Cutaneous leishmaniasis is characterized by vascular remodeling. Following infection with Leishmania parasites, the vascular endothelial growth factor A (VEGF-A)/VEGF receptor 2 (VEGFR-2) signaling pathway mediates lymphangiogenesis, which is critical for lesion resolution. Therefore, we investigated the cellular and molecular mediators involved in VEGF-A/VEGFR-2 signaling using a murine model of infection.
KEYWORDS: HIF-1α, Leishmania, VEGF-A, lymphangiogenesis, vascular remodeling
ABSTRACT
Cutaneous leishmaniasis is characterized by vascular remodeling. Following infection with Leishmania parasites, the vascular endothelial growth factor A (VEGF-A)/VEGF receptor 2 (VEGFR-2) signaling pathway mediates lymphangiogenesis, which is critical for lesion resolution. Therefore, we investigated the cellular and molecular mediators involved in VEGF-A/VEGFR-2 signaling using a murine model of infection. We found that macrophages are the predominant cell type expressing VEGF-A during Leishmania major infection. Given that Leishmania parasites activate hypoxia-inducible factor 1α (HIF-1α) and this transcription factor can drive VEGF-A expression, we analyzed the expression of HIF-1α during infection. We showed that macrophages were also the major cell type expressing HIF-1α during infection and that infection-induced VEGF-A production is mediated by ARNT/HIF activation. Furthermore, mice deficient in myeloid ARNT/HIF signaling exhibited larger lesions without differences in parasite numbers. These data show that L. major infection induces macrophage VEGF-A production in an ARNT/HIF-dependent manner and suggest that ARNT/HIF signaling may limit inflammation by promoting VEGF-A production and, thus, lymphangiogenesis during infection.
INTRODUCTION
Cutaneous leishmaniasis results from infection with the protozoan parasite Leishmania. Most dermal lesions resolve following parasite control by the Th1 immune response. However, the disease persists in some patients despite low parasite numbers, which is especially evident for patients suffering from mucocutaneous leishmaniasis due to infections caused by the Leishmania Viannia subgenus (1, 2). Additionally, patients and mouse models with the most severe disease display heightened proinflammatory cytokine responses, suggesting that the persistent inflammatory microenvironment can maintain the disease state in the absence of high parasite numbers (3–8). As a result, the parasite, as well as the inflammatory milieu, can promote pathology during infection. Thus, blockade of the pathways promoting the inflammatory microenvironment or enhancement of the pathways improving lesion resolution may provide novel therapies to alleviate the disease in patients.
Vascular remodeling is a feature of inflammatory microenvironments, and we and others have previously shown that vascular remodeling, including angiogenesis and lymphangiogenesis, occurs in both visceral and cutaneous leishmaniasis (9–13). Furthermore, vascular endothelial growth factor A (VEGF-A) and its corresponding receptor, VEGFR-2, are elevated in the skin of humans and mice infected with Leishmania parasites (11, 14, 15). Importantly, blockade of VEGFR-2 signaling during L. major infection leads to inhibition of lymphangiogenesis and larger lesions without impaired parasite control (11). Given that expansion of the lymphatic network promotes lesion resolution and inhibition of this process enhances disease, we wanted to determine the factors inducing VEGF-A/VEGFR-2 signaling during Leishmania infection.
The VEGF-A/VEGFR-2 signaling pathway stimulates vascular remodeling and has been associated with a variety of inflammatory diseases. VEGF-A/VEGFR-2 signaling promotes angiogenesis, as well as lymphangiogenesis, and this pathway is active in tumors and other pathological diseases, such as psoriasis, macular degeneration, rheumatoid arthritis, and infection (16–20). In these inflammatory settings, the massive immune infiltrate and the cellular demand for resources, including oxygen to carry out effector functions, result in a hypoxic environment. The transcription factor hypoxia-inducible factor 1α (HIF-1α) senses cellular stress, including low oxygen tensions in the tissue, leading to the transcription of target genes, including VEGF-A, that are involved in glucose metabolism, cell survival, and vascular remodeling. In addition to hypoxia, proinflammatory cytokines and Toll-like receptor (TLR) signaling can also activate HIF-1α (21). Similarly to VEGF-A during Leishmania infection, HIF-1α is expressed in the skin (11, 14, 22, 23). The site of Leishmania infection is hypoxic, which may lead to HIF-1α activation (14, 24). Alternatively, Leishmania major, Leishmania amazonensis, and Leishmania donovani can directly induce HIF activation, and this response is amplified in the presence of proinflammatory stimuli (22, 25–27). Since HIF-1α activation is associated with Leishmania infection, we hypothesize that HIF-1α mediates the expression of VEGF-A, which induces vascular remodeling at the site of infection. However, the cells producing VEGF-A at the site of infection and the molecular pathways leading to VEGF-A expression have not been examined.
In this study, we examined the cells and mediators contributing to VEGF-A expression and vascular remodeling in Leishmania-infected tissues. We found that VEGF-A expression mirrors lesion development and that macrophages are the major cell type expressing VEGF-A in infected skin during L. major infection. In leishmanial lesions, macrophages are also the predominant cell type expressing HIF-1α, with infected macrophages displaying the highest levels of HIF-1α expression. Furthermore, mice with impaired myeloid ARNT/HIF signaling also showed decreased VEGF-A expression by macrophages and a heightened inflammatory response with larger lesions. Taken together, these data suggest that L. major infection induces HIF-1α activation, leading to the production of VEGF-A by macrophages and vascular remodeling, which limits inflammation at the site of infection.
RESULTS
Given that myeloid cells produce VEGF-A in cancer and inflammation and these cells are also the major cell type infected by Leishmania parasites, we hypothesize that macrophages produce VEGF-A during L. major infection, which contributes to vascular remodeling. In order to determine the cell type expressing VEGF-A following L. major infection, C57BL/6 mice were inoculated with parasites in the dermis and lesion progression was monitored over time (Fig. 1A). After lesions were established, nonhematopoietic and immune cell populations from infected ears were sorted by fluorescence-activated cell sorting (FACS). Ears from naive mice were used as controls. Dermal cells were sorted into CD45− nonhematopoietic cells (mainly consisting of fibroblasts, endothelial cells, and keratinocytes), CD45+ CD11b− CD64− cells (including T cells, B cells, NK cells, and dendritic cells [DCs]), CD45+ CD11b+ CD64− cells (consisting of monocytes, neutrophils, and CD11b+ dermal DCs), or CD45+ CD11b+ CD64+ macrophages. As previously published, our results confirmed that infected ears displayed increased levels of VEGF-A transcript compared to the levels in naive ears (Fig. 1B). Further analysis revealed that CD45+ CD11b+ CD64+ macrophages expressed the highest levels of VEGF-A transcript following infection (Fig. 1B). In order to determine if parasites contribute to VEGF-A expression, mice were infected with parasites fluorescently labeled with DsRed, which allows the separation of infected and uninfected macrophages. After 4 weeks of infection, cell populations from naive and infected ears were sorted. Cells were sorted as follows: CD45− nonhematopoietic cells, CD45+ CD11b+/− CD64− hematopoietic nonmacrophage cells, CD45+ CD11b+ CD64+ infected (DsRed-positive [DsRed+]) macrophages, or CD45+ CD11b+ CD64+ uninfected (DsRed-negative [DsRed−]) macrophages. Real-time PCR analysis on sorted populations showed that both infected and uninfected macrophages express VEGF-A (Fig. 1C). Taken together, these results suggest that macrophages are the predominant cell type responsible for VEGF-A expression during Leishmania infection and that these cells may promote vascular remodeling in the tissue.
FIG 1.
Macrophages express VEGF-A during L. major infection in vivo. (A) C57BL/6 mice were infected with 2 × 106 L. major metacyclic parasites intradermally in the ear, and lesion development was monitored over time. Data shown are pooled from three experiments. SEM, standard error of the mean. (B) At 7 weeks postinfection (p.i.), infected ears were digested. Total cells were stained, and cell populations were sorted by fluorescence-activated cell sorting (FACS) for CD45− cells (fibroblasts, ECs, and keratinocytes), CD45+ CD11b− CD64− cells (T cells, B cells, NK cells, and DCs), CD45+ CD11b+ CD64− cells (monocytes [Monos], neutrophils, and DCs), or CD45+ CD11b+ CD64+ cells (macrophages). PMNs, polymorphonuclear leukocytes. (C) Mice were infected with DsRed-labeled fluorescent L. major parasites in the ear dermis. At 4 weeks p.i., infected ears were digested, and total live cells were stained for sorting by FACS. Cell populations were sorted as follows: CD45− cells (fibroblasts, ECs, and keratinocytes), CD45+ CD11b+/− CD64− cells (all hematopoietic nonmacrophage cells, including T cells, B cells, NK cells, DCs, monocytes, and neutrophils), CD45+ CD11b+ CD64+ DsRed+ cells (infected macrophages), or CD45+ CD11b+ CD64+ DsRed− cells (uninfected macrophages). (B and C) Following sorting, quantitative real-time PCR was performed for the expression of VEGF-A on each population. Relative mRNA expression normalized to that of the RPS11 housekeeping gene is presented as the mean value + SEM. Results shown here are from one representative experiment of two individual experiments that included 4 to 6 mice per group. *, P < 0.05, and ***, P < 0.001, by Tukey’s test for multiple comparisons.
Given that VEGF-A was increased following infection and that HIF-1α can drive VEGF-A production (11, 28), the expression of HIF-1α was examined. Confirming our previous findings, infected mice exhibited elevated HIF-1α expression compared to the expression in naive controls (11). Furthermore, the expression of VEGF-A and HIF-1α increased as lesions developed, and the kinetics of HIF-1α expression mirrored that of VEGF-A over the course of infection (Fig. 2). HIF-1α expression by nonhematopoietic and immune cell populations was subsequently determined after infection using the same strategy as described in the legend to Fig. 1B. Consistent with the VEGF-A expression (Fig. 1B), HIF-1α was predominantly expressed in the macrophage population following L. major infection (Fig. 3A). More in-depth analysis revealed that both infected and uninfected macrophages expressed HIF-1α following infection with DsRed parasites, and the highest level of expression was detected in DsRed+ parasite-infected macrophages compared to its expression in the other nonhematopoietic and immune cell populations in the lesions (Fig. 3B). Taken together, these results suggest that macrophages are the major cell type expressing HIF-1α during Leishmania infection and, thus, may be the factor responsible for increased VEGF-A production in vivo.
FIG 2.
HIF-1α and VEGF-A display similar kinetics of expression following L. major infection. C57BL/6 mice were infected with L. major parasites in the ear dermis, and ears were analyzed by quantitative real-time PCR at 3, 7, 14, and 35 days p.i. for the expression of HIF-1α (A) and VEGF-A (B). Relative mRNA expression normalized to that of the RPS11 housekeeping gene is presented as the mean value + SEM for 5 mice per group from one experiment. *, P < 0.05, **, P < 0.01, and ***, P < 0.001, by Dunnett’s test for multiple comparisons.
FIG 3.
Macrophages express HIF-1α during L. major infection in vivo. (A) C57BL/6 mice were infected with L. major parasites in the ear dermis. At 7 weeks p.i., infected ears were digested, and total cells were stained. Cell populations were sorted by FACS for CD45− cells (fibroblasts, ECs, and keratinocytes), CD45+ CD11b− CD64− cells (T cells, B cells, NK cells, and DCs), CD45+ CD11b+ CD64− cells (monocytes, neutrophils, and DCs), or CD45+ CD11b+ CD64+ cells (macrophages). (B) Mice were infected with fluorescently labeled DsRed L. major parasites. After 4 weeks of infection, cells were sorted as follows: CD45− cells (fibroblasts, ECs, and keratinocytes), CD45+ CD11b+/− CD64− cells (all hematopoietic nonmacrophage cells, including T cells, B cells, NK cells, DCs, monocytes, and neutrophils), CD45+ CD11b+ CD64+ DsRed+ cells (infected macrophages), or CD45+ CD11b+ CD64+ DsRed− cells (uninfected macrophages). Following sorting, quantitative real-time PCR was performed for HIF-1α expression on the individual populations. Relative mRNA expression normalized to that of the RPS11 housekeeping gene is presented as the mean value + SEM for 4 to 6 mice per group from two experiments. ***, P < 0.001, by Tukey’s test for multiple comparisons.
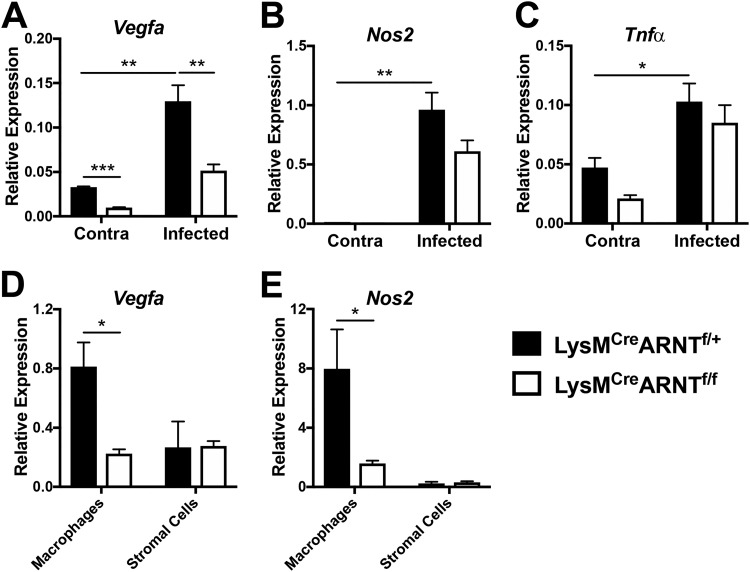
Since Leishmania infection can induce HIF activation and VEGF-A is a transcriptional target of HIF-1α (22, 25–27), we hypothesized that infection-induced VEGF-A production by macrophages is mediated by HIF signaling. To determine the mechanistic basis of infection-induced VEGF-A production, mice exhibiting ARNT deficiency in a myeloid-specific manner were infected with L. major parasites. ARNT (also known as HIF-1β) is the constitutive binding partner of the HIF-1α and HIF-2α subunits. ARNT/HIF-α dimerization and nuclear translocation leads to the transcription of HIF target genes; therefore, ARNT deficiency is a strategy to study pan-HIF inhibition in vivo (29). Following L. major infection, LysMCre ARNTflox/+ (here called LysMCre ARNTf/+) mice display increased VEGF-A expression in infected ears compared to its expression in uninfected contralateral ears (Fig. 4A). In addition, LysMCre ARNTflox/flox (here called LysMCre ARNTf/f) mice, which exhibit impaired HIF signaling in myeloid cells (29–32), showed decreased expression of VEGF-A at the site of infection compared to its expression in LysMCre ARNTf/+ control mice (Fig. 4A). Defective myeloid HIF signaling also reduced basal VEGF-A expression in the skin in the contralateral ears (Fig. 4A). Given that NOS2 is a known target of HIF-1α during L. major infection (22), the expression of NOS2 following infection was examined in the presence or absence of myeloid ARNT/HIF signaling. We found that LysMCre ARNTf/+ mice showed increased NOS2 expression with infection compared to the level in contralateral ears (Fig. 4B), similar to the results for wild-type C57BL/6 mice (33). Infected LysMCre ARNTf/f mice trended toward a lower expression of NOS2 than LysMCre ARNTf/+ mice, but this finding was not significant. Given that differences in expression levels can be masked when analyzing whole tissue, macrophages and stromal skin cells from infected ears were differentiated using FACS, and the expression of VEGF-A and NOS2 on the individual cell populations was examined. Following infection, CD45+ CD11b+ CD64+ macrophages sorted from the site of infection of LysMCre ARNTf/+ mice had higher expression of VEGF-A than CD45− stromal cells (Fig. 4D), confirming our findings shown in Fig. 1B. However, dermal macrophages sorted from infected LysMCre ARNTf/f mice had lower expression of VEGF-A than macrophages sorted from infected LysMCre ARNTf/+ controls (Fig. 4D). Along similar lines, macrophages sorted from infected LysMCre ARNTf/f mice also had lower levels of NOS2 than did controls (Fig. 4E), confirming elegant work by Schatz et al. showing that NOS2 is a target of myeloid HIF signaling during L. major infection (22). Since tumor necrosis factor alpha (TNF-α) expression by macrophages is not affected by HIF deficiency (34–36) and TNF-α is elevated during L. major infection, we analyzed TNF-α expression to examine the specificity of ARNT/HIF signaling using this model. As expected, we observed that TNF-α expression was enhanced with infection; however, there was no difference in TNF-α expression at the site of infection between LysMCre ARNTf/+ and LysMCre ARNTf/f mice (Fig. 4C). In summary, these data demonstrate that macrophage VEGF-A production during L. major infection is dependent on ARNT/HIF signaling in vivo.
FIG 4.
Macrophage VEGF-A expression is dependent on ARNT/HIF signaling during L. major infection in vivo. LysMCre ARNTf/f or LysMCre ARNTf/+ mice were infected with L. major parasites in the ear dermis. (A to C) Total ears were analyzed by quantitative real-time PCR at 4 weeks p.i. for the expression of VEGF-A (A), NOS2 (B), and TNF-α (C) genes. Data shown here are from one experiment and are representative of at least two independent experiments with 2 to 6 mice per group. (D, E) CD45+ CD11b+ Ly6G− CD64+ macrophages and CD45− stromal cells were sorted by FACS from infected and uninfected ear skin, and the expression of VEGF-A (D) and NOS2 (E) genes was analyzed for each cell population at 3 weeks p.i. by quantitative real-time PCR. Data shown here are from one experiment with 3 to 5 mice per group. Relative mRNA expression normalized to that of the RPS11 housekeeping gene is presented as the mean value + SEM. ***, P < 0.005, **, P < 0.01, and *, P < 0.05, by t test comparing the results for LysMCre ARNTf/f mice to those for LysMCre ARNTf/+ controls or comparing the results for infected ears to those for contralateral control ears.
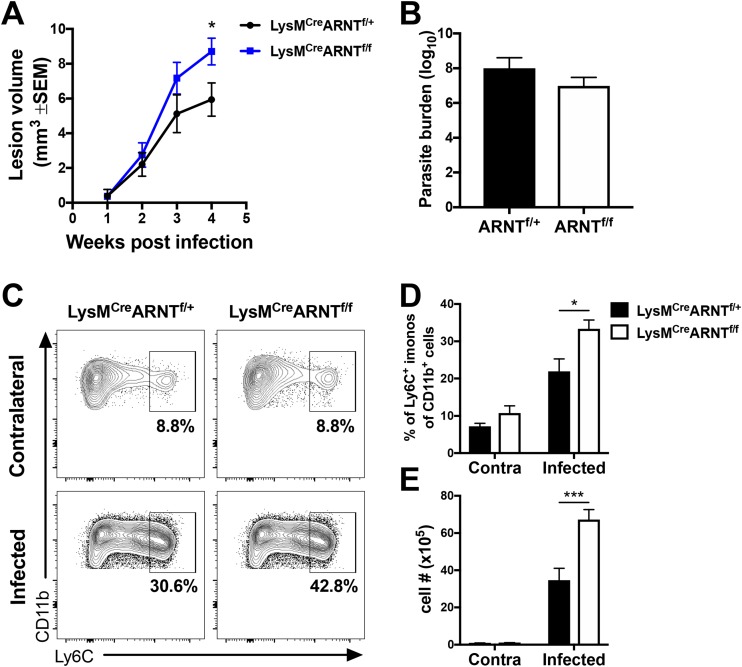
Given that infected mice with defective myeloid ARNT/HIF signaling exhibit decreased VEGF-A expression and VEGF-A/VEGFR-2 signaling contributes to lesion resolution, we examined the role of myeloid ARNT/HIF signaling in disease pathology. We found that LysMCre ARNTf/f mice developed significantly larger lesions than LysMCre ARNTf/+ control animals following L. major infection (Fig. 5A). Furthermore, the larger lesions in the LysMCre ARNTf/f mice were not associated with increased parasite burdens, as there was no difference in the numbers of parasites between infected LysMCre ARNTf/+ and LysMCre ARNTf/f mice (Fig. 5B). Alternatively, infected LysMCre ARNTf/f mice showed enhanced immunopathology with increased percentages of Ly6C+ inflammatory monocytes compared to the percentages in LysMCre ARNTf/+ control mice at the site of infection (Fig. 5C and D). Similarly, LysMCre ARNTf/f mice displayed higher numbers of Ly6C+ inflammatory monocytes in the infected ears than did LysMCre ARNTf/+ mice (Fig. 5E). Of note, prior to infection, there was no difference between the percentages or numbers of Ly6C+ inflammatory monocytes in the skin for LysMCre ARNTf/f and LysMCre ARNTf/+ mice, and the percentages and numbers of these cells increased with infection in both groups. Altogether, these data suggest that myeloid VEGF-A production mediated by ARNT/HIF signaling limits immunopathology and contributes to lesion resolution during L. major infection.
FIG 5.
Myeloid ARNT/HIF signaling limits inflammation during L. major infection in vivo. LysMCre ARNTf/f or LysMCre ARNTf/+ mice were infected with L. major parasites in the ear dermis. (A) Lesion volume was monitored over time. Data shown here are pooled from three independent experiments with totals of 17 to 23 mice per group. (B) The number of parasites in the ear was quantified at 5 weeks p.i. by limiting dilution assay (LDA). Data shown here are from one experiment and representative of two independent experiments with 5 to 7 mice per group. (C) Representative flow cytometry plots showing the percentages of Ly6C+ inflammatory monocytes after gating on total, live, singlet, and CD45+ CD11b+ Ly6G− events. (D, E) Quantification of the percentages (D) and numbers (E) of Ly6C+ cells of the CD45+ CD11b+ Ly6G− myeloid population at 5 to 6 weeks p.i. from the skin of infected and contralateral ears. Data shown here are pooled from two independent experiments with totals of 2 or 3 contralateral ears and 10 or 11 infected ears per group. Data are presented as the mean value + SEM. ***, P < 0.005, and *, P < 0.05, by t test comparing the results for LysMCre ARNTf/f mice to those for LysMCre ARNTf/+ controls.
DISCUSSION
Vascular remodeling occurs during Leishmania infection and influences the pathogenesis of disease (9, 11, 12). During infection, a majority of lymphatic vessels are dilated and lymphatic endothelial cells (ECs) exhibit enhanced proliferation, suggesting that lymphangiogenesis is occurring at the site of infection (11). Previously, we demonstrated that the VEGF-A/VEGFR-2 pathway drives lymphangiogenesis, which serves as a protective mechanism that restricts the inflammatory response and promotes lesion resolution (11). However, the cellular and molecular mechanisms leading to lymphangiogenesis remain unknown. In this article, we show that macrophages are the predominant cell type expressing VEGF-A in leishmanial lesions. Given that the transcription factor HIF-1α drives VEGF-A expression in a variety of other inflammatory diseases, we wanted to determine whether VEGF-A production is mediated by HIF-1α. We found that macrophages from infected mice were also the predominant cell type expressing HIF-1α, with HIF-1α and VEGF-A displaying similar kinetics, thus strengthening the link between these two molecules during L. major infection. These findings are relevant to human leishmaniasis because human lesions also express VEGF-A and HIF-1α, along with dilated vessels, corroborating our observations in the mouse model (15, 22). In addition, we show that mice with deficient ARNT/HIF signaling in myeloid cells exhibit decreased VEGF-A production and enhanced immunopathology during L. major infection. In conclusion, these data suggest that the ARNT/HIF-1α/VEGF-A/VEGFR-2 pathway in macrophages mediates lymphangiogenesis during L. major infection to restrict tissue inflammation.
Many cell types can express VEGF-A during inflammation. In tumors, macrophages, stromal cells, and tumor cells are the major producers of VEGF-A. During wound healing, fibroblasts and epidermal cells are the major producers of VEGF-A (37, 38). In the draining lymph node (dLN) during inflammation, B cells produce VEGF-A that mediates vascular remodeling (39, 40) and T cells can produce VEGF-A when activated (41). During L. major infection, we can detect all of these events, including aspects of the wound healing process, as well as dLN vascular remodeling and T cell activation. In our model of dermal Leishmania infection, macrophages were the major VEGF-A-expressing cell type in vivo. These results are consistent with granuloma macrophages being the major producers of VEGF-A during mycobacterial infection (42). We did not find elevated VEGF-A expression in the CD45− cell fraction (which would contain fibroblasts), suggesting that fibroblasts and epidermal cells are not the dominant VEGF-A-producing cell type during infection, despite the ongoing wound healing response following L. major infection. In conclusion, these results suggest that myeloid cells promote vascular remodeling during Leishmania infection. These findings are consistent with a previous report showing that depletion of myeloid cells in Matrigel implants containing Leishmania parasites decreased the number of LYVE-1+ lymphatic vessels in vivo (10). These investigators elegantly demonstrated that CEACAM1+ myeloid cells contribute to angiogenesis and lymphangiogenesis following infection (10).
VEGF-A expression, mediated by HIF signaling, is elevated in multiple inflammatory settings and infections. During infection, a variety of signals, including hypoxia, TLR stimulation, and the cytokine milieu in the microenvironment, activate HIF, leading to VEGF-A production (21). Given that many of these factors are associated with Leishmania infection, we speculate that multiple factors contribute to HIF-mediated VEGF-A production in vivo. Our results show that both infected and uninfected bystander macrophages express elevated levels of HIF-1α and VEGF-A. These data suggest that factors from the tissue microenvironment influence macrophage HIF-1α/VEGF-A signaling. During L. major infection, the skin microenvironment is hypoxic (24), but it is not clear if hypoxia is responsible for HIF-1α/VEGF-A activation in vivo. HIF-1α activation leads to the transcription of target genes like the NOS2 gene following L. major infection (22); however, other HIF target genes beyond NOS2 have not been investigated. Additionally, cytokines like TNF-α and interleukin-1β (IL-1β) are elevated at the site of infection in myeloid cells (43). These cytokines also induce HIF-1α/VEGF-A activation (44, 45) and may play a role in HIF-1α signaling following L. major infection. Given that L. major induces reactive oxygen species (ROS) production and ROS activates HIF under normoxic conditions, ROS may also be contributing to the HIF-mediated VEGF-A production in vivo (46). Since HIF activation occurs in response to hypoxia, cytokines, and ROS and all of these factors are present during Leishmania infection (24, 46), future experiments are required to dissect how hypoxia, the cytokine milieu, and ROS contribute to ARNT/HIF activation and subsequent VEGF-A production during leishmaniasis.
In addition to the tissue microenvironment, it has also been reported that microorganisms can directly induce VEGF-A production using HIF-dependent and HIF-independent mechanisms. Similarly to Leishmania infection in the skin, herpes simplex virus 1 (HSV-1) infection in the cornea drives inflammatory lymphangiogenesis in a VEGF-A/VEGFR-2-dependent manner (47). However, viral transcripts directly induce VEGF-A production in epithelial cells (47). Furthermore, Bartonella henselae, the bacterium responsible for cat scratch disease and bacillary angiomatosis, induces HIF-mediated VEGF-A production from myeloid cells and ECs (17, 48–52). In turn, VEGF-A-stimulated ECs also potentiate infection by promoting the growth of B. henselae (49). Similarly to Bartonella, the bacterium Clostridium difficile induces HIF-dependent VEGF-A production and vascular permeability to promote disease pathogenesis (53). Mycobacterium tuberculosis infection also induces VEGF-A production by macrophages, and the serum level of VEGF-A is elevated in tuberculosis patients (42, 54). Importantly, VEGF-A production during Mycobacterium marinum infection in zebrafish promotes bacterial growth and dissemination (55). Given the results of these studies, experiments are ongoing in the laboratory to determine if VEGF-A promotes Leishmania growth or infection in macrophages and if the parasites play a direct role in VEGF-A production. We are addressing roles for both promastigotes and amastigotes in VEGF-A production, given that the peak VEGF-A expression occurs when amastigotes are the predominant parasite stage. Furthermore, even though both mycobacterial granulomas and Leishmania lesions are hypoxic and display HIF-1α/VEGF-A activation and angiogenesis (55, 56), further work is needed to determine if VEGF-A-induced vascular remodeling requires HIF-1α during these infections.
Our findings show that myeloid ARNT/HIF signaling promotes the VEGF-A/VEGFR-2 pathway in vivo during L. major infection. Here, we found that mice deficient in myeloid ARNT/HIF signaling exhibit more severe disease without differences in parasite burdens. These data are consistent with our previous results showing that VEGF-A/VEGFR-2 blockade during L. major infection enhances pathology with no differences in parasite numbers (11). Our finding that myeloid ARNT/HIF signaling promotes disease resolution is also consistent with the disease outcome of mice deficient in myeloid HIF-1α signaling following L. major infection (22). However, our results do not replicate the increased parasite burdens seen in LysMCre HIF-1αf/f mice (22), suggesting that the transcription of other HIF targets may contribute to parasite control in our model. The observation that myeloid ARNT/HIF signaling promotes disease resolution during leishmaniasis is in line with previous reports showing that myeloid ARNT/HIF signaling encourages the resolution of inflammation in colitis and wound healing (29, 36). In both leishmaniasis and colitis, the absence of myeloid ARNT/HIF activation was associated with the increased presence of Ly6C+ inflammatory monocytes. However, these findings are in contrast to a previous study showing that defective myeloid ARNT/HIF signaling reduces cutaneous inflammation due to a chemical irritant (36). Taken together, these results suggest that the role of myeloid ARNT/HIF signaling is context dependent. In addition, our work, coupled with studies by Schatz et al. in myeloid cells, also suggests that HIF signaling in different cell types can lead to different disease outcomes in leishmaniasis (22). For example, similar to studies using LysMCre HIF-1αf/f mice, we found that myeloid ARNT/HIF signaling promotes disease resolution. In contrast, Hammami et al. showed that HIF-1α signaling in CD11c+ dendritic cells hampers DC functions and the development of a protective immune response during L. donovani infection (57, 58). A caveat to our in vivo studies is that mice deficient in myeloid HIF/ARNT activation will be defective in both HIF-1α and HIF-2α signaling. Taking into consideration the current evidence, we speculate that HIF-1α is the major HIF isoform driving resolution during leishmaniasis. Here, we showed that HIF/ARNT activation leads to expression of VEGF-A and NOS2 by macrophages. VEGF-A can result from HIF-1α or HIF-2α signaling, but NOS2 is preferentially affected by HIF-1α (30). Furthermore, HIF-2α expression was not significantly elevated at the peak of infection in the skin and the HIF-2α expression kinetics did not mirror that of VEGF-A (data not shown). Following infection, we did not detect a difference in the expression of Arg1, an HIF-2α-specific gene target (30), between LysMCre ARNTf/f and control mice, suggesting that HIF/ARNT/VEGF-A signaling is primarily associated with HIF-1α activation (data not shown). Finally, leishmanial lesions exhibit high levels of IFN-γ, which inhibits HIF-2α expression (30, 43), so the lack of HIF-2α expression may be due to regulation by IFN-γ. Regardless, these data do not refute a role for HIF-2α during infection, and a thorough analysis employing mice deficient in myeloid HIF-1α (LysMCre HIF-1αf/f) and HIF-2α (LysMCre HIF-2αf/f) signaling will be required to dissect out the roles of these individual pathways.
In summary, we showed that L. major infection activates the ARNT/HIF-1α/VEGF-A/VEGFR-2 signaling pathway in macrophages to promote lymphangiogenesis. Hypoxia in combination with proinflammatory cytokines has been reported to drive HIF-1α activation during L. major infection, leading to increased parasite control through nitric oxide production by macrophages (22). We hypothesize that, in addition to the role of HIF-1α in parasite killing, HIF-1α may also drive lymphangiogenesis through VEGF-A production during Leishmania infection. Thus, understanding and defining the cellular and molecular factors that (i) result from ARNT/HIF signaling and (ii) drive lymphangiogenesis during infection will have important implications in strategies to alleviate lesion resolution.
MATERIALS AND METHODS
Mice.
Female C57BL/6NCr mice were purchased from the National Cancer Institute. To achieve myeloid-specific Arnt conditional-knockout mice, mice with the floxed Arnt conditional allele were crossed with mice expressing the LysMCre allele as previously described (29, 59). The LysMCre ARNTf/f and LysMCre ARNTf/+ control mice were a gift from M. Celeste Simon (University of Pennsylvania, Philadelphia, PA). For in vivo experiments, all mice, including controls, were homozygous for the LysMCre allele. For infections, LysMCre ARNTf/f mice were infected alongside LysMCre ARNTf/+ controls. Mice were housed at the School of Veterinary Medicine at the University of Pennsylvania or the University of Arkansas for Medical Sciences under pathogen-free conditions. All procedures were performed in accordance with institutional guidelines approved by the Institutional Animal Care and Use Committees at the University of Pennsylvania and the University of Arkansas for Medical Sciences. Mice were used for experiments between 6 and 8 weeks of age.
Parasites.
Leishmania major (WHO/MHOM/IL/80/Friedlin) and L. major Friedlin strain parasites fluorescently labeled with DsRed were grown in vitro in Schneider’s Drosophila medium (Gibco) supplemented with 20% heat-inactivated fetal bovine serum (FBS; Invitrogen), 2 mM l-glutamine (Sigma), 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma). Ficoll density gradient separation (Sigma) was used to isolate metacyclic stationary-phase promastigotes from 4- to 5-day cultures (60).
In vivo infections.
For dermal ear infections, 2 × 106 parasites in 10 μl phosphate-buffered saline (PBS; Lonza) were injected intradermally into the ear. Following infection, lesion progression was monitored weekly, using a caliper to measure ear thickness and lesion area, and the lesion volume was calculated. To analyze cell populations in the tissue, ears were enzymatically digested in 0.25 mg/ml Liberase (Roche) with 10 μg/ml DNase I (Sigma) in incomplete RPMI 1640 medium (Life Technologies) for 90 min at 37°C. To determine parasite burdens in the tissue, limiting dilution assays were performed (61).
Flow cytometry and cell sorting.
For cell viability, cells were incubated with fixable aqua dye (Invitrogen). Fc receptors (FcRs) were blocked with 2.4G2 anti-mouse CD16/32 antibody (eBioscience or BioXCell) and 0.2% normal rat IgG (Sigma). Cells were surface stained using anti-CD45 AF700 and anti-Ly6C–peridinin chlorophyll protein (PerCP)-Cy5.5 antibodies (eBioscience), as well as anti-CD64–phycoerythrin (PE)-Cy7 and anti-CD11b–BV605 antibodies (BioLegend). For sorting, cell events were sorted on a FACSAria II flow cytometer (BD Biosciences). For flow cytometry, cell events were acquired on an LSRII Fortessa flow cytometer (BD Biosciences) and analyzed using FlowJo (Tree Star).
mRNA extraction and real-time PCR.
mRNA was extracted by using either the RNeasy plus minikit (Qiagen) or the EZNA total RNA kit I (Omega Bio-tek). RNA was reverse transcribed with high-capacity cDNA reverse transcription (Applied Biosystems). Quantitative real-time PCR was performed using SYBR green or PowerUp SYBR green PCR master mix on a ViiA 7 (Life Technologies) or QuantStudio 6 flex (Applied Biosystems) system. Mouse primer sequences were selected from the PrimerBank (http://pga.mgh.harvard.edu/primerbank/). Primer sequences are as follows: for Vegfa, forward, 5′-ATCTTCAAGCCGTCCTGTGT-3′, and reverse, 5′-GCATTCACATCTGCTGTGCT-3′; for Hif-1α, forward, 5′-TCCCCTCTCCTGTAAGCAAG-3′, and reverse, 5′-TCGACGTTCAGAACTCATCCT-3′; and for Rps11, forward, 5′-CGTGACGAAGATGAAGATGC-3′, and reverse, 5′-GCACATTGAATCGCACAGTC-3′. The results were normalized to those of the housekeeping gene encoding the ribosomal protein S14 (RPSII), using the comparative threshold cycle method (2−ΔΔCT) for relative quantification.
Statistics.
All data were statistically analyzed using GraphPad Prism 7. A P value of <0.05 was considered statistically significant. For a single comparison between groups, statistical significance was calculated using the two-tailed Student’s unpaired t test. For multiple-comparison analysis, statistical significance was determined by a one-way analysis of variance (ANOVA) followed by the post hoc Dunnett’s test (comparing to a single control group) or Tukey’s test (with no designated control group).
ACKNOWLEDGMENTS
We thank Ba Nguyen, Ciara Gimblet, and Nan Lin for their technical assistance, as well as M. Celeste Simon for her intellectual input and generosity in sharing experimental tools and mice. We also thank Lin-Xi Li and Jason Stumhofer for their critical reading of the manuscript and Andrea Harris of the UAMS flow cytometry core.
T.W. was supported by National Institutes of Health postdoctoral research grant number F32 AI 114080 and the University of Arkansas for Medical Sciences Center for Microbial Pathogenesis and Host Inflammatory Responses (funded by National Institutes of Health National Institute of General Medical Sciences Centers of Biomedical Research Excellence grant number P20-GM103625). This work was also supported by National Institutes of Health grant number R01 to P.S. (AI 106842). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Experiments were conceived by T.W. and P.S. and performed by H.R, A.B., and T.W. The paper was written by T.W. with revisions by P.S.
REFERENCES
- 1.Gutierrez Y, Salinas GH, Palma G, Valderrama LB, Santrich CV, Saravia NG. 1991. Correlation between histopathology, immune response, clinical presentation, and evolution in Leishmania braziliensis infection. Am J Trop Med Hyg 45:281–289. doi: 10.4269/ajtmh.1991.45.281. [DOI] [PubMed] [Google Scholar]
- 2.Barral A, Almeida RP, de Jesus AR, Medeiros Neto E, Santos IA, Johnson W Jr.. 1987. The relevance of characterizing Leishmania from cutaneous lesions. A simple approach for isolation. Mem Inst Oswaldo Cruz 82:579. doi: 10.1590/S0074-02761987000400018. [DOI] [PubMed] [Google Scholar]
- 3.Bacellar O, Lessa H, Schriefer A, Machado P, Ribeiro de Jesus A, Dutra WO, Gollob KJ, Carvalho EM. 2002. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun 70:6734–6740. doi: 10.1128/iai.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bittencourt AL, Barral A. 1991. Evaluation of the histopathological classifications of American cutaneous and mucocutaneous leishmaniasis. Mem Inst Oswaldo Cruz 86:51–56. doi: 10.1590/s0074-02761991000100009. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Lombana C, Gimblet C, Bacellar O, Oliveira WW, Passos S, Carvalho LP, Goldschmidt M, Carvalho EM, Scott P. 2013. IL-17 mediates immunopathology in the absence of IL-10 following Leishmania major infection. PLoS Pathog 9:e1003243. doi: 10.1371/journal.ppat.1003243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveira F, Bafica A, Rosato AB, Favali CB, Costa JM, Cafe V, Barral-Netto M, Barral A. 2011. Lesion size correlates with Leishmania antigen-stimulated TNF-levels in human cutaneous leishmaniasis. Am J Trop Med Hyg 85:70–73. doi: 10.4269/ajtmh.2011.10-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira LO, Moreira RB, de Oliveira MP, Reis SO, de Oliveira Neto MP, Pirmez C. 2017. Is Leishmania (Viannia) braziliensis parasite load associated with disease pathogenesis? Int J Infect Dis 57:132–137. doi: 10.1016/j.ijid.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 8.Terabe M, Kuramochi T, Ito M, Hatabu T, Sanjoba C, Chang KP, Onodera T, Matsumoto Y. 2000. CD4(+) cells are indispensable for ulcer development in murine cutaneous leishmaniasis. Infect Immun 68:4574–4577. doi: 10.1128/iai.68.8.4574-4577.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalton JE, Maroof A, Owens BM, Narang P, Johnson K, Brown N, Rosenquist L, Beattie L, Coles M, Kaye PM. 2010. Inhibition of receptor tyrosine kinases restores immunocompetence and improves immune-dependent chemotherapy against experimental leishmaniasis in mice. J Clin Invest 120:1204–1216. doi: 10.1172/JCI41281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horst AK, Bickert T, Brewig N, Ludewig P, van Rooijen N, Schumacher U, Beauchemin N, Ito WD, Fleischer B, Wagener C, Ritter U. 2009. CEACAM1+ myeloid cells control angiogenesis in inflammation. Blood 113:6726–6736. doi: 10.1182/blood-2008-10-184556. [DOI] [PubMed] [Google Scholar]
- 11.Weinkopff T, Konradt C, Christian DA, Discher DE, Hunter CA, Scott P. 2016. Leishmania major infection-induced VEGF-A/VEGFR-2 signaling promotes lymphangiogenesis that controls disease. J Immunol 197:1823–1831. doi: 10.4049/jimmunol.1600717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yurdakul P, Dalton J, Beattie L, Brown N, Erguven S, Maroof A, Kaye PM. 2011. Compartment-specific remodeling of splenic micro-architecture during experimental visceral leishmaniasis. Am J Pathol 179:23–29. doi: 10.1016/j.ajpath.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalton JE, Glover AC, Hoodless L, Lim EK, Beattie L, Kirby A, Kaye PM. 2015. The neurotrophic receptor Ntrk2 directs lymphoid tissue neovascularization during Leishmania donovani infection. PLoS Pathog 11:e1004681. doi: 10.1371/journal.ppat.1004681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araujo AP, Giorgio S. 2015. Immunohistochemical evidence of stress and inflammatory markers in mouse models of cutaneous leishmaniosis. Arch Dermatol Res 307:671–682. doi: 10.1007/s00403-015-1564-0. [DOI] [PubMed] [Google Scholar]
- 15.Fraga CA, Oliveira MV, Alves LR, Viana AG, Sousa AA, Carvalho SFG, De Paula AMB, Botelho AC, Guimarães ALS. 2012. Immunohistochemical profile of HIF-1alpha, VEGF-A, VEGFR2 and MMP9 proteins in tegumentary leishmaniasis. An Bras Dermatol 87:709–713. doi: 10.1590/s0365-05962012000500006. [DOI] [PubMed] [Google Scholar]
- 16.Ambati J, Atkinson JP, Gelfand BD. 2013. Immunology of age-related macular degeneration. Nat Rev Immunol 13:438–451. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dehio C. 2005. Bartonella-host-cell interactions and vascular tumour formation. Nat Rev Microbiol 3:621–631. doi: 10.1038/nrmicro1209. [DOI] [PubMed] [Google Scholar]
- 18.Kreuger J, Phillipson M. 2016. Targeting vascular and leukocyte communication in angiogenesis, inflammation and fibrosis. Nat Rev Drug Discov 15:125–142. doi: 10.1038/nrd.2015.2. [DOI] [PubMed] [Google Scholar]
- 19.McInnes IB, Schett G. 2007. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 20.Wagner EF, Schonthaler HB, Guinea-Viniegra J, Tschachler E. 2010. Psoriasis: what we have learned from mouse models. Nat Rev Rheumatol 6:704–714. doi: 10.1038/nrrheum.2010.157. [DOI] [PubMed] [Google Scholar]
- 21.Konisti S, Kiriakidis S, Paleolog EM. 2012. Hypoxia—a key regulator of angiogenesis and inflammation in rheumatoid arthritis. Nat Rev Rheumatol 8:153–162. doi: 10.1038/nrrheum.2011.205. [DOI] [PubMed] [Google Scholar]
- 22.Schatz V, Strussmann Y, Mahnke A, Schley G, Waldner M, Ritter U, Wild J, Willam C, Dehne N, Brune B, McNiff JM, Colegio OR, Bogdan C, Jantsch J. 2016. Myeloid cell-derived HIF-1alpha promotes control of Leishmania major. J Immunol 197:4034–4041. doi: 10.4049/jimmunol.1601080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werth N, Beerlage C, Rosenberger C, Yazdi AS, Edelmann M, Amr A, Bernhardt W, von Eiff C, Becker K, Schafer A, Peschel A, Kempf VA. 2010. Activation of hypoxia inducible factor 1 is a general phenomenon in infections with human pathogens. PLoS One 5:e11576. doi: 10.1371/journal.pone.0011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahnke A, Meier RJ, Schatz V, Hofmann J, Castiglione K, Schleicher U, Wolfbeis OS, Bogdan C, Jantsch J. 2014. Hypoxia in Leishmania major skin lesions impairs the NO-dependent leishmanicidal activity of macrophages. J Invest Dermatol 134:2339–2346. doi: 10.1038/jid.2014.121. [DOI] [PubMed] [Google Scholar]
- 25.Degrossoli A, Arrais-Silva WW, Colhone MC, Gadelha FR, Joazeiro PP, Giorgio S. 2011. The influence of low oxygen on macrophage response to Leishmania infection. Scand J Immunol 74:165–175. doi: 10.1111/j.1365-3083.2011.02566.x. [DOI] [PubMed] [Google Scholar]
- 26.Degrossoli A, Bosetto MC, Lima CB, Giorgio S. 2007. Expression of hypoxia-inducible factor 1alpha in mononuclear phagocytes infected with Leishmania amazonensis. Immunol Lett 114:119–125. doi: 10.1016/j.imlet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Singh AK, Mukhopadhyay C, Biswas S, Singh VK, Mukhopadhyay CK. 2012. Intracellular pathogen Leishmania donovani activates hypoxia inducible factor-1 by dual mechanism for survival advantage within macrophage. PLoS One 7:e38489. doi: 10.1371/journal.pone.0038489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor CT, Colgan SP. 2017. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol 17:774–785. doi: 10.1038/nri.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin N, Shay JES, Xie H, Lee DSM, Skuli N, Tang Q, Zhou Z, Azzam A, Meng H, Wang H, FitzGerald GA, Simon MC. 2018. Myeloid cell hypoxia-inducible factors promote resolution of inflammation in experimental colitis. Front Immunol 9:2565. doi: 10.3389/fimmu.2018.02565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KE, Simon MC. 2015. SnapShot: hypoxia-inducible factors. Cell 163:1288. doi: 10.1016/j.cell.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Lin N, Simon MC. 2016. Hypoxia-inducible factors: key regulators of myeloid cells during inflammation. J Clin Invest 126:3661–3671. doi: 10.1172/JCI84426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majmundar AJ, Wong WJ, Simon MC. 2010. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liese J, Schleicher U, Bogdan C. 2007. TLR9 signaling is essential for the innate NK cell response in murine cutaneous leishmaniasis. Eur J Immunol 37:3424–3434. doi: 10.1002/eji.200737182. [DOI] [PubMed] [Google Scholar]
- 34.Braverman J, Sogi KM, Benjamin D, Nomura DK, Stanley SA. 2016. HIF-1alpha is an essential mediator of IFN-gamma-dependent immunity to Mycobacterium tuberculosis. J Immunol 197:1287–1297. doi: 10.4049/jimmunol.1600266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B, Simon MC. 2010. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest 120:2699–2714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott C, Bonner J, Min D, Boughton P, Stokes R, Cha KM, Walters SN, Maslowski K, Sierro F, Grey ST, Twigg S, McLennan S, Gunton JE. 2014. Reduction of ARNT in myeloid cells causes immune suppression and delayed wound healing. Am J Physiol Cell Physiol 307:C349–C357. doi: 10.1152/ajpcell.00306.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, Seed B. 1998. Tumor induction of VEGF promoter activity in stromal cells. Cell 94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 38.Kishimoto J, Ehama R, Ge Y, Kobayashi T, Nishiyama T, Detmar M, Burgeson RE. 2000. In vivo detection of human vascular endothelial growth factor promoter activity in transgenic mouse skin. Am J Pathol 157:103–110. doi: 10.1016/S0002-9440(10)64522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, Randolph GJ. 2006. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity 24:203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Shrestha B, Hashiguchi T, Ito T, Miura N, Takenouchi K, Oyama Y, Kawahara K, Tancharoen S, Ki IY, Arimura N, Yoshinaga N, Noma S, Shrestha C, Nitanda T, Kitajima S, Arimura K, Sato M, Sakamoto T, Maruyama I. 2010. B cell-derived vascular endothelial growth factor A promotes lymphangiogenesis and high endothelial venule expansion in lymph nodes. J Immunol 184:4819–4826. doi: 10.4049/jimmunol.0903063. [DOI] [PubMed] [Google Scholar]
- 41.Palazon A, Tyrakis PA, Macias D, Velica P, Rundqvist H, Fitzpatrick S, Vojnovic N, Phan AT, Loman N, Hedenfalk I, Hatschek T, Lovrot J, Foukakis T, Goldrath AW, Bergh J, Johnson RS. 2017. An HIF-1alpha/VEGF-A axis in cytotoxic T cells regulates tumor progression. Cancer Cell 32:669–683.e5. doi: 10.1016/j.ccell.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harding JS, Herbath M, Chen Y, Rayasam A, Ritter A, Csoka B, Hasko G, Michael IP, Fabry Z, Nagy A, Sandor M. 2019. VEGF-A from granuloma macrophages regulates granulomatous inflammation by a non-angiogenic pathway during mycobacterial infection. Cell Rep 27:2119–2131.e6. doi: 10.1016/j.celrep.2019.04.072. [DOI] [PubMed] [Google Scholar]
- 43.Scott P, Novais FO. 2016. Cutaneous leishmaniasis: immune responses in protection and pathogenesis. Nat Rev Immunol 16:581–592. doi: 10.1038/nri.2016.72. [DOI] [PubMed] [Google Scholar]
- 44.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. 2003. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J 17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 45.Kuo HP, Lee DF, Xia W, Wei Y, Hung MC. 2009. TNFalpha induces HIF-1alpha expression through activation of IKKbeta. Biochem Biophys Res Commun 389:640–644. doi: 10.1016/j.bbrc.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Assche T, Deschacht M, da Luz RA, Maes L, Cos P. 2011. Leishmania-macrophage interactions: insights into the redox biology. Free Radic Biol Med 51:337–351. doi: 10.1016/j.freeradbiomed.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 47.Wuest TR, Carr DJ. 2010. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med 207:101–115. doi: 10.1084/jem.20091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kempf VA, Lebiedziejewski M, Alitalo K, Walzlein JH, Ehehalt U, Fiebig J, Huber S, Schutt B, Sander CA, Muller S, Grassl G, Yazdi AS, Brehm B, Autenrieth IB. 2005. Activation of hypoxia-inducible factor-1 in bacillary angiomatosis: evidence for a role of hypoxia-inducible factor-1 in bacterial infections. Circulation 111:1054–1062. doi: 10.1161/01.CIR.0000155608.07691.B7. [DOI] [PubMed] [Google Scholar]
- 49.Kempf VA, Volkmann B, Schaller M, Sander CA, Alitalo K, Riess T, Autenrieth IB. 2001. Evidence of a leading role for VEGF in Bartonella henselae-induced endothelial cell proliferations. Cell Microbiol 3:623–632. doi: 10.1046/j.1462-5822.2001.00144.x. [DOI] [PubMed] [Google Scholar]
- 50.Resto-Ruiz SI, Schmiederer M, Sweger D, Newton C, Klein TW, Friedman H, Anderson BE. 2002. Induction of a potential paracrine angiogenic loop between human THP-1 macrophages and human microvascular endothelial cells during Bartonella henselae infection. Infect Immun 70:4564–4570. doi: 10.1128/iai.70.8.4564-4570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Rourke F, Mandle T, Urbich C, Dimmeler S, Michaelis UR, Brandes RP, Flotenmeyer M, Doring C, Hansmann ML, Lauber K, Ballhorn W, Kempf VA. 2015. Reprogramming of myeloid angiogenic cells by Bartonella henselae leads to microenvironmental regulation of pathological angiogenesis. Cell Microbiol 17:1447–1463. doi: 10.1111/cmi.12447. [DOI] [PubMed] [Google Scholar]
- 52.Riess T, Andersson SG, Lupas A, Schaller M, Schafer A, Kyme P, Martin J, Walzlein JH, Ehehalt U, Lindroos H, Schirle M, Nordheim A, Autenrieth IB, Kempf VA. 2004. Bartonella adhesin A mediates a proangiogenic host cell response. J Exp Med 200:1267–1278. doi: 10.1084/jem.20040500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang J, Kelly CP, Bakirtzi K, Villafuerte Galvez JA, Lyras D, Mileto SJ, Larcombe S, Xu H, Yang X, Shields KS, Zhu W, Zhang Y, Goldsmith JD, Patel IJ, Hansen J, Huang M, Yla-Herttuala S, Moss AC, Paredes-Sabja D, Pothoulakis C, Shah YM, Wang J, Chen X. 2019. Clostridium difficile toxins induce VEGF-A and vascular permeability to promote disease pathogenesis. Nat Microbiol 4:269–279. doi: 10.1038/s41564-018-0300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Polena H, Boudou F, Tilleul S, Dubois-Colas N, Lecointe C, Rakotosamimanana N, Pelizzola M, Andriamandimby SF, Raharimanga V, Charles P, Herrmann JL, Ricciardi-Castagnoli P, Rasolofo V, Gicquel B, Tailleux L. 2016. Mycobacterium tuberculosis exploits the formation of new blood vessels for its dissemination. Sci Rep 6:33162. doi: 10.1038/srep33162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oehlers SH, Cronan MR, Scott NR, Thomas MI, Okuda KS, Walton EM, Beerman RW, Crosier PS, Tobin DM. 2015. Interception of host angiogenic signalling limits mycobacterial growth. Nature 517:612–615. doi: 10.1038/nature13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belton M, Brilha S, Manavaki R, Mauri F, Nijran K, Hong YT, Patel NH, Dembek M, Tezera L, Green J, Moores R, Aigbirhio F, Al-Nahhas A, Fryer TD, Elkington PT, Friedland JS. 2016. Hypoxia and tissue destruction in pulmonary TB. Thorax 71:1145–1153. doi: 10.1136/thoraxjnl-2015-207402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hammami A, Abidin BM, Heinonen KM, Stager S. 2018. HIF-1alpha hampers dendritic cell function and Th1 generation during chronic visceral leishmaniasis. Sci Rep 8:3500. doi: 10.1038/s41598-018-21891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammami A, Charpentier T, Smans M, Stager S. 2015. IRF-5-mediated inflammation limits CD8+ T cell expansion by inducing HIF-1alpha and impairing dendritic cell functions during Leishmania infection. PLoS Pathog 11:e1004938. doi: 10.1371/journal.ppat.1004938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. 1999. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 8:265–277. doi: 10.1023/A:1008942828960. [DOI] [PubMed] [Google Scholar]
- 60.Spath GF, Beverley SM. 2001. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol 99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- 61.Titus RG, Marchand M, Boon T, Louis JA. 1985. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol 7:545–555. [DOI] [PubMed] [Google Scholar]