Coxiella burnetii is an obligate intracellular Gram-negative bacterium which causes human Q fever. An acidified citrate cysteine medium (ACCM-2) has been developed which mimics the intracellular replicative niche of C. burnetii and allows axenic growth of the bacteria.
KEYWORDS: ACCM-derived phase I vaccine, CD4+ T cells, Coxiella burnetii, antibody isotype switching, eosinophils, granulocytes
ABSTRACT
Coxiella burnetii is an obligate intracellular Gram-negative bacterium which causes human Q fever. An acidified citrate cysteine medium (ACCM-2) has been developed which mimics the intracellular replicative niche of C. burnetii and allows axenic growth of the bacteria. To determine if C. burnetii cultured in ACCM-2 retains immunogenicity, we compared the protective efficacies of formalin-inactivated C. burnetii Nine Mile phase I (PIV) and phase II (PIIV) vaccines derived from axenic culture 7, 14, and 28 days postvaccination. PIV conferred significant protection against virulent C. burnetii as early as 7 days postvaccination, which suggests that ACCM-2-derived PIV retains immunogenicity and protectivity. We analyzed the cellular immune response in spleens from PIV- and PIIV-vaccinated mice by flow cytometry at 7 and 14 days postvaccination and found significantly more granulocytes in PIV-vaccinated mice than in PIIV-vaccinated mice. Interestingly, we found these infiltrating granulocytes to be SSChigh CD11b+ CD125+ Siglec-F+ (where SSChigh indicates a high side scatter phenotype) eosinophils. There was no change in the number of eosinophils in PIV-vaccinated CD4-deficient mice compared to the level in controls, which suggests that eosinophil accumulation is CD4+ T cell dependent. To evaluate the importance of eosinophils in PIV-mediated protection, we vaccinated and challenged eosinophil-deficient ΔdblGATA mice. ΔdblGATA mice had significantly worse disease than their wild-type counterparts when challenged 7 days postvaccination, while no significant difference was seen at 28 days postvaccination. Nevertheless, ΔdblGATA mice had elevated serum IgM with decreased IgG1 and IgG2a whether mice were challenged at 7 or 28 days postvaccination. These results suggest that eosinophils may play a role in early vaccine protection against C. burnetii and contribute to antibody isotype switching.
INTRODUCTION
Coxiella burnetii is an obligate intracellular Gram-negative bacterial pathogen and the causative agent of human Q fever. This disease manifests acutely as a flu-like illness although it can escalate to a chronic and often fatal disease. Chronic Q fever commonly presents as endocarditis (1–4) and occurs in <5% of acutely infected patients. Among those who develop chronic disease, fatality is observed in 25 to 60% of patients when the disease is left untreated (5). Long-term (≥18 months) administration of doxycycline and hydroxychloroquine is the preferred treatment (2, 6, 7). However, even with the recommended antibiotic regimen, one in three Q fever patients continues to experience diminished health 2 years postdiagnosis (4, 8, 9). This globally distributed pathogen is spread to humans via aerosols from infected ruminants (1, 2) and therefore serves as an occupational hazard for individuals working closely with livestock (10–12). The highly infectious nature of C. burnetii (13–15), coupled with its prolonged environmental stability (14) and ease of dissemination (16, 17), makes it an important zoonotic pathogen. C. burnetii is an NIH category B priority pathogen because it serves as a threat to our national security, with potential uses in bioterrorism (18). A recent outbreak in the Netherlands highlights the relevance of this disease to human health, with more than 4,000 human cases reported (19, 20). Considering the threat of chronic manifestations and the failure of antibiotic therapies, creation of a safe and effective vaccine remains an important public health and national biosecurity goal.
C. burnetii undergoes antigenic phase variation upon serial passage in eggs, tissue culture, or synthetic medium. During this process, virulent phase I organisms lose the O antigen and outer core regions of their lipopolysaccharide (LPS) and become avirulent phase II organisms (1, 21, 22). Phase I organisms are able to replicate in immunocompetent animals and cause disease, while phase II organisms are rapidly cleared and do not cause disease (13).
A formalin-inactivated whole-cell vaccine produced from the Henzerling strain in phase I (Q-VAX) has been shown to elicit long-lasting protection in animal models and human vaccinees (10, 23–25). Despite its high protective efficacy, Q-VAX is not approved for use in the United States due to adverse reactions, especially in previously sensitized individuals (10, 23, 26–29). Safe use of this vaccine requires multiple screening procedures, which precludes a mass vaccination program. Understanding what is needed to confer protection with minimal side effects is essential to developing an intervention that is both safe and effective.
Previous work suggests that both humoral and cell-mediated immunity are involved in vaccine protection against C. burnetii (25, 30–33); however, the contribution of innate immunity remains unknown. The innate immune response stimulates adaptive immunity and tailors adaptive responses to different types of microbes. As such, the innate immune system is a useful tool which can be manipulated to enhance vaccine protection. In fact, the use of Toll-like receptor agonists as immunoadjuvants has proved effective when they are incorporated into vaccines against multiple pathogens (34–39).
Here, we use the recently developed host cell-free culture system (40) and assess the immunogenicity of formalin-inactivated C. burnetii Nine Mile phase I (PIV) and phase II (PIIV) vaccines. The data indicate that PIV elicits protection superior to that of PIIV at all time points examined. Furthermore, using flow cytometry to examine the cellular immune response, we find that PIV and PIIV differ in the accumulation of eosinophils in the spleen. This accumulation appears to be CD4+ T cell dependent as PIV-vaccinated CD4-deficient mice do not have elevated eosinophils in their spleens. Increased splenomegaly and bacterial burden in PIV-vaccinated eosinophil-deficient ΔdblGATA mice compared to levels in wild-type (WT) mice challenged at 7 days postvaccination (dpv) suggests a partial role for eosinophils in early vaccine protection. Additionally, elevated serum IgM coupled with decreased IgG1 and IgG2a subclass antibodies in ΔdblGATA mice suggests a role for these cells in antibody isotype switching. These findings highlight the utility of the axenic culture system for the manufacture of C. burnetii vaccines and provide new insights into the contribution of innate immunity to vaccine protection against C. burnetii, which can be applied to Q fever vaccine development.
RESULTS
Host cell-free C. burnetii retains immunogenicity and protectivity.
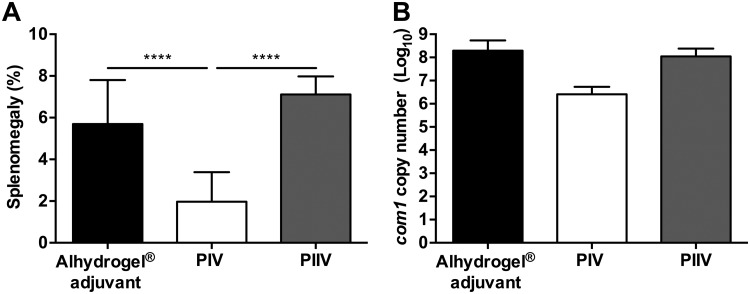
Q-VAX is produced in embryonated eggs (41), and while this allows for the generation of large quantities of material at a relatively low cost, residual egg proteins often remain in the final vaccine preparation and contribute to the high incidence of vaccine reactions (42). An acidified citrate cysteine medium (ACCM-2) has been developed which mimics the intracellular replicative niche of C. burnetii and allows axenic growth of the bacteria. This medium removes the potential for host contaminants, while still allowing the generation of large quantities of material in a short time (∼3 logs of growth in 6 days) (43). Previous reports have shown that C. burnetii strains grown in ACCM-2 as well as a newer formulation, ACCM-D, retain in vivo infectivity in mouse and guinea pig models (43, 44). To determine if ACCM-2-derived C. burnetii retains immunogenicity, we vaccinated BALB/c mice subcutaneously (s.c.) with 10 μg of PIV or PIIV with aluminum hydroxide adjuvant (Alhydrogel) and challenged mice intraperitoneally (i.p.) with 5 × 107 C. burnetii Nine Mile phase I (NMI) bacteria 28 days later. An aluminum hydroxide adjuvant was selected for these vaccination studies due to its broadly accepted use in FDA-approved vaccines for humans (45). To assess disease severity, we evaluated splenomegaly and bacterial burden in the spleen at 14 days postinfection (dpi). Similar to previous reports of vaccines generated from chicken eggs or cell culture (25), we found that PIV grown in ACCM-2 significantly reduces splenomegaly compared to results with PIIV and adjuvant controls (Fig. 1A). Additionally, we found that bacterial burden in the spleens of PIV-vaccinated animals was reduced compared to that with PIIV and adjuvant controls (Fig. 1B). These results indicate that axenically generated PIV retains immunogenicity and protectivity.
FIG 1.
Host cell-free C. burnetii retains immunogenicity and protectivity. BALB/c mice were vaccinated subcutaneously (s.c.) with 10 μg of formalin-inactivated C. burnetii Nine Mile phase I (PIV) or phase II (PIIV) vaccine and challenged via intraperitoneal (i.p.) route with 5 × 107 C. burnetii NMI bacteria at 28 days postvaccination (dpv). Mice receiving Alhydrogel adjuvant alone served as unvaccinated controls. Splenomegaly (A) and bacterial burden in the spleen (B) were evaluated at 14 days postinfection (dpi) to compare protective efficacies. Results are expressed as percent splenomegaly [(spleen weight/body weight) × 100]. Bacterial burden was determined by real-time quantitative PCR (qPCR) and is expressed as log10 C. burnetii com1 gene copy numbers. Each experimental group includes four mice, with error bars representing the standard deviations from the means. ****, P < 0.0001, as determined by one-way ANOVA with Tukey’s multiple-comparison test.
PIV protects BALB/c mice as early as 1 week postvaccination.
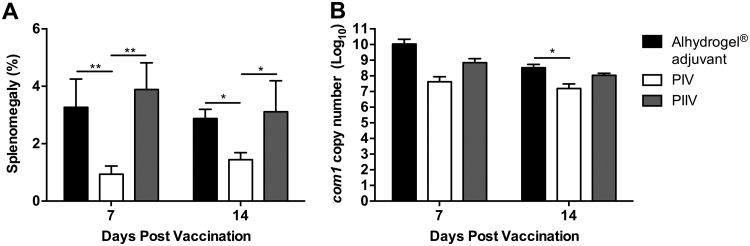
While it has been established that PIV is protective at 28 dpv, it remains unknown how early mice are protected from developing clinical disease following vaccination. To test this, we vaccinated BALB/c mice as described previously and then challenged mice at 7 or 14 dpv with 1 × 107 C. burnetii NMI bacteria by i.p. injection. Due to the high degree of splenomegaly (∼8%) observed with a challenge dose of 5 × 107 bacteria (Fig. 1A), we lowered the challenge dose to 1 × 107 bacteria for all subsequent infections. PIV had significantly reduced splenomegaly compared to that with PIIV and adjuvant controls when challenged at 7 or 14 dpv (Fig. 2A). Additionally, mice vaccinated with PIV had a reduction in splenic bacterial burden at all time points examined (Fig. 2B). Taken together, these data suggest that immunity develops as early as 1 week postvaccination.
FIG 2.
PIV protects BALB/c mice as early as 1 week postvaccination. BALB/c mice were vaccinated s.c. with 10 μg of PIV or PIIV and challenged via the i.p. route with 1 × 107 C. burnetii NMI bacteria at 7 or 14 dpv. Mice receiving Alhydrogel adjuvant alone served as unvaccinated controls. Splenomegaly (A) and bacterial burden in the spleen (B) were evaluated at 14 dpi to compare protective efficacies. Results are expressed as percent splenomegaly [(spleen weight/body weight) × 100]. Bacterial burden was determined by real-time quantitative PCR (qPCR) and is expressed as log10 C. burnetii com1 gene copy numbers. Each experimental group includes four mice, with error bars representing the standard deviations from the means. *, P < 0.05; **, P < 0.01, as determined by one-way ANOVA with Tukey’s multiple-comparison test.
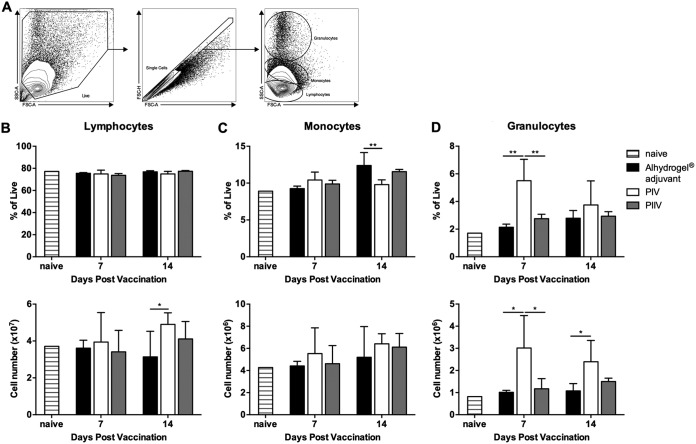
PIV significantly increases SSChigh granulocytes in the spleen.
PIV significantly reduces splenomegaly and bacterial burden in BALB/c mice challenged with virulent C. burnetii NMI. However, PIIV fails to protect mice from clinical disease. The immunological differences between PIV and PIIV that lead to such disparate outcomes remain unknown. Considering the early time point at which this difference becomes apparent (∼7 dpv), we hypothesized that innate cells may be differentially recruited to the spleen following vaccination. To test this hypothesis, we used flow cytometry to analyze cellular recruitment to the spleens of adjuvant control and PIV- or PIIV-vaccinated BALB/c mice at 7 and 14 dpv. Naïve animals were used to establish baseline values for all populations examined. Figure 3A details the gating strategy used for the initial analysis. Cellular debris and doublets were removed before gating on lymphocytes, monocytes, and granulocytes based on their forward (FSC) and side scatter (SSC) characteristics. The frequency of lymphocytes was not significantly altered at either time point though there was a significant increase in the number of lymphocytes in PIV-vaccinated mice compared to levels in the adjuvant controls at 14 dpv (Fig. 3B). While there were significant changes in the frequency of monocytes in PIV-vaccinated mice compared to levels in the PIIV-vaccinated and adjuvant control mice at 14 dpv, there were no significant differences in the numbers of monocytes at either time point (Fig. 3C). However, there was a significant increase in the frequency of granulocytes with a high SSC (SSChigh) phenotype in PIV-vaccinated mice compared to the levels in PIIV-vaccinated and adjuvant control mice at 7 dpv. Additionally, the total number of granulocytes in the spleen was significantly higher in PIV-vaccinated mice than in the PIIV-vaccinated and adjuvant control mice (Fig. 3D). Overall, these data demonstrate that PIV elicits granulocyte accumulation in the spleen early in the vaccine response.
FIG 3.
PIV significantly increases high side scatter granulocytes in the spleen. BALB/c mice were vaccinated s.c. with 10 μg of PIV or PIIV, and spleens were processed for flow cytometry as described in Materials and Methods. Mice receiving Alhydrogel adjuvant alone served as unvaccinated controls. Naïve mice were used to establish baseline values. (A) Gating strategy. Forward (FSC) and side scatter (SSC) were used to identify lymphocytes, monocytes, and granulocytes as well as to remove cell debris from the analysis. (B to D) Population frequencies and absolute cell counts were determined at 7 and 14 dpv. Each experimental group includes 4 to 6 mice, with error bars representing the standard deviations from the means. *, P < 0.05; **, P < 0.01, as determined by one-way ANOVA with Tukey’s multiple-comparison test.
SSChigh granulocytes express CD11b and the interleukin-5 receptor.
Following the observation that PIV elicits significant accumulation of granulocytes in the spleen, we further analyzed this population to determine which granulocytic cells were responding. Figure 4A details the gating strategy used for the analysis. Cellular debris and doublets were removed as previously described. PIV-vaccinated animals had a significantly higher frequency of SSChigh cells that expressed CD11b than PIIV-vaccinated or adjuvant control mice. Furthermore, the total number of SSChigh CD11b+ cells in the spleens of PIV-vaccinated animals was significantly higher than that in PIIV-vaccinated animals or adjuvant controls (Fig. 4B).
FIG 4.
SSChigh granulocytes express CD11b and the interleukin-5 receptor. BALB/c mice were vaccinated s.c. with 10 μg of PIV or PIIV, and spleens were processed for flow cytometry as previously described. Mice receiving Alhydrogel adjuvant alone served as unvaccinated controls. Naïve mice were used to establish baseline values. (A) Gating strategy. FSC and SSC were used to remove cell debris from the analysis. CD45+ leukocytes were then identified and further subgated based on SSC and expression of CD11b. CD11b+ SSChigh granulocytes were then further subgated based on expression of the interleukin-5 receptor alpha subunit (IL-5Rα, CD125). (B and C) Population frequencies and absolute cell counts were determined at 7 and 14 dpv. Each experimental group includes 4 to 6 mice, with error bars representing the standard deviation from the mean. *, P < 0.05; **, P < 0.01, as determined by one-way ANOVA with Tukey’s multiple-comparison test.
Granulocytic cells expressing CD11b include neutrophils and eosinophils. To distinguish these two populations, we next looked at interleukin-5 receptor alpha subunit (IL-5Rα, CD125) expression. CD125 is expressed on eosinophils and absent from neutrophils (46). Interestingly, PIV-vaccinated mice had significantly more CD125+ cells than PIIV-vaccinated animals or adjuvant controls (Fig. 4C). These results suggest that the granulocyte population recruited to the spleens of PIV-vaccinated mice may be eosinophils.
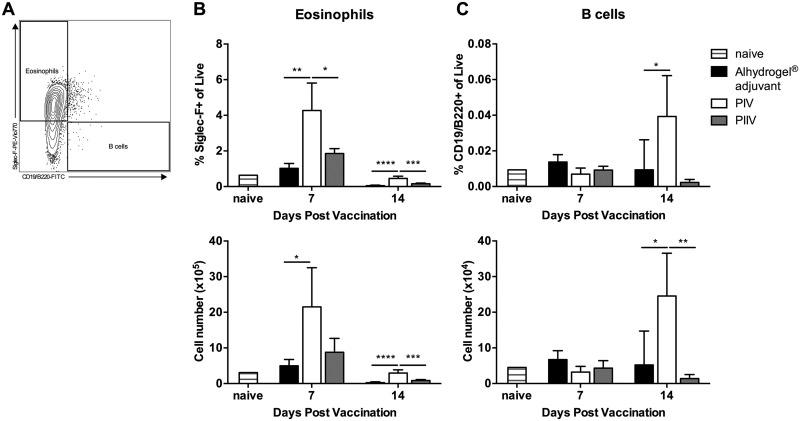
SSChigh CD11b+ CD125+ cells are Siglec-F+ eosinophils.
While CD125 is most commonly expressed on eosinophils, there are subsets of B1 cells which also express CD125 (47). To determine the phenotype of these cells, we evaluated their expression of Siglec-F, an eosinophil-specific marker, as well as CD19/B220 (Fig. 5A). PIV-vaccinated mice had significantly more Siglec-F+ positive cells at 7 dpv than PIIV-vaccinated animals and adjuvant controls (Fig. 5B). Less than 0.1% of the SSChigh CD11b+ CD125+ cells were CD19/B220+ (Fig. 5C). Taken together, these data indicate that PIV and PIIV differ in the early recruitment of eosinophils to the spleen.
FIG 5.
SSChigh CD11b+ CD125+ cells are Siglec-F+ eosinophils. BALB/c mice were vaccinated s.c. with 10 μg of PIV or PIIV, and spleens were processed for flow cytometry. Mice receiving Alhydrogel adjuvant alone served as unvaccinated controls. Naïve mice were used to establish baseline values. (A) Gating strategy. SSChigh CD11b+ CD125+ cells were examined for expression of Siglec-F (eosinophils) and CD19/B220 (B cells). (B and C) Population frequencies and absolute cell counts were determined at 7 and 14 dpv. Each experimental group includes 4 to 6 mice, with error bars representing the standard deviations from the means. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, as determined by one-way ANOVA with Tukey’s multiple-comparison test.
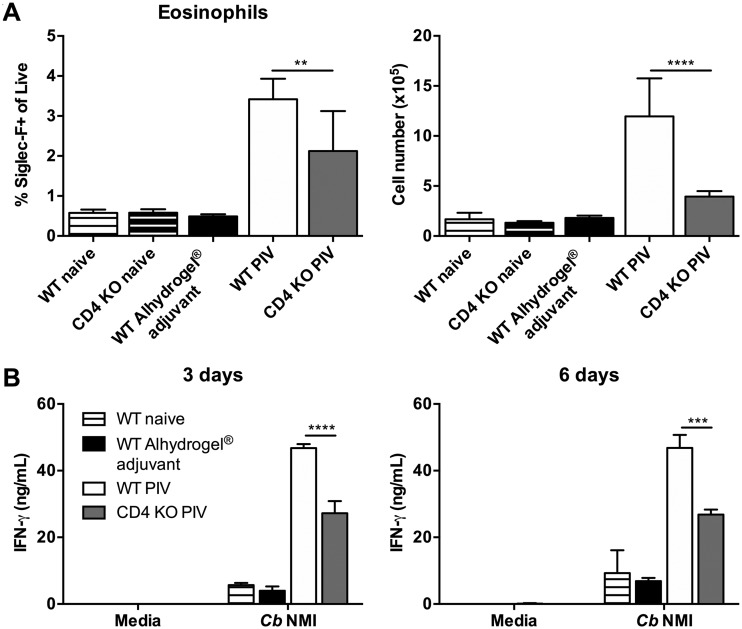
Eosinophil accumulation in PIV-vaccinated mice is CD4+ T cell dependent.
CD4+ T cells are potent producers of IL-5, which promotes eosinophil differentiation, maturation, migration, and activation (48, 49). Therefore, to determine if eosinophil accumulation in PIV-vaccinated spleens is dependent on signals from CD4+ T cells, we vaccinated CD4-deficient mice with 10 μg of PIV and assessed eosinophil numbers 7 days later. Interestingly, we observed a defect in eosinophil accumulation in PIV-vaccinated CD4-deficient mice compared to the level in PIV-vaccinated WT mice (Fig. 6A). There was no difference in the numbers of eosinophils between naive WT and CD4-deficient mice, which indicates that this phenomenon was not due to an inherent defect in CD4-deficient mice. Overall, these data suggest that eosinophil accumulation in PIV-vaccinated mice is CD4+ T cell dependent.
FIG 6.
Eosinophil accumulation in PIV-vaccinated mice is CD4+ T cell dependent. WT C57BL/6 and CD4-deficient mice were vaccinated s.c. with 10 μg of PIV, and 7 days later spleens were harvested for flow cytometry or ex vivo restimulation studies. Mice receiving Alhydrogel adjuvant alone served as unvaccinated controls. Naïve mice were used to establish baseline values. (A) SSChigh CD11b+ CD125+ Siglec-F+ eosinophils were enumerated using the gating strategy described in the legends for Fig. 4 and 5. (B) Total splenocytes were isolated and restimulated with medium or with 1 × 107 C. burnetii NMI bacteria for 3 and 6 days, and cell culture supernatants were evaluated for IFN-γ production by cytokine-specific ELISA. Each experimental group includes 5 mice, with error bars representing the standard deviations from the means. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, as determined by one-way ANOVA with Tukey’s multiple-comparison test. Cb, C. burnetii; KO, knockout.
Next, we assessed whether the loss of eosinophils in CD4-deficient mice had any effect on the production of gamma interferon (IFN-γ), an essential cytokine for C. burnetii clearance (50). To test this, we isolated total splenocytes from naive, adjuvant-treated, and PIV-vaccinated WT and CD4-deficient mice for ex vivo restimulation as described previously (51). IFN-γ levels were measured by enzyme-linked immunosorbent assay (ELISA) 3 and 6 days postrestimulation with either medium alone or 1 × 107 C. burnetii NMI bacteria. Splenocytes from PIV-vaccinated WT mice produced significant levels of IFN-γ in response to C. burnetii NMI at all time points (Fig. 6B). However, while PIV-vaccinated CD4-deficient mice were still able to respond to C. burnetii NMI, there was a significant defect in their production of IFN-γ at both 3 and 6 days postrestimulation compared to the level in PIV-vaccinated WT mice. These data suggest a CD4-dependent mechanism of IFN-γ production which could be partially related to the absence of significant eosinophil accumulation.
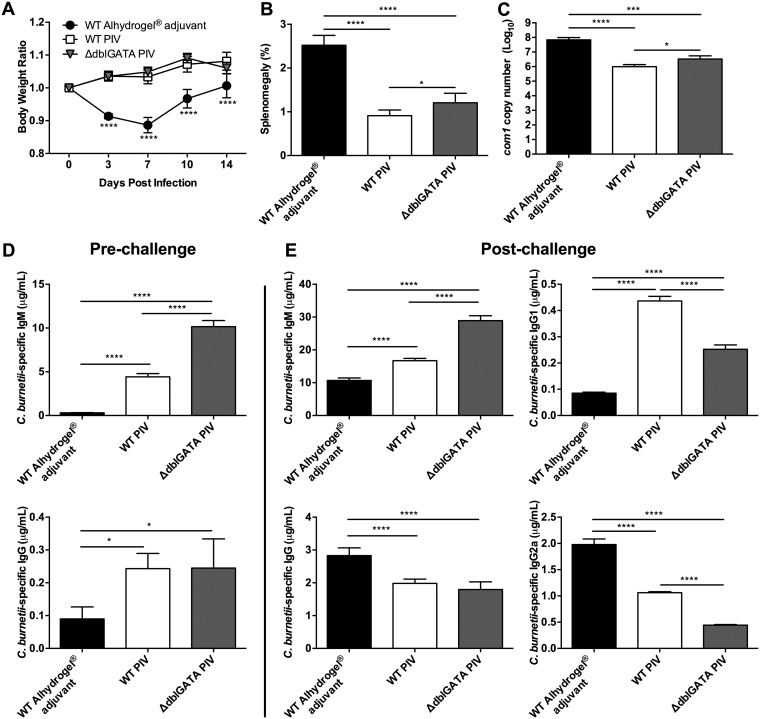
Eosinophils may partially contribute to early PIV protection.
Following the observation that eosinophils are recruited to the spleens of PIV-vaccinated mice, we evaluated whether these cells were important for vaccine-mediated protection. To test this, we vaccinated eosinophil-deficient ΔdblGATA mice with 10 μg of PIV and challenged them i.p. with 1 × 107 C. burnetii NMI bacteria 7 days later. Disease severity was evaluated by comparing body weight loss, splenomegaly, and bacterial burden at 14 dpi. Compared to WT PIV-vaccinated mice, ΔdblGATA mice displayed no significant loss in body weight throughout infection (Fig. 7A). However, ΔdblGATA mice had significantly higher splenomegaly and bacterial burden than their WT PIV-vaccinated counterparts (Fig. 7B and C). These data indicate that eosinophils may partially contribute to the early vaccine response against C. burnetii infection.
FIG 7.
Eosinophils play a role in early PIV protection. WT and eosinophil-deficient ΔdblGATA mice were vaccinated s.c. with 10 μg of PIV 7 days prior to i.p. challenge with 1 × 107 C. burnetii NMI bacteria. (A) Relative body weight was measured throughout infection. Splenomegaly (B) and bacterial burden in the spleen (C) were evaluated at 14 dpi to assess disease severity. C. burnetii NMI-specific serum IgM and IgG levels were evaluated at 7 dpv (D) or 14 dpi (E). Results are expressed as percent splenomegaly [(spleen weight/body weight) × 100]. Bacterial burden was determined by qPCR and is expressed as log10 C. burnetii com1 gene copy numbers. Each experimental group includes 5 mice, with error bars representing the standard deviations from the means. *, P < 0.05; ***, P < 0.001; ****, P < 0.0001, as determined by two-way ANOVA with Dunnett’s multiple-comparison test (for panel A) or by one-way ANOVA with Tukey’s multiple-comparison test (for panels B to E).
Eosinophils are known to play a role in priming the early IgM response as well as eliciting Th2 cells through the secretion of cytokines (49). In order to evaluate the effect of eosinophil deficiency on antibody-mediated immunity, we measured C. burnetii NMI-specific IgM and IgG by ELISA. At 7 dpv, there was significantly more NMI-specific IgM in serum from ΔdblGATA mice than in that from their WT counterparts (Fig. 7D). At this time point, however, there was no significant difference in IgG levels.
To evaluate the secondary response, we challenged ΔdblGATA mice at 7 dpv and again measured NMI-specific IgM and IgG by ELISA. At 14 dpi, there was a significant increase in the serum concentration of NMI-specific IgM in ΔdblGATA mice (Fig. 7E) compared to that in the WT. Again, no significant difference in IgG levels was measured between PIV-vaccinated cohorts. However, while there was not a significant difference in total IgG, there were significant decreases in IgG subclasses associated with eosinophil deficiency. Specifically, there was significantly less NMI-specific IgG1 and IgG2a in ΔdblGATA mice than in their WT counterparts (Fig. 7E). Collectively, these data suggest that eosinophils may partially contribute to the early vaccine response as well as antibody isotype switching.
Eosinophils contribute to antibody isotype switching in late PIV responses.
Next, we examined whether eosinophils also contribute to the late PIV response. WT and ΔdblGATA mice were vaccinated as previously described and challenged i.p. with 1 × 107 C. burnetii NMI bacteria 28 days later. Body weight loss, splenomegaly, and bacterial burden were examined to assess disease severity at 14 dpi. No body weight loss was observed in the ΔdblGATA cohort compared to WT (Fig. 8A). Additionally, there was no significant difference in levels of splenomegaly or bacterial burdens between PIV-vaccinated WT and ΔdblGATA mice (Fig. 8B and C). These results suggest that eosinophils may not play a role in later protection.
FIG 8.
Eosinophils contribute to antibody isotype switching in late PIV responses. WT and ΔdblGATA mice were vaccinated s.c. with 10 μg of PIV 4 weeks prior to i.p. challenge with 1 × 107 C. burnetii NMI bacteria. (A) Relative body weight was measured throughout infection. Splenomegaly (B) and bacterial burden in the spleen (C) were evaluated at 14 dpi to assess disease severity. (D) Serum IFN-γ levels were quantified at 14 dpi by cytokine-specific ELISA. C. burnetii NMI-specific serum IgM and IgG levels were measured weekly until 28 dpv (E) as well as at 14 dpi (F). Results are expressed as percent splenomegaly [(spleen weight/body weight) × 100]. Bacterial burden was determined by qPCR and is expressed as log10 C. burnetii com1 gene copy numbers. Each experimental group includes 4 mice, with error bars representing the standard deviations from the means. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, as determined by two-way ANOVA with Dunnett’s multiple-comparison test (panels A and D) or by one-way ANOVA with Tukey’s multiple-comparison test (panels, B, C, and E).
To determine if eosinophils play a role in the IFN-γ response, we measured IFN-γ in serum from PIV-vaccinated WT and ΔdblGATA mice by ELISA. Interestingly, there was significantly less IFN-γ present in ΔdblGATA mice at 14 dpi than in their WT counterparts (Fig. 8D). This suggests that eosinophils may contribute to the cytokine response following C. burnetii infection.
When evaluating the primary antibody response to vaccination, we found that at the time of challenge there was no significant difference in total serum IgM (Fig. 8E). Interestingly, we did find a significant decrease in total serum IgG in ΔdblGATA mice compared to the level in their WT counterparts. At 2 weeks postchallenge we detected elevated serum IgM (Fig. 8F) in ΔdblGATA mice and no difference in serum IgG, similar to the phenotype seen in ΔdblGATA mice challenged at 7 dpv. In line with previous results, we also found ΔdblGATA mice to have significantly reduced IgG1 and IgG2a subclass antibodies (Fig. 8F). Taken together, these data indicate a role for eosinophils in isotype switching for the generation of Coxiella-specific antibodies.
DISCUSSION
There is currently no FDA-approved vaccine available against C. burnetii infection due to a high incidence of adverse reactions in vaccinated populations. It remains unclear what vaccine components elicit these delayed-type hypersensitivity reactions. This, coupled with a lack of knowledge regarding the immunological mechanisms of vaccine protection, has prevented the rational design of a safe and effective new-generation vaccine against Q fever.
In this study, we sought to test the protective efficacies of PIV and PIIV generated by axenic culture methods. Reactogenicity has been attributed, in part, to residual egg proteins from the vaccine manufacturing process (41). By utilizing the axenic culture system, we removed the possibility of contamination by host-derived components. In line with previous results utilizing formalin-inactivated whole-cell vaccines derived from chicken eggs or tissue culture (25, 32, 52, 53), axenically generated PIV was able to prevent clinical disease in BALB/c mice. There was a significant reduction in splenomegaly and an approximately 2-log decrease in C. burnetii genomic equivalents. Further investigation is required to determine if there is a decrease in adverse reactions with the use of this vaccine. Baeten et al. recently published a standardized guinea pig model for Q fever vaccine reactogenicity testing that could prove useful for these studies (54).
A recent improvement on ACCM-2, ACCM-D, allows for the generation of 5- to 10-fold more bacteria with increased viability (55). Future studies examining the immunogenicity/protectivity of vaccines propagated in this defined axenic medium would be interesting, considering these changes in C. burnetii replication dynamics. Furthermore, the higher bacterial yield would improve the feasibility of this propagation method for future vaccine manufacturing.
While whole-cell inactivated C. burnetii vaccines have been demonstrated to be highly efficacious in lieu of adjuvant (10, 23, 24, 56, 57), these studies were carried out in healthy adults. It has recently been reported that children’s risk of exposure to C. burnetii in South West Queensland is similar to the high risk of abattoir workers (58); however, Q-VAX is not currently recommended for children (59). Vaccines which are successfully tested in healthy adults often fail in hypo/nonresponders such as young children and the elderly (60). Immunopotentiation in these risk groups should be the focus in designing a new-generation Q fever vaccine, and adjuvantation is one approach to this aim. Additionally, the use of adjuvant for immunopotentiation may allow a reduction in the amount of antigen required to generate protection. This “dose-sparing” approach may, in turn, reduce adverse reactions as they appear to occur in a dose-dependent manner (57).
Previous reports in human cohorts suggested that it took at least 2 to 3 weeks for protection to develop (10, 23, 24). However, this was based on the incidence of clinical Q fever cases in vaccinated groups. Considering an incubation period of 2 to 3 weeks, it is likely that these patients were exposed prior to vaccination. Using our previously established mouse splenomegaly model, we determined that axenically generated PIV is able to generate a protective response as early as 7 dpv. At this time point, there was a significant reduction in splenomegaly as well as a roughly 4-log decrease in C. burnetii genomic equivalents. This finding has important implications in the context of C. burnetii’s use as a biological weapon. A vaccine which generates protection in a short time frame is not only a great prophylactic candidate but also a potential therapeutic.
The early time point at which PIV was able to significantly reduce splenomegaly suggested that innately driven mechanisms were involved in early vaccine protection. Indeed, we found significantly more SSChigh granulocytes in the spleens of PIV-vaccinated mice at all time points. There also appeared to be an increase in the number of lymphocytes at 14 dpv. Considering the kinetics of the anti-Coxiella antibody response, it is likely that this initial increase is due to the expansion of B cells in response to antigen stimulus. We saw peak serum IgM titers at 1 week postvaccination and began to detect measurable changes to serum IgG as early as 2 weeks postvaccination.
Further characterization of the SSChigh granulocyte population indicated that the cells were CD11b+ CD125+ Siglec-F+ eosinophils. Eosinophils are typically associated with clearance of helminth infections and allergic diseases (61, 62); however, they are also capable of bridging innate and adaptive immunity (49). These granulocytic cells contain preformed cytokines, chemokines, and growth factors that are able to be released quickly following activation (63). These mediators can activate neutrophils, immature dendritic cells, B cells, and T cells (64).
Major basic protein (MBP) has been shown to increase superoxide production in neutrophils (65). Elliott et al. have shown that neutrophils are important for the secondary response against C. burnetii (66). It is possible that eosinophil activation aids in the bactericidal activity of neutrophils, which could be important for protection. Alternatively, eosinophil-derived neurotoxin (EDN) released from eosinophils stimulated with CpG DNA can elicit the maturation of immature dendritic cells (67, 68). Dendritic cells are known to play a pivotal role in generating protective adaptive responses following vaccination against many intracellular pathogens, including C. burnetii (69). Therefore, it is possible that eosinophils are the first cells to respond in a cascade involving other innate populations, which then prime naive B and T cells. However, eosinophils can also express major histocompatibility complex class II (MHC-II) and costimulatory molecules and serve as antigen-presenting cells to directly activate B and T cells (64, 70). In fact, previous studies have found that alum adjuvant induces eosinophil recruitment and activation of B cells to produce antigen-specific IgM (71).
Evaluating the role of eosinophils in the host defense against C. burnetii using a more clinically relevant aerosol model of infection is an exciting area for future research. While eosinophils are canonically known for their role in clearance of parasitic infections, these cells are frequently involved in allergic airway inflammation (61, 62). With this in mind, it is possible that eosinophils could be involved in C. burnetii lung pathology. Previous reports have shown that MBP can dampen superoxide anion production by guinea pig alveolar macrophages (72). Considering that C. burnetii possesses superoxide dismutase abilities (73–76), which promote its survival within the intracellular compartment, it is conceivable that this function of MBP would aid in bacterial survival. However, recent reports have shown that eosinophils also aid in clearance of prominent pulmonary pathogens like respiratory syncytial virus (77–82) and Mycobacterium tuberculosis (83, 84). Indeed, Shi et al. (85) have previously demonstrated that airway eosinophils, or those isolated from the peritoneal cavity of hypereosinophilic IL-5 transgenic mice, migrate to paratracheal lymph nodes following instillation into the trachea and act as antigen-presenting cells to stimulate antigen-specific CD4+ T cell responses.
In order to understand the mechanism of eosinophil accumulation, we studied potential upstream signaling events that would elicit eosinophil accumulation in PIV-vaccinated spleens. CD4+ T cells can recruit eosinophils through their secretion of chemoattractants like IL-5 and eotaxin-1. Therefore, to test whether CD4+ T cells were required for eosinophil accumulation, we vaccinated CD4-deficient mice and compared their splenic eosinophil numbers to those of WT mice. Interestingly, we found that CD4-deficient mice had a defect in tissue eosinophilia compared to that of WT mice, which suggests a CD4+ T cell-dependent role for this phenomenon. Additionally, we found that PIV-vaccinated CD4-deficient mice had a defect in IFN-γ production upon ex vivo restimulation with C. burnetii NMI. This could be due to the absence of CD4+ T cells; however, it is also possible that the lack of significant eosinophil accumulation contributes to this phenotype. While eosinophils are best known for their release of type 2 cytokines (IL-4, IL-5, and IL-13), it has become recently appreciated that these cells can also secrete many proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α) and IFN-γ (86). Indeed, PIV-vaccinated eosinophil-deficient ΔdblGATA mice had significantly lower serum IFN-γ than PIV-vaccinated WT mice at 14 dpi, which suggests that eosinophils contribute to the IFN-γ response following C. burnetii infection. Further studies are required to determine which cell population(s) serves as an early IFN-γ producer in our vaccine model.
To evaluate the contribution of eosinophils to PIV protection, we vaccinated eosinophil-deficient ΔdblGATA mice and challenged them at either 7 or 28 dpv. ΔdblGATA mice have a deletion in the GATA1 promoter, which is important for eosinophil development (87). We found that ΔdblGATA mice had a defect in protection when challenged at 7 dpv. This suggests that eosinophils may play a partial role in the early protective response against C. burnetii. Considering that the changes in splenomegaly and bacterial burden were relatively small, albeit statistically significant, it is possible that eosinophils do not make a biologically significant contribution to vaccine immunity. However, it would be interesting to evaluate vaccine protection using a hypereosinophilic IL-5 transgenic mouse model. Eosinophil recruitment to the spleen, while not sufficient for vaccine protection, could enhance the overall vaccine response. In this case, adjuvant selection targeting eosinophils would be an interesting approach to consider.
At 2 weeks postchallenge, ΔdblGATA mice vaccinated 7 days prior displayed elevated serum IgM with no difference in total IgG compared to the level of WT. However, there was a significant decrease in both IgG1 and IgG2a subclass antibodies. While there was no difference in the total IgG concentration, the fact that we saw decreases in certain IgG subclasses indicates that the absence of eosinophils likely affects the ratio of each subclass (IgG1, IgG2a, IgG2b, and IgG3), which shapes the overall antibody response. Indeed, eosinophils have been shown to promote B cell responsiveness to both T-dependent and T-independent antigens in the periphery (88). Furthermore, previous work by Chu et al. has shown that eosinophils are required for the maintenance of long-lived plasma cells in the bone marrow (89, 90). ΔdblGATA mice have normal migration of plasmablasts to the bone marrow; however, they fail to develop into mature plasma cells (91). In line with these results, we found ΔdblGATA mice challenged at 28 dpv to have elevated serum IgM with decreased IgG1 and IgG2a subclass antibodies. These data indicate that eosinophils contribute to isotype switching and help shape the humoral response.
Cumulatively, these data have led us to propose a working model (Fig. 9) to describe the role of eosinophils in PIV-mediated protection. In our proposed model, antigen-presenting cells, such as dendritic cells (92), process and present vaccine antigens to naive CD4+ T cells. These activated helper cells secrete IL-5, which is a potent chemoattractant and activator of eosinophils. Eosinophils then accumulate in the spleens of vaccinated animals, where they are poised to respond to subsequent infection. Upon secondary challenge, eosinophils can release preformed IFN-γ from their granules. IFN-γ aids in bacterial clearance through activation of macrophages and enhancement of their microbicidal activity (93), promotion of IgG2a secretion from B cells (94, 95), and expansion of Th1+ CD4+ T cells (96). While it still remains to be demonstrated that eosinophils secrete IFN-γ in response to C. burnetii, it is known that IFN-γ promotes the previously mentioned host defense mechanisms against C. burnetii (25, 31, 50, 97–101).
FIG 9.
A working model for eosinophils in PIV-mediated protection. During the primary response, vaccine antigens are taken up by antigen-presenting cells, such as dendritic cells, processed, and presented to naive CD4+ T cells. These naive cells become activated T helper cells and can secrete IL-5, a potent chemoattractant and activator of eosinophils. Eosinophils then accumulate in the spleens of vaccinated animals, where they are poised to respond to subsequent infection. Upon secondary challenge, eosinophils can release preformed IFN-γ from their granules. IFN-γ contributes to host immunity against C. burnetii through activation of macrophages and enhancement of their microbicidal activity, promotion of IgG2a secretion from B cells, and expansion of Th1+ helper cells. Steps denoted with a green check mark (✓) have been demonstrated either experimentally in the manuscript or previously in the literature. Red question marks (?) denote steps which require further experimentation.
In summary, this study provides novel information regarding the utility of axenic culture for the generation of highly efficacious vaccines against C. burnetii. Additionally, our results shed light on a previously uncharacterized mechanism of vaccine protection involving CD11b+ CD125+ Siglec-F+ eosinophils. Further understanding of the mechanisms of vaccine protection will guide future rational vaccine design and adjuvant selection.
MATERIALS AND METHODS
Animals.
Four- to 12-week-old BALB/c and C57BL/6 mice were generated in our colony for use in these studies. Eosinophil-deficient ΔdblGATA [C.129S1(B6)-Gata1tm6Sho/J] and CD4-deficient (B6.129S2-Cd4tm1Mak/J) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were housed in sterile microisolator cages in a conventional animal facility or in an animal biosafety level 3 (ABSL3) facility at the University of Missouri Laboratory for Infectious Disease Research (MU-LIDR). Animals were provided food and water ad libitum. All research involving animals was conducted in accordance with the Animal Care and Use guidelines, and all animal use protocols were approved by the Animal Care and Use Committee at the University of Missouri. All infections were conducted in an ABSL3 facility at the MU-LIDR.
Bacterial strains.
C. burnetii Nine Mile phase I (NMI) clone 7 (RSA 493) and Nine Mile phase II (NMII) clone 4 (RSA 439) were propagated in acidified citrate cysteine medium-2 (ACCM-2) as previously described (43). Bacteria were purified by centrifugation at 15,000 × g for 30 min, followed by two washes with sterile 1× phosphate-buffered saline (PBS). NMI was handled under biosafety level 3 (BSL3) conditions at the MU-LIDR.
Vaccination.
Purified C. burnetii NMI and NMII were inactivated for 48 h in 10% formalin, followed by dialysis in deionized water. Antigen concentrations were then measured using a Micro BCA protein assay kit (Pierce, Rockford, IL) per the manufacturer’s instructions. Mice were vaccinated subcutaneously (s.c.) with 10 μg of formalin-inactivated phase I (PIV) or phase II (PIIV) whole-cell vaccine in 50 μl of sterile 1× PBS with 50 μl of aluminum hydroxide adjuvant (Alhydrogel adjuvant, 2%; InvivoGen, San Diego, CA) for a final delivered volume of 100 μl. Unvaccinated control mice were given 50 μl of PBS with 50 μl of adjuvant, for a final delivered volume of 100 μl. Vaccines were prepared with a 1:1 ratio of antigen/adjuvant per the manufacturer’s instructions. Vaccinated and unvaccinated control mice were then either challenged to assess protectivity or sacrificed to assess cellular responses via flow cytometry or cytokine enzyme-linked immunosorbent assay (ELISA).
Infection.
Vaccinated and unvaccinated control mice were challenged at 7, 14, or 28 days postvaccination (dpv) by intraperitoneal (i.p.) injection with a total of 1 × 107 or 5 × 107 C. burnetii NMI bacteria in 400 μl of sterile 1× PBS. Mice were weighed throughout the course of infection. The protective efficacy of PIV and PIIV was evaluated 14 days postinfection (dpi) by comparing percent splenomegaly [(spleen weight/body weight) × 100] against that of unvaccinated controls. Bacterial burden in the spleen was also measured using real-time quantitative PCR (qPCR).
qPCR.
Spleen pieces were homogenized in 200 μl of lysis buffer (1 M Tris, 0.5 M EDTA, 7 mg/ml glucose, 28 mg/ml lysozyme) and filtered through a 100-μm-pore-size nylon mesh to remove any connective tissue. Ten microliters of proteinase K (20 mg/ml) was added to each sample prior to incubation at 60°C for 18 h. Next, 21 μl of 10% SDS was added to samples and incubated at room temperature for 1 h. Finally, DNA was extracted using a High Pure PCR Template Preparation kit (Roche, Indianapolis, IN) as directed by the manufacturer. Bacterial burden was determined by quantifying com1 gene copy numbers using a standard curve with SYBR green (Applied Biosystems, Foster City, CA) on an Applied Biosystems StepOnePlus real-time PCR system. The standard curve was generated using recombinant plasmid DNA (com1 gene ligated into pBluescript vector).
Spleen cell isolation.
Spleen cells were harvested from BALB/c, C57BL/6, and CD4-deficient mice at 7 or 14 dpv or post-adjuvant treatment. Briefly, spleens were removed and homogenized. The cell suspension was then filtered through a 100-μm-pore-size nylon mesh to remove any connective tissue. Spleen cells were pelleted by centrifugation at 500 × g for 8 min and resuspended in 5 ml of ammonium chloride-potassium (ACK) lysis buffer for 5 min at room temperature to lyse red blood cells. Remaining cells were then pelleted by centrifugation at 500 × g for 8 min and resuspended in 2 ml of fluorescence-activated cell sorting (FACS) buffer (PBS supplemented with 0.5% bovine serum albumin [BSA], 2 mM EDTA, and 0.1% sodium azide) for counting.
Antibodies.
Recombinant anti-mouse CD125 (IL-5Ra, clone REA343; conjugated to phycoerythrin [PE]), Siglec-F (clone REA798; PE-Vio770), and CD45R (B220, clone REA755; fluorescein isothiocyanate [FITC]) as well as rat anti-mouse CD19 (clone 6D5; FITC) were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). Rat anti-mouse CD45 (clone 30-F11; allophycocyanin [APC]) was purchased from ThermoFisher Scientific (Waltham, MA). Rat anti-mouse/human CD11b (clone M1/70; Brilliant Violet 785) was purchased from BioLegend (San Diego, CA).
Flow cytometry.
Approximately 1 × 106 cells were added to each well of a 96-well plate for flow cytometry. Briefly, cells were blocked for 15 min at 4°C with Fc Block (BD Biosciences) in FACS buffer. Cells were then stained in FACS buffer with an antibody cocktail containing CD45, CD125, CD11b, Siglec-F, CD19, and CD45R for 30 min at 4°C. Cells were pelleted by centrifugation at 500 × g for 8 min, followed by two washes with FACS buffer. A 2% paraformaldehyde solution was then used to fix cells for 15 min at 4°C. Finally, cells were pelleted by centrifugation at 500 × g for 8 min and resuspended in FACS buffer for analysis. A total of 500,000 cellular events were collected for each sample using a Miltenyi MACSQuant Analyzer 10 or BD LSRFortessa. Compensation controls were utilized to account for spectral overlap before data analysis was performed using FlowJo, version 10.4.2. Absolute cell counts were determined based on live cell frequency and the total number of spleen cells recovered from each animal (see Table S1 in the supplemental material).
Cytokine ELISA.
Splenocytes from naive, adjuvant-treated, or PIV-vaccinated C57BL/6 and CD4-deficient mice were restimulated ex vivo with 1 × 107 virulent C. burnetii NMI bacteria to evaluate gamma interferon (IFN-γ) levels in supernatants by ELISA as previously described (51). Briefly, 5 × 106 total splenocytes from each treatment group were added in duplicate to a 24-well plate in complete medium (RPMI 1640 medium supplemented with 10% fetal bovine serum [FBS], 10 mM HEPES buffer, 10 mM nonessential amino acids, and 10 mM sodium pyruvate). Cells were cultured alone or restimulated with C. burnetii NMI bacteria (1 × 107/ml) for 3 or 6 days at 37°C. ELISAs were performed on supernatants for IFN-γ as directed by the manufacturer (Invitrogen-ThermoFisher Scientific).
Coxiella-specific ELISA.
Sera from vaccinated and unvaccinated control mice were used for quantification of total IgM, IgG, IgG1, and IgG2a subclass antibodies. Microtiter plates (96-well) were coated with 100 μl of inactivated NMI antigen (0.5 μg/ml) or unlabeled anti-IgM or -IgG antibody (0.5 μg/ml, for the standard curve) (Southern Biotech, Birmingham, AL) in 0.05 M carbonate/bicarbonate coating buffer (pH 9.6) for 24 h at 4°C. Plates were blocked with 1% BSA in PBS-T buffer (0.05% Tween 20 in 1× PBS) and then incubated for 2 h with 200 μl of diluted sample serum (1:200 to 1:1,200) or serially diluted pure IgM, IgG, IgG1, or IgG2a (Southern Biotech) at room temperature. Plates were washed four times with PBS-T buffer and then incubated with 100 μl of diluted horseradish peroxidase (HRP)-conjugated goat anti-mouse IgM, IgG, IgG1, or IgG2a (1:4,000 to 1:8,000) at room temperature for 1 h. Plates were washed again four times with PBS-T, followed by the addition of 100 μl of 3,3',5,5'-tetramethylbenzidine (TMB) substrate (ThermoFisher Scientific). Reactions were stopped using 1 M H3PO4, and absorbance was measured at 450 nm using a SpectraMax (Molecular Devices, San Jose, CA) or Infinite F50 (Tecan, Switzerland) microplate reader.
Statistical analysis.
Prism, version 5.0 (GraphPad Software, Inc., San Diego, CA), was used for all statistical analyses. Results represent means ± standard deviations. Values were compared using one-way analysis of variance (ANOVA) with Tukey’s multiple-comparison test or two-way ANOVA with Dunnett’s multiple-comparison test. Differences were considered significant at a P value of <0.05.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by NIH/NIAID grants R01AI134681, R21AI130347, and R21AI137504 to G.Z.
We thank the staff at the University of Missouri Laboratory for Infectious Disease Research (MU-LIDR) and Cell and Immunobiology Core (MU-CIC) for their assistance with these experiments. We especially thank Ryan Metzler with Miltenyi Biotec for his assistance using the MACSQuant Analyzer 10. We also thank Elizabeth Jacobsen for lending her expertise in eosinophil biology and Jerod Skyberg for critical reading and editing of the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00376-19.
REFERENCES
- 1.Baca OG, Paretsky D. 1983. Q fever and Coxiella burnetii: a model for host-parasite interactions. Microbiol Rev 47:127–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maurin M, Raoult D. 1999. Q fever. Clin Microbiol Rev 12:518–553. doi: 10.1128/CMR.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raoult D, Marrie T, Mege J. 2005. Natural history and pathophysiology of Q fever. Lancet Infect Dis 5:219–226. doi: 10.1016/S1473-3099(05)70052-9. [DOI] [PubMed] [Google Scholar]
- 4.Parker NR, Barralet JH, Bell AM. 2006. Q fever. Lancet 367:679–688. doi: 10.1016/S0140-6736(06)68266-4. [DOI] [PubMed] [Google Scholar]
- 5.CDC. 2017. Q fever. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/qfever/.
- 6.Raoult D, Houpikian P, Tissot Dupont H, Riss JM, Arditi-Djiane J, Brouqui P. 1999. Treatment of Q fever endocarditis: comparison of 2 regimens containing doxycycline and ofloxacin or hydroxychloroquine. Arch Intern Med 159:167–173. doi: 10.1001/archinte.159.2.167. [DOI] [PubMed] [Google Scholar]
- 7.Brennan RE, Samuel JE. 2003. Evaluation of Coxiella burnetii antibiotic susceptibilities by real-time PCR assay. J Clin Microbiol 41:1869–1874. doi: 10.1128/jcm.41.5.1869-1874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moffatt JH, Newton P, Newton HJ. 2015. Coxiella burnetii: turning hostility into a home. Cell Microbiol 17:621–631. doi: 10.1111/cmi.12432. [DOI] [PubMed] [Google Scholar]
- 9.van Loenhout JA, Hautvast JL, Vercoulen JH, Akkermans RP, Wijkmans CJ, van der Velden K, Paget WJ. 2015. Q-fever patients suffer from impaired health status long after the acute phase of the illness: results from a 24-month cohort study. J Infect 70:237–246. doi: 10.1016/j.jinf.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Marmion BP, Ormsbee RA, Kyrkou M, Wright J, Worswick D, Cameron S, Esterman A, Feery B, Collins W. 1984. Vaccine prophylaxis of abattoir-associated Q fever. Lancet 2:1411–1414. doi: 10.1016/s0140-6736(84)91617-9. [DOI] [PubMed] [Google Scholar]
- 11.McQuiston JH, Holman RC, McCall CL, Childs JE, Swerdlow DL, Thompson HA. 2006. National surveillance and the epidemiology of human Q fever in the United States, 1978–2004. Am J Trop Med Hyg 75:36–40. doi: 10.4269/ajtmh.2006.75.1.0750036. [DOI] [PubMed] [Google Scholar]
- 12.Whitney EA, Massung RF, Candee AJ, Ailes EC, Myers LM, Patterson NE, Berkelman RL. 2009. Seroepidemiologic and occupational risk survey for Coxiella burnetii antibodies among US veterinarians. Clin Infect Dis 48:550–557. doi: 10.1086/596705. [DOI] [PubMed] [Google Scholar]
- 13.Moos A, Hackstadt T. 1987. Comparative virulence of intra- and interstrain lipopolysaccharide variants of Coxiella burnetii in the guinea pig model. Infect Immun 55:1144–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams JC, Thompson HA. 1991. Infectivity, virulence, and pathogenicity of Coxiella burnetii for various hosts, Q Fever: the Biology of Coxiella burnetii, vol 2 CRC Press; p 21–71. [Google Scholar]
- 15.Brooke RJ, Kretzschmar ME, Mutters NT, Teunis PF. 2013. Human dose response relation for airborne exposure to Coxiella burnetii. BMC Infect Dis 13:488. doi: 10.1186/1471-2334-13-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawker JI, Ayres JG, Blair I, Evans MR, Smith DL, Smith EG, Burge PS, Carpenter MJ, Caul EO, Coupland B, Desselberger U, Farrell ID, Saunders PJ, Wood MJ. 1998. A large outbreak of Q fever in the West Midlands: windborne spread into a metropolitan area? Commun Dis Public Health 1:180–187. [PubMed] [Google Scholar]
- 17.Tissot-Dupont H, Torres S, Nezri M, Raoult D. 1999. Hyperendemic focus of Q fever related to sheep and wind. Am J Epidemiol 150:67–74. doi: 10.1093/oxfordjournals.aje.a009920. [DOI] [PubMed] [Google Scholar]
- 18.Oyston PC, Davies C. 2011. Q fever: the neglected biothreat agent. J Med Microbiol 60:9–21. doi: 10.1099/jmm.0.024778-0. [DOI] [PubMed] [Google Scholar]
- 19.Enserink M. 2010. Infectious diseases. Questions abound in Q-fever explosion in the Netherlands. Science 327:266–267. doi: 10.1126/science.327.5963.266-a. [DOI] [PubMed] [Google Scholar]
- 20.Roest HI, Tilburg JJ, van der Hoek W, Vellema P, van Zijderveld FG, Klaassen CH, Raoult D. 2011. The Q fever epidemic in the Netherlands: history, onset, response and reflection. Epidemiol Infect 139:1–12. doi: 10.1017/S0950268810002268. [DOI] [PubMed] [Google Scholar]
- 21.Hackstadt T, Peacock MG, Hitchcock PJ, Cole RL. 1985. Lipopolysaccharide variation in Coxiella burnetii: intrastrain heterogeneity in structure and antigenicity. Infect Immun 48:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kersh GJ, Oliver LD, Self JS, Fitzpatrick KA, Massung RF. 2011. Virulence of pathogenic Coxiella burnetii strains after growth in the absence of host cells. Vector Borne Zoonotic Dis 11:1433–1438. doi: 10.1089/vbz.2011.0670. [DOI] [PubMed] [Google Scholar]
- 23.Marmion BP, Ormsbee RA, Kyrkou M, Wright J, Worswick DA, Izzo AA, Esterman A, Feery B, Shapiro RA. 1990. Vaccine prophylaxis of abattoir-associated Q fever: eight years’ experience in Australian abattoirs. Epidemiol Infect 104:275–287. doi: 10.1017/s0950268800059458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ackland JR, Worswick DA, Marmion BP. 1994. Vaccine prophylaxis of Q fever. A follow-up study of the efficacy of Q-Vax (CSL) 1985–1990. Med J Aust 160:704–708. [PubMed] [Google Scholar]
- 25.Zhang G, Russell-Lodrigue KE, Andoh M, Zhang Y, Hendrix LR, Samuel JE. 2007. Mechanisms of vaccine-induced protective immunity against Coxiella burnetii infection in BALB/c mice. J Immunol 179:8372–8380. doi: 10.4049/jimmunol.179.12.8372. [DOI] [PubMed] [Google Scholar]
- 26.Smadel JE, Snyder MJ, Robbins FC. 1948. Vaccination against Q fever. Am J Hyg 47:71–81. [DOI] [PubMed] [Google Scholar]
- 27.Meiklejohn G, Lennette EH. 1950. Q fever in California. I. Observations on vaccination of human beings. Am J Hyg 52:54–64. [PubMed] [Google Scholar]
- 28.Stoker MG. 1957. Q fever down the drain. Br Med J 1:425–427. doi: 10.1136/bmj.1.5016.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell JF, Lackman DB, Meis A, Hadlow WJ. 1964. Recurrent reaction of site of Q fever vaccination in a sensitized person. Mil Med 129:591–595. doi: 10.1093/milmed/129.7.591. [DOI] [PubMed] [Google Scholar]
- 30.Zhang G, Samuel JE. 2004. Vaccines against Coxiella infection. Expert Rev Vaccines 3:577–584. doi: 10.1586/14760584.3.5.577. [DOI] [PubMed] [Google Scholar]
- 31.Zhang G, Zhang Y, Samuel JE. 2012. Components of protective immunity. Adv Exp Med Biol 984:91–104. doi: 10.1007/978-94-007-4315-1_5. [DOI] [PubMed] [Google Scholar]
- 32.Zhang G, Peng Y, Schoenlaub L, Elliott A, Mitchell W, Zhang Y. 2013. Formalin-inactivated Coxiella burnetii phase I vaccine-induced protection depends on B cells to produce protective IgM and IgG. Infect Immun 81:2112–2122. doi: 10.1128/IAI.00297-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kersh GJ, Fitzpatrick KA, Self JS, Biggerstaff BJ, Massung RF. 2013. Long-term immune responses to Coxiella burnetii after vaccination. Clin Vaccine Immunol 20:129–133. doi: 10.1128/CVI.00613-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Surendran N, Sriranganathan N, Boyle SM, Hiltbold EM, Tenpenny N, Walker M, Zimmerman K, Werre S, Witonsky SG. 2013. Protection to respiratory challenge of Brucella abortus strain 2308 in the lung. Vaccine 31:4103–4110. doi: 10.1016/j.vaccine.2013.06.078. [DOI] [PubMed] [Google Scholar]
- 35.Dunne A, Mielke LA, Allen AC, Sutton CE, Higgs R, Cunningham CC, Higgins SC, Mills KH. 2015. A novel TLR2 agonist from Bordetella pertussis is a potent adjuvant that promotes protective immunity with an acellular pertussis vaccine. Mucosal Immunol 8:607–617. doi: 10.1038/mi.2014.93. [DOI] [PubMed] [Google Scholar]
- 36.Schaut RG, Grinnage-Pulley TL, Esch KJ, Toepp AJ, Duthie MS, Howard RF, Reed SG, Petersen CA. 2016. Recovery of antigen-specific T cell responses from dogs infected with Leishmania (L.) infantum by use of vaccine associated TLR-agonist adjuvant. Vaccine 34:5225–5234. doi: 10.1016/j.vaccine.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dowling DJ, Scott EA, Scheid A, Bergelson I, Joshi S, Pietrasanta C, Brightman S, Sanchez-Schmitz G, Van Haren SD, Ninkovic J, Kats D, Guiducci C, de Titta A, Bonner DK, Hirosue S, Swartz MA, Hubbell JA, Levy O. 2017. Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses. J Allergy Clin Immunol 140:1339–1350. doi: 10.1016/j.jaci.2016.12.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko EJ, Lee YT, Lee Y, Kim KH, Kang SM. 2017. Distinct effects of monophosphoryl lipid A, oligodeoxynucleotide CpG, and combination adjuvants on modulating innate and adaptive immune responses to influenza vaccination. Immune Netw 17:326–342. doi: 10.4110/in.2017.17.5.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coler RN, Day TA, Ellis R, Piazza FM, Beckmann AM, Vergara J, Rolf T, Lu L, Alter G, Hokey D, Jayashankar L, Walker R, Snowden MA, Evans T, Ginsberg A, Reed SG, Team T-S. 2018. The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: first-in-human trial. NPJ Vaccines 3:34. doi: 10.1038/s41541-018-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Omsland A, Cockrell DC, Howe D, Fischer ER, Virtaneva K, Sturdevant DE, Porcella SF, Heinzen RA. 2009. Host cell-free growth of the Q fever bacterium Coxiella burnetii. Proc Natl Acad Sci U S A 106:4430–4434. doi: 10.1073/pnas.0812074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seqirus. 2017. Q-VAX Q fever vaccine and Q-VAX skin test (AUST R 100517 & 100518) package insert. Seqirus, Parkville, Australia https://www.seqirus.com.au/docs/315/57/Q-VAX%20PI_v6_18Dec2017.pdf.
- 42.Lackman DB, Bell EJ, Bell JF, Pickens EG. 1962. Intradermal sensitivity testing in man with a purified vaccine for Q fever. Am J Public Health Nations Health 52:87–93. doi: 10.2105/ajph.52.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Omsland A, Beare PA, Hill J, Cockrell DC, Howe D, Hansen B, Samuel JE, Heinzen RA. 2011. Isolation from animal tissue and genetic transformation of Coxiella burnetii are facilitated by an improved axenic growth medium. Appl Environ Microbiol 77:3720–3725. doi: 10.1128/AEM.02826-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long CM, Beare PA, Cockrell DC, Larson CL, Heinzen RA. 2019. Comparative virulence of diverse Coxiella burnetii strains. Virulence 10:133–150. doi: 10.1080/21505594.2019.1575715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanicas M, Rossi S, Giudice GD, Rambe DS. 2015. Safety and mechanism of action of licensed vaccine adjuvants. Int Curr Pharm J 4:420–431. doi: 10.3329/icpj.v4i8.24024. [DOI] [Google Scholar]
- 46.Reichman H, Rozenberg P, Munitz A. 2017. Mouse eosinophils: identification, isolation, and functional analysis. Curr Protoc Immunol 119:14.43.1–14.43.22. [DOI] [PubMed] [Google Scholar]
- 47.Moon BG, Takaki S, Miyake K, Takatsu K. 2004. The role of IL-5 for mature B-1 cells in homeostatic proliferation, cell survival, and Ig production. J Immunol 172:6020–6029. doi: 10.4049/jimmunol.172.10.6020. [DOI] [PubMed] [Google Scholar]
- 48.Lee NA, McGarry MP, Larson KA, Horton MA, Kristensen AB, Lee JJ. 1997. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol 158:1332–1344. [PubMed] [Google Scholar]
- 49.Shamri R, Xenakis JJ, Spencer LA. 2011. Eosinophils in innate immunity: an evolving story. Cell Tissue Res 343:57–83. doi: 10.1007/s00441-010-1049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andoh M, Zhang G, Russell-Lodrigue KE, Shive HR, Weeks BR, Samuel JE. 2007. T cells are essential for bacterial clearance, and gamma interferon, tumor necrosis factor alpha, and B cells are crucial for disease development in Coxiella burnetii infection in mice. Infect Immun 75:3245–3255. doi: 10.1128/IAI.01767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clapp B, Yang X, Thornburg T, Walters N, Pascual DW. 2016. Nasal vaccination stimulates CD8+ T cells for potent protection against mucosal Brucella melitensis challenge. Immunol Cell Biol 94:496–508. doi: 10.1038/icb.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang GQ, Samuel JE. 2003. Identification and cloning potentially protective antigens of Coxiella burnetii using sera from mice experimentally infected with Nine Mile phase I. Ann N Y Acad Sci 990:510–520. doi: 10.1111/j.1749-6632.2003.tb07420.x. [DOI] [PubMed] [Google Scholar]
- 53.Tyczka J, Eberling S, Baljer G. 2005. Immunization experiments with recombinant Coxiella burnetii proteins in a murine infection model. Ann N Y Acad Sci 1063:143–148. doi: 10.1196/annals.1355.022. [DOI] [PubMed] [Google Scholar]
- 54.Baeten LA, Podell BK, Sluder AE, Garritsen A, Bowen RA, Poznansky MC. 2018. Standardized guinea pig model for Q fever vaccine reactogenicity. PLoS One 13:e0205882. doi: 10.1371/journal.pone.0205882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sandoz KM, Beare PA, Cockrell DC, Heinzen RA. 2016. Complementation of arginine auxotrophy for genetic transformation of Coxiella burnetii by use of a defined axenic medium. Appl Environ Microbiol 82:3042–3051. doi: 10.1128/AEM.00261-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shapiro RA, Siskind V, Schofield FD, Stallman N, Worswick DA, Marmion BP. 1990. A randomized, controlled, double-blind, cross-over, clinical trial of Q fever vaccine in selected Queensland abattoirs. Epidemiol Infect 104:267–273. doi: 10.1017/s0950268800059446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fries LF, Waag DM, Williams JC. 1993. Safety and immunogenicity in human volunteers of a chloroform-methanol residue vaccine for Q fever. Infect Immun 61:1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parker N, Robson J, Bell M. 2010. A serosurvey of Coxiella burnetii infection in children and young adults in South West Queensland. Aust N Z J Public Health 34:79–82. doi: 10.1111/j.1753-6405.2010.00478.x. [DOI] [PubMed] [Google Scholar]
- 59.Barralet JH, Parker NR. 2004. Q fever in children: an emerging public health issue in Queensland. Med J Aust 180:596–597. [DOI] [PubMed] [Google Scholar]
- 60.Wiedermann U, Garner-Spitzer E, Wagner A. 2016. Primary vaccine failure to routine vaccines: why and what to do? Hum Vaccin Immunother 12:239–243. doi: 10.1080/21645515.2015.1093263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McBrien CN, Menzies-Gow A. 2017. The biology of eosinophils and their role in asthma. Front Med (Lausanne) 4:93. doi: 10.3389/fmed.2017.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Sullivan JA, Bochner BS. 2018. Eosinophils and eosinophil-associated diseases: an update. J Allergy Clin Immunol 141:505–517. doi: 10.1016/j.jaci.2017.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weller PF, Spencer LA. 2017. Functions of tissue-resident eosinophils. Nat Rev Immunol 17:746–760. doi: 10.1038/nri.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenberg HF, Dyer KD, Foster PS. 2013. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol 13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haskell MD, Moy JN, Gleich GJ, Thomas LL. 1995. Analysis of signaling events associated with activation of neutrophil superoxide anion production by eosinophil granule major basic protein. Blood 86:4627–4637. [PubMed] [Google Scholar]
- 66.Elliott A, Schoenlaub L, Freches D, Mitchell W, Zhang G. 2015. Neutrophils play an important role in protective immunity against Coxiella burnetii infection. Infect Immun 83:3104–3113. doi: 10.1128/IAI.00042-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang D, Rosenberg HF, Chen Q, Dyer KD, Kurosaka K, Oppenheim JJ. 2003. Eosinophil-derived neurotoxin (EDN), an antimicrobial protein with chemotactic activities for dendritic cells. Blood 102:3396–3403. doi: 10.1182/blood-2003-01-0151. [DOI] [PubMed] [Google Scholar]
- 68.Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, Michalek SM, Rosenberg HF, Zhang N, Oppenheim JJ. 2008. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med 205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei Y, Wang X, Xiong X, Wen B. 2011. Coxiella burnetii antigen-stimulated dendritic cells mediated protection against Coxiella burnetii in BALB/c mice. J Infect Dis 203:283–291. doi: 10.1093/infdis/jiq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weller PF, Rand TH, Barrett T, Elovic A, Wong DT, Finberg RW. 1993. Accessory cell function of human eosinophils. HLA-DR-dependent, MHC-restricted antigen-presentation and IL-1 alpha expression. J Immunol 150:2554–2562. [PubMed] [Google Scholar]
- 71.Wang HB, Weller PF. 2008. Pivotal advance: eosinophils mediate early alum adjuvant-elicited B cell priming and IgM production. J Leukoc Biol 83:817–821. doi: 10.1189/jlb.0607392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rankin JA, Harris P, Ackerman SJ. 1992. The effects of eosinophil-granule major basic protein on lung-macrophage superoxide anion generation. J Allergy Clin Immunol 89:746–752. doi: 10.1016/0091-6749(92)90383-d. [DOI] [PubMed] [Google Scholar]
- 73.Akporiaye ET, Baca OG. 1983. Superoxide anion production and superoxide dismutase and catalase activities in Coxiella burnetii. J Bacteriol 154:520–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Akporiaye ET, Stefanovich D, Tsosie V, Baca G. 1990. Coxiella burnetii fails to stimulate human neutrophil superoxide anion production. Acta Virol 34:64–70. [PubMed] [Google Scholar]
- 75.Baca OG, Roman MJ, Glew RH, Christner RF, Buhler JE, Aragon AS. 1993. Acid phosphatase activity in Coxiella burnetii: a possible virulence factor. Infect Immun 61:4232–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hill J, Samuel JE. 2011. Coxiella burnetii acid phosphatase inhibits the release of reactive oxygen intermediates in polymorphonuclear leukocytes. Infect Immun 79:414–420. doi: 10.1128/IAI.01011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF. 1998. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis 177:1458–1464. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- 78.Rosenberg HF, Domachowske JB. 2001. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol 70:691–698. [PubMed] [Google Scholar]
- 79.Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, Foster PS, Matthaei KI. 2007. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood 110:1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 80.Davoine F, Cao M, Wu Y, Ajamian F, Ilarraza R, Kokaji AI, Moqbel R, Adamko DJ. 2008. Virus-induced eosinophil mediator release requires antigen-presenting and CD4+ T cells. J Allergy Clin Immunol 122:69–77.e2. doi: 10.1016/j.jaci.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 81.Percopo CM, Dyer KD, Ochkur SI, Luo JL, Fischer ER, Lee JJ, Lee NA, Domachowske JB, Rosenberg HF. 2014. Activated mouse eosinophils protect against lethal respiratory virus infection. Blood 123:743–752. doi: 10.1182/blood-2013-05-502443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Flores-Torres AS, Salinas-Carmona MC, Salinas E, Rosas-Taraco AG. 2019. Eosinophils and respiratory viruses. Viral Immunol 32:198–207. doi: 10.1089/vim.2018.0150. [DOI] [PubMed] [Google Scholar]
- 83.Borelli V, Vita F, Shankar S, Soranzo MR, Banfi E, Scialino G, Brochetta C, Zabucchi G. 2003. Human eosinophil peroxidase induces surface alteration, killing, and lysis of Mycobacterium tuberculosis. Infect Immun 71:605–613. doi: 10.1128/iai.71.2.605-613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Driss V, Legrand F, Hermann E, Loiseau S, Guerardel Y, Kremer L, Adam E, Woerly G, Dombrowicz D, Capron M. 2009. TLR2-dependent eosinophil interactions with mycobacteria: role of alpha-defensins. Blood 113:3235–3244. doi: 10.1182/blood-2008-07-166595. [DOI] [PubMed] [Google Scholar]
- 85.Shi HZ, Humbles A, Gerard C, Jin Z, Weller PF. 2000. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest 105:945–953. doi: 10.1172/JCI8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spencer LA, Szela CT, Perez SA, Kirchhoffer CL, Neves JS, Radke AL, Weller PF. 2009. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol 85:117–123. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH. 2002. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med 195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wong TW, Doyle AD, Lee JJ, Jelinek DF. 2014. Eosinophils regulate peripheral B cell numbers in both mice and humans. J Immunol 192:3548–3558. doi: 10.4049/jimmunol.1302241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chu VT, Frohlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, Lee JJ, Lohning M, Berek C. 2011. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol 12:151–159. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 90.Chu VT, Berek C. 2012. Immunization induces activation of bone marrow eosinophils required for plasma cell survival. Eur J Immunol 42:130–137. doi: 10.1002/eji.201141953. [DOI] [PubMed] [Google Scholar]
- 91.Chu VT, Berek C. 2013. The establishment of the plasma cell survival niche in the bone marrow. Immunol Rev 251:177–188. doi: 10.1111/imr.12011. [DOI] [PubMed] [Google Scholar]
- 92.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. 2000. Immunobiology of dendritic cells. Annu Rev Immunol 18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 93.Mosser DM. 2003. The many faces of macrophage activation. J Leukoc Biol 73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 94.Igietseme JU, Eko FO, He Q, Black CM. 2004. Antibody regulation of T-cell immunity: implications for vaccine strategies against intracellular pathogens. Expert Rev Vaccines 3:23–34. doi: 10.1586/14760584.3.1.23. [DOI] [PubMed] [Google Scholar]
- 95.Tangye SG, Hodgkin PD. 2004. Divide and conquer: the importance of cell division in regulating B-cell responses. Immunology 112:509–520. doi: 10.1111/j.1365-2567.2004.01950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schroder K, Hertzog PJ, Ravasi T, Hume DA. 2004. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 97.Turco J, Thompson HA, Winkler HH. 1984. Interferon-gamma inhibits growth of Coxiella burnetii in mouse fibroblasts. Infect Immun 45:781–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dellacasagrande J, Capo C, Raoult D, Mege JL. 1999. IFN-gamma-mediated control of Coxiella burnetii survival in monocytes: the role of cell apoptosis and TNF. J Immunol 162:2259–2265. [PubMed] [Google Scholar]
- 99.Howe D, Barrows LF, Lindstrom NM, Heinzen RA. 2002. Nitric oxide inhibits Coxiella burnetii replication and parasitophorous vacuole maturation. Infect Immun 70:5140–5147. doi: 10.1128/iai.70.9.5140-5147.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shannon JG, Heinzen RA. 2009. Adaptive immunity to the obligate intracellular pathogen Coxiella burnetii. Immunol Res 43:138–148. doi: 10.1007/s12026-008-8059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Capo C, Mege JL. 2012. Role of innate and adaptive immunity in the control of Q fever. Adv Exp Med Biol 984:273–286. doi: 10.1007/978-94-007-4315-1_14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.