo L, Shi H, He Z, Yang F, Wang X, Dong S, Li Q, Liu L. 2018. miR-1303 regulates BBB permeability and promotes CNS lesions following CA16 infections by directly targeting MMP9. Emerg Microbes Infect 7:1. doi: 10.1038/s41426-018-0157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Song J, Hu Y, Li J, Zheng H, Wang J, Guo L, Ning R, Li H, Yang Z, Fan H, Liu L. 2017. Different microRNA profiles reveal the diverse outcomes induced by EV71 and CA16 infection in human umbilical vein endothelial cells using high-throughput sequencing. PLoS One 12:e0177657. doi: 10.1371/journal.pone.0177657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu Z, Qi Y, Fan H, Cui L, Shi Z. 2016. Systematic identification and bioinformatic analysis of microRNAs in response to infections of coxsackievirus A16 and enterovirus 71. Biomed Res Int 2016:1. doi: 10.1155/2016/4302470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang X, Xie J, Jia L, Liu N, Liang Y, Wu F, Liang B, Li Y, Wang J, Sheng C, Li H, Liu H, Ma Q, Yang C, Du X, Qiu S, Song H. 2017. Analysis of miRNAs involved in mouse brain damage upon enterovirus 71 infection. Front Cell Infect Microbiol 7:133. doi: 10.3389/fcimb.2017.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang G, Wang J, Yao G, Shi B. 2017. Downregulation of CCL2 induced by the upregulation of microRNA-206 is associated with the severity of HEV71 encephalitis. Mol Med Rep 16:4620–4626. doi: 10.3892/mmr.2017.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hober D, Sauter P. 2010. Pathogenesis of type 1 diabetes mellitus: interplay between enterovirus and host. Nat Rev Endocrinol 6:279–289. doi: 10.1038/nrendo.2010.27. [DOI] [PubMed] [Google Scholar]
- 126.Engelmann I, Alidjinou EK, Bertin A, Bossu J, Villenet C, Figeac M, Sane F, Hober D. 2017. Persistent coxsackievirus B4 infection induces microRNA dysregulation in human pancreatic cells. Cell Mol Life Sci 74:3851–3861. doi: 10.1007/s00018-017-2567-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim KW, Ho A, Alshabee-Akil A, Hardikar AA, Kay TW, Rawlinson WD, Craig ME. 2016. Coxsackievirus B5 infection induces dysregulation of microRNAs predicted to target known type 1 diabetes risk genes in human pancreatic islets. Diabetes 65:996–1003. doi: 10.2337/db15-0956. [DOI] [PubMed] [Google Scholar]
- 128.Chen ZG, Liu H, Zhang JB, Zhang SL, Zhao LH, Liang WQ. 2015. Upregulated microRNA-214 enhances cardiac injury by targeting ITCH during coxsackievirus infection. Mol Med Rep 12:1258–1264. doi: 10.3892/mmr.2015.3539. [DOI] [PubMed] [Google Scholar]
- 129.Bao JL, Lin L. 2014. MiR-155 and miR-148a reduce cardiac injury by inhibiting NF-kappaB pathway during acute viral myocarditis. Eur Rev Med Pharmacol Sci 18:2349–2356. [PubMed] [Google Scholar]
- 130.Corsten MF, Papageorgiou A, Verhesen W, Carai P, Lindow M, Obad S, Summer G, Coort SL, Hazebroek M, van Leeuwen R, Gijbels MJ, Wijnands E, Biessen EA, De Winther MP, Stassen FR, Carmeliet P, Kauppinen S, Schroen B, Heymans S. 2012. MicroRNA profiling identifies microRNA-155 as an adverse mediator of cardiac injury and dysfunction during acute viral myocarditis. Circ Res 111:415–425. doi: 10.1161/CIRCRESAHA.112.267443. [DOI] [PubMed] [Google Scholar]
- 131.Liu YL, Wu W, Xue Y, Gao M, Yan Y, Kong Q, Pang Y, Yang F. 2013. MicroRNA-21 and -146b are involved in the pathogenesis of murine viral myocarditis by regulating TH-17 differentiation. Arch Virol 158:1953–1963. doi: 10.1007/s00705-013-1695-6. [DOI] [PubMed] [Google Scholar]
- 132.Fabienne B, Emilie E, Kazem Z, Marc P, Isabelle JE. 2019. The AP-1 transcriptional complex: local switch or remote command? Biochim Biophys Acta Rev Cancer 1872:11–23. doi: 10.1016/j.bbcan.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 133.Ho BC, Yu IS, Lu LF, Rudensky A, Chen HY, Tsai CW, Chang YL, Wu CT, Chang LY, Shih SR, Lin SW, Lee CN, Yang PC, Yu SL. 2014. Inhibition of miR-146a prevents enterovirus-induced death by restoring the production of type I interferon. Nat Commun 5:3344. doi: 10.1038/ncomms4344. [DOI] [PubMed] [Google Scholar]
- 134.Ho BC, Yu SL, Chen JJ, Chang SY, Yan BS, Hong QS, Singh S, Kao CL, Chen HY, Su KY, Li KC, Cheng CL, Cheng HW, Lee JY, Lee CN, Yang PC. 2011. Enterovirus-induced miR-141 contributes to shutoff of host protein translation by targeting the translation initiation factor eIF4E. Cell Host Microbe 9:58–69. doi: 10.1016/j.chom.2010.12.001. [DOI] [PubMed] [Google Scholar]