To date, the implications of interleukin 6 (IL-6) for immune responses in the context of Brucella infection are still unknown. In the present study, we found that Brucella abortus infection induced marked production of IL-6 in mice that was important for sufficient differentiation of CD8+ T cells, a key factor in Brucella clearance.
KEYWORDS: B. abortus, IL-6, cytotoxic T cells, lysosomal enzymes, SOCS3
ABSTRACT
To date, the implications of interleukin 6 (IL-6) for immune responses in the context of Brucella infection are still unknown. In the present study, we found that Brucella abortus infection induced marked production of IL-6 in mice that was important for sufficient differentiation of CD8+ T cells, a key factor in Brucella clearance. Blocking IL-6 signaling also significantly induced serum IL-4 and IL-10, together with a decreased gamma interferon (IFN-γ) level, suggesting that IL-6 is essential for priming the T-helper (Th) 1 cell immune response during Brucella infection. The IL-6 pathway also activated the bactericidal activity of primary and cultured macrophages. Bacterial killing was markedly abrogated when IL-6 signaling was suppressed, and this phenomenon was mainly associated with decreased activity of lysosome-mediated killing. Interestingly, suppressor of cytokine signaling 3 (SOCS3) was important for regulating the IL-6-dependent anti-Brucella activity through the JAK/STAT pathway. During early infection, in the absence of SOCS3, IL-6 exhibited anti-inflammatory effects and lysosome-mediated killing inhibition; however, the increase in SOCS3 successfully shifted functional IL-6 toward proinflammatory brucellacidal activity in the late stage. Our data clearly indicate that IL-6 contributes to host resistance against B. abortus infection by controlling brucellacidal activity in macrophages and priming cellular immune responses.
INTRODUCTION
Brucella abortus is a facultative intracellular Gram-negative bacterium that can invade and replicate within a number of phagocytes, such as macrophages, epithelial cells, and placental trophoblasts, leading to chronic infection (1). In macrophages, B. abortus is known to successfully avoid host lysosome-mediated killing activity and other resistant mechanisms (2, 3); however, the comprehensive view of host-Brucella interaction has been drawn from many approaches.
Inflammation, the process involving the production and function of cytokines and chemokines, is a well-known host response to microbial challenges. These cytokines are secreted to amplify and coordinate proinflammatory signals that lead to the expression of effector molecules, resulting in the modulation of diverse aspects of innate immunity against infection (4). In brucellosis, different inflammatory cytokines, including gamma interferon (IFN-γ), interleukin 2 (IL-2), IL-4, IL-10, and IL-12, have been shown to be important regulators of the host immune system. IFN-γ, IL-2, and IL-12 are beneficial molecules for host killing, whereas IL-4 and IL-10 are recognized as beneficial components of Brucella survival (5–8). However, the implications of these cytokines were only revealed by in vivo studies, whereas their contributions and the mechanisms they activate in immune cells remain to be investigated. In addition, tumor necrosis factor (TNF), which is also induced by B. abortus infection, was recently demonstrated to play a crucial role in inducing anti-Brucella effectors by regulating the function of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in macrophages (9). These reports suggest that other cytokines, including IL-6, IL-1α, IL-1β, and monocyte chemoattractant protein 1 (MCP-1), may also participate in host resistance during Brucella infection.
The proinflammatory cytokine IL-6 was initially characterized as an inducer of B cell growth and antibody production; however, IL-6 has been implicated in other immunological processes, including CD4+ T cell differentiation or proliferation and the function of cytotoxic CD8+ T cells (10–12). During viral or bacterial infection or oncogenesis, IL-6 has been demonstrated to be a crucial activator of resistant immunity (13–15). At the cellular level, IL-6 is known to bind to its receptor complex (IL-6R/Gp130) and subsequently activate different signaling cascades, including signal transducer and activator of transcription 3 (STAT3), mitogen-activated protein kinase (MAPK), and NF-κB pathways (16–19). Here, we provide novel insights on the relationship between IL-6 and different immunological aspects, including the production of other cytokines, the differentiation of T cells, and the activation of macrophages, in the context of B. abortus infection.
RESULTS
Suppression of the IL-6 pathway reduces B. abortus control associated with decreased production of antigen-presenting cell and Th1 cell cytokines in vivo.
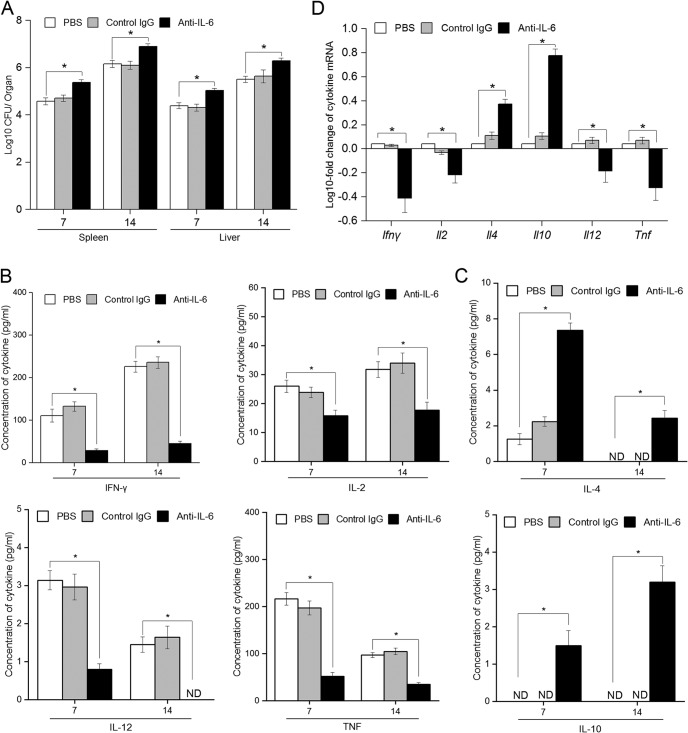
Brucella species-infected mice produce various cytokines and chemokines (20). Among them, B. abortus infection is known to induce IL-6 production in mice (21, 22). However, to date, there have been few reports that examined the immunological function of IL-6 in the context of B. abortus infection. Thus, to investigate and clarify the contribution of IL-6 to host responses to B. abortus infection, we suppressed the IL-6 pathway in a mouse model using a specific anti-IL-6 monoclonal antibody treatment during B. abortus infection and comparison of CFU in the spleen and liver at days 7 and 14 postinfection (p.i.). We observed increased CFU in both spleen and liver from IL-6-suppressed mice at day 7 p.i. compared to the control, and this increased bacterial burden was continuously observed until day 14 p.i., whereas treatment with the control antibody did not influence the survival of bacteria within host organs (Fig. 1A).
FIG 1.
Suppression of IL-6 pathway significantly reduces the resistant immunity against B. abortus infection in mice. Mice were i.p. injected with 2 mg of anti-IL-6 antibody, control IgG, or 200 μl of PBS 1 day before infection and at days 4 and 9 p.i. (A) The bacterial burdens in the spleen and liver were analyzed at days 7 and 14 p.i. (B) The concentrations of cytokines from APC (IL-12 and TNF) and Th1 cells (IFN-γ and IL-2) in serum samples were analyzed by ELISA at 7 and 14 days p.i. (C) The concentration of cytokines from Th2 cells (IL-4 and IL-10) in serum samples were analyzed by ELISA at days 7 and 14 p.i. (D) The transcriptional profiles of different cytokines were assessed by qRT-PCR at day 14 p.i. Data represent means ± the SD of triplicate experiments. The asterisk indicates a significant difference (*, P < 0.05). ND, not detectable.
Type 1 T helper (Th1) cell immunity is crucial for the clearance of B. abortus infection. Thus, to evaluate the role of IL-6 in regulating the type 1 immune response during B. abortus infection, the production of Th1 cytokines (IFN-γ and IL-2), Th2 cytokines (IL-4 and IL-10), and antigen-presenting cell (APC) cytokines (IL-12 and TNF) was assessed at days 7 and 14 p.i. We found that the levels of IFN-γ, IL-2, IL-12, and TNF in sera of IL-6-suppressed mice were significantly reduced, whereas induction of IL-4 and IL-10 was observed in these mice compared to the control (Fig. 1B and C). Again, the administration of control antibody did not influence the cytokine profiles during infection.
Moreover, to confirm this effect of IL-6, we isolated total RNA from splenic cells and subjected them to quantitative reverse transcription-PCR (qRT-PCR) to examine the expression of these cytokine genes. In agreement with these results, the expression of all Th1 and APC cytokine genes, especially IFN-γ and TNF, was abrogated when the IL-6 pathway was blocked. In contrast, blocking IL-6 signaling resulted in a significant increase in IL-4 and IL-10 in splenic cells (Fig. 1D).
These data clearly showed that induction of IL-6 is required for the production of Th1 and APC cell cytokines, which are important for the subsequent killing activity of the host in response to B. abortus infection in mice.
IL-6 is required for induction of cellular immune responses, particularly CD8+ T cell differentiation in B. abortus-infected mice.
Other than CD4+ T cells, CD8+ T cells also play a key role in cellular immunity, exerting the most important mechanism against murine brucellosis. In addition, the differentiation and activation of CD8+ T cells are known to be regulated by Th1 cytokines, especially IFN-γ and IL-2 (23, 24), and the aforementioned data indicated that inhibition of IL-6 decreased Th1 cell cytokine production, including IFN-γ and IL-2, suggesting that IL-6 might also contribute to the control of CD4+ and CD8+ T cell differentiation during Brucella infection. Thus, blood samples were collected at days 7 and 14 after infection, and the population of peripheral CD4+ and CD8+ T lymphocytes was analyzed by fluorescence-activated cell sorting (FACS) analysis. As expected, while control IgG injection did not alter the peripheral T-cell population, anti-IL-6 antibody markedly reduced CD8+ T cell differentiation as early as 7 days p.i. (Fig. 2A) and more dramatically at 14 days p.i. (Fig. 2B), with a slight inhibitory effect on CD4+ T cells. In addition to the positive regulation of IL-6 on Th1 cytokine production, the reduction of the peripheral CD8+ T cell population in IL-6-suppressed mice suggests that IL-6 is required for priming adaptive immunity, particularly a predominant cellular immune response during Brucella infection.
FIG 2.
IL-6 signaling is required for the differentiation of CD8+ T cells in B. abortus-infected mice. Mice were i.p. injected with 2 mg of anti-IL-6 antibody, control IgG, or 200 μl of PBS 1 day before infection and 4 and 9 days p.i. Blood samples were collected from tail veins, and the population of peripheral CD4+ and CD8+ T cells was analyzed by FACS assay at days 7 (A) and 14 (B) p.i. The data are presented as means ± the SD from 10,000 events (n = 5 per group). The asterisk indicates a significant difference (*, P < 0.01).
IL-6/IL-6R/Gp130 signaling activates efficient brucellacidal activity in macrophages.
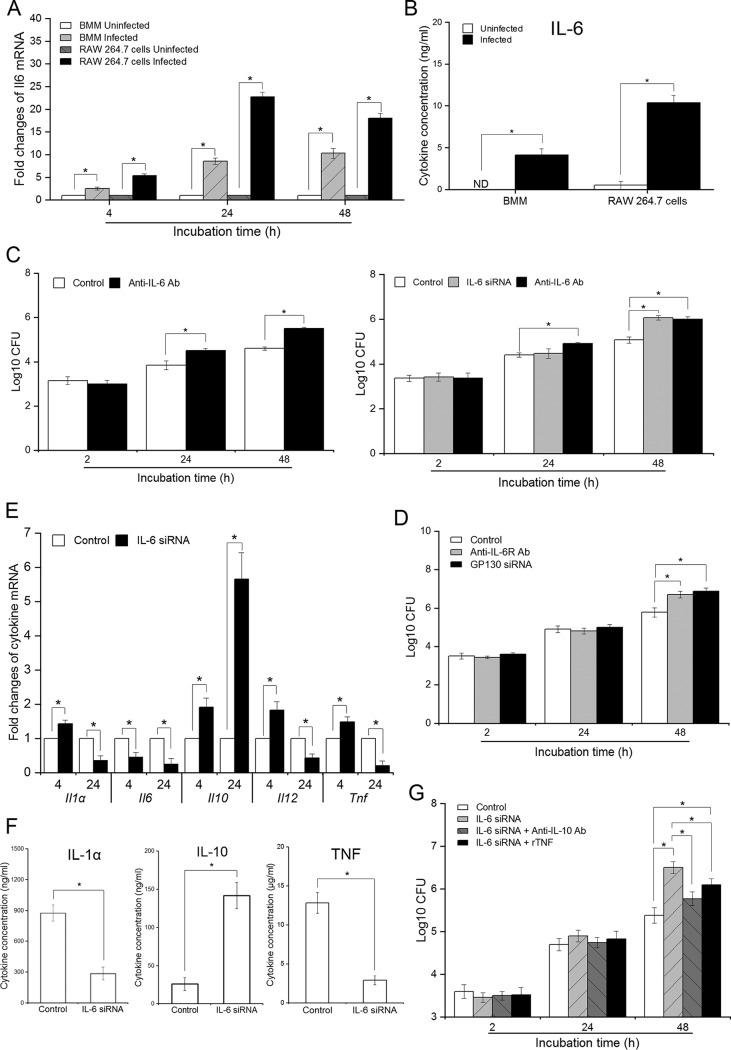
The phagocytosis and subsequent processing of Brucella by macrophages represent the initial responses that coordinate innate and adaptive immunity (3); thus, we evaluated the impact of IL-6 on the anti-Brucella activity of macrophages. We have previously shown that B. abortus infection induces marked production of IL-6 by macrophages, and in this study we reconfirmed the induction of IL-6 by Brucella infection in both primary macrophages (i.e., bone marrow-derived macrophages [BMMs]) and cultured macrophages (RAW 264.7) (Fig. 3A). To complement these data, we continuously quantified the secreted IL-6 in the culture supernatant at 48 h p.i., which showed consistent results with a large increase in IL-6 in infected cells compared to the uninfected control (Fig. 3B). In addition, with the help of IL-6 small interfering RNA (siRNA) and anti-IL-6 monoclonal antibody (MAb), we found that the induction of IL-6 required for efficient killing of B. abortus by macrophages during blockade of this pathway significantly increased the survival of intracellular Brucella within macrophages (Fig. 3C). Blocking IL-6 using a target MAb drastically reduced host defense against Brucella, which suggested that secreted IL-6 activated a subsequent immune response by binding to its receptor complex (IL-6R/Gp130). To address this hypothesis, RAW 264.7 macrophages were treated with either anti-IL-6R antibody or Gp130 siRNA. Brucella grew to larger numbers by 48 h p.i. in IL-6 antibody/siRNA-treated cells; however, differences with untreated control were not detected at early time points examined (Fig. 3D). These observations support that B. abortus strongly stimulates the secretion of IL-6, which, in turn, binds to the IL-6R/Gp130 complex to activate a subsequent resistance mechanism in murine macrophages.
FIG 3.
IL-6 mediates IL-6R/Gp130 complex to activate the anti-Brucella responses in macrophages. (A) The transcriptional profile of Il6 in BMMs and RAW 264.7 cells was evaluated by qRT-PCR at different time points. (B) IL-6 secreted from BMMs and RAW 264.7 cells was quantified by ELISA at 48 h p.i. (C) BMMs and RAW 264.7 cells were treated with either anti-IL-6 Ab or IL-6 siRNA prior to Brucella infection. Bacterial intracellular growth was evaluated at different times. (D) RAW 264.7 cells were treated with either anti-IL-6R Ab or Gp130 siRNA prior to infection. Bacterial intracellular growth was evaluated at different times. (E) RAW 264.7 cells were treated with IL-6 siRNA and infected with B. abortus. Transcriptional profiles of different cytokines were evaluated by qRT-PCR at 4 and 24 h p.i. (F) The secretion of cytokines from B. abortus-infected RAW 264.7 cells was evaluated by ELISA at 48 h p.i. (G) RAW 264.7 cells were concomitantly treated with IL-6 siRNA and either anti-IL-10 Ab or rTNF prior to infection. Bacterial growth was assessed at different times. The data represent means ± the SD of triplicate experiments. The asterisk indicates a significant difference (*, P < 0.05).
Furthermore, several studies have demonstrated that binding of IL-6 to IL-6R/Gp130 can alter the production of cytokines in macrophages (16, 17), while others have demonstrated contributions of these cytokines to brucellacidal activity (9, 25, 26), suggesting that IL-6-activated anti-Brucella immune responses might also correlate with the changes in cytokine profiles. Thus, we examined the expression of important cytokines in infected macrophages pretreated with IL-6 siRNA. We found that at an early stage, most cytokine genes, including Il1α, Il10, Il12, and Tnf, were increased when the IL-6 pathway was suppressed, except for Il6 production, which was decreased. At a late stage in the blockade of IL-6 signaling, cytokine genes Il1a, Il6, Il12 and Tnf were decreased, but Il10 was markedly increased (Fig. 3E). To complement these results, we performed an enzyme-linked immunosorbent assay (ELISA) to quantitate secreted cytokines in the culture supernatant. As shown in Fig. 3F, blockade of IL-6 signaling resulted in a remarkable increase in IL-10 but dramatically decreased the production of IL-1α and TNF during late infection. The concentration of protein at 6 h p.i. was too low to be detected (data not shown).
Furthermore, to define the contribution of these changes to IL-6 signaling, we concomitantly treated RAW 264.7 macrophages with IL-6 siRNA and either anti-IL-10 MAb or recombinant TNF (rTNF). We found that both treatments significantly recovered bacterial killing in IL-6-deficient cells, but blockade of IL-10 was more effective than supplementation with rTNF (Fig. 3G). These results indicated that the shift in IL-6 function from anti- and proinflammation in the early stage to proinflammation in the late stage was crucial for controlling intracellular growth of Brucella in macrophages.
IL-6 signaling interference alters the phagolysosome fusion event in Brucella-infected RAW 264.7 cells.
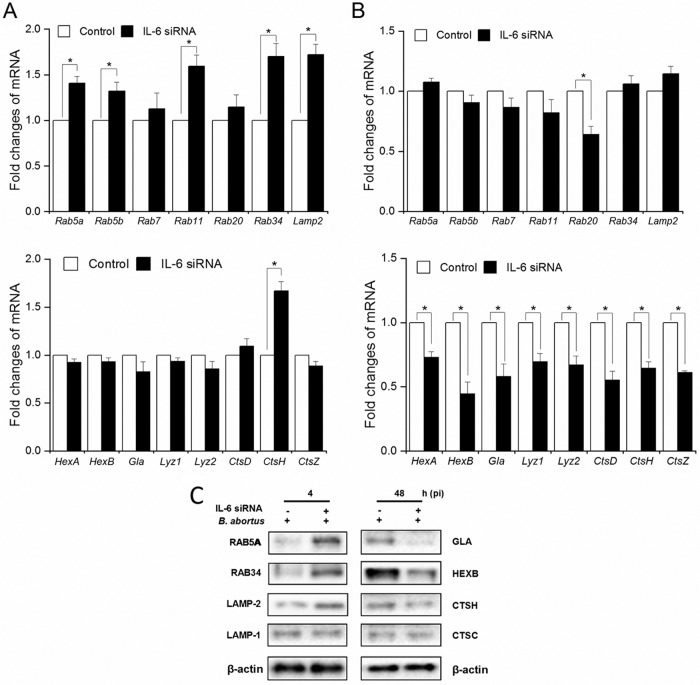
Lysosome-mediated killing, a key effector of brucellacidal immunity, has been recently revealed to be regulated by IL-10 (25, 27), which is also upregulated in IL-6-deficient cells, leading to the speculation that IL-6 influences this activity. Furthermore, Hop et al. (2) and Eskra et al. (28) provided representative trafficking regulators and lysosomal enzyme-related genes that might contribute to this regulatory process. With the help of quantitative reverse transcription-PCR (qRT-PCR), the expression of these genes was tested in the context of IL-6 signaling inhibition to examine their impact on a shift in regulatory function of IL-6 according to the time of infection. In the early stage (4 h p.i.), blockade of IL-6 signaling elevated the expression of five trafficking regulators (Rab5a, Rab5b, Rab11, Rab34, and Lamp2) and one lysosomal enzyme (Ctsh) (Fig. 4A); however, Rab20, Lyz1, Lyz2, Hexa, Hexb, Ctsd, Ctsh, Ctsz, and Gla were strongly inhibited at 48 h p.i. (Fig. 4B). In contrast, blockade of the IL-6 pathway did not cause any changes in the expression of membrane-trafficking regulators and hydrolytic enzymes at 24 h p.i., except for a decrease in Gla (data not shown).
FIG 4.
IL-6 regulates the expression of trafficking regulators and lysosomal enzymes. Cells were treated with IL-6 siRNA and infected with B. abortus. The total RNA content was isolated, and the mRNA levels of trafficking regulators and lysosomal enzymes were evaluated by qRT-PCR at 4 h (A) and 48 h (B) p.i. The data represent means ± the SD of triplicate experiments. The asterisk indicates a significant difference (*, P < 0.05). (C) The expression of representative proteins was evaluated by Western blotting at 4 and 48 h. These data are representative of triplicate experiments.
Since blocking IL-6 signaling altered the transcriptional profiles of different trafficking regulators and lysosomal enzymes, we further tested whether the expression of these proteins was also influenced by IL-6 signaling. We found that blocking IL-6 induced the expression of RAB5A, RAB34, and LAMP-2, but not LAMP-1, at 4 h p.i., whereas a decrease in GLA, HEXB, and CTSH, but not CTSC, was observed in IL-6-deficient cells compared to the control at 48 h p.i. (Fig. 4C). Our collective results revealed the regulatory functions of IL-6 on the expression of trafficking regulators, as well as hydrolytic enzymes during infection, leading to the hypothesis that IL-6 can contribute to controlling Brucella-containing phagosome (BCP)-lysosome fusion. To address this hypothesis, we evaluated the acquisition of different markers by BCP-lysosome fusion.
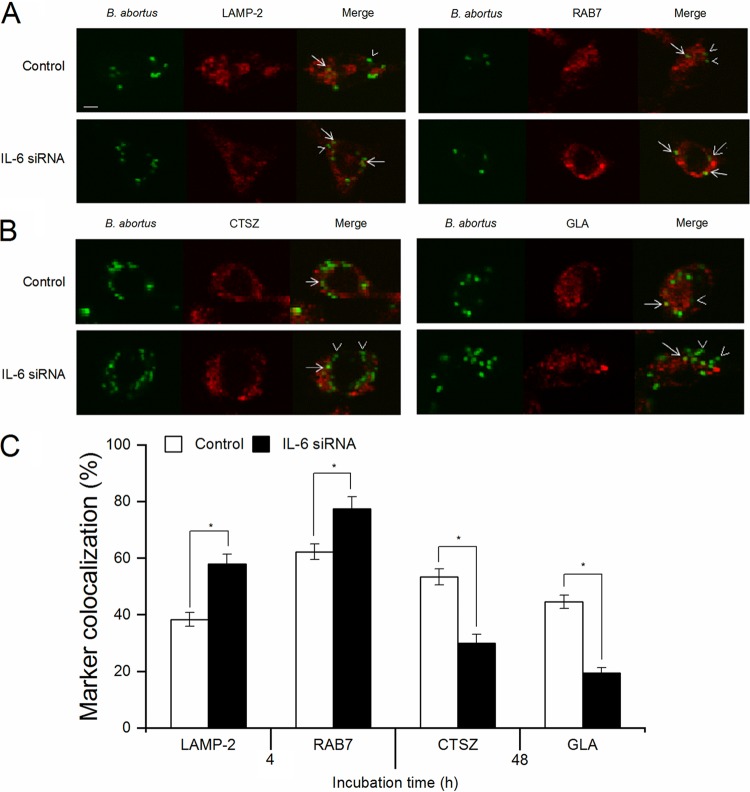
We first focused on LAMP-2 and RAB7 proteins, which have been found to be crucial effectors in the control phagosome maturation and fusion events (29, 30). As shown in Fig. 5A, treatment of macrophages with IL-6 siRNA significantly induced the fraction of BCPs labeled with these markers at 4 h p.i. compared to the control, suggesting a suppressive effect of IL-6 on phagolysosome fusion in the early stage. In contrast, monitoring the fraction of BCPs that could be labeled for CTSZ or GLA proteins at 48 h p.i. consistently showed that IL-6 inhibition markedly reduced the colocalization of these enzymes in BCPs compared to the control (Fig. 5B), indicating that IL-6 provides a great contribution to BCP-lysosome fusion in the late stage.
FIG 5.
Suppression of IL-6 signaling significantly reduces the colocalization of BCPs with trafficking regulators and lysosomal enzymes in B. abortus-infected RAW 264.7 cells. Macrophages were treated with IL-6 siRNA prior to B. abortus infection. (A) The colocalization of BCPs with LAMP-2 and RAB7 was analyzed at 4 h p.i. (B) The colocalization of BCPs with CTSZ and GLA was analyzed at 48 h p.i. (C) The percentage of markers colocalized with BCPs in 100 cells was determined. Arrow, marker positive; arrowheads, marker negative. The data represent means ± the SD of triplicate experiments. The asterisk indicates a significant difference (*, P < 0.05), Scale bars, 5 μm.
IL-6 mediates STAT1 and STAT3 for distinct functions in the early stage.
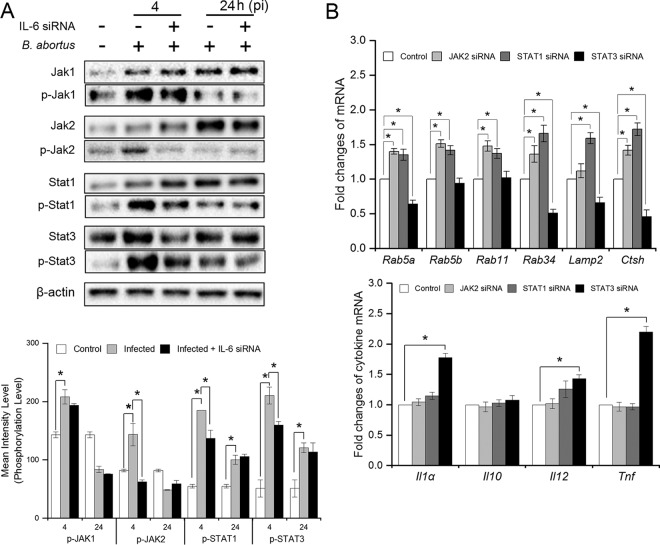
Because the regulatory functions of IL-6 in the inflammatory response and lysosome-mediated killing changed in a time-dependent manner, and because the binding of IL-6 to the IL-6R/Gp130 complex has been previously demonstrated to activate JAK/STAT pathways (16, 17), we speculated that the functional change in IL-6 in the context of Brucella infection might result from the switching of the JAK/STAT pathway. Thus, we treated cells with IL-6 siRNA prior to Brucella infection and evaluated the expression, as well as the phosphorylation, of Janus kinase 1 (JAK1), JAK2, STAT1, and STAT3 at 4 and 24 h p.i. As shown in Fig. 6A, Brucella infection induced a marked activation of these proteins at 4 h p.i., but STAT1 and STAT3 were still highly phosphorylated at 24 h p.i. In addition, IL-6 inhibition markedly reduced JAK2, STAT1, and STAT3 phosphorylation at 4 h p.i., whereas no protein influence was observed during later infection, leading to the assumption that the proinflammatory effect of IL-6 signaling in late infection is independent of JAK/STAT pathways. Moreover, IL-6 signaling was also independent of the JAK1 pathway since its interference did not alter JAK1 activation.
FIG 6.
IL-6 mediates JAK/STAT pathways that contribute in the expression of proinflammatory and phagolysosome-related genes at early infection. (A) RAW 264.7 cells were treated with IL-6 siRNA prior to B. abortus infection and total proteins were isolated at 4 and 24 h p.i. The expression and phosphorylation of JAK1, JAK2, STAT1, and STAT3 proteins were evaluated by Western blotting assay. (B) RAW 264.7 cells were treated with different siRNAs prior to Brucella infection. The total RNA was isolated, and the transcriptional profiles of IL-6-regulated genes were assessed by qRT-PCR at 4 h p.i. The data represent means ± the SD of triplicate experiments. The asterisk indicates a significant difference (*, P < 0.05).
To determine the actual roles of these proteins in IL-6 signaling during early infection, we individually treated cells with JAK2, STAT1, or STAT3 siRNAs prior to infection, and the expression of different phagolysosome-related and inflammatory genes was evaluated. We found that either JAK2 or STAT1 suppression significantly enhanced the expression of phagolysosome fusion-related genes Rab5a, Rab5b, Rab11, and Rab34 and lysosomal enzyme-related gene Ctsh, but not Lamp2, when JAK2 signaling was suppressed, whereas the opposite result was observed when STAT3 signaling was suppressed, except for Rab5b and Rab11. In addition, only STAT3 siRNA treatment elevated the expression of proinflammatory cytokines, including Il1α, Il12, and Tnf (Fig. 6B). Thus, these data suggested that IL-6 regulated the expression of different phagolysosome-related genes, which were well known as crucial factors for intracellular Brucella killing as well as anti-inflammation through two distinct pathways (JAK2/STAT1 and JAK2/STAT3, respectively). In contrast, interference of these signaling did not alter the transcriptional profile of Il10 (Fig. 6B), indicating that the proinflammation effect of IL-6 occurred through a JAK/STAT-independent pathway.
The proinflammatory brucellacidal effect of IL-6 is solely associated with the presence of SOCS3 production in macrophages.
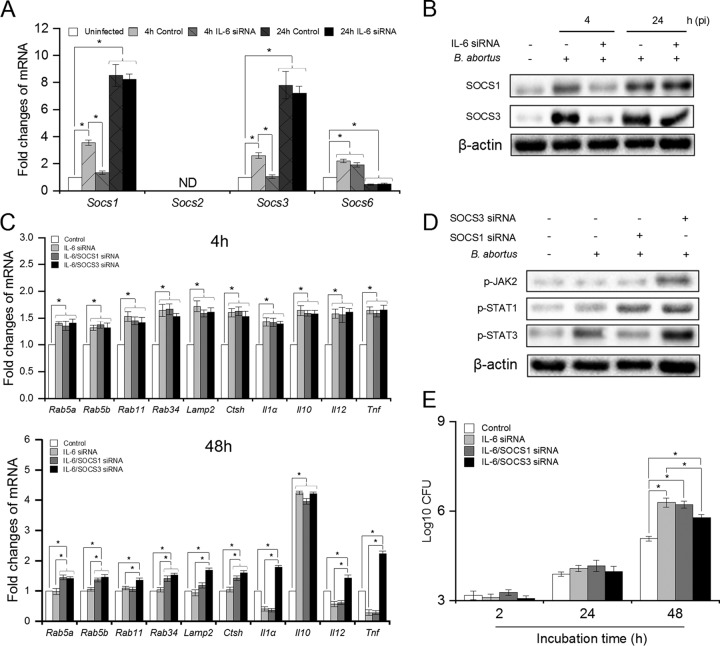
The data presented above indicated that the functions of IL-6 shifted from dual effects on inflammation to only proinflammatory action, as well as from enhancement to suppression on phagolysosome fusion, with effective inhibition of JAK/STAT pathways at the late stage; however, the molecules involved in this process remained to be investigated. SOCS3, a downstream target of the JAK1/STAT3 pathway, has been previously demonstrated to exert feedback suppression on IL-6 signaling by binding to Gp130 and inhibiting its transduction (31). Thus, we suspected that SOCS proteins would also contribute to the functional shift of IL-6 over time. To assess this phenomenon, we first checked the transcriptional profiles of Socs genes at different time points in Brucella-infected IL-6-suppressing cells. We found that Brucella continuously elevated the mRNA levels of Socs1 and Socs3 from 4 until 24 h p.i., whereas this infection first induced expression of Socs6 in the early stage but inhibited it at 24 h p.i. Moreover, blockade of the IL-6 pathway significantly reduced the transcription of Socs1 and Socs3, but not Socs6, in the early stage, whereas no influence was observed during late infection. Moreover, the expression of Socs2 was not detectable (Fig. 7A). These data were further confirmed by Western blotting assay, which also revealed a similar pattern of IL-6-dependent expression of SOCS1 and SOCS3 proteins (Fig. 7B), suggesting high potential contributions of SOCS1 and SOCS3 in the feedback regulation of IL-6 function. To further clarify this hypothesis, we concomitantly treated IL-6 siRNA with either SOCS1 or SOCS3 siRNAs and evaluated the transcription of inflammatory cytokines and phagolysosomal genes. As shown in Fig. 7C, SOCS1 and SOCS3 siRNA treatment did not alter IL-6 function during early infection; however, the anti-inflammatory action and suppressive effect of phagolysosome-related gene expression of IL-6 were still observed up to 48 h p.i. in SOCS3-lacking cells, whereas the suppression of SOCS1 only caused a prolonged suppressive effect of IL-6 on phagolysosomal and lysosomal enzyme-related genes, including Rab5a, Rab5b, Rab34, and Ctsh, during Brucella infection. In parallel, we also found that SOCS3 inhibition significantly maintained the phosphorylation of JAK2, STAT1, and STAT3 up to 24 h p.i., whereas SOCS1 only affected STAT1 activation (Fig. 7D). Moreover, the suppression of SOCS3, but not SOCS1, partially enhanced bacterial killing in IL-6-lacking cells during late infection (Fig. 7E), suggesting that SOCS3 functioned as a key regulator playing the main role in shifting the JAK/STAT-dependent anti-inflammatory and suppressive effect of phagolysosome-related gene expression to proinflammatory anti-Brucella activity of the IL-6 pathway.
FIG 7.
SOCS1 and SOCS3 play distinct roles in blocking IL-6-induced JAK/STAT pathways at late infection. (A) RAW 264.7 cells were treated with IL-6 siRNA prior to B. abortus infection and subjected to RNA isolation. The transcriptional profiles of Socs genes were checked by qRT-PCR at 4 and 24 h p.i. (B) The expression of SOCS1 and SOCS3 proteins was evaluated by Western blotting assay at 4 and 24 h p.i. (C) RAW 264.7 cells were treated with different siRNAs prior to B. abortus infection and total RNA was isolated at 4 and 48 h p.i. The mRNA level of JAK/STAT-dependent IL-6-regulated genes was checked by qRT-PCR. (D) The phosphorylation levels of JAK2, STAT1, and STAT3 were evaluated by Western blotting assay when SOCS1 or SOCS3 signaling was suppressed by siRNA treatment. (E) RAW 264.7 cells were concomitantly treated with IL-6 and either SOCS1 or SOCS3 siRNAs before Brucella infection. Bacterial growth was evaluated at different time points. The data represent means ± the SD of triplicate experiments. The asterisk indicates a significant difference (*, P < 0.05). ND, not detectable.
DISCUSSION
B. abortus, a causative agent of brucellosis, is a pathogen that acquired the ability to survive and replicate within host cells by circumventing phagosomal maturation, leading to chronic infection (32). To fight this threat, host cells activate different defense mechanisms that are still not fully understood; however, among the well-known core regulators of these processes are proinflammatory cytokines (9). To date, several studies have successfully described the immunological roles of cytokines, especially in the bactericidal activity of macrophages against Brucella infection (25, 33, 34). Thus, to identify more of the machinery involved in brucellacidal immunity, we investigated the regulatory functions of proinflammatory IL-6 in most important immunological responses, including the activation of T cells and anti-Brucella activity in macrophages.
In this study, we first reported the acquisition of IL-6 signaling for efficient restriction of B. abortus colonization in mice. The neutralization of IL-6 by target MAb treatment significantly increased the bacterial burden in both the spleen and liver, which occurred in conjunction with a drastic decrease in Th1 and APC cell cytokines but marked enhancement of Th2 cytokines in sera and splenic cells. During Brucella infection, host immunity involves both cell-mediated (Th1) and humoral (Th2) immunity, which are characterized by the production of IFN-γ and IL-10, respectively. These cytokines have been clearly proven to have opposite contributions in resistant immunity against Brucella in mice by manipulating innate and adaptive immunity. Particularly, IFN-γ is known as a key factor that stimulates Th1 cell differentiation to activate cytotoxic CD8+ T cells and elicit macrophage activation during early infection. Conversely, IL-10, which is mostly produced by Th2 cells, functions as an immune tolerance effector and is beneficial for Brucella survival (35, 36). Therefore, the balance of IFN-γ and IL-10 is crucial for determining the type of immune response that is activated and the respective outcome of infection. Other cytokines, including IL-2, IL-12, or IL-4, are also able to enhance or abrogate Brucella survival via their direct functions on immune cells or mainly by controlling the IFN-γ/IL-10 balance (5–8). Furthermore, functional TNF has been shown to link the proinflammatory response and adaptive immune response in Brucella-infected mice (8). Thus, our data suggest that IL-6 is a key regulator that bridges proinflammation and adaptive immunity by governing the production of opposite functional cytokines from Th1 and Th2 cells.
In natural Brucella infection, both macrophages and dendritic cells are able to process and present bacterial antigens to T cells via major histocompatibility complex class I (MHC-I) and MHC-II; this subsequently induces a broad range of immune responses, including the differentiation and activation of CD8+ cytotoxic T cells and CD4+ helper T cells. Th1 CD4+ T cells mediate IFN-γ and IL-2 to induce cellular immunity, such as CD8+ cytotoxic T cell activation, whereas Th2 CD4+ T cells activate a predominant humoral immune response through IL-4 and IL-10 cytokines (23, 24). Therefore, in addition to Th1 and Th2 cytokine evaluation, quantitation of blood CD4+ and CD8+ T cells can provide additional information concerning the immunological role of IL-6 in adaptive immune responses. In parallel with a decrease in IFN-γ and IL-2 production, our data showing a significant reduction in the population of peripheral CD8+ T cells but not CD4+ T cells at 7 and 14 days p.i., when IL-6 signaling was blocked, suggest that IL-6 plays an essential role in initiating the cellular immune response, which is a key effector for Brucella elimination (37).
In addition to their influence on the cellular immune response during Brucella infection, these cytokines function in macrophage activation (9, 25, 27). Thus, in this study, we also evaluated the contribution of IL-6 to bacterial killing in macrophages. In agreement with previous transcriptomic studies (28), we found that B. abortus markedly induced the expression of IL-6 during the whole course of infection in macrophages. Interestingly, Jiang and Baldwin (34) reported that IL-6 plays no role in immune responses against B. abortus infection by macrophages; however, in the present study, our data provide the first evidence of feedback regulation by Brucella-induced IL-6 in the activation of downstream anti-Brucella immunity against a real infection. We found that the binding of secreted IL-6 to its receptor complex IL-6R/Gp130 induced the signal transduction of IL-6, followed by the functional modulation of antimicrobial effectors, which was changed by the time of infection in association with the activation or inhibition of JAK/STAT pathways.
IL-6 has dual roles in inflammation depending on its interaction with the specific target receptor complex, IL-6 classic signaling and IL-6 trans-signaling, which are associated with anti-inflammatory and proinflammatory functions, respectively (38, 39). Consistent with previous studies, IL-6 exhibited three different functions, including both anti-inflammatory and proinflammatory activities, as well as the suppression of the bacterial intracellular trafficking pathway in the early stage of infection in this study. A variety of trafficking regulators and cytokines were highly induced when the IL-6 pathway was suppressed. Assessment of downstream signaling events revealed that the suppression of IL-6 abrogated JAK2, STAT1, and STAT3 activation, and the individual suppression of these genes by siRNA treatment showed their distinct functions in regulating proinflammation and phagolysosome fusion. We found that IL-6 induced JAK2/STAT3 activation aimed at regulating phagolysosome-related gene expression and proinflammatory responses. Moreover, the JAK/STAT pathway has been previously reported to be required for phagosome maturation (40). Our data suggest the novel functions of JAK1 and JAK2 during Brucella infection, since these molecules instructed the same direct downstream protein, STAT3, to exert different functions. Moreover, although interference of IL-6 signaling did not alter JAK1 activation, further investigations regarding how the IL-6/IL-6R/Gp130 pathway excludes JAK1 from its signaling may provide more information about the IL-6 transduction cascade. Interestingly, the contribution of the JAK/STAT pathway to IL-6 was eliminated during late infection, which, in turn, led to the disappearance of the anti-inflammatory effects. The function of IL-6 at this time is solely dependent on feedback regulation to other cytokines, such as TNF or IL-10, since either treatment with rTNF or blockade of IL-10 significantly recovered bacterial killing in IL-6-suppressed cells. Our data also reconfirm the immunological role of TNF and IL-10 during B. abortus infection, which has been previously observed (9, 27). Although they were induced as early as 4 h p.i., these cytokines were not required for the regulatory function of IL-6 during this stage. We could not explain this phenomenon in the present study; however, further investigations examining the cross-regulation of these cytokines in JAK/STAT pathways may be helpful for clarification.
In contrast, lysosome-mediated killing is one of the most important effectors of brucellacidal activity in macrophages, and by suppressing this process, IL-10 promotes Brucella survival (27). Here, we showed that IL-6 regulated IL-10 signaling during the whole course of Brucella infection, suggesting that IL-6 might mediate IL-10 to control this effector. However, even if induced at an early stage, IL-10 was not responsible for the inducible effect of IL-6 on bacterial trafficking at this time point. More surprisingly, IL-6 regulation of this activity was also independent of IL-10 during the late stage, since the sets of genes regulated by the IL-6 and IL-10 pathways were different. During the late stage, IL-10 has been shown to control different trafficking regulators (25), whereas only RAB20 protein is influenced when IL-6 is blocked (Fig. 4B). Furthermore, the reduced colocalization of various hydrolytic enzymes with BCPs in IL-6-suppressed cells resulted from a marked deficiency in the expression of these enzymes rather than a dysregulated recruitment of hydrolytic enzymes by BCPs as caused by IL-10.
One striking finding was the negative-feedback regulation of SOCS proteins on JAK/STAT pathways. We found that SOCS1, SOCS3, and SOCS6 were induced by Brucella but that only SOCS1 and SOCS3 were regulated by the IL-6-activated JAK2/STAT3 pathway. The induction of these proteins at an early stage is crucial for blocking JAK/STAT-dependent IL-6 signaling since their inhibition led to prolonged activation of the JAK/STAT pathway. According to previous works, SOCS3 regulates IL-6 signaling through binding and blocking Gp130 proteins (31); however, in the present study, Gp130 was required for IL-6 signaling throughout infection. Thus, the target of SOCS proteins could be a factor other than Gp130 protein in the context of Brucella infection. In agreement with this expectation, SOCS1 and SOCS3 exhibited inhibitory effects on the phosphorylation of different target proteins. SOCS1 suppression caused a prolonged activation of STAT1, whereas SOCS3 inhibited JAK2 and STAT3 phosphorylation. In addition, the regulation of SOCS3 but not SOCS1 was required to shift to the proinflammatory brucellacidal activity of IL-6 in the late stage. Although our data revealed a feedback regulation of SOCS protein on IL-6 signaling, elucidation of the fundamental mechanisms underlying this regulation requires further experiments.
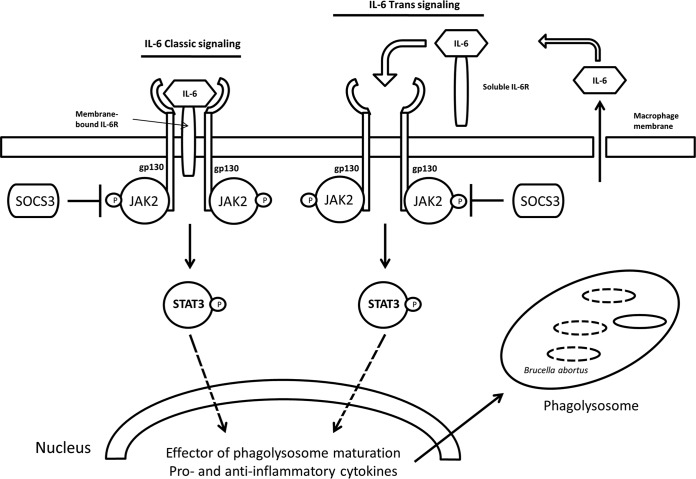
Consequently, we propose that B. abortus induces macrophages to produce IL-6. The binding of endogenous IL-6 to its receptor complex IL-6R/Gp130 elicits the activation of JAK2, and the subsequent activation of STAT3 promotes the production of effectors of phagolysosome maturation-trafficking regulators and lysosomal enzymes, as well as proinflammatory and anti-inflammatory cytokines that help in the clearance of B. abortus infection in macrophages. Importantly, this event was regulated by SOCS3 (Fig. 8).
FIG 8.
Diagram illustrating the IL-6 signaling pathway triggered by the binding of IL-6 and its receptor complex resulting in JAK2/STAT3 activation. This leads to promotion of the production of the effectors of phagolysosome maturation-trafficking regulators and lysosomal enzymes, as well as proinflammatory and anti-inflammatory cytokines, in the regulation of SOCS3. This event clears the B. abortus infection in macrophages. Lines with arrows indicate an activating reaction, and dotted lines indicate an uncertain reaction.
In summary, our findings revealed undescribed novel roles of IL-6 in the regulation of host immune responses that are crucial for B. abortus elimination. Moreover, the present study is also the first to implicate SOCS1 and SOCS3 in the immunological roles of IL-6 through the JAK/STAT pathway. Finally, further investigations of the regulation of cytokine signaling could provide further evidence and insights into inflammation activation, which is one of the most important immune response mechanisms.
MATERIALS AND METHODS
Reagents.
Mouse IL-6, SOCS1, Gp130 siRNAs, rat anti-LAMP-1 and anti-LAMP-2, mouse anti-RAB34, and goat anti-CtsA antibodies were obtained from Santa Cruz Biotechnology (USA). Rabbit anti-RAB5A, anti-CTSH, anti-GLA, anti-JAK1, anti-STAT3, anti-SOCS1, and anti-SOCS3 antibodies and mouse anti-JAK2 antibody were purchased from MyBio Source (USA), and rabbit anti-CTSC antibody was obtained from Antibodies-online (USA). Goat anti-CtsD and anti-IL-6R antibodies were obtained from R&D Systems (USA). Mouse anti-STAT3 and Texas Red–rabbit anti-goat IgG antibodies were purchased from Abcam (USA). Mouse SOCS3 siRNA antibody, rabbit anti-CTSZ, anti-HEXB, anti-phosphor-STAT1, anti-phosphor-STAT3, anti-phosphor-JAK1, and anti-phosphor-JAK2 antibodies, mouse anti-STAT1 antibody, and Texas Red–goat anti-rabbit IgG and FITC-conjugated goat anti-mouse IgG antibodies were obtained from Thermo Fisher Scientific (USA); Texas Red–goat anti-rat IgG antibody and Lipofectamine RNAiMAX were purchased from Life Technologies (USA). Finally, FITC-conjugated goat anti-rabbit IgG antibody was obtained from Sigma-Aldrich Corp (USA).
Bacterial strains and cell culture.
B. abortus 544 (ATCC 23448), a smooth, virulent biovar 1 strain used in the present study, is a standard wild type and was routinely cultured in brucella broth (BD Biosciences) at 37°C until stationary phase at a biosafety laboratory level 3 (BSL3) containment facility. Plating serial dilutions on brucella agar were performed to perform viable counting of bacteria. Murine macrophage RAW 264.7 cells were grown at 37°C in a 5% CO2 atmosphere in RPMI 1640 containing 10% heat-inactivated fetal bovine serum (FBS) with or without 100 U/ml penicillin and 100 μg/ml streptomycin.
BMM preparation.
Bone marrow-derived macrophages (BMMs) were isolated and differentiated from female BALB/c mice as previously described (2).
IL-6 inhibition in vivo.
This experiment was performed as previously described, with a few modifications (6). Briefly, eighteen 8-week-old female BALB/c mice (Japan SLC, Japan) were randomly distributed into three groups (six mice per group). Each mouse was intraperitoneally (i.p.) injected with either anti-IL-6 antibody (2 mg) or an irrelevant control antibody (2 mg) in 200 μl of phosphate-buffered saline (PBS) 1 day before infection and again at 4 and 9 days after infection. Mice in the negative-control group received 200 μl of PBS in the same manner. Mice were i.p. challenged with approximately 5 × 105 CFU of B. abortus in 100 μl of PBS. The numbers of B. abortus in the spleen and liver were determined as the log10 CFU at 2 weeks p.i. The animal facility of the Gyeongsang National University has been officially approved by a responsible authority (Ministry of Education, LML13-16, South Korea) under approval file number 613-83-00570. All performed procedures were approved by the Animal Ethical Committee of Gyeongsang National University (authorization GNU-170331-M0017).
Quantitation of peripheral blood CD4+ and CD8+ T cells.
Cellular populations in peripheral blood from the experimental mice were examined as previously described (41). The analysis was performed using a FACSCalibur flow cytometer (BD Biosciences).
Bacterial intracellular replication assay.
One day before infection, RAW 264.7 cells or BMMs (106 cells) were seeded in a 96-well plate in RPMI medium supplemented with 10% FBS. After 24 h of incubation, the cell culture was replaced with serum- and antibiotic-free RPMI medium and infected with 108 CFU of the virulent strain B. abortus for 1 h at 37°C in 5% CO2. RPMI 1640 medium containing 10% (vol/vol) FBS and gentamicin (30 μg/ml) with or without IL-6 (10 ng/ml) was subsequently added to kill extracellular bacteria. At 2, 24, and 48 h p.i., the cells were lysed, diluted, and plated on brucella agar plates for CFU determination (25).
RNA interference.
RAW 264.7 cells were grown to 50% confluence in 6- or 96-well plates and transfected with IL-6 siRNA using Lipofectamine RNAiMAX. The cells were incubated for 24 h at 37°C with 5% CO2 prior to B. abortus infection. The same concentration of negative-control siRNA was used as a control. The knockdown efficiency was quantified using qRT-PCR.
RNA extraction and qRT-PCR.
Total RNA was isolated from macrophages (uninfected or infected with B. abortus) at different time points using a Qiagen RNeasy kit. DNA was removed before final elution of the RNA sample according to Qiagen’s On-Column DNase Digestion protocol. RNA samples were then subjected to qRT-PCR analysis (Tables 1 and 2) in accordance with a previous description (9). The mRNA expression profiles were normalized with respect to β-actin. The fold increase in each gene was calculated using the 2−ΔΔCT method.
TABLE 1.
Primer sequences of cytokines and Socs family used for qRT-PCR
| Gene | Common name | Forward primer | Reverse primer |
|---|---|---|---|
| b-actin | β-Actin | 5′-CGCCACCAGTTCGCCATGGA-3′ | 5′-TACAGCCCGGGGAGCATCGT-3 |
| Il1α | Interleukin 1α | 5′-CCTTCTATGATGCAAGCTA-3′ | 5′-GTTGCTGATACTGTCACCC-3′ |
| Il2 | Interleukin 2 | 5′-CTCGCATCCTGTGTCACATTG-3′ | 5′-CTGGGGAGTTTCAGGTTCCTG-3′ |
| Il4 | Interleukin 4 | 5′-GTCATCCTGCTCTTCTTTCTC-3′ | 5′-CACCTTGGAAGCCCTACAGAC-3′ |
| Il6 | Interleukin 6 | 5′-TCCAGTTGCCTTCTTGGGAC-3′ | 5′-GTACTCCAGAAGACCAGAG-3′ |
| Il10 | Interleukin 10 | 5′-TGGCCCAGAAATCAAGGAGC-3′ | 5′-CAGCAGACTCAATACACACT-3′ |
| Il12 | Interleukin 12 | 5′-CCCAAGGTCAGCGTTCCAAC-3′ | 5′-GTCCAGAGACTGGAATGACC-3′ |
| Tnf | Tumor necrosis factor | 5′-CACAGAAAGCATGATCCGCG-3′ | 5′-CGGCAGAGAGGAGGTTGACT-3′ |
| Ifng | Interferon γ | 5′-CTGGCTGTTACTGCCACGGC-3′ | 5′-TGCTGAAGAAGGTAGTAATC-3′ |
| Socs1 | Suppressor of cytokine signaling 1 | 5′-GCCGCCAGCGCAGCCCCGGA-3′ | 5′-GGGGCTGGGACCGCCGGGCA-3′ |
| Socs2 | Suppressor of cytokine signaling 2 | 5′-GCTCGGGCGACCAGCTGTCTG-3′ | 5′-TGCGAACTATCTCTAATCAAG-3′ |
| Socs3 | Suppressor of cytokine signaling 3 | 5′-GACCAGCGCCACTTCTTCACG-3′ | 5′-GTTCCGTGGGTGGCAAAGAA-3′ |
| Socs6 | Suppressor of cytokine signaling 6 | 5′-GGCATCGCAGCCCCTCCCGA-3′ | 5′-AGAGTCATCTTTCACGAAGT-3′ |
TABLE 2.
Primer sequences of trafficking regulators and hydrolytic enzymes used for qRT-PCR
| Gene | Common name | Forward primer | Reverse primer |
|---|---|---|---|
| Rab5a | Rab5a | 5′-GTACTACCGAGGAGCACAAG-3′ | 5′-AAGCTGTTGTCATCTGCATAG-3′ |
| Rab5b | Rab5b | 5′-GACTAGCAGAAGTACAGCCAG-3′ | 5′-CAATGGTGCTTTCCTGGTATTC-3′ |
| Rab11 | Rab11 | 5′-GAGCAGTAGG TGCCTTATTGG-3′ | 5′-GAACTGCCCTGAGATGACGTA-3′ |
| Rab20 | Rab20 | 5′-CTGCTGCAGCGCTACATGGAGCG-3′ | 5′-CTCCGCGGCAGTACAGGGAGC-3′ |
| Rab34 | Rab34 | 5′-GCAAAGTGACCCCGTGTGGCGGG-3′ | 5′-GGGCGTCCCGAAGACCACTCGG-3′ |
| Lamp2 | Lysosomal membrane glycoprotein 2 | 5′-AGGGTACTTGCCTTTATGCAGAAT-3′ | 5′-GTGTCGCCTTGTCAGGTACTGC-3′ |
| Lyz1 | Lysozyme 1 | 5′-CTCTCCTGACTCTGGGACTCCTC-3′ | 5′-CTGAGCTAAACACACCCAGTCAG-3′ |
| Lyz2 | Lysozyme 2 | 5′-GGCCAAGGTCTACAATCGTTGTG-3′ | 5′-GCAGAGCACTGCAATTGATCCCA-3′ |
| HexA | Hexosaminidase A | 5′-GCCGGCTGCAGGCTCTGGGTTTC-3′ | 5′-GCGCGGCCGAACTGACATGGTAC-3′ |
| HexB | Hexosaminidase B | 5′-CCCGGGCTGCTGCTGCTGCAGG-3′ | 5′-GTGGAATTGGGACTGTGGTCGAT-3′ |
| Gla | Galactosidase, α | 5′-GGCCATGAAGCTTTTGAGCAGA-3′ | 5′-AGTCAAGGTTGCACATGAAACG-3′ |
| CtsD | Cathepsin D | 5′-CGTCTTGCTGCTCATTCTCGGCCT-3′ | 5′-CACTGGCTCCGTGGTCTTAGGCG-3′ |
| CtsH | Cathepsin H | 5′-CTGAGAACCCTTCTTCCCAAGA-3′ | 5′-AGCAGCCAGGCCCCAGCGCACA-3′ |
| CtsZ | Cathepsin Z | 5′-GGCGTCGTCGGGGTCGGTGCAG-3′ | 5′-CTGCGCCCCAGCAGAGCCAGC-3′ |
Western blot assays.
The expression level of target proteins was examined by Western blotting as previously described (25). The proteins were detected with enhanced chemiluminescence solution (Thermo Scientific).
LAMP-2, RAB7, CTSZ, and GLA staining.
The colocalization of BCPs with markers, including LAMP-2 and RAB7 (4 h p.i.) or CTSZ and GLA (48 h p.i.), was carried out to confirm phagolysosome fusion event. Briefly, RAW 264.7 cells were treated with IL-6 siRNA prior to infection. The infected RAW 264.7 cells were incubated for 4 h with anti-LAMP-2 and anti-RAB7 antibodies and for 48 h with anti-CTSZ and anti-GLA antibodies. This experiment was then performed further as previously described (25). Fluorescence images were captured using a laser scanning confocal microscope (Olympus FV1000, Japan) and processed using FV10-ASW Viewer 3.1 software. The percentage of marker colocalization with bacteria was determined from 100 random cells.
Cytokine quantitation.
The levels of IL-1α, IL-2, IL-10, TNF, and IFN-γ in serum or culture supernatants were determined by ELISA in accordance with the manufacturer’s instructions (Thermo Fisher Scientific).
Statistical analysis.
The data are expressed as means ± the standard deviations (SD). Analysis of variance with Tukey’s HSD exact test was used to statistically compare the groups. The results with a P value of <0.05 were considered significantly different.
ACKNOWLEDGMENTS
We thank the Animal, Plant, and Fisheries Quarantine and Inspection Agency in Korea for generously providing the virulent B. abortus 544 biovar 1 strain that was used in this study.
We declare that we have no conflicts of interest.
H.T.H. and T.X.N.H. carried out all experiments, contributed to data collection and analysis, and participated in drafting the manuscript. L.T.A., A.W.B.R., S.H.V., T.X.N.H., and H.T.H. contributed to revise the manuscript. W.M., C.K.K., D.H.K., D.S.T., and H.J.L. participated in the design of the study. S.K. participated in the design of the study, carried out the data analysis, conceived the experiment, and prepared the manuscript. All authors read and approved the final manuscript.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education (2018-0698).
REFERENCES
- 1.Lee JJ, Kim DG, Kim DH, Simborio HL, Min W, Lee HL, Her M, Jung SK, Watarai M, Kim S. 2013. Interplay between clathrin and Rab5 controls the early phagocytic trafficking and intracellular survival of Brucella abortus within HeLa cells. J Biol Chem 288:28049–28057. doi: 10.1074/jbc.M113.491555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hop HT, Arayan LT, Reyes AWB, Huy TXN, Min W, Lee HJ, Son JS, Kim S. 2017. Simultaneous RNA-seq based transcriptional profiling of intracellular Brucella abortus and B. abortus-infected murine macrophages. Microb Pathog 113:57–67. doi: 10.1016/j.micpath.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Kim DH, Lim JJ, Lee JJ, Kim DG, Lee HJ, Min W, Kim KD, Chang HH, Endale M, Rhee MH, Watarai M, Kim S. 2012. RGS2-mediated intracellular Ca2+ level plays a key role in the intracellular replication of Brucella abortus within phagocytes. J Infect Dis 205:445–452. doi: 10.1093/infdis/jir765. [DOI] [PubMed] [Google Scholar]
- 4.Mizgerd JP, Spieker MR, Doerschuk CM. 2001. Early response cytokines and innate immunity: essential roles for TNF receptor 1 and type I IL-1 receptor during Escherichia coli pneumonia in mice. J Immunol 166:4042–4048. doi: 10.4049/jimmunol.166.6.4042. [DOI] [PubMed] [Google Scholar]
- 5.Copin R, De Baetselier P, Carlier Y, Letesson JJ, Muraille E. 2007. MyD88-dependent activation of B220– CD11b+ LY-6C+ dendritic cells during Brucella melitensis infection. J Immunol 178:5182–5191. doi: 10.4049/jimmunol.178.8.5182. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes DM, Jiang X, Jung JH, Baldwin CL. 1996. Comparison of T cell cytokines in resistant and susceptible mice infected with virulent Brucella abortus strain 2308. FEMS Immunol Med Microbiol 16:193–203. doi: 10.1111/j.1574-695X.1996.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhan Y, Cheers C. 1995. Endogenous interleukin-12 is involved in resistance to Brucella abortus infection. Infect Immun 63:1387–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhan Y, Liu Z, Cheers C. 1996. Tumor necrosis factor alpha and interleukin-12 contribute to resistance to the intracellular bacterium Brucella abortus by different mechanisms. Infect Immun 64:2782–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hop HT, Reyes AWB, Huy TXN, Arayan LT, Min W, Lee HJ, Rhee MH, Chang HH, Kim S. 2017. Activation of NF-κB-mediated TNF-induced antimicrobial immunity is required for the efficient Brucella abortus clearance in RAW 264.7 cells. Front Cell Infect Microbiol 7:437. doi: 10.3389/fcimb.2017.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dienz O, Rincon M. 2009. The effects of IL-6 on CD4 T cell responses. Clin Immunol 130:27–33. doi: 10.1016/j.clim.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YJ, Won TJ, Hyung KE, Jang YW, Kim SJ, Lee DI, Park SY, Hwang KW. 2016. IL-6 induced proliferation and cytotoxic activity of CD8+ T cells is elevated by SUMO2 overexpression. Arch Pharm Res 39:705–712. doi: 10.1007/s12272-016-0736-6. [DOI] [PubMed] [Google Scholar]
- 12.Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. 1998. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol 161:4652–4660. [PubMed] [Google Scholar]
- 13.Dann SM, Spehlmann ME, Hammond DC, Iimura M, Hase K, Choi LJ, Hanson E, Eckmann L. 2008. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J Immunol 180:6816–6826. doi: 10.4049/jimmunol.180.10.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalrymple SA, Slattery R, Aud DM, Krishna M, Lucian LA, Murray R. 1996. Interleukin-6 is required for a protective immune response to systemic Escherichia coli infection. Infect Immun 64:3231–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ladel CH, Blum C, Dreher A, Reifenberg K, Kopf M, Kaufmann S. 1997. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect Immun 65:4843–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garbers C, Aparicio-Siegmund S, Rose-John S. 2015. The IL-6/Gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr Opin Immunol 34:75–82. doi: 10.1016/j.coi.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Rose-John S. 2012. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the proinflammatory activities of IL-6. Int J Biol Sci 8:1237–1247. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fahmi A, Smart N, Punn A, Jabr R, Marber M, Heads R. 2013. p42/p44-MAPK and PI3K are sufficient for IL-6 family cytokines/Gp130 to signal to hypertrophy and survival in cardiomyocytes in the absence of JAK/STAT activation. Cell Signal 25:898–909. doi: 10.1016/j.cellsig.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. 1998. Interleukin-6-type cytokine signalling through the Gp130/Jak/STAT pathway. Biochem J 334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhan Y, Cheers C. 1995. Differential induction of macrophage-derived cytokines by live and dead intracellular bacteria in vitro. Infect Immun 63:720–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu W, Wang X, Qiu H, Cui B, Zhao S, Zheng H, Xiao Y, Liang J, Duan R, Jing H. 2013. Comparison of cytokine immune responses to Brucella abortus and Yersinia enterocolitica serotype O:9 infections in BALB/c Mice. Infect Immun 81:4392–4398. doi: 10.1128/IAI.00856-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saunders BM, Liu Z, Zhan Y, Cheers C. 1993. Interleukin-6 production during chronic experimental infection. Immunol Cell Biol 71:275–280. doi: 10.1038/icb.1993.32. [DOI] [PubMed] [Google Scholar]
- 23.Durward-Diioia M, Harms J, Khan M, Hall C, Smith JA, Splitter GA. 2015. CD8+ T cell exhaustion, suppressed gamma interferon production, and delayed memory response induced by chronic Brucella melitensis infection. Infect Immun 83:4759–4771. doi: 10.1128/IAI.01184-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martirosyan A, Von Bargen K, Gorvel VA, Zhao W, Hanniffy S, Bonnardel J, Meresse S, Gorvel JP. 2013. In vivo identification and characterization of CD4+ cytotoxic T cells induced by virulent Brucella abortus infection. PLoS One 8:e82508. doi: 10.1371/journal.pone.0082508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hop HT, Reyes AWB, Huy TXN, Arayan LT, Min W, Lee HJ, Rhee MH, Chang HH, Kim S. 2018. Interleukin 10 suppresses lysosome-mediated killing of Brucella abortus in cultured macrophages. J Biol Chem 293:3134–3144. doi: 10.1074/jbc.M117.805556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arango Duque G, Descoteaux A. 2014. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol 5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xavier MN, Winter MG, Spees AM, Nguyen K, Atluri VL, Silva TMA, Baumler AJ, Muller W, Santos RL, Tsolis RM. 2013. CD4+ T cell-derived IL-10 promotes Brucella abortus persistence via modulation of macrophage function. PLoS Pathog 9:e1003454. doi: 10.1371/journal.ppat.1003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eskra L, Mathison A, Splitter G. 2003. Microarray analysis of mRNA levels from RAW 264.7 macrophages infected with Brucella abortus. Infect Immun 71:1125–1133. doi: 10.1128/IAI.71.3.1125-1133.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gutierrez MG. 2013. Functional role(s) of phagosomal Rab GTPases. Small GTPases 4:148–158. doi: 10.4161/sgtp.25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huynh KK, Eskelinen EL, Scott CC, Malevanets A, Saftig P, Grinstein S. 2007. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J 26:313–324. doi: 10.1038/sj.emboj.7601511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, Hanada T, Takeda K, Akira S, Hoshijima M, Hirano T, Chien KR, Yoshimura A. 2003. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol 4:551–556. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- 32.Kim S, Kurokawa D, Watanabe K, Makino S, Shirahata T, Watarai M. 2004. Brucella abortus nicotinamidase (PncA) contributes to its intracellular replication and infectivity in mice. FEMS Microbiol Lett 234:289–295. doi: 10.1016/j.femsle.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 33.Dornand J, Gross A, Lafont V, Liautard J, Oliaro J, Liautard JP. 2002. The innate immune response against Brucella in humans. Vet Microbiol 90:383–394. doi: 10.1016/S0378-1135(02)00223-7. [DOI] [PubMed] [Google Scholar]
- 34.Jiang X, Baldwin CL. 1993. Effects of cytokines on intracellular growth of Brucella abortus. Infect Immun 61:124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandes DM, Baldwin CL. 1995. Interleukin-10 downregulates protective immunity to Brucella abortus. Infect Immun 63:1130–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhan Y, Cheers C. 1993. Endogenous gamma interferon mediates resistance to Brucella abortus infection. Infect Immun 61:4899–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baldwin CL, Parent M. 2002. Fundamentals of host immune response against Brucella abortus: what the mouse model has revealed about control of infection. Vet Microbiol 90:367–382. doi: 10.1016/S0378-1135(02)00222-5. [DOI] [PubMed] [Google Scholar]
- 38.Zegeye MM, Lindkvist M, Falker K, Kumawat AK, Paramel G, Grenegard M, Sirsjo A, Ljungberg LU. 2018. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated proinflammatory response in human vascular endothelial cells. J. Cell Commun Signal 16:55. doi: 10.1186/s12964-018-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. 2011. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 40.Zhu F, Zhou Y, Jiang C, Zhang X. 2015. Role of JAK-STAT signaling in maturation of phagosomes containing Staphylococcus aureus. Sci Rep 5:14854. doi: 10.1038/srep14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Z, Chen A, Sun H, Ye Y, Fang W. 2007. Ginsenoside Rd elicits Th1 and Th2 immune responses to ovalbumin in mice. Vaccine 25:161–169. doi: 10.1016/j.vaccine.2006.05.075. [DOI] [PubMed] [Google Scholar]