Advances in defining the composition of health-associated biofilms have highlighted the important role of beneficial species in maintaining health. Comparatively little, however, has been done to address the genomic and physiological bases underlying the probiotic mechanisms of beneficial commensals. In this study, we explored the ability of a novel oral bacterial isolate, Streptococcus A12, to compete with the dental pathogen Streptococcus mutans using various gene products with diverse functions. A12 displayed enhanced competitiveness by (i) disrupting intercellular communication pathways of S. mutans, (ii) sensing and resisting antimicrobial peptides, and (iii) producing factors involved in the production of a putative antimicrobial compound. Research on the probiotic mechanisms employed by Streptococcus A12 is providing essential insights into how beneficial bacteria may help maintain oral health, which will aid in the development of biomarkers and therapeutics that can improve the practice of clinical dentistry.
Keywords: biofilm ecology, interspecies competition, dental caries, antimicrobial peptides, oral microbiome
ABSTRACT
Health-associated biofilms in the oral cavity are composed of a diverse group of microbial species that can foster an environment that is less favorable for the outgrowth of dental caries pathogens, like Streptococcus mutans. A novel oral bacterium, designated Streptococcus A12, was previously isolated from supragingival dental plaque of a caries-free individual and was shown to interfere potently with the growth and virulence properties of S. mutans. In this study, we applied functional genomics to begin to identify molecular mechanisms used by A12 to antagonize, and to resist the antagonistic factors of, S. mutans. Using bioinformatics, genes that could encode factors that enhance the ability of A12 to compete with S. mutans were identified. Selected genes, designated potential competitive factors (pcf), were deleted. Certain mutant derivatives showed a reduced capacity to compete with S. mutans compared to that of the parental strain. The A12 pcfO mutant lost the ability to inhibit comX-inducing peptide (XIP) signaling by S. mutans, while mutants with changes in the pcfFEG locus were impaired in sensing of, and were more sensitive to, the lantibiotic nisin. Loss of PcfV, annotated as a colicin V biosynthetic protein, resulted in diminished antagonism of S. mutans. Collectively, the data provide new insights into the complexities and variety of factors that affect biofilm ecology and virulence. Continued exploration of the genomic and physiological factors that distinguish commensals from truly beneficial members of the oral microbiota will lead to a better understanding of the microbiome and new approaches to promote oral health.
IMPORTANCE Advances in defining the composition of health-associated biofilms have highlighted the important role of beneficial species in maintaining health. Comparatively little, however, has been done to address the genomic and physiological bases underlying the probiotic mechanisms of beneficial commensals. In this study, we explored the ability of a novel oral bacterial isolate, Streptococcus A12, to compete with the dental pathogen Streptococcus mutans using various gene products with diverse functions. A12 displayed enhanced competitiveness by (i) disrupting intercellular communication pathways of S. mutans, (ii) sensing and resisting antimicrobial peptides, and (iii) producing factors involved in the production of a putative antimicrobial compound. Research on the probiotic mechanisms employed by Streptococcus A12 is providing essential insights into how beneficial bacteria may help maintain oral health, which will aid in the development of biomarkers and therapeutics that can improve the practice of clinical dentistry.
INTRODUCTION
Dental caries is a complex, multifactorial disease that remains the most prevalent chronic disease in children and adults, posing an enormous economic burden to patients and the health care system (1, 2). Development of dental caries is a dynamic process in which demineralization of the tooth is driven by repeated acid challenges resulting from the fermentation of dietary carbohydrates by microbes in oral biofilms. Lower pH favors the outgrowth of acidogenic, acid-tolerant species, increasing the proportions of those particular taxa in the microbiota and shifting the microbial ecological balance in the oral cavity (3, 4). With technological advances in high-throughput DNA sequencing, major strides have been made to define the diversity in oral microbiomes associated with dental caries and dental health. A group of caries-associated and health-associated taxa have been identified using metagenomic approaches, and a consensus is beginning to develop on which organisms may have the strongest influence on disease development (5–7). Dental caries pathogens belonging to the group of mutans streptococci, particularly Streptococcus mutans, along with various Lactobacillus species, have long been recognized as major contributors to the initiation and progression of caries (8, 9). With the application of metagenomic approaches, other bacterial taxa, including certain Bifidobacterium spp., Actinomyces spp., and Scardovia spp., as well as Candida albicans and certain other fungi (10, 11), have been found to be present in elevated proportions in carious tissues. On the other hand, caries-associated organisms either are absent or their proportions are much lower in health than in disease. Instead, increased proportions of a different group of organisms, including Streptococcus sanguinis, Streptococcus mitis, and Streptococcus gordonii, are present in samples from healthy subjects, and the proportions of health-associated taxa decline as the severity of caries increases (12, 13).
Polymicrobial interactions in biofilms are critical determinants of health and disease (14). While S. mutans is thought to synergize with other aciduric species to accelerate caries progression, certain microbial interactions interfere with caries pathogens and have the potential to alter the dynamics of oral biofilm behaviors in a way that is beneficial to the host. The overwhelming majority of research on caries microbiology has been focused on understanding the pathogenicity of species driving caries progression. However, a body of evidence is accumulating that supports the idea that oral bacteria that are associated with health actively promote oral health by employing various strategies that work directly or indirectly to foster an environment that is less favorable for the establishment and persistence of caries pathogens, including S. mutans. Some antagonistic strategies employed by commensal streptococci and beneficial bacteria are well defined. For example, H2O2 production by commensal oral streptococci appears to have a substantial impact on oral biofilm ecology (15); H2O2 inhibits the growth of S. mutans and many other oral pathogens at concentrations that do not appreciably affect the producing strains. In addition, the enzymatic alkalinization of oral biofilms by oral bacteria, especially through the breakdown of arginine by the arginine deiminase system (ADS), is a critical contributor to pH homeostasis in oral biofilms. Alkali production can prevent the outgrowth of caries-causing pathogens and can shift the chemical balance in favor of tooth remineralization (16, 17). Similarly, urea is secreted in millimolar quantities in saliva and can be metabolized by bacterial ureases in oral biofilms to yield ammonia, elevating biofilm pH. Both the ADS and urease release from their respective substrates one molecule of CO2 and two of ammonia, which can protect acid-sensitive bacteria from growth inhibition or killing at low pH (18, 19) by raising the cytoplasmic pH, which has bioenergetic benefits, and increasing the pH of the environment. Utilization of arginine by the ADS is particularly advantageous because it also generates ATP, which can be used by ADS-positive organisms for growth and maintenance (20).
A group of oral streptococci that commonly comprise a substantial proportion of the oral microbiome (21) express the ADS, including the highly arginolytic clinical isolate Streptococcus sp. A12 (22). Streptococcus A12 was isolated from supragingival plaque of a caries-free subject and has the ability to moderate biofilm pH and potently interfere with the growth and virulence-related properties of S. mutans. A12 was shown to have higher arginine deiminase (AD) enzyme activity than the highly arginolytic reference strain S. gordonii DL1, and much less arginine was required to induce significant levels of AD activity. A12 inhibits the growth of S. mutans through robust pyruvate oxidase (Pox)-dependent H2O2 production, expressing substantially higher Pox activity than S. gordonii DL1. Importantly, A12 was able to interfere with CSP (competence-stimulating peptide)-mediated activation of bacteriocins (mutacins) by S. mutans through a protease called Challisin that is encoded by the sgc gene, similar to S. gordonii DL1 (23). However, unlike S. gordonii, A12 also efficiently inhibited signaling by the comX- inducing peptide (XIP) through an undefined pathway (22).
The oral cavity presents tremendous opportunities for unraveling the complexities of microbial communities in humans but also an immense challenge, as it is one of the more heterogeneous microbial ecosystems in the human body. Metagenomic analyses of oral microbial communities have facilitated the identification of the microbes that may most profoundly influence health and disease (7, 21, 24), although intersubject variability in microbiome composition renders generalizing about the contribution of specific taxa challenging. Understanding the etiology of oral diseases is further complicated by the relatively recent demonstration that individual isolates of many abundant oral bacterial species display tremendous genomic and phenotypic heterogeneity. Initially, it was established that there was a high degree of genomic diversity in the dental caries pathogen S. mutans (25) and that the genomic diversity was correlated with similarly substantial diversity in a variety of phenotypic properties that are related to the pathogenic potential of the organisms (26). More recently, it was established that high levels of genomic diversity and variability in selected beneficial properties exist among the oral commensal streptococci that are the most abundant members of the oral microbiome (27, 28). Clearly, then, sequencing of DNA or RNA from human samples cannot alone predict how members of the oral microbiome function and interact with one another, and with their host, to determine the pathogenic potential of oral biofilms. Functional genomics combined with various model systems will need to be an integral part of the effort to develop a more comprehensive understanding of the spectrum of mechanisms used by beneficial bacteria to exert probiotic effects, with the ultimate goal being to ameliorate oral health by modulating the composition and activity of the oral microbiome. In this study, we employed functional genomics to begin to dissect mechanisms by which the beneficial organism Streptococcus A12 may suppress the growth and virulence of the dental caries pathogen S. mutans.
RESULTS
A12 genes that enhance competition with S. mutans.
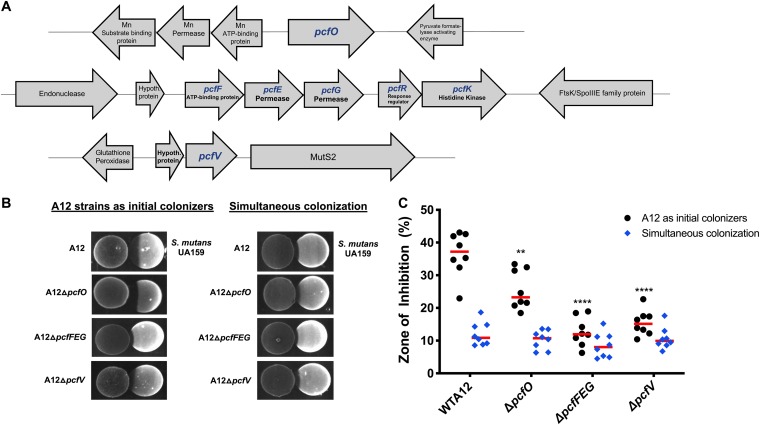
Using various bioinformatic tools (e.g., BLAST, GenBank, and Patric), three genetic loci (Fig. 1A) encoding factors that might augment the ability of A12 to compete with S. mutans were identified and designated pcf genes (potential competitive factors). The pcfO gene (ATM98_08220) was identified and selected based on its homology to a protease reported to disrupt quorum sensing in Streptococcus pyogenes (29), and the gene clusters containing pcfFEG (ATM98_05540, ATM98_05535, and ATM98_05530) and pcfV (ATM98_05815) were chosen due to their association with antimicrobial peptides. The pcfFEG genes encode a predicted three-component ABC transporter that consists of two permeases (ATM98_05535 and ATM98_05530) and a protein with the Walker motif ATP binding domain (ATM98_05540). The product of pcfV is annotated as a colicin V biosynthetic protein. To determine whether the products of these genes could influence the ability of A12 to compete with S. mutans, a collection of mutant strains was generated (Table 1) using double-crossover homologous recombination in which the genes of interest were replaced with a nonpolar kanamycin resistance (Kmr) marker. A12 and the mutants were tested in plate-based growth inhibition assays either with A12 spotted first, followed by S. mutans 24 h later, or with A12 and its derivatives spotted simultaneously with S. mutans, as detailed in Materials and Methods. As previously reported, wild-type A12 was able to inhibit the growth of S. mutans, as evinced by a large zone of inhibition when spotted first (Fig. 1B) and to a lesser extent when spotted simultaneously. When A12 strains were inoculated prior to S. mutans, deletion of pcfFEG, pcfV, and pcfO resulted in significantly less antagonism of S. mutans than of wild-type A12, although the effect of deletion of pcfO was less profound (Fig. 1C). When A12 or its derivatives were spotted simultaneously with S. mutans UA159, the abilities of all strains to inhibit S. mutans were similar. The phenotypes observed were not due to any substantial growth defects, as the growth rates of the mutants were generally comparable to that of the parental strain, as were the maximum optical densities attained and the optical densities attained after 16 h of growth (see Fig. S1 in the supplemental material).
FIG 1.
(A) Schematic diagram of loci encoding potential competitive factors (pcf genes) of A12. pcf genes are indicated in blue, and illustrated are gene order and the arrangement of neighboring genes. (B) Plate-based growth inhibition of A12 strains versus S. mutans. Bacterial cultures were grown overnight in BHI medium and adjusted to an OD600 of 0.5 with sterile BHI. Aliquots (6 μl) from each culture were spotted adjacent to the other strain on BHI agar plates whether simultaneously (right) or A12 or A12 mutant derivatives first followed by spotting of S. mutans 24 h later (left). Plates were incubated for 24 h or 48 h, respectively, at 37°C in a 5% CO2 aerobic atmosphere. Representative images are shown. (C) Zones of inhibition were captured with a digital imager and measured with NIH ImageJ analysis, which was set to a standardized scale (10.234 pixels per mm). The area that would have been occupied by an intact colony of S. mutans was measured in the same way. Then the area of growth inhibition was divided by the total area of the expected colony in square millimeters and multiplied by 100 to determine the percentage of inhibition (y axis) elicited by A12 and its derivatives. Values are the averages of two biological replicates, performed in technical quadruplicates. Red bars indicate the sample medians. Asterisks indicate statistically significant differences compared to the zone of inhibition created by wild-type A12. Statistical analysis was performed using an unpaired Student t test. **, P < 0.01; ****, P < 0.0001.
TABLE 1.
Bacterial strains and plasmid used in this study
| Strain or plasmid | Description or relevant genotypea | Reference or source |
|---|---|---|
| Strains | ||
| A12 | Clinical strain isolated from supragingival dental plaque of a caries-free subject | 22 |
| A12 ΔpcfV | ΔpcfV Kmr | This study |
| A12 ΔpcfO | ΔpcfO Kmr | This study |
| A12 ΔpcfFEG | ΔpcfFEG Kmr | This study |
| A12 ΔpcfRK | ΔpcfRK Kmr | This study |
| A12 Δpeg.1179 | Δpeg.1179 Kmr | This study |
| A12 Δsgc | Δsgc Emr | 22 |
| A12 ΔspxB | ΔspxB Kmr | 22 |
| S. gordonii DL1 | Wild-type reference strain | Laboratory stock |
| S. sanguinis SK150 | Wild-type reference strain | Laboratory stock |
| S. mutans UA159 | Wild-type reference strain | ATCC |
| S. mutans pBGS | S. mutans UA159::pBGS; Spr | This study |
| SAB358 | S. mutans UA159::PcomX-lacZ; Kmr | 76 |
| SAB249 | S. mutans UA159::PcipB-lacZ; Kmr | 22 |
| Plasmid pBGS | Streptococcus integration vector; Spr | 75 |
Kmr, kanamycin resistance; Spr, spectinomycin resistance; Emr, erythromycin resistance.
A12 mutant strains have an altered ability to tolerate antagonistic factors of S. mutans.
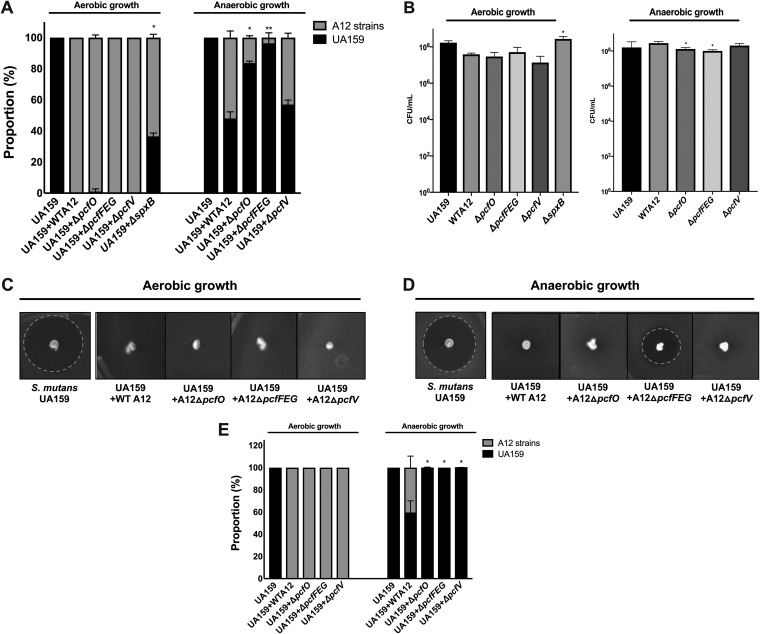
To further assess the impact of the pcf genes in the antagonism of S. mutans, interaction between A12 or A12 mutant strains and S. mutans was evaluated using a dual-species, agar plate-based system in which A12 and S. mutans were first cultured separately in planktonic culture to mid-exponential phase. The cultures were mixed in a 1:1 ratio based on optical density and inoculated onto brain heart infusion (BHI) agar by gently stabbing with a toothpick that had been dipped into the mixed culture. After 24 h of incubation, the viable proportions of A12 and S. mutans were determined by aseptically obtaining the agar plugs and dispersing the bacteria. Controls included pure cultures of A12 or S. mutans grown, inoculated into plates, and enumerated in an identical manner. When the plates were incubated under aerobic conditions, almost no S. mutans cells were recovered when cocultivated with A12 or any of the A12 pcf mutant derivates (Fig. 2A). Since a primary mechanism for commensals, including A12, to antagonize S. mutans in aerobic conditions is through the production of H2O2 (22, 27), an spxB mutant of A12 (22) was included to estimate the relative contribution of H2O2 to the observed growth inhibition; spxB encodes the H2O2-producing pyruvate oxidase that is the primary source of H2O2 produced by this organism (22). Compared to cocultures with wild-type and pcf mutants of A12, S. mutans constituted 38% of the population of viable cells recovered from the plates when coinoculated with the spxB mutant and incubated under aerobic conditions. Under anaerobic conditions, A12 strains lacking pcfO, pcfFEG, or pcfV were less competitive with S. mutans than wild-type A12, with significantly lower proportions of CFU of A12 ΔpcfO and A12 ΔpcfFEG recovered after 24 h. Of note, slightly fewer CFU were present in pure cultures of A12 ΔpcfO and A12 ΔpcfFEG when cells were grown under anaerobic conditions than for the parental strain (Fig. 2B), and the differences were statistically significant.
FIG 2.
Impact of A12 potential competitive factor. (A) Cultures of S. mutans UA159 (Spr), A12, and A12 mutant strains were grown overnight and centrifuged to collect the cells. The cells were then washed with PBS and cultures were adjusted to an OD600 of 0.5 using PBS. S. mutans was then mixed with A12 or A12 mutant strains at a ratio of 1:1. Mixed cultures were spotted on BHI plates with a sterile toothpick and incubated aerobically (5% CO2) or anaerobically at 37°C for 24 h. Pure cultures (B) were spotted on BHI plates with sterile toothpick as a control. (C and D) Mixed cultures of S. mutans UA159 (Spr) and A12 or A12 mutant strains adjusted to an OD600 of 0.5 were spotted onto BHI plates and grown aerobically or anaerobically at 37°C. After 24 h, 3 ml of soft agar overlay (1% BHI agar) was mixed with 107 cells of the indicator strain S. sanguinis SK150 and poured evenly onto the plate. All plates were incubated for an additional 24 h. (E) S. mutans and A12 cells were enumerated by plating and the proportions of each were calculated. Assays were performed at least three times, each performed with technical triplicates. Values are averages and error bars indicate standard deviations. Asterisks indicate statistically significant differences in proportions of S. mutans present when cocultured with A12 mutant strains compared with S. mutans cocultured with wild-type A12. For control assays, asterisks indicate statistical differences in CFU of A12 mutant strains compared to CFU of wild-type A12. Statistical analysis was performed using an unpaired Student t test. *, P < 0.05; **, P < 0.01.
A12 is able to interfere with the ability of S. mutans to produce bacteriocins using a protease encoded by the sgc gene that apparently degrades CSP, thereby blocking activation of bacteriocin genes by the ComDE two-component system (22). To evaluate whether the identified genes in A12 influence the ability of A12 to modulate the production of bacteriocins by S. mutans, a modified deferred antagonism assay was performed with the dual-species, plate-based cultivation system described above, in which equivalent numbers of S. mutans and A12 or its derivatives were stabbed onto an agar plate. After 24 h of incubation of the plates under aerobic or anaerobic conditions, 107 cells of S. sanguinis SK150, an indicator strain that is sensitive to the mutacins produced by S. mutans UA159, were overlaid in soft BHI agar and plates were incubated for an additional 24 h. As previously observed for aerobic conditions, A12 strains dominated the population, so growth inhibition of S. sanguinis SK150 by S. mutans mutacins did not occur, presumably because insufficient S. mutans survived to produce inhibitory quantities of mutacins (Fig. 2C). Interestingly, under anaerobic conditions, the A12 mutants lacking pcfO or pcfV behaved similarly to wild-type A12 (Fig. 2D), in that these mutant strains inhibited the ability of S. mutans to kill S. sanguinis SK150, despite the fact that the cocultures were dominated by S. mutans (Fig. 2E). In contrast, a zone of inhibition was observed when S. mutans was coinoculated with A12 ΔpcfFEG, albeit smaller than when S. mutans alone was inoculated (Fig. 2D). As was the case for the pcfO and pcfV mutants, S. mutans dominated the population when A12 ΔpcfFEG was coinoculated with S. mutans. Thus, deletion of pcf genes resulted in a markedly diminished capacity of A12 strains to kill S. mutans and/or to tolerate antagonistic factors of S. mutans.
PcfO interferes with XIP signaling by S. mutans.
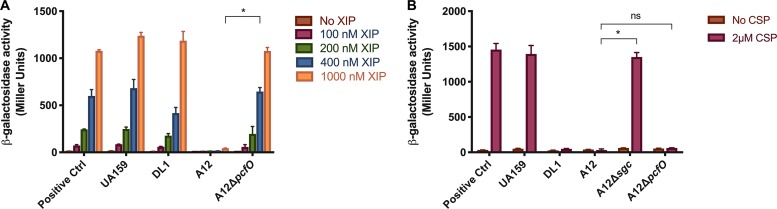
Natural genetic competence is considered an important trait for S. mutans, not only because it is a primary driver for genetic diversification (30) but also because competence overlaps with regulatory circuits controlling multiple virulence-related traits (31–33). In S. mutans, a key regulatory element of genetic competence is the comX-inducing peptide (XIP). Previously, we reported that culture supernatants of A12 are able to degrade XIP, which is not the case for S. gordonii (22) and some other commensals (34). Further, deletion of sgc in A12 did not diminish the ability of A12 supernatant fluids to block XIP-dependent activation of comX. The A12 genome contains a gene for a putative neutral endopeptidase that shares a substantial degree of homology to proteases of the neprilysin (M13) family, comprised of evolutionarily conserved zinc metallopeptidases showing functional diversities in numerous physiological processes (35). Members of the M13 protease family exist in a wide range of organisms, including mammals and bacteria, and are recognized as important regulators involved in the processing and degradation of a variety of different signaling peptides (35, 36). Having 71% identity (82% similarity) to PepO of S. pyogenes, which has been reported to degrade small hydrophobic peptides and disrupt quorum sensing (29), A12 PcfO was hypothesized to be a PepO-like protease that could degrade XIP. Culture supernatants were collected from overnight cultures of A12, A12 ΔpcfO, S. gordonii DL1, or S. mutans UA159 grown in FMC (37). Various concentrations of synthetic XIP (sXIP) were added to filter-sterilized culture supernatants and incubated overnight, as detailed in Materials and Methods. An S. mutans strain carrying a lacZ gene fusion to the comX promoter was then mixed with the supernatants and incubated for 2 h. Cells were collected and β-galactosidase assays were performed. As previously reported, A12 supernatants eliminated the ability of sXIP to activate comX expression, whereas robust XIP signaling was observed with S. gordonii DL1 supernatants, comparable to levels seen with the S. mutans control supernatants (Fig. 3A). Interestingly, deletion of pcfO in A12 effectively eliminated the ability of A12 supernatants to inhibit XIP signaling. There is cross talk in the signaling pathways that control bacteriocin expression (ComDE) and genetic competence (ComRS) in S. mutans (38), so to assess whether A12 PcfO affected CSP signaling, the ability of supernatants from A12 strains to degrade CSP was assessed using BHI medium and a derivative of S. mutans carrying a cipB promoter fused to lacZ to monitor CSP-dependent signaling through ComDE. The pcfO mutant strain retained the capacity to interfere with CSP-dependent activation of PcipB-lacZ at a level similar to that of supernatants from wild-type A12, consistent with the findings that Sgc is primarily responsible for degradation of CSP (Fig. 3B).
FIG 3.
Effects of S. gordonii or A12 supernatants on PcomX or PcipB promoter activity. S. mutans containing lacZ fused to the promoter of comX (A) or cipB (B) was grown to an OD600 of 0.15 in FMC medium (A) or BHI medium (B). Supernatants from overnight cultures of the indicated strains were obtained after centrifugation, the pH was adjusted to 7.0 with 6 N NaOH, and the solutions were filter sterilized. In the case of FMC conditions, supplemental glucose was added to increase the concentration of glucose by 25 mM. Synthetic peptides (XIP or CSP) were added to the supernatants and incubated overnight (for XIP) or for 2 h (for CSP). The reporter strains were grown to an OD of 0.15, and cells were collected by centrifugation and then were resuspended in the indicated supernatants for 2 h prior to measuring β-galactosidase activity. All assays were performed at least three times, with technical triplicates. The bars are averages and error bars represent SDs. Asterisks indicate statistically significant differences between wild-type A12 (400 nM XIP) and A12 ΔpcfO (400 nM XIP) (A) and between wild-type A12 and A12 Δsgc or A12 ΔpcfO. (B). Statistical analysis was performed using an unpaired Student t test. *, P < 0.05. ns, not significant.
PcfFEG contributes to immunity to the lantibiotic nisin.
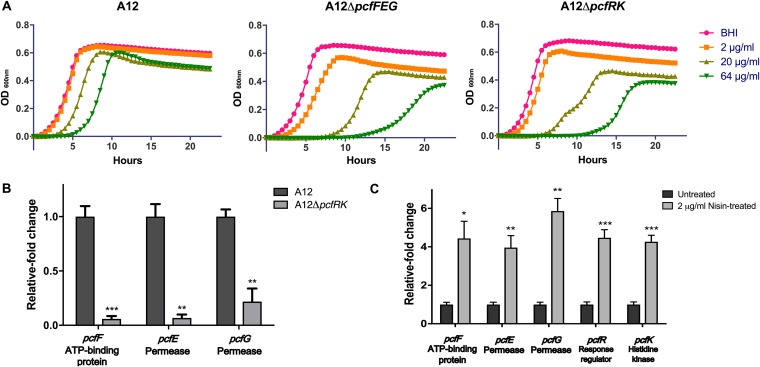
Bacteriocins appear to be highly influential ecological factors that enhance establishment and persistence of the producing bacteria. Bacteriocin-producing strains and many nonproducing strains have immunity systems to provide protection against bacteriocins produced endogenously or by competing species (39). Because the identified PcfFEG ABC transporter shared homology with a lantibiotic immunity transporter according to BLAST searches, the susceptibility of A12 to the well-characterized and commercially available lantibiotic nisin was assessed. Overnight cultures of A12 were inoculated into fresh BHI medium in the absence or presence of various concentrations of nisin, and growth was monitored over the course of 24 h (Fig. 4A). Nisin at 2 μg/ml had no effect on the growth of A12. In the presence of 20 or 64 μg/ml of nisin, the duration of the lag phase was substantially increased and the final optical density achieved was lower than without nisin, but the exponential growth rates were similar (66.5 ± 2.75 min). To assess whether PcfFEG could provide protection against nisin, the growth characteristics of the ΔpcfFEG strain were compared with those of the parental strain in the presence or absence of lantibiotic. Additionally, because these types of ABC transporters can be regulated by two-component systems (TCS), the genes for the TCS (pcfRK; ATM98_05525 and ATM98_05520) directly downstream of pcfFEG were deleted to create the ΔpcfRK strain of A12. Both the ΔpcfFEG and ΔpcfRK strains displayed a significantly greater lag phase than that of wild-type A12, even with 2 μg/ml of nisin, although the differences between the two mutant strains were minimal. Further, in the presence of nisin, the exponential-phase growth rates of the mutants were lower and the final yields were decreased compared to those of the parental strain.
FIG 4.
Deletion of pcfFEG or pcfRK increases susceptibility of A12 to the lantibiotic nisin. (A) Tolerance of A12 strains to different concentrations of nisin measured by monitoring growth OD600 over a 24-h period. Overnight cultures were subcultured into fresh BHI medium. Once the OD600 reached 0.5, cultures were diluted 1:100 into fresh BHI medium containing various concentrations of nisin and growth was monitored in a Bioscreen C. (B) Expression of pcfFEG mRNA was measured using qRT-PCR in the wild-type and pcfRK strain. Overnight cultures were inoculated into fresh BHI medium and once cultures reached OD600 0.5, cells were collected for RNA extraction and subsequent qRT-PCR. (C) Expression of pcfFEG and pcfRK in A12 in response to treatment with 2 μg/ml of nisin. RNA was extracted from mid-exponential-phase cells of A12 left untreated or treated with nisin for subsequent qRT-PCR. All experiments were done in biological triplicates with technical triplicates. Error bars represent SDs, and statistical analysis was performed using an unpaired Student t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To confirm that pcfFEG expression could be influenced by pcfRK, the pcfFEG genes were measured using quantitative reverse transcriptase PCR (qRT-PCR). As expected, in the absence of pcfRK, a significant decrease in the expression of pcfFEG was observed (Fig. 4B). To directly examine the effects of nisin on the expression of the genes for the transporter and TCS, overnight cultures of A12 were subcultured in fresh BHI medium to an optical density at 600 nm (OD600) of 0.5, and then the cultures were left untreated or treated with 2 μg/ml of nisin for 15 min (Fig. 4C). Treatment with nisin increased the expression of both the pcfRK and pcfFEG 4- to 6-fold, adding support to the idea that the function of these gene products is to respond to and confer protection against molecules that may be functionally and structurally similar to nisin. In fact, we have shown that as little as 6 nM nisin is sufficient to activate pcfFEG expression in a strain with intact pcfRK genes (data not shown).
Genetic elements involved in biogenesis of a putative antimicrobial molecule.
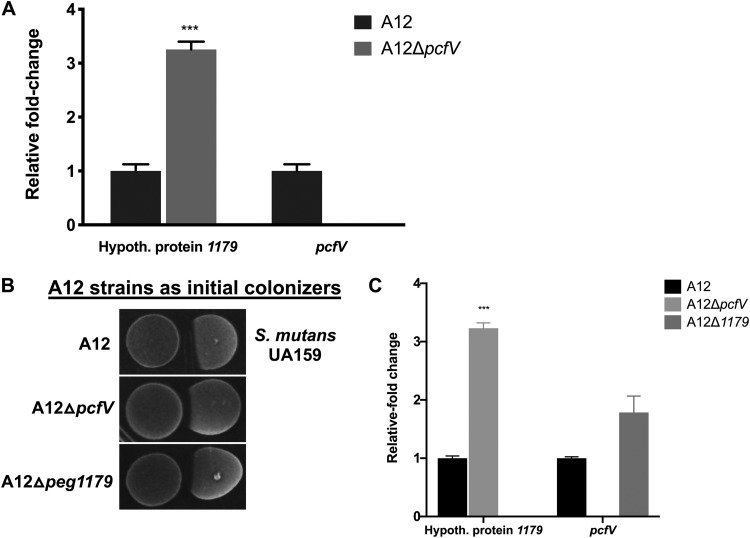
Vast diversity in composition, structure, and mechanisms of action of bacteriocins has already been described, but genome sequencing and metabolomic approaches continue to identify new molecules (40–43). The A12 genome contains a gene annotated as a colicin V production protein gene (here designated pcfV), which is similar to the cvpA gene in Escherichia coli, thought to be involved in the production of the pore-forming bacteriocin, colicin V (44). Interestingly, pcfV is located immediately downstream of the gene for an unannotated 96-amino-acid (aa) protein (A12 peg.1179; ATM98_05820) and it is cotranscribed with pcfV (data not shown). To determine if there was a connection between the 96-aa-protein open reading frame (ORF) and pcfV, RNA was extracted from A12 and A12 ΔpcfV and, after cDNA synthesis, qRT-PCR was performed. In the absence of pcfV, a 3-fold increase in the expression of the gene for the small hypothetical protein, peg.1179, was observed (Fig. 5A), compared to the level in parental A12. Next, the hypothetical protein was deleted and the mutant strain A12 Δpeg.1179 was constructed to compare its behavior with that of the pcfV mutant. Both A12 ΔpcfV and A12 Δpeg.1179 showed decreased antagonism of S. mutans compared to that of the wild-type strain (Fig. 5B). Further, deletion of pcfV resulted in a 3-fold increase in the expression of A12 peg.1179 (Fig. 5C). However, deletion of A12 peg.1179 did not affect the expression level of pcfV (P = 0.049).
FIG 5.
Expression of a hypothetical protein upstream of pcfV is upregulated in the absence of pcfV. (A) Levels of expression of peg.1179 mRNA were compared using qRT-PCR in a pcfV deletion strain of A12 and the wild type. Overnight cultures were inoculated into fresh BHI medium. When cultures reached an OD600 of 0.5, cells were collected for RNA extraction and subsequent qRT-PCR. (B) Plate-based growth inhibition of A12 strains versus S. mutans. All bacterial cultures were grown overnight in BHI medium and then adjusted to an OD600 of 0.5 with fresh BHI. Aliquots (6 μl) from A12 or A12 mutant derivatives were spotted first, followed by spotting of S. mutans 24 h later. Plates were incubated for 48 h at 37°C in a 5% CO2 aerobic atmosphere. (C) Expression of pcfV of A12 was assessed using qRT-PCR in a peg.1179 deletion A12 strain and in the wild-type strain. All experiments were done in biological triplicates with technical triplicates, and error bars represent SDs. Statistical analysis was performed using an unpaired Student t test. ***, P < 0.001.
Phylogenetic analysis of the pcf genes in other A12-like and closely related clinical isolates.
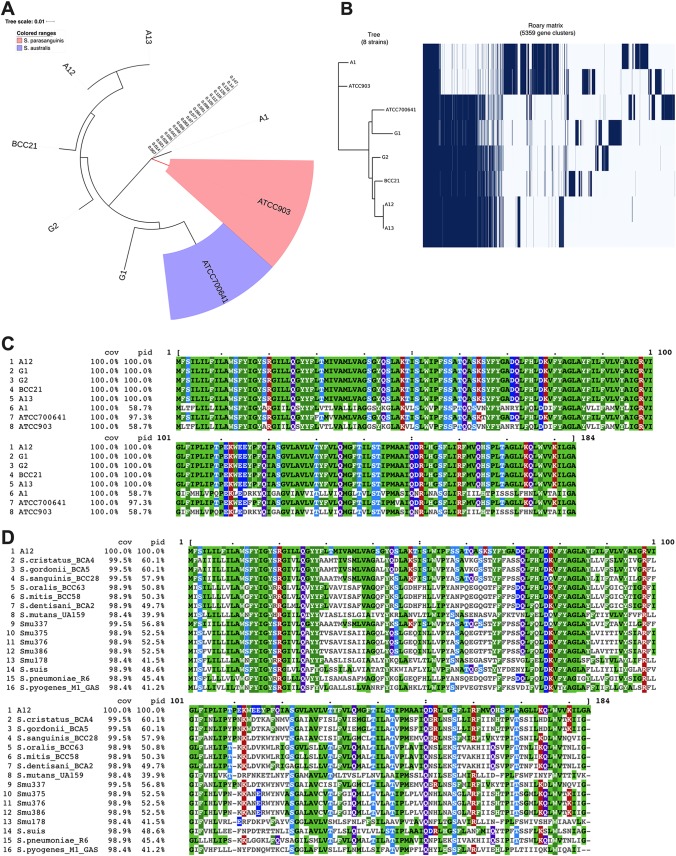
Based on comprehensive phylogenomic analyses, the closest relative of the novel streptococcus isolate A12 is Streptococcus australis (22). Certain types of streptococcus isolates that could not be appropriately classified as S. australis or Streptococcus parasanguinis (another close relative of A12) due to significant differences in their genomes are designated “A12-like isolates.” To determine whether the identified pcf genes are present and conserved in strains that are closely related to A12, the genomes of four low-passage-number S. australis/A12-like isolates were evaluated. In our analyses, the genome of S. australis ATCC 700641 deposited in the NCBI database was also included, as were the genomes of a low-passage-number isolate of S. parasanguinis, A1 (28), and S. parasanguinis ATCC 903. A phylogenetic tree (Fig. 6A) shows the overall relationship of A12 to the other strains used for comparison, and a Roary matrix depicts the presence and absence of core and accessory genes of all isolates listed (Fig. 6B). The isolate Streptococcus G1 was more closely related to S. australis, whereas the S. parasanguinis reference strain and A1 were shown to be significantly different from A12, from strains designated A12-like, and from S. australis.
FIG 6.
(A) Pangenome analysis of A12, A12-like isolates, S. australis ATCC 700641, and S. parasanguinis ATCC 903 (see text for details). (B) Presence and absence of core and accessory genes of all isolates listed. (C) Clustal Omega alignment of pcfV in A12, A12-like isolates, S. australis ATCC 700641, and S. parasanguinis ATCC 903. The reference sequence is designated A12. cov, coverage; pid, percent identity. (D) Clustal Omega alignment of A12 pcfV amino acid sequences in other oral streptococcus species.
Next, A12 pcf sequences were used to do a series of BLASTP searches among the genomes of the strains of interest. ClustalW2 sequence alignment was done using the protein sequences obtained from strains to compare the percent identity against Streptococcus A12. All analyzed isolates encoded apparent PcfO and PcfV proteins, although some sequence variation was evident. Interestingly, the PcfV sequences in isolates closely related to A12 were highly conserved, with 100% identity to that of A12, whereas the S. australis ATCC 700641 PcfV sequence showed 97.3% identity, and S. parasanguinis A1 and ATCC 903 PcfV sequences were only 58.7% identical to A12 PcfV (Fig. 6C). The percent identity patterns for the small hypothetical protein A12 peg.1179 were also similar, with the homologous sequence identified in A12-like isolates being 100% identical to A12 (Fig. S3). PcfO was more conserved, with less sequence variability among the different streptococci included in this study, consistent with what has been reported for PepO from other Streptococcus spp. (45). On the other hand, substantial sequence variations were found in PcfFEG proteins from the different strains, except for those of A13 and S. parasanguinis ATCC 903, which were 100% and ∼96% identical to A12 PcfFEG, respectively. PcfFEG sequences in other isolates were also identified by a BLAST search of A12 PcfFEG sequences against their genomes. Identified sequences were subsequently aligned, but very low degrees of identity (5.5% to 20.2%) were observed. Given the overall similarity between ATP binding cassette transporters (46), a conclusion about whether PcfFEG homologues are present in strains other than A13 cannot be reached at this time. Furthermore, PcfRK sequence alignment showed a similar pattern, in which considerable sequence variations were observed among the strains, except for A13 (100% identical to A12) and S. parasanguinis ATCC 903 (99.1% and 97.4% identical to PcfR and PcfK sequences of A12, respectively).
Because PcfV was highly conserved in A12-like isolates, we explored whether pcfV genes were present in other oral streptococci (Fig. 6D). For this analysis, the genomes of 607 streptococci (26, 28) were analyzed. PcfV was also used in BLASTP searches against three nonoral streptococcus species (S. suis, S. pneumoniae, and S. pyogenes). Sequences similarity varied among and across species, ranging from 39% to 60% identity, suggesting that PcfV homologues/orthologues/paralogues were present in most streptococci.
DISCUSSION
Intermicrobial interactions in biofilms are central drivers of homeostasis and dysbiosis and can have a powerful influence on maintenance of health or development of disease. An active role for commensal bacteria in providing protection against disease has been demonstrated in a variety of studies and environments, particularly for gut and skin infections (47–49). For example, a precisely defined consortium of commensal bacteria was reported to restore colonization resistance against vancomycin-resistant Enterococcus (VRE), a leading cause of nosocomial infection and a serious public health threat (50). This study showed that while the consortium of commensals worked cooperatively to resist VRE colonization, the previously uncharacterized bacterium Blautia producta was the key species within the consortium providing VRE-specific colonization resistance. Thus, the study of unique characteristics of certain key species in complex bacterial populations can provide considerable insights into the competitive and cooperative strategies that are employed by health-associated biofilms to persist and survive in particular ecological niches. Other examples of strain-specific inhibitory activities of commensals against pathogens are emerging (27, 51). Commensal oral streptococci, like A12, that can express high ADS activity under environmental conditions commonly occurring in oral biofilms, can directly inhibit growth of S. mutans by producing H2O2, and can disable bacteriocin production via interference with the CSP-ComDE pathway (22) may provide substantial protection to a human host against dental caries. Here we demonstrate that multiple additional factors encoded by A12 are crucial to its ability to inhibit and to tolerate the most common human dental caries pathogen, S. mutans.
Bacteria in heterogeneous biofilm environments are able to communicate through quorum sensing, which allows the coordination and regulation of genes crucial for survival (52). For the oral pathogen S. mutans, genetic competence is governed by the quorum-sensing peptides CSP and XIP. While the CSP-ComDE circuit is essential for bacteriocin production and can affect induction of the competent state in S. mutans, the regulatory system directly controlling activation of competence is ComRS, with ComS serving as the precursor to the XIP (comX-inducing peptide) signaling peptide. Here we demonstrate that an endopeptidase of A12, encoded by pcfO, interferes with the S. mutans ComRS-XIP signaling pathway. In S. mutans, comX activation via ComRS enhances cell lysis (53), which results in increased extracellular DNA (eDNA) release. The released eDNA has the potential to alter the structure of the biofilm matrix, which can impact the composition and pathogenic potential of the microbiota therein (54, 55). Additionally, autolysis of S. mutans is a primary mechanism for release of XIP, which can serve as a signal to other S. mutans to tune its competence system to assimilate exogenous DNA, providing nutritional benefits and contributing to the diversification of the species (53). It is also notable that the ComRS-XIP system in S. mutans is highly conserved among S. mutans isolates (26) and that ComR of S. mutans specifically recognizes the XIP of S. mutans but not XIP from other streptococci. Likewise, even though a pepO homologue is present in S. gordonii DL1, there is no evidence that S. gordonii can interfere with XIP signaling in S. mutans. Given the conservation of PepO in Streptococcus species and its recently discovered role in virulence-related phenotypes in pathogens, it is possible that commensal species like A12 have evolved to utilize the protease to enhance competitiveness in certain environments and/or against certain competing species. The ability of A12 to target a highly conserved and specific system of S. mutans imbues A12 with the ability to subvert key pathways of the pathogen and reduce the pathogenic potential of oral biofilm communities. Furthermore, the ability of A12 to degrade XIP using PcfO protease is particularly intriguing in light of the diverse functions of PepO being reported for Streptococcus species. For example, in addition to the ability of PepO to degrade small hydrophobic peptides (29), PepO has also been reported to affect a major virulence factor (SpeB) in S. pyogenes (56), mediate immune evasion in both S. pneumoniae and S. mutans (57–59), and even play a role in S. mutans UA140 mutacin production (45, 60). These recent findings on PepO highlight the complex roles of this peptidase in the fitness and physiology of various streptococci, including that of A12. It also raises the question of whether PepO proteins of A12 and other commensal oral streptococci may have the capacity to modulate the virulence of respiratory pathogens.
In addition to identifying the gene product that allows A12 to block XIP signaling in S. mutans, we establish here that there are multiple factors that are encoded by A12 that have a potent influence on the capacity of A12 to antagonize and to tolerate S. mutans. Under aerobic conditions (Fig. 2A), the production of H2O2 masked the effects of deletion of pcfO, pcfV, and pcfFEG on competition with S. mutans, but oxygen concentrations and redox conditions in dental plaque, particularly in mature dental plaque when conditions are favorable for caries development, are often suboptimal for the generation of H2O2 by commensals. It is also clear from our data that even when the ability of A12 to produce H2O2 is substantially impaired through the deletion of spxB, A12 is able to effectively compete with S. mutans (Fig. 2A), adding further support to the hypothesis that interspecies competition is a complex, multifactorial process. In fact, under anaerobic conditions, when A12 is unable to produce H2O2, deletion of pcfO, pcfFEG, or pcfV clearly compromises the capacity of A12 to compete with S. mutans. Furthermore, the loss of the pcf genes in A12 decreased the ability of A12 to persist and cope with antagonistic factors of S. mutans. For example, deleting pcfFEG in A12 impaired the ability of A12 to interfere with S. mutans mutacin production, largely due to the fact that the pcfFEG mutant constituted only about 4% of the viable counts recovered after 24 h of cocultivation with S. mutans. Although the deletion of pcfV and pcfO did not impair the ability of A12 to interfere with bacteriocin production by S. mutans, significantly lower proportions of those A12 mutant strains were recovered after 48 h of cocultivation. While the coculture assays whose results are shown in Fig. 2A and E were performed with minor differences, the A12 mutant strains studied in this investigation consistently performed more poorly against S. mutans than wild-type A12.
Various commensal species have been shown to have inhibitory effects on S. mutans, including S. gordonii, Streptococcus salivarius, and Streptococcus dentisani (61–63). Likewise, certain S. salivarius strains produce bacteriocin-like inhibitory substances (64, 65) that have made them potential oral probiotic candidates. While commensals may directly antagonize pathogens via the secretion of antimicrobial peptides, our findings suggest that commensals may also have genetic elements that allow them to mount a defense against other members of complex oral microbial communities. Deletion of pcfFEG in A12 resulted in a diminished ability to compete with S. mutans, and our early exploration into the mechanism of this genetic locus revealed a role in immunity to nisin, a well-characterized lantibiotic. Lantibiotics are a class of bacteriocins produced by Gram-positive bacteria that undergo posttranslational modification and contain either lanthionine or methyllanthionine ring structures with atypical amino acids (66). The producing organisms usually encode specific immunity proteins that provide protection against the deleterious effect of their own lantibiotics. Two immunity systems have been described (67), one being a dedicated ABC transporter thought to prevent accumulation of lantibiotics in the cell membrane. The second type involves lipid-anchored immunity proteins that are believed to sequester lantibiotics at the cell surface before they can cause damage (67). Various strains of S. mutans produce bacteriocins (called mutacins) that belong to the class of lantibiotics including mutacins I, II, III, K8, B-Ny266, Smb, and 1140 (60). However, not all strains of S. mutans produce lantiobiotic-type mutacins. Notably, S. mutans UA159 encodes only nonlantibiotic mutacins (68). Still, we observed a reduced persistence of A12 lacking PcfFEG when cocultured with S. mutans UA159. Thus, our hypothesis is that PcfFEG of A12 not only can function as a system that can be induced by, and confer protection against, lantibiotics but also may confer on A12 resistance to multiple antimicrobial peptides. There is precedent for broad specificity in immunity proteins, with one example being the SmbFT system of S. mutans GS5, which provides protection against a number of different but structurally similar lantibiotics (69). Further characterization of the PcfFEG system is ongoing to understand what types of molecules can induce the system through the cognate TCS identified in this study (PcfRK) and how resistance profiles to different antimicrobial peptides are affected by the PcfFEG proteins, with the overarching goal of understanding how PcfFEG can enhance the fitness of A12 in complex microbial communities.
Another novel finding reported here is that A12 harbors a gene that is homologous or orthologous to the gene for a putative colicin V biosynthetic protein: pcfV, which is similar to cvpA in E. coli. Colicin V is a small, secreted, pore-forming antimicrobial peptide produced by E. coli, and its production involves several genes, including cvpA, cvaA, and cvaB, which encode proteins required for transport of colicin V across the cytoplasmic membrane with the help of TolC and an immunity protein encoded by cvi (70). Although the role of cvpA in colicin V biogenesis of E. coli is not particularly well understood, the production of mature colicin V, which is ribosomally synthesized from a small precursor protein, is thought to require CvpA (44). Furthermore, while colicins were first studied for E. coli, they have also been found to have similarities to bacteriocins of Gram-positive bacteria (71). More recently, high-throughput screening for antimicrobial activity against Staphylococcus aureus on coagulase-negative commensal staphylococci isolated from the skin of healthy individuals disclosed previously unknown antimicrobial peptides (AMPs) encoded within the genomes of the isolates exhibiting active antimicrobial activity; among the genes identified was one annotated as “colicin V production protein” (72). Interestingly, A12 pcfV is similar in size and has 30% to 40% sequence identity to those identified in commensal Staphylococcus species. In this study, we found that the pcfV mutant of A12 has diminished capacity to antagonize S. mutans. Notably, we also show that the expression of a small hypothetical protein encoded upstream of pcfV can be altered by deletion of pcfV and that deletion of only this peptide confers a phenotype that is similar to what is observed in a strain lacking PcfV. Our working hypothesis is that this peptide may serve as a precursor that is acted on by PcfV and possibly other gene products to produce a mature antimicrobial peptide that may inhibit growth or metabolism of S. mutans and possibly other organisms in the oral microbiome. It is noteworthy that A12 encodes a gene product annotated as colicin E2 immunity protein, albeit at a site distant from pcfV. Additional studies to identify the product of PcfV and determine whether the predicted colicin E2 immunity protein is related to PcfV function or involved in protection of A12 against a colicin-like antimicrobial factor(s) are under way.
We have only begun to uncover the diverse mechanisms utilized by beneficial, health-associated organisms to persist against pathogens. Work is in progress to build a more comprehensive understanding of the interaction between A12 and S. mutans or other pathogens and how the identified pcf genes may influence the expression of other genes related to interspecies competition by A12 or the virulence of S. mutans. As reported here, other A12-like isolates have phenotypic properties similar to those of A12 (34), and many of these A12-like isolates appear to encode the competitive factors studied in A12 in this investigation. In fact, pcfV shows an exceptionally high degree of conservation in A12-like organisms and close relatives of A12, as well as being broadly distributed in oral streptococci. Another important point is that PcfFEG was only found in A12 and A13, an isolate in the group of “A12-likes.” The presence-and-absence plot in Fig. 6B suggests that A12 and A13 are most similar in their gene contents. If pcfFEG can indeed confer protection against a broad range of bacteriocins, perhaps A12 and other A12-like streptococci acquired and retained these genes to be able to persist in the hostile environment of the oral cavity. Overall, then, the mechanisms we have identified begin to provide substantive new insights into the complexities of interbacterial competition in the oral microbiome and contribute to the foundation of knowledge needed to understand how beneficial commensal species promote oral health. Continued genome-scale analysis, coupled with functional genomics and metatranscriptomics using the expanded database of sequence of commensal organisms (28), will help to understand the forces that shape and maintain the oral microbiome, and the information can be used for the rational design of novel therapies that employ pre-, pro-, or synbiotic approaches to control oral and other infectious diseases.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Table 1 lists all strains and plasmids used in this study. Streptococcus A12 was isolated from supragingival plaque of a caries-free subject (22). A12, S. mutans UA159, and genetically modified derivatives of each strain were routinely grown in brain heart infusion (BHI) broth, on BHI agar plates (Difco), or in the chemically defined medium FMC (37) at 37°C in a 5% CO2 aerobic atmosphere. To select for antibiotic-resistant A12 strains after genetic transformation or to enumerate S. mutans in dual-species cultures, BHI plates containing kanamycin (1 mg ml−1) were used. All bacterial stocks were made by diluting overnight cultures of a single colony 1:1 with 50% glycerol (25% final glycerol), and all stocks were maintained in cryovial tubes in a –80°C freezer for long-term storage.
S. mutans synthetic CSP (sCSP), corresponding to the 21-aa peptide (31), was synthesized by the Interdisciplinary Center for Biotechnology Research (ICBR) facility at the University of Florida, and its purity (97%) was confirmed by high-performance liquid chromatography (HPLC). Synthetic XIP (sXIP), corresponding to residues 11 to 17 of ComS (amino acid sequence, GLDWWSL [53]), was synthesized and purified to 96% homogeneity by NeoBioSci (Cambridge, MA). To synthesize A12 CSP to be utilized to induce competence in A12, the CSP precursor amino acid sequence (ComC) located upstream of ComDE was identified and an active 17-amino-acid peptide CSP was predicted to be derived from ComC following the cleavage near a conserved double-glycine (GG) cleavage motif. The predicted 17-aa A12 CSP was synthesized by Biomatik (96.8% purity reported by the supplier).
Construction of mutant strains and reporter gene fusions.
Mutant strains of A12 were engineered by double-crossover recombination using linear DNA assembled through a Gibson assembly kit (New England BioLabs, Beverly, MA) (73). Briefly, primer sets (Table 2) were designed to PCR amplify two DNA fragments flanking the coding sequence of the genes of interest and containing at least 25 bases of sequence that overlapped with the termini of the nonpolar kanamycin resistance cassette in pALH124 (74). The two flanking DNA fragments and the kanamycin resistance cassette were mixed in equimolar concentrations in a single isothermal ligation reaction. Overnight cultures of A12 were inoculated into fresh BHI cultures, and the ligated DNA products (0.5 μg) were used to transform A12 in BHI using 50 nM A12 sCSP to induce competence. After 3 h of incubation, cells were plated onto BHI agar with Km (1 mg ml−1) and isolated colonies were picked for PCR verification. PCR products from positive transformants were sent for DNA sequencing to ensure that the fragment was inserted into the desired locus and that no mutations were present in the flanking regions used for homologous recombination. To construct a marked strain for dual-species experiments, S. mutans was transformed with pBGS, which allowed for stable integration of a spectinomycin marker into the gtfA gene (75), yielding a spectinomycin-resistant (Spr) S. mutans strain. Construction and characteristics of strains of S. mutans carrying a lacZ gene fused to the promoter of cipB (PcipB::lacZ) or comX (PcomX::lacZ) are detailed elsewhere (22, 76).
TABLE 2.
Primers used in this study
| Strain or purpose | Primer | Sequence (5′→3′)a |
|---|---|---|
| A12 ΔpcfV | A12-pcfV F-1 | TTGGCCTCGATATGCTTCGAGGG |
| A12-pcfV R-2GA-Km | GCCATTTATTATTTCCTTCCTCTTTTAGCAACTCCCCTTAATTAGATGATCTACCG | |
| A12-pcfV F-3GA-Km | ATATTTTACTGGATGAATTGTTTTAGTAGAGGTGCGTAATCACTTCTCATCATGTC | |
| A12-pcfV R-4 | CCCTGCAACTGTGGAAGG | |
| A12 ΔpcfO | A12-pcfO F-1 | GCTGGCCGATCTGGCG |
| A12-pcfO R-2GA-Km | GCCATTTATTATTTCCTTCCTCTTTTACTTGTAAACGTACCATGATCTTCTCAC | |
| A12-pcfO F-3GA-Km | ATATTTTACTGGATGAATTGTTTTAGTAGAGGTAAATTAAGATACTAAGCCCG | |
| A12-pcfO R-4 | CCTTACCCTGTCTGGTGGAG | |
| A12 ΔpcfFEG | A12-pcfFEG F-1 | GTAAGATTAGAGTTGAATCCCCTC |
| A12-pcfFEG R-2GA-Km | GCCATTTATTATTTCCTTCCTCTTTTAATTTTGTTCCTCCTTTTTCTTCTG | |
| A12-pcfFEG F-3GA-Km | ATATTTTACTGGATGAATTGTTTTAGTAGATAGCTCTAAATGTATTTGTAAATGG | |
| A12-pcfFEG R-4 | GGCAGAAGAATCACCAATCCC | |
| A12 ΔpcfRK | A12-pcfRK F-1 | GGTGGGGTGGAAAGTCAATCAG |
| A12-pcfRK R-2GA-Km | GCCATTTATTATTTCCTTCCTCTTTTAGAAATACTATTTTGAGCG | |
| A12-pcfRK F-3GA-Km | ATATTTTACTGGATGAATTGTTTTAGTAGACAACAATTACTTTTT | |
| A12-pcfRK R-4 | GTCAAGGTCAAAGGACGGGGC | |
| A12 Δpeg.1179 | A12-peg.1179 F-1 | GGTGCTGACGAACAAATGC |
| A12-peg.1179 R-2GA-Km | GCCATTTATTATTTCCTTCCTCTTTTAGCTGGACATAAAAATCACCTCACG | |
| A12-peg.1179 F-3GA-Km | ATATTTTACTGGATGAATTGTTTTAGTAGAGGGGAGTTGCATGTTTTCAATCC | |
| A12-peg.1179 R-4 | GGGGGCTATGCTGCACC | |
| qRT-PCR | A12 pcfK 1116 F | CTAGAGCCACACTAC |
| A12 pcfK 1116 R | GCCTCTAGCATTGGCG | |
| A12 pcfR 1117 F | CGTAGCAAAGTGGATTGTCCGATTATC | |
| A12 pcfR 1117 R | CTCTTTGTTCTCTTCTCAGATGAGC | |
| A12 pcfFEG 1118 F | GTTGACATGGAAGCAAGTGCTGG | |
| A12 pcfFEG 1118 R | CAATTAACCAAGCCATAGTCCC | |
| A12 pcfFEG 1119 F | GCCGGCAACTTTCGC | |
| A12 pcfFEG 1119 R | CTCCTAGCATATGCAAAATAGC | |
| A12 pcfFEG 1120 F | CCGACAAACGGATTAGACCC | |
| A12 pcfFEG 1120 R | CTCCTTCATGTATAATTCCTATATGTTC |
Underlining indicates insertion of Km sequences.
Monitoring of growth.
Growth of A12 and A12 mutant derivatives was monitored using a Bioscreen C (Growth Curves USA, Piscataway, NJ). Overnight cultures were diluted 1:20 in BHI and were grown to an OD600 of 0.5. Subsequently, the culture was diluted 1:100 into BHI medium with or without nisin and transferred to 100-well honeycomb plates. The OD600 was measured every 20 min, with shaking for 10 s before each measurement. When noted, sterile mineral oil was overlaid in each well to reduce the exposure of cells to oxygen.
Antagonism assays on agar plates.
A plate-based antagonism assay was utilized to detect and measure growth inhibition between S. mutans UA159 and A12 or A12 derivatives (22). Briefly, bacterial cultures were grown overnight in BHI medium, and then the OD600 was adjusted to 0.5. Aliquots (6 μl) from each culture were spotted adjacent to the other strain on BHI agar plates, either simultaneously or with A12 strains inoculated first, followed by inoculation with S. mutans 24 h later. All plates were incubated for 24 or 48 h at 37°C in a 5% CO2 aerobic atmosphere. Zones of inhibition were captured with a digital imager and quantified with NIH ImageJ analysis, which was set to a standardized scale of 10.234 pixels per mm. Deferred antagonism assays were also performed to monitor the effects of A12 and its derivatives on bacteriocin production by S. mutans. Cultures of Spr S. mutans (Table 1), S. sanguinis SK150, and A12 were grown overnight, centrifuged at 10,000 × g, and washed with phosphate-buffered saline (PBS). The cells were resuspended in PBS and adjusted to an OD600 of 0.5. S. mutans was then mixed with other strains in a 1:1 ratio, based on optical density. Cultures of S. mutans or A12 alone were used as controls. Single or mixed cultures were stabbed onto BHI agar with a sterile toothpick and grown in aerobic (5% CO2) or anaerobic (GasPak; BD Life Sciences) conditions at 37°C. After 24 h of incubation, 3 ml of soft agar overlay (BHI in 1% agar) containing 107 cells of the indicator strain S. sanguinis SK150 was poured evenly onto the plate. All plates were incubated for an additional 24 h, and zones of inhibition were measured from digital images. To enumerate CFU after 48 h of incubation, agar plugs were obtained from the center of the zone, resuspended in 1 ml of PBS, and sonicated for three 30-s cycles in a sonicating water bath, with cooling on ice during intervals. Samples were then serially diluted and plated onto BHI agar or BHI agar containing spectinomycin (1 mg ml−1) or kanamycin (1 mg ml−1).
Monitoring of comX or cipB gene promoter activity.
S. mutans UA159 strains carrying a lacZ gene from Streptococcus salivarius fused to the promoter regions of comX (encodes the alternative sigma factor required for genetic competence) or cipB (encodes a bacteriocin) (77) were utilized to assess the expression of those genes in the presence of XIP or CSP, as described elsewhere (22, 76). To determine whether S. gordonii DL1 or strains of A12 had the ability to interfere with XIP or CSP signaling of S. mutans, supernatants from cultures grown in FMC medium (for XIP signaling) or BHI medium (for CSP signaling) were collected by centrifugation, the pH of the supernatant fluid was adjusted to 7.0, and, in the case of FMC medium for XIP signaling, additional glucose was added to increase the concentration by 25 mM. All supernatants were then filtered through a 0.2-μm syringe filter. Similarly prepared S. mutans UA159 supernatants were included as a positive control. Various concentrations of sXIP were then added to supernatants and incubated overnight at 37°C. Cultures of the reporter strain S. mutans UA159::PcomX-lacZ grown in FMC medium to an OD600 of 0.15 were then incubated with the supernatants for 2 h, and cells were collected to assay β-galactosidase (LacZ) activity as an indicator of comX promoter activity. For CSP signaling, 2 μM CSP was added to the supernatants and incubated for 3 h. Reporter strain S. mutans UA159::PcipB-lacZ grown in BHI medium to an OD600 of 0.15 was then incubated with the samples for an additional 2 h. Cells were then collected for LacZ activity as a measure of cipB promoter activity. β-Galactosidase activity was determined using a modification of the Miller protocol (22, 78). Briefly, cells were harvested by centrifugation, washed once with Z buffer (sodium phosphate buffer [pH 7.0], 10 mM KCl, 1 mM MgSO4, 5 mM β-mercaptoethanol), and resuspended in Z buffer. A sample aliquot was treated with toluene-acetone (1:9) for 2 min, and the remainder of each sample was used to measure OD600. The reaction was initiated by adding 80 μl of a 4-mg/ml solution of ONPG (o-nitrophenyl-β-d-galactopyranoside) and terminated by adding 500 μl of 1 M Na2CO3. Samples were then quickly centrifuged at 15,250 × g for 1 min, and the optical density of the supernatant fluid was measured at 420 and 550 nm. All assays were performed using biological triplicates, with technical triplicates for each sample. β-Galactosidase activity is reported in Miller units.
Analysis of gene expression by quantitative reverse transcriptase PCR.
Overnight cultures of A12 strains were inoculated into 7 ml of fresh BHI medium. Once cultures reached an OD600 of 0.5, they were left untreated or treated with 2 μg/ml of nisin and incubated for 15 min at 37°C. Cells were then harvested by centrifugation (5 min, 10,000 × g) and immediately suspended in 1 ml of bacterial RNAprotect (Qiagen, Germantown, MD), followed by 5 min of incubation at room temperature. Total RNA was extracted as previously described (79, 80), and 1 μg of total RNA was reverse transcribed to cDNA using the iScript Select cDNA Synthesis kit (Bio-Rad) with random hexamers. Total RNA and cDNA were prepared in biological triplicates, and subsequent technical triplicates for each cDNA template were included with appropriate controls. The obtained cDNAs were used as templates for quantitative reverse transcriptase PCR (qRT-PCR) for gene expression analysis. The levels of various mRNA transcripts were quantified using gene-specific primers (Table 2) and normalized to 16S rRNA transcripts. qRT-PCR was performed using an iCycler iQ real-time PCR detection system (CFX96; Bio-Rad) and iQ SYBR green Supermix (Bio-Rad) according to the protocols provided by the supplier.
Pangenome analysis on isolates closely related to A12.
Bioinformatic comparisons of recently isolated strains that were genetically similar to A12 (28) were conducted in two steps. First, the databases required for the analyses were built. The annotations for all the strains considered in this study were generated with the open source software Prokka (81). The protein databases for BLAST searches (82, 83) were built from the Prokka translated coding gene output. Lastly, the pangenome analysis was conducted with the Roary pipeline (84), which clustered the isolates based on gene presence. Phylogenetic maximum likelihood trees from multiple-sequence alignments from Roary were built using Phyml (85, 86) and annotated, and then they were displayed with iTol (87).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under award numbers R01 DE025832, T90 DE021990, and F30 DE028184.
We declare that there are no potential conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01335-19.
REFERENCES
- 1.Allareddy V, Rampa S, Lee MK, Allareddy V, Nalliah RP. 2014. Hospital-based emergency department visits involving dental conditions: profile and predictors of poor outcomes and resource utilization. J Am Dent Assoc 145:331–337. doi: 10.14219/jada.2014.7. [DOI] [PubMed] [Google Scholar]
- 2.Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. 2005. The global burden of oral diseases and risks to oral health. Bull World Health Organ 83:661–669. [PMC free article] [PubMed] [Google Scholar]
- 3.Marsh PD, Zaura E. 2017. Dental biofilm: ecological interactions in health and disease. J Clin Periodontol 44(Suppl 1):S12–S22. doi: 10.1111/jcpe.12679. [DOI] [PubMed] [Google Scholar]
- 4.Burne RA, Zeng L, Ahn SJ, Palmer SR, Liu Y, Lefebure T, Stanhope MJ, Nascimento MM. 2012. Progress dissecting the oral microbiome in caries and health. Adv Dent Res 24:77–80. doi: 10.1177/0022034512449462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi N. 2015. Oral microbiome metabolism: From “who are they?” to “what are they doing?” J Dent Res 94:1628–1637. doi: 10.1177/0022034515606045. [DOI] [PubMed] [Google Scholar]
- 6.Solbiati J, Frias-Lopez J. 2018. Metatranscriptome of the oral microbiome in health and disease. J Dent Res 97:492–500. doi: 10.1177/0022034518761644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richards VP, Alvarez AJ, Luce AR, Bedenbaugh M, Mitchell ML, Burne RA, Nascimento MM. 2017. Microbiomes of site-specific dental plaques from children with different caries status. Infect Immun 85:e00106-17. doi: 10.1128/IAI.00106-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowden G. 1990. Microbiology of root surface caries in humans. J Dent Res 69:1205–1210. doi: 10.1177/00220345900690051701. [DOI] [PubMed] [Google Scholar]
- 9.Hajishengallis E, Parsaei Y, Klein MI, Koo H. 2017. Advances in the microbial etiology and pathogenesis of early childhood caries. Mol Oral Microbiol 32:24–34. doi: 10.1111/omi.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, Boches SK, Dewhirst FE, Griffen AL. 2002. Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol 40:1001–1009. doi: 10.1128/jcm.40.3.1001-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philip N, Suneja B, Walsh L. 2018. Beyond Streptococcus mutans: clinical implications of the evolving dental caries aetiological paradigms and its associated microbiome. Br Dent J 224:219–225. doi: 10.1038/sj.bdj.2018.81. [DOI] [PubMed] [Google Scholar]
- 12.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. 2005. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belda-Ferre P, Alcaraz LD, Cabrera-Rubio R, Romero H, Simón-Soro A, Pignatelli M, Mira A. 2012. The oral metagenome in health and disease. ISME J 6:46–56. doi: 10.1038/ismej.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowen WH, Burne RA, Wu H, Koo H. 2018. Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol 26:229–242. doi: 10.1016/j.tim.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu L, Kreth J. 2012. The role of hydrogen peroxide in environmental adaptation of oral microbial communities. Oxid Med Cell Longev 2012:717843. doi: 10.1155/2012/717843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J, Hwang G, Liu Y, Gao L, Kilpatrick-Liverman LT, Santarpi P, Zhou X, Koo H. 2016. l-Arginine modifies the exopolysaccharide matrix and thwarts Streptococcus mutans outgrowth within mixed-species oral biofilms. J Bacteriol 198:2651–2661. doi: 10.1128/JB.00021-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nascimento MM, Browngardt C, Xiaohui X, Klepac-Ceraj V, Paster BJ, Burne RA. 2014. The effect of arginine on oral biofilm communities. Mol Oral Microbiol 29:45–54. doi: 10.1111/omi.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casiano-Colón A, Marquis RE. 1988. Role of the arginine deiminase system in protecting oral bacteria and an enzymatic basis for acid tolerance. Appl Environ Microbiol 54:1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curran TM, Lieou J, Marquis RE. 1995. Arginine deiminase system and acid adaptation of oral streptococci. Appl Environ Microbiol 61:4494–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burne RA, Marquis RE. 2000. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett 193:1–6. doi: 10.1111/j.1574-6968.2000.tb09393.x. [DOI] [PubMed] [Google Scholar]
- 21.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner ACR, Yu W-H, Lakshmanan A, Wade WG. 2010. The human oral microbiome. J Bacteriol 192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang X, Palmer SR, Ahn S-J, Richards VP, Williams ML, Nascimento MM, Burne RA. 2016. A highly arginolytic Streptococcus species that potently antagonizes Streptococcus mutans. Appl Environ Microbiol 82:2187–2201. doi: 10.1128/AEM.03887-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang BY, Kuramitsu HK. 2005. Interactions between oral bacteria: inhibition of Streptococcus mutans bacteriocin production by Streptococcus gordonii. Appl Environ Microbiol 71:354–362. doi: 10.1128/AEM.71.1.354-362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, Bonazzi V, McEwen JE, Wetterstrand KA, Deal C, Baker CC, Di Francesco V, Howcroft TK, Karp RW, Lunsford RD, Wellington CR, Belachew T, Wright M, Giblin C, David H, Mills M, Salomon R, Mullins C, Akolkar B, Begg L, Davis C, Grandison L, Humble M, Khalsa J, Roger Little A, Peavy H, Pontzer C, Portnoy M, Sayre MH, Starke-Reed P, Zakhari S, Read J, Watson B, Guyer M. 2009. The NIH Human Microbiome Project. Genome Res 19:2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornejo OE, Lefébure T, Pavinski Bitar PD, Lang P, Richards VP, Eilertson K, Do T, Beighton D, Zeng L, Ahn SJ, Burne RA, Siepel A, Bustamante CD, Stanhope MJ. 2013. Evolutionary and population genomics of the cavity causing bacteria Streptococcus mutans. Mol Biol Evol 30:881–893. doi: 10.1093/molbev/mss278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer SR, Miller JH, Abranches J, Zeng L, Lefebure T, Richards VP, Lemos JA, Stanhope MJ, Burne RA. 2013. Phenotypic heterogeneity of genomically-diverse isolates of Streptococcus mutans. PLoS One 8:e61358. doi: 10.1371/journal.pone.0061358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang X, Browngardt CM, Jiang M, Ahn SJ, Burne RA, Nascimento MM. 2018. Diversity in antagonistic interactions between commensal oral streptococci and Streptococcus mutans. Caries Res 52:88–101. doi: 10.1159/000479091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velsko IM, Chakraborty B, Nascimento MM, Burne RA, Richards VP. 2018. Species designations belie phenotypic and genotypic heterogeneity in oral streptococci. mSystems 3:e00158-18. doi: 10.1128/mSystems.00158-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkening RV, Chang JC, Federle MJ. 2016. PepO, a CovRS-controlled endopeptidase, disrupts Streptococcus pyogenes quorum sensing. Mol Microbiol 99:71–87. doi: 10.1111/mmi.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richards VP, Palmer SR, Bitar PDP, Qin X, Weinstock GM, Highlander SK, Town CD, Burne RA, Stanhope MJ. 2014. Phylogenomics and the dynamic genome evolution of the genus Streptococcus. Genome Biol Evol 6:741–753. doi: 10.1093/gbe/evu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Q, Ahn SJ, Kaspar J, Zhou X, Burne RA. 2014. Growth phase and pH influence peptide signaling for competence development in Streptococcus mutans. J Bacteriol 196:227–236. doi: 10.1128/JB.00995-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Son M, Ghoreishi D, Ahn S-J, Burne RA, Hagen SJ. 2015. Sharply tuned pH response of genetic competence regulation in Streptococcus mutans: a microfluidic study of the environmental sensitivity of comX. Appl Environ Microbiol 81:5622–5631. doi: 10.1128/AEM.01421-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li YH, Tian X. 2012. Quorum sensing and bacterial social interactions in biofilms. Sensors (Basel) 12:2519–2538. doi: 10.3390/s120302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabharwal P, Lee K, Nascimento MM, Burne RA. 2019. Characterizing clinical isolates related to Streptococcus A12. 97th Gen Session IADR/AADR/CADR, Vancouver, BC, Canada, abstr 0587. [Google Scholar]
- 35.Bland ND, Pinney JW, Thomas JE, Turner AJ, Isaac RE. 2008. Bioinformatic analysis of the neprilysin (M13) family of peptidases reveals complex evolutionary and functional relationships. BMC Evol Biol 8:16. doi: 10.1186/1471-2148-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner AJ, Elwyn Isaac R, Coates D. 2001. The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. Bioessays 23:261–269. doi:. [DOI] [PubMed] [Google Scholar]
- 37.Terleckyj B, Shockman GD. 1975. Amino acid requirements of Streptococcus mutans and other oral streptococci. Infect Immun 11:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Son M, Shields RC, Ahn SJ, Burne RA, Hagen SJ. 2015. Bidirectional signaling in the competence regulatory pathway of Streptococcus mutans. FEMS Microbiol Lett 362:fnv159. doi: 10.1093/femsle/fnv159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dawid S, Roche AM, Weiser JN. 2007. The blp bacteriocins of Streptococcus pneumoniae mediate intraspecies competition both in vitro and in vivo. Infect Immun 75:443–451. doi: 10.1128/IAI.01775-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller EL, Abrudan MI, Roberts IS, Rozen DE. 2016. Diverse ecological strategies are encoded by Streptococcus pneumoniae bacteriocin-like peptides. Genome Biol Evol 8:1072–1090. doi: 10.1093/gbe/evw055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Javan RR, van Tonder AJ, King JP, Harrold CL, Brueggemann AB. 2018. Genome sequencing reveals a large and diverse repertoire of antimicrobial peptides. Front Microbiol 9:2012. doi: 10.3389/fmicb.2018.02012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bogaardt C, van Tonder AJ, Brueggemann AB. 2015. Genomic analyses of pneumococci reveal a wide diversity of bacteriocins—including pneumocyclicin, a novel circular bacteriocin. BMC Genomics 16:554. doi: 10.1186/s12864-015-1729-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Begley M, Cotter PD, Hill C, Ross RP. 2009. Identification of a novel two-peptide lantibiotic, lichenicidin, following rational genome mining for LanM proteins. Appl Environ Microbiol 75:5451–5460. doi: 10.1128/AEM.00730-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fath MJ, Mahanty HK, Kolter R. 1989. Characterization of a purF operon mutation which affects colicin V production. J Bacteriol 171:3158–3161. doi: 10.1128/jb.171.6.3158-3161.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen T, Zhang Z, Huang IH, Wu C, Merritt J, Shi W, Qi F. 2009. Genes involved in the repression of mutacin I production in Streptococcus mutans. Microbiology 155:551–556. doi: 10.1099/mic.0.021303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidson AL, Chen J. 2004. ATP-binding cassette transporters in bacteria. Annu Rev Biochem 73:241–268. doi: 10.1146/annurev.biochem.73.011303.073626. [DOI] [PubMed] [Google Scholar]
- 47.Rea MC, Ross RP, O’Sullivan JN, O’Connor PM, Hill C. 2018. Human skin microbiota is a rich source of bacteriocin-producing staphylococci that kill human pathogens. FEMS Microbiol Ecol 95:fiy241. doi: 10.1093/femsec/fiy241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakatsuji T, Chen TH, Butcher AM, Trzoss LL, Nam SJ, Shirakawa KT, Zhou W, Oh J, Otto M, Fenical W, Gallo RL. 2018. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci Adv 4:eaao4502. doi: 10.1126/sciadv.aao4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobson A, Lam L, Rajendram M, Tamburini F, Honeycutt J, Pham T, Van Treuren W, Pruss K, Stabler SR, Lugo K, Bouley DM, Vilches-Moure JG, Smith M, Sonnenburg JL, Bhatt AS, Huang KC, Monack D. 2018. A gut commensal-produced metabolite mediates colonization resistance to Salmonella infection. Cell Host Microbe 24:296–307.e7. doi: 10.1016/j.chom.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caballero S, Kim S, Carter RA, Leiner IM, Sušac B, Miller L, Kim GJ, Ling L, Pamer EG. 2017. Cooperating commensals restore colonization resistance to vancomycin-resistant Enterococcus faecium. Cell Host Microbe 21:592–602.e4. doi: 10.1016/j.chom.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen L, Chakraborty B, Zou J, Burne RA, Zeng L. 2019. Amino sugars modify antagonistic interactions between commensal oral streptococci and Streptococcus mutans. Appl Environ Microbiol 85:e00370-19. doi: 10.1128/AEM.00370-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fontaine L, Wahl A, Fléchard M, Mignolet J, Hols P. 2015. Regulation of competence for natural transformation in streptococci. Infect Genet Evol 33:343–360. doi: 10.1016/j.meegid.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 53.Kaspar J, Underhill SAM, Shields RC, Reyes A, Rosenzweig S, Hagen SJ, Burne RA. 2017. Intercellular communication via the comX-inducing peptide (XIP) of Streptococcus mutans. J Bacteriol 199:e00404-17. doi: 10.1128/JB.00404-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rostami N, Shields RC, Yassin SA, Hawkins AR, Bowen L, Luo TL, Rickard AH, Holliday R, Preshaw PM, Jakubovics NS. 2017. A critical role for extracellular DNA in dental plaque formation. J Dent Res 96:208–216. doi: 10.1177/0022034516675849. [DOI] [PubMed] [Google Scholar]
- 55.Bayles KW. 2007. The biological role of death and lysis in biofilm development. Nat Rev Microbiol 5:721–726. doi: 10.1038/nrmicro1743. [DOI] [PubMed] [Google Scholar]
- 56.Brouwer S, Cork AJ, Ong C-L, Barnett TC, West NP, McIver KS, Walker MJ. 2018. Endopeptidase PepO regulates the SpeB cysteine protease and is essential for the virulence of invasive M1T1 Streptococcus pyogenes. J Bacteriol 200:e00654-17. doi: 10.1128/JB.00654-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agarwal V, Kuchipudi A, Fulde M, Riesbeck K, Bergmann S, Blom AM. 2013. Streptococcus pneumoniae Endopeptidase O (PepO) is a multifunctional plasminogen-and fibronectin-binding protein, facilitating evasion of innate immunity and invasion of host cells. J Biol Chem 288:6849–6863. doi: 10.1074/jbc.M112.405530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agarwal V, Sroka M, Fulde M, Bergmann S, Riesbeck K, Blom AM. 2014. Binding of Streptococcus pneumoniae endopeptidase O (PepO) to complement component C1q modulates the complement attack and promotes host cell adherence. J Biol Chem 289:15833–15844. doi: 10.1074/jbc.M113.530212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alves LA, Harth-Chu EN, Palma TH, Stipp RN, Mariano FS, Höfling JF, Abranches J, Mattos-Graner RO. 2017. The two-component system VicRK regulates functions associated with Streptococcus mutans resistance to complement immunity. Mol Oral Microbiol 32:419–431. doi: 10.1111/omi.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merritt J, Qi F. 2012. The mutacins of Streptococcus mutans: regulation and ecology. Mol Oral Microbiol 27:57–69. doi: 10.1111/j.2041-1014.2011.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeng L, Farivar T, Burne RA. 2016. Amino sugars enhance the competitiveness of beneficial commensals with Streptococcus mutans through multiple mechanisms. Appl Environ Microbiol 82:3671–3682. doi: 10.1128/AEM.00637-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu C-C, Lin C-T, Wu C-Y, Peng W-S, Lee M-J, Tsai Y-C. 2015. Inhibitory effect of Lactobacillus salivarius on Streptococcus mutans biofilm formation. Mol Oral Microbiol 30:16–26. doi: 10.1111/omi.12063. [DOI] [PubMed] [Google Scholar]
- 63.López-López A, Camelo-Castillo A, Ferrer MD, Simon-Soro Á, Mira A. 2017. Health-associated niche inhabitants as oral probiotics: the case of Streptococcus dentisani. Front Microbiol 8:379. doi: 10.3389/fmicb.2017.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wescombe PA, Upton M, Renault P, Wirawan RE, Power D, Burton JP, Chilcott CN, Tagg JR. 2011. Salivaricin 9, a new lantibiotic produced by Streptococcus salivarius. Microbiology 157:1290–1299. doi: 10.1099/mic.0.044719-0. [DOI] [PubMed] [Google Scholar]
- 65.Wescombe PA, Heng NCK, Burton JP, Chilcott CN, Tagg JR. 2009. Streptococcal bacteriocins and the case for Streptococcus salivarius as model oral probiotics. Future Microbiol 4:819–835. doi: 10.2217/fmb.09.61. [DOI] [PubMed] [Google Scholar]
- 66.Willey JM, van der Donk WA. 2007. Lantibiotics: peptides of diverse structure and function. Annu Rev Microbiol 61:477–501. doi: 10.1146/annurev.micro.61.080706.093501. [DOI] [PubMed] [Google Scholar]
- 67.Hill C, Draper L, Ross R, Cotter P. 2008. Lantibiotic immunity. Curr Protein Pept Sci 9:39–49. doi: 10.2174/138920308783565750. [DOI] [PubMed] [Google Scholar]
- 68.Ajdic D, McShan WM, McLaughlin RE, Savic G, Chang J, Carson MB, Primeaux C, Tian R, Kenton S, Jia H, Lin S, Qian Y, Li S, Zhu H, Najar F, Lai H, White J, Roe BA, Ferretti JJ. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci U S A 99:14434–14439. doi: 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biswas S, Biswas I. 2013. SmbFT, a putative ABC transporter complex, confers protection against the lantibiotic Smb in streptococci. J Bacteriol 195:5592–5601. doi: 10.1128/JB.01060-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cascales E, Buchanan SK, Duche D, Kleanthous C, Lloubes R, Postle K, Riley M, Slatin S, Cavard D. 2007. Colicin biology. Microbiol Mol Biol Rev 71:158–229. doi: 10.1128/MMBR.00036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Havarstein LS, Holo H, Nes IF. 1994. The leader peptide of colicin V shares consensus sequences with leader peptides that are common among peptide bacteriocins produced by Gram-positive bacteria. Microbiology 140:2383–2389. doi: 10.1099/13500872-140-9-2383. [DOI] [PubMed] [Google Scholar]
- 72.Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, Shafiq F, Kotol PF, Bouslimani A, Melnik AV, Latif H, Kim JN, Lockhart A, Artis K, David G, Taylor P, Streib J, Dorrestein PC, Grier A, Gill SR, Zengler K, Hata TR, Leung DYM, Gallo RL. 2017. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med 9:eaah4680. doi: 10.1126/scitranslmed.aah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeng L, Burne RA. 2019. Essential roles of the sppRA fructose-phosphate phosphohydrolase operon in carbohydrate metabolism and virulence expression by Streptococcus mutans. J Bacteriol 201:e00586-18. doi: 10.1128/JB.00586-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahn SJ, Burne RA. 2006. The atlA operon of Streptococcus mutans: role in autolysin maturation and cell surface biogenesis. J Bacteriol 188:6877–6888. doi: 10.1128/JB.00536-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wen ZT, Burne RA. 2001. Construction of a new integration vector for use in Streptococcus mutans. Plasmid 45:31–36. doi: 10.1006/plas.2000.1498. [DOI] [PubMed] [Google Scholar]
- 76.Son M, Ahn SJ, Guo Q, Burne RA, Hagen SJ. 2012. Microfluidic study of competence regulation in Streptococcus mutans: environmental inputs modulate bimodal and unimodal expression of comX. Mol Microbiol 86:258–272. doi: 10.1111/j.1365-2958.2012.08187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perry JA, Jones MB, Peterson SN, Cvitkovitch DG, Lévesque CM. 2009. Peptide alarmone signalling triggers an auto-active bacteriocin necessary for genetic competence. Mol Microbiol 72:905–917. doi: 10.1111/j.1365-2958.2009.06693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zubay G, Morse DE, Schrenk WJ, Miller J. 1972. Detection and isolation of the repressor protein for the tryptophan operon of Escherichia coli. Proc Natl Acad Sci U S A 69:1100–1103. doi: 10.1073/pnas.69.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ahn SJ, Lemos JAC, Burne RA. 2005. Role of HtrA in growth and competence of Streptococcus mutans UA159. J Bacteriol 187:3028–3038. doi: 10.1128/JB.187.9.3028-3038.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen YYM, Weaver CA, Mendelsohn DR, Burne RA. 1998. Transcriptional regulation of the Streptococcus salivarius 57.I urease operon. J Bacteriol 180:5769–5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 82.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 83.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MTG, Fookes M, Falush D, Keane JA, Parkhill J. 2015. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 86.Hordijk W, Gascuel O. 2005. Improving the efficiency of SPR moves in phylogenetic tree search methods based on maximum likelihood. Bioinformatics 21:4338–4347. doi: 10.1093/bioinformatics/bti713. [DOI] [PubMed] [Google Scholar]
- 87.Letunic I, Bork P. 2016. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.