Abstract
Background
Determining liver biomarkers can help to screen and facilitate early management of potential liver diseases. However, such studies are scarce in the present study area. Therefore, our study planned to assess the prevalence of liver function test abnormality and associated factors among Type 2 Diabetes Mellitus (T2DM) patients.
Methods
A comparative cross-sectional study was conducted at the University of Gondar Comprehensive Specialized Hospital from January 1, 2018 to May 20, 2018 among 159 T2DM patients and 159 nondiabetic controls. Clinical, lifestyle, anthropometric data and 5 ml of blood were collected from all study subjects. Liver function tests (LFTs), lipid profiles and fasting blood sugar were determined. Systematic random sampling technique was used to select the study subjects. Binary logistic regression and bivariate correlation was used to assess association of factors with outcomes and p value of ≤0.05 was considered as significant.
Results
Overall, 53 (33.3%) of T2DM had one or more liver test abnormality above the upper limit of the normal (ULN) reference range. Alanine aminotransferase was the most frequently raised liver enzyme in T2DM (n=37, 23.3%). The mean value of LFTs was significantly different between T2DM and the control group. Alcohol drink, sex and age were found to be a significant factor for impairment of LFTs.
Conclusion
The prevalence of abnormal LFTs was higher in T2DM patients than nondiabetic control group. Hence, we recommended the utilization of LFTs to monitor liver conditions in T2DM patients.
Key words: liver function tests, diabetes mellitus, liver disease
INTRODUCTION
Diabetes mellitus(DM) is a group of metabolic disorders of carbohydrates, lipids and proteins characterized by hyperglycemia (1). Globally, more than 415 million people, aged 20-79 years, were affected by DM and the figure is expected to rise up to 642 million in 2040. An epidemic growth of DM has occurred in developing countries in which 75% of patients with DM live in the low and middle-income countries. In addition, DM affects the working age in the low and middle-income countries (2).
The exact pathophysiological mechanism of DM to induce abnormalities in liver biomarkers is still unclear. The first possible explanation that DM induces liver function abnormality is the deposition of fat in the liver which is the characteristics of nonalcoholic fatty liver disease (NAFLD). The other possible assumption is the vulnerability of individuals with metabolic syndrome like DM to inflammation of the liver which alters the function of liver and induce a change in liver biomarkers (3).
Liver function tests (LFTs) are used in clinical practice to screen liver disease, to monitor the progression of a known liver disease and to monitor the effects of potentially hepatotoxic drugs. The most commonly used LFTs include the serum aminotransferases, alkaline phosphatase (ALP), bilirubin, total protein (TP), albumin, and prothrombin time. Measurement of serum aminotransferases, such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST) serve as a marker of hepatocytes injury. ALP, gamma-glutamyltranspeptidase (GGT) and bilirubin act as markers of biliary function and cholestasis whereas TP, albumin and prothrombin time reflect liver synthetic function (4).
The prevalence of abnormal LFTs among DM patients is still controversial (4). In addition, there is limited evidence about the relationship between abnormality in LFTs and DM patients in the study area. Awareness of DM as a significant risk factor for liver injury may help for early diagnosis and interventions and derive health-promoting policies which encourage action to prevent liver diseases in the future. Therefore, the objective of this study was to assess LFTs abnormality and associated factors among patients with T2DM attended at the University of Gondar Comprehensive Specialized Hospital.
MATERIALS AND METHODS
Study design and subjects
A facility based comparative cross sectional study was conducted at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, from January 1 to May 20, 2018. The study included a total of 318 participants; 159 T2DM patients and 159 non-diabetic controls. A systematic random sampling was used to select study participants. We included adult (≥18 years) T2DM and nondiabetic controls. Patients with history of liver disease, pregnant women and patients on drugs known to have effect on liver function (except antidiabetic therapy) were excluded from the study.
Data collection and laboratory methods
Socio-demographic characteristics and clinical data were collected by trained nurses using a pretested semi-structured questionnaire. Trained laboratory technologists collected five milliliter (5 ml) of blood sample. Then after centrifuging the clotted sample, the serum was analyzed for LFTs, lipid profiles and FBS by using Mindray BS-200E chemistry analyzer (Shenzhen Mindray Bio-Medical electronics Co. Ltd, China). The remaining serum was also used for HBsAg and HCV anti-body detection by using One Step Cassette Style HBsAg Rapid Test and EUGENE® anti-HCV rapid test, respectively. The quality of each test was maintained by strictly following the standard operating procedures. Quality control was run daily prior to each test. Completion, accuracy and clarity of the collected data was checked regularly.
The interpretation of test results was based on the reference range recommended by the manufacturers’ instructions. Serum level of alkaline phosphatase (ALP) >306U/L, total bilirubin >1.2mg/dl, direct bilirubin>0.2mg/dl, total protein (TP) <6.6g/L and albumin <3.5g/L was considered as abnormal. The level of ALT >32U/L and AST >31U/L for female and ALT >42U/L and AST >37U/L for male was classified as abnormal. Fasting blood sugar (FBS) >115mg/dl, triglyceride (TG) >200mg/dl, total cholesterol (TC) >190mg/dl, HDL-cholesterol (HDL-c) <40mg/dl and LDL-cholesterol (LDL-c) >100mg/dl were considered as abnormal.
Physical activity is defined as if a study participant was doing sport by allocating regular time. Sweet eating can be defined as an eating behavior in which study participants consumed carbohydrates enriched foods such as cookies and chocolate regularly.
Anthropometric measurement (weight, height, waist circumference) was measured according to WHO guideline by trained nurses. Body mass index (BMI) was calculated as weight divided by height squared (kg/ m2) and classified as underweight (BMI <18.5 kg/m2), normal weight (18.5-24.9 kg/m2), overweight (BMI=25-29.9 kg/m2) and obese (BMI ≥ 30kg/m2)(5). Waist circumference (WC) >88 centimeters for female and WC>101 centimeters for male was taken as high(central obesity)(5). Blood pressure was taken by qualified personnel using an analogue sphygmomanometer and stethoscope. Systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg or current use of blood pressure-lowering medication was used to define hypertension (6).
Data analysis and interpretation
After cleaning and coding, data was entered and analyzed with SPSS version 20 statistical package. Data is presented as mean ± standard deviation (SD) or a percentage (%). Descriptive statistics of frequency distributions, summary and variability measurements are used. Binary logistic regression and bivariate correlation were used to determine the relationship between dependent and independent variables. Factors with p-value ≤0.25 had been transferred to multiple binary logistic regression analysis. A p-value ≤0.05 was considered as statistically significant.
Ethical consideration
Ethical clearance was obtained from the School of Biomedical and Laboratory Sciences Research and Ethics committee. Written informed consent was obtained from study participants before the commencement of data collection. There was no financial compensation or provision for the study participants. To ensure confidentiality of data, study participants were identified using codes and unauthorized persons had no access to the collected data.
RESULTS
Characteristics of study subjects
The mean ± SD age of a control group was 52±13.22 years, ranging between 24 and 80 years and the mean ± SD age of T2DM was 55±11.025 years, ranging from 32 to 85 years(p=0.23). Overall, 82(51.6%) of T2DM patients and 76(47.8%) of a control group were male and 87(54.7%) of T2DM and 87(54.7%) of a control group were under the age of 55 (Table 1).
Table 1.
Sociodemographic, clinical and anthropometric characteristics of type 2 diabetes mellitus patients and nondiabetic controls, Gondar, Northwest Ethiopia, 2018
| Variables | Study subjects | Total N (%) | ||
|---|---|---|---|---|
| T2DM patients N (%) | Control group N (%) | |||
| Sex | Male | 82(51.6) | 76(47.8) | 158(49.7) |
| Female | 77(48.4) | 83(52.2) | 160(50.3) | |
| Age | <55 | 87(54.7) | 87(54.7) | 174(54.7) |
| ≥55 | 72(45.3) | 72(45.3) | 144(45.3) | |
| Alcohol drink | Yes | 56(35.2) | 65(40.7) | 121(38.1) |
| No | 103(64.8) | 94(59.3) | 191(61.9) | |
| Coffee drink | Yes | 112(70.4) | 115(72.3) | 227(71.4) |
| No | 47(29.6) | 44(27.7) | 91(28.6) | |
| Physical exercise | Yes | 40(25.2) | 47(29.6) | 87(27.4) |
| No | 119(74.8) | 112(70.4) | 231(72.6) | |
| Body mass index | Underweight | 9(5.6) | 4(2.5) | 13(4.1) |
| Normal weight | 77(48.4) | 154(96.9) | 231(72.6) | |
| Over weight | 47(29.6) | 1(0.6) | 48(15.1) | |
| Obese | 26(16.4) | - | 26(8.2) | |
| Waist circumference | Normal | 124(78) | 157(98.7) | 281(88.4) |
| Central obesity | 35(22) | 2(1.3) | 37(11.6) | |
| Duration diabetes mellitus | 0-5 | 99(62.3) | - | - |
| 6-10 | 31(19.5) | - | - | |
| >10 | 29(18.2) | - | - | |
| Systolic blood pressure | Normal | 122(76.7) | 158(99.4) | 280(88.1) |
| High | 37(23.3) | 1(0.6) | 38(11.9) | |
| Diastolic blood pressure | Normal | 142(89.3) | 149(93.7) | 291(91.5) |
| High | 17(10.7) | 10(6.3) | 27(8.5) | |
| Hypertension | Present | 67(42.1) | 11(6.9) | 78(24.5) |
| Absent | 92(57.9) | 148(93.1) | 240(75.5) | |
| Family history of diabetes mellitus | Yes | 19(11.9) | 12(7.5) | 31(9.7) |
| No | 140(88.1) | 147(92.5) | 287(90.3) | |
Note: N=number of participants; T2DM=type 2 diabetes mellitus; %=percentage.
Prevalence of abnormal liver function tests
Elevated ALT was found in 37 (23.3%) of T2DM and 4 (2.5%) of a control group. Elevated AST was observed from 34 (21.4%) of T2DM and 3 (1.9%) of a control group. On the other hand, 19 (11.9%) of T2DM patients and 1 (0.6%) of controls had decreased level of TP respectively. One or more test abnormality was observed in 53 (33.3%) of T2DM patients and 6 (3.8%) of controls whereas 30 (18.9%) of T2DM patients and 3 (19%) of controls revealed an abnormal level of LFTs in two or more tests.
DISCUSSION
Mean values of ALP, ALT, AST, total and direct bilirubin were significantly higher in T2DM than the control group (P for trend ≤0.04). On the other hand, the mean values of albumin and TP were significantly lower in T2DM than controls. A similar finding regarding ALT and AST (7-9) and TP and albumin (8) was reported.
The prevalence of abnormal LFTs was higher than the prevalence observed in control group. The most frequent abnormal LFT was ALT (23.3%, 95%Cl=17%-30.2%) which was followed by AST (21.4%, 95%Cl=14.5%-28.3%). This is in line with the studies conducted in Finland (10), Scotland (11) and England (12) reported a 17%, 23.1% and 25.6% prevalence of abnormal ALT in T2DM patients, respectively. The prevalence of abnormal ALP was 10.7% (6.3%-15.7%) which is comparable with 8.9%, reported from Algeria (13). The prevalence of elevated ALT and AST was higher than the prevalence reported from Algeria (13) which observed 13.9% for ALT and 10% for AS. The difference might be attributable to the difference in medical care, living standard and knowledge of the patients on the risk factors.
It was noted that 33.3% (95%Cl=26.4%-41%) of T2DM had one or more test abnormality. This finding is consistent with the previous study conducted in Scotland (11) which reported 29.1%. Disagreed with our result, studies from South Africa (14) and Finland (10) reported a 46% and 57% prevalence of one or more test abnormality. The possible explanation of the difference in prevalence might be due to the utilization of different cutoff values, which are influenced by sociodemographic characteristics such as sex, age and ethnicity (15). The prevalence of two or more test abnormality was 18.9% (95%Cl=12.6%=25.8%). This is similar with a finding from Sudan (8) reported 24%, but lower than the prevalence (27%) reported from Finland (10). Among T2DM patients, 11.3% (6.9%-16.4%) revealed elevated ALT and AST each beyond two times the ULN whereas 6.9% (3.1%-11.3%) and 8.2% (3.8%-12.6%) of T2DM was found with an elevated level of ALT and AST respectively beyond three times the ULN. In line with our finding, a retrospective study from South Africa (14) showed a 9% and 6% prevalence of abnormal liver enzymes over two times ULN and three times ULN in T2DM respectively.
It was noted that WC, BMI, TC, TG, LDL-c and FBS were significantly positively correlated with elevated level of ALP, ALT, AST, total bilirubin and direct bilirubin (P≤0.001). But, these were significantly negatively correlated with TP and albumin (P≤0.001). HDL-c was significantly negatively correlated with liver enzymes and bilirubin(P≤0.001) while it was significantly positively correlated with the level of TP and albumin (P≤0.001). A study from South Africa (14) has previously reported that patients with abnormal liver enzyme were significantly associated with dyslipidemia. Another study from China (16) demonstrated a significant positive correlation between elevated ALT and WC, BMI, TC, TG, LDL-c and FBS. In addition, this study noted a significant negative correlation between ALT and HDL-c (16).
Both diastolic and systolic pressure was significantly positively correlated with ALP, ALT, AST, total bilirubin and direct bilirubin (P for trend ≤0.007). Furthermore, these were negatively correlated with TP and albumin (P ≤0.001). A similar finding was reported in a study from China (16). Obstruction of the blood flow due to the deposition of fibrin in the liver sinusoids might be the probable cause of the liver damage in participants with hypertension (17).
The likelihood of having an abnormal LFTs was greater among males than females. Previous studies have shown the association between male sex and abnormal LFTs (16, 18, 19). The sex difference may be explained by differences in body fat distribution due to the presence of estrogen in females (20).
Older age, with shorter duration of DM, was significantly associated with abnormal LFTs. In contrast to our finding and others (12, 16, 18), a study from Scotland (11) and Italy (21) reported younger age with shorter duration of DM as a factor for abnormal LFTs. This difference can be related to genetic variation as ethnic difference in NAFLD progression was reported to be determined by variants in genes (22). The inverse correlation between duration of DM and liver enzyme and bilirubin may be due to survival bias (people with more severe liver injury dying earlier) and treatment effect over time. Older subjects might be less consistent with the stringent lifestyle interventions usually prescribed as part of T2DM management.
Alcohol drink was significantly associated with abnormal LFTs. A similar finding has been observed by other studies (11, 12, 16, 18, 19). Alcohol drink might bring this effect by altering lipid metabolism and direct toxicity. The breakdown of alcohol with cytosolic enzymes generates toxic metabolites such as acetaldehyde and highly reactive oxygen containing molecules evoking oxidative stress and inflammation(23).
Sweet drink was significantly associated with abnormal level of ALP (AOR=5.1(1.7-11.5)), ALT (AOR=6.2(1.7-16.0)) and TP (AOR=2.6(1.03-9.9)). The intake of sweetened beverages may affect insulin sensitivity, which results in impaired metabolism and hepatic steatosis (24). Butter consumption was significantly associated with elevated ALT (OR=1.4(1.01-2.6)) and AST (OR=2.1(1.2-3.4)). Evidences revealed that butter consumption is associated with increased risk of metabolic syndrome (25) which may induce liver function abnormality.
We have seen the inverse relationship between abnormal LFTs and coffee consumption. Coffee was reported to decrease abnormal LFTs in other studies (18, 26). Aromatic extracts (27) and chemical compounds such as cafestol, kahweol and chlorogenic acid (28, 29) has been suggested for their antioxidative function.
Strengths of this study include the comparative method we used. Consistent definitions were applied to all subjects, which we believe that help to reduce bias. Notably, based on literature search, it is the first study to report the prevalence of abnormal LFTs among T2DM patients in the study area. Despite the above strengths, our study has limitations. The study was a cross-sectional study design and thus, it is not possible to determine if diabetes preceded or followed the abnormal liver, nor is it possible to determine whether we observed chronic or transient LFTs impairment. Because of self-reporting measures of behavioral characteristics such as drinking, some error and resulting residual confounding by these covariates and others may not be excluded. Our study didn’t use imaging methods and/or histology to ascertain the association of abnormal LFTs with NAFLD. This point is particularly important because it has been demonstrated that NAFLD could be present also in absence of elevated liver enzymes (30).
CONCLUSION AND RECOMMENDATIONS
In conclusion, the prevalence of abnormal level of LFTs in T2DM was higher than a control group. The mean values of the liver enzymes and bilirubin in patients with T2DM were significantly higher than that of a control group. Moreover, T2DM patients had lower level of TP and albumin in comparison to the control group. This difference indicates that T2DM may induce liver function impairment. The high prevalence of LFTs derangement in T2DM highlights the importance of requesting LFTs in these patients as they may harbor potentially treatable co-morbid illnesses. Health education about the potential risk of liver diseases and way of prevention shall be provided to T2DM patients as well. In addition, follow up study is required to ascertain the mechanism by which liver function is impaired.
Figure 1.
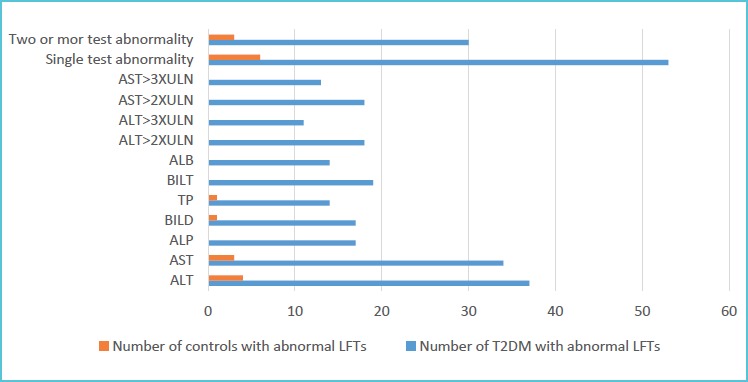
Number of study subjects with liver function tests abnormality in type 2 diabetes mellitus patients and controls, Gondar, Northwest Ethiopia, 2018
Figure 2.
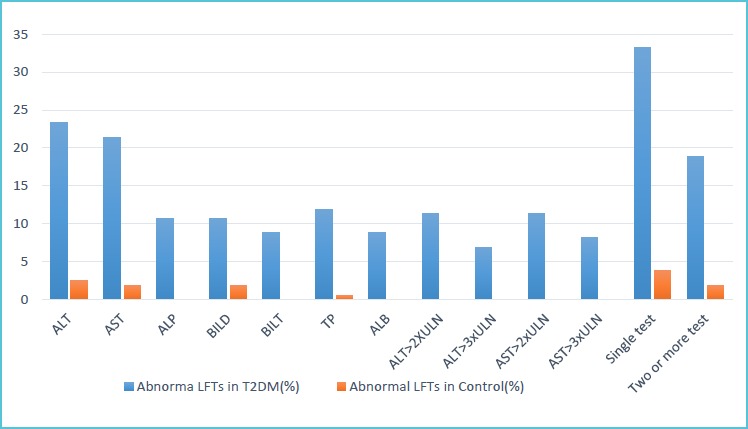
Percentage distribution of liver function tests abnormality in type 2 diabetes patients and controls, Gondar, Northwest Ethiopia, 2018
Table 2.
Comparison of clinical and biochemical parameters between T2DM patients and nondiabetic controls, Gondar, Northwest Ethiopia, 2018
| Parameters | Mean ± Standard deviation(SD) | P value | Units of measurement | |
|---|---|---|---|---|
| T2DM patients (n=159) | Controls group (n=159) |
|||
| Fasting blood sugar | 182.60±74.375 | 84.25±51.56 | ≤0.001 | mg/dl |
| Triglyceride | 188.45±103.62 | 86.58±58.84 | ≤0.001 | |
| Total cholesterol | 191.05±77.07 | 139.18±35.44 | ≤0.001 | |
| High density lipoprotein cholesterol | 44.18±11.940 | 52.28± 8.156 | ≤0.001 | |
| Low density lipoprotein cholesterol | 105.33±56.0 | 55.55±8.53 | ≤0.001 | |
| Systolic blood pressure | 135.52±16.52 | 118.72 ± 7.81 | ≤0.001 | mmHg |
| Diastolic blood pressure | 81.30±9.02 | 79.63 ± 5.2 | 0.04 | |
| Waist circumference | 94.00±8.81 | 78.14±8.26 | ≤0.001 | centimeter |
| Body mass index | 26.74±18.79 | 23.30 ± 2.53 | 0.02 | kg/m2 |
| Alkaline phosphatase | 165.55±81.610 | 118.38±23.628 | ≤0.001 | U/L |
| Alanine aminotransferase | 37.57±35.831 | 17.89±7.097 | ≤0.001 | |
| Aspartate aminotransferase | 35.2±32.278 | 17.89±7.067 | ≤0.001 | |
| Total protein | 7.469±.8461 | 8.005±.7583 | 0.003 | g/dl |
| Albumin | 4.602±.6736 | 4.930±.3262 | 0.004 | |
| Total bilirubin | .553±.3907 | .418±.2449 | 0.006 | mg/dl |
| Direct bilirubin | .169±.1680 | .140±.0575 | 0.04 | |
Note: g/dl=gram/deciliter; Kg/m2=kilogram/square meter; mg/dl=milligram/deciliter; mmHg=millimeter mercury; U/L=unit/liter.
Table 3.
Bivariate correlations among continuous variables in T2DM, Gondar, Northwest Ethiopia, 2018
| DM duration | Systolic BP | Diastolic BP | WC | BMI | TG | FBS | TC | HDL-c | LDL-c | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ALP | r | -0.245 | 0.289 | 0.273 | 0.430 | 0.329 | 0.728 | 0.772 | 0.812 | -0.628 | 0.792 |
| P value | 0.002 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | |
| AST | r | -0.200 | 0.283 | 0.289 | 0.443 | 0.327 | 0.731 | 0.786 | 0.847 | -0.680 | 0.849 |
| P value | 0.011 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | |
| ALT | r | -0.198 | 0.315 | 0.305 | 0.450 | 0.395 | 0.737 | 0.797 | 0.840 | -0.686 | 0.855 |
| P value | 0.012 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | |
| TP | r | 0.173 | -0.233 | -0.203 | -0.417 | -0.241 | -0.609 | -0.692 | -0.773 | 0.592 | -0.695 |
| P value | 0.029 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | |
| ALB | r | 0.119 | -0.261 | -0.211 | -0.424 | -0.305 | -0.671 | -0.759 | -0.822 | 0.694 | -0.784 |
| P value | 0.134 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | |
| BILT | r | -0.191 | 0.150 | 0.206 | 0.303 | 0.241 | 0.658 | 0.668 | 0.815 | -0.584 | 0.764 |
| P value | 0.016 | 0.007 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | |
| BILD | r | -0.134 | 0.162 | 0.236 | 0.254 | 0.274 | 0.528 | 0.554 | 0.690 | -0.525 | 0.690 |
| P value | 0.092 | 0.004 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | |
Note: ‘r’ denoted correlation coefficient; ALB=albumin; ALP=alkaline phosphatase; ALT=alanine aminotransferase;
AST=aspartate aminotransferase; BILD=direct bilirubin; BILT=total bilirubin; BMI=body mass index; BP=blood pressure;
FBS= fasting blood sugar; HDL-c=high density lipoprotein cholesterol; LDL-c=low density lipoprotein cholesterol;
TC=total cholesterol; TG=triglyceride; TP= total protein; WC=waist circumference.
Table 4.
Binary logistic regression analysis of associated factors with different abnormal LFTs in patients with type 2 diabetes mellitus, Gondar, Northwest, Ethiopia, 2018
| Factors | Elevated ALP | Elevated AST | Elevated ALT | Decreased TP | Decreased ALB | Elevated BILT | Elevated BILD | |
|---|---|---|---|---|---|---|---|---|
| Male | COR (95%Cl) | 8.4 (4.2-11.7) | 3.3 (1.4-6.8) | 3.9 (1.6-7.8) | 2.8 (1.1-7.2) | 6.4 (0.9-8.6) | 6.4 (2.9-8.1) | 3.4 (0.9-6.3) |
| P value | 0.038 | 0.005 | 0.002 | 0.03 | 0.08 | 0.038 | 0.068 | |
| AOR (95%Cl) | 0.6 (0.2-2.4) | 2.1 (1.2-3.8) | 1.2 (1.01-3.4) | 1.1 (0.4-3.3) | 1.2 0.4-3.6) | 1.0 (0.3-3.3) | 0.9 (0.3-2.7) | |
| P value | 0.50 | 0.014 | 0.047 | 0.80 | 0.80 | 1.0 | 0.80 | |
| Age<55 | COR (95%Cl) | 0.4 (0.2-1.1) | 0.6 (0.3-1.3) | 0.4 (0.2-0.9) | 0.5 (0.2-1.2) | 0.4 (0.1-1.05) | 0.3 (0.1-0.8) | 0.5 (0.2-1.2) |
| P value | 0.068 | 0.22 | 0.033 | 0.11 | 0.06 | 0.024 | 0.11 | |
| AOR (95%Cl) | 0.4 (0.1-1.1) | 0.2 (0.1-0.8) | 0.3 (0.1-0.9) | 0.3 (0.1-0.8) | 0.5 (0.2-1.3) | 0.3 (0.1-1.02) | 0.5 (0.2-1.4) | |
| P value | 0.054 | 0.041 | 0.035 | 0.012 | 0.13 | 0.055 | 0.22 | |
| Butter | COR (95%Cl) | 1.9 (1.1-3.1) | 2.1 (1.3-3.4) | 2.1 (1.3-3.4) | 1.5 (0.9-2.5) | - | 1.9 (1.1-3.1) | 1.9 (1.2-3.2) |
| P value | 0.019 | 0.003 | 0.003 | 0.15 | - | 0.019 | 0.012 | |
| AOR (95%Cl ) | 1.7 (0.9-3.4) | 2.1 (1.2-3.4) | 1.4 (1.01-2.6) | 1.2 (0.7-2.3) | - | 1.5 (0.8-2.8) | 1.6 (0.9-3.0) | |
| P value | 0.13 | 0.014 | 0.045 | 0.5 | - | 0.22 | 0.12 | |
| Alcohol | COR (95%Cl) | 11.7 (3.7-16.9) | 8.4 (3.7-18.9) | 11.2 (4.7-17.2) | 6.5 (2.5-16.8) | 4.1 (1.5-11.1) | 6.5 (2.4-12.3) | 7.0 (2.6-15.2) |
| P value | 0≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | 0.005 | ≤0.001 | 0≤0.001 | |
| AOR (95%Cl) | 5.9 (3.4-8.6) | 6.4 (2.6-11.2) | 6.0 (1.8-12.1) | 6.1 (2.1-12.3) | 4.2 (1.4-8.9) | 5.1 (1.6-11.6) | 6.0 (2.0-10.9) | |
| P value | ≤0.001 | .001 | ≤0.001 | .001 | .011 | .006 | 0.002 | |
| Coffee | COR (95%Cl) | 0.3 (0.1-0.8) | 0.7 (0.3-1.2) | 0.5 (0.3-1.1) | 0.4 (0.2-1.0) | 0.3 (0.1-0.8) | 0.5 (0.2-1.2) | 0.5 (0.2-1.3) |
| P value | 0.015 | 0.14 | 0.10 | 0.056 | 0.019 | 0.13 | 0.18 | |
| AOR (95%Cl) | 0.2 (0.1-0.7) | 0.4 (0.2-0.9) | 0.4 (0.2-1.2) | 0.4 (0.1-0.9) | 0.3 (0.1-0.9) | 0.1 (0.02-0.9) | 0.5 (0..2-1.4) | |
| P value | 0.01 | 0.047 | 0.09 | 0.045 | 0.028 | 0.043 | 0.16 | |
| Sweet drink | COR (95%Cl) | 5.9 (2.3-15.3) | 3.4 (1.5-7.8) | 5.1 (2.2-11.9) | 2.9 (1.1-7.3) | - | 4.6 (1.8-12.0) | 3.3 (1.3-8.6) |
| P value | 0≤0.001 | 0.004 | 0≤0.001 | 0.028 | - | 0.002 | 0.013 | |
| AOR (95%Cl) | 5.1 (1.7-11.5) | 2.2 (0.7-6.9) | 6.2 (1.7-16.0) | 2.6 (1.03-9.9) | - | 3.0 (0.8-11.5) | 2.4 (0.6-8.7) | |
| P value | 0.008 | 0.19 | 0.006 | 0.048 | - | 0.10 | 0.20 | |
| T2DM | COR (95%Cl) | - | 14.7 (4.4-18.8) | 11.8 (4.1-16.9) | 21.4 (2.8-42.2) | - | - | 6.2 (1.8-11.7) |
| P value | - | 0.001 | 0.001 | 0.003 | - | - | 0.004 | |
| AOR (95%Cl) | - | 4.2 (1.6-10.6) | 3.6 (1.8-7.1) | 5.2 (1.2-8.8) | - | 2.5 (1.2-5.2) | ||
| P value | - | 0.003 | 0.001 | 0.013 | - | - | 0.012 | |
Note:‘-’ denotes not applicable; ALB=albumin; ALP=alkaline phosphatase; ALT=alanine aminotransferase;
AOR=adjusted odds ratio; AST=aspartate aminotransferase; BILD=direct bilirubin; BILT=total bilirubin;
Cl=confidence interval; COR=crude odds ratio; TP=total protein; T2DM=type 2 diabetes mellitus.
Acknowledgments
We would like to acknowledge the University of Gondar Comprehensive Specialized Hospital for providing us permission to do this research. We would like also to thank study participants for their voluntariness.
Footnotes
Abbreviations
ALD: Alcoholic Liver Disease
ALP: Alkaline Phosphatase
ALT: Alanine Aminotransferase
AST: Aspartate Aminotransferase
BMI: Body Mass Index
DM: Diabetes Mellitus
FBS: Fasting Blood Sugar
GGT: Gamma Glutamyl Transferase
HBV: Hepatitis B Virus
HCV: Hepatitis C Virus
HDL-c: High Density Lipoprotein cholesterol
IR: Insulin Resistance
LDL-c: Low Density Lipoprotein cholesterol
LFT: Liver Function Test
NAD: Nicotinamide Adenine Dinucleotide
NAFLD: Nonalcoholic Fatty Liver Diseases
TP: Total Protein
ULN: Upper Limit of Normal
TG: Triglyceride
TC: Total Cholesterol
T2DM: Type 2 Diabetes Mellitus
WC: Waist Circumference
Data Availability
The data sets used and analyzed during the current study are available from corresponding author on a reasonable request.
Competing interest
All authors declared that there is no competing interest between all authors.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contribution
All the authors contribute equally, starting from drafting of the proposal to preparation of the manuscript.
REFERENCES
- 1.Imam K. Clinical features, diagnostic criteria and pathogenesis of diabetes mellitus. Diabetes: Springer; 2013. p. 340-355. [DOI] [PubMed] [Google Scholar]
- 2.Ogurtsova K, da Rocha Fernandes J, Huang Y, Linnenkamp U, Guariguata L, Cho N, et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes research and clinical practice. 2017;128:40-50. [DOI] [PubMed] [Google Scholar]
- 3.Hanley AJ, Williams K, Festa A, Wagenknecht LE, D’Agostino RB, Haffner SM. Liver markers and development of the metabolic syndrome. Diabetes. 2005;54(11):3140-3147. [DOI] [PubMed] [Google Scholar]
- 4.Harris EH. Elevated liver function tests in type 2 diabetes. Clinical diabetes. 2005;23(3):115-119. [Google Scholar]
- 5.de Onis M, Habicht J-P. Anthropometric reference data for international use: recommendations from a World Health Organization Expert Committee. The American journal of clinical nutrition. 1996;64(4):650-658. [DOI] [PubMed] [Google Scholar]
- 6.Kavey R-EW, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation. 2003;107(11):1562-1566. [DOI] [PubMed] [Google Scholar]
- 7.Idris AS, Mekky KFH, Abdalla BEE, Ali KA. Liver function tests in type 2 Sudanese diabetic patients. International Journal of Nutrition and Metabolism. 2011;3(2):17-21. [Google Scholar]
- 8.Elmahi H, Abdrabo A. Determinants of abnormal liver function tests in Diabetes Type 2 patients in Sudan. Journal of Science. 2014;4(1):45-49. [Google Scholar]
- 9.Salah EM, Mohammed LH, Morsi AN. Assessment of Liver Function Tests among Type 2 Sudanese Diabetic Patients in Khartoum State. [Google Scholar]
- 10.Salmela PI, Sotaniemi EA, Niemi M, Mäentausta O. Liver function tests in diabetic patients. Diabetes care. 1984;7(3):248-254. [DOI] [PubMed] [Google Scholar]
- 11.Morling J, Strachan M, Hayes P, Butcher I, Frier B, Reynolds R, et al. Prevalence of abnormal plasma liver enzymes in older people with Type 2 diabetes. Diabetic medicine. 2012;29(4):488-491. [DOI] [PubMed] [Google Scholar]
- 12.Saligram S, Williams EJ, Masding MG. Raised liver enzymes in newly diagnosed Type 2 diabetes are associated with weight and lipids, but not glycaemic control. Indian journal of endocrinology and metabolism. 2012;16(6):1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belkacemi L, Belalia M. Cross-sectional pilot study about the liver enzymes profile in type 2 diabetic patients from an Algerian west region: Wilaya of Mostaganem. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2016;10(1):S147-S50. [DOI] [PubMed] [Google Scholar]
- 14.Paruk IM, Pirie FJ, Motala AA, Kolawole BA. High prevalence of abnormal liver enzymes in South African patients with type 2 diabetes mellitus attending a diabetes clinic. Journal of endocrinology, metabolism and diabetes of South Africa. 2011;16(1):43-47. [Google Scholar]
- 15.Siest G, Schiele F, Galteau M-M, Panek E, Steinmetz J, Fagnani F, et al. Aspartate aminotransferase and alanine aminotransferase activities in plasma: statistical distributions, individual variations, and reference values. Clinical chemistry. 1975;21(8):1077-1087. [PubMed] [Google Scholar]
- 16.Liu A, Chen S, Guo X, Li Z, Zheng L, Sun Y. Relationship between the alanine aminotransferase levels and Type 2 diabetes mellitus in the general Chinese population. Journal of Diabetes. 2017. [DOI] [PubMed] [Google Scholar]
- 17.Arias F, Mancilla-Jimenez R. Hepatic fibrinogen deposits in pre-eclampsia: immunofluorescent evidence. New England Journal of Medicine. 1976;295(11):578-582. [DOI] [PubMed] [Google Scholar]
- 18.Meltzer AA, Everhart JE. Association between diabetes and elevated serum alanine aminotransferase activity among Mexican Americans. American journal of epidemiology. 1997;146(7):565-571. [DOI] [PubMed] [Google Scholar]
- 19.Erbey JR, Silberman C, Lydick E. Prevalence of abnormal serum alanine aminotransferase levels in obese patients and patients with type 2 diabetes. The American journal of medicine. 2000;109(7):588-590. [DOI] [PubMed] [Google Scholar]
- 20.Mauss S, Berg T, Rockstroh J, Sarrazin C, Wedemeyer H, Kamps BS. Hepatology-A clinical textbook. flying publisher; 2014. [Google Scholar]
- 21.Giandalia A, Romeo EL, Ruffo MC, Muscianisi M, Giorgianni L, Forte F, et al. Clinical correlates of persistently elevated liver enzymes in type 2 diabetic outpatients. Primary care diabetes. 2017;11(3):226-232. [DOI] [PubMed] [Google Scholar]
- 22.Daly AK, Ballestri S, Carulli L, Loria P, Day CP. Genetic determinants of susceptibility and severity in nonalcoholic fatty liver disease. Expert review of gastroenterology & hepatology. 2011;5(2):253-263. [DOI] [PubMed] [Google Scholar]
- 23.Lieber CS. Medical and nutritional complications of alcoholism: mechanisms and management: Springer Science & Business Media; 2012. [Google Scholar]
- 24.Assy N, Nasser G, Kamayse I, Nseir W, Beniashvili Z, Djibre A, et al. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Canadian Journal of Gastroenterology and Hepatology. 2008;22(10):811-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosseinpour-Niazi S, Mirmiran P, Hosseini-Esfahani F, Azizi F. Is the metabolic syndrome inversely associates with butter, non-hydrogenated-and hydrogenated-vegetable oils consumption: Tehran lipid and glucose study. Diabetes research and clinical practice. 2016;112:20-29. [DOI] [PubMed] [Google Scholar]
- 26.Casiglia E, Spolaore P, Inocchio G, Ambrosio B. Unexpected effects of coffee consumption on liver enzymes. European journal of epidemiology. 1993;9(3):293-297. [DOI] [PubMed] [Google Scholar]
- 27.Lee K-G, Mitchell A, Shibamoto T. Antioxidative activities of aroma extracts isolated from natural plants. Biofactors. 2000;13(1-4):173-178. [DOI] [PubMed] [Google Scholar]
- 28.Cavin C, Mace K, Offord E, Schilter B. Protective effects of coffee diterpenes against aflatoxin B1-induced genotoxicity: mechanisms in rat and human cells. Food and Chemical toxicology. 2001;39(6):549-556. [DOI] [PubMed] [Google Scholar]
- 29.Feng R, Lu Y, Bowman LL, Qian Y, Castranova V, Ding M. Inhibition of activator protein-1, NF-KB, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. Journal of Biological Chemistry. 2005;280(30):27888-27895. [DOI] [PubMed] [Google Scholar]
- 30.Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48(3):792-798. [DOI] [PubMed] [Google Scholar]


