Abstract
Background
Hyperuricemia is associated with cardiovascular disease (CVD) that presents in diabetes mellitus patients. Therefore, the aim of this study was to appraise the serum uric acid and its association with CVD risk factors among diabetes mellitus patients.
Methods
A cross-sectional study was carried out at the University of Gondar hospital from February to March, 2018. A total of 384 study participants were selected by systematic random sampling technique. Five milliliter blood sample was collected and analyzed using Mindray BS-200E machine. The data was analysed into SPSS version 20. Logistic regression model was used to investigate associated factors. A p-value <0.05 was considered statistically significant.
Results
The prevalence of hyperuricemia among type 2 diabetic patients was 31.5%. The serum uric acid concentration was higher among male (33.1%) compared to female (28.9%). Elevated systolic blood pressure (AOR: 4.4, 95%CI: 2.1-9.3), family history of DM (AOR: 1.5, 95%CI: 1.2-2.5) and BMI ≥ 25 Kg/m2 (AOR: 1.4, 95%CI: 1.1-3.7) were significantly associated with hyperuricemia. Increased BMI (52.4%), high waist circumference (63.0%) and elevated systolic blood pressure (58.2%) were the major CVD risk factors.
Conclusion
The prevalence of hyperuricemia was high in type 2 diabetes patients. The major predictors of CVD risk factors were elevated systolic blood pressure, family history of DM and BMI ≥ 25 Kg/m2 which lead to early diagnosis and treatment for hyperuricemia. Lastly, CVD risk factors are essential to reduce the disease among type 2 diabetic patients.
Key words: hyperuricemia, diabetes mellitus, cardiovascular disease
BACKGROUND
Uric acid (UA) is a final enzymatic product of purine metabolism in humans [1] and it is regulated by the xanthine-oxidoreductase enzyme, which converts hypoxanthine to xanthine and xanthine to uric acid [2]. An elevated concentration of UA is associated with a variety of cardiovascular conditions [3]. The balance between the intake endogenous synthesis, excretion ratio and metabolism of purines determines the concentrations of Serum Uric Acid (SUA). The alteration of any of these factors could cause hyperuricemia (HUA), which defined as a SUA concentration >6.8 mg/dL[4]. Currently, the prevalence of HUA is potentially attributed to recent shifts in diet and lifestyle, improved medical care and increased long life [5].
Developed countries tend to have a higher burden of gout than developing countries. Some ethnic groups are particularly vulnerable to gout, supporting the importance of genetic predisposition. Socioeconomic and dietary factors, as well as co-morbidities and medications that can impact UA levels and/or facilitate monosodium urate (MSU) crystal formation, are also important in determining the risk of developing gout [6].
Recently, SUA has received attention as a potential biomarker dependently predicting the development of hypertension, diabetes mellitus (DM), and chronic kidney disease [7]. A close relationship exists between plasma UA levels and glucose utilization in type 2 diabetes mellitus (T2DM) [8], which results from a defect in insulin secretion or action, almost always with a major contribution from insulin resistance (IR) [9]. T2DM is a corollary of the interaction between a genetic predisposition, behavioral and environmental risk factors. Obesity and physical inactivity are the main non-genetic determinants of T2DM although, the genetic basis of the disease has yet to be identified [10]. The strong relationship between UA and T2DM is due to the development of renal dysfunction in T2DM [11].
There were studies that showed a clear relationship of increased UA levels with hypertension, metabolic syndrome (MetS), abdominal obesity, endothelial dysfunction, inflammation, sub-clinical atherosclerosis and an increased risk of cardiovascular events [12]. Some other factors can also induce HUA such as hypertension, possibly by urate reabsorption, which is caused by decreased renal blood flow [13]. Dyslipidemia may also cause HUA through a negative effect on renal function [14]. According to data from the National Health and Nutrition Examination Survey (NHANES) 2007-2008, the prevalence of HUA was 21% in American adults, reaching 26% in African Americans. Recently, the prevalence of HUA has been increasing [15]. Evidence has supported the association of high level of UA with MetS, T2DM and CVD [16].
Some of the recognized risk factors of CVD are high blood pressure, rapid acculturation and step up in economic conditions, economic transition, increased tobacco use, high blood lipids, physical idleness, over-weight and obese, DM and poor dietary habit [15]. Hyperglycemia and lipid metabolism disorder is also linked to a greater risk for vascular problems, kidney disease, nerve and retinal damage resulting in challenges in managing the disease adequately, especially in the presence of immune suppression, and predisposes individual to premature mortality. Moreover, this has cost and social implications for patients, their families, communities and the healthcare system. Currently, HUA in T2DM patients has been less well investigated in sub-Saharan Africans. Until now, the pathogenic role of UA in the development of the MetS is not complete, therefore, the aim of the study was to assess the current burden of HUA and its association with CVD risk factors among T2DM patients at the University of Gondar Hospital.
METHODS AND MATERIALS
Study design, period and area
Institution-based cross-sectional study was conducted from February to March, 2018 at the University of Gondar Hospital DM clinic, Gondar, Ethiopia. The University of Gondar Hospital is one of the biggest hospitals in Amhara region that provides health service, acts as a referral center for other district hospitals and has about 400 beds.
It is expected to deliver health service for about five million people in Northwest Ethiopia. As a teaching hospital, it plays an important role in teaching, research and community service. According to the 2007 census, Gondar town has a total population of 323,900 [17].
Population
The source population was all T2DM patients who have access to be served at the University of Gondar Hospital. Moreover, the study population were all individuals with T2DM who visited the hospital during the study period and fulfilled eligibility criteria.
Inclusion and exclusion criteria
All T2DM patients > 18 years old who were willing to participate in this study were included. Pregnant women, severely ill individuals and patients on drugs known to have an effect on UA level except for anti-diabetic therapy and patients taking lipid lowering drugs were excluded from the study.
Operational definition
Study participants were classified as underweight (BMI<18.5 Kg/m2), normal weight (18.524.9 Kg/m2), overweight (BMI =25-29.9Kg/m2) and obese (BMI >30Kg/m2)(18). Waist circumference (WC) >88 centimeter for female and WC >101 centimeter for male was taken as high WC [18] Systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg or current use of blood pressure-lowering medication was used to define hypertension [19]. The interpretation of test results for fasting blood sugar (FBS), UA and lipid profiles was based on the reference range recommended by the manufacturers instruction were considered as normal.
Sample size determination and sampling technique
Single population proportion formula was used by considering the proportion of 50% prevalence among T2DM. 5% desired precision and 95% confidence interval (CI) resulting in a total sample size of 384. The study participants were selected using a systematic random sampling technique.
Data collection and laboratory methods
Socio-demographic characteristics and clinical data were collected by trained nurses using a semi-structured questionnaire. In addition to that, trained laboratory technologists collect and analyzed the blood sample. Anthropometric measurement (weight, height) was measured according to WHO stepwise approach guideline. Height was measured to the nearest 0.5 cm using standiometer and weight was recorded to the nearest 0.1 kg with the patient wearing light clothes using a balance. BMI was calculated as weight divided by height squared (kg/m2) [18].
Blood pressure was measured by nurses using an analogue sphygmomanometer. Five milliliter fasting venous blood sample was collected using serum separator test tube by following aseptic blood collection procedure. Serum glucose, lipid profiles and UA were measured by using Mindray BS-200E chemistry analyzer (Shenzhen Mindray Bio-Medical electronics Co. Ltd, China).
Data analysis and interpretation
Data was checked for its completeness, clarity and edited for its consistency and the data was entered to SPSS version 20 statistical package for analysis. Descriptive statistics were used to summarize the frequency distributions. Logistic regression analysis was used to determine the association between dependent and independent variables.
Variables with P value < 0.25 in binary logistic regression model were included into the multi-variable analysis model to identify independent predictor variables for abnormal serum uric acid concentration. In addition, Pearson’s correlation was used to determine the correlation between independent variables and serum UA.
Ethical consideration
Ethical clearance was obtained from the Research and Ethical Review Committee of School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar. Permission letter was also taken from clinical director of the Hospital and head of the DM clinic. To ensure the confidentiality of the study participant’s information, anonymous typing was applied, so that the name and any identifier of the participants were not written on the questionnaire.
RESULTS
Serum uric acid level according to socio-demographic characteristics of study participants
A total of 384 study participants were enrolled and the response rate obtained was 99.1%. In this study, a majority of 60.4% (n=232) of the study participants were males. The mean age of the study participants was 55.74 ± 9.05 years with a range of 36 to 88 years. 95% (n=365), 96.1 % (n=370), and 59.1% (n=227) study participants were urban dwellers, married and employed, respectively. The prevalence of HUA was 31.5% (n=121) with 95% CI, 27.3-36.2. The serum uric acid concentration was higher among male study participants compared to female (33.1% versus 28.9% respectively) and the prevalence was also higher among ≥45 years age group (31.8%) (Table 1).
Table 1.
Serum uric acid level of the study participants according to socio-demographic characteristics
| Variables | Category | N (%) | Uric acid level, N (%) | P-value | |
|---|---|---|---|---|---|
| Hyperuricemia | Normouricaemia | ||||
| Sex | Male | 232(60.4) | 77(33.2) | 155(66.8) | 0.382 |
| Female | 152(39.6) | 44(28.9) | 108(71.1) | ||
| Age | 36-45 | 46(12.0) | 14(30.4) | 32(69.6) | 0.001* |
| 46-55 | 148(38.5) | 28(18.9) | 120(81.0) | ||
| 56-65 | 139(36.2) | 52(37.4) | 87(62.6) | ||
| 66-75 | 41(10.7) | 24(58.5) | 17(41.5) | ||
| 76-88 | 10(2.6) | 3(30.0) | 7(70) | ||
| Marital status | Unmarried | 12(3.1) | 7(58.3) | 5(41.6) | 0.041* |
| Married | 370(96.1) | 113(30.5) | 257(69.4) | ||
| Educational level | Literate | 104(27.1) | 33(31.7) | 71(68.2) | 0.898 |
| Illiterate | 277(72.1) | 86(31.0) | 191(69.0) | ||
| Resident | Urban | 365(95.1) | 115(31.5) | 250(68.5) | 0.995 |
| Rural | 19(4.9) | 6(31.5) | 13(68.5) | ||
| Occupation | Employed | 276(59.1) | 89(32.2) | 187(67.8) | 0.524 |
| Unemployed | 104(39.8) | 30(28.8) | 74(71.1) | ||
Serum uric acid level according to clinical characteristics of study participants
The prevalence of HUA was higher among study participants with a family history of diabetes (47.1%). Higher prevalence of HUA was determined among patients with ≥5-year duration of diabetes (42.6%), overweight (BMI: 25’29.9 Kg/m2) 52.4% (n= 49), hypertensive (69.4%) T2DM patients.
The high percentage of abnormal serum uric acid concentration was determined among study participants with central obesity (63.0%), with elevated SBP (58.2%), and with family history of DM (47.1%) (Table 2).
Table 2.
Serum uric acid level according to clinical characteristics of study participants
| Variables | Category | N (%) | Uric acid level, N (%) | P-value | |
|---|---|---|---|---|---|
| Hyperuricemia | Normouricaemia | ||||
| FHDM | Yes | 121(31.5) | 57(47.1) | 64(52.9) | 0.001* |
| No | 263(68.5) | 64(24.3) | 199(75.6) | ||
| Hypertension | Present | 118(30.8) | 82(69.4) | 36(30.6) | 0.001* |
| Absent | 266(69.2) | 39(14.6) | 227(85.4) | ||
| WC | High | 92(24.0) | 58(63.0) | 34(37.0) | 0.001* |
| Normal | 292(76.0) | 63(21.5) | 229(78.4) | ||
| BMI | Normal | 239(62.2) | 46(19.2) | 193(80.7) | 0.001* |
| High | 143(37.2) | 75(52.4) | 68(47.5) | ||
| SBP | High | 79(20.6) | 46(58.2) | 33(41.7) | 0.001* |
| Normal | 305(79.4) | 75(24.6) | 230(75.4) | ||
| DBP | High | 56(14.6) | 36(64.2) | 20(35.8) | 0.26 |
| Normal | 328(85.4) | 85(25.9) | 243(74.1) | ||
| Duration of DM | <5 yr | 248(64.6) | 63(25.4) | 185(74.6) | 0.02* |
| 6-10 yr | 100(26) | 44(44.0) | 56(56.0) | ||
| >10 yr | 36(9.4) | 14(38.8) | 22(61.2) | ||
| Physical activity | No | 304(79.2) | 100(32.9) | 204(67.1) | 0.28 |
| Yes | 80(20.8) | 21(26.2) | 59(73.8) | ||
| Alcohol | Yes | 88(22.9) | 31(35.2) | 57(64.7) | 0.393 |
| No | 296(77.1) | 90(30.7) | 206(69.2) | ||
| Coffee | Yes | 265(69.0) | 81(30.5) | 184(69.5) | 0.552 |
| No | 119(31.0) | 40(33.6) | 79(66.3) | ||
FHDM: Family History of Diabetes mellitus; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; WC: Waist Circumference; BMI: Body Mass Index
*P-value < 0.05, statistically significant association.
Serum uric acid level and biochemical parameters of study participants
The HUA concentration was determined among 42.7% (n=85) study participants with hypertriglyceridemia, among 79.2% (n=61) with reduced HDL, and in 32.6% (n=118) with hyperglycemic (Table 3).
Table 3.
Serum uric acid level and biochemical parameters of the study participants
| Variables | Category | N (%) | Uric acid level, N (%) | P-value | |
|---|---|---|---|---|---|
| Hyperuricemia | Normouricaemia | ||||
| TG (mg/dl) | High | 199(51.8) | 85(42.7) | 114(57.3) | 0.001 |
| Normal | 185(48.1) | 36(19.4) | 149(80.6) | ||
| tCho (mg/dl) | High | 171(44.5) | 66(38.6) | 105(61.4) | 0.007 |
| Normal | 213(60.1) | 55(25.8) | 158(74.2) | ||
| LDL (mg/dl) | High | 131(34.1) | 73(55.7) | 58(44.3) | 0.001 |
| Normal | 253(65.8) | 48(18.9) | 208(81.1) | ||
| HDL (mg/dl) | Low | 77(20.0) | 61(79.2) | 16(20.8) | 0.001 |
| Normal | 307(79.9) | 60(19.5) | 247(80.5) | ||
| FBS (mg/dl) | High | 362(94.2) | 118(32.6) | 224(61.8) | 0.063 |
| Normal | 22(5.7) | 3(13.6) | 19(8) | ||
TG: Triglyceride; FBG: Fasting Blood Glucose; tCho: Total Cholesterol; HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein; mg/dl: milligram per deciliter.
Correlations of selected cardiovascular disease risk factors with serum uric acid level
The Pearson’s correlation coefficient had indicated significantly positive correlation between HUA and biochemical parameters like TG (r=0.3, p value=0.001), FBG (r=0.3, p value=0.063), tCho (r=0.3, p value=0.007), and significantly negative correlation with HDL (r=-0.3, p value=0.001). In addition to that, some anthropometric parameters including BMI (r=0.1), WC (r=0.3) and SBP (r=0.2) have significantly positive correlation with HUA (Table 4).
Table 4.
Pearson’s correlation of cardiovascular disease risk factors with serum uric acid level at University of Gondar Hospital, 2018
| Parameters | Mean ± SD | Correlation coefficients | P-value |
|---|---|---|---|
| TG (mg/dl) | 272.2 ± 194.6 | 0.3 | 0.001* |
| FBG (mg/dl) | 192.8 ± 66.9 | 0.3 | 0.063 |
| tCho (mg/dl) | 226 ± 152.5 | 0.3 | 0.007* |
| HDL (mg/dl) | 57.4 ± 19.8 | -0.3 | 0.001* |
| LDL (mg/dl) | 97.8 ±52.3 | 0.3 | 0.001* |
| SBP (mmHg) | 131.6 ± 13.8 | 0.2 | 0.001* |
| DBP (mmHg) | 81.9 ± 8.6 | 0.2 | 0.001* |
| WC (cm) | 94.3 ± 9.4 | 0.3 | 0.001* |
| BMI (kg/m2) | 25.4 ± 12.3 | 0.1 | 0.003* |
TG: Triglyceride; FBG: Fasting Blood Glucose; tCho: Total Cholesterol; HDL: High Density Lipoprotein;
LDL: Low Density Lipoprotein; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; WC: Waist Circumference;
BMI: Body Mass Index; mmHg: millimeter mercury; mg/dl: milligram per deciliter; kg/m2: Kilogram per meter square
* P-value < 0.05 is statistically significant.
The association between serum uric acid and cardiovascular disease risk factors among type 2 Diabetes Mellitus patients
In this study, T2DM patients with a higher Systolic BP (AOR = 4.4, 95% C.I (2.1-9.3), WC (AOR = 3.7, 95% CI (1.6-8.8), and with high BMI (AOR = 1.4, 95% C.I (1.1-3.7) were considerably associated with hyperuricemia (Table 5).
Table 5.
Logistic regression analysis of the association of serum uric acid and cardiovascular disease risk factors among T2DM patients
| Variables | N (%) | Uric acid level | COR (95% CI) | AOR | P-value | ||
|---|---|---|---|---|---|---|---|
| Hyperuricemia | Normouricaemia | ||||||
| Sex | Male | 232 (60.4) | 77 | 155 | 1.2 (0.7-1.9) | - | - |
| Female | 152 (39.6) | 44 | 108 | 1 | - | ||
| Age | >45 | 336 (87.5) | 107 | 229 | 1.1 (0.5-2.2) | - | - |
| <45 | 48 (12.5) | 14 | 34 | 1 | - | ||
| Duration of DM | 0-5 | 248 (64.6) | 63 | 185 | 1 | 1 | 0.002* |
| 6-10 | 118 (30.8) | 53 | 65 | 2.3 (1.5-3.8) | 2.4 (1.4-4.2) | ||
| >10 | 18 (4.6) | 5 | 13 | 1.1 (0.3-3.2) | - | ||
| Hypertension | Present | 118 (30.8) | 82 | 36 | 13.2 (7.8-22.2) | 13.9 (7.9-24.6) | 0.001* |
| Absent | 266 (69.2) | 39 | 227 | 1 | 1 | ||
| Systolic BP | High | 79 (20.6) | 46 | 33 | 4.2 (2.5-7.1) | 4.4 (2.1-9.3) | 0.03* |
| Normal | 305 (79.4) | 75 | 230 | 1 | 1 | ||
| Diastolic BP | High | 56 (14.6) | 36 | 20 | 5.1 (2.8-9.3) | 2.2 (0.8-5.6) | 0.089 |
| Normal | 328 (85.4) | 85 | 243 | 1 | - | ||
| Family history DM | Yes | 121 (31.5) | 57 | 64 | 2.7 (1.7-4.3) | 1.5 (1.2-2.5) | 0.05 |
| No | 263 (68.5) | 64 | 199 | 1 | 1 | ||
| WC | High | 92(24) | 58 | 34 | 6.2 (3.7-10.2) | 3.7 (1.6-8.8) | 0.001* |
| Normal | 292(76) | 63 | 229 | 1 | 1 | ||
| BMI | High | 143 (37.4) | 75 | 68 | 4.6 (2.9-7.3) | 2.0 (1.1-3.7) | 0.03* |
| Normal | 239 (62.6) | 46 | 193 | 1 | 1 | ||
| Alcohol drinking habit | Yes | 88 (22.9) | 31 | 57 | 1.2 (0.7-2.0) | - | - |
| No | 296 (77.1) | 90 | 206 | 1 | - | ||
| Coffee drinking habit | Yes | 265(69) | 81 | 184 | 0.8 (0.5-1.3) | - | - |
| No | 119(31) | 40 | 79 | 1 | - | ||
| Physical activity | Yes | 80 (20.8) | 21 | 59 | 0.7 (0.4-1.2) | - | - |
| No | 304 (79.2) | 100 | 204 | 1 | - | ||
WC: Waist Circumference; BMI: Body Mass Index; DM: Diabetes Mellitus; BP: Blood Pressure; COR: Crude Odds Ratio; AOR: Adjusted Odds Ratio
* P value < 0.05 is statistically significant.
The prevalence of cardiovascular disease risk factors among T2DM patients
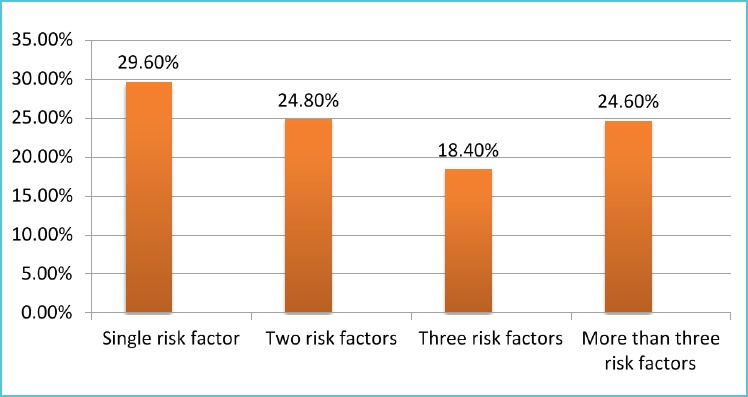
About, 29.6% (n=121) of the study, participants have single CVD risk factor, that is followed by two CVD risk factor 24.8% (n=93). At least one CVD risk factor was observed in 97.4% (n=374) of the study participants. Hypertension 58.6%; dyslipidemia 64.9%; overweight: 37.2% and central obesity: 24.0% were selected CVD risk factors (Figure 1).
Figure 1.
The overall prevalence of cardiovascular disease risk factors among T2DM patients at University of Gondar Hospital, northwest Ethiopia, 2018
DISCUSSION
A previous study has reported that moderately raised levels of SUA have been considered as a simple biochemical defect with little clinical significance. However, recently, it has become increasingly clear that moderately elevated SUA levels are independently associated with increased cardiovascular morbidity and mortality in T2DM patients [20].
The main finding of this study was high prevalence of HUA concentration among T2DM patients. There was significant association between HUA and the various types of the CVD risk factors, and an increase in number of each CVD risk factor among the study participants.
In this study, the prevalence of HUA among T2DM patients was 31.5%. The magnitude of HUA that was reported by Wang J et al. from China (32.2%), Shah P et al. from Egypt (32.0%), Woyesa et al. from Hawassa, Ethiopia (33.8%)[21-23] was comparable to our finding. In contrast to the current finding, low prevalence of HUA was reported by Moulin SR et al. from Angola (25.0%) and Mundhe et al. from India (25.3%)[24, 25], and much less prevalence was reported from US (21.0%)(26). The variation in prevalence across studies might be due to the different life style, and the existence of ethnic variation between people in different countries [27].
The magnitude of HUA in males was higher than females in our study, which was supported by the study conducted in Nigeria and India [25, 28]. These sex differences of SUA levels have been attributed to the influence of sex hormones [29], due to the mechanism of estrogen in promoting UA excretion [30].
The other possible explanation for this, could be that males are more exposed to alcohol consumption [29] since, beer contains large amounts of purine [31] and the increased renal ATP binding cassette transporter sub family G member 2 (ABCG2) expression in men compared with women. The expression of the ABCG2 protein induces HUA through the reabsorption of urate [32].
In contrast to our finding, the prevalence of HUA from China, by Wang et al [23], was high among female study participants. The difference might be due to the ethnic difference of the study participants across countries.
On the other hand, the prevalence HUA in Nigeria [33] and Taiwan [34] were comparable in both genders. Beyond dietetic factors, HUA can also be related to the genetic predisposition for higher urate reabsorption in the kidneys.
Previous studies had shown that the ABCG2 protein, a UA transporter, shows differences in its expression and function by ethnicity [27].
In our study, age greater than 45 years had high prevalence of HUA, which was similar to the study conducted in Hawassa, Ethiopia [22] and China [23]. The reason that might occur is that the effect of diuretics [35], due to ABCG2 protein, which increases as age increases and renal complications during aging [27].
The magnitude of hypertension (58.6%) in our study was comparable with the study conducted in Himalayan areas (61.5%)(36), and its prevalence was lower from Northern Catalonians (74.5%) [37]. In addition to that, the overall magnitude of dyslipidemia in our study was lower compared to the study in North Catalonia (77.7%) [37].
Similar study conducted in North Catalonia showed the different types of specific CVD risk factors, which include high BMI (>25 kg/m2) (60.9%) and hypertension (80.3%), which was higher than the current study.
On the other hand, hypertriglyceridemia (35.6%) and lower HDL (19.5%) were lower compared with our study. The possible explanation might be due to the life style, ethnicity and cultural difference between those two regions [37].
The simultaneous presence of three or more CVD risk factors in the current study was observed in 24.6% of the study participants. This was much less from the study conducted in North Catalonia (91.3) [37].
The occurrence of at least one CVD risk factor in our study was observed in 97.4%. In this study, the duration of DM and family history of diabetes had statistically significant association with HUA, which is in line with the finding reported from India (38).
The possible mechanisms to explain these associations are the use of diuretics [35] or impaired renal function [39]. Genetic predisposition could be one of the reasons for the effect of HUA because of the gross overproduction of UA which results from the inability to recycle either hypoxanthine or guanine in patients genetically deficient in Hypoxanthine-guanine phosphoribosyl transferase (HPRT), inducing a lack of feed-back control of purine synthesis, which accompanied by rapid catabolism of purines to UA [40].
Increased SBP had significantly associated with HUA, which was supported by the study conducted in Black Africans [24].
The possible factor might be the use of anti-hypertensive agents, such as diuretics, which are known to increase HUA [35] and T2DM with hypertensive patients showed a significant association with HUA compared to non-hypertensive participants which is supported by a study on Black Africans, hence, anti-hypertensive therapy contributes significantly increases HUA [41].
In this study, high WC and high BMI (>25Kg/m2) were significantly associated with HUA. This finding was supported by studies conducted in Nigerian, China and India [23, 25, 28]. The possible reason might be as a result of increase in Xanthine oxidoreductase (XOR) in obese individuals catalyzes oxidative hydroxylation of hypoxanthine to xanthine to uric acid (35).The level of HUA, accompanied with a significantly correlation with LDL, TG, TC, and HDL levels, in our study, which is agreed with study conducted in US [42]. Evidence also supported that dyslipidemia may cause HUA a negative effect on renal function [14].
Anthropometric measurements, such as high BMI, high SBP, high WC, as well as biochemical parameters, such as FBG and TG, were positively correlated with HUA.
The current study showed that, low HDL had a negative correlation with HUA, which were supported by the studies conducted in Ethiopia, China, Taiwan and India [22, 23, 34, 43], and a number of pathophysiological mechanisms have been explained to these associations including insulin resistance (IR) [44], the use of diuretics [35] or impaired renal function [39].
Patients who have IR, secrete larger amounts of insulin to maintain an adequate glucose metabolism and the kidney responds to the high insulin levels by decreasing UA clearance, probably linked to insulin-induced urinary sodium retention [45].
Due to these, the kidney has been implicated as the potential link between IR and compensatory hyperinsulinemia and the development of HUA.
The limitation of this study was cross-sectional nature of the study design that does not allow the establishment of causal relationship.
CONCLUSION AND RECOMMENDATION
The prevalence of hyperuricemia was high in type 2 diabetes patients. The major predictors of CVD risk factors were elevated systolic blood pressure, family history of DM and BMI ≥ 25 Kg/m2.
There was significantly positive correlation of HUA with hypertriglyceridemia, hypercholesterolemia, high LDL, high WC and increased BMI. Therefore, early diagnosis and treatment for hyperuricemia and CVD risk factors are essential to reduce the disease among type 2 diabetic patients.
Acknowledgement
The authors would like to acknowledge the University of Gondar, University of Gondar Hospital DM clinic staff and clinical chemistry laboratory staff of University of Gondar Hospital and Mr. Molla Abebe for cooperation and helping us during data collection. We also would like to acknowledge the study participants for their participation in this study. We thank Prof. Rana RaidaWajih Khalil, University of Philadelphia, Jordan for editing the English Language of the paper on behalf of Science Edit for the Developing World.
Footnotes
Availability of data and materials
All relevant data supporting the conclusion are within the paper. The datasets used for this manuscript are available from the corresponding author on reasonable request.
Authors’ contributions
All authors participated in data collection, analysis, and interpretation of the result, write up and reviewed the initial and final drafts of the manuscript. All authors read and approved the final manuscript.
Conflict of interest
The authors declared that there is no competing interest.
Funding statement
The authors received no specific funding for this work.
REFERENCES
- 1.Chiou W-K, Wang M-H, Huang D-H, Chiu H-T, Lee Y-J, Lin J-D. The relationship between serum uric acid level and metabolic syndrome: differences by sex and age in Taiwanese. Journal of epidemiology. 2010;20(3):219-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang D-H, Ha S-K. Uric acid puzzle: dual role as antioxidantand pro-oxidant. Electrolytes & Blood Pressure. 2014;12(1):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruggiero C, Cherubini A, Ble A, Bos AJ, Maggio M, Dixit VD, et al. Uric acid and inflammatory markers. European heart journal. 2006;27(10):1174-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bringhurst F, Demay M, Krane S, Kronenberg H. Bone and mineral metabolism in health and disease. Harrisons principles of internal medicine. 2005;16(2):2238. [Google Scholar]
- 5.Denzer C, Muche R, Mayer H, Heinze E, Debatin K-M, Wabitsch M. Serum uric acid levels in obese children and adolescents: linkage to testosterone levels and pre-metabolic syndrome. Journal of Pediatric Endocrinology and Metabolism. 2003;16(9):1225-1232. [DOI] [PubMed] [Google Scholar]
- 6.Kuo C-F, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nature reviews rheumatology. 2015;11(11):649-662. [DOI] [PubMed] [Google Scholar]
- 7.Li L, Yang C, Zhao Y, Zeng X, Liu F, Fu P. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease?: a systematic review and meta-analysis based on observational cohort studies. BMC nephrology. 2014;15(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kashinath R. Hyperuricemia in Type 2 Diabetes Mellitus. Global Journal of Medical Research. 2014;14(3). [Google Scholar]
- 9.Organization WH. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1, Diagnosis and classification of diabetes mellitus. 1999. [Google Scholar]
- 10.Dehghan A, Van Hoek M, Sijbrands EJ, Hofman A, Witteman JC. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes care. 2008;31(2):361-362. [DOI] [PubMed] [Google Scholar]
- 11.Lewis J, Neilson E. Chapter 277 Glomerular Diseases. In, Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J. (eds). Harrison’s Principles of Internal Medcine. New York, The McGraw-Hill Companies; 2008. [Google Scholar]
- 12.Gagliardi AC, Miname MH, Santos RD. Uric acid: a marker of increased cardiovascular risk. Atherosclerosis. 2009;202(1):11-17. [DOI] [PubMed] [Google Scholar]
- 13.Johnson RJ, Kang D-H, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41(6):1183-1190. [DOI] [PubMed] [Google Scholar]
- 14.Mänttäri M, Tiula E, Alikoski T, Manninen V. Effects of hypertension and dyslipidemia on the decline in renal function. Hypertension. 1995;26(4):670-675. [DOI] [PubMed] [Google Scholar]
- 15.Hyun KK, Huxley RR, Arima H, Woo J, Lam TH, Ueshima H, et al. A comparative analysis of risk factors and stroke risk for Asian and non-Asian men: the Asia Pacific cohort studies collaboration. International Journal of Stroke. 2013;8(8):606-611. [DOI] [PubMed] [Google Scholar]
- 16.Yadav D, Lee ES, Kim HM, Lee EY, Choi E, Chung CH. Hyperuricemia as a potential determinant of metabolic syndrome. Journal of lifestyle medicine. 2013;3(2):98. [PMC free article] [PubMed] [Google Scholar]
- 17.CAC. Summery and statistical report of the 2007 population and housing census addis abeba.. Population and housing cencus commision. 2008:57-60. [Google Scholar]
- 18.de Onis M, Habicht J-P. Anthropometric reference data for international use: recommendations from a World Health Organization Expert Committee. The American journal of clinical nutrition. 1996;64(4):650-658. [DOI] [PubMed] [Google Scholar]
- 19.Kavey R-EW, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation. 2003;107(11):1562-1566. [DOI] [PubMed] [Google Scholar]
- 20.Zoppini G, Targher G, Bonora E. The role of serum uric acid in cardiovascular disease in type 2 diabetic and non-diabetic subjects: a narrative review. Journal of endocrinological investigation. 2011;34(11):881-886. [DOI] [PubMed] [Google Scholar]
- 21.Shah P, Bjornstad P, Johnson RJ. Hyperuricemia as a potential risk factor for type 2 diabetes and diabetic nephropathy. Jornal Brasileiro de Nefrologia. 2016;38(4):386-387. [DOI] [PubMed] [Google Scholar]
- 22.Woyesa SB, Hirigo AT, Wube TB. Hyperuricemia and metabolic syndrome in type 2 diabetes mellitus patients at Hawassa university comprehensive specialized hospital, South West Ethiopia. BMC endocrine disorders. 2017;17(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Chen R-P, Lei L, Song Q-Q, Zhang R-Y, Li Y-B, et al. Prevalence and determinants of hyperuricemia in type 2 diabetes mellitus patients with central obesity in Guangdong Province in China. Asia Pacific journal of clinical nutrition. 2013. [DOI] [PubMed] [Google Scholar]
- 24.Moulin SR, Baldo MP, Souza JB, Luchi WM, Capingana DP, Magalhães P, et al. Distribution of serum uric acid in black africans and its association with cardiovascular risk factors. The Journal of Clinical Hypertension. 2017;19(1):45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mundhe SA, Mhasde DR. The study of prevalence of hyperuricemia and metabolic syndrome in type 2 diabetes mellitus. International Journal of Advances in Medicine. 2017;3(2):241-249. [Google Scholar]
- 26.Krishnan E, Akhras K, Sharma H, Marynchenko M, Wu E, Tawk R, et al. Relative and attributable diabetes risk associated with hyperuricemia in US veterans with gout. QJM: An International Journal of Medicine. 2013;106(8):721-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakiyama M, Matsuo H, Takada Y, Nakamura T, Nakayama A, Takada T, et al. Ethnic differences in ATP-binding cassette transporter, sub-family G, member 2 (ABCG2/BCRP): genotype combinations and estimated functions. Drug metabolism and pharmacokinetics. 2014;29(6):490-492. [DOI] [PubMed] [Google Scholar]
- 28.Ewenighi C, Dimkpa U, Ezeugwu U, Onyeanusi J, Onoh L. Prevalence of hyperuricemia and its risk factors in healthy male adults from Abakaliki metropolis, Nigeria. Journal of Molecular Pathophysiology. 2015;4(3):94-98. [Google Scholar]
- 29.Gordon T, Kannel WB. Drinking and its relation to smoking, BP, blood lipids, and uric acid: the Framingham Study. Archives of Internal Medicine. 1983;143(7):1366-1374. [PubMed] [Google Scholar]
- 30.Sumino H, Ichikawa S, Kanda T, Nakamura T, Sakamaki T. Reduction of serum uric acid by hormone replacement therapy in postmenopausal women with hyperuricaemia. The Lancet. 1999;354(9179):650. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Guo X, Liu Y, Chang Y, Sun Y, Zhu G, et al. The Relation of Moderate Alcohol Consumption to Hyperuricemia in a Rural General Population. International journal of environmental research and public health. 2016;13(7):732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dehghan A, Köttgen A, Yang Q, Hwang S-J, Kao WL, Rivadeneira F, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. The Lancet. 2008;372(9654):1953-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogbera AO, Azenabor AO. Hyperuricaemia and the metabolic syndrome in type 2 DM. Diabetology & metabolic syndrome. 2010;2(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qian Y, Shen H-c, Hu Y-c, Chen Y-f, Tung T-h. Prevalence and metabolic factors of hyperuricemia in an elderly agricultural and Ashing population in Taiwan. Archives of Rheumatology. 2017;32(2):149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savage PJ, Pressel SL, Curb JD, Schron EB, Applegate WB, Black HR, et al. Influence of long-term, low-dose, diuretic-based, antihypertensive therapy on glucose, lipid, uric acid, and potassium levels in older men and women with isolated systolic hypertension: the Systolic Hypertension in the Elderly Program. Archives of Internal Medicine. 1998;158(7):741-751. [DOI] [PubMed] [Google Scholar]
- 36.Mokta J, Mokta K, Ranjan A, Garg M. Prevalence of Cardiovascular Risk Factors among Diabetic Population and Awareness of Diabetes among Diabetic Patients: A Population Based Himalayan Study. Journal of The Association of Physicians of India. 2017;65:48. [PubMed] [Google Scholar]
- 37.Jurado J, Ybarra J, Solanas P, Caula J, Gich I, Pou JM, et al. Prevalence of cardiovascular disease and risk factors in a type 2 diabetic population of the North Catalonia diabetes study. Journal of the American Association of Nurse Practitioners. 2009;21(3):140-148. [DOI] [PubMed] [Google Scholar]
- 38.Ichida K, Matsuo H, Takada T, Nakayama A, Murakami K, Shimizu T, et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nature communications. 2012;3:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Messerli FH, Frohlich ED, Dreslinski GR, Suarez DH, Aristimuno GG. Serum uric acid in essential hypertension: an indicator of renal vascular involvement. Annals of internal medicine. 1980;93(6):817-821. [DOI] [PubMed] [Google Scholar]
- 40.Cameron JS, Simmonds HA, editors. Hereditary hyperuricemia and renal disease. Seminars in nephrology; 2005: Elsevier. [DOI] [PubMed] [Google Scholar]
- 41.Reyes AJ. Cardiovascular drugs and serum uric acid. Cardiovascular drugs and therapy. 2003;17(5-6):397-414. [DOI] [PubMed] [Google Scholar]
- 42.Peng T-C, Wang C-C, Kao T-W, Chan JY-H, Yang Y-H, Chang Y-W, et al. Relationship between hyperuricemia and lipid profiles in US adults. BioMed research international. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma N, Rajkumari R, Anil B. Prevalence of Hyperuricemia and relation of serum uric acid with diabetic risk factors. Int J Adv Res. 2015;3(5):289-296. [Google Scholar]
- 44.Vuorinen-Markkola H, Yki-Järvinen H. Hyperuricemia and insulin resistance. The Journal of Clinical Endocrinology & Metabolism. 1994;78(1):25-29. [DOI] [PubMed] [Google Scholar]
- 45.Reaven GM. The kidney: an unwilling accomplice in syndrome X. American journal of kidney diseases. 1997;30(6):928-931. [DOI] [PubMed] [Google Scholar]


