Abstract
Hericium erinaceus is a medicinal mushroom that contains many molecules promising a plethora of therapeutic properties. In this study, the strain H.e.2 (MicUNIPV, University of Pavia, Italy) was isolated from a sporophore collected in Tuscany (Italy). Mycelium, primordium, and wild type and cultivated sporophores were analyzed by HPLC-UV-ESI/MS. Erinacine A in the mycelium and hericenones C and D in the sporophores were quantified by comparison with their standard molecules. For the first time, H. erinaceus primordium was also investigated for the presence of these molecules. Comparing with the literature data, hericenes, molecules structurally similar to hericenones, were present in all our samples. The highest contents of hericenones C and D were detected in cultivated sporophores, compared to the wild type. The comparison of these data with those of another Italian H. erinaceus strain (H.e.1 MicUNIPV) was discussed. The results led us to select H. erinaceus strains more suitable for mycelium production or sporophore cultivation to obtain extracts with a higher content of bioactive compounds. This work provides a further step towards standardizing the procedures in the development of dietary supplements made from mushrooms.
Keywords: medicinal mushroom, Hericium erinaceus, bioactive compounds, mycelium, sporophore, primordium, erinacines, hericenones, hericenes
1. Introduction
Hericium erinaceus (Bull.) Pers. is a fungus belonging to Basidiomycota, Agaricomycetes, Russulales, and Hericiaceae [1]. Among all mushrooms, H. erinaceus, an edible and medicinal mushroom in traditional Chinese medicine, has been widely reported to have healthy effects on: the central nervous system [2,3]; different cancerous cell lines, such as HepG2 (hepatoma), MCF7 (breast cancer), HL-60 (human acute promyelocytic leukemia), and SGC-7901 (human gastric cancer cells) [4,5,6]; depression [7]; diabetes [8]; lipedema [9]. It also exhibited a reversion of frailty cognitive decline during aging [10]. Up to now, about 70 different secondary metabolites have been isolated from either sporophore or mycelium, or both. The investigation of chemical constituents promising for their properties is constantly being updated in the search for the discovery of a new drug source [11]. Both the high-weight metabolites (e.g., polysaccharides) and low-weight metabolites (e.g., polyketides, phenols, and terpenoids) include bioactive molecules, although each substance category provides in turn an extremely various bouquet of molecules, where only a fraction shows evidence for bioactivity [12,13,14,15]. Hericenones are low-weight aromatic compounds first isolated by Kawagishi et al. (1990) [14] from the sporophore of H. erinaceus. Up to now, eight different compounds have been recognized as hericenones (A–H) [11,16]. Hericenones C, D, and E have stimulating activity on the synthesis of nerve growth factor (NGF) [17]. Hericenone F has been reported to be responsible for an anti-inflammatory effect by reducing nitrogen monoxide (NO) release [18]. Analyses of dried sporophores have detected different volatile compounds, some of them represented by hexadecanoic acid, linoleic acid, phenylacetaldehyde, and benzaldehyde. The erinacines in H. erinaceus are a group of cyathane-type diterpenoids, including 20 members of 24 diterpenoids described by Tang et al. [19]. To date, 15 erinacines (A, B, C, D, E, F, G, H, I, P, Q, J, K, R, S) isolated from H. erinaceus mycelium have been identified and eight out of 15 show neuroprotective properties, such as enhancing NGF release (erinacines A–I), reducing amyloid-β deposition, increasing insulin-degrading enzyme (IDE) expression (erinacines A and S), and managing neuropathic pain (erinacine E), while the others have different pharmacological activities [12,20,21,22,23,24,25,26].
At present, H. erinaceus is widely used as a dietary supplement. Nevertheless, the lack of standardization strongly affects the quality and effective bioactivity of the final product. As above described, only a few molecules have been reported to stimulate NGF release, namely erinacines A–I from mycelium (the most studied being erinacine A) and hericenones C–D from sporophore. To obtain this specific target on neuroprotection, the standardization process of dietary supplements therefore relies on the selective detection and quantification of such molecules. A major problem at this concern is the availability of pure analytical standards, due to the difficulty in the isolation and achievement of suitable amounts.
The aim of this study is to analyze and compare different stages of H. erinaceus (mycelium, primordium, and sporophore) sampled in Tuscany (Italy), in order to detect the presence and to quantify the concentration of the target bioactive metabolites erinacine A and hericenones C and D. The results could be useful for suggesting optimization strategies for future dietary supplements.
2. Results
2.1. H. erinaceus Samples for Chemical Analyses
The H. erinaceus wild type (WT) sporophore analyzed in the present study was collected on a living holm oak (Quercus ilex L.) in the hilly area around Siena (Italy) in 2018. The sporophore was identified based on the macro- and micromorphological characteristics of the species [27]. The main features are reported in Table 1.
Table 1.
Characteristics of Hericium erinaceus wild type sporophore.
| H. erinaceus | |
|---|---|
| fresh weight (g) | 620 |
| dried weight (g) | 153 |
| diameter (cm) | about 20 |
| remarks on the sporophore | the collected specimen was mature, without any alteration by atmospheric or animal agents |
The strain obtained from the WT was confirmed to belong to H. erinaceus [28] and is maintained in the Fungal Research Culture Collection of Pavia University (MicUNIPV) as H.e.2.
The cultivation of H. erinaceus at the Botanical Garden of the University of Pavia (Italy) led to the collection of 44 sporophores, total weight 1344.2 g. The diameter of the collected samples was 6–15 cm.
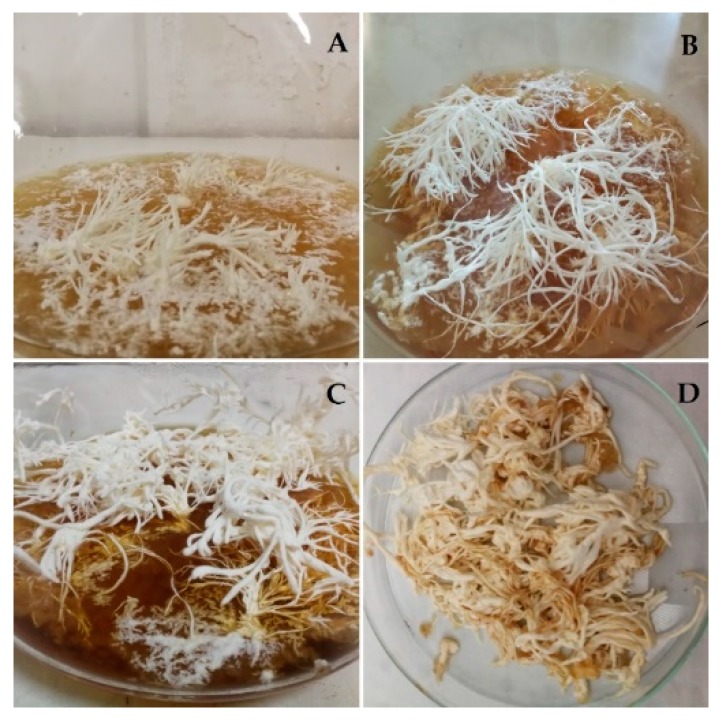
The primordium can be considered the transition stage before sporophore. It is formed by dense mycelial cords developing with negative geotropism (Figure 1). Primordia were harvested after 60 days, at the maximum of their development, and the fresh material was analyzed.
Figure 1.
Different stages of growth of the primordium. 30 (A), 45 (B), 60 (C) days of growth and fresh collected material (D).
2.2. Chemical Analyses
We processed 1 g of lyophilized mycelium, dried WT and cultivated sporophores, and fresh primordium for chemical analyses.
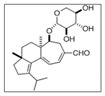
In order to identify and analyze the bioactive metabolites present in our samples, we compared them with the standard molecules of erinacine A and hericenones C and D by HPLC-UV-ESI/MS. The molecular formula, chemical structures, and molecular weight of these molecules are reported in Table 2 [29].
Table 2.
Molecular formula, chemical structures, and molecular weights of erinacine A and hericenones C and D.
| Erinacine A | Hericenone C | Hericenone D | |
|---|---|---|---|
| molecular formula | C25H36O6 | C35H54O6 | C37H58O6 |
| molecular weight (MW) (g/mol) | 432 | 570 | 598 |
| chemical structure |
|
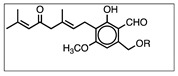 R = palmitoyl |
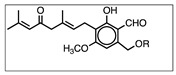 R = stearoyl |
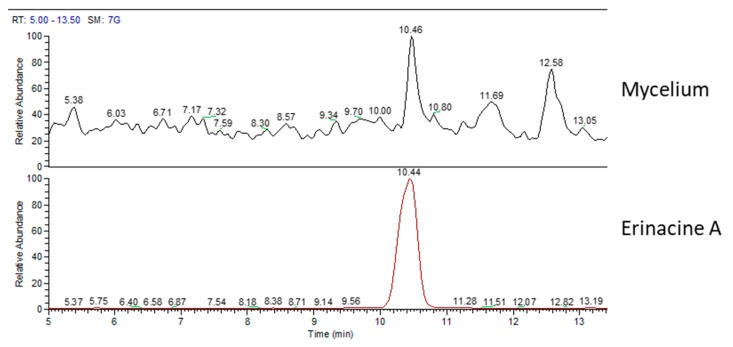
Figure 2 shows the mass spectrum (MS) chromatographic traces of H.e.2 mycelium and the standard molecule of erinacine A. The standard erinacine A was detected using HPLC-UV-ESI/MS at the retention time (RT) of 10.44 min. By comparing the RT and molecular ion or mass spectra, the presence of this molecule in the H.e.2 mycelium was detected too. Besides, the chromatographic trace of H.e.2 mycelium also showed a peak at RT 12.58 min that belongs to a molecule not yet identified with a MW of 430 Da.
Figure 2.
Mass spectrum (MS) traces of mycelium (top) and erinacine A molecule standard (bottom).
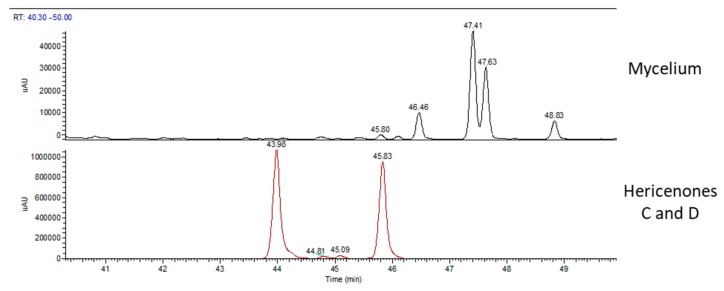
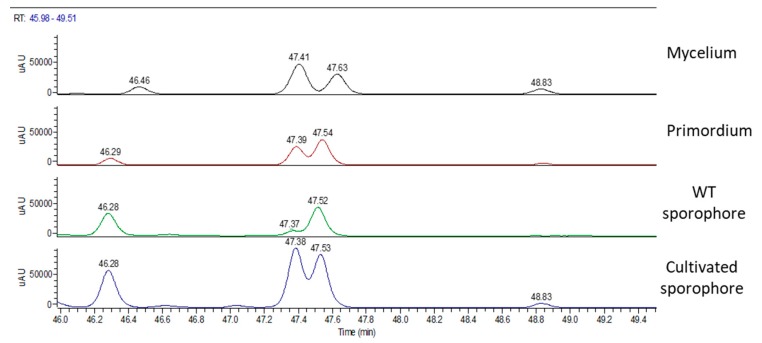
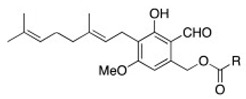
Figure 3 shows the UV chromatographic traces of H.e.2 mycelium and the standard molecules of hericenones C and D. In mycelium, hericenones C and D were not present. Moreover, the peaks at RT 46.46 min, 47.41 min, 47.63 min, and 48.83 min were supposed to be, respectively, hericene D, hericene A, hericene B, and hericene C, based on the data reported by Arnone et al. (1994) and Kobayashi et al. (2018) [30,31]. The molecular formula, chemical structures, and molecular weights of the hericenes are reported in Table 3 [29].
Figure 3.
UV traces of mycelium (top) and the standard molecules of hericenones C and D (bottom).
Table 3.
Molecular formula, chemical structures, and molecular weights of hericenes.
| Hericene A | Hericene B | Hericene C | Hericene D | |
|---|---|---|---|---|
| molecular formula | C35H56O5 | C37H58O5 | C37H60O5 | C37H56O5 |
| molecular weight (MW) (g/mol) | 556 | 582 | 584 | 580 |
| chemical structure |
|
|||
| R = palmitoyl | R = oleoyl | R = stearoyl | R = lineoyl | |
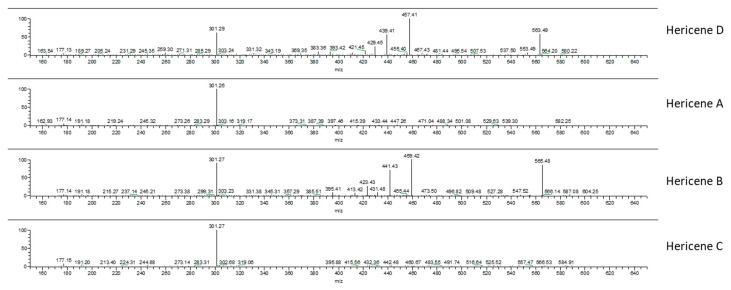
Our hypothesis was also confirmed by ion fragments in MS/MS spectra. Figure 4 reports the MS/MS-ESI spectra of hericenes: from top to bottom, hericenes D, A, B, and C are shown. The ion m/z 301 was present in all the spectra and derives from the loss of the side chain R. Spectra of hericenes D and B also show ions derived from fragmentation close to double bonds of R.
Figure 4.
MS/MS-ESI spectra of hericenes D, A, B, and C (from top to bottom).
WT and cultivated sporophores were analyzed. The data were compared with the standard molecules hericenones C and D.
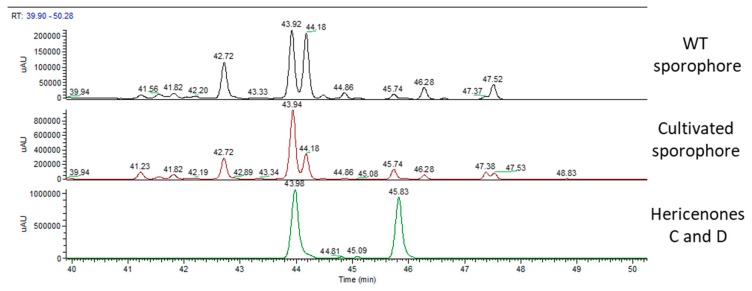
Figure 5 shows the UV chromatographic traces of the WT sporophore collected in Italy, the cultivated and the two standard molecules of hericenones: C detected at RT of 43.98 min and D detected at RT of 45.83 min. Hericenone C was detected in the WT at RT of 43.92 min and at RT of 43.94 in the cultivated sporophore. Hericenone D was detected at RT of 45.74 min in both samples. There were also other peaks close to these hericenones that could be attributed to hericenone E (RT of 42.72 min), hericenone I (RT of 44.18 min), and hericenone H (RT of 47.37 min) on the basis both of previous publications [16] and the similarity of their fragmentation pattern to that of hericenones C and D standard. In the same chromatogram (Figure 5), there were peaks that could be attributed to hericenes: from lower to higher RT hericene D (RT 46.28 min), hericene A (RT 47.37 or 47.38 min), hericene B (RT 47.52 or 47.53 min), and hericene C (RT 48.83 min).
Figure 5.
UV traces of wild type sporophore (top), cultivated sporophore (middle), and hericenones C (RT 43.98 min) and D (RT 45.83 min) standards (bottom).
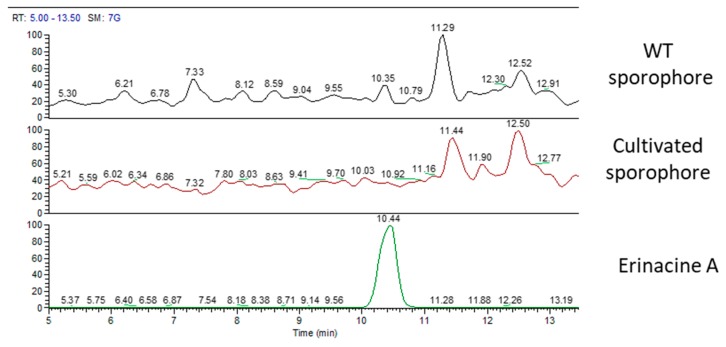
Figure 6 displays MS chromatographic traces of the two sporophores and the standard molecule of erinacine A. The erinacine A molecule was not present in both sporophores. Besides, the chromatographic traces also showed other peaks at RT of 11.29 and 12.52 min for the WT sporophores and at 11.44 and 12.50 min for the cultivated sporophores that belong to molecules not yet identified.
Figure 6.
MS traces of wild type sporophore (top), cultivated sporophore (middle), and erinacine A standard molecules (bottom).
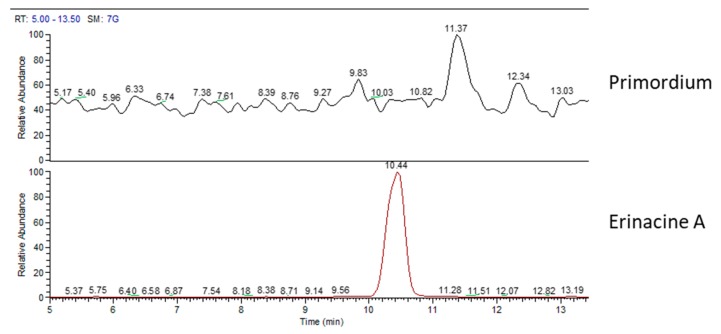
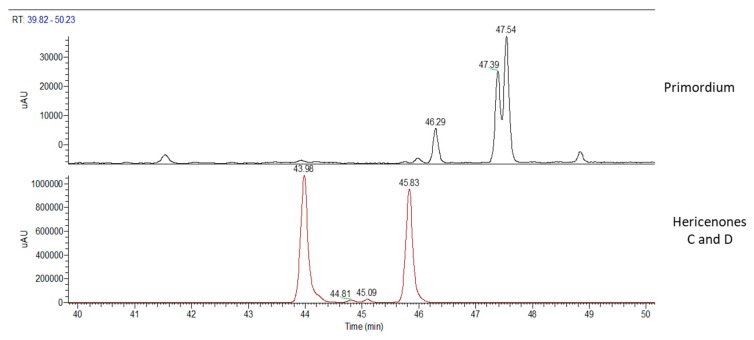
Chemical analyses of primordium showed neither erinacines (Figure 7) nor hericenones (Figure 8). MS trace of primordium (Figure 7) also showed a peak at RT of 11.37 min that has not yet been determined. Instead, the UV trace of primordium showed peaks at the same RT described for hericenes D, A, B, and C (Figure 8), as mentioned for the mycelium, and the WT and cultivated sporophores.
Figure 7.
MS (mass spectrum) traces of primordium (top) and erinacine A molecule standard (bottom).
Figure 8.
UV traces of primordium (top) and hericenones C and D standards (bottom).
Table 4 summarizes the array of metabolites present in different stages of H. erinaceus.
Table 4.
Presence of different molecules (erinacine A, hericenones C and D, and hericenes) in the lyophilized mycelium, fresh primordium, and dried sporophores.
| erinacine A | hericenone C | hericenone D | hericenes | |
|---|---|---|---|---|
| mycelium | ✓ | - | - | ✓ |
| primordium | - | - | - | ✓ |
| WT sporophore | - | ✓ | ✓ | ✓ |
| cultivated sporophore | - | ✓ | ✓ | ✓ |
The content of erinacine A in H.e.2 mycelium and of hericenone C and D in sporophores were measured by the calibration curves [10] (Table 5).
Table 5.
Content of erinacine A and hericenones C and D in H.e.2 lyophilized mycelium, fresh primordium and dried sporophores.
| Erinacine A (µg/g) | Hericenone C (µg/g) | Hericenone D (µg/g) | |
|---|---|---|---|
| mycelium | 105 | - | - |
| primordium | - | - | - |
| WT sporophore | - | 760 | 100 |
| cultivated sporophore | - | 1560 | 188 |
Figure 9 summarizes the UV chromatographic traces of different samples where it is possible to identify peaks that are attributed to hericenes. From lower to higher RT, hericene D (RT at 46.47 or 42.29 or 46.28), hericene A (RT at 47.41 or 47.39 or 47.37 or 47.38), hericene B (RT at 47.63 or 47.54 or 47.52 or 47.53), and hericene C (RT at 48.83 or 48.84) were identified. As previously reported, hericenes were present in all the samples in different amounts.
Figure 9.
UV chromatographic traces of hericenes in (from top to bottom) mycelium, primordium, and wild type and cultivated sporophores.
Table 6 reports peak area values of hericenes A, B, C, and D in different samples.
Table 6.
Content of hericenes in mycelium, primordium, and wild type (WT) and cultivated sporophores.
| Total Area 103 | Hericene A Area 103 | Hericene B Area 103 | Hericene C Area 103 | Hericene D Area 103 | |
|---|---|---|---|---|---|
| mycelium | 684 | 327 | 232 | 51 | 74 |
| primordium | 557 | 201 | 262 | 21 | 73 |
| WT sporophore | 627 | 70 | 305 | / | 252 |
| cultivated sporophore | 1685 | 645 | 588 | 42 | 410 |
3. Discussion
At present, medicinal mushrooms such as H. erinaceus are exploited as dietary foods or supplements, producing beneficial effects by daily use in a balanced and varied diet. There are different products available on the market, increasing in number year by year. Despite this, there are still unresolved issues, including standardization and safety for the production of fungal supplement. Standardization is still in its early stage because of the lack of protocols and international guidelines [32].
This study is placed within an interdisciplinary research project to draw up the steps for the production of high quality dietary supplements to improve cognitive functions. The project has been following all the stages of the supply chain: strains selection, production, extraction, chemical analysis, and finally a pre-clinical test on animal models. More specifically, this study is the first step planned to analyze the array of some metabolites present in different growth stages of the H. erinaceus collected in Italy.
Thanks to the comparison with standard molecules, we were able to identify and quantify erinacine A in mycelium, hericenones C and D, and in wild type (WT) and cultivated sporophores.
In MicUNIPV, the Fungal Research Culture Collection at the University of Pavia (Italy), two strains of H. erinaceus collected in Italy are present to date: H.e.1 and H.e.2 [10,28]. The content of erinacine A in H.e.2 (105 µg/g) is slightly less compared to H.e.1 mycelium (150 µg/g) [10]. These amounts of erinacine A are comparable to that reported by Krzyczkowski et al. (2010) in improved submerged cultivation [33].
The same comparison between the WT sporophores showed that H.e.2 contains more hericenones C and D (760 µg/g and 100 µg/g, respectively) compared to H.e.1 (500 µg/g and <20 µg/g, respectively) [10]. These values are comparable with those of some strains reported by Lee et al. (2016) [18].
Therefore, given these data, the H.e.2 strain must be used for sporophore cultivation, whereas the H.e.1 for mycelium production.
By comparing the WT and cultivated sporophores of H.e.2, hericenones C and D in the cultivated sporophores are about two folds higher (1560 µg/g vs. 760 µg/g and 188 µg/g vs. 100 µg/g, respectively). Generally, wild sporophores exhibit biological variability, depending on the different growth environment and on seasonality. Conversely, the cultivated conditions are more stable. In particular, for the mycelial colonization, for the appearance of primordia, and the development of sporophores, the medium components (nitrogen, carbon, and mineral sources) and environmental factors (pH, temperature, and relative humidity) are fundamental in order to optimize the growth and to influence the bioactive metabolites production.
The primordium is an intermediate stage of the fungus between the mycelium and the sporophore development, characterized by the formation of spider-like aerial spines that grow above the culture medium. Generally, there are still few studies concerning primordium and none for H. erinaceus [34,35]. In our primordium, only hericenes were present, without any hericenone and erinacine. Hericenes are also found in mycelium and sporophores, both WT and cultivated, in agreement to what was reported by Arnone et al. (1994) [30] and Kobayashi et al. (2018) [31]. Preliminarily, in order to obtain a relative measure among the different samples, we compared the peak areas of the single hericenes detected, which were supposed to be A, B, C, and D, by HPLC-UV-ESI/MS. In particular, it is notable that in H.e.2 mycelium and primordium, hericenes A and B are more present compared to hericenes C and D. In cultivated sporophores, all hericenes are present, with a wider peak area if compared to the other samples. In WT sporophores, hericene C is absent and hericene A is at a lower level, whereas hericenes B and D peak areas have considerable values. Hericene B is present in fairly constant quantities in all samples, whereas hericene C is present in smaller quantities.
It should be noted that the cultivated sporophore has higher content than all the hericenes compared to the WT one. Similarly, the contents of hericenones C and D are higher in the cultivated sporophores than in the WT.
We could hypothesize a chemical correlation between hericenes and hericenones. Hericene A has a side chain with palmitoyl acid similar to hericenone C. Hericene C is similar to hericenone D with a side chain with stearic acid. Hericene D is similar to hericenone H and contains a side chain with a linoleoyl acid. Thus, in these paired molecules the side chain is maintained but hericenones differ from hericenes for their oxidation state. We can speculate that hericenone C derives from the oxidation of hericene A and hericenone D from the oxidation of hericene C. Other hericenones, such as I and E, could derive from the oxidation of hericenes B and D, respectively.
Up to now, ethanolic extracts obtained from H.e.1 mycelium and sporophores, with the standardized amounts of erinacine A and hericenones C and D, have been used to evaluate the effects of oral supplementation on cognitive decline in a mice model, during physiological aging [10]. Because of the different amounts of the neuroactive metabolites present in the different strains, now it is possible to prepare the best extract blend for in vivo tests. The present study contributes as it re-addresses the selection of raw material.
Further investigation will be carried out by setting different cultivation conditions to maximize the yield of bioactive metabolites.
4. Materials and Methods
4.1. Study Area and Sampling
Samplings were conducted in the hilly area around Siena (Tuscany, Italy), where both Mediterranean and temperate environments are present. The plant communities are dominated by holm oak (Quercus ilex), strawberry tree (Arbutus unedo), heather (Erica arborea), Mediterranean buckthorn (Rhamnus alaternus), juniper (Juniperus communis), and other deciduous species such as the downy oak (Q. pubescens). Q. ilex is an important feature in the landscape, being usually prevalent and resistant to anthropic stress.
The wild type (WT) sporophore was collected from an old living specimen of Q. ilex and kept at 4 °C until experimental use.
4.2. H. erinaceus Samples for Chemical Analyses
The H. erinaceus samples processed for chemical analyses were: the WT sporophore (the sample was dried and maintained in a freezer at −20 °C for at least one month in order to avoid any further degradation); the strain isolated from it; the sporophores cultivated at the Botanical Garden of the University of Pavia (Italy) using the above mentioned isolated strain; the primordium that was the first aerial part consisting of mycelial cords.
4.3. H. erinaceus Strain Isolation
The isolation of mycelium in a pure culture from the WT was performed in accordance with the usual procedures [36,37,38]. Small pieces (up to 10 mm3) were aseptically cut off from the center of the WT and inoculated into Petri dishes containing 2% malt extract agar (MEA, Biokar Diagnostics). Chloramphenicol at 50 ppm was added in this first step. Incubation was performed at 24 °C in complete darkness. The isolated strain is maintained in the Fungal Research Culture Collection of Pavia University (MicUNIPV).
4.4. H. erinaceus Sporophores Cultivation
The cultivation of H. erinaceus sporophores was performed in the mushroom greenhouse of the Botanical Garden at the University of Pavia (Italy). As the substrate, a mix of 70% oak sawdust, 20% rice bran, and 10% wheat straw, combined with 1% sucrose and 1% calcium carbonate, was used [36,39,40,41]. The substrate was mixed and hydrated, and then 300 g were placed in polypropylene bags with filters to allow gas exchange. Each bag was sterilized twice at 120 °C for 60 min.
In parallel, the spawn with H. erinaceus was prepared: the mycelium grew in sterilized polypropylene bags containing 300 g of hydrated barley. They were taken at 24 °C with 90% relative humidity (RH) in the dark for two weeks, until complete colonization.
We aseptically put and mixed 5% of spawn into each substrate bag. The cultivation room was kept at 24 °C and bathed to maintain high relative humidity (95%-100%).
Soon after the substrate was completely colonized by the mycelium, the bags were moved to a room where it was possible to carry out the light-dark cycle, maintaining the temperature at 18 °C–24 °C, the RH of 90%–95%, and good aeration condition to induce primordia formation.
In correspondence to the appearance of primordia, holes were made in the bags to allow the development of sporophores [36,39,40,41]. Once collected, the sporophores were weighed, measured, dried, and maintained frozen.
4.5. Extraction Procedures
The procedure of alcoholic extraction described by Lee et al. (2016) and Gerbec et al. (2015) [18,42] was followed with slight modification: 1 g of lyophilized mycelium/dried WT/cultivated sporophores/fresh primordium was blended with 10 mL of ethanol 70% and left in the thermostat at 50 °C for 24 h. At the end, the material was transferred for centrifugation (4000 rpm for 3 min) and the supernatant was stored at −20 °C for HPLC analysis.
4.6. HPLC-UV-ESI/MS Method
HPLC-UV-ESI/MS analyses were carried out on a LCQ FLEET system (Thermo Fisher Scientific, San Jose, CA, USA), equipped with a PAD-UV detector working at 254 nm. The chromatographic separation was performed using an F5 HPLC column 150 × 3.0 mm, 2.7 μm particle size (Ascentis® Express, Merck KGaA, Darmstadt, Germany) maintained at 40 °C, with a flow rate of 0.3 mL/min and an injection volume of 20 µL. The mobile phase consisted of water containing 0.1% formic acid (solvent A) and acetonitrile (solvent B) (Table 7). The following gradient method was utilized: 0–9 min (30%–50% B), 9–27 min (50%–60% B), 27–54 min (60%–100% B), 54–69 min (100%–30% B), and 69–75 min (30% B).
Table 7.
The mobile phase and the gradient method.
| Time | Solvent A | Solvent B |
|---|---|---|
| 0 | 70 | 30 |
| 9 | 50 | 50 |
| 27 | 40 | 60 |
| 54 | 00 | 100 |
| 69 | 70 | 30 |
| 75 | 70 | 30 |
An Electro Spray Ionization (ESI) interface was used as an ion source, under positive ion conditions (ESI+). The Ion Spray voltage and Capillary voltage were set at 5 kV and 10 V in positive ion mode. The capillary temperature was 400 °C. Acquisition was performed both in Full Scan mode (mass range 200–2000 Da) and Dependent Scan mode. The data station utilized the Xcalibur MS Software Version 2.1.
Stock solutions of erinacine A and hericenones C and D (1 mg/mL) were prepared in 70% ethanol. Standard solutions with the final concentration range of 1–25 µg/mL for erinacine A and 20–100 µg/mL for hericenones C and D were obtained by the proper dilution of stock solutions.
Calibration curves were constructed by injecting the standard mixture solutions at five concentrations (1, 5, 10, 15, 25 µg/mL) for erinacine A and at four concentrations (20, 50, 75, 100 μg/mL) for hericenone C and D. Linear least-square regression analysis for the calibration curves showed correlation coefficients of 0.9968, 09945, and 0.9951, respectively, for erinacine A, hericenones C, and hericenones D with respect to the peak area, demonstrating a good linear relationship in the different ranges tested. Each concentration was analyzed in triplicate [10].
5. Conclusions
In this study, an array of metabolites at different growth stages of the fungus H. erinaceus collected in Italy was analyzed. In particular, for the first time we described the array of metabolites present in primordium stage, i.e., the hericenes. These molecules are also present from the formation of the mycelium to the appearance of the primordium and up to the sporophore development. Experiments in the future will focus on testing the functional role of these molecules in vitro and in vivo.
In conclusion, in our opinion this methodological approach is a necessary step for developing dietary supplements with a higher and standardized content of bioactive metabolites.
Acknowledgments
This research was supported by the Italian Ministry of Education, University and Research (MIUR): Dipartimenti di Eccellenza Program (2018–2022)—Dept. of Biology and Biotechnology “L. Spallanzani”, University of Pavia. The authors wish to thank Professor Maria Lidia Guglielminetti for her precious suggestions and Miconet S.r.l for allowing us to use its cultivation chambers.
Author Contributions
Conceptualization: P.R., E.S., F.C., V.C., H.K.; Methodology: V.C., B.M., F.C., R.M.B., C.E.G., D.R.; Samplings: C.P., V.C., C.E.G.; Supervision: P.R., E.S., F.C., A.M.P.; Writing–original draft: V.C., P.R., E.S.; Writing–review and editing: P.R., H.K., C.E.G. All authors have made a substantial contribution to the revision of the work and approved it for publication.
Funding
The research was economically supported by Department of Earth and Environmental Sciences, University of Pavia, Italy: “XXXII Ph.D. fee” V.C.’s Ph.D. project and E.S.’s “Fondo Ricerca e Giovani”.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from one of the authors (H. K.) under an MTA.
References
- 1.Mycobank. [(accessed on 24 September 2019)]; Available online: www.mycobank.org.
- 2.Rossi P., Cesaroni V., Brandalise F., Occhinegro A., Ratto D., Perrucci F., Lanaia V., Girometta C., Orrù G., Savino E. Dietary Supplementation of Lion’s Mane Medicinal Mushroom, Hericium erinaceus (Agaricomycetes), and Spatial Memory in Wild-Type Mice. Int. J. Med. Mushrooms. 2018;20:485–494. doi: 10.1615/IntJMedMushrooms.2018026241. [DOI] [PubMed] [Google Scholar]
- 3.Brandalise F., Cesaroni V., Gregori A., Repetti M., Romano C., Orrù G., Botta L., Girometta C., Guglielminetti M.L., Savino E. Dietary Supplementation of Hericium erinaceus Increases Mossy Fiber-CA3 Hippocampal Neurotransmission and Recognition Memory in Wild-Type Mice. Evid. Based Complement. Alternat. Med. 2017:3864340. doi: 10.1155/2017/3864340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y., Zhang G., Ng T.B., Wang H. A novel lectin with antiproliferative and HIV-1 reverse transcriptase inhibitory activities from dried fruiting bodies of the monkey head mushroom Hericium erinaceum. BioMed Res. Int. 2010;2010:716515. doi: 10.1155/2010/716515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W., Zhou W., Kim E.J., Shim S.H., Kang H.K., Kim Y.H. Isolation and identification of aromatic compounds in Lion’s Mane Mushroom and their anticancer activities. Food Chem. 2015;170:336–342. doi: 10.1016/j.foodchem.2014.08.078. [DOI] [PubMed] [Google Scholar]
- 6.Zan X., Cui F., Li Y., Yang Y., Wu D., Sun W., Ping L. Hericium erinaceus polysaccharide-protein HEG-5 inhibits SGC-7901 cell growth via cell cycle arrest and apoptosis. Int. J. Biol. Macromol. 2015;76:242–253. doi: 10.1016/j.ijbiomac.2015.01.060. [DOI] [PubMed] [Google Scholar]
- 7.Vigna L., Morelli F., Agnelli G.M., Napolitano F., Ratto D., Occhinegro A., Di Iorio C., Savino E., Girometta C., Brandalise F., et al. Hericium erinaceus Improves Mood and Sleep Disorders in Patients Affected by Overweight or Obesity: Could Circulating Pro-BDNF and BDNF Be Potential Biomarkers? Evid Based Complement Alternat Med. 2019;2019:1–12. doi: 10.1155/2019/7861297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Silva D.D., Rapior S., Hyde K.D., Bahkali A.H. Medicinal mushrooms in prevention and control of diabetes mellitus—A review. Fungal Divers. 2012;56:1–29. doi: 10.1007/s13225-012-0187-4. [DOI] [Google Scholar]
- 9.Yang B.K., Park J.B., Song C.H. Hypolipidemic Effect of an Exo-biopolymer Produced from a Submerged Mycelial Culture of Hericium erinaceus. Biosci. Biotech. Bioch. 2003;67:1292–1298. doi: 10.1271/bbb.67.1292. [DOI] [PubMed] [Google Scholar]
- 10.Ratto D., Corana F., Mannucci B., Priori E.C., Cobelli F., Roda E., Ferrari B., Occhinegro A., Di Iorio C., De Luca F., et al. Hericium erinaceus improves recognition memory and induces hippocampal and cerebellar neurogenesis in frail mice during aging. Nutrients. 2019;11:715. doi: 10.3390/nu11040715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman M. Chemistry, nutrition, and health-promoting properties of Hericium erinaceus (Lion’s Mane) mushroom fruiting bodies and mycelia and their bioactive compounds. J. Agric. Food Chem. 2015;63:7108–7123. doi: 10.1021/acs.jafc.5b02914. [DOI] [PubMed] [Google Scholar]
- 12.Kawagishi H., Shimada A., Shirai R., Okamoto K., Ojima F., Sakamoto H., Ishiguro Y., Furukawa S. Erinacines A, B and C strong stimulators of nerve growth factor (NGF)-synthesis from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1994;35:1569–1572. doi: 10.1016/S0040-4039(00)76760-8. [DOI] [Google Scholar]
- 13.Ma B.J., Yu H.Y., Shen J.W., Ruan Y., Zhao X., Zhou H., Wu T.T. Cytotoxic aromatic compunds from Hericium erinaceum. J. Antibiotics. 2010;63:713–715. doi: 10.1038/ja.2010.112. [DOI] [PubMed] [Google Scholar]
- 14.Kawagishi H., Ando M., Mizuno T. Hericenone A and B as cytotoxic principles from the mushroom Hericium erinaceum. Tetrahedron Lett. 1990;31:373–376. doi: 10.1016/S0040-4039(00)94558-1. [DOI] [Google Scholar]
- 15.Keong C.Y., Rashid B.A.A., Ing Y.S., Ismail Z. Quantification and identification of polysaccharide contents in Hericium erinaceus. Nutr. Food Sci. 2007;37:260–271. doi: 10.1108/00346650710774631. [DOI] [Google Scholar]
- 16.Ma B.J., Shen J.W., Yu H.Y., Ruan Y., Wu T.T., Zhao X. Hericenones and erinacines: Stimulators of nerve growth factor (NGF) biosynthesis in Hericium erinaceus. Mycology. 2010;1:2–92. doi: 10.1080/21501201003735556. [DOI] [Google Scholar]
- 17.Kawagishi H., Ando M., Sakamoto H., Yoshida S., Ojima F., Ishiguro Y., Ukai N., Furukawa S.C. Hericenones C, D and E, stimulators of nerve growth factor (NGF)-synthesis, from the mushroom Hericium erinaceum. Tetrahedron Lett. 1991;32:4561–4564. doi: 10.1016/0040-4039(91)80039-9. [DOI] [Google Scholar]
- 18.Lee D.G., Kang H.W., Park C.G., A Y.S., Shin Y. Isolation and identification of pyotochemicals and biological activities of Hericium erinaceus and their contents in Hericium strains using HPLC/UV analysis. J. Ethnopharmacol. 2016;184:219–225. doi: 10.1016/j.jep.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 19.Tang H.Y., Yin X., Zhang C.C., Jia Q., Gao J.M. Structure diversity, synthesis, and biological activity of cyathane diterpenoids in higher fungi. Curr. Med. Chem. 2015;22:2375–2391. doi: 10.2174/0929867322666150521091333. [DOI] [PubMed] [Google Scholar]
- 20.Kawagishi H., Shimada A., Hosokawa S., Mori H., Sakamoto H., Ishiguro Y., Sakemi S., Bordner J., Kojima N., Furukawa S. Erinacines E, F, and G, stimulators of nerve growth factor (NGF) synthesis from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1996;37:7399–7402. doi: 10.1016/0040-4039(96)01687-5. [DOI] [Google Scholar]
- 21.Kawagishi H., Simada A., Shizuki K., Mori H., Sakamoto H., Furukawa S. Erinacine D, a stimulator of NGF-synthesis from the mycelia of Hericium erinaceum. Heterocycl Commun. 1996;2:51–54. doi: 10.1515/HC.1996.2.1.51. [DOI] [Google Scholar]
- 22.Lee E.W., Shizuki K., Hosokawa S., Suzuki M., Suganuma H., Inakuma T., Kawagishi H. Two novel diterpenoids, erinacines H and I from the mycelia of Hericium erinaceum. Biosci. Biotech. Biochem. 2000;64:2402–2405. doi: 10.1271/bbb.64.2402. [DOI] [PubMed] [Google Scholar]
- 23.Kenmoku H., Sassa T., Kato N. Isolation of erinacine P, a new parental metabolite of cyathane-xylosides from Hericium erinaceus and its biomimetic conversion into erinacines A and B. Tetrahedron Lett. 2000;41:4389–4393. doi: 10.1016/S0040-4039(00)00601-8. [DOI] [Google Scholar]
- 24.Kenmoku H., Shimai T., Toyomasu T., Kato N., Sassa T. Erinacine Q, a new erinacine from Hericium erinaceus, and its biosynthetic route to erinacine C in the basidiomycete. Biosci. Biotech. Biochem. 2002;66:571–575. doi: 10.1271/bbb.66.571. [DOI] [PubMed] [Google Scholar]
- 25.Kawagishi H., Masui A., Tokuyamab S., Nakamurac T. Erinacines J and K from the mycelia of Hericium erinaceum. Tetrahedron Lett. 2006;62:8463–8466. doi: 10.1016/j.tet.2006.06.091. [DOI] [Google Scholar]
- 26.Ma B.J., Zhou Y., Li L.Z., Li H.M., Gao Z.M., Ruan Y. A new cyathanexyloside from the mycelia of Hericium erinaceum. Z Naturforsch. 2008;63b:1241–1242. doi: 10.1515/znb-2008-1017. [DOI] [Google Scholar]
- 27.Bernicchia A., Gorjon S.P. Corticiaceae s.l. Fungi europaei. Volume 12. Candusso; Alassio, Italy: 2010. Hericium erinaceus; pp. 318–319. [Google Scholar]
- 28.Cesaroni V., Brusoni M., Cusaro C.M., Girometta C., Perini C., Picco A.M., Rossi P., Salerni E., Savino E. Phylogenetic Comparison between Italian and Worldwide Hericium Species (Agaricomycetes) Int. J. Med. Mushrooms. 2019:accepted. doi: 10.1615/IntJMedMushrooms.2019032561. [DOI] [PubMed] [Google Scholar]
- 29.PubChem. [(accessed on 26 September 2019)]; Available online: http://pubchem.ncbi.nlm.nih.gov/
- 30.Arnone A., Cardillo R., Nasini G., De Pava O.V. Secondary mold metabolites: Part 46. Hericenes A-C and erinapyrone C, new metabolites produced by the fungus Hericium erinaceus. J. Nat. Prod. 1994;57:602–606. doi: 10.1021/np50107a006. [DOI] [Google Scholar]
- 31.Kobayashi S., Hamada Y., Yasumoto T., Hashino Y., Masuyama A., Nagai K. Total syntheses and endoplasmic reticulum stress suppressive activities of hericenes A-C and their derivatives. Tetrahedron Lett. 2018;59:1733–1736. doi: 10.1016/j.tetlet.2018.03.065. [DOI] [Google Scholar]
- 32.Wasser S.P. Medicinal Mushroom Science: Current Perspectives, Advances, Evidences, and Challenges. Biomed. J. 2014;37:345–356. doi: 10.4103/2319-4170.138318. [DOI] [PubMed] [Google Scholar]
- 33.Krzyczkowski W., Malinowska E., Herold F. Erinacine A biosynthesis in submerged cultivation of Hericium erinaceum: Quantification and improved cultivation. Eng. Life Sci. 2010;10:446–457. doi: 10.1002/elsc.201000084. [DOI] [Google Scholar]
- 34.Zhang G., Sun Z., Ren A., Shi L., Shi D., Li X., Zhao M. The mitogen-activated protein kinase GlSlt2 regulates fungal growth, fruiting body development, cell wall integrity, oxidative stress and ganoderic acid biosynthesis in Ganoderma lucidum. Fungal Genet. Biol. 2017;104:6–15. doi: 10.1016/j.fgb.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Xie C., Gong W., Zhu Z., Yan L., Hu Z., Peng Y. Comparative transcriptomics of Pleurotus eryngii reveals blue-light regulation of carbohydrate-active enzymes (CAZymes) expression at primordium differentiated into fruiting body stage. Genomics. 2018;110:201–209. doi: 10.1016/j.ygeno.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Stamets P. Growing Gourmet and Medicinal Mushrooms. 3rd ed. Ten Speed Press; Berkeley, Toronto: 2000. The lion’s mane mushroom; pp. 387–394. [Google Scholar]
- 37.Stalpers J.A. Identification of wood-inhabiting Aphyllophorales in pure culture. Stud. Mycol. 1978;16:114. [Google Scholar]
- 38.Sturini M., Girometta C., Maraschi F., Savino E., Profumo A. A preliminary investigation on Metal Bioaccumulation by Perenniporia fraxinea. Bull. Environ. Contam. Toxicol. 2017;98:508–512. doi: 10.1007/s00128-017-2038-1. [DOI] [PubMed] [Google Scholar]
- 39.Ko H.G., Park H.G., Park S.H., Choi C.W., Kim S.H., Park W.M. Comparative study of mycelial growth and basidiomata formation in seven different species of the edible mushroom genus Hericium. Bioresource Technol. 2005;96:1439–1444. doi: 10.1016/j.biortech.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Hassan F.R.H. Cultivation of the Monkey Head Mushroom (Hericium erinaceus) in Egypt. J. App. Sci. Res. 2007;3:1229–1233. [Google Scholar]
- 41.Savino E., Girometta C., Baiguera R.M., Cesaroni V., Guglielminetti M.L., Rodolfi M., Rossi P., Picco A.M. Different approaches for Hericium erinaceus spawn in the perspective of gluten free products. ISMS. 2016;19:106. [Google Scholar]
- 42.Gerbec B., Tavčar E., Gregori A., Kreft S., Berovic M. Solid State Cultivation of Hericium erinaceus Biomass and Erinacine: A Production. J. Bioproces. Biotech. 2015;5:1–5. doi: 10.4172/2155-9821.1000210. [DOI] [Google Scholar]