Abstract
The reaction of pyridines with trifluoroacetylated acetylenes was investigated. It was found that the reaction of various pyridines with two molecules of CF3CO-acetylenes proceeds under mild metal-free conditions. As a result, efficient stereoselective synthesis of 3-arylethynyl-3-trifluoromethyl-1,3-oxazinopyridines was elaborated. Target heterocycles can be prepared in up to quantitative yields.
Keywords: pyridine; CF3CO-acetylenes; 1,3-oxazines; fluorinated heterocycles
1. Introduction
Pyridine motif is the one of the most recognizable frameworks among heterocyclic molecules. A lot of attention has been paid to the chemistry of this class of heterocyclic compounds since the very beginning of its discovery. Nowadays the flow of the articles concerning pyridine is still far from the drying out. The high attractiveness of pyridine chemistry can be explained by high biological activity of pyridine derivatives both naturally occurred and prepared in the lab. Therefore, almost 300 alkaloids, having pyridine moiety (not including derivatives with fused pyridine ring, such as isoquinoline), were listed in “The Dictionary of Alkaloids” [1].
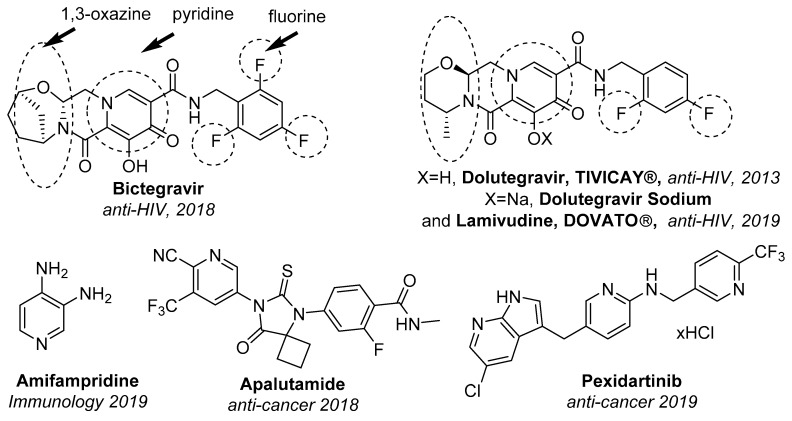
The pyridine scaffold is also a privileged structure for design of novel pharmaceuticals. Structural analysis of US FDA approved drugs showed that pyridine core is a consistent part of 62 marketed drugs (second place after piperidine) in the list of most frequent nitrogen heterocycles in structure of approved drugs [2,3]. One can also found 15 derivatives of pyridine among the “Top 200 Pharmaceutical Products by Retail Sales in 2018” which made together about $27 billion during 2018 alone [4]. Some pyridine-based drugs were approved by FDA in 2019 (for examples, see Figure 1).
Figure 1.
Selected FDA approved drugs in 2018 and 2019 containing pyridine moiety, fluorine atoms, 1,3-oxazine moiety.
On the other hand, investigation of organofluorine compounds is one of the most important trends in modern organic chemistry [5,6,7,8,9]. Due to unique physicochemical and biological properties, organofluorine compounds are widely used as construction materials, components of liquid crystalline compositions, agrochemicals and pharmaceuticals [10,11,12,13,14,15]. By some estimation, about 20–25% of currently used drugs [16,17,18,19,20,21,22,23] and agrochemicals [24,25,26,27] contain at least one fluorine atom. As for the year 2018, that value is even higher, because three out of ten drugs approved by the US FDA in 2018 contain this atom (18 out of 59 drugs) [28]. Heterocyclic compounds are also an important object for medicinal chemistry, which can be found among numerous drugs (about 59% of small-molecule drugs [2], approved by the US FDA before 2014). Last year, 35 out of 59 drugs contain any heterocyclic fragment, with 16 of them also having at least one fluorine atom, including six with fluorinated heterocyclic motif (Figure 1). It is not surprising that novel effective methodologies for the synthesis of fluorinated heterocycles have been in great demand in recent decades [29,30,31,32,33,34,35].
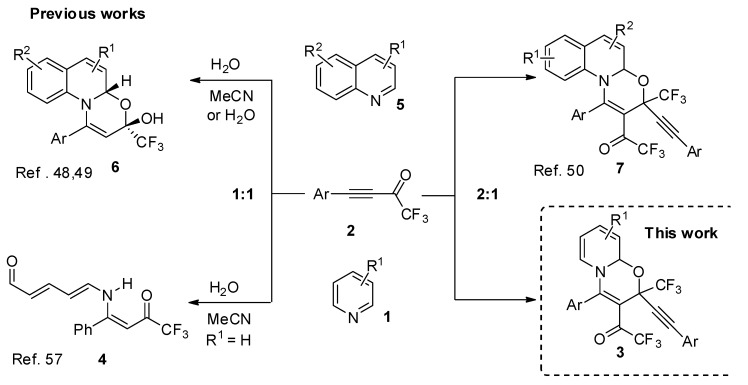
α,β-Unsaturated CF3-ketones have been shown as versatile building blocks for the synthesis of various fluorinated heterocyclic compounds [36,37,38,39,40]. In a series of works, we have demonstrated a great potential of CF3-ynones in different heterocyclizations to prepare fluorinated derivatives of diazepines [41], pyrimidines [42], thiophenes [43], triazoles [44], pyrazoles [45,46,47]. Recently, we focused our attention on the reactions of CF3-ynones with azines. It was found that, depending on nature of azine and the acetylene–azine ratio, various products can be obtained very efficiently. The reaction with quinolines opened access to 1,3-oxazinoquinolines 6 [48,49] or 7 (Scheme 1) [50]. 1,3-Oxazine moiety has been experienced a growing interest in recent years [51,52] and became perspective targets for drug design [49,53,54]. For example, Dolutegravir (Tivicay® approved in 2013 [55] and in combination with Lamivudine as Dovato® approved in 2019) and Bictegravir (Biktarvy® approved in 2018) [56] are used for treatment of patients with HIV (Figure 1).
Scheme 1.
CF3-ynones in the reactions with quinolines and pyridines.
In contrast to the reaction with quinolines, our attempt to involve pyridines into 1,3-oxazine assembling reaction with CF3-ynones has been less successful. The reaction of pyridine with equal amount of CF3-ynone in wet acetonitrile afforded the corresponding ring opening product. Polyunsaturated 5-amino-2,4-pentadienal 7 has been isolated as a result of cascade transformation (Scheme 1) [57].
2. Results and Discussion
This study is devoted to the next step of our systematic study of the reactions of fluorinated acetylenes with azines. A simple and highly efficient approach towards 3-arylethynyl-3-trifluoromethyl-1,3-oxazinopyridines 3 is presented by the reaction of CF3-ynones with pyridines in 2:1 ratio.
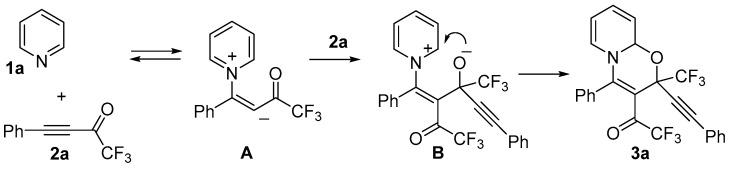
We assumed that using dry conditions and excess of ketone the reaction course could be redirected to formation of the corresponding trifluoromethylated 1,3-oxazines. Indeed, being mixed together without solvent, pyridine and CF3-ynone 2a 1:2 ratio new transformation was observed to form viscous mass in a few minutes.
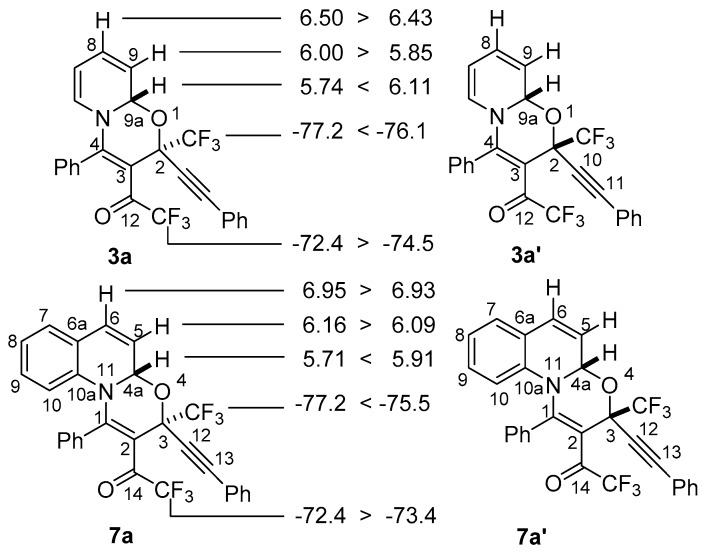
Analysis of the reaction mixture by NMR showed clean formation of 3a and unreacted starting materials. After addition of a small amount of MeCN to form homogeneous solution the reaction mixture was left overnight. As a result, oxazine 3a was isolated in 94% yield in stereoselective manner. According to NMR a 90:10 mixture of 2S*,9aS* and 2R*,9aS* diastereomers was formed. Assignment of both diastereomers was maintained by careful comparison with 3-arylethynyl-3-trifluoromethyl-1,3-oxazinoquinolines 7 having similar structures (Figure 2) [50]. Therefore, values of δ(1H-8), δ(1H-9), δ(19F-COCF3) in 2S*,9aS*-3a are larger than in 2R*,9aS*-3a′ while values of δ(1H-9a) and δ(19F-CF3) are the other way around. The same regularity can be seen in the NMR of 3S*,4aS*- and 3R*,4aS*-diastereomers of 7a.
Figure 2.
Comparison of characteristic values of chemical shifts of diastereomers of 3a in 1H- and 19F-NMR spectra with the corresponding quinoline derivative 7a.
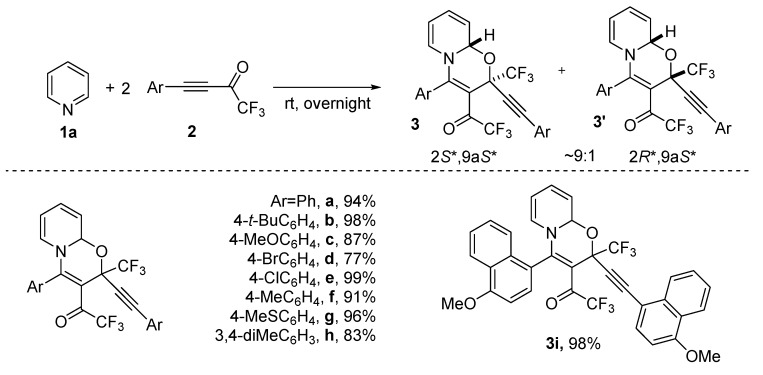
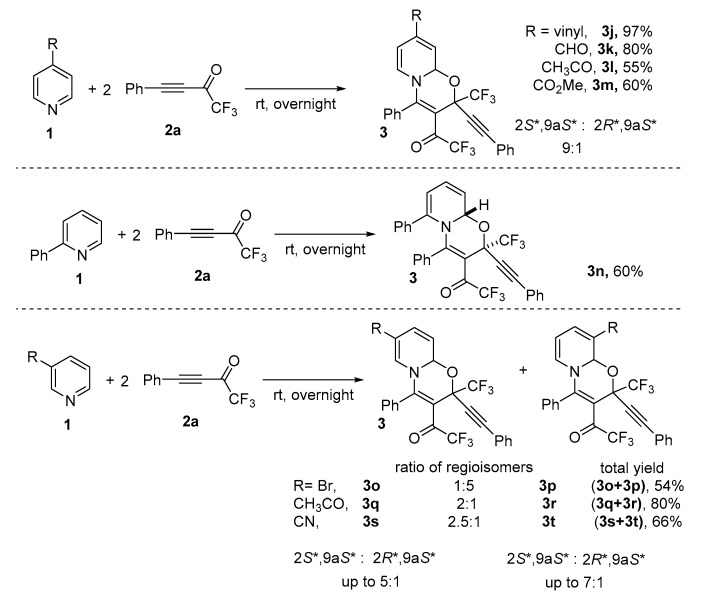
Next, the reaction scope was studied. For this aim, the interaction of parent pyridine with various CF3-ynones was investigated. To our delight, it was found that the reaction has no restrictions in terms of CF3-ynones. The corresponding 1,3-oxazinoquinolines 3a–i were isolated in 77–99% yield (Scheme 2). Similar stereoselectivity was observed for all these products. Compounds 3a–i were formed as a mixture of 2S*,9aS* and 2R*,9aS* diastereomers in near 9:1 ratio in most cases.
Scheme 2.
Reactions of pyridine with CF3-ynones 2a-i to form 1,3-oxazinopyridines 3a–i.
Next, the reaction of CF3-ynone 2a with several pyridines was studied in order to investigate the influence of nature of pyridine component of the reaction. A series of 4, 3 and 2-substituted pyridines was involved into reaction with 2a (Scheme 3).
Scheme 3.
Reactions of pyridine 1b–j with CF3-ynones 2a to form 1,3-oxazinopyridines 3j–t.
It was found that the reaction has broad scope in terms of pyridines and nature of substituents. However, the reaction is very sensitive to structure of starting pyridine. An especially important influence on the reaction is the nature of a substituent, pKa value of pyridine and its nucleophilicity, and the position of a substituent in the molecule of pyridine. Therefore, the reaction with 4-substituted pyridines afforded the corresponding oxazines 3j–m in high yields. Again, a mixture of diastereomers in near to 8:1–9:1 ratio was formed in all cases (Scheme 3, compounds 3j–m). In contrast, 2-substituted pyridines (2-phenylpyridine) reacted with CF3-ynone 2a 100% stereoselectively to form 2S*, 9aS* diastereomer exclusively (Scheme 3, compound 3n). A more complex picture was observed for the reaction with 3-substituted pyridines. Due to the presence of two possible positions for cyclization in the pyridine framework, 7- and 9-isomers were formed in about 2:1 ratio for pyridines with electron-withdrawing acetyl- and cyano groups, having strong −M effect. In contrast mostly 9-isomer (in ratio 5:1 with 7-isomer) was formed in the reaction with 3-bromopyridine having bromine atom with slight +M effect (Scheme 3, compounds 3o–t). It is noteworthy that both increase (30 °C) and decrease (7 °C) of the temperature did not change dramatically the regioselectivity of the reaction. However, the stereoselectivity of formation of compounds 3p–t was again high to give 2S*,9aS-isomer as a major one in up to 7:1 ratio with minor 2R*,9aS*-isomer.
Some restrictions were also found. We observed that pKa of azine and therefore its nucleophilicity plays a decisive role in the possibility of the reaction to occur. Therefore, pyridines with pKa lower than ~1, 2-bromopyridine (0.79), 2-fluoropyridine (−0.43), 2-methoxy-5-bromopyridine (1.04) do not react with CF3-ynone 2a.
Based on our previous mechanistic rationalizations regarding interaction of CF3-ynones with azines [48,49,58,59], the possible reaction mechanism can be proposed. The domino assembly of oxazinoquinolines 3 is initiated by the reversible formation of the intermediate zwitterion A resulted from the nitrogen nucleophilic addition to the triple bond. In contrast to 1:1 reaction, the carbanionic site of A is selectively attacked by the carbonyl group of the second molecule of 2a to form anion B. Cyclization of B undergoes by the attacks of oxygen into alpha-position of the pyridine ring to give the corresponding 1,3-oxazine 3 (Scheme 4).
Scheme 4.
Possible mechanism of the reaction of pyridines with CF3-ynones.
3. Materials and Methods
3.1. General Details
1H-, 13C- and 19F-NMR spectra were recorded on Bruker AVANCE 400 MHz spectrometer (Bruker Corp., Karlsruhe, Germany) in CD3CN and CDCl3 at 400.1, 100.6 and 376.3 MHz respectively (Supplementary Information). Chemical shifts (δ) in ppm were reported with the use of the residual CHD2CN and chloroform signals (1.94 and 7.25 for 1H and 77.0 for 13C) as internal reference. The 19F chemical shifts were referenced to C6F6, (−162.9 ppm). HRMS (ESI-TOF) spectra were measured with an Orbitrap Elite instrument (TermoFisher, Paisley, UK). TLC analysis was performed on “Merck 60 F254” plates. Visualization was accomplished by UV light (254 nm) at Vilber Lourmat UV lamp. Silica gel (silica 60, 0.063–0.2 mm, 70–230 mesh), Screw neck vials (clear, flat bottom, 4 mL) and Screw caps were purchased at MACHEREY-NAGEL (Duren, Germany). All reagents were purchased at Sigma-Aldrich (Muenchen, Germany) and Acros companies (Geel, Belgium). The reagents were of reagent grade and were used as such or distilled prior to use. CF3-ynones 2 were prepared as reported previously [46]. Melting points were determined on an Electrothermal 9100 apparatus.
3.2. Reaction of CF3-Ynones and Pyridines (General Procedure)
A 4 mL vial with a screw cap was charged with CF3-ynone 2 (1–1.05 mmol, 2–2.1 equiv.)* and then pyridine 1 (0.5 mmol, 1 equiv.) was added in one portion. After vigorous stirring for several minutes the reaction mixture became viscous due to crystallization of the product. At that moment MeCN (0.5 mL) was added to form homogeneous solution again and the reaction mixture was left overnight at stirring. Next volatiles were evaporated in vacuo, the residue was crystallized from appropriate amount of ether-hexane mixtures or purified via column chromatography on silica gel using mixtures of hexane with CH2Cl2. * In case of solid CF3-ynones 2 MeCN (0.1–0.2 mL) was added to form clear solution.
2,2,2-Trifluoro-1-(4-phenyl-2-(phenylethynyl)-2-(trifluoromethyl)-2H,9aH-pyrido[2,1-b][1,3]oxazin-3-yl)ethan-1-one (3a). Obtained from pyridine 1a (0.042 g, 0.53 mmol) and CF3-ynone 2a (0.212 g, 1.071 mmol). Yellow-brown powder, m.p. 109.4–111.8 °C (hexane), yield 0.238 g (94%). (2S*,9aS*):(2R*,9aS*)-isomers ratio is 90:10 (19F-NMR). HRMS (ESI-TOF): m/z [M + H]+ Calcd for C25H16F6NO2+: 476.1080; found: 476.1085.
(2S*,9aS*)-3a: 1H-NMR (400.1 MHz, CDCl3): δ 7.68–7.39 (m, 7H), 7.37–7.27 (m, 3H), 6.50 (dd, 3J8,9 = 9.7 Hz, 3J7,8 = 6.1 Hz, 1H, H-8), 6.46 (d, 3J6,7 = 7.7 Hz, 1H, H-6), 6.00 (dd, 3J8,9 = 9.7 Hz, 3J9a,9 = 3.9 Hz, 1H, H-9), 5.74 (d, 3J9a,9 = 3.9 Hz, 1H, H-9a), 5.50 (pseudo-td, 3J ~ 7 Hz, 4J ~ 1 Hz, 1H, H-7). 13C-NMR (100.6 MHz, CDCl3): δ 180.7 (q, 2JCF = 35.0 Hz, C-12), 160.3 (C-4), 133.2, 132.1, 131.4 (Ci′ from Ar), 129.5, 129.3, 129.2, 128.3 (C-8), 126.2 (C-6), 125.8, 122.6 (q, 1JCF = 286.6 Hz, CF3), 121.1 (Ci from Ar), 116.5 (C-9), 115.5 [q, 1JCF = 292.7 Hz, C(O)CF3], 104.0 (C-7), 88.4 (C-11), 81.3 (C-10), 79.1 (C-9a), 73.7 (q, 2JCF = 33.9 Hz, C-2). 19F-NMR (376.3 MHz, CDCl3): δ −72.5 [C(O)CF3], −77.3 (CF3).
(2R*,9aS*)-3a′: 1H-NMR (400.1 MHz, CDCl3): δ 6.43 (dd, 3J8,9 = 9.8 Hz, 3J7,8 = 6.1 Hz, 1H, H-8), 6.28 (d, 3J6,7 = 7.6 Hz, 1H, H-6), 6.11 (d, 3J9a,9 = 4.0 Hz, 1H, H-9a), 5.85 (dd, 3J8,9 = 9.8 Hz, 3J9a,9 = 4.0 Hz, 1H, H-9), 5.34 (pseudo-td, 3J ~ 7 Hz, 4J = 1 Hz, 1H, H-7). Other signals are overlapped with those of major isomer. 13C-NMR (100.6 MHz, CDCl3): δ 133.9, 132.3, 128.9, 126.5, 126.3, 115.0, 109.4, 102.2. Other signals are overlapped with those of major isomer or cannot be seen in the spectrum due to the low concentration of minor isomer. 19F-NMR (376.3 MHz, CDCl3): δ −74.6 [C(O)CF3], −76.2 (CF3).
1-(4-(4-(Tert-butyl)phenyl)-2-((4-(tert-butyl)phenyl)ethynyl)-2-(trifluoromethyl)-2H,9aH-pyrido[2,1-b][1,3]oxazin-3-yl)-2,2,2-trifluoroethan-1-one (3b). Obtained from pyridine 1a (0.041 g, 0.518 mmol) and CF3-ynone 2b (0.267 g, 1.051 mmol). Yellow powder, m.p. 130.0–132.7 °C (hexane), yield 0.300 g (98%). (2S*,9aS*):(2R*,9aS*)-isomers ratio is 90:10 (19F-NMR). HRMS (ESI-TOF): m/z [M + H]+ Calcd for C33H32F6NO2+: 588.2332; found: 588.2340.
(2S*,9aS*)-3b: 1H-NMR (400.1 MHz, CDCl3): δ 7.52 (d, 3J = 8.4 Hz, 2H), 7.47–7.36 (m, 4H), 7.32 (d, 3J = 8.4 Hz, 2H), 6.52 (d, 3J6,7 = 7.8 Hz, 1H, H-6), 6.48 (dd, 3J8,9 = 9.8 Hz, 3J7,8 = 6.1 Hz, 1H, H-8), 5.99 (dd, 3J8,9 = 9.8 Hz, 3J9a,9 = 3.9 Hz, 1H, H-9), 5.72 (d, 3J9a,9 = 3.9 Hz, 1H, H-9a), 5.49 (psedo-t, 3J ~ 7 Hz, 1H, H-7), 1.35 (s, 9H, 3Me from t-Bu), 1.29 (s, 9H, 3Me from t-Bu). 13C-NMR (100.6 MHz, CDCl3): δ 180.8 (q, 2JCF = 35.0 Hz, C-12), 160.4 (C-4), 157.2 (Cp from Ar), 152.7 (Cp′ from Ar), 131.9 (Cm,m′ from Ph), 128.6 (q, 3JCF = 2.2 Hz, C-3), 126.4, 126.3 (C-8), 126.1 (C-6), 125.3 (Co,o′ from Ar), 122.6 (q, 1JCF = 286.6 Hz, CF3), 118.1 (Ci′ from Ar), 116.5 (C-9), 115.7 [q, 1JCF = 292.5 Hz, C(O)CF3], 103.6 (C-7), 88.5 (C-11), 80.9 (C-10), 79.0 (C-9a), 73.8 (q, 2JCF = 34.6Hz, C-2), 35.2, 34.8, 31.1, 31.0. 19F-NMR (376.3 MHz, CDCl3): δ −72.5 [C(O)CF3], −77.4 (CF3).
(2R*,9aS*)-3b′: 1H-NMR (400.1 MHz, CDCl3): δ 6.43 (dd, 3J8,9 = 9.8 Hz, 3J7,8 = 6.0 Hz, 1H, H-8), 6.33 (d, 3J6,7 = 7.6 Hz, 1H, H-6), 6.10 (d, 3J9a,9 = 3.9 Hz, 1H, H-9a), 5.84 (dd, 3J8,9 = 9.8 Hz, 3J9a,9 = 3.9 Hz, 1H, H-9), 5.33 (pseudo-t, 3J ~ 7 Hz, 1H, H-7), 1.33 (s, 9H, 3Me from t-Bu), 1.30 (s, 9H, 3Me from t-Bu). Other signals are overlapped with those of major isomer. 13C-NMR (100.6 MHz, CDCl3): δ 132.0, 126.6, 126.51, 126.49, 125.2, 109.2, 35.1, 31.2, 30.9. Other signals are overlapped with those of major isomer or cannot be seen in the spectrum due to the low concentration of minor isomer. 19F-NMR (376.3 MHz, CDCl3): δ −74.6 [C(O)CF3], −76.2 (CF3).
1-(4-(4-Methoxyphenyl)-2-((4-methoxyphenyl)ethynyl)-2-(trifluoromethyl)-2H,9aH-pyrido[2,1-b][1,3]oxazin-3-yl)-2,2,2-trifluoroethan-1-one (3c). Obtained from pyridine 1a (0.041 g, 0.518 mmol) and CF3-ynone 2c (0.239 g, 1.048 mmol). Light brown powder, m.p. 117.3–118.7 °C (hexane), yield 0.242 g (87%). (2S*,9aS*):(2R*,9aS*)-isomers ratio is 90:10 (19F-NMR). HRMS (ESI-TOF): m/z [M + H]+ Calcd for C27H20F6NO4+: 536.1291; found: 536.1296.
(2S*,9aS*)-3c: 1H-NMR (400.1 MHz, CDCl3): δ 7.48–7.30 (m, 4H), 7.07–6.91 (m, 2H), 6.82 (d, 3J = 8.9 Hz, 2H), 6.49 (d, 3J6,7 = 7.0 Hz, 1H, H-6), 6.48 (dd, 3J8,9 = 9.8 Hz, 3J7,8 = 6.1 Hz, 1H, H-8), 5.99 (dd, 3J8,9 = 9.8 Hz, 3J9a,9 = 4.1 Hz, 1H, H-9), 5.71 (d, 3J9a,9 = 4.1 Hz, 1H, H-9a), 5.49 (pseudo-td, 3J ~ 7 Hz, 3J ~ 1 Hz, 1H, H-7), 3.88 (s, 3H, MeO), 3.79 (s, 3H, MeO). 13C-NMR (100.6 MHz, CDCl3): δ 180.6 (q, 2JCF = 34.7 Hz, C-12), 163.7, 160.4 (C-4), 160.3, 133.7, 126.3 (C-8), 126.0 (C-6), 123.7, 122.7 (q, 1JCF = 286.8 Hz, CF3), 116.5 (C-9), 115.7 [q, 1JCF = 293.0 Hz, C(O)CF3], 113.9, 113.2, 108.6, 103.8 (C-7), 88.3 (C-11), 80.3 (C-10), 78.9 (C-9a), 73.9 (q, 2JCF = 34.3 Hz, C-2), 55.6, 55.2. 19F-NMR (376.3 MHz, CDCl3): δ −72.4 [C(O)CF3], −77.5 (CF3).
(2R*,9aS*)-3c′: 1H-NMR (400.1 MHz, CDCl3): δ 7.52 (d, 3J = 8.9 Hz, 2H), 6.42 (dd, 3J8,9 = 9.8 Hz, 3J7,8 = 6.1 Hz, 1H, H-8), 6.31 (d, 3J6,7 = 7.5 Hz, 1H, H-6), 6.07 (d, 3J9a,9 = 4.1 Hz, 1H, H-9a), 5.84 (dd, 3J8,9 = 9.8 Hz, 3J9a,9 = 4.1 Hz, 1H, H-9), 5.33 (pseudo-t, 3J ~ 7 Hz, 1H, H-7), 3.86 (s, 3H, MeO), 3.81 (s, 3H, MeO). Other signals are overlapped with those of major isomer. 13C-NMR (100.6 MHz, CDCl3): δ 136.0, 133.8, 131.8, 126.51, 126.48, 114.4, 55.5. Other signals are overlapped with those of major isomer or cannot be seen in the spectrum due to the low concentration of minor isomer. 19F-NMR (376.3 MHz, CDCl3): δ −74.6 [C(O)CF3], −76.2 (CF3).
1-(4-(4-Bromophenyl)-2-((4-bromophenyl)ethynyl)-2-(trifluoromethyl)-2H,9aH-pyrido[2,1-b][1,3]oxazin-3-yl)-2,2,2-trifluoroethan-1-one (3d). Obtained from pyridine 1a (0.0395 g, 0.5 mmol) and CF3-ynone 2d (0.292 g, 1.054 mmol). Yellow-brown powder, m.p. 83.9–86.7 °C (hexane), yield 0.244 g (77%). (2S*,9aS*):(2R*,9aS*)-isomers ratio is 89:11 (19F-NMR). HRMS (ESI-TOF): m/z [M + H]+ Calcd for C25H14Br2F6NO2+: 633.9270; found: 633.9282.
(2S*,9aS*)-3d: 1H-NMR (400.1 MHz, CDCl3): δ 7.68–7.29 (m, 8H), 6.49 (dd, 3J8,9 = 9.8 Hz, 3J7,8 = 6.1 Hz, 1H, H-8), 6.41 (d, 3J6,7 = 7.6 Hz, 1H, H-6), 6.00 (dd, 3J8,9 = 9.8 Hz, 3J9a,9 = 3.9 Hz, 1H, H-9), 5.69 (d, 3J9a,9 = 3.9 Hz, 1H, H-9a), 5.53 (pseudo-t, 3J ~ 7 Hz, 1H, H-7). 13C-NMR (100.6 MHz, CDCl3): δ 180.4 (q, 2JCF = 35.4 Hz, C-12), 159.3 (C-4), 149.6, 133.5, 132.9, 131.6, 130.2, 128.5, 126.3 (C-8), 125.4 (C-6), 123.9, 122.4 (q, 1JCF = 286.8 Hz, CF3), 119.9, 116.7 (C-9), 115.5 [q, 1JCF = 292.7 Hz, C(O)CF3], 109.4, 104.5 (C-7), 87.4 (C-11), 82.2 (C-10), 79.2 (C-9a), 73.6 (q, 2JCF = 34.3 Hz, C-2). 19F-NMR (376.3 MHz, CDCl3): δ −72.3 [C(O)CF3], −77.3 (CF3).
(2R*,9aS*)-3d′: 1H-NMR (400.1 MHz, CDCl3): δ 6.45–6.42 (m, 1H, H-8), 6.22 (d, 3J6,7 = 7.5 Hz, 1H, H-6), 6.07 (d, 3J9a,9 = 4.0 Hz, 1H, H-9a), 5.84 (dd, 3J8,9 = 9.8 Hz, 3J9a,9 = 4.0 Hz, 1H, H-9), 5.36 (pseudo-t, 3J = 6.8 Hz, 1H, H-7). Other signals are overlapped with those of major isomer. 13C-NMR (100.6 MHz, CDCl3): δ 136.1, 135.1, 133.7, 131.6, 126.5, 126.0, 123.8, 115.1, 102.6. Other signals are overlapped with those of major isomer or cannot be seen in the spectrum due to the low concentration of minor isomer. 19F-NMR (376.3 MHz, CDCl3): δ −74.5 [C(O)CF3], −76.2 (CF3).
1-(4-(4-Chlorophenyl)-2-((4-chlorophenyl)ethynyl)-2-(trifluoromethyl)-2H,9aH-pyrido[2,1-b][1,3]oxazin-3-yl)-2,2,2-trifluoroethan-1-one (3e). Obtained from pyridine 1a (0.042 g, 0.53 mmol) and CF3-ynone 2e (0.254 g, 1.09 mmol). Yellow-brown powder, m.p. 68–70 °C (hexane), yield 0.286 g (99%). (2S*,9aS*):(2R*,9aS*)-isomers ratio is 89:11 (19F-NMR). HRMS (ESI-TOF): m/z [M + H]+ Calcd for C25H14Cl2F6NO2+: 544.0300; found: 544.0308.
(2S*,9aS*)-3e: 1H-NMR (400.1 MHz, CDCl3): δ 7.52–7.28 (m, 8H), 6.49 (dd, 3J8,9 = 9.8 Hz, 3J7,8 = 6.1 Hz, 1H, H-8), 6.41 (d, 3J6,7 = 7.6 Hz, 1H, H-6), 6.00 (dd, 3J8,9 = 9.8 Hz, 3J9a,9 = 4.0 Hz, 1H, H-9), 5.69 (d, 3J9a,9 = 4.0 Hz, 1H, H-9a), 5.53 (pseudo-t, 3J ~ 7 Hz, 1H, H-7). 1H-NMR (400.1 MHz, CD3CN): δ 7.73–7.31 (m, 8H), 6.57–6.52 (m, 2H, H-8, H-6), 6.03 (dd, 3J8,9 = 9.8 Hz, 3J9a,9 = 3.8 Hz, 1H, H-9), 5.75 (d, 3J9a,9 = 3.8 Hz, 1H, H-9a), 5.61 (pseudo-t, 3J = 7.2 Hz, 1H, H-7). 13C-NMR (100.6 MHz, CDCl3): δ 180.3 (q, 2JCF = 34.7 Hz, C-12), 159.2 (C-4), 149.6, 140.0, 135.6, 133.3, 129.9, 128.7, 126.3 (C-8), 125.4 (C-6), 122.4 (q, 1JCF = 286.8 Hz, CF3), 119.4, 116.7 (C-9), 115.5 [q, 1JCF = 292.7 Hz, C(O)CF3], 109.4, 104.5 (C-7), 87.3 (C-11), 82.0 (C-10), 79.1 (C-9a), 73.6 (q, 2JCF = 34.3 Hz, C-2). 19F-NMR (376.3 MHz, CD3CN): δ −70.0 [C(O)CF3], −75.4 (CF3). 19F-NMR (376.3 MHz, CDCl3): δ −72.2 [C(O)CF3], −77.3 (CF3).
(2R*,9aS*)-3e′: 1H-NMR (400.1 MHz, CDCl3): δ 6.22 (d, 3J6,7 = 7.6 Hz, 1H, H-6), 6.07 (d, 3J9a,9 = 4.0 Hz, 1H, H-9a), 5.85 (dd, 3J8,9 = 9.8 Hz, 3J9a,9 = 4.0 Hz, 1H, H-9), 5.36 (pseudo-t, 3J ~ 7 Hz, 1H, H-7). Other signals are overlapped with those of major isomer. 1H-NMR (400.1 MHz, CD3CN): δ 6.48–6.42 (m, 1H, H-8), 6.32 (d, 3J6,7 = 7.5 Hz, 1H, H-6), 5.89 (dd, 3J8,9 = 9.8 Hz, 3J9a,9 = 3.9 Hz, 1H, H-9), 5.42 (pseudo-t, 3J = 6.8 Hz, 1H, H-7). Other signals are overlapped with those of major isomer. 13C-NMR (100.6 MHz, CDCl3): δ 136.1, 133.5, 129.7, 128.6, 126.0, 123.9, 115.1, 102.6. Other signals are overlapped with those of major isomer or cannot be seen in the spectrum due to the low concentration of minor isomer. 19F-NMR (376.3 MHz, CD3CN): δ −72.2 [C(O)CF3], −74.1 (CF3). 19F-NMR (376.3 MHz, CDCl3): δ −74.4 [C(O)CF3], −76.1 (CF3).
1-(4-(4-Methylphenyl)-2-((4-methylphenyl)ethynyl)-2-(trifluoromethyl)-2H,9aH-pyrido[2,1-b][1,3]oxazin-3-yl)-2,2,2-trifluoroethan-1-one (3f). Obtained from pyridine 1a (0.044 g, 0.556 mmol) and CF3-ynone 2f (0.240 g, 1.13 mmol). Yellow-brown powder, m.p. 95.2–99.1 °C (hexane), yield 0.256 g (91%). (2S*,9aS*):(2R*,9aS*)-isomers ratio is 91:9 (19F-NMR). HRMS (ESI-TOF): m/z [M + H]+ Calcd for C27H20F6NO2+: 504.1393; found: 504.1401.
(2S*,9aS*)-3f: 1H-NMR (400.1 MHz, CDCl3): δ 7.54–7.10 (m, 8H), 6.50–6.47 (m, 2H, H-8, H-6), 6.00 (dd, 3J8,9 = 10.0 Hz, 3J9a,9 = 3.8 Hz, 1H, H-9), 5.74 (d, 3J9a,9 = 3.8 Hz, 1H, H-9a), 5.49 (pseudo-t, 3J = 6.7 Hz, 1H, H-7), 2.44 (s, 3H, Me), 2.33 (s, 3H, Me). 13C-NMR (100.6 MHz, CDCl3): δ 180.7 (q, 2JCF = 35.0 Hz, C-12), 160.5 (C-4), 139.5, 134.0, 132.0, 130.2, 129.0, 128.7, 126.2 (C-8), 125.9 (C-6), 122.6 (q, 1JCF = 286.8 Hz, CF3), 118.0, 116.4 (C-9), 115.6 [q, 1JCF = 293.4 Hz, C(O)CF3], 109.0, 103.8 (C-7), 88.5 (C-11), 80.8 (C-10), 79.0 (C-9a), 73.8 (q, 2JCF = 34.3 Hz, C-2), 21.6, 21.5. 19F-NMR (376.3 MHz, CDCl3): δ −72.3 [C(O)CF3], −77.2 (CF3).
(2R*,9aS*)-3f′: 1H-NMR (400.1 MHz, CDCl3): δ 6.47–6.41 (m, 1H, H-8), 6.30 (d, 3J6,7 = 7.6 Hz, 1H, H-6), 6.10 (d, 3J9a,9 = 3.8 Hz, 1H, H-9a), 5.84 (dd, 3J8,9 = 9.6 Hz, 3J9a,9 = 3.8 Hz, 1H, H-9), 5.33 (pseudo-t, 3J ~ 7 Hz, 1H, H-7), 2.42 (s, 3H, Me), 2.36 (s, 3H, Me). Other signals are overlapped with those of major isomer. 13C-NMR (100.6 MHz, CDCl3): δ 143.2, 139.4, 132.1, 126.4, 118.4, 115.0, 102.0, 79.8. Other signals are overlapped with those of major isomer or can not be seen in the spectrum due to the low concentration of minor isomer. 19F-NMR (376.3 MHz, CDCl3): δ −74.4 [C(O)CF3], −76.1 (CF3).
1-(4-(4-Methylthiophenyl)-2-((4-methylthiophenyl)ethynyl)-2-(trifluoromethyl)-2H,9aH-pyrido[2,1-b][1,3]oxazin-3-yl)-2,2,2-trifluoroethan-1-one (3g). Obtained from pyridine 1a (0.040 g, 0.506 mmol) and CF3-ynone 2g (0.256 g, 1.05 mmol). Brown powder, m.p. 120.5–123.2 °C (hexane), yield 0.274 g (96%). (2S*,9aS*):(2R*,9aS*)-isomers ratio is 92:8 (19F-NMR). HRMS (ESI-TOF): m/z [M + H]+ Calcd for C27H20F6NO2S2+: 568.0834; found: 568.0834.
(2S*,9aS*)-3g: 1H-NMR (400.1 MHz, CDCl3): δ 7.49–7.25 (m, 6H), 7.14 (d, 2H, 3J = 8.5 Hz), 6.51–6.47 (m, 2H, H-8, H-6), 6.00 (dd, 3J8,9 = 9.7 Hz, 3J9a,9 = 3.8 Hz, 1H, H-9), 5.70 (d, 3J9a,9 = 3.8 Hz, 1H, H-9a), 5.50 (pseudo-t, 3J = 6.4 Hz, 1H, H-7), 2.53 (s, 3H, Me), 2.46 (s, 3H, Me). 1H-NMR (400.1 MHz, CD3CN): δ 7.58–7.30 (m, 6H), 7.24 (d, 2H, 3J = 8.7 Hz), 6.57 (d, 3J6,7 = 7.6 Hz, 1H, H-6), 6.54 (dd, 3J8,9 = 9.8 Hz, 3J7,8 = 6.0 Hz, 1H, H-8), 6.00 (dd, 3J8,9 = 9.8 Hz, 3J9a,9 = 4.0 Hz, 1H, H-9), 5.74 (d, 3J9a,9 = 4.0 Hz, 1H, H-9a), 5.59 (pseudo-t, 3J = 6.8 Hz, 1H, H-7), 2.53 (s, 3H, Me), 2.47 (s, 3H, Me). 13C-NMR (100.6 MHz, CD3CN): δ 180.8 (q, 2JCF = 34.1 Hz, C-12), 162.6 (C-4), 148.1, 142.6, 135.2, 132.9, 131.8, 129.8, 127.1, 126.8 (C-8), 126.5 (C-6), 123.8 (q, 1JCF = 285.8 Hz, CF3), 117.8, 117.5, 116.7 [q, 1JCF = 292.2 Hz, C(O)CF3], 108.7, 105.4 (C-7), 88.4 (C-11), 82.5 (C-10), 80.1 (C-9a), 74.5 (q, 2JCF = 33.7 Hz, C-2), 15.1, 14.7. 19F-NMR (376.3 MHz, CDCl3): δ −72.3 [C(O)CF3], −77.4 (CF3). 19F-NMR (376.3 MHz, CD3CN): δ −70.0 [C(O)CF3], −75.5 (CF3).
(2R*,9aS*)-3g′: 1H-NMR (400.1 MHz, CDCl3): δ 6.47–6.41 (m, 1H, H-8), 6.30 (d, 3J6,7 = 7.5 Hz, 1H, H-6), 6.07 (d, 3J9a,9 = 4.0 Hz, 1H, H-9a), 5.84 (dd, 3J8,9 = 9.7 Hz, 3J9a,9 = 4.0 Hz, 1H, H-9), 5.34 (pseudo-t, 3J = 6.8 Hz, 1H, H-7), 2.51 (s, 3H, Me), 2.47 (s, 3H, Me). Other signals are overlapped with those of major isomer. 1H-NMR (400.1 MHz, CD3CN): δ 6.45–6.37 (m, 2H, H-8, H-9a), 6.20 (d, 3J6,7 = 7.0 Hz, 1H, H-6), 5.87 (dd, 3J8,9 = 9.8 Hz, 3J9a,9 = 4.0 Hz, 1H, H-9), 5.42 (t, 3J = 6.4 Hz, 1H, H-7), 2.52 (s, 3H, Me), 2.50 (s, 3H, Me). Other signals are overlapped with those of major isomer. 13C-NMR (100.6 MHz, CD3CN): δ 145.5, 142.8, 136.9, 133.1, 129.5, 129.0, 128.2, 126.7, 126.3, 104.4, 84.7, 15.1, 14.7. Other signals are overlapped with those of major isomer or cannot be seen in the spectrum due to the low concentration of minor isomer. 19F-NMR (376.3 MHz, CDCl3): δ −74.4 [s, 3F, C(O)CF3], −76.1 (s, 3F, CF3). 19F-NMR (376.3 MHz, CD3CN): δ −72.2 [C(O)CF3], −74.0 (CF3).
1-(4-(3,4-Dimethylphenyl)-2-((3,4-dimethylphenyl)ethynyl)-2-(trifluoromethyl)-2H,9aH-pyrido[2,1-b][1,3]oxazin-3-yl)-2,2,2-trifluoroethan-1-one (3h). Obtained from pyridine 1a (0.039 g, 0.49 mmol) and CF3-ynone 2h (0.232 g, 1.027 mmol). Yellow-brown powder, m.p. 72.6–74.6 °C (hexane), yield 0.215 g (83%). (2S*,9aS*):(2R*,9aS*)-isomers ratio is 92:8 (19F-NMR) HRMS (ESI-TOF): m/z [M + H]+ Calcd for C29H24F6NO2+: 532.1706; found: 532.1717.
(2S*,9aS*)-3h: 1H-NMR (400.1 MHz, CDCl3): δ 7.38–7.02 (m, 6H), 6.50–6.46 (m, 2H, H-8, H-6), 5.98 (dd, 3J8,9 = 10.0 Hz, 3J9a,9 = 3.9 Hz, 1H, H-9), 5.71 (d, 3J9a,9 = 3.9 Hz, 1H, H-9a), 5.47 (pseudo-t, 3J = 6.5 Hz, 1H, H-7), 2.34 (s, 3H, Me), 2.31 (s, 3H, Me), 2.24 (s, 3H, Me), 2.21 (s, 3H, Me). 13C-NMR (100.6 MHz, CDCl3): δ 180.8 (q, 2JCF = 35.0 Hz, C-12), 160.6 (C-4), 138.3, 136.6, 133.0, 130.6, 129.5, 129.1, 126.2 (C-8), 126.1 (C-6), 122.7 (q, 1JCF = 286.8 Hz, CF3), 118.3, 116.4 (C-9), 115.6 [q, 1JCF = 293.0 Hz, C(O)CF3], 109.0, 103.6 (C-7), 88.6 (C-11), 80.6 (C-10), 78.9 (C-9a), 73.8 (q, 2JCF = 34.3 Hz, C-2), 20.0, 19.7, 19.6 (br s), 19.4. 19F-NMR (376.3 MHz, CDCl3): δ −72.3 [C(O)CF3], −77.3 (CF3).
(2R*,9aS*)-3h′: 1H-NMR (400.1 MHz, CDCl3): δ 6.44–6.40 (m, 1H, H-8), 6.31 (d, 3J6,7 = 7.6 Hz, 1H, H-6), 6.08 (d, 3J9a,9 = 4.0 Hz, 1H, H-9a), 5.83 (dd, 3J8,9 = 9.9 Hz, 3J9a,9 = 4.0 Hz, 1H, H-9), 5.31 (pseudo-t, 3J = 7.2 Hz, 1H, H-7), 2.32 (s, 3H, Me), 2.28 (s, 3H, Me), 2.25 (s, 3H, Me). Other signals are overlapped with those of major isomer. 13C-NMR (100.6 MHz, CDCl3): δ 142.7, 138.1, 134.8, 133.2, 131.7, 130.3, 129.7, 126.6, 126.5, 118.7, 114.9, 108.5, 101.8, 79.7, 20.2, 19.8. Other signals are overlapped with those of major isomer or cannot be seen in the spectrum due to the low concentration of minor isomer. 19F-NMR (376.3 MHz, CDCl3): δ −74.4 [C(O)CF3], −77.3 (CF3).
2,2,2-Trifluoro-1-(4-(4-methoxynaphthalen-1-yl)-2-((4-methoxynaphthalen-1-yl)ethynyl)-2-(trifluoromethyl)-2H,9aH-pyrido[2,1-b][1,3]oxazin-3-yl)ethan-1-one (3i). Obtained from pyridine 1a (0.0405 g, 0.51 mmol) and CF3-ynone 2i (0.296 g, 1.06 mmol). Yellow-brown powder, m.p. 143.5–145.5 °C (hexane), yield 0.320 g (98%). (2S*,9aS*):(2R*,9aS*)-isomers ratio is 94:6. Rotamers ratio is (93:1):(4:2) (19F-NMR). HRMS (ESI-TOF): m/z [M + H]+ Calcd for C35H24F6NO4+: 636.1604; found: 636.1608.
(2S*,9aS*)-3i: 1H-NMR (400.1 MHz, CDCl3): δ 8.39–8.36 (m, 1H), 8.25–8.22 (m, 2H), 7.70–7.47 (m, 7H), 6.87 (d, 3J = 8.1 Hz, 1H), 6.75 (d, 3J = 8.1 Hz, 1H), 6.49 (dd, 3J8,9 = 9.7 Hz, 3J7,8 = 6.1 Hz, 1H, H-8), 6.11–6.02 (m, 3H, H-6, H-9, H-9a), 5.36 (pseudo-t, 3J = 7.2 Hz, 1H, H-7), 4.08 (s, 3H, MeO), 4.01 (s, 3H, MeO). 13C-NMR (100.6 MHz, CDCl3): δ 179.9 (q, 2JCF = 34.7 Hz, C-12), 160.8 (C-4), 160.2, 156.7, 137.4, 134.4, 132.3, 131.7, 128.8, 127.6, 126.3 (C-8), 126.2 (C-6), 125.93, 125.87, 125.8, 125.1, 124.8, 123.1 (q, 1JCF = 287.1 Hz, CF3), 123.1, 122.1, 120.7, 117.0 (C-9), 115.7 [q, 1JCF = 292.9 Hz, C(O)CF3], 111.0, 109.7, 104.0, 103.5, 103.3, 86.7 (C-11), 84.9 (C-10), 78.6 (C-9a), 74.2 (q, 2JCF = 33.9 Hz, C-2), 55.9, 55.6. 19F-NMR (376.3 MHz, CDCl3): δ major rotamer −71.6 [C(O)CF3], −76.8 (CF3); minor rotamer −73.2 [C(O)CF3], −78.1 (CF3).
(2R*,9aS*)-3i′: 1H-NMR (400.1 MHz, CDCl3): δ 8.32 (d, 3J = 8.4 Hz, 1H), 7.96 (d, 3J = 8.2 Hz, 1H), 6.86 (d, 3J = 8.1 Hz, 1H), 6.81 (d, 3J = 7.9 Hz, 1H), 4.04 (s, 3H, MeO), 4.02 (s, 3H, MeO). Other signals are overlapped with those of major isomer. 13C-NMR (100.6 MHz, CDCl3): δ 137.5, 135.0, 129.0, 126.6, 125.0, 122.9, 56.0, 55.8. Other signals are overlapped with those of major isomer or cannot be seen in the spectrum due to the low concentration of minor isomer. 19F-NMR (376.3 MHz, CDCl3): δ major rotamer −74.1 [C(O)CF3], −75.9 (CF3); minor rotamer −74.3 [C(O)CF3], −76.2 (CF3).
2,2,2-Trifluoro-1-(4-phenyl-2-(phenylethynyl)-2-(trifluoromethyl)-8-vinyl-2H,9aH-pyrido[2,1-b][1,3]oxazin-3-yl)ethan-1-one (3j). Obtained from pyridine 1b (0.054 g, 0.51 mmol) and CF3-ynone 2a (0.206 g, 1.04 mmol). Brown powder, m.p. 80–83 °C (hexane), yield 0.249 g (97%). (2S*,9aS*):(2R*,9aS*)-isomers ratio is 89:11 (19F-NMR). HRMS (ESI-TOF): m/z [M + H]+ Calcd for C27H18F6NO2+: 502.1246; found: 502.1246.
(2S*,9aS*)-3j: 1H-NMR (400.1 MHz, CDCl3): δ 7.67–7.44 (m, 7H), 7.37–7.28 (m, 3H), 6.49 (d, 3J6,7 = 8.0 Hz, 1H, H-6), 6.44 (dd, 3J = 17.6 Hz, 3J = 11.0 Hz, 1H, CH=CH2), 5.90 (d, 3J9a,9 = 4.5 Hz, 1H, H-9), 5.78 (d, 3J9a,9 = 4.5 Hz, 1H H-9a), 5.77 (dd, 3J6,7 = 8.0 Hz, 4J7,9 = 1.5 Hz, 1H, H-7), 5.56 (d, 3J = 17.6 Hz, 1H, CH=CH2), 5.33 (d, 3J = 11.0 Hz, 1H, CH2, CH=CH2). 13C-NMR (100.6 MHz, CDCl3): δ 180.9 (q, 2JCF = 34.8 Hz, C-12), 160.2 (C-4), 135.2 (C, CH=CH2), 134.3, 133.3, 132.1 (Cm,m,from Ar), 131.4 (q, 1JCF = 1.7 Hz, C-3), 129.5 (br s), 129.3, 128.2 (Co,o,from Ar), 126.0 (C-6), 122.6, (q, 1JCF = 286.9 Hz, CF3), 121.0, 116.6 (CH=CH2), 114.3 (CH=CH2), 115.5 [q, 1JCF = 292.8 Hz, C(O)CF3], 109.3, 101.7 (C-7), 88.4 (C-11), 81.3 (C-10), 79.4 (C-9a), 73.9 (q, 2JCF = 34.1 Hz, C-2). 19F-NMR (376.3 MHz, CDCl3): δ −72.5 [C(O)CF3], −77.2 (CF3).
(2R*,9aS*)-3j′: 1H-NMR (400.1 MHz, CDCl3): δ 6.31 (d, 3J6,7 = 7.9 Hz, 1H, H-6), 6.13–6.11 (m, 2H, H-9, H-9a), 5.60 (dd, 3J = 7.9 Hz, 3J = 1.6 Hz, 1H, H-7), 5.53 (d, 3J = 17.4 Hz, 1H, CH=CH2), 5.51 (d, 3J = 17.2 Hz, 1H, CH=CH2), 5.31 (d, 3J = 10.0 Hz, 1H, CH=CH2). Other signals are overlapped with those of major isomer. 13C-NMR (100.6 MHz, CDCl3): δ 137.1, 135.4, 132.4, 132.2, 129.2, 128.7, 128.2, 127.2, 126.6, 126.0, 117.6, 116.5, 113.0, 99.8. Other signals are overlapped with those of major isomer or can not be seen in the spectrum due to the low concentration of minor isomer. 19F-NMR (376.3 MHz, CDCl3): δ −74.6 [C(O)CF3], −76.2 (CF3).
4-Phenyl-2-(phenylethynyl)-3-(trifluoroacetyl)-2-(trifluoromethyl)-2H,9aH-pyrido[2,1-b][1,3]oxazine-8-carbaldehyde (3k). Obtained from pyridine 1c (0.0475 g, 0.44 mmol) and CF3-ynone 2a (0.198 g, 1 mmol). Yellow powder, m.p. 77–79 °C (hexane), yield 0.178 g (80%). (2S*,9aS*):(2R*,9aS*)-isomers ratio is 89:11 (19F-NMR). HRMS (ESI-TOF): m/z [M + H]+ Calcd for C26H16F6NO3+: 504.1029; found: 504.1035.
(2S*,9aS*)-3k: 1H-NMR (400.1 MHz, CD3CN): 9.70 (s, 1H, CHO), δ 7.75–7.25 (m, 10H), 6.81 (d, 3J = 4.2 Hz, 1H, H-9), 6.66 (d, 3J = 7.8 Hz, 1H, H-6), 6.06 (d, 3J = 4.2 Hz, 1H, H-9a), 5.94 (dd, 3J = 7.8 Hz, 3J = 1.5 Hz, 1H, H-7). 13C-NMR (100.6 MHz, CD3CN): δ 192.2 (CHO), 181.7 (q, 2JCF = 34.8 Hz, C-12), 162.0 (C-4), 137.5, 134.9, 134.7, 134.0, 132.2 (q, 3JCF = 1.8 Hz), 130.9, 130.6 (br s), 130.2, 129.8, 129.6, 128.7, 123.7 (q, 1JCF = 286.0 Hz, CF3), 121.5, 116.5 [q, 1JCF = 292.5 Hz, C(O)CF3], 110.1, 99.0, 89.5 (C-11), 82.1 (C-10), 80.1 (C-9a), 75.2 (q, 2JCF = 34.3 Hz, C-2). 19F-NMR (376.3 MHz, CD3CN): δ −70.2 [C(O)CF3], −75.2 (CF3). 19F-NMR (376.3 MHz, CDCl3): δ −71.7 [C(O)CF3], −76.0 (CF3).
(2S*,9aS*)-3k′: 1H-NMR (400.1 MHz, CD3CN): 9.66 (s, 1H, CHO), 6.52–6.46 (m, 2H), 6.37 (d, 3J = 4.2 Hz, 1H), 5.77 (dd, 3J = 7.7 Hz, 3J = 1.5 Hz, 1H). Other signals are overlapped with those of major isomer. 13C-NMR (100.6 MHz, CD3CN): δ 133.8, 132.9, 130.0, 129.7, 129.6, 129.4, 128.1, 115.1, 97.1, 83.8, 78.2. Other signals are overlapped with those of major isomer or cannot be seen in the spectrum due to the low concentration of minor isomer. 19F-NMR (376.3 MHz, CD3CN): δ −72.6 [C(O)CF3], −74.4 (CF3). 19F-NMR (376.3 MHz, CDCl3): δ −73.6 [C(O)CF3], −75.2 (CF3).
1-(8-Acetyl-4-phenyl-2-(phenylethynyl)-2-(trifluoromethyl)-2H,9aH-pyrido[2,1-b][1,3]oxazin-3-yl)-2,2,2-trifluoroethan-1-one (3l). Obtained from pyridine 1d (0.030 g, 0.25 mmol) and CF3-ynone 2a (0.101 g, 0.51 mmol). Yellow powder, m.p. 122.8–124.2 °C (hexane), yield 0.071 g (55%). (2S*,9aS*):(2R*,9aS*)-isomers ratio is 87:13 (19F-NMR). HRMS (ESI-TOF): m/z [M + H]+ Calcd for C27H18F6NO3+: 518.1185; found: 518.1214.
(2S*,9aS*)-3l: 1H-NMR (400.1 MHz, CDCl3): δ 7.66–7.45 (m, 7H), 7.38–7.29 (m, 3H), 6.72 (pseudo-d, 3J9a,9 ~ 4 Hz, 1H, H-9), 6.54 (d, 3J6,7 = 7.8 Hz, 1H, H-6), 6.04 (dd, 3J6,7 = 7.8, Hz, 3J7,9 = 1.6 Hz, 1H, H-7), 5.91 (d, 3J9a,9 = 4.2 Hz, 1H, H-9a), 2.48 (s, 3H, Me). 13C-NMR (100.6 MHz, CDCl3): δ 195.6, 180.9 (q, 2JCF = 35.4 Hz, C-12), 159.3 (C-4), 136.5, 133.4, 132.1, 131.1 (q, 1JCF = 1.7 Hz, C-3), 129.6 (br s), 129.5, 128.3 (Co,o,from Ar), 126.7 (C-6), 122.4 (q, 1JCF = 286.6 Hz, CF3), 121.2 (C-9), 120.8 (Ci from Ar), 115.4 [q, 1JCF = 292.8 Hz, C(O)CF3], 110.0, 100.3 (C-7), 89.1 (C-11), 80.9 (C-10), 78.8 (C-9a), 74.4 (q, 2JCF = 34.1 Hz, C-2), 25.3. 19F-NMR (376.3 MHz, CDCl3): δ −72.7 [C(O)CF3], −77.2 (CF3).
(2R*,9aS*)-3l′: 1H-NMR (400.1 MHz, CDCl3): δ 6.57 (pseudo-d, 3J ~ 4 Hz, 1H, H-9), 6.37 (d, 3J6,7 = 7.8 Hz, 1H, H-6), 6.28 (d, 3J9a,9 = 4.3 Hz, 1H, H-9a), 5.88 (dd, 3J6,7 = 7.8 Hz, 3J7,9 = 1.5 Hz, 1H, H-7), 2.46 (s, 3H, Me). Other signals are overlapped with those of major isomer. 13C-NMR (100.6 MHz, CDCl3): δ 136.6, 132.6, 132.3, 129.4, 128.3, 127.3, 119.9, 98.5, 29.7. Other signals are overlapped with those of major isomer or cannot be seen in the spectrum due to the low concentration of minor isomer. 19F-NMR (376.3 MHz, CDCl3): δ −74.7 [C(O)CF3], −76.3 (CF3).
Methyl 4-phenyl-2-(phenylethynyl)-3-(2,2,2-trifluoroacetyl)-2-(trifluoromethyl)-2H,9aH- pyrido[2,1-b][1,3]oxazine-8-carboxylate (3m). Obtained from pyridine 1e (0.048 g, 0.35 mmol) and CF3-ynone 2a (0.147 g, 0.74 mmol). Pale yellow powder, m.p. 115.4–116.5 °C (hexane), yield 0.112 g (60%). (2S*,9aS*):(2R*,9aS*)-isomers ratio is 87:13 (19F-NMR). HRMS (ESI-TOF): m/z [M + H]+ Calcd for C27H18F6NO4+: 534.1135; found: 534.1140.
(2S*,9aS*)-3m: 1H-NMR (400.1 MHz, CDCl3): δ 7.66–7.44 (m, 7H), 7.37–7.29 (m, 3H), 6.89 (pseudo-d, 3J ~ 4 Hz, 1H, H-9), 6.52 (d, 3J6,7 = 7.8 Hz, 1H, H-6), 5.99 (dd, 3J6,7 = 7.8 Hz, 3J7,9 = 1.5 Hz, 1H, H-7), 5.87 (d, 3J9a,9 = 4.2 Hz, 1H, H-9a), 3.85 (s, 3H, Me). 13C-NMR (100.6 MHz, CDCl3): δ 181.0 (q, 2JCF = 35.4 Hz, C-12), 164.6 (C-4), 159.2 (CO2Me), 133.4, 132.1, 131.2 (q, 1JCF = 1.7 Hz, C-3), 130.1, 129.6 (br s), 129.5 (C-8), 128.3 (Co,o,from Ar), 126.5 (C-6), 122.4, (q, 1JCF = 287.3 Hz, CF3), 121.5 (C-9), 120.8 (Ci from Ar), 115.4 (q, 1JCF = 293.0 Hz, C(O)CF3), 110.3, 101.5 (C-7), 89.0 (C-11), 80.9 (C-10), 78.8 (C-9a), 74.3 (q, 2JCF = 34.8 Hz, C-2), 52.5. 19F-NMR (376.3 MHz, CDCl3): δ −72.7 [C(O)CF3], −77.2 (CF3).
(2R*,9aS*)-3m′: 1H-NMR (400.1 MHz, CDCl3): δ 6.74 (pseudo-d, 3J ~ 4 Hz, 1H, H-9), 6.35 (d, 3J6,7 = 7.8 Hz, 1H, H-6), 6.24 (d, 3J9a,9 = 4.3 Hz, 1H, H-9a), 5.83 (dd, 3J6,7 = 7.8 Hz, 3J7,9 = 1.5 Hz, 1H, H-7), 3.84 (s, 3H, Me). Other signals are overlapped with those of major isomer. 13C-NMR (100.6 MHz, CDCl3): δ 132.6, 132.3, 130.3, 129.4, 128.3, 127.1, 120.1, 99.8, 52.5. Other signals are overlapped with those of major isomer or cannot be seen in the spectrum due to the low concentration of minor isomer. 19F-NMR (376.3 MHz, CDCl3): δ −74.7 [C(O)CF3], −76.4 (CF3).
(2S*,9aS*)-1-(4,6-diPhenyl-2-(phenylethynyl)-2-(trifluoromethyl)-2H,9aH-pyrido[2,1-b][1,3]oxazin-3-yl)-2,2,2-trifluoroethan-1-one (3n). Obtained from pyridine 1f (0.079 g, 0.51 mmol) and CF3-ynone 2a (0.204 g, 1.03 mmol). Orange powder, m.p. 90–91 °C (hexane), yield 0.168 g (60%). HRMS (ESI-TOF): m/z [M + H]+ Calcd for C31H20F6NO2+: 552.1393; found: 552.1393. (2S*,9aS*)-3o: 1H-NMR (400.1 MHz, CDCl3): δ 7.46–7.43 (m, 2H), 7.38–7.28 (m, 3H), 7.17–6.99 (m, 5H), 6.93 (br s, 5H), 6.61 (ddd, 3J8,9 = 9.7 Hz, 3J7,8 = 6.1 Hz, 4J8,9a = 0.8 Hz, 1H, H-8), 6.00 (ddd, 3J8,9 = 9.7 Hz, 3J9a,9 = 4.2 Hz, 4J7,9 = 0.7 Hz, 1H, H-9), 5.84 (d, 3J9a,9 = 4.2 Hz, 1H, H-9a), 5.43 (dd, 3J7,8 = 6.1 Hz, 3J7,9 = 0.7 Hz, 1H, H-7). 13C-NMR (100.6 MHz, CDCl3): δ 184.2 (q, 2JCF = 36.1 Hz, C-12), 157.3 (C-4), 139.2, 136.4, 134.7, 133.9, 132.5, 132.1, 131.4, 129.5, 128.9, 128.4, 128.3, 127.8, 127.7, 127.2, 122.8 (q, 1JCF = 285.7 Hz, CF3), 120.7, 115.0 [q, 1JCF = 293.6 Hz, C(O)CF3], 114.7, 106.7, 89.7 (C-11), 81.5 (C-9a), 81.2 (C-10), 74.5 (q, 2JCF = 34.8 Hz, C-2). 19F-NMR (376.3 MHz, CDCl3): δ −73.9 [C(O)CF3], −76.0 (CF3).
1-(9-Bromo-4-phenyl-2-(phenylethynyl)-2-(trifluoromethyl)-2H,9aH-pyrido[2,1-b][1,3]oxazin-3-yl)-2,2,2-trifluoroethan-1-one (3p). Major (9-Br)-regioisomer, obtained as a mixture (1:5) with minor (7-Br)-regioisomer (3o) from pyridine 1g (0.082 g, 0.52 mmol) and CF3-ynone 2a (0.208 g, 1.05 mmol). Yellow powder, m.p. 76.0–77.8 °C (hexane), yield 0.153 g (53%). (2S*,9aS*):(2R*,9aS*)-isomers ratio of 3p is 80:20 (19F-NMR). HRMS (ESI-TOF) for the mixture of 3o and 3p: m/z [M + H]+ Calcd for C25H15F6BrNO2+: 554.0185; found: 554.0190.
(2S*,9aS*)-3p: 1H-NMR (400.1 MHz, CDCl3): δ 7.65–7.30 (m, 10H), 6.80 (d, 3J6,7 = 6.6 Hz, 1H, H-6), 6.45 (d, 3J7,8 = 7.5 Hz, 1H, H-8), 5.79 (s, 1H, H-9a), 5.38 (pseudo-t, 3J ~ 7 Hz, 1H, H-7). 13C-NMR (100.6 MHz, CDCl3): δ 181.1 (q, 2JCF = 35.6 Hz, C-12), 158.7 (C-4), 133.4, 132.3, 132.1, 131.1 (q, 3JCF = 1.8 Hz, C-3), 129.6 (br s), 129.4 (C-8), 128.7 (C-6), 128.3, 125.2, 122.4 (q, 1JCF = 286.4 Hz, CF3), 121.0 (Ci from Ar), 117.4 (C-9), 115.4 [q, 1JCF = 292.6 Hz, C(O)CF3], 109.9, 103.3 (C-7), 89.2 (C-11), 82.9 (C-10), 80.5 (C-9a), 74.7 (q, 2JCF = 34.8 Hz, C-2). 19F-NMR (376.3 MHz, CDCl3): δ −72.7 [C(O)CF3], −76.8 (CF3).
(2R*,9aS*)-3p′: 1H-NMR (400.1 MHz, CDCl3): δ 6.75 (d, 3J6,7 = 6.7 Hz, 1H, H-6), 6.30 (d, 3J7,8 = 7.6 Hz, 1H, H-8), 6.16 (s, 1H, H-9a), 5.25 (pseudo-t, 3J ~ 7 Hz, 1H, H-7). Other signals are overlapped with those of major isomer. 13C-NMR of (2R*,9aS*)-3p′ and 13C-NMR of (2S*,9aS*)-3o (100.6 MHz, CDCl3): δ 158.6 (C-4), 152.3, 133.5, 132.6, 132.2, 130.8, 131.0 (q, 3JCF = 1.3 Hz, C-3), 130.5, 129.5, 129.3, 128.94, 128.91, 128.36, 128.31, 125.7, 121.5, 121.3, 120.8, 110.8, 101.8, 89.02 and 88.98 (C-11), 83.53 and 83.49 (C-10), 81.3 and 80.9 (C-9a), 77.8. Due to low concentration and equal amounts of (2R*,9aS*)-3p′ and 13C-NMR of (2S*,9aS*)-3o assignment of their signals cannot be done. 13C-NMR are reported together. Other signals are overlapped with those of major isomer 3p or cannot be seen in the spectrum due to the low concentration of minor isomers. 19F-NMR (376.3 MHz, CDCl3): δ −74.7 [C(O)CF3], −75.9 (CF3).
1-(7-Bromo-4-phenyl-2-(phenylethynyl)-2-(trifluoromethyl)-2H,9aH-pyrido[2,1-b][1,3]oxazin-3-yl)-2,2,2-trifluoroethan-1-one (3p). Minor (7-Br)-regioisomer, obtained as a mixture with major (9-Br)-regioisomer (3q) (see above). (2S*,9aS*):(2R*,9aS*)-isomers ratio is 78:22 (19F-NMR). HRMS (ESI-TOF) for the mixture of 3o and 3p: m/z [M + H]+ Calcd for C25H15F6BrNO2+: 554.0185; found: 554.0190.
(2S*,9aS*)-3o: 1H-NMR (400.1 MHz, CDCl3): δ 6.65 (s, 1H, H-6), 6.55 (d, 3J8,9 = 10.1 Hz, 1H, H-8), 5.98 (dd, 3J8,9 = 10.1 Hz, 3J9a,9 = 3.9 Hz, 1H, H-9), 5.71 (d, 3J9,9a = 3.9 Hz, 1H, H-9a). Other signals are overlapped with those of major isomer. 13C-NMR (100.6 MHz, CDCl3): See above in 3p section. 19F-NMR (376.3 MHz, CDCl3): δ −72.7 [C(O)CF3], −77.1 (CF3).
(2R*,9aS*)-3o′: 1H-NMR (400.1 MHz, CDCl3): δ 6.62 (s, 1H, H-6), 6.40 (d, 3J8,9 = 10.1 Hz, 1H, H-8), 5.84 (dd, 3J8,9 = 10.1 Hz, 3J9a,9 = 4.2 Hz, 1H, H-9), 6.06 (d, 3J9,9a = 4.2 Hz, 1H, H-9a). Other signals are overlapped with those of major isomer. 13C-NMR (100.6 MHz, CDCl3): cannot be seen in the spectrum due to the low concentration of minor isomer. 19F-NMR (376.3 MHz, CDCl3): δ −74.7 [C(O)CF3], −75.6 (CF3).
1-(7-Acetyl-4-phenyl-2-(phenylethynyl)-2-(trifluoromethyl)-2H,9aH-pyrido[2,1-b][1,3]oxazin-3-yl)-2,2,2-trifluoroethan-1-one (3q). Major (7-Ac)-regioisomer, obtained as a mixture (2:1) with minor (9-Ac)-regioisomer (3r) from pyridine 1h (0.065 g, 0.54 mmol) and CF3-ynone 2a (0.214 g, 1.08 mmol). Yellow powder, m.p. 114.4–115.3 °C (hexane), yield 0.224 g (80%). (2S*,9aS*):(2R*,9aS*)-isomers ratio is 83:17 (1H-NMR). HRMS (ESI-TOF) for the mixture of 3q and 3r: m/z [M + H]+ Calcd for C27H18F6NO3+: 518.1185; found: 518.1196.
(2S*,9aS*)-3q: 1H-NMR (400.1 MHz, CDCl3): δ 7.69–7.29 (m, 11H), 7.10 (d, 3J8,9 = 10.1 Hz, 1H, H-8), 6.00 (dd, 3J8,9 = 10.1 Hz, 3J9a,9 = 3.6 Hz, 1H, H-9), 5.88 (d, 3J9a,9 = 3.6 Hz, 1H, H-9a), 2.12 (s, 3H, Me). 13C-NMR (100.6 MHz, CDCl3): δ 193.1, 182.1 (q, 2JCF = 36.3 Hz, C-12), 156.2 (C-4), 133.7, 133.6, 132.1, 129.9, 129.7, 128.4 (C-8), 130.4 (q, 3JCF = 1.3 Hz, C-3), 124.2 (C-6), 122.2 (q, 1JCF = 286.0 Hz, CF3), 120.4 (Ci from Ar), 115.3 (C-9), 115.0 (q, 1JCF = 293.0 Hz, C(O)CF3), 102.3, 90.1 (C-11), 80.3 (C-10), 79.1 (C-9a), 74.2 (q, 2JCF = 34.8 Hz, C-2), 25.0. 19F-NMR (376.3 MHz, CDCl3): δ −73.7 [C(O)CF3], −76.8 (CF3).
(2R*,9aS*)-3q′: 1H-NMR (400.1 MHz, CDCl3): δ 7.17 (s, 1H, H-6), 7.05 (d, 3J8,9 = 10.2 Hz, 1H, H-8), 6.17 (d, 3J9a,9 = 3.6 Hz, 1H, H-9a), 5.90 (dd, 3J8,9 = 10.2 Hz, 3J9a,9 = 3.6 Hz, 1H, H-9), 2.06 (s, 3H, Me). 13C-NMR (100.6 MHz, CDCl3): δ 193.0, 131.8, 128.3, 114.5, 24.9. Other signals are overlapped with those of major isomer or cannot be seen in the spectrum due to the low concentration of minor isomer. 19F-NMR (376.3 MHz, CDCl3): δ −74.9 [C(O)CF3], −75.5 (CF3).
1-(9-Acetyl-4-phenyl-2-(phenylethynyl)-2-(trifluoromethyl)-2H,9aH-pyrido[2,1-b][1,3]oxazin-3-yl)-2,2,2-trifluoroethan-1-one (3r). Minor (9-Ac)-regioisomer, obtained as a mixture with major (7-Ac)-regioisomer (3q) (see above). (2S*,9aS*):(2R*,9aS*)-isomers ratio is 87:13 (1H-NMR). HRMS (ESI-TOF) for the mixture of 3q and 3r: m/z [M + H]+ Calcd for C27H18F6NO3+: 518.1185; found: 518.1196.
(2S*,9aS*)-3r: 1H-NMR (400.1 MHz, CDCl3): δ 7.69–7.29 (m, 11H), 6.65 (d, 3J6,7 = 7.3 Hz, 1H, H-6), 6.20 (s, 1H, H-9a), 5.62 (pseudo-t, 3J ~ 7 Hz, 1H, H-7), 2.47 (s, 3H, Me). 13C-NMR (100.6 MHz, CDCl3): δ 194.6, 181.5 (q, 2JCF = 35.8 Hz, C-12), 158.0 (C-4), 134.2, 133.5, 132.1, 131.0 (q, 3JCF = 1.7 Hz, C-3), 129.4, 128.3, (C-8), 124.9 (C-6), 122.3 (q, 1JCF = 286.6 Hz, CF3), 120.9 (Ci from Ar), 115.7 (C-9), 115.2 (q, 1JCF = 293.0 Hz, C(O)CF3), 89.6 (C-11), 80.0 (C-10), 77.7 (C-9a), 74.4 (q, 2JCF = 34.5 Hz, C-2), 25.7. 19F-NMR (376.3 MHz, CDCl3): δ −73.0 [C(O)CF3], −76.8 (CF3).
(2R*,9aS*)-3r′: 1H-NMR (400.1 MHz, CDCl3): δ 6.58 (s, 1H, H-9a), 6.51 (d, 3J6,7 = 7.4 Hz, 1H, H-6), 5.62 (pseudo-t, 3J ~ 7 Hz, 1H, H-7), 2.43 (s, 3H, Me). Other signals are overlapped with those of major isomer. 13C-NMR (100.6 MHz, CDCl3): δ 194.7, 134.3, 133.0, 132.2, 129.5, 114.9, 25.5. Other signals are overlapped with those of major isomer or cannot be seen in the spectrum due to the low concentration of minor isomer. 19F-NMR (376.3 MHz, CDCl3): δ −74.9 [C(O)CF3], −76.4 (CF3).
4-Phenyl-2-(phenylethynyl)-3-(2,2,2-trifluoroacetyl)-2-(trifluoromethyl)-2H,9aH-pyrido[2,1-b][1,3]oxazine-7-carbonitrile (3s). Major (7-CN)-regioisomer, obtained as a mixture (2.5:1) with minor (9-CN)-regioisomer (3t) from pyridine 1i (0.054 g, 0.5 mmol) and CF3-ynone 2a (0.208 g, 1.05 mmol). Yellow powder, m.p. 95–96 °C (hexane), yield 0.165 g (66%). (2S*,9aS*):(2R*,9aS*)-isomers ratio is 76:24 (19F-NMR). HRMS (ESI-TOF) for the mixture of 3s and 3t: m/z [M + H]+ Calcd for C26H15F6N2O2+: 501.1032; found: 501.1055.
(2S*,9aS*)-3s: 1H-NMR (400.1 MHz, CDCl3): δ 7.69–7.28 (m, 10H), 6.98 (s, 1H, H-6), 6.49 (d, 3J8,9 = 10.0 Hz, 1H, H-8), 6.00 (dd, 3J8,9 = 10.0 Hz, 3J9a,9 = 3.5 Hz, 1H, H-9), 5.92–5.89 (m, 1H, H-9a). 13C-NMR (100.6 MHz, CDCl3): δ 182.0 (q, 2JCF = 37.0 Hz, C-12), 154.5 (C-4), 136.0, 133.7, 132.0, 130.0, 129.9, 128.4, 130.3 (q, 4JCF = 1.7 Hz, C-3), 124.4 (C-6), 122.1 (q, 1JCF = 286.4 Hz, CF3), 120.2 (Ci from Ar), 116.3 (C-9), 114.9 [q, 1JCF = 293.0 Hz, C(O)CF3], 113.3 (CN), 101.4, 88.5 (C-11), 79.9 (C-10), 78.3 (C-9a), 74.3 (q, 2JCF = 34.7 Hz, C-2). 19F-NMR (376.3 MHz, CDCl3): δ −73.9 [C(O)CF3], −76.8 (CF3).
(2R*,9aS*)-3s′: 1H-NMR (400.1 MHz, CDCl3): δ 6.87 (s, 1H, H-6), 6.44 (d, 3J8,9 = 10.0 Hz, 1H, H-8), 6.20 (d, 3J9a,9 = 3.6 Hz, 1H, H-9a). 13C-NMR (100.6 MHz, CDCl3): δ 149.8, 136.5, 133.1, 132.2, 120.8, 115.6, 112.4 (CN), 100.3, 86.8, 80.1, 78.8 (q, 4JCF = 3.7 Hz, C-10), 72.1 (q, 2JCF = 32.4 Hz, C-2). Other signals are overlapped with those of major isomer or cannot be seen in the spectrum due to the low concentration of minor isomer. 19F-NMR (376.3 MHz, CDCl3): δ −75.0 [C(O)CF3], −76.5 (CF3).
4-Phenyl-2-(phenylethynyl)-3-(2,2,2-trifluoroacetyl)-2-(trifluoromethyl)-2H,9aH-pyrido[2,1-b][1,3]oxazine-7-carbonitrile (3t). Minor (9-CN)-regioisomer, obtained as a mixture with major (7-CN)-regioisomer (3s) (see above). (2S*,9aS*):(2R*,9aS*)-isomers ratio is 86:14 (19F-NMR). HRMS (ESI-TOF) for the mixture of 3s and 3t: m/z [M + H]+ Calcd for C26H15F6N2O2+: 501.1032; found: 501.1055.
(2S*,9aS*)-3t: 1H-NMR (400.1 MHz, CDCl3): δ 7.69–7.28 (m, 10H), 7.14 (d, 3J7,8 = 6.5 Hz, 1H, H-8), 6.64 (d, 3J6,7 = 7.5 Hz, 1H, H-6), 5.92–5.89 (m, 1H, H-9a), 5.54 (pseudo-t, 3J ~ 7 Hz, 1H, H-7). 13C-NMR (100.6 MHz, CDCl3): δ 182.5 (q, 2JCF = 37.2 Hz, C-12), 156.4 (C-4), 139.3, 133.6, 132.1, 131.3, 129.7, 129.6, 129.3, 128.3, 124.3 (C-6), 122.1 (q, 1JCF = 286.6 Hz, CF3), 120.4 (Ci from Ar), 117.2 (C-9), 115.1 [q, 1JCF = 293.2 Hz, C(O)CF3], 112.5 (CN), 98.7, 90.7 (C-11), 87.7, 79.8 (C-10), 78.2 (C-9a), 74.5 (q, 2JCF = 35.6 Hz, C-2). 19F-NMR (376.3 MHz, CDCl3): δ −73.5 [C(O)CF3], −76.8 (CF3).
(2R*,9aS*)-3t′: 1H-NMR (400.1 MHz, CDCl3): δ 7.09 (d, 3J7,8 = 6.4 Hz, 1H, H-8), 6.23 (s, 1H, H-9a). Other signals are overlapped with those of major isomer. 13C-NMR (100.6 MHz, CDCl3): δ 149.5, 132.9, 132.7, 97.8, 86.3, 90.4. Other signals are overlapped with those of major isomer or cannot be seen in the spectrum due to the low concentration of minor isomer. 19F-NMR (376.3 MHz, CDCl3): δ −74.9 [C(O)CF3], −76.5 (CF3).
4. Conclusions
In conclusion, a new efficient pathway towards to trifluoromethylated oxazinopyridines was elaborated on the base of a one-pot, metal-free 1:2 assembly of pyridines and CF3-ynones. The reaction has a broad scope in terms of both pyridines and CF3-ynones used. Therefore, pyridines with electron withdrawing as well as electron donating groups afforded corresponding products in up to 99% yield. Various CF3-ynones including bulky ones can also be involved in the reaction. High stereoselectivity (up to 100% for 2-substitueted pyridines) is the advantage of the method. However, dramatic influence of the pKa values of pyridines on the reaction course was observed. Pyridines having pKa lower than ~1 do not react with CF3-ynones. The possible mechanism of the reaction includes a cascade of ionic transformations triggered by attack of the nitrogen of pyridine molecule by electron-deficient triple bond of CF3-ynone.
Acknowledgments
The authors acknowledge partial support in measuring oF-NMR from M.V. Lomonosov Moscow State University Program of Development. The authors acknowledge Thermo Fisher Scientific Inc., MS Analytica (Moscow, Russia), and personally to A. Makarov for providing mass spectrometry equipment for this work.
Supplementary Materials
Copy of all 1H-, 13C- and 19F-NMR spectra are available online at https://www.mdpi.com/1420-3049/24/19/3594/s1.
Author Contributions
Conceptualization, V.M.M., B.A.T., and V.G.N.; investigation, V.M.M., Z.A.S., K.V.B.; writing—original draft preparation, V.M.M.; writing—review and editing, V.M.M., B.A.T., K.V.B.; V.G.N.; visualization, V.M.M.; funding acquisition, V.G.N.
Funding
This research was funded by Russian Science Foundation grant number 18-13-00136.
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Buckingham J., Baggaley K.H., Roberts A.D., Szabó L.F. Dictionary of Alkaloids. 2nd ed. CRC Press, Taylor and Francis Group; Boca Raton, FL, USA: 2010. [Google Scholar]
- 2.Vitaku E., Smith D.T., Njardarson J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014;57:10257–10274. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 3.Taylor R.D., MacCoss M., Lawson A.D. Rings in drugs: Miniperspective. Med. Chem. 2014;57:5845–5859. doi: 10.1021/jm4017625. [DOI] [PubMed] [Google Scholar]
- 4.McGrath N.A., Brichacek M., Njardarson J.T. A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives. J. Chem. Ed. 2010;87:1348–1349. doi: 10.1021/ed1003806. [DOI] [Google Scholar]
- 5.Liang T., Neumann C.N., Ritter T. Introduction of fluorine and fluorine-containing functional groups. Angew. Chem. Int. Ed. 2013;52:8214–8264. doi: 10.1002/anie.201206566. [DOI] [PubMed] [Google Scholar]
- 6.Yang X., Wu T., Phipps R.J., Toste F.D. Advances in catalytic enantioselective fluorination, mono-, di-, and trifluoromethylation, and trifluoromethylthiolation reactions. Chem. Rev. 2015;115:826–870. doi: 10.1021/cr500277b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahrens T., Kohlmann J., Ahrens M., Braun T. Functionalization of fluorinated molecules by transition metal mediated C−F bond activation to access fluorinated building blocks. Chem. Rev. 2015;115:931–972. doi: 10.1021/cr500257c. [DOI] [PubMed] [Google Scholar]
- 8.Nenajdenko V.G., Muzalevskiy V.M., Shastin A.V. Polyfluorinated ethanes as versatile fluorinated C2-building blocks for organic synthesis. Chem. Rev. 2015;115:973–1050. doi: 10.1021/cr500465n. [DOI] [PubMed] [Google Scholar]
- 9.Yerien D.E., Barata-Vallejo S., Postigo A. Difluoromethylation reactions of organic compounds. Chem. Eur. J. 2017;23:14676–14701. doi: 10.1002/chem.201702311. [DOI] [PubMed] [Google Scholar]
- 10.Kirsch P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications. Wiley-VCH; Weinheim, Germany: 2013. [Google Scholar]
- 11.Uneyama K. Organofluorine Chemistry. Blackwell Publishing; Oxford, UK: 2006. [Google Scholar]
- 12.Theodoridis G. Fluorine-containing agrochemicals: An overview of recent developments. In: Tressaud A., editor. Fluorine and the Environment: Agrochemicals, Archaeology, Green Chemistry & Water. Elsevier; Amsterdam, The Netherland: 2006. pp. 121–175. [Google Scholar]
- 13.Bégué J.P., Bonnet-Delpon D. Bioorganic and Medicinal Chemistry of Fluorine. John Wiley & Sons; Hoboken, NJ, USA: 2008. [Google Scholar]
- 14.Tressaud A., Haufe G. Fluorine and Health: Molecular Imaging, Biomedical Materials and Pharmaceuticals. Elsevier; Amsterdam, The Netherland: 2008. pp. 553–778. [Google Scholar]
- 15.Soloshonok V.A., Mikami K., Yamazaki T., Welch J.T., Honek J.F. Current Fluoroorganic Chemistry. New Synthetic Directions, Technologies, Materials, and Biological Applications. American Chemical Society; Washington, DC, USA: 2007. (ACS Symposium Series 949). [Google Scholar]
- 16.Meanwell N.A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018;61:5822–5880. doi: 10.1021/acs.jmedchem.7b01788. [DOI] [PubMed] [Google Scholar]
- 17.Gillis E.P., Eastman K.J., Hill M.D., Donnelly D.J., Meanwell N.A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 2015;58:8315–8359. doi: 10.1021/acs.jmedchem.5b00258. [DOI] [PubMed] [Google Scholar]
- 18.Zhu W., Wang J., Wang S., Gu Z., Aceña J.L., Izawa K., Liu H., Soloshonok V.A. Recent advances in the trifluoromethylation methodology and new CF3-containing drugs. J. Fluorine Chem. 2014;167:37–54. doi: 10.1016/j.jfluchem.2014.06.026. [DOI] [Google Scholar]
- 19.Purser S., Moore P.R., Swallow S., Gouverneur V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008;37:320–330. doi: 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]
- 20.Hagmann W.K. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 2008;51:4359–4369. doi: 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y., Wang J., Gu Z., Wang S., Zhu W., Aceña J.L., Soloshonok V.A., Izawa K., Liu H. Next generation of fluorine containing pharmaceuticals, compounds currently in phase II−III clinical trials of major pharmaceutical companies: New structural trends and therapeutic areas. Chem. Rev. 2016;116:422–518. doi: 10.1021/acs.chemrev.5b00392. [DOI] [PubMed] [Google Scholar]
- 22.Wang J., Sánchez-Roselló M., Aceña J.L., del Pozo C., Sorochinsky A.E., Fustero S., Soloshonok V.A., Liu H. Fluorine in pharmaceutical industry: Fluorine-containing drugs introduced to the market in the last decade (2001–2011) Chem. Rev. 2014;114:2432–2506. doi: 10.1021/cr4002879. [DOI] [PubMed] [Google Scholar]
- 23.Ilardi E.A., Vitaku E., Njardarson J.T. Data-mining for sulfur and fluorine: An evaluation of pharmaceuticals to reveal opportunities for drug design and discovery. J. Med. Chem. 2014;57:2832–2842. doi: 10.1021/jm401375q. [DOI] [PubMed] [Google Scholar]
- 24.Jeschke P. The unique role of fluorine in the design of active ingredients for modern crop protection. ChemBioChem. 2004;5:570–589. doi: 10.1002/cbic.200300833. [DOI] [PubMed] [Google Scholar]
- 25.Jeschke P. The unique role of halogen substituents in the design of modern agrochemicals. Pest Manage. Sci. 2010;66:10–27. doi: 10.1002/ps.1829. [DOI] [PubMed] [Google Scholar]
- 26.Fujiwara T., O’Hagan D. Successful fluorine-containing herbicide agrochemicals. J. Fluorine Chem. 2014;167:16–29. doi: 10.1016/j.jfluchem.2014.06.014. [DOI] [Google Scholar]
- 27.Jeschke P. Latest generation of halogen-containing pesticides. Pest Manage. Sci. 2017;73:1053–1056. doi: 10.1002/ps.4540. [DOI] [PubMed] [Google Scholar]
- 28.de la Torre B.G., Albericio F. The Pharmaceutical Industry in 2018. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules. 2019;24:809. doi: 10.3390/molecules24040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nenajdenko V.G. Fluorine in Heterocyclic Chemistry. Springer; Berlin, Germany: 2014. pp. 681–760. [Google Scholar]
- 30.Petrov V.A., editor. Fluorinated Heterocyclic Compounds: Synthesis, Chemistry, and Applications. Wiley; Hoboken, NJ, USA: 2009. [Google Scholar]
- 31.Gakh A., Kirk K.L. Fluorinated Heterocycles. Oxford University Press; Oxford, UK: 2008. [Google Scholar]
- 32.Muzalevskiy V.M., Nenajdenko V.G., Shastin A.V., Balenkova E.S., Haufe G. Synthesis of Trifluoromethyl Pyrroles and Their Benzo Analogues. Synthesis. 2009;23:3905–3929. [Google Scholar]
- 33.Serdyuk O.V., Abaev V.T., Butin A.V., Nenajdenko V.G. Synthesis of Fluorinated Thiophenes and Their Analogues. Synthesis. 2011;16:2505–2529. doi: 10.1055/s-0030-1260088. [DOI] [Google Scholar]
- 34.Serdyuk O.V., Muzalevskiy V.M., Nenajdenko V.G. Synthesis and Properties of Fluoropyrroles and Their Analogues. Synthesis. 2012;14:2115–2137. [Google Scholar]
- 35.Politanskaya L.V., Selivanova G.A., Panteleeva E.V., Tretyakov E.V., Platonov V.E., Nikul’shin P.V., Vinogradov A.S., Zonov Y.V., Karpov V.M., Mezhenkova T.V., et al. Organofluorine chemistry: Promising growth areas and challenges. Rus. Chem. Rev. 2019;88:425–569. doi: 10.1070/RCR4871. [DOI] [Google Scholar]
- 36.Shimizu M., Hiyama T. Modern Synthetic Methods for Fluorine-Substituted Target Molecules. Angew. Chem. 2005;44:214–231. doi: 10.1002/anie.200460441. [DOI] [PubMed] [Google Scholar]
- 37.Druzhinin S.V., Balenkova E.S., Nenajdenko V.G. Recent advances in the chemistry of α,β-unsaturated trifluoromethylketones. Tetrahedron. 2007;63:7753–7808. doi: 10.1016/j.tet.2007.04.029. [DOI] [Google Scholar]
- 38.Nenajdenko V.G., Sanin A.V., Balenkova E.S. Methods for the synthesis of α,β-unsaturated trifluoromethyl ketones and their use in organic synthesis. Russ. Chem. Rev. 1999;68:483–505. doi: 10.1070/RC1999v068n06ABEH000441. [DOI] [Google Scholar]
- 39.Nenajdenko V.G., Sanin A.V., Balenkova E.S. Preparation of α,β-Unsaturated Ketones Bearing a Trifluoromethyl Group and Their Application in Organic Synthesis. Molecules. 1997;12:186–232. doi: 10.3390/21200186. [DOI] [Google Scholar]
- 40.Rulev A.Y. The Wonderful Chemistry of Trifluoromethyl α-Haloalkenyl Ketones. Eur. J. Org. Chem. 2018;27–28:3609–3617. doi: 10.1002/ejoc.201800194. [DOI] [Google Scholar]
- 41.Romanov A.R., Rulev A. Yu., Ushakov I.A., Muzalevskiy V.M., Nenajdenko V.G. Synthesis of trifluoromethylated [1,4]-diazepines based on cf3-ynones. Mendeleev Commun. 2014;24:269–271. doi: 10.1016/j.mencom.2014.09.007. [DOI] [Google Scholar]
- 42.Romanov A.R., Rulev A.Y., Ushakov I.A., Muzalevskiy V.M., Nenajdenko V.G. One-Pot, Atom and Step Economy (PASE) Assembly of Trifluoromethylated Pyrimidines from CF3-Ynones. Eur. J. Org. Chem. 2017;28:4121–4129. doi: 10.1002/ejoc.201700727. [DOI] [Google Scholar]
- 43.Muzalevskiy V.M., Iskandarov A.A., Nenajdenko V.G. Reaction of CF3-ynones with methyl thioglycolate. Regioselective synthesis of 3-CF3-thiophene derivatives. J. Fluorine Chem. 2018;214:13–16. doi: 10.1016/j.jfluchem.2018.07.013. [DOI] [Google Scholar]
- 44.Muzalevskiy V.M., Mamedzade M.N., Chertkov V.A., Bakulev V.A., Nenajdenko V.G. Reaction of CF3-ynones with azides. An efficient regioselective and metal-free route to 4-trifluoroacetyl-1,2,3-triazoles. Mendeleev Commun. 2018;28:17–19. doi: 10.1016/j.mencom.2018.01.003. [DOI] [Google Scholar]
- 45.Muzalevskiy V.M., Iskandarov A.A., Nenajdenko V.G. Synthesis of dibromo substituted cf3-enones and their reactions with n-nucleophiles. Mendeleev Commun. 2014;24:342–344. doi: 10.1016/j.mencom.2014.11.009. [DOI] [Google Scholar]
- 46.Muzalevskiy V.M., Rulev A.Y., Romanov A.R., Kondrashov E.V., Ushakov I.A., Chertkov V.A., Nenajdenko V.G. Selective, Metal-Free Approach to 3- or 5-CF3-Pyrazoles: Solvent Switchable Reaction of CF3-Ynones with Hydrazines. J. Org. Chem. 2017;82:7200–7214. doi: 10.1021/acs.joc.7b00774. [DOI] [PubMed] [Google Scholar]
- 47.Topchiy M.A., Zharkova D.A., Asachenko A.F., Muzalevskiy V.M., Chertkov V.A., Nenajdenko V.G., Nechaev M.S. Mild and Regioselective Synthesis of 3-CF3-Pyrazoles by the AgOTf-Catalysed Reaction of CF3-Ynones with Hydrazines. Eur. J. Org. Chem. 2018;27–28:3750–3755. doi: 10.1002/ejoc.201800208. [DOI] [Google Scholar]
- 48.Trofimov B.A., Belyaeva K.V., Nikitina L.P., Afonin A.V., Vashchenko A.V., Muzalevskiy V.M., Nenajdenko V.G. Metal-free stereoselective annulation of quinolines with trifluoroacetylacetylenes and water: An access to fluorinated oxazinoquinolines. Chem. Commun. 2018;54:2268–2271. doi: 10.1039/C7CC09725E. [DOI] [PubMed] [Google Scholar]
- 49.Muzalevskiy V.M., Trofimov B.A., Belyaeva A.V., Nenajdenko V.G. Green, diastereoselective synthesis of CF3-oxazinoquinolines in water. Green Chem. 2019 Submitted (under revision) [Google Scholar]
- 50.Belyaeva K.V., Nikitina L.P., Afonin A.V., Vashchenko A.V., Muzalevskiy V.M., Nenajdenko V.G., Trofimov B.A. Catalyst-free 1:2 annulation of quinolines with trifluoroacetylacetylenes: An access to functionalized oxazinoquinolines. Org. Biomol. Chem. 2018;16:8038–8041. doi: 10.1039/C8OB02379D. [DOI] [PubMed] [Google Scholar]
- 51.Lazar L., Fulop F. 1,3-Oxazines and Their Benzo Derivatives. In: Katritzky A.R., Ramsden C.A., Scriven E.F.V., Taylor R.J.K., editors. Comprehensive Heterocyclic Chemistry III. Volume 8. Elsevier; Amsterdam, The Netherland: 2008. pp. 373–459. [Google Scholar]
- 52.Gaonkar S.L., Nagaraj V.U., Nayak S. A Review on Current Synthetic Strategies of Oxazines. Mini Rev. Org. Chem. 2019;16:43–58. [Google Scholar]
- 53.Sindhu T.J., Sonia D.A., Girly V., Meena C., Bhat A.R., Krishnakumar K. Biological Activities of Oxazine and Its Derivatives: A Review. Int. J. Pharm. Sci. Res. 2013;4:134–143. [Google Scholar]
- 54.Liu P., Lei M., Hu L. Synthesis of benzo-annulated 1,3-oxazine derivatives through the multi-component reaction of arynes with N-heteroaromatics and aldehydes or ketones. Tetrahedron. 2013;69:10405–10413. doi: 10.1016/j.tet.2013.09.092. [DOI] [Google Scholar]
- 55.Min S., Song I., Borland J., Chen S., Lou Y., Fujiwara T., Piscitelli S.C. Pharmacokinetics and safety of S/GSK1349572, a next-generation HIV integrase inhibitor, in healthy volunteers. Antimicrob Agents Chemother. 2010;54:254–258. doi: 10.1128/AAC.00842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsiang M., Jones G.C., Goldsmith J., Mulato A., Hansen D., Kan E., Tsai L., Bam R.A., Stepan G., Stray K.M., et al. Antiviral activity of bictegravir (GS-9883), a novel potent HIV1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrob Agents Chemother. 2016;60:7086–7097. doi: 10.1128/AAC.01474-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andriyankova L.V., Nikitina L.P., Belyaeva K.V., Mal’kina A.G., Afonin A.V., Muzalevskii V.M., Nenaidenko V.G., Trofimov B.A. Opening of the pyridine ring in the system 1,1,1-trifluoro-4-phenylbut-3-yn-2-one–water. Stereoselective synthesis of 5-{[(1Z)-4,4,4-trifluoro-3-oxo-1-phenylbut-1-en-1-yl]amino}penta-2,4-dienal. Rus. J. Org. Chem. 2016;52:1857–1860. doi: 10.1134/S1070428016120289. [DOI] [Google Scholar]
- 58.Gusarova N.K., Mikhaleva A.I., Schmidt E. Yu., Mal’kina A.G. In: Khimiya atsetilena. Novye glavy (The Chemistry of Acetylene. New Chapters) Egorov M.P., editor. Volume 92. Nauka; Novosibirsk, Russia: 2013. (In Russian) [Google Scholar]
- 59.Trofimov B.A., Belyaeva K.V., Andriyankova L.V., Nikitina L.P., Mal’kina A.G. Ring-opening of pyridines and imidazoles with electron-deficient acetylenes: En route to metal-free organic synthesis. Mendeleev Commun. 2017;27:109–115. doi: 10.1016/j.mencom.2017.03.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.