Enterotoxigenic Escherichia coli (ETEC) is a major cause of infectious diarrhea in children, travelers, and deployed military personnel. As such, development of a vaccine would be advantageous for public health. One strategy is to use subunits of colonization factors combined with antigen/adjuvant toxoids as an ETEC vaccine.
KEYWORDS: CFA/I, ETEC, LTA1, dmLT, intradermal, sublingual, vaccine
ABSTRACT
Enterotoxigenic Escherichia coli (ETEC) is a major cause of infectious diarrhea in children, travelers, and deployed military personnel. As such, development of a vaccine would be advantageous for public health. One strategy is to use subunits of colonization factors combined with antigen/adjuvant toxoids as an ETEC vaccine. Here, we investigated the intradermal (i.d.) or sublingual (s.l.) delivery of CFA/I fimbrial antigens, including CfaEB and a CfaE-heat-labile toxin B subunit (LTB) chimera admixed with double mutant heat-labile toxin (LT) LT-R192G/L211A (dmLT). In addition, we compared dmLT with other LT proteins to better understand the generation of adjuvanted fimbrial and toxoid immunity as well as the influence on any local skin reactogenicity. We demonstrate that immunization with dmLT admixed with CfaEB induces robust serum and fecal antibody responses to CFA/I fimbriae and LT but that i.d. formulations are not optimal for s.l. delivery. Improved s.l. vaccination outcomes were observed when higher doses of dmLT (1 to 5 μg) were admixed with CfaEB or, even better, when a CfaE-LTB chimera antigen was used instead. Serum anti-CFA/I total antibodies, detected by enzyme-linked immunosorbent assay, were the best predictor of functional antibodies, based on the inhibition of red blood cell agglutination by ETEC. Immunization with other LT proteins or formulations with altered B-subunit binding during i.d. immunization (e.g., by addition of 5% lactose, LTA1, or LT-G33D) minimally altered the development of antibody responses and cytokine recall responses but reduced skin reactogenicity at the injection site. These results reveal how formulations and delivery parameters shape the adaptive immune responses to a toxoid and fimbria-derived subunit vaccine against ETEC.
INTRODUCTION
Diarrheal illness contributes to malnutrition, stunted growth, impaired cognitive development, and high morbidity rates, affecting 20% of children worldwide (1). In children under age 5 years, enterotoxigenic Escherichia coli (ETEC) accounts for 14,000 to 42,000 deaths annually (2). Both travelers and deployed military personnel are also populations vulnerable to ETEC-induced diarrhea and potential disease complications (3, 4). Thus, development of an ETEC vaccine would benefit multiple populations across the world.
ETEC induces diarrheal disease through heat-stable enterotoxin (ST) or heat-labile enterotoxin (LT), but attachment to small intestinal epithelial cells via expressed colonization factors (CFs) is also critical. One vaccine strategy is to target toxin proteins and CFs, such as adhesins and pili (5). Passive protection with antibodies against the CF CFA/I minor adhesin (CfaE) can protect human volunteers from diarrheal disease (6) and nursing mouse pups (7). Immunization with CfaE antigen also elicits functional antibodies able to disrupt bacterial adherence. Moreover, CfaE administered with the mutant LT LT-R192G (mLT) protected Aotus nancymaae nonhuman primates from experimental diarrhea with CFA/I+ ETEC strain H10407, with intranasal (i.n.) immunization being better than oral immunization (8). Additionally, a newer antigen has been designed to expand antibody epitopes by fusing the major subunit of CFA/I (CfaB, pilin domain) to CfaE, creating CfaEB. CfaEB exhibits native folding properties and has a 1:1 ratio between the subunits (9), in contrast to the bacterial class 5 fimbriae, which have adhesin/pilus ratios closer to 1:1,100 (10, 11), and other fusion proteins, which can lack conformation integrity.
LT is an ADP-ribosylating protein similar to cholera toxin with an AB5 structure that is expressed by ∼60% of clinical ETEC strains and binds primarily to GM1 gangliosides on cells (12, 13). LT is both immunogenic and an adjuvant, inducing antibody responses to both itself and a codelivered antigen(s) (14). LT-producing ETEC strains (alone or in combination with ST- and LT-producing ETEC strains) exhibit important regional and seasonal contributions to the burden of diarrhea (15, 16). In studies with breast-feeding infants and natural exposure or adult experimental challenge models, anti-LT IgA or IgG antibodies are strongly associated with protection from ETEC (17, 18). In addition, the risk for recurrent ETEC diarrheal disease in areas of endemicity drops after 5 years of age (19, 20), concurrent with the development of anti-LT antibodies (21).
We and others have previously analyzed the role of LT subunits and found that the LT B subunit (LTB) is critical for protein uptake at gastrointestinal mucosal surfaces (22, 23) and promotes antitoxin antibodies (24, 25) but is not a good adjuvant by itself (14). In addition, chimera proteins consisting of genetic or chemical fusions of LTB or the cholera toxin B subunit to an ancillary antigen(s) can promote antigen uptake (26, 27). However, GM1 binding during intradermal (i.d.) immunizations in both mice and humans can contribute to minor skin reactogenicity, which is preventable through a G33D mutation in the B subunit (28, 29). In contrast, the enzymatic A subunit (LTA) and the A1 domain (LTA1) are good adjuvants when they are delivered by i.n. immunization but are weak toxoid antigens (22, 30). The development of adjuvanted immunity by LT proteins, including the induction of Th17, IgG2a, and IgA responses to a codelivered antigen, is A subunit dependent, although the magnitude of the postvaccination responses is maximized with admixed LTA and LTB or with AB5 toxoids (22, 30). The most advanced candidate for an LT toxoid adjuvant/antigen is currently an AB5 LT mutant with two mutations in the A subunit that alter the protease sensitivity while retaining the immunologic properties of native LT (referred to as the double mutant LT [dmLT]) (31, 32). This mutant LT replaces an earlier version of this molecule, mLT, since it is less enterotoxic when administered orally (32). dmLT provides both antigenic and adjuvant properties, has proved safe in oral and sublingual (s.l.) phase 1 safety studies (32, 33), and is currently being tested for i.d. delivery (ClinicalTrials.gov registration number NCT02531685).
Selection of the appropriate route for vaccine delivery is critical. While intramuscular injection is common, i.d. delivery has been suggested to be better due to the higher density of antigen-presenting cells in the skin than in muscle tissue (34) and the possibility of dose sparing (35–37). However, this strategy is not optimal in a resource-poor setting, since trained personnel to administer the injection may be limited and unsafe needle use can lead to disease outbreaks (38–41). Mucosal immunization is an attractive option, particularly given the gastrointestinal location of ETEC infection and disease; however, i.n. delivery is not a feasible option with an AB5 LT toxoid (42), and oral delivery requires large doses of antigens and risks the degradation of subunit antigens by gastric proteases and low pH. One option is to use s.l. delivery (43). A recent publication describing dmLT and ETEC adhesion protein EtpA vaccination indicated that either s.l. or oral immunization protected mice from the development of bacterial colonization in an antibiotic-ETEC challenge model, despite a 5 to 40 times lower antigen dose in the s.l. groups (44).
In the current study, we compared the use of the i.d. and s.l. delivery of two different fimbrial tip adhesin fusion proteins, CfaEB or a CfaE-CTA2/LTB chimera protein (referred to here as the chimera or CfaE-LTB), with dmLT in mouse models. In addition, we evaluated alternative forms of LT for antigenicity, adjuvanticity, and i.d. skin reactogenicity to better understand the contribution of an LT protein to immunization outcomes. Our findings have a direct impact for vaccine formulation strategies against ETEC and enhance our understanding of how LT-derived proteins interact with host cells at distinct tissue sites to promote immunologic responses.
RESULTS
The antigen/adjuvant dmLT admixed with CfaEB antigen improves i.d. immunization outcomes, but this formulation is not optimal for s.l. delivery.
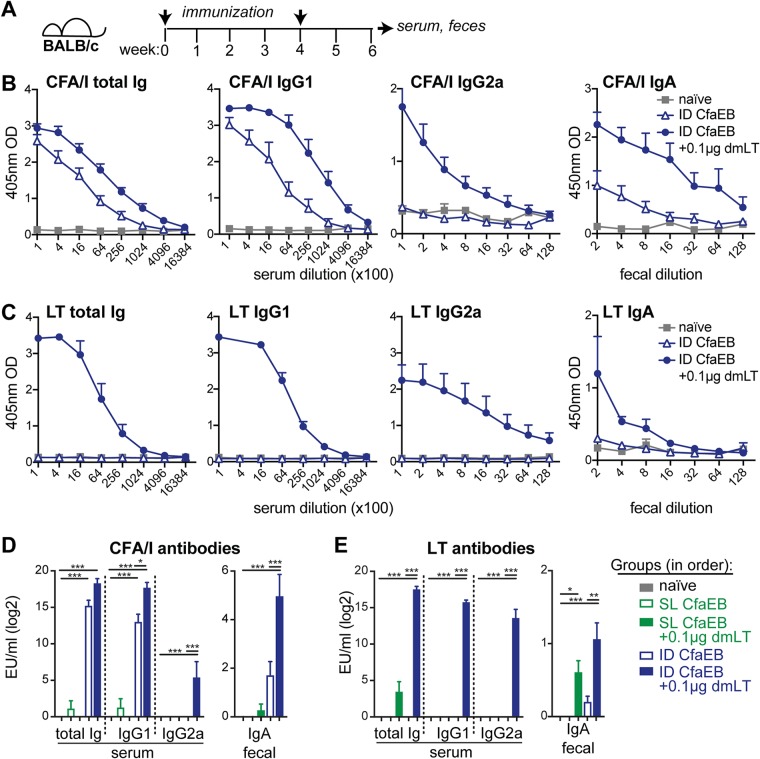
To test vaccination with CfaEB and dmLT, we immunized groups of BALB/c mice by the i.d. or s.l. route with 10 μg CfaEB alone or in combination with 0.1 μg dmLT. The animals were boosted after 4 weeks, and then serum and fecal samples were analyzed 2 weeks later for the development of anti-CFA/I or LT antibodies (Fig. 1A to E).
FIG 1.
Immunization with dmLT admixed with CfaEB induces robust antibody responses to CFA/I fimbriae and LT, but i.d. formulations are not optimal for s.l. delivery. (A) Schematic of immunization experiments. BALB/c mice were left naive or immunized with 10 μg CfaEB with or without 0.1 μg dmLT by i.d. (n = 5) or s.l. (n = 10) delivery on weeks 0 and 4, and then samples were collected at week 6. (B) Raw ELISA data for anti-CFA/I antibodies, given as the optical density (OD), for the i.d. immunized groups. (C) Raw ELISA data for serum anti-LT antibodies for the i.d. immunized groups. (D) Compiled number of ELISA units (EU) per milliliter for anti-CFA/I serum and fecal antibodies after i.d. and s.l. immunizations. (E) Compiled numbers of EU per milliliter for anti-LT serum and fecal antibodies after i.d. and s.l. immunizations. All bars are the mean + SEM. Significance was determined using a one-way ANOVA with the Bonferroni posttest for all groups compared to the naive group and for the i.d. CfaEB group compared to the i.d. CfaEB plus 0.1 μg dmLT group. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
For the groups immunized i.d., mice immunized with CfaEB developed significant levels of anti-CFA/I serum total antibodies (total Ig) and IgG1 compared to naive mice (P ≤ 0.05; Fig. 1B and D). However, as expected, mice immunized with CfaEB-dmLT developed significantly more robust and diverse responses than mice immunized with CfaEB alone, including serum IgG1, IgG2a, and fecal IgA antibody responses (P ≤ 0.05; Fig. 1B and D). In contrast, mice receiving identical formulations by s.l. delivery did not develop a significant CFA/I antibody response (Fig. 1D). All mice immunized i.d. or s.l. with CfaEB-dmLT developed anti-LT fecal IgA, but only the group immunized i.d. developed significant circulating serum antibodies (e.g., anti-LT total Ig, IgG1, and IgG2a [P ≤ 0.001]; Fig. 1C and E). Thus, as expected, mice immunized i.d. with CfaEB developed serum and fecal anti-CFA/I antibodies, and the inclusion of dmLT enhanced the magnitude of these responses. However, the same formulations were not ideal for s.l. delivery, indicating that the antigen composition, antigen dose, and/or adjuvant dose needs to be optimized to increase the antibody response elicited by this route.
Higher doses of dmLT combined with CfaEB or CfaE-LTB chimera antigen improve s.l. immunization outcomes.
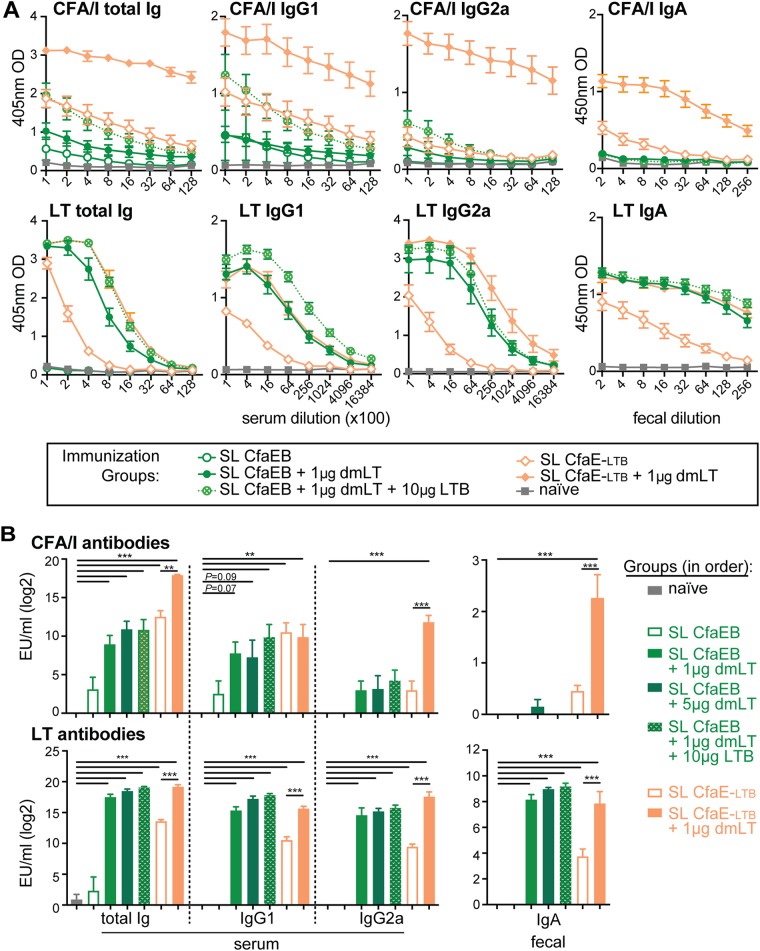
s.l. vaccination likely requires effective antigen transport to cross mucosal tissue and avoid salivary protease degradation. Older studies using dmLT in polio virus, Shigella, or EtpA ETEC vaccines utilized 0.2, 5, or 10 μg dmLT (44–47). Since our s.l. immunization with 0.1 μg dmLT failed (Fig. 1D), we next evaluated if 10 μg CfaEB combined with higher doses of dmLT alone (1 or 5 μg) or combined with excess LTB (10 μg) would improve the vaccination outcomes. Concurrently, we also evaluated whether an LTB fusion antigen would be more effective by using a chimera protein containing the minor adhesin subunit CfaE linked to LTB through a cholera toxin A2 peptide sequence (full name, CfaE-CTA2/LTB, here abbreviated CfaE-LTB). Groups of mice were immunized by prime/boost s.l. and evaluated for the development of serum and fecal CFA/I and LT antibodies.
As before, mice immunized s.l. with CfaEB did not develop significant levels of serum or fecal anti-CFA/I antibodies compared to naive mice (Fig. 2A and B, top). However, mice immunized with CfaEB admixed with 1 μg dmLT, 5 μg dmLT, or 1 μg dmLT and 10 μg LTB developed similar levels of serum anti-CFA/I total Ig, IgG1, and IgG2a. The total Ig levels were statistically significantly different from the total Ig levels in naive mice (P ≤ 0.001), but the levels of serum anti-CFA/I total Ig, IgG1, and IgG2a were not statistically significantly different from each other. No groups immunized with CfaEB antigen developed anti-CFA/I fecal IgA. In contrast, mice immunized with the CfaE-LTB chimera antigen developed robust anti-CFA/I antibodies compared to naive mice (P ≤ 0.01), and with the exception of serum IgG1, these antibodies were significantly enhanced with the addition of 1 μg dmLT (P ≤ 0.01). This CfaE-LTB–dmLT group exhibited the highest anti-CFA/I serum total Ig and IgG2a levels and fecal IgA levels observed in any s.l. group, and the antibody levels were more similar to those achieved with i.d. immunization (Fig. 1). As expected, high levels of anti-LT serum and fecal antibodies were observed in all groups receiving dmLT, CfaE-LTB, and/or LTB antigen (P ≤ 0.001; Fig. 2A and B, bottom), with similar levels being observed between these groups, except for the group receiving CfaE-LTB alone. Thus, s.l. immunization with the fimbrial antigen and optimal doses of dmLT can induce robust circulating antibodies to CFA/I. However, the generation of fecal IgA responses required an alternative antigen form that included a mucosal binding component (i.e., LTB).
FIG 2.
Higher doses (1 to 5 μg) of dmLT combined with CfaEB or the CfaE-LTB chimera improve s.l. vaccination outcomes. Mice were left naive or immunized with 10 μg CfaEB or the CfaE-LTB chimera with or without the indicated doses of dmLT and LTB by s.l. delivery (n = 9 to 10 per group) on weeks 0 and 4, and then samples were collected at week 6. (A) Raw data from ELISA testing for anti-CFA/I or anti-LT antibodies, as shown after s.l. immunizations (data for the CfaEB plus 5 μg dmLT group are not shown). (B) Compiled numbers of EU per milliliter for anti-CFA/I and anti-LT serum and fecal antibodies after s.l. immunization given as the mean + SEM. Significance was determined using a one-way ANOVA with the Bonferroni posttest for all groups compared to the naive group and the CfaE-LTB group compared to the CfaE-LTB plus dmLT group. **, P ≤ 0.01; ***, P ≤ 0.001.
CFA/I serum total antibody ELISA results are the best predictor of functional antibody responses, with CFA/I serum IgG1 and fecal IgA also being significant.
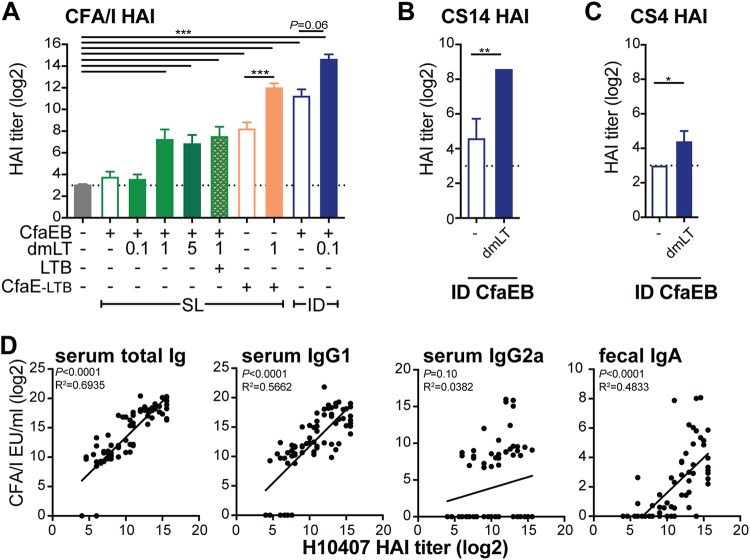
The goal of a fimbrial antigen is to elicit an antibody response to prevent ETEC bacterial attachment to mucosal tissue. We exploited the mannose-resistant hemagglutination property of the ETEC class 5 adhesins to assess the functionality of antiadhesin antibodies to inhibit the agglutination of bovine red blood cells (RBCs) using a hemagglutination inhibition (HAI) assay as a proxy for bacterial intestinal attachment (5). Using sera from all i.d. and s.l. experiments performed with a prime and a boost 4 weeks apart (Fig. 1 and 2; see also Fig. 7), we determined HAI titers using the CFA/I-expressing H10407 ETEC strain. Serum HAI titers mirrored those obtained by antibody enzyme-linked immunosorbent assays (ELISAs), with the highest levels being observed in the CfaEB-dmLT i.d. or the CfaE-LTB–dmLT s.l. group (P ≤ 0.01; Fig. 3A). Only for the groups immunized i.d., we also tested sera for in-class heterologous HAI titers using CS14- and CS4-expressing ETEC strains. We observed a significant expansion in the ability of serum antibodies from the CfaEB-dmLT i.d. group compared with those from the CfaEB i.d. group to neutralize other ETEC class 5a strains (P ≤ 0.05; Fig. 3B and C).
FIG 3.
CFA/I Ig and IgG1 are the best predictors of strain H10407 HAI responses, which were the most strongly induced in the dmLT-adjuvanted i.d. or CfaE-LTB chimera group. Sera from all i.d. and s.l. studies were assessed for HAI titers of functional antibodies. (A) HAI titers in serum of functional antibodies against CFA/I+ ETEC. (B) Serum HAI titers of antibodies against CS14+ ETEC. (C) Serum HAI titers of antibodies against CS4+ ETEC. All bars are the mean + SEM, with the dotted line indicating the assay limit of detection (LOD). Significance was determined using one-way ANOVA with the Bonferroni posttest for all groups compared to the naive group or as shown (A) or using a two-tailed t test (B and C). *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. (D) Linear regressions between H10407 HAI titers and serum CFA/I IgG, IgG1, and IgG2a and fecal IgA antibodies (log2 transformed), with P values and R2 (coefficient of determination) values indicated. Values were included in the regression analyses if the HAI titers were not below the limit of detection (which excluded 74 values).
To determine the strength of the relationship of specific antibodies to H10407 HAI titers, we performed linear regressions between all collected data (Fig. 3D). Serum total Ig and IgG1 responses and fecal IgA responses were significantly related to HAI titers (P ≤ 0.001), but serum IgG2a responses were not. R2 values indicated that the variation in HAI titers could be explained by a linear relationship with serum total Ig (69%) and IgG1 (57%) responses and fecal IgA responses (48%). Thus, immunized animals displayed functional antibodies that inhibited bacterial binding, and these were more highly related to anti-CFA/I total antibodies than serum IgG1 and fecal IgA. This indicates that total serum antibodies detected by ELISA can act as a proxy for expected functional HAI antibodies, at least for our experimental conditions.
Alteration of LT protein B-subunit binding within the i.d. or s.l. vaccine formulation modestly impacts the development of fimbrial humoral and cellular immunity but can reduce toxoid immunity.
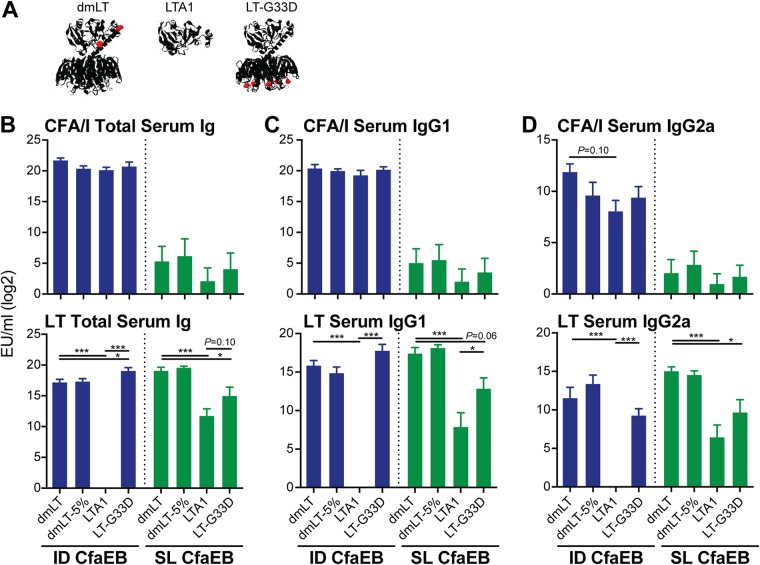
It remains an important consideration to understand how LT proteins contribute to vaccination outcomes, including efficacy and safety. We and others have compared dmLT with alternative LT protein antigens or formulations (22, 31, 48, 49), but this has yet to be performed with fimbrial adhesin antigens and by i.d. delivery. We were particularly interested in the role of B-subunit binding, since this played such an important role in s.l. vaccination responses (Fig. 2) and is known to affect skin reactogenicity after i.d. immunization (and, as such, can be prevented by the G33D mutation) (28). In addition, dmLT for clinical studies (e.g., a good manufacturing practice [GMP] product) is consistently packaged in vials with 5% lactose, which may impact dmLT function, as the B subunit is known to bind to galactose-containing residues (50). To investigate this, we immunized groups of mice by either the i.d. or s.l. route with CfaEB alone or CfaEB admixed with dmLT, dmLT in 5% lactose buffer (dmLT-5%), LTA1, or LT-G33D (Fig. 4A) in a prime/boost immunization as before and analyzed the changes to antibodies, antigen-recall cytokine responses, and skin at the injection site (the groups receiving i.d. immunizations only). All LT-derived proteins except LTA1 were delivered i.d. and at the same dose as dmLT (0.1 μg), since we have found that, without a B subunit, LTA1 requires a slightly higher dose than dmLT when delivered parenterally (22, 30; E. Valli, A. J. Harriett, M. K. Nowakowska, R. L. Baudier, W. B. Provosty, L. B. Lawson, Y. Nakanishi, and E. B. Norton, submitted for publication).
FIG 4.
Altered binding groups (dmLT-5%, LTA1, LT-G33D) exhibit fewer changes by i.d. than by s.l. immunization. Mice were left naive or immunized with 10 μg CfaEB alone or with 10 μg CfaEB admixed with 0.1 μg dmLT, 0.1 μg dmLT in 5% lactose buffer (dmLT-5%), 1 μg LTA1, or 0.1 μg LT-G33D by the i.d. route or 5 μg of protein (dmLT, LTA1, LT-G33D) by s.l. delivery on weeks 0 and 3, and then samples were collected at week 5 (n = 4 to 6 per group). (A) Ribbon diagram of dmLT, LTA1, and LT-G33D, with mutations indicated in red. (B) Total number of serum Ig ELISA units (EU) per milliliter for anti-CFA/I (top) or anti-LT (bottom). (C) Serum IgG1 for anti-CFA/I (top) or anti-LT (bottom). (D) Serum IgG2a for anti-CFA/I (top) or anti-LT (bottom). All bars are the mean + SEM. Significance was determined by a one-way ANOVA with the Bonferroni posttest for all adjuvanted groups compared to the CfaEB-dmLT group. *, P ≤ 0.05; ***, P ≤ 0.001.
i.d. immunizations with CfaEB and admixed LT proteins resulted in comparable levels of serum anti-CFA/I total Ig, IgG1, and IgG2a antibodies, although there was a nonsignificant reduction in antibody levels in the dmLT-5%, LTA1, and LT-G33D groups compared with the dmLT group (Fig. 4B to D, blue bars). Similar formulations delivered s.l. with CfaEB elicited modest or low anti-CFA/I responses, with no clear difference being observed between the groups (Fig. 4B to D, green bars), similar to the results of the previous experiments (Fig. 2). High levels of anti-LT serum total Ig and IgG1 antibodies were observed in the dmLT-5% and LT-G33D i.d. groups, similar to the findings for the dmLT i.d. group, but these responses were markedly absent in the LTA1 i.d. group (P ≤ 0.001; Fig. 4). Relatedly, both the LTA1 s.l. group and the LT-G33D s.l. group exhibited reduced anti-LT serum responses compared with the dmLT s.l. group (P ≤ 0.06). Interestingly, the anti-LT IgG2a level was lower after both the i.d. and the s.l. immunizations with the GM1-binding deficient-mutant protein LT-G33D. Altogether, these results point to the importance of the B subunit in the generation of maximal anti-LT serum responses by both the s.l. and i.d. routes and indicate that the addition of 5% lactose did not appreciably alter these responses. In addition, B-subunit GM1 binding was critical for the generation of LT serum antibodies by the s.l. route or IgG2a antibodies by any route.
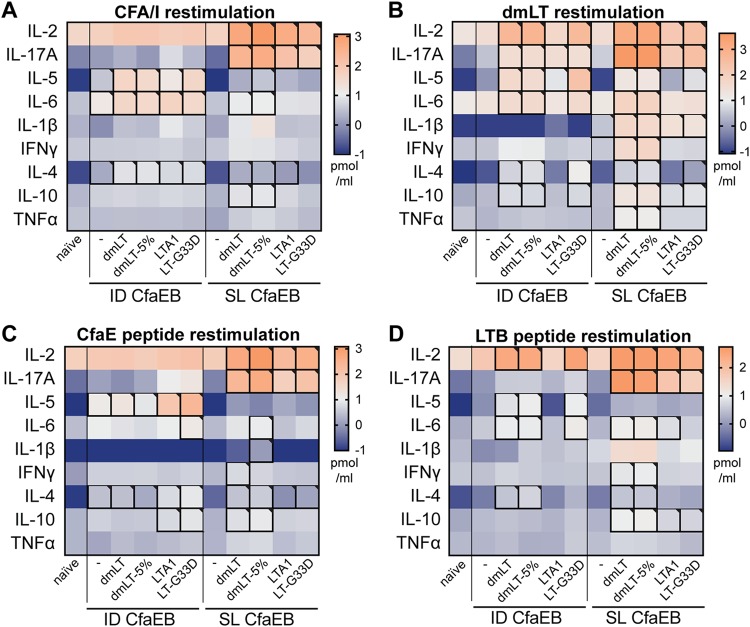
For these experiments, we also evaluated cellular immunity and memory CD4 T-cell responses by measuring cytokine secretion in splenocytes recovered from immunized animals and stimulated ex vivo with protein antigens (e.g., CFA/I, dmLT) or 15-amino-acid long peptides (e.g., CfaE or LTB peptide pools). We observed that similar cytokine levels were secreted within each immunization group in response to either protein antigens or related peptide pools, indicating that memory CD4 T cells were induced with vaccination (P ≤ 0.05; Fig. 5A to D; see also Fig. S1 in the supplemental material). In addition, the delivery route and antigen strongly influenced the generation of these responses, with the highest levels of IL-17 and IL-2 secretion being observed after s.l. immunizations compared with the levels observed after i.d. immunizations, and higher levels of IL-17 secretion were observed with dmLT or LTB peptide restimulation than with CFA/I or CfaE peptide restimulation. The highest levels of these cellular responses were observed in the groups immunized with dmLT or dmLT-5% admixed with CfaEB, although both LTA1 and LT-G33D also induced significant cytokine secretion (P ≤ 0.05). As expected, unlike the dmLT, dmLT-5%, and LT-G33D i.d. groups, the LTA1 i.d. group exhibited low levels of cytokine secretion in response to the LTB peptide; however, curiously, this was not as apparent in the LTA1 s.l. group for the interleukin-2 (IL-2), IL-17A, and IL-6 cytokines. These results reveal the surprising finding that cytokine recall responses indicative of CD4 T-cell memory responses (e.g., consistently equivalent levels of expression of IL-17 after s.l. delivery and after i.d. delivery or higher levels of expression of IL-17 after s.l. delivery than after i.d. delivery) are distinct from the observed serum antibody responses. In addition, the addition of 5% lactose, the absence of a B subunit, or GM1 binding did not inhibit the generation of CFA/I-specific cellular responses and only minimally altered LT-specific cellular responses.
FIG 5.
Recall cytokine responses after i.d. and s.l. vaccination with CFA/I or dmLT and peptide restimulation. The results obtained after ex vivo restimulation of splenocytes from naive or immunized mice 2 weeks after receiving a prime/boost of 10 μg CfaEB with or without dmLT, dmLT in 5% lactose buffer, LTA1, or LT-G33D by i.d. or s.l. delivery on weeks 0 and 3 (n = 4 to 6 per group) are shown. Cytokines were detected in culture supernatants by a Bio-Plex assay. (A) Heat map of log10 cytokine levels after CFA/I protein stimulation. (B) Heat map of log10 cytokine levels after dmLT protein stimulation. (C) Heat map of log10 cytokine levels after CfaE peptide pool stimulation. (D) Heat map of log10 cytokine levels after LTB peptide pool stimulation. Significant values (P ≤ 0.05) obtained by one-way ANOVA with Dunnett’s posttest for all groups compared to the naive group are indicated by outlining with a black box. IFN-γ, gamma interferon; TNFα, tumor necrosis factor alpha.
Alteration of LT protein B-subunit binding within i.d. vaccine formulations reduces skin reactogenicity.
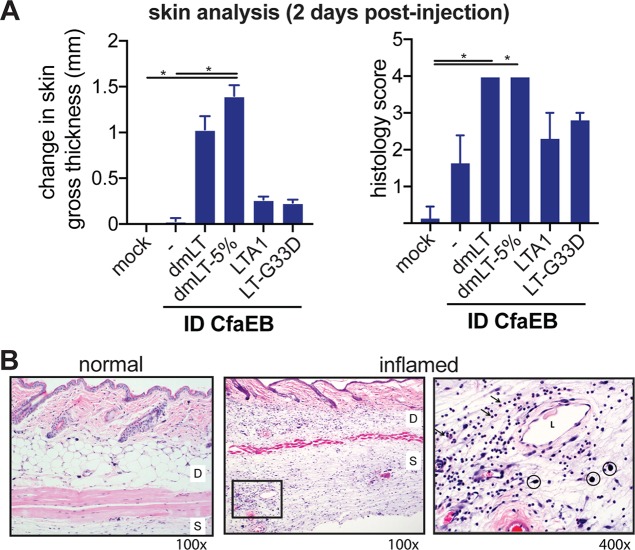
Skin reactogenicity is an important consideration of any i.d. vaccine. To examine how altered B-subunit binding or LT proteins may impact this, we repeated the prime/boost experiments and examined the responses at 2 or 45 days postimmunization. Acute reactogenicity at 2 days was selected based on previous observations that local induration and swelling peak at this time with mLT or dmLT delivered i.d. (M. Maciel, Jr., unpublished data). Skin samples from the site of immunization were evaluated for changes, including pathology scoring on a scale ranging from 0 to 5 for inflammation or skin lesions. A score of 3 (moderate dermatitis) or below is noted as an acceptable level of skin reactogenicity based on this scoring system (although any acceptable level would need to consider the severity, duration, and character of inflammation). There was a significant difference in the day 2 postimmunization skin gross thickness or histology scores between both the dmLT and dmLT-5% i.d. groups and the CfaEB-only i.d. group (P ≤ 0.05) but not the LTA1 or LT-G33D i.d. group (Fig. 6A and B). This included significant inflammation (histology median score = 4) and edema in the dmLT or dmLT-5% i.d. group, characterized by neutrophil infiltration in the dermis (i.e., dermatitis) and subcutis (i.e., panniculitis) with mild epidermal hyperplasia. The LT-G33D or LTA1 i.d. group exhibited moderate inflammation (histology median score = 3) and edema with dilated lymphatics, as well as neutrophil infiltrates admixed with macrophages and a few lymphocytes. Mice immunized with CfaEB alone (histology median score = 1.5) had no edema and only mild focal aggregates of a few neutrophils, macrophages, and lymphocytes. No dermatitis or panniculitis was observed in the mock-immunized mice (mice receiving phosphate-buffered saline [PBS] buffer only).
FIG 6.
Acute skin reactogenicity is altered by LT antigen after intradermal immunization. Mice (n = 3 per group) were immunized with PBS buffer alone (mock) or with PBS buffer containing 10 μg CfaEB with or without dmLT, dmLT in 5% lactose buffer, LTA1, or LT-G33D by i.d. delivery (all at 0.1 μg, except for 1 μg for LTA1). Skin samples were collected at 2 days postimmunization. (A) Changes to skin gross thickness, measured by the use of digital calipers, between the injection site and the adjacent back skin. The histology score for the severity of inflammation or tissue lesion is for H&E-stained skin sections and ranges from 0 to 5. Significance compares all groups to the naive or CfaEB i.d. groups and was determined using one-way ANOVA with the Kruskal-Wallis test with Dunn’s posttest. *, P ≤ 0.05. (B) Representative normal and inflamed injection sites are shown at magnifications of ×100 and ×400. D, dermis; S, subcutis; L, dilated lymphatics; arrows, neutrophil infiltrate; circles, mast cells. There were no differences in mast cells noted between groups.
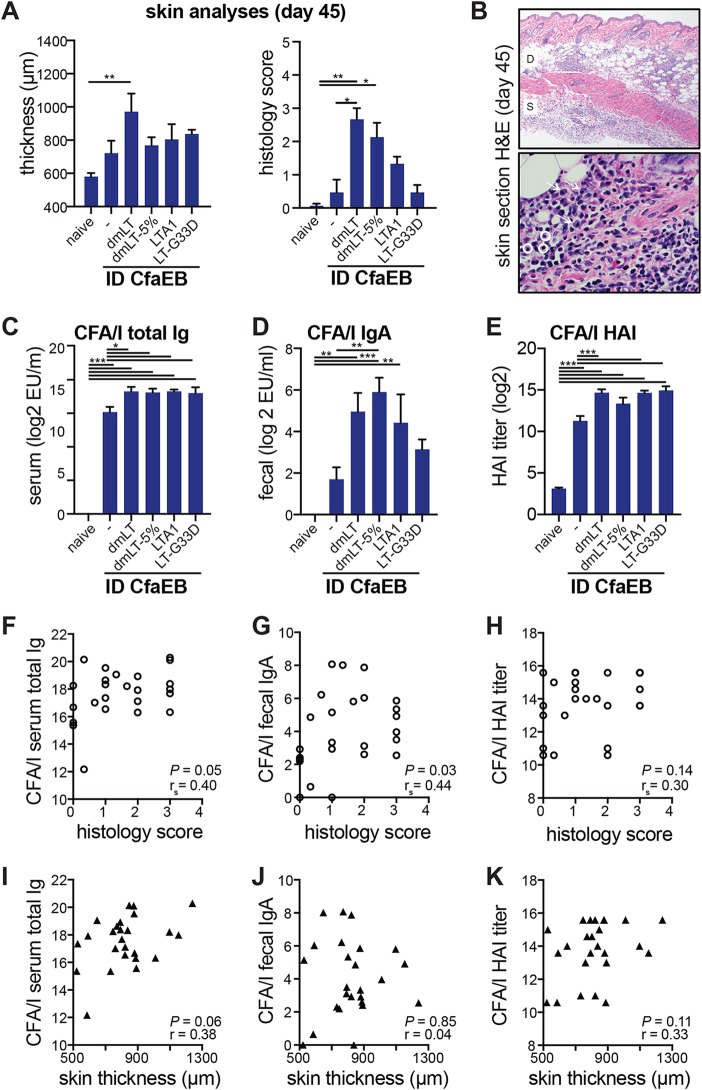
In animals prime/boost immunized and analyzed on day 45, we did not observe any changes in gross skin thickness at the primary or secondary site of immunization from that at adjacent skin samples or in naive animals. Thus, in order to more carefully examine skin changes, the thickness of the skin was measured using hematoxylin-eosin (H&E)-stained sections that were also scored as described above (Fig. 7A). We primarily observed significant skin reactions only in the dmLT group (P ≤ 0.01; histology median score = 3), but these were clearly reduced from those seen at day 2 (Fig. 6A) and were characterized by infiltrates of plasma cells and lymphocytes in the dermis with few neutrophils and minimal to no edema (Fig. 7B). Lower levels of inflammation were also present in the dmLT-5% (histology median score = 2), LTA1 (histology median score = 1), and LT-G33D (histology median score = 0.33) groups. Similar to the antibody responses observed in previous experiments (Fig. 4B), all i.d. vaccinated groups were significantly different from naive animals for the development of CFA/I total Ig and CFA/I HAI (P ≤ 0.001), but for CFA/I fecal IgA, only the responses in the dmLT (P ≤ 0.01), dmLT-5% (P ≤ 0.001), and LTA1 (P ≤ 0.01) groups were significantly different from those in the naive animals. To determine if these vaccination outcomes correlated to skin reactogenicity, we performed correlation analyses with all data from immunized animals. Except for the histology score and anti-CFA/I serum total antibodies and fecal IgA (Fig. 7F and G), most of these relationships were not significant (Fig. 7H to K). In addition, the correlation coefficients were all closer to 0 than to 1, indicative of disagreement between values. Thus, alteration of B-subunit binding reduced skin reactogenicity. Furthermore, the level of skin reactogenicity did not strongly correlate with the development of antigen-specific antibodies. Thus, our results indicate that manipulation of B-subunit binding through either buffer formulation (e.g., addition of 5% lactose) or inclusion of a different LT antigen/adjuvant (e.g., LTA1, LT-G33D) can benefit site-specific effects without compromising vaccination outcomes.
FIG 7.
Chronic skin reactogenicity is altered by LT antigen after intradermal immunization and partially correlates to vaccination outcomes. BALB/c mice were immunized with 10 μg CfaEB with or without 0.1 μg dmLT by the i.d. route on weeks 0 and 4, and skin samples from the injection sites were collected on week 6 (or day 45). (A) Histological scores are for the severity of inflammation or skin lesion on a scale of from 0 to 5 for H&E-stained skin sections and the thickness of the tissue samples. (B) Representative images of H&E-stained skin inflammation, identifying cellular infiltration of plasma cells (arrows) and lymphocytes (circles) but little edema. (C to E) Antibody analyses, including anti-CFA/I total serum Ig determined by ELISA (C), anti-CFA/I fecal IgA determined by ELISA (D), or serum HAI titers for functional antibodies against CFA/I+ ETEC (E). All bars are the mean + SEM. Significance was determined using one-way ANOVA with either the Bonferroni posttest or the Kruskal-Wallis test with Dunn’s posttest (histology score only), comparing all groups to the naive or CfaEB i.d. group. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. (F to H) Spearman correlations to histology scores, with P values and correlation coefficient (rs) indicated, using anti-CFA/I serum total Ig (F), anti-CFA/I fecal IgA (G), or CFA/I HAI titer (H). (I to K) Pearson correlation to skin thickness, with P values and correlation coefficient (r) indicated, using anti-CFA/I serum total Ig (I), anti-CFA/I fecal IgA (J), or CFA/I HAI titer (K).
DISCUSSION
An effective ETEC vaccine to protect children, travelers, and U.S. military personnel should cover about 80% of the epidemiologically relevant ETEC CFs and toxins (12). CfaEB is a fusion protein with the minor (CfaE) and major (CfaB) subunits of CFA/I, which are representative CFs from the ETEC class 5 fimbria family and which can also extend coverage against CS4 and CS14 colonization factors. Subunit protein vaccine candidates, such as CfaEB and CfaE-LTB, offer several advantages over strategies based on attenuated or killed whole-cell vaccines or purified whole fimbriae (e.g., CFA/I): (i) optimized immunogenicity, (ii) well-defined manufacturing processes, (iii) easy parenteral administration, and (iv) the possibility of combination with other vaccines on the Expanded Program on Immunization schedule. However, contrary to whole cells, which can retain their natural adjuvanticity due to pathogen-associated molecular patterns, protein-based subunit vaccines likely present low antigenicity and usually require the use of exogenous adjuvants. The use of LT-based adjuvants for a subunit-based ETEC vaccine aims at enhancing the antigenicity of the vaccine antigens as well as eliciting antitoxin immunity.
In the experiments presented here, we have shown that i.d. delivery of dmLT combined with CfaEB elicited high levels of fimbrial and toxin immunity (Fig. 1, 3, 4, 5, and 7). This is the first time that this fimbrial antigen has been evaluated with dmLT in a mouse model for the antibody and cellular response, updating older studies that utilized CfaE and mLT (8, 32, 51). We also report the first evaluation of vaccination with this combination of antigens by the s.l. route (Fig. 2 to 5), an attractive needle-free mucosal delivery route. Of note, our studies analyzed fecal IgA postimmunization. While we have previously found that fecal IgA correlates with antibody-secreting lymphocytes in intestinal tissue after parenteral immunization with the dmLT adjuvant (52), in this study, we did not specifically confirm the presence of antibody-secreting cells in the intestines. We did find that antibody responses similar to those achieved with i.d. vaccination were achieved only with the s.l. delivery of a CfaE-LTB chimera antigen in lieu of CfaEB, although as reported previously, higher levels of dmLT in s.l. formulations improve vaccine responses (44–47). This would indicate that while dmLT improves s.l. vaccines, another strategy to induce robust antibody responses is to engineer proteins to have their own mucosal binding mechanisms (e.g., by creation of an LTB fusion protein). Addition of free LTB had only a modest effect very similar to that of admixed dmLT. There is an oral ETEC vaccine in clinical trials that admixes a whole-cell killed vaccine with an LTB subunit-related toxoid (LCTBA) and small amounts of admixed dmLT (25). Our results do not show a clear advantage to this approach, at least for a subunit antigen s.l. vaccine. In contrast to our antibody responses, we observed robust cellular responses, including cytokine secretion from memory CD4+ T cells in the CfaEB-dmLT s.l. immunization group. High levels of IL-17, IL-2, and even gamma interferon in response to fimbrial and toxin antigen restimulation were observed by s.l. delivery, indicative of a Th17 or Th1 memory response. Many published ETEC vaccine studies have focused on the development of long-lasting antibody responses, but there has been less evaluation of memory T-cell responses, particularly at mucosal tissue. However, these may play a critical role in protection. A recently published study on a live-attenuated oral immunization with admixed dmLT (ACE527-dmLT) as an ETEC vaccine demonstrated significant clinical protection from moderate to severe diarrhea after oral challenge with the CFA/I-positive (CFA/I+) H10407 ETEC strain 6 months after vaccination (32, 53). However, there were no appreciable differences in serum or fecal antibody responses detected from subjects receiving ACE527 or ACE527-dmLT, despite poor disease protection in subjects receiving only ACE527. Thus, both humoral and cellular responses may play an additional, critical role in orchestrating immunity to ETEC diarrheal disease and warrant further evaluation.
There is no widely accepted challenge model for ETEC infection in rodents, as infection does not induce diarrheal disease. This is likely due to differences in attachment to intestinal cells or in vivo toxin expression, as direct LT or ST oral administration induces measurable fluid secretion in the intestines (31, 54). CfaE, the minor subunit of CFA/I, is essential to the attachment of CFA/I+ ETEC to the human small intestine. We know that the adhesive capacity of CFA/I can be assessed in vitro with differentiated Caco2 cells as well as with mammalian RBCs through HAI (55). Moreover, beads coated with CfaE but not beads coated with CfaB (the major subunit of CFA/I) are able to agglutinate RBCs. The binding site of CfaE is located in the upper pole of the molecule and consists of conserved residues, including arginine 181 (56). In fact, experimental mutagenesis of that residue and its replacement by alanine led to a loss of function (9). The HAI assay can measure the in vitro inhibition of bacterial binding by neutralizing antibodies targeting CfaE. Contrary to the evaluation of functional neutralizing antibodies with Caco2 cells, the HAI assay is high throughput and more reproducible, although the neutralization titers measured by this assay have not yet been correlated to protection in human studies. We found that the neutralization titers obtained by the HAI assay correlated to the ELISA results, particularly the serum anti-CFA/I total antibody responses (Fig. 3). Curiously, serum anti-CFA/I IgG2a responses, often related to a Th1 or Th17 response (57), did not seem to be involved in this response, although care should be taken in extrapolating these results to other models or vaccination formulations/routes.
We also report a critical evaluation of the role of the B subunit in a subunit ETEC vaccine, with in-depth analyses being performed particularly with i.d. vaccination using a 5% lactose formulation, LTA1, or LT-G33D (Fig. 4 to 7). To our knowledge, the effect of lactose on the immunologic profile of dmLT has not been previously evaluated, even though the B subunit has a low affinity of binding to blood sugars like lactose and GMP material is included in the buffer in vials with dmLT-5% (58, 59). In previous studies, we evaluated the adjuvanticity and antigenicity of LTA1 given by the i.n. route (22, 30), and others have published information on the ability of LT-G33D to reduce skin reactogenicity while maintaining i.d. adjuvanticity (28). Our major finding was that a simple change to the buffer composition by inclusion of 5% lactose in the formulation with dmLT prevented higher skin histology scores and thicknesses after the prime/boost vaccination without any changes to serum or fecal antibodies or memory T-cell responses. This is likely due to altered or delayed binding to the GM1 on cells upon immunization and would be interesting to explore further. Substitution of altered LT proteins, including LTA1 (with no B subunit) or LT-G33D (lacking GM1 binding), for dmLT further attenuated skin reactogenicity as well as acute skin changes 2 days after the prime, with no major trade-offs in CFA/I serum antibodies or memory T-cell responses being seen. However, these immunization groups did exhibit a lower magnitude of induced CFA/I fecal antibodies and LT immunity, particularly when LTA1 was used instead of dmLT. This was anticipated, as while natural infection induces A-subunit antibodies, the i.n. or s.l. delivery of LTA1 in mice has not previously induced strong anti-LT antibodies (22). It was surprising, then, that we detected cytokine recall responses with LTA1 and LT-G33D after s.l. vaccination, indicating a deficit in the movement of intact antigens across mucosal surfaces but not the prevention of immune stimulation. This will need to be investigated further.
In conclusion, we found that the i.d. or s.l. delivery route, the form of LT antigen, or the use of an LTB fusion protein played an important role in vaccination outcomes. Our findings help reveal how formulations and delivery parameters shape the adaptive immune responses to a toxoid- and fimbria-derived subunit vaccine against ETEC. In addition, these strategies can benefit vaccine design when using dmLT as an antigen or adjuvant in vaccines targeting ETEC or other pathogens, with notable findings including methods to achieve robust humoral or cellular responses by s.l. vaccination as well as approaches to i.d. vaccination to promote immunity without skin reactogenicity.
MATERIALS AND METHODS
Purification of proteins.
LT, LTB, and dmLT were produced from E. coli clones expressing recombinant protein derived from the human ETEC isolate E. coli H10407 as previously described (31, 60, 61). The LT-G33D mutation was created by site-directed mutagenesis of LT expression plasmid pCS96 using a mutagenic oligonucleotide (5′-GGAATCGATGGCAGA*CAAAAGAGAAATGGTTATCA-3′) to make a single nucleotide substitution (the underlined nucleotide in the sequence given above) at amino acid position 33 (the boldface amino acid in the sequence given above) from the N terminus of LTB. Briefly, organisms were cultured overnight in Casamino Acids yeast extract medium, the cells were lysed, the lysate was clarified by centrifugation, and LT-related proteins were purified by galactose affinity chromatography. His-tagged LTA and His-tagged LTA1 were prepared from solubilized inclusion bodies by high-performance liquid chromatography with a nickel-affinity column as previously described (30). The composition and purity of each protein were confirmed by SDS-PAGE and a Limulus amebocyte lysate assay (Lonza, Inc.). The endotoxin content of the final products was <1 endotoxin unit/mg. The CfaEB protein was expressed and purified from a BL21(DE3) expression host using previously described methods (62). Briefly, the donor strand complement of CfaE (dscCfaEB) was purified from cell lysates using a HisTrap FF column, in which the protein was eluted with a linear imidazole gradient (5 to 300 mM imidazole over 20 column volumes) in 20 mM phosphate buffer, pH 7.4, followed by a HiTrap SP HP column, in which the protein was eluted with a linear salt gradient (0 to 1 M sodium chloride) in a 20 mM MES (morpholineethanesulfonic acid) buffer, pH 5.5. The dscCfaE-CTA2/LTB chimera protein was engineered through the replacement of the A1 subunit of CT with a donor strand complement of CfaE (dsc14CfaE-sCTA2). Briefly, the fusion protein was coexpressed with LTB in an E. coli BL21 expression system, forming a noncovalently associated dsc14CfaE-sCTA2/LTB chimera (here referred to as CfaE-LTB) protein purified to ≥85% purity with endotoxin levels of <2,000 endotoxin units/mg using metal-affinity chromatography (Talon Superflow column; Clontech) and cation exchange chromatography (SP HP column; GE Healthcare). The proteins were stored lyophilized and freshly resuspended prior to use (LT, LTB, LT-G33D, dmLT) or were kept frozen at −80°C until use (LTA1, CfaEB, CfaE-LTB).
Animals, immunizations, and sample collections.
Female BALB/c mice 6 to 8 weeks of age were purchased from Charles River Laboratories and housed in sterile cages. All animal studies were approved by the Tulane University Institutional Animal Care and Use Committee. The study protocol was reviewed and approved by the Tulane University Institutional Animal Care and Use Committee and was in compliance with all applicable federal regulations governing the protection of animals in research. Immunization formulations were prepared immediately before administration by admixing antigen with or without adjuvant in sterile PBS alone or, where specified, in sterile PBS containing 5% (wt/vol) beta-lactose (MilliporeSigma). For i.d. immunizations, hind flank skin was shaved and 20 μl was injected using the Manteaux technique with a 28-gauge insulin syringe. For s.l. immunization, the mice were intraperitoneally injected with ketamine-xylazine and then held upright for 1 min while 10 μl formulation was pipetted underneath the tongue and allowed to absorb. Immunizations were performed 2 times at 3- to 4-week intervals prior to CO2 euthanasia for sample collection. Blood was collected by cardiac puncture and processed for serum. Fecal material was collected from the colon, weighed, and homogenized in 1 ml PBS containing 0.05% Tween 20 (MilliporeSigma), 1 mM EDTA (MilliporeSigma), and a protease inhibitor cocktail (Roche), followed by centrifugation and collection of supernatants. Spleens were homogenized in 3 ml of PBS containing 2% bovine serum albumin (MilliporeSigma) and 1 mM EDTA using gentleMACS C tubes and tissue dissociator (Miltenyi Biotec). RBCs were lysed using ACK lysis buffer (Gibco), and splenocytes were filtered and counted.
Antibody ELISAs.
Serum total Ig, IgG1, and IgG2a and fecal IgA antibody ELISAs were performed using CoStar 96-well flat-bottom plates as previously described (30, 31). Briefly, the wells of the plates were coated with 2 μg/ml of CFA/I (purified from wild-type ETEC strain WS1933D, as previously reported [8]), 1 μg/ml of LT, or an external recombinant mouse IgG1 or IgG2a standard (MilliporeSigma) or IgA standard (Southern Biotech), incubated with dilutions of individual serum or fecal samples, and detected using alkaline phosphatase-conjugated anti-mouse IgG (which binds nonspecifically to IgG, IgA, and IgM; MilliporeSigma) or IgG1 or IgG2a (BD Biosciences) or using horseradish peroxidase-conjugated anti-mouse IgA (MilliporeSigma). The absorbance was read at 405 nm, and the data were analyzed using a sigmoidal dose-response with least-squares fit. The results were log2 transformed after adding 1 and quantitated as the number of ELISA units (EU) per milliliter using the average for two sample dilutions closest to the midpoint of the standard curve.
ETEC HAI assay.
The hemagglutination inhibition (HAI) assay was adapted from previous studies (55). Briefly, ETEC class 5a bacterial strains expressing different CFs, namely, CFA/I (strain H10407), CS4 (strain Bang-10 SP), and CS14 (strain WS3294A), were grown overnight on colonization factor antigen (CFA) agar plates with bile salts, harvested, and resuspended in 0.5% d-mannose (MilliporeSigma) in PBS (PBS-M) to a final solution with an optical density at 650 nm (OD650) of 0.2 ± 0.02. Serum samples were initially diluted at 1:8 in PBS-M followed by 2-fold serial dilutions, for a final volume of 25 μl per well. Twenty-five microliters of the bacterial suspension was added to each well, doubling the final sample dilution, and the plate was shaken at 500 rpm for 30 min at room temperature. After the incubation, 25 μl of 1.5% bovine RBCs was added per well, the plate was shaken at 500 rpm for 30 min at 4°C, and the presence or absence of agglutination was recorded immediately after. Each sample was tested in duplicate, and the average HAI titer that completely inhibited mannose-resistant hemagglutination was calculated and log2 transformed.
Skin reactogenicity analyses.
Skin samples from the injection sites were collected at day 2 after the prime or week 2 after the boost (day 45). For acute-phase analysis, the injection site was marked at the time of immunization with a permanent marker. The gross skin thickness was directly measured from skin excised from euthanized animals using a digital caliper. For histopathological analysis, skin samples were placed in 10% neutral buffered formalin and fixed for a minimum of 24 h at room temperature. The fixed tissue was processed by ethanol dehydration and embedded in paraffin, cut into 5-μm-thick sections, and stained with hematoxylin and eosin (H&E). H&E-stained sections were scored in a blind manner by a board-certified veterinary pathologist for the severity of inflammation (noting the immune cell type) and skin thickness (in micrometers). The severity scores ranged from 0 to 5 and was classified as follows: 0, no lesions; 1, minimal; 2, mild; 3, moderate; 4, marked; and 5, severe.
Memory cell cytokine secretion.
The 30 15-amino-acid peptides (overlapping by 10 amino acids) spanning the whole LTB protein were purchased (GenScript) to create the LTB peptide pool. The 13 highest-ranked peptides from a CfaE pool created using epitope predication software (RankPep) were similarly purchased to create the CfaE pool. Splenocytes were treated at 1 × 106 cells/200 μl/well in RPMI–10% fetal bovine serum with antibiotic-antimycotic (Gibco) and 5 μg/ml CFA/I, 1 μg/ml dmLT, 4 μg/ml CfaEB, or LTB peptide pools. The cells were incubated at 37°C with 5% CO2 for 72 h, and the supernatants were collected and stored at −20°C. Freshly thawed samples were tested using a Bio-Plex Pro Th17 6-plex assay and IL-2, IL-4, and IL-5 mouse single-plex beads (Bio-Rad) and evaluated with a Bio-Plex 200 system (Bio-Rad) following the kit instructions.
Statistical analysis.
Statistical analyses were performed using Prism (v7; GraphPad Software) and SAS (v9.4; SAS Institute Inc.) software. Parametric data were analyzed by one-way analysis of variance (ANOVA) with Dunnett’s posttest for all groups compared to a control group or the Bonferroni correction for comparison of selected pairs. Nonparametric data were analyzed by the Kruskal-Wallis test with Dunn’s posttest. CfaEB and LT antibodies were tested for a linear relationship with strain H10407 HAI responses, and significant models were determined to be adequate by nonsignificant results using lack-of-fit test results. Serum total Ig and fecal IgA titers compared with HAI titers were nonsignificant for a lack of fit, indicating that the serum IgG1 titer alone does not adequately explain the variance in HAI titers. Correlations to parametric data were performed using a Pearson correlation, and correlations to nonparametric data were performed using a Spearman correlation.
Data availability.
Any materials and data that are reasonably requested by others will be made available in a timely fashion, at reasonable cost, to members of the scientific community for noncommercial purposes.
Supplementary Material
ACKNOWLEDGMENTS
The research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI114697 to E. B. Norton. This research was also supported in part by the Henry M. Jackson Foundation for the Advancement of Military Medicine and TULANE-NMRC LP-CRADA grant 9283 (5 April 2011).
S.J.S. and M.A.S. served as military service members over the course of this work, which was prepared as part of their official duties.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the U.S. Department of the Army or Navy, the U.S. Department of Defense, or the U.S. government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We all declare that there are no financial, institutional, or other relationships that might lead to bias or a conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00460-19.
REFERENCES
- 1.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AA. 2013. The impoverished gut—a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol 10:220–229. doi: 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosangadi D, Smith PG, Giersing BK. 11 October 2017. Considerations for using ETEC and Shigella disease burden estimates to guide vaccine development strategy. Vaccine. doi: 10.1016/j.vaccine.2017.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter CK, Olson S, Hall A, Riddle MS. 2017. Travelers’ diarrhea: an update on the incidence, etiology, and risk in military deployments and similar travel populations. Mil Med 182:4–10. doi: 10.7205/MILMED-D-17-00064. [DOI] [PubMed] [Google Scholar]
- 4.Steffen R, Hill DR, DuPont HL. 2015. Traveler’s diarrhea: a clinical review. JAMA 313:71–80. doi: 10.1001/jama.2014.17006. [DOI] [PubMed] [Google Scholar]
- 5.Bourgeois AL, Wierzba TF, Walker RI. 2016. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine 34:2880–2886. doi: 10.1016/j.vaccine.2016.02.076. [DOI] [PubMed] [Google Scholar]
- 6.Savarino SJ, McKenzie R, Tribble DR, Porter CK, O’Dowd A, Cantrell JA, Sincock SA, Poole ST, DeNearing B, Woods CM, Kim H, Grahek SL, Brinkley C, Crabb JH, Bourgeois AL. 2017. Prophylactic efficacy of hyperimmune bovine colostral antiadhesin antibodies against enterotoxigenic Escherichia coli diarrhea: a randomized, double-blind, placebo-controlled, phase 1 trial. J Infect Dis 216:7–13. doi: 10.1093/infdis/jix144. [DOI] [PubMed] [Google Scholar]
- 7.Luiz WB, Rodrigues JF, Crabb JH, Savarino SJ, Ferreira LC. 2015. Maternal vaccination with a fimbrial tip adhesin and passive protection of neonatal mice against lethal human enterotoxigenic Escherichia coli challenge. Infect Immun 83:4555–4564. doi: 10.1128/IAI.00858-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sincock SA, Hall ER, Woods CM, O’Dowd A, Poole ST, McVeigh AL, Nunez G, Espinoza N, Miller M, Savarino SJ. 2016. Immunogenicity of a prototype enterotoxigenic Escherichia coli adhesin vaccine in mice and nonhuman primates. Vaccine 34:284–291. doi: 10.1016/j.vaccine.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Li YF, Poole S, Rasulova F, McVeigh AL, Savarino SJ, Xia D. 2009. Crystallization and preliminary X-ray diffraction analyses of several forms of the CfaB major subunit of enterotoxigenic Escherichia coli CFA/I fimbriae. Acta Crystallogr Sect F Struct Biol Cryst Commun 65:242–247. doi: 10.1107/S1744309109001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakellaris H, Balding DP, Scott JR. 1996. Assembly proteins of CS1 pili of enterotoxigenic Escherichia coli. Mol Microbiol 21:529–541. doi: 10.1111/j.1365-2958.1996.tb02562.x. [DOI] [PubMed] [Google Scholar]
- 11.Froehlich BJ, Karakashian A, Melsen LR, Wakefield JC, Scott JR. 1994. CooC and CooD are required for assembly of CS1 pili. Mol Microbiol 12:387–401. doi: 10.1111/j.1365-2958.1994.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 12.Isidean SD, Riddle MS, Savarino SJ, Porter CK. 2011. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine 29:6167–6178. doi: 10.1016/j.vaccine.2011.06.084. [DOI] [PubMed] [Google Scholar]
- 13.Moss J, Osborne JC Jr, Fishman PH, Nakaya S, Robertson DC. 1981. Escherichia coli heat-labile enterotoxin. Ganglioside specificity and ADP-ribosyltransferase activity. J Biol Chem 256:12861–12865. [PubMed] [Google Scholar]
- 14.Clements JD, Hartzog NM, Lyon FL. 1988. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine 6:269–277. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 15.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 16.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJ, McGrath M, Olortegui MP, Samie A, Shakoor S, Mondal D, Lima IF, Hariraju D, Rayamajhi BB, Qureshi S, Kabir F, Yori PP, Mufamadi B, Amour C, Carreon JD, Richard SA, Lang D, Bessong P, Mduma E, Ahmed T, Lima AA, Mason CJ, Zaidi AK, Bhutta ZA, Kosek M, Guerrant RL, Gottlieb M, Miller M, Kang G, Houpt ER, MAL-ED Network Investigators . 2015. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 3:e564–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruz JR, Gil L, Cano F, Caceres P, Pareja G. 1988. Breast milk anti-Escherichia coli heat-labile toxin IgA antibodies protect against toxin-induced infantile diarrhea. Acta Paediatr Scand 77:658–662. doi: 10.1111/j.1651-2227.1988.tb10726.x. [DOI] [PubMed] [Google Scholar]
- 18.Porter CK, Riddle MS, Tribble DR, Bougeois AL, McKenzie R, Isidean SD, Sebeny P, Savarino SJ. 2011. A systematic review of experimental infections with enterotoxigenic Escherichia coli (ETEC). Vaccine 29:5869–5885. doi: 10.1016/j.vaccine.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Wenneras C, Erling V. 2004. Prevalence of enterotoxigenic Escherichia coli-associated diarrhoea and carrier state in the developing world. J Health Popul Nutr 22:370–382. [PubMed] [Google Scholar]
- 20.Qadri F, Saha A, Ahmed T, Al Tarique A, Begum YA, Svennerholm AM. 2007. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect Immun 75:3961–3968. doi: 10.1128/IAI.00459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Svennerholm AM. 2011. From cholera to enterotoxigenic Escherichia coli (ETEC) vaccine development. Indian J Med Res 133:188–196. [PMC free article] [PubMed] [Google Scholar]
- 22.Norton EB, Branco LM, Clements JD. 2015. Evaluating the A-subunit of the heat-labile toxin (LT) as an immunogen and a protective antigen against enterotoxigenic Escherichia coli (ETEC). PLoS One 10:e0136302. doi: 10.1371/journal.pone.0136302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anosova NG, Chabot S, Shreedhar V, Borawski JA, Dickinson BL, Neutra MR. 2008. Cholera toxin, E. coli heat-labile toxin, and non-toxic derivatives induce dendritic cell migration into the follicle-associated epithelium of Peyer’s patches. Mucosal Immunol 1:59–67. doi: 10.1038/mi.2007.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holmgren J, Bourgeois L, Carlin N, Clements J, Gustafsson B, Lundgren A, Nygren E, Tobias J, Walker R, Svennerholm AM. 2013. Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated E. coli bacteria overexpressing colonization factors CFA/I, CS3, CS5 and CS6 combined with a hybrid LT/CT B subunit antigen, administered alone and together with dmLT adjuvant. Vaccine 31:2457–2464. doi: 10.1016/j.vaccine.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 25.Lundgren A, Bourgeois L, Carlin N, Clements J, Gustafsson B, Hartford M, Holmgren J, Petzold M, Walker R, Svennerholm AM. 2014. Safety and immunogenicity of an improved oral inactivated multivalent enterotoxigenic Escherichia coli (ETEC) vaccine administered alone and together with dmLT adjuvant in a double-blind, randomized, placebo-controlled phase I study. Vaccine 32:7077–7084. doi: 10.1016/j.vaccine.2014.10.069. [DOI] [PubMed] [Google Scholar]
- 26.Jobling MG, Holmes RK. 1992. Fusion proteins containing the A2 domain of cholera toxin assemble with B polypeptides of cholera toxin to form immunoreactive and functional holotoxin-like chimeras. Infect Immun 60:4915–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeinalzadeh N, Salmanian AH, Goujani G, Amani J, Ahangari G, Akhavian A, Jafari M. 2017. A chimeric protein of CFA/I, CS6 subunits and LTB/STa toxoid protects immunized mice against enterotoxigenic Escherichia coli. Microbiol Immunol 61:272–279. doi: 10.1111/1348-0421.12491. [DOI] [PubMed] [Google Scholar]
- 28.Zoeteweij JP, Epperson DE, Porter JD, Zhang CX, Frolova OY, Constantinides AP, Fuhrmann SR, El-Amine M, Tian JH, Ellingsworth LR, Glenn GM. 2006. GM1 binding-deficient exotoxin is a potent noninflammatory broad spectrum intradermal immunoadjuvant. J Immunol 177:1197–1207. doi: 10.4049/jimmunol.177.2.1197. [DOI] [PubMed] [Google Scholar]
- 29.Behrens RH, Cramer JP, Jelinek T, Shaw H, von Sonnenburg F, Wilbraham D, Weinke T, Bell DJ, Asturias E, Pauwells HL, Maxwell R, Paredes-Paredes M, Glenn GM, Dewasthaly S, Stablein DM, Jiang ZD, Dupont HL. 2013. Efficacy and safety of a patch vaccine containing heat-labile toxin from Escherichia coli against travellers’ diarrhoea: a phase 3, randomised, double-blind, placebo-controlled field trial in travellers from Europe to Mexico and Guatemala. Lancet Infect Dis 14:197–204. doi: 10.1016/S1473-3099(13)70297-4. [DOI] [PubMed] [Google Scholar]
- 30.Norton EB, Lawson LB, Mahdi Z, Freytag LC, Clements JD. 2012. The A subunit of Escherichia coli heat-labile enterotoxin functions as a mucosal adjuvant and promotes IgG2a, IgA, and Th17 responses to vaccine antigens. Infect Immun 80:2426–2435. doi: 10.1128/IAI.00181-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norton EB, Lawson LB, Freytag LC, Clements JD. 2011. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin Vaccine Immunol 18:546–551. doi: 10.1128/CVI.00538-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clements JD, Norton EB. 2018. The mucosal vaccine adjuvant LT(R192G/L211A) or dmLT. mSphere 3:e00215-18. doi: 10.1128/mSphere.00215-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernstein DI, Pasetti MF, Brady R, Buskirk AD, Wahid R, Dickey M, Cohen M, Baughman H, El-Khorazaty J, Maier N, Sztein MB, Baqar S, Bourgeois AL. 2019. A phase 1 dose escalating study of double mutant heat-labile toxin LTR192G/L211A (dmLT) from enterotoxigenic Escherichia coli (ETEC) by sublingual or oral immunization. Vaccine 37:602–611. doi: 10.1016/j.vaccine.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hickling JK, Jones KR, Friede M, Zehrung D, Chen D, Kristensen D. 2011. Intradermal delivery of vaccines: potential benefits and current challenges. Bull World Health Organ 89:221–226. doi: 10.2471/BLT.10.079426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Program for Appropriate Technologies in Health. 2009. Intradermal delivery of vaccines: a review of the literature and potential for development for use in low- and middle-income countries. Program for Appropriate Technologies in Health, Seattle, WA. [Google Scholar]
- 36.Zehrung D, Jarrahian C, Wales A. 2013. Intradermal delivery for vaccine dose sparing: overview of current issues. Vaccine 31:3392–3395. doi: 10.1016/j.vaccine.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 37.Nelson KS, Janssen JM, Troy SB, Maldonado Y. 2012. Intradermal fractional dose inactivated polio vaccine: a review of the literature. Vaccine 30:121–125. doi: 10.1016/j.vaccine.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Coleman BL, McGeer AJ, Halperin SA, Langley JM, Shamout Y, Taddio A, Shah V, McNeil SA. 2012. A randomized control trial comparing immunogenicity, safety, and preference for self- versus nurse-administered intradermal influenza vaccine. Vaccine 30:6287–6293. doi: 10.1016/j.vaccine.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Kim YC, Jarrahian C, Zehrung D, Mitragotri S, Prausnitz MR. 2012. Delivery systems for intradermal vaccination. Curr Top Microbiol Immunol 351:77–112. doi: 10.1007/82_2011_123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh J, Bhatia R, Gandhi JC, Kaswekar AP, Khare S, Patel SB, Oza VB, Jain DC, Sokhey J. 1998. Outbreak of viral hepatitis B in a rural community in India linked to inadequately sterilized needles and syringes. Bull World Health Organ 76:93–98. [PMC free article] [PubMed] [Google Scholar]
- 41.Aylward B, Kane M, McNair-Scott R, Hu DJ, Hu DH. 1995. Model-based estimates of the risk of human immunodeficiency virus and hepatitis B virus transmission through unsafe injections. Int J Epidemiol 24:446–452. doi: 10.1093/ije/24.2.446. [DOI] [PubMed] [Google Scholar]
- 42.Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, Spyr C, Steffen R. 2004. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N Engl J Med 350:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 43.Kraan H, Vrieling H, Czerkinsky C, Jiskoot W, Kersten G, Amorij JP. 2014. Buccal and sublingual vaccine delivery. J Control Release 190:580–592. doi: 10.1016/j.jconrel.2014.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo Q, Vickers TJ, Fleckenstein JM. 2016. Immunogenicity and protective efficacy against enterotoxigenic Escherichia coli colonization following intradermal, sublingual, or oral vaccination with EtpA adhesin. Clin Vaccine Immunol 23:628–637. doi: 10.1128/CVI.00248-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White JA, Blum JS, Hosken NA, Marshak JO, Duncan L, Zhu C, Norton EB, Clements JD, Koelle DM, Chen D, Lal M. 2014. Serum and mucosal antibody responses to inactivated polio vaccine after sublingual immunization using a thermoresponsive gel delivery system. Hum Vaccin Immunother 10:3611–3621. doi: 10.4161/hv.32253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee S, Picking WL, Tzipori S. 2014. The immune response of two microbial antigens delivered intradermally, sublingually, or the combination thereof. Microbes Infect 16:796–803. doi: 10.1016/j.micinf.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 47.Sjokvist Ottsjo L, Jeverstam F, Yrlid L, Wenzel AU, Walduck AK, Raghavan S. 2017. Induction of mucosal immune responses against Helicobacter pylori infection after sublingual and intragastric route of immunization. Immunology 150:172–183. doi: 10.1111/imm.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu YJ, Yadav P, Clements JD, Forte S, Srivastava A, Thompson CM, Seid R, Look J, Alderson M, Tate A, Maisonneuve JF, Robertson G, Anderson PW, Malley R. 2010. Options for inactivation, adjuvant, and route of topical administration of a killed, unencapsulated pneumococcal whole-cell vaccine. Clin Vaccine Immunol 17:1005–1012. doi: 10.1128/CVI.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larena M, Holmgren J, Lebens M, Terrinoni M, Lundgren A. 2015. Cholera toxin, and the related nontoxic adjuvants mmCT and dmLT, promote human Th17 responses via cyclic AMP-protein kinase A and inflammasome-dependent IL-1 signaling. J Immunol 194:3829–3839. doi: 10.4049/jimmunol.1401633. [DOI] [PubMed] [Google Scholar]
- 50.Sixma TK, Pronk SE, Kalk KH, van Zanten BA, Berghuis AM, Hol WG. 1992. Lactose binding to heat-labile enterotoxin revealed by X-ray crystallography. Nature 355:561–564. doi: 10.1038/355561a0. [DOI] [PubMed] [Google Scholar]
- 51.Harro C, Gutierrez R, Riddle M, Porter C, Maciel M, Poole S, Laird R, Savarino S. 2015. Protective efficacy of an enterotoxigenic E. coli fimbrial tip adhesin vaccine given with LTR192G by intradermal vaccination against experiment challenge with CFA/I-ETEC in adult volunteers. Abstr Vaccines for Enteric Disease Conference, Edinburgh, United Kingdom http://www.meetingsmanagement.co.uk/index.php?option=com_content&view=article&id=282&Itemid=539. [Google Scholar]
- 52.Norton EB, Bauer DL, Weldon WC, Oberste MS, Lawson LB, Clements JD. 2015. The novel adjuvant dmLT promotes dose sparing, mucosal immunity and longevity of antibody responses to the inactivated polio vaccine in a murine model. Vaccine 33:1909–1915. doi: 10.1016/j.vaccine.2015.02.069. [DOI] [PubMed] [Google Scholar]
- 53.Harro C, Louis Bourgeois A, Sack D, Walker R, DeNearing B, Brubaker J, Maier N, Fix A, Dally L, Chakraborty S, Clements JD, Saunders I, Darsley MJ. 2019. Live attenuated enterotoxigenic Escherichia coli (ETEC) vaccine with dmLT adjuvant protects human volunteers against virulent experimental ETEC challenge. Vaccine 37:1978–1986. doi: 10.1016/j.vaccine.2019.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Read LT, Hahn RW, Thompson CC, Bauer DL, Norton EB, Clements JD. 2014. Simultaneous exposure to Escherichia coli heat-labile and heat-stable enterotoxins increases fluid secretion and alters cyclic nucleotide and cytokine production by intestinal epithelial cells. Infect Immun 82:5308–5316. doi: 10.1128/IAI.02496-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anantha RP, McVeigh AL, Lee LH, Agnew MK, Cassels FJ, Scott DA, Whittam TS, Savarino SJ. 2004. Evolutionary and functional relationships of colonization factor antigen I and other class 5 adhesive fimbriae of enterotoxigenic Escherichia coli. Infect Immun 72:7190–7201. doi: 10.1128/IAI.72.12.7190-7201.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baker KK, Levine MM, Morison J, Phillips A, Barry EM. 2009. CfaE tip mutations in enterotoxigenic Escherichia coli CFA/I fimbriae define critical human intestinal binding sites. Cell Microbiol 11:742–754. doi: 10.1111/j.1462-5822.2009.01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. 2010. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A 107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mudrak B, Kuehn MJ. 2010. Heat-labile enterotoxin: beyond G(m1) binding. Toxins (Basel) 2:1445–1470. doi: 10.3390/toxins2061445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toprani VM, Hickey JM, Sahni N, Toth RT IV, Robertson GA, Middaugh CR, Joshi SB, Volkin DB. 2017. Structural characterization and physicochemical stability profile of a double mutant heat labile toxin protein based adjuvant. J Pharm Sci 106:3474–3485. doi: 10.1016/j.xphs.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clements JD, Finkelstein RA. 1979. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun 24:760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clements JD, El-Morshidy S. 1984. Construction of a potential live oral bivalent vaccine for typhoid fever and cholera-Escherichia coli-related diarrheas. Infect Immun 46:564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li YF, Poole S, Nishio K, Jang K, Rasulova F, McVeigh A, Savarino SJ, Xia D, Bullitt E. 2009. Structure of CFA/I fimbriae from enterotoxigenic Escherichia coli. Proc Natl Acad Sci U S A 106:10793–10798. doi: 10.1073/pnas.0812843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any materials and data that are reasonably requested by others will be made available in a timely fashion, at reasonable cost, to members of the scientific community for noncommercial purposes.