Abstract
Objective:
The incidence rate of major depression in adolescents reaches approximately 14%. This disorder is usually recurrent, without remission of symptoms even after pharmacological treatment, and persists throughout adult life. Since the effects of antidepressants take approximately 2 weeks to begin, new pharmacological therapies are under continuous exploration. Recent evidence suggests that psychedelics could produce rapid antidepressant effects. In this study, we evaluated the potential antidepressant effects of ayahuasca in a juvenile non-human primate model of depression.
Methods:
While living with their families, juvenile marmosets (8 males; 7 females) were observed on alternate days for four weeks during a baseline phase. This was followed by 8 weeks of an induced depressive state protocol, the social isolated context (IC), in which the animals were monitored in the first and last weeks. Subsequently, five males and four females were randomly selected for treatment, first with a single administration of saline vehicle (1.67 mL/300 g of body weight, via gavage), followed by a single dose of ayahuasca (1.67 mL/300 g of body weight, via gavage). Both phases lasted 1 week and the animals were monitored daily. A third week of sampling was called the tardive-pharmacological effects phase. In all phases the marmosets were assessed for behavior, fecal cortisol levels, and body weight.
Results:
After IC, the animals presented typical hypocortisolemia, but cortisol recovered to baseline levels 24 h after an acute dose of ayahuasca; this recovery was not observed in vehicle-treated animals. Additionally, in males, ayahuasca, but not the vehicle, reduced scratching, a stereotypic behavior, and increased feeding. Ayahuasca treatment also improved body weight to baseline levels in both sexes. The ayahuasca-induced behavioral response had long-term effects (14 days). Thus, in this translational juvenile animal model of depression, ayahuasca presented beneficial effects.
Conclusions:
These results can contribute to the validation of ayahuasca as an antidepressant drug and encourage new studies on psychedelic drugs as a tool for treating mood disorders, including for adolescents with early-onset depression.
Keywords: Translational animal model, non-human primate, common marmoset, marmoset, cortisol, early-age depression, psychedelic drugs
Introduction
Major depressive disorder (MDD) is characterized by depressed mood, anhedonia, weight alterations (loss or gain), sleep disorders (insomnia or hypersomnia), and psychomotor alterations (retardation or agitation).1 Depression is currently ranked by the World Health Organization (WHO) as the greatest contributor to global disability and suicidal deaths.2
MDD has been associated with physiological dysregulation. It has been consistently linked with monoaminergic imbalance due to the decreased levels observed in dopaminergic, noradrenergic, and serotoninergic neurotransmission pathways.3 However, antidepressant drugs that target these systems and increase monoamine levels in the synaptic cleft do not, in general, totally relieve depressive symptoms, and only around 40% of patients achieve remission after several treatments. Moreover, commercially available antidepressants usually take around 2 weeks before onset of the desired therapeutic effects.3,4 Thus, enormous effort has been devoted to the search for alternative pharmacological treatments with better efficacy and faster therapeutic effects.4
Ayahuasca is a decoction of a combination of two plants from the Amazon rainforest: Psychotria viridis and Banisteriopsis caapi. Recent studies suggest that ayahuasca does not exhibit tolerance and is not addictive.5 Several different ayahuasca compounds act on the biological systems involved in the etiology of depression. For instance, N,N dimethyltriptamine (DMT) acts as a serotonergic agonist (specifically, as a serotonin receptor type 2A [5-HT2A] agonist) and modulates sigma-1 receptors (σ1Rs), which have recently been implicated in depression.6 When consumed orally, DMT is inactivated by monoamine oxidase (MAO) in the intestine and liver. However, ayahuasca also contains β-carbolines (tetrahydroharmine [THH], harmine, and harmaline), which due to their activity as reversible MAO inhibitors (MAOi) protect DMT from degradation following ingestion; this increases monoamine half-life in vivo and, consequently, residence time in the synaptic cleft space.7
Besides the MAOi properties of β-carbolines in ayahuasca tea, THH is also a selective serotonin reuptake inhibitor (SSRI),7,8 and a recent rodent model of depression indicated that harmine has significant antidepressant effects.9 It has also been observed that ayahuasca functions by modulating plasma cortisol, since healthy volunteers exhibited increased cortisol levels 2 h after ayahuasca intake.10 This finding is especially relevant, since cortisol is also involved in the etiology of depression, and patients with major depression exhibit consistently altered levels of plasma and salivary cortisol.11,12
In fact, positive health benefits have been found in individuals who regularly use ayahuasca in religious contexts.13 Furthermore, its antidepressant effects have been explored,14 with a recent randomized controlled trial suggesting a rapid onset of antidepressant effects in patients with treatment-resistant depression.4,11
The depression incidence rate among adolescents aged between 15 and 18 years reaches 14%, with approximately 40% recurrence in the 3 to 5 years following the first episode.15 The influence of sexual steroids at this age opens an important biological window of plasticity in the nervous system, which makes the brain particularly susceptible to environmental influences. If the stimulus induces maladaptive changes in brain morphology and functions, permanent impairment of cognitive, behavioral, and physiological mechanisms can result, increasing the probability of mood disorders that persist into adulthood.15 Thus, the rise of sexual hormones at puberty turns adolescents into a high-risk group for depression.
The evidence for ayahuasca’s rapid antidepressant action in adult patients raises the question of whether it might also be effective in other age groups susceptible to depression, such as adolescents. Due to this possibility and to the lack of animal studies on ayahuasca in juvenile depression, this study evaluated the acute antidepressant effects of ayahuasca on body weight and physiological (fecal cortisol) and behavioral parameters in a recently developed juvenile model of depression that involves the induction of a depressive-like state in the common marmoset (Callithrix jacchus) through chronic social isolation.16
Method
Animal maintenance
All animals were housed according to Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) guidelines (normative instruction 169: February 20, 2008) and animal care standards established by the Conselho Nacional de Controle de Experimentação Animal (CONCEA), (law 11.794: October 8, 2008). Moreover, the laboratory complies with international standards for ex situ animal maintenance as defined by the Sociedade Brasileira de Etologia (SBEt) and the International Primatological Society (IPS). The study and experimental procedures were approved by the animal research ethics committee of Universidade Federal do Rio Grande do Norte (UFRN), Natal, state of Rio Grande do Norte, Brazil (protocol 034/2014).
To facilitate genetic variation, all juvenile animals (7 to 9 months) used in this study (n=15; eight males and seven females) were randomly selected from 10 different families within a group of approximately 150 marmosets living in captivity at the Laboratório de Estudos Avançados em Primatas (LEAP) at UFRN. The marmoset colony consists of animals born in captivity, as well as some individuals that were captured in the wild and introduced to the colony.
Under baseline conditions, the animals live with their families in outdoor masonry enclosures (2.0 × 2.0 × 1.0 m3) under natural lighting, humidity, and temperature conditions. The front of each enclosure was a one-way glass wall, while the back wall contained a wire mesh door through which food and water were made available. Each enclosure contained a nest box, as well as wooden planks and tree branches to enrich the environment and allow free movement.
Depression was induced by social isolation.16 During this procedure, the animals were removed from their family groups and placed in isolation in smaller new enclosures (1.0 × 2.0 × 1.0 m3). While isolated, the animals had auditory and olfactory contact, but no visual contact with conspecifics living in the colony.
None of the animals had been used in previous scientific studies, nor had they been separated from their respective family groups for prolonged periods of time. All of the animals had been conditioned to the presence of the researchers prior to the study. Veterinary care was provided throughout the experiment. Water was available ad libitum throughout the study, and the animals were all fed the same diet, twice a day, which included seasonal fruits such as bananas, papayas, melons, and mangos, as well as potatoes and a protein mixture containing milk, oats, eggs, and bread. Twice a week, a multivitamin supplement (Glicocan) was diluted into the food. The animals were weighed every 15 days to monitor their health.
Study design
The design was based on a previous study that validated an induced depression-like state in juvenile common marmosets through isolation.16
Figure 1 shows the experimental design. During the 4-week baseline phase (BL), while living with their families the behavior of juvenile marmosets (eight males and seven females) was observed and fecal cortisol was measured on alternate days. This was followed by 8 weeks of social isolated context (IC), in which fecal cortisol and behavior were monitored in the first and last week. Subsequently, five males and four females were randomly selected for treatment, first with one administration of saline vehicle, the vehicle treatment (VE) phase, which lasted 1 week and consisted of behavioral and physiological (fecal) sampling. This was followed by pharmacological treatment (PH), also lasting 1 week, in which the animals received one dose of ayahuasca, followed by daily behavior monitoring and fecal collection. PH was followed by one more week of sampling called the tardive-pharmacological effects (tPE) phase. In addition to behavior monitoring and fecal sampling, body weight was also recorded during all five phases of the study. For a more detailed description of the protocols, see Galvão-Coelho et al.16
Figure 1. Experimental design comprising several phases: baseline (BL), 4 weeks; depression paradigm, social isolated context (IC), 8 weeks; vehicle treatment (VE), 1 week; pharmacological treatment (PH), ayahuasca, 1 week; and tardive-pharmacological effects (tPE), measured during 1 week. Marmosets were assessed for behavior, fecal cortisol levels, and body weight.
Unlike traditional antidepressants, which are usually administered daily, ayahuasca treatment consisted of a single dose, since in previous studies has been shown to induce acute increases in plasma cortisol levels.14 Thus, we decided to investigate behavioral and physiological parameters 24 and 48 h after administration of both the VE and PH treatments.
Treatments
All animals were treated with a single administration of the vehicle. Seven days later, they received a single dose of ayahuasca. In both cases, we used a dose of 1.67 mL/300 g of body weight, via gavage. The dose was based on previous human studies by our group,4,11 in which we administered 1 mL of ayahuasca/kg, which is the approximate amount commonly used in Brazilian ritualistic ceremonies. We performed allometric scaling, considering the differences in metabolic rates described by Pachaly as follows17: 1) basal metabolic rate of a typical 70-kg human individual, 70 × 700.75 = 1,694 kcal; 2) basal metabolic rate of a typical marmoset (300 g), 70 × 0.30.75 = 28.38 kcal; 3) ayahuasca dose in mL per kcal, 100 mL/1,694 kcal = 0.06 mL/kcal; and 4) equivalent dose for a typical marmoset, 0.06 mL/kcal × 28.35 kcal = 1.67 mL.
A single batch of ayahuasca was used throughout the study for all animals. The ayahuasca was produced and provided without charge by a branch of the Templo da Barquinha, Ji-Paraná, state of Rondônia, Brazil. The brew was prepared in a single batch using plants collected in a single day from the same area. Intermixed layers of 50% P. viridis leaves and 50% B. caapi bark were used in the preparation. The mixture was boiled in water (100 °C) for 60 h. As the water evaporated, more water, bark, and leaves were added to the preparation in the same proportions. The final batch was stored in glass bottles in a refrigerator. The main ayahuasca alkaloids were quantified at the beginning of the study using mass spectroscopy, resulting in values of 0.36±0.01 mg/mL DMT, 1.86±0.11 mg/mL harmine, 0.24±0.03 mg/mL harmaline, and 0.20±0.05 mg/mL THH.4,18 The tea used in this study was the same as that used in previous studies.4,18
Ayahuasca was administered via gavage. We used conventional transparent flexible 23G plastic cannulas and mineral oil lubrication to avoid internal damage to the gastrointestinal tract. We were careful not to perform the procedure on animals with a full stomach, and on the day of administration the they were fed only a small portion of fruit (papaya, mango, or banana). After feeding, we waited an additional 15 minutes and then performed the gavage procedure. To avoid handling stress, all animals had been adapted to the handling procedure and the apparatus prior to the study.
Behavioral recording
The recorded behaviors were the same as those validated by Galvão-Coelho et al.16 for juvenile marmosets and included species-specific behaviors, such as scent marking (frequency; which, in the context of stress, expresses anxiety19), individual piloerection (frequency; which, in the context of stress, expresses activation of the sympathetic nervous system16), scratching (duration; considered a stereotyped behaviour20), autogrooming (duration; considered a stereotyped behaviour21), and depressive-like behaviors that can be found across species, such as locomotion (frequency), somnolence (duration), feeding (duration), and anhedonia.16 Anhedonia was measured by frequency and duration of ingestion of an aqueous 4.16% solution of sucrose.16 Alterations in locomotion, sleep, feeding, and anhedonia are symptoms used to clinically diagnose major depression. For a more detailed description of the behavioral data and their implications in the context of stress, see Galvão-Coelho et al.16
The selected behaviors were recorded over a 30-min period between 6:30 and 7:30 a.m. to avoid the influence of circadian variation, using the focal continuous method.16
Fecal collection and cortisol assay
Fecal samples were collected between 6:30 and 8:30 a.m. to avoid circadian variation in fecal cortisol profiles.16 The enclosures were cleaned prior to fecal collection to avoid collecting samples expelled prior to 6:30 a.m. Samples were stored at –4 °C until cortisol extraction and quantification, which was performed at Laboratório de Medidas Hormonais, Departamento de Fisiologia, UFRN according to the protocol of Sousa & Ziegler.22 Fecal cortisol reflects plasma cortisol with a delay of approximately 8-10 h. The intra- and inter-assay coefficients of variation were 2.74% and 16.61%, respectively.
Statistical analysis
Hormonal data were normalized by logarithmic transformation, and the bootstrap resampling statistical technique was applied to the multivariate analysis for both hormonal and behavioral data. A generalized linear model (GLM) and Fisher's post-hoc tests (least significant difference [LSD]) were used to analyze variations in behavior, cortisol, and body weight between sexes throughout the study phases. Additionally, a parametric Student's t-test was used to analyze hormonal and behavioral data between specific phases. Differences were considered statistically significant at p < 0.05.
Results
At the end of the social IC, we observed a significant decrease, with respect to baseline, in cortisol levels (BL: µ = 2.25±0.24 ng/g; IC: µ = 1.73±0.05 ng/g; GLM test, F = 4.38, p = 0.03 degree of freedom [df] = 1), increases in autogrooming (BL: µ = 5.89±1.39; IC: µ = 44.72±9.70; GLM test, F = 15.31, p = 0.00, df = 1) and somnolence (BL: µ = 0.01±0.01; IC: µ = 0.10±0.06; GLM test, F = 3.73, p = 0.05, df = 1), and reductions in feeding (BL: µ = 227.26±14.73; IC: µ = 142.75±15.61; GLM test, F = 18.10, p = 0.00, df = 1), and sucrose ingestion (frequency: BL: µ = 2.52±0.30; IC: µ = 2.26±0.11; GLM test, F = 22.61, p = 0.00, df = 1/duration: BL: µ = 8.98± 1.67; IC: µ = 4.44±2.08; F = 5.55, p = 0.02, df = 1), which indicates anhedonia. All these changes were independent of sex. For all other values, see Table 1.
Table 1. Statistical values, generalized linear model (GLM) test and least significant difference (LSD) post-hoc, and direction of alterations of physiologic and behavior parameters in response to social isolated context compared to baseline.
| Variables | Statistical values | Alteration |
|---|---|---|
| Cortisol | F = 4.38, p = 0.03, df = 1 (P) | ↓ |
| F = 0.00, p = 0.98, df = 1 (P/S) | - | |
| Autogrooming | F = 15.31, p = 0.00, df = 1 (P) | ↑ |
| F = 0.42, p = 0.73, df = 1 (P/S) | - | |
| Somnolence | F = 3.73, p = 0.05, df = 1 (P) | ↑ |
| F = 1.47, p = 0.22, df = 1 (P/S) | - | |
| Feeding | F = 18.10, p = 0.00, df = 1 (P) | ↓ |
| F = 0.90, p = 0.34, df = 1 (P/S) | - | |
| Sucrose ingestion | ||
| Frequency | F = 22.61, p = 0.00, df = 1 (P) | ↓ |
| F = 5.69, p = 1.16, df = 1 (P/S) | - | |
| Duration | F = 5.55, p = 0.02, df = 1 (P) | ↓ |
| F = 0.12, p = 0.72, df = 1 (P/S) | - | |
| Scratching | F = 4.72, p = 0.03, df = 1 (P) | |
| F = 30.14, p = 0.00, df = 1 (P/S) | ||
| Male | p = 0.00 | ↑ |
| Female | p = 0.02 | ↑ |
| Scent marking | F = 5.49, p = 0.02, df = 1 (P) | |
| F = 9.68, p = 0.00, df = 1 (P/S) | ||
| Male | p = 0.00 | ↑ |
| Female | p = 0.15 | - |
| Body weight | F = 9.12, p = 0.00, df = 1 (P) | |
| F = 11.27, p = 0.00, df = 1 (P/S) | ||
| Male | p = 0.00 | ↓ |
| Female | p = 0.81 | - |
| Locomotion | F = 0.03, p = 0.85, df = 1 (P) | - |
| F = 0.32, p = 0.57, df = 1 (P/S) | - | |
| Individual piloerection | F = 0.03, p = 0.08, df = 1 (P) | - |
| F = 0.07, p = 0.78, df = 1 (P/S) | - |
Values in bold are statistically significant, while values in normal font are non-significant.
= statistical analysis of phase;
= statistical analysis of interaction between phase and sex.
↓ = decrease; ↑ = increase; - = no change.
For scent marking, scratching, body weight, sexual dimorphic alterations were observed. Only males showed increased scent marking (males; BL: µ = 0.98±0.18, IC: µ = 3.89±0.68; GLM test (phase*sex), F = 9.68, p = 0.00, df = 1, LSD post-hoc p = 0.00) and reduced body weight (males: BL: µ = 270.25±4.88, IC: µ = 256.75±2.68; GLM test (phase*sex), F = 11.27, p = 0.00, df = 1, LSD post-hoc p = 0.00) (Table 1). Both males and females increased scratching (BL: µ = 27.74±1.91; IC: µ = 58.11±5.00; GLM test (phase*sex), F = 30.14, p = 0.00, df = 1). No significant statistical variations were observed in locomotion or individual piloerection after the IC protocol (Table 1).
After ayahuasca, but not after vehicle, males decreased scratching (males; IC: µ = 92.05±8.91, VE: µ = 81.54±9.00, PH: µ = 65.02±11.11; GLM test (phase*sex), F = 3.59, p = 0.01, df = 3, LSD post-hoc IC*VE p = 0.27, IC*PH p = 0.00, VE*PH p = 0.08). The PH-induced reduction lasted 7 days more and was also observed in the tPE (males; tPE: µ = 61.82±6.79; GLM test (phase*sex), F = 3.59, p = 0.01, df = 3, LSD post-hoc, IC*tPE p = 0.00, VE*tPE p = 0.04, PH*tPE p = 0.73) (Figure 2A, Table 2). Again, only males showed increased feeding after treatment with ayahuasca (males; IC: µ = 134.11±17.75, VE: µ = 127.05±13.54, PH: µ = 202.31±28.12; GLM test (phase*sex), F = 3.55, p = 0.01, df = 3, LSD post-hoc IC*VE p = 0.81, IC*PH p = 0.02, VE*PH p = 0.01), which was sustained until tPE. Although this did not change after treatment with vehicle (males; tPE: µ = 225.80±29.35; GLM test (phase*sex), F = 3.55, p = 0.01, df = 3, LSD post-hoc IC*tPE p = 0.00, VE*tPE p = 0.00, PH*tPE p = 0.42) (Figure 2B, Table 2). The body weight of both sexes increased after PH, but not after VE (IC: µ = 267.88±3.03, VE: µ = 264.22±3.87, PH: µ = 274.17±4.09; GLM test, F = 15.35, p = 0.00, df = 3, LSD post-hoc IC*VE p = 0.02, IC*PH p = 0.00, VE*PH p = 0.00), and it was sustained throughout tPE (tPE: µ = 273.00±4.16; GLM test, F = 15.35, p = 0.00, df = 3, LSD post-hoc IC*tPE p = 0.00, VE*tPE p = 0.00, PH*tPE p = 0.47) (Figure 2C, Table 2). The body weight gain in PH and tPE allowed recovery to baseline weight levels (BL: µ = 267.77±3.77; PH: t = -1.68, p = 0.09/tPE: t = -1.33, p = 0.18).
Figure 2. Means ± standard error of the mean of A) scratching time, B) feeding time, and C) body weight (gram, g) in male and female juvenile Callithrix jacchus. IC = social isolated context; PH = pharmacological treatment; tPE = tardive-pharmacological effects; VE = vehicle treatment. * Statistically significant difference between respective phase and phase(s) indicated above the symbol. GLM test and post-hoc Fisher, p < 0.05.

Table 2. Statistical values, generalized linear model (GLM) test and least significant difference (LSD) post-hoc, and direction of alterations of physiologic and behavior parameters in VE, PH and tPE.
| Variables | Statistical values | VE | PH | tPE |
|---|---|---|---|---|
| Cortisol | F = 1.18, p = 0.31, df = 3 (P) | - | - | - |
| F = 0.42, p = 0. 37, df = 3 (P/S) | ||||
| Autogrooming | F = 0.93, p = 0.42, df = 3 (P) | - | - | - |
| F = 1.45, p = 0. 22, df = 3 (P/S) | ||||
| Scratching | F = 1.31, p = 0.27, df = 3 (P) | |||
| F = 3.59, p = 0.01, df = 3 (P/S) | ||||
| Male | p = 0.27 | p = 0.00 ↓ | p = 0.00 ↓ | |
| Female | p = 0.19 | p = 0.53 | p = 0.16 | |
| Somnolence | F = 0.57, p = 0.63, df = 3 (P) | - | - | - |
| F = 0.77, p = 0.51, df = 3 (P/S) | ||||
| Feeding | F = 2.23, p = 0.08, df = 3 (P) | |||
| F = 3.55, p = 0.01, df = 3 (P/S) | ||||
| Male | p = 0.81 | p = 0.02 ↑ | p = 0.00 ↑ | |
| Female | p = 0.29 | p = 0.69 | p = 0.57 | |
| Sucrose ingestion | ||||
| Frequency | F = 13.28, p = 0.80, df = 3 (P) | - | - | - |
| F = 0.78, p = 0.50, df = 3 (P/S) | ||||
| Scent marking | F = 3.14, p = 0.06, df = 3 (P) | - | - | - |
| F = 5.30, p = 0. 18, df = 3 (P/S) | ||||
| Body weight | F = 15.35, p = 0.00, df = 3 (P) | p = 0.02 ↓ | p = 0.00 ↑ | p = 0.00 ↑ |
| F = 2.00, p = 0.11, df = 3 (P/S) | ||||
| Locomotion | F = 1.01, p = 0.38, df = 3 (P) | - | - | - |
| F = 1.80 p = 0.14, df = 3 (P/S) |
Values in bold are statistically significant, while values in normal font are non-significant.
= statistical analysis of phase;
= statistical analysis of interaction between phase and sex; PH = pharmacological treatment; tPE = tardive-pharmacological effects; VE = vehicle treatment.
↓ = decrease; ↑ = increase; - = no change.
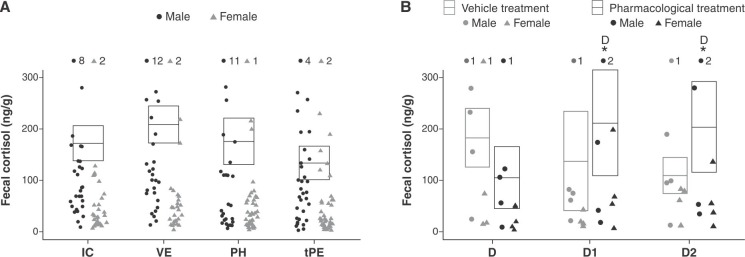
No significant alterations in response to vehicle or ayahuasca treatments were observed in fecal cortisol (Figure 3A, Table 2), or in autogrooming, scent marking, locomotion, ingestion of sucrose, or somnolence (Table 2). With respect to individual piloerection, GLM analyses could not be performed due to the large number of zero frequencies and low data variance.
Figure 3. A) Means ± standard error of the mean of fecal cortisol in IC, VE, PH, and tPE. B) Fecal cortisol at treatment (D) day 1 (D1, 24 h) and day 2 (D2, 48 h) after vehicle and ayahuasca treatment. * Statistically significant difference between respective phase and phase(s) indicated next to the symbol. GLM and post-hoc Fisher tests, p < 0.05. IC = social isolated context; PH = pharmacological treatment (ayahuasca); tPE = tardive-pharmacological effects; VE = vehicle treatment.
Cortisol levels increased 24 h (D1) and 48 h (D2) after ayahuasca ingestion, but not after vehicle, independent of sex (IC: µ = 2.08±0.06 ng/g; 24 h (D1): VE: µ = 1.93±0.20 ng/g, PH: µ = 1.59±0.21 ng/g; 48 h (D2): VE: µ = 1.67±0.19 ng/g, PH: µ = 1.93±0.21 ng/g; GLM test (Day*Treatment), F = 3.19, p = 0.05, df = 2, LSD post-hoc VE: IC*D1 p = 0.21, IC*D2 p = 0.56, PH: IC*D1 p = 0.03, IC*D2 p = 0.02) (Figure 3B, Table 3). Moreover, cortisol levels at D1 and D2 returned to baseline values (BL: µ = 2.30±0.38; D1: t = -0.55, p = 0.58/D2: t = -1.30, p = 0.21). No significant behavior alterations were found in D1 or D2 in response to vehicle or ayahuasca (Table 3). The GLM analysis of individual piloerection and sucrose ingestion was not carried out due a large number zeros and low data variance.
Table 3. Statistical values, generalized linear model (GLM) test and least significant difference (LSD) post-hoc, and direction of alterations of acute cortisol in D1 (24 h) and D2 (48 h) after treatment with vehicle and ayahuasca.
| Treatment | Statistical values | Alteration |
|---|---|---|
| Vehicle | F = 3.19, p = 0.05, df = 2 (D/T) | |
| F = 0.54, p = 0.58, df = 2 (D/T/S) | ||
| D1 (24 h) | p = 0.21 | - |
| D2 (48 h) | p = 0.56 | - |
| Ayahuasca | F = 3.19, p = 0.05, df = 2 (D/T) | |
| F = 0.54, p = 0.58, df = 2 (D/T/S) | ||
| D1 (24 h) | p = 0.03 | ↑ |
| D2 (48 h) | p = 0.02 | ↑ |
Values in bold are statistically significant, while values in normal font are non-significant.
= statistical analysis of interaction between day and treatment;
= statistical analysis of interaction among day, treatment and sex.
↓ = decrease; ↑ = increase; - = no change.
Discussion
We observed rapid, positive effects from an acute dose of ayahuasca on the expression of healthy behavior in juvenile common marmosets, which were more pronounced in males. More specifically, ayahuasca reduced scratching and depression-like behaviors, increased the feeding rate and restored body weight and fecal cortisol to baseline levels, although this did not occur after placebo.
After 8 weeks of IC, the marmosets exhibited increased self-directed stereotypic behaviors, such as scratching and autogrooming. In non-human primates, such behaviors are also expressed during psychosocial stress.20 After social isolation in both sexes, we also observed reductions in feeding behavior, increased somnolence and anhedonia (inferred here from reduced sucrose ingestion). The males also exhibited weight loss, as well as increased scent marking, which was considered an anxiety-like behavior since it occurred without a specific interest, such as territorial defense or reproductive signaling.20 The presence of anhedonia, somnolence, reduced feeding rates and body weight changes are considered depressive-like behaviors in non-human primates16; they are also consistent with symptoms observed in depressive patients and are DSM-5 guidelines for diagnosing this pathology.2
After treatment with vehicle alone, no changes regarding behavior or body weight were observed. However, a single dose of ayahuasca improved some of these depressive-like behaviors, mainly in males. In this case, a significant reduction of scratching and increased feeding rates indicated a positive effect.
Although it is recognized that serotonin acts as a pruritogen in humans and animals, the exact mechanisms of action by which it induces scratching have not yet been completely elucidated. Some studies have shown that serotonin reuptake inhibitors (such as paroxetine) or agonists of serotonin receptor type 1A (5-HT1a; such as buspirone) can improve pruritus in humans23 and can decrease scratching in non-human primates (Macaca mulata).24 Since some of the effects of ayahuasca also seem to be modulated by the serotoninergic system,8 it is possible that these changes are related to the reduced scratching behavior observed in this study.
The pathways by which serotonin may potentially act as anorectic agent are well understood, but in this case we should also consider the possibility that serotonin may modulate orexigenic neurons (neuropeptide Y/agouti-related protein) in the arcuate nucleus of the hypothalamus.25 Moreover, there is evidence that an orexigenic effect is elicited by σ1R agonists (N-allylnormetazocine) at low concentrations.26 Another important orexigenic factor increased by ayahuasca was cortisol, which induces hyperfagia via two distinct mechanisms: by reducing anorectic agents, such as corticotropin-releasing hormone (CRH), or by increasing orexigenic molecules, such as ghrelin or neuropeptide Y.27 The ayahuasca-mediated positive modulation in feeding behavior implies that the substance acts as an orexigenic modulator, although whether this is through the serotonin, σ1R, or cortisol pathways is unclear. Nevertheless, its action is potentially important, since depression produces appetite and body weight losses. The anorectic effect of many antidepressants, such as tricyclics, is considered a side-effect that induces minor tolerance. Galvão-Coelho et al.16 also observed this anorectic effect after treating common marmosets with nortriptyline.
Although no feeding changes were observed in the females, body weight in both sexes returned to baseline levels after ayahuasca treatment. In a previous study with a similar marmoset isolation protocol, the antidepressant nortriptyline induced different responses between sexes.16 The differential action of ayahuasca on the improvement of depressive-like behaviors in male and female common marmosets is probably due to the organizational and/or activational effects of sexual hormones on the serotonergic system and hypothalamus-pituitary-adrenal (HPA) axis, which may induce sexual dimorphism in these functions and the behaviors they control.28 Sex differences in response to antidepressants have been described in both human and animal models of depression. For instance, women have better outcomes than men when treated with SSRI.29 These facts point to the importance of studying both sexes in translational studies of depression, since understanding sexual dimorphism in the response to antidepressants could facilitate the development of more selective drugs. Despite this, males have been used more frequently than females in neuroscience animal models.29
To date, non-human primate models have not been used in studies on ayahuasca, particularly those involving juveniles. Studies normally use adult rodents as animal models of depression,9,30 which are phylogenetically more distant to humans. Although such studies have also observed positive antidepressant effects from ayahuasca or its components, unlike the present study their treatment protocol was 14 days and there was no continued response after treatment stopped. For example, rats treated with harmine (5, 10, and 15 mg/kg) for 14 days exhibited improvements in forced swimming and open-field tests.9 Female Wistar rats treated with ayahuasca for 14 days also performed better in a forced swimming test than a group treated with fluoxetine.30 In contrast, the single dose of ayahuasca used in the present study led to improved body weight and depression-like behaviors, which remained significant for at least 14 days.
Besides ayahuasca’s well-known pharmacological action on the serotoninergic system as an inhibitor of MAO, a transporter of serotonin, and an agonist of serotonin receptor type 2 (5-HT2),7,8 other pharmacological targets may be involved in the rapid antidepressant effect of ayahuasca observed in juvenile marmosets. Recently, some of the antidepressant effects observed in rodents, such as reduced anhedonia, have been associated with σ1R activation.6 Moreover, indirect pathways modulated by the agonist action of DMT on the 5-HT2 receptor and σ1R can stimulate molecular and cellular events involved in neural and synaptic plasticity, such as expression of transcription factors (c-fos, egr-1, egr-2), synthesis of brain-derived neurotrophic factor and the enhancement of calmodulin-dependent protein kinase II/IV, as well as protein kinase B activity in the hippocampus, which are all compatible with antidepressant action.31,32
In this study, ayahuasca treatment did not induce alterations in autogrooming, scent marking, somnolence or ingestion of sucrose solution. Using the same marmoset isolation protocol, Galvão-Coelho et al.17 also observed no improvement in such behaviors after nortriptyline treatment. The lack of behavior modulation could be related to the dose and duration of ayahuasca treatment, which might not have been sufficient to promote a significant antidepressant effect. Our data suggest that alternative protocols should be tested to determine whether substantial behavioral improvement is possible in juvenile marmosets.
The marmosets exhibited significantly lower fecal cortisol after 8 weeks of chronic social isolation. Low cortisol levels have been reported after exposure to strong stressors both in humans and small animals.33-35 Additionally, low levels of plasma cortisol have been described in juvenile common marmosets exposed to repeated separation from their families in infancy36 or to chronic social isolation during the juvenile stage.16,34 A recent study with common marmosets found that 21 days of social isolation at the juvenile stage is enough to reduce cortisol to levels below baseline.34 In other primate species, hypocortisolemia has also been correlated with a depression-like state in adult female Macaca fascicularis.33 Moreover, previous studies have reported hypocortisolemia in patients with atypical unipolar major depression and major depression with remittent conditions.11,35
During a prolonged stress response, a complex system involving the interaction of negative feedback in the HPA axis could lead to imbalance and change long-term adrenal function, which reduces cortisol synthesis.35 Keeping cortisol at low levels deregulates all adaptation systems, since cortisol is a pleiotropic hormone that regulates hormonal, neural, and immune system responses to challenging situations.35,37 Individuals with a chronic decrease in cortisol levels normally present weakness, weight loss, and immunological dysfunction.37
In this study, the low levels of cortisol observed after isolation started rising as soon as 24 after the acute dose of ayahuasca and recovered to levels similar to baseline. Homeostatic regulation was relatively rapid and did not extend into the later phases (PH and tPE). A previous study using the same animal model of juvenile depression and nortriptyline chloride treatment found increased cortisol levels only after 1 week, which then exceeded baseline. These results suggest that ayahuasca induces a faster and finer adjustment to cortisol levels than nortriptyline. Furthermore, previous human studies with ayahuasca have suggested beneficial effects in treatment-resistant depression in only one day after a single dose.4,11,14 The rapid effects of ayahuasca indicate a potential benefit over commercial antidepressants, which usually take at least 2 weeks to achieve the desired therapeutic response.38
The effects of antidepressants on the HPA axis are specially influenced by drug class (e.g. MAOi, tricyclics, SSRI, etc.).38 Serotoninergic agonists such as ayahuasca and nortriptyline might modulate the secretion of both CRH and/or adrenocorticotropic hormones at the levels of the hypothalamus and pituitary gland, respectively.38 Moreover, treatment duration is an important issue in HPA axis modulation. Normally, acute treatments increase cortisol levels, while long-term treatment protocols reduce them in peripheral blood. Reduced cortisol secretion by the adrenal gland is probably due to up-regulation of glucocorticoid receptors (GR and MR) in the brain, which could increase negative feedback in long-term treatment.39 Another pathway by which ayahuasca could modulate cortisol levels is through σ1R, since an increase in HPA axis activity has been observed in rats after acute use of the σ1R agonist N-allyl-normetazocine.40
It is important to highlight some limitations of this study. Although many studies with non-human primates have used a similar sample sizes (n=15), a larger sample size would be desirable in future studies. Additionally, the method used for ayahuasca preparation was poorly controlled. Although we used a standard traditional preparation protocol, the tea was handmade and subtle differences among preparations are probable. More standardized preparations are, therefore, desirable. Finally, this study involves the limitations inherent in all small animal models of depression.
This is the first time that the therapeutic value of ayahuasca for depression in juveniles have been investigated in an animal model. Adolescence is an important ontogenetic period of brain plasticity, and an ayahuasca treatment protocol may be a reasonable alternative to conventional pharmaceutical antidepressants. However, further follow-up studies are necessary to evaluate the safety and tolerability of ayahuasca in humans as a basis for its use as an effective antidepressant in adolescents.
In summary, depressive-like behaviors and changes in body weight and cortisol were observed in juvenile male and female common marmosets exposed to chronic social isolation for 8 weeks, and these were in large part ameliorated by a single dose of ayahuasca, the effects of which were more pronounced in males. Moreover, we observed that ayahuasca led to behavioral and physiological improvements that were rapid and long-lasting. Therefore, this study presents significant evidence of the beneficial effects of ayahuasca for depression in a common marmoset model, which is phylogenetically closer to humans than rodents.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
We would like to thank Edinolia Câmera, Antonio B. da Silva, Geniberto C. dos Santos, and Janaína Nitta for animal and veterinary care, and Raíssa Nóbrega de Almeida for hormone measurements.
Footnotes
How to cite this article: da Silva FS, Silva EAS, Sousa Jr. GM, Maia-de-Oliveira JP, Soares-Rachetti VP, de Araujo DB, et al. Acute effects of ayahuasca in a juvenile non-human primate model of depression. Braz J Psychiatry. 2019;41:280-288. http://dx.doi.org/10.1590/1516-4446-2018-0140
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) Arlington: American Psychiatric Publishing;; 2013. [Google Scholar]
- 2.World Health Organization (WHO) Depression and other common mental disorders: global health estimates. 2017. apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf;jsessionid=14A7336A2E0BDABADF55104E933C6E65?sequence=1 [Internet] [cited 2018 Aug 09] [Google Scholar]
- 3.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 4.Palhano-Fontes F, Barreto D, Onias H, Andrade KC, Novaes MM, Pessoa JA, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. 2018;Jun 15:1–9. doi: 10.1017/S0033291718001356. https://10.1017/S0033291718001356 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabregas JM, Gonzalez D, Fondevila S, Cutchet M, Fernandez X, Barbosa PC, et al. Assessment of addiction severity among ritual users of ayahuasca. Drug Alcohol Depend. 2010;111:257–61. doi: 10.1016/j.drugalcdep.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Fukunaga K, Moriguchi S. Stimulation of the sigma-1 receptor and the effects on neurogenesis and depressive behaviors in mice In: In: Smith SB, Su TP, editors. Sigma receptors: their role in disease and as therapeutic targets. New York: Springer International Publishing; 2017. pp. 201–11. [DOI] [PubMed] [Google Scholar]
- 7.Callaway JC, McKenna DJ, Grob CS, Brito GS, Raymon LP, Poland RE, et al. Pharmacokinetics of Hoasca alkaloids in healthy humans. J Ethnopharmacol. 1999;65:243–56. doi: 10.1016/s0378-8741(98)00168-8. [DOI] [PubMed] [Google Scholar]
- 8.dos Santos RG. Ayahuasca: neuroquímica e farmacologia SMAD Rev Eletr Saude Ment Alcool Drogas. 2007;3:1–11. [Google Scholar]
- 9.Fortunato JJ, Réus GZ, Kirsch TR, Stringari RB, Fries GR, Kapczinski F, et al. Chronic administration of harmine elicits antidepressant-like effects and increases BDNF levels in rat hippocampus. J Neural Transm (Vienna) 2010;117:1131–7. doi: 10.1007/s00702-010-0451-2. [DOI] [PubMed] [Google Scholar]
- 10.Dos Santos RG, Valle M, Bouso JC, Nomdedéu JF, Rodrlıguez-Espinosa J, McIlhenny EH, et al. Autonomic, neuroendocrine, and immunological effects of ayahuasca: a comparative study with d-amphetamine. J Clin Psychopharmacol. 2011;31:717–26. doi: 10.1097/JCP.0b013e31823607f6. [DOI] [PubMed] [Google Scholar]
- 11.Galvão AC, de Almeida RN, Silva EA, Freire FA, Palhano-Fontes F, Onias H, et al. Cortisol modulation by ayahuasca in patients with treatment resistant depression and healthy controls. Front Psychiatry. 2018;9:185. doi: 10.3389/fpsyt.2018.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan S, Khan RA. Chronic stress leads to anxiety and depression. Ann Psychiatry Ment Health. 2017;5:1091. [Google Scholar]
- 13.Barbosa PC, Mizumoto S, Bogenschutz MP, Strassman RJ. Health status of ayahuasca users. Drug Test Anal. 2012;4:601–9. doi: 10.1002/dta.1383. [DOI] [PubMed] [Google Scholar]
- 14.Osório Fde L, Sanches RF, Macedo LR, Santos RG, Maia-de-Oliveira JP, Wichert-Ana L, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Rev Bras Psiquiatr. 2015;37:13–20. doi: 10.1590/1516-4446-2014-1496. [DOI] [PubMed] [Google Scholar]
- 15.Hankin BL. Adolescent depression: description, causes, and interventions. Epilepsy Behav. 2006;8:102–14. doi: 10.1016/j.yebeh.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Galvão-Coelho NL, Galvão AC, da Silva FS, de Sousa MB. Common marmosets: a potential translational animal model of juvenile depression. Front Psychiatry. 2017;8:175. doi: 10.3389/fpsyt.2017.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pachaly JR. Terapêutica por extrapolação alométrica In: In: Cubas ZS, Silva JCR, Catão-Dias JL, editors. Tratado de animais selvagens-medicina veterinária. São. Paulo: Roca; 2006. pp. p. 1215–23. [Google Scholar]
- 18.Savoldi R, Polari D, Pinheiro-da-Silva J, Silva PF, Lobao-Soares B, Yonamine M, et al. Behavioral changes over time following ayahuasca exposure in zebrafish. Front Behav Neurosci. 2017;11:139. doi: 10.3389/fnbeh.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barros M, Tomaz C. Non-human primate models for investigating fear and anxiety. Neurosci Biobehav Rev. 2002;26:187–201. doi: 10.1016/s0149-7634(01)00064-1. [DOI] [PubMed] [Google Scholar]
- 20.di Sorrentino EP, Schino G, Tiddi B, Aureli F. Scratching as a window into the emotional responses of wild tufted capuchin monkeys. Ethology. 2012;118:1072–84. [Google Scholar]
- 21.Ferreira RG, Mendl M, Wagner PG, Araujo T, Nunes D, Mafra AL. Coping strategies in captive capuchin monkeys (Sapajus spp.) Appl Anim Behav Sci. 2016;176:120–7. [Google Scholar]
- 22.Sousa MB, Ziegler TE. Diurnal variation on the excretion patterns of steroids in common marmoset (Callithrix jacchus) females. Am J Primatol. 1998;46:105–17. doi: 10.1002/(SICI)1098-2345(1998)46:2<105::AID-AJP1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Siemens W, Xander C, Meerpohl JJ, Buroh S, Antes G, Schwarzer G, et al. Pharmacological interventions for pruritus in adult palliative care patients. Cochrane Database Syst Rev. 2016;11 doi: 10.1002/14651858.CD008320.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontenot MB, Padgett EE 3rd, Dupuy AM, Lynch CR, De Petrillo PB, Higley JD. The effects of fluoxetine and buspirone on self-injurious and stereotypic behavior in adult male rhesus macaques. Comp Med. 2005;55:67–74. [PubMed] [Google Scholar]
- 25.Voigt JP, Fink H. Serotonin controlling feeding and satiety. Behav Brain Res. 2015;277:14–31. doi: 10.1016/j.bbr.2014.08.065. [DOI] [PubMed] [Google Scholar]
- 26.Gosnell BA, Levine AS, Morley JE. N-allylnormetazocine (SKF-10,047): the induction of feeding by a putative sigma agonist. Pharmacol Biochem Behav. 1983;19:737–42. doi: 10.1016/0091-3057(83)90072-2. [DOI] [PubMed] [Google Scholar]
- 27.Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23:887–94. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Goel N, Bale TL. Sex differences in the serotonergic influence on the hypothalamic-pituitary-adrenal stress axis. Endocrinology. 2010;151:1784–94. doi: 10.1210/en.2009-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kokras N, Dalla C. Preclinical sex differences in depression and antidepressant response: implications for clinical research. J Neurosci Res. 2017;95:731–6. doi: 10.1002/jnr.23861. [DOI] [PubMed] [Google Scholar]
- 30.Pic-Taylor A, da Motta LG, de Morais JA, Junior WM, Santos Ad F, Campos LA, et al. Behavioural and neurotoxic effects of ayahuasca infusion (Banisteriopsis caapi and Psychotria viridis) in female Wistar rat. Behav Processes. 2015;118:102–10. doi: 10.1016/j.beproc.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Frankel PS, Cunningham KA. The hallucinogen d-lysergic acid diethylamide (d-LSD) induces the immediate-early gene c-Fos in rat forebrain. Brain Res. 2002;958:251–60. doi: 10.1016/s0006-8993(02)03548-5. [DOI] [PubMed] [Google Scholar]
- 32.González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, et al. Hallucinogens recruit specific cortical 5-HT 2A receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–52. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Willard SL, Shively CA. Modeling depression in adult female cynomolgus monkeys (Macaca fascicularis) Am J Primatol. 2012;74:528–42. doi: 10.1002/ajp.21013. [DOI] [PubMed] [Google Scholar]
- 34.de Sousa MB, Galvão AC, Sales CJ, de Castro DC, Galvão-Coelho NL. Endocrine and cognitive adaptations to cope with stress in immature common marmosets (Callithrix jacchus): sex and age matter. Front Psychiatry. 2015;6:160. doi: 10.3389/fpsyt.2015.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fries E. Hypocortisolemic disorders. In: In: Hellhammer DH, Hellhammer J, editors. Stress. Basel: Karger Publishers; 2008. pp. p. 60–77. [Google Scholar]
- 36.Dettling AC, Feldon J, Pryce CR. Early deprivation and behavioral and physiological responses to social separation/novelty in the marmoset. Pharmacol Biochem Behav. 2002;73:259–69. doi: 10.1016/s0091-3057(02)00785-2. [DOI] [PubMed] [Google Scholar]
- 37.McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, et al. Mechanisms of stress in the brain. Nat Neurosci. 2015;18:1353–63. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levinstein MR, Samuels BA. Mechanisms underlying the antidepressant response and treatment resistance. Front Behav Neurosci. 2014;8:208. doi: 10.3389/fnbeh.2014.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Budziszewska B, Siwanowicz J, Przegaliński E. The effect of chronic treatment with antidepressant drugs on the corticosteroid receptor levels in the rat hippocampus. Pol J Pharmacol. 1994;46:147–52. [PubMed] [Google Scholar]
- 40.Eisenberg RM. Plasma corticosterone changes in response to central or peripheral administration of kappa and sigma opiate agonists. J Pharmacol Exp Ther. 1985;233:863–9. [PubMed] [Google Scholar]