Abstract
Objective:
To present the essential guidelines for pharmacological management of patients with psychomotor agitation in Brazil.
Methods:
This is a systematic review of articles retrieved from the MEDLINE (PubMed), Cochrane Database of Systematic Reviews, and SciELO databases published from 1997 to 2017. Other relevant articles in the literature were also used to develop these guidelines. The search strategy used structured questions formulated using the PICO model, as recommended by the Guidelines Project of the Brazilian Medical Association. Recommendations were summarized according to their level of evidence, which was determined using the Oxford Centre for Evidence-based Medicine system and critical appraisal tools.
Results:
Of 5,362 articles retrieved, 1,731 abstracts were selected for further reading. The final sample included 74 articles that met all inclusion criteria. The evidence shows that pharmacologic treatment is indicated only after non-pharmacologic approaches have failed. The cause of the agitation, side effects of the medications, and contraindications must guide the medication choice. The oral route should be preferred for drug administration; IV administration must be avoided. All subjects must be monitored before and after medication administration.
Conclusion:
If non-pharmacological strategies fail, medications are needed to control agitation and violent behavior. Once medicated, the patient should be monitored until a tranquil state is possible without excessive sedation.
Systematic review registry number:
CRD42017054440.
Keywords: Psychomotor agitation, aggression, tranquilizing agents, emergency, mental disorders
Introduction
The proper management of agitated patients is essential for their safety and for the safety of the health care staff.1-3 In most circumstances, non-pharmacological methods of behavior control, such as a verbal intervention or de-escalation, are helpful as an initial strategy to manage agitated patients1,4-7 (see Part 1 of these Guidelines8). However, when non-pharmacological methods fail, rapid tranquilization with pharmacological agents may be indicated.
Tranquilization or “rapid tranquilization” can be understood as calming without sedation.3 This strategy allows patients to have some participation in their own care. In the acute setting, rapid tranquilization facilitates diagnosis of the underlying cause of the agitation. Moreover, patients who are not asleep are easier to discharge from the emergency department.1
The pharmacological management of acute agitation has traditionally employed three classes of medications: first-generation antipsychotics (FGA), second-generation antipsychotics (SGAs), and benzodiazepines (BZDs). Administration is usually oral (PO, from the Latin per orem), including orally dispersible tablets (ODTs), sublingual tablets (SL), oral solutions (OSs), inhaled formulations (IN), as well as intramuscular (IM) and intravenous (IV) routes.1,2,4-6,9
The present article, corresponding to Part 2 of the Brazilian guidelines for the management of psychomotor agitation, focuses on the pharmacological approach to agitated patients. It should be noted that the article covers medications that are not yet available in this country as a way of considering future options and to contextualize Brazilian and non-Brazilian psychiatrists regarding the Brazilian scenario.
Method
This project involved 14 Brazilian psychiatry professionals selected by the Psychiatric Emergency Committee of the Brazilian Psychiatric Association (Associação Brasileira de Psiquiatria [ABP]), for their experience in and knowledge of psychiatry and psychiatric emergencies. This workgroup convened in 2016-2018 to recommend best practices in the use of medication to manage agitated patients in an emergency setting, focusing on the current daily practice of the Brazilian psychiatrist.
For the development of these guidelines, 85 articles were reviewed (among 5,362 initially collected and 755 abstracts on the pharmacological approach), retrieved from MEDLINE (PubMed), Cochrane Database of Systematic Reviews, Web of Science, and SciELO, published from 1997 to 2017, in the English, Portuguese, Spanish, or French languages. The search strategy used was based on questions structured according to the PICO strategy, with definition of Patient/Population of interest, Intervention/Exposure, Control/Comparison, and Outcome, as recommended by the Guidelines Project of the Brazilian Medical Association. The use of structured clinical questions was aimed at facilitating the elaboration of strategies to search for evidence. The search terms used were psychomotor agitation AND medication.
In evaluating the literature, despite the existence of many clinical trials and reviews, analysis of the results was limited by the following: evaluation of psychomotor agitation in the context of several different diagnoses, the settings in which evaluation and follow-up of agitated patients was conducted, and procedural variables such as the evaluation of medications in a small number of patients, using different instruments and differing outcome criteria. Therefore, the following inclusion criteria were standardized for study selection: 1) studies examining psychomotor agitation in adults (18 to 65 years); 2) studies using rapid tranquilization and including an evaluation of the outcome in the first 24 hours; and 3) studies with objective assessment of the response, based on a reduction of symptoms on an objective scale or on a calm state.
Exclusion criteria were as follows: 1) studies examining special groups, such as children, adolescents, the elderly, and the pregnant (even though recommendations are presented at the end of the present article, the treatment of such groups requires separate guidelines); 2) studies examining the use and abuse of substances, which was not the focus of this review, or articles for the treatment of situations that do not involve psychomotor agitation; 3) studies with a sample including fewer than 20 participants; or 4) studies including participants with clinical conditions such as delirium and dementia.
In addition, articles that have relevance in the literature were also used in the elaboration of the guidelines. Analysis of the articles followed four steps: I) review of relevant abstracts; II) reading of relevant articles in full; III) critical analysis of the evidence; and IV) extraction of the results and grading of the quality of evidence. Levels of evidence and strength of recommendations were defined according to the system proposed by the Oxford Center for Evidence-Based Medicine.10 In the results, levels of evidence are presented with numbers and letters (1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B, 4, and 5), and strength of recommendation is presented with letters (A, B, C, or D).
Results
Despite the limitations of a slow onset of action4,11,12 and the chance of non-adherence,4,13 oral formulations are generally preferred over IM preparations as the initial treatment of agitated patients.1,4 If the patient is well enough to agree with taking the medication, and if it is possible to wait longer for an effect to occur, the use of oral medications is preferred (D). If an oral option is not possible, the IM route is recommended (D). The IV route should be avoided because it poses a greater risk of serious side effects (D). Although the literature demonstrates the efficacy of some IV medications in psychomotor agitation, our group discourages their use and recommends that IV medications only be used in settings where cardiopulmonary resuscitation equipment and trained staff are available.
First-generation antipsychotics (FGAs)
Typical FGAs have a long history of use for treatment of agitation.1 These agents act by inhibition of dopamine transmission in the human brain, especially through dopamine D2 receptor antagonism, which is associated with reduction of psychotic symptoms.14 In addition to the risk of extrapyramidal side effects (EPSs),14 FGAs’ potential blockade of muscarinic cholinergic, histaminic, and α-1-adrenergic receptors is related to additional adverse effects.14
The most common FGAs used for rapid tranquilization due to agitation are chlorpromazine,15 levomepromazine,16 haloperidol,2 and droperidol.17 Promethazine,2 primarily known as an antihistamine, but belonging to the group of phenothiazines, may also be used.18
Chlorpromazine
Chlorpromazine was mentioned in a study that reported a clinical trial showing no differences between chlorpromazine injection and haloperidol.15,19 IM chlorpromazine is related to sudden, serious hypotension, and status epilepticus.15 The authors conclude chlorpromazine should be avoided if other drugs, with a more positive evaluation, are available.15 We recommend avoiding parenteral use of chlorpromazine for rapid tranquilization, including the IM route.
Levomepromazine
Levomepromazine is another phenothiazine. In an observational study, the response of levomepromazine was similar to that of olanzapine, and better than that of haloperidol. All medications were administered as an IM formulation.16 Levomepromazine was related to EPS, hypotension, hypertension, somnolence, dizziness, paralytic ileus, and ketoacidosis (B).16
Haloperidol
The most commonly used medication in studies is haloperidol, via PO,20,21 IM,2,16,22-38 and IV routes.26,39 The efficacy of the first oral doses was similar to that of risperidone20 and olanzapine (B).20,21 Oral haloperidol was effective in combination with lorazepam administered intramuscularly (B).20 However, as will be mentioned, the use of multiple routes is not recommended by our group, since the onset of action of the medications will occur at different times.
Use of IM haloperidol is well established, either as a monotherapy (A)2,16,23-25,28-30,35-38,40 or in combination with promethazine (A),2,27,28,41-44 midazolam,2,27,42 or lorazepam (B).30,45-47 Much has been discussed with regard to the doses. Responses have been observed following administration of a first dose ranging from 2.5 up to 10 mg. It is important to note that haloperidol is associated with the same side effects as other antipsychotics, with a higher frequency of EPS.2,16,23-25,27-30,35-38,40-44
Haloperidol has also been used intravenously, with proven efficacy as compared with sodium valproate39 and BZDs26 (1A). However, the IV route amplifies the side effects, especially EPS and cardiotoxity.39 Therefore, we do not recommend IV use of haloperidol (D).
Droperidol
Droperidol, as well as haloperidol, is a butyrophenone whose efficacy has been demonstrated with IM17 and IV administration.48 In addition to the evidence regarding the efficacy and safety of the IM route (B),17,35,49-51 studies have reported fewer respiratory side effects17,50 and fewer additional medications with IM droperidol than midazolam.17 One study used a combination of IM droperidol and midazolam (B).17 IV droperidol has efficacy as monotherapy17,50,52-54 or in combination with midazolam48,53,54 or lorazepam.51 Despite the high level of evidence (1B) regarding efficacy, the combination of droperidol and BZDs increases the risk of respiratory depression and the need for ventilatory support.48 Therefore, due the risk of severe side effects such as the prolongation of the QT interval,17 EPS,49,52 and hypotension,51 we do not recommmend IV use of droperidol (D).
Loxapine
Loxapine is classified as a typical antipsychotic; however, it has atypical characteristics. Loxapine has been used in its inhaled formulation for the control of psychomotor agitation and has proven efficacy compared to placebo in randomized trials (A).55,56
Clinicians must be aware that FGA are associated with the following side effects: sudden, serious hypotension; status epilepticus, especially with chlorpromazine; headache, dizziness, and nausea; EPS; hypotension; respiratory depression (IV administration or when associated with another sedative medications); bradycardia; dry mouth; seizure; and abnormal QT interval prolongation.
Second-generation antipsychotics (SGAs)
From a pharmacological perspective, atypical antipsychotics are serotonin and dopamine antagonists, D2 partial antagonists, or serotonin partial antagonists at 5HT1A receptors.14 With these properties, they are less likely to produce EPSs (such as parkinsonism) and tardive dyskinesia.14
Atypical antipsychotics with efficacy for rapid tranquilization found in the literature were aripiprazole,36,57,58 olanzapine,2,16,42,43 risperidone,29,46,47,59 and ziprasidone.2,42,60
Risperidone
PO, ODT, or OS risperidone were effective in the control of psychomotor agitation when administered alone (A)29,61-64 or with lorazepam (B).46,47,59,61 OS risperidone together with clonazepam was as effective as IM haloperidol25 or PO risperidone in combination with PO lorazepam (B).46,47
Aripiprazole
IM aripiprazole showed efficacy for psychomotor agitation control, and equivalence compared to IM haloperidol23,36,57,58 and lorazepam.13 The quality of the evidence supporting IM aripiprazole is high (A). However, it was not available in this formulation in Brazil at the time of this review.
Olanzapine
PO or ODT olanzapine may be effective for psychomotor agitation control.21,61-63,65,66 IM olanzapine is as effective40,67 as ziprasidone,2 haloperidol,2,22,30,31,37,40,42,43 and other antipsychotics.16,27,62,68,69 The combined use of IM olanzapine and BZDs is not recommended due to the possibility of dangerous effects (e.g., hypotension, bradycardia, and respiratory depression).4,31 PO/ODT olanzapine (B), IM olanzapine (B), and IV olanzapine were shown to be effective (B).53,54 Besides, due the same reasons mentioned for haloperidol and droperidol, we do not recommend IV use of olanzapine (D).
Ziprasidone
Ziprasidone was effective at controlling psychomotor agitation.23,38,60,70,71 Ziprasidone 20 mg IM was effective when compared with a low dose (2 mg),23,60,70,71 and had positive results compared to haloperidol (and its combinations),2,27,34,38,42 olanzapine,2,42 droperidol,49 midazolam,27 and lorazepam.27 IM ziprasidone is recommended as B.
Asenapine
Finally, SL asenapine was effective for psychomotor agitation control (A).72 In a study with120 subjects, participants were randomized to receive SL asenapine or placebo. The change in Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) at 2 hours was significantly greater in asenapine-treated subjects than in score in placebo-treated subjects. The number needed to treat (NNT) for a response compared to placebo was 3 (95% confidence interval [95%CI] 2-4). The side effects reported were distorted or bad taste and akathisia.72
Clinicians must be aware of SGAs side effects, which include the following: hypotension, excessive sedation, headache, dizziness, nausea, EPS, respiratory arrest, bradycardia, dry mouth, seizure, prolongation of QTc interval, seizure, and syncope.
Benzodiazepines (BZDs)
BZDs enhance the effects of the neurotransmitter gamma-aminobutyric acid (GABA) at the GABA-A receptor, producing sedative, hypnotic (sleep-inducing), anxiolytic, anticonvulsant, and muscle relaxant effects.73 BZD side effects include the following: oversedation, dizziness, weakness, loss of orientation, headache, confusion, irritability, paradoxical effect, memory impairment, lack of coordination, risk of falls, dry mouth, blurred vision, hypotension, ataxia, respiratory depression, and cardiorespiratory arrest.
The literature search on oral BZDs for agitation control found only limited data. A systematic review concluded that there is no strong evidence to support or refute the use of BZDs (with or without antipsychotics or in combination with other drugs).26 However, considering that BZDs are inexpensive, have moderate side effects, are easy to administer, and can easily be found in most emergency departments, we will discuss the evidence from existing randomized controlled trials (RCTs) and other relevant studies.
Oral risperidone plus oral lorazepam46,47,59 and oral risperidone plus oral clonazepam were effective for psychomotor agitation (B).25 We did not find clinical trials focusing on other oral BZDs. PO diazepam, PO and OS clonazepam, and PO lorazepam may be used for mild to moderate agitation induced by alcohol withdrawal and cocaine intoxication (D).
Further evidence was found for IM lorazepam monotherapy (A),13,27,33,41,45,51,59 lorazepam plus haloperidol (B),30,45-47,59 lorazepam plus olanzapine (B),30 midazolam monotherapy (A),17,27,33,44,49,50 midazolam plus haloperidol (A),2,27,42 clonazepam monotherapy (C),74 flunitrazepam monotherapy (B),24 midazolam plus droperidol (B),17 and IM lorazepam plus oral risperidone (B).47,59,61 Regarding the IV route, evidence was obtained for midazolam plus droperidol (1B)48,53,54 and midazolam plus olanzapine (1B).48
The most frequent side effects of BZDs are respiratory depression, ataxia, excessive sedation, memory impairment, and paradoxical disinhibition.26 These effects are stronger with the IV route, in combination with other psychotropic medications, and in individuals who have used nervous system depressant drugs (such as alcohol). Finally, a word of caution against IV diazepam use (indicated for alcohol withdrawal and cocaine intoxication): its active metabolite nordazepam has a much longer elimination half-life, so that the drug accumulates when administered repeatedly, especially in elderly patients because of the drug’s effect on oxidative drug metabolism.26
Antipsychotics vs. benzodiazepines (BZDs)
Although one study concluded that there is no strong evidence to support or refute the use of BZDs for aggression and agitation,26 some studies report similar results with BZDs and antipsychotics.
We did not find any study to support the use of oral BZDs as monotherapy for rapid tranquilization. Meanwhile, oral treatment with risperidone and lorazepam appears to be a tolerable and comparable alternative to IM haloperidol and lorazepam for short-term treatment of agitated psychosis in patients who accept oral medications.46,47 Risperidone OS in combination with clonazepam is an effective treatment, comparable to IM haloperidol, and is well-tolerated for acute agitation in patients with schizophrenia.25 Therefore, we recommend the use of oral BZDs for psychomotor agitation only when administered with antipsychotics (B), except if there is contraindication for antipsychotic use (D).
Similar results have been described for IM lorazepam and IM midazolam as compared to haloperidol monotherapy,27,33 haloperidol plus promethazine,27,41,44 olanzapine,27 ziprasidone,27 and aripiprazole.13 The results obtained with midazolam were similar to those described for droperidol, however with more oversedation.17 In another study, IM lorazepam and lorazepam plus haloperidol were shown to be superior to haloperidol monotherapy and similar to olanzapine.30
The results with midazolam plus haloperidol were similar to those obtained with haloperidol monotherapy, haloperidol plus promethazine, olanzapine, and ziprasidone in the first hours after the first administration. However, after 6 to 12 hours, poor results were measured by the Overt Agitation Severity Scale (OASS) and Overt Aggression Scale (OAS).2 In the other two studies, the results were similar to those observed with antipsychotics.27,42
IM clonazepam is effective and safe, but slower-acting as compared to IM haloperidol for the treatment of agitated psychiatric patients in need of rapid tranquilization.74 IM flunitrazepam was similar to haloperidol.24 Interestingly, oral risperidone was similar to IM lorazepam.46 IM lorazepam showed similar results to IM lorazepam plus oral haloperidol or oral risperidone.59
The results described with IV midazolam are similar to those of droperidol, although there was a hypoventilation risk.50 The combination of IV midazolam plus droperidol had better results than IV droperidol or IV olanzapine.53,54 IV lorazepam was less effective in controlling agitation than droperidol.51
Thus, there is no clear difference between BZDs and antipsychotics regarding their effectiveness for rapid tranquilization. However, differences in side effects must be considered. BZDs can cause more oversedation and respiratory effects and thus should be avoided in patients with sedative drug abuse (i.e., alcohol and opioids), patients with the potential for paradoxical reactions, and patients with respiratory depression risk (D).
First-generation antipsychotics (FGAs) vs. second-generation antipsychotics (SGAs)
PO risperidone plus lorazepam was similar to IM haloperidol plus lorazepam.46 PO risperidone59 and oral olanzapine20 were as effective as PO haloperidol.59 Risperidone OS in combination with clonazepam is an effective treatment, comparable to IM haloperidol, and is well tolerated for acute agitation in patients with schizophrenia.25 There is no significant difference in effectiveness between IM olanzapine, orally disintegrating olanzapine tablets, and oral risperidone solution compared to IM haloperidol.62 Oral olanzapine was similar to oral haloperidol.27 Risperidone ODT was as effective and tolerable as IM haloperidol.29
IM aripiprazole was non-inferior compared to haloperidol.23,36,57 IM olanzapine was similar to haloperidol.22,40 IM olanzapine and ziprasidone had similar results to haloperidol and haloperidol plus promethazine.2,37,43 Low doses of haloperidol combined with midazolam can be as effective as olanzapine in reducing psychomotor agitation without increasing the risk of extrapyramidal effects.42 Olanzapine and ziprasidone were reported to be similar to haloperidol, haloperidol plus promethazine, midazolam, and haloperidol plus midazolam27; however, this article observed a quicker onset of activity with haloperidol plus promethazine and haloperidol plus midazolam, and therefore found no benefit in the use of haloperidol in monotherapy.27
Haloperidol monotherapy was less effective, or at least required additional medication compared to olanzapine with or without a BZD or haloperidol plus a BZD.30 In addition, one study suggested the possibility that the anti-agitation effects of IM olanzapine and IM levomepromazine occur more rapidly than those of IM haloperidol.16
IV olanzapine plus midazolam was similar to droperidol plus midazolam.48 In contrast, in another study, olanzapine was not superior to IV droperidol.53
Although small differences were observed, side effects were similar in the various studies evaluated to guide the choice in favor of atypical antipsychotics. Therefore, we recommend that the choice between FGAs and SGAs be based on the assessment and experience of the physician who attends each case (D).
Choice between routes of administration
Even though oral administration may be as effective as parenteral administration, it is not free of side effects. Nevertheless, since the oral route is a less invasive, it is the first option to be considered (D). However, it is important to remember that the options that were useful to treat psychomotor agitation were PO, OS, and ODT risperidone, PO and ODT olanzapine, and SL asenapine, as well as the combination of PO risperidone and lorazepam.
The oral route should be chosen if the patient is cooperative, is able to swallow, and is able to wait for the onset of effects, which takes than with parenteral administration. When the oral route is chosen, preference should be given to OS and ODT presentations, which facilitate swallowing, absorption, and the onset of effects (D). For additional details on oral medications recommended for rapid tranquilization, see Table 1.
Table 1. Recommended oral medications for rapid tranquilization in psychomotor agitation* .
| Medication | Dosage (mg) | Initial effects (h) | Half-life (h) | Can repeat (h) | Maximum dosage per 24 h (mg) | Side effects | Level of evidence | Grade of recommendation |
|---|---|---|---|---|---|---|---|---|
| Risperidone (PO/OS/ODT†) | 2-3 | 1 | 24 | 1 | 8 | Drowsiness, dizziness, EPS, hemodynamic effects, seizures, dysphagia, nausea, cardiac arrhythmia, hypotension | 1B | A |
| Asenapine (SL) | 10 | 0.5-1.5 | 24 | 12 | 20 | EPS, hemodynamic effects, seizures, dysphagia | 1B | A |
| Risperidone (OS/PO) + lorazepam (PO) | 2 + 2 | 1 | 24 | 1 | 6/6 | EPS, hemodynamic effects, seizures, dysphagia, cardiac arrhythmia, hypotension, dizziness, oversedation, respiratory depression | 2A | B |
| Olanzapine (ODT/PO) | 10 | 1-2/4-6 | 21-54 | 2/4 | 30 | EPS, hemodynamic effects, seizures, dysphagia, cardiac arrhythmia, hypotension, dizziness | 2A | B |
| Haloperidol (PO/OS) | 5-15 | 1-4 | 15-37 | 8 | 15 | EPS, hemodynamic effects, seizures, dysphagia, ECG alterations | 2B | B |
| Risperidone (OS/PO) + clonazepam (PO) | 2 + 2 | 1 h | 20-40 | 1 | 6/6 | EPS, hemodynamic effects, seizures, dysphagia, cardiac arrhythmia, hypotension, dizziness, oversedation, respiratory depression | 2B | B |
| Clonazepam (PO/OS) | 2 | 1-3 | 20-40 | 1 | 8 | Amnesia, ataxia, oversedation, dizziness, paradoxical effect | 5 | D |
| Diazepam (PO) | 10 | 0.5-1.5 | 20-80 | 1 | 60 | Amnesia, ataxia, oversedation, dizziness, paradoxical effect | 5 | D |
| Lorazepam (PO) | 2-4 | 2 | 8-16 | 2 | 4 | Amnesia, ataxia, oversedation, dizziness, paradoxical effect | 5 | D |
Only recommended formulations are included.
Not available in Brazil.
ECG = electrocardiogram; EPS = extrapyramidal side effects; ODT = oral disintegrating tablets; OS = oral solution; PO = from the Latin per orem; SL = sublingual.
The IM route accounts for most of the studies. Its absorption and onset of effects is faster than the oral route. The IM route seems best suited for patients with more severe and violent agitation. This route requires training and rigorous monitoring. Again, there are few differences justifying the use of one medication over another. The literature also shows that monotherapy may not always be a good choice, since there are issues related to the repeated administration of medications.2,17,27,30 Despite the possibility of more side effects with combined medications4,17,27 (i.e., oversedation and respiratory depression), combinations should be considered in cases of severe agitation, especially associated with violent behavior (D). For additional details on IM medications recommended for rapid tranquilization, see Table 2.
Table 2. Recommended intramuscular medications for rapid tranquilization in psychomotor agitation* .
| Medication | Dosage (mg) | Initial effects | Half-life | Can repeat | Maximum dosage per 24 h (mg) | Side effects | Level of evidence | Grade of recommendation |
|---|---|---|---|---|---|---|---|---|
| Haloperidol | 2.5-10 | 30 min | 15-37 h | 30 min | 30 | Seizure, EPS, somnolence, headache, dizziness. | 1A | A |
| Haloperidol + midazolam | 2.5 + 7.5-15 | 20 min | 15 h | 30 min | 30 for haloperidol | Excessive sedation, EPS. | 1A | A |
| Haloperidol + promethazine | 2.5-10 + 25-50 | 30 min | 15-37 h | 30 min | 30/100 | Excessive sedation, EPS, seizure | 1A | A |
| Lorazepam† | 2-4 | 20-30 min | 13-18 h | 1 h | 4 | Respiratory difficulty, nausea, dizziness with lorazepam | 1A | A |
| Midazolam | Up to 15 | 15-20 min | 90-150 min | 30 min | - | Over sedation, respiratory depression | 1A | A |
| Olanzapine | 2.5-10 | 15-45 min | Excessive sedation, EPS, orthostatic hypotension, somnolence, blood urine | 1A | A | |||
| Ziprasidone | 10-20 | 1 h | 2-5 h | 10 (2 h)/20 (4 h) | 40 | Excessive sedation, EPS | 1A | A |
| Aripiprazole† | 9.75 | 1-3 h | 75-94 h | 2 h | 30 | Headache, dizziness, nausea, insomnia, EPSs, tachycardia | 1B | A |
| Droperidol | 2.5-10 | 3-10 min | 3 h | 30 min | 20 | Abnormal QT, hypotension, dizziness, EPS | 2B | B |
| Droperidol+ midazolam | 10 + 5 | 15 min | 2 h | 30 min | 20/15 | Abnormal QT, oversedation, respiratory depression | 2B | B |
| Flunitrazepam† | 1-2 | 2 h | 18-26 h | 24 h | 2 | Respiratory arrest, oversedation, hypersalivation, drowsiness, dizziness, amnesia | 2B | B |
| Haloperidol + lorazepam† | 5 + 2 | 30 min | 18 h | 1 h | 15/4 | Excessive sedation, EPS | 2B | B |
| Levomepromazine† | 12.5-25 | 20-40 min | 30 h | 6 h | 100 | Abnormal QT, hypotension, dizziness, EPS | 2C | B |
| Clonazepam† | 1-2 | 0.5-1 h | 20-80 h | 3 h | 8 | Respiratory arrest, oversedation, hypersalivation, drowsiness, dizziness, amnesia | 4 | C |
Only recommended formulations are included. Vital signs and electrocardiogram (ECG) monitoring are recommended for patients with high cardiac risk or unknown history.
Not available in Brazil.
EPS = extrapyramidal side effects.
Table 3 describes the details of IV medications with best evidence for rapid tranquilization of agitated patients. The IV route has been proven effective but is associated with severe side effects, mainly respiratory depression. We do not recommend IV administration (D). If the clinician decides to use the IV route, it should be reserved only for severe cases, where other measures fail and only in places where monitoring of vital signs is continuous (such as with a multiparameter emergency monitor) (D).
Table 3. Best evidence for intravenous medications for rapid tranquilization in psychomotor agitation* (we do not recommend IV administration due to safety concerns).
| Medication | Dosage (mg) | Initial effects (min) | Half-life (h) | Can repeat (min) | Maximum dosage per 24 h (mg) | Side effects | Level of evidence |
|---|---|---|---|---|---|---|---|
| Droperidol | 2.5-10 | 3-10 | 4-6 | 15 | 10 | EPS, hypotension, QT prolongation | 1A |
| Haloperidol | 5 | 20 | 12-22 | 30 | 20 | EPS, hypotension | 1A |
| Midazolam | 2.5-10 | 5 | 1.5-2.5 | 15 | - | Hypoventilation requiring airway management, hypotension | 1A |
| Lorazepam | 1-4 | 1-5 | 10-20 | 15 | 10 | Hypoventilation requiring airway management, hypotension | 2B |
| Diazepam† | 10 | 1-5 | 20-80 | 30 | 40 | Hypoventilation requiring airway management, hypotension | 5 |
IV route should only be used in places with adequate equipment for cardiorespiratory support. Avoid IV administration if possible. Avoid IV administration in patients at high cardiac risk. Vital signs and electrocardiogram (ECG) monitoring are necessary for patients at high cardiac risk or whose history is unknown.
Exception: indication for alcohol withdrawal syndrome and severe cocaine intoxication (D).
EPS = extrapyramidal side effects.
At this point, special emphasis must be placed on a parameter guiding the choice of medication and the route to be used: cardiac risk. Psychotropics, especially antipsychotics, are related to the risk of events such as ventricular arrhythmia, QT prolongation (including torsade de pointes), and sudden death.75-77 The literature remains controversial, but points to greater risk with antipsychotics. Regarding the medications covered in these guidelines, risk has been described for chlorpromazine,78 droperidol,79,80 haloperidol,76,81 levomepromazine,82 olanzapine,76,78,83 risperidone,78,83 and ziprasidone.76,83 Aripiprazole showed the lowest risk.75,76 However, it is necessary to pay attention to patients at high risk of cardiac alterations, such as previous cardiac disease, family history of sudden death or ventricular tachyarrhythmia, abnormalities of ventricular repolarization, and electrolyte imbalances.77 When these risk factors are present, antipsychotic medications should be avoided, as should the parenteral route, especially the IV route. Regarding this last route, the risk will be high even in patients without the mentioned factors.77 For patients with no data or for those known to have the risk factors mentioned, consider electrocardiogram (ECG) monitoring when administering parenteral medications, especially IV medications (D). It is also recommended that the lowest dose be used (D).
A new alternative is loxapine, shown to be efficacious compared to placebo. Loxapine has been used in its IN form for the control of psychomotor agitation (B).55,56,84 Another alternative is transdermal (TD) nicotine replacement for patients with dependence (B).85
How to combine medications
One of the greatest dilemmas in rapid tranquilization is whether combinations of different medications should be used. Even if there is evidence of efficacy, administration of combined medications is challenging, and side effects could be amplified. Despite the evidence of efficacy, as discussed in the present article, we do not recommend mixing different routes of administration (for example, administering PO and IM medication at the same time), because absorption and onset of effects will occur at different times and will complicate monitoring.
Regarding the oral route, oral risperidone plus oral lorazepam (B)46,47 and oral risperidone plus oral clonazepam (B)25 were effective options compared to IM haloperidol plus IM lorazepam and IM haloperidol (Table 1).
Regarding the IM route, haloperidol was shown to be effective and safe when combined with promethazine (A),2,27,28,41-44 midazolam,2,27,42 or lorazepam (B).30,45-47 Droperidol can be combined with midazolam by the IM route (B).17 The combination of IM olanzapine and BZDs is not recommended based on the literature (D)4,31 (Table 2).
Regarding the IV route, IV droperidol, another butyrophenone, has been studied, and efficacy has been demonstrated when combined with midazolam48,53,54 or lorazepam51 (Table 3). Despite the high level of evidence (1B), it is important to underscore that combinations with BZDs can cause respiratory depression and require the need for ventilatory support.48 Therefore, this strategy requires extra care and should not be used in environments with inadequate emergency equipment (D).
Sequence of treatment
The decision to administer the drug repeatedly in cases of psychomotor agitation must be based on the individual characteristics of the patient, and therefore it is difficult to make a recommendation. We suggest that, if necessary, the same medication be used until its maximum daily dose is reached. Then, if additional medication is needed, a different medication is tried. Although there are no studies on the subject, nothing prevents the IM route from being used, but if the patient is calmer, the oral route can be considered for repeated administration (D).
Special situations
Special situations are outside the scope of the present guidelines. Each of these situations requires an individual review. However, we will briefly present the main measures to be taken in cases of agitation due to intoxication, psychiatric disorder, and delirium, and in cases of agitation in the elderly, pregnant women, and children.
Agitation due to intoxication
Unfavorable results have been reported in the literature regarding the safety of medications for rapid tranquilization in patients with acute substance intoxication. Therefore, new clinical trials and specific reviews on the subject are needed. In the presence of known substance abuse by the patient, whether the abuse is related to the agitation or not, special care should be taken. Following the use of drugs that cause central nervous system depression, such as alcohol, BZDs, and opioids, certain medications should be avoided because of the potential to compound the risk of respiratory depression (D).1,4 We recommend haloperidol as having the longest track record of safety and efficacy and minimal effects on respiration (D).1,4 SGAs, such as olanzapine, ziprasidone, and risperidone, have not been well studied in the context of alcohol intoxication, but may provide reasonable alternatives to haloperidol for agitation in this context.1,4 Of note, it is important to distinguish agitation secondary to alcohol intoxication vs. agitation secondary to alcohol withdrawal, as BZDs are preferred over antipsychotics in cases associated with alcohol withdrawal.1,4
Agitation associated with delirium
Once delirium is suspected, the first measure to be considered is an evaluation of the patient, to investigate and address the underlying cause of the delirium. Treatment of the organic cause is superior to any drug intervention, and excessive sedation without appropriate treatment should be avoided since the failure to reverse the organic factor can lead to death. When delirium associated with an underlying medical abnormality (e.g., hypoglycemia, electrolyte imbalance, or hypoxia) is the likely cause of the agitation, the most important approach is correction of the underlying medical condition.1,4 If immediate pharmacologic control of agitation is needed in a patient with delirium that is not due to alcohol, BZD withdrawal, or sleep deprivation, SGAs are the preferred agents.1 Haloperidol and risperidone are also acceptable in low doses.1,4 BZDs should be generally avoided because they can exacerbate delirium.1,4
Agitation in the elderly
In the elderly, agitation should be presumed to result from delirium until proven otherwise, especially in the presence of alterations in attention, orientation, and level of consciousness.4 If a medical etiology has been excluded, clinicians should consider affective and anxiety disorders as the most prevalent psychiatric causes of agitation in the elderly.4 After an adequate assessment of the possible etiology, it is recommended to initially try all non-pharmacological strategies. If necessary, proceed with pharmacological interventions and/or physical restraint, but do it judiciously and for a short-term period, with frequent review and close monitoring.4 As a general principle for the pharmacological treatment of psychiatric agitation in the elderly, cautious use of antipsychotics has been recommended as follows: start with low doses and slowly titrate with small increments in dose, perform an appropriate observation of the medication effects and close-meshed monitoring of the clinical situation, the risks of falls, signs of confusion and oversedation.4
Pregnancy
There is a lack of studies assessing the management and treatment of psychiatric agitation in pregnant women, and often the cohorts are too small to detect significant differences between treatment options.4 Considering the paucity of evidence on this topic, it has been suggested that clinicians should employ mainly verbal interventions in pregnant agitated patients whenever possible, and when medication is required, the minimal but effective amount of medication necessary to reduce agitation and the risk of aggression should be used.4,86 If medication is necessary, previous works have suggested haloperidol alone as first-line treatment and three agents as second-line: BZDs or risperidone monotherapy and, with less endorsement, a combination of a BZD and an FGA.4,86
Limitations
The main limitation of the present work is the difficulty in comparing the results of selected studies due to heterogeneity. Studies on agitation use different medications, different doses, and different assessment tools, and they consider different outcomes as success. To minimize this bias, we limited the discussion to studies with an observed response, i.e., a reduction in an objective scale score or being in a quiet or calm state in the first hours to a maximum of 24 hours of administration. We propose that these guidelines should be updated in the future.
Conclusions
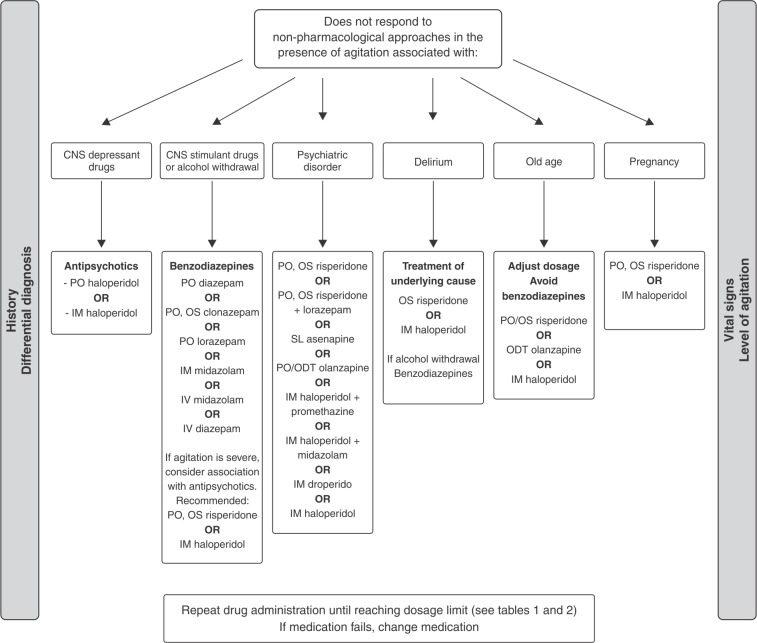
After reviewing the available evidence (online-only supplementary material, Table S1 (255.2KB, pdf) ), the following recommendations can be made: 1) pharmacologic treatment is indicated only after non-pharmacologic approaches have failed; 2) the cause of the agitation, side effects of the medications, and contraindications must guide the medication choice; 3) the oral route should be preferred for drug administration; 4) IV administration must be avoided; and 5) all subjects must be monitored before and after medication administration. We also propose a hierarchical sequence to guide the pharmacological management of psychomotor agitation, as shown in Figure 1.
Figure 1. Flow diagram of the pharmacological management of psychomotor agitation. CNS: central nervous system; IM = intramuscular; IV = intravenous; ODT = oral disintegrating tablets; OS = oral solution; PO = from the Latin per orem; SL = sublingual.
Disclosure
LB has served as consultant for Apsen and participated in a meeting from Libbs. LAP has served as speaker for Janssen-Cilag, Servier, EMS, and Libbs. LAP has received support to participate in meetings from Janssen-Cilag, Lundbeck, Abbott, Servier, and Libbs. CAM has served as speaker for Lundebeck and has performed research for Janssen-Cilag. TCT has served as member of the advisory board/consultant for Aché, Abbott, Servier, Lundbeck, and Apsen; has served as speaker for Aché, Abbott, Pfizer, EMS, Medley/Sanofi, Lundbeck, Servier, Libbs, Apsen, Torrent, and Daichii-Sankyo; has performed research for Janssen-Cilag and served as consultant for clinical/scientific related promotional articles for Aché, Abbott, Pfizer, EMS, Medley/Sanofi, Lundbeck, Servier, Supera, Janssen-Cilag, Cristália, Libbs, Apsen, Torrent, and Daichii-Sankyo. QC works in the Ministry of Health and is responsible for the elaboration of public policies in mental health in Brazil. The other authors report no conflicts of interest.
Acknowledgements
The authors wish to thank the Brazilian Psychiatric Association (ABP) for providing physical space and time during events for team meetings.
Footnotes
How to cite this article: Baldaçara L, Diaz AP, Leite V, Pereira LA, dos Santos RM, Gomes Júnior VP, et al. Brazilian guidelines for the management of psychomotor agitation. Part 2. Pharmacological approach. Braz J Psychiatry. 2019;41:324-335. http://dx.doi.org/10.1590/1516-4446-2018-0177
References
- 1.Wilson MP, Pepper D, Currier GW, Holloman GH, Jr, Feifel D. The psychopharmacology of agitation: Consensus Statement of the American Association for Emergency Psychiatry Project Beta Psychopharmacology Workgroup. West J Emerg Med. 2012;13:26–34. doi: 10.5811/westjem.2011.9.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldaçara L, Sanches M, Cordeiro DC, Jackoswski AP. Rapid tranquilization for agitated patients in emergency psychiatric rooms: a randomized trial of olanzapine, ziprasidone, haloperidol plus promethazine, haloperidol plus midazolam and haloperidol alone. Rev Bras Psiquiatr. 2011;33:30–9. doi: 10.1590/s1516-44462011000100008. [DOI] [PubMed] [Google Scholar]
- 3.McAllister-Williams RH, Ferrier IN. Rapid tranquillisation: time for a reappraisal of options for parenteral therapy. Br J Psychiatry. 2002;180:485–9. doi: 10.1192/bjp.180.6.485. [DOI] [PubMed] [Google Scholar]
- 4.Garriga M, Pacchiarotti I, Kasper S, Zeller SL, Allen MH, Vazquez G, et al. Assessment and management of agitation in psychiatry: Expert consensus. World J Biol Psychiatry. 2016;17:86–128. doi: 10.3109/15622975.2015.1132007. [DOI] [PubMed] [Google Scholar]
- 5.Zeller SL, Rhoades RW. Systematic reviews of assessment measures and pharmacologic treatments for agitation. Clin Ther. 2010;32:403–25. doi: 10.1016/j.clinthera.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani C, Migon MN, Alheira FV, Del-Ben CM. Manejo de paciente agitado ou agressivo. Rev Bras Psiquiatr. 2010;32:S96–103. doi: 10.1590/s1516-44462010000600006. [DOI] [PubMed] [Google Scholar]
- 7.Richmond JS, Berlin JS, Fishkind AB, Holloman GH, Jr, Zeller SL, Wilson MP, et al. Verbal De-escalation of the Agitated Patient: Consensus Statement of the American Association for Emergency Psychiatry Project BETA De-escalation Workgroup. West J Emerg Med. 2012;13:17–25. doi: 10.5811/westjem.2011.9.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldaçara L, Ismael F, Leite V, Pereira LA, dos Santos RM, Gomes VP, Júnior, et al. Brazilian guidelines for the management of psychomotor agitation. Part 1. Non-pharmacological approach. Braz J Psychiatry. 2019;41:153–67. doi: 10.1590/1516-4446-2018-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Citrome L, Volavka J. The psychopharmacology of violence: making sensible decisions. CNS Spectr. 2014;19:411–8. doi: 10.1017/S1092852914000054. [DOI] [PubMed] [Google Scholar]
- 10.Centre for Evidence-based Medicine (CEBM) Oxford Centre for Evidence-based Medicine – Levels of Evidence (March 2009) Oxford: CEBM; 2009. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ [Google Scholar]
- 11.Citrome L. New treatments for agitation. Psychiatr Q. 2004;75:197–213. doi: 10.1023/b:psaq.0000031791.53142.85. [DOI] [PubMed] [Google Scholar]
- 12.Ng AT, Zeller SL, Rhoades RW. Clinical challenges in the pharmacologic management of agitation. Prim Psychiatry. 2010;17:46–52. [Google Scholar]
- 13.Zimbroff DL, Marcus RN, Manos G, Stock E, McQuade RD, Auby P, et al. Management of acute agitation in patients with bipolar disorder: efficacy and safety of intramuscular aripiprazole. J Clin Psychopharmacol. 2007;27:171–6. doi: 10.1097/JCP.0b13e318033bd5e. [DOI] [PubMed] [Google Scholar]
- 14.Stahl S. New York: Cambridge University Press; 2008. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications 3rd ed. [Google Scholar]
- 15.Ahmed U, Jones H, Adams CE. Chlorpromazine for psychosis induced aggression or agitation. Cochrane Database Syst Rev. 2010:CD007445. doi: 10.1002/14651858.CD007445.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H, Gen K, Takahashi Y. A naturalistic comparison study of the efficacy and safety of intramuscular olanzapine, intramuscular haloperidol, and intramuscular levomepromazine in acute agitated patients with schizophrenia. Hum Psychopharmacol. 2014;29:83–8. doi: 10.1002/hup.2376. [DOI] [PubMed] [Google Scholar]
- 17.Isbister GK, Calver LA, Page CB, Stokes B, Bryant JL, Downes MA. Randomized controlled trial of intramuscular droperidol versus midazolam for violence and acute behavioral disturbance: the DORM study. Ann Emerg Med. 2010;56:392–401.e1. doi: 10.1016/j.annemergmed.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 18.National Center for Biotechnology Information . Phenothiazine [Internet] 2018. https://pubchem.ncbi.nlm.nih.gov/compound/7108 [cited 2018 May 29] [Google Scholar]
- 19.Man PL, Chen CH. Rapid tranquilization of acutely psychotic patients with intramuscular haloperidol and chlorpromazine. Psychosomatics. 1973;14:59–63. doi: 10.1016/S0033-3182(73)71377-3. [DOI] [PubMed] [Google Scholar]
- 20.Walther S, Moggi F, Horn H, Moskvitin K, Abderhalden C, Maier N, et al. Rapid tranquilization of severely agitated patients with schizophrenia spectrum disorders: a naturalistic, rater-blinded, randomized, controlled study with oral haloperidol, risperidone, and olanzapine. J Clin Psychopharmacol. 2014;34:124–8. doi: 10.1097/JCP.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 21.Kinon BJ, Ahl J, Rotelli MD, McMullen E. Efficacy of accelerated dose titration of olanzapine with adjunctive lorazepam to treat acute agitation in schizophrenia. Am J Emerg Med. 2004;22:181–6. doi: 10.1016/j.ajem.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Chan HY, Ree SC, Su LW, Chen JJ, Chou SY, Chen CK, et al. A double-blind, randomized comparison study of efficacy and safety of intramuscular olanzapine and intramuscular haloperidol in patients with schizophrenia and acute agitated behavior. J Clin Psychopharmacol. 2014;34:355–8. doi: 10.1097/JCP.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 23.Daniel DG, Currier GW, Zimbroff DL, Allen MH, Oren D, Manos G, et al. Efficacy and safety of oral aripiprazole compared with haloperidol in patients transitioning from acute treatment with intramuscular formulations. J Psychiatr Pract. 2007;13:170–7. doi: 10.1097/01.pra.0000271658.86845.81. [DOI] [PubMed] [Google Scholar]
- 24.Dorevitch A, Katz N, Zemishlany Z, Aizenberg D, Weizman A. Intramuscular flunitrazepam versus intramuscular haloperidol in the emergency treatment of aggressive psychotic behavior. Am J Psychiatry. 1999;156:142–4. doi: 10.1176/ajp.156.1.142. [DOI] [PubMed] [Google Scholar]
- 25.Fang M, Chen H, Li LH, Wu R, Li Y, Liu L, et al. Comparison of risperidone oral solution and intramuscular haloperidol with the latter shifting to oral therapy for the treatment of acute agitation in patients with schizophrenia. Int Clin Psychopharmacol. 2012;27:107–13. doi: 10.1097/YIC.0b013e32834fc431. [DOI] [PubMed] [Google Scholar]
- 26.Gillies D, Sampson S, Beck A, Rathbone J. Benzodiazepines for psychosis-induced aggression or agitation. Cochrane Database Syst Rev. 2013;9:CD003079. doi: 10.1002/14651858.CD003079.pub3. [DOI] [PubMed] [Google Scholar]
- 27.Huf G, Alexander J, Gandhi P, Allen MH. Haloperidol plus promethazine for psychosis-induced aggression. Cochrane Database Syst Rev. 2016;11:CD005146. doi: 10.1002/14651858.CD005146.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huf G, Coutinho ES, Adams CE, Group TC. Rapid tranquillisation in psychiatric emergency settings in Brazil: pragmatic randomised controlled trial of intramuscular haloperidol versus intramuscular haloperidol plus promethazine. BMJ. 2007;335:869. doi: 10.1136/bmj.39339.448819.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim HK, Kim JJ, Pae CU, Lee CU, Lee C, Paik IH. Comparison of risperidone orodispersible tablet and intramuscular haloperidol in the treatment of acute psychotic agitation: a randomized open, prospective study. Neuropsychobiology. 2010;62:81–6. doi: 10.1159/000315437. [DOI] [PubMed] [Google Scholar]
- 30.MacDonald K, Wilson M, Minassian A, Vilke GM, Becker O, Tallian K, et al. A naturalistic study of intramuscular haloperidol versus intramuscular olanzapine for the management of acute agitation. J Clin Psychopharmacol. 2012;32:317–22. doi: 10.1097/JCP.0b013e318253a2fe. [DOI] [PubMed] [Google Scholar]
- 31.MacDonald K, Wilson MP, Minassian A, Vilke GM, Perez R, Cobb P, et al. A retrospective analysis of intramuscular haloperidol and intramuscular olanzapine in the treatment of agitation in drug- and alcohol-using patients. Gen Hosp Psychiatry. 2010;32:443–5. doi: 10.1016/j.genhosppsych.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Man PL, Chen CH. Rapid tranquilization of acutely psychotic patients with intramuscular haloperidol and chlorpromazine. Psychosomatics. 1973;14:59–63. doi: 10.1016/S0033-3182(73)71377-3. [DOI] [PubMed] [Google Scholar]
- 33.Nobay F, Simon BC, Levitt MA, Dresden GM. A prospective, double-blind, randomized trial of midazolam versus haloperidol versus lorazepam in the chemical restraint of violent and severely agitated patients. Acad Emerg Med. 2004;11:744–9. doi: 10.1197/j.aem.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Preval H, Klotz SG, Southard R, Francis A. Rapid-acting IM ziprasidone in a psychiatric emergency service: a naturalistic study. Gen Hosp Psychiatry. 2005;27:140–4. doi: 10.1016/j.genhosppsych.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Resnick M, Burton BT. Droperidol vs. haloperidol in the initial management of acutely agitated patients. J Clin Psychiatry. 1984;45:298–9. [PubMed] [Google Scholar]
- 36.Tran-Johnson TK, Sack DA, Marcus RN, Auby P, McQuade RD, Oren DA. Efficacy and safety of intramuscular aripiprazole in patients with acute agitation: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68:111–9. doi: 10.4088/jcp.v68n0115. [DOI] [PubMed] [Google Scholar]
- 37.Wright P, Birkett M, David SR, Meehan K, Ferchland I, Alaka KJ, et al. Double-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophrenia. Am J Psychiatry. 2001;158:1149–51. doi: 10.1176/appi.ajp.158.7.1149. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Wang G, Zhao J, Xie S, Xu X, Shi J, et al. Intramuscular ziprasidone versus haloperidol for managing agitation in Chinese patients with schizophrenia. J Clin Psychopharmacol. 2013;33:178–85. doi: 10.1097/JCP.0b013e3182839612. [DOI] [PubMed] [Google Scholar]
- 39.Asadollahi S, Heidari K, Hatamabadi H, Vafaee R, Yunesian S, Azadbakht A, et al. Efficacy and safety of valproic acid versus haloperidol in patients with acute agitation: results of a randomized, double-blind, parallel-group trial. Int Clin Psychopharmacol. 2015;30:142–50. doi: 10.1097/YIC.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 40.Breier A, Meehan K, Birkett M, David S, Ferchland I, Sutton V, et al. A double-blind, placebo-controlled dose-response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Arch Gen Psychiatry. 2002;59:441–8. doi: 10.1001/archpsyc.59.5.441. [DOI] [PubMed] [Google Scholar]
- 41.Alexander J, Tharyan P, Adams C, John T, Mol C, Philip J. Rapid tranquillisation of violent or agitated patients in a psychiatric emergency setting. Pragmatic randomised trial of intramuscular lorazepam v. haloperidol plus promethazine. Br J Psychiatry. 2004;185:63–9. doi: 10.1192/bjp.185.1.63. [DOI] [PubMed] [Google Scholar]
- 42.Mantovani C, Labate CM, Sponholz A, Jr, de Azevedo Marques JM, Guapo VG, de Simone Brito dos Santos ME, et al. Are low doses of antipsychotics effective in the management of psychomotor agitation? A randomized, rated-blind trial of 4 intramuscular interventions. J Clin Psychopharmacol. 2013;33:306–12. doi: 10.1097/JCP.0b013e3182900fd6. [DOI] [PubMed] [Google Scholar]
- 43.Raveendran NS, Tharyan P, Alexander J, Adams CE, Group TR-IIC. Rapid tranquillisation in psychiatric emergency settings in India: pragmatic randomised controlled trial of intramuscular olanzapine versus intramuscular haloperidol plus promethazine. BMJ. 2007;335:865. doi: 10.1136/bmj.39341.608519.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trec Collaborative Group Rapid tranquillisation for agitated patients in emergency psychiatric rooms: a randomised trial of midazolam versus haloperidol plus promethazine. BMJ. 2003;327:708–13. doi: 10.1136/bmj.327.7417.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bieniek SA, Ownby RL, Penalver A, Dominguez RA. A double-blind study of lorazepam versus the combination of haloperidol and lorazepam in managing agitation. Pharmacotherapy. 1998;18:57–62. [PubMed] [Google Scholar]
- 46.Currier GW, Simpson GM. Risperidone liquid concentrate and oral lorazepam versus intramuscular haloperidol and intramuscular lorazepam for treatment of psychotic agitation. J Clin Psychiatry. 2001;62:153–7. doi: 10.4088/jcp.v62n0303. [DOI] [PubMed] [Google Scholar]
- 47.Currier GW, Chou JC, Feifel D, Bossie CA, Turkoz I, Mahmoud RA, et al. Acute treatment of psychotic agitation: a randomized comparison of oral treatment with risperidone and lorazepam versus intramuscular treatment with haloperidol and lorazepam. J Clin Psychiatry. 2004;65:386–94. [PubMed] [Google Scholar]
- 48.Chan EW, Taylor DM, Knott JC, Phillips GA, Castle DJ, Kong DC. Intravenous droperidol or olanzapine as an adjunct to midazolam for the acutely agitated patient: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Ann Emerg Med. 2013;61:72–81. doi: 10.1016/j.annemergmed.2012.07.118. [DOI] [PubMed] [Google Scholar]
- 49.Martel M, Sterzinger A, Miner J, Clinton J, Biros M. Management of acute undifferentiated agitation in the emergency department: a randomized double-blind trial of droperidol, ziprasidone, and midazolam. Acad Emerg Med. 2005;12:1167–72. doi: 10.1197/j.aem.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 50.Knott JC, Taylor DM, Castle DJ. Randomized clinical trial comparing intravenous midazolam and droperidol for sedation of the acutely agitated patient in the emergency department. Ann Emerg Med. 2006;47:61–7. doi: 10.1016/j.annemergmed.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Richards JR, Derlet RW, Duncan DR. Chemical restraint for the agitated patient in the emergency department: lorazepam versus droperidol. J Emerg Med. 1998;16:567–73. doi: 10.1016/s0736-4679(98)00045-6. [DOI] [PubMed] [Google Scholar]
- 52.Rosen CL, Ratliff AF, Wolfe RE, Branney SW, Roe EJ, Pons PT. The efficacy of intravenous droperidol in the prehospital setting. J Emerg Med. 1997;15:13–7. doi: 10.1016/s0736-4679(96)00259-4. [DOI] [PubMed] [Google Scholar]
- 53.Taylor DM, Yap CYL, Knott JC, Taylor SE, Phillips GA, Karro J, et al. Midazolam-Droperidol, Droperidol, or Olanzapine for Acute Agitation: A Randomized Clinical Trial. Ann Emerg Med. 2017;69:318–26.e1. doi: 10.1016/j.annemergmed.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 54.Yap CYL, Taylor DM, Knott JC, Taylor SE, Phillips GA, Karro J, et al. Intravenous midazolam-droperidol combination, droperidol or olanzapine monotherapy for methamphetamine-related acute agitation: subgroup analysis of a randomized controlled trial. Addiction. 2017;112:1262–9. doi: 10.1111/add.13780. [DOI] [PubMed] [Google Scholar]
- 55.Allen MH, Feifel D, Lesem MD, Zimbroff DL, Ross R, Munzar P, et al. Efficacy and safety of loxapine for inhalation in the treatment of agitation in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72:1313–21. doi: 10.4088/JCP.10m06011yel. [DOI] [PubMed] [Google Scholar]
- 56.Kwentus J, Riesenberg RA, Marandi M, Manning RA, Allen MH, Fishman RS, et al. Rapid acute treatment of agitation in patients with bipolar I disorder: a multicenter, randomized, placebo-controlled clinical trial with inhaled loxapine. Bipolar Disord. 2012;14:31–40. doi: 10.1111/j.1399-5618.2011.00975.x. [DOI] [PubMed] [Google Scholar]
- 57.Andrezina R, Josiassen RC, Marcus RN, Oren DA, Manos G, Stock E, et al. Intramuscular aripiprazole for the treatment of acute agitation in patients with schizophrenia or schizoaffective disorder: a double-blind, placebo-controlled comparison with intramuscular haloperidol. Psychopharmacology (Berl) 2006;188:281–92. doi: 10.1007/s00213-006-0541-x. [DOI] [PubMed] [Google Scholar]
- 58.De Filippis S, Cuomo I, Lionetto L, Janiri D, Simmaco M, Caloro M, et al. Intramuscular aripiprazole in the acute management of psychomotor agitation. Pharmacotherapy. 2013;33:603–14. doi: 10.1002/phar.1260. [DOI] [PubMed] [Google Scholar]
- 59.Veser FH, Veser BD, McMullan JT, Zealberg J, Currier GW. Risperidone versus haloperidol, in combination with lorazepam, in the treatment of acute agitation and psychosis: a pilot, randomized, double-blind, placebo-controlled trial. J Psychiatr Pract. 2006;12:103–8. doi: 10.1097/00131746-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Agid O, Kapur S, Warrington L, Loebel A, Siu C. Early onset of antipsychotic response in the treatment of acutely agitated patients with psychotic disorders. Schizophr Res. 2008;102:241–8. doi: 10.1016/j.schres.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 61.Gault TI, Gray SM, Vilke GM, Wilson MP. Are oral medications effective in the management of acute agitation? J Emerg Med. 2012;43:854–9. doi: 10.1016/j.jemermed.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 62.Hsu WY, Huang SS, Lee BS, Chiu NY. Comparison of intramuscular olanzapine, orally disintegrating olanzapine tablets, oral risperidone solution, and intramuscular haloperidol in the management of acute agitation in an acute care psychiatric ward in Taiwan. J Clin Psychopharmacol. 2010;30:230–4. doi: 10.1097/JCP.0b013e3181db8715. [DOI] [PubMed] [Google Scholar]
- 63.Hatta K, Kawabata T, Yoshida K, Hamakawa H, Wakejima T, Furuta K, et al. Olanzapine orally disintegrating tablet vs. risperidone oral solution in the treatment of acutely agitated psychotic patients. Gen Hosp Psychiatry. 2008;30:367–71. doi: 10.1016/j.genhosppsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 64.Normann C, Schmauss M, Bakri N, Gerwe M, Schreiner A. Initial treatment of severe acute psychosis with fast orally disintegrating risperidone tablets. Pharmacopsychiatry. 2006;39:209–12. doi: 10.1055/s-2006-950498. [DOI] [PubMed] [Google Scholar]
- 65.Baker RW, Kinon BJ, Maguire GA, Liu H, Hill AL. Effectiveness of rapid initial dose escalation of up to forty milligrams per day of oral olanzapine in acute agitation. J Clin Psychopharmacol. 2003;23:342–8. doi: 10.1097/01.jcp.0000085406.08426.a8. [DOI] [PubMed] [Google Scholar]
- 66.Escobar R, San L, Perez V, Olivares JM, Polavieja P, Lopez-Carrero C, et al. [Effectiveness results of olanzapine in acute psychotic patients with agitation in the emergency room setting: results from NATURA study] Actas Esp Psiquiatr. 2008;36:151–7. [PubMed] [Google Scholar]
- 67.Katagiri H, Fujikoshi S, Suzuki T, Fujita K, Sugiyama N, Takahashi M, et al. A randomized, double-blind, placebo-controlled study of rapid-acting intramuscular olanzapine in Japanese patients for schizophrenia with acute agitation. BMC Psychiatry. 2013;13:20. doi: 10.1186/1471-244X-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Castle DJ, Udristoiu T, Kim CY, Sarosi A, Pidrman V, Omar AN, et al. Intramuscular olanzapine versus short-acting typical intramuscular antipsychotics: comparison of real-life effectiveness in the treatment of agitation. World J Biol Psychiatry. 2009;10:43–53. doi: 10.1080/15622970802688051. [DOI] [PubMed] [Google Scholar]
- 69.Perrin E, Anand E, Dyachkova Y, Wagner T, Frediani S, Ballerini A, et al. A prospective, observational study of the safety and effectiveness of intramuscular psychotropic treatment in acutely agitated patients with schizophrenia and bipolar mania. Eur Psychiatry. 2012;27:234–9. doi: 10.1016/j.eurpsy.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 70.Lesem MD, Zajecka JM, Swift RH, Reeves KR, Harrigan EP. Intramuscular ziprasidone, 2 mg versus 10 mg, in the short-term management of agitated psychotic patients. J Clin Psychiatry. 2001;62:12–8. doi: 10.4088/jcp.v62n0104. [DOI] [PubMed] [Google Scholar]
- 71.Daniel DG, Potkin SG, Reeves KR, Swift RH, Harrigan EP. Intramuscular (IM) ziprasidone 20 mg is effective in reducing acute agitation associated with psychosis: a double-blind, randomized trial. Psychopharmacology (Berl) 2001;155:128–34. doi: 10.1007/s002130000658. [DOI] [PubMed] [Google Scholar]
- 72.Pratts M, Citrome L, Grant W, Leso L, Opler LA. A single-dose, randomized, double-blind, placebo-controlled trial of sublingual asenapine for acute agitation. Acta Psychiatr Scand. 2014;130:61–8. doi: 10.1111/acps.12262. [DOI] [PubMed] [Google Scholar]
- 73.Griffin CE 3rd, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13:214–23. [PMC free article] [PubMed] [Google Scholar]
- 74.Chouinard G, Annable L, Turnier L, Holobow N, Szkrumelak N. A double-blind randomized clinical trial of rapid tranquilization with I.M. clonazepam and I.M. haloperidol in agitated psychotic patients with manic symptoms. Can J Psychiatry. 1993;38(Suppl 4):S114–21. [PubMed] [Google Scholar]
- 75.Chung AK, Chua SE. Effects on prolongation of Bazett’s corrected QT interval of seven second-generation antipsychotics in the treatment of schizophrenia: a meta-analysis. J Psychopharmacol. 2011;25:646–66. doi: 10.1177/0269881110376685. [DOI] [PubMed] [Google Scholar]
- 76.Wahidi N, Johnson KM, Brenzel A, de Leon J. Two sudden and unexpected deaths of patients with schizophrenia associated with intramuscular injections of antipsychotics and practice guidelines to limit the use of high doses of intramuscular antipsychotics. Case Rep Psychiatry. 2016;2016:9406813. doi: 10.1155/2016/9406813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Acciavatti T, Martinotti G, Corbo M, Cinosi E, Lupi M, Ricci F, et al. Psychotropic drugs and ventricular repolarisation: the effects on QT interval, T-peak to T-end interval and QT dispersion. J Psychopharmacol. 2017;31:453–60. doi: 10.1177/0269881116684337. [DOI] [PubMed] [Google Scholar]
- 78.Berling I, Isbister GK. Prolonged QT risk assessment in antipsychotic overdose using the QT nomogram. Ann Emerg Med. 2015;66:154–64. doi: 10.1016/j.annemergmed.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 79.Guy JM, André-Fouet X, Porte J, Bertrand M, Lamaud M, Verneyre H. [Torsades de pointes and prolongation of the duration of QT interval after injection of droperidol] Ann Cardiol Angeiol (Paris) 1991;40:541–5. [PubMed] [Google Scholar]
- 80.Charbit B, Alvarez JC, Dasque E, Abe E, Demolis JL, Funck-Brentano C. Droperidol and ondansetron-induced QT interval prolongation: a clinical drug interaction study. Anesthesiology. 2008;109:206–12. doi: 10.1097/ALN.0b013e31817fd8c8. [DOI] [PubMed] [Google Scholar]
- 81.Vandael E, Vandenberk B, Vandenberghe J, Spriet I, Willems R, Foulon V. Development of a risk score for QTc-prolongation: the RISQ-PATH study. Int J Clin Pharm. 2017;39:424–32. doi: 10.1007/s11096-017-0446-2. [DOI] [PubMed] [Google Scholar]
- 82.Pressler J, Lucking M, Melter M, Gerling S. Nonsustained ventricular tachycardia in a patient with acquired long QT syndrome after levomepromazine injection. Intensive Care Med. 2014;40:133–4. doi: 10.1007/s00134-013-3138-y. [DOI] [PubMed] [Google Scholar]
- 83.Hasnain M, Vieweg WV. QTc interval prolongation and torsade de pointes associated with second-generation antipsychotics and antidepressants: a comprehensive review. CNS Drugs. 2014;28:887–920. doi: 10.1007/s40263-014-0196-9. [DOI] [PubMed] [Google Scholar]
- 84.Lesem MD, Tran-Johnson TK, Riesenberg RA, Feifel D, Allen MH, Fishman R, et al. Rapid acute treatment of agitation in individuals with schizophrenia: multicentre, randomised, placebo-controlled study of inhaled loxapine. Br J Psychiatry. 2011;198:51–8. doi: 10.1192/bjp.bp.110.081513. [DOI] [PubMed] [Google Scholar]
- 85.Allen MH, Debanne M, Lazignac C, Adam E, Dickinson LM, Damsa C. Effect of nicotine replacement therapy on agitation in smokers with schizophrenia: a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2011;168:395–9. doi: 10.1176/appi.ajp.2010.10040569. [DOI] [PubMed] [Google Scholar]
- 86.Allen MH, Currier GW, Carpenter D, Ross RW, Docherty JP, Expert Consensus Panel for Behavioral E. The expert consensus guideline series. Treatment of behavioral emergencies 2005. J Psychiatr Pract. 2005;11(Suppl 1):5–108. doi: 10.1097/00131746-200511001-00002. ; quiz 10-2. [DOI] [PubMed] [Google Scholar]