Abstract
Conjugation of known biologically active molecules to carbohydrate frameworks represents a valuable option for the preparation of hybrid, structurally-related families of compounds with the aim of modulating their biological response. Therefore, we present here a study on the preparation of d-galacto, d-manno, d-gluco, and d-lactose glycoconjugates of an established N-hydroxyindole-based (NHI) inhibitor of lactated dehydrogenase (LDH). Structural variations involved the sugar stereochemistry and size as well as the anchoring point of the NHI on the carbohydrate frame (either C-1 or C-6). In the case of the galactose anomeric glycoconjugate (C-1), intriguing solvent-dependent effects were observed in the glycosylation stereochemical outcome. The biological activity of the deprotected glycoconjugates in contrasting lactate formation and cancer cell proliferation are described.
Keywords: LDH inhibitors, glycoconjugates, anticancer agents
1. Introduction
Deregulation of normal cell metabolism is one of the most important hallmark of cancer cells. In fact, metabolic activities are often reprogrammed in neoplastic masses relative to healthy tissues [1]. The Warburg effect, consisting of an augmented aerobic glycolysis, represents a typical case of an altered metabolic pathway in cancer [2]. This effect is characterized by an increased glucose uptake and lactate production in cancer cells, regardless of hypoxic/normoxic conditions surrounding the cells. Such an increase in glycolytic flux produces energy and glycolytic intermediates that are needed by rapidly proliferating cells.
Numerous research lines have so far focused on the development of new therapeutic agents that can potentially take advantage of this characteristic metabolic profile present in cancer cells by acting as anti-glycolytic agents [3,4]. Furthermore, the consequential overexpression of glucose transporters (GLUTs) in the membrane of cancer cells can be either targeted by modulators of the carbohydrate flux through these proteins [5] or exploited for the preferential intracellular accumulation of glycoconjugates containing cytotoxic drugs [6].
One of the key enzymes in glycolysis is lactate dehydrogenase (LDH), which catalyzes the reversible transformation of pyruvate into lactate. This enzyme is constituted by four monomeric subunits (LDH-A and LDH-B) and may, therefore, be found in humans as five different isoforms (hLDH1-5). Cancer cells are often found to overexpress subunit LDH-A and, thus, its tetrameric form hLDH5, therefore may represent a promising therapeutic target [7]. In fact, numerous research efforts have been so far committed to the discovery of novel potent inhibitors of hLDH5 as potential anticancer drugs, even though very often these inhibitors display poor cell membrane permeability due to the high polar nature of their structures [8].
We have previously developed a class of N-hydroxyindole-based compounds (NHIs), such as 1 (Figure 1), which are able to inhibit hLDH5. Specifically, these compounds inhibit hLDH5 with Ki values in the micromolar range in enzymatic assays. Considering that the high rate of glycolysis in cancer cells is associated with a striking glucose avidity, we set out to exploit this feature in order to improve the tumor-specificity of this approach and minimize off-target effects. We recently reported that glucoconjugated NHIs, in particular 2 (Figure 1), remarkably reduced the cellular production of lactic acid in cancer cells and blocked the growth of tumor cells [9,10].
Figure 1.
Structure of NHI (1) and its known (2) and novel (3, 4) glycoconjugates.
In order to get a further insight into the potential role played by the carbohydrate structure on the inhibitor activity and on the cellular uptake through facilitative glucose transporters—namely its size, stereochemistry, and anchoring point, herein we describe a structural modulation of this class of glycoconjugate inhibitors, exemplified by 3 and 4 (Figure 1), with a N-hydroxyindole moiety linked to the anomeric or the C(6) position of monosaccharides of the d-galacto, d-manno, d-gluco series and of d-lactose.
2. Results and Discussion
2.1. Preparation of Glycoconjugates
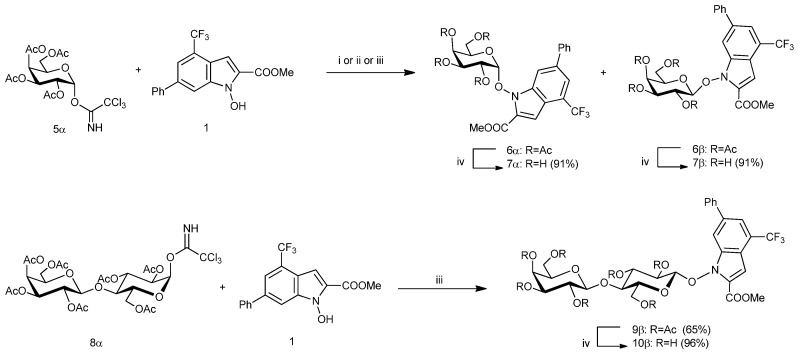
In our general approach to the synthesis of galactose glycosides 6α, 6β, and lactose glycoside 8β, known glycosyl donors 5α [11] and 8α [12,13] were reacted under several conditions with the NHI 1 [14] fragment, which was used as the glycosyl acceptor (Scheme 1 and Table 1). The glycosylation reactions have been carried out with trichloroacetimidate glycosyl donors in the presence of the two most widely used promoters (TMSOTf and BF3·Et2O) [15,16] and proceeded with good yields. Of a certain interest was observing how the nature of the promoter and the solvent both affected the α/β ratio in the formation of compound 6 (Table 1). In particular, when donors 5α and 8α were coupled with acceptors 1 using BF3·Et2O (1.3 eq) in dry CH2Cl2, a high selectivity for the beta anomer was obtained (6α:6β 5:95, 9α:9β < 1:99).
Scheme 1.
Preparation of glycosyl derivatives 6, 7 and 9, 10. Reagents and conditions: (i) TMSOTf, CH2Cl2, molecular sieves AW 300, 0 °C (30 min) and RT (3.5 h), (6α: 29%, 6β: 42%); (ii) TMSOTf, CH3CN, molecular sieves AW 300, 0 °C (30 min) and RT (2.5 h), (6α: 54%, 6β: 13%); (iii) BF3·Et2O, CH2Cl2, molecular sieves AW 300, −10 °C to RT (2 h), (6β: 78%); (iv) MeONa-MeOH 0.33M, 2:3 CH2Cl2-MeOH, 0 °C to RT.
Table 1.
Glycosylation of acceptor 1 with glycosyl donors 5α and 6α.
| Glycosyl Donor | Promoter | Solvent | Anomeric Ratio * | Isolated Yield |
|---|---|---|---|---|
| 5α | TMSOTf | CH2Cl2 | 6α:6β = 35:65 | 6α: 29% 6β: 42% |
| 5α | TMSOTf | MeCN | 6α:6β = 78:22 | 6α: 54% 6β: 13% |
| 5α | BF3·Et2O | CH2Cl2 | 6α:6β = 5:95 | 6α: 3%6β: 78% |
| 8α | BF3·Et2O | CH2Cl2 | 9α:9β < 1:99 | 9β: 65% |
* Ratios were determined by NMR analysis of the crude product on the basis of the relative H-1 signal intensities.
Moreover, when the glycosylation of NHI 1 using 5α (Scheme 1 and Table 1) was carried out with TMSOTf (0.2 eq) as the promoter in dry CH2Cl2 the reaction proceeded, surprisingly, with poor stereoselectivity (6α:6β 35:65). The use of dry MeCN as the solvent gave a more satisfactory, albeit reversed, stereoselectivity (6α:6β 78:22). Although the known “nitrile effect” [17] could be invoked when MeCN is used as the solvent, the results obtained confirm how reaction conditions and donor/acceptor structures can affect the stereochemical outcome of a glycosylation reaction. Indeed, in both 5α and 8α substrates, it can be speculated that the participation of the neighboring 2-O-acyl substituent can lead to the formation of an acyloxonium intermediate. Consequently, in the presence of BF3·Et2O, the C-O bond on the anomeric carbon of the acyloxonium intermediate is only weakened, to afford the formation of 1,2-trans linkage with a very high selectivity and a SN2-like displacement from the glycosyl acceptor NHI 1 affords 6β and 9β. Conversely, in the presence of a stronger promoter such as TMSOTf in CH2Cl2, the acyloxonium mainly evolves towards the classic oxacarbenium ion to afford 6α and 6β in 35/65 ratio.
In all cases, glycosides 6α, 6β, and 9β were efficiently separated by chromatographic means and fully characterized. NMR data confirmed the glycoside structures and, in particular, the high values (8.1 and 7.8 Hz) of the J1,2 coupling constants, which is in agreement with an axial–axial disposition of H-1 and H-2, confirmed the beta configuration of the glycosidic bonds in 6β and 9β. In the case of 6α the equatorial–axial disposition of H-1 and H-2 was confirmed by the low value (4.0 Hz) of the J1,2 coupling constant.
The deacetylation of 6α, 6β, and 9β (Scheme 1) by treatment with MeONa in CH2Cl2-MeOH afforded deprotected glycoconjugates 7α, 7β, and 10β in very good yields (91–96%).
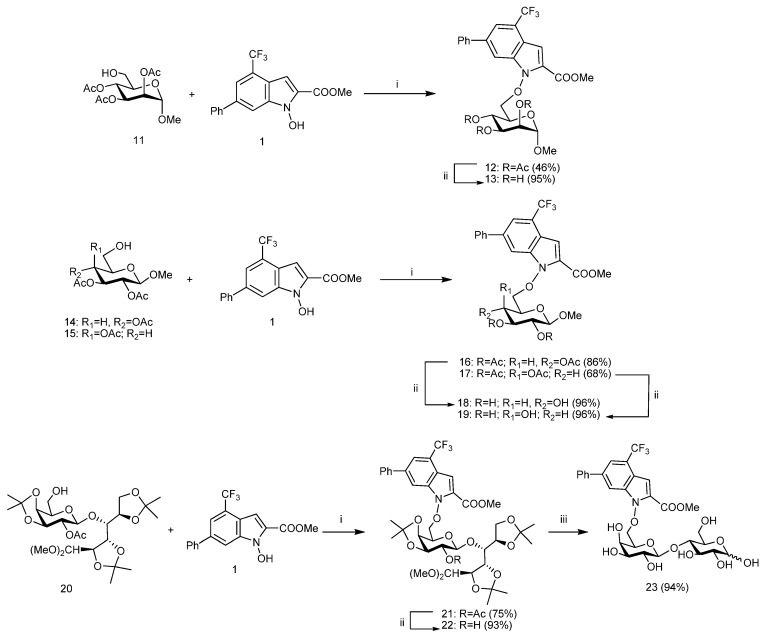
The synthesis of the protected glycoconjugates 12, 16, and 17 is described in Scheme 2. A Mitsunobu reaction [18] was performed between the N-hydroxyindole derivative 1 and known carbohydrate building blocks 11 [19], 14 [20], and 15 [21] in the presence of diisopropyl azodicarboxylate (DIAD) or di-2-methoxyethyl azodicarboxylate (DMEAD) and triphenylphosphine (PPh3) affording glycoconjugates 12 (46% yield), 16 (86% yield), and 17 (68% yield) respectively.
Scheme 2.
Preparation of glycosyl derivatives 12, 13, 16–19, and 21–23. Reagents and conditions: (i) PPh3, DIAD or DMEAD, dry THF, 0 °C to RT (3 h); (ii) MeONa-MeOH 0.33 M, 2:3 CH2Cl2-MeOH, 0 °C to RT; (iii) 80% aq AcOH, 80 °C, 4 h.
Poliacetonide lactose derivatives have been widely used as suitable protected forms of the natural disaccharide. Indeed, only one or two unprotected hydroxyl groups are left on the galacto non-reducing moiety, which allows for consecutive feasible modifications [22,23,24,25]. Herein, known poliacetonide derivatives 20 [26] was coupled with NHI 1 again through a Mitsunobu condensation with PPh3, DMEAD in dry THF giving disaccharide derivative 21 in a good yield (75%, Scheme 2).
The deacetylation of 12, 16, 18, and 21 (Scheme 2) via base-catalyzed transesterification with MeONa in CH2Cl2-MeOH afforded deprotected glycoconjugates 13, 17, 19, and 22 in very good yields (93–96%). Finally, the acetals protecting groups were cleaved from 22 by acid hydrolysis (80% aq AcOH) to afford disaccharide derivative 23 (94% yield) mostly as the α-pyranose anomer (>95%, NMR, DMSO-d6).
All compounds were characterized and their mono- and two-dimensional NMR analyses (1H, 13C, DEPT-135, COSY, and HSQC) were consistent with their structures (see experimental section).
2.2. Enzymatic Assay
The inhibitory activities of the newly synthesized sugar conjugates were measured as IC50 values on purified human enzyme isoform hLDH5 (Table 2). Galloflavin was used as reference compound, because it is a well-known phenolic LDH inhibitor which is often used in enzymatic LDH assays [27,28], since it is easily synthesizable and it is also commercially available. Among the newly synthesized sugar conjugates, compounds 7α, 7β, and 10β, which are the glyco-conjugates bearing the N-hydroxyindole moiety at the anomeric position, showed a moderate inhibition potency towards hLDH5, with IC50 values ranging from 229 to 246 μM, compared to the IC50 values of the parent gluco-conjugate 2 (IC50 = 112.8 ± 5.6 µM) and of its aglycone moiety 1 (IC50 = 10.8 ± 3.5 µM) Conversely, compounds 13, 18, 19, and 23, in which the N-hydroxyindole portion is linked to the C(6) position of mono- or disaccharide units, were inactive at the maximum tested concentration of 500 μM.
Table 2.
hLDH5 inhibition activities of glycoconjugates.
| Compound | hLDH5 (IC50, μM) a |
|---|---|
| 7α | 229 ± 16 |
| 7β | 246 ± 36 |
| 10β | 232 ± 47 |
| 13 | >500 |
| 18 | >500 |
| 19 | >500 |
| 23 | >500 |
| Galloflavin b | 110 ± 4.0 |
a Values are reported as the means ± SD of three or more independent experiments. b Reference hLDH5 inhibitor [29].
2.3. Molecular Modeling Studies
Docking studies were carried out in order to explore the binding disposition of these ligands into the hLDH5 binding site. The seven compounds were thus docked into the minimized average structure of hLDH5 previously obtained [14] by applying a robust Autodock procedure that had shown good results in virtual screening studies and in the prediction of ligands’ binding poses [30,31]. Figure 2 illustrates the predicted binding disposition of 7α. The methyl ester group shows an H-bond with R106, the phenyl ring is inserted into a lipophilic cavity mainly delimited by H193, V235, and A238, whereas the trifluoromethyl substituent shows an H-bond interaction with N138. With regards to the glycosidic portion of the molecule, it shows H-bonds to the carboxylic portion of E192 and D195 and also with the backbone nitrogen of this latter residue.
Figure 2.
Binding disposition of 7α into hLDH5.
As shown in Figure S1 compound 7β shows a binding orientation very similar to that obtained for 7α with the same interactions, except for the loss of the H-bond with the carboxylic group of D195. According to our model, the substitution of the monosaccharide with a disaccharide unit does not seem to determine important modifications of the binding orientation of the resulting compound. As shown in Figure S2, the docking analysis of 10β highlights a slight shift of the phenyl-indole fragment of the ligand and the loss of the interaction of its trifluoromethyl substituent with N138. However, the H-bond of the ligand methyl ester group with R106 and the lipophilic interactions of the phenyl ring are maintained. With regards to the disaccharide fragment, it shows H-bonds with R106, E192, and D195. The addition of the N-hydroxyindole portion to the C(6) position of mono- or disaccharide unit determines a general inactivity. This behavior, according to our model, is likely due to the different geometry of these ligands and, in particular, their impossibility to adopt the binding orientation highlighted for the anomeric derivatives; as shown in Figure S3, compounds 13–23 show a very similar binding disposition that is completely different from that shown by compounds 7α, 7β, and 10β, thus supporting their inactivity. The phenyl ring shows lipophilic interactions with I252, the methyl ester group and the trifluoromethyl substituent do not show any interaction and result to be water-exposed, whereas the glycosidic portions show H-bonds with R169, H193 and the backbone of T248.
2.4. Cancer Cell Antiproliferative Potency Assays
The cytotoxicity of glycoconjugates on HeLa and A549 cancer cells was assessed and compared to that of parent glucose derivative 2. Cells were incubated for 72 h with different concentrations of the compounds, then cell death was quantified by the Sulforhodamine B (SRB) assay [10]. The antiproliferative activities are expressed as IC50 values (Table 3). Glycoconjugates 7α and 7β showed good inhibition potencies in A549 cells, with IC50 values comparable to that of reference compound 2. Moreover, they were more effective in reducing cancer cell growth in HeLa cells (about two-fold for 7β or three-fold for 7α) than in A549 cells. Lactose-conjugated 10β was less potent in both cell lines: it was inactive at 100 μM in A549 cells, and it reached an IC50 value of 81.5 μM in HeLa cells.
Table 3.
Antiproliferative effect (72 h IC50 values, µM ± standard error, n = 3) of glycoconjugates in HeLa and A549 human cancer cell lines.
| Compound | A549 (NSCLC) | HeLa (Cervical Carcinoma) |
|---|---|---|
| IC50, μM | ||
| 7α | 20.4 ± 2.1 | 7.5 ± 1.1 |
| 7β | 17.6 ± 2.0 | 7.6 ± 1.1 |
| 10β | >100 | 81.5 ± 7.1 |
| 2 a | 17.2 ± 3.0 | 7.2 ± 0.2 |
a Data from [9].
2.5. Cellular Lactate Production Inhibition Assay
The ability of glycoconjugates 7α, 7β, and 10β to inhibit lactate production in cancer cells was evaluated using the previously reported technique [10] and the results are displayed in Figure 3. Compounds 7α and 7β were tested at two different concentrations, 25 and 50 µM, whereas the less cytotoxic derivative 10β was assayed at 100 and 200 µM for their ability to reduce lactate production in HeLa cells following an 8 h incubation. The glucose derivative 2 was able to reduce lactate production of about 50% at 50 µM, in agreement with previous results [9,10]. A noticeable dose-dependent reduction in lactate production was found with the α-galacto-conjugate 7α, which displayed a reduction of lactate production greater than 60% at 50 µM.
Figure 3.
Lactate production inhibition of glycoconjugates in HeLa human cancer cells after 8 h incubation. Averages from three or more independent experiments are depicted, with error bars depicting standard error (n = 3).
3. Materials and Methods
Melting points were determined with a Kofler hot-stage apparatus and are uncorrected. Optical rotations were measured with an ATAGO AP-300 Automatic Polarimeter at 25±2 °C. 1H NMR spectra were recorded in appropriate solvents with a Bruker Avance II operating at 250.13 MHz. 13C NMR spectra were recorded with the spectrometers operating at 62.9 MHz. The assignments were made, when possible, with the aid of DEPT, COSY, HSQC experiments. The first order proton chemical shifts δ are referenced to either residual CDCl3 (δ H 7.26, δ C 77.0), residual CD3CN (δ H 1.94, δ C 1.28), residual CD3OD (δ H 3.31, δ C 49.0) or residual DMSO-d6 (δ H 2.49, δ C 39.5) and J-values are given in Hz. Spin multiplicity was indicated by s = singlet, d = doublet, t = triplet, bt = broad triplet, q = quartet, m = multiplet. Coupling constants J were reported in Hertz (Hz). All reactions were followed by TLC on Kieselgel 60 F254 with detection by UV light and/or with ethanolic 10% phosphomolybdic or sulfuric acid, and heating. Kieselgel 60 (Merck, S.p.A., Milano, Italy, 70–230 and 230–400 mesh, respectively) was used for column and flash chromatography. Some of flash chromatography were conducted by the automated system Isolera Four SVTM (Biotage®, Uppsala, Sweden), equipped with UV detector with variable wavelength (200–400 nm). Solvents were dried by distillation according to standard procedures, and storage over 4Å molecular sieves activated for at least 24 h at 200 °C. All reagents were purchased from Aldrich Chemical Co. and were used without further purification. Molecular sieves (4Å and AW-300) and PPh3 were activated (2–8 h at 150 °C.) before use. All reactions involving air- or moisture-sensitive reagents were performed under an argon atmosphere using anhydrous solvents. Anhydrous dimethylformamide (DMF), dichloromethane (CH2Cl2), 1,2-dichloethane (DCE), and THF were purchased from Sigma-Aldrich. Other dried solvents were obtained by distillation according to standard procedure and stored over 4Å molecular sieves activated. MgSO4 or Na2SO4 were used as the drying agents for solutions. 1-Hydroxy-6-phenyl-4-(trifluoromethyl)-1H-indole-2-methyl-carboxylate (1) [14], 2,3,4,6-tetra-O-acetyl-α-d-galactopyranosyl trichloroacetimidate (5α) [11], 4-O-(2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl)-2,3,6-tri-O-acetyl-α-d-glucopyranosyl trichloroacetimidate (8α) [12,13], methyl 2,3,4-tri-O-acetyl-α-d-mannopyranoside (11) [19], methyl 2,3,4-tri-O-acetyl-β-d-glucopyranoside (14) [20], methyl 2,3,4-tri-O-acetyl-β-d-galactopyranoside (15) [21], and 4-O-(2-O-acetyl-3,4-O-isopropylidene-β-d-galactopyranosyl)-2,3:5,6-di-O-isopropylidene-aldehydo-d-glucose dimethyl acetal (20) [26] were prepared according to the reported procedure.
3.1. General Procedures for Glycosylation
3.1.1. Method A (with TMSOTf in CH2Cl2 or CH3CN)
A solution of the glycosyl donor 5α [11] (1.30 mmol, 1.3 equiv) and N-hydroxyindole derivative 1 [14] (1.00 mmol, 1.0 equiv) in anhyd toluene (10.0 mL) was concentrated under diminished pressure (2 h) for removed any traces of water. The residue was dissolved in anhyd CH2Cl2 or CH3CN (10.0 mL), molecular sieves AW 300 (1.25 g) were added and the resulting mixture was stirred under an argon atmosphere at 0 °C for 30 min. TMSOTf (36 μL, 0.20 mmol, 0.2 equiv) was added and the reaction mixture was stirred for 30 min at 0 °C and then at room temp until TLC analysis showed the complete disappearance of the N-hydroxyindole derivative 1. The reaction mixture was diluted with CH2Cl2 or CH3CN (10.0 mL), neutralized with Et3N, filtered through a short pad of Celite® and organic solution was concentrated under diminished pressure. The crude residue was subjected to flash chromatography on silica gel.
3.1.2. Method B (with BF3·Et2O in CH2Cl2)
The appropriate glycosyl donor 5α or 8α (1.50 mmol, 1.5 equiv) was dried under diminished pressure (1 h). Anhyd CH2Cl2 (26.0 mL), molecular sieves AW 300 (500 mg), the N-hydroxyindole derivative 1 (1.00 mmol, 1.0 equiv) was added and the resulting mixture was stirred under an argon atmosphere at room temp for 1 h. The suspension was cooled to −10 °C, treated with BF3·Et2O (160 μL, 1.30 mmol, 1.3 equiv) and slowly warmed to room temp. When TLC analysis showed the complete disappearance of the N-hydroxyindole derivative 1 the reaction mixture was diluted with CH2Cl2 (10.0 mL), neutralized with Et3N, filtered through a short pad of Celite® and organic solution was concentrated under diminished pressure. The crude residue was subjected to flash chromatography on silica gel.
3.2. Methyl 1-(2,3,4,6-Tetra-O-Acetyl-α-d-Galactopyranosyl)-6-Phenyl-4-Trifluoromethyl-1H-Indole-2-Carboxylate (6α) and Methyl 1-(2,3,4,6-Tetra-O-Acetyl-β-d-Galactopyranosyl)-6-Phenyl-4-Trifluoromethyl-1H-Indole-2-Carboxylate (6β)
The glycosylation of 5α [11] (100 mg, 0.20 mmol, 1.3 equiv) with N-hydroxyindole derivative 1 [14] (52 mg, 0.16 mmol, 1.0 equiv), was performed in dry CH2Cl2 (1.6 mL) in according to the general procedure (Method A). The reaction was stirred until TLC (4 h, TLC, 7:3 hexane-EtOAc, double elution) showed the complete disappearance of the acceptor 1 (Rf 0.66) and the formation of two spots at Rf 0.37 and 0.35. The crude product was constituted (NMR) by a mixture of the anomers 6α and 6β in ratio of 35:65, calculated on the basis of the relative H-1 signal intensities. Purification of crude product by flash chromatography on silica gel (7:3 hexane-EtOAc) afforded pure 6α (31 mg, 29%) and 6β (44 mg, 42%).
The glycosylation of 5α [11] (100 mg, 0.20 mmol, 1.3 equiv) with N-hydroxyindole derivative 1 [14] (52 mg, 0.16 mmol, 1.0 equiv), was performed in dry CH3CN (1.6 mL) in according to the general procedure (Method A). The reaction was stirred until TLC (3 h, TLC, 7:3 hexane-EtOAc, double elution) showed the complete disappearance of the acceptor 1 (Rf 0.66) and the formation of two spots at Rf 0.37 and 0.35. The crude product was constituted (NMR) by a mixture of the anomers 6α and 6β in ratio of 78:22, calculated on the basis of the relative H-1 signal intensities. Purification of crude product by flash chromatography on silica gel (7:3 hexane-EtOAc) afforded pure 6α (57.4 mg, 54%) and 6β (14.4 mg, 13%).
The glycosylation of 5α [11] (200 mg, 0.40 mmol, 1.5 equiv) with N-hydroxyindole derivative 1 [14] (91 mg, 0.28 mmol, 1.0 equiv) was performed in dry CH2Cl2 (7.0 mL) according to the general procedure (Method B). The reaction was stirred until TLC (2 h, TLC, 7:3 hexane-EtOAc, double elution) showed the complete disappearance of the acceptor 1 (Rf 0.66) and the formation of two spots at Rf 0.37 and 0.35. The crude product was constituted (NMR) by a mixture of the anomers 6α and 6β in ratio of 5:95, calculated on the basis of the relative H-1 signal intensities. Purification of crude product by flash chromatography on silica gel (7:3 hexane-EtOAc) afforded pure 6β (142 mg, 78%) and 6α (5 mg, 3%).
Compound 6α is a yellow foam; Rf 0.37 (7:3 hexane-EtOAc, double elution); mp 64–67 °C (crom); [α]D -10.3 (c 0.90 in CHCl3); 1H NMR (250.13 MHz, CD3CN) δ: 7.99 (m, 1H, Ar-H), 7.86 (m, 1H, Ar-H), 7.80–7.74 (m, 2H, Ar-H), 7.55–7.40 (m, 3H, Ar-H), 7.30 (qd, 1H, J 1.0 Hz, J 1.6 Hz, Ar-H), 5.91 (d, 1H, J1,2 4.0 Hz, H-1), 5.61 (ddd, 1H, J3,4 3.0 Hz, J4,5 1.4 Hz, J4,6b 0.6 Hz, H-4), 5.47 (dd, 1H, J2,3 11.2 Hz, H-2), 5.36 (dd, 1H, H-3), 5.02 (m, 1H, H-5), 4.05 (ddd, 1H, J5,6b 3.3 Hz, J6a,6b 12.1 Hz, H-6b), 4.00 (dd, 1H, J5,6a 4.7 Hz, H-6a), 3.91 (s, 3H, COOMe), 2.12, 2.11, 1.99, 1.67 (4s, each 3H, 4 × COMe); 13C NMR (62.9 MHz, CD3CN) δ: 171.2, 171.1, 170.8, 170.7 (4 × COMe), 160.6 (COOMe), 140.4, 139.7, 138.0, 130.4 (4 × Ar-C), 130.1, 129.2, 128.5 (Ar-CH), 124.8–123.2 (CF3 + Ar-C), 120.4 (Ar-CH), 117.2 (Ar-C), 113.3, 107.1 (2 × Ar-CH), 104.4 (C-1), 70.6 (C-5), 68.9 (C-4), 67.6 (C-2), 66.8 (C-3), 62.5 (C-6), 53.1 (COOMe), 21.8, 20.4 (4 × COMe). Anal. Calcd for C31H30F3NO12: C, 55.94; H, 4.54%; N, 2.10%; Found: C, 55.98; H, 4.56%; N, 2.14%.
Compound 6β is a pale yellow solid; Rf 0.35 (hexane-AcOEt 7:3, double elution); mp 139–142 °C (crom); [α]D + 26.3 (c 1.0 in CHCl3); 1H NMR (250.13 MHz, CD3CN) δ: 8.12 (m, 1H, Ar-H), 7.84 (m, 1H, Ar-H), 7.75 (m, 1H, Ar-H), 7.71 (m, 1H, Ar-H), 7.54–7.40 (m, 3H, Ar-H), 7.23 (qd, 1H, J 1.0 Hz, J 1.7 Hz, Ar-H), 5.58 (d, 1H, J1,2 8.1 Hz, H-1), 5.40 (m, 2H, 2-H, H-4), 5.27 (dd, 1H, J2,3 9.1 Hz, J3,4 3.3 Hz, H-3), 4.10–3.98 (m, 3H, H-5, H-6a, H-6b), 3.91 (s, 3H, COOMe), 2.18, 2.16, 1.98, 1.60 (4s, each 3H, 4 × COMe); 13C NMR (62.9 MHz, CD3CN) δ: 171.2, 171.1, 170.8, 170.7 (4 × COMe), 160.5 (COOMe), 140.5, 139.8, 139.5, 130.4 (4 × Ar-C), 130.1, 129.1, 128.2 (Ph-CH), 124.1–123.2 (CF3 + Ar-C), 120.3 (Ar-CH), 117.2 (Ar-C), 114.8, 106.8 (2 × Ar-CH), 106.2 (C-1), 72.1 (C-5), 71.3 (C-3), 68.4 (C-4), 68.1 (C-2), 62.4 (C-6), 52.9 (COOMe), 21.2, 20.8, 20.7, 20.4 (4 × COMe). Anal. Calcd for C31H30F3NO12: C, 55.94; H, 4.54%; N, 2.10%; Found: C, 55.97; H, 4.58%; N, 2.13%.
3.3. Methyl 1-[4-O-(2,3,4,6-Tetra-O-Acetyl-β-d-Galactopyranosyl)-2,3,6-Tri-O-Acetyl-β-d-Glucopyranosyl)]-6-Phenyl-4-Trifluoromethyl-1H-Indole-2-Carboxylate (9β)
The glycosylation of 8α [12,13] (175 mg, 0.22 mmol, 1.5 equiv) with N-hydroxyindole derivative 1 [19] (50 mg, 0.15 mmol, 1.0 equiv), was performed in dry CH2Cl2 (4.0 mL) in according to the general procedure (Method B). The reaction was stirred until TLC (2 h, TLC, 1:1 hexane-EtOAc, double elution) showed the complete disappearance of the acceptor 1 (Rf 0.76) and the formation of one spot at Rf 0.45. Purification of crude product by flash chromatography on silica gel (1:1 hexane-EtOAc) afforded pure 9β (93 mg, 65%) as solid; Rf 0.76 (7:3 hexane-EtOAc, double elution); mp 100–103 °C (crom); [α]D +49.4 (c 1.0 in CHCl3); 1H NMR (250.13 MHz, CD3CN-CDCl3) δ: 8.08 (m, 1H, Ar-H), 7.80 (m, 1H, Ar-H), 7.71–7.66 (m, 2H, Ar-H), 7.58–7.38 (m, 3H, Ar-H), 7.22 (qd, 1H, J 1.0 Hz, J 1.8 Hz, Ar-H), 5.57 (d, 1H, J1,2 8.0 Hz, 1-H), 5.33 (dd, 1H, J2,3 9.8 Hz, J3,4 8.7 Hz, 3-H), 5.30 (dd, 1H, J3′,4′ 3.5 Hz, J4′,5′ 1.3 Hz, H-4′), 5.23 (dd, 1H, H-2), 5.03 (dd, 1H, J2′,3′ 10.4 Hz, H-3′), 4.90 (dd, 1H, J1′,2′ 7.8 Hz, H-2′), 4.59 (d, 1H, H-1′), 4.19 (dd, 1H, J5,6b 2.3 Hz, J6a,6b 12.1 Hz, H-6b), 4.13–4.02 (m, 4H, H-6a, H-5′, H-6′a, H-6′b), 3.99 (dd, 1H, J4,5 9.9 Hz, H-4), 3.91 (s, 3H, COOMe), 3.75 (ddd, 1H, J5,6a 5.6 Hz H-5), 2.13, 2.09, 2.06, 2.02, 1.93, 1.89, 1.58 (7s, each 3H, 7 × COMe); 13C NMR (62.9 MHZ, CD3CN-CDCl3) δ: 171.1–170.2 (7 × COMe), 160.3 (COOMe), 140.6, 139.8, 135.5, 130.4 (4 × Ar-C), 129.9, 128.9, 128.2 (Ph-CH), 123.9–122.9 (CF3 + Ar-C), 120.3 (Ar-CH), 117.3 (Ar-C), 114.9, 106.8 (2 × Ar-CH), 105.6 (C-1), 101.4 (C-1′), 76.9 (C-4), 73.4 (C-5), 72.7 (C-3), 71.5 (C-3′, C-5′), 70.5 (C-2), 69.8 (C-2′), 68.0 (C-4′), 62.7 (C-6′), 62.0 (C-6), 52.8 (COOMe), 21.1–20.4 (7 × COMe). Anal. Calcd for C43H46F3NO20: C, 54.15; H, 4.86%; N, 1.47%; Found: C, 54.18; H, 4.89%; N, 1.50%.
3.4. General Procedures for Mitsunobu Reaction
A solution of appropriate 6-OH-sugar 11 [19], 14 [20], 15 [21], or 20 [26] (1.00 mmol, 1.0 equiv) and the N-hydroxyindole derivative 1 (1.30 mmol, 1.3 equiv) in anhyd THF (20.0 mL) was cooled to 0 °C and treated with dry PPh3 (3.50 mmol, 3.5 equiv) and di-isopropyl azodicarboxylate (DIAD) or di-2-methoxyethyl azodicarboxylate (DMEAD) (3.50 mmol, 3.5 equiv). The reaction mixture was stirred at room temp until TLC analysis showed the complete disappearance of the starting compound. The reaction mixture was diluted with CH2Cl2 (35.0 mL) and washed with satd aq Na2HCO3 (35 mL). The aqueous phases were extracted with CH2Cl2 (2 × 25 mL) and the combined organic layers were dried over MgSO4, filtered and concentrated under diminished pressure. The crude residue was subjected to flash chromatography on silica gel.
3.5. Methyl 2,3,4-Tri-O-Acetyl-6-O-(2-Methoxycarbonyl-6-Phenyl-4-Trifluoromethyl-1H-Indol-1-Yl)-α-d-Mannopyranoside (12)
Mitsunobu reaction applied to 11 (120 mg, 0.38 mmol, 1.0 equiv) was performed with DIAD (263 μL, 1.33 mmol, 3.5 equiv) in accordance with the general procedure. The reaction was stopped after 3 h and the crude product was purified by flash chromatography on silica gel (3:7 hexane-EtOAc) gave pure 12 (108 mg, 46%) as a colorless oil; Rf 0.58 (1:1 hexane-EtOAc); [α]D +34.2 (c 0.89 in CHCl3); 1H NMR (250.13 MHz, CD3CN) δ: 8.13 (m, 1H, Ar-H), 7.81 (m, 1H, Ar-H), 7.77–7.64 (m, 2H, Ar-H), 7.55–7.39 (m, 3H, Ar-H), 7.22 (qd, 1H, Ar-H), 5.33 (dd, 1H, J3,4 10.2 Hz, J4,5 9.8 Hz, H-4), 5.23 (dd, 1H, J2,3 6.2 Hz, H-3), 4.90 (dd, 1H, J1,2 1.4 Hz, H-2), 4.86 (d, 1H, H-1), 4.68 (dd, 1H, J5,6b 5.6 Hz, J6a,6b 10.1 Hz, H-6b), 4.52 (dd, 1H, J5,6a 2.3 Hz, H-6a), 4.28 (ddd, 1H, H-5), 3.92 (s, 3H, COOMe), 3.44 (s, 3H, OMe), 2.04, 2.02, 1.95 (3s, each 3H, 3 × COMe); 13C NMR (62.9 MHz, CD3CN) δ: 171.0–170.8 (3 × COMe), 160.5 (COOMe), 140.5, 139.4, 137.2, 130.1 (4 × Ar-C), 130.1, 129.1, 128.3 (Ph-CH), 124.3–123.3 (CF3 + Ar-C), 120.0 (Ar-CH), 117.6 (Ar-C), 112.7, 105.4 (2 × Ar-CH), 99.5 (C-1), 78.9 (C-6), 72.7 (C-2), 70.1 (C-3), 69.4 (C-5), 66.4 (C-4), 55.9 (OMe), 52.9 (COOMe), 22.1, 21.0, 20.9 (3 × COMe). Anal. Calcd for C30H30F3NO11: C, 56.52; H, 4.74%; N, 2.20%; Found: C, 56.55; H, 4.77%; N, 2.23%.
3.6. Methyl 2,3,4-Tri-O-Acetyl-6-O-(2-Methoxycarbonyl-6-Phenyl-4-Trifluoromethyl-1H-Indol-1-Yl)-β-d-Glucopyranoside (16)
Mitsunobu reaction applied to 14 (72 mg, 0.22 mmol, 1.0 equiv) was performed with DMEAD (186 mg, 0.80 mmol, 3.5 equiv) in accordance with the general procedure. The reaction was stopped after 4 h and the crude product was purified by flash chromatography on silica gel (65:35 hexane-EtOAc) gave pure 16 (104 mg, 86%) as a yellow solid; Rf 0.53 (1:1 hexane-AcOEt); mp 191–194 °C (crom); [α]D –11.5 (c 1.0 in CHCl3); 1H NMR (250.13 MHz, CD3CN) δ: 8.04 (1H, m, Ar-H), 7.80 (m, 1H, Ar-H), 7.76 (m, 1H, Ar-H), 7.73 (m, 1H, Ar-H), 7.59–7.40 (m, 3H, Ar-H), 7.18 (qd, 1H, J 0.9 Hz, J 1.7 Hz, Ar-H), 5.27 (dd, 1H, J2,3 9.8 Hz, J3,4 9.5 Hz, H-3), 5.10 (dd, 1H, J4,5 10.1 Hz, H-4), 4.93 (dd, 1H, J1,2 8.0 Hz, H-2), 4.63 (dd, 1H, J5,6b 5.8 Hz, J6a,6b 10.8 Hz, H-6b), 4.56 (d, 1H, H-1), 4.49 (dd, 1H, J5,6a 2.1 Hz, H-6a), 4.10 (ddd, 1H, H-5), 3.91 (s, 3H, COOMe), 3.33 (s, 3H, OMe), 2.02, 2.00, 1.95 (3s, each 3H, 3 × COMe); 13C NMR (62.9 MHz, CD3CN): δ 170.9–170.4 (3 × COMe), 160.5 (COOMe), 140.5, 139.5, 137.2, 130.4 (4 × Ar-C), 130.0, 129.1, 128.3 (Ph-CH), 124.3–123.3 (CF3 + Ar-C), 120.1 (Ar-CH), 117.5 (Ar-C), 112.5, 105.4 (2 × Ar-CH), 102.1 (C-1), 78.4 (C-6), 73.5 (C-3), 72.5 (C-5), 71.9 (C-2), 69.2 (C-4), 57.3 (OMe), 52.9 (COOMe), 21.0–20.8 (3 × COMe). Anal. Calcd for C30H30F3NO11: C, 56.52; H, 4.74%; N, 2.20%; Found: C, 56.54; H, 4.76%; N, 2.24%.
3.7. Methyl 2,3,4-Tri-O-Acety-6-O-(2-Methoxycarbonyl)-6-Phenyl-4-Trifluoromethyl-1H-Indol-1-Yl)-β-d-Galactopyranoside (17)
Mitsunobu reaction applied to 15 [5] (120 mg, 0.38 mmol, 1.0 equiv) was performed with DMEAD (306 mg, 1.30 mmol, 3.5 equiv) in accordance with the general procedure. The reaction was stopped after 4 h and the crude product was purified by flash chromatography on silica gel (7:3 hexane-EtOAc) gave pure 17 (162 mg, 68%) as a yellow solid; Rf 0.55 (1:1 hexane-EtOAc); mp 169–172 °C (crom); [α]D + 28.5 (c 0.96 in CHCl3); 1H NMR (250.13 MHz, CD3CN) δ: 7.99 (m, 1H, Ar-H), 7.78 (m, 1H, Ar-H), 7.77–7.72 (m, 2H, Ar-H), 7.54–7.38 (m, 3H, Ar-H), 7.18 (qd, 1H, J 1.0 Hz, J 1.7 Hz, Ar-H), 5.46 (dd, 1H, J3,4 3.4 Hz, J4,5 1.1 Hz, H-4), 5.10 (dd, 1H, J2,3 10.5 Hz, H-3), 5.03 (dd, 1H, J1,2 7.8 Hz, H-2), 4.64 (dd, 1H, J5,6a 7.5 Hz, J6a,6b 9.9 Hz, H-6a), 4.62 (dd, 1H, J5,6b 3.1 Hz, H-6b), 4.51 (d, 1H, H-1), 4.35 (ddd, 1H, H-5), 3.91 (s, 3H, COOMe), 3.35 (s, 3H, OMe), 2.07, 2.01, 1.92 (3s, each 3H, 3 × COMe); 13C NMR (62.9 MHz, CD3CN) δ: 171.4, 170.9, 170.7 (3 × COMe), 160.5 (COOMe), 140.5, 139.6, 137.2 130.8 (4 × Ar-C), 130.0, 129.5, 128.3 (Ph-CH), 124.3–123.2 (CF3 + Ar-C), 120.0 (Ar-CH), 117.4 (Ar-C), 112.5, 105.4 (2 × Ar-CH), 102.5 (C-1), 78.9 (C-6), 71.7 (C-3), 71.6 (C-5), 69.7 (C-2), 69.1 (C-4), 57.5 (OMe), 52.9 (COOMe), 20.9, 20.8, 20.7 (3 × COMe). Anal. Calcd for C30H30F3NO11: C, 56.52; H, 4.74%; N, 2.20%; Found: C, 56.57; H, 4.75%; N, 2.25%.
3.8. 4-O-[2-O-Acetyl-3,4-O-Isopropylidene-6-O-(2-Methoxycarbonyl-6-Phenyl-4-Trifluoromethyl-1H-Indol-1-Yl)-β-d-Galactopyranosyl]-2,3:5,6-Di-O-Isopropylidene-Aldehydo-d-Glucose Dimethyl Acetal (21)
Mitsunobu reaction applied to 20 [7] (170 mg, 0.30 mmol, 1.0 equiv) was performed with DMEAD (330 mg, 1.40 mmol, 3.5 equiv) in accordance with the general procedure. The reaction was stopped after 3.5 h and the crude product was purified by flash chromatography on silica gel (7:3 hexane-EtOAc) gave pure 21 (200 mg, 75%) as a yellow solid foam; Rf 0.63 (1:1 hexane-EtOAc); mp 77–80 °C (crom); [α]D -14.1 (c 1.0 in CHCl3); 1H NMR (250.13 MHz, CD3CN) δ: 8.05 (m, 1H, Ar-H), 7.77 (m, 1H, Ar-H), 7.76–7.72 (m, 2H, Ar-H), 7.56–7.40 (m, 3H, Ar-H), 7.25 (qd, 1H, J 0.9 Hz, J 1.7 Hz, Ar-H), 4.92 (dd, 1H, J1′,2′ 8.3 Hz, J2′,3′ 7.6 Hz, H-2′), 4.79 (d, 1H, H-1′), 4.73 (dd, 1H, J5′,6′b 6.5 Hz, J6′a,6′b 10.1 Hz, H-6′b), 4.68 (dd, 1H, J5′,6′a 5.2 Hz, H-6′a), 4.39 (dd, 1H, J1,2 5.6 Hz, J2,3 6.7 Hz, H-2), 4.30 (d, 1H, H-1), 4.29–4.21 (m, 2H, H-4′, H-5′), 4.16 (dd, 1H, J3′,4′ 5.2 Hz, H-3′), 4.15–3.96 (m, 2H, H-3, H-5), 3.99 (dd, 1H, J3,4 1.6 Hz, J4,5 3.1 Hz, H-4), 3.96 (s, 3H, COOMe), 3.83 m, (2H, H-6a, H-6b), 3.29, 3.24 (2s, each 3H, 2 × OMe), 2.06 (s, 3H, COMe), 1.49, 1.38 (2s, each 3H, CMe2), 1.28 (s, 6H, CMe2), 1.27, 1.25 (2s, each 3H, CMe2); 13C NMR (62.9 MHz, CD3CN): δ 170.3 (COMe), 160.3 (COOMe), 140.6, 139.8, 137.6, 131.4 (4 × Ar-C), 129.8, 128.9, 128.4 (Ph-CH), 124.7–123.5 (CF3 + Ar-C), 120.2 (Ar-CH), 117.3 (Ar-C), 112.3 (Ar-CH), 111.1, 110.8, 108.5 (3 × CMe2), 106.1 (C-1), 105.6 (Ar-CH), 101.0 (C-1′), 78.7 (C-6′), 78.4 (C-5), 78.2 (C-3), 77.6 (C-3′), 76.7 (C-2), 76.0 (C-4), 74.7 (C-4′), 73.3 (C-2′), 70.5 (C-5′), 65.2 (C-6), 56.2, 54.5 (2 × OMe), 52.8 (COOMe), 28.1, 27.7, 26.8, 26.5, 26.3, 24.8 (3 × CMe2), 21.1 (COMe). Anal. Calcd for C42H52F3NO15: C, 58.13; H, 6.04%; N, 1.61%; Found: C, 58.16; H, 6.07%; N, 1.64%.
3.9. General Procedures for Deacetylation
To a solution of appropriate acetylated NHI-glycoconjugates 6α, 6β, 9β, 12, 16, or 17 (1.00 mmol, 1.0 equiv) in a 2:3 mixture of CH2Cl2-MeOH (46.0 mL) was cooled at 0 °C and treated with a methanolic solution of MeONa (0.33 M, 0.575 mL). The reaction mixture was stirred at room temp until TLC analysis showed the complete disappearance of the starting material (2–7 h) and the formation of a lower moving product. The solution was neutralized with Amberlite® IR120 H resin and the suspension was filtered and concentrated under diminished pressure. Purification of crude product by crystallization or trituration with Et2O afforded pure NHI-glycoconjugates 7α, 7β, 10β, 13, 18, or 19.
3.10. Methyl 1-(α-d-Galactopyranosyl)-6-Phenyl-4-Trifluoromethyl-1H-Indole-2-Carboxylate (7α)
Deacetylation of 6α (74 mg, 0.11 mmol, 1.0 equiv) was performed in accordance with the general procedure. The reaction was stirred until the starting compound was completely reacted (2 h, TLC, 9:1 EtOAc-MeOH). The crude residue (52 mg, 91% yield) was constituted (NMR) exclusively by a title compound 7α. The trituration of the crude product (52 mg) with Et2O afforded a sample of pure 7α (28 mg, 49%) as a yellow solid; Rf 0.54 (9:1 EtOAc-MeOH), mp 159–162 °C (Et2O); [α]D -15.3 (c 0.63 in MeOH); 1H NMR (250.12 MHz, CD3OD-CDCl3) δ: 8.41 (m, 1H, Ar-H), 7.86–7.62 (m, 3H, Ar-H), 7.48–7.33 (m, 3H, Ar-H), 7.27 (bs, 1H, Ar-H), 5.37 (d, 1H, J1,2 3.9 Hz, H-1), 4.51 (m, 1H, H-5), 4.15 (m, 1H, H-4), 4.08 (dd, 1H, J2,3 10.6 Hz, H-2), 4.00 (dd, 1H, J3,4 2.8 Hz, H-3), 3.95 (s, 3H, COOMe), 3.91 (bs, 1H, OH), 3.80–3.65 (m, 4H, H-6a, H-6b, 2 × OH), 3.36 (bs, 1H, OH); 13C NMR (62.9 MHz, CD3OD-CDCl3) δ: 161.5 (CO), 140.6, 139.6, 137.5, 131.9 (4 × Ar-C), 129.7, 128.7, 128.0 (Ph-CH), 125.2–123.0 (CF3 + Ar-C), 119.8 (Ar-CH), 118.1 (Ar-C), 113.4 (Ar-CH), 109.4 (C-1), 106.4 (Ar-CH), 75.1 (C-5), 70.4 (C-2), 69.9 (C-3), 69.3 (C-4), 61.9 (C-6), 52.8 (OMe). Anal. Calcd for C23H22F3NO8: C, 55.54; H, 4.46%; N, 2.82%; Found: C, 55.57; H, 4.49%; N, 2.86%.
3.11. Methyl 1-(β-d-Galactopyranosyl)-6-Phenyl-4-Trifluoromethyl-1H-Indole-2-Carboxylate (7β)
Deacetylation of 6β (60 mg, 0.089 mmol, 1.0 equiv) was performed in accordance with the general procedure. The reaction was stirred until the starting compound was completely reacted (2 h, TLC, 9:1 EtOAc-MeOH). The crude residue (43 mg, 91% yield) was constituted (NMR) exclusively by a title compound 7β. The trituration of the crude product with Et2O afforded a sample of pure 7β (30 mg, 66%) as a white solid; Rf 0.83 (9:1 EtOAc-MeOH); mp 207–210 °C (Et2O); [α]D +39.4 (c 0.505 in MeOH); 1H NMR (250.13 MHz, CD3OD-CDCl3) δ: 8.24 (bs, 1H, Ar-H), 7.71 (m, 1H, Ar-H), 7.68–7.73 (2m, each 1H, Ar-H), 7.48–7.39 (m, 2H, Ar-H), 7.38–7.32 (m, 1H, Ar-H), 7.27 (qd, 1H, Ar-H), 5.09 (d, 1H, J1,2 8.1 Hz, H-1), 3.95 (m, 1H, H-2), 3.94 (s, 3H, COOMe), 3.81 (dd, 1H, J5,6b 6.9 Hz, J6a,6b 11.0 Hz, H-6b), 3.67 (dd, 1H, J5,6a 5.5 Hz, H-6a), 3.62 (dd, 1H, J2,3 9.4 Hz, J3,4 3.4 Hz, H-3), 3.60 (m, 1H, H-4), 3.49 (ddd, 1H, J4,5 0.8 Hz, H-5); 13C NMR (62.9 MHz, CD3OD-CDCl3) δ: 161.8 (CO), 140.5, 139.5, 137.4, 132.1 (4 × Ar-C), 129.6, 128.9, 128.4 (Ph-CH), 125.4–123.6 (CF3 + Ar-C), 119.7 (Ar-CH), 118.3 (Ar-C), 113.6 (Ar-CH), 110.3 (C-1), 106.8 (Ar-CH), 76.7 (C-5), 74.3 (C-3), 72.3 (C-2), 69.9 (C-4), 62.2 (C-6), 53.4 (COOMe). Anal. Calcd for C23H22F3NO8: C, 55.54; H, 4.46%; N, 2.82%; Found: C, 55.57; H, 4.49%; N, 2.85%.
3.12. Methyl 1-[4-O-(β-d-Galactopyranosyl)-β-d-Glucopyranosyl)]-6-Phenyl-4-Trifluoromethyl-1H-Indole-2-Carboxylate (10β)
Deacetylation of 9β (53 mg, 0.055 mmol, 1.0 equiv) was performed in accordance with the general procedure. The reaction was stirred until the starting compound was completely reacted (8 h, TLC, 9:1 EtOAc-MeOH). The crude residue (37 mg, 96% yield) was constituted (NMR) exclusively by a title compound 10β. The trituration of the crude product (37 mg) with Et2O afforded a sample of pure 10β (27 mg, 74%) as a white solid: Rf 0.10 (9:1 EtOAc-MeOH); mp 199–202 °C. [α]D +63.3 (c 0.59 in MeOH); 1H NMR (250.13 MHz, DMSO-d6) δ: 8.27 (m, 1H, Ar-H), 7.83–7.80 (m, 3H, Ar-H), 7.55–7.49 (m, 3H, Ar-H), 7.10 (bs, 1H, Ar-H), 5.78 (bd, 1H, J 5.0 Hz, OH), 5.14–5.01 (m, 2H, 2 × OH), 4.87–4.78 (m, 2H, 1-H, OH), 4.69–4.61 (m, 2H, 2 × OH), 4.56–4.43 (m, 2H, H-1′, OH), 4.31–4.16 (m, 3H, H-6a, H-6b, H-5′), 3.88 (s, 3H, COOMe), 3.46–3.22 (m, 9H, H-2, H-3, H-4, H-5, H-2′, H-3′, H-4′, H-6′a, H-6′b; 13C NMR (62.9 MHz, DMSO-d6) δ: 159.7 (CO), 139.0, 139.0, 137.0, 130.0 (4 × Ar-C), 129.2, 128.2, 127.3, 127.2 (Ph-CH), 124.0–121.8 (CF3 + Ar-C), 118.8 (Ar-CH), 116.7 (Ar-C), 113.5, 113.0 (2 × Ar-CH), 107.7 (C-1), 103.7 (C-1′), 79.4 (C-4), 75.6 (C-5′), 74.8, 74.3 (C-5, C-3), 73.3, 72.0 (C-2, C-3′), 70.5 (C-2′), 68.1 (C-4′), 60.4, 60.3 (C-6′, C-6), 52.4 (COOMe). Anal. Calcd for C29H32F3NO13: C, 52.81; H, 4.89%; N, 2.12%; Found: C, 52.84; H, 4.91%; N, 2.15%.
3.13. Methyl 6-O-(2-Methoxycarbonyl-6-Phenyl-4-Trifluoromethyl-1H-Indol-1-Yl)-α-d-Mannopyra-Noside (13)
Deacetylation of 12 (54 mg, 0.085 mmol, 1.0 equiv) was performed in accordance with the general procedure. The reaction was stirred until the starting compound was completely reacted (3.5 h, TLC, 9:1 EtOAc-MeOH). The crude residue (42 mg, 95% yield) was constituted (NMR) exclusively by a title compound 13. The crystallization (Et2O-hexane) of crude product (42 mg) afforded a sample of pure 13 (32 mg, 73%) as a pale yellow solid; Rf 0.69 (9:1 EtOAc-MeOH); mp 59–62 °C (Et2O-hexane); [α]D +21.6(c 0.92 in MeOH); 1H NMR (250.13 MHz, CD3OD-CDCl3) δ: 7.95 (bs, 1H, Ar-H), 7.67–7.61 (m, 3H, Ar-H), 7.49–7.32 (m, 3H, Ar-H), 7.16 (bs, 1H, Ar-H), 4.81 (bs, 1H, H-1), 4.64 (dd, 1H, J5,6b 3.8 Hz, J6a,6b 9.4 Hz, H-6b), 4.53 (dd, 1H, J5,6a 1.8 Hz, H-6a), 4.19 (dd, 1H, J3,4 9.6 Hz, J4,5 9.4 Hz, H-4), 4.02 (bs, 1H, H-2), 3.93–3.75 (m, 5H, H-3, H-5, 3 × OH), 3.89 (s, 3H, COOMe), 3.35 (s, 3H, OMe); 13C NMR (62.9 MHz, CD3OD-CDCl3) δ: 161.2 (CO), 140.6, 139.5, 136.8, 131.9 (4 × Ar-C), 129.7, 128.6, 128.1 (3 × Ph-CH), 124.8–122.7 (CF3 + Ar-C), 120.1 (Ar-CH), 117.2 (Ar-C), 112.0, 106.4 (2 × Ar-CH), 101.9 (C-1), 78.1 (C-6), 72.1 (C-3), 71.2 (C-2), 71.0 (C-5), 67.5 (C-4), 55.9 (OMe), 51.3 (COOMe). Anal. Calcd for C24H24F3NO8: C, 56.36; H, 4.73%; N, 2.74%; Found: C, 56.39; H, 4.75%; N, 2.77%.
3.14. Methyl 6-O-(2-Methoxycarbonyl-6-Phenyl-4-Trifluoromethyl-1H-Indol-1-Yl)-β-d-Glucopyra-Noside (18)
Deacetylation of 16 (52 mg, 0.081 mmol, 1.0 equiv) was performed in accordance with the general procedure. The reaction was stirred until the starting compound was completely reacted (3.5 h, TLC, 9:1 EtOAc-MeOH). The crude residue (40 mg, 96% yield) was constituted (NMR) exclusively by a title compound 18. The trituration of the crude product (40 mg) with Et2O afforded a sample pure 18 (18 mg, 43% yield) as a white solid; Rf 0.53 (9:1 EtOAc-MeOH); mp 211–214 °C (Et2O); [α]D -73.7 (c 0.66 in MeOH); 1H NMR (250.13 MHz, CD3OD) δ: 8.10 (m, 1H, Ar-H), 7.70–7.62 (m, 3H, Ar-H), 7.50–7.38 (m, 3H, Ar-H), 7.15 (qd, 1H, Ar-H), 4.79 (dd, 1H, J5,6b 1.6 Hz, J6a,6b 10.5 Hz, H-6b), 4.58 (dd, 1H, J5,6a 6.3 Hz, H-6a), 4.18 (d, 1H, J1,2 7.7 Hz, H-1), 3.95 (s, 3H, COOMe), 3.70 (ddd, 1H, J4,5 9.8 Hz, H-5), 3.41–3.30 (m, 2H, H-3, H-4), 3.36 (s, 3H, OMe), 3.24 (dd, 1H, J2,3 9.4 Hz, H-2); 13C NMR (62.9 MHz, CD3OD) δ: 161.2 (CO), 141.0, 140.1, 137.8, 131.8 (4 × Ar-C), 130.1, 129.1, 128.4 (Ph-CH), 124.9–123.2 (CF3 + Ar-C), 119.9 (Ar-CH), 117.8 (Ar-C), 112.7 (Ar-CH), 105.8 (C-1), 105.4 (Ar-CH), 80.1 (C-6), 77.8, 71.3 (C-3, C-4), 75.5 (C-5), 74.9 (C-2), 57.5 (OMe), 52.7 (COOMe). Anal. Calcd for C24H24F3NO8: C, 56.36; H, 4.73%; N, 2.74%; Found: C, 56.40; H, 4.76%; N, 2.78%.
3.15. Methyl 6-O-(2-Methoxycarbonyl-6-Phenyl-4-Trifluoromethyl-1H-Indol-1-Yl)-β-d-Galactopyra-Noside (19)
Deacetylation of 17 (68 mg, 0.11 mmol, 1.0 equiv) was performed in accordance with the general procedure. The reaction was stirred until the starting compound was completely reacted (3 h, TLC, 9:1 EtOAc-MeOH). The crude residue (52 mg, 96% yield) was constituted (NMR) exclusively by a title compound 19. The trituration of the crude product (52 mg) with Et2O afforded a sample pure 19 (41 mg, 76%) as a white solid; Rf 0.60 (9:1 EtOAc-MeOH); mp 191–194 °C (Et2O); [α]D –21.6 (c 0.70 in MeOH); 1H NMR (250.13 MHz, CD3OD-CDCl3) δ: 7.99 (m, 1H, Ar-H), 7.68–7.62 (m, 3H, Ar-H), 7.47–7.32 (m, 3H, Ar-H), 7.24 (m, 1H, Ar-H), 4.74 (dd, 1H, J5,6b 3.7 Hz, J6a,6b 9.5 Hz, H-6b), 4.59 (dd, 1H, J5,6a 7.2 Hz, H-6a), 4.15 (d, 1H, J1,2 7.6 Hz, H-1), 4.01 (m, 1H, H-5), 3.96 (m, 1H, H-3), 3.93 (s, 3H, COOMe), 3.53 (m, 2H, H-2, H-4), 3.44 (s, 3H, OMe); 13C NMR (62.9 MHz, CD3OD-CDCl3): δ 160.6 (CO), 140.3, 139.4, 139.3, 131.7 (4 × Ar-C), 129.2, 128.3, 127.7 (Ph-CH), 124.7–123.3 (CF3 + Ar-C), 119.6 (Ar-CH), 117.2 (Ar-C), 111.6, 105.8 (2 × Ar-CH), 104.5 (C-1), 79.0 (C-6), 73.5 (C-2), 72.5 (C-5), 71.3 (C-4), 69.5 (C-3), 57.2 (OMe), 52.4 (COOMe). Anal. Calcd for C24H24F3NO8: C, 56.36; H, 4.73%; N, 2.74%; Found: C, 56.38; H, 4.76%; N, 2.78%.
3.16. 4-O-[3,4-O-Isopropylidene-6-O-(2-Methoxycarbonyl-6-Phenyl-4-Trifluoromethyl-1H-Indol-1-Yl)-β-d-Galactopyranosyl]-2,3:5,6-Di-O-Isopropylidene-Aldehydo-d-Glucose Dimethyl Acetal (22)
Deacetylation of 21 (75 mg, 0.087 mmol, 1.0 equiv) was performed in accordance with the general procedure. The reaction was stirred until the starting compound was completely reacted (8 h, TLC, 1:1 hexane-EtOAc). Purification of the crude product by flash chromatography on silica gel (1:1 hexane-EtOAc) gave pure 22 (67 mg, 93%) as a white foam; Rf 0.43 (1:1 hexane-EtOAc); mp 70–73 °C (chrom); [α]D -23.2 (c 1.1 in CHCl3); 1H NMR (250.13 MHz, CD3CN) δ: 8.03 (m, 1H, Ar-H), 7.79 (bs, 1H, Ar-H), 7.76–7.71 (m, 2H, Ar-H), 7.54–7.39 (m, 3H, Ar-H), 7.23 (qd, 1H, J 1.0 Hz, J 1.7 Hz, Ar-H), 4.70 (dd, 1H, J5′,6′b 6.9 Hz, J6′a,6′b 9.4 Hz, H-6′b), 4.63 (dd, 1H, J5′,6′a 5.0 Hz, H-6′a), 4.51 (d, 1H, J1′,2′ 8.1 Hz, H-1′), 4.46 (dd, 1H, J1,2 5.9 Hz, J2,3 7.2 Hz, H-2), 4.30 (dd, 1H, J4′,5′ 2.2 Hz, H-5′), 4.29 (d, 1H, H-1), 4.23 (dd, 1H, J3′,4′ 5.5 Hz, H-4′), 4.15 (m, 1H, H-5), 4.14–4.00 (m, 3H, H-6a, H-6b, H-3′), 4.03 (dd, 1H, J3,4 1.6 Hz, H-3), 3.94 (s, 3H, COOMe), 3.88 (dd, 1H, J4,5 4.0 Hz, H-4), 3.55 (bs, 1H, 2-OH), 3.43 (m, 1H, H-2′), 3.27, 3.20 (2s, each 3H, 2 × OMe), 1.46, 1.37, 1.33, 1.29, 1.27, 1.26 (6s, each 3H, 3 × CMe2); 13C NMR (62.9 MHz, CD3CN) δ: 160.4 (CO), 140.7, 139.8, 137.5, 131.4 (4 × Ar-C), 130.0, 129.1, 128.6 (Ph-CH), 124.7–123.3 (CF3 + Ar-C), 120.3 (Ar-CH), 117.5 (Ar-C), 112.6 (Ar-CH), 110.7, 110.5, 109.0 (3 × CMe2), 106.2 (C-1), 105.5 (Ar-CH), 104.4 (C-1′), 80.0 (C-3), 78.6 (C-6′), 78.5 (C-3′), 78.3 (C-4), 77.8 (C-5), 74.5 (C-4′), 74.4 (C-2′), 72.6 (C-2), 71.0 (C-5′), 65.9 (C-6), 56.3, 54.2 (2 × OMe), 52.9 (COOMe), 28.4, 27.5, 27.0, 26.5, 26.3, 25.0 (3 × CMe2). Anal. Calcd for C40H50F3NO14: C, 58.18; H, 6.10%; N, 1.70%; Found: C, 58.21; H, 6.13%; N, 1.74%.
3.17. 4-O-[6-O-(2-Methoxycarbonyl-6-Phenyl-4-Trifluoromethyl-1H-Indol-1-Yl)-β-d-Galactopyra-Nosyl]-α,β-d-Glucopyranose (23)
A solution of 22 (59 mg, 0.072 mmol, 1.0 equiv) in 80% aq AcOH (1.3 mL) was stirred at 80 °C. After 4 h, the TLC analysis (9:1 EtOAc-MeOH) showed the complete disappearance of the starting material and the formation of a lower moving product (Rf 0.10). The reaction mixture was concentrated under diminished pressure and repeatedly coevaporated with toluene (4 × 20 mL). Trituration of the residue (45 mg) with Et2O afforded an amorphous white solid (23 mg, 48% yield) composed (13C NMR, DMSO-d6) by a α-anomer 23 (>95%). 1H NMR (250.13 MHz, DMSO-d6) δ: 8.15 (m, 1H, Ar-H), 7.92–7.83 (m, 3H, Ar-H), 7.57–7.41 (m, 3H, Ar-H), 7.15 (m, 1H, Ar-H), 6.40 (d, 1H, J1,OH 4.6 Hz, OH-1), 5.23 (d, 1H, J 7.2 Hz, OH), 4.92 (m, 2H, 2 × OH), 4.83 (d, 1H, J1,2 5.1 Hz, H-1), 4.72–4.67 (m, 2H, 2 × OH), 4.58–4.47 (m, 3H, H-6′a, H-6′b, OH), 4.34 (d, 1H, J1′,2′ 7.2 Hz, H-1′), 4.12 (bt, 1H, H-5′), 3.91 (s, 3H, COOMe), 3.78–3.48 (m, 5H, H-2, H-3, H-6a, H-6b, H-3′), 3.40–3.20 (m, 4H, H-4, H-5, H-4′, H-2′); 13C NMR (62.9 MHz, DMSO-d6) δ: 159.0 (CO), 138.8, 138.2, 135.8, 129.2 (4 × Ar-C), 129.3, 128.1, 127.6 (Ph-CH), 123.5–122.3 (CF3 + Ar-C), 118.8 (Ar-CH), 115.9 (Ar-C), 111.8, 104.1 (2 × Ar-CH), 104.0 (C-1′), 92.1 (C-1), 82.2 (C-4), 79.4 (C-6′), 72.7 (C-2′), 72.3 (C-5′), 72.0 (C-5), 71.7 (C-3), 70.3 (C-4′), 69.8 (C-2), 68.9 (C-3′), 60.7 (C-6), 52.4 (COOMe). Anal. Calcd for C29H32F3NO13: C, 52.81; H, 4.89%; N, 2.12%; Found: C, 52.84; H, 4.92%; N, 2.15%.
3.18. Enzymatic Assays
The LDH inhibition activities of the compounds were evaluated against purified human lactate dehydrogenase isoform 5 (Lee Biosolution, Inc.). The ‘forward’ direction (pyruvate→lactate) of the lactate dehydrogenase reaction was conducted, and the IC50 measurements were performed by fluorescence (emission wavelength at 460 nm, excitation wavelength at 340 nm) to monitor the amount of consumed NADH. Assays were carried out in wells containing 200 μL of a reagents solution, dissolved in 100 mM phosphate buffer (pH = 7.4), in the presence of 200 μM pyruvate and 40 μM NADH. For the IC50 calculations of the compounds, seven different concentrations (in duplicate for each concentration) were used to produce the concentration–response curve. As well as the sample test wells, maximum and minimum controls were also included in each plate. After 15 min of incubation, the final measurements were carried out by using a Victor X3 Microplates Reader (PerkinElmer®). IC50 values were produced using GraphPad Prism software (GraphPad, San Diego, CA, USA).
3.19. Molecular Modeling Studies
The seven compounds were built using Maestro 9.0 and were minimized using the conjugate gradient method until a convergence value of 0.05 kcal (mol Å)−1 was reached. The minimization was carried out in a water environment model (generalized-Born/surface-area model) using the MMFF force field and a distance-dependent dielectric constant of 1.0. The hLDH5 chain was extracted from the minimized average structure previously obtained by us [14]. AUTODOCK 4.2 software [32] was employed for molecular docking. The identification of the torsion angles in the ligands, the addition of the solvent model and the determination of protein and ligand atomic charges was carried out using Autodock Tools. Kollmann charges were assigned to the protein and Gasteiger charges to the ligand. A grid spacing of 0.375 Å and a distance-dependent function of the dielectric constant were used for the energetic map calculations. The compounds were subjected to a robust docking procedure by applying 200 runs of Autodock search, using the Lamarckian Genetic Algorithm with 10,000,000 steps of energy evaluations [33]. The number of individuals in the initial population was set to 500 and a maximum of 10,000,000 generations were simulated during each docking run.
3.20. Cancer Cell Antiproliferative Potency Assays
Determination of cellular IC50 values was performed under normoxic conditions as described previously [10]. Briefly, HeLa and A549 cells, grown in RPMI 1640 medium supplemented with 10% FBS and 1% Penicillin/Streptomycin, were added at a density of 5000 cells per well to 96 well plates to which 31.6 nM–200 µM compound in DMSO was already added (1% final concentration DMSO in all wells; triplicate wells at the same concentration per repetition). Plates were incubated at 37 °C in a 95% air/5% CO2 atmosphere for 72 h. Medium was removed and cells were fixed by the addition of 50 µL 10% trichloroacetic acid in water, 4 °C, to each well. Plates were incubated at 4 °C for at least one hour, and the sulforhodamine B colorimetric assay was performed to assess remaining biomass in each well. Cells treated with 1% DMSO were used as the 100% live control for biomass, and wells incubated with medium alone were used as the baseline zero biomass control. IC50 values were calculated using SoftMax Pro software (Molecular Devices, Sunnyvale, CA).
3.21. Cellular Lactate Production Inhibition Assays
This assay was performed under normoxic conditions as previously described [10]. Briefly, confluent HeLa cervical carcinoma cells (ATCC, Manassas, VA, USA) in a 96-well plate were treated with compound or vehicle control (1% DMSO final concentration in all samples) in DMEM minus phenol red + 10% dialyzed FBS + 1% Penstrep, supplemented with 10 mM glucose, 1 mM sodium pyruvate and 4 mM glutamine, in a final volume of 125 µL per well. Immediately following compound addition, plates were incubated for 8 h at 37 °C in a 95% air/5% CO2 atmosphere. Duplicate wells were prepared for each treatment. Following treatment, medium was collected, and 100 µL were added to 2 µL 50 mM chlorophenylalanine (CPA; internal standard for GC-MS analysis). Samples were concentrated, derivatized by a four-hour incubation with MTBSTFA + 1% TBDMCS (Thermo Scientific, Walthman, MA, USA) in acetonitrile at 85 °C, and immediately analyzed using GC-MS (Agilent 6890N GC/5973 MS, equipped with an Agilent DB-5 capillary column, 30 M × 320 µM × 0.25 µM, model number J&W 123–5032, Agilent Technologies, Santa Clara, CA, USA) and an electron impact ionization source. One microliter of each sample was injected using an automated injector, and a solvent delay of 8.20 min was implemented. The initial oven temperature was 120 °C, held for 5 min; then the temperature was increased at a rate of 10 °C min−1 until a temperature of 250 °C was reached. Temperature was then increased by 40 °C min−1 until a final temperature of 310 °C was reached. Total run time per sample was 22.5 min. Compounds were identified using AMDIS Chromatogram software (Amdis, freeware available from amdis.net) and programmed WIST and Niley commercial libraries. The integration area of lactate in each sample was divided by the integration area of CPA in the same sample to achieve a lactate/internal standard ratio. The ratios were averaged for duplicates, and percent lactate production over the vehicle was calculated for each independent experiment. The mean lactate production/vehicle was then averaged between three or more independent experiments.
4. Conclusions
Seven NHI glycoconjugates, which differ for the kind of sugar and for the conjugation branching point, were prepared and studied as potential LDH inhibitors. The chemical strategy was aimed at successfully accessing a wide structural variation while keeping the synthetic plan simple. Therefore, C-6 and C-1 were selected as the two anchoring options, as it is quite straightforward to functionalize these positions in a large variety of carbohydrates. For the C-6 glycoconjugation, the Mistunobu protocol was employed, while classical trichloroacetimidate precursors were employed in the C-1 glycosylation reactions. For the galactose donor, it was possible to switch the glycosylation stereochemical outcome by changing the nature of the solvent used. Indeed, despite the presence of an acetate participating group at C-2, the alpha anomer was obtained with high stereoselectivity by exploiting the nitrile solvent effect. After cleavage of the protecting groups, the biological results of the resulting glycoconjugates revealed that only those linked at the anomeric carbon inhibited hLDH5, whereas conjugates at C(6) were completely devoid of any enzyme inhibitory activity. Among the anomerically-linked conjugates, monosaccharide derivatives 7α and 7β displayed potent antiproliferative activities against lung and cervical cancer cells, which are comparable to those observed with reference gluco-conjugate 2, whereas disaccharide-linked 10β proved to be much less potent in the same assays. Finally, the configuration of the anomeric carbon atom seems to strongly affect the ability of these compounds to reduce the cellular production of lactate. In fact, an α-anomeric configuration (7α) proved to be positively correlated to a significant and dose-dependent inhibition of lactate production in HeLa cancer cells, when compared to its β- configured anomer (7β). Overall, these results shed further light about the structural motifs of glycoconjugates that can be further developed with the aim of exploiting the Warburg effect and selectively counteract the peculiar metabolism displayed by cancer cells. The studies reported here, therefore, have the potential to help develop therapeutic agents characterized by fewer side effects.
Acknowledgments
The authors would like to thank Prof. Paul J. Hergenrother and his group (University of Illinois at Urbana-Champaing–USA), for their support with the execution of the cell proliferation and lactate production assays.
Supplementary Materials
The following are available online at https://www.mdpi.com/1420-3049/24/19/3520/s1, NMR spectra of prepared compounds and binding disposition of 7β, 10β, 13, 18, 19, 23 into hLDH5.
Author Contributions
F.D.A., V.D.B. and F.M. conceived the study. C.G., L.G., G.V., T.T., G.P. and D.I. performed the experiments. F.M., F.D.A. and V.D.B. wrote the main text. All authors reviewed the manuscript.
Funding
This work was supported in part by MIUR-Italy (PRIN 2015, prot. 2015RNWJAM_003) and the University of Pisa (grant number PRA_2018_18).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.DeBerardinis R.J., Chandel N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016;2:e1600200. doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 3.Granchi C., Roy S., Del Fiandra C., Tuccinardi T., Lanza M., Betti L., Giannaccini G., Lucacchini A., Martinelli A., Macchia M., et al. Triazole-substituted N-hydroxyindol-2-carboxylates as inhibitors of isoform 5 of human lactate dehydrogenase (hLDH5) MedChemComm. 2011;2:638–643. doi: 10.1039/c1md00071c. [DOI] [PubMed] [Google Scholar]
- 4.Granchi C., Fancelli D., Minutolo F. An update on the therapeutic opportunities offered by cancer glycolytic metabolism. Bioorg. Med. Chem. Lett. 2014;24:4915–4925. doi: 10.1016/j.bmcl.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 5.Granchi C., Fortunato S., Minutolo F. Anticancer agents interacting with membrane glucose transporter. MedChemComm. 2016;7:1716–1729. doi: 10.1039/C6MD00287K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvaresi E.C., Hergenrother P.J. Glucose conjugation for the specific targeting and treatment of cancer. Chem. Sci. 2013;4:2319–2333. doi: 10.1039/c3sc22205e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granchi C., Bertini S., Macchia M., Minutolo F. Inhibitors of Lactate Dehydrogenase Isoforms and their Therapeutic Potentials. Curr. Med. Chem. 2010;17:672–697. doi: 10.2174/092986710790416263. [DOI] [PubMed] [Google Scholar]
- 8.Granchi C., Paterni I., Rani R., Minutolo F. Small-molecules inhibitors of human LDH5. Future Med. Chem. 2013;5:1967–1991. doi: 10.4155/fmc.13.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvaresi E.C., Granchi C., Tuccinardi T., Di Bussolo V., Huigens R.W., Lee H.Y., Palchaudhuri R., Macchia M., Martinelli A., Minutolo F., et al. Dual targeting of the warburg effect with a glucose-conjugated lactate dehydrogenase inhibitor. ChemBioChem. 2013;14:2263–2267. doi: 10.1002/cbic.201300562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Bussolo V., Calvaresi E.C., Granchi C., Del Bino L., Frau I., Dasso Lang M.C., Tuccinardi T., Macchia M., Martinelli A., Hergenrother P.J., et al. Synthesis and biological evaluation of non-glucose glycoconjugated N-hydroyxindole class LDH inhibitors as anticancer agents. RSC Adv. 2015;5:19944–19954. doi: 10.1039/C5RA00946D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng H., Cao X., Xian M., Fang L., Cai T.B., Ji J.J., Tunac J.B., Sun D., Wang P.G. Synthesis and enzyme-specific activation of carbohydrate-geldanamycin conjugates with potent anticancer activity. J. Med. Chem. 2005;48:645–652. doi: 10.1021/jm049693a. [DOI] [PubMed] [Google Scholar]
- 12.Fais M., Karamanska R., Allman S., Fairhurst S.A., Innocenti P., Fairbanks A.J., Donohoe T.J., Davis B.G., Russell D.A., Field R.A. Surface plasmon resonance imaging of glycoarrays identifies novel and unnatural carbohydrate-based ligands for potential ricin sensor development. Chem. Sci. 2011;2:1952–1959. doi: 10.1039/C1SC00120E. [DOI] [Google Scholar]
- 13.Koeman F.A.W., Meissner J.W.G., Van Ritter H.R.P., Kamerling J.P., Vliegenthart J.F.G. Synthesis of Structural Elements of the Capsular Polysaccharide of Streptococcus Pneumoniae Type 14. J. Carbohydr. Chem. 1994;13:1–25. doi: 10.1080/07328309408009174. [DOI] [Google Scholar]
- 14.Granchi C., Roy S., Giacomelli C., Macchia M., Tuccinardi T., Martinelli A., Lanza M., Betti L., Giannaccini G., Lucacchini A., et al. Discovery of N-Hydroxyindole-Based Inhibitors of Human Lactate Dehydrogenase Isoform A (LDH-A) as Starvation Agents against Cancer Cells. J. Med. Chem. 2011;54:1599–1612. doi: 10.1021/jm101007q. [DOI] [PubMed] [Google Scholar]
- 15.Demchenko A.V. Handbook of Chemical Glycosylation. Advances in Stereoselectivity and Therapeutic Relevance. Wiley-VCH; Weinheim, Germany: 2008. [Google Scholar]
- 16.Guazzelli L., McCabe O., Oscarson S. Synthesis of part structures of Cryptococcus neoformans serotype C capsular polysaccharide. Carbohydr. Res. 2016;433:5–13. doi: 10.1016/j.carres.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt R.R., Behrendt M., Toepfer A. Nitriles as Solvents in Glycosylation Reactions: Highly Selective β-Glycoside Synthesis. Synlett. 1990;11:694–696. doi: 10.1055/s-1990-21214. [DOI] [Google Scholar]
- 18.Swamy K.C.K., Kumar N.N.B., Balaraman E., Kumar K.V.P.P. Mitsunobu and Related Reactions: Advances and Applications. Chem. Rev. 2009;109:2551–2651. doi: 10.1021/cr800278z. [DOI] [PubMed] [Google Scholar]
- 19.Horrobin T., Tran C.H., Crout D. Esterase-catalysed regioselective 6-deacylation of hexopyranose per-acetates, acid-catalysed rearrangement to the 4-deprotected products and conversions of these into hexose 4- and 6-sulfates. J. Chem. Soc. Perkin Trans. 1. 1998:1069–1080. doi: 10.1039/a708596f. [DOI] [Google Scholar]
- 20.Terreni M., Salvetti R., Linati L., Fernandez-Lafuente R., Fernández-Lorente G., Bastida A., Guisan J.M. Regioselective enzymatic hydrolysis of acetylated pyranoses and pyranosides using immobilised lipases. An easy chemoenzymatic synthesis of alpha- and beta-d-glucopyranose acetates bearing a free secondary C-4 hydroxyl group. Carbohydr. Res. 2002;337:1615–1621. doi: 10.1016/S0008-6215(02)00113-1. [DOI] [PubMed] [Google Scholar]
- 21.Agnihotri G., Misra A.K. Synthesis of a di- and a trisaccharide related to the O-antigen of Escherichia coli O83:K24:H31. Carbohydr. Res. 2006;341:2420–2425. doi: 10.1016/j.carres.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Guazzelli L., Catelani G., D’Andrea F., Giannarelli A. Stereoselective entry into the d-GalNAc series starting from the d-Gal one: A new access to N-acetyl-d-galactosamine and derivatives thereof. Carbohydr. Res. 2009;344:298–303. doi: 10.1016/j.carres.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Guazzelli L., Catelani G., D’Andrea F. Lactose as an inexpensive starting material for the preparation of aldohexos-5-uloses: Synthesis of l-ribo and d-lyxo derivatives. Carbohydr. Res. 2010;345:369–376. doi: 10.1016/j.carres.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 24.Bianchini R., Rolla M., Isaad J., Catelani G., Guazzelli L., Corsi M., Bonanni M. Efficient double glycoconjugation to naturalize high molecular weight disperse dye. Carbohydr. Res. 2012;356:104–109. doi: 10.1016/j.carres.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 25.Pistarà V., Corsaro A., Rescifina A., Catelani G., D’Andrea F., Guazzelli L. Prevalence of Oxetanose Forms in the Tautomeric Equilibrium of β-Hydroxy-1,5-dicarbonyl Monosaccharides. J. Org. Chem. 2013;78:9444–9449. doi: 10.1021/jo400953s. [DOI] [PubMed] [Google Scholar]
- 26.Barili P.L., Catelani G., D’Andrea F., Mastrorilli E. Efficient Differentiation of the Hydroxyl Groups of 3,4-O-Isopropylidene-d-Galactopyranosides by Lipase Catalyzed Esterification and De –Esterification. J. Carbohydr. Chem. 1997;16:1001–1010. doi: 10.1080/07328309708005733. [DOI] [Google Scholar]
- 27.De Leo M., Peruzzi L., Granchi C., Tuccinardi T., Minutolo F., De Tommasi N., Braca A. Constituents of Polygala flavescens ssp. flavescens and Their Activity as Inhibitors of Human Lactate Dehydrogenase. J. Nat. Prod. 2017;80:2077–2087. doi: 10.1021/acs.jnatprod.7b00295. [DOI] [PubMed] [Google Scholar]
- 28.Bader A., Tuccinardi T., Granchi C., Martinelli A., Macchia M., Minutolo F., De Tommasi N., Braca A. Phenylpropanoids and flavonoids from Phlomis kurdica as inhibitors of human lactate dehydrogenase. Phytochemistry. 2015;116:262–268. doi: 10.1016/j.phytochem.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manerba M., Vettraino M., Fiume L., Di Stefano G., Sartini A., Giacomini E., Buonfiglio R., Roberti M., Recanatini M. Galloflavin (CAS 568-80-9): A novel inhibitor of lactate dehydrogenase. ChemMedChem. 2012;7:311–317. doi: 10.1002/cmdc.201100471. [DOI] [PubMed] [Google Scholar]
- 30.Poli G., Gelain A., Porta F., Asai A., Martinelli A., Tuccinardi T. Identification of a new STAT3 dimerization inhibitor through a pharmacophore-based virtual screening approach. J. Enzyme Inhib. Med. Chem. 2016;31:1011–1017. doi: 10.3109/14756366.2015.1079184. [DOI] [PubMed] [Google Scholar]
- 31.Dal Piaz F., Vera Saltos M.B., Franceschelli S., Forte G., Marzocco S., Tuccinardi T., Poli G., Nejad Ebrahimi S., Hamburger M., De Tommasi N., et al. Drug Affinity Responsive Target Stability (DARTS) Identifies Laurifolioside as a New Clathrin Heavy Chain Modulator. J. Nat. Prod. 2016;79:2681–2692. doi: 10.1021/acs.jnatprod.6b00627. [DOI] [PubMed] [Google Scholar]
- 32.Morris G.M., Ruth H., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granchi C., Lapillo M., Glasmacher S., Bononi G., Licari C., Poli G., El Boustani M., Caligiuri I., Rizzolio F., Gertsch J., et al. Optimization of a Benzoylpiperidine Class Identifies a Highly Potent and Selective Reversible Monoacylglycerol Lipase (MAGL) Inhibitor. J. Med. Chem. 2019;62:1932–1958. doi: 10.1021/acs.jmedchem.8b01483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.