Abstract
Aim:
The aim of this study was to compare and evaluate the efficacy of ViscoStat clear, Vasozine, and Racegel (with and without cord) with respect to the amount of lateral gingival displacement produced by them.
Settings and Design:
Comparative - In vivo study.
Material and Methods:
Thirty consented volunteers were selected in the age group of 18–22 years. Maxillary right first premolar and lateral incisor and maxillary left central incisor and canine were selected for each individual. A composite resin standard reference point was made two millimeters below the gingival margin on the midsection of the labial surface of each tooth. By simple random sampling, the agents (ViscoStat clear, Vasozine, and Racegel with cord and Racegel without cord) were used for gingival displacement on each of the selected teeth. Pre- and postgingival displacement impressions were made with medium-body polyvinyl siloxane impression material. Three-millimeter thick buccolingual slice sections were obtained of the models and measured under a stereo microscope (×20 magnification), and the amount of displacement was calculated.
Statistical Analysis used:
The Kruskal–Wallis test and the Mann–Whitney U-test were used for comparison between the amounts of gingival displacement produced by them.
Results:
Mean displacement produced (in mm2) by Racegel with cord, tetrahydrozoline, ViscoStat clear, and Racegel is 0.2256, 0.2158, 0.2069, and 0.1414, respectively.
Conclusions:
The largest mean gingival displacement was produced by Racegel with cord (0.2256 mm2) and lowest by Racegel without cord (0.1414 mm2). There was no significant statistical difference in the amount of gingival displacement produced between the four agents.
Keywords: Gingival displacement, marginal integrity, Racegel, tetrahydrozoline, Ultrapak cord, ViscoStat clear
INTRODUCTION
Gingival displacement facilitates effective impression making, fluid management, finishing and placement of tooth preparation margins, removal of excess cement, etc. Impressions made with sulcular width lesser than the critical value i.e 0.15-0.2mm, have higher incidence of voids in the marginal area and decrease in tear strength of impression material.[1] Chemicomechanical displacement is the most commonly used method.[2,3] An alternative to overcome the demerits of acidic nature of the chemical agents would be to use nasal decongestants such as tetrahydrozoline and oxymetazoline with higher pH as gingival displacement solution which is safer to the tissues. Furthermore, to overcome the shortcomings of the mechanical method of gingival displacement, newer cordless systems such as Racegel have been introduced which are less time-consuming, more comfortable to the patient, easy application, and minimally invasive. Since there is indefinite evidence regarding the efficacy of these newer agents, this study was conducted to compare and evaluate the clinical efficacy of a nonacidic agent tetrahydrozoline HCl (Vasozine) and a cordless system Racegel with a conventional agent aluminum chloride (ViscoStat clear).
SUBJECTS AND METHODS
The study was approved by Institutional Ethical Committee, ref no.ECR/1221/Inst/MH/2019.
Step I: Subject selection criteria
Thirty healthy human volunteers in the age group of 18–22 years were selected, and written informed consent was sought for the study. An approval from the ethical committee institutional review board for the procedure was followed strictly.
All the individuals were selected based on the following inclusion and exclusion criteria with reference to guidelines provided by Chaudhary et al.[4]
Inclusion criteria
Patients having healthy periodontium (gingival index of score 0).
Exclusion criteria
Patients undergoing orthodontic treatment
Patients with malocclusion or recession with anteriors and/or premolars
Pregnant and lactating women
Patients with restorations or prosthesis with anteriors and/or premolars.
Step II: Making preliminary impressions and casts
Oral prophylaxis was carried out for each participant meticulously followed by preliminary impressions of the maxillary arch (first right molar to first left molar) made in irreversible hydrocolloid impression material (Zhermack). The impression was poured in Type III gypsum (Kalabhai). A vacuum mixer and vibrator were used to avoid incorporating voids and air bubbles. The casts with their bases casts were numbered 1, 2,…. 30 for each of the participants [Figure 1]. Sixty custom trays (two for each patient) were fabricated using 2-mm spacer (two sheets of modeling wax) and 2 mm × 2 mm tissue stops on the buccal cusp of the second premolar and mesiobuccal cusp of the first molar.
Figure 1.
Diagnostic cast of participant no. 1
Step III: Marking a reference point on selected teeth with composite restoration material
Maxillary right first premolar and lateral incisor and maxillary left central incisor and canine were selected for all the participants. Each selected tooth was bisected, and 2 -mm marking from the marginal gingiva was made on this bisected line on the labial surface with an indelible pencil [Figure 2]. A small standard point of reference point made of composite resin was placed on this marking for evaluation purposes [Figure 3].
Figure 2.
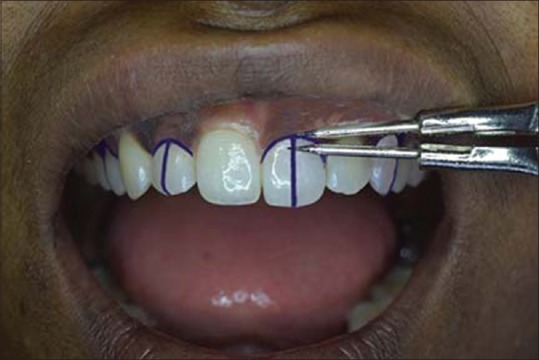
Marking on the midline of the labial surface of the selected teeth, 2 mm below the marginal gingiva
Figure 3.
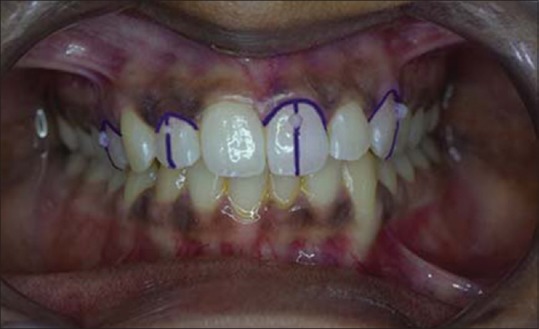
Intraoral view with standard reference point made with composite resin restoration on 14, 12, 21, and 23
Step IV: Pregingival displacement impression
Tray adhesive was applied onto the custom tray and allowed to dry for about 10 min following manufacturer's instructions. Impressions were made using medium viscosity polyvinyl siloxane (Monophase, Aquasil) impression material [Figure 4]. The impression was poured in Type IV gypsum (Ultrarock Kalabhai). The procedure was repeated for all the thirty participants and the trays, and their corresponding casts with their bases were labeled as 1B, 2B …. 30B, respectively [Figure 5].
Figure 4.
Predisplacement Impression for participant no. 1
Figure 5.

Predisplacement models for all the thirty participants
Step V: Postgingival displacement impression
The four gingival displacement agents which were used are ViscoStat clear, Vasozine, Racegel with Ultrapak knitted plain (00) cord, and Racegel without cord [Figure 6]. By simple random sampling, one of these four agents was used for gingival displacement on each of the selected teeth in the study. The cords were immersed in each of the solutions for 20 min.[5] They were removed from the sulci after 10 min,[6] and the area was washed with a jet of water [Figure 7].
Figure 6.
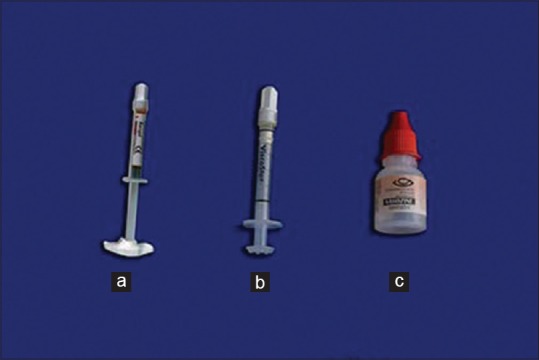
Gingival displacement agents, (a) Racegel, (b) ViscoStat, (c) Vasozine
Figure 7.

Intraoral view – Postgingival displacement
Use of Racegel without cord
After thorough isolation, Racegel was applied throughout the buccal and palatal gingival sulci as per the manufacturer's instructions. The material was washed away with a jet of water after a time interval of 10 min[6] [Figure 8].
Figure 8.
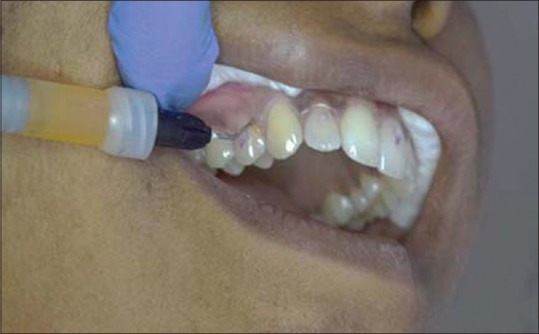
Using Racegel for gingival displacement
Postdisplacement impressions were made in a similar manner [Figure 9], and their corresponding casts and bases were labeled 1A, 2A …. 30A, respectively [Figure 10].
Figure 9.
Postdisplacement impression for participant no. 1
Figure 10.
Postdisplacement models for all the thirty participants
Step VI: Sectioning of the casts and observations
Three-millimeter thick buccolingual slice sections were made using a die sectioning lathe. The first section was made bisecting the reference point made of composite restoration and the other section was made three millimeters distal to the first section [Figure 11]. Each slice was then labeled as follows:
Figure 11.

Sectioning of samples on lathe
1) Pre -displacement model (1B,2B) or Post -displacement model (1A, 2A), 2) The tooth (maxillary right first premolar-”w,” lateral incisor-“x,” maxillary left central incisor-“y,” canine-“z”) and 3)the agent used on them (ViscoStat clear-“v,” Vasozine-“t,” Racegel with cord-“Rc,” Racegel without cord-“r”).[Figures 12 and 13]. Measuring the width of gingival sulcus, the width of the gingival sulcus was measured under a stereo microscope (×20, magnification) as the total area between three points:
Figure 12.

Three-millimeter buccolingual sections labeled (predisplacement)
Figure 13.
Three-millimeter buccolingual sections labeled (postdisplacement)
Base of the standard reference point
Deepest point in the sulcus
A tangent was drawn from the base of the standard reference point and the free gingival margin. The point of intersection determines the third point of reference for the measurements [Figure 14].
Figure 14.

Measurement of area of displacement. A: Base of standard reference point. B: Deepest point in gingival sulcus. C: Highest contour of gingival crest
The amount of displacement was calculated as the difference between the postdisplacement and the predisplacement values [Figures 15 and 16]. The data were then tabulated for each participant under four groups belonging to each of the four agents.
Figure 15.

Microscopic analysis area (predisplacement) (330,380.72)
Figure 16.

Microscopic analysis area (postdisplacement) (415,673.14)
RESULTS
The readings obtained after calculating the amount of displacement caused by each agent were divided into four groups:
Group 1: Gingival displacement caused by Racegel with cord
Group 2: Gingival displacement caused by Vasozine (tetrahydrozoline)
Group 3: Gingival displacement caused by ViscoStat
Group 4: Gingival displacement caused by Racegel.
The mean gingival displacement produced by Group 1 was the largest (0.2256 mm2) and that produced by Group 4 was the smallest (0.1414 mm2) [Master Chart 1]. The mean gingival displacement produced by Group 2 and Group 3 was 0.2158 and 0.2069 mm2, respectively.
Master Chart 1.
The amount of gingival displacement produced by each agent on each participant (μm2 and mm2)
| Serial number | Group | Area (µm2) | Group | Area (mm2) |
|---|---|---|---|---|
| 1 | rc | 795,618.6 | 1 | 0.795619 |
| 2 | rc | 134,414.2 | 1 | 0.134414 |
| 3 | rc | 507,067.8 | 1 | 0.507068 |
| 4 | rc | 206,036.9 | 1 | 0.206037 |
| 5 | rc | 372,467.8 | 1 | 0.372468 |
| 6 | rc | 183,250.9 | 1 | 0.183251 |
| 7 | rc | 66,524.05 | 1 | 0.066524 |
| 8 | rc | 342,684.4 | 1 | 0.342684 |
| 9 | rc | 248,477.1 | 1 | 0.248477 |
| 10 | rc | 78,477.7 | 1 | 0.078478 |
| 11 | rc | 99,333.83 | 1 | 0.099334 |
| 12 | rc | 342,306.2 | 1 | 0.342306 |
| 13 | rc | 66,400.15 | 1 | 0.0664 |
| 14 | rc | 224,966.3 | 1 | 0.224966 |
| 15 | rc | 290,062.4 | 1 | 0.290062 |
| 16 | rc | 66,112.08 | 1 | 0.066112 |
| 17 | rc | 188,594.3 | 1 | 0.188594 |
| 18 | rc | 9005.43 | 1 | 0.009005 |
| 19 | rc | 84,304.29 | 1 | 0.084304 |
| 20 | rc | 287,615.2 | 1 | 0.287615 |
| 21 | rc | 113,666.4 | 1 | 0.113666 |
| 22 | rc | 13,226.75 | 1 | 0.013227 |
| 23 | rc | 161,270.4 | 1 | 0.16127 |
| 24 | rc | 145,663.1 | 1 | 0.145663 |
| 25 | rc | 106,503.4 | 1 | 0.106503 |
| 26 | rc | 300,770.8 | 1 | 0.300771 |
| 27 | rc | 267,211.4 | 1 | 0.267211 |
| 28 | rc | 294,442.4 | 1 | 0.294442 |
| 29 | rc | 480,750.6 | 1 | 0.480751 |
| 30 | rc | 290,062.4 | 1 | 0.290062 |
| 1 | t | 337,356.5 | 2 | 0.337357 |
| 2 | t | 221,475.3 | 2 | 0.221475 |
| 3 | t | 100,142.5 | 2 | 0.100143 |
| 4 | t | 7452.82 | 2 | 0.007453 |
| 5 | t | 386,066.3 | 2 | 0.386066 |
| 6 | t | 429,289.2 | 2 | 0.429289 |
| 7 | t | 91,137.59 | 2 | 0.091138 |
| 8 | t | 275,438.6 | 2 | 0.275439 |
| 9 | t | 398,416.4 | 2 | 0.398416 |
| 10 | t | 140,590.8 | 2 | 0.140591 |
| 11 | t | 115,865.7 | 2 | 0.115866 |
| 12 | t | 149,385.2 | 2 | 0.149385 |
| 13 | t | 153,634.8 | 2 | 0.153635 |
| 14 | t | 783,912.7 | 2 | 0.783913 |
| 15 | t | 119,644.6 | 2 | 0.119645 |
| 16 | t | 229,962.7 | 2 | 0.229963 |
| 17 | t | 114,892.6 | 2 | 0.114893 |
| 18 | t | 64,318.57 | 2 | 0.064319 |
| 19 | t | 151,589.3 | 2 | 0.151589 |
| 20 | t | 601,114.1 | 2 | 0.601114 |
| 21 | t | 140,823.1 | 2 | 0.140823 |
| 22 | t | 315,939.7 | 2 | 0.31594 |
| 23 | t | 120,373.5 | 2 | 0.120374 |
| 24 | t | 271,761.7 | 2 | 0.271762 |
| 25 | t | 20,887.1 | 2 | 0.020887 |
| 26 | t | 147,726.9 | 2 | 0.147727 |
| 27 | t | 60,712.9 | 2 | 0.060713 |
| 28 | t | 190,347.3 | 2 | 0.190347 |
| 29 | t | 212,910.4 | 2 | 0.21291 |
| 30 | t | 119,644.7 | 2 | 0.119645 |
| 1 | v | 85,292.42 | 3 | 0.085292 |
| 2 | v | 203,264.5 | 3 | 0.203265 |
| 3 | v | 484,384.1 | 3 | 0.484384 |
| 4 | v | 464,847.5 | 3 | 0.464848 |
| 5 | v | 441,129.2 | 3 | 0.441129 |
| 6 | v | 93,758.16 | 3 | 0.093758 |
| 7 | v | 233,459.9 | 3 | 0.23346 |
| 8 | v | 115,029.4 | 3 | 0.115029 |
| 9 | v | 352,906.5 | 3 | 0.352906 |
| 10 | v | 84,418.9 | 3 | 0.084419 |
| 11 | v | 65,981.98 | 3 | 0.065982 |
| 12 | v | 232,924 | 3 | 0.232924 |
| 13 | v | 72,737.84 | 3 | 0.072738 |
| 14 | v | 490,991.3 | 3 | 0.490991 |
| 15 | v | 67,422.37 | 3 | 0.067422 |
| 16 | v | 41,297.2 | 3 | 0.041297 |
| 17 | v | 91,642.49 | 3 | 0.091642 |
| 18 | v | 146,934.7 | 3 | 0.146935 |
| 19 | v | 153,775.4 | 3 | 0.153775 |
| 20 | v | 125,872.4 | 3 | 0.125872 |
| 21 | v | 325,452.4 | 3 | 0.325452 |
| 22 | v | 200,061.6 | 3 | 0.200062 |
| 23 | v | 179,899.3 | 3 | 0.179899 |
| 24 | v | 428,271.1 | 3 | 0.428271 |
| 25 | v | 51,308.6 | 3 | 0.051309 |
| 26 | v | 168,599.3 | 3 | 0.168599 |
| 27 | v | 58,451.8 | 3 | 0.058452 |
| 28 | v | 167,285.9 | 3 | 0.167286 |
| 29 | v | 512,541.3 | 3 | 0.512541 |
| 30 | v | 67,422.4 | 3 | 0.067422 |
| 1 | r | 277,433.4 | 4 | 0.277433 |
| 2 | r | 148,644.5 | 4 | 0.148645 |
| 3 | r | 294,863.7 | 4 | 0.294864 |
| 4 | r | 76,879.33 | 4 | 0.076879 |
| 5 | r | 68,837.96 | 4 | 0.068838 |
| 6 | r | 256,406.9 | 4 | 0.256407 |
| 7 | r | 4956.16 | 4 | 0.004956 |
| 8 | r | 51,664.88 | 4 | 0.051665 |
| 9 | r | 19,1786.2 | 4 | 0.191786 |
| 10 | r | 130,656.8 | 4 | 0.130657 |
| 11 | r | 143,349.9 | 4 | 0.14335 |
| 12 | r | 60,412.49 | 4 | 0.060412 |
| 13 | r | 159,018.4 | 4 | 0.159018 |
| 14 | r | 216,754.6 | 4 | 0.216755 |
| 15 | r | 232,840.4 | 4 | 0.23284 |
| 16 | r | 51,336.52 | 4 | 0.051337 |
| 17 | r | 57,612.26 | 4 | 0.057612 |
| 18 | r | 310,268 | 4 | 0.310268 |
| 19 | r | 131,749.8 | 4 | 0.13175 |
| 20 | r | 424,868.5 | 4 | 0.424869 |
| 21 | r | 6817.81 | 4 | 0.006818 |
| 22 | r | 183,486.6 | 4 | 0.183487 |
| 23 | r | 202,112.2 | 4 | 0.202112 |
| 24 | r | 14,016.3 | 4 | 0.014016 |
| 25 | r | 15,100.8 | 4 | 0.015101 |
| 26 | r | 108,450.1 | 4 | 0.10845 |
| 27 | r | 31,809.6 | 4 | 0.03181 |
| 28 | r | 155,564.6 | 4 | 0.155565 |
| 29 | r | 1489.9 | 4 | 0.00149 |
| 30 | r | 232,840.4 | 4 | 0.23284 |
A comparison of the amount of gingival displacement was done on the whole for all four groups using the Kruskal–Wallis test for which P value obtained was 0.163305, which states that there was no statistically significant difference between all the groups [Table 1 and Graph 1]. Similarly, using the Mann–Whitney U-test, individual comparisons of area of displacement in mm2 were done between each agent (Groups 1 and 2, 1 and 3, 1 and 4, 2 and 3, 2 and 4, and 3 and 4) and P = 0.7675, 0.5946, 0.0321, 0.8016, 0.1039, and 0.1137, respectively [Tables 2–7, Graphs 2–7]. This shows that in comparison with each other, none of the groups had a statistically significant difference in the amount of area of displacement except for comparison between Groups 1 and 4 (P = 0.0321), which shows that there was a statistically significant difference between the two.
Table 1.
Descriptive comparison of area of displacement produced by Group 1, Group 2, Group 3, and Group 4
| Factor | n | Mean | SD | Median |
|---|---|---|---|---|
| Group 1 | 30 | 0.2256 | 0.1679 | 0.197 |
| Group 2 | 30 | 0.2158 | 0.1707 | 0.150 |
| Group 3 | 30 | 0.2069 | 0.1542 | 0.161 |
| Group 4 | 30 | 0.1414 | 0.1077 | 0.138 |
Graph 1.
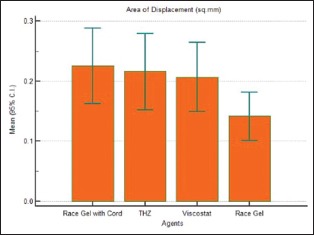
Comparison of area of displacement produced by Group 1, Group 2, Group 3, and Group 4
Table 2.
Individual comparison of area of displacement in millimeter square produced by Group 1 and Group 2
| Sample 1 | ||
| Variable Filter |
Area_mm Area of Displacement (sq.mm) Group=1 |
|
| Sample 2 | ||
| Variable Filter |
Area_mm Area of Displacement (sq.mm) Group=2 |
|
| Sample 1 | Sample 2 | |
| Sample size | 30 | 30 |
| Lowest value Highest value |
0.009005 | 0.007453 |
| 0.7956 | 0.7839 | |
| Median | 0.1973 | 0.1505 |
| 95% CI for the median Interquartile range | 0.1173-0.2896 | 0.1198-0.2285 |
| 0.09933-0.2944 | 0.1159-0.2754 | |
| Mann-Whitney test (independent samples) | ||
| Average rank of first group Average rank of second group | 31.1667 29.8333 | |
| Mann- Whitney U Large sample test statistic Z | 430.00 0.296 | |
| Two- tailed probability (P) | 0.7675 | |
Table 7.
Individual comparison of area of displacement in millimeter square produced by Group 3 and Group 4
| Sample 1 | ||
| Variable Filter |
Area_mm Area of Displacement (sq.mm) Group=3 |
|
| Sample 2 | ||
| Variable Filter |
Area_mm Area of Displacement (sq.mm) Group=4 |
|
| Sample 1 | Sample 2 | |
| Sample size | 30 | 30 |
| Lowest value Highest value |
0.04130 | 0.001490 |
| 0.5125 | 0.4249 | |
| Median | 0.1605 | 0.1375 |
| 95% CI for the median Interquartile range | 0.09201-0.2277 | 0.06189-0.1903 |
| 0.08442-0.3255 | 0.05166-0.2168 | |
| Mann- Whitney test (independent samples) | ||
| Average rank of first group Average rank of second group |
34.0667 26.9333 |
|
| Mann- Whitney U Large sample test statistic Z |
343.00 1.582 |
|
| Two- tailed probability (P) | P=0.1137 | |
Graph 2.
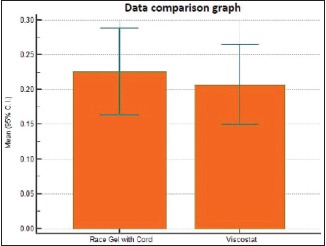
Individual comparison of area of displacement in millimeter square produced by Group 1 and Group 2
Graph 7.
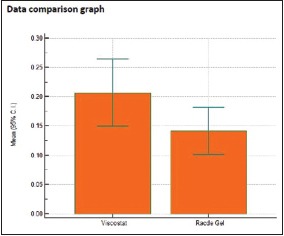
Individual comparison of area of displacement in millimeter square produced by Group 3 and Group 4
Table 3.
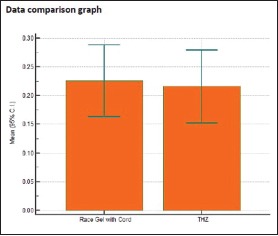
Individual comparison of area of displacement in millimeter square produced by Group 1 and Group 3
| Sample 1 | ||
| Variable Filter |
Area_mm Area of Displacement (sq.mm) Group=1 |
|
| Sample 2 | ||
| Variable Filter |
Area_mm Area of Displacement (sq.mm) Group=3 |
|
| Sample 1 | Sample 2 | |
| Sample size | 30 | 30 |
| Lowest value | 0.009005 | 0.04130 |
| Highest value | 0.7956 | 0.5125 |
| Median | 0.1973 | 0.1605 |
| 95% CI for the median Interquartile range | 0.1173-0.2896 | 0.09201-0.2277 |
| 0.09933-0.2944 | 0.08442-0.3255 | |
| Mann-Whitney test (independent samples) | ||
| Average rank of first group Average rank of second group |
31.7000 29.3000 |
|
| Mann- Whitney U Large sample test statistic Z |
414.00 0.532 |
|
| Two- tailed probability (P) | P=0.5946 | |
Table 4.
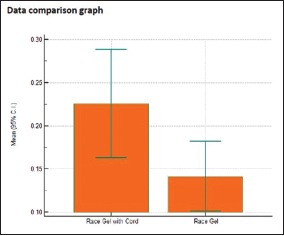
Individual comparison of area of displacement in millimeter square produced by Group 1 and Group 4
| Sample 1 | ||
| Variable Filter |
Area_mm Area of Displacement (sq.mm) Group=1 |
|
| Sample 2 | ||
| Variable Filter |
Area_mm Area of Displacement (sq.mm) Group=4 |
|
| Sample 1 | Sample 2 | |
| Sample size | 30 | 30 |
| Lowest value Highest value |
0.009005 | 0.001490 |
| 0.7956 | 0.4249 | |
| Median | 0.1973 | 0.1375 |
| 95% CI for the median Interquartile range | 0.1173-0.2896 | 0.06189-0.1903 |
| 0.09933-0.2944 | 0.05166-0.2168 | |
| Mann-Whitney test (independent samples) | ||
| Average rank of first group Average rank of second group |
35.3333 25.6667 |
|
| Mann- Whitney U Large sample test statistic Z |
305.00 2.144 |
|
| Two- tailed probability (P) | P=0.0321 | |
Table 5.
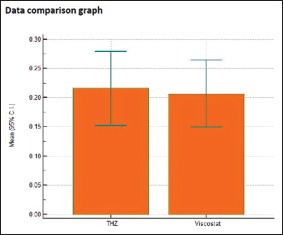
Individual comparison of area of displacement in millimeter square produced by Group 2 and Group 3
| Sample 1 | ||
| Variable Filter |
Area_mm Area of Displacement (sq.mm) Group=2 |
|
| Sample 2 | ||
| Variable Filter |
Area_mm Area of Displacement (sq.mm) Group=3 |
|
| Sample 1 | Sample 2 | |
| Sample size | 30 | 30 |
| Lowest value Highest value |
0.007453 | 0.04130 |
| 0.7839 | 0.5125 | |
| Median | 0.1505 | 0.1605 |
| 95% CI for the median Interquartile range | 0.1198-0.2285 | 0.09201-0.2277 |
| 0.1159-0.2754 | 0.08442-0.3255 | |
| Mann- Whitney test (independent samples) | ||
| Average rank of first group Average rank of second group |
31.0667 29.9333 |
|
| Mann- Whitney U Large sample test statistic Z |
433.00 0.251 |
|
| Two- tailed probability (P) | P=0.8016 | |
Table 6.
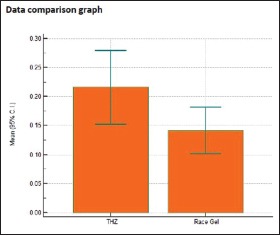
Individual comparison of area of displacement in millimeter square produced by Group 2 and Group 4
| Sample 1 | ||
| Variable Filter |
Area_mm Area of Displacement (sq.mm) Group=2 |
|
| Sample 2 | ||
| Variable Filter |
Area_mm Area of Displacement (sq.mm) Group=4 |
|
| Sample 1 | Sample 2 | |
| Sample size | 30 | 30 |
| Lowest value Highest value |
0.007453 | 0.001490 |
| 0.7839 | 0.4249 | |
| Median | 0.1505 | 0.1375 |
| 95% CI for the median Interquartile range | 0.1198-0.2285 | 0.06189-0.1903 |
| 0.1159-0.2754 | 0.05166-0.2168 | |
| Mann- Whitney test (independent samples) | ||
| Average rank of first group Average rank of second group |
34.1667 26.8333 |
|
| Mann- Whitney U Large sample test statistic Z |
340.00 1.626 |
|
| Two- tailed probability (P) | P=0.1039 | |
Graph 3.
Individual comparison of area of displacement in millimeter square produced by Group 1 and Group 3
Graph 4.
Individual comparison of area of displacement in millimeter square produced by Group 1 and Group 4
Graph 5.
Individual comparison of area of displacement in millimeter square produced by Group 2 and Group 3
Graph 6.
Individual comparison of area of displacement in millimeter square produced by Group 2 and Group 4
DISCUSSION
The rationale for tissue management is a critical aspect of impression making, whether the impression is made with a conventional impression material or by a digital scanner, so that all tooth preparation margins are captured in the impression to assure an excellent marginal integrity of a restoration.[7,8]
Historically, MJ Thompson started gingival displacement in 1959 and Benson et al.[9] in 1986, introduced the chemicomechanical method of displacement.
Today, gingival displacement procedures have been evolved from copper tubes and metal crowns filled with thermoplastic material to the present use of cotton cords and chemical agents as the most commonly used form of gingival displacement.[3,10,11,12]
The commercially available gingival displacement agents are broadly divided into astringents and vasoconstrictors. Vasoconstrictors are mainly racemic epinephrine group and sympathomimetic amine group. Racemic epinephrine group shows various systemic effects and possible cardiovascular risks.[13]
Astringents act by precipitating protein, constricting the blood vessels, and extracting the fluid from the tissues. The most commonly used astringents are 20%–25% AlCl3 and 15.5%–20% Fe2 (SO4) which leave remnants of coagulum and also stain the tissues.[3] In an endeavor to introduce better materials and techniques which are safer to the tissues and easy to use, various newer materials such as tetrahydrozoline, oxymetazoline, and xylometazoline which are commercially available as nasal decongestants and eye drops, etc., have been introduced for gingival displacement purposes.[9,14,15] Furthermore, various cordless systems such as expasyl,[16,17] Magic foam,[16,17] Merocel,[18] Traxodent Hemodent paste, GingiTrac,[19,20,21] 3M ESPE astringent retraction paste, and Racegel are introduced in the market today, which comprise using the agents in gel/paste form. This eliminates the chances of gingival trauma and maintains the health of the epithelial attachment. It is more comfortable and easy to use for the clinician.[22]
In this study, the gingival displacement agents compared were Racegel (with and without cord), ViscoStat, and tetrahydrozoline with cord. The cord (00, Ultrapak, Ultradent) used has unique knitted weave which minimizes unraveling and fraying after cutting and during cord placement. They expand when wet, opening up the sulcus greater than the original diameter of the cord. Ultrapak's interlocking loops can carry approximately 2.5 times more hemostatic solution than conventional cords. Sympathomimetic amine group of the vasoconstrictors is a better alternative to epinephrine. Tetrahydrozoline is one such member of the group which is an imidazole derivative with sympathomimetic activity. They mainly act by constricting the blood vessels but are a safer causing less systemic side effects. Nowakowska et al.[23] carried out an in vitro study to evaluate cytotoxic effects of vasoconstrictor α- and β-adrenergic group (adrenaline) versus α-adrenergic group (tetrahydrozoline, oxymetazoline, and phenylephrine) and obtained better results for α-adrenergic group.
Bowles et al.[14] showed that tetrahydrozoline is a strong retraction agent without any systemic side effect.
The study of Bowles et al.[14] showed that tetrahydrozoline is better than epinephrine in gingival retraction.
Tetrahydrozoline is not only kinder to the tissues, but it is also compatible with majority of the elastomeric impression materials. Racegel (Septodont) used in the study is a thermogelifiable gel containing 25% aluminum chloride, oxyquinol, and other excipients. It creates a clean and dry environment for the procedure. At room temperature (20°C approximately), Racegel is liquid in syringe. Its viscosity increases with temperature. When in contact with oral tissues (35°C approximately), it immediately transforms into gel form.
Participants of the age group of 20–25 years were chosen for the study to minimize the prevalence of any periodontal disease in the participants. Each participant is one's own control as before and after displacement measurements are made for the same participant. The alternate selected teeth for each participant were from the maxillary arch only. Thus, the similar age group and the same arch teeth ensured a similar gingival biotype present for each participant. The gingival displacement agents in this study were allotted to the teeth chosen, i.e., maxillary right first premolar, maxillary right lateral incisor, maxillary left central incisor, and maxillary left canine, by simple random sampling that is without any predetermined sequence, to avoid any bias. Moreover, to avoid any variance in results by subsequent displacement agents used on the same tooth in intervals, four different teeth were chosen for the same participant before and after gingival displacement. Since the gingival crest is a soft-tissue landmark, for the present study, an indigenously thought standard third point of reference was made of composite resin restoration material and placed on the midsection of each selected tooth which remained a constant point in the models before and after displacement for the measurements. The area in this study was calculated using the base of this reference point, the deepest point in the gingival sulcus, and the highest point on the height of contour of marginal gingiva.[24,25]
In this study, it was found that all the four agents produced clinically adequate and significant amount of gingival displacement required. However, Racegel with cord produced a statistically maximum amount of gingival displacement (0.2256 mm2) as compared to Racegel without cord which produced the minimum amount of gingival displacement (0.1414 mm2) compared to other agents. This may be due to the inherent property of the Racegel to expand in the sulcus into gel form in addition to the retraction cord which expands in the sulcus when wet.
This is in congruence with a study done by Dawood and Majeed in 2015 in which the results showed that the mean horizontal gingival displacement produced by Racegel was the least in comparison to Magic foam cord, astringent retraction paste, and medicated retraction cord.[26] In spite of clinically similar amount of displacement produced, statistically, the mean gingival displacement produced by Vasozine (tetrahydrozoline) (0.2158 mm2) is more than that produced by ViscoStat (0.2069 mm2). Bowles et al.[14] evaluated the efficacy of tetrahydrozoline HCl (0.05%) for gingival tissue displacement and concluded that Visine (tetrahydrozoline) produced tissue displacement greater than neosynephrine, epinephrine, and alum. In the search for a safe and easy to use material, it is found that Vasozine (tetrahydrozoline) has a neutral pH than other astringent chemical agents. Hence, it is much safer to soft tissues and avoids etching of the hard tissues. Furthermore, since it is proven to be safer systemically and produces the adequate required amount of gingival displacement, we can advocate the usage of this agent as an alternative to other conventionally and widely used chemical agents.
Racegel is a user-friendly material. In cases of thin gingival biotype, Racegel can be used effectively.
Limitations of the study
Certain clinical conditions which potentially influence the gingival displacement such as the biotype of gingiva, clinical accessibility, and compliance of the patient may impose limitations to this study
Furthermore, this study involves laboratory procedures such as pouring of the impressions and measurements made on the models. Thus, the inherent properties of the materials may have caused a difference in the results. Direct clinical evaluation would be more accurate followed by measurements made directly from the impression. Hence, further developments are needed in this regard
With each advancing day, there are newer advanced gingival displacement agents in the market such as 3M ESPE retraction systems which need to be evaluated.
Future scope of the study
This study was aimed at evaluating the efficacy of Racegel, Vasozine (tetrahydrozoline), and ViscoStat clear on the amount of gingival displacement produced by them. Other parameters such as the histological effects, time required by the agents to produce optimum gingival displacement, effect of the agents on different gingival biotypes, their ease of application, and effects on compromised periodontium were not evaluated. Further research can be done to study in depth the other parameters.
CONCLUSIONS
Thus, this study was carried out to compare and evaluate the amount of gingival displacement produced by four different gingival displacement agents/groups. The mean gingival displacement produced by Racegel with cord (Group 1) was the largest (0.2256 mm2) and that produced by Racegel without cord (Group 4) was the smallest (0.1414 mm2).
Even though clinically significant amount of gingival displacement was produced by the agents, statistically it was not significant. This explains that clinically, the agents contributed to providing an adequate amount of gingival displacement, and there is no significant difference in amount of gingival displacement produced between the newer agent Vasozine (tetrahydrozoline) and conventional agents. Thus, it can be a suitable alternative to conventional agents (ViscoStat clear – aluminum chloride).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. Kamat Varunraj (MDS Prosthodontics) for his valuable inputs in the study design.
REFERENCES
- 1.Donovan TE, Gandara BK, Nemetz H. Review and survey of medicaments used with gingival retraction cords. J Prosthet Dent. 1985;53:525–31. doi: 10.1016/0022-3913(85)90640-7. [DOI] [PubMed] [Google Scholar]
- 2.Kostić I, Najman S, Kostić M, Stojanović S. Comparative review of gingival retraction agents. Acta Med Medianae. 2012;51:81–4. [Google Scholar]
- 3.Csempesz F, Vág J, Fazekas A. In vitro kinetic study of absorbency of retraction cords. J Prosthet Dent. 2003;89:45–9. doi: 10.1067/mpr.2003.61. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhari J, Prajapati P, Patel J, Sethuraman R, Naveen YG. Comparative evaluation of the amount of gingival displacement produced by three different gingival retraction systems: An in vivo study. Contemp Clin Dent. 2015;6:189–95. doi: 10.4103/0976-237X.156043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Runyan DA, Reddy TG, Jr, Shimoda LM. Fluid absorbency of retraction cords after soaking in aluminum chloride solution. J Prosthet Dent. 1988;60:676–8. doi: 10.1016/0022-3913(88)90396-4. [DOI] [PubMed] [Google Scholar]
- 6.Shujaulla S, Tabasum S, Kumar MV. Gingival tissue retraction: A review. J Integr Dent. 2015;1:16–23. [Google Scholar]
- 7.Morgano SM, Malone WF, Gregoire SE, Goldenberg BS. Tissue management with dental impression materials. Am J Dent. 1989;2:279–84. [PubMed] [Google Scholar]
- 8.Wöstmann B, Rehmann P, Trost D, Balkenhol M. Effect of different retraction and impression techniques on the marginal fit of crowns. J Dent. 2008;36:508–12. doi: 10.1016/j.jdent.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Pandey B, Singhal MK, Nair C, Khan Z. Recent advances: Gingival retraction and fluid control. J Dent Sci Oral Rehab. 2016;7:105–9. [Google Scholar]
- 10.Houston JB, Appleby RC, DeCounter L, Funk DC, Collaghan N. Effect of epinephrine impregnated retraction cord on cardio-vascular system. J Prosthet Dent. 1970;24:376. doi: 10.1016/0022-3913(70)90077-6. [DOI] [PubMed] [Google Scholar]
- 11.Taylor AC, Campbell MM. Reattachment of gingival epithelium to the tooth. J Periodontol. 1972;43:281–93. doi: 10.1902/jop.1972.43.5.281. [DOI] [PubMed] [Google Scholar]
- 12.Ruel J, Schuessler PJ, Malament K, Mori D. Effect of retraction procedures on the periodontium in humans. J Prosthet Dent. 1980;44:508–15. doi: 10.1016/0022-3913(80)90069-4. [DOI] [PubMed] [Google Scholar]
- 13.Ramadan FA, el-Sadeek M, Hassanein el-S. Histopathologic response of gingival tissues to hemodent and aluminum chloride solutions as tissue displacement materials. Egypt Dent J. 1972;18:337–52. [PubMed] [Google Scholar]
- 14.Bowles WH, Tardy SJ, Vahadi A. Evaluation of new gingival retraction agents. J Dent Res. 1991;70:1447–9. doi: 10.1177/00220345910700111101. [DOI] [PubMed] [Google Scholar]
- 15.Kopac I, Sterle M, Marion L. Electron microscopic analysis of the effects of chemical retraction agents on cultured rat keratinocytes. J Prosthet Dent. 2002;87:51–6. doi: 10.1067/mpr.2002.119681. [DOI] [PubMed] [Google Scholar]
- 16.Prasad KD, Hegde C, Agrawal G, Shetty M. Gingival displacement in prosthodontics: A critical review of existing methods. J Interdiscip Dent. 2011;1:80. [Google Scholar]
- 17.Raghav D, Singh S, Kola MZ, Shah AH, Khalil HS, Kumar P. A comparative clinical and quantitative evaluation of the efficacy of conventional and recent gingival retraction systems: An in vitro study. Eur J Prosthodont. 2014;2:76. [Google Scholar]
- 18.Ferrari M, Cagidiaco MC, Ercoli C. Tissue management with a new gingival retraction material: A preliminary clinical report. J Prosthet Dent. 1996;75:242–7. doi: 10.1016/s0022-3913(96)90479-5. [DOI] [PubMed] [Google Scholar]
- 19.Shah MJ, Mathur S, Alkesh S. Gingival retraction methods in fixed prothodontics: A systematic review. J Dent Sci. 2008;3:4–10. [Google Scholar]
- 20.Patel P, Vaishnav K, Ganatra C, Pujara T. Evaluation of kinetic absorbency of 3 different medicaments by 5 different type of cords. J Int Oral Health. 2011;3:15–22. [Google Scholar]
- 21.Acar Ö, Erkut S, Özçelik TB, Ozdemır E, Akçil M. A clinical comparison of cordless and conventional displacement systems regarding clinical performance and impression quality. J Prosthet Dent. 2014;111:388–94. doi: 10.1016/j.prosdent.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Hong LG, Guo LP, Xue LL. Gingival retraction paste versus gingival retraction cord for fixed prosthodontics: A systematic review. Shanghai J Stomatol. 2013;22:456–61. [PubMed] [Google Scholar]
- 23.Nowakowska D, Saczko J, Kulbacka J, Choromanska A, Raszewski Z. Cytotoxic potential of vasoconstrictor experimental gingival retraction agents:In vitro study on primary human gingival fibroblasts. Folia Biol (Praha) 2012;58:37–43. [PubMed] [Google Scholar]
- 24.Thimmappa M, Bhatia M, Somani P, Kumar DRV. Comparative evaluation of three noninvasive gingival displacement systems: An in vivo study. J Indian Prosthodont Soc. 2018;18:122–30. doi: 10.4103/jips.jips_225_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gajbhiye V, Banerjee R, Jaiswal P, Chandak A, Radke U. Comparative evaluation of three gingival displacement materials for efficacy in tissue management and dimensional accuracy. J Indian Prosthodont Soc. 2019;9:173–9. doi: 10.4103/jips.jips_285_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawood ZM, Majeed MA. An evaluation of the efficacy of different gingival retraction materials on the gingival tissue displacement: A comparative in vivo study. J Baghdad Coll Dent. 2015;27:25–31. [Google Scholar]


