Abstract
Background
Current guidelines recommend rehabilitative measures to alleviate disturbances resulting from cancer and its treatment. To give cancer survivors further assistance in getting back to work, work-related medical rehabilitation is currently being tested in Germany. In this cluster-randomized, multicenter trial, we studied the efficacy of work-related medical rehabilitation compared with conventional medical rehabilitation (trial no. DRKS00007770 in the German Clinical Trials Registry).
Methods
A total of 484 cancer survivors of working age who were candidates for rehabilitation were recruited and assigned at random to either the intervention group (IG; work-related medical rehabilitation) or the control group (CG). The primary endpoint was self-assessed function in a role one year after the end of rehabilitation, as evaluated with the health-related quality of life questionnaire of the European Organisation for Research and Treatment of Cancer (EORTC QLQ-C30). Further endpoints included symptom and function scales, subjective ability to work, coping with illness, and return to work. Neither the medical personnel nor the subjects were blinded.
Results
One year after the end of rehabilitation, data from 379 subjects who participated in the last follow-up survey were evaluated. The intervention and control groups did not differ significantly either in the primary endpoint of role function (IG = 60.8 vs. CG = 57.6 out of a maximum of 100 points; p = 0.204) or in any of the secondary endpoints. A last observation carried forward analysis yielded comparable results. At 12 months, 28.5% of the subjects in the IG and 25.3% of those in the CG were still unable to work.
Conclusion
This study did not reveal any significant clinically relevant advantage of work-related medical rehabilitation at one year. Future studies should determine whether a second period of rehabilitation might be helpful and whether selected subjects might benefit from the assistance of case managers beyond the period of rehabilitation.
With an estimated 18.1 million incident cases in 2018, cancer is one of the leading causes or morbidity worldwide (1). Early detection and improved treatment options have resulted in a rise in the number of survivors (2). Some 1.3 million men and 1.3 million women currently living in Germany have received a cancer diagnosis in the past 10 years (3). Almost half of the survivors are younger than 65 and therefore still working. Work is a central part of life for most of us, not only because it provides an income and material security, but also because it enables contacts and scope for further development and self-fulfillment. For persons with cancer, the return to work is often a sign that their lives are returning to normality. It is therefore perceived as an important step in coping after this life-threatening event (4, 5). Eight out of 10 cancer patients successfully re-enter employment within the first 2 years after the diagnosis (6). In spite of this, however, cancer survivors have a higher risk for unemployment than healthy controls, and more of them report restrictions to their work participation (7, 8). Furthermore, having survived childhood cancer is associated with a greater probability of unemployment in adulthood (9).
Multidisciplinary interventions can support the return to work after cancer. A systematic review of randomized controlled trials by de Boer et al. showed that persons who participated in multidisciplinary interventions with a clear work focus had a higher probability of returning to work (87.2% versus 78.6%) after a year than those who did not take part in such interventions (5).
The current guidelines for management of oncological disorders in Germany recommend rehabilitation measures to treat the sequelae of oncological disorders and the consequences of cancer treatment (10, 11). The central objectives of such measures are the restoration of people’s ability to work, the return to work, and the prevention of health-related early retirement. Despite these objectives, traditional medical rehabilitation seemingly does not adequately address work-related issues (12). In recent years, rehabilitation programs with a stronger work-related focus have been developed (13). These are geared to people who have more permanent substantial restrictions to work participation owing to health problems and are intended to reduce health-related discrepancies in work-related skills and demands in order to enable participation in work. In order to implement such rehabilitation programs, the German Pension Insurance (Deutsche Rentenversicherung [DRV]) has developed a profile for work-related medical rehabilitation programs. This describes the target group and sets out diagnostic and therapeutic requirements (14). A meta-analysis of randomized controlled trials in persons with musculoskeletal disorders showed that people who participated in a work-related medical rehabilitation program rather than a conventional medical rehabilitation measure had shorter periods of absence (standardized mean difference [SMD] = –0.25; 95% confidence interval [–0.37; –0.12]) and were more likely to be back in gainful employment after a year. The proportion of persons with stable return to work rose from about 40% to 60% (13). Further randomized controlled trials in mental and cardiovascular disorders also showed positive effects from participation for persons who underwent work-related medical rehabilitation (15, 16). No randomized controlled trials on the effects of participating in work-related medical rehabilitation have yet been conducted in cancer patients.
The present study aimed to assess the effectiveness of work-related medical rehabilitation compared with medical rehabilitation in persons recovering from oncological disorders (17). At the end of the rehabilitation period and 3 months later, slight benefits were consistently seen for work-related medical rehabilitation (18, 19). Our study presents the results of such rehabilitation measures 1 year after completion. We followed the Consolidated Standards of Reporting Trials (CONSORT) and the recommendations for reporting cluster-randomized trials in compiling our manuscript (eTable) (20).
eTable 2. Return to work.
| IG | CG | IG | CG | ||||||
| n | % | % | HR | [95% CI] | p | AAP | AAP | ICC | |
| Regular return to work | 352 | 71.5 | 74.7 | 0.86 | [0.61; 1.19] | 0.355 | 0.697 | 0.739 | 0.04 |
| Regular return to work or graded return to work | 352 | 73.7 | 75.3 | 0.89 | [0.66; 1.21] | 0.459 | 0.736 | 0.767 | 0.00 |
We used mixed-effects models to calculate test statistics, controlled for gainful employment before the start of the rehabilitation program.
AAP, average adjusted predictions; CI, confidence interval; CG, control group; HR, hazard ratio; ICC, intracluster correlation coefficient; IG, intervention group
Methods
Study design
The effects of work-related medical rehabilitation compared with medical rehabilitation were studied in a cluster-randomized multicenter study (17). Participants were recruited from June 2015 to September 2016 in four inpatient rehabilitation centers and were followed up until October 2017. Participants who arrived and were admitted at the same time were randomly allocated to work-related medical rehabilitation (intervention group, IG) or conventional medical rehabilitation (control group, CG).
The study was approved by the ethics committee of the University of Lübeck (no. 14–289) and the data protection officer of the DRV. The study was registered in the German Clinical Trials Registry (no. DRKS00007770). A detailed explanation of the method and intervention can be found in the eMethods section and the study protocol (17).
Inclusion criteria
We included cancer patients aged 18 to 60 years from all oncological indication groups (ICD-10 codes C00–D48: Neoplasms), who had successfully completed their initial cancer treatment and for whom at least one scale of the screening instrument “Beruf und Arbeit” (Work and Occupation; SIBAR) indicated a need for work-related medical rehabilitation (SIBAR I: at least 8 points; SIBAR II: very stressful; SIBAR III: very helpful) (21). All participants had a score of = 70% on the Karnofsky Performance Status Scale (22) and a preliminary positive social medical prognosis of at least 3 h/day for the ensuing 6 months.
Intervention
The CG underwent conventional medical rehabilitation. This was provided on an inpatient basis and included exercise therapy, physiotherapy, social counseling, occupational therapy, nutritional advice, and psychological seminars and counseling, as well as medical treatment and counseling. Apart from fundamental social counseling, which is a regular component of medical rehabilitation, participants in the CG were not provided with any further work-related services. The IG underwent conventional rehabilitation plus additional work-related modules according to the requirements profile of the DRV. These included work-related diagnostic evaluation, intensive social counseling, work-related psychosocial groups, and work-related functional capacity training. In the setting of an initial implementation phase, the general conditions and minimum requirements for conducting the individual modules of the intervention were developed and defined in collaboration with the participating rehabilitation centers (17, 23).
Outcome measures
The primary outcome measure was self-assessed role functioning a year after the rehabilitation had been completed. Role functioning was captured by using the health-related quality of life questionnaire of the European Organisation for Research and Treatment of Cancer (EORTC QLQ-C30) (24). The role functioning scale employs two items to capture the degree to which affected persons feel restricted in their work and leisure activities. A minimum difference of 10 points between CG and IG was interpreted as clinically relevant (25).
The secondary outcome measures were the scales of the EORTC QLQ-C30 for physical, emotional, and cognitive functioning, general health, and pain (24), as well as the fatigue module EORTC QLQ-FA13, which records physical, emotional, and cognitive fatigue as well as problems in daily life and social consequences of fatigue (26). Furthermore, data were collected on how participants were coping with their illness (Freiburg Questionnaire of Coping with Illness [FKV] [27]), their subjective ability to work (Work Ability Score [28]), and the concrete point in time when participants returned to work. All the described outcome measures were documented by means of participants’ self-reports.
Statistical analyses
We used mixed-effects models to calculate treatment effects. We estimated the effects by controlling the relevant value in the initial survey and the rehabilitation centers, which were modeled as fixed effects. In order to describe differences between groups, we calculated standardized mean differences (SMD) (29) (small effect: SMD = 0.2; medium effect: SMD = 0.5; large effect: SMD = 0.8 [30]). To compare reintegration into work, we determined hazard ratios (HR). Furthermore, we calculated average adjusted predictions that reflected the predicted probability for the occurrence of an event (return to work) within 1 year (31). The time until return to work was depicted using Kaplan–Meier curves (32). To describe the heterogeneity that was associated with the clusters, we determined the intracluster correlation (ICC) for each outcome measure (33, 34). All participants were analyzed on an intention to treat basis (25). We analyzed the data for the participants of the final follow-up (17, 36) and conducted an analysis, for which the missing values were substituted by the value at the last available observation point (last observation carried forward, LOCF) (17, 36). Differences between groups were considered significant if the probability of a two-sided error was less than 5%. We used STATA 15 for our calculations.
Results
Sample
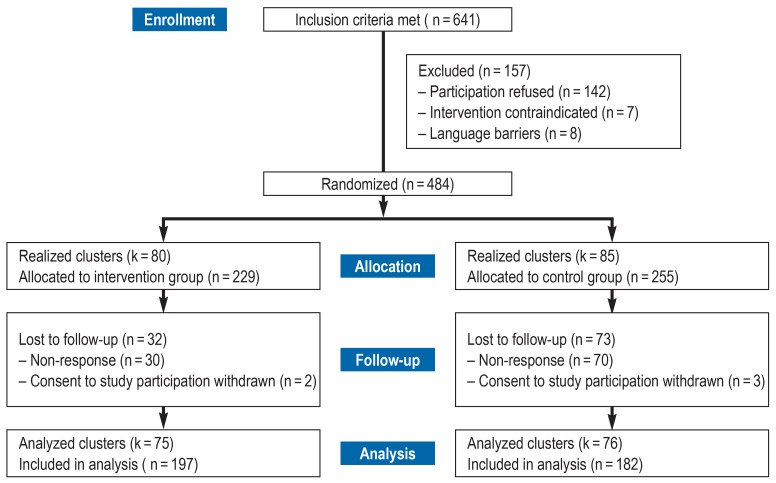
We recruited a total of 484 rehabilitants and randomized 165 groups (80 groups in the IG) (figure 1). The average cluster size in both intervention arms was three persons per week of arrival. One year after completing the rehabilitation program, 379 rehabilitants participated in the follow-up survey (78.3%) (197 persons in the IG). These persons were divided into 151 groups (75 groups in the IG). Table 1 shows the sample statistics for both treatment groups at the initial survey. The characteristics of the two groups showed no significant differences. The distributions of the scores were graphically tested and found to be symmetrical (Figure 1, Table 1).
Figure 1.
Flow diagram (CONSORT) showing participation in the study
Table 1. Sample characteristics at the start of the rehabilitation program.
| IG (n = 229) | CG (n = 255) | ||
| N | Mean (SD) or n (%) | Mean (SD) or n (%) | |
| Sociodemographic data | |||
| Age | 484 | 50.8 (7.1) | 50.3 (7.9) |
| Sex: female | 484 | 163 (71.2%) | 161 (63.1%) |
| Native language: German | 482 | 210 (92.1%) | 225 (88.6%) |
| Life partner | 478 | 160 (70.8%) | 187 (74.2%) |
| Most common diagnosis-related groups (ICD-10 code) | |||
| Breast cancer (C50) | 484 | 72 (31.4%) | 72 (28.2%) |
| Digestive tract (C15–C26) | 484 | 33 (14.4%) | 47 (18.4%) |
| Lymphatic/related tissue (C81–C96) | 484 | 35 (15.3%) | 38 (14.9%) |
| Female genitalia (C51–C58) | 484 | 35 (15.3%) | 27 (10.6%) |
| Quality of life (EORTC QLQ-C30)*1 | |||
| Role functioning | 482 | 41.4 (27.9) | 40.5 (28.3) |
| General health | 477 | 47.6 (19.2) | 43.7 (20.9) |
| Physical functioning | 483 | 66.4 (19.2) | 63.5 (21.5) |
| Emotional functioning | 480 | 43.8 (27.2) | 42.3 (26.8) |
| Cognitive functioning | 480 | 55.4 (30.5) | 56.7 (29.7) |
| Social functioning | 478 | 48.2 (28.2) | 47.7 (30.9) |
| Pain | 481 | 48.5 (28.7) | 51.9 (31.5) |
| Fatigue (EORTC FA13)*1 | |||
| Physical fatigue | 481 | 64.1 (25.3) | 64.6 (26.4) |
| Emotional fatigue | 481 | 43.6 (27.2) | 47.3 (29.3) |
| Cognitive fatigue | 481 | 28.2 (25.3) | 30.7 (26.0) |
| Problems affecting daily life | 476 | 53.3 (32.5) | 56.6 (31.6) |
| Social consequences of fatigue | 478 | 19.2 (28.9) | 28.2 (33.1) |
| Coping with illness (FKV)*2 | |||
| Active problem-oriented coping | 481 | 3.1 (0.8) | 3.1 (0.8) |
| Depressive coping | 481 | 2.3 (0.9) | 2.4 (0.9) |
| Distraction and self-encouragement | 481 | 3.1 (0.8) | 3.0 (0.8) |
| Work-related outcomes | |||
| On sick leave or unemployed | 479 | 181 (79.7%) | 194 (77.0%) |
| Work Ability Score*3 | 479 | 4.2 (2.5) | 4.2 (2.6) |
| Disability days during past 3 months | 426 | 33.6 (36.8) | 30.8 (34.5) |
EORTC, European Organization for Research and Treatment of Cancer; FA, fatigue; FKV, Freiburg Questionnaire of Coping with Illness;
ICD, International Classification of Diseases; IG, intervention group; CG, control group; QLQ, Quality of Life Questionnaire; SD, standard deviation
*1 Maximum achievable score = 100; *2 maximum achievable score = 5; *3 maximum achievable score = 10
Treatment dose delivered
In total, the difference of the therapeutic dose between intervention and control arms amounted to 16 h (IG: 79 h; CG: 63 h). The overall duration of the rehabilitation programs in treatment days was comparable in both groups (IG: 24.7 days; CG: 23.8 days) p=0.128). The rates of rehabilitants who underwent the recommended minimum dose of work-related diagnostic evaluation (at least 60 min), intensified social counseling (at least 90 min), work-related psychosocial groups (at least 240 min), and work-related functional capacity training (at least 360 min) were 99.0%, 94.4%, 87.3%, and 90.4%. In the mean, the agreed minimum doses of the work-related therapies were delivered in all centers.
Outcome measures
Role functioning (our primary outcome measure) after 1 year was rated around 4 points more favorably by the intervention group. The difference is clinically not relevant and does not attain significance. The secondary outcome measures also showed no clinically relevant advantages for the IG (Tables 2, 3, eTable 2). The differences were similar in the LOCF analysis (Tables 2, 3).
Table 2. Primary and secondary outcome measures at participants’ final follow-up.
| IG | CG | IG | CG | |||||||
| n | Mean (SD) | Mean (SD) | b | [95% CI] | p | AAP | AAP | SMD | ICC | |
| Quality of life (EORTC QLQ-C30)*1 | ||||||||||
| Role functioning | 378 | 60.83 (28.89) | 57.64 (29.05) | 3.69 | [−2.00; 9.39] | 0.204 | 61.11 | 57.42 | 0.13 | 0.01 |
| General health | 375 | 59.36 (22.09) | 57.82 (22.96) | 1.44 | [−3.18; 6.06] | 0.540 | 59.59 | 58.15 | 0.06 | 0.09 |
| Physical functioning | 379 | 76.00 (19.52) | 72.82 (21.45) | 2.89 | [−0.40; 6.18] | 0.085 | 75.86 | 72.97 | 0.14 | 0.00 |
| Emotional functioning | 377 | 55.23 (24.93) | 55.69 (25.03) | 0.38 | [−4.12; 4.88] | 0.868 | 55.64 | 55.25 | 0.02 | 0.00 |
| Cognitive functioning | 377 | 64.64 (26.97) | 66.67 (25.77) | −0.44 | [−5.00; 4.12] | 0.851 | 65.40 | 65.84 | −0.02 | 0.00 |
| Social functioning | 377 | 63.20 (29.03) | 61.94 (30.32) | 1.39 | [−4.10; 6.89] | 0.619 | 63.26 | 61.87 | 0.05 | 0.00 |
| Pain | 378 | 34.86 (29.46) | 37.11 (31.32) | −3.84 | [−9.60; 1.92] | 0.191 | 33.97 | 37.81 | −0.13 | 0.05 |
| Fatigue | 377 | 49.69 (24.34) | 50.40 (25.70) | −1.42 | [−5.96; 3.13] | 0.541 | 49.35 | 50.77 | −0.06 | 0.00 |
| Fatigue (EORTC FA13)*1 | ||||||||||
| Physical fatigue | 378 | 49.11 (26.66) | 49.63 (25.44) | −1.80 | [−6.67; 3.06] | 0.467 | 48.49 | 50.30 | −0.07 | 0.00 |
| Emotional fatigue | 378 | 35.38 (25.92) | 34.88 (26.84) | 0.19 | [−.55; 4.93] | 0.937 | 35.23 | 35.04 | 0.00 | 0.02 |
| Cognitive fatigue | 378 | 22.87 (21.53) | 20.63 (21.34) | 1.89 | [−1.83; 5.60] | 0.320 | 22.70 | 20.81 | 0.09 | 0.00 |
| Problems affecting daily life | 375 | 44.33 (30.07) | 43.26 (30.61) | 0.82 | [−4.89; 6.53] | 0.778 | 44.21 | 43.39 | 0.03 | 0.00 |
| Social consequences of fatigue | 372 | 23.54 (30.37) | 26.22 (32.07) | 1.51 | [−3.99; 7.01] | 0.590 | 25.54 | 24.03 | 0.05 | 0.00 |
| Coping with illness (FKV)*2 | ||||||||||
| Active problem-oriented coping | 378 | 2.75 (0.89) | 2.85 (0.90) | −0.12 | [−0.29; 0.05] | 0.155 | 2.74 | 2.86 | −0.14 | 0.00 |
| Depressive coping | 378 | 2.02 (0.80) | 2.03 (0.84) | −0.01 | [−0.16; 0.14] | 0.869 | 2.02 | 2.03 | −0.02 | 0.00 |
| Distraction and self-encouragement | 378 | 3.07 (0.87) | 3.02 (0.86) | 0.02 | [−0.13; 0.18] | 0.764 | 3.05 | 3.03 | 0.03 | 0.00 |
| Work-related outcomes | ||||||||||
| Work Ability Score*3 | 372 | 5.59 (2.59) | 5.85 (2.62) | −0.26 | [−0.78; 0.26] | 0.323 | 5.60 | 5.86 | −0.10 | 0.05 |
| Disability days during past 3 months | 323 | 9.44 (20.43) | 10.26 (22.48) | −0.79 | [−5.96; 4.37] | 0.764 | 9.56 | 10.35 | −0.04 | 0.12 |
We used mixed-effects models to calculate test statistics, controlled for the values of the initial survey.
AAP, Average adjusted predictions; b, non-standardized regression coefficient; CG, control group; CI, confidence interval; EORTC, European Organization for Research and Treatment of Cancer; FA, fatigue; FKV, Freiburg Questionnaire of Coping with Illness; ICC, intracluster correlation coefficient; IG, intervention group;
QLQ, Quality of Life Questionnaire; SD, standard deviation; SMD, standardized mean difference
*1 Maximum achievable score = 100; *2 maximum achievable score = 5; *3 maximum achievable score = 10
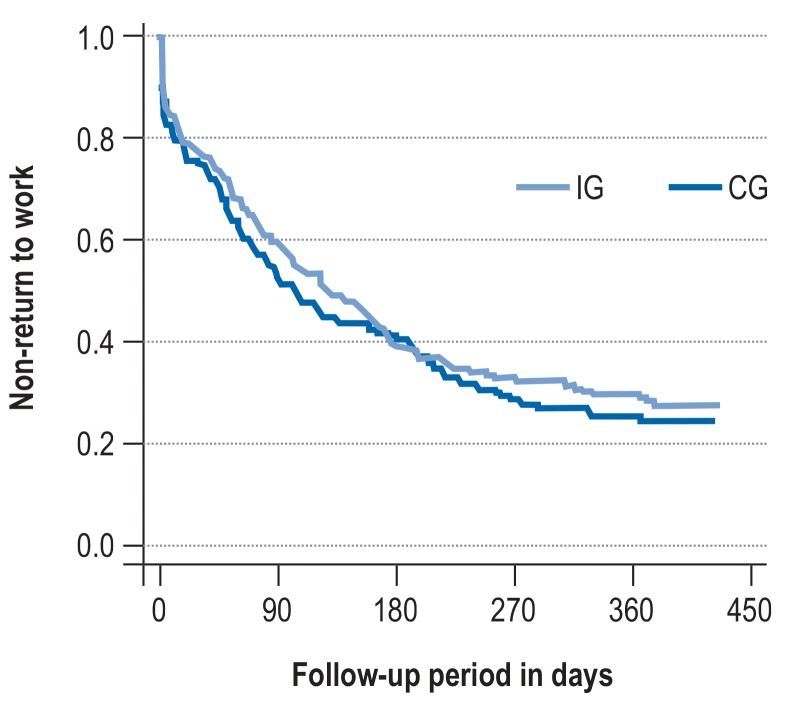
Time to return to work
The time until return to work was comparable in the two treatment arms (Figure 2, eTable 2). Half of the surveyed patients had returned to work after 3 to 4 months (IG: median 128 days; CG: median 104 days). After 1 year, 28.5% of those in the IG and 25.3% in the CG had still not returned to work (HR 0.86; 95% CI [0.61; 1.19]; p = 0.355).
Figure 2.
Time to return to work: IG, intervention group; CG, control group
Discussion
The study objective was to determine the effectiveness of work-related medical rehabilitation in rehabilitants with oncological diseases and an elevated risk of not returning to work. We compared the relative effectiveness of work-related medical rehabilitation with conventional medical rehabilitation in a multicenter study. Participants were cluster randomized to the intervention or control groups. After 1 year, no clinically relevant advantages were seen for work-related medical rehabilitation.
With regard to the effectiveness of multiprofessional interventions in inpatient oncological rehabilitation, a systematic review based on two studies did not find any difference between IG and CG with regard to health-related quality of life after 1 year (5). The effects of multidisciplinary interventions on patients’ return to work reported by Boer et al. were not confirmed by our study. The return rates in our study were similar to those in the CG in that review article (5), but it should be borne in mind that the target group of our study was greatly restricted by the defined inclusion criteria. At the start of rehabilitation, the initial scores on the function scales of the EORTC QLQ-C30 were some 35 to 50 points below those of a healthy standard population of the same age (37, 38) and self-rated work ability was extremely low (39).
Our study results reveal a fundamental challenge in oncological rehabilitation. A successful return to work mostly takes place months after the inpatient rehabilitation program was completed, and one quarter of rehabilitants had still not returned to work 1 year later. Persons who had not returned to work after a year were found to have lower quality of life and lower self-rated work ability, as well as greater symptoms of fatigue, at all four measurement points than persons who were back at work (data not shown). The early identification of persons with an unfavorable prognosis for work participation seems important (4), but the currently available programs and rehabilitation strategies—and this includes the work-related medical rehabilitation that we implemented—do not appear sufficient to enable the members of this group to return to work. Useful refinements of rehabilitation services for such persons might include provision of further support, e.g., by a case manager—particularly because the return to work will usually take place a number of months after the end of the rehabilitation program. Such ongoing support should enable the structured planning of the return to work and, wherever possible, embrace all those involved in the reintegration process, including employers and occupational physicians (5). It is also conceivable that the modules described and studied by us will achieve the desired additional effect only by virtue of a second phase of rehabilitation.
Our study results are subject to certain methodological limitations. First, the participants and physicians were not blinded. We thus cannot exclude the possibility that the more positive short-term results for patients in the IG were a result of their awareness that they were participating in a new and special program. Furthermore, transfer effects owing to joint activities during inpatient rehabilitation cannot be ruled out. Second, the work-related elements of the program comprised an additional service. The two treatment arms differed not only in content but also in intensity. The differences observed in the short term, up to 3 months after the end of rehabilitation, may also be explained by a non-specific increase in the therapeutic dose. Third, the work environment (job description and requirements, working hours) was not documented. Heterogeneity of the work environment could be a potential moderator, but this was not considered in the subgroup analyses. The same is the case for disease stage and comorbidities. Fourth, no organic and laboratory medical health parameters were captured; consequently, the effects of the rehabilitation measures on these parameters are not known.
Besides these limitations, however, we would also like to emphasize the strengths of our study. First, the chosen study design reduced the risk of biased estimated effects and ensured that the two treatment groups were structurally identical. Second, the decision in favor of cluster-randomized allocation to the IG or CG had the advantage that possible transfer effects, which are impossible to avoid in the inpatient rehabilitation setting because of common services and activities were reduced by the joint randomization of patients admitted at the same time. Third, it was possible to present the treatment dose and adherence to treatment in a transparent fashion (19).
In conclusion, implementation of a work-related medical rehabilitation program in the oncology setting—such as we did in four centers—probably does not improve cancer patients’ chances of job reintegration. Future research projects should investigate the extent to which patients who will not manage to reintegrate into the workplace can be identified early in the rehabilitation process. Furthermore, it should be determined to what extent the concrete individual workplace and occupation are actually the cause of the delayed return to work and whether it is possible that a second rehabilitation period might be indicated to support reintegration into the workplace.
Supplementary Material
eMethods
Study design
The effects of work-related medical rehabilitation were studied in a cluster-randomized multicenter trial (17). We used cluster randomization in order to reduce the risk of treatment diffusion (34). The study was set in four inpatient rehabilitation centers. Participants were recruited between June 2015 and September 2016 and followed up to October 2017. Patients with the same admission date were randomly allocated to work-related medical rehabilitation (intervention group, IG) or conventional medical rehabilitation (control group, CG) after the inclusion criteria had been checked and the patients had consented to participate in the study.
The study protocol was approved by the ethics committee at the University of Lübeck (no. 14–289) and the data protection officer of the German Pension Insurance (DRB). The study was registered in the German Clinical Trials Registry (DRKS) (DKRS-ID: DKRS00007770).
Inclusion criteria
We included all cancer patients aged 19 to 60 years from all oncological indication groups (ICD-10 codes C00–D48: Neoplasms) who had successfully completed their initial cancer treatment and for whom at least one scale of the screening instrument for work and occupation (SIBAR) indicated a need for work-related rehabilitation (SIBAR I at least 8 points; SIBAR II: very stressful; SIBAR III: very helpful) (21). The cut-off scores for assessing need on the basis of SIBAR followed the recommendations of Bürger and Deck (21).
All participants had a score = 70% on the Karnofsky Performance Status Scale (22) and a preliminary positive social medical prognosis of at least 3 h/day for the ensuing 6 months. No exclusion criteria were defined.
Control group
The CG underwent conventional medical rehabilitation. This consisted of exercise therapy, physiotherapy, social counseling, occupational therapy, nutritional advice/counseling, and psychological seminars and counseling, as well as medical treatment and advice. Apart from basic social counseling, which is a regular component of medical rehabilitation, participants in the CG group did not receive any further work-related services.
Intervention group
The IG was provided with work-related modules in addition to their conventional medical rehabilitation, based on the requirements profile of the DRB. These consisted of work-related diagnostic evaluation, intensified social counseling, work-related psychosocial groups, and work-related functional capacity training. Furthermore, participants undergoing work-related medical rehabilitation were discussed in multiprofessional team meetings, in order to do justice to the complexity of the cases. During the preceding implementation phase, the general conditions for undertaking individual intervention modules were developed and defined in collaboration with the participating rehabilitation centers (17, 23).
Work-related diagnostic evaluation
The additional work-related diagnostic evaluation was undertaken by a treating physician, a psychologist, and an occupational therapist or physiotherapist immediately after the patient’s admission. Barriers to fitness for work may arise from physical functions, physical structures, activities, or participation (e1). The physician focused primarily on physical functioning and structures. The psychologist documented environmental factors and personal factors that had positive (resources) and negative (barriers) effects on the patient’s fitness to work. On the basis of standardized tests (e.g., WorkWell Systems Functional Capacity Evaluation) (e2) and structured observations of non-standardized work-related tasks, the occupational therapist or physiotherapist assessed the patient’s capacity to undertake work-related activities. The diagnostic process was extended by at least 60 min in this way.
Intensified social counseling
Intensified social counseling was intended to clarify the patient’s work situation and potential perspectives. The social worker and the rehabilitant jointly clarified the general conditions for a return to work and identified which additional measures and services were needed and could be accessed in order to support the return to work. Possible measures included a graded return to work, adjustments of the working environment, and job-related educational achievements. Where required and desired by the rehabilitant, the social worker established contact with the employer in order to support the transition from rehabilitation back into the workplace. Intensified social counseling took at least 90 min.
Work-related psychosocial groups
Work-related psychosocial groups aimed to support the concrete return to the workplace by concentrating on modifiable personal factors and environmental factors. During the group seminars, rehabilitants were encouraged to reflect critically on their current work situation and to identify options for behavior change. They learnt more about stress and how it affects their health and fitness to work. Coping strategies were taught. Techniques for direct stress reduction, such as progressive muscle relaxation, autogenic training, and meditation, were demonstrated and practiced in order to support the coping process. Furthermore, rehabilitants were shown communications techniques to help them shape interpersonal relationships with colleagues and senior colleagues more positively. They developed concrete plans for their return to the workplace. Potential problems and barriers were considered, and strategies were developed to deal with any barriers. Plans were made in light of the general conditions elaborated during the intensified social counseling. Work-related psychosocial groups took at least 240 min.
Work-related functional capacity training
Participants learned that exercises and physical activities could be safely undertaken in spite of pain symptoms. They were trained not only in simple or one-dimensional movements, but also complex and multidimensional movement sequences, in order to simulate realistic workplace demands. Therapists integrated ergonomic corrections into the performance of the exercises. Since cancer survivors often report cognitive impairments, the module also included cognitive training units—for example, computer-assisted training sessions and group exercises as instructed by a therapist—in order to support attention, concentration, and logical thinking. The work-related functional capacity training took at least 360 min.
Multiprofessional team meetings
These meetings provided a platform for joint exchanges about the individual rehabilitants and were intended to simplify the coordination of the different modules. The first meeting took place after completion of the work-related diagnostic evaluation so as to develop—together, as a team—a treatment strategy that aimed to improve work-related functioning. During the second meeting, all team members reported on the progress achieved by the rehabilitant and his/her current situation. If necessary, treatment strategies were adjusted accordingly. During the final meeting, the individual rehabilitation objectives were evaluated. On the basis of this evaluation, the team jointly decided on discharge as fit for work or unfit for work. Furthermore, it was discussed whether additional measures or services were required to support participation. Where this was the case, requests for such services or measures were filed with the relevant organization. Each team meeting took at least 30 min.
Documentation of therapeutic services
Services provided during the rehabilitation program were documented according to the classification of therapeutic services by the DRV (e3). Each service provided during the rehabilitation program was documented, with duration and frequency. The therapeutic dose was calculated from the product of the frequency and duration of the service. This was done for each of the four treatment modules of the work-related medical rehabilitation, in order to establish the overall therapeutic dose.
Outcome measures
The primary outcome measure was self-assessed role functioning 1 year after the end of the rehabilitation program. Role functioning was documented by administering the health-related quality of life questionnaire of the European Organisation for Research and Treatment of Cancer (EORTC QLQ-C30) (24). The role functioning scale uses two items to assess limitations regarding work and leisure activities (Were you limited in your work or in other activities of daily life? Were you limited in terms of your hobbies or other leisure activities?). The response categories are “not at all” (1), “a little” (2), “moderately” (3), and “very” (4). For the evaluation, we firstly calculated the mean value of the scale items (crude value). The crude values were subsequently transformed into a range of 0 to 100 (e4). Higher scores reflected better role functioning. A difference of at least 10 points between IG and CG was interpreted as clinically relevant (25).
Further secondary outcome measures were the EORTC QLQ-C30 scales for physical, emotional, and cognitive functioning and for general health and the symptom scale pain (24). We used the fatigue module EORTC QLQ-FA13, which captures physical, emotional, and cognitive fatigue as well as problems in daily life and the social consequences of fatigue (26). All scales have a score range from 0 to 100 points, with higher scores reflecting a better health-related quality of life or a greater burden of symptoms. The reliability and validity of both instruments have been confirmed (24, 26).
Coping with the disease was captured by using three scales of the Freiburg Questionnaire of Coping with Illness (active problem-oriented coping, depressive processing, distraction, and self-encouragement. Individual scores were combined in scales of mean values (1 to 5 points) (27).
The subjective ability to work was measured by using the Work Ability Score. A score of 0 means complete unfitness for work, while a score of 10 indicates the best work ability ever achieved (28). The concrete timing when participants returned to work (if applicable, also the timing of the graded return to work) was measured after 12 months.
All outcome measures described here were based on rehabilitants’ self-assessments.
Randomization
Participants admitted at the same time were allocated to the IG or CG after their eligibility for inclusion had been confirmed and their consent for participation in the study obtained. This allocation was randomized by using four randomization lists compiled independently for the participating rehabilitation centers. The computer-generated allocation lists were produced externally by the principal investigator. The lists were produced by using block randomization (blocks of four) in order to reduce the risk of treatment arms of dissimilar size in the event that the lists were not completely used. The patients were invited to the centers without prior knowledge of the treatment arm planned for the respective day of admission.
Sample size calculation
The sample size was calculated to detect a standardized mean difference (SMD) of SMD = 0.3, with a power of 80% and a two-sided alpha error of 5%. For a t test, this requires 352 participants. Cluster randomization increases the required sample size because of the design effect: by 20% for a mean group size of five persons and an intraclass correlation of 0.05 (e5). The planned control of the baseline values, however, reduced the required sample size greatly (e6), so that the estimated case number was conservative even without considering the design effect. We assumed that 30% of participants would not respond after a year; this meant that 504 participants needed to be recruited into the sample.
Statistical analyses
Sample characteristics, the mean therapeutic dose received, and the proportion of rehabilitants who received the recommended minimum dose of work-related therapies were modeled descriptively. Treatment effects were calculated using mixed-effects models. The correlation of error terms for rehabilitants admitted during the same week, rendered possible by the cluster randomization, was considered by means of random effects. Effects were estimated while controlling the corresponding value at the first data acquisition and the rehabilitation center. The rehabilitation centers were modeled as fixed effects. SMD were calculated to describe differences between groups. To this end, the non-standardized regression coefficient of the treatment effect (29) was standardized on the pooled standard deviation of the observed measurement values (e7). SMD were interpreted as follows, adhering to Cohen’s recommendations: small effect around SMD = 0.2, moderate effect around SMD = 0.5, and large effect around SMD = 0.8. We determined hazard ratios to compare reintegration into work. Moreover, we calculated average adjusted predictions that reflected the predicted probability for the occurrence of an event (return to work) within 1 year (31). The time until return to work was plotted in Kaplan–Meier curves (32).
To describe the heterogeneity associated with the clusters, we also determined the intracluster correlation for each outcome measure. This has to be considered when planning case numbers in cluster-randomized trials (design effect), since intraclass correlation reduces the power (33). All participants were analyzed on an intention to treat basis (35). We performed an analysis for the participants of the final follow-up (17, 36) as well as an analysis for which missing values were substituted by the values from the most recent available observation (last observation carried forward, LOCF). We considered differences between groups as statistically significant if the probability of a two-sided error was less than 5%. We used STATA 15 for our calculations.
Table 3. Primary and secondary outcome measures (last observation carried forward analysis).
| IG | CG | IG | CG | |||||||
| n | Mean (SD) | Mean (SD) | b | [95% CI] | p | AAP | AAP | SMD | ICC | |
| Quality of life (EORTC QLQ-C30)*1 | ||||||||||
| Role functioning | 482 | 57.60 (30.75) | 52.49 (30.98) | 4.49 | [−0.49; 9.47] | 0.077 | 57.28 | 52.79 | 0.15 | 0.00 |
| General health | 477 | 58.63 (21.96) | 56.05 (23.11) | 0.73 | [−3.00; 4.47] | 0.700 | 57.69 | 56.95 | 0.03 | 0.01 |
| Physical functioning | 483 | 75.20 (19.33) | 70.40 (22.63) | 3.10 | [0.23; 5.96] | 0.034 | 74.30 | 71.20 | 0.15 | 0.00 |
| Emotional functioning | 480 | 56.38 (25.29) | 55.34 (26.02) | 0.34 | [−3.71; 4.39] | 0.871 | 56.03 | 55.69 | 0.01 | 0.00 |
| Cognitive functioning | 480 | 64.84 (27.37) | 65.94 (27.16) | −0.42 | [−4.08; 4.00] | 0.984 | 65.39 | 65.44 | −0.00 | 0.00 |
| Social functioning | 478 | 61.40 (29.29) | 58.33 (31.45) | 2.41 | [−2.40; 7.22] | 0.326 | 61.06 | 58.65 | 0.08 | 0.00 |
| Pain | 481 | 35.31 (29.39) | 41.30 (32.38) | −4.63 | [−9.47; 0.22] | 0.061 | 36.03 | 40.65 | −0.15 | 0.00 |
| Fatigue | 481 | 49.66 (24.03) | 52.58 (26.36) | −2.32 | [−6.32; 1.70] | 0.259 | 49.98 | 52.29 | −0.09 | 0.00 |
| Fatigue (EORTC FA13)*1 | ||||||||||
| Physical fatigue | 481 | 49.01 (26.20) | 50.76 (26.32) | −1.71 | [−6.08; 2.66] | 0.443 | 48.95 | 50.66 | −0.07 | 0.03 |
| Emotional fatigue | 481 | 34.81 (25.94) | 35.96 (27.63) | 0.47 | [−3.74; 4.67] | 0.827 | 35.63 | 35.17 | 0.02 | 0.01 |
| Cognitive fatigue | 481 | 22.98 (21.79) | 22.22 (22.70) | 2.18 | [−1.11; 5.47] | 0.195 | 23.73 | 21.55 | 0.10 | 0.00 |
| Problems affecting daily life | 476 | 44.20 (30.23) | 44.18 (32.15) | 1.27 | [−3.95; 6.49] | 0.634 | 44.82 | 43.55 | 0.04 | 0.01 |
| Social consequences of fatigue | 478 | 23.35 (30.05) | 26.29 (31.54) | 1.30 | [−3.66; 6.26] | 0.607 | 25.58 | 24.28 | 0.04 | 0.00 |
| Coping with illness (FKV)*2 | ||||||||||
| Active problem-oriented coping | 481 | 2.82 (0.91) | 2.92 (0.90) | −0.11 | [−0.27; 0.05] | 0.165 | 2.81 | 2.93 | −0.12 | 0.01 |
| Depressive coping | 481 | 2.01 (0.81) | 2.06 (0.87) | −0.01 | [−0.14; 0.12] | 0.873 | 2.03 | 2.04 | −0.01 | 0.00 |
| Distraction and self-encouragement | 481 | 3.09 (0.89) | 3.04 (0.86) | 0.02 | [−0.13; 0.16] | 0.834 | 3.07 | 3.06 | 0.02 | 0.02 |
| Work-related outcomes | ||||||||||
| Work Ability Score*3 | 479 | 5.34 (2.66) | 5.40 (2.79) | −0.10 | [−0.55; 0.35] | 0.654 | 5.32 | 5.42 | −0.04 | 0.02 |
| Disability days during past 3 months | 426 | 13.53 (25.61) | 16.59 (29.05) | −3.02 | [−8.17; 2.13] | 0.250 | 13.53 | 16.55 | −0.11 | 0.03 |
We used mixed-effects models to calculate test statistics, controlled for the values of the initial survey.
We substituted missing data with the last available observed data (last observation carried forward [LOCF]).
AAP, Average adjusted predictions; b, non-standardized regression coefficient; CG, control group; CI, confidence interval; EORTC, European Organization for Research and Treatment of Cancer; FA, fatigue; FKV, Freiburg Questionnaire of Coping with Illness; ICC, intracluster correlation coefficient; IG, intervention group; QLQ, Quality of Life Questionnaire; SD standard deviation; SMD, standardized mean difference
*1 Maximum achievable score = 100; *2 maximum achievable score = 5; *3 maximum achievable score = 10
Key Messages.
The agreed minimum dose of the work-related therapeutic modules of our work-related medical rehabilitation program was, in the mean, successfully implemented in all four rehabilitation centers included in our study.
One year after completion of the program, no clinically relevant advantages were seen for work-related medical rehabilitation compared with conventional medical rehabilitation.
After a year, 75% of rehabilitants had returned to work.
Implementing a work-related medical rehabilitation program in oncology, such as we did in the four centers, is not likely to improve the chances of reintegration for cancer patients.
Acknowledgments
Translated from the original German by Birte Twisselmann, PhD.
Acknowledgments
We thank the German Pension Insurance for financial funding and the participating rehabilitation centers Klinik Bavaria in Freyung, Paracelsus-Klinik am See in Bad Gandersheim, MediClin Rose Klinik in Horn-Bad Meinberg, and AMEOS Reha Klinikum in Ratzeburg for their support and commitment to conducting the study.
Footnotes
Conflict of interest statement Dr. Zomorodbakhsch, Dr. Schmielau, Dr. Biester, Dr. Krüger, and Dr. Presl hold positions as senior physicians or in the management in the participating rehabilitation centers. The remaining authors declare that no conflict of interest exists.
Data sharing
The authors declare that the primary data cannot be shared as the data protection concept of the study and the patient consent forms do not cover this.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Rosso T, Malvezzi M, Bosetti C, Bertuccio P, Negri E, La Vecchia C. Cancer mortality in Europe, 1970-2009: an age, period, and cohort analysis. Eur J Cancer Prev. 2018;27:88–102. doi: 10.1097/CEJ.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 3.Robert Koch-Institut. Robert Koch Institut; Berlin: 2016. Bericht zum Krebsgeschehen in Deutschland 2016 9th ed. [Google Scholar]
- 4.Rasmussen DM, Elverdam B. The meaning of work and working life after cancer: an interview study. Psycho-Oncol. 2008;17:1232–1238. doi: 10.1002/pon.1354. [DOI] [PubMed] [Google Scholar]
- 5.de Boer AG, Taskila TK, Tamminga SJ, Feuerstein M, Frings-Dresen MH, Verbeek JH. Interventions to enhance return-to-work for cancer patients. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD007569.pub3. Cd007569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehnert A. Employment and work-related issues in cancer survivors. Crit Rev Oncol Hematol. 2011;77:109–130. doi: 10.1016/j.critrevonc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Amir Z, Brocky J. Cancer survivorship and employment: epidemiology. Occup Med. 2009;59:373–377. doi: 10.1093/occmed/kqp086. [DOI] [PubMed] [Google Scholar]
- 8.de Boer AG, Taskila T, Ojajarvi A, van Dijk FJ, Verbeek JH. Cancer survivors and unemployment: a meta-analysis and meta-regression. JAMA. 2009;301:753–762. doi: 10.1001/jama.2009.187. [DOI] [PubMed] [Google Scholar]
- 9.Mader L, Michel G, Roser K. Unemployment following childhood cancer. Dtsch Arztebl Int. 2017;114:805–812. doi: 10.3238/arztebl.2017.0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft DK, AWMF) S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Mammakarzinoms. www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom/ (last accessed on 19 February 2019) [Google Scholar]
- 11.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft DK, AWMF) S3-Leitlinie Kolorektales Karzinom. www.leitlinienprogramm-onkologie.de/leitlinien/kolorektales-karzinom/ (last accessed on 19 February 2019) [Google Scholar]
- 12.Streibelt M, Brünger M. Wie viele arbeitsbezogene Leistungen bekommen Patienten mit besonderen beruflichen Problemlagen? Analyse einer repräsentativen indikationsübergreifenden Stichprobe von Rehabilitanden. Rehabilitation. 2014;53:369–375. doi: 10.1055/s-0034-1375643. [DOI] [PubMed] [Google Scholar]
- 13.Bethge M. Medizinisch-beruflich orientierte Rehabilitation. Rehabilitation. 2017;56:14–21. doi: 10.1055/s-0042-118579. [DOI] [PubMed] [Google Scholar]
- 14.Streibelt M, Buschmann-Steinhage R. Ein Anforderungsprofil zur Durchführung der medizinisch-beruflich orientierten Rehabilitation aus der Perspektive der gesetzlichen Rentenversicherung. Rehabilitation. 2011;50:160–167. doi: 10.1055/s-0031-1275721. [DOI] [PubMed] [Google Scholar]
- 15.Beutel ME, Zwerenz R, Bleichner F, Vorndran A, Gustson D, Knickenberg RJ. Vocational training integrated into inpatient psychosomatic rehabilitation - short and long-term results from a controlled study. Disabil Rehabil. 2005;27:891–900. doi: 10.1080/09638280500030464. [DOI] [PubMed] [Google Scholar]
- 16.Kittel J, Karoff M. Lässt sich die Teilhabe am Arbeitsleben durch eine berufsorientierte kardiologische Rehabilitation verbessern? Ergebnisse einer randomisierten Kontrollgruppenstudie. Rehabilitation. 2008;47:14–22. doi: 10.1055/s-2007-1004606. [DOI] [PubMed] [Google Scholar]
- 17.Wienert J, Schwarz B, Bethge M. Effectiveness of work-related medical rehabilitation in cancer patients: study protocol of a cluster-randomized multicenter trial. BMC Cancer. 2016;16 doi: 10.1186/s12885-016-2563-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wienert J, Bethge M. Medizinisch-beruflich orientierte Rehabilitation für onkologische Rehabilitanden - kurzfristige Ergebnisse einer clusterrandomisierten Multicenterstudie. Rehabilitation. 2018 doi: 10.1055/a-0604-0157. doi: 10.1055/a-0604-0157. [DOI] [PubMed] [Google Scholar]
- 19.Fauser D, Wienert J, Beinert T, et al. Work-related medical rehabilitation in cancer patients - post-rehabilitation results from a cluster-randomized multicenter trial. Cancer. 2019 doi: 10.1002/cncr.32131. [DOI] [PubMed] [Google Scholar]
- 20.Campbell MK, Piaggio G, Elbourne DR, Altman DG. Consort 2010 statement: extension to cluster randomised trials. BMJ. 2012;345 doi: 10.1136/bmj.e5661. e5661. [DOI] [PubMed] [Google Scholar]
- 21.Bürger W, Deck R. SIBAR - ein kurzes Screening-Instrument zur Messung des Bedarfs an berufsbezogenen Behandlungsangeboten in der medizinischen Rehabilitation. Rehabilitation. 2009;48:211–221. doi: 10.1055/s-0029-1231062. [DOI] [PubMed] [Google Scholar]
- 22.Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer Evaluation of chemotherapeutic agents. In: MacLeod CM, editor. Columbia University Press. New York: 1949. pp. 191–205. [Google Scholar]
- 23.Schwarz B, Wienert J, Bethge M. Development and implementation of work-related medical rehabilitation in cancer patients using organizational ethnography and action research methodology. Int J Occup Med Environ Health. 2019;32:217–228. doi: 10.13075/ijomeh.1896.01250. [DOI] [PubMed] [Google Scholar]
- 24.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer I. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 25.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 26.Weis J, Arraras JI, Conroy T, et al. Development of an EORTC quality of life phase III module measuring cancer-related fatigue (EORTC QLQ-FA13) Psycho-Oncol. 2013;22:1002–1007. doi: 10.1002/pon.3092. [DOI] [PubMed] [Google Scholar]
- 27.Dörner U, Muthny FA. Comparison of two coping questionnaires in cardiological rehabilitation - the ‚Trierer Skalen zur Krankheitsbewältigung‘ (TSK) and the ‚Freiburger Fragebogen zur Krankheitsverarbeitung‘ (FKV) Z Med Psychol. 2008;17:125–132. [Google Scholar]
- 28.Ilmarinen J. The Work Ability Index (WAI) Occup Med (Lond) 2007;57 [Google Scholar]
- 29.Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least squares means. Am Stat. 1980;34:216–221. [Google Scholar]
- 30.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 31.Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J. 2012;12:308–331. [Google Scholar]
- 32.Zwiener I, Blettner M, Hommel G. Survival analysis: part 15 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2011;108:163–169. doi: 10.3238/arztebl.2011.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorenz E, Köpke S, Pfaff H, Blettner M. Cluster-randomized studies—part 25 of a series on evaluating scientific publications. Dtsch Arztebl Int. 2018;115:163–168. doi: 10.3238/arztebl.2018.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dreyhaupt J, Mayer B, Kaluscha R, Muche R. Cluster-randomisierte Studien: Methodische und praktische Aspekte. Rehabilitation. 2019 doi: 10.1055/a-0801-5697. DOI: 10.1055/a-0801-5697. [DOI] [PubMed] [Google Scholar]
- 35.Kabisch M, Ruckes C, Seibert-Grafe M, Blettner M. Randomized controlled trials: part 17 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2011;108:663–668. doi: 10.3238/arztebl.2011.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 51.0. Wiley. 2011 [Google Scholar]
- 37.Schwarz R, Hinz A. Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur J Cancer. 2001;37:1345–1351. doi: 10.1016/s0959-8049(00)00447-0. [DOI] [PubMed] [Google Scholar]
- 38.Nolte S, Liegl G, Petersen MA, et al. General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the Unites States. Eur J Cancer. 2019;107:153–163. doi: 10.1016/j.ejca.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 39.El Fassi M, Bocquet V, Majery N, Lair ML, Couffignal S, Mairiaux P. Work ability assessment in a worker population: comparison and determinants of work ability index and work ability score. BMC Public Health. 2013;13 doi: 10.1186/1471-2458-13-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamminga SJ, Verbeek JH, Bos MM, et al. Effectiveness of a hospital-based work support intervention for female cancer patients - a multi-centre randomised controlled trial. PloS One. 2013;8 doi: 10.1371/journal.pone.0063271. e63271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Escorpizo R, Finger M, Glässel A, Gradinger F, Lückenkemper M, Cieza A. A systematic review of functioning in vocational rehabilitation using the International Classification of Functioning, Disability and Health. J Occup Rehabil. 2011;21:134–146. doi: 10.1007/s10926-011-9290-8. [DOI] [PubMed] [Google Scholar]
- E2.Bieniek S, Bethge M. The reliability of workwell systems functional capacity evaluation: a systematic review. BMC Musculoskelet Disord. 2014;15 doi: 10.1186/1471-2474-15-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Deutsche Rentenversicherung. Deutsche Rentenversicherung. Berlin: 2015. Klassifikation therapeutischer Leistungen in der medizinischen Rehabilitation. [Google Scholar]
- E4.European Organisation for Research and Treatment of Cancer. EORTC QLQ-C30 Scoring Manual. www.eortc.org/app/uploads/sites/2/2018/02/SCmanual.pdf(last accessed on 30 April 2019) [Google Scholar]
- E5.Sedgwick P. Cluster randomised controlled trials: sample size calculations. BMJ. 2013;346 [Google Scholar]
- E6.Borm GF, Fransen J, Lemmens WAJG. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60:1234–1238. doi: 10.1016/j.jclinepi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- E7.Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks: SAGE Publications; 2000 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
Study design
The effects of work-related medical rehabilitation were studied in a cluster-randomized multicenter trial (17). We used cluster randomization in order to reduce the risk of treatment diffusion (34). The study was set in four inpatient rehabilitation centers. Participants were recruited between June 2015 and September 2016 and followed up to October 2017. Patients with the same admission date were randomly allocated to work-related medical rehabilitation (intervention group, IG) or conventional medical rehabilitation (control group, CG) after the inclusion criteria had been checked and the patients had consented to participate in the study.
The study protocol was approved by the ethics committee at the University of Lübeck (no. 14–289) and the data protection officer of the German Pension Insurance (DRB). The study was registered in the German Clinical Trials Registry (DRKS) (DKRS-ID: DKRS00007770).
Inclusion criteria
We included all cancer patients aged 19 to 60 years from all oncological indication groups (ICD-10 codes C00–D48: Neoplasms) who had successfully completed their initial cancer treatment and for whom at least one scale of the screening instrument for work and occupation (SIBAR) indicated a need for work-related rehabilitation (SIBAR I at least 8 points; SIBAR II: very stressful; SIBAR III: very helpful) (21). The cut-off scores for assessing need on the basis of SIBAR followed the recommendations of Bürger and Deck (21).
All participants had a score = 70% on the Karnofsky Performance Status Scale (22) and a preliminary positive social medical prognosis of at least 3 h/day for the ensuing 6 months. No exclusion criteria were defined.
Control group
The CG underwent conventional medical rehabilitation. This consisted of exercise therapy, physiotherapy, social counseling, occupational therapy, nutritional advice/counseling, and psychological seminars and counseling, as well as medical treatment and advice. Apart from basic social counseling, which is a regular component of medical rehabilitation, participants in the CG group did not receive any further work-related services.
Intervention group
The IG was provided with work-related modules in addition to their conventional medical rehabilitation, based on the requirements profile of the DRB. These consisted of work-related diagnostic evaluation, intensified social counseling, work-related psychosocial groups, and work-related functional capacity training. Furthermore, participants undergoing work-related medical rehabilitation were discussed in multiprofessional team meetings, in order to do justice to the complexity of the cases. During the preceding implementation phase, the general conditions for undertaking individual intervention modules were developed and defined in collaboration with the participating rehabilitation centers (17, 23).
Work-related diagnostic evaluation
The additional work-related diagnostic evaluation was undertaken by a treating physician, a psychologist, and an occupational therapist or physiotherapist immediately after the patient’s admission. Barriers to fitness for work may arise from physical functions, physical structures, activities, or participation (e1). The physician focused primarily on physical functioning and structures. The psychologist documented environmental factors and personal factors that had positive (resources) and negative (barriers) effects on the patient’s fitness to work. On the basis of standardized tests (e.g., WorkWell Systems Functional Capacity Evaluation) (e2) and structured observations of non-standardized work-related tasks, the occupational therapist or physiotherapist assessed the patient’s capacity to undertake work-related activities. The diagnostic process was extended by at least 60 min in this way.
Intensified social counseling
Intensified social counseling was intended to clarify the patient’s work situation and potential perspectives. The social worker and the rehabilitant jointly clarified the general conditions for a return to work and identified which additional measures and services were needed and could be accessed in order to support the return to work. Possible measures included a graded return to work, adjustments of the working environment, and job-related educational achievements. Where required and desired by the rehabilitant, the social worker established contact with the employer in order to support the transition from rehabilitation back into the workplace. Intensified social counseling took at least 90 min.
Work-related psychosocial groups
Work-related psychosocial groups aimed to support the concrete return to the workplace by concentrating on modifiable personal factors and environmental factors. During the group seminars, rehabilitants were encouraged to reflect critically on their current work situation and to identify options for behavior change. They learnt more about stress and how it affects their health and fitness to work. Coping strategies were taught. Techniques for direct stress reduction, such as progressive muscle relaxation, autogenic training, and meditation, were demonstrated and practiced in order to support the coping process. Furthermore, rehabilitants were shown communications techniques to help them shape interpersonal relationships with colleagues and senior colleagues more positively. They developed concrete plans for their return to the workplace. Potential problems and barriers were considered, and strategies were developed to deal with any barriers. Plans were made in light of the general conditions elaborated during the intensified social counseling. Work-related psychosocial groups took at least 240 min.
Work-related functional capacity training
Participants learned that exercises and physical activities could be safely undertaken in spite of pain symptoms. They were trained not only in simple or one-dimensional movements, but also complex and multidimensional movement sequences, in order to simulate realistic workplace demands. Therapists integrated ergonomic corrections into the performance of the exercises. Since cancer survivors often report cognitive impairments, the module also included cognitive training units—for example, computer-assisted training sessions and group exercises as instructed by a therapist—in order to support attention, concentration, and logical thinking. The work-related functional capacity training took at least 360 min.
Multiprofessional team meetings
These meetings provided a platform for joint exchanges about the individual rehabilitants and were intended to simplify the coordination of the different modules. The first meeting took place after completion of the work-related diagnostic evaluation so as to develop—together, as a team—a treatment strategy that aimed to improve work-related functioning. During the second meeting, all team members reported on the progress achieved by the rehabilitant and his/her current situation. If necessary, treatment strategies were adjusted accordingly. During the final meeting, the individual rehabilitation objectives were evaluated. On the basis of this evaluation, the team jointly decided on discharge as fit for work or unfit for work. Furthermore, it was discussed whether additional measures or services were required to support participation. Where this was the case, requests for such services or measures were filed with the relevant organization. Each team meeting took at least 30 min.
Documentation of therapeutic services
Services provided during the rehabilitation program were documented according to the classification of therapeutic services by the DRV (e3). Each service provided during the rehabilitation program was documented, with duration and frequency. The therapeutic dose was calculated from the product of the frequency and duration of the service. This was done for each of the four treatment modules of the work-related medical rehabilitation, in order to establish the overall therapeutic dose.
Outcome measures
The primary outcome measure was self-assessed role functioning 1 year after the end of the rehabilitation program. Role functioning was documented by administering the health-related quality of life questionnaire of the European Organisation for Research and Treatment of Cancer (EORTC QLQ-C30) (24). The role functioning scale uses two items to assess limitations regarding work and leisure activities (Were you limited in your work or in other activities of daily life? Were you limited in terms of your hobbies or other leisure activities?). The response categories are “not at all” (1), “a little” (2), “moderately” (3), and “very” (4). For the evaluation, we firstly calculated the mean value of the scale items (crude value). The crude values were subsequently transformed into a range of 0 to 100 (e4). Higher scores reflected better role functioning. A difference of at least 10 points between IG and CG was interpreted as clinically relevant (25).
Further secondary outcome measures were the EORTC QLQ-C30 scales for physical, emotional, and cognitive functioning and for general health and the symptom scale pain (24). We used the fatigue module EORTC QLQ-FA13, which captures physical, emotional, and cognitive fatigue as well as problems in daily life and the social consequences of fatigue (26). All scales have a score range from 0 to 100 points, with higher scores reflecting a better health-related quality of life or a greater burden of symptoms. The reliability and validity of both instruments have been confirmed (24, 26).
Coping with the disease was captured by using three scales of the Freiburg Questionnaire of Coping with Illness (active problem-oriented coping, depressive processing, distraction, and self-encouragement. Individual scores were combined in scales of mean values (1 to 5 points) (27).
The subjective ability to work was measured by using the Work Ability Score. A score of 0 means complete unfitness for work, while a score of 10 indicates the best work ability ever achieved (28). The concrete timing when participants returned to work (if applicable, also the timing of the graded return to work) was measured after 12 months.
All outcome measures described here were based on rehabilitants’ self-assessments.
Randomization
Participants admitted at the same time were allocated to the IG or CG after their eligibility for inclusion had been confirmed and their consent for participation in the study obtained. This allocation was randomized by using four randomization lists compiled independently for the participating rehabilitation centers. The computer-generated allocation lists were produced externally by the principal investigator. The lists were produced by using block randomization (blocks of four) in order to reduce the risk of treatment arms of dissimilar size in the event that the lists were not completely used. The patients were invited to the centers without prior knowledge of the treatment arm planned for the respective day of admission.
Sample size calculation
The sample size was calculated to detect a standardized mean difference (SMD) of SMD = 0.3, with a power of 80% and a two-sided alpha error of 5%. For a t test, this requires 352 participants. Cluster randomization increases the required sample size because of the design effect: by 20% for a mean group size of five persons and an intraclass correlation of 0.05 (e5). The planned control of the baseline values, however, reduced the required sample size greatly (e6), so that the estimated case number was conservative even without considering the design effect. We assumed that 30% of participants would not respond after a year; this meant that 504 participants needed to be recruited into the sample.
Statistical analyses
Sample characteristics, the mean therapeutic dose received, and the proportion of rehabilitants who received the recommended minimum dose of work-related therapies were modeled descriptively. Treatment effects were calculated using mixed-effects models. The correlation of error terms for rehabilitants admitted during the same week, rendered possible by the cluster randomization, was considered by means of random effects. Effects were estimated while controlling the corresponding value at the first data acquisition and the rehabilitation center. The rehabilitation centers were modeled as fixed effects. SMD were calculated to describe differences between groups. To this end, the non-standardized regression coefficient of the treatment effect (29) was standardized on the pooled standard deviation of the observed measurement values (e7). SMD were interpreted as follows, adhering to Cohen’s recommendations: small effect around SMD = 0.2, moderate effect around SMD = 0.5, and large effect around SMD = 0.8. We determined hazard ratios to compare reintegration into work. Moreover, we calculated average adjusted predictions that reflected the predicted probability for the occurrence of an event (return to work) within 1 year (31). The time until return to work was plotted in Kaplan–Meier curves (32).
To describe the heterogeneity associated with the clusters, we also determined the intracluster correlation for each outcome measure. This has to be considered when planning case numbers in cluster-randomized trials (design effect), since intraclass correlation reduces the power (33). All participants were analyzed on an intention to treat basis (35). We performed an analysis for the participants of the final follow-up (17, 36) as well as an analysis for which missing values were substituted by the values from the most recent available observation (last observation carried forward, LOCF). We considered differences between groups as statistically significant if the probability of a two-sided error was less than 5%. We used STATA 15 for our calculations.