Interleukin-21 (IL-21), a cytokine produced by many subsets of activated immune cells, is critical for driving inflammation in several models. Using Helicobacter pylori infection as a model for chronic mucosal infection, we previously published that IL-21 is required for the development of gastritis in response to infection. Concomitant with protection from chronic inflammation, H. pylori-infected IL-21−/− mice exhibited limited Th1 and Th17 responses in their gastric mucosa.
KEYWORDS: Helicobacter pylori, dendritic cells, inflammation, interleukin-17, interleukin-21
ABSTRACT
Interleukin-21 (IL-21), a cytokine produced by many subsets of activated immune cells, is critical for driving inflammation in several models. Using Helicobacter pylori infection as a model for chronic mucosal infection, we previously published that IL-21 is required for the development of gastritis in response to infection. Concomitant with protection from chronic inflammation, H. pylori-infected IL-21−/− mice exhibited limited Th1 and Th17 responses in their gastric mucosa. Here we report that H. pylori-infected IL-21−/− mice express significantly higher levels of IL-17A than H. pylori-infected wild-type (WT) mice in the Peyer’s patches and mesenteric lymph nodes. This led us to hypothesize that IL-21 may indirectly regulate H. pylori-specific T cell responses by controlling dendritic cell (DC) functions in mucosa-associated lymphoid tissue. It was found that IL-21 treatment reduced the ability of dendritic cells to produce proinflammatory cytokines in response to H. pylori. While H. pylori increased the expression of costimulatory proteins on DCs, IL-21 reduced the expression of CD40 in the presence of H. pylori. Also, Th17 recall responses were intact when DCs were used as antigen-presenting cells in the presence of IL-21, but IL-21 did impact the ability of DCs to induce antigen-specific proliferation. These data suggest that IL-21, while proinflammatory in most settings, downregulates the proinflammatory cytokine microenvironment through modulating the cytokine expression of DCs, indirectly modifying IL-17A expression. Understanding how these proinflammatory cytokines are regulated will advance our understanding of how and why H. pylori infection may be tolerated in some individuals while it causes gastritis, ulcers, or cancer in others.
INTRODUCTION
Helicobacter pylori is a dominant member of the gastric microbiota in a majority of the world’s population (1, 2). H. pylori colonization can lead to detrimental outcomes, such as chronic active gastritis. Furthermore, this bacterium is implicated in more severe gastric diseases, including chronic atrophic gastritis (a precursor of gastric carcinomas), peptic ulceration, mucosa-associated lymphoid tissue (MALT) lymphomas, and gastric adenocarcinoma (reviewed in references 3 and 4). On the contrary, H. pylori colonization is attributed to protection from several proinflammatory diseases (5–10). Current data suggest that, in addition to bacterial virulence factors, the magnitude and types of immune responses influence the outcome of colonization (11, 12). Specifically, CD4+ T cell responses, including expression of gamma interferon (IFN-γ) and interleukin-17 (IL-17) and regulatory T (Treg) cell development, impact the pathology elicited in response to H. pylori colonization (13–19).
We identified that interleukin-21 (IL-21), a cytokine produced by many subsets of activated CD4+ T cells (especially Th17 cells) and NK cells (20, 21), is required for the development of gastritis during H. pylori colonization and infection (22). Our published data demonstrated that, concomitantly with protection from chronic inflammation, H. pylori-infected IL-21-deficient mice exhibited limited Th1 and Th17 responses in their gastric mucosa. In addition, an expression analysis of Th17-associated markers during H. pylori infection and gastric cancer demonstrated a strong positive correlation between RORγt (a transcription factor associated with Th17 responses) and IL-17A with IL-21 in both H. pylori-infected tissues and cancer tissues (23). Moreover, the expression of IL-21 was associated with H. pylori infection in a study of infected humans (24, 25).
IL-21 is a pleiotropic cytokine, and its receptor is present on a number of cell types, including lymphocytes, dendritic cells (DCs), and epithelial cells. As a member of the common gamma-chain family of cytokines, IL-21 shares a chain of its receptor with receptors for IL-2, IL-4, IL-7, IL-9, IL-13, and IL-15. The IL-21 receptor (IL-21R) has the highest amino acid sequence similarity to IL2Rβ and IL4Rα (26) and has been shown to activate the Janus kinase/signal transducers and activators of transcription signaling pathway upon ligand binding. IL-21 induces proliferation and increases cell survival and cytokine synthesis in many immune cells (26–28). In addition to directly stabilizing and expanding pathogenic T cell responses by driving strong Th1 and Th17 responses, along with their associated pathologies, IL-21 can inhibit the function and differentiation of Treg cells (29).
The major goal of this study was to define how IL-21 modulates DC responses and functions during H. pylori infection. There are data indicating that IL-21 inhibits DC activation and cytokine production (30–32), modulates DCs’ ability to enhance NK T cell IFN-γ production (33), and inhibits DC-induced T cell-mediated contact hypersensitivity (34). Therefore, we tested the hypothesis that IL-21 regulates DCs by controlling cytokine expression, modulating costimulatory molecule expression, and altering DC-mediated antigen-specific T cell responses. These DC functions were investigated in vitro and ex vivo with respect to DC-T cell interactions.
RESULTS
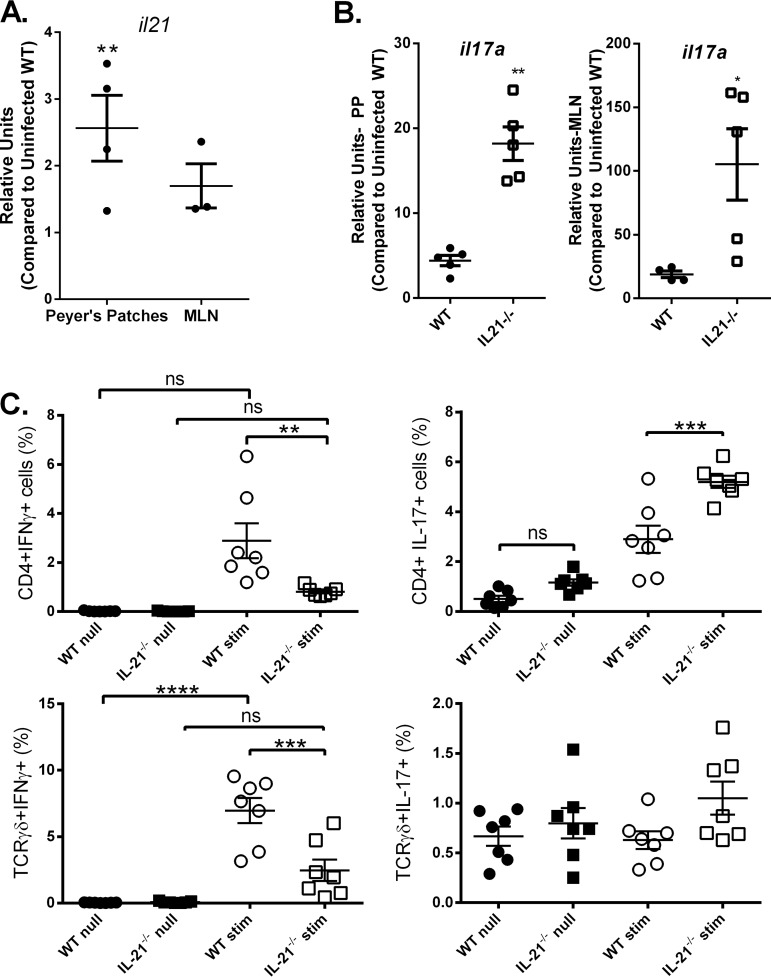
H. pylori-infected IL-21-deficient mice have an unexpected increase in IL-17A expression in the PPs and MLN.
Previously published results from our group indicate that IL-21 is required for the development or maintenance of Th1 and Th17 responses in the gastric mucosa during H. pylori infection (22). Moreover, since IL-21 is described as having a role in the maintenance of Th17 responses but not necessarily in the initial T cell activation, we sought to examine the Th17 response at sites of T cell activation and priming in lymphatic tissue during H. pylori infection. IL-21 expression in the Peyer’s patches (PPs) and mesenteric lymph nodes (MLN) of H. pylori-infected mice was measured at 1 month postinfection by real-time PCR. IL-21 expression did increase significantly in the PPs and was consistently expressed in the MLN of H. pylori-infected mice, in contrast to the findings for uninfected mice (Fig. 1A); therefore, IL-21 has the potential to signal to dendritic cells in those environments.
FIG 1.
IL-17 responses are significantly elevated in the MLN and PPs of H. pylori-infected IL-21−/− mice. Real-time RT-PCR was used to measure Il-21 (A) and Il-17a (B) expression levels in H. pylori-infected mice. (C) Intracellular cytokine staining was used to measure IFN-γ and IL-17A production by CD4+ T cells and γδ-positive T cells from the Peyer’s patches of H. pylori-infected mice (with PMA restimulation [stim] and without PMA restimulation [null]). Four to 7 mice were used for each experiment, and the data presented are representative of those from 3 experiments. (A, B) An unpaired t test was performed to test for statistical significance. (C) ANOVA followed by Dunnett’s correction for multiple comparisons was used. ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
To determine if IL-21 impacts T cell activation in the lymphoid tissue, the CD4+ cytokine responses were measured in the PPs and MLN of wild-type (WT) C57BL/6 mice and IL-21-deficient (IL-21−/−) mice. Expression of Il17a was significantly increased in both the MLN and PPs (Fig. 1B) of H. pylori-infected IL-21−/− mice compared to those of wild-type control mice. Ifng transcript levels were somewhat higher in infected mice but did not differ significantly in the MLN or PPs at this state (data not shown). In order to evaluate whether T cell receptor αβ-positive (TCRαβ+) CD4+ T cells and/or TCRγδ+ T cells were impacted by the IL-21 deficiency, intracellular cytokine staining was performed on cells from the PPs of H. pylori-infected IL-21−/− mice and H. pylori-infected WT mice (Fig. 1C). When in vitro stimulated with phorbol myristate acetate (PMA)-ionomycin, both TCRαβ+ CD4+ T cells and TCRγδ+ T cells from the PPs of H. pylori-infected IL-21−/− mice produced significantly less IFN-γ than those from the PPs of H. pylori-infected WT mice, whereas both TCRαβ+ CD4+ T cells and TCRγδ+ T cells from the PPs of H. pylori-infected IL-21−/− mice produced significantly more IL-17A than the cells from WT mice. These results suggest that IL-21 is required for the development of Th1 responses in the lymphatic tissue during H. pylori infection but that IL-21 is not a requirement for IL-17A expression in these tissues. Additionally, they suggest that IL-17A expression in lymphoid tissue may be downregulated due to indirect interactions with IL-21. Based on previous data that IL-21 may inhibit dendritic cell function, we hypothesized that IL-21 may indirectly regulate Th17 activation in the lymphoid tissue through dendritic cell function.
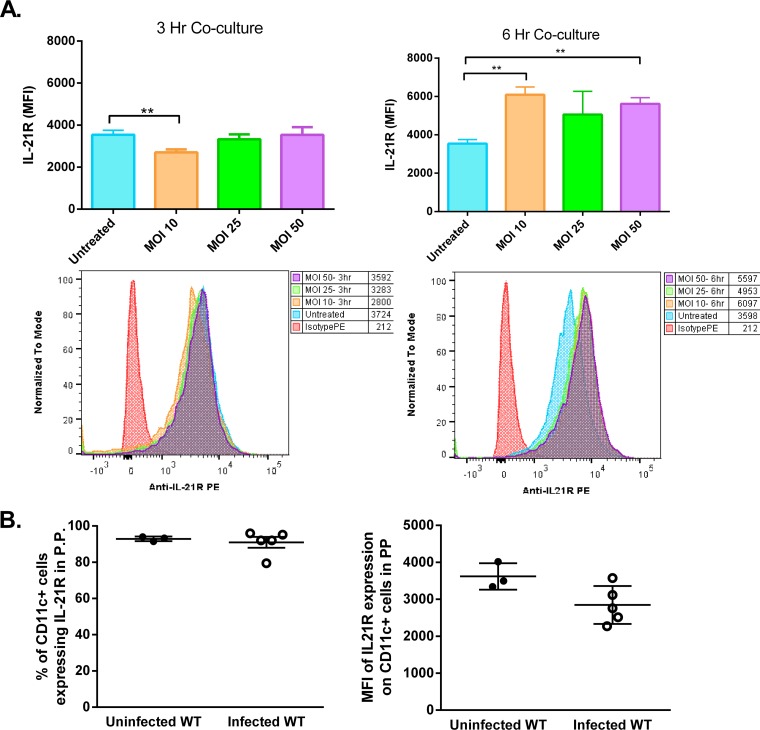
IL-21R (CD360) expression on dendritic cells and in lymphatic tissues of H. pylori-infected mice.
To investigate the potential of dendritic cells to respond to IL-21, the expression of the IL-21 receptor (IL-21R [CD360]) on dendritic cells was measured using flow cytometry. Bone marrow-derived dendritic cells (BMDCs) were challenged with H. pylori (a cag pathogenicity island-positive [cagPAI+] strain) at a multiplicity of infection (MOI) of 10, 25, or 50, and the level of expression was quantified by flow cytometry over the first 6 h of coculture with the bacterium (Fig. 2A). The results indicate that IL-21R is expressed on BMDCs and that expression is not significantly downregulated over the course of 6 h, unless the dendritic cells are undergoing cell death. This was taken into consideration for subsequent studies, and lower MOIs were used.
FIG 2.
IL-21R expression on DCs. (A) IL-21 receptor expression was measured on BMDCs cultured with and without H. pylori (MOI, 10, 25, or 50) for 3 or 6 h by flow cytometry. The mean fluorescence intensity of live BMDCs expressing IL-21R was calculated. Experimental conditions were set up in triplicate, and the data are representative of those from 3 experiments. Error bars represent the standard deviation. An unpaired t test was performed to test for statistical significance. **, P < 0.01. (B) IL-21 receptor expression was measured on live CD11c+ cells in the Peyer’s patches of uninfected and H. pylori-infected WT mice. Error bars represent standard deviations.
In chronically infected mice, IL-21R expression was also detected on the dendritic cells in PPs and MLN. Expression was present on greater than 90% of CD11c+ cells in these tissues (data for Peyer’s patches are shown in Fig. 2B; data for MLN are not shown). When measuring the level of expression by determination of the mean fluorescence intensity, there was no significant difference in IL-21R expression on dendritic cells between DCs from the Peyer’s patches of H. pylori-infected WT mice and those from the Peyer’s patches of uninfected mice (Fig. 2B).
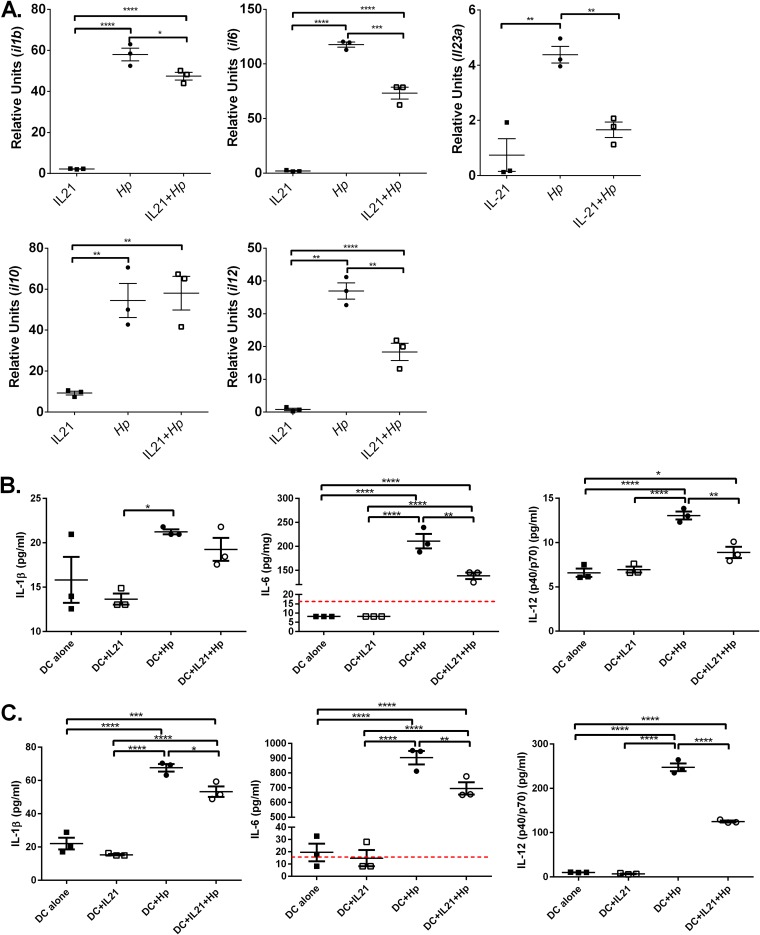
In vitro IL-21 impacts the bone marrow-derived DC response to H. pylori.
Given that IL-21 decreases the lipopolysaccharide-induced production of tumor necrosis factor alpha (TNF-α), IL-12, CCL5, and CXCL10 by monocyte-derived DCs (31), we investigated whether IL-21 modulates BMDC cytokine responses to H. pylori. BMDCs were cocultured with H. pylori with and without recombinant murine IL-21 (rmIL-21), and cytokine expression was measured at 6 h postinfection by real-time PCR. H. pylori induced a significant increase in gene expression of Il1b, Il6, Il10, Il12, and Il23a by BMDCs compared to that by unstimulated BMDCs (unstimulated BMDCs were the calibrator sample). The addition of IL-21 to cultures with H. pylori significantly reduced the levels of Il1b, Il6, Il12, and Il23a compared to those obtained by H. pylori stimulation alone, but the addition of IL-21 did not significantly impact Il10 expression in the presence of H. pylori. In the absence of H. pylori, the addition of IL-21 alone did not activate proinflammatory cytokine expression, but it did increase Il10 expression significantly (but not to the extent that H. pylori stimulated it) (Fig. 3A).
FIG 3.
IL-21 reduces the proinflammatory gene expression profile of H. pylori (Hp)-activated BMDCs. BMDCs were cultured with or without recombinant murine IL-21 and with or without H. pylori (MOI, 25) for 6 h. (A) Real time RT-PCR with the TaqMan technology was used to quantify cytokine transcription. Relative units were calculated using GAPDH as the endogenous control, and untreated BMDCs were used as the calibrator sample. Experimental conditions were set up in triplicate, and the data are representative of those from 4 experiments. ANOVA followed by Dunnett’s correction for multiple comparisons was used. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. (B, C) BMDCs were cultured with or without recombinant murine IL-21 and with or without H. pylori (MOI, 25) for 6 h (B) or 24 h (C). A Luminex assay was performed on supernatants collected at 6 h and 24 h. The dashed red lines represent the limit of detection (LOD) for the analytes, for which some of the values were below the LOD for the experimental conditions used. Experimental conditions were set up in triplicate, and the data are representative of those from 2 experiments. ANOVA followed by Dunnett’s correction for multiple comparisons was used. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
To determine whether changes in gene expression correlated with changes in protein expression in dendritic cell cultures at 6 and 24 h, a bead-based chemokine/cytokine protein expression profile was performed using a Luminex assay. The data are consistent with the gene expression data, in that H. pylori activation of dendritic cells elevated the expression of IL-1β, IL-6, and IL-12(p40/p70) by 6 h and the addition of rmIL-21 reduced the expression of IL-1β, IL-6, and IL-12(p40/p70) by 24 h of coculture (Fig. 3B and C).
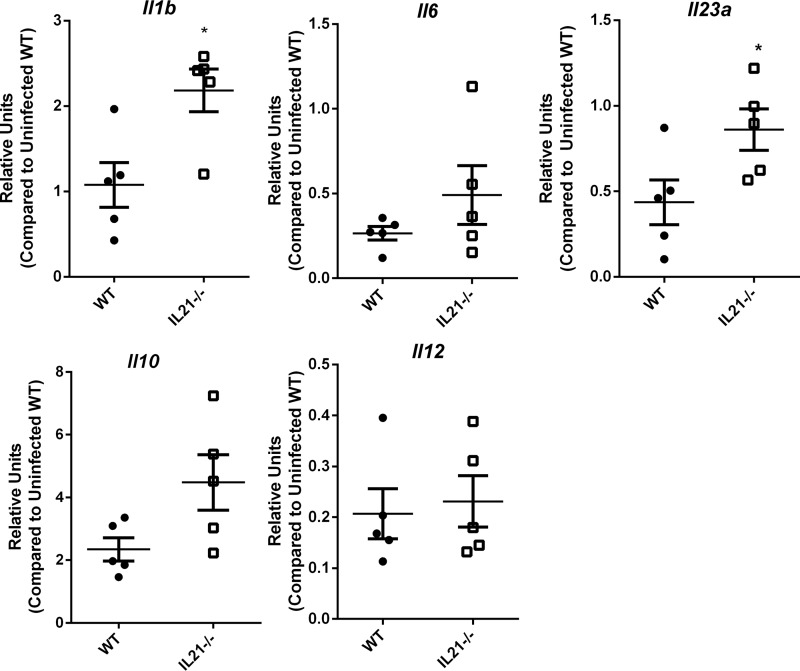
IL-21-deficient mice express higher levels of Th17-inducing cytokines in Peyer’s patches.
To determine if the impact of IL-21 on cytokines observed in vitro may occur in vivo, we focused on measuring innate cytokines which could impact Th17 cell expression in the PPs of IL-21−/− and WT mice. We measured Il1b, Il6, Il10, Il12, and Il23a in the PPs of infected H. pylori-infected mice (Fig. 4). At 1 month postinfection, H. pylori-infected IL-21−/− mice expressed significantly higher levels of Il1b and Il23a than WT mice, but there were no significant differences in Il6, Il10, or Il12 transcript levels.
FIG 4.
Proinflammatory gene expression in the PPs of H. pylori-infected mice. Real time RT-PCR was used to measure Il1b, Il6, Il10, Il12, and Il23 expression levels in PPs from H. pylori-infected mice at 1 month postinfection. Relative units were calculated using GAPDH as the endogenous control and tissue from uninfected WT mice as the calibrator sample. Five mice per genotype were measured in these assays. An unpaired Student’s t test was performed to test for statistical significance. *, P < 0.05.
Taken together, these data suggest that IL-21 expression may reduce the proinflammatory profile that is activated when H. pylori stimulates dendritic cells, and these data suggest that there is the potential for IL-21 to indirectly impact T cell activation through DC function.
In vitro IL-21 impacts the bone marrow-derived DC response to H. pylori.
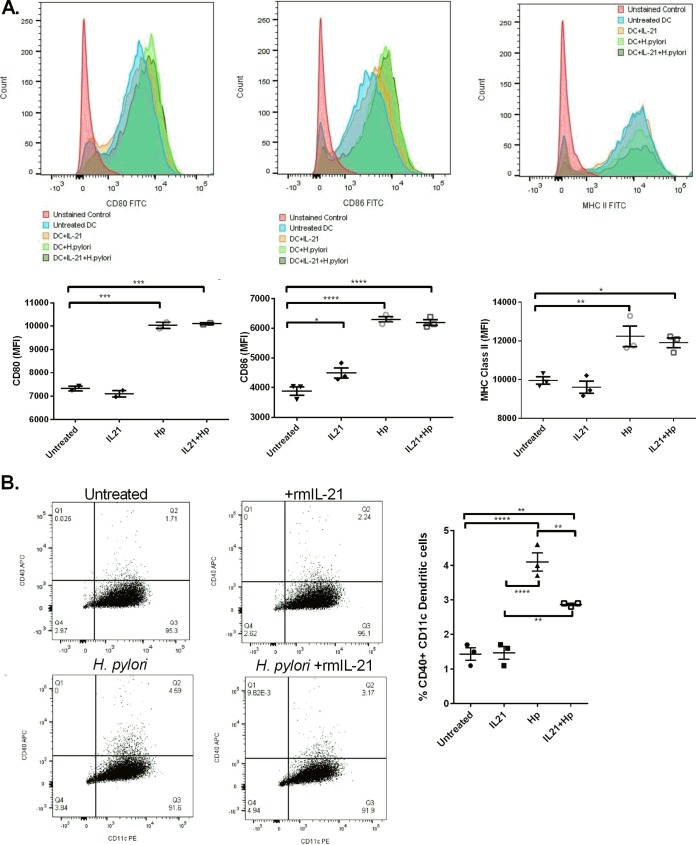
Previous studies have suggested that IL-21 inhibits the maturation of dendritic cells (30). A limitation of these published studies was that they compared the phenotype of BMDC precursors differentiated in the presence of IL-21 to that of BMDCs cultured in the presence of other cytokines with homology to IL-21 (i.e., IL-4 or IL-15). To investigate the maturation of BMDCs in the presence of H. pylori, BMDCs were cocultured with H. pylori (MOI, 25) with and without rmIL-21, and the levels of the costimulatory markers CD80 and CD86, CD40, and major histocompatibility complex (MHC) class II were measured by flow cytometry. A pattern similar to that seen for the cytokine profiles in response to H. pylori was observed. The stimulation of BMDCs with H. pylori or H. pylori and rmIL-21 significantly upregulated CD80, CD86, and MHC class II compared to no stimulation of BMDCs or activation of BMDCs with rmIL-21 (Fig. 5A). The percentage of BMDCs expressing CD40 was significantly increased with H. pylori coculture compared to that with no treatment of BMDCs. The percentage of CD40+ BMDCs decreased with the addition of rmIL-21 compared to the percentage of CD40+ BMDCs obtained with H. pylori activation (Fig. 5B). These data suggest that at the dose used in the current study, IL-21 only moderately downregulated CD40, and other costimulation molecules which impact T cell priming and activation were largely unaffected. Concurrently, the data posit that IL-21 may have a stronger influence on the cytokine microenvironment than on dendritic cell-cell surface protein expression.
FIG 5.
IL-21 does not regulate H. pylori-induced DC maturation based on surface marker expression. BMDCs were cultured with or without recombinant murine IL-21 and with or without H. pylori (MOI, 25) for 6 h. Flow cytometry was used to calculate the level of expression of CD80, CD86, and MHC class II (A) and the percentage of BMDCs expressing CD40 (B). Experimental conditions were set up in triplicate, and the data are representative of those from 4 experiments. ANOVA followed by Dunnett’s correction for multiple comparisons was used. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. APC, allophycocyanin.
IL-21 modulates T cell recall responses by acting on DCs.
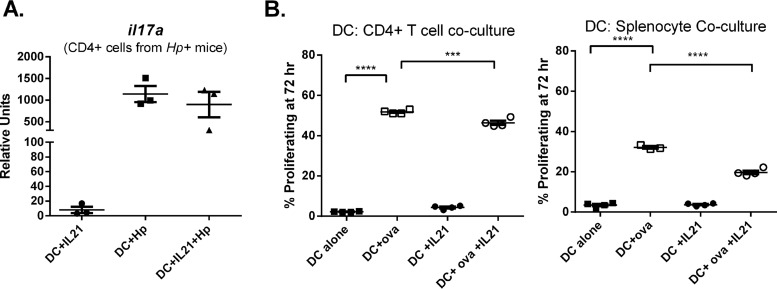
Based on the knowledge that DCs mature and migrate to local lymph nodes and Peyer’s patches to activate T cells and that IL-21 has been reported to affect DC-mediated antigen-specific tolerance in a model of contact hypersensitivity (34), we investigated how IL-21 affects DC-mediated T cell activation. IL-21 was reported to inhibit DC antigen presentation when DCs were cultured with IL-21 during their differentiation (34), and therefore, we hypothesized that IL-21 may inhibit the ability of DCs to activate T cells. To test this hypothesis, BMDCs were used as the antigen-presenting cells in proliferation and T cell cytokine-based assays. The ability of H. pylori lysate-pulsed DCs to activate and induce the proliferation of and cytokine expression by CD4+ H. pylori-specific T cells (from infected mice) in the presence and absence of IL-21 was assessed. BMDCs were differentiated, and on day 7 or 8, the BMDCs were counted and cultured with or without IL-21 and with or without H. pylori lysate (prepared from the PMSS1 strain) overnight. On the following day, the BMDCs were washed and T cells isolated from H. pylori-infected wild-type mice were added to the culture. The ability of the BMDCs to stimulate cytokine gene expression by the T cells was measured by real-time PCR. Il17a and Ifng were measured as markers of Th17 and Th1 cell activation during H. pylori infection. Il17a expression was stimulated in these cocultures only when H. pylori lysate was added, suggesting that Il17a expression was antigen specific (Fig. 6A), but the ability of DCs to activate the T cells to express Il17a was not impacted by the presence of IL-21. Ifng expression was not antigen specific in this assay (data not shown).
FIG 6.
IL-21 minimally impacts T cell recall responses and proliferative responses. (A) Recall responses were measured by the ability of T cells to express IL-17 in response to antigen-loaded DCs. BMDCs were cultured with or without recombinant murine IL-21 and with or without H. pylori lysate overnight. In a T cell restimulation assay, CD4+ T cells from H. pylori-infected mice were cultured with these BMDCs, and 24 h later, cytokine gene expression was measured by real time RT-PCR. (B) IL-21 did impact the ability of DCs to induce T cell proliferation in the coculture model. BMDCs were cultured with or without recombinant murine IL-21 and with or without ova for 4 h. In a T cell restimulation assay, CellTrace Violet-labeled CD4+ T cells from OT-II mice were cultured with these BMDCs for 72 h, and cell proliferation was measured by flow cytometry. ANOVA followed by Dunnett’s correction for multiple comparisons was used. ***, P < 0.001; ****, P < 0.0001.
To address how IL-21 impacts dendritic cell function as an antigen-presenting cell, we set up a different type of recall assay. BMDCs were treated with or without IL-21 and then pulsed with H. pylori lysate. Splenocytes from H. pylori-infected mice were prepared, and after 72 h of culture, survival was measured by flow cytometry. Both antigen presence and IL-21 prolonged the survival of the splenocytes (Table 1). Since the number of H. pylori-specific T cells could be limited in this assay, on the basis of the nonspecific nature of the IFN-γ response observed in the prior recall assay with H. pylori-infected mice, a second approach was taken to investigate how IL-21 impacts dendritic cell function as an antigen-presenting cell. Ovalbumin (ova) MHC class II TCR transgenic (Tg) (OT-II) mice were used as the source of antigen-specific T cells. These transgenic mice express the mouse α-chain and β-chain T cell receptor that pairs with the CD4 coreceptor and that is specific for the chicken ovalbumin peptide from amino acids 323 to 339 in the context of I-Ab. BMDCs were treated with or without IL-21 and then pulsed with the ova peptide. CD4+ T cells were selected from the spleens of OT-II mice, and these cells were labeled with CellTrace Violet. The proliferation of CD4+ T cells and whole splenocytes was measured after 3 days of culture. As expected, OT-II mouse CD4+ T cells proliferated in the presence of the DCs pulsed with the ova antigen (Fig. 6B). IL-21 treatment of BMDCs significantly decreased the ability of these antigen-presenting cells to induce the proliferation of CD4+ T cells at day 3, but the magnitude of the difference in proliferation may not be biologically relevant.
TABLE 1.
Splenocyte survival in coculture with BMDCa
| Condition | Mean % of surviving splenocytes at 72 h | SD | Statistical significance |
|---|---|---|---|
| Untreated DCs + splenocytes | 22.25 | 2.72 | |
| H. pylori lysate-treated DCs + splenocytes | 37.03 | 3.07 | ** |
| rmIL-21-treated DCs + splenocytes | 32.88 | 1.97 | ** |
| rmIL-21- and H. pylori lysate-treated DCs + splenocytes | 33.50 | 2.37 | NS |
The mean percentage of surviving cells after 72 h of culture was calculated based on 4 replicates of the 4 conditions. A two-way ANOVA was run with a correction for multiple comparisons. **, statistically significant difference from the results for untreated DCs and splenocytes; NS, not significant.
DISCUSSION
IL-21 is a member of the IL-10 cytokine family. It has been characterized as such due to its use of the shared γ-chain and CD360 (the IL-21 receptor). It is produced primarily by T lymphocytes but has been reported to act on a number of different cell types, including lymphocytes, myeloid cells, and epithelial cells. IL-21 expression by T follicular helper cells in lymphatic tissue has been well described and is required for the generation of germinal centers (35–37), suggesting that dendritic cells in the MALT may be responding to IL-21. In this study, we sought to understand how IL-21 impacts dendritic cell cytokine production and function.
IL-21 has a significant impact on Th1 cell differentiation and activation in the context of H. pylori infection. Th1 responses were not detected in the gastric mucosa of H. pylori-infected IL-21−/− mice even at 3 months postinfection (22). Analyses of splenic CD4+ T cell responses have demonstrated a significant reduction in the expression of tbx21, which transcribes the Th1-specific transcription factor Tbet, and significantly reduced phosphorylated STAT1 (P-STAT1) levels. Data presented in this report support this finding, as CD4+ T cell IFN-γ responses were reduced in the Peyer’s patches and MLN of H. pylori-infected IL-21−/− mice compared to those of H. pylori-infected WT mice.
On the other hand, the role that IL-21 has in activating and differentiating IL-17-producing cells is not completely clear, likely due to other cytokines, including IL-6 and IL-23, inducing the activation of STAT3 and RORγt expression (38). IL-21 increases the activation of STAT3, which provides for signals to stabilize and expand Th17 cells (39). In the H. pylori infection model, IL-17 responses are not detected in the gastric mucosa of H. pylori-infected IL-21−/− mice, whereas H. pylori-infected WT mice do activate IL-17 expression (22). Moreover, while data from CD4+ splenocytes isolated from H. pylori-infected mice demonstrated an impact on rorc expression and P-STAT3 in IL-21−/− mice, the magnitude of the impact that IL-21 had on Th17-related signaling was not as robust as the magnitude of its influence on IFN-γ responses. Even more surprising is our finding, reported here, which indicates that in MLN and PPs, H. pylori-infected IL-21−/− mice have significantly higher levels of IL-17-producing T cells than H. pylori-infected WT mice. So, while our previously published data indicate that IL-21 has important lymphocyte-intrinsic effects, the fact that IL-21R is widely expressed on cells of both the adaptive and innate arms of the immune system led us to hypothesize that IL-21 may indirectly downregulate IL-17-producing T cells. We expected that this mode of influence would be done through changing the microenvironment of the DC-T cell interaction or through modifying how DCs interact with T cells during priming in the MLN and PPs of H. pylori-infected mice.
We focused on how dendritic cells respond to IL-21 for our studies and found that IL-21 had modest but significant impacts on BMDCs. H. pylori was a major stimulus for activating the BMDC surface expression of the costimulatory proteins CD80, CD86, and CD40. Likewise, H. pylori increased the expression of MHC class II. IL-21 reduced the expression of CD40. Moreover, in our ex vivo experiments, the short-term coculture of BMDCs activated with antigen in the presence of IL-21 before adding T cells suggests that IL-21 does not significantly impact the ability of BMDCs to activate the IL-17 response and only modestly impacts the ability to induce proliferation in the Tg ova mouse model. IL-21 had a stronger impact on cytokine expression in the BMDC cultures, significantly reducing many proinflammatory cytokines, including Th17-differentiating cytokines IL-1β and IL-6. The expression of IL-1β was also significantly elevated in the Peyer’s patches of IL-21−/− mice. Taking these in vitro and in vivo data together, they suggest that IL-21 downregulates the proinflammatory cytokine microenvironment by modulating the cytokine expression of DCs in these tissues and, therefore, indirectly regulating IL-17 expression.
A limitation of this study was the inability to directly demonstrate that DCs in the Peyer’s patches were responsible for the changes in the cytokine levels or for directly impacting T cell IL-17 production. Many types of DCs have been described in the PPs of mice; therefore, no homogeneous population of DCs would suffice, and in order to isolate DCs from PPs, enzymatic digestion is required (40). Therefore, the microenvironment would be altered by the time that gene expression could be assessed, and the number of DCs was too small for a coculture-based experiment. Moreover, our experience with the DC isolation protocols was that there were a number of B cells expressing CD11c in the PPs, producing a mixed population of cells. Granulocyte-macrophage colony-stimulating factor (GM-CSF)-generated BMDCs (adherent) resemble monocyte-derived inflammatory DCs and not the steady-state population of DCs in tissue (41). More recently, it was demonstrated that the collection of both adherent and nonadherent BMDCs for culture also provides a heterologous population of DCs (42). For this study, the choice was made to use BMDCs on the same day of culture for all experiments and to use both adherent and nonadherent cells. While DC functions have been described to be divergent in different tissues, a major function of DCs is to produce cytokines in response to the microenvironment. This function is maintained in all DC populations. This study verifies that IL-21 receptor expression is present on DCs in the PPs. These studies also demonstrate that IL-21 impacts cytokine expression in vitro in DC cocultures with H. pylori.
While the relationship between H. pylori and DCs has been investigated by several groups (reviewed in reference 43), it is not fully understood. Several studies have demonstrated that H. pylori does not activate DCs to the same level as other pathogens, such as Escherichia coli or Salmonella enterica serovar Typhimurium (44, 45). H. pylori also induces IL-12, IL-10, and caspase-1 activation and therefore induces some changes in IL-1β and IL-18 production (46, 47). The relative amount of IL-12 versus IL-10 has been suggested to impact the strength of the downstream T cell response (48, 49). This has led researchers to suggest that H. pylori induces tolerizing DCs. Further, DC depletion studies using the CD11c-diphtheria toxin receptor transgenic mouse model (which expressed the diphtheria toxin receptor on all CD11c+ cells) demonstrate that even with only 50% depletion of DCs, the diphtheria toxin-treated mice had increased gastritis and increased T cell activation, suggesting that DCs do modulate proinflammatory T cells (50). Some studies have also demonstrated that H. pylori activates IL-10, which can downregulate IL-1β production (47). The experiments described in this report also saw the activation of IL-10 alongside IL-1β production. Notably, the experiments described in this report were set up to address the impact of IL-21 on DC activation. Therefore, our results do not necessarily contradict other findings; in fact, in vivo these DC responses (to both H. pylori and IL-21) could be working together to modulate the inflammatory response. H. pylori infection leads to gastritis in all persons infected with this bacterium, but only a small percentage go on to develop more severe consequences, such as atrophic gastritis and/or gastric cancer. It is important to understand which host factors impact the outcome of colonization. Elevated levels of acute-phase proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α, and proinflammatory T cell cytokines, including IL-17A, IL-21, and IFN-γ, have been associated with more detrimental outcomes and/or a poor prognosis. It is important to understand how many of the cytokines which are classified as proinflammatory may also play immune-modulatory roles in different tissues or microenvironments. The data presented in this paper combined with previous work on IL-21 suggest that this cytokine can be proinflammatory and immune regulatory.
MATERIALS AND METHODS
Mice.
Male and female C57BL/6 mice and interleukin-21+/− mice (stock number MMRRC:032800-UCD) were obtained from the NIH Consortium (UC Davis) for the establishment of a breeding colony and housed in the Vanderbilt University Animal Care Facilities. Helicobacter-free IL-21−/− and IL-21+/+ (wild-type) male littermates 8 to 10 weeks old were used in all H. pylori infection experiments. Studies were limited to male mice due to findings in previously published studies which indicate that male and female mice have differences in inflammation and that male mice are more consistently colonized with Helicobacter (51–53). The IL-21+/− breeding pairs tested negative for intestinal Helicobacter. Feces from sentinel mice housed in the same room consistently tested negative for pinworms, mouse parvovirus, and several other murine pathogens. Spleens from ova MHC class II TCR transgenic (OT-II) mice were a gift from Mark Boothby, Vanderbilt University Medical Center [they were originally purchased from The Jackson Laboratory and are also known as B6.Cg-Tg(TcraTcrb)425Cbn/J mouse strain 004194]. OT-II mice express the mouse α-chain and β-chain T cell receptor, which pairs with the CD4 coreceptor and which is specific for chicken ovalbumin from amino acids 323 to 339 in the context of I-Ab. For all in vitro dendritic cell experiments, 6- to 8-week-old mice were used. All animal studies were carried out in accordance with the Animal Welfare Act and U.S. federal law. All experiments were carried out at the Vanderbilt University Medical Center or in the U.S. Department of Veteran’s Affairs animal facility under protocol number V/15/130 and then subsequently under protocol number V1800072. The protocol was approved by the U.S. Department of Veteran’s Affairs and Vanderbilt University's Institutional Animal Care and Use Committee (IACUC), which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All animals were housed under the guidelines in an accredited research animal facility fully staffed with trained personnel.
Culture of H. pylori.
H. pylori strains 7.13 and PMSS1 were used in the experiments, as indicated in the Results and the figure legends. Bacteria were grown on Trypticase soy agar (TSA) plates containing 5% sheep blood. Alternatively, bacteria were grown in brucella broth containing 10% heat-inactivated fetal bovine serum (FBS; Atlanta Biologicals, Flowery Branch, GA, USA) and 10 μg/ml vancomycin. Cultures were grown at 37°C in room air supplemented with 5% CO2 or under microaerophilic conditions, generated by use of a GasPak EZ Campy container system (BD, Franklin Lakes, NJ, USA).
Infection of mice with H. pylori.
At 1 day prior to infection of mice, H. pylori strain PMSS1 was inoculated into liquid medium and was cultured for 18 h under microaerophilic conditions as described above. Mice were orogastrically inoculated with a suspension of 5 × 108 CFU of H. pylori (in 0.5 ml of brucella broth) twice over 3 days.
Harvesting and processing of PPs and MLN.
The mice were euthanized with carbon dioxide followed by cervical dislocation. The mice were placed in the supine position, 70% ethanol was used to sterilize the abdomen, and a midline incision was used to open the skin and abdominal wall to expose the peritoneal cavity. The small intestine was then positioned in order to expose the chain of mesenteric lymph nodes (MLN). Using forceps and scissors, the MLN were excised while being careful not to rupture the mesenteric artery. After removing and discarding the surrounding fat from the MLN, they were placed in complete RPMI medium (RPMI 1640, 10% FBS, 50 μg/ml vancomycin, 2 μg/ml amphotericin, 0.05 mM 2-mercaptoethanol) and stored on ice. The small intestine was then unfolded to expose the Peyer’s patches (PPs), which present as small white nodules that protrude from the small intestine opposite the mesenteric side. The PPs were removed using forceps to tighten the area, and then the nodule was cut off with scissors, being careful not to include intestinal tissue while cutting. Each PP was placed in complete RPMI medium and stored on ice. To create single-cell suspensions from the MLN or PPs, two sterile frosted glass slides were used to dissociate the tissues. The solution was then poured through a 70-μm-mesh-size cell strainer placed over a 50-ml tube, and a plunger from a 3-ml syringe was used to crush any visible pieces. The cell strainer was washed with complete RPMI medium to ensure that the cells were eluted from the strainer. The suspension was then centrifuged at 400 × g for 6 min at 4°C, and the supernatant was discarded. The pellet was resuspended in medium, and the number of cells was attained for further processing.
Quantification of cytokine production in mouse tissue.
The Peyer’s patches and mesenteric lymph nodes were harvested from H. pylori-infected mice at 1 month postinfection and from uninfected age- and genotype-matched controls. Tissues were processed into single-cell suspensions, and either the cells were then treated with the TRIzol reagent and subsequently subjected to RNA isolation and reverse transcription-PCR (RT-PCR) analysis or the cells were processed for intracellular cytokine staining. For intracellular cytokine staining, the single-cell suspensions were treated with GolgiPlug and Golgi Stop protein transport inhibitor (BD) and either left unstimulated or restimulated with PMA-ionomycin for 6 h. Subsequently, surface staining with fluorochrome-conjugated monoclonal antibodies specific for cell surface antigens was performed, followed by fixation with Cytofix/Cytoperm solution (BD Biosciences, San Jose, CA, USA). The cells were then stained with anti-IL-17 or anti-IFN-γ in Cell Perm/Wash buffer for 30 min and washed prior to collection. Anti-TCRγδ (clone eBioGL3), anti-TCRβ (clone H57-597), anti-CD3 (clone 145-2C11), anti-CD8α (clone 53-6.7), anti-CD8β (clone REA793), anti-CD4 (clone GK1.1), anti-IL-17 (clone TC11-18H10), anti-IFN-γ (clone XMG1.1), and anti-rat Ig (clone eBRG1) monoclonal antibodies were purchased from BD Biosciences. Cells were acquired in a FACSCalibur instrument, and data were analyzed using FlowJo software (FlowJo, LCC, a BD Company).
Generation of bone marrow-derived dendritic cells.
Dendritic cells (DCs) were derived from the bone marrow of C57BL/6 mice. Bone marrow from the femur and tibia was flushed out using a 21-gauge needle attached to a 10-ml syringe filled with cold RPMI medium. The bone marrow was passed through a 70-μm-mesh-size nylon cell strainer to remove any large debris. Bone marrow cells were pelleted by centrifugation at 1,300 rpm for 6 min at 4°C and, upon resuspension, adjusted to a concentration of 1 × 106 cells/ml in DC medium, containing RPMI 1640 medium (Thermo Fisher, Waltham, MA, USA), 5% FBS, 55 μM 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA), 1 mM HEPES buffer (Fisher Scientific, Hampton, NH, USA), and 20 ng/ml mouse granulocyte-macrophage colony-stimulating factor (GM-CSF; Tonbo Biosciences, San Diego, CA, USA). The cells are cultured in T75 non-tissue culture flasks for 8 to 9 days at 37°C with 5% atmospheric CO2. After 3 days in culture, the cells were fed 10 ml of fresh DC medium with 20-ng/ml GM-CSF. On day 6, 10 ml of cell culture was replaced with a 10 ml of fresh DC medium containing GM-CSF. On day 9, the cells were >90% confluent. The DCs were detached using a cell scraper, and after washing, viable cells were counted and treated for subsequent experiments.
Dendritic cell coculture with IL-21 and H. pylori strain.
Harvested BMDCs were centrifuged at 1,300 rpm for 8 min at 37°C, enumerated, and adjusted to a concentration of 3 × 106 cells/ml in DC medium. One million BMDCs were prepared for treatment in non-tissue-culture-treated 12-well plates or in 5-ml round-bottom polypropylene tubes. The BMDCs were then treated with 20-ng/ml IL-21 and/or infected with H. pylori at an MOI of 25 or 50 in triplicate. Recombinant IL-21 (rIL-21; 20 ng/ml) was used in these assays after higher doses (up to 100 ng/ml) did not change the response of the BMDCs, and doses ranging from 20 ng/ml to 25 ng/ml were used in other studies with rIL-21 (30, 54, 55). The BMDCs were then cultured for 6 h or overnight at 37°C with 5% atmospheric CO2. After treatment, the cells were collected for downstream experiments, including coculture with T cells, surface marker expression analysis, or gene expression analysis.
Flow cytometric analysis of dendritic cells.
BMDCs were treated for 6 h or overnight as described above. Treated samples were centrifuged to wash off the culture medium and resuspended in fluorescent-activated cell sorting (FACS) buffer, containing phosphate-buffered saline (PBS; Thermo Fisher, Waltham, MA, USA) and 1% bovine serum albumin (BSA; Research Product International, Mount Prospect, IL, USA). To exclude dead cells, DCs were treated with Ghost Dye 510 (Tonbo Bioscience, San Diego, CA) for 30 min, as directed by the manufacturer. After washing, the cells were resuspended in FACS buffer, containing PBS (Thermo Fisher, Waltham, MA, USA) and 1% BSA (Research Product International, Mount Prospect, IL, USA), and stained with cell surface markers for 20 min in the dark at 4°C. The samples were washed twice with FACS buffer, centrifuged at 1,500 rpm for 5 min, and fixed with 2% paraformaldehyde. Multiparameter analysis was performed on an LSR II flow cytometer, and dead cells were excluded in the analysis by gating on Ghost Dye 510-negative cells (BD Biosciences, San Jose, CA, USA). Follow-up analyses were performed with FlowJo software (FlowJo, LCC, a BD company). The following cell surface markers were purchased from BD Biosciences, San Jose, CA, USA: Alexa Fluor 700-conjugated anti-CD11b (clone M1/70), phycoerythrin (PE)-conjugated anti-CD11c (clone HL3), fluorescein isothiocyanate (FITC)-conjugated anti-CD80 (clone 16-10A1), FITC-conjugated anti-CD86 (clone GL1), FITC-conjugated MHC class II, PE-conjugated anti-IL21R (clone 4A9), and allophycocyanin-conjugated anti-CD40 (clone HM40-3).
RNA isolation and real-time RT-PCR analysis.
RNA was isolated from Peyer’s patch cells, mesenteric lymph nodes, or BMDCs using a TRIzol isolation protocol (Invitrogen, Carlsbad, CA, USA). Cells were homogenized in 0.75 ml of the TRIzol reagent. Then, 0.2 ml of chloroform was added for RNA extraction, followed by isopropanol precipitation. The RNA was then washed with 70% ethanol and air dried, and the pellet was resuspended with 25 to 50 μl of RNase-free water. RNA was reverse transcribed using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). For real-time PCR, the relative gene expression method was applied, where the gene for glyceraldehyde 3-phosphate dehydrogenase (Gapdh) served as the housekeeping gene. For the DC and T cell coculture experiments, Gapdh served as the normalizer, and the calibrator sample was the RNA isolated from the coculture of T cells with unstimulated/unpulsed BMDCs. All cDNA samples were set up in a 96-well plate in triplicate using TaqMan Fast Universal PCR master mix (Applied Biosystems, Foster City, CA, USA). All real-time RT-PCRs were analyzed using an Applied Biosystems StepOne Plus real-time PCR instrument. The following primers and probes were purchased as TaqMan Gene Expression Assays from Thermo Fisher: Il1b (catalog no. Mm00434228_m1), Il6 (catalog no. Mm00446190_m1), Il10 (catalog no. Mm01288386_m1), Il12 (catalog no. Mm00434165_m1), Il17a (catalog no. Mm00439619_m1), Il21 (catalog no. Mm00517640_m1), Ifng (catalog no. Mm99999071_m1), and Il23a and Gapdh (catalog no. Mm99999915_g1).
T cell isolation.
For T cell recall response experiments, H. pylori-infected C57BL/6 mice (WT) were used. These mice had been orogastrically infected with PMSS1, a cagPAI+ strain, for more than 3 months. The mice were euthanized, and their lymph nodes (popliteal, axillary, accessory axillary, superficial cervical, submandibular, and mesenteric lymph nodes) and spleen were excised. The lymph nodes and spleen were then ground into a combined single-cell suspension using the frosted edges of sterile glass slides, and red blood cells were lysed with ACK lysing buffer (Life Technologies). T cells were then isolated using a CD4+ T cell isolation kit, mouse (catalog no. 130-104-454; Miltenyi Biotec, Bergisch Gladbach, Germany), according to the protocol provided by the manufacturer.
For T cell proliferation experiments, T cells were isolated only from the spleens. Spleens were either from ova transgenic MHC class II (OT-II) mice or chronically H. pylori-infected C57BL/6 mice.
Dendritic cell and T cell coculture for H. pylori-specific recall responses.
Freshly isolated CD4+ T cells were cocultured with BMDCs that had been stimulated overnight with either IL-21 (20 ng/ml), H. pylori lysate (5 μg/ml), or a combination of IL-21 and H. pylori lysate or with BMDCs which were left unstimulated. Cells were cultured in a ratio of 10:1 (T cells [2 × 106 cells/ml] to dendritic cells [2 × 105 cells/ml]) in 24-well tissue culture plates. The coculture was incubated for 4 h at 37°C in 5% CO2. We rationalized that H. pylori-specific responses are difficult to detect, and previously published enzyme-linked immunosorbent spot assay data utilized this ratio with paragastric lymph nodes from H. pylori-infected mice (56). Samples were then transferred to microcentrifuge tubes and spun in order to collect the cells. Pelleted cells as well as those remaining on the tissue culture plates were then lysed with 1 ml the TRIzol reagent (Thermo Fisher, Waltham, MA, USA) for RNA extraction.
Dendritic cell and T cell coculture for proliferation.
BMDCs were harvested and stimulated with IL-21 (20 ng/ml) and/or ova (10 μM) or H. pylori lysate (5 μg/ml). The BMDCs were then incubated for 4 h at 37°C in 5% CO2. Meanwhile, CD4+ T cells were isolated as described above and then labeled with CellTrace Violet or carboxyfluorescein succinimidyl ester (CFSE; 5 μM; Invitrogen, Carlsbad, CA, USA) for 5 min at 37°C. Unlabeled cells were incubated with dimethyl sulfoxide only as the vehicle for CellTrace Violet or CFSE. To quench any dye remaining in solution, complete medium was added at 5 times the original staining volume, and the culture was incubated for 5 min. The labeled CD4+ T cells or labeled splenocytes were then cocultured with BMDCs at a ratio of 20:1 or 10:1, respectively (T cells [1 × 106 cells/ml] to dendritic cells [5 × 104 cells/ml] or splenocytes [1 × 106 cells/ml] to dendritic cells [1 × 105 cells/ml]), that had previously been cultured with either ova, IL-21, or a combination of IL-21 and ova or were cocultured with unstimulated DCs in 96-well tissue culture plates. The coculture was incubated for 3 days or 5 days at 37°C in 5% CO2 and protected from light. Cell proliferation was then analyzed using an LSR II flow cytometer (BD Biosciences, San Jose, CA) with a 405-nm excitation filter and a 450/40-nm bandpass emission filter.
Analysis of cytokine protein levels in cell culture supernatants.
Twenty cytokine analytes were measured in culture supernatants using a mouse cytokine magnetic 20-plex panel kit according to the manufacturer’s instructions (Invitrogen). Standards were also prepared for all 20 cytokine analytes according to the manufacturer’s instructions. Data were accumulated using a Luminex Flexmap three-dimensional assay. The concentration of each cytokine is presented as the number of picograms of protein per milliliter. IL-10 expression was below the limit of detection in these assays, and IL-23 was not part of the multiplex.
Statistical analysis.
All data are shown as the mean ± standard error of the mean. When comparing in vivo data (for IL-21−/− mice versus WT mice), Student's t test was used. For in vitro DC cultures and T cell-DC cultures, data were tested using one-way or two-way analysis of variance (ANOVA), utilizing GraphPad Prism (version 6.0) software. Statistical significance is based on the t test or one-way ANOVA with Dunnett’s correction for multiple comparisons of means. A P value of <0.05 was considered significant.
ACKNOWLEDGMENTS
We thank the NIH Consortium for providing IL-21+/− mice. The flow cytometry experiments were performed in the VUMC Flow Cytometry Shared Resource.
Core services are supported by NIH grants P30DK058404 (through Vanderbilt University Medical Center’s Digestive Disease Research Center) and P30CA068485 (through a Vanderbilt-Ingram Cancer Center support grant). We acknowledge the Translational Pathology Shared Resource, supported by NCI/NIH Cancer Center support grant 5P30 CA68485-19 and Vanderbilt Mouse Phenotyping Center grant 2 U24 DK059637-16. This work has been funded through merit review grant IBX000915A (to H.M.S.A.) from the Office of Medical Research, U.S. Department of Veterans Affairs, and NIH/NIDDK grant R01DK111671 (to D.O.-V.)
Footnotes
For a companion article on this topic, see https://doi.org/10.1128/IAI.00363-19.
REFERENCES
- 1.Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, Perez-Perez G, Blaser MJ, Relman DA. 2006. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci U S A 103:732–737. doi: 10.1073/pnas.0506655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson AF, Lindberg M, Jakobsson H, Backhed F, Nyren P, Engstrand L. 2008. Comparative analysis of human gut microbiota by barcoded pyrosequencing. PLoS One 3:e2836. doi: 10.1371/journal.pone.0002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makola D, Peura DA, Crowe SE. 2007. Helicobacter pylori infection and related gastrointestinal diseases. J Clin Gastroenterol 41:548–558. doi: 10.1097/MCG.0b013e318030e3c3. [DOI] [PubMed] [Google Scholar]
- 4.Bauer B, Meyer TF. 2011. The human gastric pathogen Helicobacter pylori and its association with gastric cancer and ulcer disease. Ulcers 2011:340157. doi: 10.1155/2011/340157. [DOI] [Google Scholar]
- 5.Vieth M, Masoud B, Meining A, Stolte M. 2000. Helicobacter pylori infection: protection against Barrett’s mucosa and neoplasia? Digestion 62:225–231. doi: 10.1159/000007820. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Blaser MJ. 2007. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med 167:821–827. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]
- 7.Konturek PC, Rienecker H, Hahn EG, Raithel M. 2008. Helicobacter pylori as a protective factor against food allergy. Med Sci Monit 14:CR452–CR458. [PubMed] [Google Scholar]
- 8.Reibman J, Marmor M, Filner J, Fernandez-Beros ME, Rogers L, Perez-Perez GI, Blaser MJ. 2008. Asthma is inversely associated with Helicobacter pylori status in an urban population. PLoS One 3:e4060. doi: 10.1371/journal.pone.0004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonnenberg A, Lash RH, Genta RM. 2010. A national study of Helicobactor [sic] pylori infection in gastric biopsy specimens. Gastroenterology 139:1894–1901.e2. doi: 10.1053/j.gastro.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Perry S, de Jong BC, Solnick JV, de la Luz Sanchez M, Yang S, Lin PL, Hansen LM, Talat N, Hill PC, Hussain R, Adegbola RA, Flynn J, Canfield D, Parsonnet J. 2010. Infection with Helicobacter pylori is associated with protection against tuberculosis. PLoS One 5:e8804. doi: 10.1371/journal.pone.0008804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gobert AP, Wilson KT. 2017. Human and Helicobacter pylori interactions determine the outcome of gastric diseases. Curr Top Microbiol Immunol 400:27–52. doi: 10.1007/978-3-319-50520-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Algood HM, Cover TL. 2006. Helicobacter pylori persistence: an overview of interactions between H. pylori and host immune defenses. Clin Microbiol Rev 19:597–613. doi: 10.1128/CMR.00006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizuno T, Ando T, Nobata K, Tsuzuki T, Maeda O, Watanabe O, Minami M, Ina K, Kusugami K, Peek RM, Goto H. 2005. Interleukin-17 levels in Helicobacter pylori-infected gastric mucosa and pathologic sequelae of colonization. World J Gastroenterol 11:6305–6311. doi: 10.3748/wjg.v11.i40.6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris PR, Smythies LE, Smith PD, Dubois A. 2000. Inflammatory cytokine mRNA expression during early and persistent Helicobacter pylori infection in nonhuman primates. J Infect Dis 181:783–786. doi: 10.1086/315257. [DOI] [PubMed] [Google Scholar]
- 15.Harris PR, Wright SW, Serrano C, Riera F, Duarte I, Torres J, Pena A, Rollan A, Viviani P, Guiraldes E, Schmitz JM, Lorenz RG, Novak L, Smythies LE, Smith PD. 2008. Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology 134:491–499. doi: 10.1053/j.gastro.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Serrano C, Wright SW, Bimczok D, Shaffer CL, Cover TL, Venegas A, Salazar MG, Smythies LE, Harris PR, Smith PD. 2013. Downregulated Th17 responses are associated with reduced gastritis in Helicobacter pylori-infected children. Mucosal Immunol 6:950–959. doi: 10.1038/mi.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smythies LE, Waites KB, Lindsey JR, Harris PR, Ghiara P, Smith PD. 2000. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J Immunol 165:1022–1029. doi: 10.4049/jimmunol.165.2.1022. [DOI] [PubMed] [Google Scholar]
- 18.Sjökvist Ottsjö LS, Flach C-F, Nilsson S, Malefyt RDW, Walduck AK, Raghavan S. 2015. Correction: defining the roles of IFN-gamma and IL-17A in inflammation and protection against Helicobacter pylori infection. PLoS One 10:e0142747. doi: 10.1371/journal.pone.0142747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sjökvist Ottsjö L, Flach C-F, Nilsson S, Malefyt RDW, Walduck AK, Raghavan S. 2015. Defining the roles of IFN-gamma and IL-17A in inflammation and protection against Helicobacter pylori infection. PLoS One 10:e0131444. doi: 10.1371/journal.pone.0131444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarra M, Pallone F, Macdonald TT, Monteleone G. 2010. Targeting interleukin-21 in immune-mediated pathologies. Curr Drug Targets 11:645–649. doi: 10.2174/138945010791011910. [DOI] [PubMed] [Google Scholar]
- 21.Sarra M, Pallone F, Monteleone G. 2013. Interleukin-21 in chronic inflammatory diseases. Biofactors 39:368–373. doi: 10.1002/biof.1105. [DOI] [PubMed] [Google Scholar]
- 22.Carbo A, Hontecillas R, Kronsteiner B, Viladomiu M, Pedragosa M, Lu P, Philipson CW, Hoops S, Marathe M, Eubank S, Bisset K, Wendelsdorf K, Jarrah A, Mei Y, Bassaganya-Riera J. 2013. Systems modeling of molecular mechanisms controlling cytokine-driven CD4+ T cell differentiation and phenotype plasticity. PLoS Comput Biol 9:e1003027. doi: 10.1371/journal.pcbi.1003027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinchuk IV, Morris KT, Nofchissey RA, Earley RB, Wu JY, Ma TY, Beswick EJ. 2013. Stromal cells induce Th17 during Helicobacter pylori infection and in the gastric tumor microenvironment. PLoS One 8:e53798. doi: 10.1371/journal.pone.0053798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caruso R, Fina D, Peluso I, Fantini MC, Tosti C, Del Vecchio Blanco G, Paoluzi OA, Caprioli F, Andrei F, Stolfi C, Romano M, Ricci V, MacDonald TT, Pallone F, Monteleone G. 2007. IL-21 is highly produced in Helicobacter pylori-infected gastric mucosa and promotes gelatinases synthesis. J Immunol 178:5957–5965. doi: 10.4049/jimmunol.178.9.5957. [DOI] [PubMed] [Google Scholar]
- 25.Bagheri N, Azadegan-Dehkordi F, Shirzad M, Zamanzad B, Rahimian G, Taghikhani A, Rafieian-Kopaei M, Shirzad H. 2015. Mucosal interleukin-21 mRNA expression level is high in patients with Helicobacter pylori and is associated with the severity of gastritis. Cent Eur J Immunol 40:61–67. doi: 10.5114/ceji.2015.50835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. 2000. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 27.Monteleone G, Caruso R, Fina D, Peluso I, Gioia V, Stolfi C, Fantini MC, Caprioli F, Tersigni R, Alessandroni L, MacDonald TT, Pallone F. 2006. Control of matrix metalloproteinase production in human intestinal fibroblasts by interleukin 21. Gut 55:1774–1780. doi: 10.1136/gut.2006.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spolski R, Leonard WJ. 2014. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov 13:379–395. doi: 10.1038/nrd4296. [DOI] [PubMed] [Google Scholar]
- 29.Battaglia A, Buzzonetti A, Baranello C, Fanelli M, Fossati M, Catzola V, Scambia G, Fattorossi A. 2013. Interleukin-21 (IL-21) synergizes with IL-2 to enhance T-cell receptor-induced human T-cell proliferation and counteracts IL-2/transforming growth factor-beta-induced regulatory T-cell development. Immunology 139:109–120. doi: 10.1111/imm.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brandt K, Bulfone-Paus S, Foster DC, Rückert R. 2003. Interleukin-21 inhibits dendritic cell activation and maturation. Blood 102:4090–4098. doi: 10.1182/blood-2003-03-0669. [DOI] [PubMed] [Google Scholar]
- 31.Strengell M, Lehtonen A, Matikainen S, Julkunen I. 2006. IL-21 enhances SOCS gene expression and inhibits LPS-induced cytokine production in human monocyte-derived dendritic cells. J Leukoc Biol 79:1279–1285. doi: 10.1189/jlb.0905503. [DOI] [PubMed] [Google Scholar]
- 32.Rothwell L, Hu T, Wu Z, Kaiser P. 2012. Chicken interleukin-21 is costimulatory for T cells and blocks maturation of dendritic cells. Dev Comp Immunol 36:475–482. doi: 10.1016/j.dci.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 33.Maeda M, Yanagawa Y, Iwabuchi K, Minami K, Nakamaru Y, Takagi D, Fukuda S, Onoe K. 2007. IL-21 enhances dendritic cell ability to induce interferon-gamma production by natural killer T cells. Immunobiology 212:537–547. doi: 10.1016/j.imbio.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Brandt K, Bulfone-Paus S, Jenckel A, Foster DC, Paus R, Rückert R. 2003. Interleukin-21 inhibits dendritic cell-mediated T cell activation and induction of contact hypersensitivity in vivo. J Invest Dermatol 121:1379–1382. doi: 10.1046/j.1523-1747.2003.12603.x. [DOI] [PubMed] [Google Scholar]
- 35.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. 2004. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol 173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 36.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. 2008. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. 2008. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. 2007. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 39.Bettelli E, Korn T, Oukka M, Kuchroo VK. 2008. Induction and effector functions of T(H)17 cells. Nature 453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadaoui KA, Corthesy B. 2007. Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer’s patches with restriction to mucosal compartment. J Immunol 179:7751–7757. doi: 10.4049/jimmunol.179.11.7751. [DOI] [PubMed] [Google Scholar]
- 41.Shortman K, Naik SH. 2007. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol 7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Dai X, Hsu C, Ming C, He Y, Zhang J, Wei L, Zhou P, Wang CY, Yang J, Gong N. 2017. Discrimination of the heterogeneity of bone marrow-derived dendritic cells. Mol Med Rep 16:6787–6793. doi: 10.3892/mmr.2017.7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiu J, Blanchard TG. 2013. Dendritic cell function in the host response to Helicobacter pylori infection of the gastric mucosa. Pathog Dis 67:46–53. doi: 10.1111/2049-632X.12014. [DOI] [PubMed] [Google Scholar]
- 44.Guiney DG, Hasegawa P, Cole SP. 2003. Helicobacter pylori preferentially induces interleukin 12 (IL-12) rather than IL-6 or IL-10 in human dendritic cells. Infect Immun 71:4163–4166. doi: 10.1128/iai.71.7.4163-4166.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hafsi N, Voland P, Schwendy S, Rad R, Reindl W, Gerhard M, Prinz C. 2004. Human dendritic cells respond to Helicobacter pylori, promoting NK cell and Th1-effector responses in vitro. J Immunol 173:1249–1257. doi: 10.4049/jimmunol.173.2.1249. [DOI] [PubMed] [Google Scholar]
- 46.Hitzler I, Sayi A, Kohler E, Engler DB, Koch KN, Hardt WD, Muller A. 2012. Caspase-1 has both proinflammatory and regulatory properties in Helicobacter infections, which are differentially mediated by its substrates IL-1beta and IL-18. J Immunol 188:3594–3602. doi: 10.4049/jimmunol.1103212. [DOI] [PubMed] [Google Scholar]
- 47.Rizzuti D, Ang M, Sokollik C, Wu T, Abdullah M, Greenfield L, Fattouh R, Reardon C, Tang M, Diao J, Schindler C, Cattral M, Jones NL. 2015. Helicobacter pylori inhibits dendritic cell maturation via interleukin-10-mediated activation of the signal transducer and activator of transcription 3 pathway. J Innate Immun 7:199–211. doi: 10.1159/000368232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kao JY, Rathinavelu S, Eaton KA, Bai L, Zavros Y, Takami M, Pierzchala A, Merchant JL. 2006. Helicobacter pylori-secreted factors inhibit dendritic cell IL-12 secretion: a mechanism of ineffective host defense. Am J Physiol Gastrointest Liver Physiol 291:G73–G81. doi: 10.1152/ajpgi.00139.2005. [DOI] [PubMed] [Google Scholar]
- 49.Kao JY, Zhang M, Miller MJ, Mills JC, Wang B, Liu M, Eaton KA, Zou W, Berndt BE, Cole TS, Takeuchi T, Owyang SY, Luther J. 2010. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology 138:1046–1054. doi: 10.1053/j.gastro.2009.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oertli M, Sundquist M, Hitzler I, Engler DB, Arnold IC, Reuter S, Maxeiner J, Hansson M, Taube C, Quiding-Järbrink M, Müller A. 2012. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J Clin Invest 122:1082–1096. doi: 10.1172/JCI61029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aebischer T, Laforsch S, Hurwitz R, Brombacher F, Meyer TF. 2001. Immunity against Helicobacter pylori: significance of interleukin-4 receptor α chain status and gender of infected mice. Infect Immun 69:556–558. doi: 10.1128/IAI.69.1.556-558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fox JG, Rogers AB, Ihrig M, Taylor NS, Whary MT, Dockray G, Varro A, Wang TC. 2003. Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer Res 63:942–950. [PubMed] [Google Scholar]
- 53.Sheh A, Lee CW, Masumura K, Rickman BH, Nohmi T, Wogan GN, Fox JG, Schauer DB. 2010. Mutagenic potency of Helicobacter pylori in the gastric mucosa of mice is determined by sex and duration of infection. Proc Natl Acad Sci U S A 107:15217–15222. doi: 10.1073/pnas.1009017107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wan CK, Li P, Spolski R, Oh J, Andraski AB, Du N, Yu ZX, Dillon CP, Green DR, Leonard WJ. 2015. IL-21-mediated non-canonical pathway for IL-1beta production in conventional dendritic cells. Nat Commun 6:7988. doi: 10.1038/ncomms8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karrich JJ, Jachimowski LC, Nagasawa M, Kamp A, Balzarolo M, Wolkers MC, Uittenbogaart CH, Marieke van Ham S, Blom B. 2013. IL-21-stimulated human plasmacytoid dendritic cells secrete granzyme B, which impairs their capacity to induce T-cell proliferation. Blood 121:3103–3111. doi: 10.1182/blood-2012-08-452995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Algood HM, Gallo-Romero J, Wilson KT, Peek RM Jr, Cover TL. 2007. Host response to Helicobacter pylori infection before initiation of the adaptive immune response. FEMS Immunol Med Microbiol 51:577–586. doi: 10.1111/j.1574-695X.2007.00338.x. [DOI] [PubMed] [Google Scholar]