Abstract
Chronic liver disease (CLD) is a condition that progresses over time toward advanced disease state which is known as liver cirrhosis. Liver cirrhosis leads to dangerous health problems among people living across the world. One such problem that observed in about 75% of cirrhotic patients is thrombocytopenia; which in turn associated with poor prognosis and recovery from CLD. Beyond these, thrombocytopenia in cirrhotic patients led to impairment of coagulation cascade and significantly influenced the utilization of effective mechanism in the management of CLD. By nature, treatment of CLD involves invasive diagnostic and treatment procedures; therefore, in the presence of thrombocytopenia implementing these methods put the lives of patients in a critical health problem due to increased risk of bleeding and mortality. Because of these reasons, prophylactic transfusion of platelets is considered to be one of the most effective options that reduce the risk of bleeding in patients with CLD that required to undergo an invasive procedure. Although platelet transfusion presented with significant advantages in facilitating the invasive procedure in patients with CLD, refractoriness with repeated use and various problems associated with its transfusion limit the continuous utilization of this important option. With these challenges and current advance in the knowledge of thrombopoiesis, the development of relatively safe and alternative drugs that enhance the production of platelets by interacting with thrombopoietin receptor agonists provides a promising option to platelet transfusion. The discovery and approval of romiplostim and eltrombopag in August 2008 and November 2008, respectively, for the treatment of chronic immune thrombocytopenia paved a way and followed by the Food and Drug Administration (FDA) approval of 2 potentially advantageous drugs, lusutrombopag, and avatrombopag, in 2018 for the treatment of thrombocytopenia in patients with CLD that required to undergo elective surgery. Therefore, this review aims to assess pathogenesis of thrombocytopenia and its challenges in the management of liver-related issues and, more importantly, gives emphasis to address the potential use of avatrombopag in the treatment of thrombocytopenia underlying CLD, its pharmacokinetics and pharmacodynamics, as well as its toxicological profiles by presenting the most commonly reported adverse events in various trials.
Keywords: Thrombocytopenia, platelets, chronic liver disease, avatrombopag, thrombopoietin receptor agonists
Introduction
Chronic liver disease (CLD) gradually causes damage and impairment of the liver that result in liver cirrhosis. It is a serious case of CLD that leads to dangerous health problems among people living across the world.1,2 As observed in 75% of patients with cirrhosis, thrombocytopenia is the most frequent problem that has seen in patients with CLD3 and limited the available treatment option for the patients.4 The proportion of thrombocytopenia in patients with CLD is about 6% to 70% for noncirrhotic and cirrhotic patients, respectively.5 Severe liver disease is related with a high risk of thrombocytopenia3; in turn, thrombocytopenic conditions also lead to poor prognosis and recovery from CLD,5,6 as well as impaired coagulation cascade that leads to increase the tendency to bleed7-9 and mortality.5,6 Prophylactic transfusion of platelets reduces the risk of bleeding in patients with CLD and platelet count <50 × 109/L that required to undergo invasive procedure.4,10,11 The degree of bleeding disorder that occurs as a result of the invasive procedure in patients with CLD is influenced by a number of factors such as coagulation status of the patients, platelet count, and procedure performed.2,7,12
Before the advent and approval of thrombopoietin (TPO) receptor agonists such as eltrombopag by Food and Drug Administration (FDA) and European Medicine Agency in 2008 and 2009 respectively; the management of thrombocytopenia includes platelet transfusion,10,13 splenic artery embolization, splenectomy, and transjugular intrahepatic portosystemic stent shunting. Although these treatments are effective in the management of thrombocytopenia, securing donor and the cost of transfusion are the major limiting factors. Despite the fact that platelet transfusion is the most effective option for the treatment of thrombocytopenia, after repeated administration of platelets, the condition becomes refractory due to human leukocyte antigen alloimmunization. Moreover, it is also associated with the development of febrile nonhemolytic reactions, increased risk of viral and bacterial infections,10,13-17 longer hospital stays, postoperative ventilation, and transfusion-related acute lung injury.13,18
Recently with the advance in the knowledge of thrombopoiesis and the role of its key regulator, TPO led to the production of novel drugs that act as TPO receptor (TPO-R) agonists that activate and enhance megakaryopoiesis which in turn increase platelet synthesis.19 Despite the fact that eltrombopag and romiplostim received FDA approval for adult patients with chronic immune thrombocytopenia (CITP),20-24 until recently there is no product approved for patients with CLD that require the invasive procedure. On the contrary, the trial conducted to evaluate the efficacy of eltrombopag for patients with CLD that need to undergo the invasive procedure was discontinued due to increased risk of thromboembolic events.24 The new second-generation TPO-R agonists approved by FDA, lusutrombopag and avatrombopag, found to provide an alternative for the management of thrombocytopenia associated with CLD. Therefore, this review is concerned with the pathogenesis of thrombocytopenia, its impact on CLD management, and evidence on the role of TPO-R agonists with particular focus on avatrombopag in the treatment of thrombocytopenia related with CLD.
Methods Used for Data Extraction
The articles were searched from July 20 to August 30, 2018, using Boolean Operators (AND, OR) to combine different key terminologies that assisted to obtain data related to the use of avatrombopag in the management of thrombocytopenia in CLD. These key terms were as follows: “avatrombopag,” “doptelet,” “AKR501,” “AKR 501,” “AKR-501,” “YM477,” “YM 477,” “YM-477,” “Thrombopoietin receptor agonist*,” “chronic liver disease,” “liver cirrho*,” “low platelet count*,” “platelet deficiency,” and “thrombocytopen*.” Truncation was utilized to extend the likelihood of securing pertinent articles/topics concerning with the use of avatrombopag in patients with thrombocytopenia. To retrieve the required information, databases indexed to MEDLINE, EMBASE, and COCHRANE library were searched using key terms and 83 articles were identified. The articles were screened by removing duplicate, titles and abstract not consistent with the topic of interest, abstract in which full text is not accessible, and full texts which are not related to the present study. Accordingly, 79 references were included in the study, and after critically reviewing, only 8 original articles were identified and used to summarize the clinical use of avatrombopag, its pharmacology, pharmacokinetics, and toxicological profiles. Regarding the drug of interest, avatrombopag, the important information linked to primary and secondary endpoints of clinical studies and preclinical data were searched. Moreover, relevant data for the undergoing study of clinical trials of avatrombopag use for various thrombocytopenias such as thrombocytopenia linked to CLD, CITP, anticancer drugs, and antiviral drugs like interferon were extracted from the clinical trial website of the National Library of Medicine (www.clinicaltrials.gov).
Factors lead to the development of thrombocytopenia
Thrombocytopenia refers to the reduction in platelet count below the level required for normal physiological function (ie, <150 × 109/L). It also indicated a condition wherein accomplishing invasive procedure consisting of liver biopsy and administration of an antiviral drug, interferon, could be fatal for the patients (ie, <50–75 × 109/L), or a situation that needs a transfusion of platelets to undertake the procedure (ie, <10 × 109/L). Thrombocytopenia causes a critical problem in patients with CLD by complicating a procedure need to be carried out or certain drug administration for the patients. Thus, it may cause interruption of chemotherapeutic drugs to be used for diverse cancerous situations and surgical procedures need to be performed for the patients.5
A number of factors are responsible for the development of thrombocytopenia; some of these causes are hepatitis C virus (HCV) infection, sequestration of platelets in spleen, impairment of bone marrow that leads to a decrease in platelet production, and reduction in the activities of TPO.12,25 In most instances, thrombocytopenia became reportedly associated with a sizable pooling of platelets in the enlarged spleen due to portal hypertension. Nevertheless, treatment targeted to portal hypertension could not always reverse thrombocytopenia, and decrement in the synthesis of platelet has been observed in the absence of hypersplenism; this could indicate the presence of other factors that are accountable for the development of thrombocytopenia. In addition to the destruction of platelet in the spleen, autoantibodies produced against platelets lead to the incidence of thrombocytopenia.12,26 Besides, a condition such as liver disease due to HCV and alcohol will deteriorate bone marrow function which ultimately causes a decrease in platelet production.27,28
In patients with chronic HCV infection, the platelet-specific immunoglobulin level was increased in a proportional manner to the degree of severity of CLD. The increase in immunoglobulin in turn associated with immune complex–coated platelets that lead to reduce the number of platelet count via accelerating its removal and destruction.29,30 Moreover, the major mechanism by which interferon increased the risk of thrombocytopenia and neutropenia is through inhibition of bone marrow function and progenitor cells of hematological lineages.4 Overall, the prominent factors associated with a low level of platelet count or thrombocytopenia in the cirrhotic patient is due to a reduction in TPO in the liver and sequestration of platelets in spleen.31
Finally, though the degrees may vary from drug to drug (eg, busulfan affects pluripotent stem cells, cyclophosphamide affects the later stage of megakaryocyte progenitors, and Bortezomib inhibits nuclear factor kappa B, a critical regulator of platelet shedding), anticancer drugs strongly influence megakaryocyte and platelet synthesis by interfering with blood cell producing machinery. Furthermore, anticancer drugs may also accelerate platelet removal through an immune-mediated mechanism that leads to the development of thrombocytopenia.32
The Impact of Thrombocytopenia in the Management of Various Liver-Related Cases
Thrombocytopenia has frequently complicated the management of patients with CLD who require treatment for their underlying disease or need to undergo invasive procedures.4 It is well known that thrombocytopenia limits the effective treatment options of the following cases observed in patients with liver cirrhosis.
HCV infection management
HCV infection is considered as one of the major problems that lead to a severe stage of liver disease as well as liver transplantation in Europe and the United States.33 To restrict the advance of the disease to a severe stage of liver cirrhosis, antiviral drugs play a sizable role to meet with a goal set to produce sustained virologic response or to eliminate HCV RNA from the serum after 6 months of treatment as well as reduce the risk of liver transplantation. Nevertheless, what is a big challenge to the successful treatment of HCV infection is antiviral drug-induced thrombocytopenia. This condition, in turn, leads to adversely affecting patient care and increasing the risk of morbidity and mortality of the patients.2,34,35 Despite the fact that clinical practice may vary, an antiviral drug such as pegylated interferon (PEG-IFN) is to be avoided when platelet counts fall below 25 to 50 000/µL or the dose need to be reduced when platelet counts fall below 5000 to 100 000/µL to reduce the risk of thrombocytopenia.2 Moreover, severe thrombocytopenia has been considered as a contraindication for PEG-IFN treatment initiation and maintenance.4
This condition was further strengthened by a retrospective multicenter study from Europe that consists of 466 patients with platelet counts <100 × 109/L in which about 184 of 466 (39.5%) were unable to take IFN-based therapy during an investigation. The management was discontinued because of various clinical problems such as liver cirrhosis (16.3%), thrombocytopenia (16.3 %), and age above 60 years (10.9%). Overall, in this study, the proportion of patients who failed to receive antiviral drugs due to thrombocytopenia is accounted for 4.9%.36 In addition, in the study involving 1538 patients with chronic HCV infection, 6.5% of the patients did not receive antiviral drugs because of the presence of thrombocytopenia.37 However, the development of PegIFN-free direct-acting antiviral agents currently reduced the challenge associated with thrombocytopenia that limits the treatment of HCV infection.38
Impeding invasive procedure
Most of the time, cirrhotic patients require medical procedures for diagnosis and treatment, some of which are invasive. However, thrombocytopenia increases the risk of bleeding from such invasive procedures and delays the routine care provided for the patients.2 Some studies have found no increase in the risk of bleeding in patients with platelet counts above 50 000/µL undergoing these procedures.2,39 Most of the physicians try to suspend invasive procedures associated with the risk of bleeding in patients with CLD until the patients’ platelet count rises to the required level to minimize the risk of potential bleeding related with the procedures. Various treatments such as dental procedures are postponed as a result of the risk of bleeding associated with thrombocytopenia.2 Taking these challenges into account, novel drugs that increase platelet counts through stimulation of TPO-Rs received more attention and are being investigated as an alternative treatment for thrombocytopenia in patients with CLD.
Challenge in the management of cancerous condition
Thrombocytopenia causes a critical problem in the management of various cancer types which in turn increased the risk of morbidity and mortality among patients suffering from cancer.40 When platelet counts become <10 000 and <50 000/µL, the conditions lead to spontaneous bleeding and obstacle the operation procedures, respectively. As chemotherapy and radiation therapy are known to impair bone marrow, initiation of these therapies in thrombocytopenic patients is the major challenge due to the risk of exacerbation of thrombocytopenia and associated with a high risk of bleeding.32 Overall, the advantage of anticancer drugs is significantly influenced by the incidence of thrombocytopenia which limits the use of appropriate doses and necessitates dose reduction that leads to the sub-therapeutic effects of the drugs.10
Biology and History of TPO-R Agonist Development
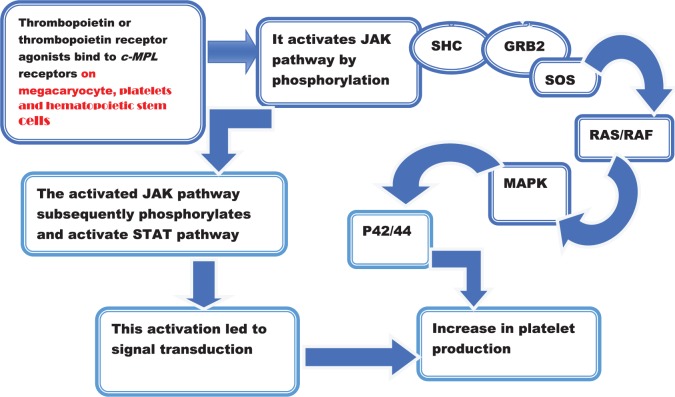
Thrombopoietin acts by interacting with specific cell surface receptor encoded by Mpl. Mpl gene was initially identified as a proto-oncogene for murine myeloproliferative leukemia virus. It is also present on the cell surface of megakaryocytes, platelets, and hematopoietic stem cells. The actual macromolecule that interacts with TPO is c-mpl, which is a type 1 homodimeric receptor existing as inactive state under normal physiologic condition.41 When the ligand, TPO, comes and interacts with c-mpl, a range of signal transduction occurs through the JAK and STAT pathways that become phosphorylated and enhance cell growth, and also involved in the activation of MAP kinase pathways,42 which contributed for the production of platelets. The effect of TPO binding to its receptors and the associated consequences were displayed in Figure 1.
Figure 1.
Activation of TPO receptors by TPO or TPO receptor agonist and subsequent cascade associated with the activated pathway. GRB2 indicates growth factor receptor–bound protein 2; RAF, rapidly accelerated fibrosarcoma; SOS, Son of Sevenless; TPO, thrombopoietin; JAK, Janus Kinase; STAT, signal transducer and activator of transcription; MAPK, Mitogen -activated protein kinase.
Thrombopoietin was first introduced in 1958 when it was known to control the production of platelets just as erythropoietin did in controlling red blood cell synthesis.42 Thrombopoietin was cloned in 1994 and considered as important hematopoietic cytokine that facilitates megakaryopoiesis.43 Recombinant full-length human TPO and pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) were the 2 first-generation TPOs that undergone clinical trials as well as used for the prevention of thrombocytopenia related to hematopoietic stem cell transplantation or anticancer drug used for hematological malignancies.44-46 Both in animals and human subjects, the aforementioned drugs were able to enhance megakaryocyte growth and platelet production. Various trials indicated the efficacy of TPO in patients with anticancer drug-induced thrombocytopenia and immune thrombocytopenia (ITP).42 Nevertheless, none of these drugs are approved and marketed for the treatment of thrombocytopenia as they increased the risk of thrombocytopenia and pancytopenia by neutralizing antibody through cross-reacting with the endogenous TPO (eTPO).46-48 These are caused by their antigenicity that halts the further investigation and limits the development of recombinant TPOs.42 However, intravenous administration of recombinant human TPO (rhTPO) has not been associated with the development of immunogenicity.49,50
Thrombopoietin plays a significant role in regulating the production of platelets by interacting with c-Mpl receptors. It is mainly produced in the liver, kidney, and bone marrow.51 The c-Mpl receptor serves as a crucial target to facilitate the investigation of new second-generation drugs such as TPO peptide, TPO nonpeptide, and TPO antibody mimetics those that are free from immunogenicity for the treatment of thrombocytopenia.52 These agents have no any common homology with the primary sequence of TPO and compete with the native molecule for c-Mpl binding. Nevertheless, they are able to bind to c-Mpl receptors and activate it.49,53,54 Thrombopoietin peptide mimetic, romiplostim (formerly, AMG 531), and TPO nonpeptide mimetic, eltrombopag (SB 497115-GR), were approved by FDA for the treatment of CITP patients who were refractory to the prior therapy in 2008.52 Currently, different drugs belonging to these categories are under investigation and also being approved for the management of some of the thrombocytopenic conditions.
Overview of TPO-R Agonists That Received FDA Approval
Romiplostim is one of the TPO-R agonists that received FDA approval by August 2008. It is an Fc-fusion protein that promotes the production of platelet by a mechanism related to that of naturally occurring eTPO. Although there is a similarity between romiplostim and eTPO in terms of their mechanism of action, romiplostim did not share same amino acid sequence homology with eTPO, and this reduces the chance of antibodies to romiplostim to bind to eTPO and inducing thrombocytopenia which is common with first-generation TPO.55 Subcutaneous administration of romiplostim in a dose that ranges from 1 to 10 µg/kg per week in patients with thrombocytopenia is reported to maintain platelet counts to >50 000/µL.56 According to the study conducted in 2 phase-III placebo-controlled studies in splenectomized and nonsplenectomized 142 patients, weekly administration of romiplostim over a period of 24 weeks in patients with CITP resulted in favorable response in about 83% of patients (ie, platelet counts ⩾50 × 109/L for a period of 4 weeks on study) and the response of platelet count to romiplostim lasts longer in about 49% of patients.20 In addition, phase I/II study carried out on pediatric patients with chronic immune thrombocytopenia (CIPT) illustrated 17 children who used romiplostim, 15 (88%) of them achieved platelet count of ⩾50 × 109/L for 2 consecutive weeks whereas 5 children who used placebo did not show any improvement (P = .0008).55 Moreover, another study conducted by administering romiplostim preoperatively for patients with chronic HCV infection and liver cirrhosis showed that out of 35 patients registered for the study, 33 of them achieved the desired target platelet count of 70 × 109/L from the baseline which was 31 × 109/L that facilitates the invasive procedure.57
Eltrombopag is an orally available, nonpeptide acting as a TPO-R agonist. The drug interacts with its corresponding TPO-R on megakaryocyte precursors and megakaryocytes, and facilitates their proliferation and differentiation to enhance the production of platelets.4 The drug is structurally unrelated to eTPO and noncompetitively interacts with TPO-R. Eltrombopag has a unique binding site from that of eTPO on c-mpl; therefore, it may have additive effects in the presence of TPO signaling which could lead to an additional proliferation of megakaryocyte.41
According to a phase-II trial conducted to evaluate the efficacy and safety of eltrombopag by involving 117 subjects, the study assigned 29 patients to placebo, whereas 30, 30, and 28 patients were assigned to 30, 50, and 75 mg of eltrombopag doses, respectively. Compared with placebo, the increment in platelet counts ⩾50 000/µL was observed in a dose-dependent fashion in which a statistically significant difference was reported with 50 and 75 mg of eltrombopag. On day 43 of the study period, the median rise in platelet count of placebo-treated group vs various doses of eltrombopag (30, 50, and 75 mg) was found to be 16 000 vs 26 000, 128 000, and 183 000/µL, respectively.58 In addition, report from 2 phase-III randomized controlled trials of ENABLE-1 (n = 715) and ENABLE-2 (n = 805) illustrated the efficacy of eltrombopag in increasing the number of platelets as well as facilitating initiation and maintenance of antiviral therapy with PEG and ribavirin (RBV) in patients with hepatitis B virus (HBV) infection. Accordingly, eltrombopag-treated group produced sustained viral response in both trials as compared with placebo (ENABLE-1, 23% vs 14%, P = .0064; ENABLE-2, 19% vs 13%, P = .0202). In ENABLE-1 and ENABLE-2, 69% and 81% of eltrombopag-treated patients vs 15% and 23% of placebo-treated patients were reported to maintain platelet counts of ⩾50 000/mL throughout the antiviral treatment phase respectively (Table 1).59
Table 1.
Some of the selected completed clinical trials of thrombopoietin receptor agonists for the treatment of various thrombocytopenias.
| Study/arms | Number of participants involved in the study (received drug of interest or placebo) | Dose | Clinical condition for which the drug is approved or under clinical trial | Baseline platelet counts | Primary endpoint | Proportion of patients who did not require platelet transfusions before invasive procedure | Reference |
|---|---|---|---|---|---|---|---|
| Romiplostim | Romiplostim received = 35 | 2 µg/kg | CITP | Mean 31 × 109/L | 70 × 109/L | 94.3% | Moussa and Mowafy57 |
| Eltrombopag | |||||||
| PETIT | Eltrombopag received = 45 Placebo received = 22 |
1-<6 years = 25 mg/day and ⩾6 years = 50 mg/day | CIPT | 30 × 109/L | ⩾50 × 109/L | 62% | Bussel et al60 |
| PETIT 2 | Eltrombopag received = 63 Placebo received = 29 |
1-5 years–1.2 mg/kg/day or 0·8 mg/kg/day for east Asian (oral suspension) | CITP | <30 × 109/L | ⩾50 × 109/L | 36% | Grainger et al61 |
| 6-11 years = 25 mg/day | CITP | <30 × 109/L | ⩾50 × 109/L | 42% | |||
| 12-17 years = 50 mg/day | CITP | <30 × 109/L | ⩾50 × 109/L | 39% | |||
| ENABLE-1 (PEG-2a) | Initiation phase = 715 In antiviral phases Eltrombopag received = 450 Placebo received = 232 |
25-100 mg/day | Thrombocytopenic patients with HCV infection and liver cirrhosis | 59 × 109/L (median baseline) | ⩾50 × 109/L (maintained sustained virologic response by preventing dose reduction for PEG-2a and RBV) | 69% | Afdhal et al59 |
| ENABLE-2 (PEG-2b) | Initiation phase = 805 In antiviral phase Eltrombopag received = 506 Placebo received = 253 |
⩾50 × 109/L (maintained sustained virologic response by preventing dose reduction for PEG-2b and RBV) | 81% | ||||
| Avatrombopag | |||||||
| ADAPT-1 | Avatrombopag received = 149 Placebo received = 82 |
90 patients received 60 mg of avatrombopag | Thrombocytopenia in patients with CLD | <40 × 109/L (mean baseline) | ⩾50 × 109/L | 62/90 = 68.9% | Terrault et al62 |
| 59 patients received 40 mg of avatrombopag | 40 to <50 × 109/L (mean baseline) | ⩾50 × 109/L | 52/59 = 88.1% | ||||
| ADAPT-2 | Avatrombopag received = 128 Placebo received = 76 |
70 patients received 60 mg of avatrombopag | Thrombocytopenia in patients with CLD | <40 × 109/L (mean baseline) | ⩾50 × 109/L | 47/70 = 67.1% | |
| 58 patients received 40 mg of avatrombopag | 40 to <50 × 109/L (mean baseline) | ⩾50 × 109/L | 54/58 = 93.1% | ||||
| Cohort A | Avatrombopag received = 51 Placebo received = 16 |
18 patients received 100mg loading dose/20 mg 16 patients received 100mg loading dose/40 mg 17 patients received |
Thrombocytopenia in patients with CLD | ⩾10 to ⩽58 × 109/L | ⩾50 × 109/L or increase by ⩾20 × 109/L from baseline | 49.0% | Terrault et al63 |
| Cohort B | Avatrombopag received = 42 Placebo received = 21 |
21 patients received 80 mg loading dose/10mg 21 patients received 80 mg loading dose/20mg |
47.6% | ||||
| Lusutrombopag | |||||||
| Lusutrombopag received = 46 Placebo received = 15 |
15 patients received 2 mg | Patients with CLD undergoing RFA | <50 × 109/L | Proportion of patients who did not require platelet transfusion before RFA (⩾50 × 109/L secondary endpoint) | 80.0% | Tateishi et al64 | |
| 16 patients received 3 mg | 81.3% | ||||||
| 15 patients received 4 mg | 93.3% | ||||||
Abbreviations: CITP indicates chronic immune thrombocytopenia; CLD, chronic liver disease; HCV, hepatitis C virus; RBV, ribavirin; RFA, radiofrequency ablation.
PETIT and PETIT-2 were phase-II/III and phase-III randomized controlled trials, respectively, that were designed to assess the safety and efficacy of eltrombopag among pediatric patients with CITP. The study involved children with the age of 1 to 17 years in whom ITP lasted for 6 months or more and a platelet count of <30 × 109/L. On the basis of results of both studies, PETIT and PETIT-2, for children ⩾6 years of age, the beginning dose is 50 mg per day whereas for those who are between 1 and 6 years, 25 mg per day of eltrombopag doses were recommended. Based on the patients’ response, the dose was titrated to reach a goal of platelet count set to be 50 × 109/L and not to go beyond 200 × 109/L, and the maximum dose of eltrombopag after dose titration is 75 mg per day. Accordingly, in phase II/III of PETIT, 62% of children with IPT achieved platelet counts of ⩾50 × 109/L from a baseline of <30 × 109/L, whereas in phase-III trials of PETIT 2, 36% of children with 1-5 years, 42% of children with 6 to 11 years, and 39% of children with 12 to 17 years obtained the desired level of platelet counts of ⩾50 × 109/L from the baseline which is <30 × 109/L (Table 1).41,60,61
Regarding adverse events (AEs), permanent discontinuation of antiviral drug therapy occurred in 27% of patients on placebo vs 19% patients on eltrombopag treatment due to AEs. With respect to ENABLE-1 study, 17% vs 28%, and in ENABLE-2, 21% vs 26% of eltrombopag and placebo-treated group discontinue antiviral treatment, respectively. On the contrary, the incidence of cataracts in ENABLE-1 was 8% vs 3% for eltrombopag and placebo, respectively, whereas the incidence was not statistically different in ENABLE-2 (eltrombopag, 7%; placebo, 6%). Moreover, headache, fatigue, gastrointestinal disorders, nausea, and diarrhea were the most commonly observed adverse effects in both studies. Nevertheless, the increase in blood bilirubin level and malignant hepatic neoplasm were among AEs assigned to be grade 3 and above in terms of their severity in both ENABLE-1 and ENABLE-2 studies.59 Overall, in PETIT and PETIT-2, the most common adverse effects reported during the trial period include headache, upper respiratory tract infection, nasopharyngitis, diarrhea, and transaminitis.60,61
In the lusutrombopag trial of phase-IIb placebo-controlled study that involved 61 patients with CLD, 15 patients were assigned to the placebo-treated group whereas 15, 16, and 15 patients were assigned to 2, 3 and 4 mg of lusutrombopag doses, respectively. The finding of the study indicated that the proportion of patients with CLD who did not require platelet transfusion before the procedure of radiofrequency ablation, as compared with placebo-treated group which is only 20%, was (80.0%, P = .0006), (81.3%, P = .0014), and (93.3%, P = .0002) with 2-, 3-, and 4-mg treated groups, respectively (Table 1). Besides the number of days (mean ± SE) in which lusutrombopag-treated patients maintained platelet count ⩾50 × 109/L and did not require platelet transfusion was 21.22 ± 1.56 for 2 mg, 21.76 ± 1.66 for 3 mg, and 24.23 ± 1.67 for 4 mg treated groups, whereas that of placebo-treated group was found to be 4.33 ± 1.57.64 In addition, a case report of a 56-year-old Japanese man showed that administration of lusutrombopag for 7 days rises platelet counts from 33 000 to 50 000/mm3.65 Similarly, administration of 3 mg of lusutrombopag 1 week prior to the initiation of hepatocellular carcinoma (HCC) treatment in a 62-year-old Japanese woman with a history of HCC treatment and HCV infection with the platelet count of 38 000/µL indicated platelet count increased to 98 000/µL from its baseline. Moreover, this report also demonsterated platelet counts went beyond the required level and led to discontinuation of treatment 5 days after the treatment was initiated. Therefore, this condition facilitated the treatment of HCC with invasive procedure of transcatheter arterial chemoembolization, and percutaneous radiofrequency ablation without the need for rescue platelet transfusion.66 In addition, the efficacy of lusutrombopag administration was also reported with the other 2 cases. Accordingly, the administration of lusutrombopag increased platelet counts from 20 000 to 40 000/µL in a 50-year-old man who developed cirrhosis due to HBV infection and alcohol consumption, whereas in a 30-year-old woman who developed cirrhosis due to HBV infection, the administration of the drug increased platelet counts from 41 000 to 68 000/µL. In both cases, the use of lusutrombopag administration led to an increase in platelet count, and partial splenic embolization was performed safely.67
Moreover, the drug of interest for this review, avatrombopag, also showed superior clinical efficacy among patients with CLD in raising platelet count to ⩾50 × 109/L from baseline as compared with placebo and reducing rescue treatment and platelet transfusion during the invasive procedure as reported by ADAPT-1, ADAPT-2, Cohort A, and Cohort B controlled trials (Table 1).62,63
Avatrombopag: Chemistry, Pharmacology, Clinical Trials, Efficacy, and Toxicological Profile
Chemistry
The international union of pure and applied chemistry (IUPAC) name of avatrombopag is 1-(3-chloro-5-{[4-(4-chlorothiophen-2-yl)-5-(4-cyclohexylpiperazin-1-yl)-1,3-thiazol-2-yl]carbamoyl}pyridin-2-yl)piperidine-4-carboxylic acid,68,69 and its chemical formula is C29H34Cl2N6O3S2 (see Figure 2).
Figure 2.
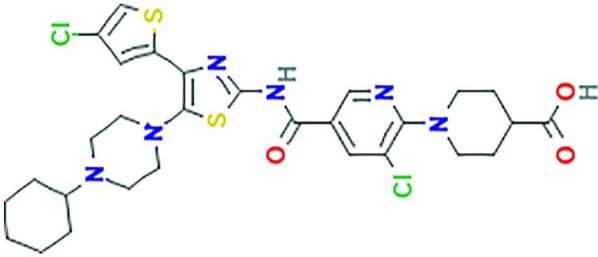
Chemical structure of avatrombopag.70
Mechanism of action of avatrombopag
Avatrombopag is a small molecule nonpeptide that stimulates TPO-R.71 Similar to other TPO-R agonists, avatrombopag is thought to interact with and stimulate TPO-R that mediates a cascade of cellular events through signal transduction by activating Janus kinase/signal transducer and activator of transcription (JAK-STAT) and Shc-Ras-Raf-ERK signaling pathways and finally promoting megakaryocyte differentiation into platelets.63 The binding site for avatrombopag on TPO-R is unique and separates from that of an eTPO-R agonist; therefore, avatrombopag does not interfere with the activity of eTPO receptors, rather it complements and produces additive effects that increase platelet production.69,71
In Vitro and In Vivo Preclinical Evaluation of the Activities of Avatrombopag
In vitro studies have shown that avatrombopag stimulates the proliferation of human c-Mpl-Ba/F3 cells with EC50 of 33 ± 2 nmol/L and enhances megakaryocytic colony formation from human CD34+ cells (EC50 24.8 ± 78 nmol/L). The maximum effect produced by AKR-501 is found to be equivalent to that of rhTPO. In a similar manner to rhTPO, AKR-501 causes stimulation that leads to tyrosine phosphorylation of STAT3 and STAT5 as well as threonine phosphorylation of ERK cells. Besides, AKR-501 mediates species-specific tyrosine phosphorylation of STAT5 in human and chimpanzee blood platelets similar to rhTPO. However, unlike rhTPO, AKR-501 did not promote tyrosine phosphorylation of blood platelets extracted from an olive baboon, cynomolgus monkey, rhesus monkey, common marmoset, squirrel monkey, beagle dog, guinea pig, rabbit, rat, and hamster.68 Another in vitro study also evaluated the effect of AKR-501 and TPO combination, AKR-501 alone, or TPO alone in a serum-free liquid culture system that composed of human peripheral blood CD34+. Following culturing, flow cytometry was used to measure the number of CD34+CD41– cells, CD34+CD41+ cells, and CD34–CD41+ cells. Based on this study, on day 14, both AKR-501 and TPO separately found to raise the number of megakaryocytes in a proportional manner to their doses, in which the ceiling effect of AKR-501 is concordant with that of TPO. Combination of 3 µM AKR-501 + 3 nM TPO found to raise the number of megakaryocytes approximately by 200% more as compared with a single use of 3 nM TPO.69
In addition, in vivo evaluation of oral avatrombopag found to increase the number of human platelets in a dose-dependent fashion in mice transplanted with human hematopoietic stem cells.72 Therefore, these in vitro and in vivo data illustrated significant therapeutic value of avatrombopag for the management of various thrombocytopenias that are associated with ITP, CLD, and cancer chemotherapeutic drugs.73
Clinical Evaluation of Avatrombopag (YM 477, AKR-501, and E5501) for Various Thrombocytopenias
From its inception to now, avatrombopag is being evaluated for various disorders of thrombocytopenia associated with CLD, immune thrombocytopenic purpura, and anticancer drugs. Therefore, this review tried to summarize the major findings of different clinical trials related to the aforementioned issues.
Phase-I study
As per the results of 2 double-blind, dose-rising, placebo-controlled phase-I studies in healthy adults reported by Kuter and Allen,73 63 individuals who fulfilled the required criteria were registered to a single-dose study of avatrombopag (1, 3, 10, 20, 50, 75, and 100 mg) and 9 participants were assigned to each of these doses, whereas in each cohort, 6 participants took avatrombopag while the remaining 3 of them used placebo. In the multiple-dose study population, 29 subjects were assigned, in which 9, 11, and 9 subjects were assigned to 3, 10, and 20 mg cohort studies, respectively. However, when the dose was increased to 20 mg, all participants of the study experienced a higher level of platelet counts than prespecified pharmacodynamic limit after 10 to 11 days of drug administration (ie, platelet counts ⩾500 × 109/L). Due to this reason, assignments of study subject to 50 or 100 mg dose cohorts were excluded. With regard to pharmacokinetics, after a single-dose administration of avatrombopag, detectable plasma concentration was achieved 0.5 to 1 hour later, whereas maximum plasma concentration was obtained at 4 to 6 hours after the drug was administered. In the multiple-dose study, after the first- and repeated-dose administration of avatrombopag, measurable plasma concentration obtained after 0.25 to 1 hour, whereas maximum plasma concentration was observed 4.5 to 6 hours after the first dose and the last dose on day 14. In terms of pharmacodynamics, in a single-dose study, avatrombopag dose less than 10 mg did not result in improvement of platelet count, while doses ranged from 10 to 100 mg were associated with an increase in platelet count significantly after 6 to 10 days of administration. On the contrary, in multiple-dose study, increment in platelet counts from baseline was demonstrated by 3, 10, and 20 mg though a significant change was detected only at a dose of 10 and 20 mg of avatrombopag after 3 to 5 days of administration.
Phase-II study
As reported by Terrault et al,63 phase-II study of avatrombopag assesses the safety and efficacy of the drug in the management of thrombocytopenia in patients with CLD who has planned to have an elective procedure. The study categorized 93 participants engaged in the study into 2 cohorts: cohort A involved 51 subjects and cohort B involved 42 participants. With respect to efficacy, the results of the 2 cohorts demonstrated that the proportion of responders among all avatrombopag-treated patients was 48.4% vs 8.1% in the combined placebo group (P < .0001). Taking into account the primary endpoint that dictates increment of platelet count to be ⩾20 × 109/L above baseline and at least 1 platelet count >50 × 109/L from days 4 to 8, when compared with placebo, the proportion of the patients who achieved primary endpoint of platelet count in cohort A was 49% vs 6.3%, whereas in cohort B, it was found to be 47.6% vs 9.5%. On the contrary, the proportion of patients who met the desired goal of platelet count were increased in both cohorts A and B when the loading dose was incorporated with the maintenance doses, in which the higher response was observed with the larger maintenance dose. Accordingly, in cohort A, the proportion of patients who obtained the required level of platelet count increased from 38.9% to 76.6% with 100/20 and 100/80 mg doses, respectively, whereas in cohort B, the proportion of patients who obtained the required level of platelet count increased from 42.9% to 52.4% with 80/10 mg arm and 80/20 mg doses, respectively. Moreover, in both cohorts A and B, avatrombopag produced a significant change in platelet count in a large number of respondents as compared with placebo (P < .01), except 100/40 mg treated group in cohort A (P = .17) which did not show statistical significance.
Another phase-II study reported by Bussel et al74 indicated that 64 patients with persistent and CITP received various doses of avatrombopag for a period of 28 days to assess the safety and efficacy of the drug, whereas in extension phase, 53 patients who completed randomized study received avatrombopag for a period of 24 weeks to assess its safety and tolerability. With regard to efficacy in a randomized study, a large number of patients who received avatrombopag obtained a higher platelet count than the placebo group. Out of 64 patients involved in the randomized study, 13%, 53%, 50%, and 80% of those who received 2.5, 5, 10, and 20 mg doses of avatrombopag, respectively, obtained a rise in platelet count of ⩾50 and ⩾20 × 109/L from a baseline at day 28 vs 0% for placebo. Moreover, treatment with 20 mg of avatrombopag resulted in significantly higher response (80%) in relation to 0% in placebo (P = .0036). In addition, the proportion of patients who achieved platelet count ⩾100 × 109/L on day 28 is 53% for those patients treated with 20 mg of avatrombopag and 6.7% for those patients treated with 2.5 mg of avatrombopag. Coming to the extension phase, of 53 individuals involved in the study, 76% of participants favorably respond to avatrombopag, whereas 53% of participants were able to produce a sustained response. Overall, in the extension study, platelet counts raised from 22 × 109/L at baseline to 56 × 109/L at week 4 (n = 51) and to 112.5 × 109/L at week 24 (n = 38) in participants engaged in the study. Among participants respond to avatrombopag in a 4-week randomized study, 72% obtained sustained response while only 36% of nonrespondents of the randomized study produced the sustained response in the extension study.
Phase-III study
The primary endpoint of 2 phase-III study reported by Terrault et al75 is the proportion of patients who did not require a platelet transfusion or rescue procedure for bleeding after randomization and up to 7 days after a scheduled procedure in patients with CLD. In ADAPT-1, a total of 149 patients were randomized to avatrombopag and 82 patients to placebo in the 2 baseline platelet count cohorts, whereas in ADAPT-2, 128 patients randomized to avatrombopag and 76 patients to placebo arms. The efficacy results indicated that in the ADAPT-1 study, 65.6% of patients who received 60 mg avatrombopag (baseline platelet counts <40 × 109/L) and 88.1% of patients who received 40 mg avatrombopag (baseline platelet counts 40 × 109/L to <50 × 109/L) met the primary endpoint compared with 22.9% and 38.2% of patients receiving placebo, respectively (P < .0001 for both). In the ADAPT-2 study, 68.6% of patients who received 60 mg avatrombopag (baseline platelet counts <40 × 109/L) and 87.9% of patients who received 40 mg avatrombopag (baseline platelet counts 40 × 109/L to <50 × 109/L) met the primary endpoint compared with 34.9% and 33.3% of patients who received placebo, respectively (P < .001 for both). Avatrombopag led to a measurable increase in platelet counts and increased the proportion of patients who achieved the target platelet count ⩾50 × 109/L on procedure day as compared with placebo. Similarly, another phase-III trial evaluating the efficacy and safety of avatrombopag is reported by Michelson et al.76 The study involved 30 adult patients whose thrombocytopenic condition is associated with CLD and registered at US sites in phase III of ADAPT-1 (NC 01972529) and ADAPT-2 (NCT01976104) studies. The results of the study demonstrated that in its once-daily dosing, avatrombopag, at both 60 and 40 mg, was able to produce a 2-fold increase in the platelet counts on day 10 as compared with the placebo-treated group. However, there is no increase in platelet activation in avatrombopag-treated patients at both 60 and 40 mg daily doses administered to the patients as compared with the placebo-treated group. This was evaluated by measuring platelet surface P-selectin and platelet surface–activated GPIIb-IIIa. Accordingly, the findings indicated that platelet reactivity at day 4 or 10, as measured by platelet surface P-selectin and platelet surface–activated GPIIb-IIIa after low and high concentrations of adenosine diphosphate (ADP) or thrombin receptor activating peptide (TRAP), was not further increased in avatrombopag-treated patients as compared with placebo-treated patients.
Another phase-III trial designed as core study and extension phase that conducted by Jurczak et al77 concerning with the efficacy and safety of avatrombopag 20 mg/day in an adult with CITP demonstrated that avatrombopag was found to be superior to placebo in the cumulative number of weeks of platelet response. Moreover, the trial showed that significantly longer duration of platelet counts ⩾50 × 109/L in the absence of rescue therapy was observed with avatrombopag as compared with placebo (median: 12.4 vs 0.0 weeks; Mean: 12.0 vs 0.1 weeks; P < .0001). In addition, more patients in the avatrombopag-treated group (21/32, 65.6%) had a platelet response at day 8 than in the placebo-treated group (0/17, 0.0%, P < .0001). Although statistically not significant (N = 22, P = .1348) because of a small number of patients who used ITP medication, patients in the avatrombopag-treated group reduced the use of concomitant ITP medication from baseline as compared with placebo-treated patients (33.3% vs 0%, respectively; 95% confidence interval, 9.48-57.19). The durable platelet response rate was significantly greater in avatrombopag-treated patients as compared with those receiving placebo (34.4% vs 0.0%; P = .009; 95% confidence interval, 17.92-50.83). The median platelet count by a visit in avatrombopag-treated patients was reliably higher than that of the placebo-treated group starting at day 8 (80.5 vs 8 × 109/L), whereas the median platelet count for the placebo-treated group remained unaltered throughout the core study.
Therefore, several clinical trials are underway in evaluating the safety and efficacy of avatrombopag in patients with CLD, ITP, anticancer-induced thrombocytopenia, as well as pharmacokinetics and pharmacokinetic/pharmacodynamic (PK/PD). The most common primary endpoints were the proportion of subjects who had platelet counts greater than or equal to 50 × 109/L or at least ⩾20 × 109/L increase from baseline (Table 2).
Table 2.
Clinical trials undergoing for avatrombopag (E5501, AKR 501, YM477) for the treatment of various thrombocytopenia (www.clinicaltrials.gov).
| Sr. No | Trial identifier (number) |
Phase of the study | Trial design | Intervention/treatment | Primary endpoints expected outcome | Status |
|---|---|---|---|---|---|---|
| 1 | NCT03326843 | Phase III | Open-label study to evaluate the efficacy and safety of avatrombopag for the treatment of subjects with thrombocytopenia scheduled for a surgical procedure | Avatrombopag 60 mg | Proportion of subjects who achieve a platelet count >100 × 109/L on procedure day | Terminated |
| 2 | NCT03471078 | Phase III | Randomized, double-blind, placebo-controlled study with an open-label extension to evaluate the efficacy and safety of avatrombopag for the treatment of chemotherapy-induced thrombocytopenia in subjects with active nonhematological cancers | Avatrombopag Placebo oral tablet |
Proportion of subjects who do not require platelet transfusion, dose reduction in chemotherapy by 15%, or chemotherapy delay by ⩾4 days | Recruiting |
| 3 | NCT03554759 | Phase IV | An observation, cohort study of the use of avatrombopag in patients with thrombocytopenia associated with CLD undergoing a procedure | Avatrombopag | Change in platelet count up to 8 days after the last dose of avatrombopag | Terminated |
| 4 | NCT02227693 | Phase II | Randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy, safety, and pharmacokinetics of once-daily oral avatrombopag in Japanese subjects with CLDs and thrombocytopenia | Avatrombopag Placebo |
Proportion of subjects who had platelet count ⩾50 × 109/L and at least 20 × 109/L increase from baseline at visit 4 | Completed |
| 5 | NCT01976104 and NCT01972529 | Phase III | Randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of once-daily oral avatrombopag for the treatment of adults with thrombocytopenia associated with liver disease prior to an elective procedure | Avatrombopag Placebo |
Percentage of participants who did not require a platelet transfusion up to 7 days following a scheduled procedure | Completed |
| 6 | NCT01438840 | Phase III | Randomized, double-blind, placebo-controlled, parallel-group trial with an open-label extension phase to evaluate the efficacy and safety of oral E5501 plus standard of care for the treatment of thrombocytopenia in adults with CITP/idiopathic thrombocytopenic purpura | Avatrombopag Placebo Standard of care |
Number of weeks with platelet count greater ⩾50 × 109/L during 6-month treatment period | Completed |
| 7 | NCT00914927 | Phase II | Randomized, placebo-controlled, double-blind, parallel-group study to evaluate the efficacy, safety, and population PKs of once-daily oral E5501 tablets used up to 7 days in subjects with CLD and thrombocytopenia prior to elective surgical or diagnostic procedures | Avatrombopag Placebo |
Percentage of participants experiencing response, ie, a participant having an increase of at least 20 000/mm3 platelet count from baseline and a platelet count >50 000/mm3 at least once during day 4 through day 8 | Completed |
| 8 | NCT02039076 | Phase I | Randomized, open-label, 5-treatment-period study to evaluate the PK and PD of avatrombopag following a single-dose administration of avatrombopag in the fed and fasted condition to healthy Japanese and white subjects | Avatrombopag | Primary outcome: PK profiles of avatrombopag—Cmax, AUC, and t1/2 | Completed |
| 9 | NCT01355289 | Phase II | Randomized, placebo-controlled, double-blind, parallel-group study, with an open-label extension, to evaluate the efficacy, safety, and pharmacokinetics of E5501 in subjects with chronic hepatitis C virus–related thrombocytopenia who are potential candidates for antiviral treatment | Avatrombopag Placebo PEG-IFN Telaprevir Ribavirin |
Number of participants who achieved platelet response ⩾100 × 109/L) by day 21 of treatment period | Completed |
| 10 | NCT01433978 | Phase III | Randomized, double-blind, active-controlled, parallel-group trial with an open-label extension phase to evaluate the efficacy and safety of oral E5501 vs eltrombopag, in adults with CITP | Eltrombopag Avatrombopag Standard of care |
Change from baseline in local platelet count for the 6-month treatment period (day 5, day 8, weeks 2, 3, 4, 6, 8, 10, 12, 14, 16, 18, 19, 20, 22, 23, 24, 25, 26) | Terminated |
| 11 | NCT00441090 | Phase II | Randomized, double-blind, placebo-controlled, dose-ranging, parallel-group study of AKR-501 tablets taken orally once daily for 28 days in patients with ITP | Placebo Avatrombopag tablets |
Response rate to avatrombopag on day 28 | Completed |
| 12 | NCT00625443 | Phase II | This is a nonrandomized study used to determine the safety and efficacy of AKR-501 administered in participants with chronic ITP who were enrolled into and completed 28 days of study treatment in Protocol 501-CL-003 (NCT00441090) | Placebo Avatrombopag tablets |
Number of participants with treatment-emergent adverse events—from day 1 to 6 months while receiving the treatment and 7 months after discontinuation of treatment Incidence of severe (Grade 3 or 4) treatment-emergent adverse events—from day 1 to 6 months while receiving the treatment and 7 months after discontinuation of treatment Incidence of drug-related treatment-emergent adverse events—from day 1 to 6 months while receiving the treatment and 7 months after discontinuation of treatment |
Completed |
Abbreviations: CITP indicates chronic immune thrombocytopenia; CLD, chronic liver disease; ITP, immune thrombocytopenia; PD, pharmacodynamics; PEG-IFN, pegylated interferon; PK, pharmacokinetics.
Pharmacokinetics of Avatrombopag
According to a phase-I study conducted to evaluate the pharmacokinetics and PK/PD of avatrombopag in Japan using Japanese and white ethnic group patients, the maximum plasma concentration of avatrombopag after administration of a single dose of 20, 40, and 60 mg in the fed state was obtained 6 to 8 hours later. There are no significant variation in terms of half-life between the Japanese (16-17 hours) and white ethnic (18 to 19 hours) groups. However, the maximum concentration (Cmax) of the drug achieved in plasma for 40 and 60 mg doses of avatrombopag was slightly larger for the Japanese ethnic group as compared with white subjects involved in the study. Accordingly, Japanese Cmax was 29% larger than that of white subjects, whereas area under the curve (AUC) is almost the same for both ethnic groups at 40 and 60 mg doses. For the doses stated, the ratios of the percentage of AUC for Japanese to white subjects were 108% and 104%, respectively. Consumption of food that is rich in fat contents tends to decrease variation in PK of avatrombopag expected to be observed within the subject and between the subjects. In Japanese subjects, when comparing fed and empty (fasted) state, the least square mean values for 40 and 60 mg doses were 83.9% vs 96.2% for Cmax, 91.9% vs 108% for AUC as well as in white subjects, 132% vs 144% for Cmax, whereas that of AUC 156% and 180%, respectively. On the contrary, tmax of avatrombopag in any of the ethnic groups did not influence by a meal that is rich in fat for both 40 and 60 mg doses.78
Although platelet response change is not sound, the polymorphism (*2, *3) observed was related to a high risk of PK variability. Avatrombopag is known to be a substrate for cytochrome P450, CYP2C9, and CYP3A. Co-administration of a single dose of 20 mg of avatrombopag with fluconazole demonstrated increment in the terminal half-life from 19.7 to 39.9 hours and causes a 1.66-fold increase in the maximum platelet count. Nevertheless, co-administration of rifampicin resulted in a 0.5-fold decrease in AUC and terminal half-life (from 20.3 to 9.84 hours).79
Toxicological Profiles of Avatrombopag
According to the Jurczak et al report of phase-III study of avatrombopag efficacy for CITP, the tendency of any bleeding event that occurred during the study was not statistically significant between the controlled group and avatrombopag-treated group (43.8% vs 52.9%, P = .5394). All observed bleeding events were graded to be World Health Organization (WHO) grade 1, except 3 patients who encountered WHO grade 2 (2 patients) and grade 3 (1 patient). Above all, 21.9% of patients who received avatrombopag and 11.8% of patients who used placebo need a rescue treatment. Nevertheless, there was no statistical difference between avatrombopag- and placebo-treated groups in terms of using rescue treatment (P = .4668). Of patients received avatrombopag, 4 of them faced grade 3 treatment-emergent adverse events (TEAEs), in which epistaxis, petechiae, headache, and platelet count reduction were considered to be severe and associated with the study drug. In the other 2 cases of grade 4 TEAEs observed, 1 patient discontinued treatment due to a cerebrovascular accident while 1 experienced worsening of ITP that was not thought to link with the study drug. Moreover, TEAEs most frequently reported in the avatrombopag received group was a headache, contusion, upper respiratory tract infection, arthralgia, epistaxis, fatigue, gingival bleeding, and petechiae, none of which were significantly different from placebo-treated groups.77
Overall, the frequency of TEAEs in low- and high-baseline platelet counts in both ADAPT-1 and ADAPT-2 between placebo- vs avatrombopag-treated groups indicated as 64.6% vs 59.6%, 56.3% vs 53.4%, 51.2% vs 51.4%, and 45.5% vs 49.1%, respectively. Therefore, in ADAPT-1 and ADAPT-2 cohorts, the occurrence of TEAEs was comparable between avatrombopag- and placebo-treated groups in both low- and high-baseline platelet count groups. Similarly, the phase-II study reported by Terrault et al indicated that the occurrence of any TEAEs in cohorts A and B were 75% vs 84.3% and 76.2% vs 83.3% between combined placebo- and avatrombopag-treated groups (for different doses), respectively. In addition, the report by Kuter and Allen showed that the development of TEAEs between placebo- and avatrombopag-treated groups was 33.3% vs 26.2%. Moreover, the most commonly encountered TEAEs in these cohorts include abdominal pain, diarrhea, nausea, headache, dizziness, dysgeusia, dyspepsia, fatigue, edema peripheral, and pyrexia, among others (Table 3).
Table 3.
Common adverse events reported by 3 trials conducted on avatrombopag.
| Adverse events | Adverse events reported from 3 selected studies |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Terrault et al62 |
Terrault et al63 |
Kuter and Allen73 |
|||||||||||||||||||||||
| ADAPT-1 |
ADAPT-2 |
Cohort A |
Cohort B |
Multiple dose |
|||||||||||||||||||||
| Low-baseline platelet count |
High-baseline platelet count |
Low-baseline platelet count |
High-baseline platelet count |
Placebo (n = 16) | 20 mg (n = 18) | 40 mg (n = 16) | 80 mg (n = 17) | Placebo (n = 21) | 10 mg (n = 21) | 20 mg (n = 21) | Placebo (n = 21) | 1 mg (n = 6) | 3 mg (n = 6) | 10 mg (n = 6) | 20 mg (n = 6) | 50 mg (n = 6) | 75 mg (n = 6) | 100 mg (n = 6) | |||||||
| Placebo (n = 48) | AVP 60 mg (n = 89) |
Placebo (n = 32) | AVP 40 mg (n = 58) |
Placebo (n = 43) | AVP 60 mg (n = 70) |
Placebo (n = 33) | AVP 40 mg (n = 57) |
||||||||||||||||||
| Patients with any TEAEs | 31 (64.6%) | 53 (59.6%) | 18 (56.25%) | 31 (53.4%) | 22 (51.2%) | 36 (51.4%) | 15 (45.5%) | 28 (49.12%) | 12 (75%) | 17 (94.4%) | 13 (81.3%) | 13 (76.5%) | 16 (76.2%) | 17 (81.0%) | 18 (85.7%) | 7 (33.3%) | 2 (33.3%) | 1 (16.7%) | 2 (33.3%) | 1 (16.7%) | 1 (16.7%) | 2 (33.3%) | 2 (33.3%) | ||
| Drug therapy-related TEAEs | 7 (14.6%) | 12 (13.5%) | 2 (6.3%) | 4 (6.9%) | 9 (20.9%) | 6 (8.6%) | 2 (6.1%) | 4 (7.0%) | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ||
| Dysgeusia | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 (9.5%) | 1 (16.7%) | 1 (16.7%) | 1 (16.7%) | 1 (16.7%) | 0 | 1 (16.7%) | 1 (16.7%) | ||
| Flatulence | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 (9.5%) | 1 (16.7%) | 0 | 0 | 0 | 0 | 0 | (16.7%) | ||
| Diarrhea | 1 (2.1%) | 4 (4.5%) | 2 (6.3%) | 1 (1.7%) | 3 (7.0%) | 3 (4.3%) | 0 | 2 (3.5%) | 0 | 2 (11.1%) | 1 (6.3%) | 1 (5.9%) | 2 (9.5%) | 0 | 3 (14.3%) | 1 (4.8%) | 0 | 0 | 0 | 0 | 0 | 0 | (16.7%) | ||
| Headache | 3 (6.3%) | 5 (5.6%) | 2 (6.3%) | 6 (10.3%) | 4 (9.3%) | 2 (2.9%) | 1 (3.0%) | 2 (3.5%) | 2 (12.5%) | 2 (11.1%) | 2 (12.5%) | 1 (5.9%) | 3 (14.3%) | 1 (4.8%) | 3 (14.3%) | ||||||||||
| Somnolence | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 (4.8%) | 0 | 0 | 0 | 1 (16.7%) | 1 (16.7%) | 0 | 0 | ||
| Nausea | 2 (4.2%) | 4 (4.5%) | 2 (6.3%) | 5 (8.6%) | 5 (11.6%) | 6 (8.6%) | 6 (8.6%) | 3 (5.3%) | 2 (12.5%) | 1 (5.6%) | 2 (12.5%) | 2 (11.8%) | 3 (14.3%) | 5 (23.8%) | 2 (9.5%) | 1 (4.8%) | 0 | 1 (16.7) | 0 | 0 | 0 | 0 | 0 | ||
| Vomiting | – | – | – | – | – | – | – | – | 0 | 1 (5.6%) | 2 (12.5%) | 3 (17.6%) | 1 (4.8%) | 0 | 1 (4.8%) | – | – | – | – | – | – | – | – | ||
| Lethargy | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 | 0 | 0 | 1 (16.7%) | 0 | 0 | 0 | 0 | ||
| Muscle contraction | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (16.7%) | ||
| Portal hypertensive gastropathy | – | – | – | – | – | – | – | – | 0 | 4 (22.2%) | 2 (12.5%) | 0 | 2 (9.5%) | 3 (14.3%) | 0 | ||||||||||
| Abdominal pain | 3 (6.3%) | 8 (9.0%) | 3 (9.4%) | 6 (10.3%) | 3 (7.0%) | 2 (2.9%) | 1 (3.0%) | 2 (3.5%) | 0 | 4 (22.2%) | 1 (6.3%) | 1 (5.9%) | 3 (14.3%) | 2 (9.5%) | 0 | ||||||||||
| Fatigue | 1 (2.1%) | 6 (6.7%) | 1 (3.1%) | 1 (1.7%) | 3 (7.0%) | 1 (1.4%) | 0 | 2 (3.5%) | 1 (6.3%) | 4 (22.2%) | 1 (6.3%) | 0 | 4 (19%) | 2 (9.5%) | 2 (9.5%) | ||||||||||
| Edema peripheral | 2 (4.2%) | 3 (3.4%) | 1 (3.1%) | 3 (5.2%) | 2 (6.1%) | 0 | 3 (9.1%) | 0 | – | – | – | – | – | – | – | ||||||||||
| Pyrexia | 6 (12.5%) | 7 (7.9%) | 2 (6.3%) | 5 (8.6%) | 2 (4.7%) | 11 (15.7%) | 4 (12.1) | 4 (7.0%) | 2 (12.5%) | 0 | 0 | 2 (11.8%) | 3 (14.3%) | 1 (4.8%) | 0 | ||||||||||
| Dyspepsia | 2 (4.2) | 0 | 2 (6.3) | 0 | 0 | – | – | – | 2 (12.5) | 0 | 0 | 2 (11.8%) | 3 (14.3%) | 1 (4.8%) | 0 | ||||||||||
| Dizziness | – | – | – | – | 3 (7.0%) | 3 (4.3%) | 1 (3.0%) | 0 | 1 (6.3%) | 2 (11.1%) | 0 | 1 (5.9%) | 1 (4.8%) | 3 (14.3%) | 1 (4.8%) | ||||||||||
Abbreviation: TEAEs indicate treatment-emergent adverse events; AVP -avatrombopag.
Conclusions
Thrombocytopenia is the most frequent problem that has been observed in patients with CLD, and this condition leads to limit the utility of available treatment option for the patients. Although platelet transfusion is considered as a gold standard option to be used to undertake invasive procedure among patients with CLD, refractoriness and various infections associated with platelet transfusion significantly reduced the use of this option. However, the advance in the knowledge of TPO-R agonists and their role as alternative agents in reducing the rescue therapy or platelet transfusion in patients with CLD who need to undergo an elective surgery received great attention. Therefore, the current review is attempted to address the potential advantages of a TPO-R agonist, avatrombopag, in the management of thrombocytopenia associated with CLD and other conditions. Avatrombopag is one of the second-generation TPO-R agonists that enhance megakaryocytes and platelet production. Currently, this drug obtained FDA approval in May 2018 as an alternative option for the treatment of thrombocytopenia in patients with CLD who need to undergo the elective procedure. This drug provides a superior clinical effect as compared with placebo in facilitating invasive diagnostic and treatment procedures by reducing the frequency of rescue therapy or platelet transfusion. It is also under clinical investigation for patients with ITP, HCV-infected patients who are on antiviral drugs, and cancer patients who are on anticancer drugs. Especially in the last 2 cases, avatrombopag was able to prevent dose reduction of antiviral and anticancer drugs to preserve their helpful impacts. Avatrombopag is known to be a substrate for cytochrome P450, CYP2C9, and CYP3A. Therefore, co-administration of drugs that inhibit or induce these isoenzymes may affect the plasma concentration of avatrombopag. This may be observed as increase or decrease in its duration of action depending on the types of drugs co-administered. Despite a few adverse effects observed in various trials, severe AEs are less commonly noticed, and in most cases, it does not significantly differ from the controlled group. Some of the commonly encountered AEs include headache, upper respiratory tract infection, arthralgia, epistaxis, fatigue, gingival bleeding, petechiae, dizziness, abdominal pain, diarrhea, and nausea.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: The author prepared key terms for searching of relevant articles, summarize it and prepared final manuscript for publication.
ORCID iD: Jemal Abdela  https://orcid.org/0000-0003-4205-8641
https://orcid.org/0000-0003-4205-8641
References
- 1. Neuberger J. An update on liver transplantation: a critical review. J Autoimmun. 2016;66:51-59. [DOI] [PubMed] [Google Scholar]
- 2. Poordad F. Thrombocytopenia in chronic liver disease. Aliment Pharmacol Ther. 2007;26:5-11. [DOI] [PubMed] [Google Scholar]
- 3. Ansari MZ, Tolstoy R, Jagadeeswaran G. A rare etiology of severe thrombocytopenia in patient with chronic liver disease. J Assoc Physicians India. 2018;66:86-87. [PubMed] [Google Scholar]
- 4. Maan R, de Knegt RJ, Veldt BJ. Management of thrombocytopenia in chronic liver disease: focus on pharmacotherapeutic strategies. Drugs. 2015;75:1981-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giannini E. Thrombocytopenia in chronic liver disease and pharmacologic treatment options. Aliment Pharmacol Ther. 2006;23:1055-1065. [DOI] [PubMed] [Google Scholar]
- 6. Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. J Hepatol. 1994;21:656-666. [DOI] [PubMed] [Google Scholar]
- 7. Giannini EG, Greco A, Marenco S, Andorno E, Valente U, Savarino V. Incidence of bleeding following invasive procedures in patients with thrombocytopenia and advanced liver disease. Clin Gastroenterol Hepatol. 2010;8:899-902, quiz e109. [DOI] [PubMed] [Google Scholar]
- 8. Seeff LB, Everson GT, Morgan TR, et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8:877-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohta M, Nishizaki T, Matsumoto T, et al. Analysis of risk factors for massive intraoperative bleeding during laparoscopic splenectomy. J Hepatobiliary Pancreat Surg. 2005;12:433-437. [DOI] [PubMed] [Google Scholar]
- 10. Demetri GD. Targeted approaches for the treatment of thrombocytopenia. Oncologist. 2001;6:15-23. [DOI] [PubMed] [Google Scholar]
- 11. Szczepiorkowski ZM, Dunbar NM. Transfusion guidelines: when to transfuse. Hematology Am Soc Hematol Educ Program. 2013;2013:638-644. [DOI] [PubMed] [Google Scholar]
- 12. Afdhal N, McHutchison J, Brown R, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000-1007. [DOI] [PubMed] [Google Scholar]
- 13. Spiess BD. Platelet transfusions: the science behind safety, risks and appropriate applications. Best Pract Res Clin Anaesthesiol. 2010;24:65-83. [DOI] [PubMed] [Google Scholar]
- 14. McCullough J. Current issues with platelet transfusion in patients with cancer. Semin Hematol. 2000;37:3-10. [DOI] [PubMed] [Google Scholar]
- 15. Perrotta PL, Snyder EL. Non-infectious complications of transfusion therapy. Blood Rev. 2001;15:69-83. [DOI] [PubMed] [Google Scholar]
- 16. Kerkhoffs JLH, Eikenboom JC, van de Watering LM, van Wordragen-Vlaswinkel RJ, Wijermans PW, Brand A. The clinical impact of platelet refractoriness: correlation with bleeding and survival. Transfusion. 2008;48:1959-1965. [DOI] [PubMed] [Google Scholar]
- 17. Novotny V. Prevention and management of platelet transfusion refractoriness. Vox Sang. 1999;76:1-13. [DOI] [PubMed] [Google Scholar]
- 18. Snyder EL, Stramer SL, Benjamin RJ. The safety of the blood supply—time to raise the bar. N Engl J Med. 2015;372:1882-1885. [DOI] [PubMed] [Google Scholar]
- 19. Rodeghiero F, Carli G. Beyond immune thrombocytopenia: the evolving role of thrombopoietin receptor agonists. Ann Hematol. 2017;96:1421-1434. [DOI] [PubMed] [Google Scholar]
- 20. Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. 2009;113:2161-2171. [DOI] [PubMed] [Google Scholar]
- 21. Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371:395-403. [DOI] [PubMed] [Google Scholar]
- 22. Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357:2237-2247. [DOI] [PubMed] [Google Scholar]
- 23. Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011;377:393-402. [DOI] [PubMed] [Google Scholar]
- 24. Afdhal NH, Giannini EG, Tayyab G, et al. Eltrombopag before procedures in patients with cirrhosis and thrombocytopenia. N Engl J Med. 2012;367:716-724. [DOI] [PubMed] [Google Scholar]
- 25. Mitchell O, Feldman DM, Diakow M, Sigal SH. The pathophysiology of thrombocytopenia in chronic liver disease. Hepatic Med. 2016;8:39-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pereira J, Accatino L, Alfaro J, Brahm J, Hidalgo P, Mezzano D. Platelet autoantibodies in patients with chronic liver disease. Am J Hematol. 1995;50:173-178. [DOI] [PubMed] [Google Scholar]
- 27. Wang C-S, Yao W-J, Wang S-T, Chang T-T, Chou P. Strong association of hepatitis C virus (HCV) infection and thrombocytopenia: implications from a survey of a community with hyperendemic HCV infection. Clin Infect Dis. 2004;39:790-796. [DOI] [PubMed] [Google Scholar]
- 28. Ballard HS. Hematological complications of alcoholism. Alcohol Clin Exp Res. 1989;13:706-720. [DOI] [PubMed] [Google Scholar]
- 29. Nagamine T, Ohtuka T, Takehara K, Arai T, Takagi H, Mori M. Thrombocytopenia associated with hepatitis C viral infection. J Hepatol. 1996;24:135-140. [DOI] [PubMed] [Google Scholar]
- 30. Doi T, Homma H, Mezawa S, et al. Mechanisms for increment of platelet associated IgG and platelet surface IgG and their implications in immune thrombocytopenia associated with chronic viral liver disease. Hepatol Res. 2002;24:23-33. [DOI] [PubMed] [Google Scholar]
- 31. Peck-Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver Int. 2017;37:778-793. [DOI] [PubMed] [Google Scholar]
- 32. Kuter DJ. Managing thrombocytopenia associated with cancer chemotherapy. Oncology (Williston Park). 2015;29:282-294. [PubMed] [Google Scholar]
- 33. Davis GL, Lau JY, Lim HL. Therapy for chronic hepatitis C. Gastroenterol Clin North Am. 1994;23:603-613. [PubMed] [Google Scholar]
- 34. Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147:677-684. [DOI] [PubMed] [Google Scholar]
- 35. Danish FIA, Yasmin S. The role of eltrombopag in the management of hepatitis C virus-related thrombocytopenia. Hepat Med. 2013;5:17-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rizzetto M, Grotzinger K, Theodore D, et al. Reasons for nonuse of antiviral treatment in patients with chronic hepatitis C infection and thrombocytopaenia: a retrospective chart review from five European countries. J Viral Hepat. 2014;21:e129-e134. [DOI] [PubMed] [Google Scholar]
- 37. Giannini EG, Marenco S, Fazio V, Pieri G, Savarino V, Picciotto A. Peripheral blood cytopaenia limiting initiation of treatment in chronic hepatitis C patients otherwise eligible for antiviral therapy. Liver Int. 2012;32:1113-1119. [DOI] [PubMed] [Google Scholar]
- 38. Bourliere M. Peripheral blood cytopenia before treatment in HCV patients: is it a limitation for HCV treatment in the era of DAA? Liver Int. 2012;32:1033-1036. [DOI] [PubMed] [Google Scholar]
- 39. Inabnet WB, Deziel DJ. Laparoscopic liver biopsy in patients with coagulopathy, portal hypertension, and ascites. Am Surg. 1995;61:603-606. [PubMed] [Google Scholar]
- 40. Skomorovski K, Harpak H, Ianovski A, et al. New TPO treatment schedules of increased safety and efficacy: pre-clinical validation of a thrombopoiesis simulation model. Br J Haematol. 2003;123:683-691. [DOI] [PubMed] [Google Scholar]
- 41. Kim TO, Despotovic J, Lambert MP. Eltrombopag for use in children with immune thrombocytopenia. Blood Adv. 2018;2:454-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kuter DJ. The biology of thrombopoietin and thrombopoietin receptor agonists. Int J Hematol. 2013;98:10-23. [DOI] [PubMed] [Google Scholar]
- 43. Kaushansky K. Thrombopoietin. N Engl J Med. 1998;339:746-754. [DOI] [PubMed] [Google Scholar]
- 44. Kizaki M, Miyakawa Y, Ikeda Y. Long-term administration of pegylated recombinant human megakaryocyte growth and development factor dramatically improved cytopenias in a patient with myelodysplastic syndrome. Br J Haematol. 2003;122:764-767. [DOI] [PubMed] [Google Scholar]
- 45. Kaushansky K. Historical review: megakaryopoiesis and thrombopoiesis. Blood. 2008;111:981-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kuter DJ. New thrombopoietic growth factors. Blood. 2007;109:4607-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Basser RL, O’Flaherty E, Green M, et al. Development of pancytopenia with neutralizing antibodies to thrombopoietin after multicycle chemotherapy supported by megakaryocyte growth and development factor. Blood. 2002;99:2599-2602. [DOI] [PubMed] [Google Scholar]
- 48. Li J, Yang C, Xia Y, et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001;98:3241-3248. [DOI] [PubMed] [Google Scholar]
- 49. Vadhan-Raj S, Cohen V, Bueso-Ramos C. Thrombopoietic growth factors and cytokines. Curr Hematol Rep. 2005;4:137-144. [PubMed] [Google Scholar]
- 50. Kuter DJ. Whatever happened to thrombopoietin. Transfusion. 2002;42:279-283. [DOI] [PubMed] [Google Scholar]
- 51. Kaushansky K. Molecular mechanisms of thrombopoietin signaling. J Thromb Haemost. 2009;7:235-238. [DOI] [PubMed] [Google Scholar]
- 52. Ikeda Y, Miyakawa Y. Development of thrombopoietin receptor agonists for clinical use. J Thromb Haemost. 2009;7:239-244. [DOI] [PubMed] [Google Scholar]
- 53. Kaushansky K. Hematopoietic growth factor mimetics. Ann New York Acad Sci. 2001;938:131-138. [DOI] [PubMed] [Google Scholar]
- 54. Dower WJ, Cwirla SE, Balasubramanian P, Schatz PJ, Baccanari DP, Barrett RW. Peptide agonists of the thrombopoietin receptor. Stem Cells. 1998;16:21-29. [DOI] [PubMed] [Google Scholar]
- 55. Bussel JB, Buchanan GR, Nugent DJ, et al. A randomized, double-blind study of romiplostim to determine its safety and efficacy in children with immune thrombocytopenia (ITP). Blood. 2011;118:28-36. [DOI] [PubMed] [Google Scholar]
- 56. Bohn J-P, Steurer M. Current and evolving treatment strategies in adult immune thrombocytopenia. Memo. 2018;11:241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Moussa MM, Mowafy N. Preoperative use of romiplostim in thrombocytopenic patients with chronic hepatitis C and liver cirrhosis. J Gastroenterol Hepatol. 2013;28:335-341. [DOI] [PubMed] [Google Scholar]
- 58. Bussel JB, Cheng G, Saleh M, et al. Analysis of bleeding in patients with immune thrombocytopenic purpura (ITP): a randomized, double-blind, placebo-controlled trial of eltrombopag, an oral platelet growth factor. Am Soc Hematology. https://www.semanticscholar.org/paper/Analysis-of-Bleeding-in-Patients-with-Immune-(ITP)%3A-Bussel-Cheng/a9364beb42293b169ffcb60f3c01c5330e9463b1. Updated 2006.
- 59. Afdhal NH, Dusheiko GM, Giannini EG, et al. Eltrombopag increases platelet numbers in thrombocytopenic patients with HCV infection and cirrhosis, allowing for effective antiviral therapy. Gastroenterology. 2014;146:442.e1-452.e1. [DOI] [PubMed] [Google Scholar]
- 60. Bussel JB, de Miguel PG, Despotovic JM, et al. Eltrombopag for the treatment of children with persistent and chronic immune thrombocytopenia (PETIT): a randomised, multicentre, placebo-controlled study. Lancet Haematol. 2015;2:e315-e325. [DOI] [PubMed] [Google Scholar]
- 61. Grainger JD, Locatelli F, Chotsampancharoen T, et al. Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;386:1649-1658. [DOI] [PubMed] [Google Scholar]
- 62. Terrault N, Chen Y-C, Izumi N, et al. Avatrombopag before procedures reduces need for platelet transfusion in patients with chronic liver disease and thrombocytopenia. Gastroenterology. 2018;155:705-718. [DOI] [PubMed] [Google Scholar]
- 63. Terrault NA, Hassanein T, Howell CD, et al. Phase II study of avatrombopag in thrombocytopenic patients with cirrhosis undergoing an elective procedure. J Hepatol. 2014;61:1253-1259. [DOI] [PubMed] [Google Scholar]
- 64. Tateishi R, Seike M, Kudo M, et al. A randomized controlled trial of lusutrombopag in Japanese patients with chronic liver disease undergoing radiofrequency ablation. J Gastroenterol. 2018;54:171-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sakamaki A, Watanabe T, Abe S, et al. Lusutrombopag increases hematocytes in a compensated liver cirrhosis patient. Clin J Gastroenterol. 2017;10:261-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sato S, Miyake T, Kataoka M, et al. Efficacy of repeated lusutrombopag administration for thrombocytopenia in a patient scheduled for invasive hepatocellular carcinoma treatment. Intern Med. 2017;56:2887-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fujita M, Abe K, Hayashi M, Okai K, Takahashi A, Ohira H. Two cases of liver cirrhosis treated with lusutrombopag before partial splenic embolization. Fukushima J Med Sci. 2017;63:165-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fukushima-Shintani M, Suzuki K, Iwatsuki Y, et al. AKR-501 (YM477) a novel orally-active thrombopoietin receptor agonist. Eur J Haematol. 2009;82:247-254. [DOI] [PubMed] [Google Scholar]
- 69. Fukushima-Shintani M, Suzuki K, Iwatsuki Y, et al. AKR-501 (YM477) in combination with thrombopoietin enhances human megakaryocytopoiesis. Exp Hematol. 2008;36:1337-1342. [DOI] [PubMed] [Google Scholar]
- 70. Kano T, Fukuhara T, Katsube T. Pharmaceutical composition containing a compound having a thrombopoietin receptor agonistic activity (Google Patents). https://patents.justia.com/inventor/takahiro-fukuhara. Updated 2016.
- 71. Shirley M. Avatrombopag: first global approval. Drugs. 2018;78:1163-1168. [DOI] [PubMed] [Google Scholar]
- 72. Abe M, Suzuki K, Sakata C, et al. Pharmacological profile of AS1670542, a novel orally-active human thrombopoietin receptor agonist. Eur J Pharmacol. 2011;650:58-63. [DOI] [PubMed] [Google Scholar]
- 73. Kuter DJ, Allen LF. Avatrombopag, an oral thrombopoietin receptor agonist: results of two double-blind, dose-rising, placebo-controlled phase 1 studies. Br J Haematol. 2018;183:466-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bussel JB, Kuter DJ, Aledort LM, et al. A randomized trial of avatrombopag, an investigational thrombopoietin-receptor agonist, in persistent and chronic immune thrombocytopenia. Blood. 2014;123:3887-3894. [DOI] [PubMed] [Google Scholar]
- 75. Terrault N, Chen YC, Izumi N, et al. Avatrombopag before procedures reduces need for platelet transfusion in patients with chronic liver disease and thrombocytopenia. Gastroenterology. 2018;155:705-718. [DOI] [PubMed] [Google Scholar]
- 76. Michelson AD, Smolensky Koganov E, Forde EE, Carmichael SL, Frelinger AL., III Avatrombopag increases platelet count but not platelet activation in patients with thrombocytopenia resulting from liver disease. J Thromb Haemost. 2018;16:2515-2519. [DOI] [PubMed] [Google Scholar]
- 77. Jurczak W, Chojnowski K, Mayer J, et al. Phase 3 randomised study of avatrombopag, a novel thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia. Br J Haematol. 2018;183:479-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nomoto M, Pastino G, Rege B, Aluri J, Ferry J, Han D. Pharmacokinetics, pharmacodynamics, pharmacogenomics, safety, and tolerability of avatrombopag in healthy Japanese and White subjects. Clin Pharmacol Drug Dev. 2018;7:188-195. [DOI] [PubMed] [Google Scholar]
- 79. Nomoto M, Zamora CA, Schuck E, et al. Pharmacokinetic/pharmacodynamic drug–drug interactions of avatrombopag when coadministered with dual or selective CYP2C9 and CYP3A interacting drugs. Br J Clin Pharmacol. 2018;84:952-960. [DOI] [PMC free article] [PubMed] [Google Scholar]