Abstract
Background
Isoform-specific histone deacetylase inhibitors (HDACIs) MC1568 and ACY1083 are comparable to the non-selective HDACI valproic acid (VPA) in improving survival in rodents undergoing lethal hemorrhage. However, the organ-specific properties of isoform-specific HDACIs have not been fully evaluated. Also, whether they can act synergistically is not known. We hypothesized that isoform-specific HDACIs are superior to VPA in attenuating intestinal injury and act synergistically when coadministered.
Methods
Sprague Dawley rats were hemorrhaged (40% of total blood volume) and randomized to receive (n=4 per group) (1) MC1568 (5 mg/kg), (2) ACY1083 (30 mg/kg), (3) MC1568+ACY1083 (combination: 5 mg/kg + 30 mg/kg, respectively), (4) VPA (250 mg/kg), or (5) normal saline (NS; vehicle; 250 μL). Animals were observed for 3 hours, after which blood samples were collected and samples of the ileum were harvested. Expression of interleukin 1 beta (IL-1β), tumor necrosis factor alpha (TNF-α), and cytokine-induced neutrophil chemoattractant 1 (CINC-1) was assessed in the tissues using enzyme-linked immunosorbent assay. Intestinal cleaved caspase 3 (c-caspase 3) levels were assessed as a marker of apoptosis, and histologic sections of the ileum were examined for signs of bowel injury. Levels of IL-1β and TNF-α were also measured in the serum as global markers of inflammation.
Results
Treatments with MC1568, ACY1083, MC1568+ACY1083, and VPA were associated with decreased IL-1β levels in the intestine and serum compared with NS. IL-1β and TNF-α levels were significantly lower in the ACY1083 group compared with the VPA group. CINC-1 levels were significantly lower in the isoform-specific HDACI groups compared with the NS; however, no significant differences were seen with VPA. All treatment groups had a lower expression of intestinal c-caspase 3 compared with NS. Furthermore, MC1568 and ACY1083 groups had lower apoptosis compared with the VPA group. Bowel injury scores were significantly lower in the isoform-specific HDACI groups compared with the NS group; however, the attenuation in the VPA-treated animals did not reach statistical significance.
Discussion
Isoform-specific HDACIs provide superior intestinal protection compared with VPA in a rodent model of hemorrhagic shock.
Level of evidence
Preclinical study.
Keywords: hemorrhagic shock, histone deacetylase inhibitors, intestine, inflammation
Background
Hemorrhage is a leading cause of preventable deaths, and it is responsible for 1.5 million trauma-related fatalities worldwide annually.1 2 Among those who survive the initial hemorrhage, long-term outcomes remain poor.2 3 During hemorrhagic shock (HS), global hypoperfusion and subsequent organ ischemia can provoke systemic inflammatory responses that can worsen clinical outcomes.4 5 Intestinal inflammation, in particular, may act as the driver of systemic inflammatory response leading to multiorgan failure in HS.6 7 A potential way to reduce the long-term damage from hemorrhage could be to control the initial intestinal inflammation and injury.8 9
Recently, post-translational modifications of both histone and non-histone proteins have emerged as a potential treatment in trauma. The acetylation and deacetylation of histones are regulated by two classes of enzymes, histone acetyltransferases and histone deacetylases (HDACs).10 HDACs remove acetyl groups from histones, encouraging tighter association of the histones with DNA and overall chromatin condensation. By preventing this, HDAC inhibitors (HDACIs) can promote gene transcription, resulting in production of proteins that are protective in trauma.11 Acetylation also alters the function of numerous cytoplasmic proteins to create a “pro-survival phenotype”.12 There are 18 isoform subtypes of HDACs that can be subgrouped into four classes: class I (HDAC 1, 2, 3, 8), class IIa (HDAC 4, 5, 7, 9) and class IIb (HDAC 6, 10), and class III (SIRT 1–7) and class IV (HDAC 11).13 Valproic acid (VPA), a non-selective HDACI that inhibits class I and IIa HDACs, has been rigorously tested in animal models of HS and injuries.14–16 Due to its non-selective nature, however, the dose of VPA required to achieve these beneficial effects is high (150–400 mg/kg). Furthermore, the non-selective inhibition may have adverse effects that can limit its clinical utility.17
Recently, our group has tested various isoform-specific HDACIs in a rodent model of lethal HS. We found that MC1568 (a class IIa inhibitor) and ACY1083 (a class IIb inhibitor) were as effective as VPA in improving survival (survival: MC1568 vs. ACY1083 vs. VPA, 75% vs. 75% vs. 87.5%; p>0.05).18 However, their effectiveness in attenuating organ injury has not been compared. Furthermore, whether isoform-specific HDACIs act synergistically when administered together has not been established.
In this study, we sought to evaluate the efficacy of isoform-specific HDACIs and the non-selective HDACI (VPA) in attenuating intestinal inflammation and injury. We hypothesized that isoform-specific HDACIs would provide superior intestinal protection compared with VPA in a rodent model of HS. We also hypothesized that isoform-specific HDACIs would act synergistically when administered in combination.
Materials and methods
Animal selection and acclimation
This study was designed in accordance with the Guide for the Care and Use of Laboratory Animals. The experiments conducted followed relevant regulations and guidelines regarding animal welfare. Male Sprague Dawley rats (240–300 g) were purchased from Charles River Laboratories (Chicago, IL). Animals underwent an acclimation period of 5 days, during which they were provided food and water ad libitum.
Anesthesia and instrumentation
Anesthesia was induced using 3% to 5% inhaled isoflurane mixed with air. A nose cone was placed for maintenance of anesthesia at 0.8% to 2.5% isoflurane, delivered through a veterinary multichannel anesthesia system and vaporizer (Kent Scientific, Torrington, CT).
A subcutaneous injection of bupivacaine (1 mg/kg of 1% solution) was administered for analgesia, and a vertical incision was made in the right groin to expose the femoral vessels. The right femoral artery and vein were both cannulated with polyethylene-50 catheters. The arterial line was used for blood withdrawal and hemodynamic monitoring (Ponemah Physiology Platform, Gould Instrument Systems, Valley View, OH), and the venous line was used for drug administration.
HS model
The model was designed to be sublethal to ensure survival until the end of the study to eliminate survival bias in tissue analyses. Total blood volume (TBV) was calculated according to the following formula: TBV (mL)=mass (g) × 0.06 (mL/g) + 0.77.19 Rats were subjected to 40% TBV hemorrhage over 20 minutes. After hemorrhage, animals were left in unresuscitated shock for 20 minutes. Arterial blood gas samples were collected at baseline and postshock timepoints; sample volumes were subtracted from the total hemorrhage volume.
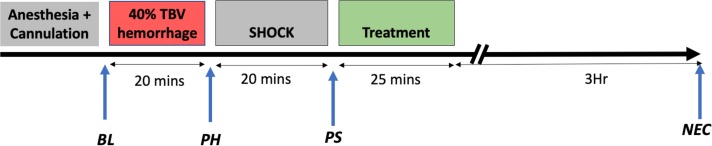
After shock, the animals were randomized to the following groups (n=4 per group): (1) sham (anesthetized and cannulated without hemorrhage or treatment), (2) vehicle (250 μL of 0.9% normal saline (NS)), (3) MC1568 (5 mg/kg in DMSO; MedChemExpress, NJ), (4) ACY1083 (30 mg/kg in cyclodextrin; Celgene, Summit, NJ), (5) MC1568+ACY1083 (combination group; 5 mg/kg in DMSO and 30 mg/kg in cyclodextrin, respectively), and (6) VPA (250 mg/kg in 0.9% NS). Drug administration was started immediately, and the doses were selected based on the manufacturer’s recommendations as well as previous studies.18 20 Treatment was administered via the femoral venous catheter over 15 minutes. In the combination group, ACY1083 was given first, followed immediately by MC1568. After drug administration, 200 µL of 0.9% NS was used to flush the catheter over 10 minutes. The total volume of drug and resuscitation was standardized between the groups. The catheters were then removed, vessels ligated, and skin closed with a running silk suture. Animals were then recovered from anesthesia and killed 3 hours post-treatment for tissue harvest (figure 1).
Figure 1.
Model of injuries. Animals were subjected to a 40% total blood volume hemorrhage, followed by a period of unresuscitated shock, and then treated with various histone deacetylase inhibitors. They were then observed for a period of 3 hours prior to euthanasia and sample collection. BL, baseline; NEC, necropsy; PH, posthemorrhage; PS, postshock; TBV, total blood volume.
Sample collection and tissue harvest
At the time of euthanasia, blood samples were collected by cardiac puncture, and sections of ileum 2 cm proximal to the ileocecal valve were harvested. Samples for biochemical analysis were rinsed with cold NS, flash-frozen in liquid nitrogen, and stored at −80°C. Samples for histologic analysis were fixed in 10% buffered formalin, embedded in paraffin, sliced into 5 µm sections, placed on slides, and stained with H&E.
Histopathologic analysis
A pathologist blinded to the group allocation of the samples quantified the intestinal histologic changes using the Chiu histopathologic injury score.21
Western blotting
Western blotting was performed as described previously.18 Tissue samples were manually homogenized with a glass homogenizer, and whole tissue extracts were prepared with radioimmunoprecipitation assay buffer (RIPA) whole cell lysis buffer supplemented with 1× phosphatase and protease inhibitors (Halt, Thermo Fisher Scientific, Waltham, MA). The supernatant was collected after centrifugation at 10 000 RPM at 4°C for 15 minutes. Prior to loading on a 12% polyacrylamide gel, whole cell extracts were balanced by spectrophotometry using the Bradford assay to ensure equal loading. Once separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, proteins were electrotransferred onto a nitrocellulose membrane. Membranes were blocked for 30 minutes using 5% bovine serum albumin (Roche, Indianapolis, IN) dissolved in Tris-buffered saline infused with 0.035% Tween 20 (TBST) and then incubated with the primary antibody diluted in TBST containing 5% bovine serum albumin at 4°C overnight. Rabbit anti-cleaved caspase 3 (c-caspase 3, 1:1000) from Cell Signaling Technology (Danvers, MA) was used as the primary antibody.
Enzyme-linked immunosorbent assay
Levels of tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), and cytokine-induced neutrophil chemoattractant 1 (CINC-1) in serum and intestine samples were quantified using TNF-α, IL-1β and CINC-1 enzyme-linked immunosorbent assay kits (R&D, Minneapolis, MN). Serum was prepared by allowing blood samples to clot for 1 hour at room temperature before centrifuging at 1500 revolutions per minute at 4°C for 10 minutes and collecting the supernatant. The intestinal tissue was homogenized as stated earlier. The concentrations of TNF-α, IL-1β, and CINC-1 were measured according to the manufacturer’s instructions.
Statistical analysis
Using pilot data comparing endpoints for the NS group and the best treatment group, effect size (d) was determined. Sample sizes (n) were calculated with 80% power and 95% confidence for each variable: injury score (d=2.3; n=4), c-caspase 3 (d=7.9; n=2), CINC-1 (d=3.1; n=3), IL-1β intestine (d=4.8; n=2), IL-1β serum (d=6.5; n=2), TNF-α intestine (d=8.8; n=2), and TNF-α serum (d=4.1; n=2). Statistical analyses were performed using GraphPad Prism V.7 (La Jolla, CA). Differences among the three groups were analyzed using a one-way analysis of variance test followed by Bonferroni post-hoc testing for multiple comparisons. All continuous variables are presented as mean±SEM. Statistical significance was defined as p<0.05.
Results
Survival and physiologic parameters
All animals survived until the end of the experiment. There were no significant differences in body weight, mean arterial pressure (MAP), and lactate levels among the groups at baseline. At the end of the shock phase, all animals had significantly increased lactate values and reduced MAP when compared with their own baseline measurements (table 1); however, there were no significant differences between the groups.
Table 1.
Select laboratory data
| Timepoint | |||
| Variable | Group | Baseline | End of shock |
| Weight (g) | Vehicle | 272.±11.4 | – |
| MC1568 | 276.3±7.4 | – | |
| ACY1083 | 279.5±10.0 | ||
| MC1568+ACY1083 | 270.5±11.7 | ||
| VPA | 278.0±8.3 | ||
| Cannulation time (min) | Vehicle | 13.3±1.7 | – |
| MC1568 | 15.0±1.6 | ||
| ACY1083 | 15.5±2.3 | – | |
| MC1568+ACY1083 | 18.5±1.0 | ||
| VPA | 13.5±1.5 | ||
| MAP (mm Hg) | Vehicle | 94.9±1.5 | 50.8±3.5* |
| MC1568 | 92.2±5.4 | 49.1±1.3* | |
| ACY1083 | 92.7±7.2 | 53.7±4.2* | |
| MC1568+ACY1083 | 93.3±4.4 | 53.8±2.3* | |
| VPA | 94.1±4.5 | 57.9±2.9* | |
| Lactate (mmol/L) | Vehicle | 1.4±0.1 | 4.3±1.0* |
| MC1568 | 1.5±0.2 | 4.9±0.6* | |
| ACY1083 | 1.3±0.1 | 4.7±0.5* | |
| MC1568+ACY1083 | 1.2±0.1 | 4.5±0.3* | |
| VPA | 1.3±0.2 | 4.3±0.6* |
*Significant difference compared with the baseline value of the same group (p<0.05).
MAP, mean arterial pressure; VPA, valproic acid.
Isoform-specific HDACIs are superior to VPA in suppressing the IL-1β levels
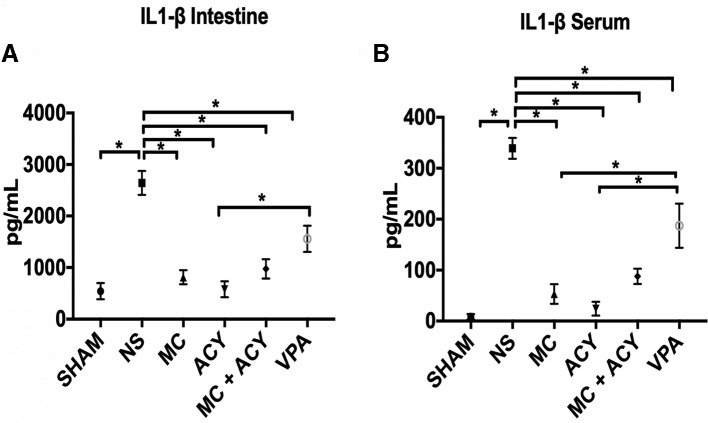
The levels of IL-1β in the intestine and serum were significantly elevated in the vehicle group as compared with sham, and treatment with all the HDACIs significantly decreased the IL-1β levels (figure 2A,B). MC1568 was superior to VPA in suppressing the IL-1β in the serum (IL-1β levels in the serum, pg/mL: MC1568, 53.3±19.3; VPA, 187.3±43.4; p=0.009), but not in the intestine (p=0.19). ACY1083 was superior to VPA in suppressing the IL-1β levels in both the intestine and serum (IL-1β levels in the intestine, pg/mL: ACY1083, 579.3±154.4; VPA, 1559±253; p=0.02; IL-1β levels in the serum, pg/mL: ACY1083, 24.3±13.5; VPA, 187.3±43.4; p=0.0012). No differences were observed between the MC1568 and MC1568+ACY1083 groups (intestine and serum, p=0.99), and the ACY1083 and MC1568+ACY1083 groups (intestine and serum, p=0.99).
Figure 2.
Effect of each HDACI treatment on HS-induced IL-1β levels in the (A) intestine and (B) serum. HS resulted in a significant increase in the levels of IL-1β in both the intestine and serum as compared with sham. In the intestine, treatment with each of MC (p<0.0001), ACY (p<0.0001), MC+ACY (p=0.0001), and VPA (p<0.05) significantly decreased the expression of IL-1β compared with the vehicle. Expression of IL-1β in the intestine was significantly lower in the animals treated with ACY compared with VPA (p<0.05). In the serum, treatment with MC (p<0.0001), ACY (p<0.0001), MC+ACY (p<0.0001), and VPA (p<0.01) significantly decreased the expression of IL-1β compared with the vehicle. Expression of IL-1β in the serum was significantly lower in each of the MC and ACY groups as compared with the VPA group (p<0.01). ACY, ACY1083; HDACI, histone deacetylase inhibitor; HS, hemorrhagic shock; IL-1β, interleukin 1 beta; MC, MC1568; NS, normal saline vehicle; VPA, valproic acid; * denotes significance between groups (p<0.05).
Isoform-specific HDACIs are superior to VPA in suppressing the TNF-α levels
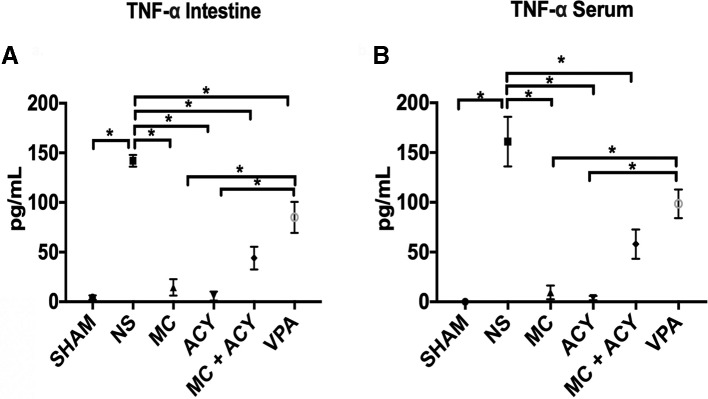
The levels of TNF-α in the intestine and serum were significantly elevated in the vehicle group compared with sham, and these levels were significantly decreased in the treatment groups (figure 3A,B). TNF-α expression was significantly lower in the intestine and serum after treatment with MC1568 and ACY1083 when compared with VPA (TNF-α levels in the intestine, pg/mL: MC1568, 14.5±8.2; VPA, 85.5±15.5; p=0.0005; TNF-α levels in the serum, pg/mL: MC1568, 9.5±6.8; VPA, 98.5±14.4; p=0.003; TNF-α levels in the intestine, pg/mL: ACY1083, 5.8±4.4; VPA, 85.5±15.5; p=0.002; TNF-α levels in the serum, pg/mL: ACY1083, 3.8±2.2; VPA, 98.5±14.4; p=0.0015). No significant differences were observed between the MC1568 and MC1568+ACY1083 groups (intestine: p=0.5; serum; p=0.7), and the ACY1083 and MC1568+ACY1083 groups (intestine; p=0.12; serum; p=0.2).
Figure 3.
Effect of each HDACI treatment on HS-induced TNF-α levels in the (A) intestine and (B) serum. HS resulted in a significant increase in the levels of TNF-α in both the intestine and serum as compared with sham. In the intestine, treatment with each of MC (p<0.0001), ACY (p<0.0001), MC+ACY (p<0.0001), and VPA (p<0.01) significantly decreased the expression of TNF-α compared with the vehicle. Expression of TNF-α in the intestine was significantly lower in the animals treated with MC and ACY as compared with VPA (p<0.001). In the serum, treatment with MC (p<0.0001), ACY (p<0.0001), and MC+ACY (p<0.001) significantly decreased the expression of TNF-α compared with the vehicle. The expression of TNF-α in the serum was significantly lower in the MC and ACY groups as compared with the VPA group (p<0.01). ACY, ACY1083; HDACI, histone deacetylase inhibitor; HS, hemorrhagic shock; MC, MC1568; NS, normal saline vehicle; TNF-α, tumor necrosis factor alpha; VPA, valproic acid; * denotes significance between groups (p<0.05).
Isoform-specific HDACIs suppress intestinal CINC-1 expression
The levels of CINC-1 in the intestine were significantly elevated in the vehicle group compared with the sham group, and MC1568, ACY1083, and MC1568+ACY1083 treatment significantly decreased the CINC-1 levels (figure 4). There was no difference in the CINC-1 levels between the MC1568 and VPA (p=0.99) groups, and in the ACY1083 and VPA (p=0.4) groups. No differences were observed in the CINC-1 levels between the MC1568 and MC1568+ACY1083 groups (p=0.99), and in the ACY1083 and MC1568+ACY1083 groups (p=0.99). CINC-1 levels were also tested in the serum, but at the 3-hour timepoint when the serum samples were collected the CINC-1 levels were elevated in all the groups without any intergroup differences.
Figure 4.

Effect of each HDACI treatment on HS-induced levels of CINC-1 in the intestine. HS significantly increased the expression of CINC-1 as compared with sham. Treatment with each of MC (p<0.01), ACY (p<0.001), and MC+ACY (p=0.01) significantly decreased intestinal CINC-1 expression compared with the vehicle. There was no significant difference in intestinal CINC-1 expression between the VPA and vehicle groups (p>0.05). ACY, ACY1083; CINC-1, cytokine-induced neutrophil chemoattractant 1; HDACI, histone deacetylase inhibitor; HS, hemorrhagic shock; MC, MC1568; NS, normal saline vehicle; VPA, valproic acid; * denotes significance between groups (p<0.05).
Isoform-specific HDACIs are superior to VPA in suppressing the intestinal c-caspase 3 expression
Western blotting was used to measure the levels of c-caspase 3 in the intestine as a marker of cellular apoptosis. The expression of c-caspase 3 was significantly higher in the vehicle group compared with sham (p<0.0001). Treatment with all the HDACIs significantly decreased the expression of c-caspase 3 (figure 5). Levels of c-caspase 3 in the rats treated with MC1568 and ACY1083 were lower than those treated with VPA (p=0.01 and p=0.001, respectively). No significant differences were observed between the MC1568 and MC1568+ACY1083 groups (p=0.09) and the ACY1083 and MC1568+ACY1083 groups (p=0.08).
Figure 5.

Effect of each HDACI treatment on HS-induced intestinal c-caspase 3 expression. HS significantly increased the expression of c-caspase 3 compared with sham. Treatment with each of MC (p<0.0001), ACY (p<0.0001), MC+ACY (p<0.0001), and VPA (p=0.0003) significantly decreased the levels of c-caspase 3 in the intestine compared with the vehicle. Animals in MC (p=0.01) and ACY (p=0.001) groups had significantly lower levels of c-caspase 3 compared with the VPA group. ACY, ACY1083; c-caspase 3, cleaved caspase 3; HDACI, histone deacetylase inhibitor; HS, hemorrhagic shock; MC, MC1568; NS, normal saline vehicle; VPA, valproic acid; * denotes significance between groups (p<0.05).
Isoform-specific HDACIs attenuate intestinal injury
Chiu histopathologic score was used to quantify the degree of intestinal injury (figure 6). As expected, we did not observe any evidence of intestinal injury in the sham rats, but significant injury was noted in the samples harvested from the vehicle group (injury score=3.8±0.9, p=0.01 compared with sham). Administration of MC1568, ACY1083, and MC1568+ACY1083 significantly attenuated the degree of intestinal injury (MC1568 vs. NS, injury score=0.5±0.3, p=0.04; ACY1083 vs. NS, injury score=0.25±0.25, p=0.02; MC1568+ACY1083 vs. NS, injury score=0.5±0.3, p=0.04). VPA administration decreased the severity of injury, but it failed to reach statistical significance (injury score=1.9±1.1, p=0.4). No differences were observed in the intestinal injury between the MC1568 and MC1568+ACY1083 groups (p=0.99), and ACY1083 and MC1568+ACY1083 groups (p=0.99).
Figure 6.
Effect of each HDACI treatment on histopathologic evidence of intestinal injury. Hemorrhagic shock resulted in significant blunting and shortening of the villi, capillary congestion, focal epithelial surface necrosis, and infiltration of inflammatory cells in the lamina propria. Treatment with each of the MC (p<0.05), ACY (p<0.05), and MC+ACY (p<0.05) groups attenuated these changes compared with the vehicle. No significant difference was seen between the VPA group and the vehicle group (p=0.4). ACY, ACY1083; HDACI, histone deacetylase inhibitor; MC, MC1568; NS, normal saline vehicle; VPA, valproic acid; * denotes significance between groups (p<0.05).
Discussion
In this study, we used a rodent model of sublethal HS to assess the effects of various HDACIs on intestinal inflammation and injury, and the efficacy of combination therapy. We found that isoform-specific HDACIs were more effective than VPA, a non-selective HDACI, in decreasing the proinflammatory cytokine levels and the activation of intestinal c-caspase 3. There was also a trend toward decreased expression of CINC-1 and attenuation of intestinal injury in animals treated with MC1568 (a conjugated pyrrole derivative, chemical formula: C17H15FN2O3) and ACY1083 (a 2-aminopyrimidine derivative, chemical formula: C₁₇H₁₈F₂N₄O₂) when compared with VPA treatment. We also found that the combination of MC1568 and ACY1083 did not confer any measurable synergistic benefits in terms of intestinal protection.
The small intestine consists of a diverse collection of inflammatory cells including resident macrophages, Paneth cells, and enterocytes. It is considered to be one of the primary sources of proinflammatory cytokines in the setting of HS as intestinal ischemia is associated with an overproduction of cytokines.22–24 During HS, there is inadequate perfusion of the bowel which leads to epithelial damage; cellular injury during hypoperfusion and subsequent reperfusion can release proinflammatory cytokines in the circulation and trigger multiorgan failure.25 After HS, we observed that rats in the vehicle group had significantly increased levels of proinflammatory cytokines IL-1β and TNF-α, and CINC-1 in the intestine and serum, which is consistent with previous studies.26–28
In this study, treatment with isoform-specific HDACIs provided better attenuation of proinflammatory cytokines compared with the non-selective HDACI. We found that the levels of IL-1β were lower in the VPA-treated animals compared with the vehicle group. However, ACY1083-treated animals had significantly lower intestinal and serum IL-1β levels compared with the VPA group, whereas MC1568-treated animals had only significantly lower serum IL-1β levels. Similarly, MC1568-treated and ACY1083-treated animals had significantly lower intestinal and serum TNF-α levels compared with the VPA-treated animals. TNF-α levels have been associated with vascular hyporeactivity to vasoconstrictor stimuli and lower blood pressure after HS.29 It is possible that the lower levels of TNF-α after isoform-specific HDACI treatment may have contributed to improved hemodynamics and thus lesser intestinal injury compared with the VPA group. However, we were not able to evaluate this as the arterial catheters were removed after treatment, which prevented us from monitoring the blood pressure in these animals. Further studies are ongoing to evaluate this possibility.
Excessive neutrophil migration and activation is a major cause of multiorgan dysfunction after trauma-induced systemic inflammation.30 CINC-1, a neutrophil-specific chemoattractant,31 is an early marker of inflammation.28 In this study, we found that treatment with VPA failed to decrease the intestinal CINC-1 expression at the 3-hour timepoint, but treatment with isoform-specific HDACIs significantly decreased the CINC-1 levels compared with the vehicle group. This suggests that MC1568 and ACY1083 may attenuate intestinal inflammation by decreasing the neutrophil migration.
Prevention of intestinal cell apoptosis could also be protective after HS. HS has been shown to cause apoptosis of intestinal cells through caspase activation.32 Caspases are cysteine proteases that are inactive in healthy cells but undergo autolytic cleavage (and activation) after HS. c-Caspase 3 is a protease that belongs to the caspase family, and is involved in mediating apoptosis by mitochondrial cytochrome-c release, caspase-9 activation, chromatin condensation, and DNA fragmentation.33 In this study, we observed that VPA-treated animals had lower expression of c-caspase 3 compared with the vehicle; however, MC1568-treated and ACY1083-treated animals had even lower levels of c-caspase 3 compared with the VPA group.
HS can cause intestinal injury within 1 hour,34 and this was the reason for focusing our attention on the early postshock period. We removed 40% TBV from the rats over a period of 20 minutes. This insult caused significant decrease in the postshock MAPs from the baseline values, and a consequential increase in lactate levels was noted (table 1). A significant injury to the intestines was observed in rats in the vehicle group demonstrated by marked focal epithelial surface necrosis, shortening and broadening of the villi, inflammatory cell infiltration, capillary congestion, and epithelial detachment. VPA treatment failed to attenuate the mean intestinal injury score, but there was significant intergroup variability. We did notice significant attenuation in intestinal injury in the rats treated with MC1568 and ACY1083 when compared with the vehicle group.
We would also like to highlight our rationale for further evaluation of isoform-specific HDACIs in trauma. VPA is Food and Drug Administration (FDA)-approved for use in epilepsy, bipolar disorder, migraines, and several other conditions.35 At high doses, it is a non-selective HDACI and has been tested successfully in animal models of HS, traumatic brain injury, sepsis, and combined insults.14 36–39 Its effectiveness in animal models has allowed us to translate it successfully into early-stage human trials.40 41 However, treatment with non-selective HDACIs such as VPA requires high doses. At these doses, VPA may carry some potentially dangerous adverse effects, including hemodynamic consequences, hypothermia, and coagulopathy. VPA also has a rare “black-box” warning issued by the FDA for teratogenicity, pancreatitis, and hepatotoxicity.42 Furthermore, VPA is known to cause dose-related toxicity through the generation of reactive free radicals.43 44 The potential of VPA for causing adverse effects can be concerning, especially in trauma patients who may have multiple physiologic derangements. These concerns prompted our group to test different isoform-specific HDACIs in a rodent model of lethal hemorrhage.18
Out of the many classes of HDACIs tested, we found that MC1568 (a class IIa inhibitor) and ACY1083 (a class IIb inhibitor) are as effective as VPA in improving survival in a lethal model of HS involving 50% TBV hemorrhage.18 We therefore decided to take these two promising compounds to the next stage of testing to evaluate how they would fare against VPA in attenuating intestinal damage. Our findings suggest that in a model of global hypoperfusion after hemorrhage, treatment with isoform-specific HDACIs may be superior to VPA in attenuating the expression of proinflammatory cytokines IL-1β and TNF-α and proapoptotic protein c-caspase 3, and in maintaining intestinal integrity.
In this study, we also observed that VPA treatment decreased the expression of proinflammatory markers but did not significantly attenuate the severity of intestinal injury. We have tested VPA in a model of intestinal ischemia reperfusion (I/R) injury in rats and have shown that VPA treatment attenuates acute lung injury (ALI) resulting from I/R to the intestine.45 This more targeted injury to the intestine was extremely severe and resulted in frank bowel necrosis that VPA treatment was unable to curtail. However, VPA still attenuated distant organ ALI and the expression of proinflammatory cytokines CINC-1 and myeloperoxidase in the lungs resulting from the insult. Furthermore, we have shown that Tubastatin A, a class IIb HDACI, can decrease the loss of intestinal tight junction proteins after HS.46 In a recent study, we also showed that ACY1083 treatment can attenuate intestinal injury.47 The current study was performed to compare MC1568 and ACY1083 with VPA in their ability to attenuate intestinal injury, and also to see if administration of these compounds together elicits a synergistic effect. The data from the current study are consistent with the previous findings.
We also compared the individual treatment with MC1568 and ACY1083 with combination therapy to determine whether there were any potential synergistic benefits. As a proof-of-concept study, we used full doses of both agents in combination. We found that the combination treatment decreased intestinal inflammation and injury; however, it was not better than either agent alone. We suspect that this may be because in the sublethal model used in the study the treatment with MC1568 and ACY1083 alone was very effective in attenuating the intestinal injury. In a more severe shock model, or with a larger sample size, there might be benefit from combined therapy. In fact, we observed that, although not statistically significant, there was a trend showing that combination treatment may be worse than individual agents in regard to the expression of inflammatory cytokines in the intestine. We think that the full doses of the tested agents in combination can be toxic, resulting in the observed result. However, the effect of combination treatment remains speculative at this stage. Nevertheless, this “proof-of-concept” study provides the hypothesis-generating preliminary data that justify more extensive follow-up mechanistic studies.
This study has several limitations. First, we used the smallest sample size that was justified by the pre-experiment sample size calculations for cost and ethical considerations. Second, only male rats were used in the study. Effects of gender dimorphism are well known in trauma, and female sex hormones are thought to be protective in shock.18 Therefore, only male rats were used to eliminate gender-dependent variation. Further studies will be required to validate these effects in females. Third, we studied the effects of isoform-specific and non-selective HDACIs on intestines only. These agents may provide protection to different extents in different organs. As such, further studies are required to establish the roles of these treatments in the attenuation of multiorgan injury in HS. Fourth, we used a single timepoint of 3-hour post-treatment to test for the effects of the treatment. This limits the ability to evaluate the long-term benefits and side effects of the agents. Fifth, the severity and duration of the shock may influence the cellular effects of treatment, and the sublethal model of hemorrhage may therefore limit the comparison. Sixth, the doses of each of the isoform-specific HDACIs have not been optimized for the treatment of HS, and therefore may vary in efficacy or adverse effects; however, the doses used in this study were based on published literature and manufacturer recommendation, and shown to have effect in this setting,18 and therefore we think this is a valid approach as a proof-of-concept study.
In summary, both non-selective and isoform-specific HDACIs provide protection against intestinal inflammation and injury after HS. However, isoform-specific HDACIs, including MC1568 and ACY1083, appear to be superior to VPA in attenuating intestinal inflammation by mitigating the expression of proinflammatory cytokines IL-1β and TNF-α. They may also provide better protection than VPA against intestinal apoptosis as evidenced by the decreased expression of c-caspase 3. MC1568 and ACY1083 may also decrease neutrophil migration to the intestines as noted by the decreased expression of intestinal CINC-1. Isoform-specific HDACIs also attenuate the severity of intestinal damage after HS.
Acknowledgments
We would like to acknowledge the work of Dr. Brian P. Coppola, Dr. Isabel Dennahy and Tian Yuzi who assisted in this project.
Footnotes
Presented at: American College of Surgeons – Michigan Chapter (2019)
Contributors: UFB and HBA conceived the study. UFB, PC, and RGK performed the animal experiments. UFB and BL performed the tissue analyses. JD graded the histology slides. UFB wrote the initial draft of the article, which was revised by AMW, RGK, PC, JZ, JD, BEB, ZW, BL, and YL. HBA performed the final review and critical revision of the article, and he also obtained funding for this work.
Funding: This study was funded by NIH RO1 GM084127 (HBA).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the University of Michigan Institutional Animal Care and Use Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Alam HB. Trauma care: finding a better way. PLoS Med 2017;14:e1002350 10.1371/journal.pmed.1002350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cannon JW, Shock H. Hemorrhagic shock. N Engl J Med Overseas Ed 2018;378:370–9. 10.1056/NEJMra1705649 [DOI] [PubMed] [Google Scholar]
- 3. Mitra B, Gabbe BJ, Kaukonen K-M, Olaussen A, Cooper DJ, Cameron PA. Long-Term outcomes of patients receiving a massive transfusion after trauma. Shock 2014;42:307–12. 10.1097/SHK.0000000000000219 [DOI] [PubMed] [Google Scholar]
- 4. Wilson M, Davis DP, Coimbra R. Diagnosis and monitoring of hemorrhagic shock during the initial resuscitation of multiple trauma patients: a review. J Emerg Med 2003;24:413–22. 10.1016/S0736-4679(03)00042-8 [DOI] [PubMed] [Google Scholar]
- 5. Sauaia A, Moore FA, Moore EE, Inflammation P, Dysfunction O. Postinjury inflammation and organ dysfunction. Crit Care Clin 2017;33:167–91. 10.1016/j.ccc.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the "motor" of critical illness. Shock 2007;28:384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carrico CJ, Meakins JL, Marshall JC, Fry D, Maier RV. Multiple-organ-failure syndrome. Arch Surg 1986;121:196–208. 10.1001/archsurg.1986.01400020082010 [DOI] [PubMed] [Google Scholar]
- 8. Dewar D, Moore FA, Moore EE, Balogh Z. Postinjury multiple organ failure. Injury 2009;40:912–8. 10.1016/j.injury.2009.05.024 [DOI] [PubMed] [Google Scholar]
- 9. Moore FA. The role of the gastrointestinal tract in postinjury multiple organ failure. Am J Surg 1999;178:449–53. 10.1016/S0002-9610(99)00231-7 [DOI] [PubMed] [Google Scholar]
- 10. Hassig CA, Schreiber SL. Nuclear histone acetylases and deacetylases and transcriptional regulation: hats off to HDACs. Curr Opin Chem Biol 1997;1:300–8. 10.1016/S1367-5931(97)80066-X [DOI] [PubMed] [Google Scholar]
- 11. Halaweish I, Nikolian V, Georgoff P, Li Y, Alam HB. Creating a "Prosurvival Phenotype" Through Histone Deacetylase Inhibition: Past, Present, and Future. Shock 2015;44(Suppl 1):6–16. 10.1097/SHK.0000000000000319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams AM, Dennahy IS, Bhatti UF, Biesterveld BE, Graham NJ, Li Y, Alam HB. Histone deacetylase inhibitors: a novel strategy in trauma and sepsis. Shock 2019;52:300–6. 10.1097/SHK.0000000000001308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 2009;10:32–42. 10.1038/nrg2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Halaweish I, Bambakidis T, Chang Z, Wei H, Liu B, Li Y, Bonthrone T, Srinivasan A, Bonham T, Chtraklin K, et al. Addition of low-dose valproic acid to saline resuscitation provides neuroprotection and improves long-term outcomes in a large animal model of combined traumatic brain injury and hemorrhagic shock. J Trauma Acute Care Surg 2015;79:911–9. discussion 919 10.1097/TA.0000000000000789 [DOI] [PubMed] [Google Scholar]
- 15. Georgoff PE, Nikolian VC, Higgins G, Chtraklin K, Eidy H, Ghandour MH, Williams A, Athey B, Alam HB. Valproic acid induces prosurvival transcriptomic changes in swine subjected to traumatic injury and hemorrhagic shock. J Trauma Acute Care Surg 2018;84:642–9. 10.1097/TA.0000000000001763 [DOI] [PubMed] [Google Scholar]
- 16. Bambakidis T, Dekker SE, Sillesen M, Liu B, Johnson CN, Jin G, de Vries HE, Li Y, Alam HB. Resuscitation with valproic acid alters inflammatory genes in a porcine model of combined traumatic brain injury and hemorrhagic shock. J Neurotrauma 2016;33:1514–21. 10.1089/neu.2015.4163 [DOI] [PubMed] [Google Scholar]
- 17. Nanau RM, Neuman MG. Adverse drug reactions induced by valproic acid. Clin Biochem 2013;46:1323–38. 10.1016/j.clinbiochem.2013.06.012 [DOI] [PubMed] [Google Scholar]
- 18. Chang P, Weykamp M, Dennahy IS, Williams AM, Bhatti UF, Liu B, Nikolian VC, Li Y, Alam HB. Histone deacetylase inhibitors: isoform selectivity improves survival in a hemorrhagic shock model. J Trauma Acute Care Surg 2018;84:795–801. 10.1097/TA.0000000000001824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med 1985;26:72–6. [PubMed] [Google Scholar]
- 20. Nikolian VC, et al. Isoform 6-selective histone deacetylase inhibition reduces lesion size and brain swelling following traumatic brain injury and hemorrhagic shock. J Trauma Acute Care Surg 2018. [DOI] [PubMed] [Google Scholar]
- 21. Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg 1970;101:478–83. 10.1001/archsurg.1970.01340280030009 [DOI] [PubMed] [Google Scholar]
- 22. Zheng X, Zheng X, Mao Y, Cai J, Li Y, Liu W, Sun P, Zhang JH, Sun X, Yuan H, et al. Hydrogen-Rich saline protects against intestinal ischemia/reperfusion injury in rats. Free Radic Res 2009;43:478–84. 10.1080/10715760902870603 [DOI] [PubMed] [Google Scholar]
- 23. Adolph TE, Tomczak MF, Niederreiter L, Ko H-J, Böck J, Martinez-Naves E, Glickman JN, Tschurtschenthaler M, Hartwig J, Hosomi S, et al. Paneth cells as a site of origin for intestinal inflammation. Nature 2013;503:272–6. 10.1038/nature12599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grotz MR, Deitch EA, Ding J, Xu D, Huang Q, Regel G. Intestinal cytokine response after gut ischemia: role of gut barrier failure. Ann Surg 1999;229:478–86. 10.1097/00000658-199904000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-Injury multiple organ failure: the role of the gut. Shock 2001;15:1–10. 10.1097/00024382-200115010-00001 [DOI] [PubMed] [Google Scholar]
- 26. Sato H, Kasai K, Tanaka T, Kita T, Tanaka N. Role of tumor necrosis factor-alpha and interleukin-1beta on lung dysfunction following hemorrhagic shock in rats. Med Sci Monit 2008;14:Br79–87. [PubMed] [Google Scholar]
- 27. He W, Zhou P, Chang Z, Liu B, Liu X, Wang Y, Li Y, Alam HB. Inhibition of peptidylarginine deiminase attenuates inflammation and improves survival in a rat model of hemorrhagic shock. J Surg Res 2016;200:610–8. 10.1016/j.jss.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fukudome EY, Li Y, Kochanek AR, Lu J, Smith EJ, Liu B, Kim K, Velmahos GC, deMoya MA, Alam HB, et al. Pharmacologic resuscitation decreases circulating cytokine-induced neutrophil chemoattractant-1 levels and attenuates hemorrhage-induced acute lung injury. Surgery 2012;152:254–61. 10.1016/j.surg.2012.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zingarelli B, Squadrito F, Altavilla D, Calapai G, Di Rosa M, Caputi AP. Role of tumor necrosis factor-alpha in acute hypovolemic hemorrhagic shock in rats. Am J Physiol Heart Circ Physiol 1994;266:H1512–H1515. 10.1152/ajpheart.1994.266.4.H1512 [DOI] [PubMed] [Google Scholar]
- 30. Junger WG, Rhind SG, Rizoli SB, Cuschieri J, Shiu MY, Baker AJ, Li L, Shek PN, Hoyt DB, Bulger EM, et al. Resuscitation of traumatic hemorrhagic shock patients with hypertonic Saline—Without Dextran—Inhibits neutrophil and endothelial cell activation. Shock 2012;38:341–50. 10.1097/SHK.0b013e3182635aca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Watanabe K, Koizumi F, Kurashige Y, Tsurufuji S, Nakagawa H. Rat CINC, a member of the interleukin-8 family, is a neutrophil-specific chemoattractant in vivo. Exp Mol Pathol 1991;55:30–7. 10.1016/0014-4800(91)90016-Q [DOI] [PubMed] [Google Scholar]
- 32. Murao Y, Hata M, Ohnishi K, Okuchi K, Nakajima Y, Hiasa Y, Junger WG, Hoyt DB, Ohnishi T. Hypertonic saline resuscitation reduces apoptosis and tissue damage of the small intestine in a mouse model of hemorrhagic shock. Shock 2003;20:23–8. 10.1097/01.shk.0000078832.57685.6c [DOI] [PubMed] [Google Scholar]
- 33. Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ 1999;6:99–104. 10.1038/sj.cdd.4400476 [DOI] [PubMed] [Google Scholar]
- 34. Thuijls G, de Haan J-J, Derikx JPM, Daissormont I, Hadfoune M'hamed, Heineman E, Buurman WA. Intestinal cytoskeleton degradation precedes tight junction loss following hemorrhagic shock. Shock 2009;31:164–9. 10.1097/SHK.0b013e31817fc310 [DOI] [PubMed] [Google Scholar]
- 35. Drug USF. Valproate information. 2015. 07/10/.
- 36. Nikolian VC, Georgoff PE, Pai MP, Dennahy IS, Chtraklin K, Eidy H, Ghandour MH, Han Y, Srinivasan A, Li Y, et al. Valproic acid decreases brain lesion size and improves neurologic recovery in swine subjected to traumatic brain injury, hemorrhagic shock, and polytrauma. J Trauma Acute Care Surg 2017;83:1066–73. 10.1097/TA.0000000000001612 [DOI] [PubMed] [Google Scholar]
- 37. Ji M-huo, Li G-min, Jia M, Zhu S-hai, Gao D-peng, Fan Y-xia, Wu J, Yang J-jun. Valproic acid attenuates lipopolysaccharide-induced acute lung injury in mice. Inflammation 2013;36:1453–9. [DOI] [PubMed] [Google Scholar]
- 38. Liu Z, Li Y, Chong W, Deperalta DK, Duan X, Liu B, Halaweish I, Zhou P, Alam HB. Creating a prosurvival phenotype through a histone deacetylase inhibitor in a lethal two-hit model. Shock 2014;41:104–8. 10.1097/SHK.0000000000000074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hwabejire JO, Lu J, Liu B, Li Y, Halaweish I, Alam HB. Valproic acid for the treatment of hemorrhagic shock: a dose-optimization study. J Surg Res 2014;186:363–70. 10.1016/j.jss.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Georgoff PE, Nikolian VC, Bonham T, Pai MP, Tafatia C, Halaweish I, To K, Watcharotone K, Parameswaran A, Luo R, et al. Safety and tolerability of intravenous valproic acid in healthy subjects: a phase I dose-escalation trial. Clin Pharmacokinet 2018;57:209–19. 10.1007/s40262-017-0553-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Georgoff PE, Halaweish I, Nikolian VC, Higgins GA, Bonham T, Tafatia C, Remmer H, Menon R, Liu B, Li Y. Alterations in the human proteome following administration of valproic acid. J Trauma Acute Care Surg 2016;81:1020–7. 10.1097/TA.0000000000001249 [DOI] [PubMed] [Google Scholar]
- 42. Selph S, Carson S, Fu R, Thakurta S, Low A, McDonagh M. Drug class review: neuropathic pain: final update 1, 2011. [PubMed] [Google Scholar]
- 43. Chang TKH, Abbott FS. Oxidative stress as a mechanism of valproic acid-associated hepatotoxicity. Drug Metab Rev 2006;38:627–39. 10.1080/03602530600959433 [DOI] [PubMed] [Google Scholar]
- 44. Oktay S, Alev B, Tunali S, Emekli-Alturfan E, Tunali-Akbay T, Koc-Ozturk L, Yanardag R, Yarat A. Edaravone ameliorates the adverse effects of valproic acid toxicity in small intestine. Hum Exp Toxicol 2015;34:654–61. 10.1177/0960327114554047 [DOI] [PubMed] [Google Scholar]
- 45. Kim K, Li Y, Jin G, Chong W, Liu B, Lu J, Lee K, Demoya M, Velmahos GC, Alam HB. Effect of valproic acid on acute lung injury in a rodent model of intestinal ischemia reperfusion. Resuscitation 2012;83:243–8. 10.1016/j.resuscitation.2011.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chang Z, Li Y, He W, Liu B, Duan X, Halaweish I, Bambakidis T, Pan B, Liang Y, Nikolian VC. Inhibition of histone deacetylase 6 restores intestinal tight junction in hemorrhagic shock. J Trauma Acute Care Surg 2016;81:512–9. 10.1097/TA.0000000000001137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chang P, Bhatti UF, Williams AM, Dennahy IS, Liu B, Li Y, Alam HB. Inhibition of histone deacetylase 6 attenuates intestinal inflammation and apoptosis in a rodent model of hemorrhagic shock. J Trauma Acute Care Surg 2019;86:874–80. [DOI] [PubMed] [Google Scholar]