Neutrophils contribute to lung injury in acute pneumococcal pneumonia. The interleukin 17 receptor E (IL-17RE) is the functional receptor for the epithelial-derived cytokine IL-17C, which is known to mediate innate immune functions. The aim of this study was to investigate the contribution of IL-17RE/IL-17C to pulmonary inflammation in a mouse model of acute Streptococcus pneumoniae pneumonia.
KEYWORDS: G-CSF, IL-17, IL-17C, IL-17RE, Streptococcus pneumoniae, host defense, lung infection, neutrophils, pneumonia, sepsis
ABSTRACT
Neutrophils contribute to lung injury in acute pneumococcal pneumonia. The interleukin 17 receptor E (IL-17RE) is the functional receptor for the epithelial-derived cytokine IL-17C, which is known to mediate innate immune functions. The aim of this study was to investigate the contribution of IL-17RE/IL-17C to pulmonary inflammation in a mouse model of acute Streptococcus pneumoniae pneumonia. Numbers of neutrophils and the expression levels of the cytokine granulocyte colony-stimulating factor (G-CSF) and tumor necrosis factor alpha (TNF-α) were decreased in lungs of IL-17RE-deficient (Il-17re−/−) mice infected with S. pneumoniae. Numbers of alveolar macrophages rapidly declined in both wild-type (WT) and Il-17re−/− mice and recovered 72 h after infection. There were no clear differences in the elimination of bacteria and numbers of blood granulocytes between infected WT and Il-17re−/− mice. The fractions of granulocyte-monocyte progenitors (GMPs) were significantly reduced in infected Il-17re−/− mice. Numbers of neutrophils were significantly reduced in lungs of mice deficient for IL-17C 24 h after infection with S. pneumoniae. These data indicate that the IL-17C/IL-17RE axis promotes the recruitment of neutrophils without affecting the recovery of alveolar macrophages in the acute phase of S. pneumoniae lung infection.
INTRODUCTION
Bacterial lung infections still have high mortality and morbidity rates (1). Immune cells and also epithelial cells drive excessive lung inflammation during pneumonia through the expression and release of inflammatory mediators, which culminate in the accumulation of neutrophils in the lung. Recruited neutrophils contribute to lung damage through different mechanisms, including the production of proteases and reactive oxygen species (2, 3). Thus, dysregulated inflammation is a hallmark of severe pneumonia and leads to tissue destruction, lung edema, and impaired pulmonary gas exchange (1, 3).
The cytokine interleukin 17C (IL-17C) signals through the interleukin 17 receptors IL-17RE and IL-17RA, with the latter receptor also serving as a functional receptor for IL-17A/F. IL-17C is expressed by epithelial cells of different organs, such as the lung, skin, and colon, whereas it is not detectable in peripheral blood mononuclear cells, alveolar macrophages, and fibroblasts (4–7). IL-17RE is expressed by epithelial cells and lymphocytes, such a Th17 cells (5, 7–10). IL-17 cytokines regulate the expression of inflammatory mediators, including proneutrophilic chemokines, and antimicrobial peptides through transcription factors and the stabilization of mRNA transcripts (11). There is evidence that IL-17C promotes the recruitment of neutrophils into the lung through IL-17RE. Tissue culture studies showed that bacteria, viruses, and inflammatory cytokines induce the expression of IL-17C in respiratory and alveolar epithelial cells and that the knockdown of IL-17RE or IL-17C in respiratory epithelial cells decreases the expression of inflammatory cytokines, chemokines, and neutrophil chemotaxis in response to viral and bacterial infection (6, 7, 10, 12–14). Moreover, administration of adenoviruses expressing IL-17C and application of recombinant IL-17C result in the recruitment of neutrophils into the lung (15, 16). We have shown that IL-17C promotes neutrophilic lung inflammation during acute Pseudomonas aeruginosa pneumonia and inflammation-induced recruitment of neutrophils into the microenvironment of lung tumors (6, 17, 18).
Streptococcus pneumoniae is a leading cause of community-acquired pneumonia (CAP) which frequently requires hospitalization and admission to an intensive care unit (19). Therefore, it is of interest to understand mechanisms that mediate tissue damaging inflammation during pneumococcal pneumonia. Here, we investigated the function of IL-17RE and IL-17C in a model of acute S. pneumoniae pneumonia. We show that IL-17RE/IL-17C promote the rapid recruitment of neutrophils into the lung without affecting the turnover of alveolar macrophages.
RESULTS
IL-17RE contributes to the recruitment of neutrophils during acute S. pneumoniae pneumonia.
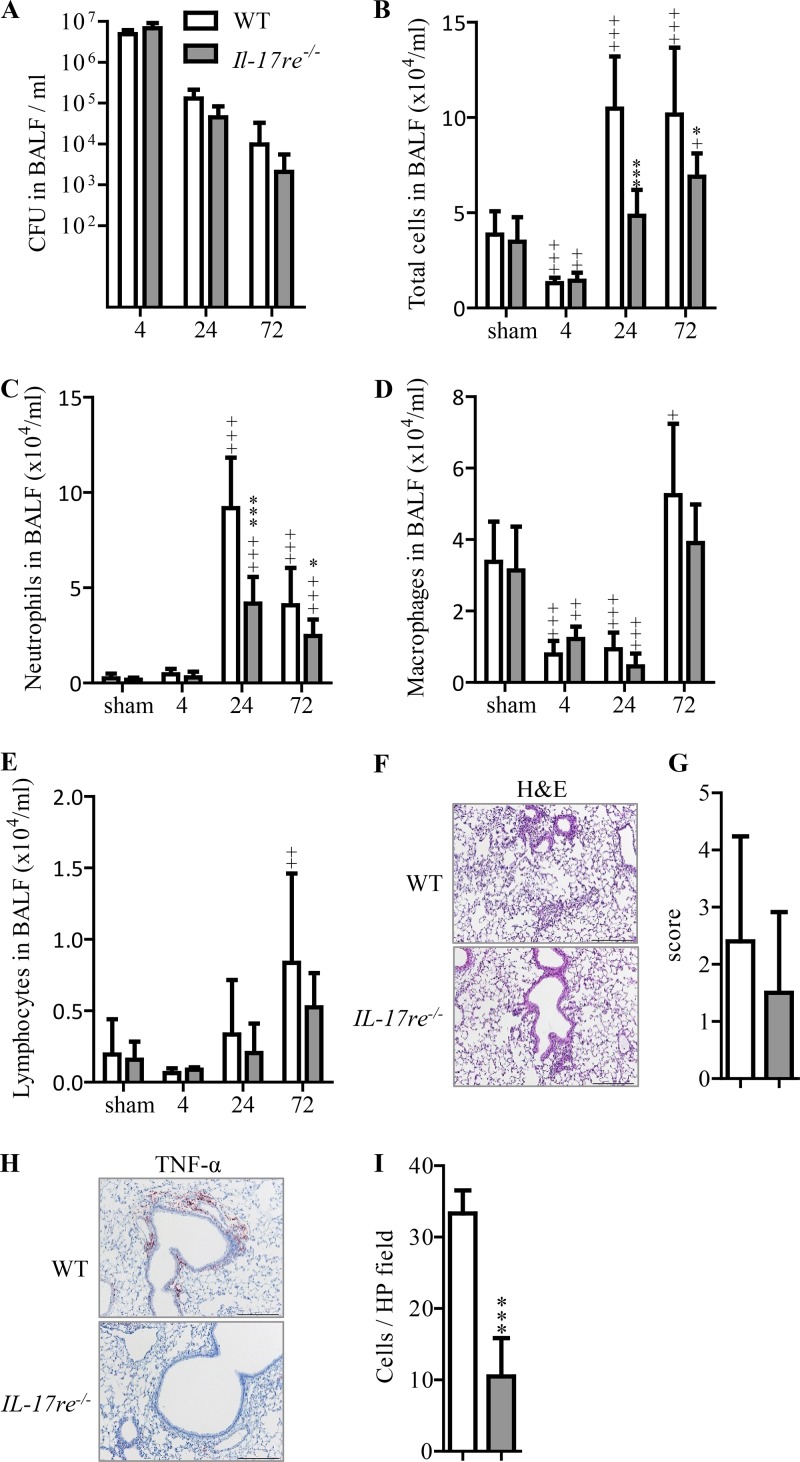
To study the role of IL-17RE in a model of experimental S. pneumoniae pneumonia, we intranasally challenged wild-type (WT) and Il-17re−/− mice with ∼5 × 106 CFU of the S. pneumoniae strain D39 or phosphate-buffered saline (PBS) as a control. WT and Il-17re−/− mice eliminated the administered bacteria from the lung to the same degree within the observation period of 72 h (Fig. 1A). Viable bacteria could be detected in the blood from 2 of 10 WT mice and from 4 of 8 Il-17re−/− mice 72 h after infection.
FIG 1.
Deficiency for Il-17RE results in decreased recruitment of neutrophils during acute S. pneumoniae pneumonia. WT and Il-17re−/− mice were intranasally infected with S. pneumoniae (∼5 × 106 CFU per mouse) or treated with PBS as a control. Numbers of viable bacteria (CFU) were determined in BAL fluids (A) at the indicated time points (hours). Numbers of total cells (B), neutrophils (C), macrophages (D), and lymphocytes (E) were determined in cytospin preparations. Data are shown as the means ± standard deviations (n = 11 and 9 for sham-infected mice, n = 8 and 5 for 4-h-infected mice, n = 9 and 5 for 24-h-infected mice, n = 10 and 8 for 72-h-infected mice). Significant differences in results for infected mice compared to those for the corresponding sham-infected mice are indicated as follows: +, P < 0.05; ++, P < 0.01; +++, P < 0.001. Significant differences in results for infected Il-17re−/− mice compared to those for the corresponding infected WT mice are indicated as follows: *, P < 0.05; ***, P < 0.001. (F to I) Representative lung histology (hematoxylin and eosin staining [H&E]), inflammatory score (n = 10 and 8 mice per group), representative immunohistochemistry of TNF-α, and quantification of TNF-α-positive cells in lung parenchyma (n = 5 and 6 mice per group) of mice infected with S. pneumoniae for 24 h (scale bar, 200 μm) were determined. Data are shown as the means ± standard deviations. ***, P < 0.001. HP, high-power field.
Bacterial infection results in a rapid recruitment of neutrophils into the lung in experimental studies (6, 20–22). Thus, we analyzed inflammatory cells in bronchoalveolar lavage (BAL) fluids of infected and sham-infected mice. Differential cell counting showed that the numbers of inflammatory cells in BAL fluids were similar between sham-infected WT and Il-17re−/− mice (Fig. 1B to E). Infection with S. pneumoniae resulted in significantly reduced numbers of total cells in BAL fluids of both WT and Il-17re−/− mice at 4 h after infection. However, BAL cells were significantly increased in infected WT mice compared to the levels in sham-infected mice at 24 and 72 h postinfection (Fig. 1B). Significantly increased numbers of BAL cells were also observed in infected Il-17re−/− mice at 72 h postinfection compared to the level in sham-infected mice. However, the total numbers of cells in BAL fluids of infected Il-17re−/− mice were significantly lower than the numbers of BAL cells found in infected WT mice at 24 and 72 h postinfection (Fig. 1B). Differential cell counting revealed that the differences in the numbers of BAL cells between infected WT and Il-17re−/− mice resulted from decreased recruitment of neutrophils in Il-17re−/− mice (Fig. 1C). Numbers of neutrophils peaked at 24 h in both infected WT and Il-17re−/− mice; however, they were significantly decreased in Il-17re−/− mice compared to the level in WT infected mice. Numbers of macrophages significantly decreased at 4 and 24 h postinfection compared to the numbers seen in sham-treated mice and recovered after 72 h without any significant difference between levels in infected WT and Il-17re−/− mice (Fig. 1D). Numbers of lymphocytes differed between sham-treated mice and WT infected mice only for the latest time point studied (Fig. 1E).
Figure 1F shows the representative histology of S. pneumoniae-infected lungs from WT and Il-17re−/− mice at 24 h postinfection. There was no significant difference in the inflammatory scores between WT and Il-17re−/− mice (Fig. 1G). However, immunohistochemical analysis for tumor necrosis factor alpha (TNF-α) (Fig. 1H) of lungs infected with S. pneumoniae for 24 h showed that numbers of cells staining positive for TNF-α were significantly increased in WT mice compared to the level in Il-17re−/− mice (Fig. 1I).
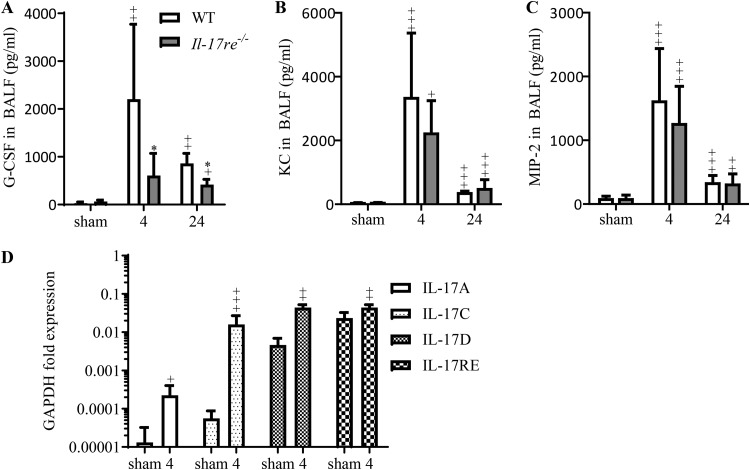
As the number of neutrophils in BAL fluids was the most prominent difference between infected WT and Il-17re−/− mice, we measured levels of cytokines known to mediate the recruitment of neutrophils. Concentrations of granulocyte colony-stimulating factor (G-CSF) were significantly reduced in BAL fluids of infected Il-17re−/− mice at 4 and 24 h after infection (Fig. 2A), whereas concentrations of keratinocyte-derived chemokine (KC) and macrophage inflammatory protein 2 (MIP-2) were not significantly affected by the IL-17RE deficiency (Fig. 2B and C). Concentrations of IL-17A and IL-17F were below the detection limit of 15 pg/ml (data not shown). Quantitative reverse transcription-PCR (qRT-PCR) with RNAs obtained from whole-lung tissue of WT mice showed that the transcription levels of genes coding for IL-17A, IL-17C, IL-17D, and IL-17RE were significantly increased at 4 h postinfection with S. pneumoniae (Fig. 2D), whereas there was no increased expression of IL-17B, IL-17E, and IL-17F detectable in infected mice at this time point (data not shown).
FIG 2.
IL-17RE promotes the expression of G-CSF during acute S. pneumoniae pneumonia. WT and Il-17re−/− mice were intranasally infected with S. pneumoniae (∼5 × 106 CFU per mouse) or treated with PBS as a control. Concentrations of G-CSF (A), KC (B), and MIP-2 (C) were measured in BAL fluids by ELISA at indicated time points (hours). Data are shown as the means ± standard deviations (n = 9 and 8 for sham-infected mice, n = 8 and 5 for 4-h-infected mice, n = 5 and 4 for 24-h-infected mice). Significant differences in results for infected mice compared to those for the corresponding sham-infected mice are indicated as follows: +, P < 0.05; ++, P < 0.01; +++, P < 0.001. *, P < 0.05 for the difference between results for infected Il-17re−/− mice and those for infected WT mice (*). (D) The expression levels of IL-17A, IL-17C, IL-17D, and IL-17RE in lung tissue of WT mice were measured by qRT-PCR at 4 h postinfection. Data are shown as the means ± standard deviations (n = 6 for sham-infected mice, n = 9 for 4-h infected mice). Significant differences in results for infected WT mice compared to those for sham-infected mice are indicated as follows: +, P < 0.05; ++, P < 0.01; +++, P < 0.001.
Deficiency for IL-17RE has a minor effect on the levels of blood granulocytes and differentiation of hematopoietic cells.
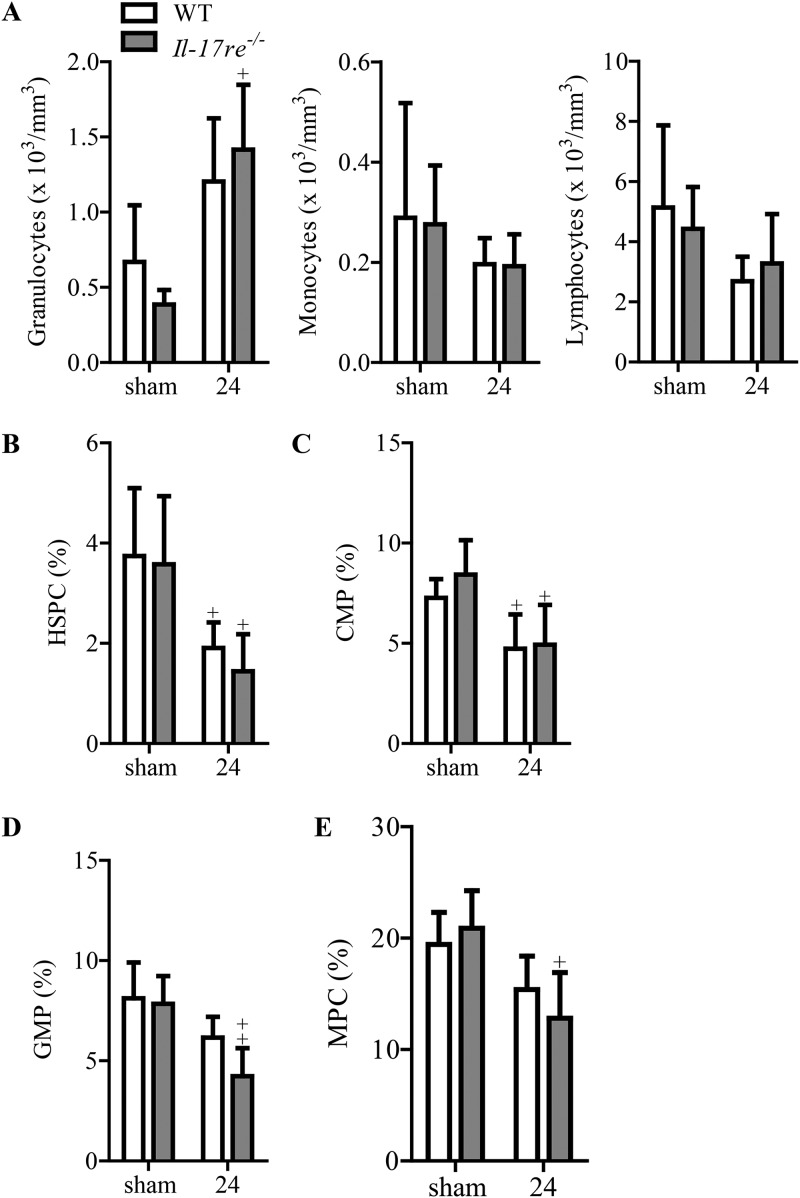
We next determined numbers of peripheral blood leukocytes of mice infected with S. pneumoniae for 24 h. Differential cell counting showed that there was no significant difference in the numbers of granulocytes, monocytes, and lymphocytes between infected and sham-infected WT and Il-17re−/− mice (Fig. 3A). As cytokines such as G-CSF regulate hematopoiesis and the mobilization of neutrophils, we further examined the differentiation of hematopoietic cells via flow cytometry analysis. Administration of S. pneumoniae into the lungs of mice resulted in significantly reduced fractions of hematopoietic stem and progenitor cells (HSPCs) and common myeloid progenitors (CMPs) in WT and Il-17re−/− mice (Fig. 3B and C). However, the fractions of granulocyte-monocyte progenitors (GMPs) and mesenchymal progenitor cells (MPCs) were significantly reduced only in infected Il-17re−/− mice (Fig. 3D and E).
FIG 3.
Evaluation of leukocytes and differentiation of hematopoietic cells during acute S. pneumoniae pneumonia. WT and Il-17re−/− mice were intranasally infected with S. pneumoniae (∼5 × 106 CFU per mouse) or treated with PBS as a control. (A) Leukocytes were differentiated by light microscopy. (B to E) Differentiation of hematopoietic cells was analyzed by flow cytometry analysis. Data are shown as the means ± standard deviations (n = 4 to 6 per group). +, P < 0.05; ++, P < 0.01, for a comparison of results in infected mice to those of corresponding sham-infected mice.
Deficiency for IL-17C contributes to the recruitment of neutrophils during acute S. pneumoniae pneumonia.
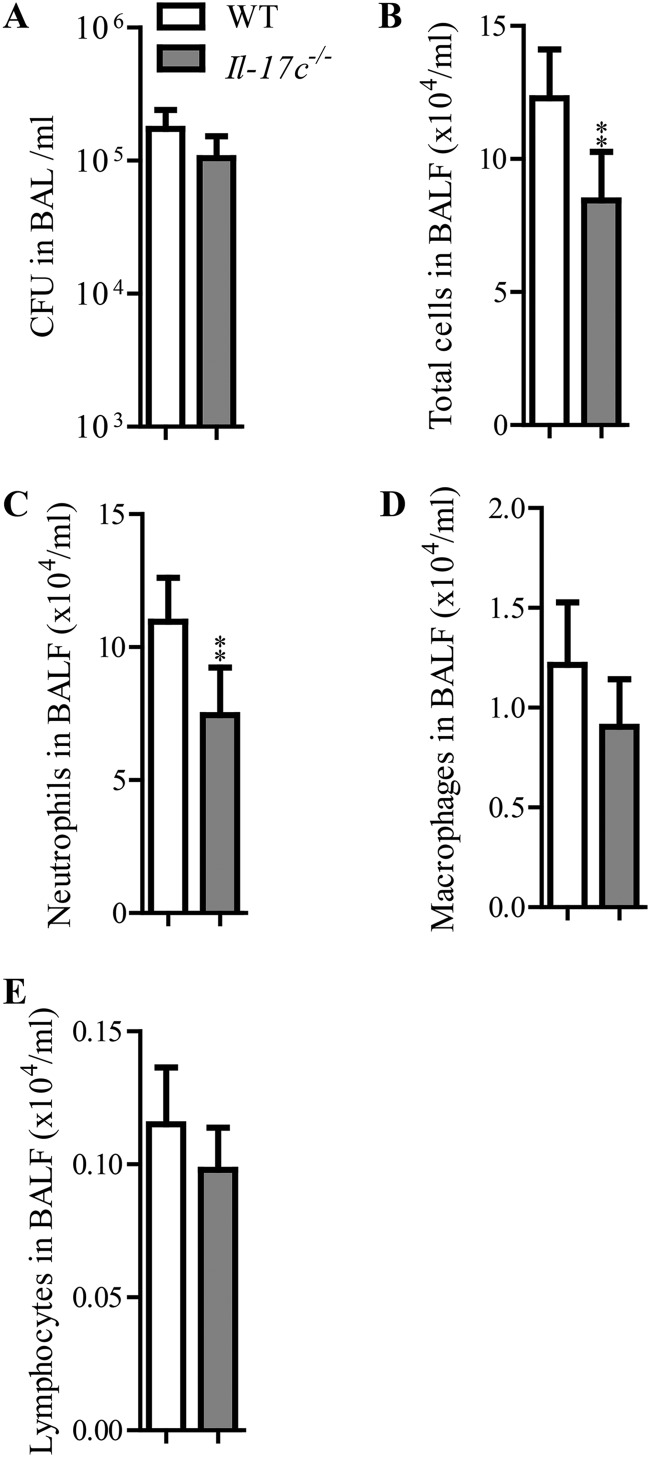
As IL-17RE has been described to be the functional receptor for IL-17C (4, 5) we intranasally challenged WT and Il-17c−/− mice with 8 × 106 CFU of S. pneumoniae and analyzed the mice after 24 h. There was no significant difference in the numbers of bacteria recovered from the lung between WT and Il-17c−/− mice (Fig. 4A). However, the deficiency for IL-17C resulted in significantly reduced numbers of total cells and neutrophils in BAL fluids (Fig. 4B and C), while the numbers of macrophages and lymphocytes were not markedly changed in WT and Il-17c−/− mice (Fig. 4D and E).
FIG 4.
IL-17C promotes the recruitment of neutrophils during acute S. pneumoniae pneumonia. WT and Il-17c−/− mice were intranasally infected with S. pneumoniae (∼8 × 106 CFU per mouse). Numbers of viable bacteria (CFU) were determined in BAL fluids (A). Numbers of total cells (B), neutrophils (C), macrophages (D), and lymphocytes (E) were determined in cytospin preparations. Data are shown as the means ± standard deviations (n = 6 per group). **, P < 0.01, for a comparison of results in infected Il-17re−/− mice to those in infected WT mice.
DISCUSSION
Tissue culture and mouse studies showed that epithelial-derived IL-17C mediates the expression of cytokines and chemokines in response to infection with Gram-negative bacteria and viruses, respectively, and the recruitment of neutrophils into the lung through the receptor IL-17RE (6, 7, 10, 12, 13, 15–17). Whether or not IL-17RE/IL-17C signaling plays a role during infection with Gram-positive bacteria is largely unknown. Thus, we analyzed the function of the IL-17RE/IL-17C axis in a model of acute pneumococcal pneumonia using mice deficient for IL-17RE and IL-17C.
Our main finding is that the IL-17RE/IL-17C axis promotes neutrophilic lung inflammation induced by S. pneumoniae. The recruitment of neutrophils into the lung was decreased in both IL-17RE- and IL-17C-deficient mice compared to the level in WT mice. We further found that levels of the cytokine G-CSF were reduced in BAL fluids of IL-17RE-deficient mice. Previous studies showed that G-CSF expression is regulated by IL-17C and mediates the recruitment and maintenance of neutrophils (5, 23–25). Thus, IL-17RE-mediated expression of G-CSF could be one mechanism by which IL-17RE contributes to the accumulation of neutrophils in the lung.
As described by other groups, numbers of alveolar macrophages declined within the first 24 h and recovered until day 3 after infection with S. pneumoniae, likely through the recruitment of monocytes from the bloodstream (20, 26, 27). In line with these studies, we also observed a decrease in macrophages at 24 h postinfection and a complete recovery of macrophage numbers at 72 h postinfection. However, in our experiments, the turnover rates of macrophages were similar between infected WT and IL-17RE- and IL-17C-deficient mice, indicating that the IL-17RE/IL-17C axis is not likely to mediate the recruitment of monocytes from the bloodstream into the lung during acute S. pneumonia lung infection.
As cytokines expressed in the lung, such as G-CSF, potentially regulate the maturation and release of neutrophils in the bone marrow, we also asked whether IL-17RE has a function in the regulation of the differentiation of hematopoietic cells and numbers of blood neutrophils. Infection with S. pneumoniae resulted in reduced percentages of GMPs in IL-17RE-deficient mice. However, there was no significant difference in the numbers of blood neutrophils, monocytes, and lymphocytes between infected WT and IL-17RE-deficient mice. Thus, our data suggest that the IL-17RE/IL-17C axis primarily acts locally in the lung and mediates the recruitment of neutrophils during acute infection with S. pneumoniae.
Excessive neutrophilic inflammation contributes to the pathology of pneumococcal pneumonia even though neutrophils are required for the elimination of S. pneumoniae from the lung (2, 3). It is therefore of interest whether attenuation of neutrophilic inflammation is a potential strategy to reduce lung damage during acute pneumococcal pneumonia or whether it promotes bacterial outgrowth and invasion (2, 3, 22). Indeed, mouse studies suggest that the reduction of neutrophil recruitment to a certain degree does not impair the host defense during acute pneumococcal pneumonia. José et al. showed that a PAR-1 antagonist attenuates the recruitment of neutrophils into the lung as well as alveolar barrier disruption without impairing the host defense, whereas systemic depletion of neutrophils resulted in bacterial outgrowth in mice intranasally infected with S. pneumoniae strain D39 (22). There is also evidence that the IL-17-mediated recruitment of neutrophils contributes to harmful lung inflammation during pneumococcal pneumonia. Ritchie et al. showed that the deficiency for IL-17RA resulted in significantly decreased numbers of BAL neutrophils and histological scores in mice that were intranasally inoculated with the S. pneumoniae strains SRL1 and TIGR4, respectively, without, however, markedly affecting the clearance of bacteria from the lung (28). The finding of our study that deficiency for IL-17RE and IL-17C results in reduced numbers of pulmonary neutrophils without impairing the clearance of S. pneumoniae from the lung is in line with the aforementioned studies and further supports the hypothesis that excessive recruitment of neutrophils does not contribute to the host defense during acute pneumococcal pneumonia. Of note, the deficiency for IL-17RA had a stronger effect on the numbers of BAL neutrophils in the study by Ritchie et al. (more than 1 order of magnitude decrease in case of infection with TIGR4) than the deficiency of IL-17RE in our study (55% ± 15.2% decrease). This can be explained by the fact that IL-17RA is the functional receptor for IL-17A and IL-17C and that the expression of both cytokines is increased during acute S. pneumoniae lung infection (11, 28). Moreover, the deficiency for IL-17RA was associated with reduced numbers of blood neutrophils and increased mortality after infection with the invasive S. pneumoniae strain TIGR4, whereas IL-17RE deficiency did not affect numbers of blood neutrophils in our infection model. This indicates that IL-17A also has a systemic effect during acute S. pneumoniae infection, whereas the function of IL-17C is more limited to the lung.
Infection with S. pneumoniae resulted in significantly increased pulmonary mRNA expression of IL-17A, IL-17C, and IL-17D. This is in line with our finding that the deficiency of IL-17RE only partially affected the pulmonary expression of G-CSF as well as neutrophil numbers in the lung. As IL-17A has been shown to regulate the recruitment of neutrophils into the lung and the numbers of blood neutrophils, as mentioned above, the expression of G-CSF IL-17A also likely contributes to the neutrophilic inflammation in our pneumonia model (28, 29).
In conclusion, our study demonstrates that the IL-17RE/IL-17C axis promotes the recruitment of neutrophils into the lung during the early phase of pneumococcal pneumonia without affecting the maintenance of alveolar macrophages and the maturation and release of neutrophils from the bone marrow. As seen in other experimental studies (22, 28), the attenuated neutrophilic inflammation did not impact the host defense in the lung. Thus, therapeutic intervention resulting in decreased neutrophilic lung inflammation without major systemic effects may be a strategy in the treatment of acute pneumococcal pneumonia. The molecular mechanism(s)that determines how IL-17-RE contributes to the protection of the host from invasive infection during pneumococcal pneumonia still needs to be identified.
MATERIALS AND METHODS
Mouse studies.
All animal experiments were approved by the Landesamt für Soziales, Gesundheit und Verbraucherschutz of the State of Saarland in accordance with the national guidelines for animal treatment. IL-17C- and IL-17RE-deficient C57BL/6 mice were obtained from the Mutant Mouse Resource and Research Center (MMRRC, Bethesda, MD, USA). S. pneumoniae strain D39 (serotype 2) bacteria were cultured in Todd Hewitt broth at 37°C for approximately 4 h, washed with PBS, and adjusted to an optical density at 600 nm (OD600) of 0.5. Mice were infected with bacteria diluted in PBS at concentrations indicated in the figure legends. Female mice, 8 to 10 weeks old, were slightly anesthetized (intraperitoneal [i.p.] injection of 90 to 120 mg/kg of ketamine hydrochloride [Ketanest; Pfizer, Germany] and 10 to 12 mg/kg of xylazine hydrochloride [Rompun; Bayer, Germany]) and infected intranasally with the indicated numbers of viable cells of S. pneumoniae strain D39 as described previously (30). A bronchoalveolar lavage (BAL) was performed with PBS flushed three times through the cannulated trachea of euthanized mice. CFU counts were determined by spreading appropriate dilutions of lung homogenates or BAL fluids on agar plates and counting the number of colonies on the agar plates after 24 h of incubation at 37°C. Neutrophils, macrophages, and lymphocytes in BAL fluids were differentiated after DiffQuick staining by light microscopy.
Determination of inflammatory mediators and histology.
Concentrations of cytokines were determined by using enzyme-linked immunosorbent assays (ELISA) (R&D Systems, Germany). For our immunohistochemical studies, lungs not subjected to BAL were fixed by instillation of 4% formalin under a hydrostatic pressure of 30 cm of H2O, embedded in agarose, cut into regular slices, embedded in paraffin, and stained (hematoxylin/eosin) as described previously (31). The inflammatory score was calculated as described previously (6). Primary antibodies for TNF-α (Abcam, UK) were used for immunohistochemistry analysis by a blinded investigator as described previously (32). Seven to 10 fields per lung were evaluated for TNF-α-positive cells. Total RNA from lung tissue was isolated using TRIzol reagent (Life Technologies, USA). Reverse transcription and real-time PCR were performed as described previously (6, 33).
Bone marrow.
Bone marrow cells were harvested from femurs and tibias by flushing the bone with ice-cold PBS. Afterwards, debris was separated by using a 70-μm-pore-size cell strainer. After lysis of red blood cells, 1 million cells were incubated with a lineage marker antibody cocktail (BioLegend, USA). After addition of the respective fluorescein isothiocyanate (FITC)-conjugated streptavidin antibodies, cells were stained with the following antibodies: c-Kit (CD117) conjugated to allophycocyanin (APC), Sca-1 (Ly6A/E)-APC/Cy7, FCγR III/II-peridinin chlorophyll protein (PerCP)/Cy5.5, and CD34-phycoerythrin (PE)/Cy7. Cells were immediately analyzed by flow cytometry (BD FACSCanto II), and results were analyzed using BD FACSDiva software.
Statistical analysis.
Differences between groups were analyzed by one-way analysis of variance (ANOVA) with a Tukey post hoc test or Student's t test using Prism software (GraphPad Software, San Diego, CA). Results were considered statistically significant at a P value of <0.05.
ACKNOWLEDGMENT
We thank Andreas Kamyschnikow for excellent technical support.
REFERENCES
- 1.Feldman C, Anderson R. 2014. Recent advances in our understanding of Streptococcus pneumoniae infection. F1000Prime Rep 6:82. doi: 10.12703/P6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pechous RD. 2017. With friends like these: the complex role of neutrophils in the progression of severe pneumonia. Front Cell Infect Microbiol 7:160. doi: 10.3389/fcimb.2017.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthay MA, Ware LB, Zimmerman GA. 2012. The acute respiratory distress syndrome. J Clin Invest 122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song X, Zhu S, Shi P, Liu Y, Shi Y, Levin SD, Qian Y. 2011. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat Immunol 12:1151–1158. doi: 10.1038/ni.2155. [DOI] [PubMed] [Google Scholar]
- 5.Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, Hackney J, Kim J, Zhou M, Lai J, Modrusan Z, Sai T, Lee W, Xu M, Caplazi P, Diehl L, de VJ, Balazs M, Gonzalez L Jr, Singh H, Ouyang W, Pappu R. 2011. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol 12:1159–1166. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]
- 6.Wolf L, Sapich S, Honecker A, Jungnickel C, Seiler F, Bischoff M, Wonnenberg B, Herr C, Schneider-Daum N, Lehr CM, Bals R, Beisswenger C. 2016. IL-17A-mediated expression of epithelial IL-17C promotes inflammation during acute Pseudomonas aeruginosa pneumonia. Am J Physiol Lung Cell Mol Physiol 311:L1015–L1022. doi: 10.1152/ajplung.00158.2016. [DOI] [PubMed] [Google Scholar]
- 7.Pfeifer P, Voss M, Wonnenberg B, Hellberg J, Seiler F, Lepper PM, Bischoff M, Langer F, Schafers HJ, Menger MD, Bals R, Beisswenger C. 2013. IL-17C is a mediator of respiratory epithelial innate immune response. Am J Respir Cell Mol Biol 48:415–421. doi: 10.1165/rcmb.2012-0232OC. [DOI] [PubMed] [Google Scholar]
- 8.Chang SH, Reynolds JM, Pappu BP, Chen G, Martinez GJ, Dong C. 2011. Interleukin-17C promotes Th17 cell responses and autoimmune disease via interleukin-17 receptor E. Immunity 35:611–621. doi: 10.1016/j.immuni.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krohn S, Nies JF, Kapffer S, Schmidt T, Riedel JH, Kaffke A, Peters A, Borchers A, Steinmetz OM, Krebs CF, Turner JE, Brix SR, Paust HJ, Stahl RAK, Panzer U. 2018. IL-17C/IL-17 Receptor E Signaling in CD4+ T cells promotes TH17 cell-driven glomerular inflammation. J Am Soc Nephrol 29:1210–1222. doi: 10.1681/ASN.2017090949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kusagaya H, Fujisawa T, Yamanaka K, Mori K, Hashimoto D, Enomoto N, Inui N, Nakamura Y, Wu R, Maekawa M, Suda T, Chida K. 2014. Toll-like receptor-mediated airway IL-17C enhances epithelial host defense in an autocrine/paracrine manner. Am J Respir Cell Mol Biol 50:30–9. doi: 10.1165/rcmb.2013-0130OC. [DOI] [PubMed] [Google Scholar]
- 11.Amatya N, Garg AV, Gaffen SL. 2017. IL-17 signaling: the yin and the yang. Trends Immunol 38:310–322. doi: 10.1016/j.it.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jamieson KC, Traves SL, Kooi C, Wiehler S, Dumonceaux CJ, Maciejewski BA, Arnason JW, Michi AN, Leigh R, Proud D. 2018. Rhinovirus and bacteria synergistically induce IL-17C release from human airway epithelial cells to promote neutrophil recruitment. J Immunol 202:160–170. doi: 10.4049/jimmunol.1800547. [DOI] [PubMed] [Google Scholar]
- 13.Bryche B, Dewaele A, Saint-Albin A, Le Poupon Schlegel C, Congar P, Meunier N. 2019. IL-17c is involved in olfactory mucosa responses to poly(I:C) mimicking virus presence. Brain Behav Immun 79:274–283. doi: 10.1016/j.bbi.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Lehmann R, Muller MM, Klassert TE, Driesch D, Stock M, Heinrich A, Conrad T, Moore C, Schier UK, Guthke R, Slevogt H. 2018. Differential regulation of the transcriptomic and secretomic landscape of sensor and effector functions of human airway epithelial cells. Mucosal Immunol 11:627–642. doi: 10.1038/mi.2017.100. [DOI] [PubMed] [Google Scholar]
- 15.Chen K, Eddens T, Trevejo-Nunez G, Way EE, Elsegeiny W, Ricks DM, Garg AV, Erb CJ, Bo M, Wang T, Chen W, Lee JS, Gaffen SL, Kolls JK. 2016. IL-17 receptor signaling in the lung epithelium is required for mucosal chemokine gradients and pulmonary host defense against K. pneumoniae. Cell Host Microbe 20:596–605. doi: 10.1016/j.chom.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G, Coffman RL. 2002. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol 169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 17.Jungnickel C, Schmidt LH, Bittigkoffer L, Wolf L, Wolf A, Ritzmann F, Kamyschnikow A, Herr C, Menger MD, Spieker T, Wiewrodt R, Bals R, Beisswenger C. 2017. IL-17C mediates the recruitment of tumor-associated neutrophils and lung tumor growth. Oncogene 36:4182–4190. doi: 10.1038/onc.2017.28. [DOI] [PubMed] [Google Scholar]
- 18.Ritzmann F, Jungnickel C, Vella G, Kamyschnikow A, Herr C, Li D, Menger MM, Angenendt A, Hoth M, Lis A, Bals R, Beisswenger C. 2019. IL-17C-mediated innate inflammation decreases the response to PD-1 blockade in a model of Kras-driven lung cancer. Sci Rep 9:10353. doi: 10.1038/s41598-019-46759-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman C, Anderson R. 2016. The role of streptococcus pneumoniae in community-acquired pneumonia. Semin Respir Crit Care Med 37:806–818. doi: 10.1055/s-0036-1592074. [DOI] [PubMed] [Google Scholar]
- 20.Herbold W, Maus R, Hahn I, Ding N, Srivastava M, Christman JW, Mack M, Reutershan J, Briles DE, Paton JC, Winter C, Welte T, Maus UA. 2010. Importance of CXC chemokine receptor 2 in alveolar neutrophil and exudate macrophage recruitment in response to pneumococcal lung infection. Infect Immun 78:2620–2630. doi: 10.1128/IAI.01169-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wonnenberg B, Tschernig T, Voss M, Bischoff M, Meier C, Schirmer SH, Langer F, Bals R, Beisswenger C. 2014. Probenecid reduces infection and inflammation in acute Pseudomonas aeruginosa pneumonia. Int J Med Microbiol 304:725–729. doi: 10.1016/j.ijmm.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 22.José RJ, Williams AE, Mercer PF, Sulikowski MG, Brown JS, Chambers RC. 2015. Regulation of neutrophilic inflammation by proteinase-activated receptor 1 during bacterial pulmonary infection. J Immunol 194:6024–6034. doi: 10.4049/jimmunol.1500124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balamayooran G, Batra S, Theivanthiran B, Cai S, Pacher P, Jeyaseelan S. 2012. Intrapulmonary G-CSF rescues neutrophil recruitment to the lung and neutrophil release to blood in Gram-negative bacterial infection in MCP-1-/- mice. J Immunol 189:5849–5859. doi: 10.4049/jimmunol.1200585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. 2010. Neutrophil kinetics in health and disease. Trends Immunol 31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knapp S, Hareng L, Rijneveld AW, Bresser P, van der Zee JS, Florquin S, Hartung T, van der Poll T. 2004. Activation of neutrophils and inhibition of the proinflammatory cytokine response by endogenous granulocyte colony-stimulating factor in murine pneumococcal pneumonia. J Infect Dis 189:1506–1515. doi: 10.1086/382962. [DOI] [PubMed] [Google Scholar]
- 26.Winter C, Taut K, Langer F, Mack M, Briles DE, Paton JC, Maus R, Srivastava M, Welte T, Maus UA. 2007. FMS-like tyrosine kinase 3 ligand aggravates the lung inflammatory response to Streptococcus pneumoniae infection in mice: role of dendritic cells. J Immunol 179:3099–3108. doi: 10.4049/jimmunol.179.5.3099. [DOI] [PubMed] [Google Scholar]
- 27.Maus UA, Janzen S, Wall G, Srivastava M, Blackwell TS, Christman JW, Seeger W, Welte T, Lohmeyer J. 2006. Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol 35:227–235. doi: 10.1165/rcmb.2005-0241OC. [DOI] [PubMed] [Google Scholar]
- 28.Ritchie ND, Ritchie R, Bayes HK, Mitchell TJ, Evans TJ. 2018. IL-17 can be protective or deleterious in murine pneumococcal pneumonia. PLoS Pathog 14:e1007099. doi: 10.1371/journal.ppat.1007099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mei J, Liu Y, Dai N, Hoffmann C, Hudock KM, Zhang P, Guttentag SH, Kolls JK, Oliver PM, Bushman FD, Worthen GS. 2012. Cxcr2 and Cxcl5 regulate the IL-17/G-CSF axis and neutrophil homeostasis in mice. J Clin Invest 122:974–986. doi: 10.1172/JCI60588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wonnenberg B, Jungnickel C, Honecker A, Wolf L, Voss M, Bischoff M, Tschernig T, Herr C, Bals R, Beisswenger C. 2016. IL-17A attracts inflammatory cells in murine lung infection with P aeruginosa. Innate Immun 22:620–625. doi: 10.1177/1753425916668244. [DOI] [PubMed] [Google Scholar]
- 31.Voss M, Wolf L, Kamyschnikow A, Wonnenberg B, Honecker A, Herr C, Lepper PM, Wegmann M, Menger MD, Bals R, Beisswenger C. 2015. Il-17A contributes to maintenance of pulmonary homeostasis in a murine model of cigarette smoke-induced emphysema. Am J Physiol Lung Cell Mol Physiol 309:L188–L195. doi: 10.1152/ajplung.00388.2014. [DOI] [PubMed] [Google Scholar]
- 32.Beisswenger C, Honecker A, Kamyschnikow A, Bischoff M, Tschernig T, Bals R. 2014. Moxifloxacin modulates inflammation during murine pneumonia. Respir Res 15:82. doi: 10.1186/1465-9921-15-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seelige R, Saddawi-Konefka R, Adams NM, Picarda G, Sun JC, Benedict CA, Bui JD. 2018. Interleukin-17D and Nrf2 mediate initial innate immune cell recruitment and restrict MCMV infection. Sci Rep 8:13670. doi: 10.1038/s41598-018-32011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]