Abstract
Objectives:
Brain imaging studies carried out in patients suffering from generalized anxiety disorder (GAD) have contributed to better characterize the pathophysiological mechanisms underlying this disorder. The present study reviews the available functional and structural brain imaging evidence on GAD, and suggests further strategies for investigations in this field.
Methods:
A systematic literature review was performed in PubMed, PsycINFO, and Google Scholar, aiming to identify original research evaluating GAD patients with the use of structural and functional magnetic resonance imaging as well as diffusion tensor imaging.
Results:
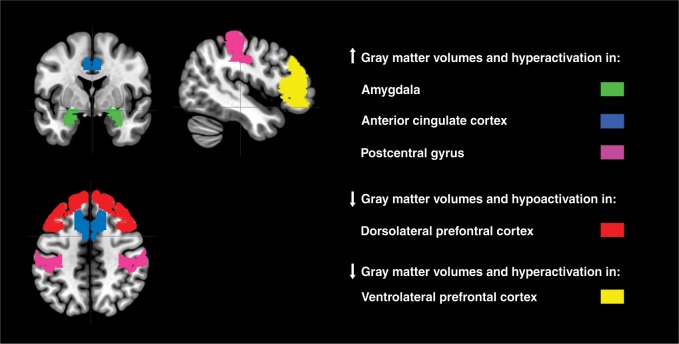
The available studies have shown impairments in ventrolateral and dorsolateral prefrontal cortex, anterior cingulate, posterior parietal regions, and amygdala in both pediatric and adult GAD patients, mostly in the right hemisphere. However, the literature is often tentative, given that most studies have employed small samples and included patients with comorbidities or in current use of various medications. Finally, different methodological aspects, such as the type of imaging equipment used, also complicate the generalizability of the findings.
Conclusions:
Longitudinal neuroimaging studies with larger samples of both juvenile and adult GAD patients, as well as at risk individuals and unaffected relatives, should be carried out in order to shed light on the specific biological signature of GAD.
Keywords: Generalized anxiety disorder, magnetic resonance imaging, functional MRI, diffusion tensor imaging, resting-state
Introduction
Anxiety disorders share common features, including excessive and irrational fear and avoidance of anxiety triggers. İn particular, generalized anxiety disorder (GAD) is a severe chronic illness characterized by symptoms including persistent and uncontrollable worry about everyday life matters and social competence, as well as autonomic hyperarousal and tension.1 The prevalence of GAD, which typically begins in young adulthood, is about 2% in the adult population; the lifetime prevalence of GAD is around 4.7%.2,3 Female sex, low socioeconomic status, and exposure to childhood adversity are considered as risk factors for the disease.4
Despite the suffering, disability, and economic burden associated with GAD,5,6 treatment options are not available for a considerable group of patients because little is known about the pathophysiology of this disease.7 Therefore, in recent years, researchers have focused their attention towards the investigation of the neurobiological underpinnings of GAD.8 In the past two decades, imaging studies have identified subtle structural, chemical, and functional brain changes in GAD that provide hints on the neurophysiologic mechanisms underlying this illness. Specifically, GAD symptoms have been linked to a disruption in the balance of activity in the emotional centers of the brain rather than in the higher cognitive centers.9,10 Indeed, the initial processing of stressful stimuli is carried out by a neural circuit composed of core limbic structures, including the amygdala, insula, periaqueductal gray matter, locus coeruleus, and hypothalamus. These structures are responsible for an early evaluation of stressful stimuli and for organizing an appropriate initial physiological and behavioral response.11 Moreover, studies have shown that in GAD patients the pattern of brain activity resembles that of animal models in which limbic circuits, particularly the amygdala, play an important role in fear response.12-14 Etkin et al.15 have also found evidence of an intra-amygdala abnormality with engagement of a compensatory fronto-parietal executive control network, consistent with cognitive theories of GAD. Interestingly, a recent review reported selective metabolic dysfunctions in regions within the prefrontal-limbic network, including the dorsolateral prefrontal cortex (DLPFC) and hippocampus.16
Furthermore, in addition to the amygdala, other cortical and subcortical regions have been found to be involved in the pathophysiology of this disabling disorder. Functional magnetic resonance imaging (fMRI) research has reported that hypoactivation of the DLPFC in GAD patients was associated with emotional dysregulation and deficits in attentional control, ultimately suggesting that the failure to engage prefrontal cortex (PFC) during emotion regulation may be part of the critical transition from dispositional high anxiety to an anxiety disorder.17-19 Additionally, evidence from structural MRI studies showed that GAD is characterized by selective impairments in the cortex, especially in regions within the prefrontal-temporal network, and subcortex, including basal ganglia and amygdala, in both adult and pediatric cohorts.20-25
With regard to neurochemistry, a number of neurotransmitter systems have been implicated in the neurobiology of GAD.16,26,27 Given the known pharmacological and clinical effects of benzodiazepines, the γ-aminobutyric acid (GABA)/benzodiazepine system has become a focus of research in GAD. Indeed, several studies showed that GAD patients have reduced central benzodiazepine receptor function, perhaps due to alterations in receptor number.28-30 However, this neurotransmitter system was not the only one found to be disrupted in GAD; other studies have also reported a key role of the norepinephrine31,32 and serotonin26 systems, resulting mainly from their involvement in neural mechanisms, such as sensitization and fear conditioning, consistently associated with stress.33,34
Considering this scenario, summarizing the available neuroimaging evidence on GAD is important to provide a consistent overview of the neural systems found to be disrupted in this disorder. The debate regarding these findings, with suggestions for future investigation in this field, may help elucidate the pathophysiological mechanisms involved in GAD and guide the development of more effective treatment strategies.35,36
Methods
A systematic literature search was performed in PubMed, PsycINFO, and Google Scholar, using the following keywords: “Generalized anxiety disorder” OR “GAD” AND (“Magnetic Resonance Imaging” OR “MRI” OR “Diffusion Tensor Imaging” OR “DTI” OR “Diffusion Weighted Imaging” OR “DWI” OR “resting-state functional Magnetic Resonance” OR “rs-FMRI,” OR “functional MRI,” OR “fMRI”). Considering the scope of the present work, the following inclusion criteria were considered: original research evaluating pediatric, adolescent, adult, and elderly GAD patients with structural\functional MRI and diffusion tensor imaging (DTI). Studies investigating the effect of psychotropic medications on the brain were also included. No time limits were set and the last search was conducted in June 2018.
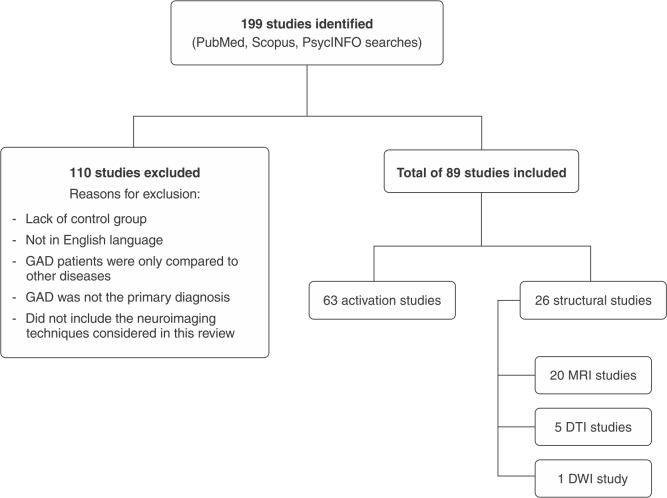
The search identified 199 articles, of which 110 were excluded for the following reasons: a) lack of control group; b) not being published in English; c) GAD patients were only compared to other diseases, including panic disorder (PD), social phobia, generalized social anxiety disorder, separation anxiety disorder, and major depressive disorder; d) GAD was not the primary diagnosis; and e) did not employ the neuroimaging techniques considered in this review. The remaining articles (n=89) were included in this review. Specifically, twenty were about structural MRI, five about DTI, one about diffusion weighted imaging, and sixty-three about fMRI. The selection of studies is summarized in Figure 1.
Figure 1. Study selection flow chart.DTI = diffusion tensor imaging; DWI = diffusion weighted imaging; GAD = generalized anxiety disorder; MRI = magnetic resonance imaging.
Putative models of anxiety pathophysiology
Three models have been proposed to describe the functional anatomy of anxiety disorders.26,37 The first is the linear model, according to which identical brain structures are thought to be hyper- or hypo- activated during the experience of anxiety in health and disease, depending on the severity of the experience. The second model is the catastrophe theory model, which assumes that, after reaching a particular set of conditions, a system is unable to maintain an equilibrium and a discontinuity occurs, leading to the activation of specific brain areas in pathological anxiety. The third and last model, named the modular model, postulates that all forms of anxiety activate a module related to the experience of autonomic arousal and fear; conversely, other areas would only be activated in the presence of specific forms of anxiety and linked to specific experiences (i.e., flight reaction as in a panic attack; immobility as in anticipatory anxiety; and awareness of one’s own body position in space as in social phobia). These three models are not mutually exclusive and may partially coexist. However, the modular theory provides an integrated explanation that is more consistent with contemporary models of brain function.38 Reviewing the neuroimaging literature can help to clarify this question.
Brain imaging studies in GAD
Structural studies
For a detailed description of MRI studies in GAD, please refer to Table 1 and Supplementary Table S1. (52.4KB, pdf) To date, several structural MRI studies have explored changes in gray matter (GM) and white matter (WM) volumes in GAD patients. Specifically for GM, the first structural MRI studies on GAD were carried out by the same research group on a small group of children and adolescents. The authors showed abnormally larger superior temporal gyrus (STG) and amygdala volumes, especially in the right hemisphere, in GAD patients compared to healthy controls (HCs).39,40 Interestingly, the same authors also reported the presence of preserved brain regions in their juvenile patient population, including intracranial volumes, total cerebral GM and WM, prefrontal and temporal lobes, corpus callosum, hippocampus, thalamus, and basal ganglia.39,40 In contrast, Strawn et al.41 found increased GM volumes in right precuneus and precentral gyrus, as well as decreased GM volumes in orbitofrontal cortex and posterior cingulate in a group of GAD adolescents. Furthermore, a more recent study from the same group,21 including an independent sample of patients with various anxiety disorders, extended their previous results by showing greater GM volumes in left cingulate gyrus and lower GM volumes in left inferior frontal gyrus, left postcentral gyrus, left precuneus, and amygdala bilaterally.41 Along this line, a study carried out by Mohlman et al.42 also reported that PFC volumes, and especially the medial orbital cortex, positively correlated with worry severity in older adults with GAD. Additionally, larger volumes of amygdala, thalamus, dorsomedial PFC (DMPFC), and putamen have been found in adolescent and adult patients suffering from GAD.23,24,43,44 In contrast, a voxel-based morphometry (VBM) investigation showed selective volume reduction in left amygdala in pediatric GAD compared to age- and gender-matched HCs.45 Further, Liao et al.46 found selective gender-related differences in left precuneus/posterior cingulate cortex, with males having larger volumes compared to female GAD patients. Hilbert et al.20 also reported significantly higher GM volumes in GAD subjects, mainly in basal ganglia and less consistently in STG, in contrast with other studies showing GM volume reductions in insula and STG in GAD patients compared to HCs.44,47,48 Additionally, two studies reported that GAD patients had lower GM volumes in supramarginal, precentral, and postcentral gyri bilaterally,44 as well as in the hypothalamus,49 as compared to HCs.49
Table 1. Structural findings.
| Study | Sample n, mean age (years) ± SD | Sex (F/M) | Comorbidity (n) | Drugs | Method | Main findings |
|---|---|---|---|---|---|---|
| De Bellis39 | GAD = 12, 12.7±2.4 HC = 24, 12.5±2.3 |
GAD=5/7 HC = 10/14 |
DDNOS = 3 MDD = 1 PD = 1 SP = 1 |
Twelve drug-naïve participants One patient on AD |
1.5 T MRI | Larger amygdala in GAD patients vs. HC: right>left |
| De Bellis40 | GAD = 13, 12.5±2.5 HC = 98, 12±2.3 |
GAD=5/8 HC = 48/50 |
DDNOS = 3 MDD = 1 PD = 1 SP = 1 |
Twelve drug-naïve participants One patient on AD |
1.5 T MRI | Larger total GM and WM STG in GAD patients vs. HC |
| Milham45 * | GAD+SP+SAD=17 (GAD = 13; SP = 9; SAD = 3), 12.9±2.3HC = 34, 14.4±2.2 | GAD+SP+ SAD=9/8 HC = 18/16 |
MDD = 4 Only three subjects had GAD without MDD or another anxiety disorder |
Two participants with previous AD exposure | 3 T MRI | GM volume reduction in left amygdala in patients with anxiety disorders vs. HC |
| Mohlman42 | GAD = 15, 67.39±5.42 HC = 15, 67.5±4.94 |
GAD = 7/8 HC = 8/7 |
Dysthymia = 3 SP = 3 Specific phobia = 3 PD = 2 |
No current psychotropic drug use (1 year before the study) | 1.5 T MRI | No difference in mOFC, DLPFC, and amygdala volumes Positive relation between mOFC and worry score in GAD patients |
| Brambilla50 | GAD = 12 HC = 15 |
GAD = 8/4 HC = 9/6 |
No psychiatric comorbidity | Seven subjects exposed to AD and six to BDZ at the moment of the study |
1.5 T DWI-MRI | Greater ADC measures in the right splenium of the corpus callosum and parietal cortex in GAD patients vs. HC |
| Schienle43 | GAD = 16, 22.9±4.1 HC = 15, 23.7±3.7 |
GAD = 16/0 HC = 15/0 |
No psychiatric comorbidity | No current psychotropic drug use | 3 T MRI | Larger GM volumes of amygdala and DMPFC in GAD patients vs. HC |
| Zhang51 | GAD = 20, 30.80±8.58 PTSD = 17, 34.06±4.97 HC = 28, 28.96±6.22 |
GAD = 8/12 PTSD = 0/17 HC = 14/14 |
No psychiatric comorbidity | No current psychotropic drug use | 1.5 T DTI-MRI | Increased FA in right postcentral gyrus in GAD vs. HC; reduced FA in right ACC in PTSD patients vs. GAD patients. |
| Tromp52 | GAD = 49, 27.10±10.61 HC = 39, 23.85±6.86 |
GAD = 30/19 HC = 19/20 |
MDD = 20 SAD = 18 |
No current psychotropic drug use | 3 T DTI-MRI | Lower FA in UF in GAD patients vs. HC |
| Terlevic49 | GAD = 12, 42.10±11.4 PD = 11, 33.54±9.94 HC = 21, 35.86±13.60 |
GAD = 8/4 PD = 6/5 HC = 14/7 |
No psychiatric comorbidity | Seven subjects from the GAD group and eight subjects from the PD group exposed to AD | 1.5 T MRI | Decreased hypothalamus volume bilaterally in GAD patients, but not in PD patients, vs. HC |
| Zhang53 | GAD = 16, 30.38±8.35 HC = 26, 30.62±8.31 |
GAD = 7/9 HC = 13/13 |
No psychiatric comorbidity | No current psychotropic drug use | 1.5 T DTI-MRI | Increased FA in right amygdala in GAD patients vs. HC Reduced FA in left caudal ACC/ midcingulate cortex in GAD patients vs. HC Positive correlation between anxiety severity scores and right amygdala FA in GAD patients vs. HC |
| Liao23 | GAD-CM = 14, 17±0.20 GAD-WCM = 12, 16.67±0.22 HC-CM =12, 16.58±0.22 HC-WCM =13, 16.85±0.21 |
GAD-CM = 7/7 GAD-WCM = 6/6 HC-CM = 6/6 HC-WCM = 6/7 |
No other current MDD or other anxiety disorder | No current psychotropic drug use | 3 T MRI | Larger right putamen GM in GAD patients vs. HC Larger left thalamic GM in GAD patients with CM vs. GAD patients WCM |
| Strawn41 | GAD = 15, 13±2 HC = 28, 13±2 |
GAD = 8/7 HC = 17/11 |
ADHD = 6 SP = 4 Specific phobia = 1 |
No current psychotropic drugs | 4 T MRI | Increased GM in right PCu and precentral gyrus in GAD patients vs. HC Decreased posterior cingulate WM and GM and orbitofrontal cortex GM gyrus in GAD patients vs. HC |
| Liao24 | GAD = 25, 16.96±0.68 HC = 24, 16.58±0.83 |
GAD = 13/12 HC = 11/13 |
No other current psychiatric disorders | No current psychotropic drugs | 3 T DTI-MRI | Reduced FA in bilateral UF, inferior fronto-occipital fasciculus, inferior longitudinal fasciculus and corona radiata in GAD patients vs. HC |
| Liao46 Gender study | GAD-M = 13, 16.77±0.73 GAD-F = 13, 16.92±0.64 HC-M = 13, 16.92±0.86 HC-F = 12, 16.50±0.80 |
GAD = 13/13 HC = 12/13 |
No other current psychiatric disorders | No current psychotropic drug use | 3 T MRI | Larger right putamen GM in GAD patients vs. HC Larger left PCu/PCC GM in M vs. F No gender-by-diagnosis interaction effect |
| Moon47 | GAD = 22, 37.0±10.7 HC = 22, 33.4± |
GAD = 9/13 GAD = 9/13 |
No other current psychiatric disorders | No current psychotropic drug use | 3 T MRI | Reduced GM in hippocampus, midbrain, thalamus, insula, and STG in GAD patients vs. HC |
| Cha54 | GAD = 32, 22.3±5.14 HC = 25, 21.3±4.56 |
GAD = 32/0 HC = 25/0 |
MDD = 17 | No current psychotropic drug use | 3 T MRI, DTI and fMRI | GAD patients with thicker VMPFC display a more discriminate VMPFC reactivity during the fear task vs. HC Increased connectivity in VMPFC in GAD patients vs. HC |
| Hilbert20 | GAD = 19, 33.47±8.9 HC = 24, 32.25±9.33 |
GAD = 16/3 HC = 17/7 |
16 of the GAD subjects had at least one comorbidity | No current psychotropic drug use | 3 T MRI | Higher GM volumes in basal ganglia structures and lower in the superior temporal pole in GAD patients vs. HC Decreased WM volumes in the DLPFC in GAD patients vs. HC |
| Moon & Jeong48 | GAD = 22, 37±10.7 HC = 22, 33.4±9.7 |
GAD = 9/13 HC = 9/13 |
All patients had primary GAD with mild depression | Eighteen patients had prescriptions for multiple psychiatric medications, including anxiolytics (buspirone [n=8]; alprazolam [n=3]; zolpidem [n=1]; tofisopam [n=2]; lorazepam [n=1]) and/or antidepressants (escitalopram [n=10]; bupropion [n=1]; fluvoxamine [n=2]; duloxetine [n=2]; paroxetine [n=3]). Four patients were taking one psychotropic medication. | 3 T MRI | Reduced WM volumes in DLPFC and anterior limb of internal capsule in GAD patients vs. HC |
| Strawn21 | GAD+SP+SAD = 38, 14.4±3.3 HC = 27, 14.8±3.9 |
GAD+SP+ SAD = 28/10 HC = 15/12 |
No other current psychiatric disorders | No current psychotropic drug use | 3 T MRI | Larger GM volumes in the dorsal ACC in GAD patients vs. HC Decreased GM volumes in the VLPFC, postcentral gyrus, and cuneus/PCu in GAD patients vs. HC |
| Moon55 | GAD = 17, 37.4±11.3 HC = 17, 35.6±6.1 |
GAD = 6/11 HC = 6/11 |
All patients had primary GAD with mild depression | Eleven patients had prescriptions for multiple psychiatric medications, including anxiolytics (buspirone [n=5]; alprazolam [n=2]; lorazepam [n=1]; tofisopam [n=2]) and/or antidepressants (escitalopram [n=9]; bupropion [n=1]; fluvoxamine [n=1]; duloxetine [n=2]; mirtazapine [n=1]; paroxetine [n=3]). | 3 T MRI and fMRI | Significant reduction in GM volumes especially in the hippocampus, midbrain, thalamus, insula, and STG in GAD patients vs. HC |
| Moon & Jeong27 | GAD = 13, 37.8±7.6 HC = 13, 35.9±9.9 |
GAD = 6/7 HC = 6/7 |
All patients had primary GAD with mild depression | Eighteen patients had prescriptions for multiple psychiatric medications, including anxiolytics (buspirone [n=5]; alprazolam [n=3]; zolpidem [n=1]; tofisopam [n=2]) and/or antidepressants (escitalopram [n=9]; bupropion [n=1]; fluvoxamine [n=1]; duloxetine [n=1]). Six patients each were taking one psychotropic medication. | 3 T MRI | Reduced WM volumes in the midbrain, precentral gyrus, DLPFC and anterior limb of the internal capsule in GAD patients vs. HC |
| Moon & Jeong56 | GAD = 17, 38.1±10.4 HC = 17, 36.9±7.7 |
GAD = 9/8 HC = 9/8 |
All patients had primary GAD with mild depression | Eleven patients had prescriptions for multiple psychiatric medications, including anxiolytics (buspirone [n=4]; alprazolam [n=1]; lorazepam [n=2]; tofisopam [n=2]) and/or antidepressants (escitalopram [n=9]; bupropion [n=1]; fluvoxamine [n=2]; duloxetine [n=1]; mirtazapine [n=1]; paroxetine [n=3]). Six patients received one psychiatric medication each. | 3 T MRI and fMRI | Reduction of WM volumes in the DLPFC, anterior limb of the internal capsule, and midbrain in GAD patients vs. HC |
| Cha57 | GAD and GAD/MDD = 32 GAD = 15, 22.1±1.20 GAD/MDD = 17, 22.5±1.12 |
GAD and GAD/ MDD= 32/0 HC = 25/0 |
MDD = 17 | No current psychotropic drug use | 3 T MRI, DWI and fMRI (multimodal MRI) | GAD patients showed significantly decreased cue repetition effects in the left anterior hippocampus Increased mean diffusivity in hippocampus in GAD patients vs. HC |
| Wang58 | GAD = 28, 32.93±4.13 HC = 28, 33.21±5.25 |
GAD = 14/14 HC = 14/14 |
No other current psychiatric disorders | No current psychotropic drug use | 3 T DTI-MRI | Decreased FA of WM in bilateral UF, corpus callosum, cingulate gyrus, bilateral anterior thalamic radiation and corona radiate, right anterior limb of internal capsule, bilateral inferior frontal-occipital fasciculus, bilateral superior, and inferior longitudinal fasciculus vs. GAD patients vs HC |
| Makovac44 | GAD = 19, 30.0±6.9 HC = 19, 29.2±9.8 |
GAD = 16/3 HC = 16/3 |
No other current psychiatric disorders | Two GAD participants were included who used long-term medications (one citalopram, one pregabalin) at the time of the study. All other patients and controls were medication free. | 1.5 T MRI | Before induction: lower GM volume within SMG, precentral, and postcentral bilaterally in GAD patients vs. HC After induction: smaller volumes of bilateral insula, bilateral opercular cortex, right SMG and precentral, anterior cingulate and paracingulate cortex in GAD patients vs. HC |
| Molent25 | GAD = 31, 43.8±14.9 HC = 31, 39.7±13.5 |
GAD = 20/11 HC = 17/14 |
MDD = 1Specific phobia = 2 (at the time of MRI) | Eighteen patients had prescriptions for multiple psychiatric medications, including antidepressants (escitalopram [n=4]; paroxetine [n=3]; sertraline [n=2]; mirtazapine [n=2]; citalopram [n=2]; venlafaxine [n=1]; duloxetine [n=1]) and antipsychotic (amisulpride [n=1]). | 3 T MRI | Reduced cortical thickness in right caudal middle frontal gyrus in GAD patients vs. HC Hyper-gyrification in right FuG, inferior temporal gyrus, superior parietal gyrus, SMG, as well as in left SMG and superior frontal gyrus in GAD patients vs. HC |
ACC = anterior cingulate cortex; AD = antidepressive medication; ADC = apparent diffusion coefficient; ADHD = attention deficit and hyperactivity disorder; BDZ = benzodiazepines; CM = childhood maltreatment; DDNOS = depressive disorder not otherwise specified; DLPFC = dorsolateral prefrontal cortex; DMPFC = dorsomedial prefrontal cortex; DTI-MRI = diffusion tensor imaging; DWI-MRI = diffusion weighted imaging; F = female; FA = fractional anisotropy; FuG = fusiform gyrus; GAD = generalized anxiety disorder; GM = gray matter; HC = healthy control; M = male; MDD = major depressive disorder; mOFC = medial orbitofrontal cortex; MRI = magnetic resonance imaging; n = patients; PCu = precuneus; PD = panic disorder; PFC = prefrontal cortex; PTSD = posttraumatic stress disorder; SAD = separation anxiety disorder; SD = standard deviation; SMG = supramarginal gyrus; SP = social phobia; STG = superior temporal gyrus; UF = uncinate fasciculus; VLPFC = ventrolateral prefrontal cortex; VMPFC = ventromedial prefrontal cortex; WCM = without childhood maltreatment; WM = white matter.
In this study GAD, SP, and SAD were considered as a whole group.
Finally, a recent MRI study25 exploring different morphological parameters, including cortical surface area, cortical thickness, GM volumes, and gyrification, reported no alterations of GM in subjects with GAD compared to HCs. However, the authors showed a hyper-gyrification in the right fusiform, right inferior temporal gyrus, right superior parietal gyrus, supramarginal gyrus bilaterally, and left superior frontal gyri, as well as reduced cortical thickness in right caudal middle frontal gyrus in GAD patients compared to HCs. Overall, this structural MRI evidence suggests that GAD is a neurobiological disorder characterized by extensive deficits in selective brain regions encompassing predominantly the prefrontal-temporo-limbic network, which has been shown to be implicated in behavioral inhibition,59 modulation of anticipatory threat,60 and attachment styles,61 abilities that are often disrupted in GAD patients.62
With regard to WM abnormalities, some DTI studies provided important evidence of WM and brain connectivity disruptions in GAD. Specifically, reduced fractional anisotropy (FA), a measure of WM coherence and organization, in left uncinate fasciculus, which is a WM pathway connecting ventral prefrontal-cortex (VPFC) and anterior cingulate cortex (ACC) to the amygdala and other limbic regions, was found in patients with GAD, particularly in subjects without comorbidity.52 Similarly, Zhang et al.53 found an augmentation of FA in the right amygdala and a lower FA in the left ACC. Furthermore, another DTI study reported that GAD patients had increased FA in right postcentral gyrus, the location of primary somatosensory cortex, which has rarely been indicated as a disrupted brain area in anxiety disorders.51 Additionally, altered microstructure coherence of the right posterior parietal cortex and the callosal splenium has been reported in adult GAD patients in a previous investigation carried out by our group.50 Interestingly, several studies from the same research group showed reduced WM volumes in the midbrain, precentral gyrus, DLPFC, and anterior limb of the internal capsule in GAD patients compared to HCs.27,48,56 Moreover, a recent DTI study by Wang et al.58 examined WM deficits in subjects with GAD and showed a reduction of FA in many other regions, including bilateral uncinate fasciculus, body of corpus callosum, left middle cingulum, bilateral anterior thalamic radiation and corona radiate, right anterior limb of internal capsule, bilateral inferior frontal-occipital fasciculus, bilateral superior and inferior longitudinal fasciculus. Finally, Cha et al.57 also found increased mean diffusivity (MD) in hippocampus in GAD patients compared to HCs.
In conclusion, despite this evidence, it is important to point out that a general consensus regarding GM and WM abnormalities in GAD has not been reached yet. Indeed, it is still not clear whether GAD is associated with increased or decreased GM and WM volumes in the abovementioned regions. These discrepancies might be associated with the inclusion of GAD patients using different psychotropic medications and/or presenting different comorbidities, especially depression or other anxiety disorders, which could have potentially influenced the results of the investigations. Indeed, depression and other anxiety disorders, such as PD, may themselves affect brain anatomy and development, as suggested by previous studies.37,63-65 Also, it has been reported that the presence of comorbidities in GAD exacerbates its clinical phenotypes by decreasing the responsiveness to treatments and worsening the outcome.66 Moreover, pharmacological treatments may have also biased the brain deficits observed in these patients. In this regard, it has been suggested that antidepressants may have significant effects on brain networks by modulating the volumes, functions, and biochemistry of brain structures.67,68 Interestingly, Dusi et al.67 showed that antidepressants seem to affect the prefrontal-limbic network by reversing the hyperactivation of limbic areas to emotional stimuli and by enhancing frontal cortex and cingulate top-down modulatory influence on subcortical structures. Similarly, it has also been reported that citalopram, a selective serotonin reuptake inhibitor (SSRI) and a first-line treatment for anxiety disorders has selective effects on specific brain regions. For instance, it has been shown that citalopram attenuated amygdala response to aversive stimuli and reduced activity in prefrontal regions, striatum, insula, and paralimbic regions in GAD patients listening to worry sentences.69 Therefore, from this evidence emerged that pharmacological treatments might have significant effects on neural processes, which may potentially explain the heterogeneity of the abovementioned results in GAD patients.
However, despite these limitations, these results highlight the importance of investigating GM and WM alterations in GAD in order to identify the neuroanatomical mechanisms associated with cognitive and emotional dysfunctions often observed in these patients. Future studies with more homogeneous samples, both from diagnostic and pharmacological perspectives, are warranted to extend the generalizability of the findings.
Functional magnetic resonance imaging (fMRI) studies
The common symptoms of GAD lead to emotional dysregulation, such as unsuppressed anger and low tolerance to frustration, and/or to cognitive deficits, including impairments of implicit and explicit memories, attention, and executive function.55,70,71 Therefore, in this section, we have tried to clarify the main functional brain abnormalities in connection with emotional regulation and cognitive function in patients with GAD.
Specifically, the studies reviewed are divided into four subsections. The first subsection encompasses the studies evaluating emotion dysregulations. The second one is related to research exploring both “emotion and cognition.” The third subsection focuses on resting-state fMRI studies, and the fourth analyzes the link between fMRI and drugs used in GAD patients. All studies evaluated in this section are summarized in Table 2 and Supplementary Table S1. (52.4KB, pdf)
Table 2. Functional MRI studies.
| Study | Sample n, mean age (years) ± SD | Sex (F/M) | Comorbidity (n) | Drugs | Method | Main findings |
|---|---|---|---|---|---|---|
| Thomas72 * | GAD + PD = 12, 12.8±2.1 HC = 12, 12.1±2.6 |
GAD + PD = 5/7 HC = 5/7 |
SP = 1 | No current psychotropic drug use | 1.5 T fMRI Task = facial emotion processing task | Greater amygdala response to fearful faces in patients with anxiety vs. HC |
| Hoehn-Saric73 | GAD = 6, 36.0 No control group |
GAD = 3/3 | No psychiatric comorbidity | No current psychotropic drug use | 1.5 T fMRI Task = verbal descriptions of a personal worry or a neutral statement Seven weeks of therapy with citalopram | Post-treatment: reduced activation in prefrontal regions, striatum, insula, and paralimbic regions during processing of worry sentences |
| Monk74 | GAD = 18, 12.28±2.0 HC = 15, 13.53±2.4 |
GAD = 8/10 HC = 8/7 |
MDD = 9 SP = 10 SAD = 8 |
No current psychotropic drug use | 3 T fMRI Task = facial emotion processing task | Greater VLPFC response to angry faces in GAD patients vs. HC |
| McClure75 | GAD = 15, 11.67±1.97 HC = 20, 12.19±2.1 |
GAD = 7/8 HC = 11/9 |
SAD = 5 SP = 6 Specific phobia = 3 ADHD = 3 Other = 5 |
Unknown | 3 T fMRI Task = facial emotion processing task | Greater response to fearful faces in amygdala, vPFC, ACC in GAD patients vs. HC |
| Krain76 * | GAD + SP = 16, 15.2±1.3 HC = 13, 15.4±1.4 |
GAD + SP = 7/9 HC = 8/5 |
SP = 10 SP without GAD = 5 SAD = 1 Specific phobia = 3 OCD = 1 ADHD = 3 Oppositional defiant disorder = 2 Dysthymia = 2 |
Unknown | 3 T fMRI Task = decision-making task | No brain hyperactivation in patients with anxiety vs. HC High level of intolerance to uncertainty associated with higher activation of amygdala, rostral, and subgenual ACC in patients with anxiety vs. HC |
| Monk77 | GAD = 17, 13.12±2.09 HC = 12, 14.33±1.67 |
GAD = 6/11 HC = 6/6 |
MDD = number not known | No current psychotropic drug use | 3 T fMRI Task = facial emotion processing task | Greater response in amygdala while viewing masked angry faces in GAD patients vs. HC. Amygdala and VLPFC showed strong negative coupling specifically to masked angry faces in GAD patients vs. HC |
| Blair78 | GAD = 17, 35.0±10.6 HC = 17, 31.2±9.1 GSAD = 17, 29.0±8.7 |
GAD = 11/6 HC = 8/9 GSAD = 8/9 |
SP = 7 MDD = 1 | No current psychotropic drug use | 1.5 T fMRI Task = facial emotion processing task | Greater response in angry expression in right middle frontal gyrus in GAD patients vs. HC |
| Whalen69 | GAD = 15, 27.0±7.0 HC = 15, 33.0±11.0 |
GAD = 12/3 HC = 12/3 |
No other current disorder | No current psychotropic drug use | 3 T fMRI Task = facial emotion processing task eight weeks of therapy with venlafaxine | No different amygdala and rACC reactivity to threatening stimuli in GAD patients vs. HC Individual pre-treatment responsivity (greater rACC and lesser amygdala) predicted by treatment response in GAD patients vs. HC |
| Etkin15 | GAD = 16, 30.6±1.7 GAD-controls = 17, 32.5±2.0 HC = 31, 20.5±0.2 |
GAD = 14/2 GAD-controls = 15/2 HC = 13/18 |
MDD = 4 SP = 5 PD = 2 Dysthymia = 2 OCD = 1 | Four subjects exposed to AD at the moment of the study | 3 T rs-fMRI and MRI | BLA and CMA disconnectivity in GAD patients vs. HC with: DLPFC and posterior parietal cortex (increased);insula and cingulate (decreased); larger amygdalar GM (in particular right CMA) |
| Nitschke79 | GAD = 14, 33.7±10.2 HC = 14, 33.1±10.3 |
GAD = 12/2 HC = 12/2 |
No other current disorder Past MDD = 10 | No current psychotropic drug use |
3 T fMRI Tasks = warning cue that preceded aversive pictures and a second cue that preceded neutral pictures Eight weeks of therapy with venlafaxine |
Greater bilateral dorsal amygdala activation during anticipation of both aversive and neutral pictures in GAD patients (aversive>neutral) vs. HCGreater pre-treatment pgACC activation during anticipation of both aversive and neutral trials predicted better treatment response in GAD patients vs. HC |
| Paulesu80 | GAD = 8, 24.1±6.6 HC = 12, 23.6±3.4 |
GAD = 5/3 HC = 6/6 |
Not known | No current psychotropic drug use | 1.5 T fMRI Task = mood induction paradigm | Hyperactivation of ACC and DMPFC after worry stimulation in both GAD patients and HC Persistent activation after stimulation in GAD patients only |
| Etkin[81] | GAD = 17, 31.5±9.9 HC = 24, 36.5±11.8 |
GAD = 11/6 HC = 18/6 |
Dysthymia = 2 SP = 6 PD = 2 OCD = 1 |
Five subjects had never received AD No psychotropic drugs within at least 48 h | 3 T fMRI Task = emotional conflict task | Failure to activate pgACC during emotional conflict in GAD patients vs. HC |
| Maslowsky82 | GAD-CBT = 7, 13.4±1.7 GAD-fluoxetine = 7, 13.3±2.5 HC = 10, 14.5±1.4 |
GAD-CBT = 4/3 GAD-fluoxetine = 3/4 HC = 6/4 |
SP = 6 SAD = 10 ADHD = 4 MDD = 8 |
No current psychotropic drug use |
3 T fMRI Task = facial emotion processing task Eight weeks of therapy with fluoxetine or CBT |
Increased right VLPFC activation after treatment (CBT and SSRI) in GAD patients vs. HC |
| Guyer83 | GAD = 18, 12.91±2.67 SP = 14, 13.13±3.02 HC = 26, 13.99±2.44 |
GAD = 10/8 SP = 9/5 HC = 11/15 |
MDD = 5 SAD = 8 Specific phobia = 8 |
No current psychotropic drug use | 3 T fMRI Task = monetary incentive delay task | Hyperactivation of putamen in response to valence in GAD patients vs. SP patients and HC No caudate increased activation as incentive increased in GAD patients and HC vs. SP patients |
| Etkin & Schatzberg84 | GAD = 18, 31.3±9.5 MDD = 14, 32.2 ± 11.7 HC = 32, 35.6±11.1 |
GAD = 11/7 MDD = 10/4 HC = 23/9 |
Dysthymia = 2 SP = 5 PD = 2 OCD = 1 |
No current psychotropic drug use | 3 T fMRI Task = emotional conflict task | Hyperactivation of ventral ACC and amygdala in GAD and MDD patients vs. HC |
| Palm85 | GAD = 15, 34±13 HC = 16, 34±13 |
n=15/0 HC = 16/0 |
Past MDD = 1 Past alcohol dependence = 1 Other anxiety disorder (PD, SP, specific phobia) = 10 | Three subjects exposed to AD at the moment of the study | 1.5 T fMRI Task = facial emotion processing task | Hypoactivation of VLPFC, MPFC, and ACC across emotional expression, especially happy faces in GAD patients vs. HC No amygdala dysregulation in GAD patients vs. HC |
| Andreescu86 | GAD = 7, 63.3±3.9 HC = 10, 76.3±4.0 |
GAD = 5/2 HC = 6/4 |
SP = 3 Specific phobia = 2 OCD = 1 |
No current psychotropic drug use | fMRI-pulse arterial spin labelling perfusion at rest and during task Task = worry modulation task | Resting-state: increased activity during worry induction in the associative temporo-occipital areas, but not in the insulaor the amygdala in GAD patients vs. HC Worry suppression: Increased activity in dorsal ACC, but not in PFC in GAD patients vs. HC |
| Blair87 | - EER GAD = 17, 36.1±11.75 GSAD = 19, 29.4±8.7 GAD/GSAD = 17, 35.7±9.54 HC = 18, 33.4±9.65 - TAC GAD = 17, 34.9±10.93 GSAD = 18, 31.8±9.10 GAD/GSAD = 15, 33.5±10.57 HC = 18, 30.4±6.86 |
- EER GAD = 13/4 GSAD = 10/8 GAD/GSAD = 12/5 HC = 10/8 - TAC GAD = 10/7 GSAD = 8/10 GAD/GSAD = 10/5 HC = 9/9 |
No other current psychiatric disorders | No current psychotropic drug use | 1.5 T fMRI Task1 = EER Task2 = TAC | HC showed significantly increased recruitment during emotion regulation, relative to emotion- picture viewing. GAD, SP, and SP/GAD patients showed no such increases. Decreased amygdala activation to negative stimuli only for GAD patients vs. HC |
| Strawn88 | GAD = 10, 14±2.2 HC = 10, 14.5±2.3 |
GAD = 6/4 HC = 6/4 |
No other current psychiatric disorders | No current psychotropic drug use | 4 T fMRI Task = continuous performance task with emotional and neutral distractors | Increased activation in left medial PFC and right VLPFC in response to emotional images in GAD patients vs. HC Decreased connectivity between VLPFC and bilateral medial PFC in GAD patients vs. HC Decreased correlation between right amygdala and PCC in GAD patients vs. HC Increased correlation between left amygdala and ipsilateral PCu in GAD patients vs. HC |
| Yassa89 | GAD = 15, 34.7±9.51 HC = 15, 32.4±8.7 |
GAD = 12/3 HC = 9/6 |
No other current psychiatric disorders | No current psychotropic drug use | 3 T fMRI Task = gambling game with non-contingent monetary loss | Decreased amygdala and BNST activity in GAD patients vs. HC |
| Roy90 | GAD = 15, 14.9±1.7 HC = 20, 14.8±1.7 |
GAD = 10/5 HC = 13/7 |
SP = 5 Specific phobia = 2 PD = 1 OCD = 1 SAD = 2 MDD = 3 ADHD = 1 |
No current psychotropic drugs | 3 T rs-fMRI | Disruption in amygdala connectivity with medial PFC, insula, and cerebellum in GAD patients vs. HC Positive correlation between anxiety severity scores and connectivity between amygdala, insula, and STG in GAD patients vs. HC |
| Chen & Etkin91 | GAD = 39, 32.4±1.5 HC = 38, 30.7±2.6 PTSD = 17, 34.4±3.4 |
GAD = 27/12 HC = 27/11 PTSD = 13/14 |
MDD = 23 | No current psychotropic drug use | 3 T fMRI resting-state + fMRI Task 1 = flashing checker board Task 2 = facial emotion processing task | Disturbed posterior hippocampus and default-mode network connectivity during resting-state and task execution in PTSD, but not in GAD patients or HC |
| Hölzel92 | GAD = 26, 37.9±12.2 HC = 26, 35.7±9.3 |
GAD = 14/12 HC = 16/10 |
MDD = 4 PD = 1 SP = 6 |
Four subjects exposed to AD at the moment of the study |
1.5 T fMRI Task = facial emotion processing task Eight weeks of MBSR and SME therapy |
Pre-treatment: higher amygdala activation in response to neutral, but not angry faces in GAD patients vs. HC Post-treatment: reduction of amygdala activation in response to neutral faces in GAD patients vs. HC Higher VLPFC activation after MBSR vs. SME in GAD patients vs. HC Increased connectivity between amygdala and PFC after MBSR in GAD patients vs. HC Correlation between activation/ connectivity and symptoms in GAD patients vs. HC |
| Ball18 | GAD = 23, 35±11 PD = 18, 29±7 HC = 22, 27±9 |
GAD = 17/5 PD = 13/6 HC = 11/11 |
GAD group: MDD=2 OCD=2 GSAD=8 PD group: MDD =2 OCD=1 GSAD=2 |
No current psychotropic drug use | 3 T fMRI Task: negative emotion processing task | PFC hypoactivation in GAD and PD patients vs. HC HC demonstrated greater activation during both reappraisal and maintenance of negative emotions then GAD patients in DMPFC, bilateral dorsolateral and ventrolateral PFC, and dorsal ACC |
| Andreescu93 | GAD-old = 15, 67.4±6.52 GAD-young = 9, 31.67±10.06 HC-old = 21, 70.76±7.33 HC-young = 10, 33.6±10.49 |
GAD-old = 11/4 GAD-young = 8/1 HC-old = 12/9 HC-young = 6/4 |
PD = 4 SP = 1 MDD past = 5 |
No current psychotropic drug use | 3 T rs-fMRI | Greater anxiety effect on connectivity between PCC and MPFC in older GAD patients vs. young subjects Positive correlation between duration of illness and greater connectivity between PCC and insula |
| Andreescu94 | Elderly GAD = 28, 64±6.75 HC = 31, 69±12.50 |
GAD = 17/11 HC = 19/12 |
SP = 1 PD = 2 PTSD = 1 |
No current psychotropic drug use | 3 T rs-fMRI | Higher connectivity in GAD patients in the DLPFC and several prefrontal regions during worry reappraisal, after 12 weeks of treatment vs. HC |
| Fonzo95 | GAD = 21, 34.29±11.27 HC = 12, 27.58±3.00 |
GAD = 16/5 HC = 7/5 |
Anxiety or mood disorder comorbidity permitted for GAD participants | No current psychotropic drug use | 3 T fMRI Task: facial emotion processing task | Pre-treatment: GAD patients showed blunted responses in amygdala, insula, and ACC to the happy faces and greater amygdalo-insular connectivity vs. HC Post-treatment: CBT attenuated amygdalar and subgenual ACC activation by fear/angry faces and heightened insular responses to the happy face comparison condition, but no apparent effects on connectivity in GAD patients vs. HC |
| Cha96 | GAD = 32, 22.3±5.14 HC = 25, 21.3±4.56 |
GAD = 32/0 HC = 25/0 |
MDD = 17 | No current psychotropic drug use | 3 T fMRI Task: fear generalization task | Higher and less discriminating VTA reactivity to generalized stimuli in GAD patients vs. HC Increased connectivity in VTA-nucleus accumbens in GAD patients vs. HC |
| Cha54 | GAD = 32, 22.3±5.14 HC = 25, 21.3±4.56 |
GAD = 32/0 HC = 25/0 |
MDD = 17 | No current psychotropic drug use | 3 T fMRI, DTI, and MRI Task: fear generalization task | GAD patients with thicker VMPFC display more discriminate VMPFC reactivity during the fear task vs. HC Increased connectivity VMPFC in GAD vs. HC |
| Brown97 Double-blind design | GAD = 30 BDZ = 19 PLC = 111 8-64 years |
BDZ = 9/10 PLC = 9/2 |
No other current psychiatric disorders | No current psychotropic drug use | 3 T fMRI Task: EFMT and affective STIMEX were performed at baseline, 1 hour after initial drug administration and 28 days later | Activation of amygdala during EFMT in GAD patients vs. HC Decreased activation of amygdala after treatment with BDZ in GAD patients vs. HC Significant treatment differences in brain activity during the STIMEX on day 28 in frontal lobe, caudate nucleus, middle temporal gyrus, secondary visual cortex, and SMG in GAD patients vs. HC |
| Liu98 | GAD = 26, 15.54±1.53 HC = 20, 15.55±1.67 |
GAD = 16/10 HC = 11/9 |
No other current psychiatric disorders | No current psychotropic drug use | 3 T rs-fMRI | Decreased functional connectivity between left amygdala and left DLPFC in GAD patients vs. HC Increased functional connectivity between right amygdala and right posterior and anterior lobes of the cerebellum, insula, STG, and putamen in GAD patients vs. HC |
| Makovac99 | GAD = 19, 29.58±6.93 HC = 21, 28.67±9.45 |
GAD = 17/2 HC = 18/3 |
No other current psychiatric disorders | No current psychotropic drug use | 1.5 T fMRI resting-state + task Task = visuomotor tracking task | Before induction: lower connectivity between right amygdala and right SFG, right paracingulate ACC, and right SMG and decreased connectivity between VLPFC and amygdala in GAD patients vs. HC After induction: connectivity increased in GAD patients but decreased in HC |
| Moon55 | GAD = 17, 37.4±11.3 HC = 17, 35.6±6.1 |
GAD = 6/11 HC = 6/11 |
All patients had primary GAD with mild depression | Eleven patients had prescriptions for multiple psychiatric medications, including anxiolytics (buspirone [n=5/; alprazolam [n=2]; lorazepam [n=1]; tofisopam [n=2]) and/or antidepressants (escitalopram [n=9]; bupropion [n=1], fluvoxamine [n=1], duloxetine [n=2], mirtazapine [n=1], paroxetine [n=3]). | 3 T MRI + fMRI Task: explicit verbal memory tasks with emotionally neutral and anxiety-inducing words | In response to neutral words, GAD patients showed lower activity in hippocampus, middle cingulate gyrus, putamen, and head of caudate nucleus vs. HC In response to anxiety-inducing words, GAD patients showed higher activities in VLPFC and precentral gyrus vs. HC Significant reduction in GM volumes, especially in the hippocampus midbrain, thalamus, insula, and STG in GAD patients vs. HC |
| Park100 | GAD = 15, 36.4±11.2 No control group |
GAD = 7/8 | No other current psychiatric disorders | Fourteen patients had prescriptions for multiple psychiatric medications, including antidepressants (escitalopram [n=8]; paroxetine [n=2], bupropion [n=1]; fluvoxamine [n=1]; duloxetine [n=1]; mirtazapine [n=1]) and/or anxiolytics (buspirone [n=6], alprazolam [n=5], zolpidem [n=1]). | 3 T fMRI Task: explicit verbal memory tasks with emotionally neutral and anxiety-inducing words | Increased activation with anxiety-provoking pictures vs. neutral pictures in: VLPFC, middle temporal gyrus, inferior occipital gyrus, inferior temporal gyrus, middle occipital gyrus, hippocampus, PHG, FuG, DLPFC, STG amygdala, cerebellar cortex, LiG, PCu, SPG |
| Buff101 | GAD = 20, 28.10±8.73 PD = 21, 27.10±6.40 SAD = 21, 29.86±8.12 HC = 21, 27.33±3.84 |
GAD = 16/4 PD = 17/4 SAD = 17/4 HC = 17/4 |
No other current psychiatric disorders | Six to seven patients per group took long-term medication (antidepressive medication, one GAD patient used Pregabalin) and had been stabilized on such medication for at least 4 weeks prior to study participation. | 3 T fMRI Task: explicit verbal memory tasks with emotionally neutral and anxiety-inducing words | Elevated activity with threat vs. neutral in cingulate cortex, dorsal anterior insula/frontal operculum, and posterior DLPFC in GAD patients vs. other groups Increased functional connectivity between posterior DLPFC and VLPFC, between cingulate cortex and amygdala, between cingulate cortex and anterior insula in GAD patients vs. other groups |
| Cui102 | GAD = 21, 39.95±12.24 PD = 18, 37.17±11.31 HC = 22, 38.05±10.32 |
GAD = 7/13 PD = 6/12 HC = 8/14 |
No other current psychiatric disorders | No current psychotropic drug use | 3 T rs-fMRI | Increased functional connectivity between hippocampus/parahippocampus and FuG in GAD patients vs. PD patients and HC |
| Kujawa103 * | GAD+SAD+GSAD = 4 17-19 years | Not specified | Participants with secondary comorbidity were included: 70.7% of the sample had current diagnoses of GAD, 58.5% GSAD, and 17.1% SAD, 7.3% had comorbid PD, 9.8% OCD, 24.4% specific phobia, 2.4% PTSD, 2.4% depression, and 12.2% ADHD | No current psychotropic drug use | 3 T fMRI Task: flanker task to elicit error-related negativity Twelve weeks of SSRI or 18 sessions (max) of CBT | Greater activation of inferior and SFG, including DLPFC and VLPFC, precentral/postcentral gyri during processing of threating faces predicted greater response to CBT and SSRI treatment |
| Makovac104 | GAD = 16, 29.62±7.51 HC = 16, 28.12±10.11 |
GAD = 14/2 HC = 13/3 |
No other current psychiatric disorders | Two GAD patients using long-term medications (one citalopram, one pregabalin) at both sessions of the study. All other patients and controls were medication free. | 1.5 T fMRI resting-state + task Task = visuomotor tracking task | After induction: reduction in connectivity between right amygdala and VMPFC in GAD patients vs. HC At follow-up: enhanced coupling between left amygdala and VTA Amplified physiological response to induction predicted increased connectivity between right amygdala and thalamus in GAD patients vs. HC |
| Wang105 | GAD = 28, 32.93±4.13 HC = 28, 33.21±5.25 |
GAD = 14/14 GAD = 14/14 |
No other current psychiatric disorders | No current psychotropic drug use | 3 T rs-fMRI |
Higher amplitude of low frequency fluctuations in bilateral DMPFC and DLPFC, as well as left PCu/PCC in GAD patients vs. HC Lower connectivity in prefrontal gyrus, in prefrontal-limbic and cingulate and higher prefrontal-hippocampus resting-state functional connectivity was correlated with symptom severity in GAD patients |
| Moon & Jeong56 | GAD = 17, 38.1±10.4 HC = 17, 36.9±7.7 |
GAD = 9/8 HC = 9/8 |
All patients had primary GAD with mild depression | Eleven patients had prescriptions for multiple psychiatric medications, including anxiolytics (buspirone [n=4]; alprazolam [n=1]; lorazepam [n=2]; tofisopam [n=2]) and/or antidepressants (escitalopram [n=9]; bupropion [n=1]; fluvoxamine [n=2]; duloxetine [n=1]; mirtazapine [n=1]; paroxetine [n=3]), and six patients used one psy-qj;chiatric medication each. | 3 T MRI and fMRI Task: verbal working memory task with emotionally neutral and anxiety-inducing words |
Lower activity in FuG, SPG, PCu, SOG, LiG, Cun, CaC, PHG, and Cb in GAD patients vs. HC In response to anxiety-inducing distractors, higher activity in hippocampus and lower activities in DLPFC, FuG, SPG, PCu, SOG, and Cb in GAD patients vs. HC |
| Moon106 | GAD = 14, 36.6±8.8 HC = 14, 37.8±7.8 |
GAD = 6/8 HC = 6/8 |
All patients had primary GAD with mild depression | Eleven patients had prescriptions for multiple psychiatric medications including anxiolytics (buspirone [n=7]; alprazolam [n=3]; tofisopam [n=1]) and/or antidepressants (escitalopram [n=10]; bupropion [n=3]; fluvoxamine [n=2]; duloxetine [n=1]) and three patients used a single psychiatric medication. | 3 T fMRI Task: working memory task with emotion- inducing distractors | In response to emotional distractors higher activity in the hippocampus and lower activities in the SOG, SPG, DLPFC, and precentral gyrus was found in GAD patients vs. HC |
| Cha57 | GAD + GAD/MDD = 32 GAD = 15, 22.1±1.20 GAD/MDD = 17, 22.5±1.12 HC = 25, 21.5±1.07 |
GAD = 32/0 HC = 25/0 |
MDD = 17 | No current psychotropic drug use | 3 T MRI, DTI and fMRI (multimodal MRI) Task: threat-associative learning task | Decreased activity in left anterior hippocampus to cue repetition in GAD patients (with or without comorbidity with MDD) during a threat-associative learning task |
| Burkhouse107 | GAD + GSAD = 37 (7-19 age) SSRI = 21, 14.43±2.87 CBT = 16, 14.50±3.03 |
GAD + GSAD = 22/15 SSRI = 11/10 CBR = 11/5 |
10.8% SAD; 10.8% PD; Specific phobia 18.9%, MDD 5.4%, ADHD 16.2% | No current psychotropic drug use | 3 T fMRI Task: emotional faces shifting attention tasksTen weeks (minimum) of SSRI or CBT | Reduced activation in SFG, ACC, and DMPFC during implicit processing of emotional faces after treatment (both CBT and SSRI) |
| Meeten108 | GAD = 19, 29.58±6.93 HC = 21, 28.67±9.45 |
GAD = 17/2 HC = 18/3 |
Patients had primary GAD with mild depression | Two GAD patients with long-term medication (one citalopram, one pregabalin) | 3 T rs-fMRI | Increased connectivity between right amygdala and brainstream in GAD patients vs. HC |
| Moon109 | GAD = 15, 37.6±11.9 HC = 15, 35.4±5.6 |
GAD = 5/10 HC = 5/10 |
All patients had primary GAD with mild depression | Eight patients had prescriptions for multiple psychiatric medications, including anxiolytics (buspirone [n=2]; alprazolam [n=2]; lorazepam [n=1]; and tofisopam [n=1]) and/or antidepressants (escitalopram [n=7]; bupropion [n=1]; fluvoxamine [n=1]; duloxetine [n=1]; mirtazapine [n=1]; and paroxetine [n=3]). Seven patients were taking one psychotropic medication. | 3 T fMRI Task: explicit memory task with neutral and anxiety-inducing words |
Decreased activity in putamen, head of the caudate nucleus, hippocampus, and middle cingulate gyrus during the memory tasks with the neutral and anxiety-inducing words in GAD patients vs. HC Increased activity in VLPFC and precentral gyrus only in the memory task with the anxiety-inducing words in GAD patients vs. HC |
| Li110 | GAD = 21, 39.90±12.24 HC = 22, 38.05±10.32 |
GAD = 7/13 HC = 8/14 |
No other current psychiatric disorders | No current psychotropic drug use | 3 T rs-fMRI | Increased functional connectivity between left amygdala and temporal pole in GAD patients vs. HC In both eyes-open and eyes-closed conditions, the brain regions showed altered FC with amygdala/DLPFC in GAD patients vs. HC |
| Karim111 | GAD = 17, 64±6 HC = 20, 67.5±8.5 |
GAD = 10/7 HC = 10/10 |
Patients with other anxiety disorders were included if GAD was the principal diagnosis | No current psychotropic drug use | 3 T fMRI Task: facial emotion processing task |
Positive association between the faces > shapes and global anxiety in PHG, precuneus, and superior occipital gyrus Negative association between the faces > shapes and worry severity in precuneus |
| Diwadkar112 | GAD = 10, 41.65±12.47 HC = 10, 44.10±15.33 |
GAD = 6/4 HC = 7/3 |
No other current psychiatric disorders | Seven patients receiving antidepressants at the time of MRI (three on venlafaxine, one on amitriptyline, three on SSRIs: sertraline, citalopram, and escitalopram). Five patients were taking benzodiazepines, two patients were medication free. | 3 T fMRI Task: explicit verbal memory tasks with emotionally neutral and anxiety-inducing words | Hypoactivation in dorsal ACC, VLPFC, and cerebellum, more pronounced during suppression than retrieval memories in GAD patients vs. HC |
| Ellard113 | GAD = 21, 29.48±8.44 No control group |
GAD = 21/0 | No other current psychiatric disorders | No current psychotropic drug use | 3 T fMRI Task: worry or emotion suppression | Emotion acceptance resulted in lower ratings of distress than worry and was associated with increased dorsal ACC activation and increased VLPFC-amygdala functional connectivity Worry showed greater distress ratings than acceptance or suppression and was associated with increased PCu, VLPFC, amygdala, and hippocampal activation |
| Fitzgerald114 | GAD = 30, 27.20±7.57 HC = 30, 25.43±10.02 |
GAD = 18/12 HC = 23/7 |
GAD = 13 MDD = 6 PD = 3 Specific phobia =3 PTSD = 2 OCD = 2 |
No current psychotropic drug use | 3 T fMRI Task: negative emotion processing task | GAD patients exhibited over-engagement of amygdala and frontal regions during the viewing of negative images vs. HC |
| MacNamara115 | Patients = 142, 25.9±6.9 GAD = 20 SAD = 79 MDD = 43 HC = 57, 27.5± 9.8 |
HC = 40/17 Patients = 104/38 No other specifications |
No other current psychiatric disorders | No current psychotropic drug use | 3 T fMRI Task: facial emotion processing task | Anxiety symptom scores were associated with increased anger>shape activation in bilateral insula, anterior/midcingulate, and DLPFC, while depressive symptom scores were associated with reduced DLPFC activation for Angry > Shapes across the three disorders |
| Carlson116 | GAD = 17, 23±4.47 MDD = 15, 26±9.08 GAD/MDD = 15, 21±2.63 HC = 13, 20±1.07 |
GAD = 17/0 MDD = 15/0 GAD/MDD = 15/0 HC = 13/0 |
MDD = 15 | No current psychotropic drug use | 3 T fMRI Task: entire pilot episode of ABC's TV- series Lost | Decreased activity of amygdala in GAD and MDD patients vs. HC Higher coupling with negative valence in PCC, DMPFC, PCu in HC vs. GAD patients and MDD |
| Blair117 | GAD = 18, 31.0±7.78 SAD = 18, 32.4±9.48 HC 18, 31.9 ±7.78 |
GAD = 8/10 SAD = 13/5 HC = 10/8 |
No other current psychiatric disorders | No current psychotropic drugs | 1.5 T fMRI Task: fear generalization and positive tasks | Reduced mPFC in GAD patients vs. HC and SAD patients Increased activity for low relative to high positive and negative impact events in rostral mPFC, right IFG/anterior insula, bilateral frontal cortex in GAD patients vs. HC |
| White118 | GAD = 46, 30.78±9.69 HC = 32, 28.85±9.69 |
GAD = 35/11 HC = 22/10 |
GSAD = 18 | No current psychotropic drug use | 3 T fMRI Task: passive avoidance task |
Reduced correlation between prediction error within VMPFC, ventral striatum, dACC/DMPCF, anterior insular cortex, and PCC in GAD patients vs. HC Lower correlations within lentiform nucleus/putamen in GAD patients vs. HC |
| Mohlman119 | GAD = 20, 67.85±4.55 HC = 16, 67.38±5.46 |
GAD = 75/25% HC = 69/31% |
No other current psychiatric disorders | Three participants (two in the GAD group and one in the control group) were taking medication for sleep problems. | 3 T fMRI Task: neutral and worry tasks |
Activation in frontal regions, amygdala, and insula in GAD patients vs. HC Effective connectivity analysis in GAD from DLPFC, VMPFC until amygdala in GAD patients vs. HC |
| Buff120 | GAD = 19, 28.2±8.9 HC = 19, 27.6±8.3 |
GAD = 14/5 HC = 14/5 |
All patients had primary GAD with mild depression | No current psychotropic drugs | 3 T fMRI Task: disorder-related vs. neutral scripts | Increased activation in amygdala, DMPFC, VLPFC, and thalamus in GAD patients vs. HC Reduced activation in VMPFC/ subgenual ACC in GAD vs. HC |
| Rabany121 | GAD = 10, 41.90±5.1 GSAD = 8, 33.0±3.5 HC = 19, 40.47±3.5 |
GAD = 2/8 GSAD = 3/5 HC = 6/13 |
No other current psychiatric disorders | No current psychotropic drug use | 3 T rs-fMRI | Altered connectivity between amygdala and all regions of the default mode network and salience network in GAD patients vs. HC |
| Yin122 | GAD = 20, 15.7±1.7 HC = 14, 15.5±1.7 |
GAD = 15/5 HC = 8/6 |
Specific phobia = 4 SP = 3 Agoraphobia = 3 PD = 2 SAD = 2 Oppositional defiant = 1 | No current psychotropic drug use | 3 T fMRI Task = emotional valence-evaluation tasks |
Evaluation of negative vs. neutral stimuli: increased activation of bilateral amygdala in GAD patients; reduced activation of right IFG in GAD patients vs. HC; magnitude of IFG activity negatively correlated with symptom severity Psychophysiological interaction analysis: decreased functional interaction in right IFG, ACC, and VMPFC in GAD vs. HC |
| Qiao123 | GAD = 20, 41.5±10.7 HC = 20, 40.1±9.8 |
GAD = 13/7 HC = 13/7 |
No other current psychiatric disorders | No current psychotropic drug use | 3 T rs-fMRI |
Increased functional connectivity in amygdala, insula, putamen, thalamus, and PCC in GAD patients vs. HC Decreased functional connectivity in frontal and temporal cortex in GAD patients vs. HC |
| Xia124 | GAD = 31, 36.87±9.16 HC = 36, 39.53±8.83 |
GAD = 16/15 HC = 23/13 |
No other current psychiatric disorders | No current psychotropic drugs | 3 T rs-fMRI | Decreased ReHo values in right orbital middle frontal gyrus, left ACC, right middle frontal gyrus and supplementary motor areas as well as decreased ReHo values in left middle temporal gyrus, left superior temporal gyrus, and right superior occipital gyrus in GAD patients vs. HC |
| Buff125 | GAD = 19, 28.26±8.93 HC = 19, 28± 5.28 |
GAD = 15/4 HC = 15/4 |
MDD = 3 PTSD = 1 Specific phobia =1eating disorder=1 | Seven GAD patients took long-term medication | 3 T fMRI Task: fear conditioning tasks | Increased activation of amygdala and BNST during threat anticipation in GAD patients vs. HC |
| Karim126 | GAD = 20, 67 HC = 20, 68 |
GAD = 13/7 HC = 10/10 |
Patients with other anxiety disorders or unipolar depression were included if GAD was the principal diagnosis, but participants with current MDD at the time of scanning were excluded. Patients with a past history of alcohol or substance abuse who were in full remission for at least 3 months were included. | No current psychotropic drug use | 3 T fMRI Task: worry induction task |
Increased activation of visual cortex, thalamus, caudate, and medial frontal cortex in GAD patients vs. HC Decreased activation during reappraisal in supplemental motor area, middle cingulate gyrus, insula, and putamen in GAD patients vs. HC |
| Burkhouse127 * | GAD+SAD+ GSAD = 25, 15.28±2.65 HC = 25, 15.92±2.81 |
GAD+SAD+ GSAD = 16/9 HC = 17/8 |
No other current psychiatric disorders | No current psychotropic drug use |
3 T fMRI Task: facial emotion processing task Between scans, anxious youth were treated with SSRI or CBT |
Pre-treatment: Increased activation in rostral ACC when matching shapes in the context of threat distractors in the anxious group vs HC. Post-treatment (SSRI or CBT): activation in the rostral ACC increased in the anxious group but not in HC |
ACC = anterior cingulate cortex; AD = antidepressive medication; ADHD = attention deficit and hyperactivity disorder; BDZ = benzodiazepines; BLA = basolateral amygdala; BNST = bed nucleus of the stria terminalis; CaC = calcarine cortex; Cb = cerebellar cortex; CBT = cognitive-behavioral therapy; CMA = centromedial amygdala; Cun = cuneus; dACC = dorsal anterior cingulate cortex; DLPFC = dorsolateral prefrontal cortex; DMPFC = dorsomedial prefrontal cortex; EER = explicit emotion regulation; EFMT = emotion face matching task; F = female; fMRI = functional magnetic resonance imaging; FuG = fusiform gyrus; GAD = generalized anxiety disorder; GSAD = generalized social anxiety disorder; HC = healthy control; IFG = inferior frontal gyrus; LiG = lingual gyrus; M = male; MBSR = mindfulness based stress reduction; MDD = major depressive disorder; MPFC = medial prefrontal cortex; MRI = magnetic resonance imaging; n = patients; OCD = obsessive-compulsive disorder; PCC = posterior cingulate cortex; PCu = precuneus; PD = panic disorder; PFC = prefrontal cortex; pgACC = pregenual anterior cingulate cortex; PHG = parahippocampal gyrus; PLC = placebo; PTSD = posttraumatic stress disorder; ReHo= regional homogeneity; rs-fMRI = resting-state fMRI; SAD = separation anxiety disorder; SD = standard deviation; SFG = superior frontal gyrus; SME = stress management education; SMG = supramarginal gyrus; SOG = superior occipital gyrus; SP = social phobia; SPG = superior parietal gyrus; SSRI = selective serotonin reuptake inhibitors; STG = superior temporal gyrus; STIMEX = stimulus expectancy task; TAC = top-down attentional control; VLPFC = ventrolateral prefrontal cortex; VMPFC = ventromedial prefrontal cortex; vPFC = ventral prefrontal cortex; VTA = ventral tegmental area.
In this study patients with different diagnoses were considered as a whole group.
Emotion regulation deficits in GAD
Emotion dysregulation, a common symptom of GAD, consists of two separate, yet related, abnormalities: atypical emotional reactivity (ER) and dysregulation of reactivity.128
Specifically, it has been reported that patients with GAD a) often experience emotions with heightened intensity compared to individuals without GAD; b) have marked difficulties identifying, describing, and clarifying their emotional experiences; and c) are prone to greater negative reactivity to emotions by holding catastrophic beliefs about the consequences of both negative and positive emotions.129
Therefore, in recent years, fMRI studies have been carried out to investigate neural activation deficits in GAD while processing emotional tasks. An fMRI study by Guyer et al.83 carried out on pediatric patients with social phobia and GAD during a monetary incentive delay task reported an hyperactivation of the putamen in response to positive-valenced stimuli in GAD patients compared to HCs and patients with social phobia. Moreover, Cha et al.96 also described alteration of the nucleus accumbens together with high reactivity of the ventral tegmental area and mesocorticolimbic system in patients with GAD compared to HCs during a fear generalization task.
Interestingly, Krain et al.76 explored the intolerance of uncertainty (IU), a trait associated with worry, in pediatric GAD patients, and showed that high levels of IU were associated with increased activation in frontal and limbic regions in these patients compared to HCs. This further suggests that an altered emotion regulation strategy is a key disability characterizing GAD.
Furthermore, an exaggerated right amygdala response to fearful faces was reported in three small fMRI studies in children with GAD.72,75,77 Similarly, a recent study carried out by Buff et al.125 showed a link between hyperactivation of the amygdala and bed nucleus of the stria terminalis and the mechanism of threat anticipation in GAD patients, ultimately underlining the importance of these two structures in the regulation of anticipatory anxiety. Interestingly, another study from the same research group120 further reported hyperactivation of the amygdala, along with DMPFC, ventrolateral PFC (VLPFC), and thalamus, as well as reduced activity in ventromedial PFC (VMPFC) during an ER task in GAD patients compared to HCs.
In contrast, fMRI studies have also demonstrated decreased amygdala activation in the presence of negative stimuli in GAD patients compared with HCs.87,89
Additionally, Greenberg et al.130 showed decreased recruitment of VMPFC during a fear conditioned stimulus in adult GAD patients compared to HCs. Similarly, Cha et al.54 found that VMPFC thickness, functional and structural connectivity within the corticolimbic system predicted individual variability of VMPFC threat assessment in an independent fashion. This is not surprising, especially because VMPFC has been shown to be involved in the regulation and inhibition of fear response in other anxiety disorders, such as PTSD and phobias.131
Moreover, increased activation has been observed in the left medial PFC and right VLPFC in response to emotional images in a juvenile GAD population.88 Right VLPFC activity was also examined by Monk et al.74 in a population of young people with GAD in response to angry stimuli. The authors describe increased VLPFC activation during trials containing angry faces in GAD patients compared to HCs as well as an inverse association between VLPFC activation and the severity of anxiety symptoms, ultimately suggesting that VLPFC activation may serve as a compensatory response.
In other studies, GAD patients had increased activation in prefrontal regions, including the DMPFC and ACC, in response to worry and neutral stimuli,80 as well as in a lateral region of the middle frontal gyrus in response to angry expressions.78 Hyperactivity in prefrontal regions was further confirmed by more recent studies in which GAD patients had elevated activity specifically in response to threat vs. neutral pictures in cingulate cortex, dorsal anterior insula/frontal operculum, and posterior DLPFC.101 Also, anxiety symptom scores were associated with increased angry > shapes activation in the bilateral insula, anterior/midcingulate, and DLPFC compared to HCs.115 In contrast, Yin et al.122 found a significant reduction of inferior frontal gyrus activity in GAD patients, which was also negatively correlated with symptom severity.
Furthermore, altered activation and dysfunctional connectivity in and between selective brain regions, including amygdala, DLPFC, cingulate, insula, posterior parietal cortex, pregenual ACC (pgACC), and cerebellum, were consistently found in adolescents and adults with GAD,15,81,84,90,95,122 especially during tasks involved in self-referential processing132,133 and mentalization,134 abilities often found altered in GAD.135 Similarly, a recent study exploring brain coupling within regions of the default-mode network (DMN), including ACC and DMPFC, showed that in GAD patients affective numbing was associated with weaker coupling between these regions, with decreased amygdala activity.116
Fitzgerald et al.114 demonstrated that emotion regulation disturbances in GAD involve excessive reactivity to negative stimuli and a failure to effectively downregulate negative affective states. The authors reported that GAD patients engaged the left amygdala to a greater extent while viewing negative images, which suggests that these patients are more responsive to negative stimuli. Further, Ellard et al.,113 by exploring the mechanisms of emotion acceptance as an alternate emotion regulation strategy to worry or emotion suppression in GAD, found that emotion acceptance resulted in lower ratings of distress than worry and was associated with increased dorsal ACC activation and increased VLPFC-amygdala functional connectivity. Interestingly, two studies also reported that worry was associated with hyperactivation of amygdala, in both adult and elderly GAD patients,108,119 as well as of insula and frontal regions, only in elderly GAD patients,119 compared to HCs. Similarly, Karim et al.126 showed that worry induction was associated with increased activation in middle and superior frontal gyrus as well as in the visual and parietal cortices compared to non-anxious patients. These results seemed to be partially in contrast with a previous study showing the lack of involvement of PFC in suppressing worry in elderly GAD patients86 and in women with GAD.85 Additionally, an fMRI study carried out by Karim et al,.111 exploring ER in a group of elderly GAD patients, showed a positive association between ER and global anxiety in the left parahippocampus, left and right precuneus, and right superior occipital gyrus, as well as a negative association between ER and worry severity in precuneus bilaterally. Finally, Blair et al.117 showed that GAD patients had significantly reduced neural modulation in medial PFC and caudate during the processing of positive events, as well as increased neural responses to low-impact events in rostral medial PFC.
In conclusion, the abovementioned findings suggest that the biological signature of GAD might be related to deficits in brain regions within the emotional processing network, which may ultimately result in increased threat sensitivity paralleled by maladaptive appraisal and exaggerated attention allocation, presumably resulting in over-interpretation and overestimation of threat.
The impact of emotion on cognition in GAD
In recent years, some studies have investigated the effects of emotion on cognition by means of specific fMRI tasks. Moon et al.109 explored functional neural activity in GAD patients using an explicit emotional verbal memory task with neutral and anxiety-inducing words. The authors showed that patients with GAD had significantly decreased activity in limbic regions (hippocampus and middle cingulate gyrus) and basal ganglia (i.e., the putamen and head of the caudate nucleus) during processing of both neutral and anxiety-inducing words, as well as increased activity in VLPFC and precentral gyrus during processing of anxiety-inducing words. Interestingly, these results only partially confirmed the evidence reported by a previous study by the same group, which employed a similar fMRI task.106 Indeed, in this study, the authors reported higher activity in the hippocampus and lower activity in the superior occipital gyrus, superior parietal gyrus, DLPFC, and precentral gyrus in GAD patients vs. HCs in response to the emotional distractors in a working memory task.
Similarly, Park et al.100 found impaired performance in a working memory task during emotional distracters in GAD patients, reporting greater activation in brain regions responsible for the maintenance of goal relevant information, including DLPFC, VLPFC, amygdala, and hippocampus, in these patients compared to HCs.
Moreover, Ball et al.18 explored the cognitive modulation of emotion in GAD patients by employing a task that required them to reappraise or maintain emotional responses to negative images. The authors showed that GAD patients had less PFC activation than HCs, and those with the least PFC activation reported the greatest anxiety severity and impairment, confirming a potential common neural basis of emotion dysregulation in anxiety disorders. Furthermore, Cha et al.57 found that during a threat-associative learning task GAD patients had a significant decrease in left anterior hippocampus with cue repetition compared to HCs. Interestingly, a recent fMRI study carried out by White et al.118 also showed the presence of selective deficits in reinforcement-based decision-making in GAD patients compared to HCs. Indeed, the authors reported that patients with GAD had a reduced correlation between reinforcement prediction error and activity within the VMPFC, the ventral striatum, and other structures involved in decision-making.
Finally, Diwadkar et al.112 found that GAD patients have selective impairments in the suppression of aversive memories through memory control. Specifically, the authors showed that when asked to suppress or retrieve memories, GAD patients had hypoactivation in a large network of brain regions, especially in the dorsal ACC, the ventral PFC and the cerebellum, compared to HCs.
In conclusion, although several studies demonstrated the impact of emotion on cognition in GAD patients, a general consensus of the brain regions involved in the interaction between emotional regulation and cognitive function in this disorder has not been reached yet. Indeed, a mixture of hypo- and hyper-activations in selective subcortical and cortical areas has been observed. However, overall these studies reported the involvement of amygdala and hippocampus, two interacting brain regions consistently found to be involved in emotional and cognitive processing.136 Additionally, from the abovementioned studies also emerged selective deficits in regions within the PFC, including the DLPFC and VLPFC, areas found to be a part of the neurobiological models of both emotion and cognition.137 Therefore, overall, this evidence suggests that GAD patients have selective deficits in key regions of two well-known interactive systems controlling affective and cognitive processing, the dorsal executive and the ventral emotional control systems.138
Resting-state fMRI alterations in GAD
Resting-state fMRI (rs-fMRI) is a sound approach to study neuropsychiatric disorders.139 Specifically for GAD, a recent rs-fMRI study showed the presence of aberrant connectivity between fusiform gyrus and hippocampus/parahippocampus in GAD patients.102 Also, the authors reported a positive correlation between abnormal cuneus/postcentral gyrus and precentral gyrus-calcarine cortex connections and symptom severity. Similarly, another rs-fMRI study carried out by Wang et al.105 showed that GAD patients have 1) higher amplitude of low-frequency fluctuation in the bilateral DMPFC, bilateral DLPFC, and left precuneus/posterior cingulate cortex; 2) lower connectivity in prefrontal gyrus; and 3) abnormal seed-based resting-state functional connectivity within prefrontal-limbic and cingulate regions coupled with decreased connectivity in prefrontal gyrus. Notably, the authors reported that decreased prefrontal-limbic and cingulate connectivity and increased prefrontal-hippocampal connectivity were correlated with clinical symptoms. Andreescu et al.93 investigated the resting-state functional connectivity patterns in the DMN in a population of adult and young GAD patients and showed an anxiety effect on the functional connectivity between the posterior cingulate and the medial PFC for the older group relative to the younger participants. Interestingly, the authors also reported that longer duration of illness was positively correlated with greater functional connectivity between the posterior cingulate cortex and insula, whereas worry severity was inversely correlated with the functional connectivity between the posterior cingulate cortex and the medial PFC. Also, Qiao et al.123 highlighted stronger functional connectivity in many key regions, including the amygdala and poster cingulate cortex, as well as weaker connectivity in frontal and temporal cortex compared with controls. Furthermore, the connectivity between the amygdala and all regions of the DMN and salience networks has been the subject of a recent investigation which highlighted that, in GAD patients, deficits in emotional regulation were associated with altered connectivity between the amygdala and both these networks.121 Similarly, Liu et al.98 also found decreased functional connectivity between the left amygdala and left DLPFC, as well as increased functional connectivity between the right amygdala and right posterior and anterior lobes of the cerebellum, insula, STG, and putamen. Li et al.110 reported increased functional connectivity between the amygdala and the temporal pole, as well as decreased connectivity between the amygdala and DLPFC in GAD patients compared to HCs. In contrast, preserved connectivity was found between posterior hippocampus and regions within the DMN in adult GAD patients.91 Furthermore, Xia et al.124 showed that GAD patients had decreased regional homogeneity (ReHo) in middle frontal gyrus, ACC, and supplementary motor areas, as well as increased ReHo in temporal and occipital cortices. Finally, Makovac et al. carried out two studies99,104 demonstrating that GAD subjects have lower connectivity between the right amygdala and right superior frontal gyrus, right paracingulate/ACC, and right supramarginal gyrus compared with HCs.
In conclusion, overall these findings suggest that GAD is characterized by widely disturbed network connectivity, mainly between the amygdala and other brain regions. This supports the hypothesis of a connectivity-based neural disorder, which may ultimately explain some of the symptoms observed in GAD patients, including decreased spontaneity, initiative, insight, judgment, abstraction, perseverance, and response inhibition.
fMRI alteration in association with drug use in GAD
In the past decades, several randomized controlled studies have demonstrated that symptoms of anxiety respond better to antidepressants with relevant serotonin reuptake inhibitory properties, such as imipramine,140 venlafaxine,141 paroxetine,142 duloxetine,143 and sertraline127 compared to benzodiazepines. However, neuro-functional changes underlying the effects of anti-anxiety treatments have not been fully characterized, although they would help to understand the neural basis involved in anxiety symptoms. In this regard, fMRI studies have become essential to accurately observe and detail the pharmacological effects of therapies in GAD.
The first fMRI study exploring the effect of a pharmacological treatment in GAD patients was carried out by Hoehn-Saric et al.,73 who employed an auditory task with statements describing a personal worry. Specifically, the authors demonstrated that treatment with citalopram reduced anxiety, psychic, and somatic symptoms. Additionally, they also reported that after treatment, worry sentences elicited reduced responses in prefrontal regions, striatum, insula, and paralimbic regions. A similar approach was employed by Whalen et al.,69 who explored the effect of venlafaxine on brain activity in GAD patients while performing an emotional processing task. The authors suggested that the magnitude of the treatment response was predicted by higher pre-treatment reactivity to fearful faces in rostral ACC and lower reactivity in the amygdala. Similarly, greater activation in the right VLPFC was also seen after psychotherapy and fluoxetine treatment.82,92 Nitschke et al.79 showed exaggerated amygdala and rostral ACC activation during the anticipation of aversive stimuli in GAD patients prior to treatment with venlafaxine. Interestingly, the authors also reported that greater rostral ACC was also associated with a more favorable response to venlafaxine. Moreover, Andreescu et al.94 explored the functional connectivity in the salience network and the executive control network during worry induction and worry reappraisal in GAD patients before and after pharmacological treatment. The authors observed that patients with GAD, after 12 weeks of treatment with drug therapy, showed greater connectivity between the DLPFC and several prefrontal regions during worry reappraisal compared to pre-treatment.
Interestingly, Kujawa et al.103 reported that greater activation in prefrontal regions, involved in appraising and regulating responses to social signals of threat, predicts better response to SSRI treatment and cognitive-behavioral therapy (CBT) in anxious youth. This has also been highlighted by a study carried out by Burkhouse et al.,107 who showed that in some patients less recruitment of superior frontal gyrus, encompassing the dorsal ACC and DMPFC, during implicit emotion processing, predicts greater reduction in youth anxiety symptoms after treatment with SSRI. Additionally, a more recent study carried out by the same research group reported that after treatment with sertraline or CBT, GAD patients showed increased activation in rostral ACC, a region responsible for better response to stimuli relevant to the threat.127 Finally, Brown et al.97 investigated the temporal pattern of brain response to emotional stimuli during 28 days of alprazolam (ALZ) treatment among GAD patients. Brain activation in the amygdala, during an emotion face-matching task, and in the insula, during an affective stimulus expectancy task, was reduced 1 hour after ALZ administration, but returned to baseline levels at day 28. These results are consistent with the notion that the neural mechanisms supporting sustained treatment effects of benzodiazepines in GAD differ from those underlying their acute effects. However, reductions in blood oxygen level dependent response in these regions did not persist over the 28 days of ALZ administration despite continued improvement of symptoms.
In conclusion, these findings suggest that neuroimaging investigations may be a useful tool for predicting how GAD patients will respond to treatment. Specifically, they suggest that GAD is amenable to psychopharmacologic treatment and that benzodiazepines are not as effective in controlling psychic symptoms of anxiety as antidepressants such as venlafaxine and paroxetine.141,142
Specifically, from the available evidence, it emerges that threat processing and the underlying neurocircuitry are relevant to treatment response in anxiety. Interestingly, the abovementioned studies investigated either the role of the brain in response to treatment or the effects exerted by specific pharmacological treatments on brain structures. With regard to the first working hypothesis, the available evidence, regardless of the treatment employed, supported the idea that specific brain regions, including the DLPFC and VLPFC, are associated with better treatment response, ultimately suggesting that the identification of key brain structures associated with treatment response may, therefore, allow the development of more targeted and efficient treatments for GAD. Finally, with regard to the second working hypothesis, which explores the effect that some drugs have on brain structures, overall the results suggest that the first line treatments of GAD, e.g., antidepressants, seem to have similar effects on specific brain structures involved in emotional processing, an ability that is consistently shown to be impaired in GAD patients. Please see Figure 2 depicting the neural regions consistently found altered in GAD in structural and functional MRI studies.
Figure 2. Brain regions consistently found to be involved in generalized anxiety disorder from structural and functional MRI studies.
Limitations
It should be noted that most available findings are preliminary and limited by major methodological issues. First, most studies enrolled small groups of patients, often with comorbid depression or other anxiety disorders (i.e., PD or social phobia), which may limit the generalizability of the findings. However, although larger and more homogenous samples of GAD patients would have been beneficial, the exclusion of patients with comorbid diagnoses would have been a difficult challenge, especially because comorbidities frequently occur in GAD. Second, age differences among GAD patients in individual studies also complicate the interpretation of results. However, it is important to point out that the presence of neuroimaging investigations in both adults and pediatric populations is also an important strength, since the combination of results from these age groups could provide important insights on the pathophysiology of GAD. Indeed, overall, neuroimaging studies with adult and pediatric populations reported converging evidence of deficits in emotional processing, mainly due to prefrontal-limbic dysfunctions in GAD patients. Third, many studies used different medications, and pre- and post-treatment evaluation was not always possible. Fourth, most studies had a cross-sectional design and therefore it was not possible to determine whether brain abnormalities are an epiphenomenon of current psychiatric states. Fifth, functional studies were conducted using different activation paradigms (auditory or visual stimuli, emotional conflicts, monetary incentive delay task, decision-making task), therefore producing heterogeneous results. Finally, differences in acquisition equipment (1.5 vs. 3 Tesla scanner) and pre-processing methodologies may explain the high heterogeneity in terms of the results reported by original studies.
Conclusions
To date, several imaging studies have been carried out in GAD. These studies have produced a better understanding of the pathophysiological mechanisms involved in this disorder by suggesting abnormalities in key brain regions within the prefrontal-limbic network, including the DLPFC, ACC, amygdala, and VLPFC, ultimately strengthening a modular model for anxiety symptoms. From this perspective, one should bear in mind that the amygdala, cingulate, and PFC are strictly connected99,104,144,145 and are mainly involved in modulating social processing, recognition of emotional expressions, and fear-related behavior.36,145-149
However, a general consensus on the morpho-functional alterations characterizing GAD has not yet been reached, although hypoactivation of the PFC18,85,113 and hyperactivation of the amygdala98,112,115,149 are among the most consistent findings. These results are not surprising, since these two regions are closely intertwined. Indeed, the PFC regulates emotional distraction and maintains ongoing performance via its modulatory interactions with the amygdala; the PFC is also thought to implement controls that minimize performance disturbances from threat-induced anxiety and target distracting stimuli by modulating activity in regions involved in threat detection, such as the amygdala.92,113,115 This implies that the amygdala-PFC functional connectivity may help maintain performance in the presence of anxiety induced by threat. Therefore, the integrity of this modulation may help maintain the performance when threat-induced anxiety stimuli occur, by minimizing alterations and malfunctions caused by the activation of anxiety circuits. Interestingly, animal models seem to further confirm the importance of these structures in mediating emotional states, including anxiety. Indeed, they consistently suggest an important role played by limbic circuits, and especially the amygdala, in the response to fear.9,12,14
Furthermore, from the abovementioned neuroimaging studies it also emerges that GAD seems to be characterized by abnormalities in selective brain regions, including amygdala, ACC, DLPFC, and VLPFC, within the right hemisphere. Various studies demonstrate a functional lateralization of the amygdala, with the functional link between the right amygdala and PFC being preferentially involved in anxiety.53 For example, Lee et al.150 showed that viewing negative emotional images correlated with greater activation of the right hemisphere than left hemisphere. Right hemisphere involvement was predominantly observed by structural studies, ultimately supporting the hypothesis that right hemisphere alterations may be crucial for the pathophysiology of GAD, even during neurodevelopment.
In conclusion, brain imaging investigations are useful to elucidate in vivo brain correlates of GAD, which may ultimately be beneficial for more targeted pharmacological interventions. However, future cross-sectional and longitudinal neuroimaging studies, coupled with cognitive and genetic investigations, on un-medicated juvenile and adult patients, children at high risk of GAD as well as unaffected family members are warranted to further characterize the link between neural circuitries and the behavioral phenotype.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
PB was partly supported by grants from the Fondazione Cariplo (Bando Ricerca Biomedica sulle Malattie Legate All’invecchiamento, 2015-2018 – AnchorAge project, grant 2014:0664).
Footnotes
How to cite this article: Madonna D, Delvecchio G, Soares JC, Brambilla P. Structural and functional neuroimaging studies in generalized anxiety disorder: a systematic review. Braz J Psychiatry. 2019;41:336-362. http://dx.doi.org/10.1590/1516-4446-2018-0108
References
- 1.American Psychiatric Association . Arlington: American Psychiatric Publishing; 2013. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). [Google Scholar]
- 2.Balestrieri M, Marcon G, Samani F, Marini M, Sessa E, Gelatti U, et al. Mental disorders associated with benzodiazepine use among older primary care attenders--a regional survey. Soc Psychiatry Psychiatr Epidemiol. 2005;40:308–15. doi: 10.1007/s00127-005-0899-9. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–84. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonzo GA, Ramsawh HJ, Flagan TM, Simmons AN, Sullivan SG, Allard CB, et al. Early life stress and the anxious brain: evidence for a neural mechanism linking childhood emotional maltreatment to anxiety in adulthood. Psychol Med. 2016;46:1037–54. doi: 10.1017/S0033291715002603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman DL, Dukes EM, Wittchen HU. Human and economic burden of generalized anxiety disorder. Depress Anxiety. 2008;25:72–90. doi: 10.1002/da.20257. [DOI] [PubMed] [Google Scholar]
- 6.Grecucci A, Giorgetta C, Brambilla P, Zuanon S, Perini L, Balestrieri M, et al. Anxious ultimatums: how anxiety disorders affect socioeconomic behaviour. Cogn Emot. 2013;27:230–44. doi: 10.1080/02699931.2012.698982. [DOI] [PubMed] [Google Scholar]
- 7.Worthington JJ, 3rd, Kinrys G, Wygant LE, Pollack MH. Aripiprazole as an augmentor of selective serotonin reuptake inhibitors in depression and anxiety disorder patients. Int Clin Psychopharmacol. 2005;20:9–11. doi: 10.1097/00004850-200501000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci. 2015;17:327–35. doi: 10.31887/DCNS.2015.17.3/bbandelow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin EI, Ressler KJ, Binder E, Nemeroff CB. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatr Clin North Am. 2009;32:549–75. doi: 10.1016/j.psc.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Visser L, van der Knaap LJ, van de Loo AJ, van der Weerd CM, Ohl F, van den Bos R. Trait anxiety affects decision making differently in healthy men and women: towards gender-specific endophenotypes of anxiety. Neuropsychologia. 2010;48:1598–606. doi: 10.1016/j.neuropsychologia.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Etkin A. Functional neuroanatomy of anxiety: a neural circuit perspective. Curr Top Behav Neurosci. 2010;2:251–77. doi: 10.1007/7854_2009_5. [DOI] [PubMed] [Google Scholar]
- 12.Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–6. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 13.Izumi T, Inoue T, Kato A, Kitaichi Y, Nakagawa S, Koyama T. Changes in amygdala neural activity that occur with the extinction of context-dependent conditioned fear stress. Pharmacol Biochem Behav. 2008;90:297–304. doi: 10.1016/j.pbb.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 14.Hilbert K, Lueken U, Beesdo-Baum K. Neural structures, functioning and connectivity in generalized anxiety disorder and interaction with neuroendocrine systems: a systematic review. J Affect Disord. 2014;158:114–26. doi: 10.1016/j.jad.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66:1361–72. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delvecchio G, Stanley JA, Altamura AC, Brambilla P. Metabolic alterations in generalised anxiety disorder: a review of proton magnetic resonance spectroscopic studies. Epidemiol Psychiatr Sci. 2017;26:587–95. doi: 10.1017/S2045796017000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price RB, Eldreth DA, Mohlman J. Deficient prefrontal attentional control in late-life generalized anxiety disorder: an fMRI investigation. Transl Psychiatry. 2011;1:e46. doi: 10.1038/tp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ball TM, Ramsawh HJ, Campbell-Sills L, Paulus MP, Stein MB. Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorders. Psychol Med. 2013;43:1475–86. doi: 10.1017/S0033291712002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mochcovitch MD, da Rocha Freire RC, Garcia RF, Nardi AE. A systematic review of fMRI studies in generalized anxiety disorder: evaluating its neural and cognitive basis. J Affect Disord. 2014;167:336–42. doi: 10.1016/j.jad.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 20.Hilbert K, Pine DS, Muehlhan M, Lueken U, Steudte-Schmiedgen S, Beesdo-Baum K. Gray and white matter volume abnormalities in generalized anxiety disorder by categorical and dimensional characterization. Psychiatry Res. 2015;234:314–20. doi: 10.1016/j.pscychresns.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strawn JR, Hamm L, Fitzgerald DA, Fitzgerald KD, Monk CS, Phan KL. Neurostructural abnormalities in pediatric anxiety disorders. J Anxiety Disord. 2015;32:81–8. doi: 10.1016/j.janxdis.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 2002;52:1066–78. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- 23.Liao M, Yang F, Zhang Y, He Z, Song M, Jiang T, et al. Childhood maltreatment is associated with larger left thalamic gray matter volume in adolescents with generalized anxiety disorder. PloS One. 2013;8:e71898. doi: 10.1371/journal.pone.0071898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao M, Yang F, Zhang Y, He Z, Su L, Li L. White matter abnormalities in adolescents with generalized anxiety disorder: a diffusion tensor imaging study. BMC Psychiatry. 2014;14:41. doi: 10.1186/1471-244X-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molent C, Maggioni E, Cecchetto F, Garzitto M, Piccin S, Bonivento C, et al. Reduced cortical thickness and increased gyrification in generalized anxiety disorder: a 3 T MRI study. Psychol Med. 2018;48:2001–10. doi: 10.1017/S003329171700352X. [DOI] [PubMed] [Google Scholar]
- 26.Nutt DJ. Neurobiological mechanisms in generalized anxiety disorder. J Clin Psychiatry. 2001;62:22–7. discussion 28. [PubMed] [Google Scholar]
- 27.Moon CM, Jeong GW. Brain morphological alterations and cellular metabolic changes in patients with generalized anxiety disorder: a combined DARTEL-based VBM and 1 H-MRS study. Magn Reson Imaging. 2016;34:429–36. doi: 10.1016/j.mri.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Kroboth PD, Folan MM, Bauer KS, Tullock W, Wright CE, Sweeney JA. Do alprazolam-induced changes in saccadic eye movement and psychomotor function follow the same time course? J Clin Pharmacol. 1998;38:337–46. doi: 10.1002/j.1552-4604.1998.tb04433.x. [DOI] [PubMed] [Google Scholar]
- 29.Malizia AL, Cunningham VJ, Bell CJ, Liddle PF, Jones T, Nutt DJ. Decreased brain GABA (A)-benzodiazepine receptor binding in panic disorder: preliminary results from a quantitative PET study. Arch Gen Psychiatry. 1998;55:715–20. doi: 10.1001/archpsyc.55.8.715. [DOI] [PubMed] [Google Scholar]
- 30.Lydiard RB. The role of GABA in anxiety disorders. J Clin Psychiatry. 2003;64:21–7. [PubMed] [Google Scholar]
- 31.Kelly CB, Cooper SJ. Differences in variability in plasma noradrenaline between depressive and anxiety disorders. J Psychopharmacol. 1998;12:161–7. doi: 10.1177/026988119801200208. [DOI] [PubMed] [Google Scholar]
- 32.Karege F, Bovier P, Gaillard JM, Tissot R. The decrease of erythrocyte catechol-O-methyltransferase activity in depressed patients and its diagnostic significance. Acta Psychiatr Scand. 1987;76:303–8. doi: 10.1111/j.1600-0447.1987.tb02899.x. [DOI] [PubMed] [Google Scholar]
- 33.Jetty PV, Charney DS, Goddard AW. Neurobiology of generalized anxiety disorder. Psychiatr Clin North Am. 2001;24:75–97. doi: 10.1016/s0193-953x(05)70207-0. [DOI] [PubMed] [Google Scholar]
- 34.Katzman MA. Current considerations in the treatment of generalized anxiety disorder. CNS Drugs. 2009;23:103–20. doi: 10.2165/00023210-200923020-00002. [DOI] [PubMed] [Google Scholar]
- 35.Kinrys G, Wygant LE. Anticonvulsants in anxiety disorders. Curr Psychiatry Rep. 2005;7:258–67. doi: 10.1007/s11920-005-0079-3. [DOI] [PubMed] [Google Scholar]
- 36.Newman MG, Llera SJ, Erickson TM, Przeworski A, Castonguay LG. Worry and generalized anxiety disorder: a review and theoretical synthesis of evidence on nature, etiology, mechanisms, and treatment. Annu Rev Clin Psychol. 2013;9:275–97. doi: 10.1146/annurev-clinpsy-050212-185544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brambilla P, Barale F, Caverzasi E, Soares JC. Anatomical MRI findings in mood and anxiety disorders. Epidemiol Psichiatr Soc. 2002;11:88–99. doi: 10.1017/s1121189x00005558. [DOI] [PubMed] [Google Scholar]
- 38.Malizia AL. What do brain imaging studies tell us about anxiety disorders? J Psychopharmacol. 1999;13:372–8. doi: 10.1177/026988119901300418. [DOI] [PubMed] [Google Scholar]
- 39.De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, et al. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2000;48:51–7. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- 40.De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Dahl RE, Axelson DA, et al. Superior temporal gyrus volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2002;51:553–62. doi: 10.1016/s0006-3223(01)01375-0. [DOI] [PubMed] [Google Scholar]
- 41.Strawn JR, Wehry AM, Chu WJ, Adler CM, Eliassen JC, Cerullo MA, et al. Neuroanatomic abnormalities in adolescents with generalized anxiety disorder: a voxel-based morphometry study. Depress Anxiety. 2013;30:842–8. doi: 10.1002/da.22089. [DOI] [PubMed] [Google Scholar]
- 42.Mohlman J, Price RB, Eldreth DA, Chazin D, Glover DM, Kates WR. The relation of worry to prefrontal cortex volume in older adults with and without generalized anxiety disorder. Psychiatry Res. 2009;173:121–7. doi: 10.1016/j.pscychresns.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schienle A, Ebner F, Schafer A. Localized gray matter volume abnormalities in generalized anxiety disorder. Eur Arch Psychiatry Clin Neurosci. 2011;261:303–7. doi: 10.1007/s00406-010-0147-5. [DOI] [PubMed] [Google Scholar]
- 44.Makovac E, Meeten F, Watson DR, Garfinkel SN, Critchley HD, Ottaviani C. Neurostructural abnormalities associated with axes of emotion dysregulation in generalized anxiety. Neuroimage Clin. 2015;10:172–81. doi: 10.1016/j.nicl.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milham MP, Nugent AC, Drevets WC, Dickstein DP, Leibenluft E, Ernst M, et al. Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biol Psychiatry. 2005;57:961–6. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 46.Liao M, Yang F, Zhang Y, He Z, Su L, Li L. Lack of gender effects on gray matter volumes in adolescent generalized anxiety disorder. J Affect Disord. 2014;155:278–82. doi: 10.1016/j.jad.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 47.Moon CM, Kim GW, Jeong GW. Whole-brain gray matter volume abnormalities in patients with generalized anxiety disorder: voxel-based morphometry. Neuroreport. 2014;25:184–9. doi: 10.1097/WNR.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 48.Moon CM, Jeong GW. Alterations in white matter volume and its correlation with clinical characteristics in patients with generalized anxiety disorder. Neuroradiology. 2015;57:1127–34. doi: 10.1007/s00234-015-1572-y. [DOI] [PubMed] [Google Scholar]
- 49.Terlevic R, Isola M, Ragogna M, Meduri M, Canalaz F, Perini L, et al. Decreased hypothalamus volumes in generalized anxiety disorder but not in panic disorder. J Affect Disord. 2013;146:390–4. doi: 10.1016/j.jad.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 50.Brambilla P, Como G, Isola M, Taboga F, Zuliani R, Goljevscek S, et al. White-matter abnormalities in the right posterior hemisphere in generalized anxiety disorder: a diffusion imaging study. Psychol Med. 2011;42:427–34. doi: 10.1017/S0033291711001255. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, Zhang Y, Li L, Li Z, Li W, Ma N, et al. Different white matter abnormalities between the first-episode, treatment-naive patients with posttraumatic stress disorder and generalized anxiety disorder without comorbid conditions. J Affect Disord. 2011;133:294–9. doi: 10.1016/j.jad.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 52.Tromp DP, Grupe DW, Oathes DJ, McFarlin DR, Hernandez PJ, Kral TR, et al. Reduced structural connectivity of a major frontolimbic pathway in generalized anxiety disorder reduced connectivity of major frontolimbic pathway. Arch Gen Psychiatry. 2012;69:925–34. doi: 10.1001/archgenpsychiatry.2011.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Li L, Yu R, Liu J, Tang J, Tan L, et al. White matter integrity alterations in first episode, treatment-naive generalized anxiety disorder. J Affect Disord. 2013;148:196–201. doi: 10.1016/j.jad.2012.11.060. [DOI] [PubMed] [Google Scholar]
- 54.Cha J, Greenberg T, Carlson JM, Dedora DJ, Hajcak G, Mujica-Parodi LR. Circuit-wide structural and functional measures predict ventromedial prefrontal cortex fear generalization: implications for generalized anxiety disorder. J Neurosci. 2014;34:4043–53. doi: 10.1523/JNEUROSCI.3372-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moon CM, Yang JC, Jeong GW. Explicit verbal memory impairments associated with brain functional deficits and morphological alterations in patients with generalized anxiety disorder. J Affect Disord. 2015;186:328–36. doi: 10.1016/j.jad.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 56.Moon CM, Jeong GW. Functional and morphological alterations associated with working memory dysfunction in patients with generalized anxiety disorder. Acta Radiol. 2017;58:344–52. doi: 10.1177/0284185116649794. [DOI] [PubMed] [Google Scholar]
- 57.Cha J, Greenberg T, Song I, Blair Simpson H, Posner J, Mujica-Parodi LR. Abnormal hippocampal structure and function in clinical anxiety and comorbid depression. Hippocampus. 2016;26:545–53. doi: 10.1002/hipo.22566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang W, Qian S, Liu K, Li B, Li M, Xin K, et al. Reduced white matter integrity and its correlation with clinical symptom in first-episode, treatment-naive generalized anxiety disorder. Behav Brain Res. 2016;314:159–64. doi: 10.1016/j.bbr.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 59.Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, Kalin NH. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci. 2010;30:7023–7. doi: 10.1523/JNEUROSCI.5952-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drabant EM, Kuo JR, Ramel W, Blechert J, Edge MD, Cooper JR, et al. Experiential, autonomic, and neural responses during threat anticipation vary as a function of threat intensity and neuroticism. Neuroimage. 2011;55:401–10. doi: 10.1016/j.neuroimage.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suslow T, Kugel H, Rauch AV, Dannlowski U, Bauer J, Konrad C, et al. Attachment avoidance modulates neural response to masked facial emotion. Hum Brain Mapp. 2009;30:3553–62. doi: 10.1002/hbm.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatr Clin North Am. 2009;32:483–524. doi: 10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sala M, Perez J, Soloff P, Ucelli di Nemi S, Caverzasi E, Soares JC, et al. Stress and hippocampal abnormalities in psychiatric disorders. Eur Neuropsychopharmacol. 2004;14:393–405. doi: 10.1016/j.euroneuro.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 64.Gunning FM, Cheng J, Murphy CF, Kanellopoulos D, Acuna J, Hoptman MJ, et al. Anterior cingulate cortical volumes and treatment remission of geriatric depression. Int J Geriatr Psychiatry. 2009;24:829–36. doi: 10.1002/gps.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM. Widespread reductions in gray matter volume in depression. Neuroimage Clin. 2013;3:332–9. doi: 10.1016/j.nicl.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noyes R., Jr Comorbidity in generalized anxiety disorder. Psychiatr Clin North Am. 2001;24:41–55. doi: 10.1016/s0193-953x(05)70205-7. [DOI] [PubMed] [Google Scholar]
- 67.Dusi N, Barlati S, Vita A, Brambilla P. Brain structural effects of antidepressant treatment in major depression. Curr Neuropharmacol. 2015;13:458–65. doi: 10.2174/1570159X1304150831121909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bellani M, Dusi N, Yeh PH, Soares JC, Brambilla P. The effects of antidepressants on human brain as detected by imaging studies. Focus on major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1544–52. doi: 10.1016/j.pnpbp.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 69.Whalen PJ, Johnstone T, Somerville LH, Nitschke JB, Polis S, Alexander AL, et al. A functional magnetic resonance imaging predictor of treatment response to venlafaxine in generalized anxiety disorder. Biol Psychiatry. 2008;63:858–63. doi: 10.1016/j.biopsych.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caudle DD, Senior AC, Wetherell JL, Rhoades HM, Beck JG, Kunik ME, et al. Cognitive errors, symptom severity, and response to cognitive behavior therapy in older adults with generalized anxiety disorder. Am J Geriatr Psychiatry. 2007;15:680–9. doi: 10.1097/JGP.0b013e31803c550d. [DOI] [PubMed] [Google Scholar]
- 71.Coles ME, Turk CL, Heimberg RG. Memory bias for threat in generalized anxiety disorder: the potential importance of stimulus relevance. Cogn Behav Ther. 2007;36:65–73. doi: 10.1080/16506070601070459. [DOI] [PubMed] [Google Scholar]
- 72.Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, et al. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–63. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 73.Hoehn-Saric R, Schlund MW, Wong SH. Effects of citalopram on worry and brain activation in patients with generalized anxiety disorder. Psychiatry Res. 2004;131:11–21. doi: 10.1016/j.pscychresns.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 74.Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163:1091–7. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- 75.McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 76.Krain AL, Gotimer K, Hefton S, Ernst M, Castellanos FX, Pine DS, et al. A functional magnetic resonance imaging investigation of uncertainty in adolescents with anxiety disorders. Biol Psychiatry. 2008;63:563–8. doi: 10.1016/j.biopsych.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 77.Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro H, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–76. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blair K, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, et al. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am J Psychiatry. 2008;165:1193–202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, et al. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166:302–10. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paulesu E, Sambugaro E, Torti T, Danelli L, Ferri F, Scialfa G, et al. Neural correlates of worry in generalized anxiety disorder and in normal controls: a functional MRI study. Psychol Med. 2010;40:117–24. doi: 10.1017/S0033291709005649. [DOI] [PubMed] [Google Scholar]
- 81.Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167:545–54. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maslowsky J, Mogg K, Bradley BP, McClure-Tone E, Ernst M, Pine DS, et al. A preliminary investigation of neural correlates of treatment in adolescents with generalized anxiety disorder. J Child Adolesc Psychopharmacol. 2010;20:105–11. doi: 10.1089/cap.2009.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez-Edgar K, et al. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. Am J Psychiatry. 2012;169:205–12. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Etkin A, Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry. 2011;168:968–78. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- 85.Palm ME, Elliott R, McKie S, Deakin JF, Anderson IM. Attenuated responses to emotional expressions in women with generalized anxiety disorder. Psychol Med. 2011;41:1009–18. doi: 10.1017/S0033291710001455. [DOI] [PubMed] [Google Scholar]
- 86.Andreescu C, Gross JJ, Lenze E, Edelman KD, Snyder S, Tanase C, et al. Altered cerebral blood flow patterns associated with pathologic worry in the elderly. Depress Anxiety. 2011;28:202–9. doi: 10.1002/da.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blair KS, Geraci M, Smith B, Hollon N, DeVido J, Otero M. Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder and comorbid generalized social phobia/generalized anxiety disorder. Biol Psychiatry. 2012;72:476–82. doi: 10.1016/j.biopsych.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strawn JR, Bitter SM, Weber WA, Chu WJ, Whitsel RM, Adler C, et al. Neurocircuitry of generalized anxiety disorder in adolescents: a pilot functional neuroimaging and functional connectivity study. Depress Anxiety. 2012;29:939–47. doi: 10.1002/da.21961. [DOI] [PubMed] [Google Scholar]
- 89.Yassa MA, Hazlett RL, Stark CE, Hoehn-Saric R. Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. J Psychiatr Res. 2012;46:1045–52. doi: 10.1016/j.jpsychires.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roy AK, Fudge JL, Kelly C, Perry JS, Daniele T, Carlisi C, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:290–9. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen AC, Etkin A. Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology. 2013;38:1889–98. doi: 10.1038/npp.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hölzel BK, Hoge EA, Greve DN, Gard T, Creswell JD, Brown KW, et al. Neural mechanisms of symptom improvements in generalized anxiety disorder following mindfulness training. Neuroimage Clin. 2013;2:448–58. doi: 10.1016/j.nicl.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Andreescu C, Sheu LK, Tudorascu D, Walker S, Aizenstein H. The ages of anxiety--differences across the lifespan in the default mode network functional connectivity in generalized anxiety disorder. Int J Geriatr Psychiatry. 2014;29:704–12. doi: 10.1002/gps.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andreescu C, Sheu LK, Tudorascu D, Gross JJ, Walker S, Banihashemi L, et al. Emotion reactivity and regulation in late-life generalized anxiety disorder: functional connectivity at baseline and post-treatment. Am J Geriatr Psychiatry. 2015;23:200–14. doi: 10.1016/j.jagp.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fonzo GA, Ramsawh HJ, Flagan TM, Sullivan SG, Simmons AN, Paulus MP, et al. Cognitive-behavioral therapy for generalized anxiety disorder is associated with attenuation of limbic activation to threat-related facial emotions. J Affect Disord. 2014;169:76–85. doi: 10.1016/j.jad.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cha J, Carlson JM, Dedora DJ, Greenberg T, Proudfit GH, Mujica-Parodi LR. Hyper-reactive human ventral tegmental area and aberrant mesocorticolimbic connectivity in overgeneralization of fear in generalized anxiety disorder. J Neurosci. 2014;34:5855–60. doi: 10.1523/JNEUROSCI.4868-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brown GG, Ostrowitzki S, Stein MB, Von Kienlin M, Liu T, Simmons A, et al. Temporal profile of brain response to alprazolam in patients with generalized anxiety disorder. Psychiatry Res. 2015;233:394–401. doi: 10.1016/j.pscychresns.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 98.Liu WJ, Yin DZ, Cheng WH, Fan MX, You MN, Men WW, et al. Abnormal functional connectivity of the amygdala-based network in resting-state fMRI in adolescents with generalized anxiety disorder. Med Sci Monit. 2015;21:459–67. doi: 10.12659/MSM.893373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Makovac E, Meeten F, Watson RA, Herman D, Garfinkel SN, D Critchley H, et al. Alterations in amygdala-prefrontal functional connectivity account for excessive worry and autonomic dysregulation in generalized anxiety disorder. Biol Psychiatry. 2016;80:786–95. doi: 10.1016/j.biopsych.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 100.Park JI, Kim GW, Jeong GW, Chung GH, Yang JC. Brain activation patterns associated with the effects of emotional distracters during working memory maintenance in patients with generalized anxiety disorder. Psychiatry Investig. 2016;13:152–6. doi: 10.4306/pi.2016.13.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Buff C, Brinkmann L, Neumeister P, Feldker K, Heitmann C, Gathmann B, et al. Specifically altered brain responses to threat in generalized anxiety disorder relative to social anxiety disorder and panic disorder. Neuroimage Clin. 2016;12:698–706. doi: 10.1016/j.nicl.2016.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cui H, Zhang J, Liu Y, Li Q, Li H, Zhang L, et al. Differential alterations of resting-state functional connectivity in generalized anxiety disorder and panic disorder. Hum Brain Mapp. 2016;37:1459–73. doi: 10.1002/hbm.23113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kujawa A, Swain JE, Hanna GL, Koschmann E, Simpson D, Connolly S, et al. Prefrontal reactivity to social signals of threat as a predictor of treatment response in anxious youth. Neuropsychopharmacology. 2016;41:1983–90. doi: 10.1038/npp.2015.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Makovac E, Watson DR, Meeten F, Garfinkel SN, Cercignani M, Critchley HD, et al. Amygdala functional connectivity as a longitudinal biomarker of symptom changes in generalized anxiety. Soc Cogn Affect Neurosci. 2016;11:1719–28. doi: 10.1093/scan/nsw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang W, Hou J, Qian S, Liu K, Li B, Li M, et al. Aberrant regional neural fluctuations and functional connectivity in generalized anxiety disorder revealed by resting-state functional magnetic resonance imaging. Neurosci Lett. 2016;624:78–84. doi: 10.1016/j.neulet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 106.Moon CM, Sundaram T, Choi NG, Jeong GW. Working memory dysfunction associated with brain functional deficits and cellular metabolic changes in patients with generalized anxiety disorder. Psychiatry Res Neuroimaging. 2016;254:137–44. doi: 10.1016/j.pscychresns.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 107.Burkhouse KL, Kujawa A, Klumpp H, Fitzgerald KD, Monk CS, Phan KL. Neural correlates of explicit and implicit emotion processing in relation to treatment response in pediatric anxiety. J Child Psychol Psychiatry. 2017;58:546–54. doi: 10.1111/jcpp.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meeten F, Davey GC, Makovac E, Watson DR, Garfinkel SN, Critchley HD, et al. Goal directed worry rules are associated with distinct patterns of amygdala functional connectivity and vagal modulation during perseverative cognition. Front Hum Neurosci. 2016;10:553. doi: 10.3389/fnhum.2016.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moon CM, Yang JC, Jeong GW. Functional neuroanatomy associated with the interaction between emotion and cognition in explicit memory tasks in patients with generalized anxiety disorder. Acta Radiol. 2017;58:98–106. doi: 10.1177/0284185116633915. [DOI] [PubMed] [Google Scholar]
- 110.Li W, Cui H, Zhu Z, Kong L, Guo Q, Zhu Y, et al. Aberrant functional connectivity between the amygdala and the temporal pole in drug-free generalized anxiety disorder. Front Hum Neurosci. 2016;10:549. doi: 10.3389/fnhum.2016.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karim H, Tudorascu DL, Aizenstein H, Walker S, Good R, Andreescu C. Emotion reactivity and cerebrovascular burden in late-life GAD: a neuroimaging study. Am J Geriatr Psychiatry. 2016;24:1040–50. doi: 10.1016/j.jagp.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Diwadkar VA, Re M, Cecchetto F, Garzitto M, Piccin S, Bonivento C, et al. Attempts at memory control induce dysfunctional brain activation profiles in generalized anxiety disorder: an exploratory fMRI study. Psychiatry Res Neuroimaging. 2017;266:42–52. doi: 10.1016/j.pscychresns.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 113.Ellard KK, Barlow DH, Whitfield-Gabrieli S, Gabrieli JD, Deckersbach T. Neural correlates of emotion acceptance vs worry or suppression in generalized anxiety disorder. Soc Cogn Affect Neurosci. 2017;12:1009–21. doi: 10.1093/scan/nsx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fitzgerald JM, Phan KL, Kennedy AE, Shankman SA, Langenecker SA, Klumpp H. Prefrontal and amygdala engagement during emotional reactivity and regulation in generalized anxiety disorder. J Affect Disord. 2017;218:398–406. doi: 10.1016/j.jad.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.MacNamara A, Klumpp H, Kennedy AE, Langenecker SA, Phan KL. Transdiagnostic neural correlates of affective face processing in anxiety and depression. Depress Anxiety. 2017;34:621–31. doi: 10.1002/da.22631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carlson JM, Rubin D, Mujica-Parodi LR. Lost emotion: disrupted brain-based tracking of dynamic affective episodes in anxiety and depression. Psychiatry Res Neuroimaging. 2017;260:37–48. doi: 10.1016/j.pscychresns.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 117.Blair KS, Otero M, Teng C, Geraci M, Ernst M, Blair RJ, et al. Reduced optimism and a heightened neural response to everyday worries are specific to generalized anxiety disorder, and not seen in social anxiety. Psychol Med. 2017;47:1806–15. doi: 10.1017/S0033291717000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.White SF, Geraci M, Lewis E, Leshin J, Teng C, Averbeck B, et al. Prediction error representation in individuals with generalized anxiety disorder during passive avoidance. Am J Psychiatry. 2017;174:110–7. doi: 10.1176/appi.ajp.2016.15111410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mohlman J, Eldreth DA, Price RB, Staples AM, Hanson C. Prefrontal-limbic connectivity during worry in older adults with generalized anxiety disorder. Aging Ment Health. 2017;21:426–38. doi: 10.1080/13607863.2015.1109058. [DOI] [PubMed] [Google Scholar]
- 120.Buff C, Schmidt C, Brinkmann L, Gathmann B, Tupak S, Straube T. Directed threat imagery in generalized anxiety disorder. Psychol Med. 2018;48:617–28. doi: 10.1017/S0033291717001957. [DOI] [PubMed] [Google Scholar]
- 121.Rabany L, Diefenbach GJ, Bragdon LB, Pittman BP, Zertuche L, Tolin DF, et al. Resting-state functional connectivity in generalized anxiety disorder and social anxiety disorder: evidence for a dimensional approach. Brain Connect. 2017;7:289–98. doi: 10.1089/brain.2017.0497. [DOI] [PubMed] [Google Scholar]
- 122.Yin D, Liu W, Zeljic K, Lv Q, Wang Z, You M, et al. Failure in cognitive suppression of negative affect in adolescents with generalized anxiety disorder. Sci Rep. 2017;7:6583. doi: 10.1038/s41598-017-07063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qiao J, Li A, Cao C, Wang Z, Sun J, Xu G. Aberrant functional network connectivity as a biomarker of generalized anxiety disorder. Front Hum Neurosci. 2017;11:626. doi: 10.3389/fnhum.2017.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xia L, Li S, Wang T, Guo Y, Meng L, Feng Y, et al. Spontaneous alterations of regional brain activity in patients with adult generalized anxiety disorder. Neuropsychiatr Dis Treat. 2017;13:1957–65. doi: 10.2147/NDT.S133853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Buff C, Brinkmann L, Bruchmann M, Becker MP, Tupak S, Hermann MJ, et al. Activity alterations in the bed nucleus of the stria terminalis and amygdala during threat anticipation in generalized anxiety disorder. Soc Cogn Affect Neurosci. 2017;12:1766–74. doi: 10.1093/scan/nsx103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Karim HT, Tudorascu DL, Butters MA, Walker S, Aizenstein HJ, Andreescu C. In the grip of worry: cerebral blood flow changes during worry induction and reappraisal in late-life generalized anxiety disorder. Transl Psychiatry. 2017;7:e1204. doi: 10.1038/tp.2017.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Burkhouse KL, Kujawa A, Hosseini B, Klumpp H, Fitzgerald KD, Langenecker SA, et al. Anterior cingulate activation to implicit threat before and after treatment for pediatric anxiety disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84:250–6. doi: 10.1016/j.pnpbp.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lewis AR, Zinbarg RE, Durbin CE. Advances, problems, and challenges in the study of emotion regulation: a commentary. J Psychopathol Behav Assess. 2010;32:83–91. [Google Scholar]
- 129.McLaughlin KA, Mennin DS, Farach FJ. The contributory role of worry in emotion generation and dysregulation in generalized anxiety disorder. Behav Res Ther. 2007;45:1735–52. doi: 10.1016/j.brat.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 130.Greenberg T, Carlson JM, Cha J, Hajcak G, Mujica Parodi LR. Ventromedial prefrontal cortex reactivity is altered in generalized anxiety disorder during fear generalization. Depress Anxiety. 2013;30:242–50. doi: 10.1002/da.22016. [DOI] [PubMed] [Google Scholar]
- 131.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- 133.Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 134.Vollm BA, Taylor AN, Richardson P, Corcoran R, Stirling J, McKie S, et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2006;29:90–8. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 135.Beesdo K, Lau JY, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry. 2009;66:275–85. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 137.Ray RD, Zald DH. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci Biobehav Rev. 2012;36:479–501. doi: 10.1016/j.neubiorev.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 139.Chen JJ, Jann K, Wang DJ. Characterizing resting-state brain function using arterial spin labeling. Brain Connect. 2015;5:527–42. doi: 10.1089/brain.2015.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hoehn-Saric R, McLeod DR, Zimmerli WD. Differential effects of alprazolam and imipramine in generalized anxiety disorder: somatic versus psychic symptoms. J Clin Psychiatry. 1988;49:293–301. [PubMed] [Google Scholar]
- 141.Allgulander C, Hackett D, Salinas E. Venlafaxine extended release (ER) in the treatment of generalised anxiety disorder: twenty-four-week placebo-controlled dose-ranging study. Br J Psychiatry. 2001;179:15–22. doi: 10.1192/bjp.179.1.15. [DOI] [PubMed] [Google Scholar]
- 142.Pollack MH, Zaninelli R, Goddard A, McCafferty JP, Bellew KM, Burnham DB, et al. Paroxetine in the treatment of generalized anxiety disorder: results of a placebo-controlled, flexible-dosage trial. J Clin Psychiatry. 2001;62:350–7. doi: 10.4088/jcp.v62n0508. [DOI] [PubMed] [Google Scholar]
- 143.Alaka KJ, Noble W, Montejo A, Dueñas H, Munshi A, Strawn JR, et al. Efficacy and safety of duloxetine in the treatment of older adult patients with generalized anxiety disorder: a randomized, double-blind, placebo-controlled trial. Int J Geriatr Psychiatry. 2014;29:978–86. doi: 10.1002/gps.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brierley B, Shaw P, David AS. The human amygdala: a systematic review and meta-analysis of volumetric magnetic resonance imaging. Brain Res Brain Res Rev. 2002;39:84–105. doi: 10.1016/s0165-0173(02)00160-1. [DOI] [PubMed] [Google Scholar]
- 145.Brambilla P, Harenski K, Nicoletti M, Sassi RB, Mallinger AG, Frank E, et al. MRI investigation of temporal lobe structures in bipolar patients. J Psychiatr Res. 2003;37:287–96. doi: 10.1016/s0022-3956(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 146.Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci. 2002;14:1264–74. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- 147.Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–23. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 148.Abu-Akel A. A neurobiological mapping of theory of mind. Brain Res Brain Res Rev. 2003;43:29–40. doi: 10.1016/s0165-0173(03)00190-5. [DOI] [PubMed] [Google Scholar]
- 149.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–91. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee GP, Meador KJ, Loring DW, Allison JD, Brown WS, Paul LK, et al. Neural substrates of emotion as revealed by functional magnetic resonance imaging. Cogn Behav Neurol. 2004;17:9–17. doi: 10.1097/00146965-200403000-00002. [DOI] [PubMed] [Google Scholar]